Summary
Background
Early prediction of childhood type 1 diabetes reduces ketoacidosis at diagnosis and provides opportunities for disease prevention. However, only highly efficient approaches are likely to succeed in public health settings. We sought to identify efficient strategies for initial islet autoantibody screening in children younger than 15 years.
Methods
We harmonised data from five prospective cohorts from Finland (DIPP), Germany (BABYDIAB), Sweden (DiPiS), and the USA (DAISY and DEW-IT) into the Type 1 Diabetes Intelligence (T1DI) cohort. 24 662 children at high risk of diabetes enrolled before age 2 years were included and followed up for islet autoantibodies and diabetes until age 15 years, or type 1 diabetes onset, whichever occurred first. Islet autoantibodies measured included those against glutamic acid decarboxylase, insulinoma antigen 2, and insulin. Main outcomes were sensitivity and positive predictive value (PPV) of detected islet autoantibodies, tested at one or two fixed ages, for diagnosis of clinical type 1 diabetes.
Findings
Of the 24 662 participants enrolled in the Type 1 Diabetes Intelligence cohort, 6722 total were followed up to age 15 years or until onset of type 1 diabetes. Type 1 diabetes developed by age 15 years in 672 children, but did not develop in 6050 children. Optimal screening ages for two measurements were 2 years and 6 years, yielding sensitivity of 82% (95% CI 79–86) and PPV of 79% (95% CI 75–80) for diabetes by age 15 years. Autoantibody positivity at the beginning of each test age was highly predictive of diagnosis in the subsequent 2–5 99 year or 6–15-year age intervals. Autoantibodies usually appeared before age 6 years even in children diagnosed with diabetes much later in childhood.
Interpretation
Our results show that initial screening for islet autoantibodies at two ages (2 years and 6 years) is sensitive and efficient for public health translation but might require adjustment by country on the basis of population-specific disease characteristics.
Funding
Juvenile Diabetes Research Foundation.
Introduction
Islet autoantibodies are useful biomarkers of future type 1 diabetes, although the time from the appearance of autoimmunity to clinical diagnosis is highly variable (ie, from weeks to decades). In young children, especially those younger than 6 years, many studies have shown that most diabetic ketoacidosis at onset of type 1 diabetes can be prevented by surveillance of islet autoantibodies, with subsequent patient education and monitoring of deteriorating glucose metabolism.1,2 Prevention therapy to delay the clinical onset of type 1 diabetes in people with islet autoantibodies was also shown to be successful in a trial of teplizumab.3 The prospective study of children at preclinical stages of type 1 diabetes is also essential to refine markers of progression, and to better understand disease mechanisms.
Both genetic screening and islet-autoantibody surveillance have become less expensive4,5 and have been shown to be accurate in predicting type 1 diabetes.6,7 However, substantial challenges must be met before public health adoption of population-wide prediction of paediatric type 1 diabetes. To prevent the most severe cases of diabetic ketoacidosis and to provide opportunity for prevention therapy to delay the onset of clinical diabetes, islet-autoantibody detection must occur early enough in life to precede the highest incidence period between age 2 years and 15 years. Childhood type 1 diabetes is a severe disease, but its incidence of approximately 1 in 300 children is low enough that decreasing diabetic ketoacidosis, delaying onset of hyperglycaemia, and improving disease course after onset together yield only moderate aggregate medical cost savings. To achieve commensurate low costs for a prediction programme, highly efficient strategies with limited testings are needed.8 Prescreening using advances in genetic risk assessment can greatly improve efficiency4 by defining a high risk subset, but efficient initial islet autoantibody testing also has a key role. Fewer tests inevitably bring sensitivity losses, therefore it is essential to optimise initial screening strategies to maximise sensitivity. After the screening is completed, subsequent follow-up autoantibody surveillance testing with greater specificity might then lead to glycaemic monitoring, education on symptoms to prevent diabetic ketoacidosis, and consideration of prevention therapy.
The Type 1 Diabetes Intelligence (T1DI) study9 offers a uniquely large and harmonised dataset combining several birth-cohort studies that tested children frequently through adolescence. Using T1DI, we sought to identify optimum testing strategies to identify paediatric islet autoantibody to efficiently reveal future risk of type 1 diabetes, ultimately for translation to public health and medical care.
Methods
Study design and cohort
The T1DI prospective cohort study9 incorporated participants from five prospective longitudinal cohorts: the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study;10 the Swedish Diabetes Prediction in Skåne Study (DiPiS);11 the Diabetes Autoimmunity Study in the Young (DAISY) from Colorado, USA;12 the Diabetes Evaluation in Washington (DEW-IT) from Washington State, USA;13 and BABYDIAB from Germany.14 Local institutional review board approval, parental informed consent, and child assent when relevant, were obtained for all study participants. Key eligibility criteria were elevated genetic risk of type 1 diabetes per study requirements, and first screening by age 2·5 years; full eligibility details have been described previously.9 All DIPP, DiPiS, and DEW-IT, and some DAISY participants underwent HLA screening before enrolment, and all BABYDIAB and some DAISY children were enrolled on the basis of having a first degree relative with type 1 diabetes. Because of enrolment of children with relatives with type 1 diabetes, and because low-resolution HLA genotyping was used in some centres, the resultant combined cohort, although weighted towards HLA genotypes conferring high risk of type 1 diabetes, also contained some individuals with lower HLA risk. To analyse this broad variation in underlying risk, four HLA genetic risk groups were defined for T1DI,9 on the basis of published odds ratios (ORs) for type 1 diabetes (appendix p 10). In summary, 1163 children were in group A (very high risk; OR>50), 3678 in group B (high risk; OR 10–50), 951 in group C (slightly elevated risk; OR 3–9·99), 911 in group D (average to very low risk; OR <3), and 19 were unassignable due to ambiguity in HLA typing.9
Screening test
At each screening timepoint, serum or plasma was tested for islet autoantibodies against glutamic acid decarboxylase, insulinoma antigen 2, and insulin using workshop-validated methods as described previously.9–14 Zinc transporter 8 antibodies were not systematically measured in all samples and were not included in this analysis.9 To model the use of islet autoantibodies tested at a specific age, we considered people who had at least one blood sample drawn within a window from 6 months before to 6 months after the specified test age. Within each annual age window, a participant was designated as testing positive, negative, or having no result available. We analysed two screening endpoints. The first endpoint was the presence of more than one of the three islet autoantibodies against glutamic acid decarboxylase, insulinoma antigen 2, and insulin (these individuals were deemed to have multiple islet autoantibodies). The second endpoint was the presence of any autoantibody, including those with single or multiple islet autoantibodies. We did tests every year from age 1–14 years, to identify screening ages with the best performance. To maximise testing efficiency, we considered screening at only one or two ages. Because of diagnosis of type 1 diabetes being made earlier in life, the number of participants followed up at older ages was less than those followed up earlier. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used as time-dependent metrics to establish screening at each age.15 If a participant had not been diagnosed with diabetes in the specified age period, they were placed in the control group regardless of their diabetes status after 15 years of age. Given that compliance with testing varied with age, a meaningful comparison of several age combinations required the calculation of cumulative sensitivity,15 in which children with diabetes with missing tests were included in the denominator of sensitivity calculations. These sensitivities are herein termed comparative sensitivity. However, once an optimum strategy was chosen, its true sensitivity among children completing the testing and observation was also calculated and referred to as the observed sensitivity.
Outcomes
Our primary outcomes were the sensitivity and positive predictive value (PPV) of detected islet autoantibodies, tested at one or two fixed ages. In addition, to identify whether optimal screening performed differently for differing HLA risk levels for type 1 diabetes, the higher risk groups A plus B were combined and were compared with the combined lower risk groups C plus D. To test whether results from the combined T1DI cohort apply to different populations, we compared results between the Finnish DIPP study10 and the Colorado DAISY study,12 the two largest T1DI subcohorts from different continents. We used the any islet autoantibodies endpoint to calculate the comparative sensitivity of two-age screening at varying ages.
Statistical analysis
Inverse-probability censoring weighting was applied to data from all 24 662 participants (appendix p 2) to account for non-random loss to follow-up.16 Each 95% CI was computed as mean ± (SD × 1·96) from 1000 total runs using a bootstrap resampling technique selecting a random sample of participants with replacement. Two-sided t tests were used for comparisons. The bootstrap method was also used to objectively identify the best screening-age pairs. Using a 10 000-run bootstrap set for each screening-age pair, we computed sensitivity and PPV. The best pair was defined as having the minimum distance (1 − sensitivity)2 + (1 − PPV)2 to the point representing 1 0 sensitivity and 1 0 PPV at the top-left corner of a sensitivity versus 1 − PPV plot, an approach analogous to the Youden index in standard receiver operating characteristic curve (ROC) analysis.17 Pearson correlation was used to assess the relationship between the age at seroconversion and onset age.
Observed sensitivity of the optimum screening strategy was high. To compare performance of various two-age screening pairs in a dataset with variable numbers of participants tested at each specified age, comparative sensitivities (using the cumulative sensitivity method)15 were calculated by a formula in which cases with missing tests were included in the denominator even though the testing strategy could not be applied to those cases. This strategy was necessary to allow comparison of screening results from all age pairs. However, this method led to lower sensitivity estimates than the directly-observed sensitivity, which considered only participants actually tested at the specified ages. Therefore, the optimum strategy identified by comparative methods was then assessed by direct observation.
Role of the funding source
The non-profit funder supported the creation of the harmonised T1DI dataset and our data analyses. An employee of the funder served on the team that analysed and interpreted the data and wrote the report.
Results
A total of 24662 participants were followed up from early childhood (DIPP n=11 652 [47%] of 24662; DiPiS n=4359 [18%]; DAISY n=2539 [10%]; DEW-IT n=3748 [15%]; BABYDIAB n=2364 [10%]) and of these 6722 total (3605 female participants) were followed up to age 15 years or until onset of type 1 diabetes. These participants included 6050 children who did not develop type 1 diabetes and 672 who were diagnosed with type 1 diabetes by age 15 years (appendix p 3). Overall, about 74% of the cohort did not have a first-degree relative with type 1 diabetes (appendix p 11). A median of 18 (IQR 14–24) samples per participant were analysed for islet autoantibodies. The median age at first test was 4·2 months (2·4–9·6), the median follow-up time was 15·4 years (15·0–17·5), and the median age for first appearance of islet autoantibodies was 4 5 years (2·0–8·6). For the prediction of diabetes, we found that screening at two ages was better than screening at a single age and that the presence of any islet autoantibodies had greater comparative sensitivity but lower PPV than the presence of multiple islet autoantibodies. The comparative sensitivities and PPVs of multiple islet autoantibodies and any islet autoantibodies are shown for all ages from 1 year to 14 years and for all two-age combinations within this range (figure 1). Corresponding specificities and NPVs for these age combinations are shown in the appendix (pp 5–6). Not surprisingly, a more extensive two-age strategy was more sensitive than screening at only one age, with the same PPV at the maximum sensitivity. This finding was true for both the multiple islet autoantibodies (51% vs 33% comparative sensitivity) and any islet autoantibodies (66% vs 46%; figure 1). For two-age screening, at ages 2 years and 6 years, the comparative sensitivity of 66% (95% CI 63–69) for any islet autoantibodies was relatively high, and came with a PPV of 54% (51–58; appendix p 11). This result contrasts with the strategy of using multiple islet autoantibodies at the same ages, whose lower comparative sensitivity of 51% (47–54) came with a higher PPV of 74% (69–78). This tradeoff is expected and is often observed when comparing a lower stringency test to one of higher stringency.
Figure 1: Comparative sensitivity and PPV for any islet autoantibodies and multiple islet autoantibodies endpoints.
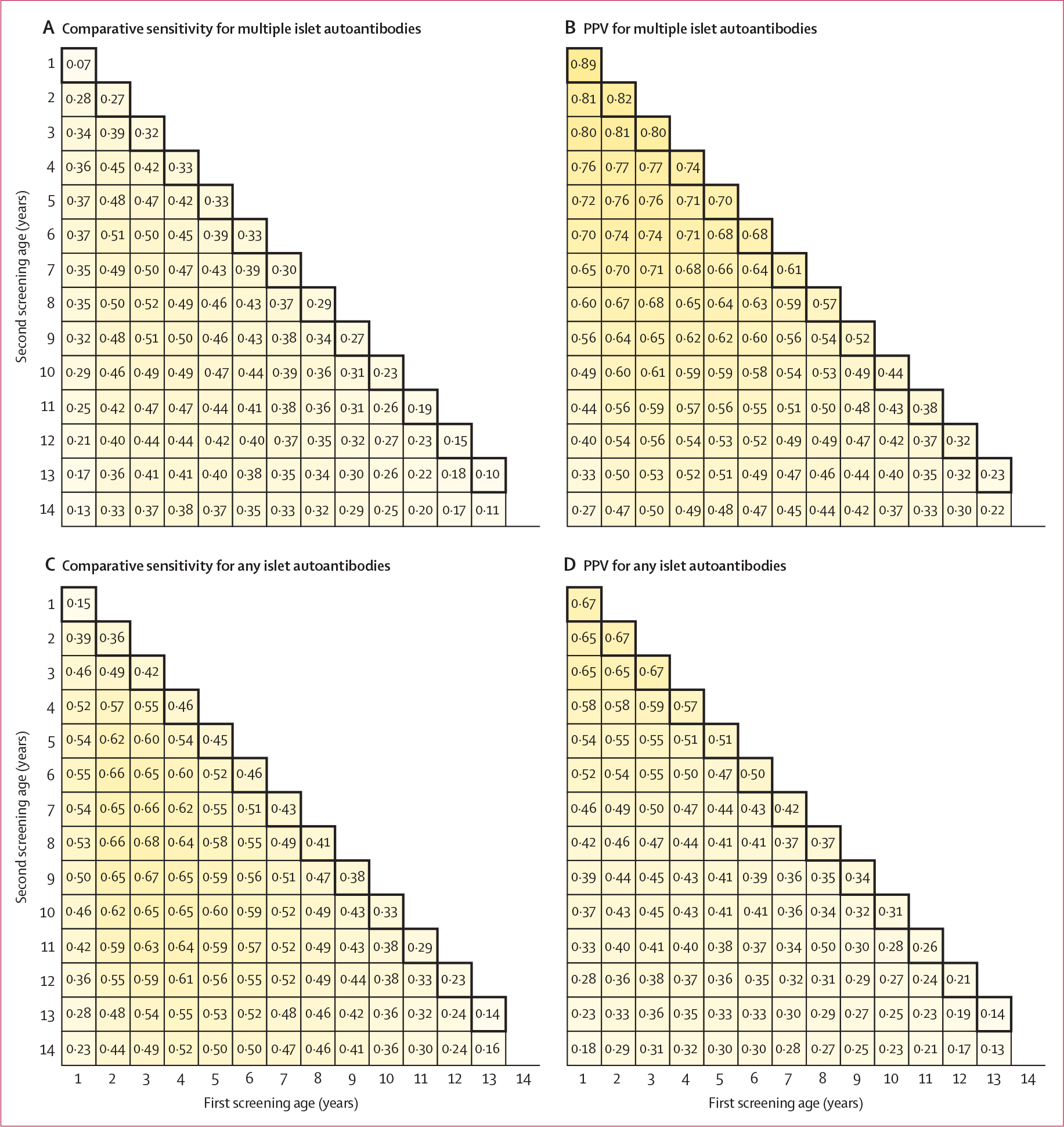
Comparative sensitivity (A) and PPV (B) from screening multiple islet autoantibodies at two ages for type 1 diabetes risk by age 15 years in the entire T1DI cohort. Comparative sensitivity (C) and PPV (D) from screening of any islet autoantibodies for risk of type 1 diabetes by age 15 years in the entire T1DI cohort. The comparative analyses herein were used to identify optimum screening age or ages for later evaluation by direct observation. The diagonal numbers highlighted within black squares represent performance of screening at a single age. For example, in panel C, the comparative sensitivity of a result from screening of any islet autoantibodies at one age (4 years) was 46% (95% CI 43–49), whereas comparative sensitivity from screening at two ages (2 years and 6 years) was 66% (63–69). PPV=positive predictive value. T1DI=Type 1 Diabetes Intelligence.
For both multiple islet autoantibodies and any islet autoantibodies, our analyses indicated the age optimum for a single screening was 4 years, whereas for a two-age screening there appeared to be broad optimum sensitivity with first screening at ages 2–4 years and second screening at ages 6–9 years (figure 1). PPVs were best at the youngest ages within these ranges, and choosing the youngest screening ages within the optimum range also enabled the screening to be undertaken earlier in life to precede more cases of type 1 diabetes onset. We used bootstrap internal replication and an approach using plots similar to ROC curves for further confirmation of optimum two-age screening pairs (figure 2A). These analyses identified 2 years and 6 years as the best ages for two-age screening in our cohort (figure 2B).
Figure 2: Bootstrap replication for optimal screening ages.
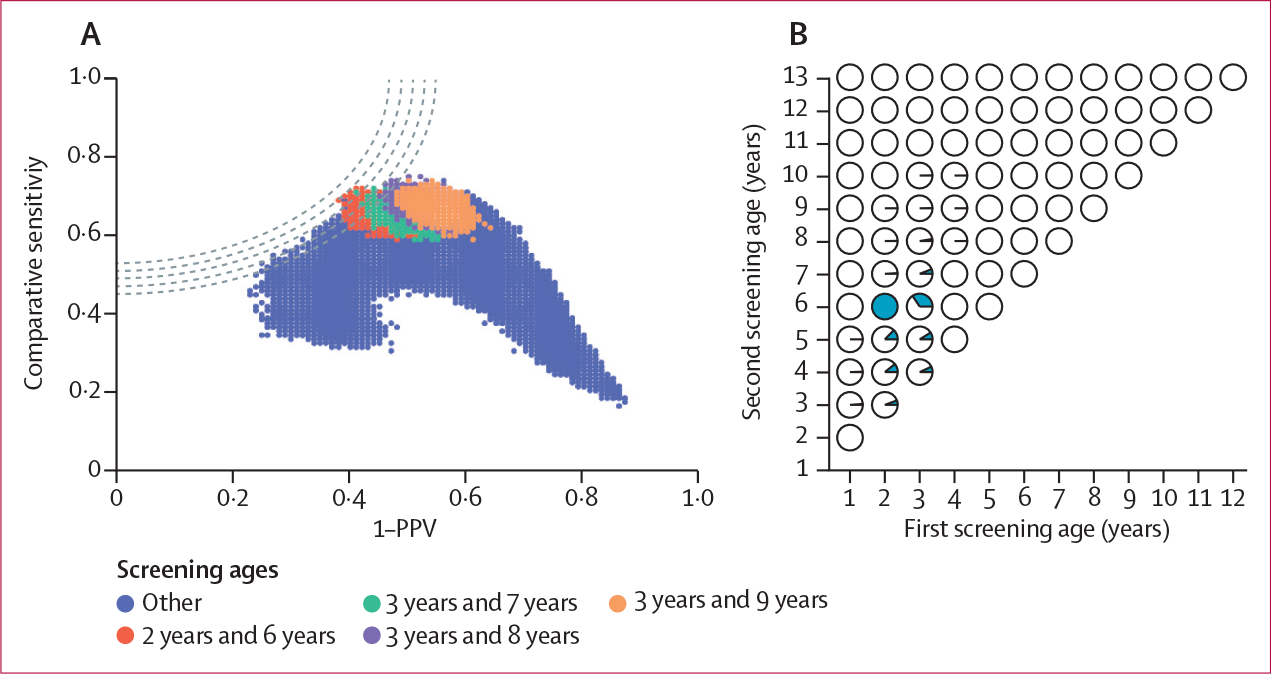
(A) Analyses of optimal ages for screening of any islet autoantibodies for risk of type 1 diabetes by age 15 years in the T1DI cohort using bootstrap internal replication for each age pair. The four testing-age combinations with the greatest comparative sensitivities are shown. Testing at ages 2 years and 6 years performed best (smallest distance to the upper-left corner of the plot). (B) Using the testing age pair of 2 years and 6 years from panel A as a reference, we compared all other age combinations using internal bootstrapping replication. For each age combination, the dark area of the pie chart represents the proportion of screening tests resulting in a distance to the top-left corner in panel A at or below that of the age 2-year and 6-year test pair. No other combination was superior to testing at age 2 years and 6 years. T1DI=Type 1 Diabetes Intelligence.
We found that comparative sensitivity of the screening did not change on the basis of HLA risk of the underlying population and that the differences in optimum screening ages between these two groups were not significant. For example, screening at ages 2 years and 6 years for any islet autoantibodies had comparative sensitivity to detect 67% (95% CI 64–70) of cases of type 1 diabetes for HLA groups A plus B versus 64% (57–71) for HLA groups C plus D (figure 3A; appendix p 7). PPV was greater for HLA groups A plus B at 59% (54–63) than for HLA groups C plus D at 45% (38–53; appendix p 11).
Figure 3: Comparison of performance metrics by HLA background and study cohort.
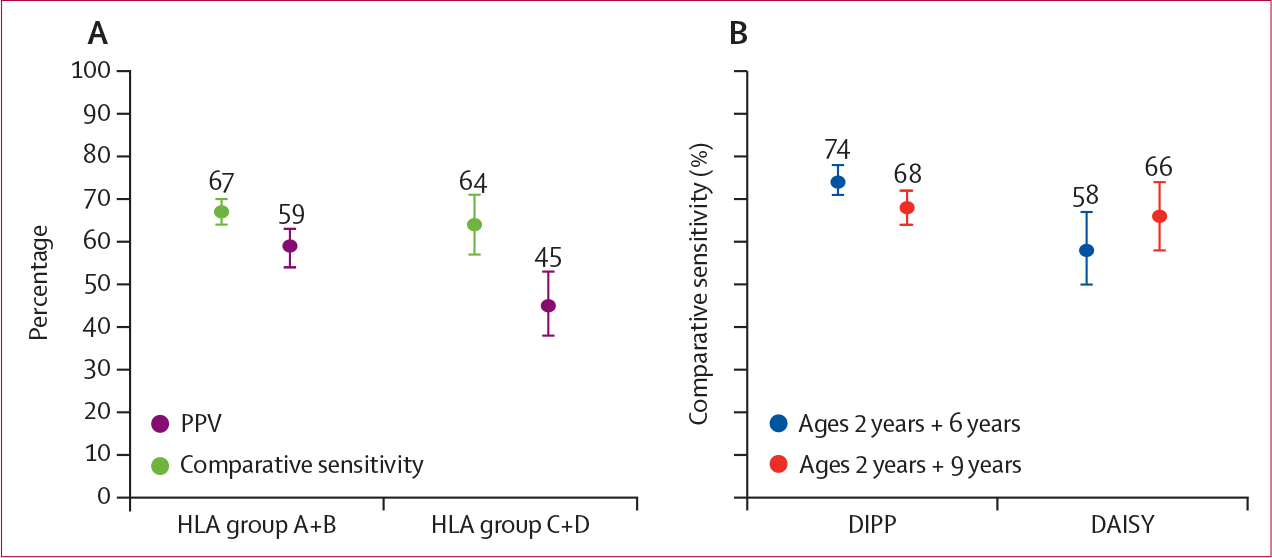
(A) Comparative sensitivity (green) and PPV (purple) with 95% CIs of any islet antibodies at ages 2 years and 6 years for high-risk combined HLA groups A plus B versus lower-risk combined HLA groups C plus D. (B) Comparative sensitivity with 95% CIs of any islet autoantibodies with a two-age strategy using different age pairs and different study populations. Maximum sensitivity was at age pair 2 years and 6 years (blue) for DIPP (Finland), but age 2 years and 9 years (red) for DAISY (Colorado, USA). PPV=positive predictive value. DIPP=Diabetes Prediction and Prevention. DAISY=Diabetes Autoimmunity Study in the Young.
Prediction performance differed by country. In DIPP participants, comparative sensitivity was highest (74%, 95% CI 71–78) at screening ages 2 years and 6 years with a 57% PPV (54–61; figure 3B; appendix p 8, 11). At ages 2 years and 6 years, DAISY data showed lower comparative sensitivity (58%, 50–67) at a similar 58% (49–67) PPV. For DAISY, prediction with any islet autoantibodies appeared to perform better at ages 2 years and 9 years, with sensitivity of 66% (58–74) and a 54% (45–62) PPV.
We first displayed observed risk by islet autoantibodies result (negative for islet autoantibodies, not tested in the age window, one islet autoantibody present, or multiple islet autoantibodies present). A single islet autoantibody at age 2 years indicated a 31% (95% CI 23–39) 4-year risk of developing type 1 diabetes by age 5·99 years, whereas multiple islet autoantibodies at age 2 years carried a 55% (50–62) 4-year risk (figure 4A). A single islet autoantibody at age 6 years conferred, over the next 9 years, an overall risk of 39% (95% CI 29–48) if the islet autoantibody test at age 2 years was negative, but an overall weight risk of 70% (95% CI 63–76) if testing at age 2 years detected any islet autoantibodies. Of participants developing only one persistent islet autoantibody in the T1DI cohort, the majority developed type 1 diabetes. Multiple islet autoantibodies at age 6 years indicated an overall risk of 83% (78–88) by age 15 years regardless of islet autoantibody status at age 2 years (figure 4B). Finally, the observed sensitivity of various screening strategies and onset intervals of type 1 diabetes is summarised in figure 4C, for children actually tested at the indicated ages and followed up to age 15 years. Each screening test (at age 2 years or age 6 years) was highly sensitive for onset of type 1 diabetes in the time interval following the test, with sensitivity of 82% (79–86) and PPV of 79% (75–80) of the combined screening ages of 2 years plus 6 years to detect onset of type 1 diabetes occurring between ages 2 years and 15 years.
Figure 4: Directly observed development of type 1 diabetes by islet autoantibody test results and by test ages and outcome horizons.
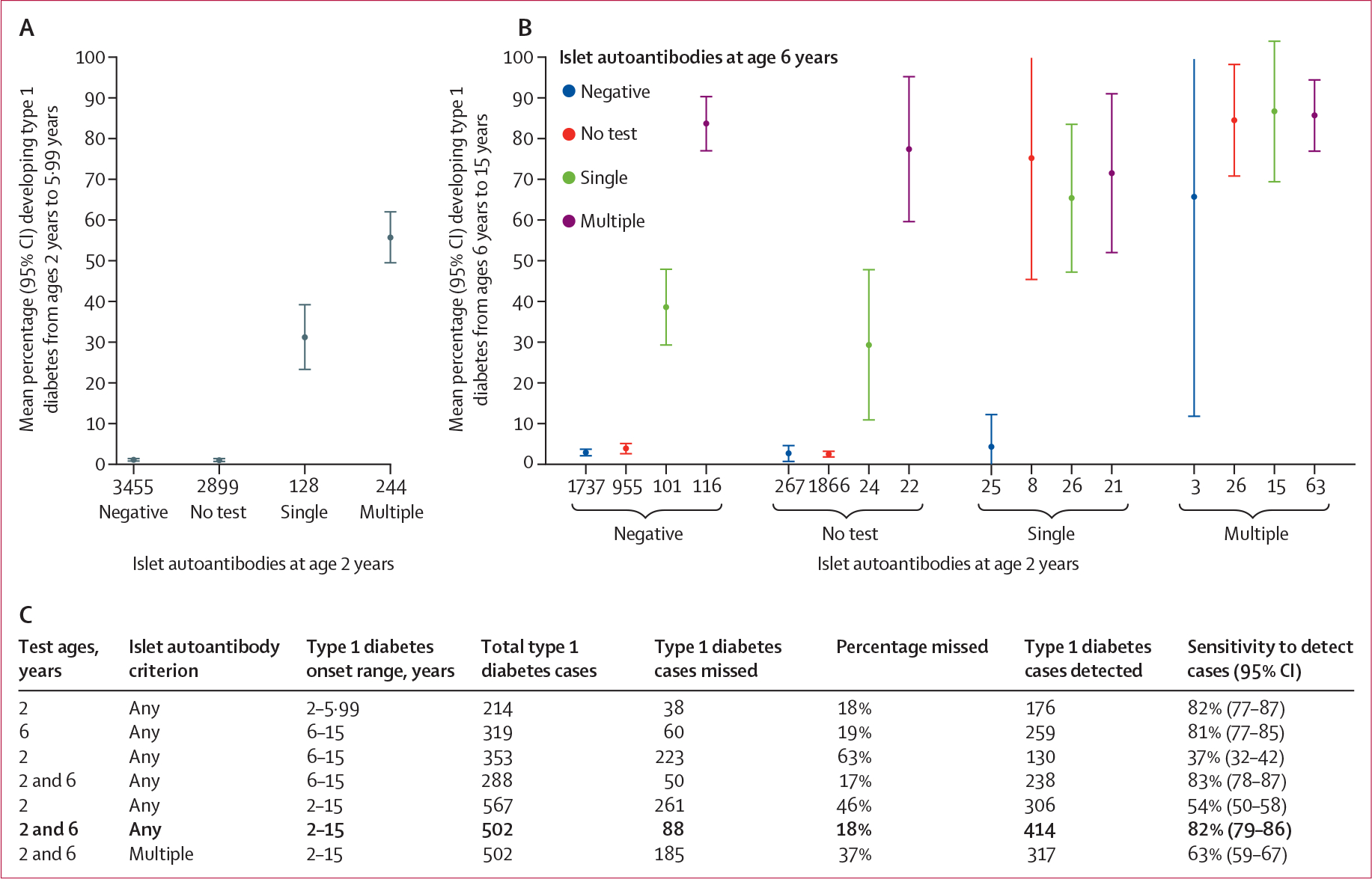
(A) Observed mean proportion of children with the indicated number of islet autoantibodies who developed type 1 diabetes between the ages of 2 years and 5·99 years. (B) Observed mean proportion of children with the indicated number of islet autoantibodies who developed type 1 diabetes between the ages of 6 years and 15 years. This analysis considers only participants observed for the entire disease-onset range shown. (C) Observed sensitivity to detect cases of type 1 diabetes among the screened cohort, considering only participants tested at the indicated ages and observed to age 15 years. The observed PPV of any islet autoantibodies tested at ages 2 years and 6 years (bolded line) was 79% (95% CI 75–80). PPV=positive predictive value.
Progression from islet autoantibodies to type 1 diabetes was slower for onset at older ages. We sought to understand why early testing (at ages 2 years and 6 years) was so efficient at detecting most children developing type 1 diabetes much later, up to age 15 years. Analysis of the T1DI dataset indicated that as age at type 1 diabetes diagnosis increases, the average time interval between islet autoantibody seroconversion and type 1 diabetes diagnosis increases substantially (p<0 0001; appendix p 9). Autoantibodies often appeared early in life even in people progressing slower to late-childhood onsets. This effect is present throughout the peak years of type 1 diabetes incidence as indicated by annual incident cases (appendix p 9).
Discussion
Early detection of islet autoantibodies in children has been widely shown to prevent diabetic ketoacidosis at diagnosis1,2 and provides an opportunity to apply prevention therapies.3 For this reason, islet-autoantibody screening strategies have been extensively studied in paediatric populations.18,19 However, the relatively low prevalence of type 1 diabetes makes it difficult to accomplish paediatric screening at a cost acceptable for public health translation.8,20 The combined T1DI cohort has a large sample size, several measurements per individual, and a long follow-up to allow comprehensive evaluation of several testing strategies. We found that screening at only two ages (2 years and 6 years) in childhood can identify a large majority of children who will develop type 1 diabetes by age 15 years. Fewer tests entails lower screening costs and greater accessibility.
Many studies focus on children who are positive for islet autoantibodies as a primary target of detection. However, a major goal of screening is to be sensitive enough to not miss potential cases. The specificity and predictive value then rise during follow-up confirmation and further testing. Of participants developing only one persistent islet autoantibody in the T1DI cohort, the majority were confirmed and persistent, consistent with high confirmation rates among children with single islet autoantibodies described in another study.18 Specificity might also be increased with higher autoantibody titres, higher affinities, or disease-specific epitopes,21 and of course monitoring for glycaemia and early symptoms. The number of paediatric participants in whom follow-up testing is needed is small, and thus not a substantial part of population-wide prediction costs. For example, the German FR1DA study found the prevalence of multiple islet autoantibodies in all children aged 2–5 years to be 0 31% (280 of 90632 children).19 Before these findings, other birth-cohort studies had found that multiple islet autoantibodies were present in 585 (55%) of 1059 individuals with any islet autoantibodies.6 This result implies that the overall prevalence of any islet autoantibodies is estimated to be 0·56% (0·31% divided by 55%), of which nearly half (about 1 in 400 children) have a single islet autoantibody. In our cohort, even a single islet autoantibody at age 2 years marked a high risk of developing future type 1 diabetes. Of children with a single islet autoantibody at age 2 years, two-thirds developed type 1 diabetes by age 15 years. Of children with a single islet antibody first appearing at age 6 years, more than a third had developed type 1 diabetes by age 15 years, a substantial risk for a single autoantibody first appearing in a school-age child. Taken together, these results are consistent with our 79% PPV for type 1 diabetes by age 15 years for any islet autoantibodies in the two-age screening strategy. We believe this PPV is acceptably high for initial paediatric screening, especially when complemented by timely follow-up evaluation.
Screening at young ages carries some advantages. Islet autoantibodies appearing earlier have long been known to indicate a higher risk of type 1 diabetes risk,22,23 and in the T1DI cohort, the youngest age combinations indeed had the highest PPVs. Early screening at age 2 years increased the number of children identified before diagnosis, at which time diabetic ketoacidosis can be prevented and type 1 diabetes prevention therapies can be offered. Likewise, the second test at age 6 years occurs at the end of the largest wave of seroconversions,24 capturing most children who are positive for islet autoantibodies early enough to offer prevention therapy. Previous studies suggest that parental anxiety is usually not increased when a child is at increased genetic risk;25 parental anxiety does increase when children are positive for islet autoantibodies, but then decreases towards baseline over time.26
Importantly, we observed similar screening sensitivity across a spectrum of high and moderate HLA risk, implying that the pattern of islet autoantibody development might be sufficiently similar to be amenable to a uniform screening protocol under a variety of genetic risk scenarios.27 Although any genetic prescreening results in loss of some future cases, steadily improving genetic methods (eg, genetic risk scores) have reduced these losses (eg, by selecting the 21% of the paediatric population that contains 89% of the future cases).4 Although we did not do a formal cost-benefit analysis, the most efficient genetic testing methods cost less than a third of the typical cost of sampling and measuring a relevant islet autoantibody panel.28 These relative costs imply that a genetic pretest followed by screening for islet autoantibodies in children with the 21% highest risk twice during childhood might detect most (but not all) future cases at a lower cost than a single cross-sectional islet autoantibodies screening of all children.
Our study has some limitations. The studies comprising our combined dataset had different risk criteria and sampling schedules. Compliance with these schedules also varied, although an average of 18 samplings per child implies good coverage of the surveillance interval in most cases. We did not consider zinc transporter-8 autoantibodies, a test previously noted to provide a small increase in the number of children with multiple antibodies identified during follow-up, but does not add substantially to identifying single autoantibody positivity.6 Our combined cohort included approximately 26% of children with first-degree relatives who had diabetes.9 Children with relatives with type 1 diabetes are known to have on average a younger age at autoantibody appearance, which could make the two-age screening strategy appear to perform better. Further, in our cohort, children who were first-degree relatives of people with diabetes formed a significantly greater proportion of the HLA with higher risk than lower risk, which might have affected the performance of the two-age screening when comparing those groups. Our results have not yet been validated in a separate cohort, because we are not aware of any cohort covering similar testing ages and frequency of follow-up. However, the TEDDY Consortium24 covering similar populations of entirely different children will be ideal for validation in the coming years. Ultimately, replication must occur in general populations unselected for family history of type 1 diabetes (with or without genetic preselection) and especially in populations with greater geographical and ancestral diversity.
Public health implementation in different countries should take into account varying genetic features4,29 and different environmental exposures.30 Environmental exposures might elicit different subtypes of type 1 diabetes,23 a possible explanation for the different optimal screening ages seen in the Finnish DIPP study10 as compared with the Colorado DAISY study.12 Screening ages and strategies must also fit in with health-care systems that vary because of local or regional politics and resources, differing glycaemic monitoring during the presymptomatic period, and different treatment practices at onset. Islet-autoantibody assay quality should be reasonably high, as in the current dataset in which all laboratories took part in periodic international proficiency testing. Regions that implement efficient web-based or cloud-based coordination of testing, reporting, and treatment will also benefit from improved cost efficiency.
We showed that screening at just two early ages detected four of five cases of future childhood type 1 diabetes and might be practical for public health implementation. When used after genetic prescreening, the majority of future cases should be detected, all at a net investment of less than one islet-autoantibody measurement per child. Following children with any islet autoantibodies rather than just multiple islet autoantibodies increases case detection with acceptable predictive value. The screening strategy appears to work in participants with varied HLA background risk, but optimum screening ages differ between countries.
Supplementary Material
Research in context.
Evidence before this study
To search for the evidence published before this study, we searched PubMed using the search terms “pediatric islet autoantibodies”, “public health screening islet autoantibodies”, and “population-based prediction of type 1 diabetes” for articles published between Jan 1, 2012, and May 1, 2022 (date of last search). Most childhood type 1 diabetes cases appear in people with high HLA genetic risk, but about a quarter of cases have lower HLA risk. Islet autoantibodies are known to precede diagnosis and can reveal people at greatest future risk of type 1 diabetes. Knowing islet autoantibodies status in advance can prevent most diabetic ketoacidosis at onset, and immunotherapy applied in individuals who are islet autoantibodies positive can significantly delay onset. Multiple-islet autoantibodies mark the greatest risk as do islet autoantibodies appearing in early childhood. However, autoimmunity might evolve over time, and people initially with single-islet autoantibodies, or with islet autoantibodies appearing later in childhood, can also progress to clinical disease. Most large studies to date have followed up children at high HLA or familial risk via frequent islet autoantibodies testing throughout childhood. Although sensitive and specific, these approaches are not cost-effective for prediction of population-wide type 1 diabetes.
Added value of this study
Our results show that testing at only two ages (2 years and 6 years) is sufficient to detect a large majority of cases occurring by age 15 years. We found that including children who were positive for single-islet autoantibodies provided a key part of this sensitivity, especially children younger than 6 years. Our results, although primarily from children who were prescreened for elevated genetic risk, suggest that this strategy might apply even in those at lower HLA risk. Another key finding was that two-age islet-autoantibody testing might have different optimal ages in different geographical regions.
Implications of all the available evidence
Our results show that an efficient, initial autoantibody testing strategy might be sufficient in predicting type 1 diabetes diagnosis before the age of 15 years and raises the possibility that similar population-wide screening for future type 1 diabetes is possible.
Acknowledgments
This work was supported by funding from the Juvenile Diabetes Research Foundation (IBM, 1-RSC-2017-368-I-X and 1-IND-2019-717-I-X; DAISY, 1-SRA-2019-722-I-X, 1-RSC-2017-517-I-X, and 5-ECR-2017-388-A-N; DiPiS, 1-SRA-2019-720-I-X and 1-RSC-2017-526-I-X; DIPP, 1-RSC-2018-555-I-X and 1-SRA-2019-721-I-X; DEW-IT, 1-RSC-2017-516-I-X and 1-SRA-2019-719-I-X).
Footnotes
Declaration of interests
MG and VA are employees of IBM. JLD did this work as an employee of the Juvenile Diabetes Research Foundation and is now an employee of Janssen Research and Development. All other authors declare no competing interests.
The Type 1 Diabetes Intelligence study group
Anette G Ziegler, Ezio Bonifacio, Peter Achenbach, Christiane Winkler, Marian Rewers, Brigitte I Frohnert, Jill Norris, Andrea Steck, Kathleen Waugh, Liping Yu, William A Hagopian, Michael Killian, Claire Crouch, Jocelyn Meyer, Shreya Roy, Åke Lernmark, Helena Elding Larsson, Markus Lundgren, Marlena Maziarz, Lampros Spiliopoulos, Josefin Jönsson, Riitta Veijola, Jorma Toppari, Jorma Ilonen, Mikael Knip, Vibha Anand, Mohamed Ghalwash, Kenney Ng, Zhiguo Li, Harry Stravopolous, Eileen Koski, Ashwani Malhotra, Shelley Moore, Jianying Hu, Jessica Dunne, Bin Liu, Ying Li, Olivia Lou, Frank Martin.
Contributor Information
Mohamed Ghalwash, Center for Computational Health, IBM Research, Yorktown Heights, NY, USA; Faculty of Science, Ain Shams University, Cairo, Egypt.
Jessica L Dunne, Juvenile Diabetes Research Foundation, New York, NY, USA.
Markus Lundgren, Department of Clinical Sciences Malmö, Lund University/Clinical Research Centre, Skåne University Hospital, Malmö, Sweden.
Marian Rewers, Barbara Davis Center for Diabetes, University of Colorado, Denver, CO, USA.
Anette-G Ziegler, Forschegruppe Diabetes and Institute of Diabetes Research, Helmholtz Zentrum München, German Research Centre for Environmental Health, Munich-Neuherberg, Germany der TU München, Munich, Germany.
Vibha Anand, Center for Computational Health, IBM Research, Yorktown Heights, NY, USA.
Jorma Toppari, Institute of Biomedicine, Research Centre for Integrative Physiology and Pharmacology, and Centre for Population Health Research, University of Turku, Turku, Finland; Department of Paediatrics, Turku University Hospital, Turku, Finland.
Riitta Veijola, Department of Paediatrics, PEDEGO Research Unit, University of Oulu and Oulu University Hospital, Oulu, Finland.
William Hagopian, Pacific Northwest Research Institute, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Data sharing
The data supporting these study findings are available through the Type 1 Diabetes Intelligence Consortium upon reasonable request to the corresponding author. The data are not publicly available because of privacy regulations.
References
- 1.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004; 27: 1399–404. [DOI] [PubMed] [Google Scholar]
- 2.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes 2014; 15: 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herold K, Bundy B, Krischer J, et al. Teplizumab in relatives at risk for type 1 diabetes. N Engl J Med 2019; 381: 1879–81. [DOI] [PubMed] [Google Scholar]
- 4.Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 2019; 42: 200–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amoroso M, Achenbach P, Powell M, et al. 3 Screen islet cell autoantibody ELISA: A sensitive and specific ELISA for the combined measurement of autoantibodies to GAD65, to IA-2 and to ZnT8. Clin Chim Acta Int J Clin Chem 2016; 462: 60–64. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013; 309: 2473–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrat L, Vehik K, Sharp S, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 2020; 26: 1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meehan C, Fout B, Ashcraft J, et al. Screening for type 1 diabetes risk to reduce diabetic ketoacidosis is not economically viable. Pediatr Diabetes 2015; 16: 565–72. [DOI] [PubMed] [Google Scholar]
- 9.Anand V, Li Y, Liu B, et al. Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: joint analyses of prospective cohort studies in Finland, Germany, Sweden, and the US. Diabetes Care 2021; 44: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kupila A, Muona P, Simell T, et al. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001; 44: 290–97. [DOI] [PubMed] [Google Scholar]
- 11.Larsson HE. A Swedish approach to the prevention of type 1 diabetes. Pediatr Diabetes 2016; 17: 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996; 39: 807–12. [DOI] [PubMed] [Google Scholar]
- 13.Wion E, Brantley M, Stevens J, et al. Population-wide infant screening for HLA-based type 1 diabetes risk via dried blood spots from the public health infrastructure. Ann NY Acad Sci 2003; 1005: 400–03. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler AG, Hummel M, Schenker M, et al. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999; 48: 460–68. [DOI] [PubMed] [Google Scholar]
- 15.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol 2017; 17: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vock DM, Wolfson J, Bandyopadhyay S, et al. Adapting machine learning techniques to censored time-to-event health record data: a general-purpose approach using inverse probability of censoring weighting. J Biomed Inform 2016; 61: 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr 2007; 96: 644–47. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen C, Rewers M, Baxter J, et al. Population screening for type 1 diabetes and celiac disease—Autoimmunity Screening for Kids (ASK). Diabetes 2018; 67 (suppl 1): 182.29208633 [Google Scholar]
- 19.Ziegler A-G, Kick K, Bonifacio E, et al. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 2020; 323: 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQueen RB, Geno Rasmussen C, Waugh K, et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care 2020; published online April 23. DOI: 10.2337/dc19-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So M, Speake C, Steck A, et al. Advances in type 1 diabetes prediction using islet autoantibodies: beyond a simple count. Endo Rev 2021. 42: 584–604. [DOI] [PubMed] [Google Scholar]
- 22.Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes. Diabetes Care 2011; 34: 1397–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifacio E, Weiss A, Winkler C, et al. An age-related exponential decline in the risk of multiple islet autoantibody seroconversion during childhood. Diabetes Care 2021; 44: 2260–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krischer JP, Lynch KF, Lernmark Å, et al. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017; 40: 1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson S, Baughcum A, Carmichael S, et al. Maternal anxiety associated with newborn genetic screening for type 1 diabetes. Diabetes Care 2004; 27: 392–97. [DOI] [PubMed] [Google Scholar]
- 26.Johnson SB, Lynch KF, Roth R, et al. My child is islet autoantibody positive: impact on parental anxiety. Diabetes Care 2017; 40: 1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilonen J, Kiviniemi M, Lempainen J, et al. Genetic susceptibility to type 1 diabetes in childhood estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity. Pediatr Diabetes 2016; 17 (suppl 22): 8–16. [DOI] [PubMed] [Google Scholar]
- 28.Locke JM, Latten MJ, Datta RY, et al. Methods for quick, accurate and cost-effective determination of the type 1 diabetes genetic risk score (type 1 diabetes-GRS). Clin Chem Lab Med 2020; 58: e102–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagopian W, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421,000 infants. Pediatr Diabetes 2011; 12: 733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vehik K, Lynch KF, Wong MC, et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med 2019; 25: 1865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting these study findings are available through the Type 1 Diabetes Intelligence Consortium upon reasonable request to the corresponding author. The data are not publicly available because of privacy regulations.


