Abstract
Exercise is an effective strategy to prevent and improve obesity and related metabolic diseases. Exercise increases the metabolic demand in the body. Although many of the metabolic health benefits of exercise depend on skeletal muscle adaptations, exercise exerts many of its metabolic effects through the liver, adipose tissue, and pancreas. Therefore, exercise is the physiological state in which inter-organ signaling is most important. By contrast, circadian rhythms in mammals are associated with the regulation of several physiological and biological functions, including body temperature, sleep-wake cycle, physical activity, hormone secretion, and metabolism, which are controlled by clock genes. Glucose and lipid tolerance reportedly exhibit diurnal variations, being lower in the evening than in the morning. Therefore, the effects of exercise on substrate metabolism at different times of the day may differ. In this review, the importance of exercise timing considerations will be outlined, incorporating a chrono-exercise perspective.
Keywords: Chrono-exercise, Exercise timing, Inter-organ communication, Circadian rhythm, Energy metabolism
Abbreviations
- SCN
suprachiasmatic nucleus
- CREB
cAMP response element binding protein
- AMPK
adenosine monophosphate-activated protein kinase
- TG
triglycerides
- LDL-C
low-density lipoprotein cholesterol
- HDL-C
high-density lipoprotein cholesterol
- FFA
free fatty acids
- GLP-1
glucagon-like peptide 1
- LPL
lipoprotein lipase
- SCFAs
short-chain fatty acids
- ZMP
5-aminimidazole-4-carboxamide ribonucleotide
- AICAR
5-aminimidazole-4-carboxamide ribonucleoside
- ATP
Adenosine triphosphate
- BCAA
branched-chain amino acid
- BHBA
β-hydroxybutyrate
- HIF1α
hypoxia-inducible factor 1α
Introduction
Physical inactivity is a risk factor for lifestyle-related diseases. One in four people worldwide does not reach the amount of physical activity recommended by the exercise guidelines.1 Physical activity (exercise and daily physical activity) is recognized as the basis for the prevention, management, and treatment of chronic diseases such as obesity and type 2 diabetes.2,3 Physical activity is a movement that is carried out by the skeletal muscles that require energy. In other words, any movement one does is physical activity. Exercise, however, is planned, a structured, repetitive, and intentional movement intended to improve or maintain physical fitness. Exercise is a subcategory of physical activity. Most daily physical activity is considered light to moderate in intensity. However, certain health benefits can only be accomplished with more strenuous physical activity. Improvement in cardiovascular fitness is one example. Jogging or running provides greater cardiovascular benefits than walking at a leisurely pace.4 This recognition is premised on the axiomatic understanding that “exercise is medicine.” Lifestyle interventions that incorporate increased physical activity are a major preventive approach to metabolic diseases, such as obesity and type 2 diabetes mellitus, and a possible reason why exercise is effective in preventing and improving them is the increased energy expenditure caused by exercise.5 In addition, exercise increases the metabolism in the skeletal muscles used for exercise. Repeated and appropriate exercises have been shown to improve the ability of skeletal muscles to uptake glucose.6 Thus, exercise is a powerful tool for metabolic disease prevention and improves the metabolic phenotype of the skeletal muscle, liver, adipose tissue, and pancreas. In addition, these tissue adaptations are important in the body's response to exercise, not only because exercise activates signaling intrinsic to each tissue, but also because interorgan communication by various signaling molecules, hormones, myokines, cytokines, adipokines, and other exercise-induced soluble factors plays an important role in the body's response to exercise.7 These humoral factors secreted into circulation by the tissues in response to exercise are collectively referred to as "Exerkine".8 Exerkine is released in response to acute and chronic exercise and exerts its effects through endocrine, paracrine, and/or autocrine pathways. Therefore, in this review, we provide a well-established broad concept rather than delve into the specific mechanism of action. In addition, we introduce a new perspective on chrono-exercise and outline the effects on inter-organ communication during exercise execution at different times.
Chrono-exercise
Exercise guidelines, which are effective in promoting health and preventing or improving metabolic diseases, have recommended exercise volume, frequency, and intensity.2,3 However, these guidelines do not mention the timing of the exercise. One reason is the insufficient evidence regarding the biological effects of exercise timing. By contrast, the endocrine and nervous systems associated with metabolic responses exhibit diurnal variations.9,10 These include various cytokines that play an important role in the development of obesity and diabetes and catecholamines (adrenaline and noradrenaline) related to energy metabolism.11,12 Previous studies have reported that catecholamines are affected by diurnal variations, with concentrations increasing from morning to midday.11 Thus, physiological responses may differ depending on the timing of the exercise.
In 2017, the Nobel Prize in Physiology or Medicine was awarded for the elucidation of the molecular mechanisms of the biological clock. Interest in the biological clock is growing in the applied research field. Specifically, it has been applied in the field of sports science, leading to a new field called chrono-exercise, which considers exercise timing “when”.13 Since then, chrono-exercise has emerged as a research field focused on the study of biological rhythms and interactions between metabolic processes. Sports science focuses on the type and intensity of exercise. On the contrary, chrono-exercise is a discipline that aims to maintain and improve people's health by adding factors such as “when” people exercise throughout the day. Chrono-exercise is considered to have two major aspects. The first is a regulatory effect that contributes to changing or resetting the biological clock through exercise. The biological clock is important in forming a rhythm of approximately 24 h per day, and disruption of the biological clock contributes to various metabolic abnormalities, including obesity and diabetes.14 Hence, the idea is to promote health by correcting disturbances in the biological clock through exercise (Fig. 1A). The second is the concept of devising exercise timing based on circadian rhythm. Diurnal variations exist in the secretion of various hormones that are involved in energy metabolism. The idea is to use this time information for efficient exercise, energy substrate utilization, or metabolic enhancement (Fig. 1B).
Fig. 1.
Schematic diagram of chrono-exercise.
There are two aspects to chrono-exercise. The effect of exercise timing on a biological clock (A), Considering the diurnal variation of substrate metabolism, hormones, and other factors regulated by the biological rhythm, exercise timing is considered (B).
Overview of circadian rhythm on substrate metabolism
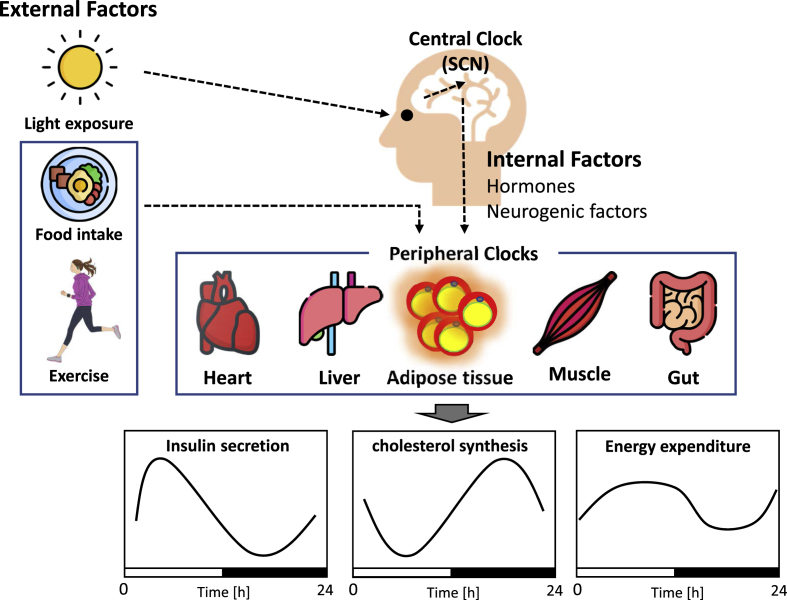
The biological clock provides a constant cycle (biological rhythm) in the body and is related to the regulation of various physiological and biological functions, including digestion, absorption, metabolism, and endocrine functions.15 The biological clock of mammals, including humans, is broadly classified into a central clock in the suprachiasmatic nucleus (SCN), which resides in the microscopic neuronal nucleus in the hypothalamus of the brain, and a peripheral clock in other brain regions and all tissues in the body, including the liver, kidney, adipose tissue, and skeletal muscle. Photic stimulation is the most important stimulus for central clock synchronization. The photic signal information received from the retina is transmitted to the SCN, and synchronization is initiated by promoting the transcription of Per1 (Period1) and Per2 through phosphorylation of cAMP response element binding protein (CREB). Subsequently, the phases of downstream peripheral tissues are adjusted by signals from humoral factors such as neurogenic factors and hormones in the body.16,17 By contrast, peripheral organs are also entrained by stimuli other than the central clock (e.g., exercise and diet).18,19 The mechanism that generates these circadian rhythms is thought to be a negative feedback loop via the transcription and translation of clock genes in cells.
This clock system collectively regulates a wide range of metabolic targets, including glucocorticoids, the master energy sensor adenosine monophosphate-activated protein kinase (AMPK), the rate-limiting step in fatty acid and cholesterol synthesis, and the hepatic CREB, which regulates gluconeogenesis.20, 21, 22, 23, 24 As a result, various metabolic processes, such as insulin sensitivity, insulin secretion, cholesterol synthesis, fat oxidation, and energy expenditure, are in rhythm throughout the 24-h daily cycle25,26 (Fig. 2). In addition, the disruption of circadian rhythms is associated with the development of metabolic diseases, such as obesity and diabetes.14 Therefore, exercise timing should be considered to reduce the risk of obesity, diabetes, and other metabolic diseases.
Fig. 2.
The architecture of the circadian system
The biological clock is divided into the central clock and the peripheral clock. The central clock resides in the suprachiasmatic nucleus (SCN) in the hypothalamus of the brain. Light is the most powerful stimulus that regulates the central clock, and this stimulus is transmitted through the optic nerve of the eye to the SCN, where the internal clock is synchronized. Signals from the central clock are transmitted to peripheral tissues by humoral and neural factors such as hormones to synchronize the peripheral clocks. On the other hand, exercise and food intake synchronize the body clock without mediating the central clock. Also, the synchronization of clocks in different tissues creates a coordinated circadian rhythm of metabolic processes such as insulin secretion, cholesterol synthesis, and energy expenditure.
The first evidence of circadian rhythms in glucose metabolism was reported in the late 1960s.27 Since then, glucose tolerance in humans has been shown to exhibit diurnal variation and has been reported to be lower in the evening than in the morning.28, 29, 30 The diurnal variation in glucose tolerance is surprisingly large, and even adults with normal glucose tolerance in the morning have been reported to be metabolically comparable to prediabetics in the evening.28,31 Furthermore, oral glucose tolerance studies in prediabetics have shown that blood glucose levels are 40 mg/dL higher in the evening than in the morning and are metabolically comparable to prediabetics and early-stage diabetics at dinner.32 In addition, previous studies have shown that insulin sensitivity and β-cell reactivity to glucose, which are thought to contribute to impaired glucose tolerance, are decreased at dinner compared with that at breakfast.33 Therefore, postprandial blood glucose levels are more likely to be higher after dinner than after breakfast.34,35 Postprandial hyperglycemia is an independent risk factor for diabetes and cardiovascular disease.36 Therefore, it is important to control postprandial hyperglycemia and fasting blood glucose levels.
Lipid metabolism also exhibits circadian rhythm. Previous studies have shown that triglyceride (TG) and cholesterol syntheses exhibit diurnal variations and are higher at night.37,38 In other words, lipid tolerance is also lower in the evening than in the morning. Most studies have shown that TGs exhibit diurnal variations.39,40 TGs vary by 33%–63% during the day, with peaks in the late afternoon and evening.39,41 However, the diurnal variability in cholesterol levels was small, and there was no consistency in the peak time. The diurnal variation in low-density lipoprotein cholesterol (LDL-C) levels is less than 10%, and total cholesterol and high-density lipoprotein cholesterol (HDL-C) levels do not show diurnal variation or exhibit rhythms of very small amplitude.38, 39, 40,42,43 However, the synthesis rate of cholesterol has been reported to peak at approximately 22:00 and fluctuates widely.44
Effect of exercise on metabolic health
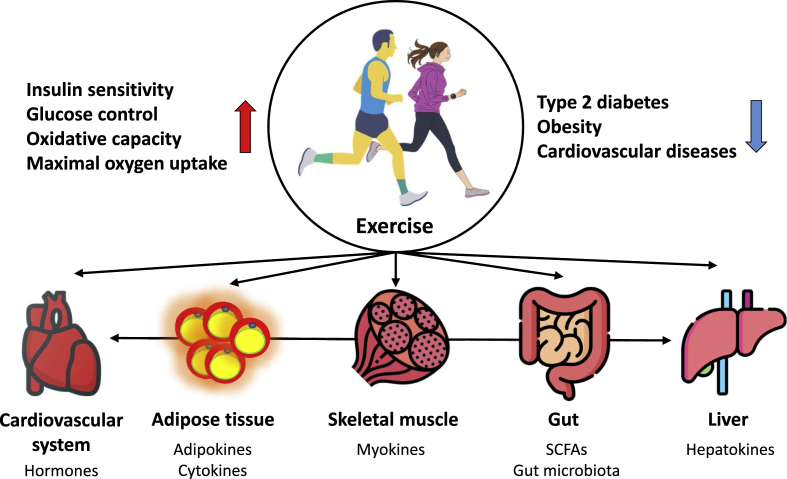
Adaptation to exercise is a complex process involving diverse changes in transcriptional and translational responses, mitochondrial function, metabolic regulation, and the signaling pathways that govern these changes.6 Briefly, molecular and metabolic responses to exercise can be categorized into acute and chronic exercises. Acute exercise alters the expression of various genes and protein phosphorylation to promote muscle adaptation.45,46 The effect of acute exercise on the major peripheral organs involved in the regulation of energy homeostasis is that muscles immediately mobilize stored glucose and fatty acids and uptake glucose and fatty acids from the plasma to meet energy demands. In endurance exercise, adipose tissue and the liver mobilize fatty acids and synthesize glucose, respectively, to keep muscles fueled.47 However, the acute exercise response is not sufficient to alter muscle phenotype. Rather, phenotypic adaptation in response to chronic exercise training requires the accumulation of repeated transient exercise-induced stimuli (Fig. 3).
Fig. 3.
Organs and tissues affected by exercise
Exercise has tremendous health benefits, including reducing the incidence and severity of metabolic diseases and increasing healthy life expectancy. In addition, peripheral organs and tissues engage in inter-organ crosstalk through bioactive substances such as hormones and cytokines.
Exercise training changes protein levels and subsequent enzyme functions, resulting in improved exercise capacity. Exercise training increases maximal oxygen uptake, decreases resting heart rate and blood pressure, and increases muscle mass.5,6,48 Blood glucose uptake by the muscle, adipose tissue, and liver is increased, peripheral tissue insulin sensitivity is improved, and β-cell function is improved.49,50 The ability to mobilize free fatty acids (FFA) from adipose tissue is improved, as is the ability of the liver to produce glucose and decrease lipogenesis.47 The ability to oxidize lipids in the muscle and liver is improved along with improved mitochondrial oxidative capacity, biosynthesis, and dynamics. As a result, the accumulation of visceral adipose tissue is reduced. These structural, functional, and metabolic adaptations improve aerobic capacity, systemic insulin sensitivity, glucose control, and oxidative capacity and decrease dyslipidemia and chronic inflammation.51,52 These changes reduce the risk of insulin resistance, obesity, type 2 diabetes, and cardiovascular diseases (Fig. 3).
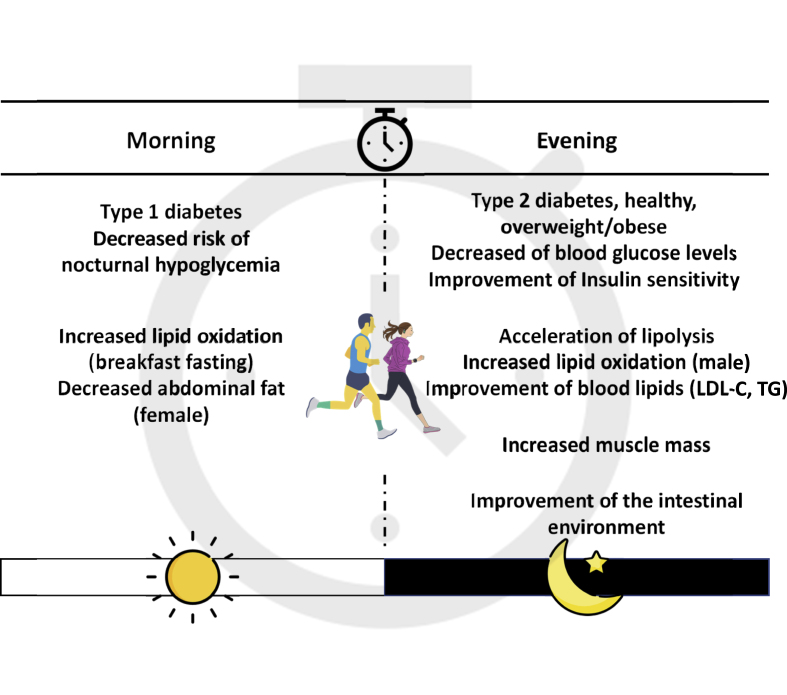
Effect of different exercise timings on metabolic health (Fig. 4, Table 1)
Fig. 4.
The effect of different exercise timings on metabolic health.
Table 1.
Changes in metabolism-related indicators in exercise timing.
| Morning | Evening | Metabolic effects | |
|---|---|---|---|
| IL-6 |  |
Increase glucose uptake, lipolysis, fat oxidation, and hepatic glucose production | |
| GH |  |
Improve visceral adipose tissue, circulating lipid levels, and insulin resistance | |
| Catecholamines |  |
Increase lipolysis and glycogenolysis | |
| Corticosterone |  |
Increase in free fatty acid consumption and β-oxidation | |
| SCFAs | Regulates metabolic and immune function |
IL-6, interleukin 6; GH, growth hormone; SCFAs, short-chain fatty acids.
Skeletal muscle
The skeletal muscle is the largest anatomical organ, occupying approximately 40% of the total body, and plays various physiological roles as a functional tissue that regulates metabolism.6 Skeletal muscle accounts for 75% of insulin-stimulated glucose uptake, and insulin-stimulated skeletal muscle glucose uptake is important for maintaining normal glucose homeostasis.53 IL-6 is a myokine produced and released by skeletal muscle cells (muscle fibers) in response to muscle contraction.46 IL-6 stimulates glucose transporter type 4 translocation to the plasma membrane by activating AMPK in muscle cells, thereby enhancing skeletal muscle insulin sensitivity.54 IL-6 promotes pancreatic α-cell expansion and improves insulin secretion and hyperglycemia by stimulating glucagon-like peptide 1 (GLP-1) secretion from intestinal L cells and pancreatic α cells.55,56 By contrast, IL-6 responds differently depending on the timing of exercise. Changes in blood IL-6 levels after acute endurance exercise were greater in the evening than in the morning.57 Therefore, the effect of exercise timing on glucose metabolism may differ; however, the detailed mechanism remains unclear. Recently, several previous studies in humans have examined the effects of different exercise timings on blood glucose levels and insulin sensitivity and have shown that evening exercise is more effective than morning exercise in improving blood glucose control and insulin sensitivity.58, 59, 60, 61 By contrast, a previous study in patients with type 1 diabetes reported that morning exercise lowers the risk of delayed hypoglycemia occurring later in the evening and improves metabolic control on the following day compared with evening exercise.62 These findings suggest that the effects of exercise timing on the prevention and improvement of diabetes may vary depending on the type and presence of diabetes.
Evening exercises are more effective in improving muscle function.63,64 A previous study showed that evening exercise produces greater increases in muscle mass than morning exercise.64 The physiological context of the time-dependent effects on muscle performance varies. Specifically, elevated body temperature, inflammatory profile, muscle metabolism, muscle tone, and resting and exercise circadian rhythms may contribute to muscle function.65,66 In previous studies, high-throughput gene expression and metabolic profiling in skeletal muscle revealed metabolic pathways that are activated in a diurnal time-dependent manner during exercise.67,68 The endogenous AMPK activator, 5-aminimidazole-4-carboxamide ribonucleotide (ZMP), increases in concentration during exercise, with a peak observed in the evening ZMP is produced during de novo purine and histidine biosynthesis and is known to act as it is known to act as an allosteric activator of AMPK.69 It acts endogenously or, when administered as the corresponding cell-permeable ribonucleoside (5-aminimidazole-4-carboxamide ribonucleoside, AICAR), exogenously.70,71 The latter has been shown to enhance exercise performance in both mice and humans. Thus, ZMP is induced by exercise in a time-dependent manner and may enhance athletic performance by regulating key steps in the glycolytic and fatty acid oxidation pathways.72,73 AMPK is also a master metabolic sensor that activates a myriad of pathways to replenish cellular Adenosine triphosphate (ATP) levels through the inhibition of anabolic pathways and induction of catabolic pathways.74 Hence, nutrient utilization during exercise timing may be regulated by ZMPs via the activation of AMPK. On the other hand, one previous study has confirmed that morning exercise has a greater metabolic impact than evening exercise by increasing muscle acylcarnitine, branched-chain amino acid (BCAA), and ketone body has been shown to have a significant impact on metabolism by increasing levels of β-hydroxybutyrate (BHBA).66 In particular, activation of the glycolytic system is specific to morning exercise. At the molecular level, it has been shown that hypoxia-inducible factor 1α (HIF1α), a central regulator of glycolysis during hypoxia, is activated in a time-dependent manner during exercise, leading to carbohydrate exhaustion, use of alternative energy sources, and adaptation of systemic energy expenditure. While it is true that the time of day is a major regulator of metabolic pathways associated with exercise capacity as described above, the issue of time-specific effects of exercise on metabolic pathways remains largely unexplored and requires further investigation.
Adipose tissue
Exercise is physiologic stress that stimulates lipid metabolism in adipose tissue and skeletal muscle. The mechanism by which exercise promotes increased lipid metabolism is thought to be the activation of the sympathetic nervous system, whereby catecholamines stimulate β-adrenergic receptors in adipocytes, resulting in intracellular lipolysis and increased fatty acid release into the blood, which is then oxidized by mitochondria in the muscle.75 However, the effects of exercise timing remain unclear. By contrast, we examined the effects of exercise timing on blood metabolism-related hormones and cytokines in healthy young men.57,76 The participants performed 60 min of endurance exercise on a treadmill in the morning or evening. The results showed that evening exercise significantly increased blood adrenaline, growth hormone, and IL-6 levels immediately after exercise compared with morning exercise. Furthermore, evening exercise showed a significant increase in blood FFA levels after exercise compared with morning exercise, indicating accelerated lipolysis. The increases in blood adrenaline, growth hormone, and IL-6 caused by exercise promote lipolysis, suggesting that evening exercise is more effective in promoting lipolysis.54,75,77 However, these were acute studies, and the effects of exercise with chronic intervention have not been evaluated. Therefore, more detailed studies are needed.
The effect of exercise timing on blood lipid levels varied. Cholesterol synthesis shows diurnal variation and is higher at night.78 In fact, the effect of exercise timing on blood lipids may also differ, as some reports have suggested that nighttime statin drug intake is more effective.79,80 In a previous study, the effects of different exercise timings over 12 weeks on blood lipid and inflammatory marker levels were examined in patients with cardiovascular disease.81 The results showed greater improvement in LDL-C with evening exercise than with morning exercise. We also examined the effects on blood lipid levels in healthy young men who underwent short-duration endurance exercise for 1 week.61 As a result, evening exercise was more effective than morning exercise in improving TG levels and TG/HDL-C ratios. This decrease in TG levels may be due to lipoprotein lipase (LPL). LPL modulates blood TG levels by promoting the uptake of fatty acids into tissues. In a previous study, exercise was reported to increase LPL mRNA expression and contribute to decreased blood TG.82 Furthermore, LPL activity in response to meal load was lower in the evening than in the morning.83 Therefore, the effect of exercise on TG levels may have been more pronounced by promoting LPL activity through endurance exercise in the evening. However, the contribution of LPL to blood TG levels at different exercise timings has not been examined and requires further investigation.
By contrast, the effect of exercise timing on lipid oxidation may vary according to sex. In a previous study of healthy adults, morning exercise was shown to be more effective than evening exercise in reducing abdominal fat in women. However, in men, evening exercise increased lipid oxidation compared with morning exercise.84 In addition, the effect of exercise timing on lipid oxidation varied before and after meals. In a previous study examining the effect of pre-breakfast exercise on lipid oxidation, 60 min of exercise was performed before and after breakfast under conditions of equal daily energy balance (difference between energy intake and consumption). The results revealed that while energy expenditure over 24 h was comparable, lipid oxidation was significantly increased in the pre-breakfast exercise compared with the post-breakfast exercise.85 Because the pre-breakfast period is the longest fasting period of the day, it is thought to be due to an increase in lipid oxidation caused by the depletion of liver and muscle glycogen.86 Thus, exercise during the longest fasting period of the day and before breakfast may be more effective in promoting lipid oxidation. However, given that exercise after prolonged fasting may cause a rapid increase in blood fatty acid concentrations and increase the risk of heart failure and that myocardial infarction and other conditions occur more frequently in the morning, exercise before breakfast should be performed with great caution.87,88
Gut
The gut microbiota is closely related to various physiological functions of the host, and associations between disturbances in the gut microbiota and various diseases have become apparent.89 For example, Firmicutes are increased and Bacteroidetes are decreased in obese patients.90 Short-chain fatty acids (SCFAs) are produced when the intestinal microflora ferments and degrades indigestible food components. SCFAs inhibit the growth of pathogenic and putrefactive bacteria in the gut and act as regulators of metabolism and immune function.91,92 Hence, the association between gut microbiota and the physiological state of the host is thought to be related to SCFAs. A previous study showed that patients with type 2 diabetes have fewer SCFAs than healthy individuals.93 Thus, the maintenance of improved gut microbiota is important for host health.
Exercise alters the gut microbiota. Exercise training in humans and animal models alters the composition and functional capacity of the gut microbiota, independent of diet.94 In humans, regular exercise was reported to alter gut microbiota and increase the utilization of SCFAs.95 Furthermore, the effects of exercise timing on gut microbiota have recently been examined in mice.96 Evening exercise decreased fecal pH, increased SCFAs, and altered microflora compared with morning exercise. SCFAs promoted GLP-1 secretion in the intestinal tract.97 GLP-1 is secreted by intestinal L cells and binds to receptors on pancreatic β cells to stimulate insulin secretion.97 In a previous study, a brief 2-week period of endurance exercise was reported to increase GLP-1 levels and improve β-cell function.98 Therefore, changes in the gut microbiota at different exercise timings may influence the improvement in pancreatic β-cell function.
Liver
Exercise increases the mobilization of hepatic glycogen to the plasma and the rate of glycogenesis during prolonged exercise.99 Exercise also increases the uptake of the glycogenic precursors lactate, pyruvate, and glycerol to fuel these processes.100 On the contrary, changes in liver metabolism during exercise are regulated, in part, by myokines released during exercise; IL-6 was shown to enhance hepatic fat oxidation and glucose production during exercise.101 Thus, through adaptive responses in glucose and fatty acid metabolism, there is a well-regulated crosstalk between the liver and muscle to exchange substrates and maintain metabolic homeostasis during exercise. In addition, recent studies have suggested that hepatokines, proteins secreted by the liver, directly influence metabolic diseases by modulating signaling pathways related to energy metabolism.102,103 Hepatokines are important regulators of metabolic organs such as the skeletal muscle, heart, and brain, and have been shown to enhance mitochondrial function and reduce the risk of developing chronic diseases such as obesity and type 2 diabetes.104,105 However, the mechanisms underlying the effects of exercise on the energy metabolism of hepatokines remain unclear. Therefore, future studies that include exercise timing are warranted.
Effect of exercise on clock genes
Disruption of the biological clock (circadian rhythm) disrupts glucose and lipid metabolism and increases the risk of metabolic diseases, such as obesity and type 2 diabetes.14 In a previous study, peripheral blood samples were collected from patients with and without type 2 diabetes, and the mRNA levels of clock genes were compared. The results showed that non-diabetics had a rhythmic expression of some clock genes, whereas diabetic patients had reduced, attenuated, or lost expression of these genes.106 In addition, BMAL1 in the visceral adipose tissue of patients with metabolic syndrome showed reduced function.107 Therefore, the biological clock may be impaired in humans with poor metabolic function.
Exercise is effective for regulating the biological clock.108,109 After mice were subjected to repetitive exercise during the light period when they were not normally moving, the rhythms of the clock gene Per2 in peripheral tissues, such as the lungs and skeletal muscles, were observed under ex vivo conditions, and a change in the phase of the clock gene due to exercise was observed.108 This effect was not observed in the central clock, indicating that exercise might modulate the peripheral clock. We also examined the effects of exercise on the peripheral biological clocks (liver, kidney, and submandibular gland) using an in vivo imaging system. We observed that exercise during the inactive period advances the phase of the biological clock and that corticosterone secretion and sympathetic activation are important for this mechanism.109
In a previous study in humans, acute endurance exercise of moderate intensity using a bicycle ergometer was performed at 7:00 a.m. for morning exercise and 4:00 p.m. for evening exercise, and the effects on the rhythm of clock gene expression were compared with those without exercise.110 The results showed that the BMAL1 expression rhythm peaked at 15:00 without exercise, at 9:00 in morning exercise, and at 18:00 in evening exercise, indicating an advanced trend in the rhythm during morning exercise and a delayed trend during evening exercise. Therefore, the results indicate that transient endurance exercise also influences the rhythm of the expression of peripheral clock genes and that this influence depends on the timing of exercise. In addition, biopsies of skeletal muscles subjected to resistance exercise showed changes in clock gene expression, indicating the possibility of exercise-induced regulation of the biological clock in humans.111 We evaluated clock genes using beard follicle cells in older people112 and observed rhythms in the expression of clock genes. Furthermore, a positive correlation was observed between the amplitude of the expression rhythm of clock genes and the amount of physical activity above moderate intensity and maximal oxygen uptake, indicating that the biological clock and physical function are related. Thus, increasing physical activity and daily fitness levels may be important for maintaining good body rhythm. However, the relationship between the biological clock and metabolism during exercise remains unclear. Therefore, further studies are warranted.
Conclusions
In this review, time-dependent physiological responses to exercise are outlined, incorporating new findings of chrono-exercise. Exercise has many beneficial effects on various organs. Although there have been reports on physiological responses during exercise timing, the mechanisms have not been fully elucidated, and these investigations have only just begun. Therefore, accumulated knowledge of chrono-exercise may elucidate the molecular mechanisms leading to the interaction between the biological clock and exercise timing effects and lead to the development of more effective timing or new approaches to exercise.
Submission statement
The work described has not been published previously, it is not under consideration for publication elsewhere, and all authors approved its publication. If accepted, it will not be published elsewhere including electronically in the same form, in English, or any other language, without the written consent of the copyright holder.
Authors' contributions
H.-K. K was involved in the conceptualization and writing of the manuscript. Z.R., M.T., T.I., and S.S. conceptualized and edited the manuscript. All the authors have read and approved the final manuscript for publication.
Conflict of interest
All authors declare no other competing interests.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (KAKENHI grant numbers 20K19689 and 18K17940 to H.-K. K. and 19H01089 to S.S.) and the JST-Mirai Program (grant number JMPJM120D5) to S.S.
References
- 1.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Global Health. 2018;6(10):e1077–e1086. doi: 10.1016/s2214-109x(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 2.Jakicic JM, Otto AD. Treatment and prevention of obesity: what is the role of exercise? Nutr Rev. 2006;64(2 Pt 2):S57–S61. doi: 10.1111/j.1753-4887.2006.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 3.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the american college of sports medicine and the american heart association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 4.Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15(6):659–668. doi: 10.1007/s11906-013-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159(4):738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia. 2020;63(8):1464–1474. doi: 10.1007/s00125-020-05177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8(2):169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 10.Akerstedt T, Levi L. Circadian rhythms in the secretion of cortisol, adrenaline and noradrenaline. Eur J Clin Invest. 1978;8(2):57–58. doi: 10.1111/j.1365-2362.1978.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107(47):20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman SA, Castanon-Cervantes O, Scheer FA, et al. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav Immun. 2015;47:4–13. doi: 10.1016/j.bbi.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata S, Sasaki H, Ikeda Y. [chrono-nutrition and chrono-exercise] Nihon Rinsho. 2013;71(12):2194–2199. [PubMed] [Google Scholar]
- 14.Doi M. Circadian clock-deficient mice as a tool for exploring disease etiology. Biol Pharm Bull. 2012;35(9):1385–1391. doi: 10.1248/bpb.b12-00364. [DOI] [PubMed] [Google Scholar]
- 15.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythm. 2003;18(3):250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 17.Shibata S. Neural regulation of the hepatic circadian rhythm. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):901–909. doi: 10.1002/ar.a.20095. [DOI] [PubMed] [Google Scholar]
- 18.Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci. 2017;67(1):1–10. doi: 10.1007/s12576-016-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoyama S, Shibata S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci. 2017;11:63. doi: 10.3389/fnins.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 21.Jordan SD, Lamia KA. Ampk at the crossroads of circadian clocks and metabolism. Mol Cell Endocrinol. 2013;366(2):163–169. doi: 10.1016/j.mce.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitaula S, Zhang J, Ruiz F, Burris TP. Rev-erb regulation of cholesterologenesis. Biochem Pharmacol. 2017;131:68–77. doi: 10.1016/j.bcp.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, Liu T, Sun Z, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang EE, Liu Y, Dentin R, et al. Cryptochrome mediates circadian regulation of camp signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MD, Li CM, Wang Z. The role of circadian clocks in metabolic disease. Yale J Biol Med. 2012;85(3):387–401. [PMC free article] [PubMed] [Google Scholar]
- 26.Marcheva B, Ramsey KM, Peek CB, Affinati A, Maury E, Bass J. In: Circadian Clocks. Handb Exp Pharmacol. Kramer A, Merrow M, editors. Springer; 2013. Circadian clocks and metabolism; pp. 127–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett RJ, Keen H. Diurnal variation of oral glucose tolerance: a possible pointer to the evolution of diabetes mellitus. Br Med J. 1969;2(5653):341–344. doi: 10.1136/bmj.2.5653.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarrett RJ, Baker IA, Keen H, Oakley NW. Diurnal variation in oral glucose tolerance: blood sugar and plasma insulin levels morning, afternoon, and evening. Br Med J. 1972;1(5794):199–201. doi: 10.1136/bmj.1.5794.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojtczak-Jaroszowa J. Physiological and clinical aspects of circadian variations in glucose tolerance. Chronobiologia. 1977;4(4):363–384. [PubMed] [Google Scholar]
- 30.Hulmán A, Færch K, Vistisen D, et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the whitehall ii study. Diabetologia. 2013;56(2):294–297. doi: 10.1007/s00125-012-2770-3. [DOI] [PubMed] [Google Scholar]
- 31.Carroll KF, Nestel PJ. Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes. 1973;22(5):333–348. doi: 10.2337/diab.22.5.333. [DOI] [PubMed] [Google Scholar]
- 32.Sonnier T, Rood J, Gimble JM, Peterson CM. Glycemic control is impaired in the evening in prediabetes through multiple diurnal rhythms. J Diabet Complicat. 2014;28(6):836–843. doi: 10.1016/j.jdiacomp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 34.Leung GKW, Huggins CE, Bonham MP. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: a crossover trial in healthy volunteers. Clin Nutr. 2019;38(1):465–471. doi: 10.1016/j.clnu.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi M, Ozaki M, Kang MI, et al. Effects of meal timing on postprandial glucose metabolism and blood metabolites in healthy adults. Nutrients. 2018;10(11):1763. doi: 10.3390/nu10111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 37.Bremner WF, Sothern RB, Kanabrocki EL, et al. Relation between circadian patterns in levels of circulating lipoprotein(a), fibrinogen, platelets, and related lipid variables in men. Am Heart J. 2000;139(1 Pt 1):164–173. doi: 10.1016/s0002-8703(00)90324-7. [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Coll A, Fuentes-Arderiu X, Díez-Noguera A. Circadian rhythmic variations in serum concentrations of clinically important lipids. Clin Chem. 1994;40(8):1549–1553. [PubMed] [Google Scholar]
- 39.Sennels HP, Jørgensen HL, Fahrenkrug J. Diurnal changes of biochemical metabolic markers in healthy young males - the bispebjerg study of diurnal variations. Scand J Clin Lab Invest. 2015;75(8):686–692. doi: 10.3109/00365513.2015.1080385. [DOI] [PubMed] [Google Scholar]
- 40.van Kerkhof LW, Van Dycke KC, Jansen EH, et al. Diurnal variation of hormonal and lipid biomarkers in a molecular epidemiology-like setting. PLoS One. 2015;10(5):e0135652. doi: 10.1371/journal.pone.0135652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Moorsel D, Hansen J, Havekes B, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metabol. 2016;5(8):635–645. doi: 10.1016/j.molmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Statland BE, Winkel P, Bokelund H. Factors contributing to intra-individual variation of serum constituents. 2. Effects of exercise and diet on variation of serum constituents in healthy subjects. Clin Chem. 1973;19(12):1380–1383. [PubMed] [Google Scholar]
- 43.Demacker PN, Schade RW, Jansen RT, Van ’t Laar A. Intra-individual variation of serum cholesterol, triglycerides and high density lipoprotein cholesterol in normal humans. Atherosclerosis. 1982;45(3):259–266. doi: 10.1016/0021-9150(82)90227-1. [DOI] [PubMed] [Google Scholar]
- 44.Cella LK, Van Cauter E, Schoeller DA. Diurnal rhythmicity of human cholesterol synthesis: normal pattern and adaptation to simulated ”jet lag”. Am J Physiol. 1995;269(3 Pt 1):E489–E498. doi: 10.1152/ajpendo.1995.269.3.E489. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 2005;98(5):1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman NJ, Parker BL, Chaudhuri R, et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and ampk substrates. Cell Metabol. 2015;22(5):922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92(1):157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- 48.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens. 2010;24(3):175–182. doi: 10.1038/jhh.2009.51. [DOI] [PubMed] [Google Scholar]
- 49.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 50.Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13(3):133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 51.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity. 2011;19(6):1131–1136. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009;296(5):E1164–E1171. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13c nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322(4):223–228. doi: 10.1056/nejm199001253220403. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 55.Ellingsgaard H, Ehses JA, Hammar EB, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci U S A. 2008;105(35):13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from l cells and alpha cells. Nat Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HK, Konishi M, Takahashi M, et al. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS One. 2015;10(9):e0137567. doi: 10.1371/journal.pone.0137567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savikj M, Gabriel BM, Alm PS, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62(2):233–237. doi: 10.1007/s00125-018-4767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mancilla R, Brouwers B, Schrauwen-Hinderling VB, Hesselink MKC, Hoeks J, Schrauwen P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Phys Rep. 2021;8(24):e14669. doi: 10.14814/phy2.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moholdt T, Parr EB, Devlin BL, Debik J, Giskeødegård G, Hawley JA. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: a randomised trial. Diabetologia. 2021;64(9):2061–2076. doi: 10.1007/s00125-021-05477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HK, Furuhashi S, Takahashi M, et al. Late-afternoon endurance exercise is more effective than morning endurance exercise at improving 24-h glucose and blood lipid levels. Front Endocrinol. 2022;13:957239. doi: 10.3389/fendo.2022.957239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez AM, Gomez C, Aschner P, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol. 2015;9(3):619–624. doi: 10.1177/1932296814566233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chtourou H, Souissi N. The effect of training at a specific time of day: a review. J Strength Condit Res. 2012;26(7):1984–2005. doi: 10.1519/JSC.0b013e31825770a7. [DOI] [PubMed] [Google Scholar]
- 64.Küüsmaa M, Schumann M, Sedliak M, et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metabol. 2016;41(12):1285–1294. doi: 10.1139/apnm-2016-0271. [DOI] [PubMed] [Google Scholar]
- 65.Ammar A, Chtourou H, Souissi N. Effect of time-of-day on biochemical markers in response to physical exercise. J Strength Condit Res. 2017;31(1):272–282. doi: 10.1519/jsc.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 66.Basti A, Yalçin M, Herms D, et al. Diurnal variations in the expression of core-clock genes correlate with resting muscle properties and predict fluctuations in exercise performance across the day. BMJ Open Sport Exerc Med. 2021;7(1):e000876. doi: 10.1136/bmjsem-2020-000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ezagouri S, Zwighaft Z, Sobel J, et al. Physiological and molecular dissection of daily variance in exercise capacity. Cell Metabol. 2019;30(1):78–91.e4. doi: 10.1016/j.cmet.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Sato S, Basse AL, Schönke M, et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metabol. 2019;30(1):92–110.e4. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Burkewitz K, Zhang Y, Mair WB. Ampk at the nexus of energetics and aging. Cell Metabol. 2014;20(1):10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asby DJ, Cuda F, Beyaert M, Houghton FD, Cagampang FR, Tavassoli A. Ampk activation via modulation of de novo purine biosynthesis with an inhibitor of atic homodimerization. Chem Biol. 2015;22(7):838–848. doi: 10.1016/j.chembiol.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating amp-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 72.Fan W, Evans RM. Exercise mimetics: impact on health and performance. Cell Metabol. 2017;25(2):242–247. doi: 10.1016/j.cmet.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narkar VA, Downes M, Yu RT, et al. Ampk and ppardelta agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mihaylova MM, Shaw RJ. The ampk signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 76.Kim HK, Takahashi M, Konishi M, et al. The effects of acute endurance exercise performed either in the morning or evening on metabolic and hormone responses. Jpn J Clin Sports Med. 2014;22:497–505. [Google Scholar]
- 77.Thomas GA, Kraemer WJ, Comstock BA, Dunn-Lewis C, Maresh CM, Volek JS. Obesity, growth hormone and exercise. Sports Med. 2013;43(9):839–849. doi: 10.1007/s40279-013-0064-7. [DOI] [PubMed] [Google Scholar]
- 78.Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018;84:11–27. doi: 10.1016/j.metabol.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saito Y, Yoshida S, Nakaya N, Hata Y, Goto Y. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb. 1991;11(4):816–826. doi: 10.1161/01.atv.11.4.816. [DOI] [PubMed] [Google Scholar]
- 80.Plakogiannis R, Cohen H. Optimal low-density lipoprotein cholesterol lowering--morning versus evening statin administration. Ann Pharmacother. 2007;41(1):106–110. doi: 10.1345/aph.1G659. [DOI] [PubMed] [Google Scholar]
- 81.Lian XQ, Zhao D, Zhu M, et al. The influence of regular walking at different times of day on blood lipids and inflammatory markers in sedentary patients with coronary artery disease. Prev Med. 2014;58:64–69. doi: 10.1016/j.ypmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Seip RL, Angelopoulos TJ, Semenkovich CF. Exercise induces human lipoprotein lipase gene expression in skeletal muscle but not adipose tissue. Am J Physiol. 1995;268(2 Pt 1):E229–E236. doi: 10.1152/ajpendo.1995.268.2.E229. [DOI] [PubMed] [Google Scholar]
- 83.Arasaradnam MP, Morgan L, Wright J, Gama R. Diurnal variation in lipoprotein lipase activity. Ann Clin Biochem. 2002;39(Pt 2):136–139. doi: 10.1258/0004563021901883. [DOI] [PubMed] [Google Scholar]
- 84.Arciero PJ, Ives SJ, Mohr AE, et al. Morning exercise reduces abdominal fat and blood pressure in women; evening exercise increases muscular performance in women and lowers blood pressure in men. Front Physiol. 2022;13:893783. doi: 10.3389/fphys.2022.893783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimada K, Yamamoto Y, Iwayama K, et al. Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism. 2013;62(6):793–800. doi: 10.1016/j.metabol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Izumida Y, Yahagi N, Takeuchi Y, et al. Glycogen shortage during fasting triggers liver-brain-adipose neurocircuitry to facilitate fat utilization. Nat Commun. 2013;4:2316. doi: 10.1038/ncomms3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoak JC, Connor WE, Warner ED. Toxic effects of glucagon-induced acute lipid mobilization in geese. J Clin Invest. 1968;47(12):2701–2710. doi: 10.1172/jci105953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marsh EE, 3rd, Biller J, Adams HP, Jr., et al. Circadian variation in onset of acute ischemic stroke. Arch Neurol. 1990;47(11):1178–1180. doi: 10.1001/archneur.1990.00530110032012. [DOI] [PubMed] [Google Scholar]
- 89.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 91.Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okada T, Fukuda S, Hase K, et al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat Commun. 2013;4:1654. doi: 10.1038/ncomms2668. [DOI] [PubMed] [Google Scholar]
- 93.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 94.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47(2):75–85. doi: 10.1249/jes.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 95.Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(2):747–757. doi: 10.1249/mss.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 96.Sasaki H, Miyakawa H, Watanabe A, et al. Evening rather than morning increased physical activity alters the microbiota in mice and is associated with increased body temperature and sympathetic nervous system activation. Biochim Biophys Acta, Mol Basis Dis. 2022;1868(6):166373. doi: 10.1016/j.bbadis.2022.166373. [DOI] [PubMed] [Google Scholar]
- 97.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor ffar2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malin SK, Francois ME, Eichner NZM, et al. Impact of short-term exercise training intensity on β-cell function in older obese adults with prediabetes. J Appl Physiol (1985) 2018;125(6):1979–1986. doi: 10.1152/japplphysiol.00680.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl Sci. 2015;135:203–225. doi: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu C, Hoene M, Plomgaard P, et al. Muscle-liver substrate fluxes in exercising humans and potential effects on hepatic metabolism. J Clin Endocrinol Metab. 2020;105(4):1196–1209. doi: 10.1210/clinem/dgz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care. 2016;19(4):270–275. doi: 10.1097/mco.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 102.Ingerslev B, Hansen JS, Hoffmann C, et al. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and camp. Mol Metabol. 2017;6(10):1286–1295. doi: 10.1016/j.molmet.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Misu H, Takayama H, Saito Y, et al. Deficiency of the hepatokine selenoprotein p increases responsiveness to exercise in mice through upregulation of reactive oxygen species and amp-activated protein kinase in muscle. Nat Med. 2017;23(4):508–516. doi: 10.1038/nm.4295. [DOI] [PubMed] [Google Scholar]
- 104.Browning MG, Khoraki J, DeAntonio JH, et al. Protective effect of black relative to white race against non-alcoholic fatty liver disease in patients with severe obesity, independent of type 2 diabetes. Int J Obes. 2018;42(4):926–929. doi: 10.1038/ijo.2017.309. [DOI] [PubMed] [Google Scholar]
- 105.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ando H, Takamura T, Matsuzawa-Nagata N, et al. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia. 2009;52(2):329–335. doi: 10.1007/s00125-008-1194-6. [DOI] [PubMed] [Google Scholar]
- 107.Gómez-Abellán P, Hernández-Morante JJ, Luján JA, Madrid JA, Garaulet M. Clock genes are implicated in the human metabolic syndrome. Int J Obes. 2008;32(1):121–128. doi: 10.1038/sj.ijo.0803689. [DOI] [PubMed] [Google Scholar]
- 108.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc. 2012;44(9):1663–1670. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sasaki H, Hattori Y, Ikeda Y, et al. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in per2::Luc mice. Sci Rep. 2016;6:27607. doi: 10.1038/srep27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanaka Y, Ogata H, Kayaba M, et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J Appl Physiol (1985) 2020;128(4):847–854. doi: 10.1152/japplphysiol.00891.2019. [DOI] [PubMed] [Google Scholar]
- 111.Zambon AC, McDearmon EL, Salomonis N, et al. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003;4(10):R61. doi: 10.1186/gb-2003-4-10-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takahashi M, Haraguchi A, Tahara Y, et al. Positive association between physical activity and per3 expression in older adults. Sci Rep. 2017;7:39771. doi: 10.1038/srep39771. [DOI] [PMC free article] [PubMed] [Google Scholar]