Abstract
High-intensity and sprint interval training (HIIT and SIT, respectively) enhance insulin sensitivity and glycemic control in both healthy adults and those with cardiometabolic diseases. The beneficial effects of intense interval training on glycemic control include both improvements seen in the hours to days following a single session of HIIT/SIT and those which accrue with chronic training. Skeletal muscle is the largest site of insulin-stimulated glucose uptake and plays an integral role in the beneficial effects of exercise on glycemic control. Here we summarize the skeletal muscle responses that contribute to improved glycemic control during and following a single session of interval exercise and evaluate the relationship between skeletal muscle remodelling and improved insulin sensitivity following HIIT/SIT training interventions. Recent evidence suggests that targeting skeletal muscle mechanisms via nutritional interventions around exercise, particularly with carbohydrate manipulation, can enhance the acute glycemic benefits of HIIT. There is also some evidence of sex-based differences in the glycemic benefits of intense interval exercise, with blunted responses observed after training in females relative to males. Differences in skeletal muscle metabolism between males and females may contribute to sex differences in insulin sensitivity following HIIT/SIT, but well-controlled studies evaluating purported muscle mechanisms alongside measurement of insulin sensitivity are needed. Given the greater representation of males in muscle physiology literature, there is also a need for more research involving female-only cohorts to enhance our basic understanding of how intense interval training influences muscle insulin sensitivity in females across the lifespan.
Keywords: HIIT, Insulin sensitivity, Type 2 diabetes, Nutrition, Sex differences
Abbreviations
- Akt
protein kinase B
- AMPK
adenosine monophosphate activated protein kinase
- AUC
area under the curve
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CGM
continuous glucose monitoring
- G6P
glucose-6-phosphate
- GLUT4
glucose transporter 4
- GS
glycogen synthase
- HbA1c
hemoglobin A1c
- HIIT
high-intensity interval training
- HKII
hexokinase II
- HRmax
maximum heart rate
- IRS-1
insulin receptor substrate 1
- MAPK
mitogen-activated protein kinase
- MICT
moderate-intensity continuous training
- OGTT
oral glucose tolerance test
- PI3K
phosphatidylinositol 3-kinase
- SIT
sprint interval training
- T2D
type 2 diabetes
- TBC1D1
TBC1 domain family member 1
- TBC1D4
TBC1 domain family member 4
- O2peak
peak oxygen uptake
Introduction
Skeletal muscle represents the largest glycogen reserve within the human body and the primary site for insulin-stimulated glucose disposal in the post-prandial state.1 Accordingly, skeletal muscle insulin sensitivity is paramount to the maintenance of whole-body glucose homeostasis and muscle insulin resistance represents an early event in the progression toward type 2 diabetes (T2D).2 Exercise is a cornerstone in the prevention and treatment of T2D and improvements in muscle insulin sensitivity are proposed to partly mediate the beneficial effects of exercise on glycemic control.3,4 However, the optimal exercise prescription for improving muscle insulin sensitivity remains an area of active research and the associated mechanisms have not been fully resolved.
High-intensity and sprint interval training (HIIT and SIT, respectively) have emerged as efficacious and time-efficient alternatives to traditional moderate-intensity continuous training (MICT) for improving indices of cardiometabolic health. Several recent meta-analyses conclude that HIIT and/or SIT promote similar (and sometimes superior) improvements in cardiorespiratory fitness,5,6 exercise performance,7 body composition,8 and cardiometabolic disease risk factors9 compared to MICT. Consistent with large-scale randomized controlled trials10 and meta-analyses11,12 demonstrating the importance of exercise intensity for glycemic control, HIIT and SIT improve insulin sensitivity and glycemic control in both healthy adults and people with T2D13, 14, 15, 16, 17 potentially to a greater extent than MICT.13 Nevertheless, several important questions surrounding the impact of HIIT and SIT on insulin sensitivity – particularly those related to the acute and chronic effects on skeletal muscle and how they contribute to whole-body glycemic control – remain unanswered.
The purpose of this brief review is to summarize findings from studies examining the impact of HIIT and SIT on whole-body glycemic control and to provide a critical appraisal of the underlying mechanisms. Specifically, we highlight acute changes in exercised skeletal muscle that enhance glucose uptake in the hours to days following a single session of HIIT and SIT and evaluate the relationship between chronic changes in skeletal muscle phenotype and insulin sensitivity after training. For this review – and consistent with the terminology used in the literature6,18, 19, 20 – we broadly define HIIT and SIT as short, repeated bouts of intense submaximal and supramaximal exercise, respectively, that are separated by periods of rest or active recovery. MICT was used to describe protocols involving prolonged (≥ 30 min) continuous exercise performed at a submaximal intensity that typically elicit ∼65%–75% of maximum heart rate (HRmax) or ∼55%–75% of peak oxygen uptake (O2peak). A greater understanding of the muscle mechanisms that mediate the insulin-sensitizing effects of intense interval exercise should help optimize exercise prescription for maximizing the therapeutic potential of exercise for glycemic control and aid in the prevention and treatment of chronic diseases like T2D.
Acute effects of HIIT and SIT on glycemic control
An acute bout of HIIT involving ten, 1-min intervals eliciting ∼90% of HRmax improves post-prandial glycemic control measured over 24 h post-exercise using continuous glucose monitoring (CGM) in people with T2D compared to a no-exercise control condition.21 When compared to an acute bout of MICT (30 min at ∼65% peak heart rate) in adults with overweight/obesity, the same HIIT protocol is equally effective at reducing post-prandial hyperglycemia on the day of exercise but boasts an added benefit of persistent post-prandial glycemic improvements into the next day.22 These findings have been supported by subsequent research demonstrating that acute HIIT elicits more pronounced improvements in post-prandial glucose on the day of exercise23 as well as nocturnal and fasting glucose on the day after exercise24 compared to volume-matched MICT in people with T2D. Because impaired post-prandial glycemic control is hypothesized to associate more strongly with muscle as opposed to hepatic insulin resistance,25 improvements in post-prandial glycemia following HIIT are suggestive of an increase in muscle insulin sensitivity. Indeed, insulin-sensitizing benefits have been reported using the gold standard hyperinsulinemic-euglycemic clamp initiated 1 h post-exercise following a HIIT protocol involving 16 min of hard exercise (4 × 4-min cycling intervals at 95% HRmax with 2-min recovery periods) in males with obesity.26,27 More recently, improvements in insulin sensitivity have also been reported the day following (∼16 h post-exercise) a session of low-volume HIIT (10 × 1 min at 90% HRmax) in adults with obesity who recently underwent a 12-week exercise training program.28 On the other hand, a lower volume HIIT protocol involving three, 1-min bouts of stair climbing lowered capillary glucose immediately after exercise but was insufficient to alter 24 h glycemic control in adults with T2D, highlighting the potential of a minimum threshold required to elicit changes in glycemic control with acute HIIT.29 Taken together, an acute bout of HIIT involving 10 min or more of hard exercise improves glycemic control for up to 24 h post-exercise in people with and without T2D. HIIT also appears equipotent to MICT for lowering post-prandial hyperglycemia in people with T2D despite a lower exercise volume, with superior effects of HIIT observed when exercise volume is matched. The minimum effective dose of HIIT for improving glycemic control – particularly in people with T2D – requires further investigation.
The impact of an acute bout of low-volume SIT on insulin sensitivity appears to be relatively less clear. Ortega and coworkers30 compared the “classic” repeated Wingate SIT protocol (4 × 30 s “all out” cycling sprints) to two different MICT protocols in healthy young males and found a greater improvement in insulin sensitivity following SIT when assessed 30 min post-exercise using an intravenous glucose tolerance test. The superior effects of SIT dissipated over the post-exercise period, however, with similar improvements in insulin sensitivity relative to baseline in both HIIT and MICT observed for up to 48 h.30 In contrast, amongst mixed cohorts of males and females, neither 5 × 30 s31 nor 2 × 20 s32 “all out” cycling sprints elicited improvements in insulin sensitivity when measured the next day (∼14–16 h post-exercise) using oral glucose tolerance tests (OGTT). Collectively, the few existing studies investigating the impact of acute SIT on insulin sensitivity have produced mixed results and the influence on glycemic control remains unclear. Discrepancy within the literature may be explained by differences in participant characteristics, the SIT protocols implemented, the timing of post-exercise measurements, and/or the method for assessing insulin sensitivity.
Acute skeletal muscle responses
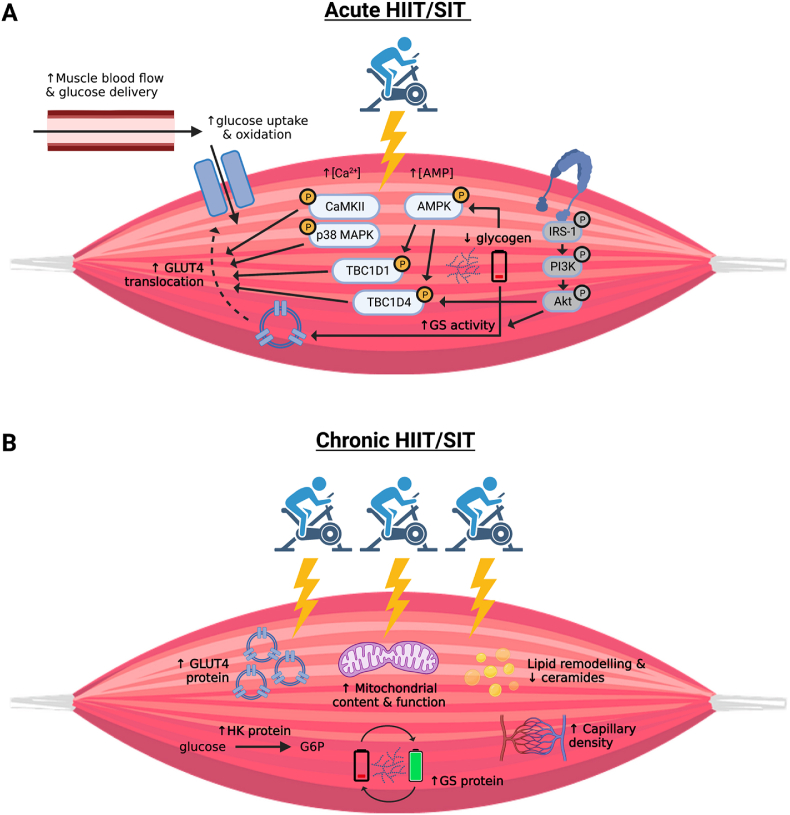
Mechanisms by which exercise enhances skeletal muscle glucose uptake during and immediately post-exercise include enhanced delivery, uptake, and intracellular utilization of glucose within the muscle (summarized in Fig. 1A).33 Given the intensity-dependent nature of muscle hyperemia during exercise34 and superior flow mediated dilation following an acute bout of HIIT compared to MICT,35 delivery of insulin and glucose to muscle would presumably be greater during HIIT and SIT compared to lower intensity protocols. To our knowledge, the impact of acute HIIT or SIT on the translocation of the glucose transporter 4 (GLUT4) to the muscle sarcolemma – as demonstrated with MICT36,37 – has not been directly assessed; however, an upregulation of key intracellular signaling pathways implicated in contraction- and insulin-mediated GLUT4 translocation has been reported. For example, phosphorylation of AMP activated protein kinase (AMPK), p38 mitogen activated protein kinase (MAPK), Ca2⁺/calmodulin-dependent protein kinase (CaMK)II, TBC1 domain family member 1 and 4 (TBC1D1/4), has been demonstrated in response to an acute bout of intense interval exercise26,27,38, 39, 40, 41, 42, 43 and likely contribute to the immediate glucose lowering effects of HIIT and SIT. The phosphorylation of AMPK and downstream targets TBC1D1 and TBC1D4 have been demonstrated to be higher in type II compared to type I muscle fibers immediately following a single session of 6 × 1.5 min cycling bouts at 95% O2peak,39 suggesting that greater muscle activation44 and/or type II fibre recruitment45,46 during intense interval exercise may contribute to the immediate glucose lowering benefits.
Fig. 1.
Proposed mechanisms mediating increases in skeletal muscle glucose uptake and insulin sensitivity following HIIT and SIT. A) During an acute bout of HIIT/SIT, increases in skeletal muscle blood flow promote glucose delivery to active muscle. Increases in glucose delivery coupled with increased intracellular glucose utilization via contraction-mediated mechanisms promotes an increased glucose diffusion gradient across the muscle sarcolemma. Glucose uptake is facilitated by the activation of contraction-mediated signaling proteins that promote GLUT4 translocation to the muscle membrane though direct evidence demonstrating an increase in GLUT4 translocation with HIIT/SIT is currently lacking (as indicated by the dashed arrow). In the hours to days following exercise when contraction-mediated mechanisms have subsided, insulin-mediated glucose uptake is enhanced for up to ∼24–48 h. Increased insulin sensitivity post-exercise has been attributed to increased activity of distal (but not proximal; in grey) proteins in the insulin signaling cascade (TBC1D4) and glycogen synthase activity to facilitate glycogen resynthesis. B) HIIT/SIT training interventions (weeks to months) promote numerous metabolic adaptations in skeletal muscle that are implicated in improved insulin sensitivity following training. This includes increased mitochondrial content and function, capillary density, and the expression of proteins involved in glucose uptake, utilization, and storage within muscle, as well as reductions in lipid intermediates. Glycogen utilization and re-synthesis with acute exercise bouts, including the final training session, may also contribute to improved insulin sensitivity observed in the days following HIIT/SIT training. Note: HIIT: high-intensity interval training; SIT: sprint interval training; Akt: Protein kinase B; AMP: adenosine monophosphate; AMPK: AMP-activated protein kinase; Ca2+: calcium; CaMKII: calcium calmodulin-dependent protein kinase II; G6P: glucose-6-phosphate; GLUT4: glucose transporter 4; GS: glycogen synthase; HK: hexokinase; IRS-1: insulin receptor substrate-1; PI3K: phosphatidylinositol 3 kinase; TBC1D1/4: TBC1 domain family member 1/4.
Contraction-mediated glucose uptake generally subsides within hours following exercise, but insulin-stimulated glucose uptake remains upregulated into the late post-exercise period and coincides with improvements in whole-body insulin sensitivity.27 The enhanced capacity for insulin-stimulated glucose uptake in the post-exercise period following HIIT is not well characterized, particularly with respect to low-volume exercise protocols. While proximal components of the insulin signaling cascade (IRS-1, PI3K, and Akt) are generally unaltered by prior exercise,47 activation of the Akt target TBC1D4 during a hyperinsulinemic-euglycemic clamp has been demonstrated to be greater when performed following a single session of HIIT involving 4 × 4 min cycling bouts at 95% HRmax in males with obesity.27 This finding, which is consistent with others,48 suggests that increased phosphorylation of distal proteins in the insulin signaling cascade may contribute to the insulin-sensitizing and glucose-lowering effects of high-volume interval training protocols. However, to our knowledge, it remains to be determined if changes to insulin signaling contribute to the robust improvements in insulin sensitivity observed for hours to days following time-efficient and low-volume HIIT and SIT. In addition to changes in molecular signaling, increases in muscle glucose delivery via enhanced insulin-stimulated microvascular perfusion are required for post-exercise improvements in insulin sensitivity.49 It is anticipated that increased delivery of glucose and insulin to skeletal muscle contributes to the protracted effects of HIIT on insulin sensitivity, but studies evaluating muscle blood flow and microvascular perfusion in recovery are needed. Possibly related, a single session of HIIT (4 × 4 min intervals at 95% HRmax) has been demonstrated to enhance endothelial function assessed by flow mediated dilation alongside reductions in fasting glucose for 72 h in adults with metabolic syndrome, an effect that was larger and longer than with volume-matched moderate-intensity continuous exercise.35 These improvements in endothelial function with HIIT may contribute to the enhanced delivery of glucose and insulin during the post-exercise period.
The depletion and subsequent restoration of muscle glycogen appear linked, at least in part, to enhanced glucose uptake and insulin sensitivity following exercise.50, 51, 52 Given the increased reliance on muscle glycogen with increasing exercise intensity,53 rapid glycogen depletion and subsequent resynthesis may partly explain the insulin-sensitizing effects of acute HIIT and SIT. Indeed, despite only involving 1–2 min of intense intermittent exercise, 3 × 20 s54 and 4 × 30 s55 ‘all-out’ SIT have been demonstrated to lower muscle glycogen content by ∼20%–25% in healthy adults. Similarly, a single session of low-volume HIIT involving 10 × 1 min cycling bouts at > 80% HRmax lowered muscle glycogen by 30% in adults with and without T2D.56 There is also evidence of increased hexokinase II (HKII) mRNA in the hours following low-volume SIT54 and HIIT,57 which may contribute to sustained skeletal muscle glucose uptake by phosphorylating intramyocellular glucose and increasing the concentration gradient for glucose transport across the muscle sarcolemma. While these mechanisms are plausible, the aforementioned studies did not assess insulin sensitivity in recovery from acute low-volume interval training and thus a relationship between the two is yet to be demonstrated. Given the limited available literature, more research that simultaneously measures insulin sensitivity and/or glycemic control following acute HIIT and SIT alongside measurement of indices regulating skeletal muscle glucose uptake, storage, and intracellular metabolism is needed. This includes studies that directly compare HIIT and/or SIT to MICT to understand how exercise intensity vs. volume influences the skeletal muscle insulin sensitizing effects of acute exercise.
Chronic effects of HIIT and SIT on glycemic control
As little as six sessions of HIIT involving ten, 1-min vigorous cycling bouts per session is sufficient to lower 24-h average glucose concentrations and post-prandial hyperglycemic excursions assessed using CGM in adults with T2D.58 Subsequent work has confirmed the glucose-lowering benefits of the same HIIT protocol performed over 8–12 weeks in people with T2D using CGM, fasting glucose, OGTT, and/or HbA1c.59,60 When compared to MICT protocols involving larger exercise volumes, HIIT promotes similar28,61 and sometimes greater62 improvements in insulin sensitivity in people with obesity. When total exercise volume is matched, HIIT is either equipotent63 or superior to MICT for improving markers of insulin sensitivity and glycemic control in healthy older adults,64 patients with metabolic syndrome,65 and people with T2D.13,66, 67, 68 The glycemic benefits of HIIT in people who are inactive or obese appear to extend beyond controlled laboratory settings, as studies show that virtually supervised HIIT performed at home69 or in a gym-setting70 promotes similar improvements in insulin sensitivity to MICT, albeit with greater adherence to HIIT in the real world.70 Collectively, training interventions involving low-volume HIIT appear to be effective for improving glycemic control in both healthy and clinical cohorts, with the observed benefits of HIIT being superior to MICT when exercise volume is matched.
Babraj and colleagues71 were the first to report improved insulin sensitivity calculated using the Cederholm index from an OGTT following two weeks of classic Wingate-based SIT (4–6, 30 s “all out” cycling bouts) in healthy active young males – findings that were later corroborated using the hyperinsulinemic-euglycemic clamp.72 The efficacy of Wingate-based SIT for improving glycemic control following a two-week period was also reported in sedentary men with obesity,73 but two weeks was seemingly insufficient to improve glycemic control in the same population when a lower volume SIT protocol involving 8-12 × 10 s “all out” sprints was implemented.74 Given the very low volume of exercise involved in the aforementioned study, a longer intervention may be required. Indeed when performed thrice weekly over 6 weeks, 2–3, ≤ 20 s “all out” sprints improved insulin sensitivity during an OGTT in sedentary males75 and 24-h average glucose concentration assessed with CGM in males with overweight/obesity.76 Most direct comparisons between SIT and MICT appear to suggest similar improvements in markers of insulin sensitivity over 6–12-week intervention periods in sedentary young males77, 78, 79 and males with obesity,80 despite up to a 5-fold less training volume and time commitment associated with SIT.79 Unlike HIIT, relatively few studies examining the glycemic benefits of SIT in T2D exist, and the literature available is conflicting. Whereas Ruffino and coworkers81 found no benefit of 8 weeks of low-volume SIT (2 × 20 s “all out” sprints) or MICT on markers of insulin sensitivity assessed with an OGTT in males with T2D, Sjoros et al.82 reported robust improvements in insulin-stimulated glucose uptake during the hyperinsulinemic-euglycemic clamp following two weeks of Wingate-based SIT in adults with prediabetes or T2D. Again, the interplay between exercise volume, intervention duration, and measurement techniques may explain the discrepant findings. Taken together, while most evidence suggests that low-volume SIT promotes similar improvements in indices of glycemic control as higher volumes of MICT, future work is needed to clarify the effects of SIT on glycemic control in people with T2D.
An important caveat to many existing training studies demonstrating improved insulin sensitivity and glycemic control following HIIT and SIT is that post-training assessment is most often performed within 72 h of the last exercise bout, with some as early as 2461,73 or 48 h56,58,59,66 following training cessation. While improvements in insulin sensitivity in these studies are often interpreted to reflect basal improvements associated with chronic training, measurement at these time points may also reflect acute (and transient) effects stemming from the last exercise bout.
Chronic skeletal muscle adaptations
Skeletal muscle mechanisms proposed to mediate training-induced improvements in glycemic control include enhanced capillarization, GLUT4 protein content, glycogen synthesis, and oxidative enzymes coupled with reduced levels of intramuscular lipids (Fig. 1B).83,84 Collectively, these adaptations enhance the delivery, uptake and oxidation of glucose, and improve insulin signaling in skeletal muscle.33 Accordingly, several HIIT/SIT training interventions that enhance insulin sensitivity also demonstrate accompanying improvements in skeletal muscle capillary density,69,77,80 GLUT4,58,76,79 glycogen synthase,56,85 and hexokinase85 protein expression, markers of mitochondrial content and/or function,28,58,62,76,79,86, 87, 88 phosphorylation of insulin signaling proteins,65,67,89 and reduced intramuscular lipids88 and ceramides.85,87 However, causal relationships between skeletal muscle remodelling and training-induced improvements in insulin sensitivity has not been established and the importance of some of these adaptations for glycemic control have been questioned. For example, despite the existence of correlational relationships between muscle mitochondrial phenotype and whole-body insulin sensitivity,90 the contribution of mitochondrial “deficiency” to the development of insulin resistance and T2D is still debated.91,92 It also remains unclear whether training-induced improvements in mitochondrial content or function – amongst the most frequently reported muscle adaptations following HIIT/SIT18 – contribute to the observed improvements in insulin sensitivity. In support of this supposition, a lack of glycemic benefit despite enhanced markers of skeletal muscle mitochondrial remodelling93,94 (or vice versa;67) have been reported following HIIT. The well-known “athletes’ paradox” further exemplifies the lack of causal link between intramuscular lipids and insulin sensitivity,95 pointing to the influence of contributing factors beyond training-induced adaptations in skeletal muscle.
Uncertainty surrounding the relationship between training-induced changes in skeletal muscle phenotype and insulin sensitivity is exemplified in an elegant study by Ryan and colleagues28 that compared metabolic responses to 12 weeks of training involving either 4 sessions per week of low-volume HIIT (10 × 1 min at ∼90% HRmax) or MICT (45 min at 70% HRmax) in adults with obesity. The authors measured the time course of metabolic responses following training by obtaining skeletal muscle biopsies and assessing insulin sensitivity with the hyperinsulinemic-euglycemic clamp at both 1 day and 4 days following training cessation (∼16 and ∼90 h following the last training bout, respectively). When measured the day following either HIIT or MICT, improvements in insulin sensitivity were observed alongside increases in skeletal muscle oxidative capacity and increased abundance of many proteins involved in carbohydrate and lipid metabolism. However, despite persistent elevations in these skeletal muscle metabolic markers after participants abstained from exercise for 4 days, training-induced improvements in insulin sensitivity at this time point had returned to pre-training levels. Thus, while the observed increase in mitochondrial capacity is clearly beneficial for skeletal muscle health and exercise tolerance,96 it does not appear to directly explain exercise training-induced changes to insulin sensitivity. Intriguingly, muscle glycogen content was 40% lower when assessed 1 day vs. 4 days following training, presumably owing to incomplete glycogen resynthesis the day following exercise. These findings indicate that glycogen resynthesis tracks with the reversal of post-exercise improvements in insulin sensitivity, corroborating the notion that oscillations in muscle glycogen content with acute HIIT contribute to improvements in insulin sensitivity and glycemic control.
The findings of Ryan et al.28 highlight the transient nature of exercise training-induced improvements in insulin sensitivity and corroborate early reports of unchanged insulin sensitivity measured 4–10 days following cessation of exercise training.97,98 These observations are also in line with the rapidly diminished insulin sensitivity in athletes to levels of sedentary individuals within 60 h of detraining.99 An important caveat to interpreting the findings of Ryan et al.28 is that body mass was strictly controlled throughout the training period in an effort to isolate the independent effects of exercise per se on insulin sensitivity without the confounding and independent influence of weight (fat) loss. It is possible that the chronic effect of exercise training on insulin sensitivity is different if fat loss is not prevented. Nonetheless, in the absence of measurable weight/fat loss, a continued exercise stimulus may be required to maintain the glycemic benefits of exercise training. In this regard, the glycogen depleting nature of HIIT/SIT protocols may contribute to a longer-lasting improvement in glycemic control compared to MICT,22 allowing for greater rest in between training sessions without a diminishment in insulin sensitivity. Determining the minimum weekly frequency of HIIT/SIT required to maintain a consistent glycemic benefit between exercise sessions is an important area for future research.
Can targeting skeletal muscle mechanisms with nutrition enhance glycemic benefits?
The importance of muscle glycogen depletion/resynthesis for stimulating insulin sensitivity50, 51, 52 and the enhanced glucose-lowering effects of endurance training performed in the fasted state100,101 suggest that coupling HIIT/SIT with carbohydrate and/or energy restriction may maximize their glycemic benefits.102 Mechanistically, exercise performed under fasted or low-carbohydrate conditions can potentiate skeletal muscle glycogenolysis103 and AMPK activation,104,105 which are known stimulants of improved insulin sensitivity in exercise recovery. Accordingly, Terada and colleagues24 reported greater reductions in postprandial glycemic excursions over 24 h when a single session of HIIT was performed in the fasted versus fed state in adults with T2D. More recently, Estafanos and colleagues106 reported that ingestion of a post-exercise carbohydrate beverage following a single session of low-volume HIIT blunted next-day glycemic control relative to when a non-caloric post-exercise drink was consumed, possibly owing to faster repletion of muscle glycogen stores with post-exercise carbohydrate intake. These two studies demonstrate that targeting muscle mechanisms that are linked to HIIT-induced improvements in insulin sensitivity with nutritional manipulation around exercise can augment acute improvements in glycemic control. However, when HIIT performed in the fasted vs. fed state was evaluated over a 6-week training program in females with overweight and obesity, no differences in training-induced changes in OGTT-derived insulin sensitivity or skeletal muscle remodelling were observed.92 Similarly, no difference in fasting insulin sensitivity (HOMA-IR) was reported following 6 weeks of low-volume SIT performed in the fed or fasted state among recreationally active males and females.107
Combining intense interval exercise with protein ingestion has also been explored as a strategy to enhance the cardiometabolic benefits of HIIT and SIT. Based on the temporal association between enhanced muscle protein synthesis and improvements in insulin sensitivity following HIIT in older adults86 Francois and colleagues59 compared the effect of 12 weeks of HIIT combined with post-exercise protein ingestion on glycemic control in people with T2D. However, improvements in glycemic control (24 h mean CGM glucose and HbA1c) following HIIT were not potentiated with the addition of post-exercise milk or protein ingestion. The combination of pre-exercise protein ingestion with SIT has also been recently explored to mitigate the potential catabolic effects of exercise performed in the fasted on skeletal muscle,108 but how glycemic control is impacted with this approach remains unknown. Considering the limited body of evidence investigating the combined effects of acute and chronic nutritional interventions with HIIT/SIT, future work is needed to clarify if low carbohydrate/calorie and/or high protein diets can optimize glycemic benefits of intense interval exercise.
Does sex influence skeletal muscle mechanisms contributing to enhanced glycemic control?
A limited number of studies have demonstrated sex-based differences in the insulin-sensitizing effects of low-volume HIIT and SIT. In response to 6 weeks of SIT involving thrice weekly 2-3 × 20 s ‘all-out’ sprints, OGTT-derived insulin sensitivity72 and 24 h glycemic control73 were improved in males but not females. More recently, Sogaard et al.85 reported similar improvements in insulin sensitivity (hyperinsulinemic-euglycemic clamp) among older males and females following 18 sessions of 5 × 1 min cycling intervals at ∼125% O2peak over 6 weeks; however, there was an 11% improvement in males and a 1% improvement in females, a difference which did not achieve statistical significance. These sex differences are corroborated by studies in full cohorts of females with overweight and obesity that have failed to demonstrate an improvement in insulin sensitivity following 6–14 weeks of low-volume HIIT assessed with OGTTs92 or the hyperinsulinemic-euglycemic clamp.109 While there is an apparent lack of sex comparisons in response to acute HIIT or SIT, a number of studies in mixed cohorts of males and females have failed to demonstrate an improvement in OGTT-derived insulin sensitivity following a single session of SIT.31,32,72
Differences between males and females with respect to the insulin-sensitizing effects of HIIT and SIT may relate to sex differences in skeletal muscle metabolism. Females use less muscle glycogen during high-intensity exercise,110, 111, 112 and AMPK activity is reportedly blunted following work-matched exercise when compared to males.113 Relatedly, muscle glycogen use during HIIT can vary across the menstrual cycle,114 with greater glycogen use observed during the early-as opposed to late-follicular phase. The menstrual cycle phase has not been controlled for in many studies examining glycemic responses to HIIT/SIT, which may confound the interpretation of findings. In response to training, many markers of skeletal muscle remodelling linked to insulin sensitivity are reportedly similar between males and females, including glycogen synthase and hexokinase protein expression85 and mitochondrial content93; although, one study has observed greater increases in GLUT4 protein content with training in males compared to females.93 However, as has been noted previously,20 studies simultaneously measuring sex differences in insulin sensitivity and associated muscle mechanisms with interval training are scarce. Taken together, while the available literature demonstrates greater improvements in insulin sensitivity following intense interval exercise in males compared to females (particularly with low-volume SIT), additional research is needed to elucidate potential mechanisms. Importantly, future studies should involve larger sample sizes and control for factors that may confound sex-based comparisons including proper matching between males and females, menstrual cycle phase, and oral contraceptive use. Given the greater proportion of male-only cohorts in muscle physiology research115 including amongst studies within the HIIT/SIT literature,20,116 more research involving exclusively females is also warranted to enhance our basic understanding of how exercise influences muscle insulin sensitivity in females.
Exercise snacks – a more practical variation of HIIT/SIT for improving glycemic control?
Emerging research points to the efficacy of more practical variations of HIIT/SIT for improving glycemic control. Early work on “exercise snacks” by Francois and colleagues117 demonstrated the ability of high-intensity interval walking (6 × 1-min intervals with 1-min recovery periods) performed before each meal to improve 3-h post-prandial and 24-h glucose in people with insulin resistance. More recent studies have defined exercise snacks as brief (≤ 1 min) isolated bouts of hard exercise performed periodically throughout the day.118, 119, 120, 121 Although the direct effects of this approach on glycemic control remain to be determined, interrupting prolonged sitting with hourly bouts of vigorous stair climbing (∼15–30 s each) reduced post-prandial insulin AUC in adults with overweight/obesity.121 Thus, exercise snacks may hold promise as an additional exercise strategy for improving glycemic control with the added benefit of interrupting prolonged sedentary time.
Concluding remarks and future directions
The available literature supports the efficacy of HIIT and/or SIT for improving glycemic control across populations ranging from healthy inactive adults to individuals diagnosed with cardiometabolic disease. Although many of the hallmark skeletal muscle adaptations that are observed following HIIT/SIT interventions may contribute to enhanced muscle insulin sensitivity, a causal link between muscle remodelling and altered insulin sensitivity has not been established. Several lines of evidence point to the acute effects of HIIT/SIT – primarily the depletion and subsequent resynthesis of muscle glycogen – being of importance for mediating interval training-induced improvements in insulin sensitivity. Future work is needed to clarify the relative importance of acute responses versus chronic adaptations for improvements in insulin sensitivity following HIIT/SIT, but establishing causal relationships without the ability to induce gain- or loss-of-function remains a challenge for mechanistic human research. The optimal pre- and post-exercise nutritional strategies for maximizing the glycemic benefits of HIIT/SIT also represent a fruitful area for future work, but it does appear that undertaking HIIT in the fasted state or limiting post-exercise carbohydrate intake may potentiate the acute glycemic benefits of HIIT. Based on observations of potentially blunted glycemic benefits of HIIT/SIT in females and the overall greater emphasis on males in existing studies, well-controlled and appropriately powered studies examining the influence of biological sex on glycemic control – and the associated mechanisms that underpin a potential sexual dimorphism – are needed. Finally, in light of studies demonstrating improved markers of insulin sensitivity following brief repeated117 or isolated bouts of vigorous exercise spread over the course of the day (i.e., exercise “snacks”),118,121 research into more practical variations of HIIT for improving glycemic control – and their associated impact on skeletal muscle – is warranted.
Authors’ contributions
Both authors contributed to the conceptualization and design, drafting, critical review, and revisions of the manuscript. Both authors approved the final version of the manuscript.
Submission statement
Both authors have read and agree with manuscript content. The manuscript has not been published nor is consideration for publication elsewhere.
Funding
JBG is supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2020-05779).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Thiebaud D., Jacot E., DeFronzo R.A., Maeder E., Jequier E., Felber J.P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colberg S.R., Sigal R.J., Yardley J.E., et al. Physical activity/exercise and diabetes: a position statement of the American diabetes association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanaley J.A., Colberg S.R., Corcoran M.H., et al. Exercise/Physical activity in individuals with type 2 diabetes: a consensus statement from the American college of sports medicine. Med Sci Sports Exerc. 2022;54(2):353–368. doi: 10.1249/MSS.0000000000002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gist N.H., Fedewa M.V., Dishman R.K., Cureton K.J. Sprint interval training effects on aerobic capacity: a systematic review and meta-analysis. Sports Med. 2014;44(2):269–279. doi: 10.1007/s40279-013-0115-0. [DOI] [PubMed] [Google Scholar]
- 6.Weston K.S., Wisløff U., Coombes J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 7.Sloth M., Sloth D., Overgaard K., Dalgas U. Effects of sprint interval training on VO 2max and aerobic exercise performance: a systematic review and meta-analysis: effects of sprint interval training on VO 2max. Scand J Med Sci Sports. 2013;23(6):e341–e352. doi: 10.1111/sms.12092. [DOI] [PubMed] [Google Scholar]
- 8.Keating S.E., Johnson N.A., Mielke G.I., Coombes J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev Off J Int Assoc Study Obes. 2017;18(8):943–964. doi: 10.1111/obr.12536. [DOI] [PubMed] [Google Scholar]
- 9.Batacan R.B., Duncan M.J., Dalbo V.J., Tucker P.S., Fenning A.S. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017;51(6):494–503. doi: 10.1136/bjsports-2015-095841. [DOI] [PubMed] [Google Scholar]
- 10.Ross R., Hudson R., Stotz P.J., Lam M. Effects of exercise amount and intensity on abdominal obesity and glucose tolerance in obese adults: a randomized trial. Ann Intern Med. 2015;162(5):325–334. doi: 10.7326/M14-1189. [DOI] [PubMed] [Google Scholar]
- 11.Boulé N.G., Kenny G.P., Haddad E., Wells G.A., Sigal R.J. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- 12.Liubaoerjijin Y., Terada T., Fletcher K., Boulé N.G. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53(5):769–781. doi: 10.1007/s00592-016-0870-0. [DOI] [PubMed] [Google Scholar]
- 13.Jelleyman C., Yates T., O'Donovan G., et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis: the effects of HIIT on metabolic health. Obes Rev. 2015;16(11):942–961. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 14.Campbell W.W., Kraus W.E., Powell K.E., et al. High-intensity interval training for cardiometabolic disease prevention. Med Sci Sports Exerc. 2019;51(6):1220–1226. doi: 10.1249/MSS.0000000000001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird S.R., Hawley J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2(1):e000143. doi: 10.1136/bmjsem-2016-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattioni Maturana F., Martus P., Zipfel S., Nieß A.M. Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med Sci Sports Exerc. 2021;53(3):559–573. doi: 10.1249/MSS.0000000000002506. [DOI] [PubMed] [Google Scholar]
- 17.Jiménez-Maldonado A., García-Suárez P.C., Rentería I., Moncada-Jiménez J., Plaisance E.P. Impact of high-intensity interval training and sprint interval training on peripheral markers of glycemic control in metabolic syndrome and type 2 diabetes. Biochim Biophys Acta, Mol Basis Dis. 2020;1866(8):165820. doi: 10.1016/j.bbadis.2020.165820. [DOI] [PubMed] [Google Scholar]
- 18.MacInnis M.J., Gibala M.J. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibala M.J., Gillen J.B., Percival M.E. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med Auckl NZ. 2014;44(Suppl 2):S127–S137. doi: 10.1007/s40279-014-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skelly L.E., Bailleul C., Gillen J.B. Physiological responses to low-volume interval training in women. Sports Med - Open. 2021;7(1):99. doi: 10.1186/s40798-021-00390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillen J.B., Little J.P., Punthakee Z., Tarnopolsky M.A., Riddell M.C., Gibala M.J. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metabol. 2012;14(6):575–577. doi: 10.1111/j.1463-1326.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- 22.Little J.P., Jung M.E., Wright A.E., Wright W., Manders R.J.F. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2014;39(7):835–841. doi: 10.1139/apnm-2013-0512. [DOI] [PubMed] [Google Scholar]
- 23.Karstoft K., Christensen C.S., Pedersen B.K., Solomon T.P.J. The acute effects of interval- vs continuous-walking exercise on glycemic control in subjects with type 2 diabetes: a crossover, controlled study. J Clin Endocrinol Metab. 2014;99(9):3334–3342. doi: 10.1210/jc.2014-1837. [DOI] [PubMed] [Google Scholar]
- 24.Terada T., Wilson B.J., Myette-Côté E., et al. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metab, Clin Exp. 2016;65(5):599–608. doi: 10.1016/j.metabol.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani M.A., Jenkinson C.P., Richardson D.K., Tripathy D., DeFronzo R.A. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55(5):1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 26.Levinger I., Jerums G., Stepto N.K., et al. The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men: EXERCISE, ucOC, and insulin sensitivity in obese men. J Bone Miner Res. 2014;29(12):2571–2576. doi: 10.1002/jbmr.2285. [DOI] [PubMed] [Google Scholar]
- 27.Parker L., Stepto N.K., Shaw C.S., et al. Acute high-intensity interval exercise-induced redox signaling is associated with enhanced insulin sensitivity in obese middle-aged men. Front Physiol. 2016;7:411. doi: 10.3389/fphys.2016.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan B.J., Schleh M.W., Ahn C., et al. Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 2020;105(8):e2941–e2959. doi: 10.1210/clinem/dgaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godkin F.E., Jenkins E.M., Little J.P., Nazarali Z., Percival M.E., Gibala M.J. The effect of brief intermittent stair climbing on glycemic control in people with type 2 diabetes: a pilot study. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2018;43(9):969–972. doi: 10.1139/apnm-2018-0135. [DOI] [PubMed] [Google Scholar]
- 30.Ortega J., Fernández-Elías V., Hamouti N., García-Pallarés J., Mora-Rodriguez R. Higher insulin-sensitizing response after sprint interval compared to continuous exercise. Int J Sports Med. 2014;36(3):209–214. doi: 10.1055/s-0034-1389942. [DOI] [PubMed] [Google Scholar]
- 31.Brestoff J.R., Clippinger B., Spinella T., von Duvillard S.P., Nindl B., Arciero P.J. An acute bout of endurance exercise but not sprint interval exercise enhances insulin sensitivity. Appl Physiol Nutr Metabol. 2009;34(1):25–32. doi: 10.1139/H08-126. [DOI] [PubMed] [Google Scholar]
- 32.Metcalfe R., Fawkner S., Vollaard N. No acute effect of reduced-exertion high-intensity interval training (REHIT) on insulin sensitivity. Int J Sports Med. 2016;37(5):354–358. doi: 10.1055/s-0035-1564258. [DOI] [PubMed] [Google Scholar]
- 33.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake — regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13(3):133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 34.Joyner M.J., Casey D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95(2):549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tjønna A.E., Rognmo Ø., Bye A., Stølen T.O., Wisløff U. Time course of endothelial adaptation after acute and chronic exercise in patients with metabolic syndrome. J Strength Condit Res. 2011;25(9):2552–2558. doi: 10.1519/JSC.0b013e3181fb4809. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy J.W., Hirshman M.F., Gervino E.V., et al. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48(5):1192–1197. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- 37.Kristiansen S., Hargreaves M., Richter E.A. Progressive increase in glucose transport and GLUT-4 in human sarcolemmal vesicles during moderate exercise. Am J Physiol. 1997;272(3 Pt 1):E385–E389. doi: 10.1152/ajpendo.1997.272.3.E385. [DOI] [PubMed] [Google Scholar]
- 38.Parker L., Trewin A., Levinger I., Shaw C.S., Stepto N.K. The effect of exercise-intensity on skeletal muscle stress kinase and insulin protein signaling. PLoS One. 2017;12(2):e0171613. doi: 10.1371/journal.pone.0171613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristensen D.E., Albers P.H., Prats C., Baba O., Birk J.B., Wojtaszewski J.F.P. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593(8):2053–2069. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias I.S., Lazauskas K.K., Siu J., Costa P.B., Coburn J.W., Galpin A.J. Sex and fiber type independently influence AMPK, TBC1D1, and TBC1D4 at rest and during recovery from high-intensity exercise in humans. J Appl Physiol. 2020;128(2):350–361. doi: 10.1152/japplphysiol.00704.2019. [DOI] [PubMed] [Google Scholar]
- 41.Gibala M.J., McGee S.L., Garnham A.P., Howlett K.F., Snow R.J., Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106(3):929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 42.Little J.P., Safdar A., Bishop D., Tarnopolsky M.A., Gibala M.J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 43.Combes A., Dekerle J., Webborn N., Watt P., Bougault V., Daussin F.N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Phys Rep. 2015;3(9):e12462. doi: 10.14814/phy2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgett B.A., Foster W.S., Hankinson P.B., et al. Dissociation of increases in PGC-1α and its regulators from exercise intensity and muscle activation following acute exercise. PLoS One. 2013;8(8):e71623. doi: 10.1371/journal.pone.0071623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gollnick P.D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vøllestad N.K., Blom P.C. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125(3):395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- 47.Frøsig C., Richter E.A. Improved insulin sensitivity after exercise: focus on insulin signaling. Obes Silver Spring Md. 2009;17(Suppl 3):S15–S20. doi: 10.1038/oby.2009.383. [DOI] [PubMed] [Google Scholar]
- 48.Treebak J.T., Frøsig C., Pehmøller C., et al. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009;52(5):891–900. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjøberg K.A., Frøsig C., Kjøbsted R., et al. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 2017;66(6):1501–1510. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 50.Devlin J.T., Hirshman M., Horton E.D., Horton E.S. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36(4):434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 51.Bogardus C., Thuillez P., Ravussin E., Vasquez B., Narimiga M., Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest. 1983;72(5):1605–1610. doi: 10.1172/JCI111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen J., Rustad P., Kolnes A., Lai Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romijn J.A., Coyle E.F., Sidossis L.S., et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265(3 Pt 1):E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 54.Skelly L.E., Gillen J.B., MacInnis M.J., et al. Effect of sex on the acute skeletal muscle response to sprint interval exercise. Exp Physiol. 2017;102(3):354–365. doi: 10.1113/EP086118. [DOI] [PubMed] [Google Scholar]
- 55.Cochran A.J.R., Percival M.E., Tricarico S., et al. Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations: muscle adaptations to high-intensity exercise training. Exp Physiol. 2014;99(5):782–791. doi: 10.1113/expphysiol.2013.077453. [DOI] [PubMed] [Google Scholar]
- 56.Dela F., Ingersen A., Andersen N.B., et al. Effects of one-legged high-intensity interval training on insulin-mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol. 2019;226(2):e13245. doi: 10.1111/apha.13245. [DOI] [PubMed] [Google Scholar]
- 57.Edge J., Mündel T., Pilegaard H., et al. Ammonium chloride ingestion attenuates exercise-induced mRNA levels in human muscle. PLoS One. 2015;10(12):e0141317. doi: 10.1371/journal.pone.0141317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Little J.P., Gillen J.B., Percival M.E., et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 59.Francois M.E., Durrer C., Pistawka K.J., Halperin F.A., Chang C., Little J.P. Combined interval training and post-exercise nutrition in type 2 diabetes: a randomized control trial. Front Physiol. 2017;8:528. doi: 10.3389/fphys.2017.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madsen S.M., Thorup A.C., Overgaard K., Jeppesen P.B. High intensity interval training improves glycaemic control and pancreatic β cell function of type 2 diabetes patients. PLoS One. 2015;10(8):e0133286. doi: 10.1371/journal.pone.0133286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisher G., Brown A.W., Bohan Brown M.M., et al. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One. 2015;10(10):e0138853. doi: 10.1371/journal.pone.0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Strijcker D., Lapauw B., Ouwens D.M., et al. High intensity interval training is associated with greater impact on physical fitness, insulin sensitivity and muscle mitochondrial content in males with overweight/obesity, as opposed to continuous endurance training: a randomized controlled trial. J Musculoskelet Neuronal Interact. 2018;18(2):215–226. [PMC free article] [PubMed] [Google Scholar]
- 63.Earnest C., Lupo M., Thibodaux J., et al. Interval training in men at risk for insulin resistance. Int J Sports Med. 2012;34(4):355–363. doi: 10.1055/s-0032-1311594. [DOI] [PubMed] [Google Scholar]
- 64.Hwang C.L., Yoo J.K., Kim H.K., et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–119. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tjønna A.E., Lee S.J., Rognmo Ø., et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karstoft K., Winding K., Knudsen S.H., et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36(2):228–236. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karstoft K., Winding K., Knudsen S.H., et al. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia. 2014;57(10):2081–2093. doi: 10.1007/s00125-014-3334-5. [DOI] [PubMed] [Google Scholar]
- 68.Karstoft K., Clark M.A., Jakobsen I., et al. The effects of 2 weeks of interval vs continuous walking training on glycaemic control and whole-body oxidative stress in individuals with type 2 diabetes: a controlled, randomised, crossover trial. Diabetologia. 2017;60(3):508–517. doi: 10.1007/s00125-016-4170-6. [DOI] [PubMed] [Google Scholar]
- 69.Scott S.N., Shepherd S.O., Hopkins N., et al. Home-hit improves muscle capillarisation and eNOS/NAD(P)Hoxidase protein ratio in obese individuals with elevated cardiovascular disease risk. J Physiol. 2019;597(16):4203–4225. doi: 10.1113/JP278062. [DOI] [PubMed] [Google Scholar]
- 70.Shepherd S.O., Wilson O.J., Taylor A.S., et al. Low-volume high-intensity interval training in a gym setting improves cardio-metabolic and psychological health. PLoS One. 2015;10(9):e0139056. doi: 10.1371/journal.pone.0139056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babraj J.A., Vollaard N.B.J., Keast C., Guppy F.M., Cottrell G., Timmons J.A. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richards J.C., Johnson T.K., Kuzma J.N., et al. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to β-adrenergic stimulation: short-term sprint interval training and insulin sensitivity. J Physiol. 2010;588(15):2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whyte L.J., Gill J.M.R., Cathcart A.J. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Skleryk J.R., Karagounis L.G., Hawley J.A., Sharman M.J., Laursen P.B., Watson G. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes Metabol. 2013;15(12):1146–1153. doi: 10.1111/dom.12150. [DOI] [PubMed] [Google Scholar]
- 75.Metcalfe R.S., Babraj J.A., Fawkner S.G., Vollaard N.B.J. Towards the minimal amount of exercise for improving metabolic health: beneficial effects of reduced-exertion high-intensity interval training. Eur J Appl Physiol. 2012;112(7):2767–2775. doi: 10.1007/s00421-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 76.Gillen J.B., Percival M.E., Skelly L.E., et al. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS One. 2014;9(11):e111489. doi: 10.1371/journal.pone.0111489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cocks M., Shaw C.S., Shepherd S.O., et al. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males: microvascular adaptations to training in sedentary males. J Physiol. 2013;591(3):641–656. doi: 10.1113/jphysiol.2012.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shepherd S.O., Cocks M., Tipton K.D., et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5: perilipin expression and IMTG metabolism. J Physiol. 2013;591(3):657–675. doi: 10.1113/jphysiol.2012.240952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gillen J.B., Martin B.J., MacInnis M.J., Skelly L.E., Tarnopolsky M.A., Gibala M.J. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS One. 2016;11(4):e0154075. doi: 10.1371/journal.pone.0154075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cocks M., Shaw C.S., Shepherd S.O., et al. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase protein ratio in obese men. J Physiol. 2016;594(8):2307–2321. doi: 10.1113/jphysiol.2014.285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruffino J.S., Songsorn P., Haggett M., et al. A comparison of the health benefits of reduced-exertion high-intensity interval training (REHIT) and moderate-intensity walking in type 2 diabetes patients. Appl Physiol Nutr Metabol. 2017;42(2):202–208. doi: 10.1139/apnm-2016-0497. [DOI] [PubMed] [Google Scholar]
- 82.Sjöros T.J., Heiskanen M.A., Motiani K.K., et al. Increased insulin-stimulated glucose uptake in both leg and arm muscles after sprint interval and moderate-intensity training in subjects with type 2 diabetes or prediabetes. Scand J Med Sci Sports. 2018;28(1):77–87. doi: 10.1111/sms.12875. [DOI] [PubMed] [Google Scholar]
- 83.Wojtaszewski J.F.P., Richter E.A. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 2006;42:31–46. doi: 10.1042/bse0420031. [DOI] [PubMed] [Google Scholar]
- 84.Richter E.A., Sylow L., Hargreaves M. Interactions between insulin and exercise. Biochem J. 2021;478(21):3827–3846. doi: 10.1042/BCJ20210185. [DOI] [PubMed] [Google Scholar]
- 85.Søgaard D., Lund M.T., Scheuer C.M., et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 2018;222(4):e13009. doi: 10.1111/apha.13009. [DOI] [PubMed] [Google Scholar]
- 86.Robinson M.M., Dasari S., Konopka A.R., et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metabol. 2017;25(3):581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shepherd S.O., Cocks M., Meikle P.J., et al. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int J Obes. 2017;41(12):1745–1754. doi: 10.1038/ijo.2017.170. [DOI] [PubMed] [Google Scholar]
- 88.Koh H.C.E., Ørtenblad N., Winding K.M., Hellsten Y., Mortensen S.P., Nielsen J. High-intensity interval, but not endurance, training induces muscle fiber type-specific subsarcolemmal lipid droplet size reduction in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2018;315(5):E872–E884. doi: 10.1152/ajpendo.00161.2018. [DOI] [PubMed] [Google Scholar]
- 89.de Matos M.A., Vieira D.V., Pinhal K.C., et al. High-intensity interval training improves markers of oxidative metabolism in skeletal muscle of individuals with obesity and insulin resistance. Front Physiol. 2018;9:1451. doi: 10.3389/fphys.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goodpaster B.H. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62(4):1032–1035. doi: 10.2337/db12-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holloszy J.O. Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes. 2013;62(4):1036–1040. doi: 10.2337/db12-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Genders A.J., Holloway G.P., Bishop D.J. Are alterations in skeletal muscle mitochondria a cause or consequence of insulin resistance? Int J Mol Sci. 2020;21(18):6948. doi: 10.3390/ijms21186948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillen J.B., Percival M.E., Ludzki A., Tarnopolsky M.A., MartinJ Gibala. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women: interval Training Improves Body Composition. Obesity. 2013;21(11):2249–2255. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 94.Apostolopoulou M., Mastrototaro L., Hartwig S., et al. Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin-resistant males. Sci Adv. 2021;7(41):eabi9551. doi: 10.1126/sciadv.abi9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goodpaster B.H., He J., Watkins S., Kelley D.E. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 96.Hood D.A., Memme J.M., Oliveira A.N., Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol. 2019;81:19–41. doi: 10.1146/annurev-physiol-020518-114310. [DOI] [PubMed] [Google Scholar]
- 97.Segal K.R., Edano A., Abalos A., et al. Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol. 1991;71(6):2402–2411. doi: 10.1152/jappl.1991.71.6.2402. [DOI] [PubMed] [Google Scholar]
- 98.King D.S., Dalsky G.P., Clutter W.E., et al. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol. 1988;64(5):1942–1946. doi: 10.1152/jappl.1988.64.5.1942. [DOI] [PubMed] [Google Scholar]
- 99.Burstein R., Polychronakos C., Toews C.J., MacDougall J.D., Guyda H.J., Posner B.I. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes. 1985;34(8):756–760. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 100.Van Proeyen K., Szlufcik K., Nielens H., et al. Training in the fasted state improves glucose tolerance during fat-rich diet. J Physiol. 2010;588(21):4289–4302. doi: 10.1113/jphysiol.2010.196493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edinburgh R.M., Bradley H.E., Abdullah N.F., et al. Lipid metabolism links nutrient-exercise timing to insulin sensitivity in men classified as overweight or obese. J Clin Endocrinol Metab. 2020;105(3):dgz104. doi: 10.1210/clinem/dgz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Francois M.E., Gillen J.B., Little J.P. Carbohydrate-restriction with high-intensity interval training: an optimal combination for treating metabolic diseases? Front Nutr. 2017;4:49. doi: 10.3389/fnut.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Bock K., Derave W., Ramaekers M., Richter E.A., Hespel P. Fiber type-specific muscle glycogen sparing due to carbohydrate intake before and during exercise. J Appl Physiol. 2007;102(1):183–188. doi: 10.1152/japplphysiol.00799.2006. [DOI] [PubMed] [Google Scholar]
- 104.Bartlett J.D., Louhelainen J., Iqbal Z., et al. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;304(6):R450–R458. doi: 10.1152/ajpregu.00498.2012. [DOI] [PubMed] [Google Scholar]
- 105.Yeo W.K., McGee S.L., Carey A.L., et al. Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp Physiol. 2010;95(2):351–358. doi: 10.1113/expphysiol.2009.049353. [DOI] [PubMed] [Google Scholar]
- 106.Estafanos S., Friesen B., Govette A., Gillen J.B. Carbohydrate-energy replacement following high-intensity interval exercise blunts next-day glycemic control in untrained women. Front Nutr. 2022;9:868511. doi: 10.3389/fnut.2022.868511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan V., Lim I., Tan P.T., Tan F., Aziz A.R. Comparison of physiological and clinical markers for chronic sprint-interval training exercise performed either in the fasted or fed states among healthy adults. Curr Res Physiol. 2021;4:192–201. doi: 10.1016/j.crphys.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aird T.P., Farquharson A.J., Bermingham K.M., O'Sulllivan A., Drew J.E., Carson B.P. Divergent serum metabolomic, skeletal muscle signaling, transcriptomic, and performance adaptations to fasted versus whey protein-fed sprint interval training. Am J Physiol Endocrinol Metab. 2021;321(6):E802–E820. doi: 10.1152/ajpendo.00265.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arad A.D., DiMenna F.J., Thomas N., et al. High-intensity interval training without weight loss improves exercise but not basal or insulin-induced metabolism in overweight/obese African American women. J Appl Physiol. 2015;119(4):352–362. doi: 10.1152/japplphysiol.00306.2015. [DOI] [PubMed] [Google Scholar]
- 110.Esbjörnsson-Liljedahl M., Sundberg C.J., Norman B., Jansson E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J Appl Physiol. 1999;87(4):1326–1332. doi: 10.1152/jappl.1999.87.4.1326108. [DOI] [PubMed] [Google Scholar]
- 111.Esbjörnsson-Liljedahl M., Bodin K., Jansson E. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J Appl Physiol. 2002;93(3):1075–1083. doi: 10.1152/japplphysiol.00732.1999109. [DOI] [PubMed] [Google Scholar]
- 112.Impey S.G., Jevons E., Mees G., et al. Glycogen utilization during running: intensity, sex, and muscle-specific responses. Med Sci Sports Exerc. 2020;52(9):1966–1975. doi: 10.1249/MSS.0000000000002332. [DOI] [PubMed] [Google Scholar]
- 113.Roepstorff C., Thiele M., Hillig T., et al. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006;574(Pt 1):125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matsuda T., Takahashi H., Nakamura M., et al. Influence of menstrual cycle on muscle glycogen utilization during high-intensity intermittent exercise until exhaustion in healthy women. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2022;47(6):671–680. doi: 10.1139/apnm-2021-0532. [DOI] [PubMed] [Google Scholar]
- 115.O'Halloran K.D. Mind the gap: widening the demographic to establish new norms in human physiology. J Physiol. 2020;598(15):3045–3047. doi: 10.1113/JP279986. [DOI] [PubMed] [Google Scholar]
- 116.Bishop D.J., Botella J., Genders A.J., et al. High-intensity exercise and mitochondrial biogenesis: current controversies and future research directions. Physiol Bethesda. 2019;34(1):56–70. doi: 10.1152/physiol.00038.2018. [DOI] [PubMed] [Google Scholar]
- 117.Francois M.E., Baldi J.C., Manning P.J., et al. Exercise snacks” before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57(7):1437–1445. doi: 10.1007/s00125-014-3244-6. [DOI] [PubMed] [Google Scholar]
- 118.Islam H., Gibala M.J., Little J.P. Exercise snacks: a novel strategy to improve cardiometabolic health. Exerc Sport Sci Rev. 2022;50(1):31–37. doi: 10.1249/JES.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 119.Jenkins E.M., Nairn L.N., Skelly L.E., Little J.P., Gibala M.J. Do stair climbing exercise “snacks” improve cardiorespiratory fitness? Appl Physiol Nutr Metabol. 2019;44(6):681–684. doi: 10.1139/apnm-2018-0675. [DOI] [PubMed] [Google Scholar]
- 120.Little J.P., Langley J., Lee M., et al. Sprint exercise snacks: a novel approach to increase aerobic fitness. Eur J Appl Physiol. 2019;119(5):1203–1212. doi: 10.1007/s00421-019-04110-z. [DOI] [PubMed] [Google Scholar]
- 121.Rafiei H., Omidian K., É Myette-Côté, Little J.P. Metabolic impact of breaking up prolonged sitting with stair climbing exercise snacks. Med Sci Sports Exerc. 2021;53(1):150–158. doi: 10.1249/MSS.0000000000002431. [DOI] [PubMed] [Google Scholar]