Abstract
Whole-genome sequencing (WGS) is being used in research and clinical settings in cancer genomics. Studies show that cancer patients generally have positive attitudes toward tumor profiling tests; however, few works revealed their attitudes toward WGS. This study clarifies the expectations, concerns, and result preferences of cancer patients (CPs), family members (FMs) and general adults (GAs) regarding WGS study in Japan. We conducted an anonymous survey with 1204 CPs, 5958 FMs, and 2915 GAs in 2021. Despite low awareness of the WGS studies, CPs had the highest expectations for it. FMs had a higher level of concern than CPs and GAs; feeling anxious by knowing the results, being treated unfavorably if germline findings were detected. Both the FMs and CPs were highly concerned about the protection of genetic information. CPs preferred results with actionability, however, only half preferred to know germline findings. Given the possibility of detecting variants across multidisciplinary diseases and the long-term continuity of WGS research, a system is needed in which study participants can consult and receive decision-making support at any time according to their needs.
Subject terms: Ethics, Health policy
Whole genome sequencing (WGS), which analyzes entire genomes using next-generation sequencing (NGS), is increasingly introduced in research and clinical settings in cancer genomics.
Several studies have revealed people’s attitudes toward cancer genomics. Advanced cancer patients participate in WGS studies and profiling tests in the hope of gaining new insights into their condition, in addition to improving treatment and contributing to the research [1]. A review indicated that patients generally have positive attitudes toward tumor NGS, and their expectations often exceed the reality of low clinical utility [2]. Patients prefer to know information about cancer and treatable noncancer conditions compared to those with predisposition to untreatable noncancer conditions in whole-exome sequencing (WES), which is one of the comprehensive analysis methods covering protein-coding regions of the genome using NGS [3].
A survey of public attitudes toward WGS study in Japan clarified that those interested in gene-related information had high levels of concern, but this did not affect their willingness to participate in such research, though for people who were not interested in their gene-related information, concerns about WGS negatively impacted their willingness to participate [4]. Another study showed that family members had higher expectations than cancer patients from genomic tumor profiling tests (GTPTs) [5]. However, cancer patients’ attitudes regarding WGS have seldom studied. This study clarifies the expectations, concerns, and result preferences of cancer patients, their families, and the public regarding WGS study, and identifies the issues to be addressed.
Cross-sectional anonymous online surveys were conducted among 5376 cancer patients and family members of cancer patients aged 20–79, and another 35,146 adults in the general Japanese population aged 20–69 in March 2021. These groups were extracted from a database of 3.6 million people compiled by INTAGE Inc. based on national census data or their sub-panel. Cancer patients and family members were registered to the sub-panel as people who were currently going to a hospital for cancer treatment or were living with a person who had undergone cancer treatment within the last year. Before answering, respondents were given a brief explanation of WGS with diagrams, which it analyzed the entire genome, instead of only specific targeted genes as single gene testing (companion diagnostics) and GTPTs.
The number of respondents was 10,731 (response rate: 26.5%). After excluding those aged 70 years or older, the remaining 10,077 respondents were divided into those with a history of cancer (CPs, n = 1204), those with family members with cancer (FMs, n = 5958), and general adults with no personal history or family history of cancer (GAs, n = 2915) based on their responses. It found that 56.6% of CPs, 61.2% of FMs, and 70.6% of GAs had never heard of the WGS study. A total of 30.5% of CPs, 27.2% of FMs, and 18.2% of GAs were willing to participate in the WGS study (Table 1).
Table 1.
Respondent characteristics, and awareness of and attitudes toward WGS studies
| CPs (n = 1204) | FMs (n = 5958) | GAs (n = 2915) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Total | 504 | 41.9 | 700 | 58.1 | 2807 | 47.1 | 3151 | 52.9 | 1719 | 59.0 | 1196 | 41.0 |
| Age group (years)* | ||||||||||||
| 20–29 | 17 | 3.4 | 14 | 2.0 | 225 | 8.0 | 373 | 11.8 | 359 | 20.9 | 270 | 22.6 |
| 30–39 | 21 | 4.2 | 47 | 6.7 | 374 | 13.3 | 573 | 18.2 | 366 | 21.3 | 253 | 21.2 |
| 40–49 | 39 | 7.7 | 193 | 27.6 | 667 | 23.8 | 773 | 24.5 | 430 | 25.0 | 284 | 23.7 |
| 50–59 | 120 | 23.8 | 254 | 36.3 | 830 | 29.6 | 760 | 24.1 | 305 | 17.7 | 208 | 17.4 |
| 60–69 | 307 | 60.9 | 192 | 27.4 | 711 | 25.3 | 672 | 21.3 | 259 | 15.1 | 181 | 15.1 |
| Marital status | ||||||||||||
| Unmarried | 87 | 17.3 | 133 | 19.0 | 936 | 33.3 | 905 | 28.7 | 776 | 45.1 | 396 | 33.1 |
| Married | 417 | 82.7 | 567 | 81.0 | 1871 | 66.7 | 2246 | 71.3 | 943 | 54.9 | 800 | 66.9 |
| Do you have any children | ||||||||||||
| No | 171 | 33.9 | 270 | 38.6 | 1361 | 48.5 | 1388 | 44.0 | 990 | 57.6 | 567 | 47.4 |
| Yes | 333 | 66.1 | 430 | 61.4 | 1446 | 51.5 | 1763 | 56.0 | 729 | 42.4 | 629 | 52.6 |
| Education background | ||||||||||||
| Junior high school | 7 | 1.4 | 14 | 2.0 | 75 | 2.7 | 72 | 2.3 | 50 | 2.9 | 43 | 3.6 |
| High school | 133 | 26.4 | 228 | 32.6 | 735 | 26.2 | 924 | 29.3 | 492 | 28.6 | 399 | 33.4 |
| Occupational school | 51 | 10.1 | 122 | 17.4 | 367 | 13.1 | 536 | 17.0 | 255 | 14.8 | 230 | 19.2 |
| Junior college | 7 | 1.4 | 147 | 21.0 | 48 | 1.7 | 608 | 19.3 | 34 | 2.0 | 186 | 15.6 |
| University or graduate school | 306 | 60.7 | 189 | 27.0 | 1582 | 56.4 | 1011 | 32.1 | 888 | 51.7 | 338 | 28.3 |
| Annual household income, JPY | ||||||||||||
| <3,000,000 | 110 | 21.8 | 168 | 24.0 | 541 | 19.3 | 837 | 26.6 | 427 | 24.8 | 360 | 30.1 |
| 3,000,000–5,000,000 | 124 | 24.6 | 195 | 27.9 | 706 | 25.2 | 904 | 28.7 | 432 | 25.1 | 339 | 28.3 |
| 5,000,000–7,000,000 | 99 | 19.6 | 141 | 20.1 | 511 | 18.2 | 611 | 19.4 | 352 | 20.5 | 228 | 19.1 |
| 7,000,000–10,000,000 | 96 | 19.0 | 128 | 18.3 | 628 | 22.4 | 524 | 16.6 | 336 | 19.5 | 197 | 16.5 |
| ≧10,000,000 | 75 | 14.9 | 68 | 9.7 | 421 | 15.0 | 275 | 8.7 | 172 | 10.0 | 72 | 6.0 |
| Awareness of genetic testing | ||||||||||||
| Understand what it means | 138 | 27.4 | 170 | 24.3 | 658 | 23.4 | 684 | 21.7 | 237 | 13.8 | 181 | 15.1 |
| Have heard of it | 318 | 63.1 | 480 | 68.6 | 1809 | 64.4 | 2152 | 68.3 | 1055 | 61.4 | 770 | 64.4 |
| Have never heard of it | 48 | 9.5 | 50 | 7.1 | 340 | 12.1 | 315 | 10.0 | 427 | 24.8 | 245 | 20.5 |
| Experience with genetic testing | ||||||||||||
| Have taken it | 51 | 11.2 | 63 | 9.7 | 76 | 3.1 | 81 | 2.9 | 33 | 2.6 | 27 | 2.8 |
| Have never taken it | 395 | 86.6 | 580 | 89.2 | 2363 | 95.8 | 2716 | 95.8 | 1225 | 94.8 | 904 | 95.1 |
| Don’t recall | 10 | 2.2 | 7 | 1.1 | 28 | 1.1 | 39 | 1.4 | 34 | 2.6 | 20 | 2.1 |
| Awareness of WGS studies | ||||||||||||
| Know about it | 89 | 17.7 | 46 | 6.6 | 380 | 13.5 | 188 | 6.0 | 148 | 8.6 | 44 | 3.7 |
| Have heard of it | 173 | 34.3 | 215 | 30.7 | 941 | 33.5 | 797 | 25.3 | 445 | 25.9 | 221 | 18.5 |
| Have never heard of it | 242 | 48.0 | 439 | 62.7 | 1486 | 52.9 | 2166 | 68.7 | 1126 | 65.5 | 931 | 77.8 |
| Participation in WGS studies | ||||||||||||
| Want to participate | 163 | 32.3 | 204 | 29.1 | 856 | 30.5 | 765 | 24.3 | 331 | 19.3 | 199 | 16.6 |
| Don’t want to participate | 107 | 21.2 | 138 | 19.7 | 614 | 21.9 | 693 | 22.0 | 579 | 33.7 | 384 | 32.1 |
| Cannot decide | 234 | 46.4 | 358 | 51.1 | 1337 | 47.6 | 1693 | 53.7 | 809 | 47.1 | 613 | 51.3 |
WGS Whole genome sequencing, CPs cancer patients, FMs family members of cancer patients, GAs general adults
*The mean age (males/females) was 58.6/52.9 years for CPs, 49.7/47.2 years for FMs, 43.1/42.5 years for GAs, and 48.4/46.9 years overall. The median age (males/females) was 62.0/53.0 years for CPs, 51.0/48.0 years for FMs, 43.0/42.0 years for GAs, and 50.0/48.0 years overall
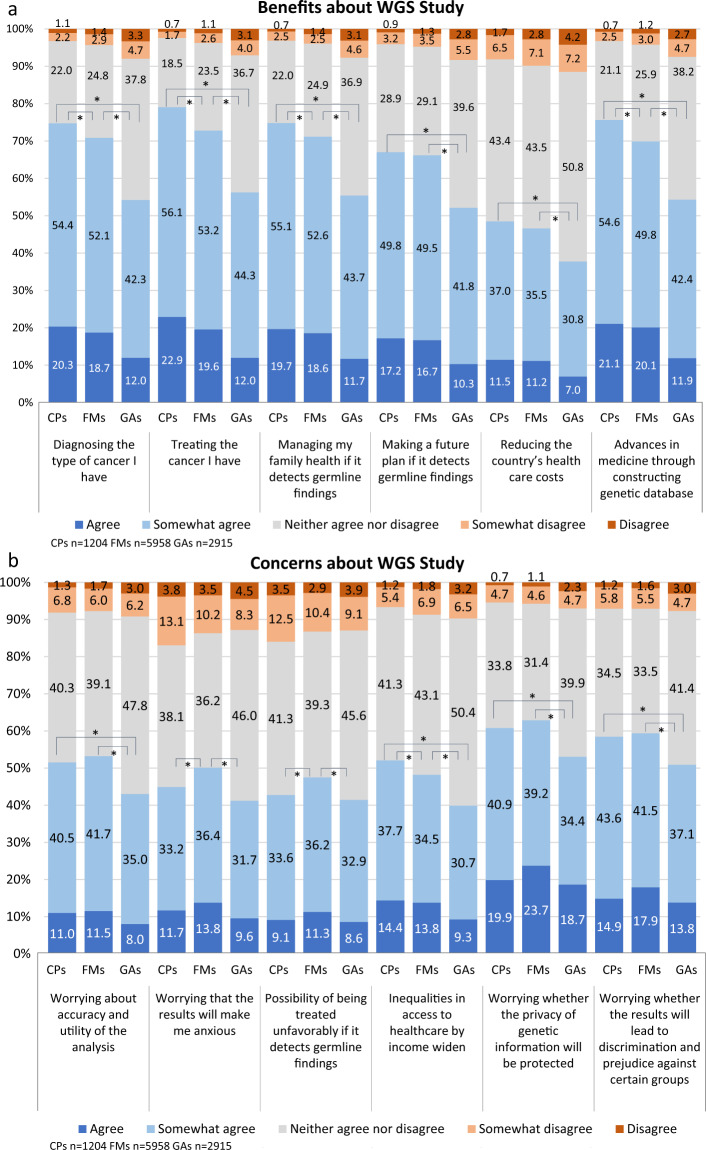
Expectations of CPs were the highest among the three groups for all items, especially for diagnosis, treatment, managing the health of their family, and advances in medicine through development of database (Fig. 1a). GAs expectations were lower than those of CPs and FMs. Among CPs, 74.7% (the sum of “somewhat agree” and “agree”) expected it to be beneficial in diagnosing their disease, 79% expected it to lead to a cure, and 75.7% expected it to lead to the development of medicine by building a large-scale database.
Fig. 1.
Perception of benefits and concerns about WGS study. * Indicates statistical significance for the sum of “agree” and “somewhat agree” (p < 0.05). The Bonferroni method is used in the adjustment of multiple comparisons. 5-point Likert scale was used to measure the respondents’ perception of benefits (a) and concerns (b) about WGS study. WGS Whole genome sequencing, CPs cancer patients, FMs family members of cancer patients, GAs general adults
Approximately 60% of both CPs and FMs were concerned about the privacy of genetic information will be protected and the possibility of discrimination and prejudice against certain groups, while about 50% of them were concerned about the accuracy and utility of WGS (Fig. 1b). FMs were especially more worried than CPs and GAs about the results making them anxious, and the possibility of being treated unfavorably if germline findings were detected.
Regarding result disclosure preference of those who answered that they would like to participate in the WGS study (Fig. 2), CPs were most interested in information that would lead to the diagnosis and treatment of their disease (85.3%), and FMs were in the possibility of developing preventable or treatable disease (80.9%). About 70% of both CPs and FMs wanted to know about life-threatening and urgent diseases. The possibility of developing non-preventable or untreatable diseases was less preferred to that of preventable or treatable diseases. A total of 54.5% of CPs and 59.8% of FMs wanted to know germline findings.
Fig. 2.
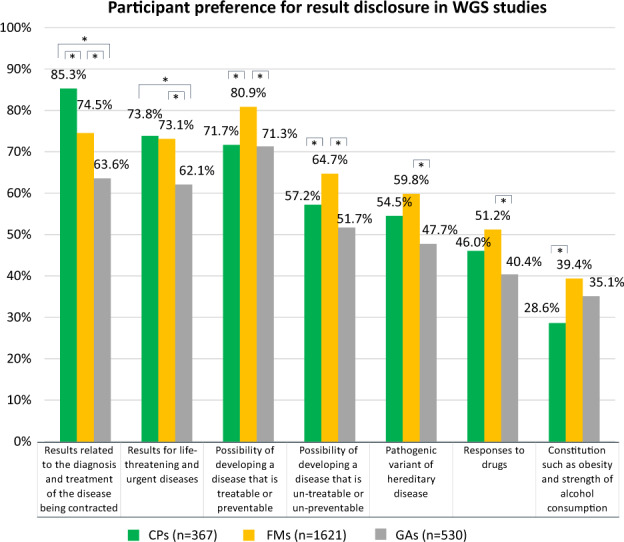
Participant preference for result disclosure in WGS studies * Indicates statistical significance (p < 0.05). The Bonferroni method is used in the adjustment of multiple comparisons. Multiple answer selection was used to measure the respondents’ preference for result disclosure in WGS studies. WGS Whole genome sequencing, CPs cancer patients, FMs family members of cancer patients, GAs general adults
Although CPs preferred to know the results with clinical utility or actionability, only about half of them preferred to know inherited diseases. This is in contradiction with a previous study that about 86% of CPs were interested in germline findings in the tumor profiling test [6], and about 70% of CPs opted for such results in WES in Japan [7]. These findings may contribute to the ongoing discussion about whether secondary findings (SFs) should be revealed to patients. Since patients’ preferences on this were influenced by their disease experience, knowledge, and life context [8, 9], the process of such disclosure is suggested to be tailored to a patient’s individual circumstances [10]. Careful consideration should be given to the benefits and burdens of knowing SFs on conditions that are not related to the patient’s current illness or symptoms, so they can make individual choices.
This study has several limitations. It could not provide a specific description of WGS study and a price for clinical test when the study translated to clinical practice. It should be noted that the survey was conducted with low awareness of WGS study. In addition, qualitative research and dialogue are needed to explore how people distinguish WGS from existing genetic testing, and the specific reasons regarding their expectations and concerns. Despite such limitations, this survey is the first to show the attitudes of CPs and FMs in Japan.
CPs had higher expectations for WGS study leading to diagnosis and treatment, as compared to the previous survey on GTPT which was about 50% [5]. This is presumably a reflection of the high expectations from novel technologies. Thus, explaining the limitations in clinical utility, and the probability of reaching a diagnosis or treatment to avoid excessive expectations or “diagnostic and therapeutic misconception” is required. In addition, the scheme of informed consent and genetic counseling based on two points in time of pre/post testing may not be suitable for a nationwide, long-term WGS study. For example, in a survey of participants in the UK’s 100,000 Genomes Project, approximately 20% of cancer patients did not recall their initial consent decision for disclosure of additional findings [11]. A survey of GTPT institutions in Japan showed that although almost all of participants preferred disclosing SFs, when presumed germline pathogenic variants (PGPVs) were detected, only about 20% of them proceeded to confirmatory testing because they prioritized their cancer treatment [12]. In WGS study, variants may be detected across multidisciplinary disease, and the interpretations may change through the study development. Given that study participants may not remember what they gave consent to, and their life circumstances and life stages may change over the years, portal sites that enable them to check study progress and key details, and consulting services that allow them to seek support from experts in decision-making at any time are required.
As both CPs and FMs were highly concerned about the genetic privacy, ensuring transparency and infrastructure for secure access to data are crucial. Creating an infrastructure that promotes the use of data provided by the participants while ensuring their rights of access and proactive control over them, as proposed by the European Health Data Space (EHDS) released by the European Commission [13] is one way to ensure it.
FMs had concerns about being treated unfavorably based on genetic characteristics, while there are no laws or regulations against genetic discrimination in Japan [14, 15]. Several studies showed despite the implementation of The Genetic Information Nondiscrimination Act (GINA) in the US, low awareness and insufficient understanding persists [16, 17]; even in a survey conducted 10 years later, the respondents answered incorrectly and indicated that they would refuse genetic testing due to fairness of discrimination in employment and insurance [18]. It suggests that mere enactment of legislation will not necessarily alleviate or dispel their concerns. Steps should be taken to inform the public and organizations involved to prevent disadvantages. An international systematic review revealed that several contexts exist for genetic discrimination; insurance, employment, familial, social, and public sector [19]. Adverse treatment such as employment and insurance, can be prevented to some extent by law or regulation, while social relations, stigmatization, and prejudice, are more subtle and difficult to prove or prevent. Ongoing fears of discrimination are the possibility of re-identifying genetic relatives of the data donor from the database, and the presence of genetic markers of disease within specific population groups, which will be used to stigmatize an already vulnerable population [20]. Although it is beyond the scope of this study, further investigation is needed to explore in detail the perceptions and experiences of those who participate in WGS study.
Supplementary information
Acknowledgements
We express our gratitude to all the survey participants.
Funding
This work was supported by AMED under Grant Number JP21cm0106001, JP22ama221001, MHLW Health and Labour Sciences Research Grants Number JPMH20AD0101, JSPS KAKENHI Grant Number JP18K09940.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: “5968 FMs” should have been “5958 FMs”.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/16/2022
“5968 FMs” should have been “5958 FMs”.
Change history
12/20/2022
A Correction to this paper has been published: 10.1038/s10038-022-01108-y
Supplementary information
The online version contains supplementary material available at 10.1038/s10038-022-01100-6.
References
- 1.Rohrmoser A, Pichler T, Letsch A, Westphalen CB, Keilholz U, Heinemann V, et al. Cancer patients’ expectations when undergoing extensive molecular diagnostics-A qualitative study. Psychooncology. 2020;29:423–9. doi: 10.1002/pon.5282. [DOI] [PubMed] [Google Scholar]
- 2.Shirdarreh M, Aziza O, Pezo RC, Jerzak KJ, Warner E. Patients’ and oncologists’ knowledge and expectations regarding tumor multigene next-generation sequencing: A narrative review. Oncologist. 2021;26:e1359–e1371. doi: 10.1002/onco.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray S, Park E, Najita J, Martins Y, Traeger L, Bair E, et al. Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: results from the CanSeq study. Genet Med. 2016;18:1011–9. doi: 10.1038/gim.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okita T, Ohashi N, Kabata D, Shintani A, Kato K. Public attitudes in Japan toward participation in whole genome sequencing studies. Hum Genomics. 2018;12:21. doi: 10.1186/s40246-018-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai A, Ri I, Muto K. Attitudes toward genomic tumor profiling tests in Japan: patients, family members, and the public. J Hum Genet. 2019;64:481–5. doi: 10.1038/s10038-018-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best M, Butow P, Jacobs C, Juraskova I, Savard J, Meiser B, et al. Advanced cancer patient preferences for receiving molecular profiling results. Psychooncology. 2020;29:1533–9. doi: 10.1002/pon.5446. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi Y, Matsubayashi H, Kiyozumi Y, Nishimura S, Higashigawa S, Kado N, et al. Disclosure of secondary findings in exome sequencing of 2480 Japanese cancer patients. Hum Genet. 2021;140:321–31. doi: 10.1007/s00439-020-02207-6. [DOI] [PubMed] [Google Scholar]
- 8.Mackley M, Fletcher B, Parker M, Watkins H, Ormondroyd E. Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: a systematic review of quantitative and qualitative studies. Genet Med. 2017;19:283–93. doi: 10.1038/gim.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houdayer F, Putois O, Babonneau ML, Chaumet H, Joly L, Juif C, et al. Secondary findings from next generation sequencing: Psychological and ethical issues. Family and patient perspectives. Eur J Med Genet. 2019;62:103711. doi: 10.1016/j.ejmg.2019.103711. [DOI] [PubMed] [Google Scholar]
- 10.Kaphingst KA, Ivanovich J, Elrick A, Dresser R, Matsen C, Goodman MS. How, who, and when: preferences for delivery of genome sequencing results among women diagnosed with breast cancer at a young age. Mol Genet Genom Med. 2016;4:684–95. doi: 10.1002/mgg3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard LM, Horton RH, Dheensa S, Fenwick A, Lucassen AM. Exploring broad consent in the context of the 100,000 Genomes Project: a mixed methods study. Eur J Hum Genet. 2020;28:732–41. doi: 10.1038/s41431-019-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minamoto A, Yamada T, Shimada S, Kinoshita I, Aoki Y, Oda K, et al. Current status and issues related to secondary findings in the first public insurance covered tumor genomic profiling in Japan: multi-site questionnaire survey. J Hum Genet. 2022;67:557–63. doi: 10.1038/s10038-022-01028-x. [DOI] [PubMed] [Google Scholar]
- 13.European Commission. European Health Data Space. 2022. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the European Health Data Space COM(2022)197. 2022. https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space_en)
- 14.Iida H, Muto K. Japanese insurers’ attitudes toward adverse selection and genetic discrimination: a questionnaire survey and interviews with employees about using genetic test results. J Hum Genet. 2021;66:539–42. doi: 10.1038/s10038-020-00873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Ho CWL, Ho CH, Athira PS, Kato K, De Castro L, et al. Genetic discrimination: introducing the Asian perspective to the debate. NPJ Genom Med. 2021;6:54. 10.1038/s41525-021-00218-4 [DOI] [PMC free article] [PubMed]
- 16.Parkman AA, Foland J, Anderson B, Duquette D, Sobotka H, Lynn M, et al. Public awareness of genetic nondiscrimination laws in four states and perceived importance of life insurance protections. J Genet Couns. 2015;24:512–21. doi: 10.1007/s10897-014-9771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. N. Engl J Med. 2015;372:397–9. doi: 10.1056/NEJMp1404776. [DOI] [PubMed] [Google Scholar]
- 18.Lenartz A, Scherer AM, Uhlmann WR, Suter SM, Anderson Hartley C, Prince AER. The persistent lack of knowledge and misunderstanding of the Genetic Information Nondiscrimination Act (GINA) more than a decade after passage. Genet Med. 2021;23:2324–34. doi: 10.1038/s41436-021-01268-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wauters A, Van Hoyweghen I. Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet. 2016;61:275–82. doi: 10.1038/jhg.2015.151. [DOI] [PubMed] [Google Scholar]
- 20.Joly, Y, Dalpe, G. Genetic discrimination still casts a large shadow in 2022. Eur J Hum Genet. 2022;30:1320–2. 10.1038/s41431-022-01194-8 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.