Abstract
Background
Diastasis rectus abdominis (DRA) involves the separation of the midline abdominal muscles and linea alba and affects more than half of postpartum women. This study aimed to assess the effect of a split tummy exercise program (STEP) on DRA closure in postpartum mothers.
Methods
A randomized controlled trial was conducted from 2008 to 2020 at the Obstetrics and Gynaecology Clinic of the Universiti Kebangsaan Malaysia Medical Centre. Primigravida mothers diagnosed with DRA were selected and randomly assigned to the intervention (n=21) or control (n=20) group. The intervention group underwent a home-based STEP consisting of three phases of nine abdominal exercises. DRA size was assessed at baseline and at 8 weeks postpartum using two-dimensional ultrasound.
Results
The mean age of the participants was 28 years (standard deviation, 3.6), with the majority of Malay ethnicity (87.8%) and working mothers (78%). After 8 weeks, the intervention group showed a significant reduction in DRA size of up to 27% (mean difference, 6.17 mm; 95% confidence interval, 3.7–8.7; P<0.001). No significant intergroup DRA changes were observed after 8 weeks of follow-up.
Conclusion
Early postpartum screening for DRA should be advocated to allow early STEP intervention to ensure favorable outcomes. STEP intervention is an effective postnatal training program for managing DRA.
Keywords: Diastasis Recti, Postpartum Period, Exercise Therapy, Randomized Controlled Trial, Intervention
INTRODUCTION
Diastasis rectus abdominis (DRA) affects more than half of postpartum women [1] and involves separation or stretching of the abdominal muscles and linea alba on the anterior abdominal wall in the midline [2]. DRA is diagnosed when the split at any point along its length, exceeds the normal width, i.e., more than a two-finger width by finger palpation [1], with the widest split occurring at 2 cm above the umbilicus [3]. Increased levels of relaxin, progesterone, and estrogen during the first trimester results in relaxation and softening of the muscles around the trunk and pelvis to accommodate the growing fetus [4]. Resolution of DRA occurs at varying rates and stages [5] and natural resolution with maximum recovery may happen days after delivery or up to 6 to 8 weeks postpartum [6,7]. Fifty-three percent of women have DRA at 4–8 weeks and 39% of women still have DRA at 6 months postpartum [1,6,8,9]. DRA may even persist up to 1 year after delivery in 30%–60% of postpartum mothers [10].
Weakening of the abdominal muscles and DRA reduces a mother’s ability to generate force during functional activities such as lifting and bending [7,11], predisposing the trunk and pelvic region to pressurization activities. These eventually contribute to pelvic instability, back pain, pelvic girdle pain, and umbilical hernia [12-14]. Pelvic floor muscle dysfunction may result in stress and urge urinary incontinence, pelvic organ prolapse, urinary frequency, and urgency [13-15].
Conservative management of DRA is preferred over surgery, involving a combination of aerobic exercises, strength training of the extremities, and electrotherapy in hot and cold modalities [16,17]. In many countries, this is part of antenatal and postpartum programs for mothers. Nevertheless, the primary concern in these programs is preventing back pain and urinary incontinence instead of focusing on DRA and the positive effects of postpartum exercise on DRA have been shown [16,18,19]. However, there is no consensus on customized postpartum exercise programs for improving DRA. This study aimed to investigate the effect of a progressive postpartum split tummy exercise program (STEP) on the size of DRA in postpartum mothers. We hypothesized that the STEP group would demonstrate a significant reduction in DRA size compared with the non-intervention group.
METHODS
1. Participant Recruitment and Data Collection
This study was approved by the Universiti Sains Malaysia Institutional Ethics Committee (USM/JEPeM/17090395). This single-blinded, twoarm randomized controlled trial (RCT) conducted at a tertiary teaching hospital, from February 2018 to February 2020, in accordance with the Helsinki Declaration, and was registered in the Thai clinical trial (TCTR20190904005). All participants provided written informed consent prior to data collection.
This study included primigravidae of a non-athletic population, older than 18 years, diagnosed with DRA at 34–40 weeks of gestation, delivering a singleton pregnancy via spontaneous vertex, assisted vaginal breech, or instrumental delivery. Women without DRA, multiple pregnancies, polyhydramnios, uterine fibroids during pregnancy, previous abdominal surgery, caesarean delivery, and known collagen deficiencies were excluded.
Sample size was calculated using Power and Sample Size software version 24 for comparison of two means using a standard deviation (SD, 0.72 mm) of DRA size in postpartum women [2]. The study power, type 1 error, and the ratio between the intervention and control groups were set at 0.8, 0.05, and 1, respectively. Based on a 0.5 mm estimated mean difference in DRA among postpartum women between groups, a sample size of 24/group, i.e., 48 altogether was estimated. Considering a 30% dropout rate, a total sample size of 62 (31/group) is required. Therefore, our recruitment rate was 41 participants which is 85.4% intended sample size. A computerized random number was used to allocate participants to the intervention or control groups. Two physiotherapists, who were not involved in the study and were blinded to the patient’s involvement, DRA size, and allocation, were assigned to impart the exercises allocated to participants.
Data were collected at 34 weeks of pregnancy (baseline) and 8 weeks postpartum, i.e., following 8 weeks of exercise (post-intervention). At baseline, the presence of DRA was assessed along the linea alba using a single assessor. Demographic and obstetric data, including pelvic girdle pain, were obtained through a standardized, non-validated clinical interview. Pelvic girdle pain was assessed as a categorical response of “yes” or “no.” Transabdominal two-dimensional ultrasound imaging was used to measure the DRA size (Figure 1).
Figure. 1.
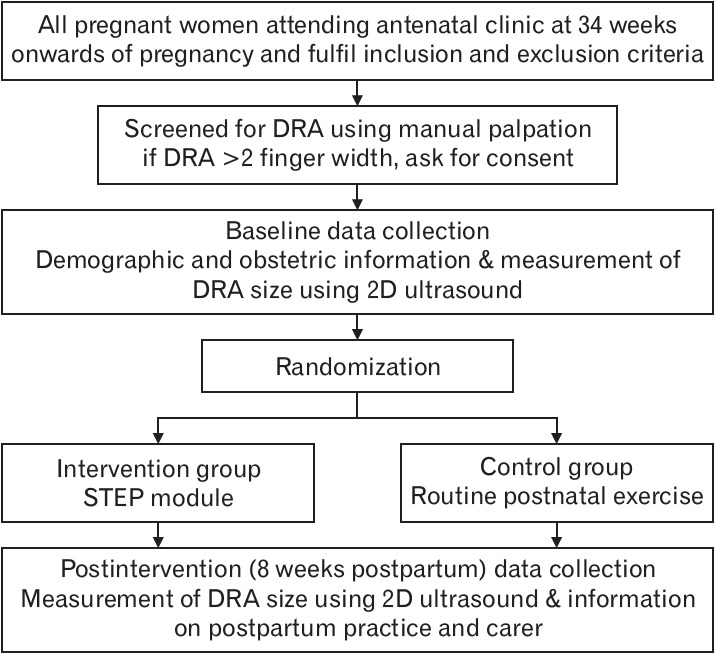
Participants’ recruitment and data collection. DRA, diastasis rectus abdominis; 2D, two-dimensional; STEP, split tummy exercise program.
Measurements were performed by a qualified physiotherapist (trained and supervised for sonographic DRA measurement by a consultant radiologist), utilizing a 7.5 MHz linear array transducer (Ultrasonic APLIO 500; Toshiba, Tokyo, Japan), which was placed transversely on two points along the linea alba, 2.5 cm above and below the umbilicus, as previously described [20]. DRA measurements were taken at rest, at the end of exhalation with the patient in the supine position, knees flexed at 90°, feet rested on the examination couch, and arms alongside the body. Excessive pressure on the abdomen by the transducer was avoided by observing any responses from the participants. DRA was defined as the transverse linear distance between the medial borders of both rectus muscles (right and left) at the same level. In this study, DRA at 2.5 cm above the umbilicus was used as the outcome parameter, as this was previously described as the best location to measure DRA accurately [21]. Obstetric data were obtained from hospital medical records and electronic databases. Information concerning postpartum practices was obtained during the post-intervention visits.
2. Intervention
STEP content development was based on a specific literature review on physical activity interventions for postpartum women diagnosed with DRA antenatally [22-24]. A consensus from six experts was obtained to evaluate the appropriateness and operational feasibility of the exercise program. The drafted STEP module was validated by having five antenatal and five postnatal mothers perform the program to ensure comprehensible content and participants’ compliance with the exercise module. The STEP module consists of three phases, facilitation, integration, and strengthening of abdominal muscles, to be implemented during the 8 weeks postpartum, at a minimum frequency [25] of 3 times a week involving at least three sets at 10 repetitions each. No specific time frame or regression of exercises in case the participant was unable to perform the proposed exercises, were given. Refer to Supplement 1 for a detailed STEP module.
Phase 1 began on day 1 of delivery and ended at the end of the fourth week postpartum. The aim of this phase was to facilitate isometric abdominal muscle contraction without exerting any load on the pelvis or spine. The participants were required to perform three exercises: (1) isometric abdominal exercise, (2) upper limb movements with isometric abdominal exercise, and (3) alternate lower limb movements. For isometric abdominal exercises, participants were instructed to contract the abdominal muscles slowly and hold for 3 seconds, followed by normal breathing. For the second exercise, participants were required to move both hands upward while contracting the abdominal muscles, as in Exercise 1. Finally, participants were asked to straighten their legs alternately while contracting the abdominal muscles, as in Exercise 1. All exercises were performed in the supine position, with both legs bent.
During the 5th to 6th weeks postpartum, all participants were required to perform posterior pelvic tilts, pelvic clocks, and bridging exercises, which sought to integrate abdominal and pelvic muscle functions. These exercises were performed in the supine position, with both legs bent. For the pelvic clock exercise, the participants were instructed to gently bend their back and return to the original position for a posterior pelvic tilt exercise to move the pelvic bones clockwise from 6 to 12 o’clock. For the bridging exercise, participants had to lift the buttocks gently and hold for 3 seconds.
Finally, between weeks 7 and 8 postpartum, the exercises focused on abdominal muscle strengthening with crunches, planks, and Russian twists. For the crunch exercise, participants were asked to lift their head and focus both eyes between both thighs while in a supine position, with both legs bent. For the plank exercise, participants were required to lie down using the support of both elbows and to hold the position as long as possible. Finally, to complete a Russian twist, the participant had to sit back slightly while keeping her spine straight and both legs bent and then turning the body to the right and left.
A trained physiotherapist provided STEP training within 24 hours after delivery in one-on-one sessions while the participants were still in the hospital. The inability to fully supervise the STEP exercise performed by the participants at home was our main limitation. To enhance compliance, participants were supplied with STEP pamphlets consisting of a pictorial representation of the exercises, an exercise log table (to record the exercises performed), a short video on STEP (shared via WhatsApp), and a weekly electronic message reminder and telephone call. The control group underwent routine postnatal exercises in the ward consisting of general exercise and ambulation.
3. Data Analysis
Statistical analyses were performed using IBM SPSS for Windows ver. 25.0 (IBM Corp., Armonk, NY, USA). The Mann-Whitney U test was applied for non-normally distributed continuous data, and the association between categorical data was assessed using the chi-square and Fisher’s exact tests. Mean differences in DRA size within and between groups were assessed using paired and independent t-tests, respectively. Statistical significance was set at P<0.05 for all tests.
RESULTS
1. Participant Recruitment and Characteristics
Of the 114 primigravidae identified, 57 were included in this study and randomly assigned to the intervention (n=28) or control (n=29) groups. However, seven and nine participants in the intervention and control groups, respectively, were excluded due to defaulting on the postpartum follow-up, leaving a total of 41 participants for further analysis (Figure 2).
Figure. 2.
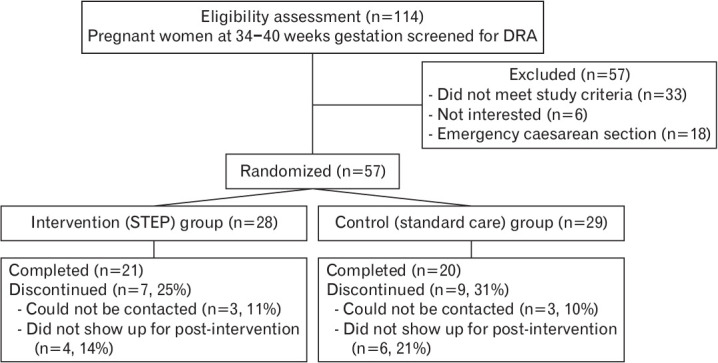
CONSORT diagram explaining the participant enrolment process. DRA, diastasis rectus abdominis; STEP, split tummy exercise program.
Ninety percent of participants in the intervention group completed the three phases of the exercise regime within 8 weeks postpartum, at a minimum of three exercise sessions per week. Non-compliant participants attributed this to being preoccupied with caring for their neonates. No subgroup analysis was performed because of the small proportion of noncompliance. The majority (87.8%, n=36) were Malay, followed by Chinese (7%), and others (5%). The mean (SD) age was 28±3.6 years, most (80.5%) had completed tertiary education, that is, college/university, and 78% were working mothers. In terms of health status, 85% of the participants were healthy and 15% were affected by diabetes or hypertensive disease. Sixty-eight percent of women complained of pelvic girdle pain in the third trimester of pregnancy. There were no significant differences in health status or demographic characteristics between the intervention and control groups (Table 1).
Table 1.
Characteristics of the primigravidae with diastasis recti abdominal in the study (n=41)
| Characteristic | Control group | Interventional group | P-value | |
|---|---|---|---|---|
| No. of participants | 20 | 21 | ||
| Maternal age (yr) | 29±4.06 | 27±2.96 | 0.185* | |
| Height (cm) | 156±6.84 | 158±4.96 | 0.647* | |
| Body weight (kg) | ||||
| Weight at pregnancy | 68±17.36 | 68±9.53 | 0.465* | |
| Weight at postpartum | 56±16.69 | 58±9.15 | 0.267* | |
| BMI (kg/m2) | ||||
| BMI at pregnancy | 26±9.52 | 26±6.77 | 0.676* | |
| BMI at postnatal | 21±8.91 | 22±5.69 | 0.375* | |
| Ethnicity | ||||
| Malay | 17 (85.0) | 19 (90.5) | 0.810† | |
| Chinese | 2 (10.0) | 1 (4.8) | ||
| Other | 1 (5.0) | 1 (4.7) | ||
| Education level | 0.454‡ | |||
| Secondary | 5 (25.0) | 3 (14.3) | ||
| Tertiary | 15 (75.0) | 18 (85.7) | ||
| Work status | 0.454‡ | |||
| Working | 17 (85.0) | 15 (71.4) | ||
| Not working | 3 (15.0) | 6 (28.6) | ||
| Antenatal health status | 0.158† | |||
| Healthy | 15 (75.0) | 20 (95.0) | ||
| Hypertension | 2 (10.0) | - | ||
| Diabetes | 3 (15.0) | 1 (5.0) | ||
| Pelvic girdle pain during pregnancy | 13 (65.0) | 15 (71.4) | 0.658† | |
| Pelvic girdle pain during postpartum | 7 (35.0) | 10 (50.0) | 0.412† | |
| Intrapartum history | ||||
| Duration of second stage of labor (min) | 24±15.81 | 26±19.73 | 0.814* | |
| Neonatal birth weight (kg) | 3±0.38 | 3±0.27 | 0.273* | |
| Episiotomy | 10 (50.0) | 16 (76.0) | 0.082† | |
| Tear | 5 (25.0) | 3 (14.0) | 0.454‡ | |
| Postpartum practice | ||||
| Massage | 8 (38.0) | 6 (30.0) | 0.796† | |
| Wearing a corset | 15 (71.4) | 15 (75.0) | 0.440† | |
| Breastfeeding | 17 (80.9) | 16 (80.0) | 0.697‡ | |
| Carer during postpartum | 0.743† | |||
| Husband | 4 (19.0) | 4 (19.0) | ||
| Mother | 12 (57.1) | 12 (57.1) | ||
| Mother-in-law | 4 (19.0) | 4 (19.0) | ||
Values are presented as mean±standard deviation or number (%).
BMI, body mass index.
By Mann-Whitney U test.
By chi-square test.
By Fisher’s exact test.
2. Changes in Diastasis Rectus Abdominis Size Following Split Tummy Exercise Program Intervention
At baseline, the mean (SD) DRA size was 21.6±5.17 mm. Table 2 presents the differences in the DRA size between the control and intervention groups. Prior to the STEP intervention, the DRA size was 2.54 mm larger in the intervention group than in the control group. Following the intervention, mothers who followed the STEP intervention showed a 1.96 mm reduction in DRA size. However, these differences were not statistically significant.
Table 2.
Mean diastasis rectus abdominis differences between the control and intervention groups (n=41)
| Time | Mean±SD* |
Mean size difference (95% CI) | t-statistic | df | P-value† | |
|---|---|---|---|---|---|---|
| Control (n=20) | Intervention (n=21) | |||||
| Baseline | 20.3±4.82 | 22.9±5.31 | 2.54 (-0.66 to 5.75) | 1.60 | 39 | 0.117 |
| Postintervention | 18.7±5.35 | 16.7±3.55 | -1.96 (-4.82 to 0.88) | -1.39 | 39 | 0.171 |
SD, standard deviation; CI, confidence interval; df, degrees of freedom.
Measured in mm as mean diastasis rectus abdominis at 2.5 cm above the umbilicus.
By independent t-test.
Table 3 shows the results of the paired t-test analysis for intragroup changes in DRA size. There was a significant reduction of 6.17 mm in DRA size in the intervention group following the STEP module, signifying a reduction of up to 27% (P<0.001). In contrast, the control group showed a reduction of 1.66 mm, i.e., 8.2% from the baseline. The magnitude of change between groups was 4.51 mm and statistically significant (P=0.20).
Table 3.
Comparison of mean diastasis rectus abdominis size within each group (n=41)
| Group | Mean±SD* |
Mean difference† (95% CI) | t-statistic | df | P-value‡ | |
|---|---|---|---|---|---|---|
| Baseline | Postintervention | |||||
| Intervention (n=21) | 22.9±5.30 | 16.7±3.55 | 6.17 (3.7 to 8.7) | 5.2 | 20 | <0.001 |
| Control (n=20) | 20.3±4.82 | 18.7±5.34 | 1.66 (-1.3 to 4.6) | 1.2 | 19 | 0.260 |
SD, standard deviation; CI, confidence interval; df, degrees of freedom.
Measured in mm as mean diastasis rectus abdominis at 2.5 cm above the umbilicus.
Mean difference between groups=4.51 (SD=1.85), P=0.20 (using independent t-test).
By paired t-test.
DISCUSSION
DRA is a known impairment of the anterior abdominal wall, and the best method for restoring the wall is abdominal exercises [15]. Strong abdominal muscles are associated with improved function and increased recruitment of muscle fibers [6,26]. Focusing on abdominal training (as in the STEP module) is believed to shorten the anterior and lateral fibers of the abdominal muscles, thereby reducing the DRA size. However, Lee and Hodges [12] in 2016 reported that shortening of muscle fibers does not always reduce DRA, which is attributed to distortion of the linea alba.
At baseline, the DRA size in the STEP intervention group was significantly larger than that in the control group. Although both groups showed a reduction in DRA size after 8 weeks, the 8.2% reduction observed in the control group (8.2% reduction) was not statistically significant between baseline and post-intervention. The STEP intervention group, however, showed a more substantial reduction of 27% in the mean DRA size, from 22.9 to 16.7 mm after 8 weeks, which was statistically significant. The reduction in DRA size observed in our study was slightly lower than the reduction reported by El-Mekawy et al. [23] in 2013. Their study demonstrated a 33% reduction in mean DRA size after 6 weeks of abdominal exercises performed over 30 minutes for three sessions per week. A study conducted by Walton et al. [19] in 2016 showed only a 0.2% reduction in mean DRA. The reduction in DRA size in our study was more substantial. Differences in DRA severity at baseline, inclusion criteria, and the content of the prescribed intervention might have contributed to the different results. The authors included a small sample (nine participants), the mean baseline DRA size was less than 15 mm, which was considered normal by Beer et al. [27] in 2009. Moreover, the participants involved in the previously cited studies were parous women with more than one child [19,23].
Currently, evidence on the most efficient abdominal exercise for reducing DRA size during the postpartum period is scarce. Most studies have combined a few types of abdominal exercises [18,19,22,23] to be completed within a specified duration, such as 2 weeks [22], 6 weeks [19,23], and 8 weeks [18]. The significant reduction in DRA size following STEP intervention performed from day 1 of delivery to 8 weeks postpartum suggests an alternative non-surgical solution for DRA in postnatal mothers. STEP does not require the use of equipment and is therefore more feasible in clinical and home settings.
The strengths of the study include the RCT design of this study and the inclusion of primigravidae with DRA who delivered vaginally. Bias in the study was eliminated by randomization and blinding, in which two trained physiotherapists were blinded to the conduct of the DRA assessment with no knowledge of the group allocations. The short postpartum interval of 8-weeks at which we reassessed the DRA size should be acknowledged as a limitation of our study and that may explain the small magnitude of reduction observed in our study. This is expected at 8-weeks postpartum, as DRA closure may take more than 6 months or even years [21]. To control for this issue, we have compared the effect of intervention with the control group. However, future studies with longer postpartum intervals are warranted. Another limitation is that the baseline DRA measurement was performed at 34 to 40 weeks of gestation, instead of on day 1 postpartum which is ideal as the latter was not logistically feasible for many of the participants. In this study, we used the DRA measurements above the umbilicus. However, since linea alba properties above and below the umbilicus are different, we recommend the measurement of DRA along the linea alba in the future. Studies involving longer postpartum intervals and incorporating the distortion index of linea alba in relation to abdominal exercise would also be beneficial. However, ethnic variances in skeletal muscle characteristics have been reported [28]. The smaller DRA size at baseline in our study, compared to other studies, may be attributed to potential racial differences in muscular characteristics. Our study population was Asian, mainly of the Malay ethnicity. Hence, our results cannot be directly extrapolated to populations of other ethnicities. Future research using our exercise protocol with different ethnic backgrounds is recommended.
In conclusion, the large reduction in DRA size demonstrated in the STEP intervention group at 8 weeks postpartum suggests that STEP intervention is effective as part of a postpartum training program in managing DRA. Postnatal mothers should be screened for DRA as early as possible during postnatal follow-up to allow early prescription of STEP interventions, resulting in favorable outcomes.
Acknowledgments
We would like to thank the head of nursing and nurses at the Department of Obstetrics & Gynecology, Universiti Kebangsaan Malaysia Medical Centre, for their technical support.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This research was funded by the Universiti Sains Malaysia Short Term grant with Project Code: 304/PPSP/6315119.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4082/kjfm.22.0035. Supplement 1. Split tummy exercise program (STEP) module.
REFERENCES
- 1.Fernandes da Mota PG, Pascoal AG, Carita AI, Bo K. Prevalence and risk factors of diastasis recti abdominis from late pregnancy to 6 months postpartum, and relationship with lumbo-pelvic pain. Man Ther. 2015;20:200–5. doi: 10.1016/j.math.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Liaw LJ, Hsu MJ, Liao CF, Liu MF, Hsu AT. The relationships between inter-recti distance measured by ultrasound imaging and abdominal muscle function in postpartum women: a 6-month follow-up study. J Orthop Sports Phys Ther. 2011;41:435–43. doi: 10.2519/jospt.2011.3507. [DOI] [PubMed] [Google Scholar]
- 3.Mota P, Pascoal AG, Carita AI, Bo K. Normal width of the inter-recti distance in pregnant and postpartum primiparous women. Musculoskelet Sci Pract. 2018;35:34–7. doi: 10.1016/j.msksp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Dehghan F, Haerian BS, Muniandy S, Yusof A, Dragoo JL, Salleh N. The effect of relaxin on the musculoskeletal system. Scand J Med Sci Sports. 2014;24:e220–9. doi: 10.1111/sms.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsia M, Jones S. Natural resolution of rectus abdominis diastasis: two single case studies. Aust J Physiother. 2000;46:301–7. doi: 10.1016/s0004-9514(14)60291-9. [DOI] [PubMed] [Google Scholar]
- 6.Boissonnault JS, Blaschak MJ. Incidence of diastasis recti abdominis during the childbearing year. Phys Ther. 1988;68:1082–6. doi: 10.1093/ptj/68.7.1082. [DOI] [PubMed] [Google Scholar]
- 7.Coldron Y, Stokes MJ, Newham DJ, Cook K. Postpartum characteristics of rectus abdominis on ultrasound imaging. Man Ther. 2008;13:112–21. doi: 10.1016/j.math.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Bo K, Hilde G, Tennfjord MK, Sperstad JB, Engh ME. Pelvic floor muscle function, pelvic floor dysfunction and diastasis recti abdominis: prospective cohort study. Neurourol Urodyn. 2017;36:716–21. doi: 10.1002/nau.23005. [DOI] [PubMed] [Google Scholar]
- 9.Gilleard WL, Brown JM. Structure and function of the abdominal muscles in primigravid subjects during pregnancy and the immediate postbirth period. Phys Ther. 1996;76:750–62. doi: 10.1093/ptj/76.7.750. [DOI] [PubMed] [Google Scholar]
- 10.Turan V, Colluoglu C, Turkyilmaz E, Korucuoglu U. Prevalence of diastasis recti abdominis in the population of young multiparous adults in Turkey. Ginekol Pol. 2011;82:817–21. [PubMed] [Google Scholar]
- 11.Hernandez-Gascon B, Mena A, Pena E, Pascual G, Bellon JM, Calvo B. Understanding the passive mechanical behavior of the human abdominal wall. Ann Biomed Eng. 2013;41:433–44. doi: 10.1007/s10439-012-0672-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee D, Hodges PW. Behavior of the linea alba during a curl-up task in diastasis rectus abdominis: an observational study. J Orthop Sports Phys Ther. 2016;46:580–9. doi: 10.2519/jospt.2016.6536. [DOI] [PubMed] [Google Scholar]
- 13.Sapsford RR, Hodges PW. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch Phys Med Rehabil. 2001;82:1081–8. doi: 10.1053/apmr.2001.24297. [DOI] [PubMed] [Google Scholar]
- 14.Spitznagle TM, Leong FC, Van Dillen LR. Prevalence of diastasis recti abdominis in a urogynecological patient population. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:321–8. doi: 10.1007/s00192-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 15.Sapsford R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man Ther. 2004;9:3–12. doi: 10.1016/s1356-689x(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin DR, van de Water AT, Peiris CL. Effects of exercise on diastasis of the rectus abdominis muscle in the antenatal and postnatal periods: a systematic review. Physiotherapy. 2014;100:1–8. doi: 10.1016/j.physio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Keeler J, Albrecht M, Eberhardt L, Horn L, Donnelly C, Lowe D. Diastasis recti abdominis: a survey of women’s health specialists for current physical therapy clinical practice for postpartum women. J Womens Health Phys Ther. 2012;36:131–42. [Google Scholar]
- 18.Khandale SR, Hande D. Effects of abdominal exercises on reduction of diastasis recti in postnatal women. Int J Health Sci Res. 2016;6:182–91. [Google Scholar]
- 19.Walton LM, Costa A, LaVanture D, McIlrath S, Stebbins B. The effects of a 6 week dynamic core stability plank exercise program compared to a traditional supine core stability strengthening program on diastasis recti abdominis closure, pain, Oswestry Disability Index (ODI) and Pelvic Floor Disability Index scores (PFDI) Phys Ther Rehabil. 2016;3:3. [Google Scholar]
- 20.Candido G, Lo T, Janssen PA. Risk factors for diastasis of the recti abdominis. J Assoc Chart Physiother Womens Health. 2005;97:49–54. [Google Scholar]
- 21.Mendes Dde A, Nahas FX, Veiga DF, Mendes FV, Figueiras RG, Gomes HC, et al. Ultrasonography for measuring rectus abdominis muscles diastasis. Acta Cir Bras. 2007;22:182–6. doi: 10.1590/s0102-86502007000300005. [DOI] [PubMed] [Google Scholar]
- 22.Acharry N, Kutty RK. Abdominal exercise with bracing, a therapeutic efficacy in reducing diastasis-recti among postpartal females. Int J Physiother Res. 2015;3:999–1005. [Google Scholar]
- 23.El-Mekawy HS, Eldeeb AM, El-Lythy MA, El-Begawy AF. Effect of abdominal exercises versus abdominal supporting belt on post-partum abdominal efficiency and rectus separation. Int J Med Health Sci. 2013;7:75–9. [Google Scholar]
- 24.Jung H, Jung S, Joo S, Song C. Comparison of changes in the mobility of the pelvic floor muscle on during the abdominal drawing-in maneuver, maximal expiration, and pelvic floor muscle maximal contraction. J Phys Ther Sci. 2016;28:467–72. doi: 10.1589/jpts.28.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine . In: ACSM’s guidelines for exercise testing and prescription. 8th ed. American College of Sports Medicine; Thompson WR, Gordon NF, Pescatello LS, editors. Philadelphia (PA): Lippincott Williams & Wilkins; 2010. Benefits and risk associated with physical activity; pp. 2–30. [Google Scholar]
- 26.Lee DG, Lee LJ, McLaughlin L. Stability, continence and breathing: the role of fascia following pregnancy and delivery. J Bodyw Mov Ther. 2008;12:333–48. doi: 10.1016/j.jbmt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Beer GM, Schuster A, Seifert B, Manestar M, Mihic-Probst D, Weber SA. The normal width of the linea alba in nulliparous women. Clin Anat. 2009;22:706–11. doi: 10.1002/ca.20836. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


