Abstract
Early skin-to-skin contact (SSC), beginning in the delivery room, provides myriad health benefits for mother and baby. Early SSC in the delivery room is the standard of care for healthy neonates following both vaginal and cesarean delivery. However, there is little published evidence on the safety of this practice in infants with congenital anomalies requiring immediate postnatal evaluation, including critical congenital heart disease (CCHD). Currently, the standard practice following delivery of infants with CCHD in many delivery centers has been immediate separation of mother and baby for neonatal stabilization and transfer to a different hospital unit or a different hospital altogether. However, most neonates with prenatally diagnosed congenital heart disease, even those with ductal-dependent lesions, are clinically stable in the immediate newborn period. Therefore, we sought to increase the percentage of newborns with prenatally diagnosed CCHD who are born in our regional level II–III delivery hospitals who receive mother-baby SSC in the delivery room. Using quality improvement methodology, through a series of Plan-Do-Study-Act cycles we successfully increased mother-baby skin-to-skin contact in the delivery room for eligible cardiac patients born across our city-wide delivery hospitals from a baseline 15% to greater than 50%.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00246-023-03149-2.
Keywords: Skin-to-skin contact, Quality improvement, Congenital heart disease, Perinatal period
Introduction
One important means of supporting mother-baby bonding is skin-to-skin contact (SSC), the placement of a neonate onto mother’s chest or abdomen to permit direct ventral-to-ventral skin-to-skin contact [1]. SSC is known to confer health benefits for both mother and baby, improving maternal stress and anxiety, promoting healthy mother–child interactions, and increasing maternal breastmilk intention rates [2–9]. For baby, SSC supports autonomic regulation, weight gain, and improved immunological functioning, as well as promotes attachment and healthy emotional and cognitive development [4, 5, 10–12]. “Early SSC” refers to the initiation of SSC shortly after birth—ideally immediately after delivery and lasting through an initial breastfeeding attempt [1], however a consensus definition is lacking regarding the exact time frame for SSC initiation to qualify as “early”.
Specific benefits of early SSC are well-established and include improved fetal-to-neonatal transition and cardiorespiratory stability with enhanced autonomic regulation [2, 13, 14]. Published studies support that early SSC in healthy term and preterm neonates following both vaginal and cesarean delivery is both safe and effective [15–19], and early SSC is recommended by the American Academy of Pediatrics and World Health Organizations [20]. Further, researchers have hypothesized that this immediate postnatal period represents a critical window for neuronal programming, somatosensory system development, parent-infant bonding, social development, and reward processing and learning, suggesting the potential for longer-term neurodevelopmental benefits of early SSC [5]. For mothers, early SSC decreases analgesia requirements and risk of peripartum hemorrhage [13, 21]. Additionally, early SSC confers positive birthing experiences, decreased maternal stress, anxiety and pain, and increased rates of breastfeeding exclusivity and duration [7, 22, 23]. While data detailing contemporary early SSC rates in United States hospitals is inadequate at best, in Pennsylvania’s experience of deliveries at 34–42 weeks gestation, 56% of mothers reported being able to hold their baby within five minutes of delivery [23].
Neonates and infants with critical congenital heart disease are at risk for a number of postnatal complications, including growth failure, feeding and breastfeeding challenges, and necrotizing enterocolitis [24–28]. They are also known to be at risk for neurodevelopmental impairments [29, 30]. Ongoing SSC in an intensive care setting can be challenging, and many parents have expressed fears around holding their infant in the ICU as well as significant stress throughout the ICU stay [31, 32]. However, among neonates with CCHD, SSC around the time of cardiac surgery improved neonatal comfort and decreased maternal stress and anxiety [33–35].
Despite the strong of evidence demonstrating beneficial effects of early SSC, the separation of mother and baby following delivery remains routine in many circumstances, particularly for neonates with prenatally diagnosed congenital anomalies requiring timely postnatal evaluation [36–38]. Historically, standard practice following delivery of neonates with critical cardiac anomalies has been immediate separation of mother and baby to permit perinatal assessment and intervention by a neonatal resuscitation team, followed by transfer to a specialized unit or separate children’s hospital, while the mother remains in the delivery center post-partum unit to recover. Unique amongst other congenital anomalies, most neonates with prenatally diagnosed CCHD, even those with ductal-dependent lesions, are clinically stable in the immediate newborn period and urgent postnatal interventions are not typically required [39]. Donofrio and colleagues have published “Levels of Care” guidelines for the resuscitation of infants by cardiac lesion, supporting routine delivery care for many infants with CHD [40]. While SSC is not specifically addressed in these guidelines, in any other context routine DR care would include early SSC. Despite the overwhelming evidence of the benefits of early SSC, we are aware of only one center that has ventured to explore SSC in the delivery room (DR) for neonates with CHD [41], and we are not aware of any published guidelines specifically addressing early SSC for cardiac neonates. Utilizing quality improvement (QI) methodology, we sought to increase the percentage of newborns prenatally diagnosed with eligible CCHD lesions who receive SSC in the DR from our baseline of 15% to greater than or equal to 50% within 18 months following project initiation and sustain for 12 months.
Methods
Context
This QI project was implemented across eight regional delivery hospitals within the neonatal service line at Nationwide Children’s Hospital (NCH) in Columbus, Ohio from 2018 through 2021. NCH itself is a large, not for profit, quaternary care, freestanding pediatric teaching hospital located in the Midwest with affiliation to regional delivery hospitals. The NCH Fetal Center performs regional multidisciplinary prenatal consultation for all families with prenatally diagnosed significant CHD through collaboration with fetal cardiology, neonatology, high-risk maternal fetal medicine, and other specialists. At our centers, a modified version of the Donofrio et al. Level of Care designation is utilized following a fetal cardiac diagnosis to develop an individualized delivery and perinatal care plan, with regional delivery hospital sites representing a spectrum of volume and acuity[42]. Across our service line, delivery hospitals range from Level I-III regarding neonatal intensive care capacities and vary significantly with regards to delivery volume and acuity. Generally, high-risk fetal deliveries are cohorted to delivery at one of the two highest-level delivery hospitals.
Medical Ethics Approval
The Institutional Review Board (IRB) at NCH determined that this project was QI and not human subjects research. Therefore, IRB review and approval was not required per institutional policy.
Preintervention
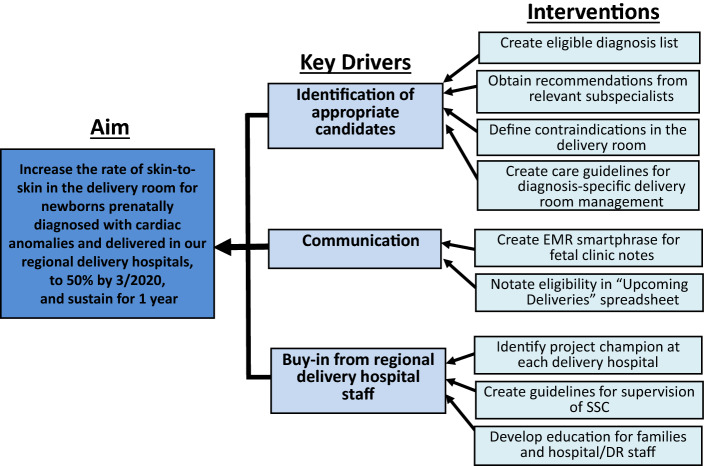
In January 2018 we assembled a multidisciplinary working group composed of key stakeholders: physicians trained in neonatology and pediatric cardiology; Fetal Center nurse coordinator; at least one neonatal nurse or nurse practitioner to champion the project at each delivery hospital (“project champion”). The team utilized strategies from the Institute for Healthcare Improvement (IHI) model for improvement, including Aim statement, Key Driver Diagram (KDD), and Plan-Do-Study-Act (PDSA) cycles to achieve project aims. After a review of baseline data, the project team developed a KDD (Fig. 1) with identification of several key drivers for success including identification of appropriate candidates, communication, and buy-in from regional delivery hospital staff to support project engagement. These drivers informed development of specific interventions which were implemented sequentially through PDSA cycles.
Fig. 1.
Key driver diagram identifies study aim, key drivers, and interventions. DR delivery room, SSC skin-to-skin care, EMR electronic medical record
Interventions
Our workgroup focused initial efforts on development of an eligible cardiac diagnosis list. We included all mother-baby dyads referred for prenatal consultation with a prenatal diagnosis of CHD determined to require postnatal admission to a neonatal or cardiac intensive care unit (NICU, CTICU). We incorporated recommendations from relevant subspecialists to define eligibility criteria based on both cardiac diagnosis and factors at delivery defining safety in the perinatal transition, and created standardized cardiac-diagnosis specific guidelines for perinatal management (such as target saturation goals, postnatal hospital unit and monitoring requirements, empiric prostaglandin need) (Table 1). Inclusion criteria supporting an expected normal perinatal transition required successfully meeting each of the following: delivery at full term gestational age (defined as minimum 37 weeks gestational age), minimum Apgar score of eight at both one and five minutes, and absence of a positive pressure ventilation requirement during delivery resuscitation.
Table 1.
DR SSC eligibility criteria
| Fetal diagnosis |
Critical congenital heart disease expected to require NICU/CTICU admission with mother-baby separation Exclusions: CHD with increased risk for cardiopulmonary instability TGA/IVS HLHS/IAS TAPVR Complete Heart block Severe Ebstein’s anomaly Any condition with increased risk for clinical deterioration in the first few hours of life |
| Delivery |
Planned delivery within neonatal service line Exclusions: clear plan in place for perinatal palliative care |
| Perinatal stability | No positive pressure ventilation needed |
| Minimum Apgar score of 8 at 1 min AND 8 at 5 min | |
| Gestational age ≥ 37 weeks |
Eligibility criteria for delivery room skin-to-skin (SSC)
NICU neonatal intensive care unit, CTICU cardiac intensive care unit, CHD congenital heart disease, TGA/IVS transposition of the great arteries with intact ventricular septum, HLHS/IAS hypoplastic left heart syndrome with intact atrial septum, TAPVR total anomalous pulmonary venous return
Next, we defined contraindications in the DR. Exclusion criteria were identified to prioritize safety in the DR. CHD diagnoses with a high chance of early clinical instability were excluded: transposition of the great arteries with intact ventricular septum, fetal heart block, hypoplastic left heart syndrome with intact or highly restrictive atrial septum, severe Ebstein’s anomaly, and total anomalous pulmonary venous return. Additionally excluded were neonates with a planned delivery outside of our neonatal delivery hospital network (since those delivery hospitals would not have received relevant education or have supervision processes established) and any patient with a non-cardiac condition for which fetal teams deemed a reasonable chance for clinical deterioration in the first few hours of life, such as patients with airway anomalies. Finally, we excluded patients with a clear perinatal palliative care plan directing that baby would exclusively remain with family to focus on bonding, given that this situation would not be expected to result in mother-baby separation for delivery room stabilization and ICU admission and management.
Our multidisciplinary team developed a generalized algorithm for SSC that allowed for center-specific individualizations based on workflow, personnel and other unit factors. We additionally created guidelines for supervision of SSC, for which all eligible patients receiving DR SSC would be directly monitored by a NICU nurse, nurse practitioner or physician who would remain physically present with baby throughout the SSC period, and cardiopulmonary stability would be evaluated via direct visual assessment and continuous pulse oximetry monitoring. Finally, standardized documentation (Data Collection Sheet) was developed to support delivery teams’ recording key elements of the SSC experience (Supplemental Fig. 3).
Initial project education included written study materials distributed across delivery centers and to members of the Fetal Center, as well as monthly virtual project meetings with open question and answer sessions. For our Fetal Center families, education regarding DR SSC was incorporated into their multidisciplinary fetal counseling session. Evaluating barriers to S2S adoption across the first two data collection quarters, it was discovered that there were varying degrees of provider comfort in caring for neonates with CHD across delivery centers. Therefore, in January of 2019 we created targeted education for DR staff across all eight regional delivery hospitals in the form of PowerPoint slides focused on perinatal instability risk by cardiac diagnosis as well as the expected course of PDA closure postnatally. For each delivery center, the project champion was tasked with dissemination and assessment of comprehension, although no formal measure was assessed.
Multiple iterations of communication pathways to effectively identify eligible patients and disseminate perinatal SSC plans across the neonatal service line were ultimately required based on our network of delivery hospitals. At the time of project go live in 2018, we implemented a first formal process to track eligible fetal patients for DR SSC, as well as an initial process for dissemination to all delivery hospital personnel via biweekly email listserv. Beginning in July of 2019, we updated this documentation process and our fetal team began to document individual patient eligibility in the electronic medical record (EMR) at the time of prenatal consultation, which was then standardized through an EMR “SmartPhrase” template in 2020. This allowed for eligible patients to be added to a DR SSC specific fetal list which was maintained by the Fetal Center nurse coordinator for tracking purposes.
Measures and Definitions
Outcome Measure:
Percentage of eligible neonates successfully receiving SSC in the delivery room: Calculated quarterly by taking all eligible patients who received SSC in the DR and dividing by the total number of eligible patients. We defined “early SSC” as direct mother-baby direct contact in the delivery room but did not formally designate a required time-to-SSC initiation or required duration.
Process Measure:
Percentage of eligible patients with a prenatally identified SSC plan: This process measure was chosen as we believed that effective development and dissemination of an SSC plan for all fetal patients would be one critical input required for delivery teams to achieve our desired outcome.
Balancing Measures:
Adverse events in the DR: Defined as provider concern for clinical decompensation, need for escalation of cardiorespiratory support or early termination of SSC attributable to any provider perceived concerns related to DR SSC.
Percent of neonates receiving DR SSC with hypothermia upon arrival to NICU: Hypothermia was defined as temperature at or below 36.0°F while hyperthermia was defined as temperature greater than or equal to 38°F.
Data Analysis and Study of Interventions
Data for analysis were obtained from July 2018 through March 2021. Our primary outcome measure was tracked on a p-chart, a type of Statistical Process Control (SPC) chart with application of established rules to identify signals of special cause variation, or nonrandom change [43]. Given relatively small denominators, we plotted data quarterly. Our SPC chart was generated using QI Macros SPC Software Version 2020.10, an add-in to Microsoft Excel. Observed improvements were felt to be directly related to the interventions implemented as described given temporal relationship of interventions to improvements and no other known changes to the system. Compliance with our process measure was gathered by performing chart review on all eligible patients during the study period with documentation of a DR SSC plan required for compliance. Adverse events were assessed by documentation within the EMR DR summary or as reported in the Data Collection Sheet. Hypothermia and hyperthermia were assessed by EMR review to determine initial recorded temperature upon NICU arrival. Both our process and balancing measures were analyzed utilizing descriptive statistics.
Results
Our baseline percentage of eligible patients who received DR S2S was 15%. Following project initiation, 124 total patients met eligibility criteria based on cardiac diagnosis alone. Two of these patients were excluded as they were delivered outside our network of delivery hospitals and another 2 had incomplete DR information to assess eligibility and were therefore excluded. Of the remaining 120 patients, 60% (72/120) met the DR transition criteria necessary for safe SSC. Of patients not meeting transition inclusion criteria (N = 48, 40%), the most common reasons were premature delivery (< 37 weeks gestational age) and Apgar score below threshold.
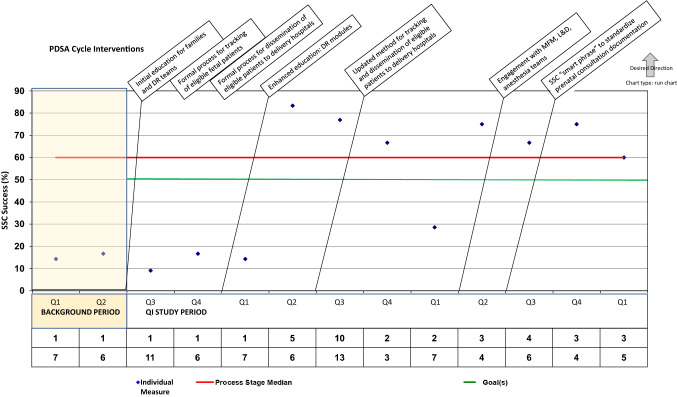
Our target SSC success rate greater than 50% was achieved around 9 months into the intervention period, with ultimate SSC success rate for our target population stabilizing around 70%. This SSC success rate was sustained for our QI project goal of 12 months. Figure 2 details percentage of eligible newborns successfully receiving SSC in the DR. Data points are presented by quarter. The isolated drop below 30% once target SSC had been established represents months during the emergence of SARS COV 2 pandemic, which across our delivery centers undoubtedly impacted factors responsible for successful SSC performance.
Fig. 2.
Percentage of eligible prenatally diagnosed congenital heart disease patients successfully achieving skin-to-skin care following delivery. Run chart depicting successful skin-to-skin (SSC) of eligible patients, data presented quarterly (Q1–4) throughout background and study periods. PDSA Plan-Do-Study-Act, DR delivery room, MFM Maternal–Fetal-Medicine subspecialty, L&D Labor and delivery
Regarding our process measure, of the 72 eligible patients, 49 (68%) had documentation of a prenatal plan for SSC, while 23 (32%) did not. This was noted as a large barrier to successful SSC early in the project; 87% (20/23) of patients with absent plan occurred in the first twelve months of study implementation, and none of these patients achieved successful SSC. PDSA cycles targeting barriers for this process measure proved critical for success.
Next, we explored SSC success by individual factors including delivery hospital and delivery modality, presented in Table 2. While delivery hospital within our regional neonatal network hospitals did not appear to significantly impact likelihood of success, delivery via cesarean section was associated with an overall SSC success rate of only 28%. Even when considering SSC success during the second half of the project, assuming a now-established culture of DR SSC, SSC was achieved only 25% of the time following cesarean delivery but 72% of the time following vaginal birth.
Table 2.
Delivery room SSC success by identified key factors
| Delivery Factor | Eligible deliveries (N) | SSC success (%, N) | ||
|---|---|---|---|---|
| Overall half | Project Second half | |||
| 1 | 38 | 50 (19/38) | 58 (7/12) | |
| 2 | 14 | 50 (7/14) | 50 (2/4) | |
| 3 | 9 | 56 (5/9) | 80 (4/5) | |
| 4 | 4 | 50 (2/4) | 100 (1/1) | |
| 5 | 4 | 0 (0/4) | 0 (0/2) | |
| 6 | 1 | 100 (1/1) | 100 (1/1) | |
| 7 | 1 | 0 (0/1) | 0 (0/1) | |
| 8 | 1 | 100 (1/1) | N/A | |
| Delivery modality | Vaginal birth | 51 (70%, 51/72) | 57 (29/51) | 72 (13/18) |
| Cesarean birth | 21 (29%, 21/72) | 29 (6/21) | 25 (2/8) | |
Delivery room SSC success by identified key factors including delivery location and delivery modality
SSC skin-to-skin care, N number of patients
Regarding study balancing measures, no deaths occurred in the DR during the study period, and every patient achieved admission to the NICU or CTICU as per their prenatal care plan. We did not identify any adverse event in the delivery room reportedly attributable to DR SSC. Evaluation of NICU admission temperature identified an overall low frequency of admission temperature outside of physiologic range. Admission hypothermia was reported in 2 (3%, 2/70 with 2 patients missing data, lowest admission temperature 35.9°F) neonates following SSC and zero neonates for whom DR SSC was not performed. Admission hyperthermia was reported in only 1 (1%, 1/70) neonate and did not represent a neonate completing DR SSC.
Discussion
Our QI project successfully developed and implemented a plan to increase DR SSC for mothers and infants with a prenatal diagnosis of CCHD. This initiative was very well received by our fetal families. While single study in nature, our results are important in that they demonstrate both feasibility and safety for this notable change in peripartum culture. Additionally, this multi-site project involved eight delivery sites across our regional neonatal network, encompassing a spectrum of NICU levels, resources, and healthcare provider teams. Out of an abundance of safety, our eligibility criteria were intentionally stringent, with the clear goal of limiting SSC to the most stable neonates with CHD. We had no reported adverse events. The dialog created by our project improved the peripartum culture in our delivery hospitals and has allowed for us to consider expanding our eligibility to permit inclusion of additional populations. Specifically, we propose that moving forward we may be able to expand eligibility to consider additional diagnoses and more lenient gestational age and one minute Apgar score, such as considering CHD neonates born at 36 weeks gestational age or with a lower one minute Apgar score, provided they meet the five minute Apgar score threshold.
Table 3 reports our group’s identified sources of challenge. Observed early barriers to successful implementation included perceptions around inadequate staffing, insufficient project awareness, and lack of DR team education. Consistent with our experience, lack of project awareness has been cited by other groups as a key barrier to successful SSC [21, 37]. Thus, early PDSA cycles targeted family and delivery provider engagement through enhanced education to improve comfort with CHD physiology, collaborative development of CCHD care and SSC supervision guidelines, and development of process for tracking eligible fetal patients/families with effective information distribution to key individuals across delivery centers. Achieving comfort, proficiency, and buy-in across our diverse regional delivery hospitals required an individualized approach at each hospital, which was most effectively achieved by partnering with the institutionally based project champion. Without question, individual delivery hospitals each identified unique site-specific barriers to implementation, including staffing model concerns, time limitations, comfort level with culture change, and multiprovider and multisubspecialty communication challenges. Additionally, we believe that family engagement through SSC education provided prenatally contributed to successful SSC; families were encouraged and empowered to request skin-to-skin following delivery.
Table 3.
Identified Barriers to Successful DR SSC
| Identified barriers to successful DR SSC |
|---|
| Lack of comfort with CHD and expected pathophysiology |
| Incomplete/inadequate prenatal documentation or communication for delivery hospital team |
| Individual delivery hospital implementation challenges |
| Staffing model concerns |
| Time limitations |
| Comfort level with culture change |
| Multiprovider and multisubspecialty communication |
| Insufficient team education/project awareness |
| Maternal cesarean section |
| Unexpected delivery outside expected regional delivery hospital |
Identified barriers to achieving successful DR SSC for patients meeting eligibility requirements
DR delivery room, SSC skin-to-skin care, CHD congenital heart disease
As we overcame early barriers, cesarean delivery emerged as the most persistent and significant barrier to DR SSC success. Similarly, cesarean delivery has been a well-documented barrier to early SSC across diverse neonatal populations [11, 36, 38, 44]. Recently, Crenshaw et al. reported that despite positive core beliefs by health care providers regarding neonatal and maternal benefits of SSC, cesarean section related concerns limiting early SSC included risk of hypothermia due to cold OR, contamination of sterile field, and inability to assess newborn with potential for maternal and/or neonatal instability [44]. However, clinical studies specifically evaluating safety of early SSC following cesarean delivery suggest this intervention not only appears safe, but also decreases maternal pain, improves maternal birth experience, and supports fetal-to-neonatal transition [14, 21, 45, 46]. Moving forward, partnering with Labor and Delivery front line providers to improve engagement and support will undoubtedly be critical for sustained SSC improvement and evolution of the perinatal culture.
For our initial QI project, we prioritized achievement of any DR SSC as our first outcome, regardless of duration, as this felt most realistically attainable within our service line culture. By large database review, no outcome differences were observed when SSC duration was stratified into less than 60 min versus more than one hour [1]. While this suggests that any period of early SSC may be beneficial, a more complete understanding of the effects of early SSC duration on desired mother-baby outcomes would be clinically important. Additionally, incorporating routine DR breastfeeding will be an important target outcome as we aim to improve mother-baby bonding and optimize longer-term maternal and neonatal health.
Important for both families and caregivers, prioritizing mother-baby bonding and breastmilk use may be important contributors in optimizing care and outcomes specific to the CHD population. Additionally, fathers and partners undoubtedly represent yet another important contributor in early bonding and family health. While our study was limited in scope to birth mothers, moving forward a more expansive consideration exploring potential benefits in SSC between neonate and additional parent/caregiver would undoubtedly prove valuable for optimizing family-centered care and family bonding.
Considering the big picture, early skin-to-skin care following delivery represents one easily achievable piece of a much larger puzzle mapping opportunities to optimize family-centered care, family bonding, and health and wellness outcomes for both families and babies. This may be particularly important when considering challenges of families affected by high-risk congenital anomalies such as CCHD. Our strong belief is that this comprehensive, collaborative, and family-centered approach must start prenatally, continue perinatally, and extend postnatally throughout hospitalization and homegoing.
Limitations of our study include relatively small patient numbers, owning to pilot exclusively within our Nationwide Children’s Hospital delivery network. Additionally, we acknowledge that assessment of measures and outcome was dependent upon their inclusion within documentation in the EMR or data sheet. Further, our endpoint of SSC success was believed to be a first important outcome, however additional details around the SSC experience such as age of life at initiation, SSC duration, and opportunity to attempt initial breastfeed will be important to explore moving forward.
Conclusion
We demonstrated safety and efficacy of early mother-baby skin-to-skin contact following delivery of neonates prenatally diagnosed with high-risk cardiac anomalies for which postnatal care required separation of mother-baby for neonatal intensive care. Overwhelmingly, delivery via cesarean section represents the most persistent and significant barrier to SSC success. We are hopeful that evolution of DR culture supporting SSC will have “trickle down” effects on pediatric health and maternal and family wellbeing; this may be particularly impactful for families already facing a potentially life-threatening condition for their child.
Larger-scale studies and exploration of early SSC effect on expanded clinical and longitudinal outcomes, such as feeding, growth and necrotizing enterocolitis, or duration of maternal breastfeeding and breastmilk use, as well as maternal wellbeing and mother-baby bonding would be important next steps. Additionally, incorporating early direct breastfeeding efforts into this SSC experience could prove beneficial for both mother and baby.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Regional Delivery Center Project Champions. Armbruster D, Fosselman K, Holley W, Lewis C, Jagger A, Jalbuena A, Saft S, Thiel A, Thiel L, Thomas L, Thomas T.
Author contributions
MB, RC, KT conceived of study design. RS, CS, KN, AM, LB, AB assisted with further study refinement and implementation as well as with data collection. RS, JM, RB, TC, KT assisted with QI design, data interpretation, and Fig. 2 development. RS prepared Fig. 1 and Fig. 3. MB wrote the main manuscript text and prepared Tables and Figures, while TC wrote substantive portions of the methodology and analysis section. All authors reviewed the manuscript at multiple key timepoints.
Funding
The authors have no financial relationships relevant to this article to disclose.
Declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moore ER, Bergman N, Anderson GC, Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11:CD003519. doi: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi Y, Tamakoshi K, Matsushima M, Kawabe T. Comparison of salivary cortisol, heart rate, and oxygen saturation between early skin-to-skin contact with different initiation and duration times in healthy, full-term infants. Early Hum Dev. 2011;87(3):151–157. doi: 10.1016/j.earlhumdev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Cong S, Wang R, Fan X, Song X, Sha L, Zhu Z, et al. Skin-to-skin contact to improve premature mothers' anxiety and stress state: a meta-analysis. Matern Child Nutr. 2021;17(4):e13245. doi: 10.1111/mcn.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigelow AE, Power M. Mother-infant skin-to-skin contact: short- and long-term effects for mothers and their children born full-term. Front Psychol. 2020;11:1921. doi: 10.3389/fpsyg.2020.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carozza S, Leong V. The role of affectionate caregiver touch in early neurodevelopment and parent-infant interactional synchrony. Front Neurosci. 2020;14:613378. doi: 10.3389/fnins.2020.613378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs. 2018;20(1):54–62. doi: 10.1177/1099800417735633. [DOI] [PubMed] [Google Scholar]
- 7.Bramson L, Lee JW, Moore E, Montgomery S, Neish C, Bahjri K, et al. Effect of early skin-to-skin mother–infant contact during the first 3 hours following birth on exclusive breastfeeding during the maternity hospital stay. J Hum Lact. 2010;26(2):130–137. doi: 10.1177/0890334409355779. [DOI] [PubMed] [Google Scholar]
- 8.Sakala C, Romano AM, Buckley SJ. Hormonal Physiology of Childbearing, an Essential Framework for Maternal-Newborn Nursing. J Obstet Gynecol Neonatal Nurs. 2016;45(2):264–75. doi: 10.1016/j.jogn.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Bigelow A, Power M, MacLellan-Peters J, Alex M, McDonald C. Effect of mother/infant skin-to-skin contact on postpartum depressive symptoms and maternal physiological stress. J Obstet Gynecol Neonatal Nurs. 2012;41(3):369–382. doi: 10.1111/j.1552-6909.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 10.Samra NM, Taweel AE, Cadwell K. Effect of intermittent kangaroo mother care on weight gain of low birth weight neonates with delayed weight gain. J Perinat Educ. 2013;22(4):194–200. doi: 10.1891/1058-1243.22.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong X, Cusson RM, Walsh S, Hussain N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012;13(7):636–645. doi: 10.1016/j.jpain.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Marín Gabriel MA, Llana Martín I, López Escobar A, Fernández Villalba E, Romero Blanco I, Touza PP. Randomized controlled trial of early skin-to-skin contact: effects on the mother and the newborn. Acta Paediatr. 2010;99(11):1630–1634. doi: 10.1111/j.1651-2227.2009.01597.x. [DOI] [PubMed] [Google Scholar]
- 14.Billner-Garcia R, Spilker A, Goyal D. Skin to skin contact: newborn temperature stability in the operating room. MCN Am J Matern Child Nurs. 2018;43(3):158–163. doi: 10.1097/NMC.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 15.Brimdyr K, Cadwell K, Stevens J, Takahashi Y. An implementation algorithm to improve skin-to-skin practice in the first hour after birth. Matern Child Nutr. 2018;14(2):e12571. doi: 10.1111/mcn.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyqvist KH, Rosenblad A, Volgsten H, Funkquist EL, Mattsson E. Early skin-to-skin contact between healthy late preterm infants and their parents: an observational cohort study. PeerJ. 2017;5:e3949. doi: 10.7717/peerj.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Espino LF, Zuniga-Villanueva G, Ramirez-GarciaLuna JL. An educational intervention to implement skin-to-skin contact and early breastfeeding in a rural hospital in Mexico. Int Breastfeed J. 2019;14:8. doi: 10.1186/s13006-019-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd MM. Implementing skin-to-skin contact for cesarean birth. AORN J. 2017;105(6):579–592. doi: 10.1016/j.aorn.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Stevens J, Schmied V, Burns E, Dahlen H. Immediate or early skin-to-skin contact after a Caesarean section: a review of the literature. Matern Child Nutr. 2014;10(4):456–473. doi: 10.1111/mcn.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.USA B-F (2018) The 10 steps to successful breastfeeding
- 21.Wagner DL, Lawrence S, Xu J, Melsom J. Retrospective chart review of skin-to-skin contact in the operating room and administration of analgesic and anxiolytic medication to women after cesarean birth. Nurs Womens Health. 2018;22(2):116–125. doi: 10.1016/j.nwh.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Lau Y, Tha PH, Ho-Lim SST, Wong LY, Lim PI, Citra Nurfarah BZM, et al. An analysis of the effects of intrapartum factors, neonatal characteristics, and skin-to-skin contact on early breastfeeding initiation. Matern Child Nutr. 2018;14(1):e12492. doi: 10.1111/mcn.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brubaker LH, Paul IM, Repke JT, Kjerulff KH. Early maternal-newborn contact and positive birth experience. Birth. 2019;46(1):42–50. doi: 10.1111/birt.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman V, Zühlke L, Murray K, Morrow B. Prevalence of feeding and swallowing disorders in congenital heart disease: a scoping review. Front Pediatr. 2022;10:843023. doi: 10.3389/fped.2022.843023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello CL, Gellatly M, Daniel J, Justo RN, Weir K. Growth restriction in infants and young children with congenital heart disease. Congenit Heart Dis. 2015;10(5):447–456. doi: 10.1111/chd.12231. [DOI] [PubMed] [Google Scholar]
- 26.Brief F, Guimber D, Baudelet JB, Houeijeh A, Piéchaud JF, Richard A, et al. Prevalence and associated factors of long-term growth failure in infants with congenital heart disease who underwent cardiac surgery before the age of one. Pediatr Cardiol. 2022;43(8):1681–1687. doi: 10.1007/s00246-022-02933-w. [DOI] [PubMed] [Google Scholar]
- 27.Jones CE, Desai H, Fogel JL, Negrin KA, Torzone A, Willette S, et al. Disruptions in the development of feeding for infants with congenital heart disease. Cardiol Young. 2021;31(4):589–596. doi: 10.1017/S1047951120004382. [DOI] [PubMed] [Google Scholar]
- 28.Kashif H, Abuelgasim E, Hussain N, Luyt J, Harky A. Necrotizing enterocolitis and congenital heart disease. Ann Pediatr Cardiol. 2021;14(4):507–515. doi: 10.4103/apc.apc_30_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 30.Ortinau CM, Smyser CD, Arthur L, Gordon EE, Heydarian HC, Wolovits J, Nedrelow J, Marino BS, Levy VY (2022) Optimizing neurodevelopmental outcomes in neonates with congenital heart disease. Pediatrics. 150(Suppl 2):e2022056415L. 10.1542/peds.2022-056415L [DOI] [PMC free article] [PubMed]
- 31.Lisanti AJ, Golfenshtein N, Medoff-Cooper B. The pediatric cardiac intensive care unit parental stress model: refinement using directed content analysis. ANS Adv Nurs Sci. 2017;40(4):319–336. doi: 10.1097/ANS.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood E, Karpyn A, Demianczyk AC, Ryan J, Delaplane EA, Neely T, et al. Mothers and fathers experience stress of congenital heart disease differently: recommendations for pediatric critical care. Pediatr Crit Care Med. 2018;19(7):626–634. doi: 10.1097/PCC.0000000000001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisanti AJ, Buoni A, Steigerwalt M, Daly M, McNelis S, Spatz DL. Kangaroo care for hospitalized infants with congenital heart disease. MCN Am J Matern Child Nurs. 2020;45(3):163–168. doi: 10.1097/NMC.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisanti AJ, Demianczyk AC, Costarino A, Vogiatzi MG, Hoffman R, Quinn R, et al. Skin-to-skin care is associated with reduced stress, anxiety, and salivary cortisol and improved attachment for mothers of infants with critical congenital heart disease. J Obstet Gynecol Neonatal Nurs. 2021;50(1):40–54. doi: 10.1016/j.jogn.2020.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lisanti AJ, Demianczyk AC, Costarino A, Vogiatzi MG, Hoffman R, Quinn R, et al. Skin-to-skin care is a safe and effective comfort measure for infants before and after neonatal cardiac surgery. Pediatr Crit Care Med. 2020;21(9):e834–e841. doi: 10.1097/PCC.0000000000002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdulghani N, Edvardsson K, Amir LH. Worldwide prevalence of mother-infant skin-to-skin contact after vaginal birth: a systematic review. PLoS ONE. 2018;13(10):e0205696. doi: 10.1371/journal.pone.0205696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahsina T, Hossain AT, Ruysen H, Rahman AE, Day LT, Peven K, et al. Immediate newborn care and breastfeeding: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021;21(Suppl 1):237. doi: 10.1186/s12884-020-03421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee D, Chandra Shaw S, Venkatnarayan K, Dudeja P. Skin-to-skin contact at birth for vaginally delivered neonates in a tertiary care hospital: a cross-sectional study. Med J Armed Forces India. 2020;76(2):180–184. doi: 10.1016/j.mjafi.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson BA, Ades A. Delivery room and early postnatal management of neonates who have prenatally diagnosed congenital heart disease. Clin Perinatol. 2005;32(4):921–46. doi: 10.1016/j.clp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 41.Barker PCA, Tatum GH, Campbell MJ, Camitta MGW, Milazzo AS, Hornik CP, et al. Improving maternal-infant bonding after prenatal diagnosis of CHD. Cardiol Young. 2018;28(11):1306–1315. doi: 10.1017/S104795111800121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donofrio MT, Levy RJ, Schuette JJ, Skurow-Todd K, Sten MB, Stallings C, et al. Specialized delivery room planning for fetuses with critical congenital heart disease. Am J Cardiol. 2013;111(5):737–747. doi: 10.1016/j.amjcard.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 43.Provost LP, Murray SK. The health care data guide : learning from data for improvement. 1. San Francisco: Jossey-Bass; 2011. [Google Scholar]
- 44.Crenshaw JT, Adams ED, Gilder RE, Nolte HG. Measuring health professionals' beliefs about skin-to-skin care during a cesarean. Matern Child Nutr. 2021;17(4):e13219. doi: 10.1111/mcn.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolan A, Lawrence C. A pilot study of a nursing intervention protocol to minimize maternal-infant separation after Cesarean birth. J Obstet Gynecol Neonatal Nurs. 2009;38(4):430–442. doi: 10.1111/j.1552-6909.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 46.Schneider LW, Crenshaw JT, Gilder RE. Influence of immediate skin-to-skin contact during cesarean surgery on rate of transfer of newborns to NICU for observation. Nurs Womens Health. 2017;21(1):28–33. doi: 10.1016/j.nwh.2016.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.