Summary
This is a phase II study of PD-1 blockade plus chemoradiotherapy as preoperative therapy for patients with locally advanced or borderline resectable pancreatic cancer (LAPC or BRPC, respectively). Twenty-nine patients are enrolled in the study. The objective response rate (ORR) is 60%, and the R0 resection rate is 90% (9/10). The 12-month progression-free survival (PFS) rate and 12-month overall survival (OS) rate are 64% and 72%, respectively. Grade 3 or higher adverse events are anemia (8%), thrombocytopenia (8%), and jaundice (8%). Circulating tumor DNA analysis reveals that patients with a >50% decline in maximal somatic variant allelic frequency (maxVAF) between the first clinical evaluation and baseline have a longer survival outcome and a higher response rate and surgical rate than those who are not. PD-1 blockade plus chemoradiotherapy as preoperative therapy displays promising antitumor activity, and multiomics potential predictive biomarkers are identified and warrant further verification.
Keywords: preoperative therapy, PD-1 blockade, circulating tumor DNA, locally advanced pancreatic cancer, borderline resectable pancreatic cancer
Graphical abstract
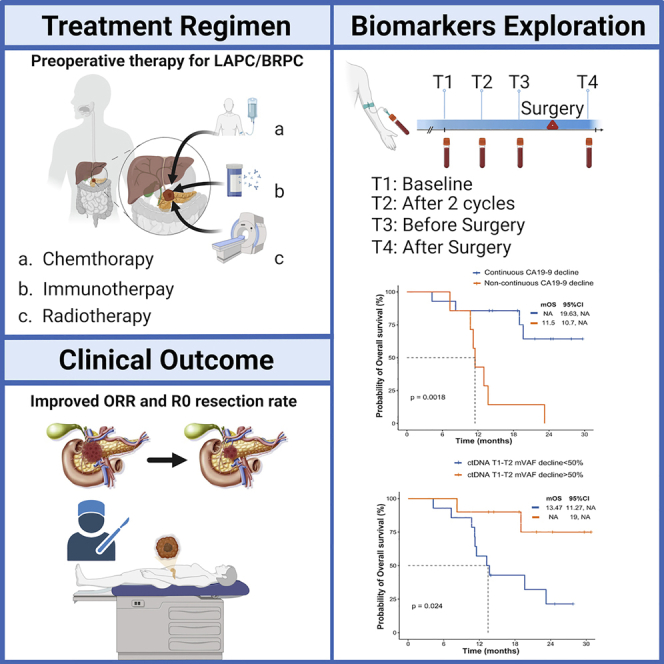
Highlights
-
•
PD-1 blockade plus chemoradiotherapy shows promising antitumor activity for LAPC/BRPC
-
•
PD-1 blockade plus chemoradiotherapy raises ORR and R0 resection rates for LAPC/BRPC
-
•
Patients with a >50% ctDNA decline in maxVAF have a better survival outcome
Du et al. show the efficacy, safety, and predictive biomarkers of PD-1 blockade plus chemotherapy followed by concurrent SBRT with SIB as preoperative therapy for LAPC and BRPC. They report that ctDNA dynamic changes or continuous CA19-9 decline may predict tumor response and survival outcomes.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignant tumor with an overall 5-year survival rate of <10%.1 Research indicates that PDAC may become the second leading cause of cancer-related death by 2030.2 As PDAC is usually occult in onset, its diagnosis is difficult; it is resectable in only 20% of cases.3 Even after surgery, the 5-year survival is low, and the recurrence rate is high, which are associated with surgical margin status and postoperative pathological stage.4 Several clinical trials have demonstrated that adjuvant therapy can prolong the survival of patients after resection.5,6 Meanwhile, R0 resection still appears relevant prognostic after pretreatment, and various studies encourage neoadjuvant and induction therapy that may increase the R0 resection rate and further improve prognosis.7,8 The phase II LAPACT clinical trial demonstrated the efficacy and safety of gemcitabine plus nab-paclitaxel (AG) as induction therapy to enable locally advanced pancreatic cancer (LAPC) to be surgically resectable.9 Compared with immediate surgery, neoadjuvant AG for borderline resectable pancreatic cancer (BRPC) significantly prolonged survival,10 and a systematic review and meta-analysis concluded that neoadjuvant chemotherapy with AG was safe and effective in patients with BRPC and LAPC.11
Abundant chemoradiotherapy regimens have been explored in the hope of improving survival. A phase II study observed that patients with LAPC treated with stereotactic body radiotherapy (SBRT) followed by FOLFIRINOX had an unexpectedly high resectability rate compared with that in the non-SBRT group.12 In the PREOPANC trial, compared with those in the immediate surgery group, neoadjuvant chemoradiotherapy improved the disease-free survival (DFS) and R0 resection rate of patients with BRPC.8 Furthermore, intraoperative radiotherapy (IORT) is reportedly well tolerated without causing any serious postoperative complications.13
Immunotherapy is also not a routine treatment option for PDAC owing to the low tumor mutational burden (TMB) and typical characteristics of “cold” tumors, and the combination of neoadjuvant chemotherapy or chemoradiation and cancer vaccine has shown no optimistic clinical benefit.14,15 Immune checkpoint inhibitors (ICIs) applied as monotherapies, such as PD-1 blockade, have not yielded clinical improvement.16 However, the combination of immunotherapy and radiotherapy appeared to significantly improve treatment efficiency.17 A patient with LAPC achieved near-pathologic complete response (pCR) after the combination of pembrolizumab and radiation therapy.18 Moreover, the result of the CheckPAC clinical trial showed that SBRT plus nivolumab and ipilimumab was a promising therapy for patients with metastatic pancreatic cancer.19
Given the successful regimens and promising ongoing clinical trials, we aimed to conduct a clinical trial combining PD-1 blockade with chemoradiotherapy as preoperative therapy for patients with LAPC and BRPC for improving the resection rate and prolonging the survival outcome. Additionally, we sought to investigate some peripheral blood- and tumor-specific biomarkers for predicting the prognostic outcome and disease monitoring.
Results
Study flow
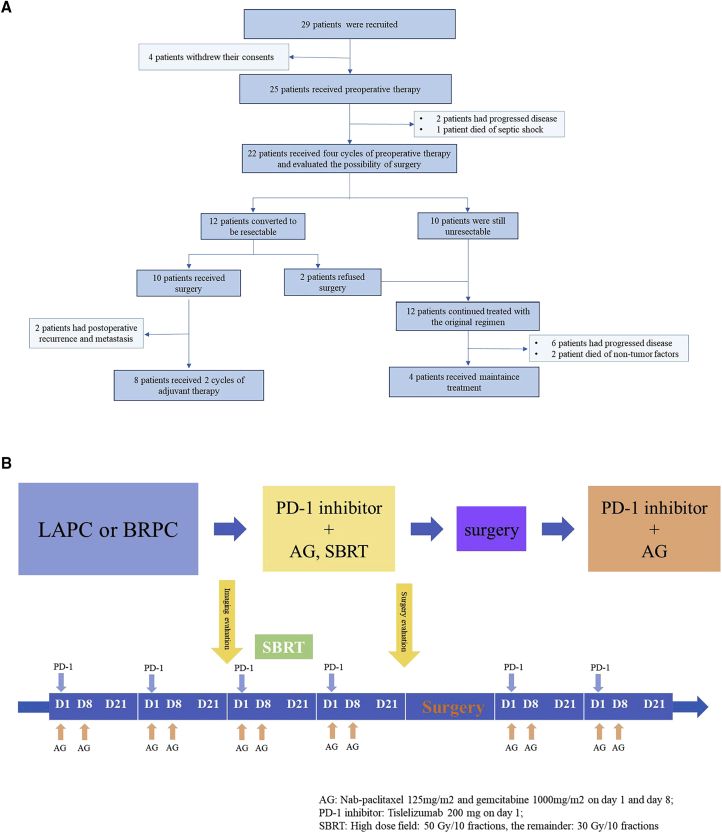
Between May 2020 and October 2021, 29 patients with LAPC or BRPC were enrolled. Until the last follow-up time (November 30, 2022), 25 of them included in the intention-to-treat (ITT) analysis who completed at least two cycles of tislelizumab plus AG and underwent concurrent radiotherapy. In the ITT populations, one patient died after three cycles of treatment, which was not related to the treatment drugs. Twelve patients exhibited surgical indications after the treatment, but two patients refused surgery for personal reasons. Finally, ten patients received surgical resection, while two of them exhibited disease progression after surgery. A flowchart of the enrolled patients is shown in Figure 1A. At least two cycles of adjuvant therapy with a combination of tislelizumab and AG chemotherapy were administrated 1 month after the operation. The treatment scheme was altered for eight patients owing to disease progression (PD) during the preoperative treatment. The main inclusion and exclusion criteria are listed in Table 1, and the timeline of the treatment is shown in Figure 1B.
Figure 1.
Study design
(A) Flowchart of the enrolled patients.
(B) Timeline of the treatment.
Table 1.
Inclusion and exclusion criteria of enrolled patients
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Characteristics of the patients
The detailed baseline demographics of the enrolled patients are presented in Table 2. The median age of patients was 62 (range: 40–75) years, comprising six women (24%). The Eastern Cooperative Oncology Group (ECOG) score of the patients was 0–1. All patients were diagnosed with BRPC (40%) or LAPC (60%). Pancreas head/uncinate (n = 13, 52%) was the most common primary tumor site, followed by body/tail (n = 8, 32%) and neck (n = 4, 16%).
Table 2.
Baseline characteristic of patients
| Characteristic | n=25 |
|---|---|
| Median age, years (range) | 62 (40,75) |
| Sex, n (%) | |
| Male | 19 (76) |
| Female | 6 (24) |
| ECOG PS score, n (%) | |
| 0 | 19 (76) |
| 1 | 6 (24) |
| Tumor location, n (%) | |
| Head/uncinate | 13 (52) |
| Neck | 4 (16) |
| Body/tail | 8 (32) |
| Tumor type, n (%) | |
| BRPC | 10 (40) |
| LAPC | 15 (60) |
| Baseline CA19-9, n (%) | |
| ≤27 U/mL, normal | 6 (24) |
| ≥27 U/mL, elevated | 19 (76) |
| Vascular involvement, n (%) | |
| Arterial alone | 6 (24) |
| Venous alone | 10 (40) |
| Arterial + venous | 9 (36) |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; BRPC, Borderline Resectable Pancreatic Cancer; LAPC, Locally Advanced Pancreatic Cancer; CA19-9, Carbohydrate Antigen 19-9.
Treatment response
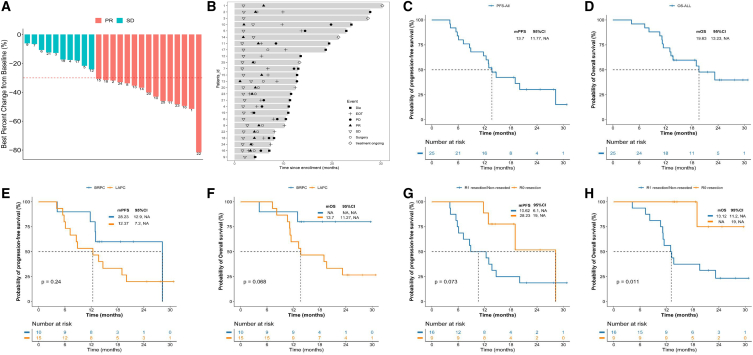
Treatment efficacy was evaluated every two cycles of the preoperative therapy based on the investigator’s assessment using RECIST 1.1. Among the patients who had completed at least one clinical response evaluation, 15 patients (60%) had a best response of partial response (PR) and 10 (40%) had stable disease (SD). The objective response rate (ORR) was 60% (95% confidence interval [CI]: 38.7%–78.9%), and the disease control rate (DCR) was 100%. The best changes compared with the baseline tumor size are shown in Figure 2A, and the overall treatment results are presented using swimmer charts in Figure 2B.
Figure 2.
Treatment response and survival analysis
(A) Best percentage change from baseline on the basis of radiologic response.
(B) Duration of responses of patients in the ITT population. The length of each bar represents the duration of treatment of each patient.
(C and D) The Kaplan-Meier curves of (C) PFS and (D) OS in all enrolled patients.
(E and F) The Kaplan-Meier curves of (E) PFS and (F) OS stratified by tumor type.
(G and H) The Kaplan-Meier curves of (G) PFS and (H) OS stratified by surgical margin.
PR, partial response; SD, stable disease; PD, disease progression; EOT, end of treatment; OS, overall survival; PFS, progression-free survival; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer.
Survival and subgroup analyses
At the last follow up (November 30, 2022), six patients had died from PD, and three patients died from non-tumorous diseases not related to treatment drugs. The median follow up and progression-free survival (PFS) were 23.9 (95% CI: 18.4–27.3) and 13.7 (95% CI: 11.7–NR) months, respectively, whereas median OS was not reached. The 12-month OS and PFS rates were 72% (95% CI: 56.3%–91.9%) and 64% (95% CI: 47.6%–85.8%), respectively (Figures 2C and 2D). No significant association was observed in survival outcomes in BRPC and LAPC groups (PFS, hazard ratio [HR], 0.54; 95% CI: 0.21–1.41; p = 0.24; Figure 2E; OS, HR, 0.28; 95% CI: 0.09–0.84; p = 0.068; Figure 2F). The medium PFS (mPFS) was 28.23 months in patients with R0 resection vs. 10.62 months in patients with R1 resection or without surgery (HR, 0.38; 95% CI: 0.14–0.98; p = 0.073; Figure 2G). The mOS was not reached in patients with R0 resection vs. 13.12 months in patients with R1 resection or without surgery (HR, 0.11; 95% CI: 0.04–0.34; p = 0.011; Figure 2H).
Toxicities
Hematological and non-hematological toxicities during initial preoperative therapy are summarized in Table 3. We did not observe any grade 5 adverse events (AEs) in our study. The most common grade 3–4 hematological and non-hematological toxicities were anemia (8%), thrombocytopenia (8%), and jaundice (8%), respectively. There were no serious immune-related AEs such as autoimmune myocarditis, pneumonitis, and so on.
Table 3.
Summary of adverse events
| Toxicities | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|
| Hematologic toxicities | ||||
| Anemia | 8 (32) | 12 (48) | 2 (8) | 0 (0) |
| Leukopenia | 6 (24) | 9 (36) | 0 (0) | 1 (4) |
| Neutropenia | 5 (20) | 5 (20) | 0 (0) | 0 (0) |
| Thrombocytopenia | 8 (32) | 3 (12) | 1 (4) | 1 (4) |
| Nonhematologic toxicities | ||||
| ALT↑ | 9 (36) | 5 (20) | 2 (8) | 0 (0) |
| AST↑ | 5 (20) | 5 (20) | 2 (8) | 0 (0) |
| Jaundice | 1 (4) | 1 (4) | 2 (8) | 0 (0) |
| Nausea | 4 (16) | 0 (0) | 0 (0) | 0 (0) |
| Anorexia | 6 (24) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 2 (8) | 2 (8) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Rash: Dermatitis | 2 (8) | 1 (4) | 0 (0) | 0 (0) |
| Hyperthyroidism | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pneumonia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Conversion surgery and postoperative complications
Surgical resection
Pancreatoduodenectomy, distal pancreatectomy, and total pancreatectomy were performed in five, one, and four patients, respectively. En bloc vascular resection was required in seven patients, with venous resections in six and combined venous/arterial resections in one. The median operative time and estimated blood loss (EBL) were 528 min and 1,170 mL, respectively.
Pathologic evaluation
All tumors have been completely enclosed by pathologists. Regional lymph node metastases were identified in one patient. Negative (R0) margin resection was achieved in nine patients. Two of the ten patients achieved pCR, whereas one patient achieved major pathological response (MPR). The patient who received R1 resection was found to exhibit a poor response.
Perioperative complications
We found that postoperative complications developed in eight patients, with four patients experiencing major complications (Clavien-Dindo classification ≥ 3). Pancreas-specific complications included postoperative infectious complications (POICs), postoperative pancreatic fistula (POPF), and chyle leak in four, two, and two patients, respectively. We did not observe any delayed gastric emptying (DGE), postpancreatectomy hemorrhage (PPH), and bile leakage. The median length of postoperative stay was 22.5 days, with one patient requiring subsequent 90-day readmissions. The overall 90-day mortality rate was 0. The surgical outcomes of the patients are summarized in Table S1.
Association between peripheral blood biomarkers and tumor response
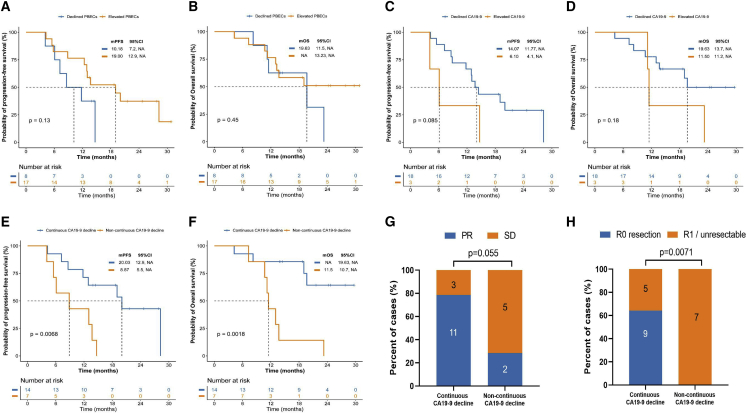
In the prespecified exploratory analysis, we first assessed the correlation between clinical response and peripheral blood biomarkers that are associated with response to immunotherapy. Reportedly, elevated peripheral blood eosinophil counts (PBECs) are associated with a better response during immunotherapy for metastatic triple-negative breast cancer.20 Therefore, we intended to explore the association between changes in PBECs during treatment and the clinical response. Survival analysis revealed a statistically significant association between elevated PBECs during treatment and longer survival outcome. The mPFS was 19 months in patients with elevated PBECs vs. 10.18 months in patients with declined PBECs (HR, 0.48; 95% CI: 0.15–1.56; p = 0.13; Figure 3A). The mOS was not reached in patients with elevated PBECs vs. 19.63 months in patients with declined PBECs (HR, 0.65; 95% CI: 0.20–2.16; p = 0.45; Figure 3B). These findings support the predictive role of elevated PBECs in immunotherapy-based preoperative therapy for pancreatic cancer.
Figure 3.
Association between peripheral blood biomarkers and treatment response
(A and B) The Kaplan-Meier curves of (A) PFS and (B) OS of patients stratified by PBEC (declined vs. elevated).
(C and D) The Kaplan-Meier curves of (C) PFS and (D) OS of patients stratified by CA19-9 change between baseline and after four treatment cycles (decline vs. elevated).
(E and F) The Kaplan-Meier curves of (E) PFS and (F) OS of patients stratified by CA19-9 decline from baseline, two treatment cycles, and four treatment cycles (continuous decline vs. non-continuous decline).
(G) Clinical response of patients stratified by CA19-9 decline from baseline, two treatment cycles, and four treatment cycles (continuous decline vs. non-continuous decline).
(H) Surgery margin of the patients stratified by CA19-9 decline from baseline, two treatment cycles, and four treatment cycles (continuous decline vs. non-continuous decline).
A high neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in the baseline are markers of host inflammation and are associated with worse survival outcomes in immunotherapy for several tumors21,22 but have not yet been extensively analyzed in pancreatic cancer. Thus, we investigated whether high baseline NLR or PLR was associated with clinical response in our study. We observed no significant differences in either survival outcomes or clinical response in high baseline NLR or PLR (Figures S1A–S1F). This result highlighted the heterogeneity of pancreatic cancer compared with that of other solid tumors.
Association between CA19-9 decline and tumor response
As a predictor biomarker, CA19-9 is the best-validated biomarker and an indicator of aberrant glycosylation in pancreatic cancer.23 Normal baseline CA19-9 and declined CA-19-9 levels are associated with long-term survival in pancreatic cancer. We found that CA19-9 levels decreased after two cycles of treatment in all treated patients. Furthermore, changes in CA19-9 levels at baseline and after four treatment cycles showed a better PFS and OS trend but were not statistically significant (PFS: HR, 0.35, 95% CI: 0.06–2.26; declined vs. elevated: 14.07 vs. 6.1 months; p = 0.085; OS: HR, 0.42, 95% CI: 0.07–2.35; declined vs. elevated: 19.63 vs. 11.5 months; p = 0.18; Figures 3C and 3D). Notably, continuous CA19-9 decline during four treatment cycles was associated with improved survival outcomes and clinical response in our study. Patients with continuous CA19-9 decline did not reach mOS, whereas those without continuous CA19-9 decline reached a mOS of 11.5 months (HR, 0.18; 95% CI: 0.05–0.72; p = 0.0018), and patients with continuous CA19-9 decline reached a mPFS of 20.03 vs. 8.87 months in patients without continuous CA19-9 decline (HR, 0.28; 95% CI: 0.08–1; p = 0.0068). Concurrently, the ORR was 78.6% [11/14] vs. 28.6% [2/7]; p = 0.055) for these two groups. Moreover, continuous CA19-9 decline significantly improved the R0 resection rate after NAT (64.2% [9/14] vs. 0% [0/7]; p = 0.0071) (Figures 3E–3H).
Association between serial ctDNA dynamic changes and tumor response
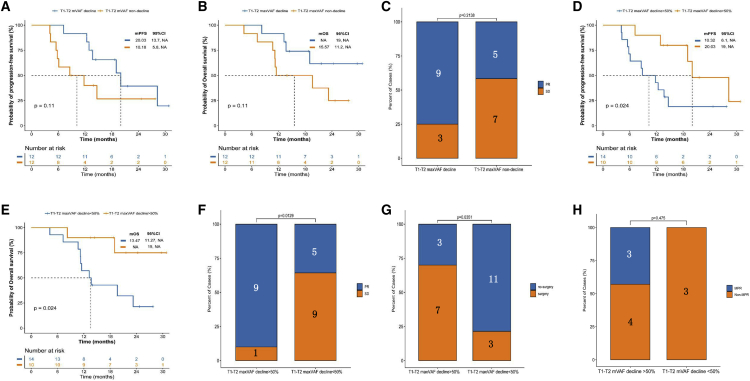
We assessed serial circulating tumor DNA (ctDNA) dynamic changes in predicting tumor responses and survival outcomes by performing next-generation sequencing testing on 539 genes. Patients with a decline in maximal somatic variant allelic frequency (maxVAF) between the first clinical evaluation (T2 [two cycles after therapy]) and baseline (T1) were assessed. We observed a better survival benefit trend without statistically significant association in the decline and non-decline groups (PFS: HR, 0.46, 95% CI: 0.16–1.33; decline vs. non-decline groups: 20.03 vs. 10.18 months, p = 0.11; OS: HR, 0.39, 95% CI: 0.13–1.22; decline vs. non-decline groups: not reached vs. 15.57 months, p = 0.11; ORR: 75% vs. 41.7%, p = 0.21) (Figures 4A–4C). Furthermore, patients with a >50% decline in maxVAF between T2 and T1 had longer survival outcomes and higher response rates than those who did not (PFS: HR, 0.33, 95% CI: 0.12–0.89; decline vs. non-decline groups: 20.03 vs. 10.32 months, p = 0.024; OS: HR, 0.21, 95% CI: 0.07–0.65; decline vs. non-decline groups: not reached vs. 13.47 months, p = 0.024; ORR: 90% vs. 35.7%, p = 0.013) (Figures 4D–4F). Moreover, maxVAF decline (T1-T2, >50%) significantly improved the surgical rate after preoperative theapy (70% vs. 21.4%; p = 0.035) (Figure 4G). Notably, no statistically significant difference was observed between the maxVAF decline (T1-T2, >50%) and MPR beneficiaries (42.9% vs. 0%; p = 0.48) (Figure 4H). To reduce the impact between extremely low or negative baseline ctDNA status and maxVAF change, VAFmean was also applied to investigate the correlation between VAF changes and patient outcomes. The results showed that patients with a >50% decline in VAFmean between T2 and T1 also demonstrated a significantly longer PFS (28.2 vs. 11.7 months; HR = 0.38; p = 0.048) and a trend for longer OS (not reached vs. 13.7 months; HR = 0.27; p = 0.058) (Figures S2A–S2D). Interestingly, we found that four patients harbored ctDNA clearance after T2, and patients with ctDNA clearance demonstrated a significantly prolonged PFS and OS (28.2 vs. 12.3 months; HR = 0.18; p = 0.036) and a longer OS (not reached vs. 19 months; HR = 0.27; p = 0.049) (Figures S2E and S2F). We further investigated the changes in maxVAF between the second clinical evaluation (T3 [four cycles after therapy]) and baseline. No association was observed in the PFS or ORR in the decline and non-decline groups (Figures S3A–S3F).
Figure 4.
ctDNA dynamics and correlation with treatment response
(A and B) The Kaplan-Meier curves of (A) PFS and (B) OS of patients stratified by change of ctDNA (T1-T2 mVAF decline vs. non-decline).
(C) Treatment response of patients stratified by change of ctDNA (T1-T2 mVAF decline vs. non-decline).
(D and E) The Kaplan-Meier curves of (D) PFS and (E) OS of patients stratified by decline of ctDNA (T1-T2 mVAF > 50% vs. < 50%).
(F) Treatment response of patients stratified by decline of ctDNA (T1-T2 mVAF > 50% vs. < 50%).
(G and H) Clinical outcome (G) and postoperation pathological stage (H) of the patients stratified by decline of ctDNA (T1-T2 mVAF > 50% vs. < 50%).
ctDNA, circulating tumor DNA; T1, baseline; T2, two cycles after therapy; mVAF, maximal somatic variant allelic frequency; PR, partial response; SD, stable disease; MPR, major pathological response.
The baseline genetic alterations in the cohort are depicted in Figure S4A, and the average number of genomic alterations was 1.5 mutations in each patient. KRAS was the most frequently altered gene, occurring in 12 (50%) patients with a missense mutation, followed by TP53 (30%). We found that no association was observed between the TMB-high and -low groups (mPFS: HR, 1.07, 95% CI: 0.39–2.91; high vs. low: 13.70 vs. 13.83 months; p = 0.9). Similarly, the baseline mVAF (mPFS: HR, 0.49, 95% CI: 0.18–1.31; mVAF > 1% vs. < 1%: 28.23 vs. 13 months; p = 0.14) and KRAS mutation status in PFS (HR, 1.27, 95% CI: 0.48–3.38; mutation vs. wild type: 13.35 vs. 14.67 months; p = 0.62) did not exhibit any association (Figures S4B–S4D).
Comparison of clinical features and biomarker changes in patients with resectable vs. unresectable cancer
Owing to the poor prognosis of patients with inoperable pancreatic cancer, one of the goals of preoperative therapy is to increase the surgical rate. Thus, we compared the clinical features and biomarker changes in patients stratified by the eligibility for resection after preoperative therapy. Baseline characteristics for patients with resectable and unresectable cancer were similar for age (median values: 60.4 vs. 60.1 years; p = 0.93; Figure S5A) and the longest tumor diameter (median values: 38.8 vs. 34.6 mm; p = 0.36; Figure S5B). Compared with patients with unresectable cancer, patients with resectable cancer exhibited a greater response in decreasing the size of the longest tumor diameter from baseline to after four cycles of preoperative therapy (−40.32% vs. −14.39%; p = 0.0012; Figure S5C). Additionally, the proportion of patients with BRPC who became resectable after preoperative therapy was higher than in those with unresectable patients (66.7% vs. 15.3%, p = 0.041; Figure S5D). Moreover, resectable participants compared with non-resectable patients had greater response in maxVAF decline (T1-T2 > 50% maxVAF decline, 63.6% vs. 16.7%, p = 0.036; Figure S5E) and CA19-9 change (continuous CA19-9 decline, 100% vs. 45.4%; p = 0.012; Figure S5F).
Discussion
This single-arm, phase II trial was designed to examine the efficacy and safety of PD-1 inhibitors in combination with chemotherapy and concurrent SBRT in patients with LAPC and BRPC. Neoadjuvant and induction therapy are being increasingly applied for LAPC and BRPC, with higher resection rates and better tumor responses.24 At the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, data of the AIO-NEONAX trial indicated improved survival and R0 resection rate in patients treated with perioperative chemotherapy compared with adjuvant therapy, respectively.25 However, the role of neoadjuvant radiotherapy remains uncertain. Upon comparing neoadjuvant chemoradiotherapy vs. chemotherapy in patients with LAPC or BRPC, a study found that preoperative chemoradiotherapy was associated with improved treatment response and increased survival.26 Furthermore, data of the PREOPANC trial, a multicenter randomized clinical trial, concluded that there is no significant difference in the incidence of surgical complications or mortality in patients who received preoperative chemoradiotherapy or underwent surgery immediately. Preoperative chemoradiotherapy has even been found to reduce the rate of POPF.27
Recently, immunotherapy is of great interest in cancer treatment. Although single-agent PD-1 inhibitor is yet to show a substantial clinical benefit in PDAC treatment, PD-1 blockade plus chemotherapy or radiotherapy provides various options for treating patients with BRPC, LAPC,18 or metastatic PDAC.19 In a melanoma mouse model, the combination of radiation and ICI resulted in higher response rates and improved survival.28 Tislelizumab combined with chemotherapy as neoadjuvant therapy has shown promising efficacy in esophageal squamous cell carcinoma29 and locally advanced gastric/gastroesophageal junction cancer.30 Radiotherapy or chemotherapy can upregulate PD-L1 expression,31 thereby giving rise to a mode of treatment. Our study is a prospective evaluation to demonstrate the clinical benefit and safety of preoperative therapy that combined chemoradiotherapy and PD-1 inhibitor in patients with LAPC or BRPC. Our data revealed that this regimen was potentially effective, which contributed to superior ORR and outstanding R0 resection rates without serious adverse reactions or postoperative complications. The incidence of pCR is higher in our study (20%) than in patients with LAPC or BRPC treated with neoadjuvant chemoradiation (10%),32 which may be an effect of the immunotherapy plus a high radiation dose, and more samples are required to demonstrate the correlation.
To the best of our knowledge, no effective predictive biomarkers have been identified for pancreatic cancer therapy; neither PD-L1 expression nor TMB has been verified to predict the response to immunochemotherapy.33,34 CA19-9 is the best-validated predictor biomarker and an indicator of aberrant glycosylation in pancreatic cancer. In our study, continuous CA19-9 decline during four treatment cycles was associated with superior survival outcomes and clinical response, providing a viable predictive biomarker. Additionally, one of the significant findings in our study was that elevated PBEC was associated with clinical benefits in survival benefits and tumor response. Eosinophils influence the function of other leukocytes by expressing major histocompatibility complex class II costimulatory molecules, releasing cytokines, and stimulating T cell proliferation.24 Moreover, eosinophils secrete chemokines, such as CCL5, CXCL9, and CXCL10, that attract CD8+ T cells into the tumor.35,36 These are all possible reasons for the increase in eosinophil levels during treatment to be associated with better clinical benefits. Notably, a positive association between eosinophil invasion of tumor tissue or an increase in PBEC and superior response to ICIs in several types of cancer has been reported.20,37 Although the underlying mechanism is not fully understood, there is strong evidence that eosinophils exhibit antitumor effects. Thus, eosinophils affect the immune response to diseases such as cancer, and predictive biomarkers that reflect this inflammatory response to treatment may be useful for clinical decision-making in the management of patients with cancer.
Genomic features are believed to hold great potential to predict tumor response to cancer therapy. A large sample analysis has demonstrated that ctDNA may be a feasible biomarker for various solid tumor types.38 Moreover, ctDNA could provide longitudinal and dynamic surveillance of the tumor-specific genetic characteristics without having to repeatedly perform invasive tumor biopsies that cost more time and money.39 In our study, serial ctDNA dynamic changes in predicting tumor responses and survival outcomes revealed that patients with a >50% decline in maxVAF between the first clinical evaluation and baseline had longer survival outcomes and higher response rates than those who did not. Additionally, maxVAF decline significantly improved the surgical rate after preoperative therapy. This rapid decline in the maxVAF of ctDNA-positive patients from baseline to postchemoradiation reflects the substantial downstaging achieved with induction treatment. Consistent with some studies, ctDNA has potential value in predicting immunotherapy efficacy in patients with non-small cell lung cancer28 and gastric cancer40; it may be an accurate dynamic biomarker reflecting real-time tumor volume.
In summary, this is a prospective clinical trial that adopts a regimen of preoperative therapy for patients with BRPC or LAPC. The findings of this trial demonstrate the effectiveness and safety of the combination of PD-1 inhibitors and neoadjuvant chemoradiotherapy. It shows the potential of this treatment in improving the R0 resection rate without causing serious postoperative complications.
Limitations of the study
This study has some limitations. This is a single-arm study lacking a comparator treatment arm so that selection bias could not be ruled out. Furthermore, the small sample size and relatively short follow-up time reduced the certainty of effectiveness observed and restricted the interpretation of definite conclusions. In addition, most enrolled patients only had endoscopic ultrasound fine-needle aspiration (EUS-FNA) for diagnosis so there were not enough tissue samples for immunohistochemistry (IHC) to evaluate microsatellite instability/mismatch repair deficiency (MSI/dMMR) status and expression of PD-L1, which restricted us to analyze the association between these biomarkers and the benefits of our induction therapy. We did not conduct exploratory analyses on the resected specimens to evaluate the effect of preoperative therapy on the tumor microenvironment given the small sample size of resected patients. The study data aided in interpreting the treatment effect; nevertheless, further research on the elucidation of the underlying mechanisms is necessary.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Blood samples for analysis were collected from 29 patients recruited in the trial | This manuscript | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Gemcitabine | Hengrui, Jiangsu, China | https://www.hengrui.com/ |
| Nab-paclitaxel | Lilly Pharmaceutical, IN, USA | https://www.lilly.com.cn/ |
| Tislelizumab | BeiGene, Beijing, China | https://www.beigene.com.cn/ |
| Software and algorithms | ||
| SAS software version 9.4 | SAS Institute, Cary, NC, USA | https://support.sas.com/software/94 |
| Prism version 8.0.2 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Baorui Liu (baoruiliu@nju.edu.cn).
Materials availability
This study did not generate new, unique reagents.
Experimental model and subject details
Human subject
Chinese adults with histologically confirmed locally advanced pancreatic cancer or borderline resectable pancreatic cancer were enrolled in the study. Demographic information along with the key inclusion criteria and exclusion criteria were provided. To determine the sample size for this clinical trial, ORR improvement with standard of care chemotherapy was assumed and estimated. In this study, 29 treatment-naive patients were enrolled and 25 of them were analyzed, comprising six female (24.0%). The Eastern Cooperative Oncology Group (ECOG) score of the patients was 0 (76%) and 1 (24%). All patients provided written informed consent prior to enrollment. The study was performed per the Declaration of Helsinki and was reviewed and approved by the Medical Ethics Committee of Drum Tower Hospital Affiliated to Nanjing University Medical School (2020-088-01).
Method details
Study design and patients
This is a phase II, single-arm, prospective study of PD-1 inhibitor plus chemotherapy and concurrent SBRT as preoperative therapy for patients with LAPC or BRPC between May 2020 and October 2021 at the Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School. The patients were screened within one week before the initial treatment, and all the patients involved in this study provided written informed consent. The protocol of the study has already been published.41
Treatment
Patients who met the inclusion criteria received two treatment cycles of PD-1 inhibitor plus chemotherapy firstly. Briefly, each cycle lasted for 3 weeks, including gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) administered intravenously (IV) on days 1 and 8, with tislelizumab (200 mg) IV on day 1. After two cycles of treatment, an imaging examination was performed to assess the prognosis, and patients without PD received SBRT with SIB (high dose field: 50 Gy/10 fractions; the remainder: 30 Gy/10 fractions) during the third cycle. On completion of four cycles of treatment and radiotherapy, multiple disciplinary team (MDT), comprising medical oncologists, pancreatic surgeons, gastroenterologists, radiologists, pathologists et al., would reassess the surgical possibility according to NCCN Version 2.202142 for resection following neoadjuvant therapy, including imaging checkups, positron emission computed tomography (PET-CT), changes in tumor markers, and the health status of patients. Patients whose CA19-9 was stable or decreased and radiographic findings didn’t demonstrate clear progression, or the standardized uptake value (SUV) max parameter of the lesion decreased in PET-CT, were eligible for resection. Adjuvant therapy including a combination of tislelizumab and AG was administered in at most four cycles after resection according to the patient’s physical condition. Patients who were not suitable for surgery continued the treatment until investigator-assessed PD, unacceptable toxicity, withdrawal of consent, investigator decision, or study completion.
Endpoints and assessments
The primary endpoint was the R0 resection rate and ORR. Resection status (R0, R1, or R2) is graded according to institutional guidelines. ORR in this trial refers to the best overall response during four courses of the preoperative therapy which was calculated as complete response (CR) rate plus partial response (PR) rate under CT according to RECIST 1.1.43 CR was defined as total tumor regression, while PR was defined as greater than 30% reduction. And DCR was defined as the proportion of patients with CR, PR and SD The secondary objectives included safety, median overall survival (mOS), median progression-free survival (mPFS), and postoperative pathological stage. OS is defined as the time from the date of enrollment to the date of death owing to any cause. PFS is defined as the time from initial treatment to the first evidence of PD based on RECIST 1.1 or disease-related death. Two individual pathologists blinded to the clinical outcome assessed the tumor response. MPR defined as 90%–99% tumor necrosis in resected tissue and pCR defined as no residual cancer cells in the resected tissue. Adverse events (AEs) were evaluated according to Adverse Events (CTCAE) version5. Clavien–Dindo classification was applied for postoperative complications, with major complications defined as grade ≥ III.44 CR-POPF (Grade B/C), biliary leakage (BL), chylous fistula, delayed gastric emptying (DGE), and post-pancreatectomy hemorrhage (PPH) were diagnosed according to the International Study Group of Pancreatic Surgery (ISGPS).45,46,47 Wound infection, intra-abdominal infection, bacteremia, pneumonia and urinary tract infection were all included. Multiomics biomarkers associated with clinical response were assessed as exploratory objectives.
Peripheral blood biomarker collection and evaluation
According to the protocol designs, peripheral blood biomarkers were measured at baseline and before each cycle of treatment. Peripheral neutrophil, lymphocyte, thrombocyte, and PBECs and carbohydrate antigen 19-9 (CA19-9) levels were measured. NLR was calculated by division of absolute neutrophil and lymphocyte counts, while PLR was calculated by division of thrombocyte and lymphocyte counts. Patients with normal baseline CA19-9 levels (<27 U/mL) were excluded from the CA19-9 response evaluation because they were less likely to exhibit a significant decline in CA19-9 levels. In addition, if the CA19-9 value was greater than 27 U/mL at any time point, the patient was included in the analysis.
ctDNA sequencing and bioinformatics analysis
Plasma samples were collected at the following time points: before preoperative therapy (baseline), two cycles after the initiation of preoperative therapy, 4 cycles after preoperative therapy or before surgery (preop), and within 1 month after surgery (postop) or at time of progression. For each sample, 10 mL of peripheral (intravenous) blood was collected and stored in a 10 mL BD EDTA-K2 anticoagulation tube. Double centrifugation was applied to eliminate leukocyte contamination. Plasma was isolated within 2 h by centrifugation (1200g, 15 min), and then the collected plasma was removed into a 1.5 mL low-adsorption centrifuge tube (Eppendorf DNA Lobind tube, 12000g, 10 min).
A total of 86 dynamic plasma samples and 25 leukocyte germline control samples were collected and subjected to panel sequencing of 539 cancer-related genes. The 539-gene panel includes genes associated with targeted medicines approved by Food and Drug Administration (FDA) or recommended by the NCCN guideline, genes involved in the major signaling pathways regulating cancer cell survival and proliferation, and potential cancer driver genes. Clonal hematopoiesis of indeterminate potential (CHIP) interference variants could be distinguished and excluded using the paired whole-blood control first. ctDNA positive is defined as detectable somatic mutations, we tracked the dynamic change of the mutation with the highest variant allele frequency (maxVAF) at baseline and predefined points in each patient. maxVAF change is defined as the change in maxVAF during the treatment, calculated by subtracting maxVAF at baseline from maxVAF at different points. Also, VAFmean is defined as the mean of the VAF(s) of somatic mutated genes in each patient. VAFmean change is defined as the change in VAFmean during the treatment, calculated by subtracting VAFmean at baseline from VAFmean at different points. ctDNA clearance is defined as lack of detectable mutation from this panel covering 539 cancer-related genes at predefined points, with an average sequencing depth of 15000× and 0.2% detection limit.
DNA library and corresponding cDNA library were standardized using the library homogenization method, purified by magnetic beads, and sequenced using the Illumina NextSeq 550Dx platform. Before library normalization, the next-generation sequencing libraries enriched by hybridization capture were quantified using the Qubit dsDNA HS Assay Kit. The fastp tool (V.2.20.0) was used for adapter pruning and to filter low-quality sequencing reads.48 Cleaned reads were alignd to the human reference genome (hg19) using the BWA-mem algorithm.49 Somatic mutations including point mutations, small insertions, and deletions were identified and annotated using VarDict and Inter-Var, respectively.50,51 We screened for germline variations using the internal database. Copy number variation involved amplification and deletion were identified by CNVkit.52 bTMB was defined as the number of somatic SNVS and indels in examined coding region. All SNVs and indels in the coding region of targeted genes, including missense, silent, stop gain, stop loss, in-frame and frameshift mutations, are initially considered. Known germline SNVs, defined as population frequency more than 0.015, in dbSNP, 1000 genome, and ESP6500 were filtered. Variants with allele frequencies more than 30%, which are more likely germline mutations, were not counted. TMB high was defined as the median value in this study.
Quantification and statistical analysis
Historical data showed that the ORR rate after neoadjuvant chemotherapy (LAPACT) in patients with LAPC was 33.6%. We estimated that a sample size of 26 patients would detect approximately 26% improvement (60%) in ORR rate with a power of 80%, using a one-sided alpha of 0.025. Assuming a 10% drop-out rate, a total of 29 treatment-naive patients with LAPC/BRPC were planned to accrue in our study.
Statistical analysis was conducted using SAS statistical software (V.9.4,SAS Institute). Efficacy analyses were performed in patients who underwent one or more post-treatment scans in the intention-to-treat populations. Safety outcomes were analyzed in patients who received at least one of the aforementioned doses of the study regimen. Categorical variables, as the proportions of patients with an objective response or adverse events were summarized by descriptive statistics with 95% confidence interval Wilson score (CIs). Continuous variables were expressed as median (range). Response differences (ORR) and other binary outcomes among clinical subgroups were assessed with the Fisher’s exact test. Furthermore, we provided Kaplan-Meier plots for PFS and OS, the log rank test was used to compare the survival functions among different subgroups. For all analyzes, p value < 0.05 was considered to be statistically significant.
Additional resources
This study has been registered on https://www.chictr.org.cn/, ID: ChiCTR2000032955.
Acknowledgments
We gratefully thank the patients and their families for participating in this study. We thank Fan Tong for collection of the data. This study was funded by National Key Research and Development Program of China (2020YFA0713804) and Special Fund of Health Science and Technology Development of Nanjing (YKK20080).
Author contributions
D.J., C.L., and L.M. have contributed equally to this work. J.D., B.L., Y.Q., and L.W. were responsible for the design of the project and writing articles. D.J., C.L., and L.M. were responsible for all data sorting and writing articles. Y.Z., K.W., S.S., and X.Q. were involved in the diagnosis and the instruction of the treatment. M.T., J.H., and A.L. were responsible for imaging evaluation. S.B., H.C., and G.L. were responsible for surgery. J.C. and Q.L. were responsible for pathological evaluation. Q.X., Q.G., D.C., C.Q., and Y.S. were responsible for sample sequencing and data analysis. All authors have agreed to the final version of the manuscript.
Declaration of interests
D.C., Y.S., and C.Q. were employed by Jiangsu Simcere Diagnostics Co., Ltd.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: March 7, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.100972.
Contributor Information
Lei Wang, Email: 867152094@qq.com.
Yudong Qiu, Email: yudongqiu510@163.com.
Baorui Liu, Email: baoruiliu@nju.edu.cn.
Supplemental information
Data and code availability
-
•
The raw sequencing data are available under restricted access due to data privacy laws. Data are available on request sharing by sending requests to the corresponding author Baorui Liu (baoruiliu@nju.edu.cn), which will need the approval of the institutional ethical committees. Clinical data were not publicly available due to involving patient privacy, but can be accessed from the corresponding author, upon request for 3 years; individual de-identified patient data will be shared for clinical study analyses. The remaining data are available in the manuscript, supplemental information, or Source Data file. The study protocol is provided in the supplemental information file.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA. Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Barugola G., Partelli S., Marcucci S., Sartori N., Capelli P., Bassi C., Pederzoli P., Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann. Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 4.Kimura K., Amano R., Nakata B., Yamazoe S., Hirata K., Murata A., Miura K., Nishio K., Hirakawa T., Ohira M., Hirakawa K. Clinical and pathological features of five-year survivors after pancreatectomy for pancreatic adenocarcinoma. World J. Surg. Oncol. 2014;12:360. doi: 10.1186/1477-7819-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Choné L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 6.van Roessel S., van Veldhuisen E., Klompmaker S., Janssen Q.P., Abu Hilal M., Alseidi A., Balduzzi A., Balzano G., Bassi C., Berrevoet F., et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6:1733–1740. doi: 10.1001/jamaoncol.2020.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosumeck N., Timmermann L., Klein F., Bahra M., Stintzig S., Malinka T., Pelzer U. Induction chemotherapy for primarily unresectable locally advanced pancreatic adenocarcinoma-who will benefit from a secondary resection? Medicina (Kaunas) 2021;57:77. doi: 10.3390/medicina57010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versteijne E., Suker M., Groothuis K., Akkermans-Vogelaar J.M., Besselink M.G., Bonsing B.A., Buijsen J., Busch O.R., Creemers G.J.M., van Dam R.M., et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philip P.A., Lacy J., Portales F., Sobrero A., Pazo-Cid R., Manzano Mozo J.L., Kim E.J., Dowden S., Zakari A., Borg C., et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet. Gastroenterol. Hepatol. 2020;5:285–294. doi: 10.1016/s2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y., Saiura A., Oba A., Ono Y., Mise Y., Ito H., Sasaki T., Ozaka M., Sasahira N., Takahashi Y. Neoadjuvant gemcitabine and nab-paclitaxel for borderline resectable pancreatic cancers: intention-to-treat analysis compared with upfront surgery. J. Hepatobiliary Pancreat. Sci. 2021;28:143–155. doi: 10.1002/jhbp.844. [DOI] [PubMed] [Google Scholar]
- 11.Damm M., Efremov L., Birnbach B., Terrero G., Kleeff J., Mikolajczyk R., Rosendahl J., Michl P., Krug S. Efficacy and safety of neoadjuvant gemcitabine plus nab-paclitaxel in borderline resectable and locally advanced pancreatic cancer-A systematic review and meta-analysis. Cancers. 2021;13:4326. doi: 10.3390/cancers13174326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teriaca M.A., Loi M., Suker M., Eskens F.A.L.M., van Eijck C.H.J., Nuyttens J.J. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): long-term outcome. Radiother. Oncol. 2021;155:232–236. doi: 10.1016/j.radonc.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Cho Y., Kim J.W., Kim H.S., Park J.S., Lee I.J. Intraoperative radiotherapy for resectable pancreatic cancer using a low-energy X-Ray Source: postoperative complications and early outcomes. Yonsei Med. J. 2022;63:405–412. doi: 10.3349/ymj.2022.63.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawlor R.T., Mattiolo P., Mafficini A., Hong S.M., Piredda M.L., Taormina S.V., Malleo G., Marchegiani G., Pea A., Salvia R., et al. Tumor mutational burden as a potential biomarker for immunotherapy in pancreatic cancer: systematic review and still-open questions. Cancers. 2021;13:3119. doi: 10.3390/cancers13133119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt D.B., Nissen N., Hatoum H., Musher B., Seng J., Coveler A.L., Al-Rajabi R., Yeo C.J., Leiby B., Banks J., et al. A phase 3 randomized clinical trial of chemotherapy with or without algenpantucel-L (HyperAcute-Pancreas) immunotherapy in subjects with borderline resectable or locally advanced unresectable pancreatic cancer. Ann. Surg. 2022;275:45–53. doi: 10.1097/SLA.0000000000004669. [DOI] [PubMed] [Google Scholar]
- 16.Wandmacher A.M., Letsch A., Sebens S. Challenges and future perspectives of immunotherapy in pancreatic cancer. Cancers. 2021;13:4235. doi: 10.3390/cancers13164235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X., Cao Y., Liu W., Ju X., Zhao X., Jiang L., Ye Y., Jin G., Zhang H. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23:e105–e115. doi: 10.1016/s1470-2045(22)00066-3. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy P.M., Rendo M.J., Uy M.D., Adams A.M., O'Shea A.E., Nelson D.W., Fenderson J.L., Cebe K.M., Krell R.W., Clifton G.T., et al. Near complete pathologic response to PD-1 inhibitor and radiotherapy in a patient with locally advanced pancreatic ductal adenocarcinoma. OncoTargets Ther. 2021;14:3537–3544. doi: 10.2147/OTT.S311661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen I.M., Johansen J.S., Theile S., Hjaltelin J.X., Novitski S.I., Brunak S., Hasselby J.P., Willemoe G.L., Lorentzen T., Madsen K., et al. Randomized phase II study of nivolumab with or without ipilimumab combined with stereotactic body radiotherapy for refractory metastatic pancreatic cancer (CheckPAC) J. Clin. Oncol. 2022;40:3180–3189. doi: 10.1200/JCO.21.02511. [DOI] [PubMed] [Google Scholar]
- 20.Ghebeh H., Elshenawy M.A., AlSayed A.D., Al-Tweigeri T. Peripheral blood eosinophil count is associated with response to chemoimmunotherapy in metastatic triple-negative breast cancer. Immunotherapy. 2022;14:189–199. doi: 10.2217/imt-2021-0149. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Li S., Zhang S., Liu Y., Ma L., Zhu J., Xin Y., Wang Y., Yang C., Cheng Y. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 2019;33:e22964. doi: 10.1002/jcla.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo A., Russano M., Franchina T., Migliorino M.R., Aprile G., Mansueto G., Berruti A., Falcone A., Aieta M., Gelibter A., et al. Neutrophil-to-Lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv. Ther. 2020;37:1145–1155. doi: 10.1007/s12325-020-01229-w. [DOI] [PubMed] [Google Scholar]
- 23.Luo G., Jin K., Deng S., Cheng H., Fan Z., Gong Y., Qian Y., Huang Q., Ni Q., Liu C., Yu X. Roles of CA19-9 in pancreatic cancer: biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer. 2021;1875:188409. doi: 10.1016/j.bbcan.2020.188409. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg H.F., Dyer K.D., Foster P.S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ettrich T.J., Uhl W., Kornmann M., Algül H., Friess H., Koenig A., Gallmeier E., Lutz M.P., Wille K., Schimanski C.C., et al. Perioperative or adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer: updated final results of the randomized phase II AIO-NEONAX trial. J. Clin. Oncol. 2022;40:4133. doi: 10.1200/JCO.2022.40.16_suppl.4133. [DOI] [Google Scholar]
- 26.Murphy J.E., Wo J.Y., Ryan D.P., Jiang W., Yeap B.Y., Drapek L.C., Blaszkowsky L.S., Kwak E.L., Allen J.N., Clark J.W., et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dongen J.C., Suker M., Versteijne E., Bonsing B.A., Mieog J.S.D., de Vos-Geelen J., van der Harst E., Patijn G.A., de Hingh I.H., Festen S., et al. Surgical complications in a multicenter randomized trial comparing preoperative chemoradiotherapy and immediate surgery in patients with resectable and borderline resectable pancreatic cancer (PREOPANC trial) Ann. Surg. 2022;275:979–984. doi: 10.1097/SLA.0000000000004313. [DOI] [PubMed] [Google Scholar]
- 28.Ricciuti B., Jones G., Severgnini M., Alessi J.V., Recondo G., Lawrence M., Forshew T., Lydon C., Nishino M., Cheng M., Awad M. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC) J. Immunother. Cancer. 2021;9:e001504. doi: 10.1136/jitc-2020-001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan X., Duan H., Ni Y., Zhou Y., Wang X., Qi H., Gong L., Liu H., Tian F., Lu Q., et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE) Int. J. Surg. 2022;103:106680. doi: 10.1016/j.ijsu.2022.106680. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y., Lin Y., Yang M., Lv J., Liu J., Wu K., Liu K., Li A., Shuai X., Cai K., et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: early results of a phase 2, single-arm trial. Front. Oncol. 2022;12:959295. doi: 10.3389/fonc.2022.959295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevtsov M., Sato H., Multhoff G., Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front. Oncol. 2019;9:156. doi: 10.3389/fonc.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J., Blair A.B., Groot V.P., Javed A.A., Burkhart R.A., Gemenetzis G., Hruban R.H., Waters K.M., Poling J., Zheng L., et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann. Surg. 2018;268:1–8. doi: 10.1097/SLA.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeber A., Zimmer K., Kocher F., Puccini A., Xiu J., Nabhan C., Elliott A., Goldberg R.M., Grothey A., Shields A.F., et al. Molecular characteristics of BRCA1/2 and PALB2 mutations in pancreatic ductal adenocarcinoma. ESMO Open. 2020;5:e000942. doi: 10.1136/esmoopen-2020-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karamitopoulou E., Andreou A., Wenning A.S., Gloor B., Perren A. High tumor mutational burden (TMB) identifies a microsatellite stable pancreatic cancer subset with prolonged survival and strong anti-tumor immunity. Eur. J. Cancer. 2022;169:64–73. doi: 10.1016/j.ejca.2022.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Cheng J.N., Luo W., Sun C., Jin Z., Zeng X., Alexander P.B., Gong Z., Xia X., Ding X., Xu S., et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci. Adv. 2021;7:eabc7609. doi: 10.1126/sciadv.abc7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grisaru-Tal S., Dulberg S., Beck L., Zhang C., Itan M., Hediyeh-Zadeh S., Caldwell J., Rozenberg P., Dolitzky A., Avlas S., et al. Metastasis-entrained eosinophils enhance lymphocyte-mediated antitumor immunity. Cancer Res. 2021;81:5555–5571. doi: 10.1158/0008-5472.CAN-21-0839. [DOI] [PubMed] [Google Scholar]
- 37.Furubayashi N., Minato A., Negishi T., Sakamoto N., Song Y., Hori Y., Tomoda T., Harada M., Tamura S., Miura A., et al. Association between immune-related adverse events and efficacy and changes in the relative eosinophil count among patients with advanced urothelial carcinoma treated by pembrolizumab. Cancer Manag. Res. 2022;14:1641–1651. doi: 10.2147/CMAR.S360473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broccard S.P., Kasbi A.A., Bagaria S.P., Jones J., Shoudry M., Gabriel E.M. Liquid biopsies for colorectal cancer: a narrative review of ongoing clinical trials and the current use of this technology at a comprehensive cancer center. J. Gastrointest. Oncol. 2022;13:438–449. doi: 10.21037/jgo-21-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herberts C., Wyatt A.W. Technical and biological constraints on ctDNA-based genotyping. Trends Cancer. 2021;7:995–1009. doi: 10.1016/j.trecan.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Lengyel C.G., Hussain S., Trapani D., El Bairi K., Altuna S.C., Seeber A., Odhiambo A., Habeeb B.S., Seid F. The emerging role of liquid biopsy in gastric cancer. J. Clin. Med. 2021;10:2108. doi: 10.3390/jcm10102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu C., Zhu Y., Kong W., Yang J., Zhu L., Wang L., Tang M., Chen J., Li Q., He J., et al. Study protocol for a prospective, open-label, single-arm, phase II study on the combination of tislelizumab, nab-paclitaxel, gemcitabine, and concurrent radiotherapy as the induction therapy for patients with locally advanced and borderline resectable pancreatic cancer. Front. Oncol. 2022;12:879661. doi: 10.3389/fonc.2022.879661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [DOI] [PubMed]
- 43.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besselink M.G., van Rijssen L.B., Bassi C., Dervenis C., Montorsi M., Adham M., Asbun H.J., Bockhorn M., Strobel O., Büchler M.W., et al. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161:365–372. doi: 10.1016/j.surg.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 46.Bassi C., Marchegiani G., Dervenis C., Sarr M., Abu Hilal M., Adham M., Allen P., Andersson R., Asbun H.J., Besselink M.G., et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years after. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Wente M.N., Veit J.A., Bassi C., Dervenis C., Fingerhut A., Gouma D.J., Izbicki J.R., Neoptolemos J.P., Padbury R.T., Sarr M.G., et al. Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai Z., Markovets A., Ahdesmaki M., Chapman B., Hofmann O., McEwen R., Johnson J., Dougherty B., Barrett J.C., Dry J.R. VarDict: a novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016;44:e108. doi: 10.1093/nar/gkw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q., Wang K. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am. J. Hum. Genet. 2017;100:267–280. doi: 10.1016/j.ajhg.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talevich E., Shain A.H., Botton T., Bastian B.C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 2016;12:e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The raw sequencing data are available under restricted access due to data privacy laws. Data are available on request sharing by sending requests to the corresponding author Baorui Liu (baoruiliu@nju.edu.cn), which will need the approval of the institutional ethical committees. Clinical data were not publicly available due to involving patient privacy, but can be accessed from the corresponding author, upon request for 3 years; individual de-identified patient data will be shared for clinical study analyses. The remaining data are available in the manuscript, supplemental information, or Source Data file. The study protocol is provided in the supplemental information file.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.