Abstract
This study examined electromyographic amplitude (EMGRMS)-force relationships during repeated submaximal knee extensor muscle actions among chronic aerobically-(AT), resistance-trained (RT), and sedentary (SED) individuals. Fifteen adults (5/group) attempted 20 isometric trapezoidal muscle actions at 50% of maximal strength. Surface electromyography (EMG) was recorded from vastus lateralis (VL) during the muscle actions. For the first and last successfully completed contractions, linear regression models were fit to the log-transformed EMGRMS-force relationships during the linearly increasing and decreasing segments, and the b terms (slope) and a terms (antilog of y-intercept) were calculated. EMGRMS was averaged during steady force. Only the AT completed all 20 muscle actions. During the first contraction, the b terms for RT (1.301 ± 0.197) were greater than AT (0.910 ± 0.123; p = 0.008) and SED (0.912 ± 0.162; p = 0.008) during the linearly increasing segment, and in comparison to the linearly decreasing segment (1.018 ± 0.139; p = 0.014), respectively. For the last contraction, the b terms for RT were greater than AT during the linearly increasing (RT = 1.373 ± 0.353; AT = 0.883 ± 0.129; p = 0.018) and decreasing (RT = 1.526 ± 0.328; AT = 0.970 ± 0.223; p = 0.010) segments. In addition, the b terms for SED increased from the linearly increasing (0.968 ± 0.144) to decreasing segment (1.268 ± 0.126; p = 0.015). There were no training, segment, or contraction differences for the a terms. EMGRMS during steady force increased from the first- ([64.08 ± 51.68] μV) to last-contraction ([86.73 ± 49.55] μV; p = 0.001) collapsed across training statuses. The b terms differentiated the rate of change for EMGRMS with increments in force among training groups, indicating greater muscle excitation to the motoneuron pool was necessary for the RT than AT during the linearly increasing and decreasing segments of a repetitive task.
Keywords: Electromyography, Fatigue, Isometric trapezoidal muscle action, Motor unit control properties, Natural log-transformed model, Vastus lateralis
Abbreviations:
- a term
antilog of the y-intercept
- ANOVA
Analysis of Variance
- AT
Aerobically Trained
- b term
slope
- CI
Confidence Interval
- cm
Centimeter
- EMG
Electromyography
- EMGRMS
Electromyographic Amplitude
- h
Hour
- Hz
Hertz
- Kg
Kilogram
- kHz
Kilohertz
- MHC
Myosin Heavy Chain
- MU
Motor Unit
- MUAP
Motor Unit Action Potential
- MVC
Maximal Voluntary Contraction
- RMS
Root-Mean-Square
- RT
Resistance Trained
- SD
Standard Deviation
- SED
Sedentary
- SPSS
Statistical Package for the Social Sciences
- VL
Vastus Lateralis
Introduction
Surface electromyography (EMG) is the recording of myoelectric signals from the skin overlying active motor units (MUs).1,2 The time domain (amplitude) of the EMG signal is influenced by both the recruitment of MUs and their firing rates.2 Thus, surface EMG can provide information regarding MU control strategies.
It is well documented training elicits adaptations that are dependent on the mode of exercise.3,4 Numerous studies have reported acute and chronic aerobic- (AT) and resistance-training (RT) alters MU: firing rates,5, 6, 7, 8 synchronization,9,10 recruitment thresholds,11,12 excitability,13,14 and MU pool output6,15 during submaximal voluntary contractions. Therefore, it is plausible that EMG amplitude (EMGRMS) would be sensitive to alterations in MU control strategies as a function of chronic training status; however, previous literature has been mixed. For example, Herda et al.16 reported no differences in the b terms (slopes) calculated from the natural log-transformed EMGRMS-force relationships during a linearly increasing muscle action up to 90% maximal voluntary contraction (MVC) among chronic training statuses (AT, RT, sedentary [SED]) with known fiber area differences for the vastus lateralis (VL). Conversely, Trevino and Herda17 reported b term differences among chronic training statuses for an isometric trapezoidal muscle action at 60% MVC that included a linearly increasing, steady force, and linearly decreasing segment. Therefore, the ability to differentiate muscle excitation to the motoneuron pool among chronic training statuses may depend on the targeted intensity and muscle action being examined.
Chronic training also elicits structural alterations; such as greater type Ⅰ- and type IIA-% myosin heavy chain (MHC) expression for AT-16,18 and RT-individuals,19,20 respectively, which would influence the fatigability21 and twitch forces of the MU pool.22 During fatiguing contractions, increases in MU recruitment and firing rates in conjunction with decreases in recruitment thresholds have been observed.23,24 In addition, there is evidence suggesting that muscle excitation to the motoneuron pool will adjust in response to MU twitch forces when producing a desired force output.24 Thus, examining the EMGRMS patterns of responses among AT, RT, and SED during a fatigue inducing task may indicate chronic training related specific adjustments in MU control strategies (MU recruitment and/or firing rates) when maintaining a targeted force. In addition, investigating MU control strategy responses during fatigue as a result of specific chronic training may provide information for strength and conditioning coaches and clinicians that allows for better exercise and rehabilitation programming, respectively. Furthermore, investigating a series of isometric trapezoidal muscle actions that includes a linearly increasing, steady force, and decreasing segment, mimics the MU activation, force maintenance, and MU deactivation-strategies performed during the cyclical movement patterns that humans perform during activities of daily living more so than a single, sustained contraction to failure. However, we are aware of only one study that has examined the influence of chronic training status on the EMGRMS patterns of response for the VL during a fatiguing isometric muscle action. Beck et al.25 reported no differences for absolute and normalized EMGRMS between chronic AT and RT individuals with known MHC expression differences during a 30 s step contraction of the knee extensors at 50% MVC. Recently, our group reported greater fatigability for chronic RT and SED individuals in comparison to AT, which was associated with differences in mechanical behavior (mechanomyographic amplitude patterns) of the VL during a series of repetitive muscle actions.26 Subsequently, based on the findings of Trevino and Herda17 and Olmos et al.,26 a series of isometric trapezoidal muscle actions may be sensitive to adjustments in the electrophysiological behavior during fatigue among chronic training statuses. However, this has yet to be examined and warrants further investigation.
The patterns of response for EMGRMS-force relationships during linearly increasing muscle actions have typically been examined with polynomial regression27,28 or analysis of variance (ANOVA) models of composite EMGRMS values at discrete %MVC levels. However, due to large variability among individuals,29 it has been suggested that linearly varying muscle actions should be examined on a subject-by-subject basis2,30,31 to better describe the individual patterns of response.32 Subsequently, Herda et al.16 proposed log-transforming the EMGRMS and force values and calculating b terms to investigate possible changes in the individual patterns. In addition, the 95% confidence intervals (CI) calculated around the b terms provide insight on the linearity of the relationship.32 For example, if the b term is equal to 1 or the 95% CI include 1, the relationship between EMGRMS and force is linear. If the b term is greater than 1 and the 95% CI do not include 1, the relationship accelerates across the force spectrum as the rate of change is greater for the Y variable (EMGRMS) than the X variable (force). Furthermore, the y-intercepts (a terms) of the log-transformed relationships reflect upward or downward shifting of the overall exponential relationship without changes to the EMGRMS patterns. Previously, the b terms calculated from EMGRMS-force relationships have identified MU activation- and muscle action-related differences among chronic training statuses during a 60% MVC.17 Thus, examining the b and a terms during repetitive isometric trapezoidal muscle actions may provide insight on differences in MU control strategies among chronically trained individuals during fatigue.
Therefore, the purpose of this study was to examine EMGRMS-force relationships during repetitive muscle actions at 50% MVC that contained linearly increasing, steady force, and linearly decreasing segments for AT, RT, and SED individuals. Based off the findings of Trevino and Herda,17 we hypothesized greater b terms for the RT compared to the AT and SED during the linearly increasing segment of the first contraction. In addition, we hypothesized muscle action-related differences for the first contraction, such as greater b terms for the RT during the increasing-in comparison to the decreasing-segment and the converse for the AT.17 During fatiguing contractions, it has been reported that as MU twitch forces decline, MU firing rates increase, MU recruitment thresholds decrease, and muscle excitation from the central nervous system to motoneuron pool increases to maintain the target force level.33 Thus, for the last contraction, we hypothesized greater b terms for the RT compared to the AT during the linearly increasing and decreasing segments due to the accumulation of fatigue. Based on the findings of Beck et al.25 and Trevino and Herda,17 we hypothesized EMGRMS from the steady force segment would not differentiate training statuses.
Material and methods
Subjects
Fifteen healthy adults (mean ± standard deviation [SD]; age = (21.80 ± 3.67) years [yr]; body weight= (73.59 ± 22.79) kg; height = (172.85 ± 11.71) cm participated in this study. Based on training status, participants were categorized as AT (five participants; age = [19.20 ± 0.45] yr; body weight = [59.02 ± 11.98] kg; height = [171.89 ± 15.81] cm, RT (five participants; age = [25 ± 4.53] yr; body weight = [99.22 ± 17.87] kg; height = [178.74 ± 8.09] cm or SED (five participants; age = [21.20 ± 2.17] yr; body weight = [62.52 ± 10.69] kg; height = [167.90 ± 9.45] cm) for further statistical analysis. Individuals in the AT group participated in a structured running program for at least 3 years prior to the study, completing an average of (61 ± 15) miles per week for 7–10 h per week, and none of them reported engaging in resistance training. RT individuals reported engaging in a structured resistance training program for at least 4 years prior to the study and performed 4–8 h per week of resistance training without engaging in any type of aerobic activity and self-reported a one-repetition back squat of at least twice their body mass. SED individuals reported no participation in any form of structured physical activity or exercise for 3 years prior to this study. According to Herda et al.16 and Fry et al.,34 differences in %MHC isoform expression for the VL have been reported among individuals with similar training histories, such as a greater type Ⅰ %MHC isoform expression for AT than the RT and SED. The sample size (n = 5 for each group) was based on previous investigations with similar study designs that examined the log transformed EMGRMS-force relationships of the VL for AT, RT, and SED groups.16,17 In addition, power calculation software (G∗power 3.1.9.7, Heinrich-Heine-Universität Düsseldorf, Dusseldorf, Germany) indicated 5 participants per group were sufficient for detecting training status related differences. Prior to experimental testing, participants completed an informed consent and health and exercise status questionnaire. No participants reported any current or previous neuromuscular diseases or musculoskeletal injuries specific to the ankle, knee, or hip joints. This study was approved by the University Institutional Review Board for human subject research. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Kansas (10-30-2012/HSCL #20495).
Isometric testing
Each participant was seated on a Biodex dynamometer (Biodex Medical System, Inc., Shirley, NY, USA) with restraining straps over the pelvis, trunk, and left thigh. The right femur was aligned with the input axis of the Biodex and all isometric knee extensor strength assessments were performed on the right leg at a knee joint angle of 90° (Biodex Pro Manual, Application/Operations, 1998). The force output of knee extensors was measured using a load cell (LC402, Omegadyne, Inc., Sunbury, OH, USA) that was fitted to the Biodex System 3 isokinetic dynamometer.
Participants visited the laboratory for one experimental visit. During experimental testing, participants performed three isometric MVCs for the knee extensors with 3 min rest between muscle actions. Following a 5-min rest period, participants were asked to complete 20 repetitive submaximal isometric trapezoidal muscle actions at 50% MVC. The trapezoid trajectory contained a 5 s baseline, a linearly increasing segment from baseline at a rate of 10% MVC/s, a 12 s steady force segment at the targeted %MVC, a linearly decreasing segment to baseline at rate of 10% MVC/s, and a 3 s baseline (Fig. 1). Thus, participants were given an 8–9 s rest period between contractions. Participants repeatedly performed the muscle actions until they completed the 20 repetitions or the average force decreased by > 5% MVC from the 50% MVC target.24,26 Prior to the repeated trapezoidal muscle actions at 50% MVC, participants practiced the isometric trapezoidal muscle actions at 20% MVC.17 A computer monitor was provided to display the target force template and real-time force output, and participants were asked to maintain their force as close as possible to target force template during the testing.
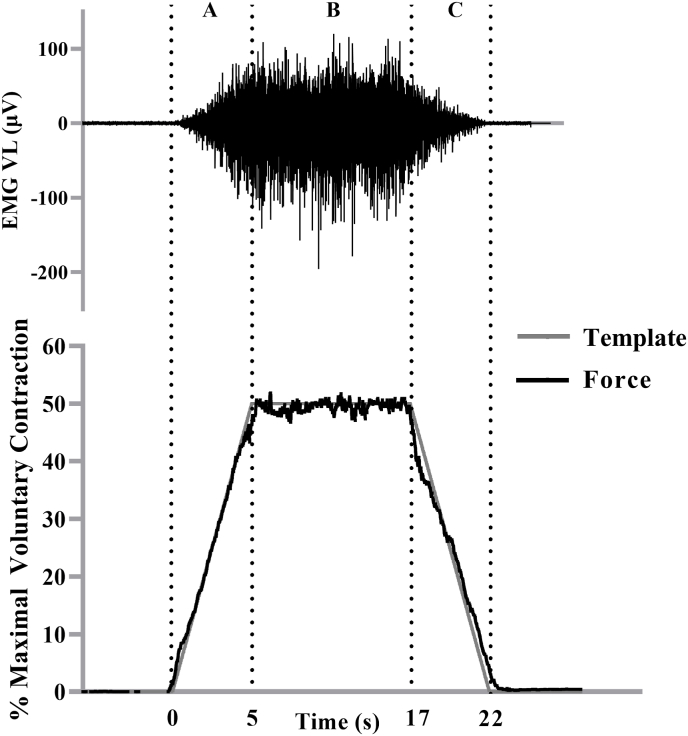
Fig. 1.
The electromyographic (EMG) signal recorded from the vastus lateralis during a 50% isometric trapezoidal contraction from one participant. The force signal (bottom) is overlaid onto the trapezoidal template as it appeared for the participant during the trial. The vertical dotted lines indicate the (A) linear force increase, (B) the steady force, and (C) the linear force decrease segments of the 50% isometric trapezoidal contraction. The EMG signal that corresponded with the contraction segments (A-C) was selected for analysis.
Electromyography
Surface EMG signals were recorded from the VL during the trapezoidal muscle action with a 5 pin array sensor (Delsys, Boston, MA, USA). Each pin had a 0.5 mm diameter, with 4 pins located at the corners of a 5 × 5 mm square and the 5th pin at the center of the square. Before sensor placement, the surface of the skin was shaved, adhesive tape was used to remove superficial dead skin, and the skin was sterilized with alcohol. The sensor was placed on the muscle belly of the VL at half the distance between the greater trochanter and lateral condyle of the femur with adhesive tape. A reference electrode was place over the left patella after superficial skin was shaved, cleaned, and sterilized. The EMG signals from the 4 pins of the sensor were differentially amplified and filtered with a bandwidth of 20 Hz to 9.5 kHz. The EMG signal recorded from channel 1 was used for all subsequent analyses and statistical comparisons.
Signal processing
Both EMG (μV) and force (N) signals were sampled at 20 kHz with a Delsys data acquisition system (Bagnoli-16 channel EMG system, Delsys, Inc., Boston, MA) during each muscle action. All subsequent signals were stored and processed off-line with a customized LabVIEW program (LabVIEW, version 11; National Instruments, Austin, TX). The EMG signals were bandpass filtered (fourth-order Butterworth) at 10–500 Hz. The force and EMG signals were analyzed with consecutive, non-overlapping 0.25 s epochs during the submaximal isometric trapezoidal muscle actions. The amplitude of the EMG signal was calculated as the root-mean-square (RMS).
Skinfold thickness
Skinfold thickness measurements were taken at the location of the EMG sensor placement for the VL. An experienced investigator performed the measurements with a calibrated Harpenden caliper (John Bull, UK) in accordance to the recommendations of Jackson and Pollock.35 Three measurements were recorded and the average was defined as the representative skinfold thickness for each participant. It has previously been suggested that subcutaneous fat may low-pass filter the EMG signals.16,36
Statistical analyses
For the linearly increasing (Fig. 1–A) and decreasing (Fig. 1–C) segments of the trapezoid, simple linear regression models were fit to log-transformed EMGRMS-force relationships.16,17 The equations were represented as:
| ln[Y] = b(ln[X]) + ln[a] | (1) |
Where ln[Y] = the natural log of the EMGRMS values, ln[X] = the natural log of the force values, b = slope, and ln[a] = the natural log of the y-intercept. This can also be expressed as an exponential equation after antilog transformation:
| Y = aXb | (2) |
Where Y = the predicted EMGRMS values, X = force, b = slope of equation (1), and a = the antilog of the y-intercept from equation (1). Slopes (b) were calculated using Microsoft Excel (Microsoft Excel, version 2010; Microsoft, Inc., Redmond, WA).
For the steady force segment of the trapezoid (Fig. 1–B), EMGRMS was calculated by averaging the values for each 0.25 s epoch from the entire 12 s targeted %MVC.
Two separate three-way mixed factorial ANOVAs (training status [AT vs. RT vs. SED] × segment [linear increase vs. linear decrease] × contraction [first vs. last]) was used to examine differences in the b and a terms from the log-transformed EMGRMS-force relationships during linear increasing and linear decreasing segments of submaximal isometric trapezoid muscle actions. A two-way mixed factorial ANOVA (training status [AT vs. RT vs. SED] × contraction [first vs. last]) was used to examine possible differences in EMGRMS among training statuses during the steady force segment of the isometric trapezoid muscle action. In addition, Pearson's product moment correlation coefficients were calculated comparing skinfold thicknesses among the b and a terms and EMGRMS during the steady force segments. When appropriate, follow-up tests included one-way ANOVAs and paired sample t-tests for the b terms of log-transformed EMGRMS-force relationships with Bonferroni corrections. The partial η2 statistics were calculated with values of 0.01, 0.06, and 0.14 corresponding to small, medium, and large effect size, respectively. In addition, Hedges' g was calculated for paired comparisons, with 0.2, 0.5, and 0.8 corresponding to small, medium, and large effect size, respectively. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) (version 24, IBM Corporation, Armonk, New York, USA) with alpha set at 0.05.
Results
Table 1 contains the individual values for EMGRMS at steady-force and the a and b terms from the log-transformed EMGRMS-force relationships during the linearly increasing and decreasing segments of the first and last successfully completed isometric trapezoidal contraction.
Table 1.
Electromyographic amplitude (EMGRMS) during steady-force and the a and b terms calculated from the log-transformed EMGRMS-force relationship during linearly increasing and decreasing segments for the first and last successfully completed isometric trapezoidal contraction for the aerobically- (AT), resistance-trained (RT) and sedentary (SED) individuals.
| Group | Subject | First contraction |
Last contraction |
Coefficients | First contraction |
Last contraction |
||
|---|---|---|---|---|---|---|---|---|
| EMGRMS (μV) | EMGRMS (μV) | Increase | Decrease | Increase | Decrease | |||
| AT |
1 | 56.27 | 70.00 | a term | 0.213 | 0.270 | 0.222 | 0.564 |
| b term | 1.013a | 1.000a | 1.084a | 0.919a | ||||
| 2 | 51.05 | 60.44 | a term | 0.530 | 0.542 | 0.679 | 0.366 | |
| b term | 0.838a | 0.855a | 0.832b | 0.998b | ||||
| 3 | 80.65 | 98.14 | a term | 0.634 | 0.240 | 0.798 | 1.175 | |
| b term | 0.769a | 0.962a | 0.827a | 0.722a | ||||
| 4 | 59.57 | 78.65 | a term | 0.172 | 0.126 | 0.529 | 0.069 | |
| b term | 1.062a | 1.165a | 0.926a | 1.325a | ||||
| 5 |
36.02 |
37.27 |
a term | 0.366 | 0.285 | 0.734 | 0.337 | |
|
b term |
0.866a |
0.886a |
0.748a |
0.885b |
||||
| RT |
6 | 34.08 | 47.47 | a term | 0.023 | 0.042 | 0.389 | 0.016 |
| b term | 1.249a | 1.105b | 0.806a | 1.319a | ||||
| 7 | 63.29 | 88.54 | a term | 0.004 | 0.022 | 0.002 | 0.005 | |
| b term | 1.494b | 1.208a | 1.636a | 1.479a | ||||
| 8 | 50.40 | 111.74 | a term | 0.011 | 0.142 | 0.006 | 0.001 | |
| b term | 1.405b | 0.985a | 1.697a | 2.098a | ||||
| 9 | 93.78 | 133.40 | a term | 0.019 | 0.254 | 0.025 | 0.037 | |
| b term | 1.373b | 0.925a | 1.391a | 1.306a | ||||
| 10 |
41.66 |
77.54 |
a term | 0.071 | 0.141 | 0.016 | 0.009 | |
|
b term |
0.986b |
0.865b |
1.333b |
1.428b |
||||
| SED | 11 | 27.95 | 60.70 | a term | 0.426 | 0.518 | 0.111 | 0.040 |
| b term | 0.744b | 0.815a | 1.152b | 1.371b | ||||
| 12 | 26.48 | 39.39 | a term | 0.293 | 0.069 | 0.430 | 0.035 | |
| b term | 0.794b | 1.166a | 0.810a | 1.355b | ||||
| 13 | 23.42 | 32.80 | a term | 0.111 | 0.022 | 0.056 | 0.017 | |
| b term | 0.979a | 1.387a | 1.174b | 1.538a | ||||
| 14 | 234.04 | 215.42 | a term | 1.161 | 0.299 | 1.996 | 0.264 | |
| b term | 0.891a | 1.131a | 0.834a | 1.127a | ||||
| 15 | 82.59 | 149.52 | a term | 0.075 | 0.066 | 0.390 | 0.175 | |
| b term | 1.153a | 1.156b | 1.032b | 1.133a | ||||
Indicates relationship was linear.
Indicates relationship was curvilinear.
Linearly increasing and decreasing segments EMGRMS-force relationships
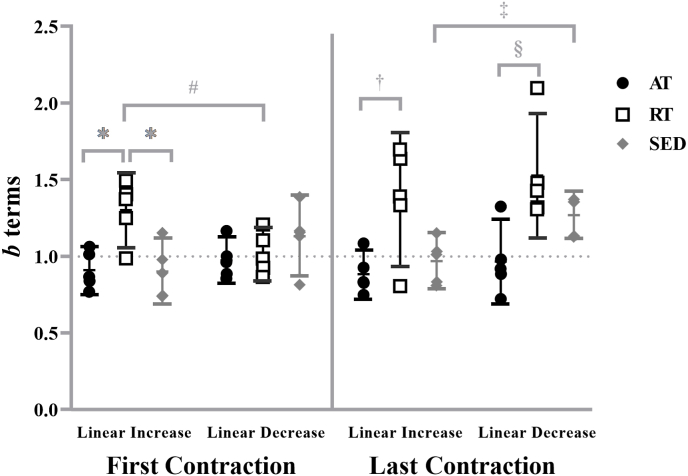
For the b terms, the analyses indicated a significant three-way interaction (training status × segment × contraction; F = 4.926, p = 0.027, partial η2 = 0.451). The b terms for RT during the linearly increasing segment of the first contraction (1.301 ± 0.197) were greater than the AT (0.910 ± 0.123; p = 0.008, g = 2.381) and SED (0.912 ± 0.162; p = 0.008, g = 2.157), the b terms for the RT during the first contraction were greater during the linearly increasing segment than decreasing segment (1.018 ± 0.139; p = 0.014, g = 1.660), the b terms for the RT during the linearly increasing (1.373 ± 0.353) and decreasing segment (1.526 ± 0.328) of the last contraction were greater than the AT (linear increase = 0.883 ± 0.129, p = 0.018, g = 1.844; linear decrease = 0.970 ± 0.223, p = 0.010, g = 1.983), and the b terms for SED during the last contraction were less during the linearly increasing (0.968 ± 0.144) than decreasing segment (1.268 ± 0.126; p = 0.015, g = 2.217) (Fig. 2). There were no other differences reported among training statuses, between segments, or between repetitions. Fig. 3 illustrates the mean EMGRMS patterns for the AT, RT, and SED during the linearly increasing and decreasing segments of the first and last contractions.
Fig. 2.
Individual values for the b terms (slopes) from the electromyographic amplitude (EMGRMS)-force relationship for the aerobically- (AT), resistance-trained (RT), and sedentary (SED) from the linearly increasing and decreasing segments of the isometric trapezoidal contractions. Horizontal bars indicate the means and 95% confidence intervals for the respective groups. ∗ indicates greater b terms for RT than AT (p = 0.008) and SED (p = 0.008) during the linearly increasing segment of the first contraction. # indicates greater b terms for RT during the linearly increasing segment than decreasing segment of first contraction (p = 0.014). † indicates greater b terms for RT than AT during the linearly increasing segment of the last contraction (p = 0.018). § indicates greater b terms for RT than AT for the linearly decreasing segment of the last contraction (p = 0.010). ‡ indicates lower b terms for SED during the linearly increasing segment than decreasing segment of last contraction (p = 0.015).
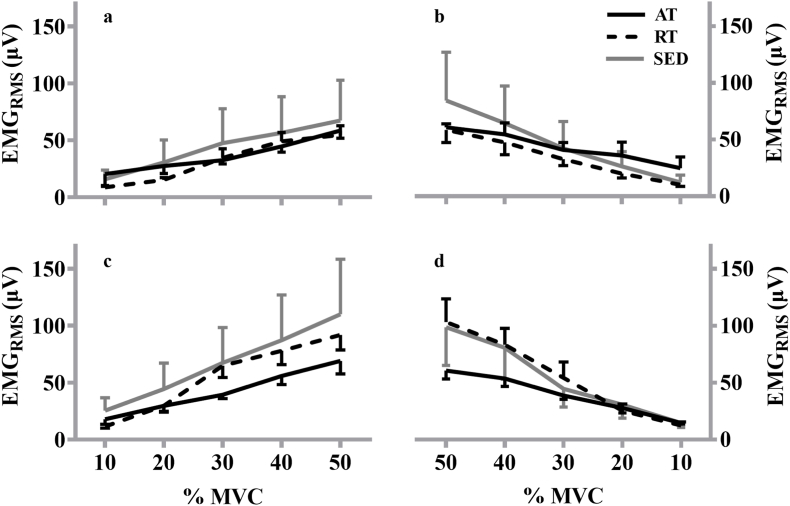
Fig. 3.
Plotted means and standard error of the mean for the aerobically- (AT; black line), resistance-trained (RT; black dashed line), and sedentary (SED; gray line) during the linearly increasing (A, C) and decreasing (B, D) segments of electromyographic amplitude (EMGRMS)-force relationship from 10% to 50% maximal voluntary isometric contraction (%MVC) for the first (top) and last (bottom) contraction.
For the a terms, the analyses indicated no significant three-way interaction (training status × segment × contraction; F = 0.715, p = 0.509, partial η2 = 0.107), no two-way interactions (segment × contraction, F = 2.529, p = 0.138, partial η2 = 0.174; training status × segment, F = 1.601, p = 0.242, partial η2 = 0.211; training status × contraction, F = 1.432, p = 0.277, partial η2 = 0.193) or main effects for training status (F = 3.677, p = 0.057, partial η2 = 0.380), segment (F = 2.860, p = 0.117, partial η2 = 0.192), or contraction (F = 1.985, p = 0.184, partial η2 = 0.142).
Steady force segments
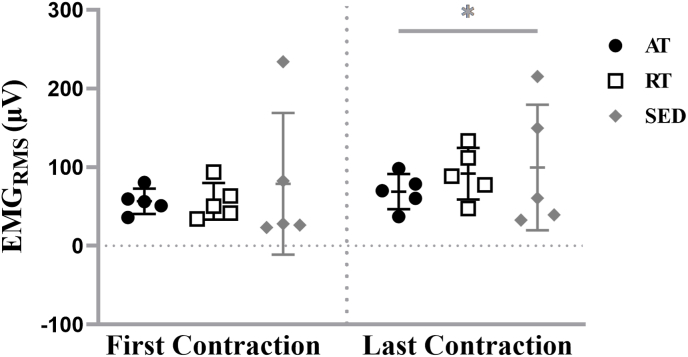
For the steady force segments, the analyses indicated neither a two-way interaction (training status × contraction; F = 1.463, p = 0.270, partial η2 = 0.196) nor a main effect for training status (F = 0.325, p = 0.729, partial η2 = 0.051). There was a main effect for contraction (F = 16.787, p = 0.001, partial η2 = 0.583). EMGRMS during the steady force segment was greater for the last contraction ([86.73 ± 49.55] μV) than the first contraction ([64.08 ± 51.68] μV) when collapsed across training status (Fig. 4).
Fig. 4.
Individual values for electromyographic amplitude (EMGRMS) from the steady force segment of the first and last isometric trapezoidal contraction for the aerobically- (AT), resistance-trained (RT), and sedentary (SED). Horizontal bars represent the means and standard deviations for the respective groups. ∗ Indicates greater EMGRMS for the last contraction when collapsed across groups (p = 0.001).
Correlations
Pearson's product moment correlations were not significant for skinfold thickness with the b terms from the linearly increasing and decreasing segments for the first and last contractions (p = 0.114–0.733, r = −0.425 to 0.327). In addition, there were no correlations for skinfold thickness among the a terms for the linearly increasing or decreasing segments for the first contraction (p = 0.114–0.855, r = −0.052 to −0.379), or the linearly decreasing segment for the last contraction (p = 0.114, r = −0.425). Skinfold thickness was correlated with the a terms during the linearly increasing segment of the last contraction (p = 0.025, r = −0.576). Therefore, only 1 of 8 (12.5%) correlations were significant for skinfold thickness among the coefficients, which is in agreement with previous examinations37,38 and provides further confidence that training status related differences for the b terms were not influenced by skinfold thicknesses. Skinfold thickness was correlated with EMGRMS from the steady force segments for the first (p = 0.014, r = −0.617) and the last contractions (p = 0.040, r = −0.535).
Discussion
All 60 log-transformed EMGRMS-force relationships were significant for the linearly increasing (p < 0.05; r range = 0.808–0.988) and decreasing segments (p < 0.05; r range = 0.899–0.988) of the first and last completed isometric trapezoidal muscle action. As previously reported by our group,26 only the AT were able to successfully complete all 20 isometric trapezoidal muscle actions, as well as maintain maximal strength following the repeated muscle actions. Additionally, during the first isometric trapezoidal muscle action, the RT exhibited greater b terms than the AT and SED during the linearly increasing segment, and only the RT displayed muscle action-related differences.17 Significant and novel findings during the last contraction in response to fatigue include greater b terms for the RT during the linearly increasing and decreasing segments in comparison to the AT, and muscle action-related differences for the SED where the b terms were greater during the linearly decreasing in comparison to the linearly increasing segment.
During the repetitive submaximal isometric trapezoidal muscle actions, there were numerous differences as a function of training status and muscle action. For the linearly increasing segment of the first contraction, the b terms for the RT were greater than the AT and SED. Previously, Trevino and Herda17 reported similar findings during an isometric trapezoidal contraction performed at 60% MVC. It is suggested that EMGRMS-force relationships reflect changes in MU recruitment and firing rates to modulate force production.1,2 Thus, it was hypothesized the larger b terms for the RT indicated a greater amount of MU activation were necessary to match the targeted force compared to the AT and SED.17 However, it has recently been reported that EMGRMS is primarily explained by the size of the motor unit action potentials (MUAPs).39 Numerous studies have reported chronic resistance-training increases muscle cross-sectional area,40,41 particularly due to the hypertrophy of type II muscle fibers.40 In addition, MUAPs are positively correlated to muscle fiber size.42,43 Therefore, the greater b terms for the RT compared to the AT and SED during the linearly increasing segment of the first contraction is likely due to the recruitment of MU comprised of larger muscle fibers, rather than greater MU activation as fatigue was unlikely accumulating during the 5 s linearly increasing portion of the isometric trapezoidal contraction.39
During the first contraction, the b terms for RT were greater during the linearly increasing than decreasing segment, which is agreement with Trevino and Herda.17 The differences in MU activation and deactivation strategies for the RT may be a result of the 12 s steady force segment between the linearly increasing and decreasing segments. MU potentiation of the VL was previously reported for chronic RT individuals during a 40% and 70% isometric trapezoidal muscle action.44 MU potentiation would increase MU twitch force output during the contraction, allowing potentiated MUs to be derecruited at higher force levels during the linearly decreasing segment than which they were initially recruited at during the increasing segment.5,44,45 Thus, the amount of muscle excitation to the motoneuron pool and the size of the largest activated MUs would be less at the same relative force level (% MVC) during the decreasing segment, which is supported by signficant decrease in b terms for the RT. Conversely, the AT and SED displayed no significant differences in the b terms between increasing and decreasing segments. Mettler and Griffin46 and Herda et al.44 reported a lack of MU potentiation for the adductor pollicis and VL following endurance training and for chronic AT individuals, respectively. In addition, no differences in b terms from EMGRMS-force relationships were previously reported for SED between linearly increasing and decreasing muscle actions.17 Thus, the findings suggest alterations in MU potentiation may require chronic resistance training.
There were additional chronic training status related differences during the last contraction, such as greater b terms for the RT during the linearly increasing and decreasing segments in comparison to the AT. The chronic training-related differences in MU activation and deactivation strategies may be due to differences in fatigue resistance. During muscular fatigue, it is well understood that MU twitch forces decrease.33 Consequently, the central nervous system increases the amount of excitation to the motoneuron pool and the recruitment thresholds decrease for higher-threshold MUs to compensate for the decline in twitch forces of the fatiguing MUs.24 Aerobic training elicits relatively higher amounts of type I % MHC expression,47,48 whereas the converse is true for resistance training.40 Thus, the VL for the RT is likely comprised of a greater percentage of MUs that possess lower fatigue resistance than the AT, and the larger b terms indicate a greater increase in MU recruitment and/or firing rates to match the same %MVC during the linearly increasing and decreasing segments of the last contraction. Conversely, the SED showed no significant differences in the b terms during the linearly increasing and decreasing segments compared with the RT and AT, although it has been reported the proportion of total type II% MHC for SED-is similar with the RT-individuals.16,34 Therefore, it would be expected the RT and SED would possess similar fatigability, which has previously been reported in this cohort of participants.26 However, the lack of differences for the SED in comparison to the AT and RT may suggest that neuromuscular and muscle structure training-related adaptions are both necessary for the b terms to differentiate MU activation and deactivation strategies among chronic training status during fatigue. There were also muscle action related differences for the SED during the last contraction, such as greater b terms during the linearly decreasing in comparison to the linearly increasing segment. It is well documented that muscle excitation and descending drive from central nervous system increases with muscular fatigue.49 Thus, the greater b terms for the SED during the linearly decreasing indicates increased muscle excitation in comparison to the linearly increasing segment when trying to produce force. Nonetheless, the findings suggest the log-transformed EMGRMS-force relationships may be sensitive to chronic training induced alterations in motor control strategies during fatigue.
For the steady force segment, EMGRMS was greater during the last contraction in comparison to the first contraction when collapsed across training statuses. The amplitude of the EMG signal is influenced by the number of recruited MUs and their firing rates.1,2 Thus, the change in EMGRMS likely resulted from the increase in muscle excitation that occurs in response to muscular fatigue (e.g., increase in firing rates, accelerated recruitment of higher-threshold MUs).24 EMGRMS during steady force did not differentiate among groups (p = 0.051), which supports the findings of Beck et al.25 that reported no difference in EMGRMS of the VL between AT- and RT-individuals during a fatiguing 30 s submaximal isometric muscle action of the leg extensors at 50% MVC. These findings further support that utilizing the b terms from the log-transformed EMGRMS-force relationships during linearly increasing and decreasing segments of an isometric trapezoidal contraction may provide more insight than EMGRMS recorded during steady force to elucidate neuromuscular adaptations resulting from chronic training.17,25 It should be noted that EMGRMS recorded during the 12 s steady force segments was negatively correlated with skinfold thickness, which can serve as a low-pass filter.16 However, it was previously reported that skinfold thickness differences did not exist among groups for this cohort of individuals,26 which may suggest low-pass filtering was not responsible for the lack of training-related differences in EMGRMS during the 12 s steady force segment. Nonetheless, EMGRMS at steady force was not able to differentiate among AT, RT, and SED during the first and last successfully completed muscle action.
We would like to acknowledge this study did not collect muscle biopsies, which is a limitation. Due to our strict inclusion criteria based of Herda et al.,16 Fry et al.,34 and Beck et al.,25 we were confident our participants would possess differences in MHC isoform expression. For example, 2 AT and 1 RT individual participated in another study for our laboratory that included MHC analysis of the VL. The participants exhibited training status related differences for MHC isoforms of the VL, such as the type I MHC isoform expression percentages for the AT individuals were 55% and 68%, respectively, whereas the type I MHC isoform expression percentage for the RT individual was 31%. However, since we did not collect muscle biopsies for every participant in our study, we cannot confirm that differences for type I MHC isoform expression existed between the chronic training groups.
In conclusion, the b terms from the log-transformed EMGRMS-force relationships during linearly increasing and decreasing muscle actions differentiated training statuses during pre- and late-fatigue isometric trapezoidal contractions, such as greater acceleration in EMGRMS throughout the force spectrum for the RT than AT. The greater acceleration in EMGRMS for RT during the first contraction may reflect the recruitment of larger sized MUs compared to the AT as fatigue was likely not present yet, whereas it is plausible the group differences during the last contraction is due to accelerated recruitment of additional higher-threshold MUs and/or greater firing rates of the recruited MUs as fatigue was accumulating to a greater extent for the RT. In addition, there were muscle-action related differences (linearly increasing vs. decreasing) among training statuses. The RT exhibited greater acceleration during the linearly increasing muscle action in comparison to the linearly deceasing for the first contraction, whereas the opposite was true for the SED during the last contraction. Conversely, there were no muscle-action related difference for the AT. Therefore, different modes of chronic training elicited specific EMGRMS patterns of response (i.e. muscle excitation to motoneuron pool) during a fatiguing-type task. The findings suggest strength coaches and practitioners should consider the athletic demands of their athletes and the goals of their patients when designing exercise programs, as solely engaging in resistance training resulted in a significantly greater amount of necessary muscle excitation to produce a relative targeted force during repetitive MU activation and deactivation tasks compared to individuals who strictly aerobically train. Thus, athletes or individuals who currently resistance train but have task demands lasting longer than a few seconds (repetitive or continuous) may see increased performance in the latter stages of the activity by incorporating some aerobic training. Future research should utilize EMG signal decomposition techniques to examine the influence of chronic training on MU control strategies (e.g., MU recruitment and firing rates) during fatigue.
Submission statement
The work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or any other language, without the written consent of the copyright-holder.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SJ, SAS, TJH, and MAT. The first draft of the manuscript was written by SJ and MAT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethical approval statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Kansas (10-30-2012/HSCL #20495). Written informed consents were collected from participants.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the participants who took time out of their schedules to help with these projects.
References
- 1.De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13(2):135–163. doi: 10.1123/jab.13.2.135. [DOI] [Google Scholar]
- 2.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 3.Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002;29(3):218–222. doi: 10.1046/j.1440-1681.2002.03623.x. [DOI] [PubMed] [Google Scholar]
- 4.Jukic I, Van Hooren B, Ramos AG, et al. The effects of set structure manipulation on chronic adaptations to resistance training: a systematic review and meta-analysis. Sports Med. 2021;51(5):1061–1086. doi: 10.1007/s40279-020-01423-4. [DOI] [PubMed] [Google Scholar]
- 5.De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol. 1982;329(1):113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vila-Chã C, Falla D, Farina D. Motor unit behavior during submaximal contractions following six weeks of either endurance or strength training. J Appl Physiol. 2010;109(5):1455–1466. doi: 10.1152/japplphysiol.01213.2009. [DOI] [PubMed] [Google Scholar]
- 7.Dimmick HL, Miller JD, Sterczala AJ, Trevino MA, Herda TJ. Vastus lateralis muscle tissue composition and motor unit properties in chronically endurance-trained vs. sedentary women. Eur J Appl Physiol. 2018;118(9):1789–1800. doi: 10.1007/s00421-018-3909-9. [DOI] [PubMed] [Google Scholar]
- 8.Trevino MA, Dimmick HL, Parra ME, et al. Effects of continuous cycling training on motor unit firing rates, input excitation, and myosin heavy chain of the vastus lateralis in sedentary females. Exp Brain Res. 2022;240(3):825–839. doi: 10.1007/s00221-021-06278-3. [DOI] [PubMed] [Google Scholar]
- 9.Milner-Brown HS, Lee R. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- 10.Semmler J, Nordstrom M. Motor unit discharge and force tremor in skill-and strength-trained individuals. Exp Brain Res. 1998;119(1):27–38. doi: 10.1007/s002210050316. [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513(1):295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Vecchio A, Casolo A, Negro F, et al. The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol. 2019;597(7):1873–1887. doi: 10.1113/JP277250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perot C, Goubel F, Mora I. Quantification of T-and H-responses before and after a period of endurance training. Eur J Appl Physiol Occup Physiol. 1991;63(5):368–375. doi: 10.1007/BF00364464. [DOI] [PubMed] [Google Scholar]
- 14.Latella C, Teo WP, Harris D, Major B, VanderWesthuizen D, Hendy AM. Effects of acute resistance training modality on corticospinal excitability, intra-cortical and neuromuscular responses. Eur J Appl Physiol. 2017;117(11):2211–2224. doi: 10.1007/s00421-017-3709-7. [DOI] [PubMed] [Google Scholar]
- 15.Vila-Chã C, Falla D, Correia MV, Farina D. Changes in H reflex and V wave following short-term endurance and strength training. J Appl Physiol. 2012;112(1):54–63. doi: 10.1152/japplphysiol.00802.2011. [DOI] [PubMed] [Google Scholar]
- 16.Herda TJ, Housh TJ, Fry AC, et al. A noninvasive, log-transform method for fiber type discrimination using mechanomyography. J Electromyogr Kinesiol. 2010;20(5):787–794. doi: 10.1016/j.jelekin.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Trevino MA, Herda TJ. The effects of training status and muscle action on muscle activation of the vastus lateralis. Acta Bioeng Biomech. 2015;17(4):107–114. [PubMed] [Google Scholar]
- 18.McKendry J, Joanisse S, Baig S, et al. Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults. J Gerontol A Biol Sci. 2020;75(6):1079–1088. doi: 10.1093/gerona/glz142. [DOI] [PubMed] [Google Scholar]
- 19.Campos GE, Luecke TJ, Wendeln HK, et al. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88(1):50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- 20.Shoepe TC, Stelzer JE, Garner DP, Widrick JJ. Functional adaptability of muscle fibers to long-term resistance exercise. Med Sci Sports Exerc. 2003;35(6):944–951. doi: 10.1249/01.MSS.0000069756.17841.9E. [DOI] [PubMed] [Google Scholar]
- 21.Shenkman B. From slow to fast: hypogravity-induced remodeling of muscle fiber myosin phenotype. Acta naturae. 2016;8(4):47–59. doi: 10.32607/20758251-2016-8-4-47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garnett RA, O’Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979;287(1):33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stock MS, Beck TW, Defreitas JM. Effects of fatigue on motor unit firing rate versus recruitment threshold relationships. Muscle Nerve. 2012;45(1):100–109. doi: 10.1002/mus.22266. [DOI] [PubMed] [Google Scholar]
- 24.Contessa P, De Luca CJ, Kline JC. The compensatory interaction between motor unit firing behavior and muscle force during fatigue. J Neurophysiol. 2016;116(4):1579–1585. doi: 10.1152/jn.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck TW, Housh T, Fry A, et al. The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyogr Clin Neurophysiol. 2007;47(4–5):221–232. [PubMed] [Google Scholar]
- 26.Olmos AA, Herda TJ, Sontag SA, Trevino MA. The influence of chronic training status on the mechanical behavior of the vastus lateralis during repetitive trapezoidal contractions. J Musculoskelet Neuronal Interact. 2022;22(2):161–171. [PMC free article] [PubMed] [Google Scholar]
- 27.Perry S, Housh T, Johnson G, et al. Mechanomyography, electromyography, heart rate, and ratings of perceived exertion during incremental cycle ergometry. J Sports Med Phys Fit. 2001;41(2):183. [PubMed] [Google Scholar]
- 28.Zuniga JM, Malek MH. Electromyographic responses of the superficial quadriceps femoris muscles during incremental treadmill running. Muscle Nerve. 2013;48(6):938–944. doi: 10.1002/mus.23842. [DOI] [PubMed] [Google Scholar]
- 29.Ryan ED, Beck TW, Herda TJ, et al. Mechanomyographic amplitude and mean power frequency responses during isometric ramp vs. step muscle actions. J Neurosci Methods. 2008;168(2):293–305. doi: 10.1016/j.jneumeth.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Orizio C, Perini R, Veicsteinas A. Muscular sound and force relationship during isometric contraction in man. Eur J Appl Physiol Occup Physiol. 1989;58(5):528–533. doi: 10.1007/BF02330708. [DOI] [PubMed] [Google Scholar]
- 31.Zwarts MJ, Keidel M. Relationship between electrical and vibratory output of muscle during voluntary contraction and fatigue. Muscle Nerve. 1991;14(8):756–761. doi: 10.1002/mus.880140810. [DOI] [PubMed] [Google Scholar]
- 32.Herda TJ, Weir JP, Ryan ED, et al. Reliability of absolute versus log-transformed regression models for examining the torque-related patterns of response for mechanomyographic amplitude. J Neurosci Methods. 2009;179(2):240–246. doi: 10.1016/j.jneumeth.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Davis MP, Walsh D. Mechanisms of fatigue. J Support Oncol. 2010;8(4):164–174. [PubMed] [Google Scholar]
- 34.Fry AC, Housh TJ, Cramer JB, et al. Noninvasive assessment of skeletal muscle myosin heavy chain expression in trained and untrained men. J Strength Condit Res. 2017;31(9):2355–2362. doi: 10.1519/JSC.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 35.Jackson AS, Pollock ML. Practical assessment of body composition. Physician Sportsmed. 1985;13(5):76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 36.Bartuzi P, Tokarski T, Roman-Liu D. The effect of the fatty tissue on EMG signal in young women. Acta Bioeng Biomech. 2010;12(2):87–92. [PubMed] [Google Scholar]
- 37.Cooper MA, Herda TJ, Vardiman JP, Gallagher PM, Fry AC. Relationships between skinfold thickness and electromyographic and mechanomyographic amplitude recorded during voluntary and non-voluntary muscle actions. J Electromyogr Kinesiol. 2014;24(2):207–213. doi: 10.1016/j.jelekin.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Herda TJ, Cooper MA. Muscle-related differences in mechanomyography frequency–force relationships are model dependent. Med Biol Eng Comput. 2015;53(8):689–697. doi: 10.1007/s11517-015-1261-3. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Valdes E, Negro F, Falla D, De Nunzio AM, Farina D. Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol. 2018;124(4):1071–1079. doi: 10.1152/japplphysiol.01115.2017. [DOI] [PubMed] [Google Scholar]
- 40.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107(5):1655–1662. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterczala AJ, Miller JD, Dimmick HL, Wray ME, Trevino MA, Herda TJ. Eight weeks of resistance training increases strength, muscle cross-sectional area and motor unit size, but does not alter firing rates in the vastus lateralis. Eur J Appl Physiol. 2020;120(1):281–294. doi: 10.1007/s00421-019-04273-9. [DOI] [PubMed] [Google Scholar]
- 42.Hakansson CH. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand. 1956;37(1):14–34. doi: 10.1111/j.1748-1716.1956.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 43.Trevino M, Sterczala A, Miller J, et al. Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol. 2019;225(4):e13151. doi: 10.1111/apha.13151. [DOI] [PubMed] [Google Scholar]
- 44.Herda TJ, Siedlik JA, Trevino MA, Cooper MA, Weir JP. Motor unit control strategies of endurance-versus resistance-trained individuals. Muscle Nerve. 2015;52(5):832–843. doi: 10.1002/mus.24597. [DOI] [PubMed] [Google Scholar]
- 45.De Luca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol. 2010;104(2):1034–1046. doi: 10.1152/jn.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mettler JA, Griffin L. Muscular endurance training and motor unit firing patterns during fatigue. Exp Brain Res. 2016;234(1):267–276. doi: 10.1007/s00221-015-4455-x. [DOI] [PubMed] [Google Scholar]
- 47.Klitgaard H, Mantoni M, Schiaffino S, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140(1):41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 48.Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim JS. The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Condit Res. 2012;26(6):1724–1729. doi: 10.1519/JSC.0b013e318234eb6f. [DOI] [PubMed] [Google Scholar]
- 49.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]