Abstract
Background:
Translocation between chromosomes 4 and 14, t(4;14), has been reported in 15% of patients with multiple myeloma (MM), and is considered a high-risk cytogenetic abnormality that is associated with inferior outcomes. Autologous hematopoietic stem cell transplantation (auto-HCT) is standard of care for patients with high-risk MM (HRMM), yet there is scarce data on post-transplant outcomes of patients with t(4;14) MM patients.
Objective:
The aim of the study was to evaluate outcomes of MM patients with t(4;14) who underwent an auto-HCT, and received contemporary anti-myeloma agents for induction and post-transplant maintenance.
Study design:
We conducted a retrospective analysis of MM patients with t(4;14), detected by fluorescence in situ hybridization (FISH), who underwent auto-HCT between 2008-2018 at MD Anderson Cancer Center. Primary endpoints were progression free survival (PFS) and overall survival (OS), and secondary endpoints were hematological response and minimal residual disease (MRD) status after auto-HCT. MRD status on the bone marrow biopsy was evaluated using 8-color next generation flow cytometry with a sensitivity of 1/10−5 cells.
Results:
Seventy nine patients were included, with a median age of 60 (range: 32-78) years, and 52% were male. Forty-four (56%) patients had an additional HR cytogenetic abnormality. Fifty patients (63%) achieved ≥VGPR prior to auto-HCT and 20 (25%) had minimal residual disease (MRD) negative ≥VGPR. At best post-transplant evaluation, 90% and 63% had ≥VGPR and MRD-negative ≥VGPR, respectively. Median follow-up for survivors was 35.7 (range 7.7-111.6) months. Median PFS and OS for the entire cohort were 22.9 months and 60.4 months, respectively. Patients with MRD negative ≥VGPR prior to transplant had improved PFS and OS on both univariate analysis (UVA) and multivariate analysis (MVA): (HR [95% CI] 0.35 [0.16-0.76], p=0.008) and (0.12 [0.03-0.44], p=0.002), respectively. The presence of additional high-risk cytogenetic abnormalities was not associated with inferior PFS (p=0.57) or OS (p=0.70). Post-transplant lenalidomide-based combinations were associated with improved OS in both UVA and MVA (0.14 [0.04-0.45], p=0.001), while their impact on PFS was not statistically significant (p=0.37).
Conclusions:
Our results consolidate t(4;14) as a high-risk abnormality associated with poor outcomes despite novel-agent induction, auto-HCT and post-transplant maintenance. Despite some inherent study design limitations including a relatively small cohort and heterogeneity in treatment, we observed that deeper pre-transplant response and post-transplant maintenance with lenalidomide-based combination were associated with improved outcomes. Novel immune- and cellular therapies are needed to improve the outcomes in patients with t(4;14).
Keywords: Multiple myeloma, autologous hematopoietic cell transplantation, translocation (4;14)
Introduction
Translocation between chromosomes 4 and 14, t(4;14), is one of the high-risk (HR) aberrations seen in multiple myeloma (MM), occurring in 15% of MM patients1. It is associated with worse outcomes, including shorter progression-free survival (PFS) and overall survival (OS)2, and is one of the three chromosomal abnormalities that defines high-risk disease in the R-ISS3. The translocation results in the generation of derivative chromosomes that place two genes under the control of IgH enhancers: Fibroblast Growth Factor Receptor 3 (FGFR3) and Nuclear Receptor SET Domain Protein 2 (NSD2)4. The exact mechanism through which dysregulation of these putative oncogenes contributes to the aggressive phenotype of t(4;14) MM is not completely understood. The majority of patients with t(4;14) have FGFR3 overexpression, yet the adverse risk also persists in non-expressors5. A recently published report by Stong et al. found that the breakpoint location and expression of NSD2 fusion transcripts are the key molecular features that distinguish high-risk patients within the t(4;14) population6.
There is a paucity of data on post-transplantation outcomes for patients with t(4;14). A study comparing the outcomes following autologous hematopoietic stem cell transplantation (auto-HCT) in MM patients with or without t(4;14) showed that patients with t(4;14) had inferior PFS (median 9.9 months vs. 25.8 months, p=0.0003) and OS (median 18.3 months vs. 48.1 months, p < 0·0001) compared to patients without t(4;14)7. However, only 15 of the 128 patients reported in that study had t(4;14). A report from the Center for International Blood and Marrow Transplant Research (CIBMTR) compared post-transplantation outcomes of HRMM to non-HRMM patients, where patients with t(4;14) had a significantly lower three-year rates for PFS (28% vs. 49%) and OS (60% vs. 85 %) compared to non-HRMM patients8. However, among the 125 HRMM patients studied, only 28 had t(4;14). Another study evaluated the outcomes of 17 patients with t(4;14) compared to 76 without this translocation. The patients received various induction regimens, and six of the patients with t(4;14) underwent auto-HCT. Median PFS after auto-HCT was shorter for the t(4;14) group compared to those without this translocation (24.4 months vs. unreached, respectively, p=0.088). There was no statistically significant difference in median OS or overall response rate (ORR) in patients with or without t(4;14) in that study9.
Given the scarcity of data and the need for a larger study focusing on MM patients with t(4;14), here we report the outcomes of these patients, who received induction with contemporary anti-myeloma agents, auto-HCT and post-transplant maintenance therapy.
Materials and Methods
Study Design and Participants
We conducted a retrospective, single-center, chart review study of patients with newly diagnosed MM and t(4;14) who received auto-HCT between 2008-2018. Data was obtained from our institution’s transplant database and chart-based review. The primary endpoints were PFS and OS, and the secondary endpoints were hematological response and minimal residual disease (MRD) status after auto-HCT. The study was conducted after approval by our institutional review board (IRB) and in accordance with the declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act (HIPAA).
Response definitions and MRD evaluation
The International Myeloma Working Group (IMWG) criteria were used to evaluate response as well as progression10. Patients were categorized as having complete response (CR), stringent CR (sCR), very good partial response (VGPR), partial response (PR), stable disease (SD), or progressive disease (PD).
We also evaluated MRD status, which was assessed via 8-color next-generation flow cytometry (NGF) at our institution. The sensitivity of our assay is 1/10−5 cells (0.001%) based on acquisition and analysis of at least 2 million events.
Fluorescence in situ hybridization analysis
Fluorescence in situ hybridization (FISH) analysis was performed for the detection of known high-risk cytogenetic alterations, namely t(4;14), t(14;16), del(17p), and 1q21 gain or amplification by using the following FISH probe sets: IGH::FGFR3 dual-color dual-fusion probes; IGH::MAF dual-color dual-fusion probes; TP53/CEP17 dual-color and CDKN2C/CKS1B dual-color probes. 1q21 gain was defined as having 3~4 copies of CKS1B, while 1q21 amplification was identified as having ≥5 copies of CKS1B. Plasma cell enrichment was not performed on majority of the cases during this study period. The cut-off value for common abnormal signal patterns was established by our clinical cytogenetics laboratory as follows: 7.9% for 1q21 gain, 0% 1q21 amplification, 4.7% for deletion of TP53, 0.4% for t(4;14), and 0% for t(14;16).
Statistical methods
PFS was computed from the date of auto-HCT to the date of disease progression or death (if died without disease progression) or the last follow-up date. Patients who were alive and did not experience progression of disease at the last follow-up date were censored. OS was measured from the date of auto-HCT to the last known vital sign. Patients alive at the last follow-up date were censored. PFS and OS were estimated using the Kaplan-Meier method and differences between groups were evaluated by the log-rank test. Associations between PFS and OS and measures of interest were determined using Cox proportional hazards regression models. Measures that occur after auto-HCT (i.e., response and MRD after auto-HCT as well as maintenance treatment) were included in the regression models as time-dependent covariates.
Statistical analyses were performed using SAS 9.4 for Windows (Copyright © 2002-2012 by SAS Institute Inc., Cary, NC). All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Patient, disease, and treatment characteristics
Our analysis included 79 MM patients with t(4;14) who received auto-HCT at our institution. Median age was 60 (range 32-78) years and 52% (n=41) were male. Forty-four (56%) patients had additional HR cytogenetic abnormalities. The most commonly used induction and conditioning regimens were bortezomib, lenalidomide (Len), and dexamethasone (VRD) (n=34, 43%) and melphalan (n=54, 68%), respectively. Sixty-one patients (77%) received maintenance therapy post-transplantation, including 32% with Len-based combinations, and 29% with Len alone or with dexamethasone. Table 1 summarizes patient characteristics.
Table 1.
Clinical and treatment characteristics
| Measure | N=79 |
|---|---|
| Gender, n (%) | |
| Male | 41 (52) |
| Female | 38 (48) |
| Age at auto-HCT (years) | |
| Median (Range) | 60.4 (31.7 - 77.5) |
| Presence of Other High-risk Cytogenetics, n (%) | |
| No | 35 (44) |
| Yes | 44 (56) |
| Induction Regimen, n (%) | |
| VRD | 34 (43) |
| VCD | 8 (10) |
| KRD | 15 (19) |
| VD | 15 (19) |
| Other | 7 (9) |
| KPS, n (%) | |
| < 90 | 32 (43) |
| ≥ 90 | 43 (57) |
| Unknown | 4 |
| R-ISS, n (%) | |
| II | 46 (70) |
| III | 20 (30) |
| Unknown | 13 |
| HCT-CI Score, n (%) | |
| ≤ 3 | 58 (73) |
| > 3 | 21 (27) |
| Response at auto-HCT, n (%) | |
| sCR/CR | 14 (18) |
| nCR/VGPR | 36 (46) |
| PR | 23 (29) |
| SD | 2 (3) |
| PD | 4 (5) |
| MRD status at auto-HCT, n (%) | |
| Negative | 26 (36) |
| Positive | 47 (64) |
| No analysis performed | 6 |
| Conditioning Regimen, n (%) | |
| Busulfan/Melphalan | 14 (18) |
| Melphalan | 54 (68) |
| Other | 11 (14) |
| Maintenance Therapy, n (%) | |
| No | 18 (23) |
| Yes | 61 (77) |
| Len alone/with Dex | 23 (29) |
| Len-based combo* | 25 (32) |
| PI alone** | 11 (14) |
Abbreviations: n/N=Number, auto-HCT=Autologous hematopoietic stem cell transplant, KPS=Karnofsky Performance Scale, HCT-CI=Hematopoietic Cell Transplantation-specific Comorbidity Index, VRD=Bortezomib/Revlimid/dexamethasone, VCD=Bortezomib/cyclophosphamide/dexamethasone, KRD=Carfilzomib/Revlimid/dexamethasone, VD=Bortezomib/dexamethasone, sCR=Stringent complete response, CR=Complete response, nCR=Near complete response, VGPR=very good partial response, PR=Partial response, SD=Stable disease, PD=Progression of disease, MRD=Minimal residual disease, R-ISS= Revised International multiple myeloma staging system.
Revlimid/Carfilzomib/Dexamethasone (n=7), Revlimid/Bortezomib/Dexamethasone (n=4), Revlimid/Ixazomib (n=2), Revlimid/Elotuzumab (n=7), Ixazomib/Revlimid/Dexamethasone (n=5).
Bortezomib (n=7), Ixazomib (n=4).
Responses and MRD outcomes
After completing induction therapy and prior to transplantation, 14 (18%) and 50 (63%) patients achieved ≥CR or ≥VGPR, respectively, and 20 (25%) achieved MRD negative ≥VGPR. Eighty-four percent and 90% of patients had ≥VGPR at day 100 and at best response post- auto-HCT, respectively. Thirty-nine of 62 patients (63%) had MRD negative ≥VGPR at best response after auto-HCT. Pre- and post-transplant responses are depicted in Figure 1.
Figure 1.
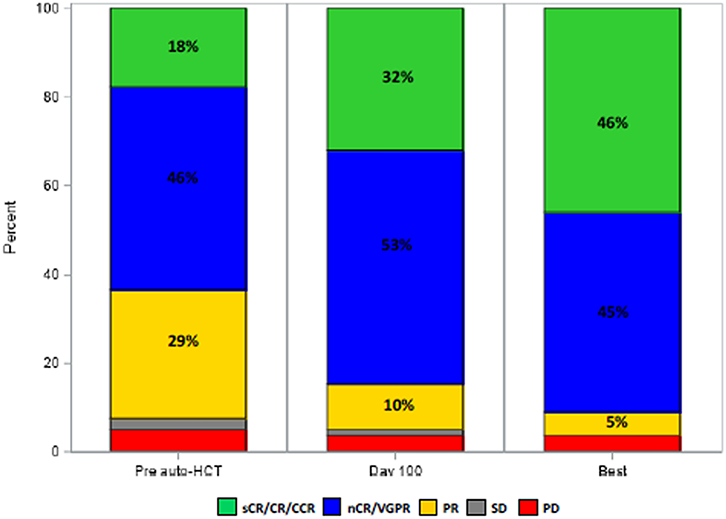
Disease response prior to transplant, at day 100 post-transplant and at best response post-transplant.
Survival outcomes
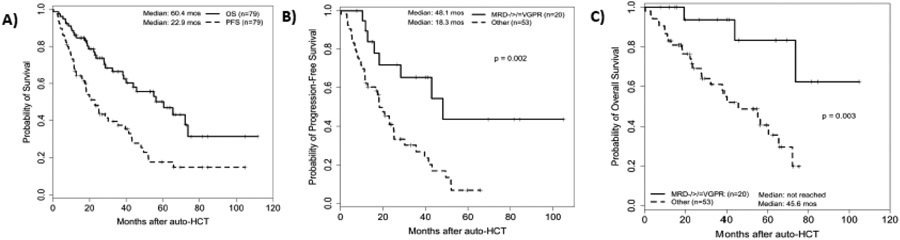
The median follow-up of survivors was 35.7 (range 7.7-111.6) months and for all patients was 28.3 (range 0.3-111.6) months. For the entire cohort, the median PFS and OS were 22.9 (95% CI 16.5- 35.6) months and 60.4 (95% CI 38.7- 73.7) months, respectively (Figure 2A). Three-year PFS and OS rates of the entire cohort were 38% and 67%, respectively.
Figure 2.
(A) Kaplan-Meier curves of progression free survival (PFS) and overall survival (OS) for the entire cohort; Outcomes of patients with or without MRD negative ≥VGPR: (B) PFS and (C) OS.
On univariable analysis (UVA), achieving ≥VGPR before auto-HCT was associated with better PFS (hazard ratio [95% CI] 0.52 [0.30-0.90], p=0.019) and OS (0.45 [0.23-0.89], p=0.022) that was not maintained on multivariable analysis (MVA). Notably, the presence of an additional HR cytogenetic abnormality beyond t(4;14) was not associated with inferior PFS (0.86 [0.50-1.47], p=0.57) or OS (0.87 [0.44-1.73], p=0.70). Achieving MRD negative ≥VGPR prior to transplant was associated with improved PFS and OS on both UVA and MVA (0.35 [0.16-0.76], p=0.008) and (0.12 [0.03-0.44], p=0.002), respectively. Patients who achieved MRD negative ≥VGPR prior to auto-HCT had a median PFS of 48.1 months and median OS was not reached, compared to a median PFS of 18.3 months (p=0.002) and a median OS of 45.6 months (p=0.003) in patients not achieving that response (Figure 2B-C).
Len-based combination for post-transplant maintenance was associated with improved OS in both UVA and MVA (MVA: 0.14 [0.04-0.45], p=0.001), though the difference in PFS did not reach a statistical significance (UVA: 0.74 [0.39-1.41], p=0.37). Female gender was associated with worse OS in both UVA and MVA (MVA: 4.70 [1.98-11.14], p<0.001). The type of induction regimen did not impact PFS (p=0.65) or OS (p=0.42). Similarly, the conditioning regimen, busulfan+melphalan vs. melphalan alone, also did not influence PFS (p=0.31) or OS (p=0.66). Table 2 presents the MVA for PFS and Table 3 presents the MVA for OS.
Table 2:
Multivariable Analysis for Progression-Free Survival.
| Measure | Progression-Free Survival | |
|---|---|---|
| Hazard Ratio (95% CI) | p-Value | |
| Model 1 | ||
| Age at auto-HCT | 1.04 (1.00, 1.07) | 0.032 |
| HCT-CI | ||
| ≤ 3 | ref | |
| > 3 | 1.52 (0.83, 2.78) | 0.17 |
| MRD negative ≥VGPR at auto-HCT | ||
| No | ref | |
| Yes | 0.35 (0.16, 0.77) | 0.008 |
| Unknown | 1.50 (0.57, 3.98) | 0.42 |
| Model 2 | ||
| Age at auto-HCT | 1.05 (1.01, 1.09) | 0.013 |
| HCT-CI | ||
| ≤ 3 | ref | |
| > 3 | 1.54 (0.84, 2.83) | 0.16 |
| Response at auto-HCT | ||
| ≥VGPR | ref | |
| ≤PR | 1.45 (0.79, 2.63) | 0.23 |
| MRD at auto-HCT | ||
| Negative | ref | |
| Positive | 2.60 (1.31, 5.15) | 0.006 |
| Unknown | 4.20 (1.41,12.51) | 0.010 |
Abbreviations: CI=Confidence interval, ref=Reference, auto-HCT=Autologous stem cell transplant, HCT-CI=Hematopoietic Cell Transplantation-specific Comorbidity Index, VGPR=Very good partial response, PR=Partial response, MRD=Minimal residual disease.
Table 3:
Multivariable Analysis for Overall Survival.
| Measure | Overall Survival | |
|---|---|---|
| Hazard Ratio (95% CI) | p-value | |
| Model 1 | ||
| Gender | ||
| Male | ref | |
| Female | 4.70 (1.98, 11.14) | < 0.001 |
| Age at auto-HCT | 1.01 (0.97, 1.05) | 0.65 |
| HCT-CI | ||
| ≤ 3 | ref | |
| > 3 | 1.77 (0.86, 3.64) | 0.12 |
| MRD negative ≥VGPR at auto-HCT | ||
| No | ref | |
| Yes | 0.12 (0.03, 0.44) | 0.002 |
| Unknown | 1.17 (0.39, 3.54) | 0.78 |
| MRD negative after auto-HCT a | ||
| No vs. Yes | 1.35 (0.62, 2.93) | 0.45 |
| Len-based Combination a | ||
| Yes vs. No | 0.21 (0.07, 0.65) | 0.006 |
| Model 2 | ||
| Gender | ||
| Male | ref | |
| Female | 4.84 (1.93, 12.15) | < 0.001 |
| Age at auto-HCT | 1.03 (0.98, 1.08) | 0.28 |
| HCT-CI | ||
| ≤ 3 | ref | |
| > 3 | 1.67 (0.77, 3.61) | 0.19 |
| Response at auto-HCT | ||
| ≤PR | ref | |
| ≥VGPR | 0.52 (0.23, 1.17) | 0.11 |
| MRD at auto-HCT | ||
| Positive | ref | |
| Negative | 0.12 (0.04, 0.36) | < 0.001 |
| Unknown | 1.21 (0.37, 3.98) | 0.76 |
| MRD negative after auto-HCT a | ||
| No vs. Yes | 1.44 (0.64, 3.23) | 0.38 |
| Len-based Combination a | ||
| Yes vs. No | 0.14 (0.04, 0.45) | 0.001 |
Abbreviations: CI=Confidence interval, ref=Reference, auto-HCT=Autologous stem cell transplant, HCT-CI=Hematopoietic Cell Transplantation-specific Comorbidity Index, VGPR=Very good partial response, PR=Partial response, MRD=Minimal residual disease, Len-based=Lenalidomide based.
Included in the model as a time-dependent covariate.
Discussion
To the best of our knowledge, this is the largest report of contemporary outcomes of MM patients with t(4;14) who received induction therapy followed by auto-HCT. Overall, survival outcomes were similar to those previously reported for HRMM, with a median PFS and OS of 23 months and 60 months, respectively. A prior report by our group showed a lower median PFS and OS in patients with HRMM (25 and 70 months, respectively), compared to standard-risk patients (57 and 102 months, respectively)11. Similarly, a CIBMTR report on patients with HRMM undergoing auto-HCT, showed a 3-year rates for PFS of 37% and OS of 72%, comparable to the 3-year rates for PFS and OS of 38% and 67%, respectively, in our study8.
Earlier reports have suggested that the proteasome inhibitor, bortezomib, could abrogate the negative prognostic impact of t(4;14) in patients with MM. Induction with bortezomib and dexamethasone (VD) has been shown to improve PFS compared to traditional chemotherapy-based induction prior to auto-HCT in patients with t(4;14) MM 12,13. However, we demonstrate poor survival outcomes in our cohort, despite all patients receiving proteasome inhibitor–based induction regimens, and 29 (37%) also receiving maintenance that included a proteasome inhibitor. This observation suggests that current treatment modalities for patients with t(4;14) MM remain suboptimal.
Small retrospective studies have previously shown the negative impact of t(4;14) on outcomes of MM patients who undergo auto-HCT. Compared to the present study, Chang et al. reported a shorter median post-transplantation OS of 18.3 months for the t(4;14) patient population, yet the study was conducted prior to the widespread use of contemporary anti-myeloma therapies such as Len. MRD assessment was not provided in that report7. In the study by Sato et al., pretransplant hematological responses were similar for MM patients with and without t(4;14) (p=0.171)9. MRD assessment was not detailed, though the authors did comment that only one of the 17 patients in the t(4;14) cohort achieved MRD negativity, but it was not clear at what stage of treatment this occurred. In an analysis of participants of three IFM-99 trials with t(4;14), patients received chemotherapy-based induction treatment followed by either tandem auto-HCT or single auto-HCT followed by allogeneic HCT14. The authors found that patients with lower levels of β2-microglobulin and higher hemoglobin at diagnosis had improved survival outcomes.
We observed improved OS with Len-based combination therapy in our cohort. The optimal maintenance regimen for patients with HRMM is unknown15, yet in the IFM 2005-02 trial, single-agent Len maintenance did not overcome the poor prognosis of t(4;14), compared to no maintenance (PFS of 27 vs 24 months, respectively)16. This is in line with our finding that Len-based combinations were required to improve survival. This is also consistent with another report which showed that Len-based combinations improved PFS in HRMM patients, albeit only 2 patients had t(4;14) in that study17. The FORTE trial showed that the Len-based combination (KR) resulted in better PFS in HRMM, except for those with 1q+18. A recent report from our group had similar findings, with a trend towards improved PFS in HRMM without 1q+ who received Len-based combination maintenance19. Risk-adapted combination maintenance was evaluated in a large study of 1000 consecutive newly diagnosed MM patients, including 25% with high-risk cytogenetics20. Seven hundred and fifty-one patients received upfront auto-HCT and the majority (76%) subsequently proceeded to Len monotherapy maintenance, while 16% received Len plus PI. This approach led to a median PFS and OS of 65 months and 78 months, respectively, underscoring the value of Len-based maintenance regimens, as well as a potential for modelling a risk-adapted therapeutic approach for patients with MM20.
Our results highlight the importance of achieving a deeper response prior to transplant in patients with t(4;14). MVA showed that those who achieved MRD negative ≥VGPR had superior OS and PFS, compared to patients with a lesser response. Hematological response prior to auto-HCT has been shown to correlate with post-transplant outcomes. An analysis of the Intergroupe Francophone du Myelome 99-02 and 99-04 trials revealed that patients who achieved at least a VGPR prior to autoHCT had better 5-year event free survival (EFS) (43% vs. 26%, p=0.005) and OS (74% vs. 61%, p=0.0017) rates compared to those who achieved less than VGPR21. The prognostic impact of hematological response was also observed in the subset of patients with t(4;14) and/or del17p. In our study, a response of ≥VGPR was associated with improved PFS in UVA, but not in MVA, yet the addition of MRD status improved the predictive impact of pretransplant response. A small study by Korthals et al. evaluated real-time quantitative (RQ) PCR–based pre-transplant MRD status in 53 patients with MM22. Patients with MRD negative status prior to transplant had better median EFS (35 vs. 20 months, p=0.001) and OS (70 vs. 45 months, p=0.04) compared to those with MRD positive status. The study did not provide data on the impact of MRD status in the subset of HRMM patients.
Novel therapeutic modalities have recently been added to the armamentarium against MM, including chimeric antigen receptor T-cell (CAR-T) therapy and bispecific T-cell engagers, with the ability to induce deep responses in heavily pre-treated patients. The CARTITUDE-123 and KarMMa 24 trials demonstrated impressive response rates with B-cell maturation antigen (BCMA)–directed CAR-Ts, ciltacabtagene autoleucel and idecabtagene vicleucel, respectively. Similarly, teclistamab, a bispecific antibody, showed remarkable results in the MajesTEC-1 clinical trial25. However, only a quarter of participants in the CARTITUDE-1 and MajesTEC-1 trials had HRMM, and very few patients had t(4;14) (n=3 and n=16, respectively). The KarMMa clinical trial had a greater representation of patients with high-risk cytogenetics (35%), including 23 patients (18%) with t(4;14). Sub-analysis of both the KarMMa and MajesTEC-1 clinical trials showed that the efficacy of idecabtagene vicleucel and teclistamab was preserved in patients with HR cytogenetics24,25. Similarly, in the CARTITUDE-1 trial, the high-risk population benefited from ciltacabtagene autoleucel, with a median duration of response of ~20 months23. Further studies are necessary to elucidate the optimal way to incorporate these new modalities into the treatment algorithm of HRMM patients, including those with t(4;14). One option could be to use these potent therapies to induce deep responses prior to auto-HCT, which, as our study suggests, may provide long-term PFS.
Previous studies have shown that MM patients with multiple high-risk chromosomal abnormalities have inferior outcomes. In a CIBMTR analysis of HRMM patients undergoing auto-HCT, those with more than one HR cytogenetic abnormality had inferior 3-year PFS (27%) compared to patients who had either del17p (43%) or 1q gain (50%) as their only cytogenetic abnormality, yet for patients with only t(4;14) the PFS was comparable (28%)8. A recent report showed that MM patients with t(4;14) had worse OS if they also had either del17p or del1p (Z score of 3.12 and 2.94 in MVA, respectively)6. Only 61% of the t(4;14) patients included in that analysis underwent auto-HCT. In the present study, having an additional HR chromosomal abnormality was not associated with inferior outcomes. This observation could be partially explained by the relatively small number of patients in our cohort.
Our study has several limitations inherent to its retrospective nature, including variabilities in care and unknown confounders that were not accounted for, despite the use of multivariable cox regression analysis. Furthermore, since we only included patients who had received auto-HCT, this selection bias precludes conclusions regarding outcomes of all patients with t(4:14).
In conclusion, our study reinforces t(4;14) as a high-risk chromosomal aberration with poor outcomes despite the use of contemporary anti-myeloma agents for induction, auto-HCT and post-transplant maintenance. Despite the relatively small cohort and heterogeneity in treatment, we observed improved outcomes in patients who achieved deeper pre-transplant responses and post-transplant Len-based combination for maintenance. Recently developed CAR T and T cell engagers are being incorporated in the management of patients with MM, and will hopefully improve the outcomes of patients with high-risk cytogenetics, including t(4;14).
Highlight.
Outcomes of patients with t(4;14) MM remain poor, despite novel-agent induction, auto-HCT and post-transplant maintenance
Achieving MRD-negative ≥VGPR prior to auto-HCT was associated with improved PFS and OS
Post-transplant lenalidomide-based combination maintenance was associated with improved OS
Funding
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
R.Z.O., the Florence Maude Thomas Cancer Research Professor, would like to acknowledge support from the Leukemia & Lymphoma Society (SCOR-12206-17), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the Riney Family Multiple Myeloma Research Fund at MD Anderson from the Paula and Rodger Riney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: MRG has consulting from The Dedham Group and Guidepoint Global, LLC and serves on an advisory board for Bristol Myers Squibb. HCL has consulting from Bristol Myers Squibb, Celgene, Genentech, Karyopharm, Legend Biotech, GlaxoSmithKline, Sanofi, Pfizer, Monte Rosa Therapeutics, Oncopetides, Takeda Pharmaceuticals, and Allogene Therapeutics and research funding from Amgen, Bristol Myers Squibb, Janssen, GlaxoSmithKline, Regeneron, and Takeda Pharmaceuticals. The other authors have not declared a conflict of interest.
References
- 1.Avet-Loiseau H, Facon T, Grosbois B, et al. Oncogenesis of multiple myeloma: 14q32 and 13q chromosomal abnormalities are not randomly distributed, but correlate with natural history, immunological features, and clinical presentation. Blood. 2002;99(6):2185–2191. [DOI] [PubMed] [Google Scholar]
- 2.Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high-risk multiple myeloma. Leukemia. 2015;29(11):2119–2125. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keats JJ, Reiman T, Belch AR, Pilarski LM. Ten years and counting: so what do we know about t(4;14)(p16;q32) multiple myeloma. Leuk Lymphoma. 2006;47(11):2289–2300. [DOI] [PubMed] [Google Scholar]
- 5.Keats JJ, Reiman T, Maxwell CA, et al. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101(4):1520–1529. [DOI] [PubMed] [Google Scholar]
- 6.Stong N, Ortiz Estevez M, Towfic F, et al. Location of the t(4;14) translocation breakpoint within the NSD2 gene identifies a subset of high-risk NDMM patients. Blood. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang H, Sloan S, Li D, et al. The t(4;14) is associated with poor prognosis in myeloma patients undergoing autologous stem cell transplant. Br J Haematol. 2004;125(1):64–68. [DOI] [PubMed] [Google Scholar]
- 8.Scott EC, Hari P, Sharma M, et al. Post-Transplant Outcomes in High-Risk Compared with Non-High-Risk Multiple Myeloma: A CIBMTR Analysis. Biol Blood Marrow Transplant. 2016;22(10):1893–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Kamata W, Okada S, Tamai Y. Clinical and prognostic significance of t(4;14) translocation in multiple myeloma in the era of novel agents. Int J Hematol. 2021;113(2):207–213. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. [DOI] [PubMed] [Google Scholar]
- 11.Gaballa MR, Ma J, Tanner MR, et al. Real-world long-term outcomes in multiple myeloma with VRD induction, Mel200-conditioned auto-HCT, and lenalidomide maintenance. Leuk Lymphoma. 2022;63(3):710–721. [DOI] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634. [DOI] [PubMed] [Google Scholar]
- 13.El-Ghammaz AM, Abdelwahed E. Bortezomib-based induction improves progression-free survival of myeloma patients harboring 17p deletion and/or t(4;14) and overcomes their adverse prognosis. Ann Hematol. 2016;95(8):1315–1321. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, Attal M, Garban F, et al. Heterogeneity of t(4;14) in multiple myeloma. Long-term follow-up of 100 cases treated with tandem transplantation in IFM99 trials. Leukemia. 2007;21(9):2020–2024. [DOI] [PubMed] [Google Scholar]
- 15.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2012;366(19):1782–1791. [DOI] [PubMed] [Google Scholar]
- 17.Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28(3):690–693. [DOI] [PubMed] [Google Scholar]
- 18.Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22(12):1705–1720. [DOI] [PubMed] [Google Scholar]
- 19.Pasvolsky O, Milton DR, Rauf M, et al. Lenalidomide-Based Maintenance after Autologous Hematopoietic Stem Cell Transplantation for Patients with High-Risk Multiple Myeloma. Transplant Cell Ther. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2020;38(17):1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009;27(34):5720–5726. [DOI] [PubMed] [Google Scholar]
- 22.Korthals M, Sehnke N, Kronenwett R, et al. The level of minimal residual disease in the bone marrow of patients with multiple myeloma before high-dose therapy and autologous blood stem cell transplantation is an independent predictive parameter. Biol Blood Marrow Transplant. 2012;18(3):423–431 e423. [DOI] [PubMed] [Google Scholar]
- 23.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet. 2021;398(10297):314–324. [DOI] [PubMed] [Google Scholar]
- 24.Munshi NC, Anderson LD Jr., Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384(8):705–716. [DOI] [PubMed] [Google Scholar]
- 25.Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine. 2022;387(6):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]