Abstract
Objectives.
Epidermal growth factor EGF-like domain multiple-6 (EGFL6) is highly expressed in high grade serous ovarian cancer and promotes both endothelial cell proliferation/angiogenesis and cancer cell proliferation/metastasis. As such it has been implicated as a therapeutic target. As a secreted factor, EGFL6 is a candidate for antibody therapy. The objectives of this study were to create and validate humanized affinity-matured EGFL6 neutralizing antibodies for clinical development.
Methods.
A selected murine EGFL6 antibody was humanized using CDR grafting to create 26 variant humanized antibodies. These were screened and the lead candidate was affinity matured. Seven humanized affinity-matured EGFL6 antibodies were screened for their ability to block EGFL6 activity on cancer cells in vitro, two of which were selected and tested their therapeutic activity in vivo.
Results.
Humanized affinity matured antibodies demonstrated high affinity for EGFL6 (150 pM to 2.67 nM). We found that several humanized affinity-matured EGFL6 antibodies specifically bound to recombinant, and native human EGFL6. Two lead antibodies were able to inhibit EGFL6-mediated (i) cancer cell migration, (ii) proliferation, and (iii) increase in ERK phosphorylation in cancer cells in vitro. Both lead antibodies restricted growth of an EGFL6 expressing ovarian cancer patient derived xenograft. Analysis of treated human tumor xenografts indicated that anti-EGFL6 therapy suppressed angiogenesis, inhibited tumor cell proliferation, and promoted tumor cell apoptosis.
Conclusions.
Our studies confirm the ability of these humanized affinity-matured antibodies to neutralize EGFL6 and acting as a therapeutic to restrict cancer growth. This work supports the development of these antibody for first-in-human clinical trials.
Keywords: therapeutic antibody, antibody humanization, affinity maturation, EGFL6, angiogenesis, ovarian cancer, in vivo xenograft tumor model
1. Introduction
Ovarian cancer is a deadly disease with a high mortality:incidence ratio. Thus, there is an urgent need to develop new therapies for ovarian cancer. Monoclonal antibody (mAb)-based therapies have been proven effective in many cancers, and compared to standard chemotherapies, antibody therapies are generally less toxic [1].
One potential target for ovarian cancer mAb therapy is the epidermal growth factor-like domain multiple-6 (EGFL6) protein. EGFL6, is a secreted protein that plays a critical role in development, is downregulated in the adult, and upregulated in many cancers including high grade serous ovarian cancer [2–4]. EGFL6 was first identified as a tumor vascular specific antigen in ovarian cancer [2, 5]. Subsequently EGFL6 was noted to also be expressed in tumor cells[6]. Functional studies indicate EGFL6 acts on endothelial cells to promote tumor specific angiogenesis [7–9]. In addition, EGFL6 acts on tumor cells to promote cancer stem-like cell asymmetric division and migration resulting in increased metastasis [6]. Following studies in ovarian cancer, EGFL6 has been reported to play a critical protumorigenic role in multiple cancer types, including breast, colorectal, and gastric cancers [10–13]. EGFL6 increases cancer cell proliferation, epithelial mesenchymal transition, and metastasis [10, 11, 14–16].
As EGFL6 is a secreted factor, like EGF and VEGF, EGFL6 is an ideal target for antibody-based therapy. Indeed, murine antibodies targeting EGFL6 have been found to reduce both cancer growth and metastasis [11, 12]. While murine antibodies can be used in the clinic, they have a major limitation related to immunogenic responses [17, 18]. Even chimeric antibodies consisting of rodent variable regions and human constant regions are still capable of eliciting a significant immune response [17, 18]. An approach to overcoming this limitation is “humanization” of the murine antibody [19, 20]. Furthermore, antibody affinity maturation is an important strategy to increase affinity of therapeutic antibody candidates to their targets [18, 19, 21–23]. In the present study, we describe the humanization of murine EGFL6 antibody, followed by in vitro affinity maturation to enhance binding affinity of the selected humanized antibody. Seven humanized affinity-matured EGFL6 antibody candidates were created, expressed and purified. We screened these EGFL6 antibody candidates for their ability to block EGFL6 activity on cancer cells in vitro. We then tested the therapeutic activity of two humanized affinity-matured antibodies in vivo. Humanized affinity-matured EGFL6 antibodies significantly inhibited growth of EGFL6-expressing human patient derived xenografts. Treatment response was associated with a reduction in angiogenesis, and reduced Ki67 expression in tumor cells, and reduced metastasis. As such these antibodies represent candidates for first-in-human clinical trials in ovarian cancer.
2. Materials and Methods
2.1. 2D and 3D cell culture
The ovarian cancer cell line SKOV3 was purchased from ATCC and the OVCAR3 cell line was obtained from Dr. Kathleen Cho at the University of Michigan and validated with STR testing with ATCC. EGFL6-expressing cell line E33 was established in our lab [11]. SKOV3 cells were also transiently transfected with control vector (p3xFlag) and EGFL6-expressing vector (EGFL6-p3xFLAG) using FuGENE® HD Transfection Reagent (Promega, Catalog number: E2312) per protocol. Primary cultured ovarian cancer cells from a high grade serous ovarian cancer (HGSOC) patient (Pt. 366) and carcinoma-associated mesenchymal stem cells (CAMSC) were cultured as described previously[24, 25]. Cell lines were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C and 5% CO2. HEK293 cell line was purchased from Life Technologies (Carlsbad, CA) and maintained in DMEM with 10% FBS. Recombinant human EGFL6 protein was purchased from GenScript USA Inc (Piscataway, NJ) or Sino Biological US Inc (Wayne, PA). All cell lines were tested for mycoplasma infection at least every 6 months.
Sphere/3D cell culture was performed as previously described[26]. Briefly, OVCAR3 and SKOV3 were plated in triplicate in ultra-low attachment 24-well plates (Corning™ 3473) at a density of 5,000 cells/well in serum-free mammary epithelial cell growth medium (MEBM) (ATCC or Lonza). Medium was changed every 2-3 days. For EGFL6 protein and EGFL6 antibody treatment, control human immunoglobulin G (IgG) (10 μg/ml), each of the selected EGFL6 antibodies (10 μg/ml) were incubated with EGFL6 protein (0.4 μg/ml) for 2 hours at 4°C prior to adding to sphere culture. For control group, human IgG (10 μg/ml) without EGFL6 protein and EGFL6 antibody was treated the same way as did for the treatment groups. The cell number in sphere culture was assessed 7 days after seeding the cells. All the cells were digested into single cells by trypsin and counted with a hemocytometer.
2.2. Generation of humanized affinity-matured EGFL6 antibodies
Based on our previous discovery of mouse monoclonal antibodies with neutralizing activity[11], we selected one mouse monoclonal antibody for humanization using complementarity determining region (CDR) grafting method[27–30]. The heavy and light chain CDRs of the selected mouse monoclonal antibody were grafted into the human acceptors to obtain five humanized light chains and five humanized heavy chains. Twenty-six humanized antibodies were expressed in HEK293 cells, and the antibodies were assayed for affinity ranking by enzyme-linked immunosorbent assay (ELISA). The humanized antibody with better binding affinity than chimeric antibody was selected for affinity maturation as previously described [31, 32].
For antibody affinity maturation, key residues that affected antibody expression and binding affinity were evaluated by GenScript’s FASEBA (Fast Screening for Expression, Biophysical-properties and Affinity) platform. After key residues were selected, partially randomized NNK libraries (where N = A/C/G/T and K = G/T) for each of key residues were constructed. For each NNK library, over 90 clones were randomly selected, grown, and tested for antigen binding activity by ELISA. Based on “beneficial mutants” identified by NNK library screening, the combinatory libraries were designed and constructed with all desirable mutations randomly induced at 50% frequency. About 400 clones were randomly selected, grown, and tested for antigen-binding activity by ELISA. Clones were ranked and sequenced. Finally, lead clones were selected, expressed, and purified. The affinities of the selected antibodies were measured by ELISA and by surface plasmon resonance (SPR) using Biacore 8K (GE Healthcare).
Both antibody humanization and affinity maturation were performed by GenScript USA Inc (Piscataway, NJ) following GenScript’s standard operating procedure (SOP).
ELISA and SPR are detailed in Supplementary Materials and Methods.
2.3. Expression and purification of selected humanized affinity-matured antibodies.
After antibody humanization and affinity maturation, 7 humanized affinity-matured antibodies including #9 (VH2+VL1), #10 (VH2+VL2), #11 (VH3+VL2), #12 (VH4+VL2), #13 (VH5+VL3), #15 (VH1+VL2) and #16 (VH1+VL1) were selected and expressed in HEK293 cells. Co-transfection of plasmid pairs containing full-length antibody genes for heavy and light chains at a 1:1 ratio was performed in HEK293 cells using FuGENE 6 reagent (Promega, Catalog number: E2692). As comparison, one parental humanized antibody CBM (VH1-CBM+VL1-CBM) that was used for affinity maturation and one chimeric antibody were also expressed in HEK293 cells. Antibodies in the conditioned media were purified using Protein G Agarose, Fast Flow (MilliporeSigma, Catalog number: 16-266). Purified antibodies were used for in vitro and in vivo experiments.
2.4. RT-qPCR
Total RNA was isolated from cells using RNeasy-mini kit (Qiagen). cDNA was synthesized using Superscript™ III First-Strand Synthesis System (ThermoFisher Scientific, Cat#: 18080051). Quantitative PCR (qPCR) reactions were performed in triplicate with SYBR Green Supermix (Bio-Rad, Cat#: 1725271). Hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) was used as an internal control. PCR primers were: EGFL6 (forward, 5′-AGAAACAGCAAGGGAGTCTG-3′ reverse, 5′-GGTTTCATTCCACACTCATTCAC-3′); and HPRT1 (forward, 5′-ACCGTGTGTTAGAAAAGTAAGAAG -3′; reverse, 5′-AGGGAACTGCTGACAAAGATTC -3′).
2.5. Western blot analysis
Western blot analysis was conducted as described previously[11] and is detailed in Supplementary Materials and Methods.
2.6. Wound healing assay
SKOV3 cells (5×105) were plated in a 6-well plate and incubated for 24 hours in RPMI-1640 containing 10% FBS. The monolayer was disrupted by a single scratch using a 200 μl yellow pipette tip. The media were then changed to serum-free media. Images were captured at 0 hour and 24 hours post-scratch using a phase-contrast inverted microscope (Olympus USA, Waltham, MA). The pretreatments were the same as described in Cell Culture section. Treatments started at the same time as the cell scratches were made. The gap distance was quantitatively evaluated using ImageJ, and the rate of migration of the cells was calculated by measuring the distance traveled toward the center of the wound. Wound closure %= (0 h gap distance – 24 h gap distance)/0 h gap distance × 100%. Five views of each well were documented, and each group was repeated in three wells. Experiment was repeated three times independently.
2.7. Immunofluorescence (IF)
E33 cells (5×105/well) were plated on sterilized coverslips in six-well plates. After washing with PBS, the cells in coverslips were fixed in 4% paraformaldehyde for 30 min and washed in PBS, followed by permeabilizing with ice-cold acetone for 10 min and blocked in 10% bovine serum albumin. The cells were then incubated with #9 and #16 EGFL6 antibodies for 30 min at 37°C. After washing, Alexa Fluor 594-conjugated goat anti-human IgG (H+L), cross-adsorbed secondary antibody (Invitrogen, Cat#: A-11014) was added at 10 μg/ml in a blocking buffer and incubated for 30 min at RT. Following further washing, cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, CA, USA), and viewed with the Leica DM4 B upright microscope (Leica Chicago, IL). Digital images were collected with Leica LAS X 3.3 Software. For a negative control, primary antibody was omitted.
2.8. In vivo therapeutic studies
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. For EGFl6-expressiong ovarian cancer PDX model, six -week-old female NSG mice (NOD.Cg-PrkdcSCID) (Jackson Laboratories, USA) were randomly divided into 3 groups. Mice were treated with human control IgG, #9 or #16 humanized affinity-matured EGFL6 antibody (10 mg/kg) every day for 3 days prior to xenografting Pt. 366 PDX cells and CAMSC. As we have not validated IP tumor initiation with our PDX, PDX/CA-MSC were injected into the flank. In addition, the use of flank tumors allows monitoring of metastasis to the ovary. Dosing was selected based upon (i) prior studies with the mouse monoclonal antibody from which the humanized antibody was derived [6], and pharmacokinetic studies and toxicity studies (performed by Jackson laboratories). Pretreatment was used to potentially neutralize any endogenous murine EGFL6. The Pt366 PDX cells (2×106) and CAMSC (1×106) mixture were mixed 1:1 with chilled Matrigel and then injected subcutaneously into the bilateral flanks of each NSG mouse. Human control IgG, #9 or #16 EGFL6 antibody (10 mg/kg) were administered biweekly using intraperitoneal injections. Tumor volume was measured by digital caliper and calculated using the modified ellipsoid formula (L*W*W/2). Tumor growth curves were graphed in Graphpad Prism version 9 (GraphPad Software, Inc., San Diego, CA, USA). All mice were euthanized 90 days post cancer cell and CAMSC injection, and tumor tissues were collected for Hematoxylin and Eosin (H&E) staining and immunohistochemistry (IHC).
For intraperitoneal tumor metastasis model, NIHOVCAR3 ovarian cancer cells (5x106/mouse) were injected IP into NSG mice. The mice were divided into two groups and treated with either EGFL6 ab #16 or human IgG control 10mg/kg twice weekly (N=10/group) for 9 weeks.
2.9. H&E staining
Tumor tissues were paraffin embedded. Paraffin-embedded tissues were sectioned at 5 μm thickness. H&E staining was performed at the Pathology Cores for Animal Research at the Magee-Womens Research Institute, University of Pittsburgh. Cell death areas were quantified by ImageJ (http://imagej.nih.gov/ij/docs/index.html).
2.10. Immunohistochemistry
Immunohistochemistry was modified based on a previous publication[33] and is detailed in in Supplementary Materials and Methods.
2.11. Statistical Analysis
Data are presented as means ± standard deviation (SD) and were analyzed using Graphpad Prism version 9. Statistical significance was evaluated by two-tailed Student’s t test or one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
3.1. Generation of humanized affinity-matured EGFL6 antibodies
Given the encouraging results of our previous studies showing that a mouse mAb against EGFL6 inhibited the growth and metastasis of ovarian cancer cells in vivo [11], we proceeded to humanize one mouse EGFL6 antibody using CDR grafting method [27–29]. The humanized variable domains of five light chains and five heavy chains were denoted as VL1, VL2, VL3, VL4 and VH1, VH2, VH3, VH4 and VH5. respectively. Plasmid pairs containing full-length antibody genes with VH/VL CDR combinations were expressed in HEK293 with a total of 26 IgGs were expressed successfully in the HEK293 cells (Fig. S1). The antibody generated were then evaluated for binding to purified human EGFL6 protein by ELISA (with EGFL6 purified from either cell culture media (secreted EGFL6) or cell bodies (intracellular EGFL6) (Fig. S2)).
The clone CBM demonstrated the strongest binding signal with binding affinity greater than chimeric antibody (arrow in Fig. S2) CBM was next used for in vitro affinity maturation. In conjunction with GenScript using the FASEBA platform, 34 residues that could affect the parental CBM antibody expression and binding affinity were identified. 34 NNK codon libraries were constructed and 90 clones from each of NNK libraries were tested for antigen-binding via ELISA. Based on these results combinatory libraries of the highest binders were screened. Surface plasmon resonance (SPR) was used to measure the binding kinetics and affinity of purified antibodies to human EGFL6. Compared with the parental humanized antibody CBM that had a KD of 3.97 nM, 7 humanized affinity-matured antibodies demonstrated improved affinity with KD ranged from 150 pM to 2.67 nM (Table 1).
Table 1.
Affinity measurement of chimeric, humanized and humanized affinity-matured EGFL6 antibodies
| Ab Name | Capture | Analyte | ka (1/Ms) | kd (1/s) | KD (nM) | Chi2 (RU2) |
|---|---|---|---|---|---|---|
| Chimeric | Chimeric | Human EGFL6 | 2.15E+04 | 1.27E−03 | 59.20 | 3.74E+00 |
| CBM | VH1-CBM + VL1-CBM | Human EGFL6 | 5.30E+05 | 2.10E−03 | 3.97 | 3.39E+00 |
| #9 | VH2+VL1 | Human EGFL6 | 2.57E+05 | 5.46E−05 | 2.13 | 6.38E−01 |
| #10 | VH2+VL2 | Human EGFL6 | 2.92E+05 | 6.15E−04 | 2.11 | 1.26E+00 |
| #11 | VH3+VL2 | Human EGFL6 | 3.11E+05 | 5.29E−04 | 1.70 | 7.79E−01 |
| #12 | VH4+VL2 | Human EGFL6 | 1.93E+05 | 5.15E−04 | 2.67 | 7.34E−01 |
| #13 | VH5+VL3 | Human EGFL6 | 3.74E+05 | 1.11E−04 | 0.30 | 4.84E−01 |
| #15 | VH1+VL2 | Human EGFL6 | 4.04E+05 | 1.30E−04 | 0.32 | 1.44E−01 |
| #16 | VH1+VL1 | Human EGFL6 | 3.83E+05 | 5.66E−05 | 0.15 | 2.21E−01 |
3.2. Humanized affinity-matured EGFL6 antibodies bind to both denatured and native human EGFL6
To confirm reactivity via Western blotting, we first evaluated control and EGFL6 transiently transfected SKOV3 cells. We chose SKOV3 as SKOV3 cells express little or no EGFL6 at baseline yet are EGFL6 responsive [6]. Humanized affinity-matured antibodies #9, #13, #15 and #16 recognized a major band of between the 51 and 64 kDa molecular weight markers in EGFL6-transiently transfected SKOV3 cell line, but not mock transfected SKOV3 controls (Fig. 1A).
Figure 1. Characterization of chimeric, humanized, and humanized affinity-matured EGFL6 antibodies.

(A) Western blot of EGFL6-negative cell line SKOV3 cells transiently transfected with empty vector (vector) or EGFL6-expressing vector (EGFL6). Whole-cell lysates were prepared and immunoblotted with humanized antibodies #9, #13, #15 and #16. (B) Western blot of whole-cell lysates from EGFL6-expressing SKOV3 cells were prepared and immunoblotted with #9 or #16 antibody preincubated with or without soluble recombinant EGFL6 protein (blocking). Arrow indicates the position of EGFL6 protein detected. (C) Immunofluorescent staining of (i) E33 cells (ii) and Pt. 366 tumor xenograft with #9 or #16 EGFL6 antibodies. Nuclei were labelled by 4′,6-diamidino-2-phenylindole (DAPI). NC, negative control. Arrows indicate the expression of EGFL6 protein in blood vessels. Scale bars, 20 μm.
To further confirm the specificity of the antibody binding to EGFL6, we determined if antibody binding could be blocked by recombinant human EGFL6 protein. EGFL6-transiently transfected SKOV3 samples were loaded for Western blotting analysis. Antibody incubation was done in the absence or presence of human EGFL6 fusion protein. Antibody incubation in the presence of the EGFL6 fusion protein reduced binding by mAb #9 antibody and nearly eliminated binding by mAb #16 (Fig. 1B).
To test whether humanized affinity-matured EGFL6 antibodies could bind to non-denatured EGFL6, we performed immunofluorescence staining of cultured EGFL6-expressing E33 cells. Both humanized affinity-matured EGFL6 antibodies (#9 and #16) recognized EGFL6 protein in E33 cells (Fig. 1C, i). Similarly, we confirmed detection of expression in tumor sample using a patient derived xenograft (Pt366) with confirmed high level EGFL6 mRNA expression (Fig. S3). Both humanized affinity-matured EGFL6 antibodies (#9 and #16) detected EGFL6 protein in blood vessels (arrows) and tumor cells in the Pt.366 tumor xenografts (Fig. 1C, ii).
Together, these studies indicate that the humanized affinity-matured antibodies can detect denatured EGFL6 protein as well as native EGFL6 protein expressed in EGFL6-expressing cells and tumor tissues.
3.3. Humanized affinity-matured EGFL6 antibodies block EGFL6-mediated ovarian cancer cell migration, proliferation and ERK phosphorylation in vitro.
We next used multiple assays to determine if any of the humanized affinity-matured anti-EGFL6 antibodies could neutralize EGFL6 function. We first used a wound healing migration assay to evaluate antibody effect on EGFL6-driven cancer cell migration [11]. Consistent with prior studies, treatment with EGFL6 protein increased “wound healing” in a standard scratch assay (Fig. 2A). The non-affinity matured CBM antibody showed a modest, but not statistically significant inhibition of EGFL6-mediated cancer cell migration (Fig. 2B). In contrast affinity matured antibodies #9, #11, #13, #15 and #16 resulted in a >90% reduction in EGFL6-mediated increases in cancer cell migration (Fig. 2B). Based on these results and the results above, we chose antibodies #9, #13 and #16 (all of which inhibited migration with p values < 0.01) for further testing.
Figure 2. Functional validation of humanized affinity-matured EGFL6 antibodies.

(A) Representative images and (B) quantification of wound healing assay in SKOV3 cells treated as indicated. (C) Effects of humanized affinity-matured EGFL6 antibodies (#9, #13 and #16) on EGFL6-induced cell growth in 3D culture of SKOV3 cells (left panel) and OVCAR3 cells (right panel). All assays were performed three times and error bars indicate standard deviation (SD). *p < 0.05, ** p < 0.01. Scale bar is 100 μm.
We next tested the ability of the antibodies to block EGFL6 induced cancer cell proliferation. We compared cell proliferation of both SKOV3 (non EGFL6-expresing), and OVCAR3 (EGFL6 expressing) ovarian cancer cell lines grown as tumor spheres in the presence and absence of EGFL6 and treated with IgG control or humanized affinity-matured EGFL6 antibodies. While recombinant EGFL6 protein significantly increased cancer cell number growth in the presence of control IgG, humanized affinity-matured EGFL6 antibodies #9 and #16 abolished EGFL6-mediated cell number increases in both cell lines tested (Fig. 2C). EGFL6 antibody #13, significantly reduced proliferation in SKOV3 cells and led a nonstatistically significant reduction of growth in OVCAR3 cells (Fig. 2C).
Our group and others have shown that EGFL6 increases ERK phosphorylation to mediate proliferation and migration effects [11, 34]. We therefore assessed the impact of humanized affinity-matured EGFL6 antibodies on EGFL6-mediated increases in pERK. Western blot analysis of whole cell lysates from SKOV3 cells demonstrated that all three humanized affinity-matured EGFL6 antibodies (#9, #13 and #16) completely blocked EGFL6-induced phosphorylation of ERK (Fig. 3), with pERK levels observed to be below that of non-EGFL6 treated controls.
Figure 3. Suppression of EGFL6-mediated ERK phosphorylation by humanized affinity-matured EGFL6 antibodies.

(A) Representative images of Western blot analysis of whole cell lysates from SKOV3 cells treated as indicated. (B) Quantification of phosphorylation of ERK (pERK) in EGFL6-treated SKOV3 cells in the absence or presence of EGFL6 antibodies. Experiments were repeated three times and error bars indicate standard deviation (SD). *p < 0.05.
3.4. Humanized affinity-matured EGFL6 antibodies suppress tumor growth and metastasis in vivo
Antibodies #9 and #16 demonstrated the greatest ability to restrict EGFL6 mediated protumorigenic effects in vitro. We evaluated the impact of these two antibodies on ovarian tumor growth in vivo. We performed these studies using a stringent human PDX model which incorporates human cancer associated mesenchymal stem cells (CA-MSC). We previously demonstrated that incorporated CA-MSC differentiate to create human cancer associated fibroblasts and increase cancer growth, metastasis, and therapeutic resistance [24, 25, 35]. PDX were initiated in the flank, and we initiated treatment with intraperitoneal injections of human IgG (control group), #9 or #16 EGFL6 antibody (10 mg/kg biweekly). Dosing was based on prior murine studies and PK studies indicating 10 mg/kg dosing-maintained antibody levels 10 ug/ml (optimal dose to inhibit EGFL6 activity in vitro) for ~3 days (FigS4a). The PK of these EGFL6 neutralizing antibodies mirrors that of other clinically approved antibody therapeutics [36].
Treated animals demonstrated normal weight gain (Fig. S4) and no gross evidence of toxicity. Both humanized antibodies significantly reduced tumor growth compared with the control group (Fig. 4A). Humanized affinity-matured antibodies #9 and #16 reduced tumor growth by 45.2% and 64.7% respectively at day 90. At the time of euthanasia, we evaluated animals for evidence of metastasis. Notably, metastases were evident in 75% of IgG treated controls, while no metastases were detectable in the anti-EGFL6 treated animals. Human cytokeratin immunofluorescence confirmed these nodules were metastatic disease (Fig. 4B). Extensive necropsy of the treated animals revealed that anti-EGFL6 treated animals had a mild reduction in splenic size/weight relative to IgG treated controls (Fig. 4C). Otherwise, no other toxicities were noted.
Figure 4. Suppression of tumor growth and metastasis by humanized affinity-matured EGFL6 antibodies in vivo.

(A) Tumor growth curves of EGFL6 expressing PDX treated with 10 mg/kg human IgG, #9 Ab and #16 Ab twice a week using intraperitoneal injections for 3 months. (B) Human cytokeratin IF of ovaries from control tumors. Human cytokeratin (CK; green)-positive cells were found in small clusters (left) and around blood vessels (right). (C) Images of spleens (left) and quantification of spleen weights (right) from control IgG-, #9 Ab- and #16 Ab-treated NSG mice. Data are expressed as mean ± SD. *p < 0.05, **p < 0.01. Scale bars, 20 μm.
Traditional H&E staining of control tumors indicated large tumor islets with proliferative cancer cells. In contrast, mice treated with #9 Ab or #16 Ab demonstrated a significant loss of cellularity with large areas of necrosis (Fig. 5A). Cleaved caspase-3 (Asp175) IHC indicated significantly increased levels of apoptosis in anti-EGFL6 treated tumors; antibodies #9 and #16 increased cleaved caspase-3-positive epithelial cells by 45.2% and 64.7% respectively (Fig. 5B; p < 0.001 for both antibodies). Consistent with an increase in apoptosis, DAPI labeling showed condensed/fragmented nuclei in the anti-EGFL6 treated tumor (Figs. 5B & Fig. S5). In-line with increased death and reduced growth both antibodies significantly suppressed tumor cell proliferation as measured by Ki67 IHC (Fig. 5C; p < 0.01 for both antibodies). Consistent with the known angiogenic effect of EGFL6, immunofluorescent staining showed a statistically significant reduction in CD31 microvascular density in tumors treated with either of the anti-EGFL6 antibodies (Fig. 5D; p < 0.05 for #9 Ab, p < 0.001 for #16 Ab). Finally, phosphorylation of ERK (p-ERK) was significantly inhibited in tumors treated with EGFL6 antibodies (Fig. 5E), indicating reduced EGFL6 signaling.
Figure 5. Promotion of tumor cell death and suppression of angiogenesis and tumor cell proliferation by humanized affinity-matured EGFL6 antibodies in vivo.

(A) Representative H&E images (top panels) and quantification (bottom panel) of cell death area per high-power field (HPF) in tumors from control IgG-, #9 Ab and #16 Ab-treated NSG mice. (B) IF images (top panels) and quantification (bottom panel) of cleaved caspase-3 (cleaved casp-3; an apoptosis marker) and human cytokeratin (hCK; an epithelial marker) in tumors from NSG mice treated with control IgG, #9 Ab and #16 Ab. (C) Representative IF images (top panels) and quantification (bottom panel) of Ki67 (a cell proliferation marker) in tumors from NSG mice treated with human IgG, #9 Ab and #16 Ab. Nuclei were labelled by DAPI. (D) Representative IF images (top panels) and quantification (bottom panel) of CD31 (a marker of endothelial cells) in tumors from NSG mice treated with control IgG, #9 Ab and #16 Ab. Data are expressed as mean ± SD. *p < 0.05, ** p < 0.01, *** p < 0.001. Scale bars, 20 μm. (E) Representative IHC images (left panels) and quantification (right panel) of p-ERK (44/42) in tumors from NSG mice treated with control IgG, #9 Ab and #16 Ab (n=6/group). Scale bars, 100 μm. Graph represents the average of p-ERK (44/42) positive cell counts per 40 high power fields in each group. Results were analyzed using Student t test. Data are presented as mean ± SEM. *P < 0.05, ***P < 0.001.
To further evaluate the impact of humanized EGFL6 antibody on tumor metastasis to the peritoneal cavity we used intraperitoneal injection of EGFL6-expressing NIHOVCAR3 ovarian cancer cells. Following cancer cell injections, mice were then treated with either EGFL6 ab#16 or human IgG control twice weekly for 9 weeks (a time at which 100% engraftment in controls and gross macrometastases in untreated controls is anticipated) and then euthanized. Untreated control mice demonstrated large metastases to the ovarian surface oviducts and peritoneum (Fig 6A). Anti-EGFL6 treatment was associated with a reduction in the number of tumor nodules (p=0.087), and a significant reduction in overall metastatic tumor volume (as assessed by tumor weight, p=0.004) (Fig. 6C).
Figure 6. Inhibition of peritoneal tumor metastasis by humanized affinity-matured EGFL6 antibodies in vivo.
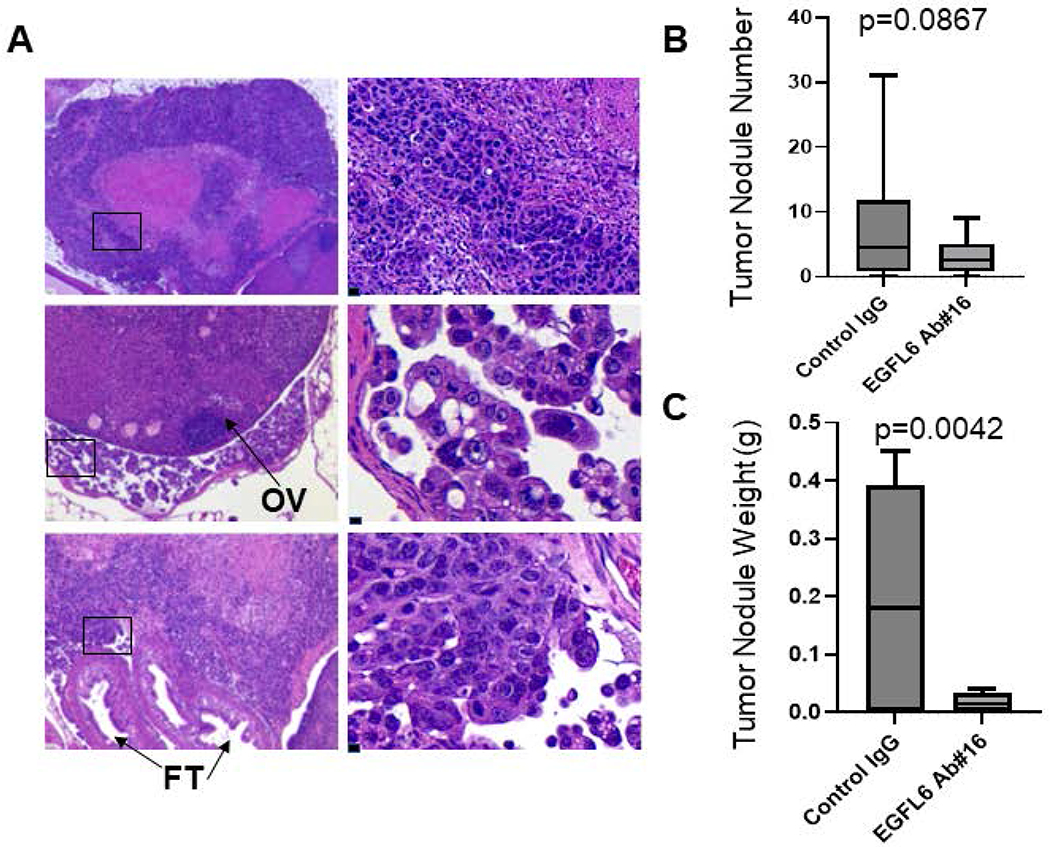
(A) H&E images of NIHOVCAR3 metastatic tumors in fat tissue (top left panels), the ovary surface (middle left panels), and oviduct (bottom left panels) of control mice. Left panel scale bar 50 μm. top right 20 μm, middle and bottom right 10 μm. (B-C) Tumor nodule numbers (B) and weights (C) in the peritoneum in EGFL6 Ab-treated vs control mice.
Together, these data indicate that the humanized affinity-matured EGFL6 antibodies effectively inhibit tumor growth and represent a potential therapeutic to move into first-in-human clinical trials.
4. Discussion
EGFL6 is a biomarker of poor prognosis in numerous cancers including ovarian, breast, lung, and head and neck cancers [10, 11, 37, 38]. Consistent with this, there are an increasing number of studies indicating a critical role for EGFL6 in cancer growth and progression [6, 9, 10, 16, 39]. As a secreted factor, EGFL6 represents an ideal candidate for antibody targeted therapy. Furthermore, as EGFL6 is primarily active in development, with limited expression in the adult, as a therapeutic target it is less likely to have significant side-effects.
This study describes generation and characterization of humanized affinity-matured antibodies for therapeutic EGFL6 neutralization. Of 26 humanized antibodies generated, one humanized antibody that demonstrated the strongest EGFL6 binding signal with better binding affinity than the chimeric antibody was selected for affinity maturation. By using FASEBA platform, constructing and screening NNK libraries and combinatory libraries, we finally identified and purified 7 humanized affinity-matured EGFL6 antibodies for further characterization in vitro. Five humanized affinity-matured antibodies were found to block EGFL6-mediated ovarian cancer cell migration. Three humanized affinity-matured antibodies were found to block EGFL6-mediated increases in pERK, two of which inhibited EGFL6 driven increase in tumor sphere growth across cell lines.
The study has several strengths. PK studies were completed in mice expressing the human FC receptor. Results in these mice more accurately mirror results in humans [36]. Further, the two humanized affinity-matured EGFL6 antibodies that were most effective in multiple assays in vitro were therapeutically effective in NSG mice bearing human HGSOC PDX and IP injected cell line xenografts. The PDX model we used, incorporated human CA-MSC which generate human tumor stroma and increase therapeutic stringency. During 3-month treatment regimen, mice looked healthy, maintained similar weight increases to control group and no obvious side effects were observed. The only side effected noted on necropsy was a mild reduction in splenic size. Of note, while the use of a PDX with endogenous human EGFL6 expression is a strength, it is also a weakness as PDX are grown in immune-incompetent mice thus any effects of EGFL6 antibody on the immune system are not known. Additional work, including testing these antibodies in humanized mice or syngeneic mouse models will be important to understand the impact of the antibodies on tumor immunity.
Finally, there is growing interest in antibody for cancer therapy as antibodies tend to have limited toxicity and can be relatively tumor specific. In particular antibody drug conjugates have begun to show activity in the clinic. While EGFL6 is a secreted factor, immunofluorescence with the newly developed anti-EGFL6 antibodies showed strong stain on the tumor cell bodies. This raises the possibility an EGFL6 targeted antibody drug conjugate could be developed.
In conclusion, we generated and characterized novel humanized affinity-matured EGFL6 antibodies which inhibited EGFL6-mediated biological functions in vitro and tumor growth and metastasis in vivo. Humanized affinity-matured EGFL6 antibodies generated by us produce a promising therapeutic agent for first-in-human clinical trials in targeted cancer therapy.
Supplementary Material
Supplementary Figure 1. SDS-PAGE Analysis of 26 humanized IgGs under non-reducing conditions. Cell culture supernatants were collected on day 2 (d2), day 4 (d4), and day 6 (d6) post-transfection. About 20 μl of supernatant was loaded in each lane. M, marker.
Supplementary Figure 2. Generation of humanized EGFL6 antibodies. Binding validation of humanized antibodies to antigen EGFL6 purified from intracellular (blue bars) and medium (dark red bars) by ELISA. Blank is the HEK293 medium control; NC represents negative control of capture antibody with secondary antibody only. Arrow indicates the CBM clone (VH1-CBM+VL1-CBM) with better binding affinity than chimeric antibody. ELISA, enzyme-linked immunosorbent assay.
Supplementary Figure 3. RT-qPCR analysis of EGFL6 mRNA expression in ovarian cancer cells. EGFL6 mRNA was expressed in patient 366 (Pt. 366) tumor cells, but not in SKOV3 cells. Data was normalized to a housekeeping gene, hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1).
Supplementary Figure 4. Body weight change in NSG mice treated with IgG, #9 and #16 humanized affinity-matured EGFL6 antibodies. (A) Antibody concentration in serum following a single injection of antibody (10 mg/kg) in Tg32 SCID mice (expressing human FC receptor) (B) Changes in mouse body weights from days 0 to 90 post-injection. (C) Average mouse body weight change at day 90 the end point of experiments. Compared with mice treated with IgG, both humanized antibodies had no effects on NSG mouse body weights, indicating that the two humanized affinity-matured antibodies were not toxic during the 3-month treatment regimen.
Supplementary Figure 5. Condensed/fragmented nuclei induced in humanized affinity-matured EGFL6 antibody-treated tumors. Nuclei were labeled by DAPI. Fluorescent images displayed non-condensed nuclei in control IgG group, whereas tumors from #9 Ab- and #16 Ab-treated mice showed condensed/fragmented nuclei, a marker of apoptosis. Scale bars, 20 μm.
Highlights.
We created and validated seven humanized affinity-matured EGFL6 antibodies.
These EGFL6 antibodies blocked EGFL6-mediated effects on ovarian cancer cells in vitro.
Lead EGFL6 antibodies suppressed tumor growth and metastasis in vivo.
EGFL6 antibody treatment reduced inhibited angiogenesis, reduced tumor cell proliferation, and increased tumor cell death.
Acknowledgements
This work was supported by National Cancer Institute Grants R01CA218026. Use of affinity matured antibody variants was enabled by an NIH STTR grant R41CA250757 to Tradewind BioScience, Inc., Daly City, CA, and the University of Pittsburgh.
Competing Interests
Dr. Buckanovich is co-founder of Tradewinds Bioscience which supported antibody affinity maturation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292–302. [DOI] [PubMed] [Google Scholar]
- [2].Buckanovich RJ, Sasaroli D, O’Brien-Jenkins A, Botbyl J, Hammond R, Katsaros D, et al. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol. 2007;25:852–61. [DOI] [PubMed] [Google Scholar]
- [3].Wei JP, Gong Y, Zhong HB, Hua Wang T, Liao XH. EGFL6 expression in hair follicle central isthmus is dependent on the presence of terminal Schwann cells. Exp Dermatol. 2020;29:400–3. [DOI] [PubMed] [Google Scholar]
- [4].Yeung G, Mulero JJ, Berntsen RP, Loeb DB, Drmanac R, Ford JE. Cloning of a novel epidermal growth factor repeat containing gene EGFL6: expressed in tumor and fetal tissues. Genomics. 1999;62:304–7. [DOI] [PubMed] [Google Scholar]
- [5].Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer research. 2007;67:1757–68. [DOI] [PubMed] [Google Scholar]
- [6].Bai S, Ingram P, Chen YC, Deng N, Pearson A, Niknafs Y, et al. EGFL6 Regulates the Asymmetric Division, Maintenance, and Metastasis of ALDH+ Ovarian Cancer Cells. Cancer research. 2016;76:6396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Noh K, Mangala LS, Han H-D, Zhang N, Pradeep S, Wu SY, et al. Differential Effects of EGFL6 on Tumor versus Wound Angiogenesis. Cell Reports. 2017;21:2785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wu B, Zhang L, Yu Y, Lu T, Zhang Y, Zhu W, et al. miR-6086 inhibits ovarian cancer angiogenesis by downregulating the OC2/VEGFA/EGFL6 axis. Cell Death Dis. 2020;11:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhu W, Liu C, Lu T, Zhang Y, Zhang S, Chen Q, et al. Knockout of EGFL6 by CRISPR/Cas9 Mediated Inhibition of Tumor Angiogenesis in Ovarian Cancer. Front Oncol. 2020;10:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].An J, Du Y, Fan X, Wang Y, Ivan C, Zhang XG, et al. EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene. 2019;38:2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bai S, Ingram P, Chen YC, Deng N, Pearson A, Niknafs YS, et al. EGFL6 Regulates the Asymmetric Division, Maintenance, and Metastasis of ALDH+ Ovarian Cancer Cells. Cancer research. 2016;76:6396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Noh K, Mangala LS, Han HD, Zhang N, Pradeep S, Wu SY, et al. Differential Effects of EGFL6 on Tumor versus Wound Angiogenesis. Cell Rep. 2017;21:2785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song K, Su W, Liu Y, Zhang J, Liang Q, Li N, et al. Identification of genes with universally upregulated or downregulated expressions in colorectal cancer. J Gastroenterol Hepatol. 2019;34:880–9. [DOI] [PubMed] [Google Scholar]
- [14].Huo FC, Zhu WT, Liu X, Zhou Y, Zhang LS, Mou J. Epidermal growth factor-like domain multiple 6 (EGFL6) promotes the migration and invasion of gastric cancer cells by inducing epithelial-mesenchymal transition. Invest New Drugs. 2021;39:304–16. [DOI] [PubMed] [Google Scholar]
- [15].Zhang QW, Zhang XT, Tang CT, Lin XL, Ge ZZ, Li XB. EGFL6 promotes cell proliferation in colorectal cancer via regulation of the WNT/beta-catenin pathway. Mol Carcinog. 2019;58:967–79. [DOI] [PubMed] [Google Scholar]
- [16].Zhu Z, Ni H, You B, Shi S, Shan Y, Bao L, et al. Elevated EGFL6 modulates cell metastasis and growth via AKT pathway in nasopharyngeal carcinoma. Cancer Med. 2018;7:6281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gonzales NR, De Pascalis R, Schlom J, Kashmiri SV. Minimizing the immunogenicity of antibodies for clinical application. Tumour Biol. 2005;26:31–43. [DOI] [PubMed] [Google Scholar]
- [19].Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qu ZX, Griffiths GL, Wegener WA, Chang CH, Govindan SV, Horak ID, et al. Development of humanized antibodies as cancer therapeutics. Methods. 2005;36:84–95. [DOI] [PubMed] [Google Scholar]
- [21].Kennedy PJ, Oliveira C, Granja PL, Sarmento B. Monoclonal antibodies: technologies for early discovery and engineering. Crit Rev Biotechnol. 2018;38:394–408. [DOI] [PubMed] [Google Scholar]
- [22].Tabasinezhad M, Talebkhan Y, Wenzel W, Rahimi H, Omidinia E, Mahboudi F. Trends in therapeutic antibody affinity maturation: From in-vitro towards next-generation sequencing approaches. Immunol Lett. 2019;212:106–13. [DOI] [PubMed] [Google Scholar]
- [23].Vajda S, Porter KA, Kozakov D. Progress toward improved understanding of antibody maturation. Curr Opin Struct Biol. 2021;67:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 2016;7:6916–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. The Journal of clinical investigation. 2011;121:3206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer research. 2011;71:3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hanf KJ, Arndt JW, Chen LL, Jarpe M, Boriack-Sjodin PA, Li Y, et al. Antibody humanization by redesign of complementarity-determining region residues proximate to the acceptor framework. Methods. 2014;65:68–76. [DOI] [PubMed] [Google Scholar]
- [28].Kettleborough CA, Saldanha J, Heath VJ, Morrison CJ, Bendig MM. Humanization of a mouse monoclonal antibody by CDR-grafting: the importance of framework residues on loop conformation. Protein Eng. 1991;4:773–83. [DOI] [PubMed] [Google Scholar]
- [29].O’Brien S, Jones T. Humanization of monoclonal antibodies by CDR grafting. Methods in molecular biology (Clifton, NJ). 2003;207:81–100. [DOI] [PubMed] [Google Scholar]
- [30].Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rajpal A, Beyaz N, Haber L, Cappuccilli G, Yee H, Bhatt RR, et al. A general method for greatly improving the affinity of antibodies by using combinatorial libraries. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang WP, Green K, Pinzsweeney S, Briones AT, Burton DR, Barbas CF. Cdr Walking Mutagenesis for the Affinity Maturation of a Potent Human Anti-Hiv-1 Antibody into the Picomolar Range. J Mol Biol. 1995;254:392–403. [DOI] [PubMed] [Google Scholar]
- [33].Yang D, Pan A, Swaminathan A, Kumar G, Hughes BA. Expression and localization of the inwardly rectifying potassium channel Kir7.1 in native bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3178–85. [DOI] [PubMed] [Google Scholar]
- [34].Chim SM, Qin A, Tickner J, Pavlos N, Davey T, Wang H, et al. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. The Journal of biological chemistry. 2011;286:22035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fan H, Atiya HI, Wang Y, Pisanic TR, Wang TH, Shih IM, et al. Epigenomic Reprogramming toward Mesenchymal-Epithelial Transition in Ovarian-Cancer-Associated Mesenchymal Stem Cells Drives Metastasis. Cell Rep. 2020;33:108473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haraya K, Tachibana T, Nanami M, Ishigai M. Application of human FcRn transgenic mice as a pharmacokinetic screening tool of monoclonal antibody. Xenobiotica. 2014;44:1127–34. [DOI] [PubMed] [Google Scholar]
- [37].Cao YQ, Li Z, Wang LF, Li N, Chang H. High EGFL6 expression is associated with clinicopathological characteristics in colorectal cancer. Int J Clin Exp Pathol. 2018;11:5893–900. [PMC free article] [PubMed] [Google Scholar]
- [38].Chang CC, Sung WW, Hsu HT, Yeh CM, Lee CH, Chen YL, et al. Validation of EGFL6 expression as a prognostic marker in patients with lung adenocarcinoma in Taiwan: a retrospective study. BMJ Open. 2018;8:e021385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sung TY, Huang HL, Cheng CC, Chang FL, Wei PL, Cheng YW, et al. EGFL6 promotes colorectal cancer cell growth and mobility and the anti-cancer property of anti-EGFL6 antibody. Cell Biosci. 2021;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. SDS-PAGE Analysis of 26 humanized IgGs under non-reducing conditions. Cell culture supernatants were collected on day 2 (d2), day 4 (d4), and day 6 (d6) post-transfection. About 20 μl of supernatant was loaded in each lane. M, marker.
Supplementary Figure 2. Generation of humanized EGFL6 antibodies. Binding validation of humanized antibodies to antigen EGFL6 purified from intracellular (blue bars) and medium (dark red bars) by ELISA. Blank is the HEK293 medium control; NC represents negative control of capture antibody with secondary antibody only. Arrow indicates the CBM clone (VH1-CBM+VL1-CBM) with better binding affinity than chimeric antibody. ELISA, enzyme-linked immunosorbent assay.
Supplementary Figure 3. RT-qPCR analysis of EGFL6 mRNA expression in ovarian cancer cells. EGFL6 mRNA was expressed in patient 366 (Pt. 366) tumor cells, but not in SKOV3 cells. Data was normalized to a housekeeping gene, hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1).
Supplementary Figure 4. Body weight change in NSG mice treated with IgG, #9 and #16 humanized affinity-matured EGFL6 antibodies. (A) Antibody concentration in serum following a single injection of antibody (10 mg/kg) in Tg32 SCID mice (expressing human FC receptor) (B) Changes in mouse body weights from days 0 to 90 post-injection. (C) Average mouse body weight change at day 90 the end point of experiments. Compared with mice treated with IgG, both humanized antibodies had no effects on NSG mouse body weights, indicating that the two humanized affinity-matured antibodies were not toxic during the 3-month treatment regimen.
Supplementary Figure 5. Condensed/fragmented nuclei induced in humanized affinity-matured EGFL6 antibody-treated tumors. Nuclei were labeled by DAPI. Fluorescent images displayed non-condensed nuclei in control IgG group, whereas tumors from #9 Ab- and #16 Ab-treated mice showed condensed/fragmented nuclei, a marker of apoptosis. Scale bars, 20 μm.


