Abstract
Background
Widespread implementation of the minimally invasive technique in pancreatic surgery has proven to be challenging. The aim of this study was to compare the perioperative outcomes of minimally invasive (laparoscopic and robotic) pancreatic surgery with open pancreatic surgery using data obtained from RCTs.
Methods
A literature search was done using Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Web of Science; all available RCTs comparing minimally invasive pancreatic surgery and open pancreatic surgery in adults requiring elective distal pancreatectomy or partial pancreatoduodenectomy were included. Outcomes were mortality rate, general and pancreatic surgery specific morbidity rate, and length of hospital stay.
Results
Six RCTs with 984 patients were included; 99.0 per cent (486) of minimally invasive procedures were performed laparoscopically and 1.0 per cent (five) robotically. In minimally invasive pancreatic surgery, length of hospital stay (−1.3 days, −2 to −0.5, P = 0.001) and intraoperative blood loss (−137 ml, −182 to −92, P < 0.001) were reduced. In the subgroup analysis, reduction in length of hospital stay was only present for minimally invasive distal pancreatectomy (−2 days, −2.3 to −1.7, P < 0.001). A minimally invasive approach showed reductions in surgical site infections (OR 0.4, 0.1 to 0.96, P = 0.040) and intraoperative blood loss (−131 ml, −173 to −89, P < 0.001) with a 75 min longer duration of surgery (42 to 108 min, P < 0.001) only in partial pancreatoduodenectomy. No significant differences were found with regards to mortality rate and postoperative complications.
Conclusion
This meta-analysis presents level 1 evidence of reduced length of hospital stay and intraoperative blood loss in minimally invasive pancreatic surgery compared with open pancreatic surgery. Morbidity rate and mortality rate were comparable, but longer duration of surgery in minimally invasive partial pancreatoduodenectomy hints that this technique in partial pancreatoduodenectomy is technically more challenging than in distal pancreatectomy.
This systematic review and meta-analysis presents level 1 evidence of randomized clinical trials (RCTs) comparing minimally invasive (laparoscopic and robotic) with open pancreatic surgery. A minimally invasive approach in pancreatic surgery seems feasible and safe with comparable general as well as pancreatic surgery specific morbidity. Further, a minimally invasive approach was associated with reduced length of hospital stay, reduced intraoperative blood loss, and longer duration of surgery.
Introduction
Beginning with the first laparoscopic appendectomy and cholecystectomy performed in the 1980s, the minimally invasive technique has become increasingly common in abdominal surgery over the past decades. Today, the laparoscopic approach represents the standard of care in many basic surgical procedures1,2. Recognizing the advantages of minimally invasive surgery, such as reduced postoperative pain, reduction in length of hospital stay (LOS), and faster return to daily activities, the indications for the laparoscopic approach have extended to increasingly complex procedures. Meanwhile, enhanced recovery after surgery protocols broadly incorporated laparoscopic technique as an essential part in bariatric, colorectal and upper gastrointestinal surgery3–5 and focus of research has shifted to the implementation of robotic surgery.
However, widespread implementation of the laparoscopic and robotic technique in pancreatic surgery has proven to be more challenging. Despite considerable improvements in operative techniques and perioperative care, the rate of postoperative complications and subsequent impairment in patients’ quality of life remain high in open pancreatic surgery. For increased comparability of postoperative results and improved complication monitoring, several attempts to define benchmark outcomes have been made. The benchmark for mortality rate is reported as 2 per cent for partial pancreatoduodenectomy (PD) and less than 1 per cent for distal pancreatectomy (DP) by the Evidence Map of Pancreatic Surgery6, with an overall complication rate of 53 per cent for PD and 59 per cent for DP. The benefit of a minimally invasive approach in pancreatic surgery remains unclear; however, an increasing amount of high-level evidence in the form of RCTs comparing laparoscopic with open pancreatic resection is available.
The aim of this systematic review and meta-analysis was to compare the perioperative outcomes of minimally invasive (laparoscopic and robotic) pancreatic surgery with open pancreatic surgery using data obtained from RCTs only. Furthermore, subgroup analysis of open versus minimally invasive PD and DP was performed.
Methods
This systematic review and meta-analysis was conducted according to an a priori-developed review protocol predefined by Probst et al.7 and is in line with the PRISMA statement8. Study selection, data extraction, and critical appraisal were performed according to Evidence Map of Pancreatic Surgery protocols. A detailed methodological description has been published by Probst et al.6,7.
Systematic literature search
A systematic literature search was done using Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (via PubMed), and Web of Science for all RCTs on pancreatic surgery. The detailed search strategy of Probst et al.7 was applied and is displayed in Table S1. The last database search was performed on 24 April 2022. No date or language restrictions were applied.
Study selection
RCTs comparing minimally invasive (laparoscopic or robotic) versus open pancreatic resection for benign, premalignant, or malignant pancreatic disease that met the following PICOS criteria were considered eligible for inclusion: P (patients) - patients over 18 years of age with benign, premalignant, or malignant disease, who required elective PD or DP; I (intervention) - minimally invasive (laparoscopic or robotic) PD or DP; C (control) - open PD or DP; O (outcome) - predefined outcome parameters as described in the ‘Data extraction’ section; and S (study design) - RCTs only.
Study selection was performed according to the recommendations of The Cochrane Collaboration9. Further, the World Health Organization trial registry10 was systematically searched for ongoing RCTs and unpublished terminated RCTs, which were regularly incorporated in the analysis as results became available.
Data extraction
The screening of titles, abstracts, and full texts was performed by two independent reviewers. Disagreements were resolved by consensus or a third party. Outcomes of interest were mortality rate, complications greater than or equal to grade III according to Clavien–Dindo classification11, postoperative pancreatic fistula (POPF)12, post-pancreatectomy haemorrhage (PPH)13, delayed gastric emptying (DGE)14, bile leakage15, surgical site infection (SSI), reoperations, readmissions, R0 resection, lymph node yield (LNY), and LOS. Safety outcomes were examined 90 days after surgery. Definitions of the International Study Group of Pancreatic Surgery for pancreatic surgery specific complications were applied.
Critical appraisal
The assessment of methodological quality of included trials was done according to The Cochrane Collaboration tool for assessing risk of bias 2.09. In summary, five different domains were assessed for all types of bias that are currently understood: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in selection of the reported result.
Each domain was assigned one of three levels of risk of bias (‘low-risk’, ‘some concern’, and ‘high-risk’), determined by an algorithm based on answers to signalling questions. In conclusion, an overall risk-of-bias assessment was achieved. For assessment of certainty in the body of evidence, the GRADE approach was used16.
Statistical analysis
Data pooling and statistical analysis were performed using review manager software (Review Manager (RevMan) (computer program), Version 5.4, The Cochrane Collaboration, 2020). All categorical data were analysed using the Mantel–Haenszel model and are presented as odds ratio (OR) and 95 per cent c.i. For all continuous data, the mean difference (MD) and 95 per cent c.i. were calculated using the inverse variance model. Non-normally distributed data were converted to mean and standard deviation (s.d.) according to Hozo et al.17. Heterogeneity among trials was assessed using the I2 test and random-effects model was used. All reported P-values are two-sided.
Results
Study selection
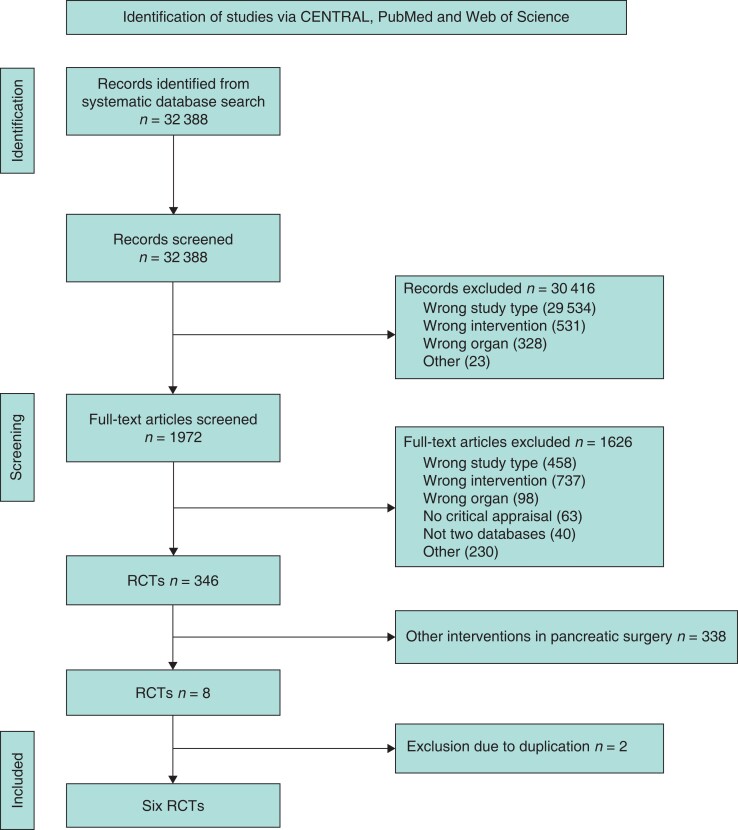
In a systematic literature search, a total of 32 388 studies were found and screened for eligibility. After title, abstract, and full-text screening, six RCTs were included for further analysis18–23. Reasons for exclusion included wrong study type, wrong intervention, and wrong organ investigated. Two studies were excluded due to data duplication24,25. The flow chart in Fig. 1 depicts the process of study selection according to PRISMA6,7. An overview of study characteristics is displayed in Table 1.
Fig. 1.
PRISMA flow chart
Table 1.
Study characteristics
| First author | Year published | Country | Design | Primary endpoint | Sample size | Method of analysis | Centre experience | Surgeon experience |
|---|---|---|---|---|---|---|---|---|
| DP | ||||||||
| Björnsson | 2020 | Sweden | RCT | Length of postoperative hospital stay | 58 | ITT | ns | ≥37 lap. DP |
| de Rooij | 2019 | Netherlands | RCT | Time to functional recovery | 108 | ITT | ≥20 PDs/year | ≥50 advanced MI procedures ≥20 DP (MI or open) ≥5 MI DP |
| PD | ||||||||
| Palanivelu | 2017 | India | RCT | Length of hospital stay | 64 | ITT | ≥40 PDs/year | ≥25 open PD ≥25 lap. PD |
| Poves | 2018 | Spain | RCT | Length of hospital stay | 61 | ITT | Expert surgeon | Expert surgeon |
| van Hilst | 2019 | Netherlands | RCT | Time to functional recovery | 99 | ITT | ≥20 PDs/year (of which ≥10 lap.) | ≥50 advanced lap. procedures ≥50 PD (lap. or open) ≥20 lap. PD |
| Wang | 2021 | China | RCT | Length of postoperative stay | 594 | mITT | ≥50 PDs/year (of which ≥20 lap.) | ≥104 lap. PD ≥104 open PD |
DP, distal pancreatectomy; ITT, intention to treat; lap., laparoscopic; PD, partial pancreatoduodenectomy; MI, minimally invasive; mITT, modified intention to treat; ns, not specified.
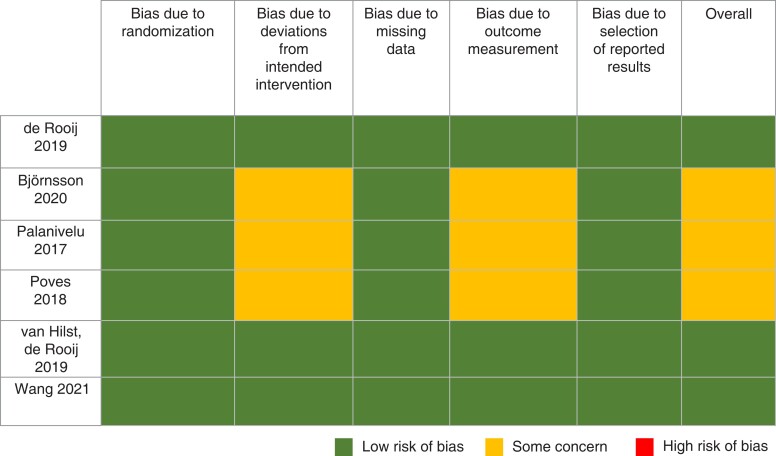
Qualitative analysis—bias
An overview of risk-of-bias assessment according to bias domains and overall assessment is displayed in Fig. 2. None of the studies was considered at high overall risk of bias. In three of six studies the overall risk of bias was considered of some concern, whereas three showed a low overall risk of bias. ‘Deviations from intended intervention’ and ‘outcome measurement’ represented the main sources of bias and received an assessment of some concern in three out of six studies. In one study25, the randomization process revealed some concern for bias.
Fig. 2.
Risk-of-bias assessment
Quantitative analysis
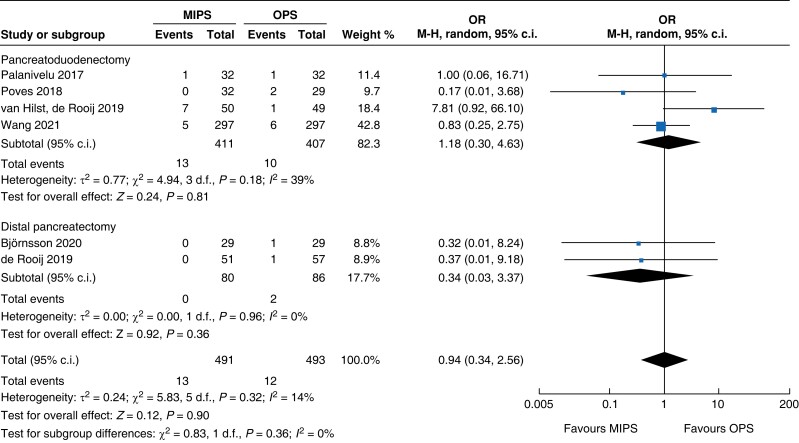
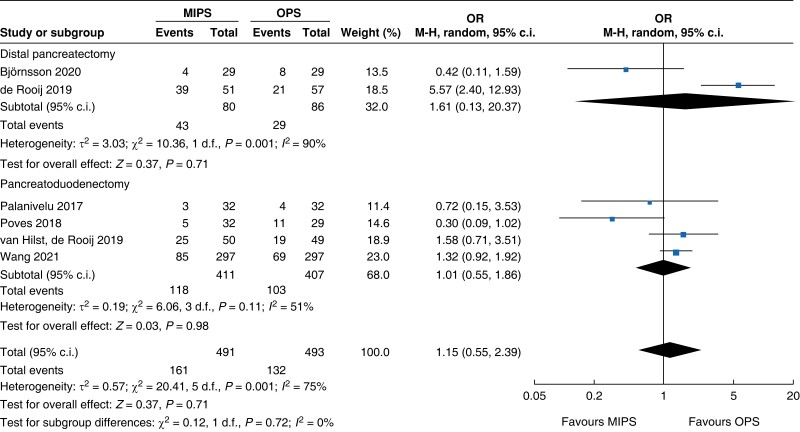
A summary of quantitative outcomes is shown in Table 2. A total of 166 patients for DP (80 minimally invasive pancreatic surgery versus 86 open pancreatic surgery) in two RCTs and 818 patients for PD (411 minimally invasive pancreatic surgery versus 407 open pancreatic surgery) in four RCTs were analysed.
Table 2.
Quantitative outcomes
| 90-day mortality rate |
|
| Certainty of evidence: low |
| Clavien–Dindo ≥grade III |
|
| Certainty of evidence: low |
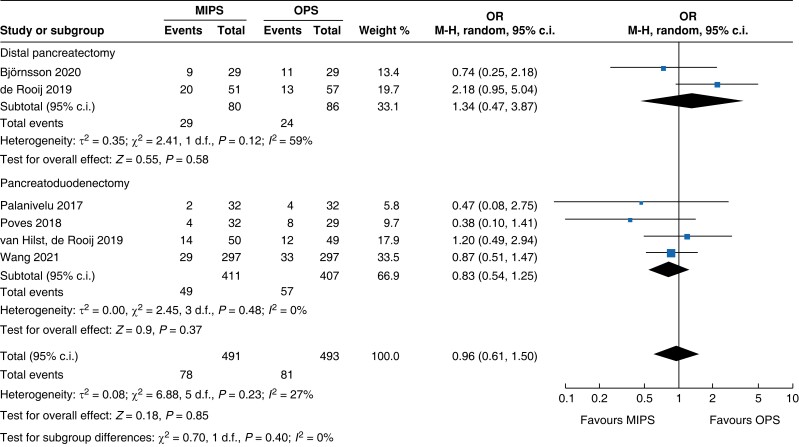
| Postoperative pancreatic fistula |
|
| Certainty of evidence: moderate |
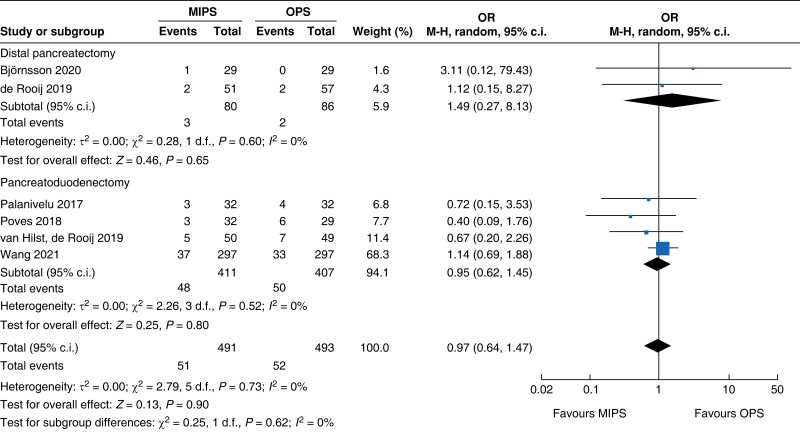
| Post-pancreatectomy haemorrhage |
|
| Certainty of evidence: moderate |
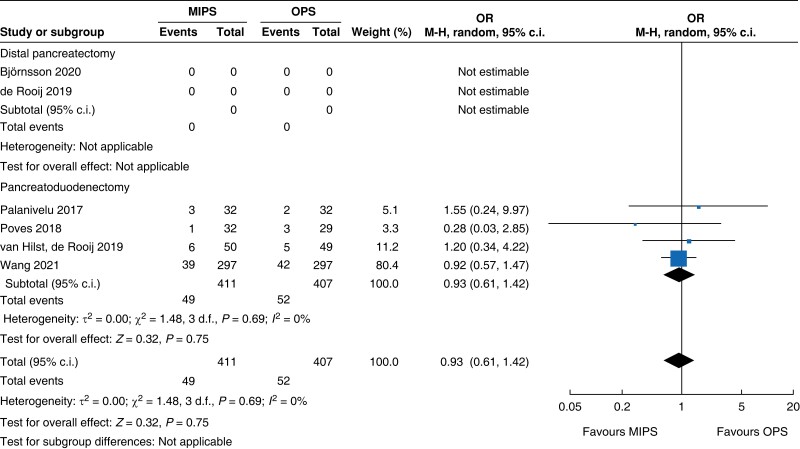
| Bile leakage |
|
| Certainty of evidence: moderate |
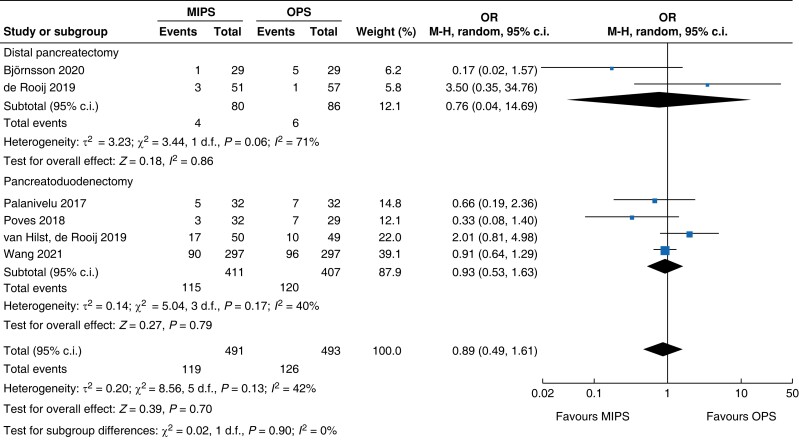
| Delayed gastric emptying |
|
| Certainty of evidence: low |
| Surgical site infection |
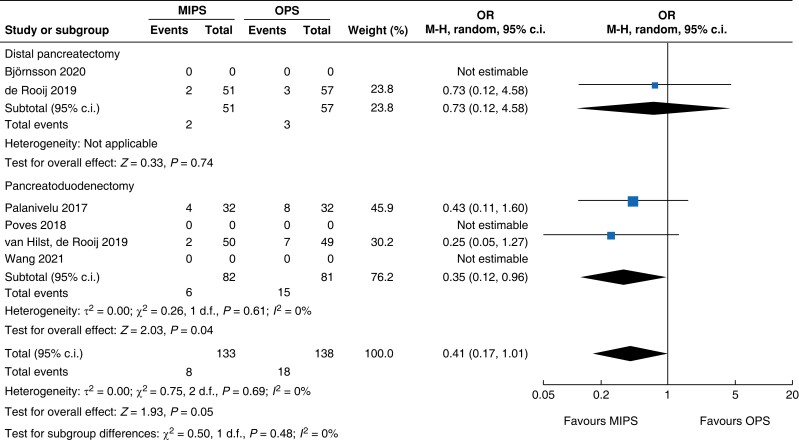
|
| Certainty of evidence: low |
| Reoperation |
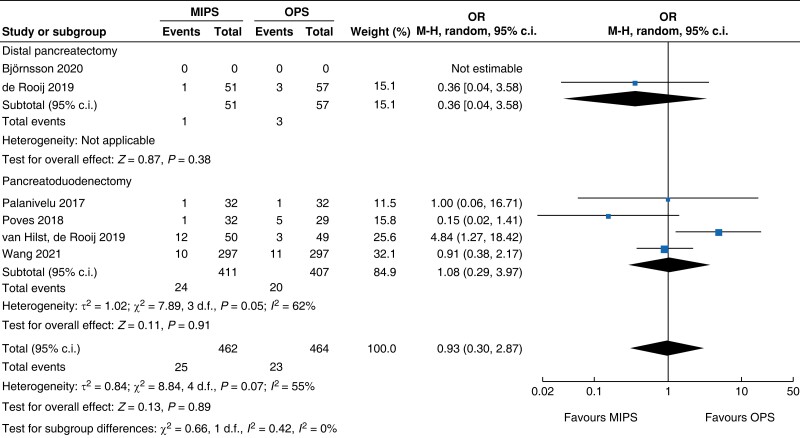
|
| Certainty of evidence: low |
| Readmission |
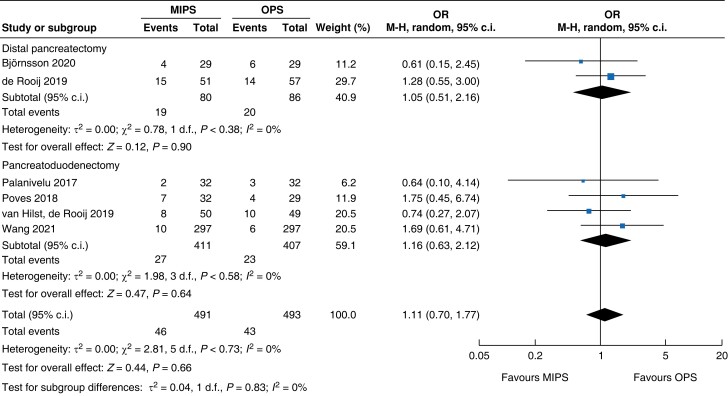
|
| Certainty of evidence: low |
| Blood loss |
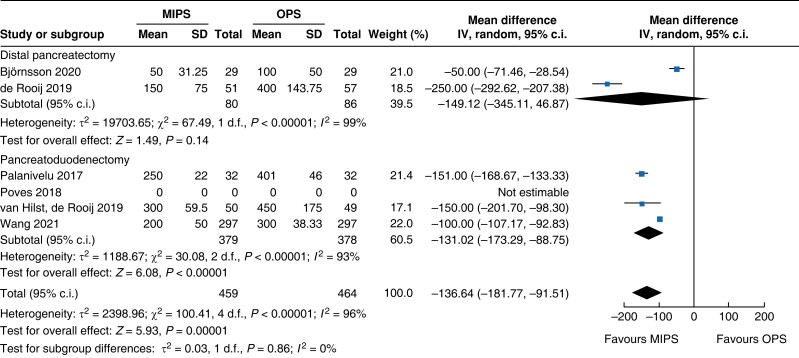
|
| Certainty of evidence: low |
| Duration of surgery |
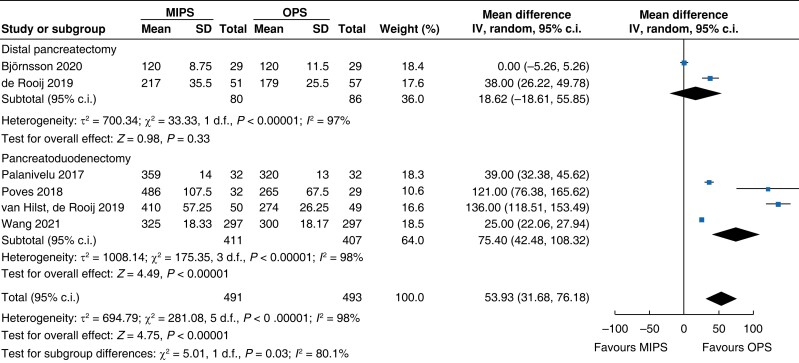
|
| Certainty of evidence: low |
| R0 resection |
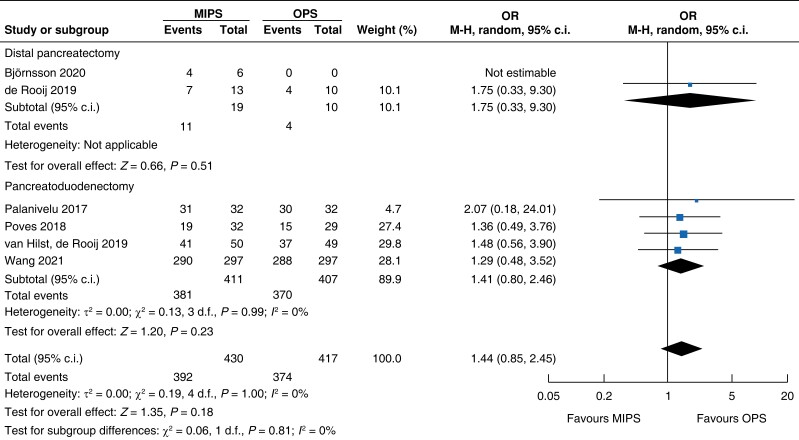
|
| Certainty of evidence: low |
| Lymph node yield |
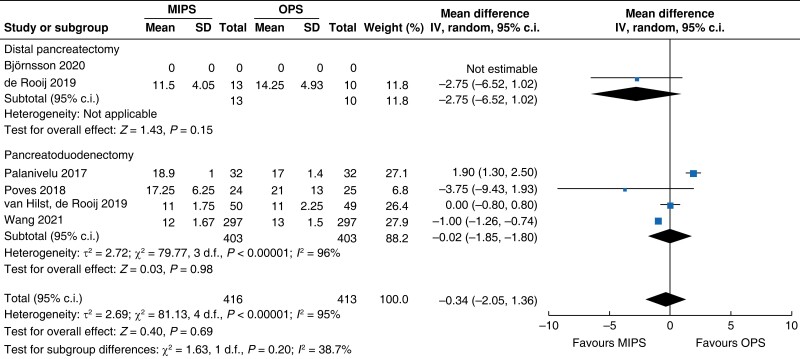
|
| Certainty of evidence: low |
| Length of hospital stay |
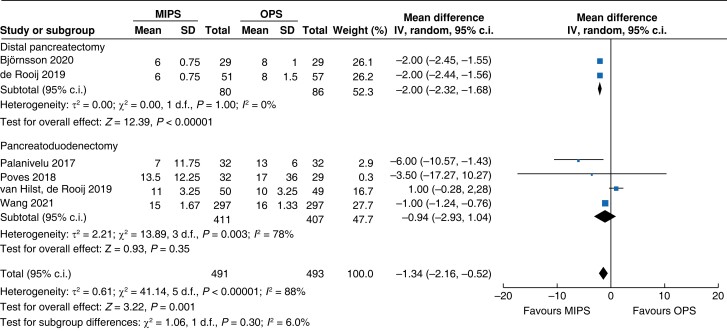
|
| Certainty of evidence: low |
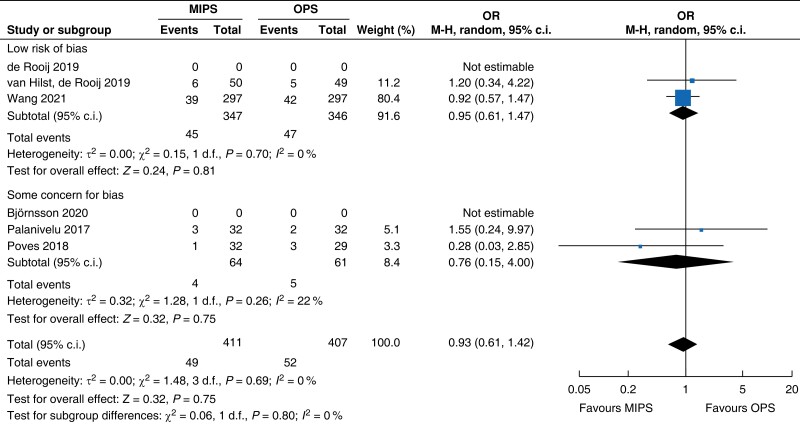
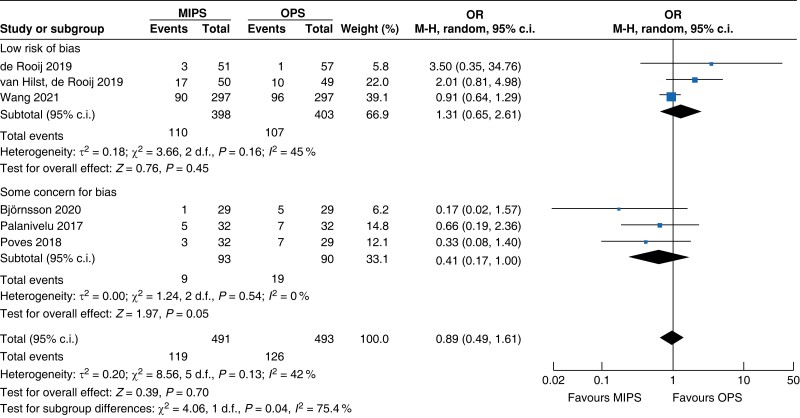
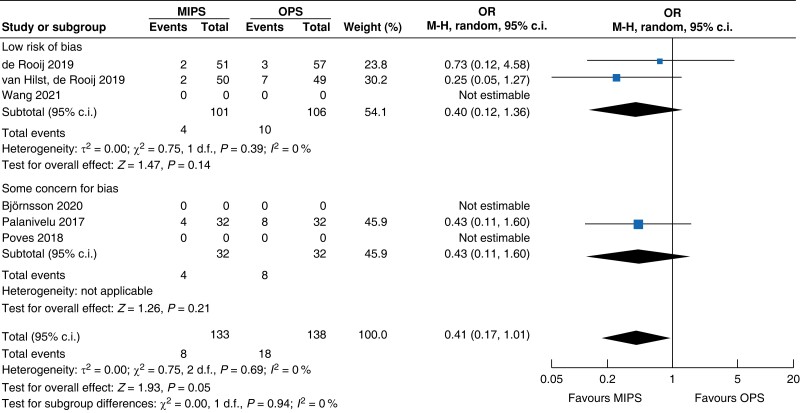
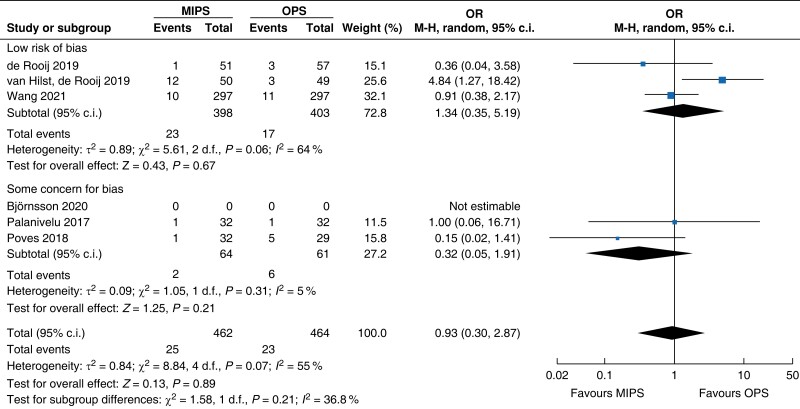
MIPS, minimally invasive pancreatic surgery; OPS, open pancreatic surgery; OR, odds ratio.
Intraoperative outcomes
Intraoperative blood loss was decreased by 137 ml (−182 to −92, P < 0.001, I2 = 96 per cent, GRADE: low) in minimally invasive surgery. Duration of surgery increased in minimally invasive pancreatic surgery by 54 min (32 to 76, P < 0.001, I2 = 98 per cent, GRADE: low) compared with open pancreatic surgery.
Postoperative outcomes
An overall 90-day mortality rate of 2.6 per cent (13) for minimally invasive pancreatic surgery and 2.4 per cent (12) for open pancreatic surgery without a significant difference between the two groups was reported (OR (95 per cent c.i.) 0.94 (0.34 to 2.56), P = 0.900). Neither overall nor pancreatic surgery specific complications differed between the two groups. The incidence of complications of greater than or equal to grade III according to the Clavien–Dindo classification was 32.8 per cent (161) in the minimally invasive group and 26.8 per cent (132) in the open group (OR (95 per cent c.i.) 1.15 (0.55 to 2.39), P = 0.710). Overall, readmissions and reoperations were necessary in 9.0 per cent (89; minimally invasive pancreatic surgery 9.4 per cent, 46; and open pancreatic surgery 8.7 per cent, 43) and 5.2 per cent (48; minimally invasive pancreatic surgery 5.4 per cent, 25; and open pancreatic surgery 5.0 per cent, 23) of patients respectively. No significant difference between minimally invasive pancreatic surgery and open pancreatic surgery was seen (OR (95 per cent c.i.) 1.11 (0.70 to 1.77) (P = 0.660) and OR (95 per cent c.i.) 0.93 (0.30 to 2.87) (P = 0.890) respectively). Meta-analysis showed a reduction in LOS of 1.3 days (−2 to −0.5, P < 0.001) in the minimally invasive group compared with the open group.
Oncologic outcomes
Surgical oncologic outcomes did not differ between the two groups. R0 resection was achieved in 91.2 per cent (392) in the minimally invasive pancreatic surgery group and 89.7 per cent (374) in the open pancreatic surgery group (OR (95 per cent c.i.) 1.44 (0.85 to 2.45), P = 0.180, I2 = 0 per cent, GRADE: low); no difference in LNY was seen (MD (95 per cent c.i.) 0.0 (−2 to 1), P = 0.690, I2 = 95 per cent, GRADE: low). Overall, the mean (s.d.) number of lymph nodes resected was 13 (3) in the minimally invasive pancreatic surgery group and 14 (4) in the open pancreatic surgery group.
Subgroup analysis
An overview of the main outcomes and procedural subgroup analysis is shown in Table 3 and Table 4. In the subgroup analysis, reduction in LOS was only present in minimally invasive DP (−2 days, −2.3 to −1.7, P < 0.01, I2 = 0 per cent, GRADE: low). A minimally invasive approach showed reductions in SSI (OR 0.4 (0.1 to 0.96), P = 0.04, I2 = 0 per cent, GRADE: low) and intraoperative blood loss (−131 ml, −173 to −89, P < 0.01, I2 = 93 per cent, GRADE: low) only in PD. However, the duration of surgery was about 75 min longer in minimally invasive PD (42 to 108 min, P < 0.01, I2 = 99 per cent, GRADE: low), but not in DP. Analysis showed no further significant differences between subgroups with regards to mortality rate, general and pancreatic surgery specific complications, and intraoperative and oncologic outcomes.
Table 3.
Subgroup analysis of categorical outcomes
| Outcomes | PD | DP | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | MIPS | OPS | OR (95% c.i.) | n | MIPS | OPS | OR (95% c.i.) | n | MIPS | OPS | OR (95% c.i.) | |
| Mortality rate | 818 | 13 (3.2) | 10 (2.5) | 1.18 (0.30 to 4.63) | 166 | 0 (0) | 2 (2.3) | 0.34 (0.03 to 3.37) | 984 | 13 (2.6) | 12 (2.4) | 0.94 (0.34 to 2.56) |
| Clavien–Dindo ≥grade III | 818 | 118 (28.7) | 103 (25.3) | 1.01 (0.55 to 1.86) | 166 | 43 (53.8) | 29 (33.7) | 1.61 (0.13 to 20.37) | 984 | 161 (32.8) | 132 (26.8) | 1.15 (0.55 to 2.39) |
| POPF | 818 | 49 (11.9) | 57 (14.0) | 0.83 (0.54 to 1.25) | 166 | 29 (36.3) | 24 (27.9) | 1.34 (0.47 to 3.87) | 984 | 78 (15.9) | 81 (16.4) | 0.96 (0.61 to 1.50) |
| PPH | 818 | 48 (11.7) | 50 (12.3) | 0.95 (0.62 to 1.45) | 166 | 3 (3.8) | 2 (2.3) | 1.49 (0.27 to 8.13) | 984 | 51 (10.4) | 52 (10.5) | 0.97 (0.64 to 1.47) |
| DGE | 818 | 115 (27.9) | 120 (29.5) | 0.93 (0.53 to 1.63) | 166 | 4 (5.0) | 6 (7.0) | 0.76 (0.04 to 14.69) | 984 | 119 (24.2) | 126 (25.6) | 0.89 (0.49 to 1.61) |
| Bile leakage | 818 | 49 (11.9) | 52 (12.8) | 0.93 (0.61 to 1.42) | ns | ns | ns | ns | 818 | 49 (11.9) | 52 (12.8) | 0.93 (0.61 to 1.42) |
| SSI | 163 | 6 (7.3) | 15 (18.5) | 0.35 (0.12 to 0.96) | 108 | 2 (3.9) | 3 (5.3) | 0.73 (0.12 to 4.58) | 271 | 8 (6) | 18 (13.0) | 0.41 (0.17 to 1.01) |
| Readmission | 818 | 27 (6.6) | 23 (5.7) | 1.16 (0.63 to 2.12) | 166 | 19 (23.8) | 20 (23.3) | 1.05 (0.51 to 2.16) | 984 | 46 (9.4) | 43 (8.7) | 1.11 (0.70 to 1.77) |
| Reoperation | 818 | 24 (5.8) | 20 (4.9) | 1.08 (0.29 to 3.97) | 108 | 1 (2.0) | 3 (5.3) | 0.36 (0.04 to 3.58) | 926 | 25 (5.4) | 23 (5.0) | 0.93 (0.30 to 2.87) |
| R0 resection | 818 | 381 (92.7) | 370 (90.9) | 1.41 (0.80 to 2.46) | 29 | 11 (57.9) | 4 (40.0) | 1.75 (0.33 to 9.30) | 847 | 392 (91.2) | 374 (89.7) | 1.44 (0.85 to 2.45) |
Values are n (%) unless otherwise indicated. PD, partial pancreatoduodenectomy; DP, distal pancreatectomy; MIPS, minimally invasive pancreatic surgery; OPS, open pancreatic surgery; OR, odds ratio; POPF, postoperative pancreatic fistula; PPH, post-pancreatectomy haemorrhage; DGE, delayed gastric emptying; SSI, surgical site infection; ns, not specified.
Table 4.
Subgroup analysis of continuous outcomes
| Outcomes | PD | DP | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | MD (95% c.i.) | P | n | MD (95% c.i.) | P | n | MD (95% c.i.) | P | |
| Duration of surgery (min) | 818 | 75 (42 to 108) | <0.001 | 166 | 19 (−19 to 56) | 0.330 | 984 | 54 (32 to 76) | <0.001 |
| Blood loss (ml) | 757 | −131 (−173 to −89) | <0.001 | 166 | −149 (−345 to 47) | 0.140 | 923 | −137 (−182 to −92) | <0.001 |
| LNY (n) | 806 | 0.0 (−2.0 to 2.0) | 0.980 | 23 | −3 (−7 to 1) | 0.150 | 829 | 0.0 (−2 to 1) | 0.20 |
| LOS (days) | 818 | −0.9 (−2.9 to 1.0) | 0.610 | 166 | −2.0 (−2.3 to −1.7) | <0.001 | 984 | −1.3 (−2.0 to −0.5) | <0.001 |
PD, partial pancreatoduodenectomy; DP, distal pancreatectomy; MD, mean difference; LNY, lymph node yield; LOS, length of hospital stay.
Further subgroup analysis was performed stratifying for studies assessed with a low risk of bias and some concern for bias. In Table 5, detailed results after risk-of-bias stratification are displayed. A separate analysis of studies with a low risk of bias showed no differences between minimally invasive pancreatic surgery and open pancreatic surgery regarding general and pancreatic surgery specific morbidity rate, as well as oncologic outcomes. Reduction in blood loss in minimally invasive pancreatic surgery compared with open pancreatic surgery could be confirmed in low-risk studies by a mean of −165 ml (−262 to −69, P < 0.001, I2 = 96 per cent, GRADE: low), with longer operative time by a mean of 66 min (14 to 117, P = 0.010, GRADE: low). The reduction in LOS previously reported in the minimally invasive group compared with the open group could not be confirmed in the low-risk subgroup (−0.9 days, −1.9 to 0.2, P = 0.100, I2 = 92 per cent, GRADE: low).
Table 5.
Subgroup analysis of risk-of-bias stratification
| 90-day mortality rate |
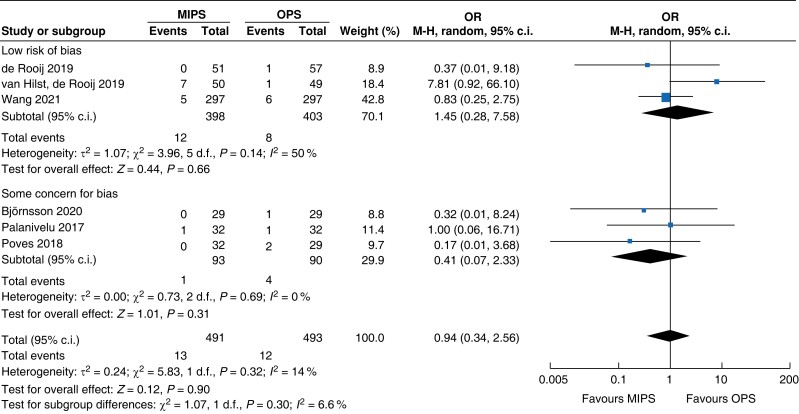
|
| Certainty of evidence: low |
| Clavien–Dindo ≥3 grade III |
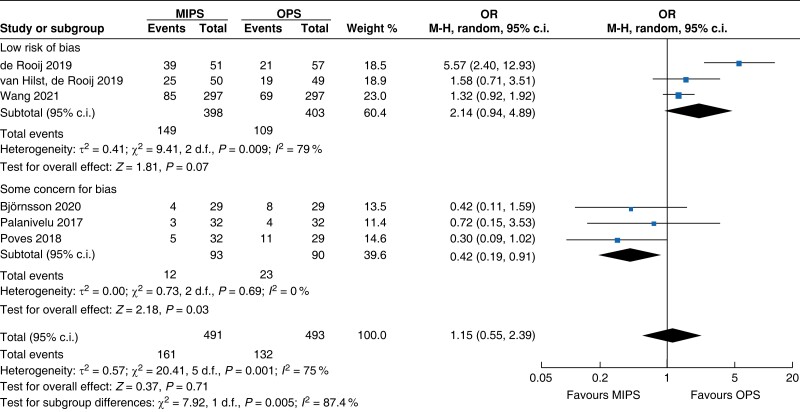
|
| Certainty of evidence: low |
| Postoperative pancreatic fistula |
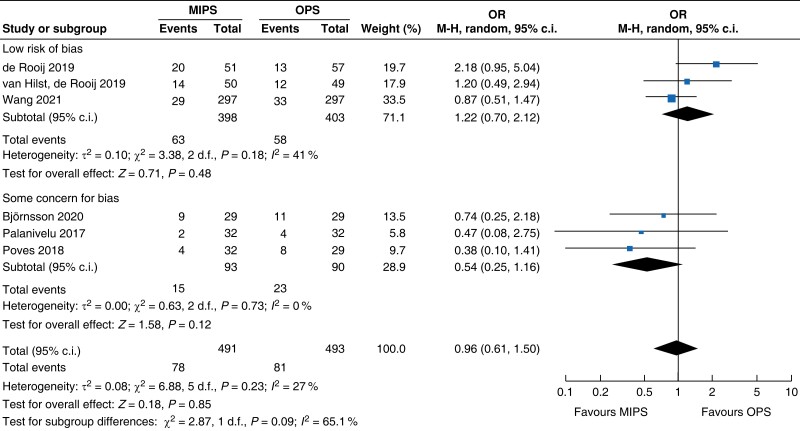
|
| Certainty of evidence: moderate |
| Post-pancreatectomy haemorrhage |
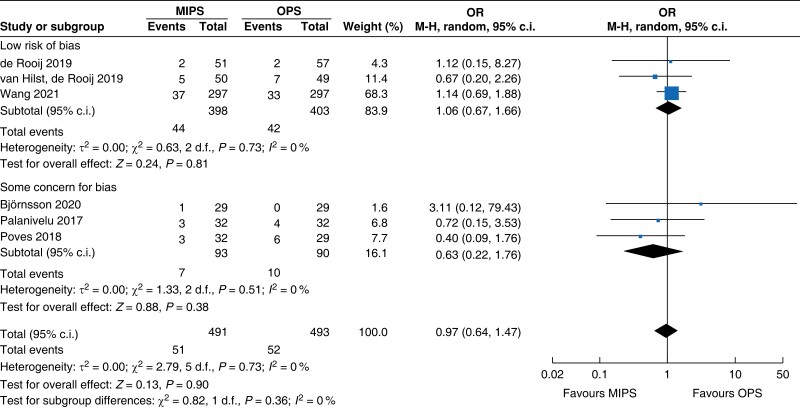
|
| Certainty of evidence: moderate |
| Bile leakage |
|
| Certainty of evidence: moderate |
| Delayed gastric emptying |
|
| Certainty of evidence: low |
| Surgical site infection |
|
| Certainty of evidence: low |
| Reoperation |
|
| Certainty of evidence: low |
| Readmission |
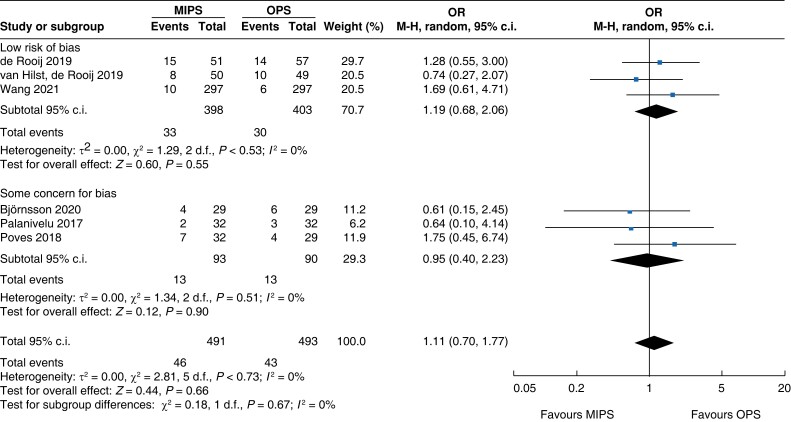
|
| Certainty of evidence: low |
| Blood loss |
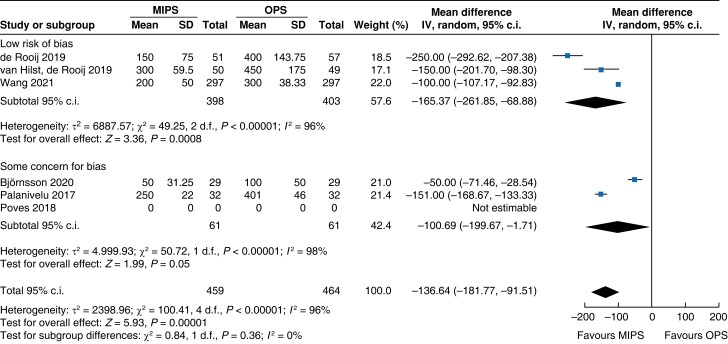
|
| Certainty of evidence: low |
| Duration of surgery |
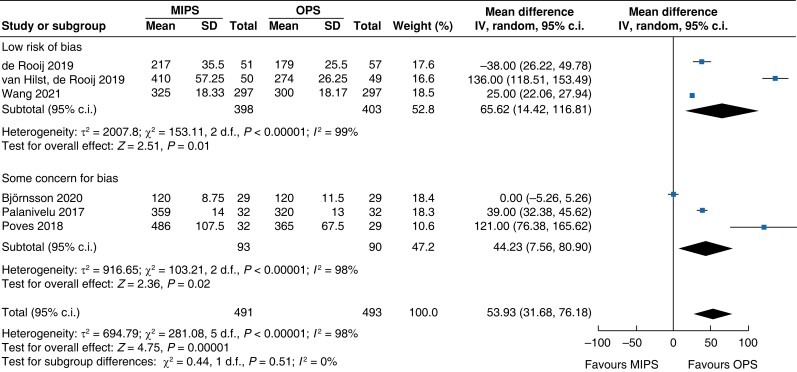
|
| Certainty of evidence: low |
| R0 resection |
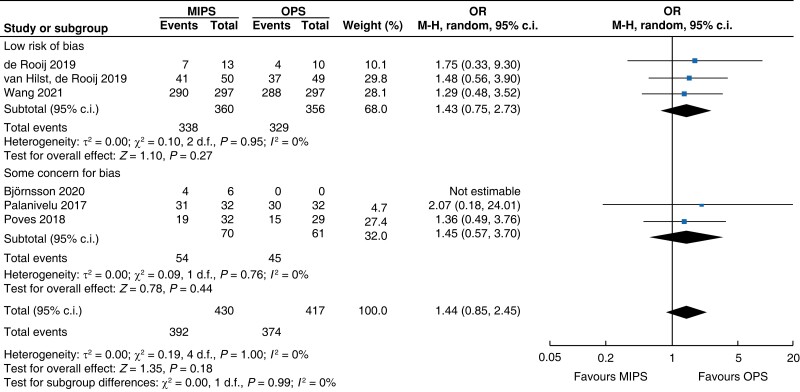
|
| Certainty of evidence: low |
| Lymph node yield |
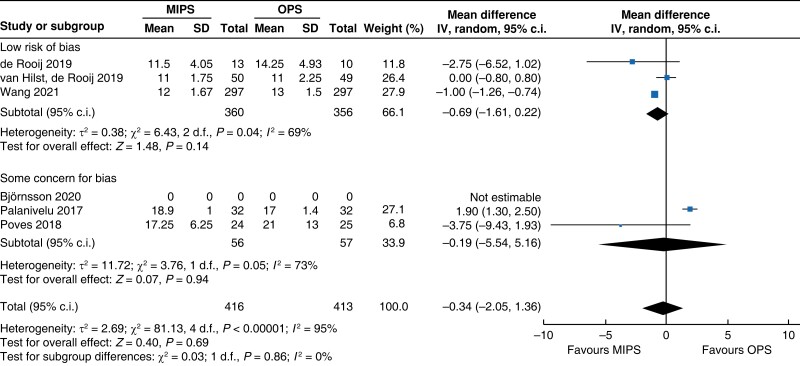
|
| Certainty of evidence: low |
| Length of hospital stay |
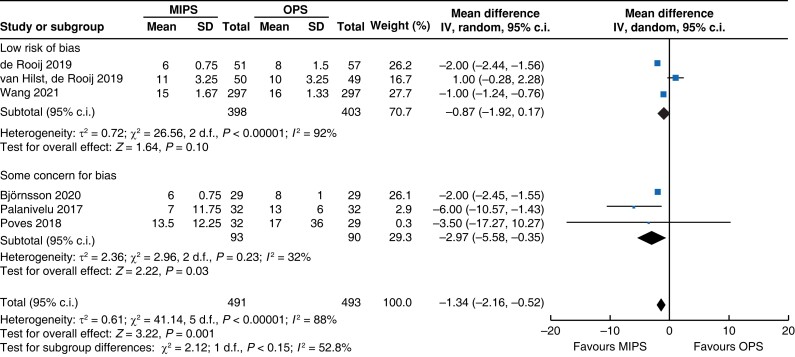
|
| Certainty of evidence: low |
MIPS, minimally invasive pancreatic surgery; OPS, open pancreatic surgery; OR, odds ratio.
Ongoing trials
A total of 12 ongoing RCTs comparing minimally invasive with open PD and eight ongoing RCTs comparing minimally invasive with open DP were found in a systematic search in the World Health Organization trial registry. An overview of trials and their expected termination is shown in Table 6.
Table 6.
Ongoing RCTs and unpublished terminated RCTs
| Identifier | Title | Expected end of trial |
|---|---|---|
| Partial pancreatoduodenectomy | ||
| NCT03785743 | Comparing laparoscopic and open surgery for pancreatic carcinoma | 1 March 2026 |
| NCT04171440 | Comparison of perioperative outcomes between minimally invasive and open pancreaticoduodenectomy | 1 July 2024 |
| ChiCTR1900024788 | Robotic pancreaticoduodenectomy (RPD) versus open pancreaticoduodenectomy (OPD) in the long-term oncologic outcomes (LR301PD1): a randomized controlled trial | 1 September 2021 |
| NCT03870698 | Comparison of functional recovery between laparoscopic and open pancreaticoduodenectomy | 1 July 2021 |
| NCT03747588 | The comparison of laparoscopic and open pancreaticoduodenectomy for pancreatic cancer (LOPA) | 30 December 2020 |
| NCT03138213 | Comparing total laparoscopic versus open pancreaticoduodenectomy | 1 September 2020 |
| NCT03722732 | Comparison of blood loss in laparoscopic versus open pancreaticoduodenectomy in patients with periampullary carcinoma | 1 December 2019 |
| DRKS00020407 | Evaluation of robotic versus open partial pancreaticoduodenectomy of a randomized controlled trial (EUROPA) | Not reported |
| NCT04400357 | Robotic versus open pancreaticoduodenectomy for pancreatic and periampullary tumours (PORTAL) | Not reported |
| ChiCTR1900028686 | A prospective randomized controlled trial for the effects of laparoscopic and non-laparoscopic surgery on pancreas islet function | Not reported |
| ChiCTR2000038932 | Robotic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, patient-blinded, randomized controlled trial | Not reported |
| Distal pancreatectomy | ||
| NCT03957135 | Laparoscopic versus open distal pancreatectomy for pancreatic cancer: a multicentre randomized controlled trial | 30 November 2025 |
| NCT04483726 | Distal pancreatectomy, minimally invasive or open, for malignancy (DIPLOMA) | 9 July 2025 |
| ISRCTN44897265 | Distal pancreatectomy, minimally invasive or open, for malignancy | 1 May 2024 |
| KCT0004176 | Multicentre prospective randomized controlled clinical trial for comparison between laparoscopic and open distal pancreatectomy for ductal adenocarcinoma of the pancreatic body and tail | 30 November 2023 |
| NCT03792932 | Laparoscopic versus open pancreatectomy for body and tail pancreatic cancer | 31 January 2022 |
| ChiCTR1900024648 | A randomized controlled study for the short-term oncologic outcomes of robot-assisted radical and open anterograde modular pancreaticosplenectomy | 30 November 2020 |
| DRKS00014011 | Distal pancreatectomy of a randomized controlled trial to compare open versus laparoscopic resection (DISPACT 2-TRIAL) | Not reported |
| ChiCTR2000038933 | Robotic versus open radical antegrade modular pancreatosplenectomy for pancreatic cancer of the body and tail: a multicentre, randomized controlled trial | Not reported |
Discussion
The present systematic review and meta-analysis of all currently available RCTs comparing minimally invasive (laparoscopic and robotic) and open pancreatic surgery showed no significant difference between the minimally invasive and open approaches regarding 90-day mortality rate, as well as general and pancreatic surgery specific morbidity rate. However, overall reductions in intraoperative blood loss and LOS using the minimally invasive approach were seen. On the other hand, a longer duration of surgery was reported for minimally invasive pancreatic surgery. Taking the subgroup analysis into account, decreased intraoperative blood loss, reduction in SSI, and longer duration of surgery were only present in PD. Similarly, reduction in LOS was reported only in DP, not PD. Apart from reduced LOS using the minimally invasive approach, these results were confirmed in a subgroup of studies with a low risk of bias.
None of the studies was considered to be at high overall risk of bias. Some concern for bias was present in the domains ‘deviations from intended intervention’ and ‘outcome measurement’ in half of the studies. In the other half of the studies, the overall risk of bias was considered low. After critical appraisal, the certainty of evidence of outcomes was only low to moderate.
In the past, safety concerns, especially regarding minimally invasive PD, have been expressed in line with the results of early observational studies26,27. The highly complex surgical technique and consecutive long learning curves were regarded as presumable reasons28. On the other hand, higher mortality rate in minimally invasive PD was considered the result of a surmountable learning curve with supposedly comparable safety outcomes after its completion29. Meanwhile, centre volume and surgeon experience remain crucial for favourable postoperative safety and efficacy outcomes in high-risk and pancreatic surgery30–33. In a study performed by Sharpe et al.29 reporting data from a nationwide database in the USA comparing laparoscopic with open pancreatoduodenectomy, a more than two-fold increased risk of mortality rate was found for the laparoscopic approach compared with the open approach in centres performing fewer than ten laparoscopic PDs/2 years. In larger-volume centres (greater than or equal to ten laparoscopic PDs/2 years) no difference in 30-day mortality rate between the laparoscopic and open approaches was reported29. All studies in this meta-analysis subjectively reported high levels of surgeon experience in pancreatic and minimally invasive surgery. The minimal number of laparoscopic DPs required was 3718 and five19. In PD, at least 2520, 2022 and 10423 minimally invasive procedures per participating surgeon were required before the start of the study. Further, several authors described standardized training programmes as eligibility criteria34,35. A centre volume of at least 20–50 PDs annually was reported, with a minimum of 10–20 being done laparoscopically.
Whereas the importance of centre and surgeon volume is already undebated in successful open pancreatic surgery, its role in minimally invasive surgery appears to be at least as essential in achieving acceptable postoperative results29. Further, data on the standardized assessment of a learning curve are still missing. An attempt to define median learning curves for minimally invasive pancreatic surgery was recently done in a systematic review by Fung et al.,36 reporting a median learning curve in DP of 17 (10–30) and 23.5 (7–40) cases for laparoscopic and robotic procedures respectively. The median learning curve for PD was reported to have been achieved at 30 (4–60) cases for the laparoscopic approach and 36.5 (20–80) cases for the robotic approach36. Comparing surgeon experience reported in the included studies with extrapolated learning curves from the literature28,36, it becomes evident that not all surgeons reached sufficient expertise in minimally invasive pancreatic surgery before the start of the investigation. Nevertheless, postoperative mortality rate and morbidity rate between minimally invasive and open pancreatic surgery seem to be comparable. Taking surrogate outcomes into account, a minimally invasive approach even seems to be beneficial compared with open surgery, with regard to intraoperative blood loss, SSI, and LOS. A word of caution is necessary with regards to intraoperative blood loss, as inconsistency between different evaluation methods has been reported in pancreatic surgery37.
However, data on compelling benefits of a minimally invasive approach known from other fields of surgery, such as in general, bariatric, and lower and upper gastrointestinal surgery, are still missing in pancreatic surgery. Meanwhile, the question of safe implementation of the minimally invasive technique in pancreatic surgery remains a matter of debate. Müller et al.28 suggested a stepwise introduction of different pancreatic resections according to the procedural complexity and standardized reporting of learning curves to reduce learning curve-related bias. Adequate assessment of baseline surgeon experience and skill level, and standardized reporting of learning curves within a three-phase model (competency, proficiency, and mastery) was suggested. Meanwhile, adequate case selection with regards to favourable anatomical (low BMI) and disease specific (no vessel involvement) features in a first learning phase under supervision to reach competency level seems of paramount importance. With increasing experience, proficiency and mastery levels are reached and more complex procedures may be introduced with the goal to achieve benchmark outcomes28,38. Furthermore, several initiatives for collaborative research on safe implementation of the minimally invasive technique in pancreatic surgery, such as the European Consortium of Minimally Invasive Pancreatic Surgery (E-MIPS), have emerged39,40. Additionally, efforts to provide international evidence-based guidelines for minimally invasive pancreatic resections and the implementation of the minimally invasive approach to obtain optimal patient outcomes and safety have been made41.
Additionally, in comparison with benchmark criteria in open pancreatic surgery defined by Sánchez-Velázquez et al.42 and Probst et al.6, postoperative safety outcomes from the current meta-analysis appear within benchmark cut-offs. Recently published analyses further report benchmark criteria for minimally invasive and open DP38,43,44. Benchmark cut-off values for mortality rate in PD were set at less than or equal to 1.6 per cent and 2 per cent by Sánchez-Velázquez et al.42 and Probst et al.6 respectively. In DP, Probst et al.6 reported a benchmark cut-off of 1 per cent. A slightly elevated overall 90-day mortality rate in minimally invasive PD (3.2 per cent), as well as in the open procedure (2.5 per cent), was found in the current study. On the other hand, reduced mortality rate compared with the benchmark cut-off was reported in minimally invasive DP (0 per cent), though without a significant difference compared with open DP in the current study. The discrepancy can most likely be explained by insufficient surgeon and centre experience, as described above. Interestingly, when examining only the two studies with completed learning curves before study start18,23, postoperative safety outcomes even undercut benchmark cut-offs. Furthermore, 90-day mortality rate cannot be directly compared with in-hospital mortality rate, as presented by Sánchez-Velázquez et al.42 Other general and pancreatic surgery specific morbidity rate outcomes met the predefined benchmark cut-offs. In the current study, a POPF incidence of 11.9 per cent was seen in minimally invasive PD. The benchmark cut-off is defined as less than or equal to 19 per cent by Sánchez-Velázquez et al.42 and 14 per cent by Probst et al.6 In DP, an elevated POPF incidence of 36.3 per cent compared with the benchmark outcome (less than or equal to 8.3 per cent to less than or equal to 32 per cent) without a significant difference compared with open DP was reported. Björnsson et al.18 postulated that this difference derives from the multicentre nature of the study with the inclusion of low-volume centres. de Rooij et al19. included a subgroup of patients with prolonged percutaneous drainage due to biochemical leakage, leading to an increased POPF incidence.
Focusing on data comparing different minimally invasive techniques (laparoscopic versus robotic), superior results in terms of spleen preservation rate, conversion rate, blood loss, and LOS, at the price of higher economic burden, have been reported in DP45–48. However, no RCTs on this topic are available and high-quality data are still missing. Data are even more scarce on robotic versus laparoscopic PD. In low-quality evidence no difference in clinically relevant outcomes has been seen so far49–51. Even though no definite conclusion can be drawn from low-quality evidence, robotic surgery might represent a valid alternative minimally invasive approach to laparoscopy in DP in experienced hands. However, head-to-head comparison in RCTs is required in the future to further address this question.
The current study has some limitations. First, data from only two RCTs with a small sample size in DP were available. Additionally, not all outcomes were investigated in all the studies, leading to a further decrease in reported events for certain outcome parameters (SSI and oncologic outcomes). Inconsistent definitions of R0 resection in the different RCTs further limit a conclusive statement on oncologic outcome parameters. Moreover, the high grade of heterogeneity between the RCTs needs to be considered when interpreting the results. As minimally invasive data of the current meta-analysis mainly related to the laparoscopic approach (99 per cent, 491), possible benefits resulting from robotic surgery are not displayed.
In summary, data from the current meta-analysis support the assumption that a minimally invasive (laparoscopic and robotic) approach in pancreatic surgery seems feasible and safe. Even though high-quality data after surgeons have surmounted the learning curve are still missing, one might expect superior postoperative outcomes in minimally invasive pancreatic surgery compared with open pancreatic surgery, like in other fields of surgery. However, a tailored approach regarding surgical technique might represent the preferred strategy in the future. Whereas for procedures of high complexity, including multi-visceral resections and vascular reconstructions, the open approach may appear favourable, low-risk patients might benefit from the advantages of minimally invasive surgery in less complex cases. The spectrum of the minimally invasive approach is increasing and, as evidence from mainly retrospective analyses suggests, robotic surgery with its benefits compared with laparoscopy may play a fundamental role in pancreatic resections in the future, tackling the technical issues brought forward in early laparoscopic experience in pancreatic surgery. Furthermore, despite comparable oncologic outcomes between minimally invasive pancreatic surgery and open pancreatic surgery, such as R0 resection and LNY, the minimally invasive technique might be able to improve the oncologic big picture in the future. When projecting improved recovery after surgery on patients undergoing pancreatic procedures for malignant disease, more patients might be able to benefit from adjuvant therapy. Nevertheless, the certainty of evidence remains low to moderate and more RCTs after the learning curve are needed to clarify the role of a minimally invasive approach in pancreatic surgery.
Supplementary Material
Contributor Information
Matthias Pfister, Department of Surgery, Cantonal Hospital Thurgau, Frauenfeld, Switzerland; Department of Surgery and Transplantation, University Hospital Zurich, Zurich, Switzerland.
Pascal Probst, Department of Surgery, Cantonal Hospital Thurgau, Frauenfeld, Switzerland.
Philip C Müller, Department of Surgery and Transplantation, University Hospital Zurich, Zurich, Switzerland.
Pia Antony, Department of Surgery, Cantonal Hospital Thurgau, Frauenfeld, Switzerland.
Rosa Klotz, The Study Centre of the German Surgical Society (SDGC), University of Heidelberg, Heidelberg, Germany.
Eva Kalkum, The Study Centre of the German Surgical Society (SDGC), University of Heidelberg, Heidelberg, Germany.
Daniela Merz, The Study Centre of the German Surgical Society (SDGC), University of Heidelberg, Heidelberg, Germany.
Pietro Renzulli, Department of Surgery, Cantonal Hospital Thurgau, Münsterlingen, Switzerland.
Fabian Hauswirth, Department of Surgery, Cantonal Hospital Thurgau, Münsterlingen, Switzerland.
Markus K Muller, Department of Surgery, Cantonal Hospital Thurgau, Frauenfeld, Switzerland; Department of Surgery, Cantonal Hospital Thurgau, Münsterlingen, Switzerland.
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The data that support the findings of this study are openly available in CENTRAL, MEDLINE, and Web of Science.
References
- 1. Jaschinski T, Mosch C, Eikermann M, Neugebauer EA. Laparoscopic versus open appendectomy in patients with suspected appendicitis: a systematic review of meta-analyses of randomised controlled trials. BMC Gastroenterol 2015;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;8:CD006231. [DOI] [PubMed] [Google Scholar]
- 3. Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer 2012;3:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet Det al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 2019;380:152–162 [DOI] [PubMed] [Google Scholar]
- 5. Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JRet al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 6. Probst P, Hüttner FJ, Meydan Ö, Abu Hilal M, Adham M, Barreto SGet al. Evidence Map of Pancreatic Surgery-A living systematic review with meta-analyses by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2021;170:1517–1524 [DOI] [PubMed] [Google Scholar]
- 7. Probst P, Hüttner FJ, Meydan Ö, Kalkum E, Kretschmer R, Jensen Ket al. Evidence map of pancreatic surgery: protocol for a living systematic review and meta-analysis. BMJ Open 2019;9:e032353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green SP, Cochrane C. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration, 2021 [Google Scholar]
- 10. World Health Organization . International Clinical Trials Registry Platform (ICTRP). https://www.who.int/clinical-trials-registry-platform (accessed 29 April 2022)
- 11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham Met al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591 [DOI] [PubMed] [Google Scholar]
- 13. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJet al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25 [DOI] [PubMed] [Google Scholar]
- 14. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JRet al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768 [DOI] [PubMed] [Google Scholar]
- 15. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti Let al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688 [DOI] [PubMed] [Google Scholar]
- 16. Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave 2021;21:e8109. [DOI] [PubMed] [Google Scholar]
- 17. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Björnsson B, Larsson AL, Hjalmarsson C, Gasslander T, Sandström P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: randomized controlled trial. Br J Surg 2020;107:1281–1288 [DOI] [PubMed] [Google Scholar]
- 19. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams Fet al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 2019;269:2–9 [DOI] [PubMed] [Google Scholar]
- 20. Palanivelu C, Senthilnathan P, Sabnis SC, Babu NS, Srivatsan Gurumurthy S, Anand Vijai Net al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg 2017;104:1443–1450 [DOI] [PubMed] [Google Scholar]
- 21. Poves I, Burdío F, Morató O, Iglesias M, Radosevic A, Ilzarbe Let al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg 2018;268:731–739 [DOI] [PubMed] [Google Scholar]
- 22. van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MGet al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199–207 [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Li D, Chen R, Huang X, Li J, Liu Yet al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:438–447 [DOI] [PubMed] [Google Scholar]
- 24. Korrel M, Roelofs A, van Hilst J, Busch OR, Daams F, Festen Set al. Long-term quality of life after minimally invasive vs open distal pancreatectomy in the LEOPARD randomized trial. J Am Coll Surg 2021;233:730–739.e9 [DOI] [PubMed] [Google Scholar]
- 25. van Hilst J, Brinkman DJ, de Rooij T, van Dieren S, Gerhards MF, de Hingh IHet al. The inflammatory response after laparoscopic and open pancreatoduodenectomy and the association with complications in a multicenter randomized controlled trial. HPB (Oxford) 2019;21:1453–1461 [DOI] [PubMed] [Google Scholar]
- 26. Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski Pet al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 2015;220:831–838 [DOI] [PubMed] [Google Scholar]
- 27. Fong ZV, Chang DC, Ferrone CR, Lillemoe KD, Fernandez Del Castillo C. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: is this really a short learning curve? J Am Coll Surg 2016;222:209. [DOI] [PubMed] [Google Scholar]
- 28. Müller PC, Kuemmerli C, Cizmic A, Sinz S, Probst P, de Santibanes Met al. Learning curves in open, laparoscopic, and robotic pancreatic surgery: a systematic review and proposal of a standardization. Ann Surg Open 2022;3:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJet al. Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg 2015;221:175–184 [DOI] [PubMed] [Google Scholar]
- 30. Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG. Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg 2017;266:333–338 [DOI] [PubMed] [Google Scholar]
- 31. Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125:250–256 [PubMed] [Google Scholar]
- 32. Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RAet al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg 2011;98:485–494 [DOI] [PubMed] [Google Scholar]
- 33. Macedo FIB, Jayanthi P, Mowzoon M, Yakoub D, Dudeja V, Merchant N. The impact of surgeon volume on outcomes after pancreaticoduodenectomy: a meta-analysis. J Gastrointest Surg 2017;21:1723–1731 [DOI] [PubMed] [Google Scholar]
- 34. de Rooij T, van Hilst J, Boerma D, Bonsing BA, Daams F, van Dam RMet al. Impact of a nationwide training program in minimally invasive distal pancreatectomy (LAELAPS). Ann Surg 2016;264:754–762 [DOI] [PubMed] [Google Scholar]
- 35. de Rooij T, van Hilst J, Topal B, Bosscha K, Brinkman DJ, Gerhards MFet al. Outcomes of a multicenter training program in laparoscopic pancreatoduodenectomy (LAELAPS-2). Ann Surg 2019;269:344–350 [DOI] [PubMed] [Google Scholar]
- 36. Fung G, Sha M, Kunduzi B, Froghi F, Rehman S, Froghi S. Learning curves in minimally invasive pancreatic surgery: a systematic review. Langenbecks Arch Surg 2022;407:2217–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perri G, Marchegiani G, Reich F, Casetti L, Fontana M, Esposito Aet al. Intraoperative blood loss estimation in hepato-pancreato-biliary surgery-relevant, not reported, not standardized: results from a systematic review and a worldwide snapshot survey. Ann Surg 2022; DOI: 10.1097/SLA.0000000000005536[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38. Müller PC, Breuer E, Nickel F, Zani S, Kauffmann E, De Franco Let al. Robotic distal pancreatectomy, a novel standard of care? Benchmark values for surgical outcomes from 16 international expert centers. Ann Surg 2022; DOI: 10.1097/SLA.0000000000005601[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39. van Hilst J, de Rooij T, Klompmaker S, Rawashdeh M, Aleotti F, Al-Sarireh Bet al. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-European propensity score matched study. Ann Surg 2019;269:10–17 [DOI] [PubMed] [Google Scholar]
- 40. Klompmaker S, van Hilst J, Wellner UF, Busch OR, Coratti A, D'Hondt Met al. Outcomes after minimally-invasive versus open pancreatoduodenectomy: a pan-European propensity score matched study. Ann Surg 2020;271:356–363 [DOI] [PubMed] [Google Scholar]
- 41. Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi Aet al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 2020;271:1–14 [DOI] [PubMed] [Google Scholar]
- 42. Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli Net al. Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg 2019;270:211–218 [DOI] [PubMed] [Google Scholar]
- 43. Giani A, van Ramshorst T, Mazzola M, Bassi C, Esposito A, de Pastena Met al. Benchmarking of minimally invasive distal pancreatectomy with splenectomy: European multicentre study. Br J Surg 2022;109:1124–1130 [DOI] [PubMed] [Google Scholar]
- 44. Durin T, Marchese U, Sauvanet A, Dokmak S, Cherkaoui Z, Fuks Det al. Defining benchmark outcomes for distal pancreatectomy: results of a French multicentric study. Ann Surg 2022; DOI: 10.1097/SLA.0000000000005539[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45. Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Assessment of laparoscopic versus open distal pancreatectomy: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol 2022;31:350–358 [DOI] [PubMed] [Google Scholar]
- 46. Rompianesi G, Montalti R, Ambrosio L, Troisi RI. Robotic versus laparoscopic surgery for spleen-preserving distal pancreatectomies: systematic review and meta-analysis. J Pers Med 2021;11:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu YH, Qin YF, Yu DD, Li X, Zhao YM, Kong DJet al. Meta-analysis of short-term outcomes comparing robot-assisted and laparoscopic distal pancreatectomy. J Comp Eff Res 2020;9:201–218 [DOI] [PubMed] [Google Scholar]
- 48. Mavrovounis G, Diamantis A, Perivoliotis K, Symeonidis D, Volakakis G, Tepetes K. Laparoscopic versus robotic peripheral pancreatectomy: a systematic review and meta-analysis. J BUON 2020;25:2456–2475 [PubMed] [Google Scholar]
- 49. Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal Met al. Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol 2020;46:6–14 [DOI] [PubMed] [Google Scholar]
- 50. Kamarajah SK, Bundred JR, Marc OS, Jiao LR, Hilal MA, Manas DMet al. A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB (Oxford) 2020;22:329–339 [DOI] [PubMed] [Google Scholar]
- 51. Aiolfi A, Lombardo F, Bonitta G, Danelli P, Bona D. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg 2021;73:909–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in CENTRAL, MEDLINE, and Web of Science.