Summary
Under the ever-present threat of a pandemic influenza strain, the evolution of a broadly reactive, neutralizing, functional, humoral immune response may hold the key to protection against both circulating and emerging influenza strains. We apply a systems approach to profile hemagglutinin- and neuraminidase-specific humoral signatures that track with the evolution of broad immunity in a cohort of vaccinated individuals and validate these findings in a second longitudinal cohort. Multivariate analysis reveals the presence of a unique pre-existing Fcγ-receptor-binding antibody profile in individuals that evolved broadly reactive hemagglutination inhibition activity (HAI), marked by the presence of elevated levels of pre-existing FCGR2B-binding antibodies. Moreover, vaccination with FCGR2B-binding antibody-opsonized influenza results in enhanced antibody titers and HAI activity in a murine model. Together, these data suggest that pre-existing FCGR2B binding antibodies are a key correlate of the evolution of broadly protective influenza-specific antibodies, providing insight for the design of next-generation influenza vaccines.
Keywords: antibody, influenza, Fc receptor, vaccine, FCGR2B, hemagglutination inhibition
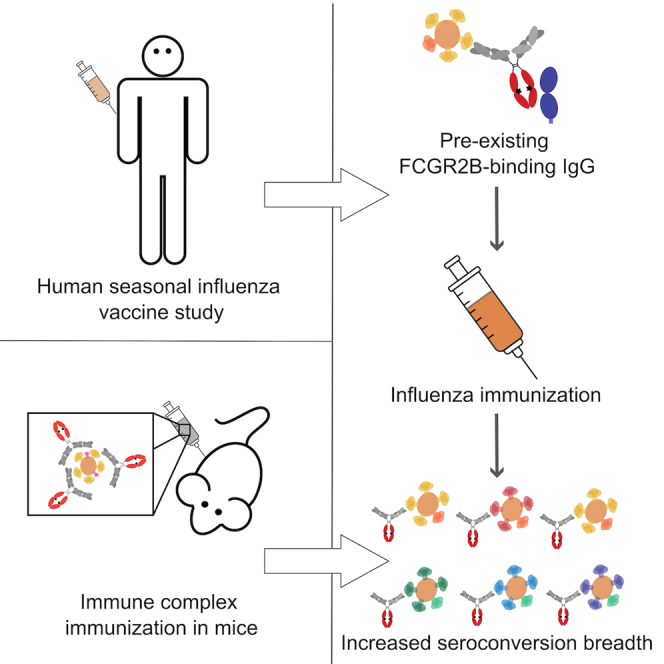
Graphical abstract
Highlights
-
•
FcγR2-binding antibodies develop with neutralizing antibodies after flu vaccination
-
•
FcγR2B binding prior to vaccination leads to broad neutralizing influenza immunity
-
•
FcγR2B phenotype is observed across two orthogonal patient cohorts
-
•
Pre-existing FcγR2B-binding antibodies increase neutralizing antibody titers in mice
Boudreau et al. study the response to influenza vaccination in humans and find that pre-existing antibodies capable of binding to inhibitory Fc γ receptor 2B enhance cross-strain neutralizing immunity after vaccination. This shaping of post-vaccine immunity is mirrored in a mouse model using influenza-specific antibody variants with enhanced binding to Fc receptors.
Introduction
Despite the availability of seasonal vaccines, influenza remains a major global health concern due to the limited effectiveness of vaccination (10%–60% depending on the season1). Vaccine breakthroughs are speculated to be caused by antigenic drift, unexpected strain mismatches, limited vaccination rates,2 and limited immune response durability. Furthermore, our incomplete understanding of the correlates of immunity against disease hinders evaluation of novel vaccine strategies. While our current vaccination strategies provide some level of annual protection against circulating viruses, current vaccines do not consistently induce broad immunity across historical and future influenza strains but rather induce strain-specific immunity.3 To this end, the development of a broad, “universal” influenza vaccine has been named a top priority by the National Institutes of Health.4
The ability of vaccine-induced antibodies to block viral receptor binding to the host cell is regarded as the correlate of protection of influenza vaccines.5 This neutralizing antibody function can be measured using a hemagglutination inhibition (HAI) assay.6 Notably, broad HAI has been documented in a small fraction of individuals,7 suggesting that broad HAI can evolve in response to vaccination and natural infection. However, vaccine design efforts to date have inconsistently induced robust breadth of immunity,7 largely due to a persistent failure in overcoming immunological imprinting. While vaccine design strategies have targeted conserved epitopes8 as a strategy to focus immunity on broadly reactive antigenic determinants across strains, it remains unclear how natural HAI breadth evolves and whether other key immunological mechanisms may be leveraged to drive broadly protective immunity to influenza.
To begin to define whether unique immunological mechanisms may underlie the evolution of HAI activity, here we applied an agnostic, comprehensive antibody-profiling strategy to a Discovery cohort of 108 individuals sampled before and 21 days after influenza vaccination and validated these findings in an additional Investigation cohort of 137 individuals followed over 4 years of seasonal influenza vaccination. HAI was performed on all subjects across 50 strains, providing a comprehensive opportunity to define the predictors of HAI conversion to the vaccine strain, as well as breadth of HAI to non-vaccine strains. Multivariate humoral profiling across the groups pointed to the presence of a unique Fc-effector signature among individuals that evolved HAI, with a selective FCGR2B binding signature among individuals that evolved broad HAI activity confirmed across the second Investigation cohort of vaccinees. Moreover, immunization of mice with FCGR2B-binding antibody-opsonized influenza virus resulted in potent humoral titers and HAI activity, providing unexpected insights into the potentially critical adjuvating activity of pre-existing influenza-specific antibodies that may be required for the evolution of broadly protective influenza-specific immune responses.
Results
Response to seasonal influenza vaccination is highly heterogeneous
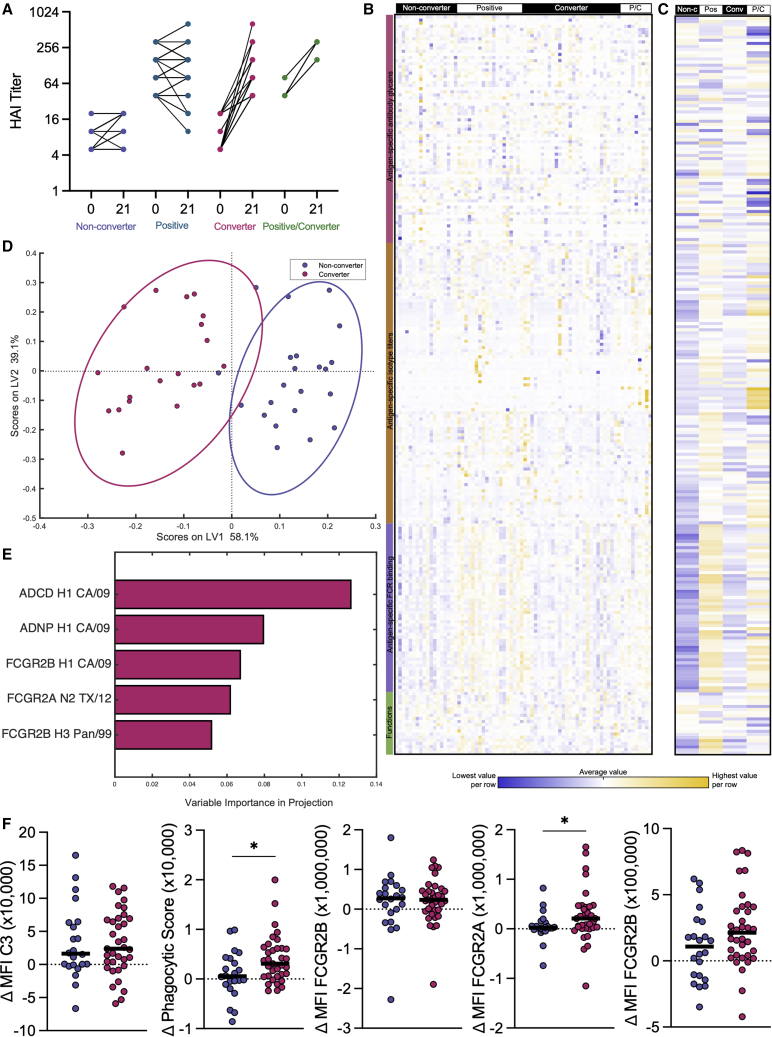
A Discovery cohort of 108 healthy adults was sampled prior to and 21 days following inactivated influenza vaccination (2013/2014 season).9 Serum samples were profiled for hemagglutinin inhibition (HAI) across 50 influenza strains (Table S1). Individual samples remaining from the clinical study9 were selected for inclusion in this in-depth analysis based on their HAI conversion profiles against H1N1 A/California/07/2009 and H3N2 A/Texas/50/2012, the World Health Organization-recommended influenza A vaccine strains for the 2013/2014 season. Annual HAI conversion profiles for each influenza strain could be divided into four patterns for each strain/subtype (Figure 1A for H1N1 A/California/07/2009): (1) individuals who were HAI negative (titer <1:40) who did not have at least a 4-fold increase in HAI titer (non-converters), (2) individuals who were HAI positive (titer ≥1:4010) prior to vaccination and did not experience significant (at least a 4-fold) increase in HAI titer (positive), (3) individuals who had sub-protective HAI titers (<1:40) prior to vaccination but seroconverted following vaccination (converters), and (4) individuals who were HAI positive prior to vaccination but also experienced a greater than or equal to 4-fold increase in HAI titer following vaccination (positive/converters). Samples were chosen so that the Discovery cohort represented all four of these response profiles equally for each year of the study across H1N1 and H3N2. Samples were matched, to the extent possible, for demographics (age, gender, comorbidities).
Figure 1.
Seasonal influenza vaccine produces highly heterogeneous responses in healthy adults
(A–F) Line plot (A) shows before and after HAI titers against H1N1 A/California/07/2009, with individuals broken out into groups based on HAI seroconversion as described. Non-converter n = 25, Positive n = 43, Converter n = 31, Positive/Converter n = 9. Heatmap (B) shows normalized Δ day 21 – day 0 values for all antibody biophysical and functional features measured with rows grouped by type of antibody feature measured. All features are listed in Table S2. Each column represents an individual subject, grouped by H1N1 A/California/07/2009 HAI response to vaccination. Yellow represents high values and blue represents low values. Smaller heatmap (C) shows median value for each H1N1 seroconversion group by column and the same antibody features in (B) by row. Dot plot (D) shows PLSDA of H1N1 A/California/07/2009 converters and non-converters graphed across latent variables 1 and 2. Percentages indicate amount of variation explained by each latent variable. Multivariate modeling in this analysis used Δ values calculated as the change from pre- to post-vaccination. Bar graph (E) shows LASSO-elastic net selected features contributing to the separation in (D) graphed by variable importance in projection (VIP) score. VIP scores indicate contribution to the model. Dot plots (F) show LASSO-elastic net selected features (Δ day 21 – day 0) as univariate plots. Significance was tested by Kruskal-Wallis test followed by Dunn’s multiple comparisons correction. ∗p < 0.05. This figure is composed of data from the Discovery cohort.
See also Figure S1.
To begin to define the humoral immune profile changes that could potentially provide mechanistic insights into these different vaccine response profiles, Systems Serology11 was used to deeply profile the biophysical and functional characteristics of the influenza-specific antibodies elicited by vaccination. Over 200 measurements were taken per serum sample, mapped across 23 influenza antigens (Table S2), resulting in a comprehensive analysis of the polyclonal humoral immune response to influenza vaccination. For each individual evaluated, a delta from pre- to post-vaccination was calculated to account for pre-existing immunity, enabling the comparison of vaccine-induced immunity. Striking heterogeneity in influenza-specific humoral immune responses was observed across the vaccinees, where no two individuals possessed an identical response (Figure 1B). The data were therefore collapsed into the four groups to begin to visualize potential differences in profiles across HAI seroconversion to the vaccine strain H1N1 A/California/07/2009 (Figure 1C). As expected, non-converters exhibited the lowest overall reactivity (in blue) compared with the other three groups (yellow). Conversely, robust induction of humoral immune responses was noted in HAI converters and positive/converters. Surprisingly, those individuals who had pre-existing HAI titers and did not respond with increased HAI-mediating antibodies to vaccination (HAI positive) still experienced a marked increase in influenza-specific activating FCGR binding antibody levels (Figures S1A–S1C). Distinct correlations were observed between HAI levels and FCGR-binding levels against the same H1N1 A/California/07/2009 virus (Figure S1D) across all HAI conversion groups. Specifically, while pre-existing levels of total binding antibodies, HAI, and FCGR-binding antibodies were highly correlated (Figure S1E), correlations between vaccine-induced changes, while still present, were not as strong and were variable across the FCGRs (Figure S1F). This suggests that while the development of HAI titers and FCGR binding are linked, vaccination can subtly and differentially tune HAI or FCGR-binding activities.
To gain a precise understanding of the specific vaccine-induced features that distinguished converters from non-converters to the vaccine strain H1N1 A/California/07/2009, all systems serology data were combined and a least absolute shrinkage and selection operator (LASSO)-elastic net12,13 was used to define the minimal set of antibody features required to discriminate converters and non-converters. Briefly, LASSO-elastic net algorithms attempt to explain the separation between groups using latent variables (LVs), which are mathematical combinations of multiple measured features. To prevent over-fitting in high dimensional datasets, such as this one, LASSO algorithms penalize the inclusion of additional features, which ultimately excludes highly correlated features from selection. The robustness of these models is evaluated using repeated 5-fold cross validations.12,13 Following this analysis, partial least-squares discriminant analysis (PLSDA), a supervised clustering algorithm, was then used to visualize the differences across the groups using the LVs identified by LASSO.
Significant discrimination was observed by HAI seroconversion status using antibody Fc features (Figure 1D) compared with random size-matched or permuted label models12,13 (p < 0.001, Figure S1G). The features selected by the LASSO algorithm can be ranked in their overall importance in the model depicted as a variable importance in projection (VIP) score, which includes each feature’s contribution/weight to separation across the groups. VIP scores illustrate the direction and enrichment score of a given feature that is differentially expressed; however, these scores are not a reflection of correlation. As few as five of the total 239 humoral features captured for each serum sample were sufficient to build this model (Figures 1E and 1F), pointing to a highly focused humoral signature of conversion. Interestingly, no features were enriched in the non-converters, pointing to limited negative predictors of HAI conversion. Conversely, while antibody titers were not a correlate of conversion, the “converter signature” included vaccine strain-specific antibody-dependent complement deposition, antibody-dependent neutrophil phagocytosis, and binding to both the activating and inhibitory FCGR2 receptors. Given that univariate differences across the five “correlates of conversion” were modest (Figure 1E), these data point to the importance of a functional multivariate signature of conversion, indicating that the evolution of HAI occurs in concert with a significant shift in Fc-biology.
Defining correlates for the evolution of breadth of HAI
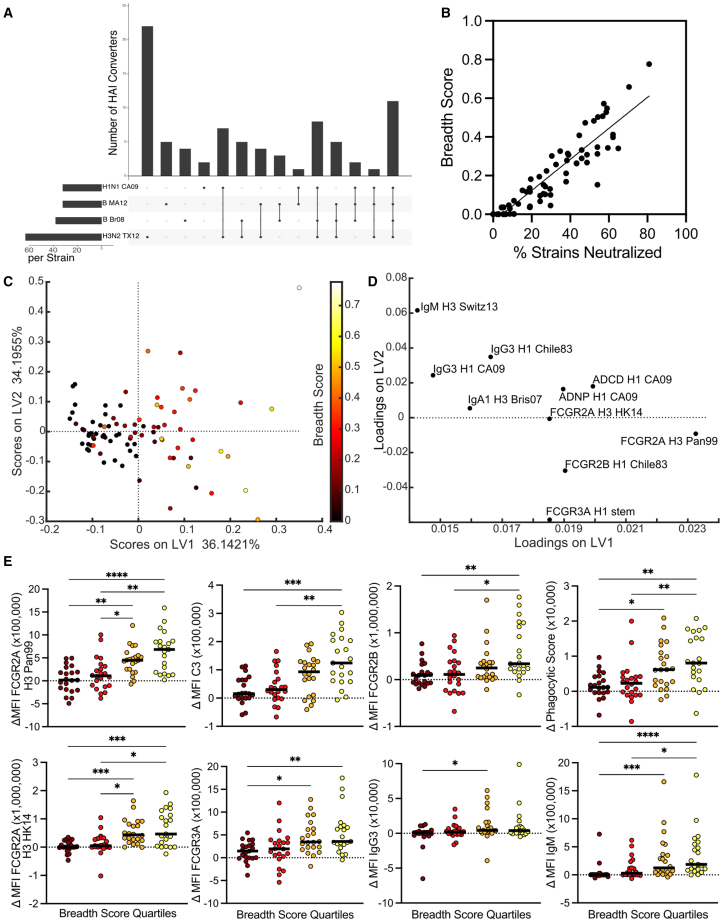
Across this Discovery cohort, differences were seen in seroconversion to different viral strains (Figures 1A and S1H–S1J), with more individuals seroconverting to the H3N2 strain A/Texas/50/2012 than to the H1N1 A/California/07/2009 (Figure 2A). However, interestingly, when we examined how many individuals converted their HAI responses to both strains, only a small fraction (∼25%) of individuals converted simultaneously to both strains (Figure 2A). Furthermore, an even smaller fraction (∼10%) showed seroconversion to all four strains included in the quadrivalent vaccine (Figure 2A). Despite a mild effect of age/immunological imprinting in the magnitude of pre-existing HAI titers across tested strains (Figure S2A), there was no striking difference in vaccine response by initially imprinted subtypes (Figure S2B).
Figure 2.
FCGR-binding titers are correlates of the development of neutralization breadth
(A–E) UpSet chart (A) shows number of individuals who seroconvert to a given vaccine strain or strains (n = 108). Vertical bars show seroconversion groups across all possible combinations of four vaccine strains. Horizontal bars show total individuals seroconverting to each strain, regardless of other strains seroconverted. Dot plot (B) shows correlation between percentage of strains seroconverted and breadth score across all strains included in Table S1. Dot plot (C) represents PLSR for delta values regressed against breadth in the test cohort. Loadings plot (D) represents relative contributions of LASSO-elastic net selected features for the model, with features plotted to represent where they have highest values if overlaid on the PLSR plot. Dot plots (E) show significantly different LASSO-elastic net selected features as univariate plots binned by quartile of breadth score. Significance (E) was tested by Kruskal-Wallis test followed by Dunn’s multiple comparisons correction. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. This figure shows data from the Discovery cohort.
See also Figure S2.
Given the ultimate goal of defining the predictors of broad seroconversion across vaccine and emerging strains, we calculated a breadth score, encompassing the normalized average pairwise HA genetic sequence distance between strains to which an individual seroconverted (including a panel of historical strains not included in the vaccine). A breadth score of 0 indicated seroconversion to no or only one strain, whereas higher scores indicated increased breadth of seroconversion. Furthermore, conversion of HAI to two strains that have low sequence similarity resulted in a higher breadth score than conversion of HAI to two genetically similar strains. A seroconversion breadth score was generated for each individual and compared with percentage of strains neutralized. Individuals with percentage values from 0% to 20% represented those with low total number of strains neutralized; however, within this group the breadth score and the percentage neutralized were poorly correlated, as some individuals converted to a small number of genetically distant strains leading to a low percentage but relatively higher breadth (Figures 2B and S2C). On the high end, percentages above 60% indicated individuals who seroconverted to a large number of strains, and again within this group the breadth score allowed discrimination between those who converted to a large number of genetically similar strains (e.g., breadth scores <0.5) and those who converted to a large number of genetically different strains. Because of the strains included (Table S1), the breadth score here largely represented retrospective breadth. Future analysis with a larger panel of strains could probe prospective predictors of the evolution of breadth.
Using the seroconversion breadth score, we next explored which humoral features developed in concert with the evolution of breadth using LASSO-elastic net and partial least-squares regression (PLSR). Using the change in antibody humoral features from pre- to post-vaccination (delta), individuals with broader responses were separated across LV1 (Figure 2C, model p < 0.001, Figure S2D). Specifically, 10 of a total of 239 analyzed features per plasma sample were sufficient to separate individuals who evolved broad neutralizing responses. The features overlapped with features captured in the single HAI conversion model (Figure 1) and included FCGR2A, FCGR2B, FCGR3A, and functional titers to both vaccine strains and additional strains not included in the vaccine (Figures 2D, 2E, and S2E). Interestingly, the strains in the breadth panel included in this analysis reflected a wide diversity of H3N2 influenza HA antigens, spanning nearly 20 years of antigenic drift. For H1N1, a more focused response on the 2009 pandemic strain was observed. This accumulation of Fc features, rather than simply binding titers, again emphasized the involvement of the Fc-region in Fab evolution.
Validation of neutralizing breadth correlates in an orthogonal Investigation cohort of vaccinees
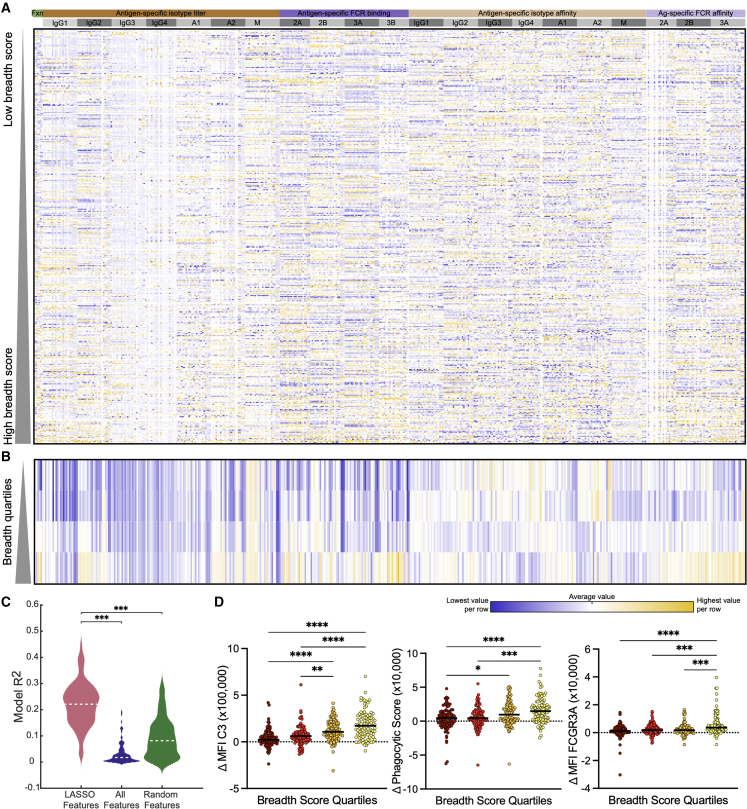
Given the potential role of Fc profiles in tuning both conversion to a single HA as well as in the development of breadth, we next aimed to validate these signatures in an orthogonal cohort of vaccinated individuals (Investigation cohort). Specifically, influenza-specific systems serology was performed in the second Investigation cohort of 137 vaccinated individuals followed over multiple years for a total of 430 pairs of before and after vaccination samples. Over 450 distinct humoral features, including antigen-specific antibody-dependent functions, subclass/isotype titers, FCGR binding titers, and isotype-and FCGR-specific affinities, were mapped in the Investigation cohort across 23 influenza antigens (HA and NA) representing 15 distinct strains (Table S2). Like the first Discovery cohort, the Investigation cohort showed a broad heterogeneity in response to vaccination across the antibody functional and biophysical features measured (Figure 3A). Patterns emerged across individuals, with visibly enhanced functionality and Fc-receptor binding among individuals with higher breadth scores. This was more apparent when individuals were grouped into breadth quartiles (Figure 3B). To test the generalizability of the correlates of breadth defined in the first Discovery cohort, we built a model using the features selected using the Discovery cohort but used the data from the second cohort. The five features selected in the Discovery model were able to significantly separate the converters from non-converters in the second, independent Investigation cohort (Figure 3A).
Figure 3.
Validation of breadth findings in an independent Investigation cohort
(A–D) Heatmap (A) shows normalized Δ day 21 – day 0 values for all antibody biophysical and functional features measured with columns grouped by type of antibody feature measured. Each row represents a specific season of an individual subject, ordered by HAI conversion breadth score (n = 387). Yellow represents high values and blue represents low values. Smaller heatmap (B) shows median value for each breadth score quartile by row and the same antibody features in (A) by column. Violin plot (C) shows validation model system in the Investigation cohort, comparing the LASSO-elastic net selected features, to all features, to a random equal-sized subset of features. Dot plots (D) show a subset of LASSO-elastic net selected features as univariate plots binned by quartile of breadth score. Significance in (C) and (D) was tested by Kruskal-Wallis test followed by Dunn’s multiple comparisons correction. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
See also Figure S3.
In addition, breadth scores were calculated for the Investigation cohort using the same approach used on the Discovery cohort, with breadth scores ranging from 0 to 0.73, which reflected a wide range of neutralizing breadth (Figures S3B and S3C). To validate our previous findings, a model was built for the evolution of breadth of neutralization based on the 10 features selected using the Discovery cohort (Figure 2D). When applied to the Investigation cohort, these 10 features were able to robustly differentiate individuals across neutralizing breadth (Figure 3C). These data suggest that conserved Fc profiles are linked to the development of broad neutralizing responses across individuals, with orthogonal validation highlighting that these features are not unique to one cohort but represent conserved features of a broadly neutralizing polyclonal humoral immune response. Furthermore, analysis of these humoral features on a univariate basis shows significant differences developing in coordination with neutralizing breadth (Figures 3D and S3D). This emphasized and reinforced the findings from the Discovery cohort, showing that a broad neutralizing response to vaccination develops in concert with extra-neutralizing functions driven by the binding of influenza-specific antibodies to FCGRs.
Pre-existing FCGR-binding antibody titers predict a broad neutralizing vaccine response
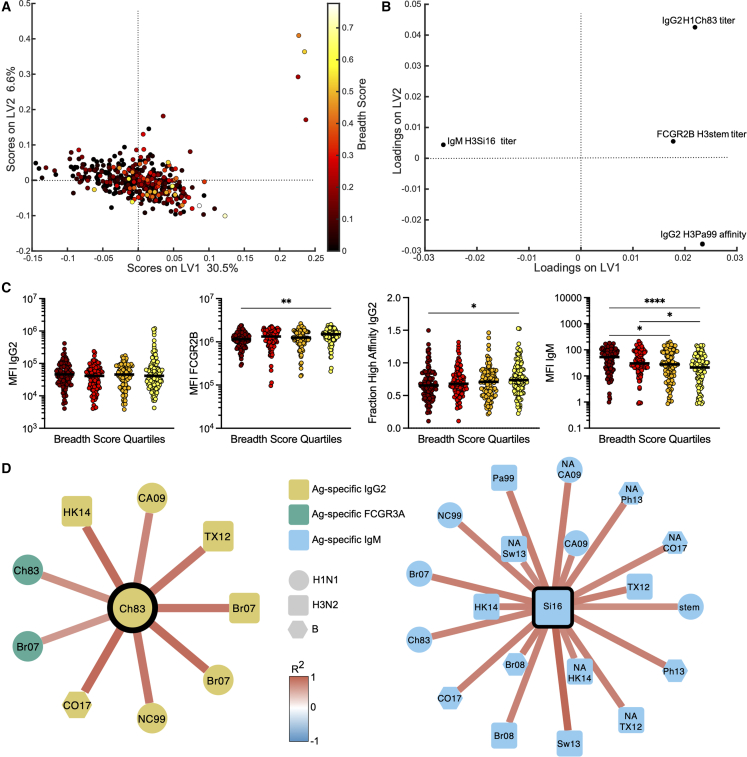
Collectively, these data pointed to the existence of conserved Fc-signatures that co-evolve with breadth of neutralization across cohorts. However, whether any Fc features exist prior to immunization that could help predict or explain the response to vaccination remains incompletely understood. Thus, we next aimed to determine whether the evolution of breadth could be predicted from pre-vaccine humoral profiles. LASSO-PLSR was performed on day 0 systems serology data from the Investigation cohort, where more samples were tested to increase power to see predictive differences. The model successfully discriminated individuals who evolved broad neutralizing antibody responses (Figure 4A, p < 0.001, Figure S4) using only pre-existing humoral profiles.
Figure 4.
FCGR2B-binding antibodies are a predictor of neutralizing breadth
(A–D) Dot plot (A) represents PLSR for day 0 values regressed against breadth score in the Investigation cohort (n = 387). Loadings plot (B) represents relative contributions of LASSO-elastic net selected features for each model. Dot plots (C) represent univariate values for the LASSO-elastic net selected features, with individual samples binned by quartile of breadth score. Significance was tested by Kruskal-Wallis test followed by Dunn’s multiple comparisons correction. ∗p < 0.05. Networks (D) show significant (R > 0.6 and false discovery rate-corrected Q < 0.01) co-correlates of LASSO-elastic net selected features as shown in (B), where stronger red edges indicate stronger correlations and node shape and color reflects strain and measurement type. All antigens are HA unless otherwise noted. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
See also Figure S4.
Among the 524 features analyzed per sample in the Investigation cohort, as few as four features were required to predict the evolution of breadth. Specifically, these features included titers of immunoglobulin (Ig)G2 antibodies specific to H1 A/Chile/1/1983, FCGR2B binding antibody titers to conserved stem of H3, affinity of IgG2 antibodies for H3 A/Panama/2007/1999, and IgM titers specific to H3 A/Panama/2007/1999. Among the predictive features, HA-specific FCGR2B binding antibodies were selectively enriched among individuals who elicited the broadest responses (Figures 4B and 4C). FCGR2B-binding antibody levels, rather than IgG levels, were positively associated with breadth of seroconversion highlighting the importance of quality, not just quantity, of pre-existing antibodies in the evolution of breadth. While FCGR2A and FCGR2B share high-sequence homology,14 emerging studies have highlighted differences in antibody binding profiles across the receptors,15,16,17 even enabling the evolution to monoclonal antibodies able to bind to these receptors with different affinities.18 In addition, pre-existing avidity/affinity and IgG2 antibody titers were also among the predictors that bind with lower affinity to Fc receptors,19 potentially protecting antigens from rapid uptake and degradation and were also hypothesized to preferentially drive presentation on pDCs, as opposed to other IgG subclasses that drive antigen destruction by monocytes/neutrophils.20 Conversely, IgM antibody titers were a negative predictor of neutralizing breadth (Figures 4B and 4C), possibly related to the overly efficient role of IgM in opsonization and rapid antigen clearance prior to deposition in germinal centers.
Because LASSO aims to select a minimal set of features to avoid statistical over-fitting, the model only selects a single feature that may represent a group of highly correlated features. These correlated, but not LASSO-selected, features potentially provide additional biologically relevant insights on antibody mechanism of action. Thus, we next probed the additional co-correlates of the model-selected features to gain a deeper appreciation of the biological differences across the groups. Thirty highly significant (R > 0.6 and FDR-corrected Q < 0.01) co-correlates were identified (Figure 4D). Co-correlates of the positive predictor IgG2 titer included a variety of H1N1, H3N2, and influenza B antigen-specific IgG2 titers, as well as FCGR3A binding antibodies to the two H1 antigens (Figure 4D, left), indicating coordination across both the Fab and Fc domains. Interestingly, IgG2 levels to CH83 HA marked a larger cluster of IgG2 responses to H1, H3, and B strains and H1-FCGR3A, highlighting a network of IgG2-responses, rather than CH83-specificity alone as a critical marker of neutralization breadth. Similarly, the negative IgM co-correlate was linked to a broad array of H1N1, H3N2, and flu B antigen IgM titers, emphasizing that the antigenic specificity is not the relevant consideration here but rather that an enrichment of IgM responses may trap vaccine antigen and drive narrower immunity, potentially via rapid antigen destruction. Together, these linked features suggest that a broad pre-existing IgM repertoire may inhibit the development of a broad neutralizing response. Conversely, the existence of pre-existing IgG antibodies capable of binding to FCGRs may enhance the developing immune response.
Pre-existing FCGR2B-binding antibodies drive HAI in mice
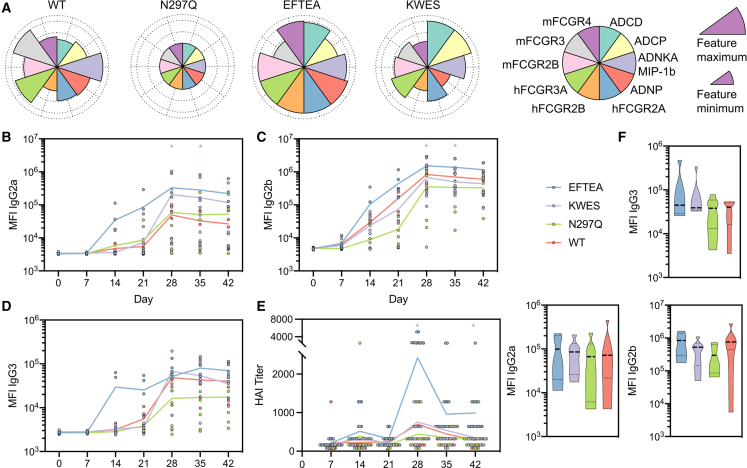
Given the enrichment of particular IgG subclasses with FCGR2B-binding properties both as pre- and post-vaccine predictors of evolution of HAI and breadth of HAI, we next aimed to test whether these features were directly linked to the evolution of HAI. While subclasses differ across humans and mice, we assessed whether binding to FCGR2B was key to the evolution of HAI. Previous data have suggested that pre-existing antibodies could “block” epitopes21,22 by obscuring key sites on the immune-complexed antigen. Thus, here, an HA stem-specific binding antibody, CR9114,23 and not an HA head binding antibody, was selected to ensure that the head was unobscured at the time of delivery to the lymph node. A panel of antibody Fc variants was engineered on a broadly cross-reactive HA stem-specific CR9114 monoclonal antibody,24,25 including a wild-type (WT) human IgG1 backbone; an IgG1-Fc with an N297Q mutation,25 known to ablate FCGR binding and complement activity; a KWES mutant IgG1 Fc,26 known to exhibit increased complement and monocyte phagocytic activity; and an EFTEA mutant IgG1 Fc,27 known to increase FCGR2B and general FCGR binding. Each variant was tested in our systems serological assays for antibody-mediated innate immune functions and binding across both mouse and human FCGRs (Figures 5A, S5A, and S5B). WT IgG1 CR9114 showed moderate phagocytic and complement activity and strong natural killer (NK) cell activation, while N297Q decreased binding to all FCGRs and functions to background levels, as expected. The KWES mutant showed the highest level of complement and monocyte phagocytosis, but with decreased overall FCGR binding. Finally, the EFTEA mutant showed high levels of binding to human and mouse FCGR2s and generally mid to high binding of FCGRs across both mouse and human. Furthermore, the EFTEA mutant enhanced neutrophil phagocytosis and complement fixation. Importantly, we observed a strong correlation between human and mouse FCGR binding, with EFTEA demonstrating high human and mouse FCGR2, and especially FCGR2B, binding. The use of these engineered stem-specific monoclonal antibodies, with differential FCGR binding activities, allowed for the controlled hypothesis testing on the role of particular FCGR-binding and innate immune functionality on shaping the evolution of HAI, although the use of differential FCGR binding polyclonal antibodies could also provide additional insights.28,29
Figure 5.
Presence of FCGR-binding antibodies in immunization drives improved humoral immune response in mice
(A–C) Flower plots (A) show relative capacity of each Fc-mutant of CR9114 to bind to FCGRs (both mouse and human) and elicit antibody-dependent functionalities, normalized to the minimum and maximum observed values for each feature. Line graphs show HAI titers (B) of mouse serum against vaccine strain H3N2 X-31 and antibody isotype Luminex MFIs (C–E) against vaccine strain recombinant H3 A/Aichi/2/1968 (n = 5–6 mice per group). Significance tested by two-way ANOVA with Dunnett’s multiple comparison test, ∗p < 0.5 for that isotype compared with WT IgG1. Violin plots (F) show antibody isotype Luminex MFIs at day 28 against vaccine strain recombinant N2 A/Aichi/2/1968.
See also Figure S6.
Using this panel of Fc-variant antibodies that bound to HA equally (Figure S5C) but differentially to Fc receptors/complement (Figure 5A), immune complexes were generated with inactivated influenza virus to mimic the current vaccine, then incubated with mAbs to mimic immune complexes formed with pre-existing antibodies. Mice were then immunized and serum samples were collected weekly to measure the development of both antigen-specific antibody titers (Figures 5B–5D) and HAI (Figure 5E). Mice immunized with WT immune complexes exhibited a robust response to vaccination following boosting across antibody subclasses (Figures 5B–5D), with similar performance by all Fc variants, except for the EFTEA variant, which induced higher titers across subclasses. The WT and KWES variants induced comparable HAI (Figure 5E), with a trend toward reduced HAI with the N297Q variant, confirming the importance of Fc-binding to improved Fab qualitative evolution. Conversely, the EFTEA variant, which exhibited enhanced binding to both human and mouse Fcg2B, exhibited the most rapid antibody titer evolution across subclasses (Figures 5B–5D), as well as the most robust HAI induction (Figure 5E). Importantly, no differences were noted in the evolution of NA-specific antibody titers, further suggesting that the target of the pre-existing monoclonal antibody specifically focuses the response to precise immunological targets (Figure 5F). These data clearly confirm the importance of pre-existing antibodies in shaping the evolution of the humoral immune response, and suggest that broad FCGR binding, and specifically FCGR2B binding, by pre-existing antibodies may accelerate the speed of class switching and the evolution of HAI.
Discussion
The rapid spread and unpredictable disease caused by SARS-CoV-2 and its variants has clearly illustrated the urgent need for vaccines against pathogens that have the potential to cause pandemics. While multiple vaccines exist for influenza, these vaccines largely confer strain-specific immunity, requiring annual redesign and delivery. However, emerging data suggest that broadly reactive influenza-specific immune responses can evolve in rare individuals.23 Thus, understanding the mechanisms by which these individuals are able to induce protective immune responses may provide critical insights for the design of next-generation vaccines that capitalize on this biology to drive enhanced immunity to influenza and protection against future emerging strains. Both in vitro and in vivo data presented here point to the critical role for pre-existing FCGR-binding IgG antibodies as potential adjuvants for the evolution of qualitatively superior humoral immune responses against influenza. Thus, next-generation vaccines able to harness the immunomodulatory role of pre-existing antibodies or deliver similar signals may improve the breadth and quality of the vaccine-induced immune response to influenza and beyond.
The development of a broad and effective humoral immune response to vaccination is dependent on both antigen specificity and Fc-functionality. Here, we observed that HAI seroconversion to a single vaccine antigen occurred in concert with the evolution of several opsonophagocytic functions. Specifically, A/California/07/2009 HAI evolved in tandem with increased levels of H1 A/California/07/2009-specific antibody-mediated complement deposition, neutrophil activation, and FCGR2B binding. In addition, HAI conversion also evolved in concert with FCGR2A-binding neuraminidase (NA) responses, highlighting the presence of broader, more holistic responses to the vaccine among individuals who seroconverted in response to the vaccine. Given the emerging appreciation for the importance of NA-specific responses to protection against influenza,30 these data suggest that functional humoral immunity to both targets may contribute to enhanced HAI conversion, potentially via enhanced vaccine antigen-trapping. Moreover, both complement and neutrophil functions have been implicated in protective immunity against influenza,31,32 thus co-evolution of HAI and opsonophagocytosis to multiple antigenic targets may collectively drive the broadest immunity. These data highlight the co-evolution of multiple lines of protective humoral immunity, pointing to an overall enhanced immune response to influenza.
“Original antigenic sin”33 has been proposed to prevent the evolution of neutralization breadth via the selective expansion of responses to a limited set of antigens targeted by pre-existing responses to influenza.34,35,36 The agnostic nature of systems serology allowed us to broadly profile the humoral immune response across many historical strains, contemporaneous strains, and “future” strains relative to when the samples were drawn. Interestingly, IgM titers to H3 A/Singapore/INFIMH/16–0019/2016 was the sole feature negatively associated with the development of vaccine-induced breadth. Importantly, IgM titers to H3 A/Singapore/INFIMH/16–0019/2016 were correlated with IgM responses across several tested virus antigens, arguing that IgM responses prior to vaccination may directly interfere with the evolution of breadth or mark a less responsive immune profile. IgM deposition on antigens/pathogens results in rapid complement activation37 and opsonophagocytic uptake via complement receptors38 that may lead to rapid antigen degradation.39 Conversely, Fcg2B binding and IgG2 antibodies were enriched in individuals who developed breadth. IgG2 antibodies have poor affinity for complement and Fc receptors40 and thus likely create complexes that are poorly phagocytosed and not readily cleared by immune cells. Whether the observed enhanced binding to FCGR2B is related to differences in Fc glycosylation differences or related to differences in the geometry of antibody binding to the target remains unclear but represents an unexplored area of immunology. However, mechanistically, FCGR2B-binding immune complexes may bind to FCGR2B on the surface of circulating B cells, enabling rapid traffic into germinal centers. In the germinal center, FCGR2B expression on follicular dendritic cells (FDCs) likely competes for immune complexes on incoming B cells and ultimately captures immune complexes that can then be presented to evolving B cells.29,41 Thus, the ability of pre-existing antibodies to redirect the response to either myeloid or follicular DCs may contribute mechanistically to shifting the response to vaccination, increasing affinity maturation in germinal centers and the evolution of broader neutralization.42,43,44
To define whether these observed changes in Fc profiles were simple biomarkers or mechanistic players in the evolution of HAI breadth, immune complex-based immunization was performed in mice. Given the homology of the human and murine Fc-receptor system, where human IgG1 binds murine FCGRs and FcRn,45,46 we were able to select a set of Fc-modifications that tuned human and murine Fc-receptor binding in an analogous manner. However, some differences exist between murine and human FCGR function and affinity for antibodies. ADCC in mice is primarily mediated by monocytes/macrophages via FCGR4,47 rather than exclusively by FCGR3A on NK cells in humans.45 In addition, mice do not express FCGR2A, instead relying on FCGR3 as a more generalized activating receptor.19 In contrast, FCGR2B expression and function are relatively conserved across mice and humans, with this lone inhibitory FCGR serving to limit the development of auto-antibodies, heighten the affinity threshold in germinal centers, maintain tolerance, and decrease other pro-inflammatory functions of cells.48,49,50
Mimicking our observations in these human studies, the EFTEA Fc variant, which displays the most robust FCGR2B binding, exhibited rapid induction of antibody titers as well as HAI. Despite the fact that the EFTEA variant exhibited the single highest binding to both the murine and human FCG2B receptors, the EFTEA antibody also showed enhanced binding to a wide array of Fc receptors. In contrast, the KWES mutant enhanced complement but not neutrophil phagocytosis or FCGR binding and did not enhance the development of HAI. Combined with previous studies indicating that IgG1 and FCGR2A-binding variants resulted in comparable antibody responses and HAI activity following challenge,51,52 these data suggest that FCGR2B specifically, rather than the fixation of complement or activating Fc receptors, is likely to be key in the adjuvanting activity of antibodies. This observed mechanism supports previous studies indicating that FCGR2B-binding antibodies are important to the development of neutralization against influenza,29 likely acting through its dual role in the germinal center, both raising the B cell activation threshold and trapping antigen-containing immune complexes on FDCs.41 As IgM was a negative predictor of the evolution of HAI breadth in humans, the functional comparison of EFTEA to the KWES mutant also provides important insights. While IgM also fixes complement, similar to EFTEA, IgM does not interact with the FCGRs. Like IgM, KWES likely drives rapid clearance of the antigen via complement-mediated opsonophagocytic pathways, resulting in delayed antibody evolution compared with EFTEA. The fact that KWES also did not accelerate the induction of humoral immunity, supporting the notion that the complement-mediated opsonophagocytic functions of IgM may not augment humoral immunity. Instead, while EFTEA also is able to fix complement and drive opsonophagocytosis, it is plausible that its higher affinity for FCGR, and specifically FCGR2B, may be the key to driving enhanced humoral immunity. Thus, future studies using larger panels of variants may further dissect the precise Fc-receptor binding patterns required for optimal evolution of the Fab and the immunological mechanisms occurring in the germinal center following seasonal influenza vaccination, including in populations with FCGR2 polymorphisms previously linked to susceptibility to systemic lupus erythematosus53 and malaria.54 Furthermore, these studies may probe the interaction between the Fab and Fc portions of the antibody as epitope specificity has already been shown to play a critical role in ADCC activity.55,56 Future studies may further elucidate the interplay between multi-FCGR binding profiles, using polyclonal antibody transfers.
Altogether, the in vitro and in vivo findings from this study emphasize the importance of systems-level immunological dissection, together with mechanistic studies of the humoral immune response, to identify unexpected insights into the key mechanics that may be required to achieve broadly protective immunity to influenza. Those individuals in this study who exhibited enhanced neutralizing breadth and elevated FCGR binding antibody/activity benefited by potentially being protected against strains of influenza arising in the years following vaccination. Furthermore, the presence of these antibodies at the time of vaccination may be sufficient to drive an enhanced vaccine response. Emerging data argue that distinct inflammatory responses, induced, for example, by adjuvants,57 could shape antibody Fc profiles and thereby drive enhanced Fab activity. Thus, whether next-generation vaccines seek to include existing or emerging adjuvants,58 prime-boost strategies with chimeric antigens,59 or targeted exploitation of pre-existing antibodies,60 several avenues now exist to rationally exploit the correlates of breadth identified here. These data suggest that future influenza vaccine studies, in particular those exploiting repeated boosting, should include some considerations for the recruitment and elicitation of FCGR-binding antibodies.
Limitations of the study
Although this study focused on the identification of signatures of the evolution of breadth across two orthogonal cohorts, whether the same signatures would evolve across geographic cohorts as well as in the setting of distinct vaccine platforms is not yet known. Moreover, the involvement of memory B cells and T cells that may also contribute to original antigenic sin, was not addressed in this study. Whether adjuvants or other alternative vaccine strategies may overcome the influence of pre-existing antibodies also remains unclear. Thus, future systems immunology studies able to probe the importance of pre-existing IgM and FCGR immunity across vaccine platforms and capturing both cellular and humoral drivers of antigenic competition may provide critical insights for the design of future vaccines able to drive universal immunity across populations.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-human IgG1 PE | Southern Biotech | Cat#9052-09; RRID: AB_2796621 |
| Mouse anti-human IgG2 PE | Southern Biotech | Cat#9060-09; RRID:AB_2796635 |
| Mouse anti-human IgG3 PE | Southern Biotech | Cat#9210-09; RRID:AB_2796701 |
| Mouse anti-human IgG4 PE | Southern Biotech | Cat#9200-09; RRID: AB_2796693 |
| Mouse anti-human IgG-Fc PE | Southern Biotech | Cat#9040-09; RRID: AB_2796601 |
| Mouse anti-human IgA1 PE | Southern Biotech | Cat#9130-09; RRID: AB_2796656 |
| Mouse anti-human IgA2 PE | Southern Biotech | Cat#9140-09; RRID: AB_2796664 |
| Mouse anti-human IgM PE | Southern Biotech | Cat#9020-09; RRID: AB_2796577 |
| Mouse anti-human CD66b Pacific Blue | BioLegend | Cat#305112; RRID: AB_2563294 |
| Goat anti-guinea pig C3 FITC | MP Biomedical | Cat#0855371 |
| CD107a PE-Cy5 | BD Biosciences | Cat#555802; RRID: AB_396136 |
| CD56 PE-Cy7 | BD Biosciences | Cat#557747; RRID: AB_396853 |
| CD16 APC-Cy7 | BD Biosciences | Cat#557758; RRID: AB_396864 |
| CD3 Pacific Blue | BD Biosciences | Cat#558117; RRID: AB_397038 |
| MIP-1β PE | BD Biosciences | Cat#550078; RRID: AB_393549 |
| IFN-Ɣ FITC | BD Biosciences | Cat#340449; RRID: AB_400425 |
| HLA-DR FITC | Biolegend | Cat#307604; RRID: AB_314682 |
| CD86 PE-Cy7 | BD Biosciences | Cat#561128; RRID:AB_10563077 |
| CD83 APC-Cy7 | Biolegend | Cat#305330; RRID: AB_2566393 |
| Goat Anti-Mouse IgG1, Human ads-PE | Southern Biotech | Cat#1070-09S; RRID: AB_2794415 |
| Goat Anti-Mouse IgG2a, Human ads-PE | Southern Biotech | Cat#1080-09S; RRID: AB_2794415 |
| Goat Anti-Mouse IgG2b, Human ads-PE | Southern Biotech | Cat#1090-09S; RRID: AB_2794524 |
| Goat Anti-Mouse IgG3, Human ads-PE | Southern Biotech | Cat#1100-09S; RRID: AB_2794577 |
| Goat Anti-Mouse IgM, Human ads-PE | Southern Biotech | Cat#1020-09S; RRID: AB_2794205 |
| CR9114 Fc variant antibodies | This manuscript | N/A |
| Bacterial and virus strains | ||
| H3N2 X-31 | BEI Resources | Cat#NR-3483 |
| Stellar competent cells | Takara | Cat#636766 |
| Biological samples | ||
| Lyophilized Guinea Pig Complement | Cedarlane | Cat#CL-4051 |
| Chicken Red Blood Cells | Rockland | Cat#R302-0050 |
| Chemicals, peptides, and recombinant proteins | ||
| H1 A/California/07/2009 | Laboratory of Dr. Ted Ross | N/A |
| N1 A/California/07/2009 | Laboratory of Dr. Ted Ross | N/A |
| H1 A/Brisbane/59/2007 | Laboratory of Dr. Ted Ross | N/A |
| H1 A/Chile/1983 | Laboratory of Dr. Ted Ross | N/A |
| H1 A/New Caledonia/20/1999 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Texas/50/2012 | Laboratory of Dr. Ted Ross | N/A |
| N2 A/Texas/50/2012 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Brisbane/10/2007 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Hong Kong/4108/2014 | Laboratory of Dr. Ted Ross | N/A |
| N2 A/Hong Kong/4108/2014 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Panama/2007/1999 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Singapore/19/2016 | Laboratory of Dr. Ted Ross | N/A |
| H3 A/Switzerland/9715293/2013 | Laboratory of Dr. Ted Ross | N/A |
| HA B/Brisbane/60/2008 | Laboratory of Dr. Ted Ross | N/A |
| HA B/Phuket/3073/2013 | Laboratory of Dr. Ted Ross | N/A |
| HA B/Colorado/06/2017 | Laboratory of Dr. Ted Ross | N/A |
| H1 stem | Laboratory of Dr. Daniel Lingwood | N/A |
| H3 stem | Laboratory of Dr. Daniel Lingwood | N/A |
| N1 A/California/07/2009 | Laboratory of Dr. Florian Krammer | N/A |
| EBOV GPdTM | Mayflower Bioscience | Cat#0501-016 |
| H3 A/Aichi/2/1968 | Immune Technology Corp. | Cat# IT-003-0043ΔTMp |
| Human IL-15 recombinant protein | ThermoFisher Scientific | Cat#BMS319 |
| Golgistop | BD Biosciences | Cat#554724 |
| FIX & PERM Cell Permeabilization Kit | ThermoFisher Scientific | Cat#GAS004 |
| Streptavidin-R-Phycoerythrin | Prozyme | Cat#PJ31S |
| Human FCGR2A (R) | Duke Human Vaccine Institute | N/A |
| Human FCGR2B | Duke Human Vaccine Institute | N/A |
| Human FCGR3A (V) | Duke Human Vaccine Institute | N/A |
| Human FCGR3B | Duke Human Vaccine Institute | N/A |
| Human FCRN | Duke Human Vaccine Institute | N/A |
| Brefeldin A | Sigma-Aldrich | Cat#B7651-5MG |
| Gelatin Veronal Buffer with Magnesium & Calcium | Boston Bioproducts | Cat#IBB-300 |
| IdeZ Protease | New England Biolabs | Cat# P0770S |
| Imject Alum | ThermoFisher Scientific | Cat#77161 |
| Critical commercial assays | ||
| RosetteSep Human NK Cell Enrichment Kit | Stem Cell Technologies | Cat#15065 |
| GlycanAssure APTS Kit | ThermoFisher Scientific | Cat#A28676 |
| Experimental models: Cell lines | ||
| THP-1 cells | ATCC | Cat#TIB-202 RRID: CVCL_0006 |
| Expi293 cells | ThermoFisher Scientific | Cat#A14635 |
| Experimental models: Organisms/strains | ||
| Mouse: Female C57BL6/J | The Jackson Laboratories | Strain#000664 |
| Recombinant DNA | ||
| pUC19 vector | New England Biolabs | Cat#N3041S |
| Fc variant Golden Gate Cloning reagents | Laboratory of Dr. Galit Alter | Gunn et al.21 |
| Software and algorithms | ||
| Forecyt | Sartorius (Intellicyt) | https://www.sartorius.com/en/products/flow-cytometry/flow-cytometry-software |
| GraphPad Prism | GraphPad | https://www.graphpad.com/ |
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| R | R Project | https://www.r-project.org |
| Cytoscape | Cytoscape Consortium | https://cytoscape.org/ |
| GlycanAssure | ThermoFisher Scientific | N/A |
| Other | ||
| MagPlex Microspheres | Luminex Corp. | Cat#MC12001-01, MC12003-01, MC12005-01, MC10008-YY, MC10012-YY, MC10015-YY, MC10020-YY, MC102024-01, MC10026-YY, MC10044-YY, MC10045-YY, MC10055-YY |
| FluoSpheres Carboxylate-modified Microspheres, 1.0 μm, yellow-green fluorescent | ThermoFisher Scientific | Cat#F8776 |
| FluoSpheres Carboxylate-modified Microspheres, 1.0 μm, red fluorescent | ThermoFisher Scientific | Cat#F8775 |
| CD14 microbeads | Miltenyi | Cat#130-050-201 |
| Protein A/G Plus Agarose | ThermoFisher Scientific | Cat#20423 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Dr. Galit Alter (ragonsystemserology@mgh.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human subjects
Samples for the initial dataset were selected from a pool of available samples from a previous study61 conducted in Pittsburgh, Pennsylvania and Stuart, Florida and approved by the Western Institutional Review Board and the Institutional Review Boards of the University of Pittsburgh and the University of Georgia. Participants were enrolled with written informed consent. Samples were collected at the time of vaccination (day 0) and 21-28 days post-vaccination (day 21). Samples were chosen for inclusion in this study’s initial Discovery cohort to answer key questions regarding breadth and durability of the humoral immune response following seasonal vaccination. To address breadth, subjects were selected to include extremes in terms of breadth of both seroprotection and seroconversion. To address durability, subjects were selected who seroconvert to vaccination and either maintain a protective titer to the next season or drop below protective HAI titers (1:40) by the next season.
Samples were chosen from vaccinees in the 2013-2015 influenza seasons. The vaccine formulations consisted of three or four strains of influenza virus as specified by the U.S. Food and Drug Administration for inclusion each year. For the 2013-2014 and 2014-2015 seasons, the strains included a trivalent formulation of A/California/7/2009 (H1N1), A/Texas/ 50/2012 (H3N2), B/Massachusetts/2/2012 (Yamagata lineage). In the 2015-2016 season, a quadrivalent influenza vaccine formulation composed of A/California/7/2009 (H1N1), A/Switzerland/ 9715293/2013 (H3N2), B/Phuket/3073/2013 (Yamagata lineage), and B/Brisbane/60/2008 (Victoria lineage) was used for vaccination. In the 2016-2017 season, vaccine composition included A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2), B/Phuket/3073/2013 (Yamagata lineage), and B/Brisbane/60/2008 (Victoria lineage).
The current studies were approved by the Massachusetts General Hospital Institutional Review Board.
Discovery cohort
24 subjects were included with a minimum of two samples each for a total of 198 samples over the three-year study period. For all day 0 samples (n=105), demographics of the samples are: 75% female and 25% male; 19% ages 18-34, 15% ages 35-49, 38% ages 50-64, and 28% 65+; 74% received regular dose (RD) inactivated vaccine, 17% received high dose (HD) inactivated vaccine, and 9% received intradermal (ID) inactivated vaccine.
Investigation cohort
Samples were chosen for inclusion based on the availability of samples for at least three out of four influenza seasons from 2013-2016. Overall, 136 subjects were included with a minimum of six samples each for a total of 835 samples. For all included individuals, demographics of the samples are: 74% female and 26% male; 9% ages 18-34, 9% ages 35-49, 37% ages 50-64 and 46% ages 65+ at study end. Breakdown of vaccine types varied by year: year 1 had 466% RD, 9% HD, 26% ID; year 2 had 89% RD, 11% HD; year 3 had 76% RD, 24% HD; and year 4 had 63% RD, 37% HD.
Animals
7-week-old female C57BL6/J naïve mice were sourced from Jackson Laboratory for the immune complex immunization experiments. The mice were group housed in the animal facility at the Ragon Institute of MGH, MIT and Harvard under specific pathogen-free conditions. Mice were monitored daily. During the course of the study, one mouse was single housed due to barbering concerns. The study was approved by the Mass General Brigham IACUC, Protocol # 2017N000134.
Cell lines
THP-1 cells (cell line isolated from a 1-year-old male) were grown in R10 medium (RPMI plus 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin) supplemented with 0.01% β-mercaptoethanol at 37°C, seeded at 200,000 cells/ml and split prior to reaching 1,000,000 cells/ml.
Expi293 cells (Thermo Fisher) were cultured in Optim-MEM I Reduced Serum Medium (Gibco) and used for protein expression in Expi293 Expression medium (Gibco) with transfection via ExpiFectamine 293 Transfection Kit (Gibco).
Viruses
H3N2 X-31 is a lab-adapted seasonal influenza virus that has the HA and NA gene segments from A/Aichi/2/1968 and other gene segments from A/Puerto Rico/8/1934. This virus was obtained from the lab of Dr. Daniel Lingwood at the Ragon Institute of MGH, MIT and Harvard and was originally sourced from BEI (https://www.beiresources.org/Catalog/animalViruses/NR-3483.aspx).
Method details
Influenza proteins
HA trimer and NA tetramer antigens (H1 A/California/07/2009, N1 A/California/07/2009, H1 A/Brisbane/59/2007, H1 A/Chile/1983, H1 A/New Caledonia/20/1999, H3 A/Texas/50/2012, N2 A/Texas/50/2012, H3 A/Brisbane/10/2007, H3 A/Hong Kong/4108/2014, N2 A/Hong Kong/4108/2014, H3 A/Panama/2007/1999, H3 A/Singapore/19/2016, H3 A/Switzerland/9715293/2013, HA B/Brisbane/60/2008, HA B/Phuket/3073/2013, HA B/Colorado/06/2017) were produced for this project by the laboratory of Dr. Ted Ross at the University of Georgia and provided by Sanofi Pasteur were produced and validated according to protocols described in.62 Briefly, they were produced in Expi293F cells, purified by His-tag affinity column, and validated by SDS-PAGE, ELISA, and HAI/ELLA (as appropriate). HA stem antigens were produced for this project by the laboratory of Dr. Daniel Lingwood at the Ragon Institute of MGH, MIT and Harvard. They were produced in Expi293F cells and purified by FPLC and binding confirmed by appropriate monoclonal stem-specific antibodies. An additional NA monomeric antigen (N1 A/California/07/2009) was provided by the laboratory of Dr. Florian Krammer was produced in insect cells and purified by Nickel affinity columns and size exclusion chromatography, according to protocols described in.63,64 It was validated by binding ELISA, and previous productions from the same system were subject to X-ray crystallography.
Antibody isotyping/subclassing
HA and NA antigens as described above and EBOV GPdTM (Mayflower Biosciences) were coupled via carboxyl chemistry to Magplex® fluorescently bar-coded beads (Luminex Corp.) with sulfo-NHS and EDC (Thermo Fisher) per manufacturer’s instructions. These beads were then incubated with diluted, heat inactivated serum samples overnight at 4C, shaking. To detect total IgG, IgG1, and FcgR binding, samples were incubated with beads at a final dilution of 1:500. To detect IgG3, IgA1, and IgM, samples were incubated with beads at a final dilution of 1:100. Each sample was assayed in duplicate. Beads were then washed to remove unbound sample and incubated with PE-labeled secondary detection reagents (anti-Ig isotypes from Southern Biotechnology and FcgRs from the Duke University Protein Production Facility). Excess detection antibody was washed away, and samples were quantified on the iQue Screener Plus using Forecyt software (Intellicyt).
For the Investigation cohort, urea wash affinity measurements were taken for antigen-specific antibodies. Protocol was as above, with each sample run four times. One set of duplicates was washed with 7M urea for 15 minutes while the other was washed with buffer. Affinity was quantified as the fraction of antibodies remaining after urea wash by dividing the urea wash median fluorescence intensity (MFI) by the buffer wash MFI.
Antibody-dependent cellular phagocytosis
HA antigens (H1 A/California/07/2009 and H3 A/Texas/50/2012) were biotinylated with Sulfo-NHS-LC-LC Biotin (Thermo Fisher) and excess biotin was removed using a Zeba size exclusion column (Thermo Fisher). Biotinylated antigens were coupled to Neutravidin fluorescent beads (Thermo Fisher). Antigen-coupled beads were incubated with 1:200 diluted serum samples for two hours at 37C, then washed to remove unbound sample. THP-1 cells (ATCC) were added to immune complexes and incubated for 16 hours at 37C. Cells were then washed to remove unbound immune complexes, fixed, and quantified on the iQue Screener Plus using Forecyt software (Sartorius). Phagocytic scores were calculated by multiplying the percentage of bead positive cells by the bead fluorescence GMFI of bead positive cells and dividing by 10,000. Each sample was assayed in two independent replicates.
Antibody-dependent neutrophil phagocytosis
Antibody-dependent neutrophil phagocytosis was adapted from the previously published protocol.65 HA-coated beads were created as for ADCP. Antigen-coupled beads were incubated with 1:50 diluted serum samples for two hours at 37C, then washed to remove unbound sample. Fresh primary human white blood cells, isolated by ACK lysis, were incubated with immune complexes for one hour at 37C. Cells were then washed to remove unbound immune complexes, labeled for CD66b (anti-human CD66b fluorescent antibody from Biolegend), fixed, and quantified on the iQue Screener Plus using Forecyt software (Intellicyt). Neutrophils were identified as CD66b positive. Phagocytic scores were calculated by multiplying the percentage of bead positive cells by the bead fluorescence GMFI of bead positive cells and dividing by 10,000. Each sample was assayed for two healthy blood donors.
Antibody-dependent complement deposition
Antibody-dependent complement deposition was adapted from the previously published protocol.66 HA-coated beads were created as for ADCP. Antigen-coupled beads were incubated with 1:25 diluted heat inactivated serum samples for two hours at 37C, then washed to remove unbound sample. Immune complexes were then incubated with reconstituted lyophilized guinea pig complement (Cedarlane) for 20 minutes at 37C, and excess complement washed off. Immune complexes were stained with anti-guinea pig C3 fluorescent antibody (MP Biomedicals). Excess staining antibody was removed by washing and immune complexes were quantified on the iQue Screener Plus using Forecyt software (Intellicyt). Complement deposition was indicated by the C3 GMFI of immune complexes. Each sample was assayed in two independent replicates.
Antibody-dependent NK cell activation
ELISA plates (NUNC, Thermo Fisher) were coated with HA antigens (H1 A/California/07/2009 and H3 A/Texas/50/2012), washed to remove unbound antigen, and blocked with 5% bovine serum albumin (Sigma) in PBS. Primary human NK cells were isolated from fresh buffy coats using the RosetteSep NK cell enrichment kit (StemCell) and rested overnight at 37C with 1 ng/ml IL-15 (StemCell). 1:25 diluted serum samples were added to the washed, HA-coated plates and incubated for 2 hours at 37C. Immediately prior to use, brefeldin A (Sigma), GolgiStop (BD), and fluorescent anti-CD107a (BD) were added to primary NK cells. Immune complexed plates were washed, and NK cells were added for 5 hours at 37C. After incubation, NK cells were removed and stained with fluorescent antibodies for cell surface markers CD3, CD56, and CD16 (BD). NK cells were fixed and permeabilized using Fixation & Permeabilization Media A & B (BD). Permeabilized NK cells were stained for intracellular makers MIP-1β and IFN-ɣ (BD). NK cells were quantified on the iQue Screener Plus using Forecyt software (Intellicyt). NK cells were defined as CD3 negative, CD16 positive. Each sample was assayed on two buffy coat donors.
Antibody-dependent dendritic cell phagocytosis
Primary human Dendritic Cells (DCs) were derived from buffy coats through isolation with CD14 microbeads (Miltenyi) and culture in MoDC media (Miltenyi) for 6-7 days. HA antigens (H1 A/California/07/2009 and H3 A/Texas/50/2012) were coupled to fluorescently-labeled beads (Thermo Fisher) through carboxyl chemistry as for antibody isotyping/subclassing. Antigen-coupled beads were incubated with 1:50 diluted serum samples for two hours at 37C, then washed to remove unbound sample. Immune complexes were then incubated with differentiated DCs for 4 hours at 37C. DCs were then fixed and stained for cell surface activation markers HLA-DR (Biolegend), CD86 (BD), and CD83 (Biolegend). DCs were quantified on the iQue Screener Plus using Forecyt software (Intellicyt). Phagocytic scores were calculated by multiplying the percentage of bead positive cells by the bead fluorescence GMFI of bead positive cells and dividing by 10,000. Other activation markers were quantified as GMFI of each marker as a percentage of total cells. Each sample was assayed on two buffy coat donors.
Antigen-specific antibody Fc glycan analysis
HA and NA antigens (H1 A/California/07/2009, H3 A/Texas/50/2012, N2 A/Texas/50/2012) were coated onto magnetic beads through biotin-streptavidin as for ADCP. Serum samples were pre-cleared with non-coupled magnetic beads to remove nonspecific serum binding components. Pre-cleared samples were incubated with antigen-coated beads for 2 hours at 37C to form immune complexes. Beads were washed and Fc regions were cleaved off from beads by IDEZ digestion (New England Biolabs). Resulting solubilized Fc regions were then deglycosylated and labeled with APTS per GlycanAssure kit instructions (Thermo Fisher). Labeled glycans were analyzed by capillary electrophoresis (GeneScan 3500XL, Applied Biosciences) and GlycanAssure software (ThermoFisher). Peaks were called based on known labeled glycan standards and reported as a percentage of total glycan peak area.
Production of CR9114 Fc variants
Fc variant mutations were introduced into the sequence of CR911423 through Golden Gate cloning using the library created in.25
Antibodies were expressed in Expi293 cells (Thermo Fisher). Plasmids were transfected into Expi293 suspension cells grown in Opti-MEM I Reduced serum Medium (Gibco). Cells were seeded two days pre-transfection at a density of 0.4x106 cells/mL. On the day of transfection, cells were counted again and the cell density was adjusted to 1.2x106 cells/mL in 50 mL media. Total DNA (25 mg per 50ml of initial cell culture) was transfected into cells using the ExpiFectamine 293 Transfection Kit (Gibco). Cell culture supernatants were harvested 5 days post-transfection. Cell culture supernatants were harvested 5 days post-transfection and incubated overnight at 4C with Pierce Protein A/G Plus Agarose (Thermo Fisher). Agarose resin was collected by pouring the supernatant over a Econo-Column Chromatography Column (Bio-rad). Resin was washed with PBS before antibody was eluted with Pierce IgG Elution Buffer (Thermo Fisher) directly into Tris-HCl pH 8.0 at a ratio of 1:10. Antibody eluates were concentrated using Amicon Ultra-15 Centrifugal Filter Units with a 50 kDa molecular weight cut off. Final concentrations were measured and adjusted by Nanodrop (Thermo Fisher). Antibody expression and purity were confirmed by SDS-PAGE. Binding was confirmed by influenza HA ELISA.
Immune complex immunizations
Immune complexes were prepared using inactivated influenza virus H3N2 X-31. The virus was inactivated with Triton X-100 (Millipore Sigma), followed by purification to remove detergent contaminants, according to published protocol.67 Inactivated virus was then incubated with Fc variant antibodies at a 1:1 molar ratio to form immune complexes under sterile conditions. Immune complexes were stored at 4C for up to three days prior to immunization.
Mice were intraperitoneally immunized with 100ul of immune complex mixture containing 20ug of inactivated influenza and 20ul alum (Imject) on days 0 and 21. Weekly starting on day 0, blood was collected from mice from the facial vein using sterile lancets (Medipoint) into serum separator tubes (BD Biosciences). Serum was isolated by allowing to clot in SST tube for a minimum of 30 minutes, centrifuging for 2 minutes at 10,000g, and removing the serum layer for storage at -80C. At day 42, all mice were euthanized by carbon dioxide inhalation, at which point terminal bleeds were collected. Terminal bleeds were processed in the same manner as facial vein bleeds.
HAI assay
Chicken red blood cells (Rockland) were washed and resuspended at a standard concentration (0.5% v/v).68 HAI assay protocol was adapted from.69 Virus was prepared at 4x hemagglutination titer and incubated with an equal volume of serially diluted antibody. Plates were incubated at room temperature for 30 minutes prior to addition of prepared red blood cells. Plates were then incubated again at room temperature for 30 minutes and hemagglutination inhibition titer evaluated by two independent operators.
Serum antibody level measurements
Murine serum samples were evaluated by Luminex bead-based assay as described above. Luminex fluorescently barcoded microspheres were coupled to strain-matched recombinant H3N2 A/Aichi/2/1968 HA (Immune Technology Corp.), H3 stem, and inactivated virus. Antibodies were detected with goat anti-mouse isotype-specific PE-coupled antibodies (Southern Biotech). Samples were quantified on the iQue Screener Plus using Forecyt software (Intellicyt).
Quantification and statistical analysis
Breadth score
Sequences were aligned and pairwise distances between the amino acids of the sequences were generated (Figure S6). Pairwise distances of amino acid changes were used to calculate a normalized overall breadth score of all neutralized viruses. This score was calculated by summing the pairwise distances between neutralized strains and dividing by the total number of strains assayed. This number was then normalized to set 1 as the level for a hypothetical individual who neutralizes all strains included in the analysis.
Multivariate and statistical analyses
Univariate visualizations and statistics were generated using Prism 8 (Graphpad Software, LLC) unless otherwise indicated. Figure 2A was generated in RStudio 2021.09.0 (R Version 4.1.1). Statistical tests are described in figure legends.
Multivariate analyses were completed using MATLAB (The Mathworks, Inc.) as previously described.12,13 For Elastic Net-Partial Least Squares analyses, a dataset containing antigen-specific antibody isotypes, FcgR binding, neutralization, antibody-dependent functional data, and age/gender for study subjects was used. Missing datapoints were imputed using K-nearest neighbor and data were normalized using z-scoring. Elastic Net machine learning was performed in a 5-fold cross-validation framework. The Elastic Net lambda coefficient was chosen within this cross-validation framework. A minimum of 3 antibody features were selected in each replicate. Random size-matched and label permuted datasets were compared for each model within the cross-validation framework. Networks were created from highly significant (false discovery rate adjusted q value < 0.01 and absolute value of r greater than 0.6) co-correlates of LASSO-selected features and visualized using Cytoscape. Code files and datasets required to recreate the analyses presented here are included as supplemental information.
Acknowledgments
The authors dedicate this paper to the memory of Dr. Todd J. Suscovich, who provided invaluable guidance and scientific input to this study but sadly passed away prior to publication. The authors also thank C. Luedemann, M. Davis, and S. Taylor for administrative assistance. This work was supported by the following: Sanofi Pasteur, Inc, the Ragon Institute, the Samana Cay Massachusetts General Hospital scholar program, and NIH grants R37AI080289, R01AI146785, and R01AI153098, as well as NIH T32 AI007245. We also acknowledge Harvard CFAR for ongoing support through P30 AI060354-02.
Author contributions
C.M.B., H.K., T.M.R., and G.A. designed the study. S.M., S.S., T.R., and H.K. were involved in the original clinical study and provided samples. C.M.B., J.S.B., and M.J.G. conducted experiments. D.L. provided influenza virus and expertise. C.M.B. and A.L.R. analyzed data. C.M.B., H.K., S.S., and G.A. wrote the manuscript. All authors contributed to the final manuscript.
Declaration of interests
G.A. is an equity holder in Seromyx Systems and Leyden Labs and is an employee of Moderna. C.M.B. is an employee and equity holder of Leyden Labs. S.M., S.S., and H.K. were/are employees of Sanofi Pasteur, Inc.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community.
Published: March 14, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.100975.
Supplemental information
Data and code availability
Data to replicate machine learning analyses are available in the supplemental information. All additional data reported in this paper will be shared by the lead contact upon request.
Original code for machine learning models is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Centers for Disease Control and Prevention: National Center for Immunization and Respiratory Diseases . Seasonal Influenza (Flu); 2020. Past Seasons Vaccine Effectiveness Estimates.https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html [Google Scholar]
- 2.CDC . Influenza (Flu); 2018. Disease Burden of Influenza. [Google Scholar]
- 3.Broadbent A.J., Subbarao K. Influenza virus vaccines : lessons from the 2009 H1N1 pandemic. Curr. Opin. Virol. 2011;1:254–262. doi: 10.1016/j.coviro.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erbelding E.J., Post D.J., Stemmy E.J., Roberts P.C., Augustine A.D., Ferguson S., Paules C.I., Graham B.S., Fauci A.S. A universal influenza vaccine: the strategic plan for the National Institute of allergy and infectious diseases. J. Infect. Dis. 2018;218:347–354. doi: 10.1093/infdis/jiy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson D., Curry R.L., Beare A.S., Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. 1972;70:767–777. doi: 10.1017/S0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohmit S.E., Petrie J.G., Cross R.T., Johnson E., Monto A.S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis. 2011;204:1879–1885. doi: 10.1093/infdis/jir661. [DOI] [PubMed] [Google Scholar]
- 7.Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019;19:383–397. doi: 10.1038/s41577-019-0143-6. [DOI] [PubMed] [Google Scholar]
- 8.Thulin N.K., Wang T.T. The role of Fc gamma receptors in broad protection against influenza viruses. Vaccines. 2018;6:36. doi: 10.3390/vaccines6030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiazGranados C.A., Dunning A.J., Kimmel M., Kirby D., Treanor J., Collins A., Pollak R., Christoff J., Earl J., Landolfi V., et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 10.Hannoun C., Megas F., Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Chung A.W., Ghebremichael M., Robinson H., Brown E., Choi I., Lane S., Dugast A.-S., Schoen M.K., Rolland M., Suscovich T.J., et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014;6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman M.E., Das J., Pittala S., Broge T., Linde C., Suscovich T.J., Brown E.P., Bradley T., Natarajan H., Lin S., et al. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat. Med. 2018;24:1590–1598. doi: 10.1038/s41591-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn B.M., Yu W.H., Karim M.M., Brannan J.M., Herbert A.S., Wec A.Z., Halfmann P.J., Fusco M.L., Schendel S.L., Gangavarapu K., et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against ebola virus. Cell Host Microbe. 2018;24:221–233.e5. doi: 10.1016/j.chom.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks D.G., Qiu W.Q., Luster A.D., Ravetch J.V. Structure and expression of human IgG (FcRII(CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J. Exp. Med. 1989;170:1369–1385. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman M.E., Dugast A.-S., McAndrew E.G., Tsoukas S., Licht A.F., Irvine D.J., Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 2013;87:5468–5476. doi: 10.1128/jvi.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C., Li Y., Kaplonek P., Gentili M., Fischinger S., Bowman K.A., Sade-Feldman M., Kays K.R., Regan J., Flynn J.P., et al. The kinetics of SARS-CoV-2 antibody development is associated with clearance of RNAemia. mBio. 2022;13:e0157722. doi: 10.1128/mbio.01577-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittala S., Bagley K., Schwartz J.A., Brown E.P., Weiner J.A., Prado I.J., Zhang W., Xu R., Ota-Setlik A., Pal R., et al. Antibody Fab-Fc properties outperform titer in predictive models of SIV vaccine-induced protection. Mol. Syst. Biol. 2019;15:e8747. doi: 10.15252/msb.20188747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu S.Y., Vostiar I., Karki S., Moore G.L., Lazar G.A., Pong E., Joyce P.F., Szymkowski D.E., Desjarlais J.R. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcγRIIb with Fc-engineered antibodies. Mol. Immunol. 2008;45:3926–3933. doi: 10.1016/j.molimm.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Bruhns P., Jönsson F. Mouse and human FcR effector functions. Immunol. Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 20.French M.A., Abudulai L.N., Fernandez S. Isotype diversification of IgG antibodies to HIV gag proteins as a therapeutic vaccination strategy for HIV infection. Vaccines. 2013;1:328–342. doi: 10.3390/vaccines1030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dugan H.L., Guthmiller J.J., Arevalo P., Huang M., Chen Y.Q., Neu K.E., Henry C., Zheng N.Y., Lan L.Y.L., Tepora M.E., et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci. Transl. Med. 2020;12:eabd3601. doi: 10.1126/scitranslmed.abd3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarnitsyna V.I., Lavine J., Ellebedy A., Ahmed R., Antia R. Multi-epitope models explain how pre-existing antibodies affect the generation of broadly protective responses to influenza. PLoS Pathog. 2016;12:e1005692. doi: 10.1371/journal.ppat.1005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreyfus C., Laursen N.S., Kwaks T., Zuijdgeest D., Khayat R., Ekiert D.C., Lee J.H., Metlagel Z., Bujny M.V., Jongeneelen M., et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Impagliazzo A., Milder F., Kuipers H., Wagner M.V., Zhu X., Hoffman R.M.B., Van Meersbergen R., Huizingh J., Wanningen P., Verspuij J., et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 25.Gunn B.M., Lu R., Slein M.D., Ilinykh P.A., Huang K., Atyeo C., Schendel S.L., Kim J., Cain C., Roy V., et al. A Fc engineering approach to define functional humoral correlates of immunity against Ebola virus. Immunity. 2021;54:815–828.e5. doi: 10.1016/j.immuni.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steurer W., Nickerson P.W., Steele A.W., Steiger J., Zheng X.X., Strom T.B. Ex vivo coating of islet cell allografts with murine CTLA4/Fc promotes graft tolerance. J. Immunol. 1995;155:1165–1174. [PubMed] [Google Scholar]
- 27.Moore G.L., Chen H., Karki S., Lazar G.A. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. mAbs. 2010;2:181–189. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lofano G., Gorman M.J., Yousif A.S., Yu W.-H., Fox J.M., Dugast A.-S., Ackerman M.E., Suscovich T.J., Weiner J., Barouch D., et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci. Immunol. 2018;3:eaat7796. doi: 10.1126/sciimmunol.aat7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T.T., Maamary J., Tan G.S., Bournazos S., Davis C.W., Krammer F., Schlesinger S.J., Palese P., Ahmed R., Ravetch J.V. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell. 2015;162:160–169. doi: 10.1016/j.cell.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krammer F., Fouchier R.A.M., Eichelberger M.C., Webby R.J., Shaw-Saliba K., Wan H., Wilson P.C., Compans R.W., Skountzou I., Monto A.S. NAction! how can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio. 2018;9:e02332-17. doi: 10.1128/mBio.02332-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullarkey C.E., Bailey M.J., Golubeva D.A., Tan G.S., Nachbagauer R., He W., Novakowski K.E., Bowdish D.M., Miller M.S., Palese P. Broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an Fc-dependent manner. mBio. 2016;7:e01624-16. doi: 10.1128/mBio.01624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien K.B., Morrison T.E., Dundore D.Y., Heise M.T., Schultz-Cherry S. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza a virus infection. PLoS One. 2011;6:e17377. doi: 10.1371/journal.pone.0017377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis T., Jr. On the doctrine of original antigenic sin. Proc. Am. Phil. Soc. 1960;104:572–578. [Google Scholar]
- 34.Andrews S.F., Kaur K., Pauli N.T., Huang M., Huang Y., Wilson P.C. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J. Virol. 2015;89:3308–3317. doi: 10.1128/jvi.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang K.Y.A., Chang S.C., Huang Y.C., Chiu C.H., Lin T.Y. Antibody responses to trivalent inactivated influenza vaccine in health care personnel previously vaccinated and vaccinated for the first time. Sci. Rep. 2017;7:40027. doi: 10.1038/srep40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Boutz D.R., Chromikova V., Joyce M.G., Vollmers C., Leung K., Horton A.P., DeKosky B.J., Lee C.H., Lavinder J.J., et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat. Med. 2016;22:1456–1464. doi: 10.1038/nm.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown D.L., Harkiss G.D. In vivo generation and clearance of soluble immune complexes containing IgM antibodies in normal and decomplemented rabbits. Clin. Exp. Immunol. 1981;43:231–239. [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani B. Phagocytosis of immune complexes mediated by IgM and C3 receptors by macrophages from mice treated with glycogen. J. Immunol. 1981;126:127–130. [PubMed] [Google Scholar]
- 39.Litvack M.L., Post M., Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS One. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith K.G.C., Clatworthy M.R. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heesters B.A., Chatterjee P., Kim Y.A., Gonzalez S.F., Kuligowski M.P., Kirchhausen T., Carroll M.C. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38:1164–1175. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tew J.G., Mandel T.E. Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology. 1979;37:69–76. [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y., Zhang Z., Sheehan J., Avnir Y., Ridenour C., Sachnik T., Sun J., Hossain M.J., Chen L.M., Zhu Q., et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat. Commun. 2016;7:12780. doi: 10.1038/ncomms12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121.5640. [DOI] [PubMed] [Google Scholar]
- 46.Dekkers G., Bentlage A.E.H., Stegmann T.C., Howie H.L., Lissenberg-Thunnissen S., Zimring J., Rispens T., Vidarsson G. Affinity of human IgG subclasses to mouse Fc gamma receptors. mAbs. 2017;9:767–773. doi: 10.1080/19420862.2017.1323159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overdijk M.B., Verploegen S., Ortiz Buijsse A., Vink T., Leusen J.H.W., Bleeker W.K., Parren P.W.H.I. Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. 2012;189:3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 48.Takai T., Ono M., Hikida M., Ohmori H., Ravetch J.V. Augmented humoral and anaphylactic responses in FcyRll-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 49.Brownlie R.J., Lawlor K.E., Niederer H.A., Cutler A.J., Xiang Z., Clatworthy M.R., Floto R.A., Greaves D.R., Lyons P.A., Smith K.G.C. Distinct cell-specific control of autoimmunity and infection by FcγRIIb. J. Exp. Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F., Smith P., Ravetch J.V. Inhibitory Fcγ receptor is required for the maintenance of tolerance through distinct mechanisms. J. Immunol. 2014;192:3021–3028. doi: 10.4049/jimmunol.1302934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bournazos S., Corti D., Virgin H.W., Ravetch J.V. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature. 2020;588:485–490. doi: 10.1038/s41586-020-2838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiLillo D.J., Ravetch J.V. Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161:1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsang-A-Sjoe M.W.P., Nagelkerke S.Q., Bultink I.E.M., Geissler J., Tanck M.W.T., Tacke C.E., Ellis J.A., Zenz W., Bijl M., Berden J.H., et al. Fc-gamma receptor polymorphisms differentially influence susceptibility to systemic lupus erythematosus and lupus nephritis. Rheumatology. 2016;55:939–948. doi: 10.1093/rheumatology/kev433. [DOI] [PubMed] [Google Scholar]
- 54.Willcocks L.C., Carr E.J., Niederer H.A., Rayner T.F., Williams T.N., Yang W., Scott J.A.G., Urban B.C., Peshu N., Vyse T.J., et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA. 2010;107:7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He W., Tan G.S., Mullarkey C.E., Lee A.J., Lam M.M.W., Krammer F., Henry C., Wilson P.C., Ashkar A.A., Palese P., Miller M.S. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc. Natl. Acad. Sci. USA. 2016;113:11931–11936. doi: 10.1073/pnas.1609316113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leon P.E., He W., Mullarkey C.E., Bailey M.J., Miller M.S., Krammer F., Palese P., Tan G.S. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc. Natl. Acad. Sci. USA. 2016;113:E5944–E5951. doi: 10.1073/pnas.1613225113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arunachalam P.S., Walls A.C., Golden N., Atyeo C., Fischinger S., Li C., Aye P., Navarro M.J., Lai L., Edara V.V., et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594:253–258. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- 58.Zhu W., Dong C., Wei L., Wang B.Z. Promising adjuvants and platforms for influenza vaccine development. Pharmaceutics. 2021;13:68. doi: 10.3390/pharmaceutics13010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nachbagauer R., Feser J., Naficy A., Bernstein D.I., Guptill J., Walter E.B., Berlanda-Scorza F., Stadlbauer D., Wilson P.C., Aydillo T., et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021;27:106–114. doi: 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- 60.Andersen T.K., Huszthy P.C., Gopalakrishnan R.P., Jacobsen J.T., Fauskanger M., Tveita A.A., Grødeland G., Bogen B. Enhanced germinal center reaction by targeting vaccine antigen to major histocompatibility complex class II molecules. npj Vaccines. 2019;4:9. doi: 10.1038/s41541-019-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuñez I.A., Carlock M.A., Allen J.D., Owino S.O., Moehling K.K., Nowalk P., Susick M., Diagle K., Sweeney K., Mundle S., et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS One. 2017;12:e0185666. doi: 10.1371/journal.pone.0185666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ecker J.W., Kirchenbaum G.A., Pierce S.R., Skarlupka A.L., Abreu R.B., Cooper R.E., Taylor-Mulneix D., Ross T.M., Sautto G.A. High-yield expression and purification of recombinant influenza virus proteins from stably-transfected mammalian cell lines. Vaccines. 2020;8:462. doi: 10.3390/vaccines8030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margine I., Palese P., Krammer F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp. 2013;81:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X., Zhu X., Dwek R.A., Stevens J., Wilson I.A. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J. Virol. 2008;82:10493–10501. doi: 10.1128/jvi.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karsten C.B., Mehta N., Shin S.A., Diefenbach T.J., Slein M.D., Karpinski W., Irvine E.B., Broge T., Suscovich T.J., Alter G. A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis. J. Immunol. Methods. 2019;471:46–56. doi: 10.1016/j.jim.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischinger S., Fallon J.K., Michell A.R., Broge T., Suscovich T.J., Streeck H., Alter G. A high-throughput, bead-based, antigen-specific assay to assess the ability of antibodies to induce complement activation. J. Immunol. Methods. 2019;473:112630. doi: 10.1016/j.jim.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jonges M., Liu W.M., Van Der Vries E., Jacobi R., Pronk I., Boog C., Koopmans M., Meijer A., Soethout E. Influenza virus inactivation for studies of antigenicity and phenotypic neuraminidase inhibitor resistance profiling. J. Clin. Microbiol. 2010;48:928–940. doi: 10.1128/JCM.02045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balish A.L., Katz J.M., Klimov A.I. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. 2013;29:15G.1.1–15G.1.24. doi: 10.1002/9780471729259.mc15g01s29. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y., Cho M., Shore D., Song M., Choi J., Jiang T., Deng Y.-Q., Bourgeois M., Almli L., Yang H., et al. Hemagglutination inhibition (HI) assay of influenza viruses with monoclonal antibodies. Bio-Protocol. 2016;6:4–9. doi: 10.21769/BioProtoc.1828. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data to replicate machine learning analyses are available in the supplemental information. All additional data reported in this paper will be shared by the lead contact upon request.
Original code for machine learning models is available in this paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.