Abstract
Background
Esophageal cancer is a malignant tumor with a poor prognosis and high incidence. Circular RNAs (circRNAs) have been shown to be involved in the pathogenesis of cancers, including esophageal cancer. Here, we explored the precise role of circ_0003340 in esophageal cancer development.
Methods
The expression levels of circ_0003340, miR‐874‐3p and enabled homolog (ENAH) were detected by quantitative real‐time polymerase chain reaction and western blot. Subcellular localization and RNase R assays were used to characterize circ_0003340. Cell Counting Kit 8, flow cytometry, transwell assays were used to analyze cell proliferation, apoptosis, migration and invasion. The effect of circ_0003340 on tumor growth was assessed by tumor experiments in vivo. Dual‐luciferase reporter assay was used to analyze the relationship between miR‐874‐3p and circ_0003340 or ENAH.
Results
Circ_0003340 was mainly located in the cytoplasm and was upregulated in esophageal cancer tissues and cells. Circ_0003340 knockdown inhibited cell proliferation, migration, invasion, glucose consumption, and lactate production and induced cell apoptosis in esophageal cancer cells. Moreover, circ_0003340 knockdown suppressed tumor growth in vivo. MiR‐874‐3p was reduced in esophageal cancer tissues and cells, and it was a molecular mediator of circ_0003340 function in esophageal cancer cells. ENAH was identified as a direct and functional target of miR‐874‐3p in esophageal cancer cells. The promotion effect of circ_0003340 on ENAH was ameliorated by miR‐874‐3p.
Conclusion
The data demonstrated that circ_0003340 promoted the progression of esophageal cancer through miR‐874‐3p/ENAH axis, which might provide novel therapeutic targets for esophageal cancer intervention.
Keywords: circ_0003340, ENAH, esophageal cancer, miR‐874‐3p
Circ_0003340 promotes the progression of esophageal cancer through miR‐874‐3p/enabled homolog axis.

INTRODUCTION
Esophageal cancer, with a poor prognosis, is the eighth most common form of cancer in humans, usually characterized by dysphagia, unintentional weight loss, and other symptoms. 1 , 2 Esophageal squamous carcinoma (ESCC) was the most common mold of esophageal cancer, accounting for ~90%. 3 Long non‐coding RNAs (lncRNAs), messenger RNAs (mRNAs), microRNAs (miRNAs), and circular RNAs (circRNAs) are reported to be associated with cancers and are biological targets for cancer therapy. 4 , 5
Using microarray, gene ontology, and other bioinformatics tools to find 1384 lncRNAs, 2046 circRNAs, and 936 mRNAs involved in the pathogenesis and development of ESCC. 6 CircGSK3β expression was upregulated in ESCC, which promoted migration and invasion of ESCC. 7 Hou et al. 8 found that circ_0003340 sponged miR‐564 and regulated the expression of TPX2 to promote esophageal cancer cell proliferation. Silencing of circZNF292 reduced the activity, migration, and invasion ability of esophageal cancer cells and induced cell apoptosis through miR‐206. 9 Circ‐OGDH (circ_0003340) was upregulated in ESCC and regulated PDX1 expression by targeting miR‐615‐5p to promote glutamine metabolism and tumor growth in ESCC cells. 10 Nonetheless, our understanding of the molecular basis of circ_0003340 in ESCC remains limited.
MiRNAs are non‐coding RNAs that regulate various physiological functions of the body. 11 MiRNAs are associated with cancer treatment and drug resistance according to reports. 12 MiR‐203a mediated the phosphoinositide 3‐kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and had a connection with the treatment and growth of ESCC patients. 13 LncFAM83A‐AS1 was drastically upregulated in esophageal cancer tissues and cells, and it promoted lymph node metastasis in esophageal cancer patients by targeting miR‐495‐3p. 14 It is reported that miR‐874‐3p was related to osteosarcoma, 15 epithelial ovarian cancer, 16 nasopharyngeal carcinoma, 17 and so on. Yuan et al. 18 found that miR‐874‐3p was remarkably hindered in ESCC tissues and cells, and miR‐874‐3p overexpression inhibited the growth of ESCC cells, which was correlated with lymph node metastasis, overall survival, and clinical stage.
Enabled homolog (ENAH) is a member of the Ena/vasodilator‐stimulated phosphoproteome. 19 ENAH expression was up‐regulated in ESCC, and ENAH knockdown inhibited proliferation, invasion, migration, tumor sphere formation of ESCC cells, and induced cell apoptosis. 20 Wang et al. 21 found that ENAH knockdown abolished the promoting effects of circ_0030018 silencing on proliferation, migration and epithelial‐mesenchymal transition (EMT) of esophageal cancer cells. However, the function of circ_0003340/miR‐874‐3p/ENAH axis in esophageal cancer has not been reported.
The effects of circ_0003340, miR‐874‐3p and ENAH on glycolysis metabolism in esophageal cancer cells were demonstrated for the first time. This study opens up a new possibility for the clinical treatment and prognosis of esophageal cancer patients.
MATERIALS AND METHODS
Clinical tissue collection
The Ethics Committee of Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University recognized this experiment. In this study, 30 patients from Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University voluntarily signed an informed consent form and provided their esophageal cancer tissues and para‐cancer tissues. Once in isolation, these tissues were refrigerated in liquid nitrogen and stored in a −80°C refrigerator until use.
Cell lines and cell culture
Esophageal cancer cell lines (ECA109 and EC9706) and the normal esophageal epithelium cell line (HET‐1A) were purchased from Chuan Qiu Biotechnology. All cells were fostered in Roswell Park Memorial Institute (RPMI)‐1640 medium (Beijing Warbisson Technology) with 10% fetal bovine serum (FBS) (Zhengzhou Jiulong Biological Products) and 1% penicillin–streptomycin solution (Invitrogen), and trained in a 37°C incubator supplemented with 5% CO2.
RNA extraction and quantitative real‐time polymerase chain reaction
The overall RNA was segregated with TRIzol reagent (Invitrogen). The ABI Prism 7700 Sequence Detection System (Applied Biosystems, Thermo Fisher Scientific) were used to quantify the amount of RNA. We analyzed circ_0003340 and mRNA expression using the SuperScript VILO cDNA Synthesis Kit (Invitrogen) for complementary DNA (cDNA) synthesis and SYBR Green Mix (Invitrogen) for cDNA amplification. We analyzed miR‐874‐3p expression using BON‐miR miRNA cDNA Synthesis Kit (Bonyakhteh) and SYBR Green Mix for cDNA synthesis and amplification, respectively. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) or small nuclear RNA U6 (U6) was used as the internal gene. The 2−ΔΔCt method was used to calculate the fold change. The primers used in this experiment were all synthesized by Tianjin Jinweizhi Biotechnology. Primer sequences were shown in Table 1.
TABLE 1.
Sequences used for RT‐qPCR
| Name | Primers sequences for PCR (5′‐3′) | |
|---|---|---|
| hsa_circ_0003340 | Forward | CGTGCCCGCTGACATTATCT |
| Reverse | GGGGAAGGGGACTCTGGTAG | |
| ENAH | Forward | ACATTCAGAGTGGTGGGCAG |
| Reverse | ATGTTGGCCCTGTTTCCTGT | |
| miR‐847‐3p | Forward | GCCGAGCTGCCCTGGCCCGA |
| Reverse | CTCAACTGGTGTCGTGGA | |
| Linear 0003340 | Forward | CGTGTCACCGACAGGAACAT |
| Reverse | CTTGACGATCTGCCTTGCAC | |
| GAPDH | Forward | AAGGCTGTGGGCAAGGTCATC |
| Reverse | GCGTCAAAGGTGGAGGAGTGG | |
| β‐actin | Forward | CTCCATCCTGGCCTCGCTGT |
| Reverse | GCTGTCACCTTCACCGTTCC | |
| U6 | Forward | CTCGCTTCGGCAGCACATA |
| Reverse | CGAATTTGCGTGTCATCCT |
Abbreviations: PCR, polymerase chain reaction; ENAH, enabled homolog; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; U6, small nuclear RNA U6.
Subcellular localization assay
Cytoplasmic and nuclear RNA was separated by the Cytoplasmic and Nuclear RNA Isolation Kit (Tiangen Biochemical Technology). Next, quantitative real‐time polymerase chain reaction (qRT‐PCR) was executed to estimate the levels of circ_0003340 in the cytoplasm and nucleus. GAPDH and U6 were used as cytoplasmic and nuclear controls, respectively.
RNase R digestion assay
Ten units of RNase R (20 U/μL; Epicenter) was added to 2.5 μg total RNA and incubated at 37°C for 30 min to identify the circular structure of circ_0003340. Next, the expression levels of circ_0003340 and linear 0003340 were monitored by qRT‐PCR, respectively
Cell transfection
Lentiviruses expressing short hairpin RNA (shRNA)‐circ_0003340 (sh‐circ_0003340) and shRNA‐control (sh‐con) negative control, and circ_0003340 expression plasmid and control vector were acquired from Shanghai Integrated Biotech Solutions. Oligonucleotides (Shanghai Integrated Biotech Solutions) used were: si‐circ_0003340 (si‐circ_0003340#1, si‐circ_0003340#2 and si‐circ_0003340#3), control mock si‐con, miR‐874‐3p mimic, mimic control (miR‐con), anti‐miR‐874‐3p and inhibitor control anti‐miR‐con. ENAH expression plasmid pcDNA3.1‐ENAH and plasmid cloning DNA (pcDNA) vector control were also purchased from Shanghai Integrated Biotech Solutions and 2 × 105 cells were plated into 6‐well cell culture plates. When the cells reached 70%–80% confluence, the lipid complex was prepared and transfected into the cells based on the specification of the Lipofectamine 2000 liposome transfection reagent (Shanghai Haoran Biotechnology). At 48 h after transfection, qRT‐PCR was used to measure the transfection efficiency.
Cell counting kit 8 assay
Cell proliferation of ECA109 and EC9706 was evaluated with the Cell Counting Kit‐8 (CCK8) (GLPBIO). Approximately 2 × 104 cells were added to per well of the cell culture plates for transfection and hatched with 10 μL of CCK8 solution for 3 h at 37°C. The proliferation ability of cells was detected with a microplate reader at 0 h, 24 h, 48 h, and 72 h after transfection, respectively.
Flow cytometry
ECA109 and EC9706 cells (3 × 105 cells/well) were placed in 6‐well cell culture plates and transfected. After transfection for 48 h, cells were collected and tinted with 5 μL Annexin V‐FITC at 2°C –8°C in dark for 15 min; the cells then were fostered with 10 μL propidium iodide (PI) at 2°C –8°C in dark for 5 min following the protocols of the Annexin V‐FITC/PI Apoptosis Kit (Solarbio). The apoptotic cells and living cells were distinguished by Cytoflex flow cytometry (BECKMA COULTER) within 1 h.
Transwell migration and invasion assays
The capacities of cell migration and invasion were surveyed by transwell chambers (Corning) without (for migration) or with (for invasion) Matrigel (BD Biosciences). Transfected cells with serum‐free were appended to the above chamber of transwell chamber at 5 × 105 (in migration assay) or 2 × 106 (in invasion assay) cells/well, and the nether chamber was added with 800 μL complete medium containing 20% serum for incubation at 37°C and 5% CO2 for 24 h. During incubation, the cells moved to the nether chamber containing serum. After 24 h, cells located on the lower surface of the supernatant chamber were fixed and colored with crystal violet (Solarbio). The migration and invasion abilities of cells were analyzed under a microscope.
Glycolysis metabolism analysis
The levels of glucose consumption and lactate production in the cell culture medium were determined in line accordance with the instructions of the glucose assay kit (Shanghai Rongsheng Biological Pharmaceutical) and lactate assay kit (Biovision), respectively.
Western blot
Proteins were extracted using radio immunoprecipitation assay lysis buffer (Beyotime Biotechnology). Dodecyl sulfonate polyacrylamide gel electrophoresis Kit (Beijing Dingguo Changsheng Biotechnology) and the polyvinylidene fluoride (PVDF) membranes (Merck) were used to separate the protein, and PVDF membranes were closed with 5% non‐fat powdered milk (Solarbio) for 2 h. The primary antibodies to Bcl2‐associated X (Bax) (ab32503, 1:1000; Abcam), cyclin D1 (2978, 1:1000; Cell Signaling Technology), and ENAH (ab124685, 1:1000; Abcam) and immunoglobulin G secondary antibody conjugated with horseradish peroxidase (HRP) (ab201718, 1:5000; Abcam) were incubated with the membranes. The membranes were added with Immobilon Western Chemiluminescent HRP Substrate (Merck) and photographed in a gel imaging analyzer (UVITEC).
Tumor xenograft mice model construction
Two groups of 7‐week‐old nude mice (n = 3 per group) were purchased from Beijing Vital River Laboratory Animal Technology. A total of 1 × 106 ECA109 and EC9706 cells stably transfected with sh‐con or sh‐circ_0003340 were subcutaneously injected into the mice. Tumor volume was assessed weekly. After 4 weeks, the mice were euthanized, after subcutaneous tumor removal, part of frozen nitrogen was used for extraction of protein and RNA, and part of formalin was fixed for immunohistochemistry. The Animal Welfare and Research Ethics Committee of Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University agreed with all animal experiments in this study.
Immunohistochemistry assay
Tumor tissue was fastened with 4% paraformaldehyde (Solarbio) at room temperature for 20 min and encapsulated in paraffin to prepare paraffin sections. The sections were hatched overnight with diluted Ki67 antibody (ab15580, 1:100; Abcam) at 4°C, and hatched with the secondary antibody for 30 min. The samples were stained with diaminobenzidine (Solarbio) and observed under a microscope and photographed.
Dual‐luciferase reporter assay
Dual‐fluorescent reporter gene vectors (circ_0003340‐WT, circ_0003340‐MUT, ENAH‐WT, and ENAH‐MUT) were constructed for dual‐luciferase analysis. The segments of circ_0003340 and ENAH 3'UTR containing the predicted miR‐874‐3p pairing sequence or miss‐matched seed sequence were inserted in the pMIR‐REPORTER vector (YouBio). These vectors and miR‐874‐3p mimics or miR‐con were transfected into ECA109 and EC9706 cells, respectively. The luciferase activity in each group was measured according to the Dual‐Lucy Assay Kit (Solarbio) after transfection for 48 h.
Statistical analysis
GraphPad Prism 7.0 software (GraphPad) was serviced for data analysis. The Student's t‐test and one‐way analysis of variance (ANOVA) were used to compare the diversities between two groups or multiple sets of data, respectively. Results were displayed as mean ± standard deviation from at least three independent biological repeats, and p < 0.05 indicated statistically significant differences in data. Pearson's correlation coefficients were used to analyze the expression correlation of circ_0003340, miR‐874‐3p and ENAH in tumor samples.
RESULTS
Circ_0003340 was upregulated in esophageal cancer
To analyze the regulatory mechanism of circ_0003340 in esophageal cancer, we first detected the expression level of circ_0003340 in esophageal cancer tissues (n = 30) and cells, and the results showed that circ_0003340 in esophageal cancer tissues and cells was significantly higher than that in normal tissues and normal esophageal epithelial cells (Figure 1(a), (b)). The results of subcellular localization experiments confirmed that circ_0003340 was located in the cytoplasm (Figure 1(c), (d)). The data of RNase R experiments revealed that circ_0003340 was not digested by RNase R, and linear 0003340 level was reduced after RNase R digestion, indicating that circ_0003340 had a more stable ring structure (Figure 1(e), (f)). In summary, circ_0003340 was increased in esophageal cancer.
FIGURE 1.
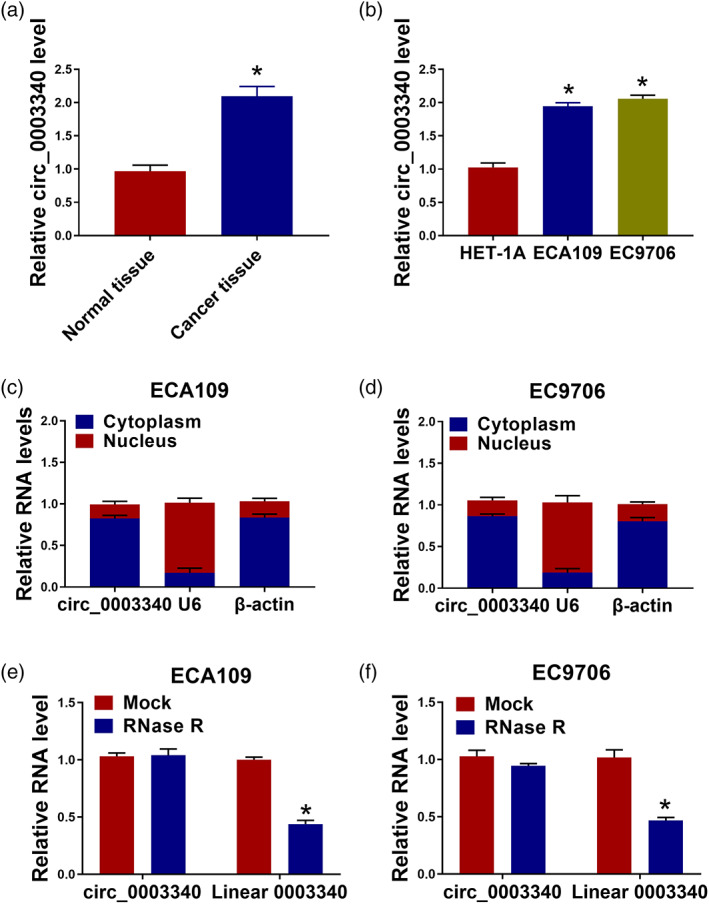
The expression of circ_0003340 was increased in esophageal cancer tissues and cells. (a), (b) The expression level of circ_0003340 in esophageal cancer tissues (n = 30) and cells was detected by qRT‐PCR. (c), (d) The localization of circ_0003340 in cells was detected by subcellular localization assay. (e), (f) The ring structure of circ_0003340 was identified by RNase R assay. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” qRT‐PCR, quantitative real‐time polymerase chain reaction
Effects of circ_0003340 knockdown on migration, invasion, proliferation, and apoptosis of esophageal cancer cells
To explore the role of circ_0003340 in esophageal cancer, CCK8, flow cytometry, and other assays were used to detect the effects of si‐circ_0003340 on esophageal cancer cells. The transfection efficiency of si‐circ_0003340 was tested with qRT‐PCR; the results discovered that circ_0003340 was reduced in ECA109 and EC9706 cells with si‐circ_0003340 transfection (Figure 2(a)). Owing to the most significant suppression of si‐circ_0003340#1 (called circ_0003340 below) on circ_0003340 expression, we selected it for further research (Figure S1). The results of CCK8 found that cell proliferation in ECA109 and EC9706 cells was significantly inhibited by si‐circ_0003340 (Figure 2(b), (c)). Flow cytometry showed that si‐circ_0003340 induced cell apoptosis of ECA109 and EC9706 cells (Figure 2(d)–(f)). The migration and invasion were decreased in ECA109 and EC9706 cells with circ_0003340 knockdown (Figure 2(g)–(i)). The glucose consumption and lactate production of ECA109 and EC9706 cells were restrained in the si‐circ_0003340 group compared with the si‐con group (Figure 2(j), (k)). After ECA109 and EC9706 cells were transfected with si‐circ_0003340, the expression of Bax was boosted, whereas the expression of cyclin D1 was suppressed (Figure 2(l)–(n)). In a word, circ_0003340 knockdown inhibited the growth of esophageal cancer cells by inhibiting the glycolysis process.
FIGURE 2.
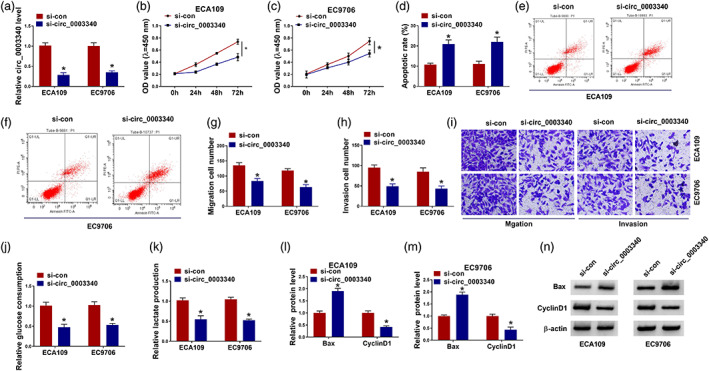
Circ_0003340 knockdown suppressed cell proliferation, migration, invasion and glycolysis and elevated cell apoptosis in esophageal cancer cells. ECA109 and EC9706 cells were transfected with si‐circ_0003340 or si‐con. (a) qRT‐PCR was performed to detect the efficiency of si‐circ_0003340 in ECA109 and EC9706 cells. (b), (c) CCK8 assay was used to test cell viability at different time points after si‐circ_0003340 transfection. (d)–(f) Flow cytometry was used to examine the cell apoptosis of esophageal cancer cells after transfection with si‐circ_0003340. (g)–(i) The ability of transfected cells to migrate and invade was detected by the transwell assay. (j), (k) The effect of si‐circ_0003340 on glucose consumption and lactate production in ECA109 and EC9706 cells was detected. (l)–(n) Western blot assay was used to detect the expression of Bax and cyclin D1 in ECA109 and EC9706 cells transfected with si‐circ_0003340. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” qRT‐PCR, quantitative real‐time polymerase chain reaction; CCK8, Cell Counting Kit‐8; Bax, Bcl2‐associated X
MiR‐874‐3p was a target gene of circ_0003340 in esophageal cancer cells
To study the mechanism of circ_0003340 in regulating esophageal cancer, we used Circular RNA Interactome (https://circinteractome.nia.nih.gov/) to predict the target of circ_0003340 and found that miR‐874‐3p was a downstream regulatory gene of circ_0003340. Their binding sites were shown in Figure 3(a). The data of dual‐luciferase reporter experiments indicated the luciferase activity of ECA109 and EC9706 cells was specially constrained in circ_0003340‐WT and miR‐874‐3p group compared with circ_0003340‐WT and miR‐con group (Figure 3(b), (c)). We discovered that miR‐874‐3p was reduced in esophageal cancer tissues and cells (Figure 3(d), (e)). The data implied that circ_0003340 participated in the development of esophageal cancer by targeting miR‐874‐3p.
FIGURE 3.

MiR‐874‐3p was regulated by circ_0003340. (a) Circular RNA interactome was used to predict the binding site between circ_0003340 and miR‐874‐3p. (b), (c) Dual‐luciferase reporter assays were performed to verify the targeting relationship between circ_0003340 and miR‐874‐3p in ECA109 and EC9706 cells. (d), (e) qRT‐PCR was used to detect the expression of miR‐874‐3p in esophageal cancer tissues and cells. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” qRT‐PCR, quantitative real‐time polymerase chain reaction
The effect of si‐circ_0003340 on esophageal cancer cells was reversed by anti‐miR‐874‐3p
Functional recovery experiments were used to explore the interaction between circ_0003340 and miR‐874‐3p in esophageal cancer cells. Si‐circ_0003340 boosted the expression of miR‐874‐3p in ECA109 and EC9706 cells, whereas anti‐miR‐874‐3p overturned this effect (Figure 4(a)). MiR‐874‐3p knockdown significantly reversed circ_0003340 reduction‐driven anti‐proliferation (Figure 4(b), (c)), pro‐apoptosis (Figure 4(d)), anti‐migration (Figure 4(e)), and anti‐invasion (Figure 4(f)) effects. Moreover, miR‐874‐3p knockdown abolished the effect of si‐circ_0003340 on glucose consumption and lactate production in ECA109 and EC9706 cells (Figure 4(g), (h)). Moreover, miR‐874‐3p knockdown reversed the effect of si‐circ_0003340 on the protein expression of Bax and cyclin D1 in ECA109 and EC9706 cells (Figure 4(i)–(k)). In a word, miR‐874‐3p downregulation ameliorated the effect of si‐circ_0003340 on esophageal cancer cells.
FIGURE 4.

Anti‐miR‐874‐3p reversed the effect of si‐circ_0003340 on esophageal cancer cells. ECA109 and EC9706 cells were transfected with si‐circ_0003340, si‐con, si‐circ_0003340 + anti‐miR‐con or si‐circ_0003340 + anti‐miR‐874‐3p. (a) qRT‐PCR was performed to detect the expression of miR‐874‐3p. (b), (c) CCK8 assay was used to test cell viability. (d) Flow cytometry was used to examine the cell apoptosis. (e), (f) The ability of transfected cells to migrate and invade was detected by the transwell assay. (g), (h) The levels of glucose consumption and lactate production in transfected ECA109 and EC9706 cells were detected. (i)–(k) Western blot assay was used to detect the expression of Bax and cyclin D1 in transfected ECA109 and EC9706 cells. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” qRT‐PCR, quantitative real‐time polymerase chain reaction; CCK8, Cell Counting Kit‐8; Bax, Bcl2‐associated X
ENAH was regulated by miR‐874‐3p in esophageal cancer cells
Starbase (https://starbase.sysu.edu.cn/) was used to predict the downstream gene of miR‐874‐3p, and ENAH was found to be a target gene of miR‐874‐3p (Figure 5(a)). The results of dual‐luciferase reporter experiments proved the luciferase activity was prominently suppressed in ENAH‐WT and miR‐874‐3p co‐transfected group, and the luciferase activity of ENAH‐MUT and miR‐874‐3p co‐transfected cells showed no significant change (Figure 5(b), (c)). The results of qRT‐PCR and western blot exhibited that ENAH was dramatically intensified in esophageal cancer tissues and cells (Figure 5(d)–(f)). These data indicated that miR‐874‐3p regulated the growth of esophageal cancer by targeting ENAH.
FIGURE 5.

ENAH was regulated by miR‐874‐3p in esophageal cancer cells. (a) Target sites of miR‐874‐3p in ENAH 3′‐UTR were analyzed by Starbase. (b), (c) Dual‐luciferase reporter assays were performed to verify the targeting relationship between miR‐874‐3p and ENAH in ECA109 and EC9706. (d)–(f) The expression of ENAH in esophageal cancer tissues and cells was detected by qRT‐PCR and western blot. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” ENAH, enabled homolog; qRT‐PCR, quantitative real‐time polymerase chain reaction
The effect of miR‐874‐3p on esophageal cancer cells was recovered by ENAH
ENAH was negatively regulated by miR‐874‐3p, and the inhibitory effect of miR‐874‐3p on ENAH was recuperated after ENAH overexpression (Figure 6(a)). ENAH upregulation restored the effects of miR‐874‐3p on proliferation, apoptosis, migration, invasion, glucose consumption, and lactate production of ECA109 and EC9706 cells (Figure 6(b)–(h)). MiR‐874‐3p elevated the expression of Bax, and repressed the expression of cyclin D1 in ECA109 and EC9706 cells, whereas upregulated ENAH reversed these effects (Figure 6(i)–(k)). To sum up, ENAH regained the effect of miR‐874‐3p on esophageal cancer cells.
FIGURE 6.
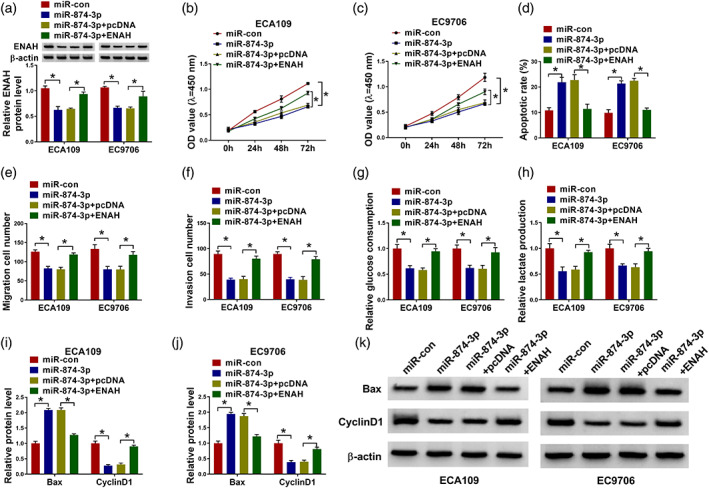
ENAH overexpression overturned the effect of miR‐874‐3p on the progression of esophageal cancer cells. ECA109 and EC9706 cells were transfected with miR‐con mimic, miR‐874‐3p mimic, miR‐874‐3p mimic+pcDNA or miR‐874‐3p mimic+ENAH. (a) Western blot was performed to detect the expression of ENAH protein. (b), (c) CCK8 assay was used to test cell viability. (d) Flow cytometry was used to examine the cell apoptosis. (e), (f) The ability of transfected cells to migrate and invade was detected by the transwell assay. (g), (h) The levels of glucose consumption and lactate production in transfected ECA109 and EC9706 cells were detected. (i)–(k) Western blot assay was used to detect the expression of Bax and cyclin D1 in transfected ECA109 and EC9706 cells. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” ENAH, enabled homolog; CCK8, Cell Counting Kit‐8; Bax, Bcl2‐associated X
Circ_0003340 regulated ENAH expression through miR‐874‐3p in esophageal cancer cells
In esophageal cancer tissues, miR‐874‐3p expression inversely correlated with circ_0003340 and ENAH mRNA levels and ENAH mRNA expression positively correlated with circ_0003340 expression (Figure 7(a)–(c)). The overexpression efficiency of circ_0003340 was detected by qRT‐PCR, and it was found that circ_0003340 was significantly increased in esophageal cancer cells after transfection with a circ_0003340 expression plasmid (Figure 7(d)). Moreover, the expression of ENAH was distinctly increased in ECA109 and EC9706 cells with circ_0003340 upregulation, whereas these effects were overturned by miR‐874‐3p overexpression (Figure 7(e),(f)). In general, circ_0003340 targeted miR‐874‐3p to regulate the expression of ENAH.
FIGURE 7.

Circ_0003340 regulated ENAH gene expression through miR‐874‐3p. (a)–(c) Expression correlation of variables in esophageal cancer tissues. (d) The transfected efficiency of circ_0003340 expression plasmid was detected by qRT‐PCR. (e), (f) The ENAH protein level was measured by western blot assay after transfected with pcDNA, circ_0003340, circ_0003340 + miR‐con and circ_0003340 + miR‐874‐3p in ECA109 and EC9706 cells. *p < 0.05. All cellular experiments were independently repeated three times, and the data were presented in the format of “mean ± standard deviation.” ENAH, enabled homolog; qRT‐PCR, quantitative real‐time polymerase chain reaction
Circ_0003340 knockdown inhibited the growth of xenograft tumors in vivo
To analyze the effect of circ_0003340 on esophageal cancer tumor growth and metastasis in vivo, we constructed a xenograft tumor mice model by transplanting EC9706 and ECA109 cells pre‐transfected with sh‐circ_0003340 into the nude mice. As shown in Figure 8(a)–(f), tumor volume and weight in the sh‐circ_0003340 group were distinctly retarded compared to the sh‐con group. The expression levels of circ_0003340 and ENAH were greatly attenuated and miR‐874‐3p expression was elevated in the sh‐circ_0003340 group when compared with the sh‐con group (Figure 8(g)–(j)). The results of immunohistochemistry (IHC) exhibited that Ki67 was substantially abated in the sh‐circ_0003340 group (Figure 8(k)). These results suggested that sh‐circ_0003340 could inhibit tumor growth in vivo.
FIGURE 8.

Circ_0003340 silence inhibited the tumor growth of esophageal cancer in vivo. (a), (b) Tumor volume was detected in each group every week. (c)–(f) Tumor weight was detected in each group at the ending point. (g), (h) The expression of circ_0003340 and miR‐874‐3p was examined in each group by qRT‐PCR. (i), (j) ENAH expression was assessed by western blot. (k) The protein expression of Ki67 in mouse tissues was detected by IHC. *p < 0.05 qRT‐PCR, quantitative real‐time polymerase chain reaction; ENAH, enabled homolog; immunohistochemistry, IHC
DISCUSSION
Esophageal cancer is one of the most deadly and fastest occurring cancers in the world. 21 This research confirmed that circ_0003340 was upregulated in esophageal cancer and our results indicated that circ_0003340 facilitated esophageal cancer progression through the miR‐874‐3p/ENAH axis.
CircRNAs are non‐coding RNAs that have been shown to be involved in cancer treatment, prognosis, and drug resistance. 22 Overexpression of circRNA_001275 increased the activity of esophageal cancer cells and inhibited apoptosis. 23 As an oncogene, circPVT1 was significantly upregulated in esophageal carcinoma tissues and cells, promoting the growth of esophageal cancer cells. 24 We had discovered that circ_0003340 was mainly located in the cytoplasm and was strikingly boosted in esophageal cancer tissues and cells. The results of function experiments exhibited that, circ_0003340 knockdown retarded cell proliferation, migration, invasion, and augmented cell apoptosis in esophageal cancer cells. The results are the same as those of Hou et al. 8 and Liu et al. 10 who displayed that the expression of circ_0003340 was upregulated in ESCC and esophageal carcinoma cells, and silencing circ_0003340 hampered cell proliferation and invasion, and intensified cell apoptosis. We also suggested that silence of circ_0003340 evidently restrained the levels of glucose consumption and lactate production in esophageal carcinoma cells. The protein expression of Bax was exceptionally reinforced, and the protein expression of cyclin D1 was abnormally repressed in esophageal cancer cells after si‐circ_0003340 was transfected. In vivo experiments indicated circ_0003340 silence substantially repressed the growth of tumors. The data manifested that circ_0003340 knockdown could inhibit the proliferation of esophageal cancer cells and elevate their apoptosis by inhibiting the glycolysis metabolism of esophageal carcinoma cells.
Many studies have shown that miRNAs are involved in the regulatory process of circRNAs in cancer and are downstream regulatory factors of circRNAs. 25 The increased expression of miR‐21 in esophageal carcinoma cells could negatively regulate the expression of programmed cell death 4 (PDCD4) and reduce the cisplatin sensitivity of the cells. 26 MiR‐483‐5p was upregulated in esophageal cancer cells, negatively regulated the expression of KCNQ1, and facilitated the growth of esophageal carcinoma cells. 27 In our study, we proved that miR‐874‐3p was a target gene of circ_0003340 by dual‐luciferase reporter experiment. MiR‐874‐3p was drastically hampered in esophageal cancer tissues and cells, consistent with the results of Yuan et al. 18 The data of function rescue experiments uncovered that the effect of circ_0003340 silence on cell proliferation, apoptosis, migration, invasion, glucose consumption, lactate production, Bax, and cyclin D1 in esophageal carcinoma cells was reversed by anti‐miR‐874‐3p co‐transfection. In short, circ_0003340 regulated the development of esophageal cancer by negatively regulating miR‐874‐3p.
Similar to the research data of He et al. 20 we uncovered that ENAH was upregulated in esophageal carcinoma tissues and cells. We also revealed that ENAH was negatively regulated by miR‐874‐3p. Moreover, we elucidated that ENAH overexpression regained the effect of miR‐874‐3p on cell proliferation, apoptosis, migration, invasion, glucose consumption, lactate production, Bax, and cyclin D1 in esophageal cancer cells. We also demonstrated that circ_0003340 increase promoted the expression of ENAH in esophageal cancer cells and this effect was abated by miR‐874‐3p overexpression.
CONCLUSION
In this study, we found that circ_0003340 was enhanced in esophageal cancer tissues and cells. The data of function rescue experiments disclosed that circ_0003340 regulated the progression and glycolysis metabolism of esophageal cancer through the miR‐874‐3p/ENAH axis for the first time.
AUTHOR CONTRIBUTIONS
Jingyi Wang wrote the manuscript. Ning Zhao designed and performed the research. Shengzu Peng and Tao Zhang analyzed the data. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Figure S1. Relative circ_0003340 expression by qRT‐PCR in ECA109 and EC9706 cells transfected as indicated. *p < 0.05.
Wang J, Zhao N, Peng S, Zhang T. Circ_0003340 regulates the expression of ENAH to affect the development of esophageal cancer through miR‐874‐3p. Thorac Cancer. 2023;14(9):815–826. 10.1111/1759-7714.14812
Contributor Information
Ning Zhao, Email: 13834562256@163.com.
Shengzu Peng, Email: pengshengzu@163.com.
REFERENCES
- 1. Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician. 2017;95(1):22–8. [PubMed] [Google Scholar]
- 2. Dupuis O, Ganem G, Béra G, Pointreau Y, Pradier O, Martin P, et al. Esophageal cancer. Cancer Radiother. 2010;14(Suppl 1):S74–83. [DOI] [PubMed] [Google Scholar]
- 3. Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–21. [DOI] [PubMed] [Google Scholar]
- 4. Hou X, Wen J, Ren Z, Zhang G. Non‐coding RNAs: new biomarkers and therapeutic targets for esophageal cancer. Oncotarget. 2017;8(26):43571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu F, Jiang L, Zhao Q. Whole‐transcriptome and proteome analyses identify key differentially expressed mRNAs, miRNAs, lncRNAs and circRNAs associated with HCC. Oncogene. 2021;40(29):4820–31. [DOI] [PubMed] [Google Scholar]
- 6. Song J, Lu Y, Sun W, Han M, Zhang Y, Zhang J. Changing expression profiles of lncRNAs, circRNAs and mRNAs in esophageal squamous carcinoma. Oncol Lett. 2019;18(5):5363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu X, Wu D, He X, Zhao H, He Z, Lin J, et al. circGSK3β promotes metastasis in esophageal squamous cell carcinoma by augmenting β‐catenin signaling. Mol Cancer. 2019;18(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hou Y, Liu H, Pan W. Knockdown of circ_0003340 induces cell apoptosis, inhibits invasion and proliferation through miR‐564/TPX2 in esophageal cancer cells. Exp Cell Res. 2020;394(2):112142. [DOI] [PubMed] [Google Scholar]
- 9. Liu Z, Hu G, Zhao Y, Xiao Z, Yan M, Ren M. Silence of cZNF292 suppresses the growth, migration, and invasion of human esophageal cancer Eca‐109 cells via upregulating miR‐206. J Cell Biochem. 2020;121(3):2354–62. [DOI] [PubMed] [Google Scholar]
- 10. Liang Z, Zhao B, Hou J, Zheng J, Xin G. CircRNA circ‐OGDH (hsa_circ_0003340) acts as a ceRNA to regulate glutamine metabolism and esophageal squamous cell carcinoma progression by the miR‐615‐5p/PDX1 Axis. Cancer Manage Res. 2021;13:3041–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwakawa HO, Tomari Y. The functions of MicroRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015;25(11):651–65. [DOI] [PubMed] [Google Scholar]
- 12. Malhotra A, Sharma U, Puhan S, Chandra Bandari N, Kharb A, Arifa PP, et al. Stabilization of miRNAs in esophageal cancer contributes to radioresistance and limits efficacy of therapy. Biochimie. 2019;156:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Zhang Z, Yu X, Li Q, Wang Q, Chang A, et al. SOX9/miR‐203a axis drives PI3K/AKT signaling to promote esophageal cancer progression. Cancer Lett. 2020;468:14–26. [DOI] [PubMed] [Google Scholar]
- 14. Huang GM, Zang HL, Geng YX, Li YH. LncRNA FAM83A‐AS1 aggravates the malignant development of esophageal cancer by binding to miR‐495‐3p. Eur Rev Med Pharmacol Sci. 2020;24(18):9408–15. [DOI] [PubMed] [Google Scholar]
- 15. Liu WG, Zhuo L, Lu Y, Wang L, Ji YX, Guo Q. miR‐874‐3p inhibits cell migration through targeting RGS4 in osteosarcoma. J Gene Med. 2020;22(9):e3213. [DOI] [PubMed] [Google Scholar]
- 16. Xia B, Lin M, Dong W, Chen H, Li B, Zhang X, et al. Upregulation of miR‐874‐3p and miR‐874‐5p inhibits epithelial ovarian cancer malignancy via SIK2. J Biochem Mol Toxicol. 2018;32(8):e22168. [DOI] [PubMed] [Google Scholar]
- 17. Feng X, Xue H, Guo S, Chen Y, Zhang X, Tang X. MiR‐874‐3p suppresses cell proliferation and invasion by targeting ADAM19 in nasopharyngeal carcinoma. Panminerva Med. 2021;63(2):238–9. [DOI] [PubMed] [Google Scholar]
- 18. Yuan RB, Zhang SH, He Y, Zhang XY, Zhang YB. MiR‐874‐3p is an independent prognostic factor and functions as an anti‐oncomir in esophageal squamous cell carcinoma via targeting STAT3. Eur Rev Med Pharmacol Sci. 2018;22(21):7265–73. [DOI] [PubMed] [Google Scholar]
- 19. Roussos ET, Wang Y, Wyckoff JB, Sellers RS, Wang W, Li J, et al. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle‐T transgenic mouse mammary tumors. Breast Cancer Res. 2010;12(6):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Z, Li W, Zheng T, Liu D, Zhao S. Human umbilical cord mesenchymal stem cells‐derived exosomes deliver microRNA‐375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J Exp Clin Cancer Res. 2020;39(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Tang D, Wang H, Hu G, Hu S, Li L, et al. Circular RNA hsa_circ_0030018 acts as a sponge of miR‐599 to aggravate esophageal carcinoma progression by regulating ENAH expression. J Cell Biochem. 2019;121(8–9):3730–8. [DOI] [PubMed] [Google Scholar]
- 22. Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou FW, Yang SZ, Li WY, Liu CY, Liu XH, Hu CH, et al. [Erratum] circRNA_001275 upregulates Wnt7a expression by competitively sponging miR‐370‐3p to promote cisplatin resistance in esophageal cancer. Int J Oncol. 2020;57(6):1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong R, Chen Z, Mo T, Li Z, Zhang P. Potential role of circPVT1 as a proliferative factor and treatment target in esophageal carcinoma. Cancer Cell Int. 2019;19:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verduci L, Strano S, Yarden Y, Blandino G. The circRNA‐microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13(4):669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang YC, Liu GJ, Yuan DF, Li CQ, Xue M, Chen LJ. Influence of exosome‐derived miR‐21on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci. 2019;23(4):1513–9. [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Wang H, Zhu S, Lan X. miR‐483‐5p promotes esophageal cancer progression by targeting KCNQ1. Biochem Biophys Res Commun. 2020;531(4):615–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relative circ_0003340 expression by qRT‐PCR in ECA109 and EC9706 cells transfected as indicated. *p < 0.05.


