Abstract
Background
The prevalence of hypothyroidism among older patients hospitalized for COVID-19 and its association with mortality is unclear. This study aims to investigate the prevalence of hypothyroidism in older COVID-19 inpatients and verify if this comorbidity is associated with a specific pattern of onset symptoms and a worse prognosis.
Methods
COVID-19 inpatients aged ≥ 60 years, participating in the GeroCovid acute wards cohort, were included. The history of hypothyroidism was derived from medical records and the use of thyroid hormones. Sociodemographic data, comorbidities, symptoms/signs at the disease onset and inflammatory markers at ward admission were compared between people with vs without history of hypothyroidism. The association between hypothyroidism and in-hospital mortality was tested through Cox regression.
Results
Of the 1245 patients included, 8.5% had a history of hypothyroidism. These patients were more likely to present arterial hypertension and obesity compared with those without an history of hypothyroidism. Concerning COVID-19 clinical presentation, patients with hypothyroidism had less frequently low oxygen saturation and anorexia but reported muscle pain and loss of smell more commonly than those without hypothyroidism. Among the inflammatory markers, patients with hypothyroidism had higher lymphocytes values. At Cox regression, hypothyroidism was associated with reduced in-hospital mortality only in the univariable model (HR = 0.66, 95% CI 0.45–0.96, p = 0.03); conversely, no significant result were observed after adjusting for potential confounders (HR = 0.69, 95% CI 0.47–1.03, p = 0.07).
Conclusions
Hypothyroidism does not seem to substantially influence the prognosis of COVID-19 in older people, although it may be associated with peculiar clinical and biochemical features at the disease onset.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-023-02048-w.
Keywords: Hypothyroidism, COVID-19, Prognosis, Aged, GeroCovid study
Introduction
Coronavirus disease (COVID-19) pandemic, caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has probably represented the most crucial health challenge of our century [1, 2].
Since the beginning of the COVID-19 pandemic, epidemiological data underlined that individuals in the oldest age classes and those with multimorbidities had a more severe course of the disease. Further scientific reports uncovered that both the number and the type of pre-existing diseases could substantially influence the prognosis of SARS-CoV-2 infection. Among these, metabolic conditions, such as obesity and diabetes mellitus, and cardiovascular diseases, including hypertension and heart failure, have been identified as risk factors for more severe COVID-19 presentation and worse outcomes [1, 2].
Concerning the impact of thyroid dysfunctions on the COVID-19 course, evidence is still scarce [3, 4]. In particular, contrasting results have emerged both on the prevalence of hypo- and hyperthyroidism among patients with COVID-19 and the impact of these multimorbidities on mortality [3, 5]. Moreover, only a few of the available works published on this topic focused on older adults, representing the population most strongly struck by the pandemic [1]. At the base of the possible influence of thyroid pathologies on COVID-19, there is the frequent co-occurrence of this condition with diseases like obesity, kidney and liver dysfunctions, associated with an increased risk and severity of COVID-19 infection. Moreover, elevated levels of thyroid hormones have been linked to a greater expression of the ACE2 protein, which promotes the entering of SARS-CoV-2 into the host cells [6]. As a further mechanism, thyroid hormones are involved in the modulation of the immune system. For instance, hypermetabolism linked to thyrotoxicosis can enhance oxidative stress and impair the individual resilience against adverse events, such as infections [7, 8]. Interestingly, previous studies have shown that oxidative stress can act not only at the host level by altering the immune system function but can also increase the pathogenesis of some viruses. However, more marked oxidative stress and alterations in both the innate and adaptive immune system have also been observed in hypothyroidism status [9].
To further investigate the association between thyroid dysfunction and COVID-19, this study aimed to evaluate the prevalence of hypothyroidism in older patients hospitalized for COVID-19 and investigate whether this comorbidity was associated with a specific pattern of onset symptoms and a different prognosis of SARS-CoV-2 infection. We hypothesized that this condition might influence the clinical presentation of SARS-CoV-2 infection and its prognosis in term of oxygen need and mortality.
Methods
Study population
GeroCovid Observational is a multicenter retrospective-prospective study promoted by the Italian Society of Gerontology and Geriatrics, in collaboration with the Norwegian Society of Geriatrics, involving older people with or at risk of COVID-19 in different care settings. This initiative involved individuals aged ≥ 60 years with or at risk of COVID-19 in different care settings, consecutively enrolled (for details, please see Trevisan et al. Assessing the impact of COVID-19 on the health of geriatric patients: The European GeroCovid Observational Study [10]. The data collection was performed retrospectively for most of the participants (98%) and prospectively only for a minority of them, depending on the availability of time and personnel resources at the study sites. Enrollment was performed between March and December 2020 and reported in a structured e-Registry developed by Bluecompanion Ltd (London, UK). For the present study, we considered the GeroCovid acute wards cohort, involving inpatients hospitalized for SARS-CoV-2 infection in 19 Italian acute and post-acute wards. Eleven centres involved were tertiary, six were secondary and two were primary.
Enrollment was performed between March and December 2020 and reported in a structured e-Registry developed by Bluecompanion Ltd (London, UK).This study involves participants in the GeroCovid acute wards cohort, i.e. individuals aged 60 years or older hospitalized for SARS-CoV-2 infection. From the initial sample of 1276 participants involved in this cohort, we excluded those with missing data on age, symptoms at the disease onset, and clinical status at ward admission (defined according to the World Health Organization [WHO] classification). The statistical analysis was performed on a final sample of 1245 inpatients.
Data collection
For each GeroCovid participant, we considered the demographic characteristics (sex, age, race), living arrangements, smoking habits, and pre-COVID-19 mobility (categorized as moving independently, using walking aid/moving with a wheelchair, moving with assistance in a wheelchair/bedridden).
The presence of the following chronic diseases was derived from medical records: arterial hypertension, cardiovascular diseases (including cardiomyopathies, ischemic heart diseases, heart failure, atrial fibrillation), chronic obstructive pulmonary disease (COPD), diabetes, obesity, chronic renal failure, depressive mood, and cognitive disorders.
As the main exposure, we considered the history of hypothyroidism based on medical documentation (recording disease diagnosis through the Medical Dictionary for Regulatory Activities [MedDRA] coding) and the use of thyroid hormones (recording the drugs chronically used through the Anatomical Therapeutic Chemical [ATC] system). Therefore, we considered the diagnosis of hypothyroidism before the hospitalization [11].
Moreover, based on hypothyroidism etiology, we categorized patients into three groups: congenital (including autoimmune etiology), acquired (including post-surgical etiology), and unspecified (including participants with no information on etiology) hypothyroidism.
Information on COVID-19 phenotype at the ward admission was collected regarding signs/symptoms, WHO clinical status, oxygen saturation (SpO2) in ambient air, and inflammatory markers. In particular, the presence of the following symptoms and signs at the disease onset was recorded from medical data collected from the patient (or the caregiver) and the clinical examination at ward admission: fever, pharyngodynia, cough, sneezing, dyspnea, tachypnoea, low oxygen saturation after walking or at rest (SpO2 ≤ 90%), weakness/prostration, fall or faint, muscle aching, delirium, loss of smell or taste, anorexia, gastrointestinal symptoms, and sudden health worsening. Based on the WHO classification [12], COVID-19 severity at hospital admission was classified into three main groups: mild disease (WHO classes 1, 2, and 3, i.e. no oxygen therapy required), moderate disease (WHO class 4, i.e. need for low-flow oxygen support), and severe disease (WHO classes 5, 6, 7, i.e. high-flow oxygen or organ support needed). Among biochemical parameters, we considered the following inflammatory markers routinely assessed at ward admission: white blood cell count (WBC; normal range: 4–10 × 10^9/l), neutrophils proportion (normal range: 45–70%), lymphocytes proportion (normal range: 20–45%), neutrophils/lymphocytes (NL) ratio, lactate dehydrogenase (LDH; normal range: < 247 U/l), C-reactive protein (CRP; normal range: < 5–10 mg/l), and procalcitonin (normal range: > 0.5 ng/ml).
The clinical outcomes over the observation period were obtained from medical documentation and classified as a clinical improvement with hospital discharge, clinical worsening, transfer to other care settings (at lower or higher intensity of care), and death.
Statistical analysis
Statistical analyses were performed using the software R (version 4.0.2). We used mean and standard deviation to describe continuous variables normally distributed, while count and percentages for the categorical ones. Sociodemographic characteristics, pre-COVID-19 mobility level, main comorbidities, signs/symptoms, and inflammatory markers were compared between patients with vs without a history of hypothyroidism using the Student t-test or the Chi-square tests. To compare the frequency of smoking habits and mobility level between patients with vs without history of hypothyroidism, we considered a p value of < 0.017 (0.05/3) as statistically significant based on Bonferroni correction for multiple comparisons. To take into account the possible confounding effect of age and sex on the differences of the characteristics of patients with vs without hypothyroidism, we performed appropriate generalized linear models and logistic regressions adjusted for age and sex.
The association of hypothyroidism with in-hospital mortality was assessed through Cox regression analysis, and the strength of such association was expressed as Hazard Ratios (HRs) and 95% Confidence Intervals (95%CIs). First, we ran unadjusted analyses (Model 1), and second, we adjusted for potential confounders (age, sex, number of chronic diseases, pre-admission mobility level, smoking habits, and admission WHO clinical status, Model 2). As a secondary analysis, we run further Cox regressions to assess the association between different types of hypothyroidism and in-hospital mortality. For all analyses, statistical significance was set at a p value < 0.05.
Results
The sample consisted of 1245 individuals, including 106 (8.5%) with hypothyroidism history (Table 1). Of the patients with hypothyroidism, 20% had a congenital and 24% had an acquired etiology, while for 30% data on hypothyroidism type was unavailable. The mean age of the participants was 78.6 (min–max: 60–105) years. Patients in the hypothyroidism group were more likely to be women (76.5% vs 45.3%), be non-smokers and to have a higher mobility level as compared with those with no history of hypothyroidism. The most common chronic diseases in the sample were arterial hypertension (78%), cardiovascular diseases (63%), diabetes mellitus (27.3%). Patients with hypothyroidism had a higher prevalence of arterial hypertension (78% vs 65%) and obesity (21.2% vs 13.3%) than their counterparts with no history of hypothyroidism, while marginally significant results were found for chronic kidney disease. No significant differences were observed in the frequency of other comorbidities.
Table 1.
Characteristics of the study sample as a whole and by a history of hypothyroidism
| All n = 1245 | History of hypothyroidism | p value | ||
|---|---|---|---|---|
| No n = 1113 | Yes n = 132 | |||
| Age | 78.6 (9.3) | 78.6 (9.4) | 78.4 (8.3) | 0.859 |
| Sex (female) | 605 (48.6) | 504 (45.3) | 101 (76.5) | < 0.001 |
| Smoking habits* | ||||
| Never | 433 (34.8) | 374 (33.6) | 59 (44.7) | 0.015 |
| Former | 178 (14.3) | 166 (14.9) | 12 (9.1) | 0.094 |
| Current | 33 (2.7) | 31 (2.8) | 2 (1.5) | 0.567 |
| Mobility level* | ||||
| Moves indipendently | 708 (56.9) | 620 (55.7) | 88 (66.7) | 0.021 |
| Uses walking aid/moves with wheelchair | 181 (14.5) | 160 (14.4) | 21 (15.9) | 0.732 |
| Wheelchair assisted/bedridden | 271 (21.8) | 249 (22.4) | 22 (16.7) | 0.164 |
| Living arrangements | ||||
| Lives at home, autonomous | 842 (67.6) | 738 (66.3) | 104 (78.8) | 0.01 |
| Lives at home assisted/NH | 220 (17.7) | 202 (18.1) | 18 (13.6) | |
| Chronic diseases | ||||
| Hypertension | 828 (66.5) | 725 (65.1) | 103 (78.0) | 0.004 |
| Cardiovascular diseases | 714 (57.3) | 630 (56.6) | 84 (63.6) | 0.147 |
| Diabetes | 304 (24.4) | 268 (24.1) | 36 (27.3) | 0.484 |
| COPD | 189 (15.2) | 164 (14.7) | 25 (18.9) | 0.252 |
| Chronic kidney disease | 159 (12.8) | 135 (12.1) | 24 (18.2) | 0.067 |
| Depression | 211 (16.9) | 183 (16.4) | 28 (21.2) | 0.208 |
| Cognitive disorders | 207 (16.6) | 187 (16.8) | 20 (15.2) | 0.721 |
| Obesity | 176 (14.1) | 148 (13.3) | 28 (21.2) | 0.02 |
Missing in smoking habits n = 601, in mobility n = 85, in living arrangements n = 183
Numbers are count (%)
COPD chronic obstructive pulmonary disease, NH nursing home
p values < 0.05 are shown in bold
*A p value < 0.017 should be considered as statistically significant according to Bonferroni correction for multiple comparison
In Table 2, patients with vs without an history of hypothyroidism are compared in terms of signs/symptoms, inflammatory markers, and oxygen requirements at ward admission. Patients in the hypothyroidism group presented lesser frequently with low oxygen saturation (42% vs 49.5%, p = 0.03) and anorexia (4.5% vs 10.5%, p = 0.01), while they were more likely to report loss of smell (2.3% vs 0.7%, p = 0.01) and muscle aching (9.1% vs 4.3%, p = 0.02). No substantial differences between groups were observed in oxygen requirements while, among the inflammatory markers, patients with hypothyroidism had higher lymphocytes levels and tended to have lower NL ratios. The differences described above were confirmed also after adjusting for age and sex (data not shown).
Table 2.
Frequency of COVID-19 signs and symptoms at the disease onset in the sample as a whole and by a history of hypothyroidism
| All (n = 1245) | History of hypothyroidism | p value | ||
|---|---|---|---|---|
| No (n = 1112) | Yes (n = 75) | |||
| Symptoms/signs of COVID-19 | ||||
| Fever | 708 (56.9) | 633 (56.9) | 75 (56.8) | 0.317 |
| Pharyngodynia | 48 (3.9) | 42 (3.8) | 6 (4.5) | 0.152 |
| Cough | 443 (35.6) | 395 (35.5) | 48 (36.4) | 0.456 |
| Sneezing | 52 (4.2) | 46 (4.1) | 6 (4.5) | 0.117 |
| Dyspnoea* | 610 (49.0) | 550 (49.4) | 60 (45.5) | 0.200 |
| Low oxygen saturation* | 607 (48.8) | 551 (49.5) | 56 (42.4) | 0.031 |
| Tachypnea* | 420 (33.7) | 378 (34.0) | 42 (31.8) | 0.08 |
| Weakness | 479 (38.5) | 436 (39.2) | 43 (32.6) | 0.07 |
| Fall or fainted | 45 (3.6) | 37 (3.3) | 8 (6.1) | 0.109 |
| Muscle aching | 60 (4.8) | 48 (4.3) | 12 (9.1) | 0.02 |
| Delirium | 86 (6.9) | 78 (7.0) | 8 (6.1) | 0.342 |
| Loss of smell | 11 (0.9) | 8 (0.7) | 3 (2.3) | 0.013 |
| Loss of taste | 14 (1.1) | 10 (0.9) | 4 (3.0) | 0.06 |
| Anorexia | 123 (9.9) | 117 (10.5) | 6 (4.5) | 0.01 |
| Gastrointestinal symptoms | 134 (10.8) | 123 (11.1) | 11 (8.3) | 0.164 |
| Sudden health worsening | 240 (19.3) | 213 (19.1) | 27 (20.5) | 0.221 |
| Inflammatory markers | ||||
| WBC [× 109/l] | 7.6 (6.0) | 7.7 (6.2) | 6.9 (4.2) | 0.28 |
| Neutrophils [%] | 74.2 (14.9) | 74.5 (15.2) | 71.7 (12.7) | 0.15 |
| Lymphocytes [%] | 15.7 (10.2) | 15.3 (10.0) | 19.5 (11.1) | 0.001 |
| NLratio | 8.4 (11.6) | 8.7 (12.1) | 6.0 (5.9) | 0.078 |
| LDH [U/l] | 362.1 (204.7) | 366.8 (211.0) | 322.9 (136.9) | 0.126 |
| CRP [mg/l] | 108.6 (344.7) | 112.1 (361.0) | 76.7 (122.1) | 0.426 |
| Procalcitonin [ng/ml] | 1.4 (7.0) | 1.4 (7.3) | 1.1 (3.8) | 0.801 |
| Disease severity (WHO) | 0.164 | |||
| No oxygen therapy | 313 (25.1) | 276 (24.8) | 37 (28.0) | 0.482 |
| Low-flow oxygen therapy | 476 (38.2) | 422 (37.9) | 54 (40.9) | 0.566 |
| High-flow oxygen therapy, NIV or intubation | 295 (23.7) | 274 (24.6) | 21 (15.9) | 0.034 |
Missing in fever n = 314, pharyngodynia n = 434, cough n = 376, sneezing n = 431, dyspnea n = 336, low SpO2 n = 371, tachypnea n = 437, weakness n = 437, fall or fainted n = 486, muscle aching n = 507, delirium n = 460, loss of smell n = 590, loss of taste n = 591, anorexia n = 497, gastrointestinal symptoms n = 462, sudden health worsening n = 462, WBC n = 823, neutrophils n = 507, lymphocytes n = 628, NLratio n = 620, CRP n = 725, procalcitonin n = 587, oxygen requirements n = 161
CRP C-reactive protein, LDH lactic dehydrogenase, NLratio neutrophil-to-lymphocyte ratio, WBC white blood cell count
p values < 0.05 are shown in bold
*Data are in ambient air
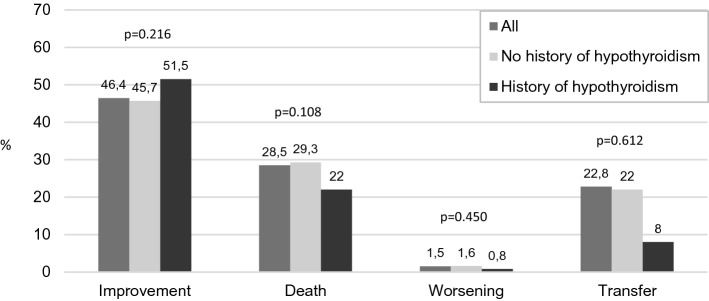
Figure 1 compares the clinical outcomes of COVID-19 patients with vs without hypothyrodism history. During the hospitalization, 342 (28.5%) patients died, 313 in the non-hypothyroidism group (28.5%) and 29 (22%) in the hypothyroidism group (p = 0.108). Among the 556 (46.4%) COVID-19 patients with a clinical improvement, 488 (45.7%) were non-hypothyroid, and 68 (51.5%) were hypothyroid (p = 0.216). Among the 18 (1.5%) patients with a clinical worsening during the hospitalization, 17 had not a hypothyroidism history and only 1 (0.8%) was in the hypothyroidism group (p = 0.450). Finally, concerning the 273 (22.8%) patients who were moved to a different setting, patients without hypothyroidism were 240 and 33 (25%) COVID-19 patients had a hypothyroidism history (p = 0.612).
Fig. 1.
Clinical outcomes of older COVID-19 inpatients with and without history of hypothyroidism
At Cox regression (Supplementary Table 1), hypothyroidism history was associated with a 34% lower risk of in-hospital mortality in the unadjusted model (HR = 0.66, 95% CI 0.45–0.96 p = 0.03). Conversely, no significant results were observed after adjusting for potential confounders (HR = 0.69, 95% CI 0.47–1.03, p = 0.07). Similar non-significant results emerged at our secondary analysis, especially for congenital hypothyroidism, despite the small number of patients in each hypothyroidism group (Supplementary Table 2).
Discussion
This multicenter observational study suggests that having a history of hypothyroidism does not substantially impact in-hospital mortality of older patients with COVID-19.
Although not all the study participants had available information on hypothyroidism etiology, the results of our main analysis seemed to be confirmed, especially for those with congenital hypothyroidism. However, further studies with larger sample sizes are needed to explore this issue better.
The prevalence of hypothyroidism in our sample was 8.5%, slightly higher than other cohorts [5, 13–15] probably for the older mean age of the involved participants. In line with other studies, patients with hypothyroidism, compared to the without hypothyroidism ones, were more frequently females, non-smokers, and had greater self-sufficiency and mobility levels. Among the main comorbidities, we found a higher prevalence of arterial hypertension and obesity in the hypothyroidism group but no differences in cardiovascular diseases or other chronic conditions. Although these findings are not in line with some studies underlining an association between hypothyroidism and increased cardiovascular risk, they comply with other works that failed to show a higher risk of cardiovascular diseases linked to subclinical hypothyroidism in advanced age [13]. Moreover, patients with hypothyroidism tended to present more frequently kidney dysfunctions, supporting the well-known influence that thyroid hormones have on renal growth, glomerular filtration rate, and renal transport systems [14, 15].
When considering the pattern of signs/symptoms at COVID-19 onset, we found that the hypothyroidism group was less likely to present anorexia and low oxygen saturation [16], which is a well-known red flag for COVID-19 greater respiratory involvement and poor prognosis. The latter aspect could be ascribed to a milder cytokine response in patients with thyroid disorders, which may less strongly affect pulmonary function [16, 17]. Moreover, the lower prevalence of anorexia could be due to the fact that hypothyroidism is often characterized by reduced resting energy expenditure, weight gain, and appetite loss. Therefore, patients with this condition may be less sensitive to COVID-19-related anorexia than individuals without hypothyroidism [18]. Conversely, they reported more frequently muscle aching and, despite the small number of cases in our sample, loss of smell. Concerning muscle aching, several studies have found that myopathic pain is a frequent symptom of either hypo- or hyperthyroidism and that thyroid hormones modulate many pathways at the skeletal muscle level [19]. In light of this effect, we could argue that people with thyroid disorders may exacerbate muscle symptoms in response to acute diseases like COVID-19. As regards smell loss, although the few cases with this symptom do not allow us to draw any solid conclusions, our results are in line with those of a published case series, which found an association between hypothyroidism and persistent olfactory dysfunction in COVID-19 patients [3]. As for muscle pain, olfactory deficits have already been reported in hypothyroidism and its subclinical forms [20].
The biochemical examinations at ward admission showed a significant difference in the lymphocytes level between hypothyroid andnon-hypothyroid individuals. Indeed, the former presented higher values with a subsequent decrease in the NL ratio. As known, lymphopenia can be considered a negative prognostic factory of COVID-19 [21] and may be related both to the direct infection of the lymphocytes, expressing the ACE2 receptor, and, most likely, to the apoptosis generated by inflammatory cytokines. This finding, along with the features of clinical presentation, suggests that hypothyroidism in older COVID-19 patients may be associated with favourable outcomes. In keeping with these data, the history of hypothyroidism tended to be associated with lower in-hospital mortality, although this relationship is not significant when adjusting for potential confounders.
In contrast with our results, some recent evidence showed that COVID-19 patients with thyroid dysfunctions, ranging from thyrotoxicosis to hypothyroidism [1, 22–24] had an increased risk of developing severe disease. Concerning the mechanisms that may mediate this effect, some studies demonstrated that the serum concentration of thyroid hormones influences the tissue distribution of ACE2 receptors for host-cell entry. In addition, alterations of the thyroid gland and its function have been linked to cytokine storm and dysregulated inflammation [23].
On the other hand, in some works reporting a correlation between thyroid dysfunctions and poor COVID-19 prognosis, such alterations were due to non-thyroidal illnesses (NTIS) rather than of underlying thyroid disease [4]. Other authors did not find any significant difference in the prognosis of non-hypothyroid and hypothyroid patients with COVID-19 [25, 26]. Among these, the British Thyroid Association/Society for Endocrinology (BTA/SFE) concluded that controlled hypothyroidism did not significantly increase the risk or severity of viral infections [27]. Journy et al. observed that infectious diseases did not increase the mortality in hypothyroid patients despite the higher prevalence of cardiovascular diseases and diabetes mellitus in those individuals [28]. Moreover, the review of Horisberger et al. suggested that autoimmune diseases did not increase the complications of COVID-19, despite the limited evidence on this topic [25]. In another analysis, hypothyroidism was not associated with an increased risk of mechanical ventilation or death in people with COVID-19, but this study included only a limited number of hospitalized people and did not focus specifically on older patients [29].
This study has some limitations. First, data collection took place during the first waves of the pandemic; therefore, it was mostly retrospective due to the availability of limited resources. For this reason, it was not possible to routinely collect biochemical parameters related to thyroid function and verify if the hypothyroidism was compensated at the hospitalization time. Moreover, we had incomplete data on the type of hypothyroidism; therefore, we when assessing the possible differential impact of congenital and acquired hypothyroidism on COVID-19-related mortality, we had low statistical power that could affect our results. Future investigation are needed to verify and confirm our findings for the more recent COVID-19 outbreaks, evaluating causes of hypothyroidism and biochemical data on thyroid hormones. On the other hand, the study’s strengths include the multicenter design, the involvement of a large sample of older patients and the broad set of clinical and biochemical parameters to compare people with and without hypothyroidism.
Conclusion
In this study, hypothyroidism history does not seem to substantially influence the prognosis of COVID-19 in older inpatients. Still, it might be associated with a milder clinical and biochemical presentation of the disease at ward admission. Since the role of thyroid disorder in infectious diseases is unclear, further studies, including more extensive bio-humoral data about thyroid function, are needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Complete list of the GeroCovid acute ward working group (alphabetical order): Rachele Antognoli (Azienda Ospedaliero Universitaria Pisana), Raffaele Antonelli Incalzi (Policlinico Universitario Campus Bio-Medico, Roma), Maria Paola Antonietti (Ospedale Regionale di Aosta), Viviana Bagalà (Azienda Ospedaliero-Universitaria di Ferrara), Giulia Bandini (USL Toscana Centro, Ospedale San Jacopo, Pistoia), Giuseppe Bellelli (Ospedale San Gerardo, Monza), Enrico Benvenuti (USL Toscana Centro, Ospedale Santa Maria Annunziata, Bagno a Ripoli (FI)), Marina Bergamin (Azienda Ospedaliero-Universitaria di Parma), Marco Bertolotti (Azienda Ospedaliero-Universitaria di Modena), Carlo Adriano Biagini (USL Toscana Centro, Ospedale San Jacopo, Pistoia), Angelo Bianchetti (Istituto Clinico Sant'Anna, Brescia), Alessandra Bianchi (Spedali Civili, Montichiari (BS)), Mariangela Bianchi (Policlinico Sant'Orsola-Malpighi, Bologna), Silvia Bignamini (Casa di Cura San Francesco, Bergamo), Damiano Blandini (Policlinico Sant'Orsola-Malpighi, Bologna), Stefano Boffelli (Fondazione Poliambulanza, Brescia), Maura Bugada (Casa di Cura San Francesco, Bergamo), Valeria Calsolaro (Azienda Ospedaliero Universitaria Pisana), Donatella Calvani (USL Toscana Centro, Presidio Misericordia e Dolce, Prato), Elisiana Carpagnano (Ospedale Giovanni XXIII Policlinico di Bari), Barbara Carrieri (IRCCS INRCA, Ancona), Viviana Castaldo (Presidio Ospedaliero Universitario Santa Maria della Misericordia, Udine), Alessandro Cavarape (Presidio Ospedaliero Universitario Santa Maria della Misericordia, Udine), Ilaria Cazzulani (Ospedale San Gerardo, Monza), Carilia Celesti (Policlinico Universitario Campus Bio-Medico, Roma), Chiara Ceolin (Azienda Ospedale Università di Padova), Maria Giorgia Ceresini (Azienda Ospedaliero-Universitaria di Ferrara), Antonio Cherubini (IRCCS INRCA, Ancona), Anita Chizzoli (Istituto Clinico Sant'Anna, Brescia), Erika Ciarrocchi (IRCCS INRCA, Ancona), Paola Cicciomessere (Azienda Ospedaliero Universitaria di Foggia), Alessandra Coin (Azienda Ospedale Università di Padova), Annalisa Corsi (USL Toscana Centro, Ospedale San Jacopo, Pistoia), Carlo Custodero (Ospedale Giovanni XXIII Policlinico di Bari), Federica D’Agostino (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Maria Maddalena D’Errico (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Aurelio De Iorio (Azienda Ospedaliero-Universitaria di Parma), Alessandro De Marchi (Policlinico Sant'Orsola-Malpighi, Bologna), Giovambattista Desideri (Ospedale di Avezzano (AQ)), Evelyn Di Matteo (Policlinico Universitario Campus Bio-Medico, Roma), Emma Espinosa (Azienda Ospedali Riuniti Marche Nord, Fano (PU)), Luigi Esposito (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Chiara Fazio (Azienda Ospedaliero-Universitaria di Parma), Chiara Filippini (Spedali Civili, Montichiari (BS)), Lucia Fiore (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Caterina Fontana (Azienda Ospedaliero-Universitaria di Modena), Lina Forte (Ospedale di Avezzano (AQ)), Riccardo Franci Montorzi (Azienda Ospedaliero Universitaria Careggi, Firenze), Carlo Fumagalli (Azienda Ospedaliero Universitaria Careggi, Firenze), Stefano Fumagalli (Azienda Ospedaliero Universitaria Careggi, Firenze), Pietro Gareri (CDCD Catanzaro Lido, ASP Catanzaro), Antonella Giordano (Azienda Ospedaliero Universitaria Careggi, Firenze), Evelina Giuliani (USL Toscana Centro, Ospedale Santa Maria Annunziata, Bagno a Ripoli (FI)), Antonio Greco (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Andrea Herbst (Azienda Ospedaliero Universitaria Careggi, Firenze), Giuseppe Ielo (Azienda Ospedaliero-Universitaria di Parma), Antonella La Marca (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Umberto La Porta (Azienda Ospedaliero-Universitaria di Parma), Ilaria Lazzari (Policlinico Sant'Orsola-Malpighi, Bologna), Diana Lelli (Policlinico Universitario Campus Bio-Medico, Roma), Yari Longobucco (Azienda Ospedaliero-Universitaria di Parma), Flaminia Lucchini (Azienda Ospedaliero Universitaria Careggi, Firenze), Daniela Lucente (Spedali Civili, Montichiari (BS)), Lorenzo Maestri (Policlinico Sant'Orsola-Malpighi, Bologna), Marcello Maggio (Azienda Ospedaliero-Universitaria di Parma), Paola Mainquà (Azienda Ospedali Riuniti Marche Nord, Fano (PU)), Alessandra Marengoni (Spedali Civili, Montichiari (BS)), Benedetta Martin (Ospedale di Avezzano (AQ)), Valentina Massa (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Liliana Mazza (Policlinico Sant'Orsola-Malpighi, Bologna), Carmela Mazzoccoli (Ospedale Giovanni XXIII Policlinico di Bari), Fabio Monzani (Azienda Ospedaliero Universitaria Pisana), Enrico Mossello (Azienda Ospedaliero Universitaria Careggi, Firenze), Federica Morellini (Azienda Ospedaliero-Universitaria di Modena), Chiara Mussi (Azienda Ospedaliero-Universitaria di Modena), Chukwuma Okoye (Azienda Ospedaliero Universitaria Pisana), Giuseppe Orio (Policlinico Sant'Orsola-Malpighi, Bologna), Annalisa Paglia (Azienda Ospedaliero Universitaria di Foggia), Giulia Pelagalli (Azienda Ospedaliero Universitaria Careggi, Firenze), Laura Pelizzoni (Policlinico Sant'Orsola-Malpighi, Bologna), Alessandro Picci (Presidio Ospedaliero Universitario Santa Maria della Misericordia, Udine), Anette Hylen Ranhoff (University of Bergen, Norway), Francesca Remelli (Azienda Ospedaliero-Universitaria di Ferrara), Onofrio Resta (Ospedale Giovanni XXIII Policlinico di Bari), Antonella Riccardi (Policlinico Sant'Orsola-Malpighi, Bologna), Daniela Rinaldi (Ospedale di Comunità (Camposampiero), Distretto Alta Padovana, ULSS 6 Euganea, Padova), Renzo Rozzini (Fondazione Poliambulanza, Brescia), Carlo Sabbà (Ospedale Giovanni XXIII Policlinico di Bari), Leonardo Sacco (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Mariateresa Santoliquido (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Mariella Savino (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Francesco Scarso (Azienda Ospedaliero-Universitaria Sant'Andrea, Roma), Giuseppe Sergi (Azienda Ospedale Università di Padova), Gaetano Serviddio (Azienda Ospedaliero Universitaria di Foggia), Chiara Sidoli (Ospedale San Gerardo, Monza), Vincenzo Solfrizzi (Ospedale Giovanni XXIII Policlinico di Bari), Benedetta Soli (Azienda Ospedaliero-Universitaria di Modena), Laura Tafaro (Azienda Ospedaliero-Universitaria Sant'Andrea, Roma), Andrea Tedde (Azienda Ospedaliero-Universitaria di Modena), Giuseppe Dario Testa (USL Toscana Centro, Ospedale San Jacopo, Pistoia), Maria Giulia Tinti (Casa Sollievo della Sofferenza, San Giovanni Rotondo (FG)), Francesco Tonarelli (USL Toscana Centro, Presidio Misericordia e Dolce, Prato), Elisabetta Tonon (USL Toscana Centro, Ospedale San Jacopo, Pistoia), Caterina Trevisan (Ospedale di Comunità (Camposampiero), Distretto Alta Padovana, ULSS 6 Euganea, Padova; Azienda Ospedale Università di Padova), Aurora Vitali (Azienda Ospedaliero-Universitaria di Ferrara), Stefano Volpato (Azienda Ospedaliero-Universitaria di Ferrara), Francesca Zoccarato (Azienda Ospedale Università di Padova), Sonia Zotti (Policlinico Universitario Campus Bio-Medico, Roma).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and material
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The Gerocovid Observational study involved human participants. The study protocol was approved by the Campus Bio-Medico University Ethical Committee in April 2020. All participating centers received approval by their local Ethical Committee.
Informed consent
Informed consent was obtained from the patient included in GeroCovid Observational study.
Footnotes
The complete list of the GeroCovid-acute wards group members is shown in the supplementary materials.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
V. Bagalà and A. Sala equally contributed to the work.
Contributor Information
V. Bagalà, Email: viviana.bagala@edu.unife.it
the GeroCovid acute ward working group:
Rachele Antognoli, Raffaele Antonelli Incalzi, Maria Paola Antonietti, Viviana Bagalà, Giulia Bandini, Giuseppe Bellelli, Enrico Benvenuti, Marina Bergamin, Marco Bertolotti, Carlo Adriano Biagini, Angelo Bianchetti, Alessandra Bianchi, Mariangela Bianchi, Silvia Bignamini, Damiano Blandini, Stefano Boffelli, Maura Bugada, Valeria Calsolaro, Donatella Calvani, Elisiana Carpagnano, Barbara Carrieri, Viviana Castaldo, Alessandro Cavarape, Ilaria Cazzulani, Carilia Celesti, Chiara Ceolin, Maria Giorgia Ceresini, Antonio Cherubini, Anita Chizzoli, Erika Ciarrocchi, Paola Cicciomessere, Alessandra Coin, Annalisa Corsi, Carlo Custodero, Federica D’Agostino, Maria Maddalena D’Errico, Aurelio De Iorio, Alessandro De Marchi, Giovambattista Desideri, Evelyn Di Matteo, Emma Espinosa, Luigi Esposito, Chiara Fazio, Chiara Filippini, Lucia Fiore, Caterina Fontana, Lina Forte, Riccardo Franci Montorzi, Carlo Fumagalli, Stefano Fumagalli, Pietro Gareri, Antonella Giordano, Evelina Giuliani, Antonio Greco, Andrea Herbst, Giuseppe Ielo, Antonella La Marca, Umberto Porta, Ilaria Lazzari, Diana Lelli, Yari Longobucco, Flaminia Lucchini, Daniela Lucente, Lorenzo Maestri, Marcello Maggio, Paola Mainquà, Alessandra Marengoni, Benedetta Martin, Valentina Massa, Liliana Mazza, Carmela Mazzoccoli, Fabio Monzani, Enrico Mossello, Federica Morellini, Chiara Mussi, Chukwuma Okoye, Giuseppe Orio, Annalisa Paglia, Giulia Pelagalli, Laura Pelizzoni, Alessandro Picci, Anette Hylen Ranhoff, Francesca Remelli, Onofrio Resta, Antonella Riccardi, Daniela Rinaldi, Renzo Rozzini, Carlo Sabbà, Leonardo Sacco, Mariateresa Santoliquido, Mariella Savino, Francesco Scarso, Giuseppe Sergi, Gaetano Serviddio, Chiara Sidoli, Vincenzo Solfrizzi, Benedetta Soli, Laura Tafaro, Andrea Tedde, Giuseppe Dario Testa, Maria Giulia Tinti, Francesco Tonarelli, Elisabetta Tonon, Caterina Trevisan, Aurora Vitali, Stefano Volpato, Francesca Zoccarato, and Sonia Zotti
References
- 1.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilev M, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsivgoulis G, et al. Hypothyroidism is associated with prolonged COVID-19-induced anosmia: a case–control study. J Neurol Neurosurg Psychiatry. 2021;92(8):911–912. doi: 10.1136/jnnp-2021-326587. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi SS, Kalidoss VK. Is there an association between hypothyroidism and COVID 19? Wien Klin Wochenschr. 2021;133(7–8):414–415. doi: 10.1007/s00508-021-01813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murugan AK, Alzahrani AS. SARS-CoV-2: emerging role in the pathogenesis of various thyroid diseases. J Inflamm Res. 2021;14:6191–6221. doi: 10.2147/JIR.S332705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venditti P, di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci. 2006;63(4):414–434. doi: 10.1007/s00018-005-5457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann N Y Acad Sci. 2006;917(1):906–912. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 9.de Vito P, Incerpi S, Pedersen JZ, Luly P, Davis FB, Davis PJ. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21(8):879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 10.Trevisan C, et al. Assessing the impact of COVID-19 on the health of geriatric patients: the European GeroCovid observational study. Eur J Intern Med. 2021;87:29–35. doi: 10.1016/j.ejim.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monzani F (2022) Infezione da SARS-COV-2 e funzione tiroidea. Conference proceedings of 67° national conference of S.I.G.G., Rome, Italy, p 5–6
- 12.WHO R&D Blueprint (2020) novel Coronavirus COVID-19 - therapeutic trial synopsis. Geneva, Switzerland. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed Jan 2022
- 13.Decandia F. Risk factors for cardiovascular disease in subclinical hypothyroidism. Ir J Med Sci. 2018;187(1):39–43. doi: 10.1007/s11845-017-1617-9. [DOI] [PubMed] [Google Scholar]
- 14.Narasaki Y, Sohn P, Rhee CM. The interplay between thyroid dysfunction and kidney disease. Semin Nephrol. 2021;41(2):133–143. doi: 10.1016/j.semnephrol.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18(1):131–144. doi: 10.1007/s11154-016-9395-7. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, et al. Thyrotropin alters T cell development in the thymus in subclinical hypothyroidism mouse model. Scand J Immunol. 2017;85(1):35–42. doi: 10.1111/sji.12507. [DOI] [PubMed] [Google Scholar]
- 17.Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830(7):3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Amin A, Dhillo WS, Murphy KG. The central effects of thyroid hormones on appetite. J Thyroid Res. 2011;2011:1–7. doi: 10.4061/2011/306510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid hormones and skeletal muscle—new insights and potential implications. Nat Rev Endocrinol. 2014;10(4):206–214. doi: 10.1038/nrendo.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baskoy K, et al. Is there any effect on smell and taste functions with levothyroxine treatment in subclinical hypothyroidism? PLoS One. 2016;11(2):e0149979. doi: 10.1371/journal.pone.0149979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz Y, Percik R, Oberman B, Yaffe D, Zimlichman E, Tirosh A. Sick euthyroid syndrome on presentation of patients with COVID-19: a potential marker for disease severity. Endocr Pract. 2021;27(2):101–109. doi: 10.1016/j.eprac.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggeri RM, et al. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17(7):737–759. doi: 10.1080/1744666X.2021.1932467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik J, et al. Association of hypothyroidism with acute COVID-19: a systematic review. Expert Rev Endocrinol Metab. 2021;16(5):251–257. doi: 10.1080/17446651.2021.1968830. [DOI] [PubMed] [Google Scholar]
- 25.Horisberger A, Moi L, Ribi C, Comte D. Autoimmune diseases in the context of pandemic COVID-19. Rev Med Suisse. 2020;16(N° 691–2):827–830. [PubMed] [Google Scholar]
- 26.Pereira DN, et al. Hypothyroidism does not lead to worse prognosis in COVID-19: findings from the Brazilian COVID-19 registry. Int J Infect Dis. 2022;116:319–327. doi: 10.1016/j.ijid.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Society for Endocrinology (2020) BTA/SFE statement regarding issues specific to thyroid dysfunction during the COVID-19 pandemic. https://www.british-thyroid-association.org/sandbox/bta2016/management-of-thyroid-dysfunction-during-covid-19_final.pdf. Accessed Feb 2022
- 28.Journy NMY, Bernier M-O, Doody MM, Alexander BH, Linet MS, Kitahara CM. Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid. 2017;27(8):1001–1010. doi: 10.1089/thy.2017.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naymagon L, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res. 2020;196:99–105. doi: 10.1016/j.thromres.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.