Abstract
Skeletal muscle anabolism is driven by numerous stimuli such as growth factors, nutrients (i.e., amino acids, glucose), and mechanical stress. These stimuli are integrated by the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) signal transduction cascade. In recent years, work from our laboratory and elsewhere has sought to unravel the molecular mechanisms underpinning the mTOR-related activation of muscle protein synthesis (MPS), as well as the spatial regulation of these mechanisms within the skeletal muscle cell. These studies have suggested that the skeletal muscle fiber periphery is a region of central importance in anabolism (i.e., growth/MPS). Indeed, the fiber periphery is replete with the substrates, molecular machinery, and translational apparatus necessary to facilitate MPS. This review provides a summary of the mechanisms underpinning the mTOR-associated activation of MPS from cell, rodent, and human studies. It also presents an overview of the spatial regulation of mTORC1 in response to anabolic stimuli and outlines the factors that distinguish the periphery of the cell as a highly notable region of skeletal muscle for the induction of MPS. Future research should seek to further explore the nutrient-induced activation of mTORC1 at the periphery of skeletal muscle fibers.
Keywords: Skeletal muscle, mTOR, Periphery, Muscle protein synthesis, Hypertrophy, Translation
Abbreviations:
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- AA
amino acids
- DEPTOR
DEP containing mTOR interacting protein
- EAA
essential amino acids
- eIF3F
eukaryotic initiation factor 3F
- eNOS
endothelial nitric oxide synthase
- FAK
Focal Adhesion Kinase
- FLCN
folliculin tumor suppressor
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- IGF-1
Insulin-like growth factor-1
- LAMP2
Lysosome associate membrane protein 2
- LAT1
Large neutral amino acid transporter 1
- LEAA
leucine enriched essential amino acids
- MPS
Muscle protein synthesis
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR Complex 1
- mTORC2
mTOR Complex 2
- PI3K
phosphatidylinositol 3-kinase
- PtdIns3P
phosphatidylinositol 3-phosphate
- RAPTOR
regulator associated protein of mTOR
- Rheb
Ras homologue enriched in brain
- RICTOR
rapamycin insensitive companion of mTOR
- S6K
ribosomal protein S6 Kinase
- SAM
S-adenosylmethionine
- SAMTOR
S-adenosylmethionine sensor upstream of mTORC1
- SNAT2
Sodium-coupled neutral amino acid transporter 2
- TSC
Tuberous sclerosis
- v-ATPase
vacuolar-type H+ ATPase
- VPS34
vacuolar protein sorting 34
- WGA
wheat germ agglutinin
Introduction
In 1968 Goldberg reported that mechanical loading of rat plantaris and soleus via tenotomy of the gastrocnemius resulted in increased incorporation of [14C]-Leucine into muscle proteins (i.e. protein synthesis), and the increase in muscle protein synthesis (MPS) was correlated with muscle hypertrophy over time.1 Extensive research has since been conducted to characterize the molecular mechanisms through which mechanical loading and unloading influence skeletal muscle protein turnover. In the years since Goldberg's findings, much has been learned about skeletal muscle anabolism, and the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), has emerged as a candidate central regulator of skeletal muscle anabolism.
mTOR is a serine/threonine protein kinase found in two protein complexes, mTORC1 and mTOR complex 2 (mTORC2), across a variety of cell types.2 mTORC1 is composed of regulator associated protein of mTOR (RAPTOR),3 mammalian lethal with sec13 protein 8 (mLST8, also known as GβL), and two negative regulators, proline rich substrate of Akt of 40 kDa (PRAS40), and DEP containing mTOR interacting protein (DEPTOR).4, 5, 6, 7 Similar to mTORC1, mTORC2 contains mLST8, and DEPTOR. However, mTORC2 also contains rapamycin insensitive companion of mTOR (RICTOR), mSIN1, and Protor1/2, which are distinct from mTORC1.8 mTORC1 positively regulates mRNA translation (protein synthesis) through the phosphorylation of ribosomal protein S6 kinase 1 (S6K1, also called p70S6K), and eukaryotic initiation factor 4E binding protein 1 (4E-BP1).9 Furthermore, mTORC1 negatively regulates autophagy by phosphorylating and inactivating transcription factor EB, and Unc-51 like autophagy activating kinase, further promoting net cellular anabolism.2 Although mTORC2 influences substrate metabolism,2 mTORC1 has been the primary complex characterized for skeletal muscle anabolism, and thus will be the focus of the two kinase complexes for this review.
Seminal work by Baar & Esser10 demonstrated that the degree of hypertrophy following resistance-type exercise was highly correlated with S6K1 phosphorylation. It was subsequently shown that the mTORC1 inhibitor rapamycin completely prevented both protein synthesis ([14C]-Phenylalanine incorporation) and S6K1T389 phosphorylation following stretch in an ex vivo model of skeletal muscle.11 The role of mTOR as the rapamycin-sensitive element in mechanically-induced protein synthesis was later confirmed using transgenic mice and C2C12 myotubes with skeletal muscle-specific expression of a rapamycin-resistant mTOR.12,13 Indeed, mTORC1 is required for the acute (< 3 h) in vivo increases in mixed MPS following resistance exercise in rodents,14,15 and humans,16 as evidenced by rapamycin administration preventing the immediate rise in mixed MPS following resistance exercise. These findings have been corroborated in several rodent models showing that rapamycin administration prevents resistance exercise-induced MPS17 and largely blunts (∼90%) the hypertrophic response to resistance exercise training.14,18 These findings highlight the importance of mTORC1 following mechanical stimulation on skeletal muscle anabolism.
mTORC1 is involved in the regulation of many anabolic pathways across a variety of cell types, and mTORC1 dysregulation has been implicated in a variety of diseases such as cancer, metabolic disease, and skeletal muscle anabolic resistance.2,19 Therefore, understanding the molecular mechanisms governing mTORC1 can help facilitate the development of clinical interventions. Many studies have attempted to determine the upstream mechanisms which lead to mTORC1 activation in response to a variety of cellular inputs such as nutrient availability, growth factors and mechanical stimulation. The upstream regulation of mTORC1 by nutrients, specifically amino acids (AA) and growth factors (e.g., insulin, insulin-like growth factor 1 [IGF-1]), has been well characterized, in part due to their role in cancer biology. This ‘canonical’ pathway of mTORC1 activation by AA and growth factors is far better understood than mechanical stimulation-induced activation of mTORC1, although significant progress has been made in recent years. The aim of this review is to discuss mTOR-related anabolism in skeletal muscle. Specifically, we will discuss the canonical understanding of mTORC1 activation from in vitro studies, the emerging data on the spatial regulation of mTORC1 in human skeletal muscle, and how the periphery of skeletal muscle fibers acts as a nexus of anabolism.
Canonical understanding of mTORC1 activation in vitro
The two canonical upstream arms that converge to activate mTORC1 are the growth factor- and AA-responsive pathways. The growth factor pathway functions to ultimately allow mTORC1 to interact with its activator, GTP-loaded Ras homologue enriched in brain (Rheb) on the lysosome. Separately, the AA pathway serves to promote mTORC1 translocation to the lysosome during periods of AA sufficiency. The subsequent section will discuss the understanding of these two upstream arms and their roles in mTORC1 activation from in vitro studies across several cell types. Importantly, these upstream arms can act synergistically to stimulate mTORC1.
Growth factor-mediated activation of mTORC1
Growth factor-mediated activation of mTORC1 functions by promoting GTP-loading of the small GTPase, Rheb.20,21 Although Rheb is generally stated to be localized on the lysosome,21 some studies have shown that Rheb is localized to other cellular organelles such as the Golgi apparatus, and the endoplasmic reticulum.22,23 Nonetheless, Rheb is able to activate mTORC1 from both the lysosome,21 and the Golgi apparatus, particularly when the latter is adjacent to lysosomes,24 and where mTOR is localized in response to AA. Rheb can bind to mTOR regardless of its guanylyl nucleotide binding state. However, Rheb-mediated activation of mTOR requires Rheb to be GTP-bound,20 which suggest that regulation of mTOR kinase activity can occur through modulating Rheb's GTP-binding status.
Growth factor activation of mTORC1 occurs through insulin or IGF-1 signalling, both of which activate their respective receptor tyrosine kinases. Activation of these receptors results in the recruitment and phosphorylation of insulin receptor substrate proteins,25 which then bind and activate phosphatidylinositol 3-kinase (PI3K).26 PI3K subsequently phosphorylates plasma membrane phospholipids, creating phosphoinositol-(3,4,5)-triphosphate (PIP3), which interact with and recruit Akt (also called protein kinase B) and 3-phosphoinositide dependent protein kinase 1 to the plasma membrane.27 Akt kinase activity is subsequently promoted by phosphorylation at Thr308 and Ser473 by PDK1 and mTORC2, respectively.28,29 Active Akt is then able to phosphorylate proline-rich Akt substrate of 40 kDa, a negative regulator of mTORC1.30 Furthermore, active Akt phosphorylates and deactivates the negative regulator of Rheb, the tuberous sclerosis (TSC) complex.31 The TSC complex is a heterotrimeric complex containing TSC1, TSC2 and TBC1D7.32 Specifically, TSC2 acts as a GTPase activating protein (GAP) towards Rheb, promoting its GDP-bound form, and preventing Rheb from activating mTORC1.33 TSC2 activity towards Rheb is regulated by its cellular localization, whereby TSC2 phosphorylation by Akt results in TSC dissociating from Rheb and the lysosome,31 allowing Rheb to become GTP-bound and activate mTORC1 in response to growth factors. Rheb activates mTORC1 allosterically by binding to and inducing conformation change through closure of the catalytic cleft and reorienting active-site residues to promote mTOR kinase activity.34
AA-mediated activation of mTORC1
As discussed, mTORC1 becomes activated on the lysosome through interaction with its allosteric activator, GTP-bound Rheb.20,33,35,36 mTORC1-lysosome localization in vitro is regulated by intracellular AA availability.37 In vitro, mTORC1 localization to the lysosome is both necessary and sufficient for mTORC1 activation by AA and forced localization of mTOR to lysosomes renders mTORC1 active even in the absence of AA,38 thus demonstrating the importance of lysosomes in cellular anabolism.
mTORC1-lysosomal recruitment is mediated by a heterodimer of Rag GTPases during periods of AA sufficiency, whereby the Rags are in an active conformation with RagA/B GTP-bound and RagC/D GDP-bound (GTPRagA/B-RagC/DGDP). This conformation allows them to recruit mTORC1 to the lysosome via their interaction with RAPTOR.37 Several upstream AA sensors, located both in the cytosol and on the lysosome, converge on the Rag GTPase heterodimer to control mTORC1 localization in response to changes in intracellular AA concentrations.
The putative cytosolic AA sensors that regulate mTORC1 are the protein complexes GATOR1, GATOR2, Sestrin2, CASTOR1, and SAMTOR, which all interact to regulate the GTP/GDP loading status of the Rag GTPases. Briefly, during AA starvation, GATOR1 acts as a GAP towards RagA/B, promoting it's inactive GDP-bound form, and preventing mTOR translocation to the lysosome.39 Upstream of GATOR1, GATOR2 negatively regulates GATOR1 during periods of AA sufficiency, promoting mTORC1 activity.39 Sestrin2 and CASTOR1 negatively regulate mTORC1 signalling by acting on GATOR2 to prevent its interaction with GATOR1 in the absence of leucine and arginine, respectively.40,41 SAMTOR (S-adenosylmethionine sensor upstream of mTORC1) is a S-adenosylmethionine (SAM) sensor that acts to promote the activity of GATOR1, also negatively regulating the mTORC1 pathway. SAM sufficiency, resulting from increased cellular methionine, prevents SAMTOR from activating GATOR1, and therefore positively regulates mTORC1 signalling.42
In addition to anchoring the Rag heterodimer to the lysosome, it was originally reported that the Ragulator complex acted as a guanine nucleotide exchange factor (GEF) for RagA/B.43 However, recent findings from the same lab with improved assays have shown that Ragulator instead acts to induce conformational changes in the Rag GTPase heterodimer, promoting the GTP off-rate of RagC and allowing its active GDP bound state through a non-canonical GEF mechanism.44 Similarly, the folliculin tumor suppressor (FLCN)-FNIP2 complex acts as a GAP towards RagC, promoting its active GDP-bound form at the lysosomal surface in the presence of AA, facilitating mTORC1 recruitment.45
SLC38A9, a lysosomal AA transporter, also plays a central role in AA-induced activation of the Rag GTPase heterodimer. SLC38A9 acts as a GEF for RagA in the presence of AA, specifically lysosomal arginine.44 SLC38A9 GEF activity towards RagA promotes GDP dissociation from RagA and therefore opens the binding pocket for GTP. Importantly, SLC38A9 cannot bind RagA when it is bound to RAPTOR, suggesting SLC38A9 binds and activates the inactive Rags, and then dissociates prior to mTORC1 recruitment, possibly activating other Rags.44 Furthermore, SLC38A9 can sense lysosomal cholesterol levels, activating Rag-mediated recruitment of mTOR to the lysosome in the presence of cholesterol, but not several other oxysterols.46 As SLC38A9 senses lysosomal AA levels, it is important in activating mTORC1 during periods of catabolism, to promote cellular AA homeostasis and preventing excessive protein breakdown.47 Another protein, the vacuolar-type H+ ATPase (v-ATPase) is also required for AA-induced mTORC1 activation and localization to the lysosome.48 V-ATPase does not interact directly with Rags but rather acts upstream of them, where Ragulator connects them.48 While the exact function of the v-ATPase has yet to be determined, although it is evident that it senses intra-lysosomal AA concentration and acts to promote mTORC1 recruitment to the lysosome, similar to SLC38A9.
Overall, it is evident that mTORC1 regulation by AA is multi-faceted and involves several integrated signalling pathways and numerous upstream protein regulators (Fig. 1). These upstream proteins respond to cellular AA levels (both in the cytosol and in lysosomes), and ultimately function to regulate mTORC1 through modulating the GTP/GDP loading status of the Rag GTPase heterodimer through a variety of mechanisms, thereby controlling mTORC1 recruitment to the lysosome and its activity.
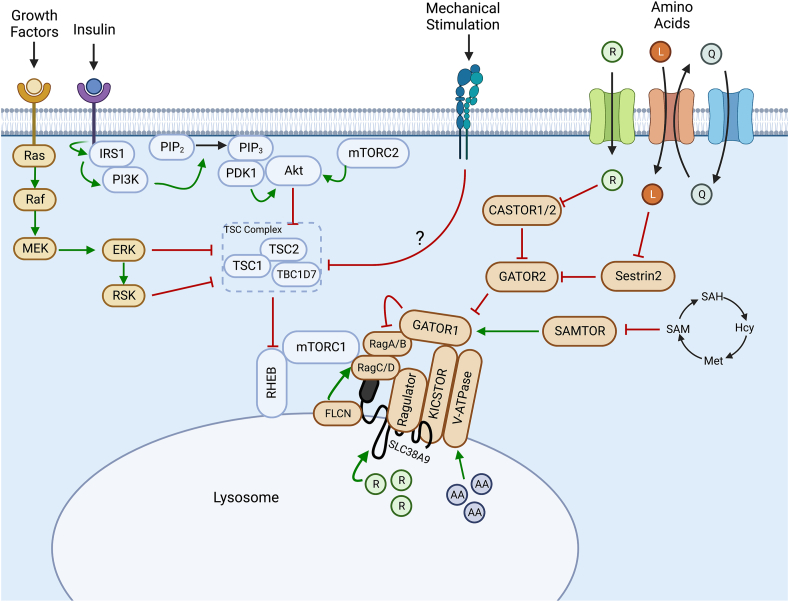
Fig. 1.
Canonical understanding of activation of mTORC1 in a variety of cell types. Growth factor and insulin stimulation results in signalling cascades through the Ras/Raf/ERK and the PI3K/Akt pathways, respectively, resulting in the inhibitory phosphorylation of the TSC complex, alleviating inhibition on Rheb. This allows Rheb to allosterically activate mTORC1. Amino acids function to promote mTORC1 recruitment to the lysosome by the Rag GTPases through a variety of upstream regulators, such that it can be activated by lysosomal Rheb. R, Arginine; L, Leucine; Q, Glutamine; AA, Amino acids; SAM, S-adenosylmethionine; Hcy, homocysteine; Met, methionine; SAH, S-adenosylhomocysteine. Images were created using BioRender.com.
Mechanically induced activation of mTORC1
Mechanical stimulation (e.g., resistance exercise, ex vivo stretching, synergist ablation) of skeletal muscle is a potent stimulator of mTORC1 activity and promotes acute increases in protein synthesis via increased translation efficiency (i.e., greater protein synthetic capacity per ribosome).11 Compared to growth factor and AA-induced signalling, the mechanical regulation of mTORC1 is relatively less characterized as many in vitro models/cell lines place relatively low importance on mechanical stimulation as an anabolic stimulus.
Despite the importance of PI3K/Akt signaling in growth factor-mediated mTORC1 activation, inhibition of PI3K by Wortmannin does not prevent mechanically induced activation of mTORC113,49 or elevated protein synthesis.11 Instead, it appears that mechanical stimulation results in increased activity of membrane-associated diacylglycerol kinase ζ, resulting in increased concentrations of phosphatidic acid, a direct activator of mTOR kinase activity.50,51 Furthermore, eccentric contractions and synergist ablation in mice can also induce phosphorylation of TSC2,52 and increase mTOR association with the lysosome,52, 53, 54 in a manner that required the presence of RAPTOR.54 Importantly, the sites phosphorylated on TSC2 differ between insulin-stimulation and eccentric contraction-induced stimulation.52 Indeed, mutating the mechanically sensitive phosphorylation sites on TSC2 to alanine prevents eccentric contraction-induced, but not insulin-induced, activation of mTORC1 signalling in skeletal muscle.52 Together, these results suggest that TSC2 phosphorylation and inactivation is an important event in eccentric-contraction induced activation of mTORC1, and that the upstream mechanism is divergent from growth factor induced inactivation of TSC2.
Interestingly, inducible muscle-specific RAPTOR knockout mice have similar protein synthetic responses after 7 days of myotenectomy as control mice,54 and electrical stimulation of rat gastrocnemius is only partially inhibited by rapamycin at 6 h post-stimulation.55 However, the mTOR ATP-competitive inhibitor AZD8055 abolished the protein synthetic response to electrical stimulation at 1 h and 6 h in rat gastrocnemius, suggesting that mTORC2, or rapamycin-resistant mTOR substrates, may be responsible for regulating the longer-term protein synthetic response to mechanical stimulation. However, the loss of RAPTOR, and therefore mTORC1 kinase activity, renders skeletal muscle unable to hypertrophy in response to chronic mechanical stimulation.54 While future work should attempt to elucidate the mechanisms underlying the divergent response between MPS and hypertrophy with mTOR inhibition in skeletal muscle, it is clear that mTORC1 plays a prominent role in the response to anabolic stimuli, both acute and chronic. As such, further understanding of the mechanisms by which mTORC1 is regulated in human skeletal muscle will provide vital information that will inform potential therapeutic targets in populations who exhibit anabolic resistance.
mTORC1 translocation in human skeletal muscle following anabolic stimuli
As discussed, many in vitro investigations have observed mTORC1 translocation to the lysosome to be an integral event in the activation of this kinase complex, particularly following increased AA availability.38,48 However, other investigations have suggested that other mechanisms are involved in mTORC1 activation following anabolic stimuli. Specifically, Korolchuk et al.,56 observed no dissociation of mTORC1 and the lysosome when a less severe nutrient deprivation protocol, more akin to postabsorptive conditions in vivo, was applied to cells. Moreover, when nutrients were re-introduced mTORC1-lysosome complexes were seen to translocate to peripheral regions of cells, and if this movement was inhibited mTORC1 could no longer be activated.56 As such, in addition to localization at the lysosomal surface, it is also well accepted that mTORC1 translocation to peripheral regions of cells supports its activation.
Our laboratory first began to investigate these mechanisms in human skeletal muscle several years ago. In our initial investigation, no changes in mTOR colocalization with LAMP2 (lysosomal marker) were observed following resistance exercise alone or when combined with protein-carbohydrate feeding.57 In these settings, readouts of mTORC1 kinase activity were elevated following each intervention, and therefore the lack of changes in mTOR-LAMP2 colocalization suggest that this may not be a mechanism of mTORC1 activation in human skeletal muscle. Conversely, our findings were in concordance with the data presented from Korolchuk et al.,56 as mTOR and LAMP2 colocalization with a sarcolemmal marker (WGA) was elevated in response to anabolic stimuli indicative of translocation of these complexes to peripheral regions of fibres.57 Although dystrophin is regarded as a more “specific” marker of the sarcolemma, work from our laboratory has demonstrated a high level of agreement between WGA and dystrophin staining in human skeletal muscle (r = 0.77).58 Moreover, similar patterns and direction of change were observed when we compared mTOR-WGA colocalization to the peripheral staining intensity of mTOR protein, in response to anabolic stimuli, suggesting this is a reliable measure of peripheral mTOR translocation. Our observations were then extended in a unilateral exercise model whereby protein-carbohydrate ingestion alone or following acute resistance exercise elicited small, non-significant reductions in mTOR-LAMP2 colocalization, whereas mTOR-WGA colocalization was elevated across the postprandial period, and to a greater extent when exercise was undertaken.59 RAPTOR-WGA colocalization followed a similar pattern to this while RICTOR-WGA colocalization remained unchanged,59 confirming that it was likely mTORC1 which is most mobile following anabolic stimuli in human skeletal muscle. These initial data suggested the predominant mechanism of mTORC1 activation in human skeletal muscle was likely mTORC1-lysosome translocation to peripheral regions (reviewed in detail elsewhere60), however, it must be noted that these conclusions were founded upon a small evidence base.
Acute changes in mTOR translocation in human skeletal muscle
Recently, a greater focus has been placed on understanding mTOR translocation in human skeletal muscle research leading to several more investigations involving these measurements stemming from a range of laboratories. These investigations have produced somewhat varying results to those initially observed,57,59,60 which indicates that the regulation of mTORC1 activity in human skeletal muscle may be more complex than previously thought based on early investigations (summarized in Table 1). Nevertheless, there have been several reports in agreement with the earlier work on this topic. For example, our laboratory reported that, following protein-carbohydrate feeding alone, mTOR-WGA colocalization was elevated 1 h into the postprandial period, which coincided with elevated S6K1 kinase activity (readout of mTORC1 activity).61 Moreover, in that investigation, no alterations in mTOR-LAMP2 colocalization were observed, consistent with the notion that, in vivo, postabsorptive nutrient deprivation does not influence mTOR's lysosomal association.57
Table 1.
Summary of human studies investigating mTOR localization in skeletal muscle. Estimated percent change is expressed for studies when possible. n.a., Not applicable (i.e. not measured); T, Resistance Exercise Trained; UT, Untrained; R.Ex, Resistance exercise; CHO, Carbohydrate; EAA, Essential amino acids; LEAA, Leucine-enriched EAA; RT, Resistance training.
| Investigation | Stimulus/Intervention | mTOR-LAMP2 | mTOR-WGA | LAMP2-WGA | mTOR-Rheb |
|---|---|---|---|---|---|
| Abou Sawan et al., (2018) 66 | R.Ex with whole egg or egg white | ↑ (Whole-egg) | n.a. | n.a. | ↑↑ |
| Abou Sawan et al., (2022) 69 | Pre & Post RT (8 weeks) with acute R.Ex. | ↓ (Trained only) | ↑ (T vs UT) | n.a. | n.a. |
| D'Lugos et al., (2018) 67 | Acute R.Ex | ↑ (Type II Fibers) | n.a. | n.a. | n.a. |
| de Hart et al., (2021) 62 | Cheese or milk protein | ↔ | ↑ (Cheese only) | n.a. | n.a. |
| Hannaian et al., (2020) 64 | R.Ex with LEAA or carb placebo | ↓ | ↔ | ↔ | ↔ |
| Hodson et al., (2017) 59 | Protein & CHO ± R.Ex | ↔↓ | ↑↑↑↑ | n.a. | n.a. |
| Hodson et al., (2020) 61 | Protein & CHO Ingestion | ↔ | ↑ | n.a. | n.a. |
| Hodson et al., (2022) 58 | Protein & CHO ± R.Ex | n.a. | ↑↑(EX-FED only) | n.a. | n.a. |
| Holowaty, Lees, et al., (2022; In Review) | Leucine ingestion | ↑ | ↑↑↑ | n.a. | ↔ |
| Moro et al., (2018) 68 | EAA consumption Pre & Post-RT (12 weeks) | ↑ (Trained only) | n.a. | n.a. | n.a. |
| Song et al., (2017) 57 | R.Ex ± Protein & CHO | ↔ | ↑↑ | ↑↑↑ | ↑↑↑ |
Protein-dense whole foods offer distinct amino acid profiles and anabolic responses that are not contingent on the protein dose or extent of aminoacidemia.62,63 For instance, no changes in mTOR-LAMP2 colocalization were observed in individuals consuming either cheese or milk containing 20 g protein.62 Again, mTOR-WGA colocalization was elevated, indicative of peripheral translocation, albeit only following cheese ingestion.62 It is therefore possible that the source of protein ingested may impact mTOR translocation, albeit more clarifying research is needed in this area. More intriguingly, although cheese ingestion induced mTOR translocation, markers of mTORC1 activity (p-S6K1Thr389, p-RPS6Ser240/244) were only elevated when milk was consumed.62 This divergence between translocation events and kinase activity is counterintuitive based on in vitro gain/loss of function studies38,48,56 but may be explained by the static nature of human skeletal muscle sampling (i.e. biopsy sampling only provides a snapshot of the given timepoint and may miss precise temporal variations in measures). Such discord warrants further study in order to ascertain if alterations in mTOR translocation are always linked to kinase activity or if congruence only occurs under certain cellular conditions. It is, however, important to acknowledge that another investigation failed to observe measurable changes in mTOR-WGA localization following resistance exercise combined with leucine-enriched AA (LEAA) supplementation,64 even when mTORC1 activity is elevated. Further, LEAA ingestion seemed to maintain the peripheral location of mTOR further into the postprandial period (4 h) when compared to a cohort who completed resistance exercise but only ingested carbohydrates.64 As such, it was hypothesised that the localization of mTOR may also be implicated in the mechanically-induced sensitisation of skeletal muscle to further anabolic stimuli.65 Therefore, although some questions remain, recent research had provided further evidence of mTORC1 translocation occurring in human skeletal muscle following anabolic stimuli.
Acute changes in mTOR-lysosome colocalization in human skeletal muscle
While there is evidence of peripheral mTOR translocation corroborating the notions we have previously proposed,60 other investigations have produced varying findings. In particular, several studies have reported increases in mTOR-LAMP2 colocalization following anabolic stimuli, suggestive of the canonical mechanism of mTOR translocation to the lysosomal surface previously identified in vitro and in rodent skeletal muscle. Abou Sawan et al.,66 investigated the effects of post-resistance exercise whole egg or egg white ingestion (protein-matched) on mTOR colocalization in human skeletal muscle. The authors reported that elevated mTOR-LAMP2 colocalization was only observed when individuals consumed whole eggs following resistance exercise, suggesting a non-protein substance within the egg yolk may have contributed to mTOR translocation to the lysosomal surface.66 Based on in vitro evidence in non-muscle cells it is possible that cholesterol contained in the egg yolk may have contributed to these results as it can drive mTOR recruitment to the lysosomal surface via a SLC38A9-specific mechanism.3 Importantly, Abou Sawan et al.,66 found that mTOR-LAMP2 colocalization was positively associated with a readout of mTORC1 activity (S6K1Thr389 phosphorylation) and myofibrillar protein synthesis which highlights a potential contribution of this cellular event to these downstream processes. Post-resistance exercise elevations in mTOR-LAMP2 colocalization were also observed by D'Lugos et al.,67 where colocalization was seen to increase 3 h following exercise cessation whereas markers of mTORC1 activity were predominantly elevated at 1 h post-exercise. These elevations only occurred in type II fibres, suggesting a fibre-type specific regulation of mTOR translocation/activity, and were impaired by prior acetaminophen consumption, indicating that some drugs may contribute to mTOR cellular movement as well.67 Whilst these two studies indicate mTOR translocation to the lysosomal surface occurs following anabolic stimuli in human skeletal muscle, it is important to note here that both investigations did not assess/report mTOR colocalization with the sarcolemma, and therefore whether mTOR also translocated to peripheral regions is unknown. Our laboratory has observed one occasion where mTOR colocalization with both LAMP2 and WGA was elevated. Here, participants consumed 2 g leucine (considered the most anabolic AA) at rest, and it was found that both mTOR-LAMP2 and mTOR-WGA colocalization were elevated for 60 min post-ingestion (Holowaty, Lees, Abou Sawan et al., 2022; in review). Thus, it seems possible that following acute anabolic stimuli, both lysosomal recruitment of mTOR and the translocation of these complexes to peripheral regions of muscle fibres could be implicated in subsequent cellular anabolism. However, additional research is needed to confirm the roles of these events in this tissue.
Changes in mTOR localization in human skeletal muscle from chronic anabolic stimuli
In addition to the effects of acute anabolic stimuli on mTOR translocation and protein -protein interactions, several recent investigations have also examined the effects of more chronic anabolic stimuli on these outcome measures. The first of these investigations was conducted by Moro et al.68 and studied the effects of a 12-week progressive resistance exercise program on acute anabolic responses to essential AA ingestion in older individuals. mTOR-LAMP2 colocalization was assessed with the only difference observed being a greater colocalization 3 h following AA ingestion in the trained state compared to untrained state. As such, it could be inferred that a period of resistance training increases the sensitivity of this mechanism to anabolic stimuli in such a population, however this has yet to be replicated. Our laboratory has attempted to understand the influence of chronic anabolic stimuli on these mechanisms in young individuals.69 Here, both young males and females completed an 8-week progressive resistance training program which elicited significant hypertrophy of 8%–20% depending on measure and sex. Immunofluorescent microscopy analysis of mTOR colocalization displayed that, irrespective of sex and acute exercise status, resistance training caused mTOR to be located in more peripheral regions of muscle fibres and in closer proximity to lysosomes.1 We hypothesised that these spatial changes would have the effect of placing mTOR in a location it can be more efficiently activated by subsequent acute anabolic stimuli. Additionally, sexually dimorphic findings were also observed, whereby mTOR-LAMP2 colocalization was lower in females compared to males across all timepoints assessed, a phenomenon which may underpin the reduced rates of MPS observed in females.69 Together these recent studies suggest that a period of resistance training can alter mTOR cellular location, which could impact subsequent downstream anabolism. However, as this evidence base is still in its infancy, future investigations are required to determine the role of these training-mediated changes particularly in populations exhibiting anabolic resistance.
The recent increase in attention on the spatial regulation of mTORC1 in human skeletal muscle has solidified these mechanisms as integral to anabolic responses in this tissue, whilst asking several further questions regarding their role. As findings regarding mTOR translocation to the lysosomal surface appear to be inconsistent, it is important for future research to attempt to understand what components of anabolic stimuli initiate these events (e.g., resistance exercise, AA, or non-protein dietary components). The mechanism, however, that seems to be most consistently observed in response to anabolic stimuli, under both acute and chronic conditions, is the translocation of mTORC1-lysosome complexes to peripheral regions of fibres (Fig. 2). This notion is compounded by our recent observations that mTOR-mediated phosphorylation events (i.e., kinase activity) seems to occur in the periphery of fibres,58 and implies that this area is the primary site of anabolism in human skeletal muscle. A summary of all research articles studying mTOR translocation in human skeletal muscle can be seen in Table 1. The next section of this review will focus on the potential reasons why mTOR translocation and subsequent activation to these areas would increase cellular anabolism as well as the other associated proteins/cellular events that have been observed there.
Fig. 2.
Overview of the regulation of mTOR action at the skeletal muscle fiber periphery (A) Post-absorptive (i.e., fasted). state. During the post-absorptive period, there are less circulating amino acids, the mTOR-Lysosome complexes are mostly colocalized, but dispersed throughout the cytoplasm, as is VPS34 (B) The cellular state during the post-prandial (i.e., fed). period and following resistance exercise. The influx of amino acids and mechanical stimulation results in the mTOR-lysosome complex moving to the periphery of the cell, where the translational machinery is located. In some instances, certain stimuli (e.g., whole-egg ingestion and leucine ingestion) may stimulate an increase in mTOR-lysosome colocalization. Mechanical stimulation of muscle may also promote phosphorylation of TSC2 and its dissociation from the lysosome, further promoting mTORC1 activation. L, Leucine; Q, Glutamine; P, Phosphorylation modification.
The importance of peripheral regions in skeletal muscle anabolism
The translocation of the mTORC1-lysosome complex toward the periphery of skeletal muscle fibers is of significant importance for mTORC1 activation and the stimulation of MPS.70 Indeed, the periphery appears to be the predominant site of MPS, as new proteins are synthesized here in vivo.71 Emerging evidence using immunofluorescence microscopy approaches in humans suggests that the periphery of the cell represents a protein synthetic ‘hub’, replete with the nutrients (e.g., AA transporters, nutrient sensors, capillaries) and molecular apparatus (e.g., ribosomal RNA72) that facilitate skeletal muscle anabolism.
Proximity to the microvasculature
The microvasculature appears to be a central component underpinning the fiber periphery as a region of importance in skeletal muscle anabolism. Feeding and resistance exercise are important anabolic stimuli that redistribute mTOR-LAMP2 complexes toward the sarcolemmal membrane,57,59,62,66,73 in close proximity to capillaries.57,73 Repositioning of mTORC1 to the microvasculature in this manner could ostensibly suggest a greater ability to “sense” extracellular AA in the post-training state.73These complexes are themselves enriched with growth factor receptors, AA transporters, and integrins, suggesting that they could be a nexus where anabolic stimuli convene to regulate mTORC1 activity.58
AA transporters
In the first instance, the periphery of skeletal muscle fibers contains AA transporters that govern access of AAs from the interstitial fluid into the sarcoplasm.74,75 This is an indispensable aspect of cell survival76 that promotes AA availability as substrates for MPS.77, 78, 79 It is proposed that AA transporters might also serve a dual function as receptors, or ‘transceptors’, that detect extracellular AA concentrations and convert this availability into intracellular signals.80, 81, 82, 83
The large neutral AA transporter 1 (LAT1) mediates the influx of essential AA (EAAs), particularly leucine but also isoleucine, valine, tyrosine, and phenylalanine,84 into skeletal muscle.80 Concomitantly, LAT1 transports glutamine out of the cell, working in tandem with the sodium-coupled neutral AA transporter 2 (SNAT2).80,85 Leucine is generally regarded as distinct among the AAs due to its role as a substrate for MPS and its ability to directly stimulate mTORC1-associated signaling in skeletal muscle.86,87 Moreover, LAT1 is the most highly expressed large neutral AA transporter in skeletal muscle,88 and is required for normal myogenesis in vitro.79
In humans, LAT1 is observed in close proximity to the sarcolemmal membrane in skeletal muscle, as shown in immunofluorescence studies,89,90 with greater abundance in the sarcoplasmic regions of type II fibers.91 At rest, LAT1 is located close to the microvasculature, as revealed by endothelial nitric oxide synthase (eNOS)-positive staining,89 which might support the efficient transport of these substrates for MPS.60 Evidence for the acute modulation of LAT1 and SNAT2 protein expression and trafficking by dietary protein ingestion and exercise in humans is equivocal.64,73,90,92, 93, 94 However, chronic total-body resistance training, irrespective of daily dietary protein supplementation, was shown to increase LAT1 protein content per muscle fiber after 12 weeks.85 This increase occurred primarily in the sarcoplasm, suggesting that membrane LAT1 content remains unaltered.85 Nevertheless, the sarcolemmal localization of indispensable AA transporters, such as LAT1, underlines the importance of the periphery in the upstream activation of MPS by EAAs.
Nutrient sensors
The stimulation of mTORC1 activity by nutrients in human skeletal muscle is still a poorly understood phenomenon. Nonetheless, one potential nutrient-sensitive activator of mTORC1 is the vacuolar protein sorting 34 (Vps34),61,95, 96, 97 a class III PI3Kinase that has recently been explored by our laboratory in C2C12 myotubes and human skeletal muscle.61 The proposed mechanism of Vps34 nutrient sensing holds that in the postabsorptive state, mTORC1 and Vps34 are located independently in the cytosol, with mTORC1 and the lysosome in close association. Following the provision of protein and carbohydrates (and thus high AA availability and the elevation of plasma insulin concentrations), the mTORC1-lysosome complex and Vps34 translocate to the periphery of the cell, upon which they colocalize.61 This process then brings about an elevation in mTORC1 activity, and stimulation of MPS, whereas Vps34 activity remains comparable to that in the postabsorptive state.61 One proposed mechanism of Vps34-induced mTORC1-lysosome redistribution to the periphery concerns several products of Vps34 activity itself.98 AA stimulate phosphatidylinositol 3-phosphate (PtdIns3P) production via Vps34 which is eventually bound by the PtdIns3P effector Protrudin. Under conditions of Vps34 inhibition or depletion of Protrudin, the mTORC1-lysosome complex is located perinuclearly. Hence, its translocation to the periphery and subsequent activation is PtdIns3P-dependent.98 This adds further credence to our findings61 suggesting the increased peripheral colocalization of mTOR and Vps34 may have resulted from PtdIns3P-dependent lysosomal translocation to aid mTORC1 activation.
mTORC2-mediated glucose uptake
mTORC2 is thought to directly regulate insulin-mediated skeletal muscle glucose uptake, insulin sensitivity, and glucose tolerance.99,100 In mice lacking RICTOR, insulin-stimulated glucose uptake was decreased and there was evidence of glucose intolerance.101 The insulin-stimulated, mTORC2-specific phosphorylation of AktSer473 was also significantly reduced. Elsewhere, in mouse skeletal muscle lacking mTORC2 activity, exercise-induced glucose uptake was also decreased.102 Thus, mTORC2 appears to act as an important regulator of glucose homeostasis in rodent skeletal muscle.
In humans, previous work by the lead author has revealed that mTORC2 is in a constant state of association with the sarcolemma and is largely unaffected by nutrient provision or resistance exercise.103 This is noteworthy, given that leucine has been shown to mediate insulin signaling and glucose uptake in rat primary skeletal muscle not only through mTORC1, but mTORC2 as well.104 Hence, the subcellular localization of mTORC2 in peripheral regions,103 in close proximity to LAT1,89,90 capillaries,57 and growth factors seems to carry substantial physiological relevance, however, it may be unaffected by acute anabolic stimuli. In this region, the presence of mTORC2 might potentially facilitate enhanced insulin-stimulated glucose uptake, an important process during periods of hypertrophy wherein skeletal muscle reprograms its metabolism, and diverts at least some of this glucose into anabolic pathways.105 It must be stated however, that our laboratory has only explored the subcellular localization of mTORC2 in the acute phase (i.e., 3 h post-exercise/feeding).103 Therefore, the effects of chronic stimuli on mTORC2 subcellular distribution and protein-protein interactions in human skeletal muscle remain to be elucidated. Although not in skeletal muscle, there is evidence to suggest that mTORC2-lysosome translocation to peripheral regions regulates mTORC2 kinase activity. Jia and Bonifacino106 showed that mTORC2-rich lysosomes are redistributed to peripheral regions in cells following nutrient replenishment and this was essential for downstream Akt phosphorylation (HeLa, HeLa-derived KO and HEK293T lines). Such data further solidifies the view that the periphery of cells/fibres is a hub of anabolism.
Focal adhesions and “Mechanosensing” proteins
Focal adhesion complexes are discrete plasma membrane hubs that appear to govern growth factor signaling and AA transport into the cell, and are implicated in the peripheral and intracellular induction of mTORC1.107 They are constructed from proteins such as talin, paxillin, vinculin, and focal adhesion kinase (FAK).108,109 In skeletal muscle, focal adhesion complexes form part of the costamere108,110 and serve as major interfaces between extracellular mechanical stimuli and intracellular biochemical signaling, a process referred to as mechanotransduction.111,112
Immunofluorescence studies in humans have shown that focal adhesion complexes are situated in sarcolemmal regions113 and in proximity with the microvasculature.114 Following feeding, mTORC1-mediated phosphorylation events are acutely elevated within paxillin-positive regions, with or without resistance exercise,58 and the in vitro targeting of mTORC1 to focal adhesions enhances mTORC1 activity regardless of the position of the lysosome.107 FAK may modulate mTOR through its inhibition of TSC2 a negative regulator of mTOR,110 but at present this area remains largely unexplored in human skeletal muscle. Nevertheless, inhibition of FAK in an ovarian cancer cell line suppressed the activation of the PI3K/Akt/mTOR pathway and blunted cell proliferation and migration.115 Accordingly, the movement of mTORC1 to, and subsequent activation in, peripheral regions rich in focal adhesion complexes may provide a mechanism by which mTORC1 is able to integrate multiple anabolic stimuli.
Translational apparatus
The translocation of mTOR-LAMP2 to the sarcolemma occurs in tandem with enhanced activity of mTORC1 and mTORC1-associated proteins involved in translation initiation and elongation.57,59 In vivo research in rodents using puromycin incorporation and visualization methods has indicated that MPS occurs in the periphery of skeletal muscle fibers.71 Indeed, there is an abundance of ribosomal RNA in proximity to the sarcolemma,116 and a subsarcolemmal pool of ribosomes has been revealed.117 We previously observed that mTOR colocalizes with eukaryotic translation initiation factor 3 subunit F (eIF3F) at the cell membrane post-exercise in the fasted or fed state, with a greater response in the fed condition.57 eIF3F is an important subunit of the eukaryotic initiation factor 3 complex that serves as a scaffold for the interaction between mTOR and S6K1, ultimately leading to the formation of the pre-initiation complex, a crucial process in protein synthesis.118,119 Therefore, it follows that MPS is likely to occur in the regions wherein mTORC1, its associated proteins, and the translational apparatus coincide,70 confirming that the functional consequences of mTORC1 activation also occur in peripheral regions of the cell.
Conclusion
This review has presented contemporary findings on the physiological relevance of the peripheral regions of skeletal muscle within the context of MPS. Indeed, these regions appear to serve as a protein synthetic ‘nexus’ that integrates the molecular machinery and materials needed to drive anabolism. This comprises the apparatus that facilitates the ingress of substrates (i.e., AAs, glucose), as well as that needed to translate mechanical stimuli into biochemical signals and AAs into functional proteins. Future work should more cogently elucidate these pertinent topics, particularly with respect to the stimulation of mTORC1 activity by nutrients in human skeletal muscle.
Submission statement
We can also confirm that this review is not under consideration for publication elsewhere and all authors have approved this submission.
Authors’ contributions
All authors contributed to the writing and editing of the article.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Alexa Govette for her insights and discussion during the preparation of the manuscript.
References
- 1.Goldberg A.L. Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol. 1968;36(3):653–658. doi: 10.1083/jcb.36.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara K., Maruki Y., Long X., et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110(2):177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.H., Sarbassov D.D., Ali S.M., et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.H., Sarbassov D.D., Ali S.M., et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11(4):895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 6.Sancak Y., Thoreen C.C., Peterson T.R., et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Peterson T.R., Laplante M., Thoreen C.C., et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon M.S. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9(11):1176. doi: 10.3390/nu9111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X.M., Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 10.Baar K., Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1):C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 11.Hornberger T.A., Stuppard R., Conley K.E., et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;380(Pt 3):795–804. doi: 10.1042/bj20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman C.A., Frey J.W., Mabrey D.M., et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589(Pt 22):5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornberger T.A., Sukhija K.B., Wang X.R., Chien S. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr(389) in p70(S6k) FEBS Lett. 2007;581(24):4562–4566. doi: 10.1016/j.febslet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogasawara R., Fujita S., Hornberger T.A., et al. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep. 2016;6:31142. doi: 10.1038/srep31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West D.W., Baehr L.M., Marcotte G.R., et al. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol. 2016;594(2):453–468. doi: 10.1113/jp271365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drummond M.J., Fry C.S., Glynn E.L., et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(Pt 7):1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubica N., Bolster D.R., Farrell P.A., Kimball S.R., Jefferson L.S. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280(9):7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- 18.Bodine S.C., Stitt T.N., Gonzalez M., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson D.J., Babraj J., Leese G., Siervo M. Anabolic resistance does not explain sarcopenia in patients with type 2 diabetes mellitus, compared with healthy controls, despite reduced mTOR pathway activity. Clin Nutr. 2017;36(6):1716–1719. doi: 10.1016/j.clnu.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Long X., Lin Y., Ortiz-Vega S., Yonezawa K., Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15(8):702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Menon S., Dibble C.C., Talbott G., et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buerger C., DeVries B., Stambolic V. Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun. 2006;344(3):869–880. doi: 10.1016/j.bbrc.2006.03.220. [DOI] [PubMed] [Google Scholar]
- 23.Hanker A.B., Mitin N., Wilder R.S., et al. Differential requirement of CAAX-mediated posttranslational processing for Rheb localization and signaling. Oncogene. 2010;29(3):380–391. doi: 10.1038/onc.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao F., Kondo K., Itoh T., et al. Rheb localized on the Golgi membrane activates lysosome-localized mTORC1 at the Golgi-lysosome contact site. J Cell Sci. 2018;131(3):jcs208017. doi: 10.1242/jcs.208017. [DOI] [PubMed] [Google Scholar]
- 25.Sun X.J., Rothenberg P., Kahn C.R., et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 26.Myers M.G., Jr., Backer J.M., Sun X.J., et al. IRS-1 activates phosphatidylinositol 3’-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992;89(21):10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frech M., Andjelkovic M., Ingley E., Reddy K.K., Falck J.R., Hemmings B.A. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272(13):8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 28.Alessi D.R., James S.R., Downes C.P., et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Kovacina K.S., Park G.Y., Bae S.S., et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278(12):10189–10194. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 31.Manning B.D., Tee A.R., Logsdon M.N., Blenis J., Cantley L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10(1):151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 32.Dibble C.C., Elis W., Menon S., et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47(4):535–546. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoki K., Li Y., Xu T., Guan K.L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17(15):1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H., Jiang X., Li B., et al. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature. 2017;552(7685):368–373. doi: 10.1038/nature25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fawal M.A., Brandt M., Djouder N. MCRS1 binds and couples Rheb to amino acid-dependent mTORC1 activation. Dev Cell. 2015;33(1):67–81. doi: 10.1016/j.devcel.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Saito K., Araki Y., Kontani K., Nishina H., Katada T. Novel role of the small GTPase Rheb: its implication in endocytic pathway independent of the activation of mammalian target of rapamycin. J Biochem. 2005;137(3):423–430. doi: 10.1093/jb/mvi046. [DOI] [PubMed] [Google Scholar]
- 37.Sancak Y., Peterson T.R., Shaul Y.D., et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar-Peled L., Chantranupong L., Cherniack A.D., et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340(6136):1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chantranupong L., Wolfson R.L., Orozco J.M., et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014;9(1):1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chantranupong L., Scaria S.M., Saxton R.A., et al. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell. 2016;165(1):153–164. doi: 10.1016/j.cell.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu X., Orozco J.M., Saxton R.A., et al. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science. 2017;358(6364):813–818. doi: 10.1126/science.aao3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar-Peled L., Schweitzer L.D., Zoncu R., Sabatini D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150(6):1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen K., Sabatini D.M. Ragulator and SLC38A9 activate the Rag GTPases through noncanonical GEF mechanisms. Proc Natl Acad Sci U S A. 2018;115(38):9545–9550. doi: 10.1073/pnas.1811727115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsun Z.Y., Bar-Peled L., Chantranupong L., et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52(4):495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellano B.M., Thelen A.M., Moldavski O., et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science. 2017;355(6331):1306–1311. doi: 10.1126/science.aag1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Remaileh M., Wyant G.A., Kim C., et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science. 2017;358(6364):807–813. doi: 10.1126/science.aan6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Neil T.K., Duffy L.R., Frey J.W., Hornberger T.A. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587(Pt 14):3691–3701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.You J.S., Lincoln H.C., Kim C.R., et al. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem. 2014;289(3):1551–1563. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon M.S., Sun Y., Arauz E., Jiang Y., Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J Biol Chem. 2011;286(34):29568–29574. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs B.L., McNally R.M., Kim K.J., et al. Identification of mechanically regulated phosphorylation sites on tuberin (TSC2) that control mechanistic target of rapamycin (mTOR) signaling. J Biol Chem. 2017;292(17):6987–6997. doi: 10.1074/jbc.M117.777805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobs B.L., You J.S., Frey J.W., Goodman C.A., Gundermann D.M., Hornberger T.A. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol. 2013;591(18):4611–4620. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.You J.S., McNally R.M., Jacobs B.L., et al. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. Faseb J. 2019;33(3):4021–4034. doi: 10.1096/fj.201801653RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogasawara R., Suginohara T. Rapamycin-insensitive mechanistic target of rapamycin regulates basal and resistance exercise-induced muscle protein synthesis. Faseb J. 2018 doi: 10.1096/fj.201701422R. fj201701422R. [DOI] [PubMed] [Google Scholar]
- 56.Korolchuk V.I., Saiki S., Lichtenberg M., et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13(4):453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Z., Moore D.R., Hodson N., et al. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep. 2017;7(1):5028. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodson N., Mazzulla M., Holowaty M.N.H., Kumbhare D., Moore D.R. RPS6 phosphorylation occurs to a greater extent in the periphery of human skeletal muscle fibers, near focal adhesions, after anabolic stimuli. Am J Physiol Cell Physiol. 2022;322(1):C94–C110. doi: 10.1152/ajpcell.00357.2021. [DOI] [PubMed] [Google Scholar]
- 59.Hodson N., McGlory C., Oikawa S.Y., et al. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol. 2017;313(6):C604–C611. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodson N., Philp A. The importance of mTOR trafficking for human skeletal muscle translational control. Exerc Sport Sci Rev. 2019;47(1):46–53. doi: 10.1249/jes.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodson N., Dent J.R., Song Z., et al. Protein-carbohydrate ingestion alters Vps34 cellular localization independent of changes in kinase activity in human skeletal muscle. Exp Physiol. 2020;105(12):2178–2189. doi: 10.1113/ep088805. [DOI] [PubMed] [Google Scholar]
- 62.de Hart N., Mahmassani Z.S., Reidy P.T., et al. Acute effects of cheddar cheese consumption on circulating amino acids and human skeletal muscle. Nutrients. 2021;13(2):614. doi: 10.3390/nu13020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985) 2009;107(3):987–992. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 64.Hannaian S.J., Hodson N., Abou Sawan S., et al. Leucine-enriched amino acids maintain peripheral mTOR-Rheb localization independent of myofibrillar protein synthesis and mTORC1 signaling postexercise. J Appl Physiol (1985) 2020;129(1):133–143. doi: 10.1152/japplphysiol.00241.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Churchward-Venne T.A., Burd N.A., Phillips S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: strategies to enhance anabolism. Nutr Metab. 2012;9(1):40. doi: 10.1186/1743-7075-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abou Sawan S., van Vliet S., West D.W.D., et al. Whole egg, but not egg white, ingestion induces mTOR colocalization with the lysosome after resistance exercise. Am J Physiol Cell Physiol. 2018;315(4):C537–C543. doi: 10.1152/ajpcell.00225.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Lugos A.C., Patel S.H., Ormsby J.C., et al. Prior acetaminophen consumption impacts the early adaptive cellular response of human skeletal muscle to resistance exercise. J Appl Physiol (1985) 2018;124(4):1012–1024. doi: 10.1152/japplphysiol.00922.2017. [DOI] [PubMed] [Google Scholar]
- 68.Moro T., Brightwell C.R., Deer R.R., et al. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr. 2018;148(6):900–909. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abou Sawan S., Hodson N., Malowany J.M., et al. Trained integrated post-exercise myofibrillar protein synthesis rates correlate with hypertrophy in young males and females. Med Sci Sports Exerc. 2022;54(6):953–964. doi: 10.1249/mss.0000000000002878. [DOI] [PubMed] [Google Scholar]
- 70.Hodson N., Philp A. The importance of mTOR trafficking for human skeletal muscle translational control. Exerc Sport Sci Rev. 2019;47(1):46–53. doi: 10.1249/JES.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman C.A., Mabrey D.M., Frey J.W., et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. Faseb J. 2011;25(3):1028–1039. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horne Z., Hesketh J. Increased association of ribosomes with myofibrils during the skeletal-muscle hypertrophy induced either by the beta-adrenoceptor agonist clenbuterol or by tenotomy. Biochem J. 1990;272(3):831–833. doi: 10.1042/bj2720831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abou Sawan S., Hodson N., Tinline-Goodfellow C., et al. Incorporation of dietary amino acids into myofibrillar and sarcoplasmic proteins in free-living adults is influenced by sex, resistance exercise, and training status. J Nutr. 2021;151(11):3350–3360. doi: 10.1093/jn/nxab261. [DOI] [PubMed] [Google Scholar]
- 74.Broer S., Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem J. 2017;474(12):1935–1963. doi: 10.1042/bcj20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roberson P.A., Mobley C.B., Romero M.A., et al. LAT1 protein content increases following 12 Weeks of resistance exercise training in human skeletal muscle. Front Nutr. 2020;7:628405. doi: 10.3389/fnut.2020.628405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kilberg M.S., Pan Y.X., Chen H., Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr. 2005;25:59–85. doi: 10.1146/annurev.nutr.24.012003.132145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biolo G., Tipton K.D., Klein S., Wolfe R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 78.Tipton K.D., Gurkin B.E., Matin S., Wolfe R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10(2):89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 79.Collao N., Akohene-Mensah P., Nallabelli J., et al. The role of L-type amino acid transporter 1 (Slc7a5) during in vitro myogenesis. Am J Physiol Cell Physiol. 2022;323(2):C595–C605. doi: 10.1152/ajpcell.00162.2021. [DOI] [PubMed] [Google Scholar]
- 80.Dickinson J.M., Rasmussen B.B. Amino acid transporters in the regulation of human skeletal muscle protein metabolism. Curr Opin Clin Nutr Metab Care. 2013;16(6):638–644. doi: 10.1097/MCO.0b013e3283653ec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyde R., Cwiklinski E.L., MacAulay K., Taylor P.M., Hundal H.S. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282(27):19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- 82.Pinilla J., Aledo J.C., Cwiklinski E., Hyde R., Taylor P.M., Hundal H.S. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed). 2011;3:1289–1299. doi: 10.2741/e332. [DOI] [PubMed] [Google Scholar]
- 83.Taylor P.M. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr. 2014;99(1):223S–230S. doi: 10.3945/ajcn.113.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dickens D., Chiduza G.N., Wright G.S.A., Pirmohamed M., Antonyuk S.V., Hasnain S.S. Modulation of LAT1 (SLC7A5) transporter activity and stability by membrane cholesterol. Sci Rep. 2017;7:43580. doi: 10.1038/srep43580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberson P.A., Mobley C.B., Romero M.A., et al. LAT1 protein content increases following 12 Weeks of resistance exercise training in human skeletal muscle. Front Nutr. 2021;7:628405. doi: 10.3389/fnut.2020.628405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atherton P.J., Smith K., Etheridge T., Rankin D., Rennie M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 87.Hodson N., West D.W.D., Philp A., Burd N.A., Moore D.R. Molecular regulation of human skeletal muscle protein synthesis in response to exercise and nutrients: a compass for overcoming age-related anabolic resistance. Am J Physiol Cell Physiol. 2019;317(6):C1061–C1078. doi: 10.1152/ajpcell.00209.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poncet N., Mitchell F.E., Ibrahim A.F., et al. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One. 2014;9(2):e89547. doi: 10.1371/journal.pone.0089547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodson N., Brown T., Joanisse S., et al. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients. 2017;10(1):23. doi: 10.3390/nu10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazzulla M., Hodson N., Lees M., et al. LAT1 and SNAT2 protein expression and membrane localization of LAT1 are not acutely altered by dietary amino acids or resistance exercise nor positively associated with leucine or phenylalanine incorporation in human skeletal muscle. Nutrients. 2021;13(11):3906. doi: 10.3390/nu13113906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hodson N., Brown T., Joanisse S., et al. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients. 2017;10(1):23. doi: 10.3390/nu10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drummond M.J., Glynn E.L., Fry C.S., Timmerman K.L., Volpi E., Rasmussen B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(5):E1011–E1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beals J.W., Sukiennik R.A., Nallabelli J., et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am J Clin Nutr. 2016;104(4):1014–1022. doi: 10.3945/ajcn.116.130385. [DOI] [PubMed] [Google Scholar]
- 94.Agergaard J., Bülow J., Jensen J.K., et al. Effect of light-load resistance exercise on postprandial amino acid transporter expression in elderly men. Phys Rep. 2017;5(18):e13444. doi: 10.14814/phy2.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gran P., Cameron-Smith D. The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol. 2011;11:10. doi: 10.1186/1472-6793-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirsch D.S., Shen Y., Dokmanovic M., et al. Insulin activation of vacuolar protein sorting 34 mediates localized phosphatidylinositol 3-phosphate production at lamellipodia and activation of mTOR/S6K1. Cell Signal. 2014;26(6):1258–1268. doi: 10.1016/j.cellsig.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 97.MacKenzie M.G., Hamilton D.L., Murray J.T., Taylor P.M., Baar K. mVps34 is activated following high-resistance contractions. J Physiol. 2009;587(1):253–260. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong Z., Pedersen N.M., Wang L., Torgersen M.L., Stenmark H., Raiborg C. PtdIns3P controls mTORC1 signaling through lysosomal positioning. J Cell Biol. 2017;216(12):4217–4233. doi: 10.1083/jcb.201611073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamming D.W., Ye L., Katajisto P., et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao Z., Zhang W. Role of mTOR in glucose and lipid metabolism. Int J Mol Sci. 2018;19(7):2043. doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar A., Harris T.E., Keller S.R., Choi K.M., Magnuson M.A., Lawrence J.C., Jr. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28(1):61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kleinert M., Parker B.L., Fritzen A.M., et al. Mammalian target of rapamycin complex 2 regulates muscle glucose uptake during exercise in mice. J Physiol. 2017;595(14):4845–4855. doi: 10.1113/JP274203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodson N., McGlory C., Oikawa S.Y., et al. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol. 2017;313(6):C604–C611. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu H., Liu R., Xiong Y., et al. Leucine facilitates the insulin-stimulated glucose uptake and insulin signaling in skeletal muscle cells: involving mTORC1 and mTORC2. Amino Acids. 2014;46(8):1971–1979. doi: 10.1007/s00726-014-1752-9. [DOI] [PubMed] [Google Scholar]
- 105.Wackerhage H., Vechetti I.J., Baumert P., et al. Does a hypertrophying muscle fibre reprogramme its metabolism similar to a cancer cell? Sports Med. 2022;52(11):2569–2578. doi: 10.1007/s40279-022-01676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jia R., Bonifacino J.S. Lysosome positioning influences mTORC2 and AKT signaling. Mol Cell. 2019;75(1):26–38.e3. doi: 10.1016/j.molcel.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rabanal-Ruiz Y., Byron A., Wirth A., et al. mTORC1 activity is supported by spatial association with focal adhesions. J Cell Biol. 2021;220(5):e202004010. doi: 10.1083/jcb.202004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flück M., Ziemiecki A., Billeter R., Müntener M. Fibre-type specific concentration of focal adhesion kinase at the sarcolemma: influence of fibre innervation and regeneration. J Exp Biol. 2002;205(Pt 16):2337–2348. doi: 10.1242/jeb.205.16.2337. [DOI] [PubMed] [Google Scholar]
- 109.Andresen B., de Marees M., Schiffer T., Bloch W., Suhr F. Skeletal muscle fiber type-specific expressions of mechanosensors integrin-linked kinase, talin, and vinculin and their modulation by loading and environmental conditions in humans. Faseb J. 2022;36(8):e22458. doi: 10.1096/fj.202101377RR. [DOI] [PubMed] [Google Scholar]
- 110.Graham Z.A., Gallagher P.M., Cardozo C.P. Focal adhesion kinase and its role in skeletal muscle. J Muscle Res Cell Motil. 2015;36(4–5):305–315. doi: 10.1007/s10974-015-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burkholder T.J. Mechanotransduction in skeletal muscle. Front Biosci. 2007;12(1):174–191. doi: 10.2741/2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seong J., Wang N., Wang Y. Mechanotransduction at focal adhesions: from physiology to cancer development. J Cell Mol Med. 2013;17(5):597–604. doi: 10.1111/jcmm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilson O.J., Shaw C.S., Sherlock M., Stewart P.M., Wagenmakers A.J. Immunofluorescent visualisation of focal adhesion kinase in human skeletal muscle and its associated microvasculature. Histochem Cell Biol. 2012;138(4):617–626. doi: 10.1007/s00418-012-0980-x. [DOI] [PubMed] [Google Scholar]
- 114.Wilson O.J., Bradley H., Shaw C.S., Wagenmakers A.J. Paxillin and focal adhesion kinase colocalise in human skeletal muscle and its associated microvasculature. Histochem Cell Biol. 2014;142(3):245–256. doi: 10.1007/s00418-014-1212-3. [DOI] [PubMed] [Google Scholar]
- 115.Li H., Gao Y., Ren C. Focal adhesion kinase inhibitor BI 853520 inhibits cell proliferation, migration and EMT process through PI3K/AKT/mTOR signaling pathway in ovarian cancer. Discov Oncol. 2021;12(1):29. doi: 10.1007/s12672-021-00425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Habets P.E., Franco D., Ruijter J.M., Sargeant A.J., Pereira J.A., Moorman A.F. RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem. 1999;47(8):995–1004. doi: 10.1177/002215549904700803. [DOI] [PubMed] [Google Scholar]
- 117.Horne Z., Hesketh J. Immunological localization of ribosomes in striated rat muscle. Evidence for myofibrillar association and ontological changes in the subsarcolemmal:myofibrillar distribution. Biochem J. 1990;268(1):231–236. doi: 10.1042/bj2680231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harris T.E., Chi A., Shabanowitz J., Hunt D.F., Rhoads R.E., Lawrence J.C. mTOR-dependent stimulation of the association of eIF4G and eIF3 by insulin. EMBO J. 2006;25(8):1659–1668. doi: 10.1038/sj.emboj.7601047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holz M.K., Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280(28):26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]