Abstract
Background
Immune levels were observed by giving vitamin D supplements to vitamin D deficient women who received the COVID-19 vaccine.
Methods
In the research, there were volunteer women who had received two doses of the COVID-19 vaccine who participated for a mean of more than 65 days. Group D (n=14 Pfizer-BioNTech, 2 Sinovac) received 150,000 IU of vitamin D supplementation, but group C (n=14 Pfizer-BioNTech), 3 Sinovac) no support was provided.
Results
When the consumption of vitamin D ends (D group), serum 25-Hydroxy Vitamin D levels were found to increase regularly in the (W3) last measurements (p=0.001). There was no significant difference in immunoglobulin M levels between groups D and C (Control group) (p=0.063). It was observed that the immunoglobulin G levels reached the peak level between the W1 and W2 measurements of the D group (P<0.001) and there were significant differences between the three sizes. Also, no correlation was found between the D group's initial serum immunoglobulin G and 25-Hydroxy Vitamin D levels. However, when the final measurements were examined, a significant positive correlation was found between immunoglobulin G and 25-Hydroxy Vitamin D levels (r=0.558, p=0.031).
Conclusion
It was determined that serum IgG levels increased significantly depending on the duration between those who used vitamin D and those who did not and it was above the initial level for a long time. A positive and significant relationship was found between the last measured immunoglobulin G and 25(OH) D levels while vitamin D supplementation continued.
Trial Registration: This study registered under ClinicalTrials.gov (Identifier no. NCT05447065)
Keywords: COVID-19 vaccine, Antibody, IgG/IgM, Nutritional, Vitamin D, Supplements
1. Introduction
The coronavirus disease (COVID-19) pandemic is known to have caused millions of deaths globally as of February 2021 [1].
Compared to other coronaviruses, it was understood that there is an instant need for a vaccine to prevent SARS-CoV-2 because of its faster transmission [2]. However, it is very important to determine that the COVID-19 vaccine is immune to individuals, and it has been emphasized that Immunoglobulin G (IgG) levels are checked for immune testing and it is important that these levels are high [3]. People’s immune systems or nutritional levels are influential in the high rate or efficiency of immunoglobin levels [4]. Recommendations were made for healthy individuals to give importance to their nutrition and active physical life during this pandemic process [5].
Since a treatment process is not clear at the beginning of the COVID-19 disease, these supplements, such as D, C, and B vitamin groups and zinc minerals, have been given to COVID-19 patients in the clinic [6], [7]. However, the pathogenesis relationship of 25(OH) vitamin D with the COVID-19 pandemic is consistently seen in the clinical data [8]. In addition, 25(OH) vitamin D deficiency hurts people with COVID-19. For this reason, it is thought that hospitalizations due to COVİD-19 are increasing [9]. In a way, it is claimed to be an important factor in the severe course of the disease [10], [11]. With these results, the views of the severe course of the disease in COVID-19 patients with low vitamin D levels are getting stronger. It is also stated that 25(OH) vitamin D supplementation reduces the mortality rate due to Covit-19 infection [8].
It has been stated that serum 25(OH) vitamin D levels are not related to antibody formation in individuals with the COVID-19 vaccine [12], but it is found to be beneficial in another study [13].
Although the process of vaccinating individuals worldwide gained momentum in 2021, most countries have difficulties in reaching the vaccine. The relationship between studies of vitamin D intake, nutritional status, or other supplements on the antibody of COVID-19 in vaccinated individuals is not well known or not available in the current literature.
By analyzing a few immune biomarkers, this trial aimed to evaluate the effects of vitamin D supplementation on the reply to COVID-19 vaccination in vitamin D-deficient women.
2. Methods
There are 2 types of vaccines (Sinovac and Pfizer-BioNTech) that are widely applied in our country
The study participated with 33 individuals who ınformed consent with positive antibody levels according to the measurement results. The sample sizes in the studies conducted are similar [14]. The effect size was measured by performing Post Hoc analysis with G-Power v. 3.1.9.4 software (d=1.71), and the power was 99% with a=0.05 significance.
These volunteer participants were divided into 2 groups: experimental (14 Pfizer-BioNTech, 2 Sinovac) and control (14 Pfizer-BioNTech, 3 Sinovac) groups. Participants in the experimental group were given 150,000 IU (10 ml) of COLEMAN-D3 (Each 1 ml oral drop contains 0.375 mg of vitamin D3, equivalent to 15,000 IU) supplementation. It was used regularly at 3,200 IU per day for two months. No supplement was given to the control group.
2.1. The study group
The study was conducted from May 2022 to July 2022 on healthy female (having a vitamin D deficiency) volunteers aged between 18-23. In addition, individuals participating in the research who were pregnant, had physician-diagnosed cardiovascular diseases, had kidney or liver failure, and had chronic obstructive pulmonary disease were not included in the study. It consisted of volunteer participants who completed 2 doses of COVID-19 vaccines, did not receive the 3rd dose, and spent a mean of 65 days over the 2nd dose. Participants were included in the study when the IgM value was negative, the IgG value was positive, and serum 25(OH) vitamin D was below 30 ng/mL. A total of 39 people participated in the study and antibody measurements were made with the standard F200 (SD Biosensor) analyzer device. According to the results of these measurements, those who were negative for antibody formation (2), those who did not want to continue the study (3), and those who were found to have osteomyelitis (1) were excluded from the study. A total of 33 people continued the study (Fig. 1 ).
Fig. 1.
CONSORT flow chart. The flow of participants through the trial is represented by a diagram, as suggested by the CONSORT group. C group, Control no supplementation; D group, Vit-D supplementation
From the first measurement, 2 more measurements were taken, 28 days apart, and a total of 3 blood measurements were taken from the participants. In each measurement, antibody level, 25(OH) vitamin D level, anthropometric information, and food consumption registration form were taken.
To control the use of the vitamin D supplement given to the experimental group, information was exchanged every day via the SMS method. In addition, a picture of the supplement bottles was taken to control the use of the supplements given to the experimental group a day before the measurements. The pictures of each participant were visually examined with each other and it was understood that there was no difference in the reduction levels.
2.2. Routine biochemical results
The e-Nabız system (Ministry of Health of the Republic of Turkey) information of the participants was questioned and it was determined that they were healthy and routine biochemistry results were taken to define it.
2.3. Measurement of serum antibody level
Antibody levels were measured with SD Biosensor F200 analyzer fluorescent immunological device (FIA) and Standard F COVID-19 IgM/IgG Combo FIA tests. In addition, the high sensitivity in IgG measurements was 76.9%; It was stated that the specificity was 100%, and it was emphasized that the measurements showed 100% sensitivity after 3 weeks [15].
Venous blood was taken from the participants by the nurse after 6-8 hours of fasting, and these blood samples were centrifuged at 4000 rpm for 10 minutes and separated into blood serum.
20 µl of serum was taken with the help of a micropipette (Eppendorf Research plus 100 µl) and poured into the test cassette. 20 µl of buffer was added to it and left for 15 minutes. Then, the test cassette was given to the device and the results were obtained from the analyzer immediately. A value of IgG/IgM COI ≥1.0 was considered positive, and a value of COI <1.0 was considered negative.
2.4. Measurement of serum 25-hydroxy vitamin D level
25(OH) Vitamin D level was measured with the Standard F200 Analyzer device. Firstly, to measure 25(OH) vitamin D, the incubator was opened and the temperature was set to 37 °C. When the incubator reaches the desired temperature, the test cassette is placed in the device and defined. Then, 175 µl of buffer solution and 35 µl of serum sample were taken with a micropipette and added to reaction tablet 1, respectively. The resulting solution and the defined test cassette were left to incubate for 30 minutes. After incubation for 30 minutes, 1 piece of reaction tablet 2 was taken and added to the resulting solution. When homogeneity was achieved, the whole sample was placed in the test cassette with a micropipette and incubated for 15 minutes. Finally, the incubated test cassette was introduced into the instrument and the result was immediately obtained from the analyzer.
2.5. Data collection methods
Food consumption record and food frequency form was recorded with the 24-hour food consumption record meal separation. A 3-day food consumption record, 2 days on weekdays and 1 day on weekends, was taken by dietitians. Nutritional habits were determined with the nutrition frequency form taken from the participants. The food consumption registration form taken from the participants was repeated every 3 measurements (every 4 weeks for three days). The feeding frequency form was taken once. Before starting the study, the participants were asked about the frequency of food consumption daily, every other day, once a week, every two weeks, and once a month basis. Consumption frequencies were calculated as a percentage.
2.6. Anthropometric measurements
Weight, waist-hip ratio, waist circumference, basal metabolic rate (BMR), muscle, lubrication level, body fat percentage, mineral, and protein ratio measurements were taken with the Bioelectrical Impedance Analysis (BIA) method (TANITA MC-780MA). Height measurement was made with a stadiometer (TANITA brand).
The IPAQ (International Physical Activity Questionnaire) questionnaire was applied to the participants and their physical activity (PA) levels were calculated.
Socio-demographic information, the type of vaccine that was shot, the date of the vaccination, etc are collected via questionnaires methods. Questionnaires were filled in face-to-face by a dietitian.
2.7. Ethical principles
The ethics committee approval of this study was approved by the E-69268593-050-4141 Ethical Committee of Avrasya University, dated 29.06.2021, and numbered 02. This study was registered under ClinicalTrials.gov (Identifier no. NCT05447065).
2.8. Statistics evaluation
Statistical analysis was done in Rstudio version 0.98.501 software with R language. The suitability of variables to normal distribution was examined using analytical methods (Kolmogorov-Smirnov / Shapiro-Wilk tests) in all individuals. Continuous variables were presented with mean ± standard deviation (SD) and classified variables were presented with number-% tables. When all individuals’ data values did not show a normal distribution, the Wilcoxon Signed Ranks Test was used for dependent groups. When comparison data levels of the D and C groups did not show normal distribution, the Mann-Whitney U test was used for independent groups. Friedman Variance analysis was used for triple comparisons' dependent groups. Food consumption record and food frequency form was analyzed by BEBİS 8.2. Values, where the p-value was below 0.05, were considered statistically significant, and G-Power v. 3.1.9.4 software was used to determine the number of samples.
3. Results
The study consisted of volunteers who took vitamin D supplements (16 women) and did not consume vitamin D (17 women), with a total of 33 women participants. An Anthropometric, questionnaire (IPAQ-SF (The International Physical Activity Questionnaire-Short Form), sociodemographic), food consumption records, and routine biochemical information were obtained and analyzed.
There was no significant difference between group D and group C in terms of age, start-up times to study, routine biochemical values (except NEUTROPHIL and MONOCYTE), and anthropometric values. In addition, significant decreases were observed in the consumption of butter, sunflower oil, eggs, yogurt, cheese, and white bread in Group D compared to Group C (Table 1 ).
Table 1.
Characteristics, Anthropometrics, Comparison of Frequency of Food Consumption, and Routine Biochemical Findings of healthy volunteers.a
| D Group (n:16) | C Group (n:17) | pb | |
|---|---|---|---|
| Age (years) | 20.31 (1.40) | 20.47 (1.37) | 0.781 |
| 2. Time after vaccination (days) | 65.63 (10.50) | 66.59 (10.70) | 0.652 |
| PA (Met-min/week) | 2744.40 (2944.13) | 2473.94 (1953.27) | 0.773 |
| Weight (kg) | 61.87 (9.43) | 61.74 (10.75) | 0.829 |
| Height (cm) | 161.77 (3.07) | 162.06 (6.52) | 0.914 |
| BMI (kg/m2) | 23.62 (3.41) | 23.45 (3.32) | 0.801 |
| Waist/Hip | 0.78 (0.05) | 0.79 (0.04) | 0.718 |
| Waist(cm) | 76.10 (9.38) | 74.94 (12.29) | 0.857 |
| Visceral Fat | 1.85 (1.09) | 1.82 (1.09) | 0.939 |
| Muscle (%) | 70.33 (6.09) | 70.03 (5.45) | 0.971 |
| Mineral (%) | 4.83 (0.86) | 4.78 (0.86) | 0.928 |
| Protein (%) | 15.77 (1.48) | 15.69 (1.25) | 0.719 |
| PBF (%) | 25.90 (6.43) | 26.23 (5.74) | 0.971 |
| BMR (Kcal) | 1412.19 (119.24) | 1405.52 (140.41) | 0.666 |
| ALT (U/L) | 12.81 (5.63) | 13.35 (4.91) | 0.664 |
| BASOPHIL (10*3/uL) | 0.07 (0.04) | 0.06 (0.04) | 0.621 |
| NEUTROPHIL (10*3/uL) | 5.46 (1.78) | 4.08 (1.14) | 0.013 |
| EOSINOPHIL (10*3/uL) | 0.20 (0.13) | 0.11 (0.08) | 0.207 |
| LYMPHOCYTE (10*3/uL) | 2.62 (0.72) | 2.31 (0.91) | 0.497 |
| MONOCYTE (10*3/uL) | 0.75 (0.25) | 0.56 (0.26) | 0.037 |
| RBC (10*6/uL) | 4.67 (0.39) | 4.60 (0.53) | 0.311 |
| HGB (g/dL) | 12.76 (1.48) | 12.77 (1.33) | 0.725 |
| MCV (fL) | 81.27 (7.95) | 86.06 (9.89) | 0.065 |
| MCH (pg) | 27.18 (3.71) | 28.33 (4.12) | 0.357 |
| MCHC (g/dL) | 33.40 (2.11) | 32.89 (2.44) | 0.394 |
| RDW (%) | 15.92 (4.19) | 12.88 (0.81) | 0.568 |
| RDWCD (%) | 13.60 (1.27) | 14.86 (3.32) | 0.243 |
| RDWSD (fL) | 40.85 (3.89) | 42.90 (2.62) | 0.355 |
| HCT (%) | 37.98 (3.21) | 38.51 (3.31) | 0.857 |
| PLT (10*3/uL) | 291.69 (91.42) | 273.01 (81.83) | 0.356 |
| MPV (fL) | 8.43 (1.10) | 8.86 (1.68) | 0.437 |
| PDW (fL) | 16.67 (3.99) | 18.04 (8.17) | 0.758 |
| PCT (%) | 0.25 (0.11) | 0.24 (0.08) | 0.586 |
| WBC (10*3/uL) | 8.89 (1.88) | 8.09 (2.56) | 0.247 |
| Comparison of Frequency of Food Consumption | |||
| Turkish coffee (%) | 2.61 (3.69) | 3.42 (4.43) | 0.912 |
| Instant coffee (%) | 3.72 (5.08) | 2.38 (4.80) | 0.317 |
| Herbal teas (%) | 2.08 (3.51) | 3.93 (6.16) | 0.150 |
| Fizzy Drinks (cola, soda, etc.) (%) | 3.01 (3.94) | 3.05 (5.66) | 0.769 |
| Mineral Water (soda) (%) | 2.67 (4.61) | 3.37 (5.73) | 0.911 |
| Instant Juice (%) | 2.60 (5.43) | 3.44 (6.35) | 0.453 |
| Alcoholic beverages (%) | 0.00 (0.00) | 0.00 (0.00) | 1.000 |
| Bagel- Pastry (%) | 2.63 (4.17) | 3.40 (6.68) | 0.524 |
| Rice (%) | 2.58 (2.91) | 3.45 (4.54) | 0.970 |
| Bulgur Rice (%) | 3.10 (7.46) | 2.96 (3.98) | 0.161 |
| Pasta (%) | 3.78 (4.61) | 2.33 (2.37) | 0.680 |
| Cake- Biscuit (%) | 2.51 (3.40) | 3.52 (3.53) | 0.175 |
| Diet Biscuits (%) | 5.74 (17.06) | 0.48 (1.51) | 0.507 |
| Dumplings (%) | 2.19 (2.98) | 3.82 (5.52) | 0.348 |
| Milky desserts (%) | 1.74 (2.23) | 4.24 (6.22) | 0.200 |
| Chocolate (%) | 3.95 (4.24) | 2.16 (1.67) | 0.383 |
| Jam / Honey / Molasses (%) | 3.22 (3.61) | 2.85 (6.88) | 0.313 |
| Olives (%) | 2.38 (4.03) | 3.64 (3.56) | 0.114 |
| Butter (%) | 1.20 (2.95) | 4.75 (8.53) | 0.044 |
| Margarine (%) | 1.85 (7.39) | 4.14 (10.58) | 0.194 |
| Olive oil (%) | 2.28 (3.92) | 3.73 (4.20) | 0.153 |
| Sunflower oil (%) | 1.36 (3.17) | 4.60 (3.80) | 0.005 |
| Red meat (%) | 2.84 (1.83) | 3.21 (5.78) | 0.179 |
| Chicken meat (%) | 2.41 (2.95) | 3.62 (3.99) | 0.704 |
| The fish (%) | 2.24 (2.70) | 3.78 (3.22) | 0.120 |
| Egg (%) | 1.73 (2.48) | 4.25 (3.66) | 0.021 |
| Salami / Sausage etc. (%) | 2.37 (3.01) | 3.66 (5.67) | 0.485 |
| Dry beans (%) | 2.82 (4.07) | 3.22 (3.74) | 0.441 |
| Oilseeds (walnuts, hazelnuts) (%) | 2.44 (3.21) | 3.59 (3.78) | 0.242 |
| Offal (liver, kidney, brain) (%) | 1.77 (6.12) | 4.22 (12.12) | 0.644 |
| Milk (%) | 1.54 (2.29) | 4.43 (6.67) | 0.149 |
| Yogurt (%) | 1.87 (1.88) | 4.12 (2.60) | 0.011 |
| Buttermilk (%) | 2.49 (2.47) | 3.53 (5.02) | 0.700 |
| Kefir (%) | 1.29 (2.93) | 4.67 (14.20) | 0.686 |
| Cheese (%) | 2.36 (3.58) | 3.66 (3.23) | 0.038 |
| Green Leafy Vegetables (%) | 2.61 (2.89) | 3.42 (3.27) | 0.319 |
| Other Vegetables (%) | 3.05 (2.81) | 3.01 (2.77) | 0.769 |
| Dried Fruits (%) | 5.21 (11.40) | 0.98 (1.23) | 0.738 |
| Fresh Fruits (%) | 2.80 (2.93) | 3.24 (3.62) | 0.613 |
| Freshly Squeezed Fruit Juice (%) | 2.54 (4.92) | 3.49 (7.53) | 0.659 |
| White bread (%) | 0.86 (1.28) | 5.08 (8.31) | 0.013 |
| Whole Wheat Bread (%) | 1.41 (3.11) | 4.55±7.08 | 0.315 |
| Whole Grain Bread (%) | 3.96 (5.00) | 2.15±4.19 | 0.119 |
D group, Vit-D supplemented group; C group, No supplemented group. Physical activity: PA, Body mass index: BMI, Body fat percentage: PBF, Basal metabolic rate: BMR, WBC= White blood cells; RBC = Red blood cells; HGB= Hemoglobin; HCT= Hematocrit; MCV = Mean corpuscular volume; MCH = Mean corpuscular hemoglobin; MCHC = Mean corpuscular hemoglobin concentration; Plt= Platelet; PDW= Platelet distribution width; MPV= Mean platelet volume; PCT= plateletcrit,
aData are expressed as mean ± SD.
bp-values were determined using a Mann-Whitney U-test.
The mean 12-day food consumption was taken and analyzed. Although there was a significant decrease in sodium, alcohol, and water consumption in Group D compared to Group C, it was observed that there were no significant differences in energy, carbohydrate, protein, fat, vitamin, and mineral group (except sodium) values. In addition, it was observed that there was a significant decrease in energy, protein, fat, MUFA, PUFA, oleic acid, linoleic acid, a-linolenic acid, cholesterol, Riboflavin, vitamin B12, vitamin E, sodium, and phosphorus values in Group D compared to Group C (Table 2 ).
Table 2.
Daily Mean Levels of Energy and Nutrient Consumption and Comparison of Frequency of Food Consumption in the Groups a
| FCR | FCF | |||||
|---|---|---|---|---|---|---|
| D Group (n:16) | C Group(n:17) | D Group (n:16) | C Group (n:17) | pb | pc | |
| Energy (kcal) | 1055.90 (268.32) | 1270.47 (372.41) | 1389.38 (668.84) | 1759.34 (647.73) | 0.121 | 0.028 |
| Water (g) | 671.13 (168.71) | 820.16 (178.14) | 950.69 (414.38) | 1068.45 (505.64) | 0.017 | 0.368 |
| Protein (g) | 43.63 (12.05) | 47.72 (10.49) | 37.98 (16.64) | 52.88 (22.43) | 0.235 | 0.034 |
| Oil (g) | 52.25 (14.29) | 59.56 (17.75) | 63.72 (37.00) | 85.46 (19.54) | 0.280 | 0.002 |
| CHO (g) | 99.93 (32.94) | 133.20 (48.92) | 163.47 (83.35) | 191.39 (107.61) | 0.066 | 0.449 |
| Fiber (g) | 9.17 (2.74) | 10.87 (3.38) | 14.24 (6.91) | 17.01 (10.22) | 0.121 | 0.439 |
| Alcohol (g) | 0.02 (0.05) | 0.06 (0.08) | 0.01 (0.03) | 0.05 (0.12) | 0.015 | 0.390 |
| SFA (g) | 21.43 (7.56) | 25.90 (8.78) | 28.59 (15.67) | 33.50 (11.76) | 0.331 | 0.150 |
| MUFA (g) | 17.37 (5.30) | 19.84 (5.58) | 22.94 (14.23) | 30.39 (8.89) | 0.280 | 0.008 |
| Oleic asit (g) | 14.80 (4.64) | 17.02 (4.75) | 21.23 (13.81) | 28.13 (8.74) | 0.235 | 0.007 |
| PUFA (g) | 9.40 (3.77) | 9.33 (3.53) | 7.82 (6.51) | 14.45 (4.74) | 1.000 | 0.001 |
| Linoleic asit (g) | 6.92 (3.13) | 6.81 (2.71) | 6.34 (6.03) | 12.58 (4.66) | 0,928 | 0.001 |
| a-linolenic asit (g) | 1.12 (0.64) | 1.01 (0.60) | 0.65 (0.32) | 0,97 (0.38) | 0,349 | 0.014 |
| Cholesterol (mg) | 223.27 (122.99) | 270.94 (90.28) | 152.28 (106.52) | 268.73 (134.36) | 0.098 | 0.005 |
| VITAMINS | ||||||
| D (g) | 3.33 (3.75) | 3.82 (1.87) | 2.77 (1.80) | 4.84 (3.62) | 0.105 | 0.080 |
| A (µg) | 748.50 (712.61) | 692.89 (278.04) | 731.78 (457.55) | 1011.91 (732.40) | 0.564 | 0.160 |
| Carotene (mg) | 1.85 (1.38) | 1.61 (0.91) | 2.32 (1.51) | 2.51 (1.87) | 0.564 | 0.551 |
| E (mg) | 6.04 (2.96) | 6.92 (2.44) | 6.28 (7.07) | 13.67 (5.79) | 0.264 | <0.001 |
| Thiamine (mg) | 0.47 (0.11) | 0.55 (0.13) | 0.60 (0.31) | 0.75 (0.38) | 0.200 | 0.087 |
| Riboflavin (mg) | 0.76 (0.28) | 0.92 (0.21) | 1.00 (0.43) | 1.40 (0.51) | 0.097 | 0.019 |
| Niacin (mg) | 10.19 (3.10) | 10.17 (2.09) | 6.79 (2.41) | 8.31 (4.18) | 0.857 | 0.517 |
| Pyridoxine (mg) | 0.86 (0.24) | 0.85 (0.24) | 0.64 (0.19) | 0.78 (0.37) | 0.914 | 0.173 |
| Folate (µg) | 148.25 (54.18) | 167.74 (57.64) | 180.50 (95.41) | 239.45 (126.76) | 0.349 | 0.105 |
| B12 (µg) | 3.17 (2.17) | 3.34 (1.24) | 2.29 (1.37) | 3.72 (2.34) | 0.589 | 0.015 |
| C (mg) | 48.52 (27.70) | 62.96 (31.52) | 62.57 (31.61) | 70.75 (46.64) | 0.235 | 0.719 |
| MINERALS | ||||||
| Sodium (mg) | 1856.36 (735.34) | 2361.16 (584.39) | 1055.03 (428.21) | 1632.95 (838.71) | 0.037 | 0.021 |
| Potassium (mg) | 1409.63 (329.81) | 1553.86 (366.10) | 1556.24 (657.61) | 1850.42 (787.60) | 0.296 | 0.090 |
| Calcium (mg) | 364.65 (154.05) | 447.86 (145.60) | 528.34 (216.35) | 709.51 (302.90) | 0.207 | 0.105 |
| Magnesium (mg) | 150.69 (25.24) | 176.07 (43.62) | 200.94 (99.90) | 229.56 (97.94) | 0.090 | 0.171 |
| Phosphorus (mg) | 630.99 (155.02) | 726.68 (155.83) | 742.72 (324.29) | 952.85 (384.56) | 0.121 | 0.034 |
| Iron (mg) | 5.88 (1.39) | 6.69 (1.63) | 5.48 (2.78) | 6.37 (3.26) | 0.171 | 0.304 |
| Zinc (mg) | 6.17 (1.76) | 6.86 (1.73) | 5.94 (2.55) | 7.25 (2.85) | 0.368 | 0.077 |
| D group, Vit-D supplemented group; C group, No supplemented group. SFA, Monounsaturated fatty acid: MUFA, Polyunsaturated fatty acid: PUFA, Food Consumption Record: FCR, Food Consumption Frequency: FCFa Data are expressed as mean ± SD.b Comparison of FCR between D and C values, p values were determined using the Mann-Whitney U-test.c Comparison of FCF between D and C values, p values were determined using the Mann-Whitney U-test. | ||||||
In the D group, a positive and moderately strong correlation was found between the W2 (second measurement) of 25(OH) D values and in the consumption of sunflower oil (r:0.545, p:0.029), white bread (r:0.556, p:0.025), linoleic acid (r:0.549, p:0.028), a-linolenic acid (r:0.503, p:0.033), and vitamin E (r:0.607, p:0.013). Also, a positive and weak correlation was found between the W2 of 25(OH) D values and the consumption of eggs (r:0.499, p:0.049). In addition, a positive and moderately strong correlation was found between the W3 (final measurement) of 25(OH) D values and the consumption of eggs (r:0.514, p:0.041). Last, a positive and moderately strong correlation was found between the W3 (final measurement) of IgG values and the consumption of cholesterol (r:0.584, p:0.022).
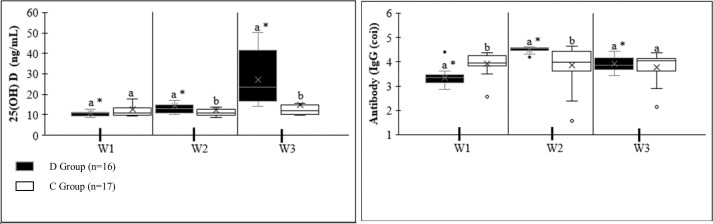
The result of all values in IgG measurements was found to be >1 COI and was considered positive (Table 3 ). There was a significant difference between the first (W1-W1) and second (W2-W2) measurements of IgG values of groups D and C. It was observed that the W1 values of the D group were lower than those of the C group, and there was a significant increase in the W2 values. However, there was no significant difference between the third measurement (W3-W3) of the groups. There was no significant difference in IgG values in the three measurements of group C (W1, W2, and W3). A significant increase in W2 compared to W1 was found between the first and second measurements (W1 and W2) of the IgG values of the D group. In addition, it was observed that there was a significant decrease in W3 compared to W2 between the second-third (W2-W3) measurements. It was stated that there was a significant increase in W3 compared to W1 between the first-third (W1-W3) measurements (Fig. 2 ).
Table 3.
Antibody response to COVID-19 vaccine in all-volunteer women.a
| D Group (n=16) | C Group (n=17) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | pb | pc | pd | W1 | W2 | W3 | pe | pf | p7 | p8 | |
| IgG (COI) | 3.36 (0.34) | 4.51 (0.12) | 3.91 (0.31) | <0.001 | 0.001 | 0.004 | 3.94 (0.43) | 3.86 (0.81) | 3.78 (0.61) | 0.779 | <0.001 | 0.001 | 1.000 |
| 25 (OH) D (ng/mL) | 10.97 (2.41) | 14.37 (4.63) | 27.18 (12.08) | 0.021 | <0.001 | 0.001 | 12.60 (4.10) | 12.20 (3.77) | 14.78 (10.75) | 0.646 | 0.121 | 0.036 | <0.001 |
| Pre-Study Values of Volunteers experiencing COVID-19 Disease | |||
| D Group (n=6) | C Group (n=6) | pi | |
| IgG (COI) | 3.53 (0.45) | 3.98 (0.31) | 0.078 |
| 25(OH) D (ng/mL) | 11.05 (1.39) | 13.90 (6.28) | 0.575 |
| The time between antibody measurement and the disease (days) | 353.50 (80.02) | 242.00 (177.79) | 0.423 |
D group, Vit-D supplemented group; C group, No supplemented group. Immunoglobulin G: IgG, Immunoglobulin M: IgM, 25-Hydroxy(25(OH)) vitamin D: 25(OH) D,
aAb titers are expressed as mean ± SD.
b, c, d Determined using a paired Wilcoxon test for intra-group differences between Antibody titers in the D group (W1-W2 (pb)), (W2-W3 (pc)), and (W1-W3 (pd)).
eDetermined using Friedman test for intra-group differences between Antibody titers in the C group (pe).
e,f,g,h Determined using Mann-Whitney U-test for inter-group differences in W1-W1 Antibody titers (pf), W2-W2 Antibody titers (pg), and W3-W3 Antibody titers (ph) between D and C groups.
ip-values were determined using a Mann-Whitney U-test.
Fig. 2.
Antibody IgG levels and 25(OH) D levels, Vitamin D serum levels. W1, baseline (measurement within this first week or mean 65 days after vaccination.); W2, 4 weeks (28 days) after W1 measurement; W3, 4 weeks (28 days) after W2 measurement. Data are expressed as mean ± SD. *Significantly different between vaccination titers in the same group using paired Wilcoxon test (p <0.05); a,b Significantly different for the same period between D group and C group using Mann-Whitney U-test (p < 0.05).
There were no significant differences in 25(OH) vitamin D values in the first measurements (W1-W1) between groups D and C. In addition, a significant difference was observed between the second (W2-W2) and third (W3-W3) measurements of the D and C groups. W2 and W3 values of group D were found to be higher than in group C. There is a significant regular increase between W1, W2, and W3 in Group D. However, no significant difference was found between the measurements within the C group (Fig. 2).
It is known that a total of 12 people in the D (n=6) and C (n=6) groups had COVID-19 disease before starting the research. There was no significant difference between the IgM, IgG, 25(OH) Vitamin D, and the time between antibody measurement and the disease in the D and C groups (Table 3).
In the D group, a positive and moderately strong correlation was found between the W3 (third or final measurement) measurements of IgG and 25(OH) D values, and no significant correlation was found between the other measurements (W1, W2). Also, no significant correlation was found between the first measurements of IgG and 25(OH) D values of the D and C groups (Fig. 3 ).
Fig. 3.
25(OH) D with IgG Correlate, W1, baseline (measurement within this first week or mean 65 days after vaccination.); W2, 4 weeks (28 days) after W1 measurement; W3, 4 weeks (28 days) after W2 measurement. Analyzed by Spearman's Correlation.
4. Discussion
By analyzing a few immune biomarkers, this trial evaluated the effects of vitamin D supplementation on the reply to COVID-19 vaccination in vitamin D-deficient women.
The groups were studied extensively and as a result, it was understood that they were close to each other. There were no patients who had an illness or showed any symptoms during the study.
When similar studies are examined, it is seen that the groups that are formed are not different [14], [16].
In a study by Goncalves-Mendes et al., volunteers were supplemented with 200,000 IU of vitamin D monthly [14]. It has also been stated that high-level vitamin D supplementation does not cause side effects [17]. In this study, the vitamin D dosage was given as 150,000 IU in total for 8 weeks. It is seen that the level of vitamin D supplementation is low and at a normal level compared to other studies. In addition, no side effects were followed in any of the participants.
It is known that vitamin D has other effects on the immune system [18]. It has been reported that the hazard of acute viral respiratory infections is reduced with vitamin D supplementation, and the preventive effects of vitamin D supplementation have been lengthily stated [19]. When the relationships between 25(OH) D status and COVID-19 are examined, it continues to arise with the suggestion that there is an inverse correlation between 25(OH) D levels in serum and COVID-19 positivity [20]. In addition, vitamin D supplementation has been studied to improve the efficacy of vaccines in infectious diseases such as tuberculosis, pneumococcal, influenza (A/H1N1, A/H3N2), rubella, hepatitis B, measles, and meningococcal disease [14], [21]. In addition, it was recommended to use vitamin D daily, and it was stated that there is a significant and strong correlation between COVID-19 IgG levels when serum 25(OH) D level is above 50 nmol/L [13]. In this study, it was observed that serum 25(OH) D levels increased in women who took vitamin D supplements. In addition, although there was no significant relationship among serum 25(OH) D and IgG in the first measurements of those taking vitamin D supplements, a significant, moderately strong correlation was reported in the last measurements.
Studies have emphasized that IgG levels of vaccinated individuals peak within 3 weeks and remain stable for 6 months [22]. On the other hand, in another study, it was reported that IgG levels of vaccinated individuals reached the highest peak on the 42nd day [2]. The study is done on a mean of 65 days after vaccination because IgG levels remain at a certain level at the end of this period. When the volunteers who did not take vitamin D supplements were examined, it was examined that there was no significant increase or decrease in IgG levels.
In addition, it has been stated that although a higher peak occurs after vaccination in people with COVID-19 disease, serum IgG levels begin to decrease after 3 weeks and remain stable for a while [1], [23]. In this study, there was no significant difference in IgG levels in volunteers who had COVID-19 disease (duration: 353.50±80.02/242.00±177.79) before starting the study, especially between women using and not using supplements.
Adequate findings and clear information were not obtained among the measurements of serum 25(OH) D and COVID-19 IgG levels [16], [24]. However, routine nutritional supplementation information is currently insufficient to avert acute respiratory infections or COVID-19 [25]. In another study, it was stated that there was no significant relationship among serum 25(OH) D and IgG of COVID-19-vaccinated individuals [12]. However, a low-protein diet has been reported to have a lowering effect on IgG levels [26]. In addition, it has been stated that a diet rich in arginine and tryptophan has a positive effect on IgG levels [27]. It has been reported that a PUFA-rich diet affects IgG or antibody levels [28]. In addition, studies have emphasized that sunflower oil and vitamin E affect IgG [29]. And also, it was examined that there was a significant difference in the initial value between serum IgG, and those who used vitamin D supplements had lower serum IgG values. The reason for this was; It was determined that the consumption of butter, eggs, yogurt, and cheese was low before starting the study. It has been thought that nutrition low in PUFA (linoleic acid and α-linolenic acid), vitamin E, and protein may also affect IgG levels. When vitamin D intake was made, it was observed that IgG levels reached a peak level, but decreased towards the last measurements. However, it can be said that IgG levels remained high according to the first measurements.
The study has limitations. The vitamin D intakes of the volunteers were monitored remotely and their consumption was evaluated based on declaration (the bottles they used were checked). Also, the sample size and duration of the study (8 weeks) were insufficient to provide further insight.
5. Conclusions
In the study, it was observed that the use of vitamin D significantly increased serum IgG levels compared to those who did not use vitamin D, depending on the duration, and in the long term, it was observed that the study remained above the baseline. It has been determined that there is a positive and significant correlation between the last measurement of serum IgG and 25(OH) vitamin D levels during the use of vitamin D.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to thank all the participants.
Fınancıal disclosure
This work was supported by the Research Promotion Program of Avrasya University 2021/02 number.
Data availability
Data will be made available on request.
References
- 1.Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and Neutralization Antibody Titers after a Single Vaccine Dose in Health Care Workers Previously Infected with SARS-CoV-2. JAMA - J Am Med Assoc. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reifer J., Hayum N., Heszkel B., Klagsbald I., Streva V.A. SARS-CoV-2 IgG antibody responses in New York City. Diagn Microbiol Infect Dis. 2020;98 doi: 10.1016/j.diagmicrobio.2020.115128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Quintela A., Milton-Laskibar I., Trepiana J., Gómez-Zorita S., Kajarabille N., Léniz A., et al. Key aspects in nutritional management of covid-19 patients. J Clin Med. 2020;9:1–24. doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filgueira T.O., Castoldi A., Santos L.E.R., de Amorim G.J., de Sousa Fernandes M.S., de Anastácio W., et al. The Relevance of a Physical Active Lifestyle and Physical Fitness on Immune Defense: Mitigating Disease Burden, With Focus on COVID-19 Consequences. Front Immunol. 2021;12:1. doi: 10.3389/fimmu.2021.587146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junaid K., Ejaz H., Abdalla A.E., Abosalif K.O.A., Ullah M.I., Yasmeen H., et al. Effective immune functions of micronutrients against sars-CoV-2. Nutrients. 2020;12:1–14. doi: 10.3390/nu12102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullin GE, Limektkai B, Wang L, Hanaway P, Marks L, Giovannucci E. Dietary Supplements for COVID-19. Adv. Exp. Med. Biol., vol. 1318, Springer; 2021, p. 499–515. https://doi.org/10.1007/978-3-030-63761-3_29. [DOI] [PubMed]
- 8.Ismailova A., White J.H. Vitamin D, infections and immunity. Rev Endocr Metab Disord. 2022;23:265–277. doi: 10.1007/s11154-021-09679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chetty V.V., Chetty M. Potential benefit of vitamin D supplementation in people with respiratory illnesses, during the COVID-19 pandemic. Clin Transl Sci. 2021;14:2111–2116. doi: 10.1111/cts.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Eltriki M., Hopefl R., Wright J.M., Deb S. Association between Vitamin D Status and Risk of Developing Severe COVID-19 Infection: A Meta-Analysis of Observational Studies. J Am Coll Nutr. 2021 doi: 10.1080/07315724.2021.1951891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemian R., Shamshirian A., Heydari K., Malekan M., Alizadeh-Navaei R., Ebrahimzadeh M.A., et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int J Clin Pract. 2021;75:e14675. doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chillon T.S., Demircan K., Heller R.A., Hirschbil-Bremer I.M., Diegmann J., Bachmann M., et al. Relationship between vitamin d status and antibody response to covid-19 mrna vaccination in healthy adults. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piec I., Cook L., Dervisevic S., Fraser W.D., Ruetten S., Berman M., et al. Age and vitamin D affect the magnitude of the antibody response to the first dose of the SARS-CoV-2 BNT162b2 vaccine. Curr Res Transl Med. 2022;70 doi: 10.1016/J.RETRAM.2022.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves-Mendes N., Talvas J., Dualé C., Guttmann A., Corbin V., Marceau G., et al. Impact of Vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: A randomized placebo-controlled trial. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamkhi S., Dhaouadi T., Sfar I., Mokni S., Jebri A., Mansouri D., et al. Comparative study of six SARS-CoV-2 serology assays: Diagnostic performance and antibody dynamics in a cohort of hospitalized patients for moderate to critical COVID-19. Int J Immunopathol Pharmacol. 2022;36 doi: 10.1177/20587384211073232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinaci S., Ocal D.F., Yucel Yetiskin D.F., Uyan Hendem D., Buyuk G.N., Goncu Ayhan S., et al. Impact of vitamin D on the course of COVID-19 during pregnancy: A case control study. J Steroid Biochem Mol Biol. 2021;213 doi: 10.1016/j.jsbmb.2021.105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Janet B.-L. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/AJCN/77.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Terrier B., Derian N., Schoindre Y., Chaara W., Geri G., Zahr N., et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther. 2012;14:R221. doi: 10.1186/ar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadarangani S.P., Whitaker J.A., Poland G.A. “let there be light”: The role of Vitamin D in the immune response to vaccines. Expert Rev Vaccines. 2015;14:1427–1440. doi: 10.1586/14760584.2015.1082426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50:2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gobbi F., Buonfrate D., Moro L., Rodari P., Piubelli C., Caldrer S., et al. Antibody response to the bnt162b2 mrna covid-19 vaccine in subjects with prior sars-cov-2 infection. Viruses. 2021;13 doi: 10.3390/v13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darren A., Osman M., Masilamani K., Habib Ali S., Kanthimathinathan H.K., Chikermane A., et al. Vitamin D status of children with paediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (PIMS-TS) Br J Nutr. 2022;127:896–903. doi: 10.1017/S0007114521001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NICE. Overview | COVID-19 rapid guideline: vitamin D | Guidance | NICE 2021. https://www.nice.org.uk/guidance/ng187 (accessed May 31, 2022).
- 26.Lin G.Y., Chan H.Y., Cheng C.A., Lin L.P., Peng G.S., Hsiao P.M., et al. Open-labelled observations of language dysfunction in old ischemic stroke patients with aphasia when given plant and marine-based nutrient supplements for 12 weeks. Asia Pac J Clin Nutr. 2016;25:265–272. doi: 10.6133/apjcn.2016.25.2.27. [DOI] [PubMed] [Google Scholar]
- 27.Emadi M., Jahanshiri F., Kaveh K., Hair-Bejo M., Ideris A., Alimon A.R. Nutrition and immunity: the effects of the combination of arginine and tryptophan on growth performance, serum parameters and immune response in broiler chickens challenged with infectious bursal disease vaccine. Avian Pathol. 2011;40:63–72. doi: 10.1080/03079457.2010.539590. [DOI] [PubMed] [Google Scholar]
- 28.Stupin A., Cvetko A., Kralik G., Mihalj M., Šušnjara P., Kolobarić N., et al. The effect of n-3 polyunsaturated fatty acids-enriched hen eggs consumption on IgG and total plasma protein N-glycosylation in healthy individuals and cardiovascular patients. Glycobiology. 2021;31:1163–1175. doi: 10.1093/glycob/cwab051. [DOI] [PubMed] [Google Scholar]
- 29.Parmentier H.K., Awati A., Nieuwland M.G.B., Schrama J.W., Sijben J.W.C. Different sources of dietary n-6 polyunsaturated fatty acids and their effects on antibody responses in chickens. Br Poult Sci. 2002;43:533–544. doi: 10.1080/0007166022000004444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.