Summary
Background
Lower respiratory tract infections are frequently treated with antibiotics, despite a viral cause in many cases. It remains unknown whether low procalcitonin concentrations can identify patients with lower respiratory tract infection who are unlikely to benefit from antibiotics. We aimed to compare the efficacy and safety of azithromycin versus placebo to treat lower respiratory tract infections in patients with low procalcitonin.
Methods
We conducted a randomised, placebo-controlled, double-blind, non-inferiority trial at five health centres in the USA. Adults aged 18 years or older with clinically suspected non-pneumonia lower respiratory tract infection and symptom duration from 24 h to 28 days were eligible for enrolment. Participants with a procalcitonin concentration of 0·25 ng/mL or less were randomly assigned (1:1), in blocks of four with stratification by site, to receive over-encapsulated oral azithromycin 250 mg or matching placebo (two capsules on day 1 followed by one capsule daily for 4 days). Participants, non-study clinical providers, investigators, and study coordinators were masked to treatment allocation. The primary outcome was efficacy of azithromycin versus placebo in terms of clinical improvement at day 5 in the intention-to-treat population. The non-inferiority margin was −12·5%. Solicited adverse events (abdominal pain, vomiting, diarrhoea, allergic reaction, or yeast infections) were recorded as a secondary outcome. This trial is registered with ClinicalTrials.gov, NCT03341273.
Findings
Between Dec 8, 2017, and March 9, 2020, 691 patients were assessed for eligibility and 499 were enrolled and randomly assigned to receive azithromycin (n=249) or placebo (n=250). Clinical improvement at day 5 was observed in 148 (63%, 95% CI 54 to 71) of 238 participants with full data in the placebo group and 155 (69%, 61 to 77) of 227 participants with full data in the azithromycin group in the intention-to-treat analysis (between-group difference −6%, 95% CI −15 to 2). The 95% CI for the difference did not meet the non-inferiority margin. Solicited adverse events and the severity of solicited adverse events were not significantly different between groups at day 5, except for increased abdominal pain associated with azithromycin (47 [23%, 95% CI 18 to 29] of 204 participants) compared with placebo (35 [16%, 12 to 21] of 221; between-group difference −7% [95% CI −15 to 0]; p=0·066).
Interpretation
Placebo was not non-inferior to azithromycin in terms of clinical improvement at day 5 in adults with lower respiratory tract infection and a low procalcitonin concentration. After accounting for both the rates of clinical improvement and solicited adverse events at day 5, it is unclear whether antibiotics are indicated for patients with lower respiratory tract infection and a low procalcitonin concentration.
Funding
National Institute of Allergy and Infectious Diseases, bioMérieux.
Trial Registration:
ClinicalTrials.gov, NCT03341273, https://clinicaltrials.gov/ct2/show/NCT03341273
INTRODUCTION
Lower respiratory tract infections (LRTIs) are among the most common reasons for acute healthcare visits.(1) Bacterial and viral LRTI have similar clinical manifestations resulting in high rates of inappropriate antibiotic utilization even among LRTI syndromes such as acute bronchitis where guidelines do not recommend antibiotics.(2, 3) Whereas social and behavioral interventions have improved rates of inappropriate antibiotic use, rates remain high. (3) Strategies are needed to identify patients unlikely to benefit from antibacterial therapy, mitigating the development and spread of resistant pathogens and reducing antibiotic-related adverse effects.
Procalcitonin is a host-derived inflammatory marker whose expression correlates with the presence of bacterial infection and tracks with infection severity.(4, 5) Procalcitonin-driven algorithms that recommend initiating or withholding antibacterials have been evaluated for safety and efficacy in multiple trials.(6-8) Results from these and other studies supported the 2017 Food and Drug Administration decision to clear procalcitonin as an aid in decision making on antibiotic use for inpatients or emergency department (ED) patients with LRTI.(9) A subsequent U.S.-based study using the same algorithms, however, did not show a difference in antibiotic use compared to usual care.(10) Moreover, some antibiotics such as azithromycin have independent anti-inflammatory effects, which can mitigate LRTI symptoms. (11) One common feature of procalcitonin clinical trials conducted to date is that the algorithm recommends, but does not require, the specified therapeutic approach. This allows clinicians to accommodate conflicting clinical data in deciding on antibiotic use. Since clinicians in these studies could overrule the procalcitonin algorithm (as high as 56% of the time), prior studies do not directly address whether procalcitonin identifies a patient population for which antibacterials offer no benefit, which is the assumption underlying its use.(6) The primary hypothesis of this study is that placebo is non-inferior in efficacy to azithromycin in terms of clinical improvement when administered to adults with suspected lower respiratory tract infection (LRTI) and a procalcitonin level ≤0·25 ng/mL. We therefore conducted a randomized, double-blinded, placebo-controlled, non-inferiority multicenter clinical trial of azithromycin vs. placebo in adults presenting as outpatients with suspected LRTI and a procalcitonin level of ≤0·25 ng/mL, as a strategy for reducing inappropriate antibiotic prescriptions.
METHODS
Trial design and oversight
The Targeted Reduction of Antibiotics Using Procalcitonin in a Multi-center, Randomized, Double-Blinded, Placebo-Controlled Non-Inferiority Study of Azithromycin Treatment in Outpatient Adults with Suspect LRTI and a Procalcitonin Level of ≤0·25 ng/mL (TRAP-LRTI) was approved by the institutional review board at each site. Written informed consent was obtained prior to any study procedures. An independent data safety monitoring board provided safety oversight. The study is registered with ClinicalTrials.gov (NCT03341273). The protocol can be found at https://www.clinicaltrials.gov/ProvidedDocs/73/NCT03341273/Prot_000.pdf, Appendix page 12.
Sites and patients
The five sites included the Atlanta Veterans Affairs Health Care System (VAHCS), The Hope Clinic of Emory University, Duke University Hospital, Durham VAHCS, and the Michael E. DeBakey VAMC. Enrollment occurred from December 8, 2017 to March 9, 2020 at outpatient clinics and EDs. None of the sites used procalcitonin as part of routine LRTI management.
Adults with a clinician-suspected LRTI of ≥24 hours and ≤28 days duration were eligible. Inclusion required at least two qualifying patient-reported symptoms or one qualifying symptom and at least one qualifying vital sign. Qualifying symptoms included new or worsening cough, new sputum production, increased volume or purulence of chronic sputum production, chest pain, and difficulty breathing. Qualifying vital signs included temperature ≥37·8°C or patient-reported feverishness, heart rate ≥90 beats/minute, or respiratory rate >20 breaths/minute. Exclusion criteria were hospitalization prior to enrollment, known or suspected infections at other anatomic sites requiring antibacterial therapy, known or suspected pneumonia based on chest imaging and clinician diagnosis, immune compromise, azithromycin use two weeks prior or any systemic antibiotic 24 hours prior, or a contraindication to azithromycin use. The type of LRTI (e.g., acute bronchitis, asthma exacerbation, or acute exacerbation of chronic obstructive pulmonary disease) was not recorded due to imprecision in the diagnostic criteria and overlapping clinical syndromes.(12)
Randomisation and masking
Enrolled subjects had serum or plasma collected for procalcitonin measurement within two hours using the VIDAS B.R.A.H.M.S. according to manufacturer instructions (bioMérieux, Marcy-l'Étoile, France). All other clinical evaluations including laboratory testing and radiography were performed at the treating clinician’s discretion. At enrollment, a nasopharyngeal swab was collected and banked for subsequent batched testing by the Respiratory Panel 2 (BioFire Diagnostics, Salt Lake City, UT). Subjects with a procalcitonin value ≤0·25 ng/mL were block randomized with stratification by site to receive over-encapsulated oral azithromycin 250mg or matching placebo (2 capsules on day 1 followed by one capsule daily for four days) in a 1:1 ratio. The Emmes Corporation generated treatment numbers for pill bottles, which were shipped to sites in multiples of six (three placebo, three azithromycin). After study staff confirmed eligibility, Emmes assigned a treatment number for the pharmacist to dispense. The first dose was to be taken within 24 hours of randomization. Participants, providers, investigators, and study coordinators remained blind to treatment allocation.
Cytokine measurements
A post-hoc analysis was performed to assess the potential mechanisms by which azithromycin impacted clinical outcomes. Ten cytokines (IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, and TNF-α) were measured in duplicate by the Biomarkers Core Facility at the Duke Molecular Physiology Institute using the manufacturer’s protocol (VPLEX PLUS Proinflammatory Panel 1, Meso Scale Diagnostics, Rockville MD) for subjects with plasma available at Days 1 and 5.
Outcome measures
The primary outcome was the efficacy of azithromycin versus placebo on Day 5based on clinical improvement. Clinical improvement was defined as improvement in ≥2 qualifying patient-reported symptoms (or one symptom and one vital sign abnormality); no deterioration in any qualifying symptom nor new vital sign abnormality; absence of fever in the 24-hours prior to the Day 5 visit; and no medically attended visits for persistent or worsening LRTI requiring antibacterial therapy. Absence of fever at Day 5 was based on participants’ measurement of their temperatures using a study-provided thermometer in the preceding 24 hours as well as temperature measurement by study staff at the Day 5 in-person visit. This efficacy definition was selected because of its use in prior studies.(13) Secondary outcomes included the individual efficacy components for azithromycin versus placebo at Days 11 and 28. The composite efficacy outcome to determine non-inferiority was added during a protocol amendment on July 24, 2018. Secondary outcomes also compared azithromycin to placebo with respect to the Response Adjusted for Days of Antibiotic Risk (RADAR).(14) RADAR was determined by categorizing each participant’s clinical outcome according to a Desirability of Outcome Ranking (DOOR), an 8-level ordinal clinical outcome encompassing three components: clinical improvement, the presence of patient-reported antibiotic-related solicited adverse events (AEs) (abdominal pain, vomiting, diarrhea, allergic reactions, and candidiasis), and their severity (mild, moderate, or severe) (Supplementary Table 1, Appendix page 2).(14) For RADAR, the desirability of the overall patient experience was further ranked by the observed treatment duration (not treatment assignment) with fewer days of antibiotic receiving a more desirable rank. Post-hoc subgroup analyses were performed to explore treatment interactions by baseline characteristics, selected due to the possible contributions they might make to the underlying LRTI or its response to treatment. This analysis was performed in a similar manner as the primary analysis: We fit a linear regression model on clinical improvement at the Day 5 visit for each level of the subgroup variable while adjusting for the actual day of the Day 5 visit and ignoring missing data. An interaction test was performed to assess whether the effect of treatment is different among the categories of the subgroup analysis. This was performed for the Day 11 visit as well. Additional secondary and exploratory endpoints are listed in the Statistical Analysis Plan (ClinicalTrials.gov NCT03341273).
Sample size
The study planned to enroll 840 subjects to randomize 420 (210/group) with 80% power at a 2·5% significance level for a one-sided hypothesis to demonstrate noninferiority (NI) of placebo to azithromycin with a 12·5% NI margin, assuming a Day 5 clinical improvement rate of 80%. The size of the NI margin was chosen to match existing FDA guidance for the study of community-acquired bacterial pneumonia.(15) Although individuals known to have bacterial pneumonia were excluded from this study, the FDA guidance document offered a well-established precedent for other types of LRTI studied here. At a planned interim analysis, assumptions underlying the targeted sample size were assessed resulting in a recalculated sample size of 674 enrolled subjects to randomize 560 for the according-to-protocol (ATP) analysis given an observed Day 5 clinical improvement rate of 57·6% (102/177). Due to the COVID-19 pandemic, enrollment closed with 514 subjects enrolled and 499 randomized to azithromycin (n=249) or placebo (n=250), resulting in 77% power.
Statistical analysis
The primary analyses were conducted using an intent-to-treat (ITT) approach, including all randomized participants. Baseline characteristics in the two treatment groups were reported using frequency distributions and descriptive statistics. As recommended by FDA guidance (16), assessment of non-inferiority at the Day 5 primary endpoint utilized a two-sided 95% confidence interval (CI) of the difference in the rates (placebo - azithromycin), with the noninferiority of placebo to azithromycin concluded if the lower limit of the 95% CI was less than the NI margin of −12·5%. (i.e., if the NI was concluded, it could be interpreted that azithromycin treatment would be unnecessary). Wald type confidence intervals were calculated based on proportions from a linear regression with multiple imputation and adjusting for the actual day of the study visit (since study visits could occur within a predefined window) in the final model. Assuming a missing-at-random process, multiple imputation methodology with linear regression was used to impute missing primary outcomes for the ITT population. An ATP analysis was conducted to evaluate the robustness of the results from the ITT analysis. ATP analyses by definition required complete data and so did not require imputation. Similar analyses were conducted for Days 11 and 28. For all analyses, rates and differences in rates of clinical improvement were estimated from linear regression adjusting for the actual day of the designated study visit (e.g., the Day 5 visit could occur on Days 5, 6, 7, or 8) instead of being calculated directly from absolute numbers.
The DOOR, a key secondary endpoint, was analyzed by the RADAR method.(14) When calculating the probability of having a more desirable outcome with placebo compared to azithromycin (Wilcoxon-Mann-Whitney statistic) as a summary contrast measure, the probability was adjusted by the number of observed days of antibiotic as a tie-breaker, where a shorter duration of antibiotic use was considered more desirable when comparing participants with the same DOOR rank. The unadjusted probabilities were also calculated. Given the composite nature of the DOOR, its individual components were analyzed separately using similar methods.
Cytokine measurements below the lower limit of detection were imputed at half that value. Multiplicity for cytokine data analyses were adjusted using Holm’s procedure.(17) All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) or R statistical software version R 3.2 or later (R Foundation for Statistical Computing, Vienna, Austria).
The full statistical analysis plan can be accessed at https://www.clinicaltrials.gov/ct2/show/NCT03341273.
Role of the funding source
This project was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services. Additional support was provided by the NIAID by way of the Antibacterial Resistance Leadership Group (ARLG). The NIAID and ARLG helped develop the study design, data collection, analysis, interpretation, and manuscript preparation. bioMérieux provided study product (azithromycin and placebo), instruments and reagents to perform procalcitonin and Respiratory Panel-2 testing, and provided input on study conception and design but had no role in the decision to publish these results.
RESULTS
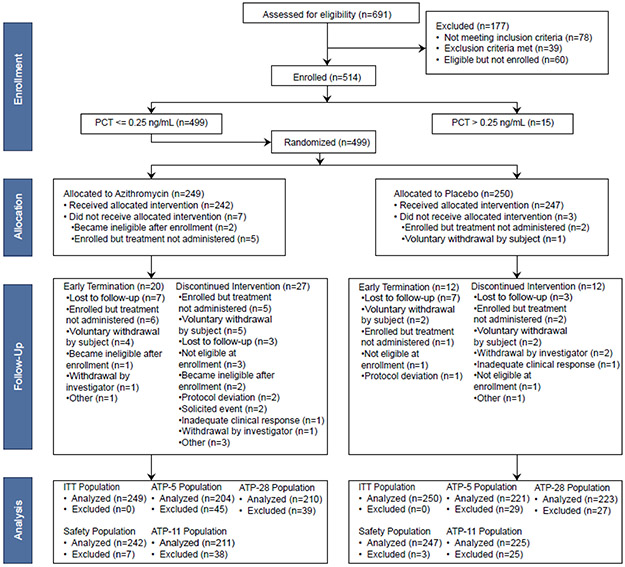
From December 2017 to March 2020, 514 patients were enrolled and 499 with procalcitonin levels ≤0·25 ng/mL were randomized (249 to azithromycin and 250 to placebo) (Figure 1). These 499 subjects comprised the ITT population. The ATP population ranged from 204-211 azithromycin-treated subjects and 221-225 placebo-treated subjects, depending on the timepoint (Day 5, 11, or 28). The azithromycin and placebo-treated groups had similar demographics (Table 1). The majority of subjects were Black (305/499, 61%), non-Hispanic (461/499, 91%), and male (323/499, 65%) with a mean age of 52 years (median 55, range 18-93). Six individuals had taken non-azithromycin antibiotics (two in the azithromycin group and four in the placebo group) >24 hours but within two weeks prior to enrollment. Pre-existing medical conditions were reported in 91% of all subjects and were similar in frequency between the two groups. This included 158/499 (32%) with asthma and 57/499 (11%) with COPD. All five doses of study product were taken by 221/249 (89% of subjects in the azithromycin group and 237/250 (95%) in the placebo group. In the ITT population, routine clinical testing and supplemental multiplex respiratory pathogen testing identified seven subjects with bacteria and 261 with viruses (53·7% of the cohort). These were evenly distributed across placebo (3 bacterial, 133 viral) and azithromycin (4 bacterial, 128 viral) groups. The bacterial pathogens included Mycoplasma pneumoniae (n=2), Streptococcus pyogenes (n=2), and one case each of Chlamydia pneumoniae, Haemophilus influenzae, and Bordatella parapertussis. The most commonly detected viruses were rhinovirus/enterovirus (n=99) and influenza (n=76).
Figure 1. CONSORT flow diagram.
Table 1:
Baseline Characteristics for the Intention to Treat Population
| Characteristic | Azithromycin (N=249) |
Placebo (N=250) |
All Subjects (N=499) |
|---|---|---|---|
| Age in years, mean (standard deviation) | 52·8 (15·9) | 51·7 (15·0) | 52·2 (15·5) |
| Sex, n (%) | |||
| Male | 169 (68) | 154 (62) | 323 (65) |
| Female | 80 (32) | 96 (38) | 176 (35) |
| Race, n (%) | |||
| White | 81 (33) | 89 (36) | 170 (34) |
| Black or African American | 159 (64) | 146 (58) | 305 (61) |
| Asian | 4 (2) | 1 (<1) | 5 (1) |
| American Indian or Alaska Native | - | 3 (1) | 3 (<1) |
| Native Hawaiian or Other Pacific Islander | - | 1 (<1) | 1 (<1) |
| Multiracial | 2 (<1) | 8 (3) | 10 (2) |
| Unknown | 3 (1) | 2 (<1) | 5 (1) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 228 (92) | 233 (93) | 461 (92) |
| Hispanic or Latino | 17 (7) | 16 (6) | 33 (7) |
| Unknown | 4 (2) | 1 (<1) | 5 (1) |
| Pre-existing medical disorders, n (%)1 | |||
| Any | 225 (90) | 230 (92) | 455 (91) |
| Vascular | 136 (55) | 112 (45) | 248 (50) |
| Metabolic | 118 (47) | 108 (43) | 226 (45) |
| Respiratory | 102 (41) | 116 (46) | 218 (44) |
| Psychiatric | 66 (27) | 69 (28) | 135 (27) |
| Gastrointestinal | 54 (22) | 64 (26) | 118 (24) |
| Musculoskeletal/connective tissue | 58 (23) | 56 (22) | 114 (23) |
| Baseline LRTI Symptoms/Signs, n (%) | |||
| Chest Pain | 151 (61) | 157 (63) | 308 (62) |
| Cough | 243 (98) | 245 (98) | 488 (98) |
| Difficulty Breathing | 177 (71) | 182 (73) | 359 (72) |
| Fever | 117 (47) | 115 (46) | 232 (46) |
| Sputum Production | 219 (88) | 205 (82) | 424 (85) |
Only pre-existing condition categories with >15% prevalence are listed.
The primary outcome of clinical improvement at Day 5 was observed in 155/227 (69%, 95% CI 61,77%) of azithromycin-treated subjects and 148/238 (63%, 95% CI 54,71%) of placebo-treated subjects in the ITT analysis (Table 2). The difference in rates relative to azithromycin treatment was −6% (95% CI - 15,2%). Since the lower bound of this difference was less than −12·5%, non-inferiority cannot be concluded. An ATP analysis at Day 5 also failed to demonstrate non-inferiority [65% (136/221) for improvement with placebo vs. 70% (136/204) for azithromycin; rate difference −5% (95% CI −14,4%)]. Although placebo was not non-inferior to azithromycin at Day 5, there was no statistically significant difference in the rates of any of the individual parameters comprising the clinical improvement composite outcome (Figure 2).
Table 2:
Rates of Clinical Improvement
| Timepoint | Analysis Population |
Treatment Group | Rate of Clinical Improvement, n [%, (95% CI)] |
Difference in Rates, % (95% CI) |
Non- inferior1 |
|---|---|---|---|---|---|
| Day 5 | |||||
| ITT-5 | Azithromycin (N=249) | 155 [69, (61, 77)] | −6 (−15,2) | No | |
| Placebo (N=250) | 148 [63, (54, 71)] | ||||
| ATP-5 | Azithromycin (N=204) | 136 [70, (62, 79)] | −5 (−14,4) | No | |
| Placebo (N=221) | 136 [65, (57, 74)] | ||||
| Day 11 | |||||
| ITT-11 | Azithromycin (N=249) | 187 [81, (74, 87)] | −4 (−12,3) | Yes | |
| Placebo (N=250) | 184 [76, (70, 83)] | ||||
| ATP-11 | Azithromycin (N=211) | 174 [80, (73, 87)] | −4 (−11,4) | Yes | |
| Placebo (N=225) | 177 [77, (70, 83)] | ||||
| Day 28 | |||||
| ITT-28 | Azithromycin (N=249) | 202 [88, (83, 93)] | −7 (−13,0) | No | |
| Placebo (N=250) | 194 [82, (77, 86)] | ||||
| ATP-28 | Azithromycin (N=210) | 185 [88, (83, 93)] | −6 (−12,1) | Yes | |
| Placebo (N=223) | 184 [82, (77, 87)] | ||||
ITT = Intention to treat. ATP = According to protocol. n = Number of subjects with clinical improvement.
Non-inferiority of placebo is concluded if the lower bound of the 95% CI for the difference in proportions is greater in below −12·5%.
Figure 2. Clinical improvement and solicited events among azithromycin-treated and placebo-treated subjects at Day 5.
The desirability of outcome ranking (DOOR) incorporates elements of clinical improvement and solicited adverse events while simultaneously considering the severity of those adverse events. Presented values indicate the probability of a more desirable DOOR when assigned to placebo vs. azithromycin. Probabilities above 50% indicate a more favorable DOOR with placebo treatment. Rather than reporting probabilities of adequate clinical improvement (a study endpoint), we report its absence to align the treatment-dependent directionality show in the figure.
At later timepoints, placebo was non-inferior to azithromycin [Day 11: 184/235 (76%, 95% CI 70,83%) vs. 187/226 (81%, 95% CI 74,87%), respectively]; difference in rates −4% (95% CI −12,3%) (Table 2). The ATP analysis at Day 11 showed similar results. Whereas placebo was non-inferior to azithromycin at Day 11, the placebo group reported higher rates of worsening sputum production [14/225 (6%, 95% CI 3·7,10·2%) vs. 3/211 (1%, 95% CI 0·5,4·1%); difference 5% (95% CI 1,9%); P=0·012] and worsening difficulty breathing [8/225 (4%, 95% CI 1·8,6·9%) vs. 1/211 (<1%, 95% CI 0·1,2·6%); difference 3% (95% CI 1,6%); P=0·038].
Results at Day 28 were mixed: the ITT population failed to show non-inferiority [azithromycin 88% (202/229) vs. placebo 82% (194/238), difference in rates of −7% (95% CI −13,0%)] but the ATP analysis indicated non-inferiority [differences in rate of −6% (95% CI −12,1%)] (Table 2).
DOOR rankings incorporate clinical improvement and AEs (Supplementary Table 1, Appendix page 2; Supplementary Figure 1, Appendix page 3). The probability of a more desirable rank when assigned to placebo was 48·7% (95% CI 43·7,53·7%) for DOOR (Figure 2) and 62% (95% CI 56,67%) for RADAR at Day 5, and 50·5% (95% CI 45·5,55·5%) for DOOR (Supplementary Figure 2, Appendix page 4) and 65% (95% CI 60,70%) for RADAR at Day 11. Individual elements of clinical improvement, solicited AEs, and the severity of solicited AEs were not significantly different between arms at Day 5 except for increased abdominal pain associated with azithromycin treatment [47/204 (23%, 95% CI 18,29% vs. 35/221 (16%, 95% CI 12,21%) for placebo; difference −7% (95% CI −15,0%); P=0·066]. No participants died. Four subjects were hospitalized for persistent or worsening LRTI in the ITT population although none required ICU care (three in the placebo group and one in the azithromycin group).
In a post-hoc analysis, we measured the change in levels of ten cytokines from enrollment to Day 5 to identify associations with treatment or clinical improvement. Samples were available at both timepoints for 374 subjects (172/249 in the azithromycin group and 202/250 in the placebo group). IL-1β, IL-2, IL-4, IL-12p70, and IL-13 had high rates of undetectable levels (68-94% of samples) and were excluded from analysis. In contrast, IFN-γ, IL-6, IL-8, IL-10, and TNF-α had undetectable levels in only 0-1·9% of samples. The changes in Day 1-Day 5 cytokine levels were not statistically different when stratified by clinical improvement (Supplementary Table 2, Appendix page 5). However, IL-8 demonstrated significantly greater declines in azithromycin-treated subjects than in placebo-treated subjects [median change - 1·5pg/ml, IQR (−3·4,0·0) vs −0·4pg/ml, IQR (−2·8,1·9), respectively; P=0·002]. Moreover, this difference was only significant among individuals with clinical improvement [median change -1·8pg/ml, IQR (−4·0,−0·1) for azithromycin vs −0·9pg/ml, IQR (−2·7,1·2) for placebo; P=0·004] (Supplementary Table 3, Appendix page 6). No treatment-related differences in IL-8 were observed among subjects without adequate clinical improvement [median change −1·1pg/ml, IQR (−2·3,0·3) for azithromycin vs 0·1pg/ml, IQR (−2·8,2·5) for placebo; P=0·099].
There were no pre-existing medical conditions or concomitant medications associated with clinical improvement at Days 5 or 11 (Supplementary Tables 4-5, Appendix pages 7-8). In a post-hoc analysis, logistic regression identified subjects with ‘difficulty breathing’ at enrollment as more likely to improve with an odds ratio of 2·29 (95% CI 1·40,3·73) at Day 5 (Supplementary Figure 3, Appendix page 9) and 2·24 (95% CI 1·25,4·00) at Day 11 (Supplementary Figure 4, Appendix page 10). A second post-hoc analysis evaluated whether baseline variables were associated with clinical improvement in a treatment-dependent manner. No statistically significant treatment interactions were observed for sex, race, pre-existing asthma, pre-existing COPD, identification of a viral etiology, chest pain at enrollment, or sputum production at enrollment. However, a statistically significant treatment effect was observed in participants with or without difficulty breathing at enrollment as well as participants with or without fever at enrollment (Figure 3). Those without difficulty breathing at enrollment had higher rates of clinical improvement in the azithromycin group (62%) compared to the placebo group (42%) (p<0.0001 for the interaction of difficulty breathing and treatment). Participants with fever at enrollment had higher rates of clinical improvement in the azithromycin group (79%) compared to the placebo group (58%) (P=0.006 for the interaction of fever and treatment). Similar results were seen at Day 11 (Supplementary Figure 5, Appendix page 11). There was also a significant treatment effect among non-smokers and a significant interaction between treatment effect and smoking status, but this was only present at Day 5 (P=0.019 for the interaction of smoking status and treatment; Figure 3) and not at Day 11 (P=0.407; Supplementary Figure 5, Appendix page 11).
Figure 3. Forest plot of baseline characteristics by clinical improvement in the intent-to-treat Day 5 analysis population.
Each point estimate and error bars represent the estimate of the difference in rates of clinical improvement at Day 5 and the associated 95% CI obtained from a linear regression model. A difference in rates greater than zero favors azithromycin. Interaction p-values are obtained from an interaction Type III test where the interaction term between subgroup variable and treatment is being added to the linear regression model.
DISCUSSION
Procalcitonin is a host response biomarker used to guide antibacterial prescribing in patients with LRTI based on its ability to discriminate bacterial from non-bacterial disease. The studies supporting this indication were randomized clinical trials of a procalcitonin-based algorithm to recommend administering or withholding antibacterials, which clinicians adhered to at rates of 44-97% across 18 trials.(6) Furthermore, procalcitonin-based algorithms variably decreased antibacterial use with some studies showing no effect.(10) Consequently, uncertainty persists as to whether a low procalcitonin identifies patients for whom antibacterials can be safely withheld. To answer this question, we carried out the TRAP-LRTI trial, which asked whether a low procalcitonin level identifies adults with non-pneumonia LRTI who derive no benefit from azithromycin compared to placebo. This non-inferiority trial had no algorithm or treatment recommendation. Instead, subjects with low procalcitonin levels were randomized to azithromycin or placebo. At the early Day 5 endpoint, we could not conclude that placebo was statistically non-inferior to azithromycin although there was no individual component of the composite endpoint that significantly differed between groups. This included no increase in patient harm as measured by medically attended visits, hospitalization, ICU admission, or death. The failure to demonstrate non-inferiority may have been affected by the premature termination of enrollment due to COVID-19 and decreased power (77% compared to the planned 80%). However, at later time points, when recovery from viral infection is expected(18, 19), placebo was non-inferior to azithromycin on Day 11 and the Day 28 ATP population. DOOR, a global patient outcome based on clinical benefits and harms,(14) revealed no significant difference in treatment groups although azithromycin was associated with more solicited adverse events, particularly abdominal pain. RADAR extends the DOOR outcome by accounting for antibiotic utilization. In doing so, we observed superiority of placebo to azithromycin primarily due to reduced antibiotic use in the placebo group.
This study’s primary endpoint of five days was chosen based on established treatment endpoints for community-acquired bacterial pneumonia and to identify clinical deterioration due to lack of needed antibacterial treatment.(15) However, we observed rates of clinical improvement that were lower at five days than would be expected for community-acquired bacterial pneumonia, consistent with the largely viral nature of LRTI observed in this study. In addition, higher rates of clinical improvement in the azithromycin group could have been due to the drug’s anti-inflammatory effects rather than its antibacterial properties. Azithromycin lowers cytokine responses, most notably IL-8, which is elevated in respiratory viral infections.(11, 20-23) These anti-inflammatory properties of azithromycin supported its selection above other antibiotics. For example, demonstrating non-inferiority compared to an antibiotic such as amoxicillin could have left persistent questions about whether antibiotics with anti-inflammatory properties, such as azithromycin, would have produced different results. Azithromycin is also a guideline-recommended antibiotic for the treatment of acute exacerbations of COPD associated with bacterial triggers.(24) To further explore the anti-inflammatory properties of azithromycin, we measured levels of multiple inflammatory cytokines at enrollment and Day 5 to assess treatment-related cytokine changes. We observed decreases in IL-8 among azithromycin-treated subjects, which was only statistically significant in subjects demonstrating clinical improvement. This is consistent with the hypothesis that azithromycin-associated reductions in IL-8 could contribute to the higher Day 5 clinical improvement rates observed. A common clinical manifestation of inflammation is fever. An exploratory analysis suggested that participants with fever were more likely to clinically improve when treated with azithromycin. This might suggest that fever should prompt antibacterial therapy although fever is a poor discriminator of bacterial or viral infection. Results of this study do not distinguish whether it is the antibacterial, anti-inflammatory, or both properties of azithromycin that explain this observation. They also do not address whether procalcitonin identifies patients who might benefit from anti-inflammatory therapy. Additional research specifically targeting patients with fever would help address this question.
Prior studies using a procalcitonin algorithm included diverse populations spanning children to adults, colds to pneumonia, and outpatients to the critically ill.(8, 10, 25-27) This study focused on outpatient adults enrolled in clinics or EDs who had non-pneumonia LRTI. We excluded cases of pneumonia to minimize risks to participants and because prior evidence showed that reductions in antibiotics using procalcitonin algorithms were greatest in non-pneumonia LRTI.(7) The cohort had few subjects with elevated procalcitonin levels, suggesting some bias in subject selection. We also did not categorize the type of LRTI (e.g., acute bronchitis, asthma exacerbations, or acute exacerbations of chronic obstructive pulmonary disease) given imprecision in the diagnostic criteria and overlapping clinical syndromes. This is underscored by persistently high rates of inappropriate antibiotic use in patients with syndromes for whom antibiotics are not otherwise recommended.(3) As such, we are unable to determine if particular subtypes of LRTI are more suitable for a procalcitonin-driven strategy. No pathogen was identified in 46% of the cohort, raising the possibility that some participants did not have an infection at all and representing another opportunity to limit unnecessary antibiotic use.
Future procalcitonin studies (or those of other biomarkers) should consider a similar design to TRAP-LRTI in which subjects are randomized to antibiotic or placebo therapy when a bacterial infection is unlikely. In particular, studies of other acute respiratory infections such as pneumonia as well as other age groups such as infants and children would be an important extension of this study.
Among the study limitations are that most screened participants had low procalcitonin levels along with a low prevalence of bacterial infection. It is unclear how well this represents a typical non-pneumonia LRTI population. The study does not address the utility of procalcitonin in pneumonia or immunocompromised patients, which were excluded. LRTI was not further stratified into conditions where antibiotics are routinely or occasionally prescribed (e.g., acute exacerbation of COPD versus acute bronchitis). We did not compare or combine multiple diagnostic approaches (e.g., host gene expression testing or microbial testing) alongside procalcitonin.(28, 29) Another antibiotic without notable anti-inflammatory properties could have been chosen. However, azithromycin is a frequently prescribed antibiotic for non-pneumonia LRTI, in part because of its pleiotropic effects.(30) Furthermore, we cannot assume that similar results would have been observed using an antibiotic with a different spectrum of activity than azithromycin. All of the bacterial pathogens identified in this study are generally azithromycin susceptible. However, it is possible we enrolled (but did not identify) patients with azithromycin-resistant infections, which would lower rates of clinical improvement in both arms. The study did not achieve the pre-specified target enrollment due to COVID-19, although the study still had 77% power to demonstrate non-inferiority compared to the planned 80%. The study’s primary endpoint was based on Day 5 clinical improvement, which was likely too early given the viral etiology in most cases.
In conclusion, placebo was not non-inferior to azithromycin in terms of clinical improvement at day 5 for adults with non-pneumonia lower respiratory tract infection and a low procalcitonin concentration, but was non-inferior at day 11 and showed mixed findings at day 28. However, the placebo group showed better outcomes using a RADAR analysis that accounted for azithromycin-related solicited adverse events and the inherent harms of unnecessary antibiotic use. Furthermore, patients with non-pneumonia lower respiratory tract infection have high rates of clinical improvement through 28 days, regardless of antibacterial treatment.
Supplementary Material
Research in context.
Evidence before this study:
Inappropriate prescribing of antibiotics for viral acute respiratory infection (ARI) contributes to increased healthcare costs, unnecessary drug-related adverse effects, and is a major contributor to antimicrobial resistance. This is among the reasons why developing improved diagnostics that can be applied in the outpatient setting has been recommended by numerous entities including the World Health Organization. As a complement to current pathogen-focused testing, host-based biomarkers can provide useful diagnostic information. This is predicated on the observation that patients respond differently to viral and bacterial infection. No biomarker has received more attention in this regard than procalcitonin (PCT). PCT was first described in the setting of sepsis, where concentrations were increased compared to non-infectious conditions. Moreover, PCT has been used to distinguish bacterial from viral infection because interferon gamma production induced by viral infections inhibits PCT production. However, studies assessing the performance characteristics in pneumonia revealed only moderate accuracy with a 55% sensitivity and 76% specificity in a meta-analysis published by Kamat et al. Nevertheless, PCT-guided management of lower respiratory tract infections (LRTI) has been used to withhold antibiotics or shorten the duration of antibiotic therapy without adversely affecting outcomes in several European studies. Specifically, low PCT values (≤0.25 ng/mL) were used to generate recommendations that antibiotics be withheld. Conversely, high PCT values prompted recommendations that antibiotics be provided. The results of these various studies are highlighted in a Cochrane database systematic review and showed that antibacterial use decreased by 50% without adversely affecting clinical outcomes. However, the large randomized ProACT clinical trial performed in the U.S. did not observe antibiotic reductions using a PCT algorithm. Moreover, rates of adherence to the PCT algorithm were variable and as low as 72.9% in the ProACT trial. Given these findings, it remains unclear whether low PCT levels can be used to safely withhold antibiotics in patients with LRTI. A search for ‘procalcitonin’ and ‘clinical trial’ on PubMed on 15 August, 2022 did not identify any results demonstrating the clinical impact of an enforced PCT algorithm. A search on the same day for ‘procalcitonin’ and ‘placebo’ on ClinicalTrials.gov identified one feasibility study involving randomization to placebo in 5 participants, but results were not posted.
Added value of this study:
This placebo-controlled, randomized, double blinded study represents an approach to the evaluation of PCT in LRTI that has not previously been evaluated. Based on the above evidence, we hypothesized that patients with suspected LRTI and a PCT value ≤0.25ng/mL are unlikely to have a bacterial infection, and hence, will not benefit from antibacterial therapy. Therefore, we hypothesised that treatment of these patients with placebo would be non-inferior to antibiotic treatment. By randomly assigning participants to antibiotics or placebo, this study design eliminated the possibility of algorithm non-adherence. We found that placebo was not non-inferior to azithromycin treatment in terms of clinical improvement at day 5. There was no increase in any adverse events among those treated with placebo. Later timepoints, including days 11 and 28, showed placebo to be non-inferior to azithromycin.
Implications of all the available evidence:
The clinical community has long sought a means to identify which patients require antibiotic therapy and which can be safely managed without. Despite the abundance of PCT-related literature, there is no consensus on its reliability in distinguishing bacterial from viral infection or guiding antibiotic use. The results of this study clarify many previous unknowns but do not address all scenarios. Specifically, this study excluded patients where the prescriber was unwilling to randomize to placebo. Therefore, it remains unknown whether PCT can safely and effectively guide antibiotic use on its own (independent of additional clinical information). These study results show that low procalcitonin concentrations in patients with lower respiratory tract infection are unable to identify when patients could benefit from azithromycin in terms of clinical improvement at day 5, possibly due to the drug’s anti-inflammatory effects. At later timepoints, placebo was non-inferior to azithromycin. The placebo group also had lower rates of solicited adverse events and an intrinsically lower antibiotic use rate. Clinicians should weigh these factors (clinical improvement, reduced adverse events, and lower antibiotic use) when deciding whether to use procalcitonin as a guide for antibiotic initiation.
Acknowledgements:
The TRAP-LRTI Study Group includes the following additional authors: Ghina Alaaeddine MD, Jennifer J. Zreloff MD, Nina McNair BS, Colleen S. Kraft MD, David L. Roberts MD, Sharon H. Bergquist MD, Nour Beydoun MD, Jesse J. Waggoner MD, Merin E. Kalangara MD, Matthew H. Collins MD PhD, Alexandra W. Dretler MD, Amer R. Bechnak MD, Laura Oh MD, Zhihong Yuan PhD, Brian J. Burrows MD, Emily R. Ko MD, Weixiao Dai MS, Lijuan Zeng MPH Their affiliations are as follows:
GA: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
JJZ: Division of General Internal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, GA
NM: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia; Atlanta VA Health Care System, Atlanta, GA
CSK: Department of Pathology, Emory University School of Medicine, Atlanta, GA
DLR: Division of General Internal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, GA
SHB: Division of General Internal Medicine, Department of Medicine, Emory University School of Medicine, Atlanta, GA
NB: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
JJW: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
MEK: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
MHC: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
AWD: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
ARB: Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA
LO: Department of Emergency Medicine, Emory University School of Medicine, Atlanta, GA; Emergency Medicine Service, Atlanta VA Health Care System, Atlanta, GA
ZY: Medical Service, Atlanta VA Health Care System, Atlanta, GA; Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE
BJB: Department of Emergency Medicine, Duke Regional Hospital, Durham, NC
ERK: Department of Hospital Medicine, Duke Regional Hospital, Durham, NC
WD: The Biostatistics Center, Milken Institute School of Public Health, George Washington University, Rockville, MD; Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Rockville, MD
LZ: The Biostatistics Center, Milken Institute School of Public Health, George Washington University, Rockville, MD; Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Rockville, MDThe authors would like to acknowledge the study participants, the institutions that contributed to participant recruitment, members of the Data and Safety Monitoring Board (David Carlin, Eric Meissner, Emanuel Rivers, Eugene Shapiro, and Ellen Wald), bioMérieux (Samuel Bozzette), and the following individuals who supported the TRAP-LRTI study:
Emory University and Atlanta VA Health Care System: Dilshad Rafi Ahmed, Mary Bower, Ana Drobeniuc, Lisa Harewood, Christopher Huerta, Jessica Ingersoll, Jesse Jacob, Brandi Johnson, Colleen Kelley, Dean Kleinhenz, Lilin Lai, Pamela Lankford-Turner, Hollie Macenczak, Michele McCullough, Candace Miller, Juliet Morales, Varun Phadke, Youssef Saklawi, Amy Sherman, Taé Stallworth, Jessica Traenkner, Cynthia Whitney, and Zanthia Wiley.
Duke University and Durham VA Health Care System: Jack Anderson, Brian Antczak, Luis Ballon, Thomas Burke, Katherine Frankey, Joyce Gandee, Kristen Gunnell, Lynn Harrington, Sara Hoffman, Pearce Jackson, Ally Johnson, Alexander Limkakeng, Tyffany Locklear, Anna Mazur, Beth McLendon-Arvik, Ellen Poulsen, Cathy Sampey, Liz Schmidt, Stephanie Smith, and Rachel Toler.
Michael E. DeBakey VA Medical Center: Katharine Breaux, Biju Johnson, Bashir Lengi, Dena Mansouri, and Tehquin Tanner.
Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Disease, National Institutes of Health: Jane Knisley and Janie Russell.
Emmes Corporation: Tom Conrad, Aya Nakamura, Randoph Oler, Michelle Serock, Alison Wall, and Katie Wiegand.
Funding sources:
This project was funded in part by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services under contracts to the Vaccine and Treatment Evaluation Units at Duke University (HHSN272201300017I, PI: EBW), Emory University (HHSN272201300018I, PI: NGR) and Baylor College of Medicine (HHSN27220130015l, PI: HMES). Additional support was provided by NIAID award UM1AI104681 to the Antibacterial Resistance Leadership Group (ARLG). bioMérieux provided study product (azithromycin and placebo), instruments and reagents to perform procalcitonin and Respiratory Panel-2 testing, and provided input on study conception and design. Statistics and data management support for the trial was provided by the EMMES corporation under NIAID award HHSN272201500002C. RTS was supported by grants R01HL144478 and VA MR BX001786.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: ELT and CWW report consulting for Biomeme, Inc. ELT, CWW, and MTM have a patent pending for Methods to Diagnose and Treat Acute Respiratory Infections (US20180245154A1). Following completion of the study, ELT became an employee of Danaher Diagnostics. EBW is a principal investigator for Pfizer-funded studies of COVID-19 vaccine, a co-investigator for a vaccine study funded by Moderna, and a member of an advisory board for Vaxcyte. NGR serves as a safety consultant for the Emmes Corporation and ICON-I, and receives research funds from Pfizer, Merck, Sanofi, Quidel, and Lilly. All remaining authors report no conflicts of interest.
Contributor Information
Ephraim L. Tsalik, Division of Infectious Diseases, Department of Medicine, Duke University School of Medicine, Durham, NC; Center for Applied Genomics and Precision Medicine, Department of Medicine, Duke University School of Medicine, Durham, NC; Emergency Medicine Service, Durham VA Health Care System, Durham, NC.
Nadine G. Rouphael, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA.
Ruxana T. Sadikot, Atlanta VA Health Care System, Atlanta, Georgia; Medical Service, VA Nebraska-Western Iowa Health Care System, Omaha, NE; Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE.
Maria C. Rodriguez-Barradas, Infectious Diseases Section, Michael E. DeBakey VA Medical Center and Department of Medicine, Baylor College of Medicine, Houston, TX.
Micah T. McClain, Medical Service, Durham VA Health Care System, Durham NC; Division of Infectious Disease, Department of Medicine, Duke University School of Medicine, Durham, NC; Center for Applied, Genomics and Precision Medicine, Department of Medicine, Duke University School of Medicine, Durham, NC.
Dana M. Wilkins, bioMérieux, Inc., Durham, NC; Rho, Inc., Durham, NC.
Christopher W. Woods, Division of Infectious Diseases, Department of Medicine, Duke University School of Medicine, Durham, NC; Center for Applied Genomics and Precision Medicine, Department of Medicine, Duke University School of Medicine, Durham, NC; Medical Service, Durham VA Health Care System, Durham NC.
Geeta K. Swamy, Department of Obstetrics & Gynecology, Duke University School of Medicine, Durham, NC; Duke Human Vaccine Institute, Duke University School of Medicine, Durham, NC.
Emmanuel B. Walter, Department of Pediatrics and Duke Human Vaccine Institute, Duke University School of Medicine, Durham, NC.
Hana M. El Sahly, Department of Molecular Virology & Microbiology, Baylor College of Medicine, Houston, TX; Department of Medicine, Baylor College of Medicine, Houston, TX.
Wendy A. Keitel, Department of Molecular Virology & Microbiology, Baylor College of Medicine, Houston, TX; Department of Medicine, Baylor College of Medicine, Houston, TX.
Mark J. Mulligan, Hope Clinic of the Emory Vaccine Center, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia; Division of Infectious Diseases and Immunology, NYU Langone Health, New York, NY.
Bonifride Tuyishimire, The Emmes Company, LLC, Rockville, MD.
Elisavet Serti, The Emmes Company, LLC, Rockville, MD.
Toshimitsu Hamasaki, The Biostatistics Center, Milken Institute School of Public Health, George Washington University, Rockville, MD; Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Rockville, MD.
Scott R. Evans, The Biostatistics Center, Milken Institute School of Public Health, George Washington University, Rockville, MD; Department of Biostatistics and Bioinformatics, Milken Institute School of Public Health, George Washington University, Rockville, MD.
Varduhi Ghazaryan, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville MD.
Marina S. Lee, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville MD.
Ebbing Lautenbach, Division of Infectious Diseases, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Data sharing:
Individual deidentified participant data (and supporting documentation, data dictionaries, and protocol) that underlie the results in this Article can be made available to investigators following submission of a plan for data use, approval by the ARLG or designated entity, and execution of required institutional agreements. Provision might be contingent upon the availability of funding for data preparation and deidentification. More information can be found at https://arlg.org/how-to-apply/requestdata/. The full protocol and statistical analysis plan can be accessed at https://www.clinicaltrials.gov/ct2/show/NCT03341273.
REFERENCES
- 1.Disease Injury IP GBD, Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open. 2018;1(2):e180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr., et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315(17): 1864–73. [DOI] [PubMed] [Google Scholar]
- 4.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to Distinguish Viral From Bacterial Pneumonia: A Systematic Review and Meta-analysis. Clin Infect Dis. 2020;70(3):538–42. [DOI] [PubMed] [Google Scholar]
- 6.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews. 2017(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600–7. [DOI] [PubMed] [Google Scholar]
- 8.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. Jama. 2009;302(10): 1059–66. [DOI] [PubMed] [Google Scholar]
- 9.FDA. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. In: Services USDoHH, editor. www.accessdata.fda.gov2017. [Google Scholar]
- 10.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CH, Doi Y, et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N Engl J Med. 2018;379(3):236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82(11):1345–50. [PubMed] [Google Scholar]
- 13.Williams DJ, Creech CB, Walter EB, Martin JM, Gerber JS, Newland JG, et al. Short- vs Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: The SCOUT-CAP Randomized Clinical Trial. JAMA Pediatrics. 2022;176(3):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans SR, Rubin D, Follmann D, Pennello G, Huskins WC, Powers JH, et al. Desirability of Outcome Ranking (DOOR) and Response Adjusted for Days of Antibiotic Risk (RADAR). Clinical Infectious Diseases. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA. Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment. 2014. [Google Scholar]
- 16.FDA. Non-Inferiority Clinical Trials to Establish Effectiveness. 2016. [Google Scholar]
- 17.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- 18.Bruyndonckx R, Coenen S, Butler C, Verheij T, Little P, Hens N, et al. Respiratory syncytial virus and influenza virus infection in adult primary care patients: Association of age with prevalence, diagnostic features and illness course. Int J Infect Dis. 2020;95:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and Safety of the Oral Neuraminidase Inhibitor Oseltamivir in Treating Acute InfluenzaA Randomized Controlled Trial. JAMA. 2000;283(8): 1016–24. [DOI] [PubMed] [Google Scholar]
- 20.Yang J Mechanism of azithromycin in airway diseases. The Journal of international medical research. 2020;48(6):300060520932104-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClain MT, Henao R, Williams J, Nicholson B, Veldman T, Hudson L, et al. Differential evolution of peripheral cytokine levels in symptomatic and asymptomatic responses to experimental influenza virus challenge. Clin Exp Immunol. 2016;183(3):441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature medicine. 2020;26(10):1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venditto VJ, Haydar D, Abdel-Latif A, Gensel JC, Anstead MI, Pitts MG, et al. Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19. Frontiers in immunology. 2021;12:574425-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 Report 2021 [Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
- 25.Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93. [DOI] [PubMed] [Google Scholar]
- 26.Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Muller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. [DOI] [PubMed] [Google Scholar]
- 27.Baer G, Baumann P, Buettcher M, Heininger U, Berthet G, Schafer J, et al. Procalcitonin Guidance to Reduce Antibiotic Treatment of Lower Respiratory Tract Infection in Children and Adolescents (ProPAED): A Randomized Controlled Trial. PLoS ONE. 2013;8(8):e68419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsalik EL, Henao R, Montgomery JL, Nawrocki JW, Aydin M, Lydon EC, et al. Discriminating Bacterial and Viral Infection Using a Rapid Host Gene Expression Test. Crit Care Med. 2021;49(10): 1651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko ER, Henao R, Frankey K, Petzold EA, Isner PD, Jaehne AK, et al. Prospective Validation of a Rapid Host Gene Expression Test to Discriminate Bacterial From Viral Respiratory Infection. JAMA Netw Open. 2022;5(4):e227299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durkin MJ, Jafarzadeh SR, Hsueh K, Sallah YH, Munshi KD, Henderson RR, et al. Outpatient Antibiotic Prescription Trends in the United States: A National Cohort Study. Infect Control Hosp Epidemiol. 2018;39(5):584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual deidentified participant data (and supporting documentation, data dictionaries, and protocol) that underlie the results in this Article can be made available to investigators following submission of a plan for data use, approval by the ARLG or designated entity, and execution of required institutional agreements. Provision might be contingent upon the availability of funding for data preparation and deidentification. More information can be found at https://arlg.org/how-to-apply/requestdata/. The full protocol and statistical analysis plan can be accessed at https://www.clinicaltrials.gov/ct2/show/NCT03341273.