Abstract
Objectives:
Assess cardiovascular health (CVH) during early childhood using the American Heart Association’s recently-updated construct, Life’s Essential 8 (LE8); examine concordance in CVH status per LE8 vs. Life’s Simple 7 (LS7); and identify perinatal correlates of high CVH per LE8.
Methods:
We applied LE8 and LS7 to data from 305 children aged 4–7 years in Denver, CO; estimated % low, moderate, high, and optimal CVH; assessed concordance in CVH status based on LE8 and LS7 using contingency tables; and used multivariable logistic regression to identify early-life correlates of high CVH per LE8.
Results:
Average age of children was 4.7±0.6 years; 44.6% were female. No participants had low or optimal CVH; 43.9% had high and 56.1% had moderate CVH per LE8, whereas 33.4% had high and 66.6% had moderate CVH per LS7. Twenty-two percent had high CVH based on both constructs. Correlates of high CVH were maternal prenatal diet quality (ORHealthy Eating Index score > vs. ≤57 =1.90 [1.12, 3.21]) and child age (ORper 1 year=1.58 [1.04. 2.42]).
Conclusions:
LE8 yielded higher prevalence of high CVH than LS7 during early childhood, though there is modest concordance between the two constructs. Maternal diet is a potential modifiable target to optimize early-life CVH.
Keywords: cardiovascular health, pediatrics, prenatal correlates, epidemiology, optimal cardiovascular health, ideal cardiovascular health, primordial prevention
INTRODUCTION
Ten years ago, the American Heart Association (AHA) put forth a cardiovascular health (CVH) construct known as Life’s Simple 7 (LS7) 1,2 to facilitate primordial prevention of cardiovascular disease (CVD). LS7 comprises three behavioral (no cigarette smoking, healthy diet, physical activity) and four health factors (normal body mass index [BMI], blood pressure, cholesterol, and glucose) that predict lower risk of CVD 3,4, longer life span 5, and better quality of life 6. Each LS7 metric comprises strata of poor, intermediate, and ideal CVH and the composite score ranges from 0 to 14, with 14 indicating “ideal” CVH. Studies from the last decade revealed low prevalence (%) of ideal CVH in adults (<1%) 3,7 and children (<2%) 4,8–19. In 2022, the AHA introduced Life’s Essential 8 (LE8) 20, an updated construct that includes an eight metric of sleep and holds promise to enhance CVH assessment across the lifespan. One advantage of LE8 over LS7 is the method of calculating the composite CVH score, which ranges from 0 to 100 regardless of the number of available metrics. This scoring system facilitates comparisons of CVH across populations and enables assessment of change in CVH at the individual and population levels. Moreover, the distinction between high vs. optimal CVH per LE8, as opposed to a single category of ideal CVH per LS7, may allow for greater sensitivity in assessing differences in CVH that are especially relevant during early-life.
At present, we are aware of only one study that assessed CVH in children using LE8. Leveraging data from 9,888 children and adolescents ages 2–19 years in the National Health and Nutrition Examination Survey (NHANES) 2013–2018, Lloyd-Jones et al. 21 found that 2.2% of youth had optimal CVH and 29.1% had high CVH. Importantly, prevalence of high CVH declined with age, emphasizing a need to identify determinants of early-life CVH to home in on opportunities for primordial prevention of CVD.
Here, we sought to assess and describe CVH status at ages 4–7 years based on LE8 and LS7, evaluate concordance in CVH status across the two constructs, and identify perinatal correlates of high CVH per LE8. Given the relatively scant literature on this topic, the data science tasks 22 of interest in this analysis are: (1) description – that is, describing and comparing CVH status based on LE8 and LS7; and (2) prediction, referring to the identification of perinatal factors that are independently associated with high early-life CVH – findings that will inform future studies investigating causal determinants of CVH in young children.
METHODS
Study population.
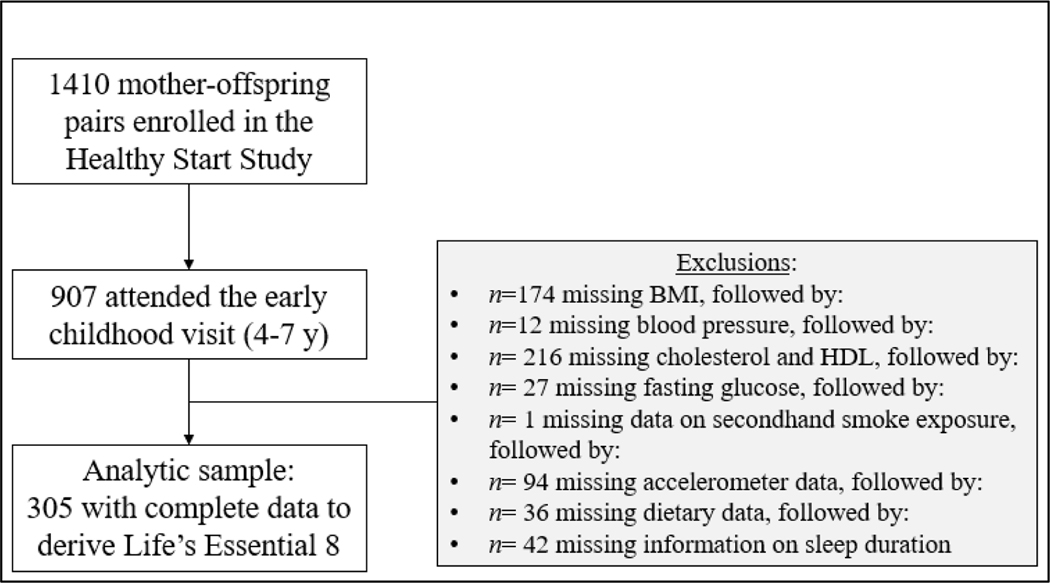
Study participants were from the Healthy Start Study, a prospective cohort of 1,410 racially/ethnically diverse pregnant women enrolled at ≤24 gestational weeks from prenatal clinics at the University of Colorado Hospital from 2009–2014 23,24. Women were excluded if they were expecting multiple births; had a previous stillbirth or delivery <25 gestational weeks; had pre-existing diabetes, asthma managed with steroids, cancer, or psychiatric illness; were younger than 16 y of age; or had already completed 24 gestational weeks. Of the 1,410 women enrolled, 907 mother-offspring pairs returned for the early childhood at ages 4–7 years. Of them, the analytical sample comprised 305 mother-child pairs with complete data on LE8 metrics.
As shown in Supplemental Table 1, participants included in this analysis are similar to those not included, except for slightly older maternal age (~2 years), higher prevalence of non-Hispanic White women (62.0% vs. 51.0%), higher maternal education (30.2% vs. 19.2% college graduates), and higher household income (26.1% vs. 39.6% with ≤$40,000). All mothers provided written informed consent. The study protocol was approved by the Colorado Multiple Institutional Review Board.
Assessment of Life’s Essential 8
BMI and blood pressure assessment
Research assistants (RAs) measured the children’s weight on an electronic scale and height via a stadiometer. We calculated BMI as weight(kg)/height(m)2 and standardized values as percentiles using the World Health Organization (WHO) growth reference 25,26.
We measured systolic (SBP) and diastolic blood pressure (DBP) twice in the seated position using an oscillometric monitor (Dinamap V100, GE Carescape; Waukesha, WI) using an appropriately-sized cuff. For the analysis, we took the average across the two measures and converted values to age, sex, and height-specific z-scores 27.
Fasting glucose and lipid profile
We assayed fasting glucose using an enzymatic approach (Olympus America, Center Valley, PA). Serum concentrations of total cholesterol, high-density lipoprotein (HDL), and triglycerides were measured via a radioimmunoassay (Millipore Corporation, Burlington, MA). We calculated non-HDL cholesterol as (total cholesterol–HDL).
Physical activity
After the research visit, the children wore a waist-worn accelerometer (ActiGraph wGT3X-BT, ActiGraph, Pensacola, FL) for seven days. For each child, we used data from the four days with the longest wear-time, with a minimum of 8 hours/day, to derive intensity of physical activity and defined moderate-to-vigorous physical activity (MVPA) as a vector count ≥3908 28. These data were used to estimate minutes of MVPA/day.
Diet quality, sleep duration, and nicotine exposure
Mothers reported their child’s dietary intake via two Automated Self-Administered 24-hour Dietary Recalls (ASA24). We used the USDA Food and Nutrient Database to extract nutrient and caloric intake, and calculated the Healthy Eating Index (HEI)-2015 score 29, an assessment of diet quality 30 used in LE8. Mothers also reported on their child’s usual sleep duration via questionnaire.
LE8’s metric for nicotine exposure comprises first- and secondhand exposure. Given the young age of the participants, we assumed that none had ever smoked a cigarette. Exposure to secondhand smoke was assessed via a questionnaire inquiring on whether an adult in the household smoked.
Perinatal characteristics.
At enrollment, we administered a questionnaire inquiring on the women’s birthdate, reproductive history, educational attainment, and annual household income. We also inquired on the women’s self-identified race and ethnicity, for which we provided the options of American Indian or Alaska Native; Asian, Native Hawaiian or Pacific Islander; non-Hispanic Black or African American; non-Hispanic White, and >1 race. Due to small cell sizes for some of the categories, we combined American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander and >1 race into a single category of “non-Hispanic other.” We view race and ethnicity as social constructs that reflect the effects of lived experiences on health.
We obtained information on the women’s diet via two ASA24s administered across pregnancy and used the same procedure as for the children to calculate prenatal HEI score 29, which we dichotomized as >57 vs. ≤57 29,31. We derived the women’s pre-pregnancy BMI based on weight and height, and dichotomized values as < vs. ≥25 kg/m2. Medical records provided information on gestational diabetes mellitus diagnoses during the index pregnancy. The women reported on cigarette smoking via questionnaires during pregnancy and at delivery.
We abstracted data on offspring sex, gestational age at delivery, and birthweight from medical records. We dichotomized gestational age at delivery as <37 vs. ≥37 gestational weeks as an indicator of preterm birth, which is associated with poor CVH 32. We calculated birthweight-for-gestational-age z-scores using the WHO standard 25 and categorized values as small (<10th), appropriate (10th-90th), and large (>90th percentile) for gestational age to reflect the U-shaped relationship between birth size and CVH 33,34. Finally, we created a breastmilk-months variable reflecting duration and exclusivity of breastfeeding 35, dichotomized as ≥6 vs. <6 months.
Data analysis
Step 1: Derive CVH score and assess CVH status per LE8
We applied the LE8 scoring system to data on individual CVH metrics using weights proposed by Lloyd-Jones et al. 20 (Supplemental Table 2). We then calculated a composite CVH score as the average value across all metrics and created separate scores for the health and behavioral factors. We assessed the overall composite CVH score continuously, and as low (<50), moderate (50-<80), high (80-<100), and optimal (100) 21.
Step 2: Assess concordance in CVH status per LE8 vs. LS7
We derived LS7 metrics as previously described 19. Each metric comprises strata of poor (0 points), intermediate (1 point), and ideal (2 points) based on clinically-accepted thresholds. We took the sum across all seven metrics and derived CVH status categories based on thresholds suggested by Perak et al. for CVH assessment in children 36: <7 of 14 points (0%-<50%) as low CVH; 7-<10.5 (50%-<75%) as moderate CVH; 10.5-<14 (75%-<100%) as high CVH; and 14 (100%) as ideal CVH. We then evaluated concordance in CVH status based on LE8 vs. LS7 via contingency tables and assessed Pearson’s correlation between the continuous scores. We also repeated the above steps after removing sleep from LE8.
Step 3: Perinatal correlates of high CVH per LE8
We examined bivariate associations of the perinatal characteristics with prevalence (%) of LE8’s definition of high CVH, using alpha=0.10 as the threshold for statistical significance. We entered statistically significance correlates identified in the prior step, plus child age and sex, as predictors into a logistic regression model where high CVH was the outcome. We tested for an interaction between all characteristics and child sex to assess need for sex-stratified models (P-interaction<0.10). In sensitivity analyses, we re-ran all models with the addition of characteristics that were marginally significant in bivariate analyses (P=0.10-<0.15) and assessed whether their inclusion altered the estimates. We carried out all analyses using SAS 9.4 (Cary, NC, USA).
RESULTS
Average age of the children was 4.7±0.6 years; 45% were female. The majority (62%) of mothers identified as non-Hispanic White, 23.3% as Hispanic, 11.8% as non-Hispanic Black, and 2.9% were classified as non-Hispanic Other. Table 1 shows additional mother-child characteristics.
Table 1.
Background characteristics of 305 mother-offspring pairs in the Healthy Start pre-birth cohort.
| Mean ± SD or % (N) | |
|---|---|
| Maternal and child sociodemographic characteristics | |
|
| |
| Maternal age at delivery, years | 29.4 ± 5.7 |
| Maternal race/ethnicity | |
| Hispanic | 23.3% (71) |
| Non-Hispanic White | 62.0% (189) |
| Non-Hispanic Black | 11.8% (36) |
| Non-Hispanic Other | 2.9% (9) |
| Parity (not including index) | |
| 0 | 42.6% (130) |
| 1 to 2 | 52.8% (161) |
| ≥3 | 4.6% (14) |
| Preterm delivery | 6.2% (19) |
| Birth size | |
| Small-for-gestational age | 11.6% (34) |
| Appropriate-for-gestational age | 80.5% (235) |
| Large-for-gestational age | 7.9% (23) |
| Maternal education level | |
| Less than high school | 22.0% (67) |
| Some college/associate degree | 23.3% (71) |
| College graduate | 24.6% (75) |
| Graduate degree | 30.2% (92) |
| Annual household income <$40,000 | 26.1% (68) |
| Child is female | 44.6% (136) |
| Child’s age at the early childhood visit, years | 4.7 ± 0.6 |
|
| |
| Maternal perinatal metabolic characteristics | |
|
| |
| Pre-pregnancy BMIa | |
| BMI <25 kg/m2 | 55.1% (168) |
| BMI ≥25 kg/m2 | 44.9% (137) |
| Pre-pregnancy BMI, kg/m2 | 25.7 ± 6.4 |
| Gestational weight gainb | |
| Inadequate | 23.9% (73) |
| Adequate | 29.2% (89) |
| Excessive | 46.9% (143) |
| Gestational diabetes mellitus (GDM dx) | 4.9% (14) |
|
| |
| Maternal and infant behaviors | |
|
| |
| Cigarette smoking during pregnancy | 5.3% (16) |
| High diet quality during pregnancy (HEI >57) | 45.3% (136) |
| ≥150 min/week of MVPA during pregnancy | 61.0% (186) |
| Child was breastfed for ≥6 months | 15.8% (38) |
Abbreviations: BMI: body mass index; HEI: Healthy Eating Index-2010; MVPA: moderate-to-vigorous physical activity
Includes 6 women classified as underweight (BMI <18.5 kg/m2).
According to Institute of Medicine 2009 guidelines.
No children had low or optimal CVH; 43.9% had high CVH and 56.1% had moderate CVH. Average CVH score was 78.2±6.7. Boys had higher prevalence of high CVH than girls (46.8% vs. 40.4%) and, accordingly, higher average CVH score (Table 2). When assessing individual metrics, boys had higher scores for diet and physical activity, but a lower score for BMI (Table 2). Generally, children had high scores for the health factors, and low-to-moderate scores for the behavioral factors, except for nicotine which ranged from 80 (high) to 100 (optimal).
Table 2.
Mean ± SD scores and % of cardiovascular health (CVH) status categories based on Life’s Essential 8 among 305 children aged 4–7 years in the Healthy Start pre-birth cohort.
| All children | Girls | Boys | P a | |
|---|---|---|---|---|
| n = 305 | n = 136 | n = 169 | ||
|
|
|
|||
| Behavioral factor scores | ||||
| Nicotine exposure | ||||
| Mean ± SD | 97.4 ± 6.8 | 97.6 ± 6.6 | 97.3 ± 6.9 | 0.78 |
| Min, Max | 80, 100 | 80, 100 | 80, 100 | |
| Diet quality | ||||
| Mean ± SD | 47.6 ± 15.3 | 45.8 ± 14.5 | 49.1 ± 15.8 | 0.05 |
| Min, Max | 25, 100 | 25, 80 | 25, 100 | |
| Physical activity | ||||
| Mean ± SD | 34.3 ± 15.4 | 30.0 ± 11.0 | 37.8 ± 17.5 | <0.0001 |
| Min, Max | 0, 80 | 25, 70 | 0, 80 | |
| Sleep duration | ||||
| Mean ± SD | 73.7 ± 34.0 | 74.2 ± 34.6 | 73.3 ± 33.6 | 0.81 |
| Min, Max | 0, 100 | 0, 100 | 0, 100 | |
| Biological factor scores | ||||
| Body mass index | 0.08 | |||
| Mean ± SD | 92.3 ± 22.3 | 94.8 ± 18.2 | 90.3 ± 25.0 | |
| Min, Max | 0, 100 | 0, 100 | 0, 100 | |
| Blood pressure | 0.11 | |||
| Mean ± SD | 99.8 ± 2.0 | 99.6 ± 3.0 | 100 | |
| Min, Max | 75, 100 | 75, 100 | 100, 100 | |
| Non-HDL cholesterol | 0.67 | |||
| Mean ± SD | 81.0 ± 23.2 | 81.6 ± 22.7 | 80.5 ± 23.7 | |
| Min, Max | 0, 100 | 0, 100 | 20, 100 | |
| Fasting glucose | 0.69 | |||
| Mean ± SD | 99.6 ± 4.0 | 99.7 ± 3.4 | 99.5 ± 4.3 | |
| Min, Max | 60, 100 | 60, 100 | 60, 100 | |
| Overall CVH score b | ||||
| Mean ± SD | 78.2 ± 6.7 | 77.9 ± 6.4 | 78.5 ± 6.9 | 0.46 |
| Min, Max | 54.4, 93.8 | 54.4, 88.1 | 58.8, 93.8 | |
| CVH status | 0.27 | |||
| Low (score <50) | 0% (0) | 0% (0) | 0% (0) | |
| Moderate (score: 50-<80) | 56.1% (171) | 59.6% (81) | 53.3% (90) | |
| High (score: 80-<100) | 43.9% (134) | 40.4% (55) | 46.8% (79) | |
| Optimal (score = 100) | 0% (0) | 0% (0) | 0% (0) | |
From a Wald test for continuous variables; from the Chi-squared test for % participants by CVH status.
Average of score across the eight Life’s Essential 8 components: nicotine exposure, physical activity, healthy diet, sleep duration, body mass index, non-HDL cholesterol, blood pressure, and fasting glucose.
Table 3 shows the % of participants categorized as having moderate and high CVH based on LE8 and LS7. LE8 yielded greater % high CVH than LS7 (43.9% vs. 33.4%). The Wald chi-square test indicated a difference in the proportion of participants in each category based on LE8 and LS7, with 22.3% of children categorized as high and 44.9% categorized as moderate CVH based on both definitions. Pearson’s R2 for continuous CVH scores was 0.56. After removing sleep from LE8, 4.3% of participants classified as high CVH were re-classified as moderate CVH. The % of participants categorized as high CVH based on both definitions increased to 26.6% and Pearson’s R2 increased to 0.67.
Table 3.
Concordance (% [N]) of cardiovascular health (CVH) status and correlation between CVH scores based on Life’s Essential 8 vs. Life’s Simple 7 definitions among 305 children aged 4–7 years in the Healthy Start pre-birth cohort.
| Life’s Essential 8 (LE8) | ||||
|
|
||||
| Life’s Simple 7 (LS7) | Low | Moderate | High | Ideal/Optimal |
| Low | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Moderate | 0% (0) | 44.9% (137) | 21.6% (66) | 0% (0) |
| High | 0% (0) | 11.2% (34) | 22.3% (68) | 0% (0) |
| Ideal/Optimal | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Wald chi-square test statistic:32.2; P <0.0001 | ||||
| Pearson’s R2 for continuous CVH scores = 0.56; P <0.0001 | ||||
|
|
||||
| LE8 without sleep | ||||
|
|
||||
| LS7 | Low | Moderate | High | Ideal/Optimal |
| Low | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Moderate | 0% (0) | 44.9% (137) | 21.6% (66) | 0% (0) |
| High | 0% (0) | 6.9% (21) | 26.6% (81) | 0% (0) |
| Ideal/Optimal | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Wald chi-square test statistic: 59.8; P <0.0001 | ||||
| Pearson’s R2 for continuous CVH scores = 0.67; P <0.0001 | ||||
Table 4 shows crude associations of perinatal characteristics with % high CVH per LE8. Older maternal age, non-Hispanic White maternal ethnicity, higher maternal education and household income, maternal BMI <25 kg/m2, maternal HEI score >57, and older child age were each associated with high CVH. We noted marginal associations of lower parity (0 or 1–2 prior births), household income (≥$40,000), and breastfeeding duration (≥6 months) with high CVH.
Table 4.
Percentage (% [N]) of participants with high cardiovascular health (CVH) according to Life’s Essential 8 criteria across categories of perinatal characteristics among 305 children aged 4–7 years in the Healthy Start pre-birth cohort.
| N a | % (N) with high CVH | P c | |
|---|---|---|---|
|
|
|||
| 305 | 43.9% (134) | ||
| Maternal and child sociodemographic characteristics | |||
|
| |||
| Maternal age at delivery | 0.002 | ||
| 16–24 y | 65 | 24.6% (16) | |
| 25–29 y | 79 | 43.0% (34) | |
| 30–34 y | 101 | 54.5% (55) | |
| ≥35 y | 60 | 48.3% (29) | |
| Maternal education level | 0.003 | ||
| Less than high school | 27 | 33.3% (9) | |
| High school graduate | 40 | 22.5% (9) | |
| Some college/associate degree | 71 | 38.0% (27) | |
| College graduate | 75 | 50.7% (38) | |
| Graduate degree | 92 | 55.4% (51) | |
| Annual household income | 0.08 | ||
| <$40,000 | 68 | 36.8% (25) | |
| ≥$40,000 | 193 | 49.2% (95) | |
| Maternal race/ethnicity | 0.002 | ||
| Hispanic | 71 | 31.0% (22) | |
| Non-Hispanic White | 189 | 52.4% (99) | |
| Non-Hispanic Black | 36 | 27.8% (10) | |
| Non-Hispanic Other | 9 | 33.3% (3) | |
| Parity (not including index) | 0.12 | ||
| 0 | 130 | 41.5% (54) | |
| 1 to 2 | 161 | 47.8% (77) | |
| ≥3 | 14 | 21.4% (3) | |
| Child’s sex | 0.27 | ||
| Female | 136 | 40.4% (55) | |
| Male | 169 | 46.8% (79) | |
| Preterm delivery | 0.76 | ||
| No (≥37 weeks) | 286 | 43.7% (125) | |
| Yes (<37 weeks) | 19 | 47.4% (9) | |
| Birth size | 0.58 | ||
| Small-for-gestational age | 34 | 38.2% (13) | |
| Appropriate-for-gestational age | 235 | 45.1% (106) | |
| Large-for-gestational age | 23 | 52.2% (12) | |
| Child’s age at the time of CVH assessment | 0.04 | ||
| Q1 (median: 4.1 y) | 50 | 35.1% (27) | |
| Q2 (median: 4.5 y) | 45 | 40.8% (31) | |
| Q3 (median: 4.6 y) | 37 | 51.3% (39) | |
| Q4 (median: 5.3 y) | 39 | 48.7% (37) | |
|
| |||
| Maternal prenatal metabolic characteristics | |||
|
| |||
| Pre-pregnancy BMI a | 0.06 | ||
| BMI <25 kg/m2 | 168 | 48.4% (82) | |
| BMI ≥25 kg/m2 | 137 | 38.0% (52) | |
| Gestational weight gain b | 0.28 | ||
| Inadequate | 73 | 49.3% (36) | |
| Adequate | 89 | 47.2% (42) | |
| Excessive | 143 | 39.2% (45) | |
| Gestational diabetes | 0.12 | ||
| No | 271 | 43.2% (117) | |
| Yes | 14 | 64.3% (9) | |
|
| |||
| Maternal and infant behaviors | |||
|
| |||
| Cigarette smoking during pregnancy | 0.29 | ||
| No | 289 | 44.6% (129) | |
| Yes | 16 | 31.3% (5) | |
| Maternal diet quality during pregnancy | 0.0003 | ||
| Low quality (HEI ≤57) | 164 | 34.2% (56) | |
| High quality (HEI >57) | 136 | 55.2% (75) | |
| Maternal MVPA during pregnancy | 0.68 | ||
| <150 min/week | 119 | 45.4% (54) | |
| ≥150 min/week | 186 | 43.0% (80) | |
| Infant feeding practices | 0.09 | ||
| <6 breastmilk months | 202 | 43.1% (87) | |
| ≥6 breastmilk months | 38 | 57.9% (22) | |
Abbreviations: HEI: Healthy Eating Index-2010; MVPA: moderate-to-vigorous physical activity
Includes 6 women who are underweight (n = 3 each when stratified by sex).
According to Institute of Medicine 2009 guidelines.
P-value represents a Wald chi-squared test with the exception of ordinal variables (age, education level, and pre-pregnancy weight status) where the P-value represents a test for linear trend.
We did not find evidence of an interaction between any perinatal characteristics and child sex, so the multivariable model included all children. Table 5 shows odds ratios (ORs) and 95% confidence intervals (CI) from a multivariable logistic regression model in which maternal age, race/ethnicity, education, BMI, and HEI score; and child age and sex were predictors; and high CVH was the outcome. Children whose mothers had HEI score >57 vs. ≤57 during pregnancy had nearly twice the odds of high CVH (OR: 1.90, [95% CI: 1.12, 3.20]), and each 1-year increment in child age corresponded with 1.58 (95% CI: 1.04, 2.42) times greater odds of high CVH.
Table 5.
Odds ratios (OR [95% CI]) of having high cardiovascular health (CVH) based on Life’s Essential 8 with respect to perinatal characteristics among 305 children aged 4–7 years in the Healthy Start pre-birth cohort.
| OR (95% CI)a of having high CVH during early childhoodb | |
|---|---|
|
|
|
| Maternal and child sociodemographic characteristics | |
| Maternal age, per 1 year | 1.02 (0.96, 1.07) |
| Race/ethnicity | |
| Non-Hispanic White | 1.00 (Reference) |
| Hispanic | 0.72 (0.33, 1.59) |
| Non-Hispanic Black | 0.65 (0.26, 1.62) |
| Non-Hispanic Other | 0.62 (0.14, 2.83) |
| Type 3 P-valueb | 0.69 |
| Maternal education level | |
| Less than high school | 1.00 (Reference) |
| Some college/associate degree | 1.51 (0.68, 3.35) |
| College | 1.62 (0.64, 4.12) |
| Graduate school | 1.62 (0.60, 4.38) |
| P-trend | 0.48 |
| Child sex | |
| Female | 1.00 (Reference) |
| Male | 1.14 (0.69, 1.88) |
| Child’s age, per 1 year | 1.58 (1.04, 2.42) |
| Maternal perinatal characteristics | |
| Maternal pre-pregnancy weight status | |
| BMI <25 kg/m2 | 1.41 (0.83, 2.37) |
| BMI ≥25 kg/m2 | 1.00 (Reference) |
| Maternal prenatal behaviors | |
| Maternal diet quality during pregnancy | |
| Low quality (HEI ≤57) | 1.00 (Reference) |
| High quality (HEI >57) | 1.90 (1.12, 3.21) |
Abbreviations: BMI: body mass index; HEI: healthy eating index; OR: odds ratio; CI: confidence interval
From a multivariable logistic regression model comprising for all variables in table as predictors.
CVH score of 80 to <100.
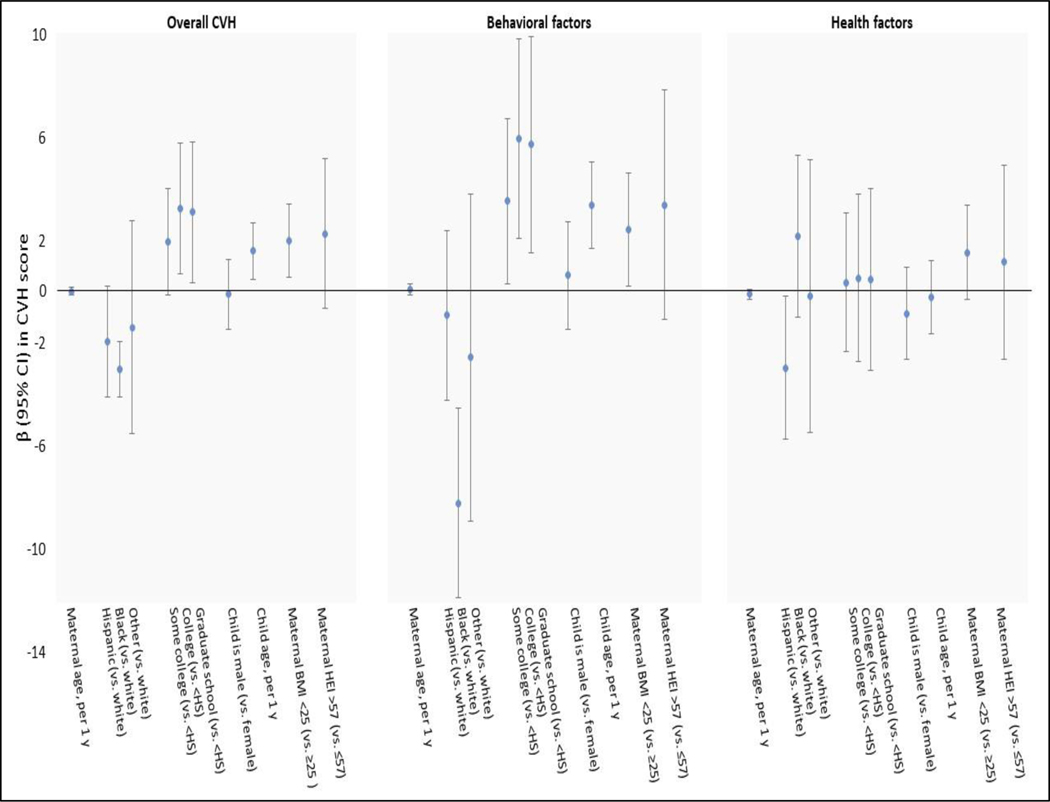
Figure 2 shows β (95% CI) for associations of the same predictors described above with continuous CVH scores. Maternal education, BMI, and HEI score; and child age were each positively associated with the overall CVH score, whereas children with mothers of non-Hispanic Black race/ethnicity had lower CVH scores. These associations were driven by associations with the behavioral factors, though we noted that Hispanic maternal race/ethnicity corresponded with a lower health factor score (−2.96 [95% CI: −5.75, −0.17]). Including parity, breastfeeding duration, and household income in the model did not appreciably change results (data available upon request).
Figure 2.
Associations (β[95% CI]) of perinatal characteristics with overall cardiovascular health (CVH), behavioral factors, and health factors scores based on Life’s Essential 8 among 305 children aged 4–7 years in the Healthy Start pre-birth cohort. Estimates are from a multivariable logistic regression model that included all variables shown in the figure.
Abbreviations: CVH – cardiovascular health; HS – high school; BMI – body mass index; HEI – Healthy Eating Index
DISCUSSION
In this study of 305 diverse children, we compared early-life CVH status based on Life’s Essential 8 (LE8) vs. Life’s Simple 7 (LS7); and identified perinatal correlates of high CVH per LE8. None of the participants had low or optimal CVH according to LE8, though 43.9% had high CVH. We observed modest concordance in % high CVH based LE8 and LS7, with 22.3% classified as high CVH based on both definitions. Maternal diet quality and older child age were the strongest correlates of high CVH at age 4–7 years. When we assessed CVH continuously, higher maternal educational attainment and prenatal diet quality, and lower pre-pregnancy BMI predicted high CVH in offspring; these findings were driven by behavioral, rather than biological, CVH components.
CVH status in children per LE8
To date, we are aware of seven studies that assessed CVH in youth, six of which applied LS7. These studies include 8-to-17 year-olds in Minnesota (n=300) 15; youth in China (n=5,596 youth ages 6–18 years in Beijing 14; n ~15,000 youth ages 6–16 years in Beijing 37; n=12,618 youth ages 6–18 years in northern and southern provinces 17); 2-to-11-year-olds in NHANES (n=8,946) 16; 12-to-15-year-olds in the Cardiovascular Risk in Young Finns Study (n=856) 4; and Finnish adolescents in the STRIP trial followed at ages 15–19 years 38. Our finding of 0% optimal CVH aligns with very low prevalence of ideal CVH (0% to 1.7%) reported in the above earlier studies based on LS7 13,15–17,38.
We know of only one paper that applied LE8 in youth, in which Lloyd-Jones et al. 21 reported an average CVH score of 65.5 of 100 points among 2-to-19-year-olds in NHANES. In this analysis, 2.2% and 29.1% of youth had optimal and high CVH, respectively. Prevalence of high CVH declined with age. Scores were lowest for diet (unitless CVH component score = 40.6) and physical activity (75.1); and highest for blood pressure (96.0), glucose (92.2), and nicotine exposure (85.6).
In the present study, average CVH score (78.2) was higher than in NHANES. This may be due to the younger and narrower age range of Healthy Start participants given that cardiometabolic profile typically worsens with age. As in NHANES, we found a low average score for diet (47.6) – though in our study sample, physical activity was the lowest-scoring metric (34.3); and high scores for blood pressure (99.8), glucose (99.6), and nicotine exposure (97.4). When assessing CVH by sex, we noted higher scores for physical activity in boys than girls (37.8 vs. 30.0), as was the case in NHANES 21. However, contrary to Lloyd-Jones et al.’s findings of a higher diet score in girls, boys had a higher diet score than girls (49.1 vs. 45.8) – a pattern that may reverse later in life 39–41.
We observed a modest correlation (Pearson’s R2=0.56) between LE8 and LS7 CVH scores, which was lower than in NHANES (R2=0.88). After removing sleep from LE8, the correlation increased to 0.67. When comparing CVH status with vs. without inclusion of sleep in the LE8 definition, 4.3% of children were reclassified from high to moderate CVH after including sleep, whereas both upward (7.9%) and downward reclassification (8.4%) across quartiles of LE8 score occurred in NHANES 21. The downward reclassification of CVH status in this study highlights the impact of sleep on CVH in young children, though we note that beyond the inclusion of sleep, other differences in LS7 1,2 vs. LE8 20 may influence concordance in CVH status. Specifically, the LS7 diet metric was based on AHA’s recommendations for a healthy diet 1,2 whereas LE8 employs HEI 2015 20. Additionally, LS7 focused on firsthand cigarette smoking as an indicator for nicotine exposure whereas LE8 considers first and secondhand cigarette smoke exposure 1,2.
Perinatal correlates of high CVH during early childhood per LE8
A key finding of this analysis is that children whose mothers had an HEI score >57 vs. ≤57 during pregnancy had nearly twice the odds of having high CVH. Additionally, each 1-year increment in child age corresponded with ~60% greater odds of high CVH. The association of higher prenatal diet quality with offspring CVH aligns with a growing literature 31,42,43, but the positive association between age and CVH was unexpected in light of findings of Lloyd-Jones et al. 21 and others 38. However, within the narrow age range of our study sample, the older children (6–7 years) may exhibit healthier behaviors than younger ones (4–5 years), such as higher diet diversity and quality 44, which may then drive the overall CVH score. Indeed, disaggregation into the behavioral and health components of CVH revealed that the above associations are driven by the relationship between perinatal characteristics and the behavioral factors. We also noted that children born to non-Hispanic Black women had the lowest behavioral score – an association that reflects myriad social determinants of health, including but not limited access to healthful foods, built environment characteristics, and cultural differences in health behaviors. Regardless, these results indicate that improving children’s diet and physical activity may be key to optimizing early-life CVH.
Strengths & limitations
Strengths of this study include use of the most updated construct to assess CVH in early childhood, a sensitive period for development of cardiovascular risk 45–47; ability to compare concordance between LE8 and LS7; and the rich prospective data.
A key limitation of this study is that only 22% of the mother-offspring dyads enrolled during pregnancy had complete data on early childhood CVH components. Because mothers included in this study were more likely to be White, college-educated, and with higher annual income than those not included, our findings may not be generalizable to the overall cohort.
Future studies in larger, representative cohorts are warranted to investigate the extent to which the perinatal correlates identified herein are causally involved in shaping early-life CVH.
Supplementary Material
Figure 1.
shows the study participant flow.
ACKNOWLEDGEMENTS
We are grateful to the LEAD Center research staff and Healthy Start participants.
Funding/support:
The Healthy Start Study is funded by the National Institutes of Health (NIH) R01DK076648 and UH3OD023248. Dr. Perng is supported by the Center for Clinical and Translational Sciences Institute KL2-TR002534 and ADA-7-22-ICTSPM-08. Dr. Aris is supported by U2COD02337. The funders/sponsors did not participate in the work.
Abbreviations:
- CVH
cardiovascular health
- HEI
healthy eating index
- BMI
body mass index
- CVD
cardiovascular disease
- LE8
Life’s Essential 8
- LS7
Life’s Simple 7
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest disclosure: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Steinberger J, Daniels Stephen R, Hagberg N, et al. Cardiovascular Health Promotion in Children: Challenges and Opportunities for 2020 and Beyond: A Scientific Statement From the American Heart Association. Circulation. 2016/09/20 2016;134(12):e236–e255. doi: 10.1161/CIR.0000000000000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. Feb 2 2010;121(4):586–613. doi: 10.1161/circulationaha.109.192703 [DOI] [PubMed] [Google Scholar]
- 3.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. Apr 24 2012;125(16):1971–8. doi: 10.1161/circulationaha.111.073585 [DOI] [PubMed] [Google Scholar]
- 5.Ford Earl S, Greenlund Kurt J, Hong Y. Ideal Cardiovascular Health and Mortality From All Causes and Diseases of the Circulatory System Among Adults in the United States. Circulation. 2012/02/28 2012;125(8):987–995. doi: 10.1161/CIRCULATIONAHA.111.049122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen Norrina B, Ning H, Huffman Mark D, Liu K, Reis J, Lloyd-Jones D. Abstract 15499: Maintenance of Ideal Cardiovascular Health Over 20 Years and Its Impact on Health Related Quality of Life: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation. 2013/11/26 2013;128(suppl_22):A15499-A15499. doi: 10.1161/circ.128.suppl_22.A15499 [DOI] [Google Scholar]
- 7.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. Mar 1 2011;123(8):850–7. doi: 10.1161/circulationaha.110.980151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aatola H, Hutri-Kähönen N, Juonala M, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. Journal of the American Heart Association. Mar 10 2014;3(2):e000532. doi: 10.1161/jaha.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrignani MG, Lucà F, Favilli S, et al. Lifestyles and Cardiovascular Prevention in Childhood and Adolescence. Pediatric cardiology. Aug 2019;40(6):1113–1125. doi: 10.1007/s00246-019-02152-w [DOI] [PubMed] [Google Scholar]
- 10.Agostinis-Sobrinho C, García-Hermoso A, Ramírez-Vélez R, et al. Longitudinal association between ideal cardiovascular health status and muscular fitness in adolescents: The LabMed Physical Activity Study. Nutrition, metabolism, and cardiovascular diseases : NMCD. Sep 2018;28(9):892–899. doi: 10.1016/j.numecd.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Liu RS, Wake M, Grobler A, et al. Cross-sectional associations between Ideal Cardiovascular Health scores and vascular phenotypes in 11- to 12-year-olds and their parents: The Longitudinal Study of Australian Children. International journal of cardiology. Feb 15 2019;277:258–265. doi: 10.1016/j.ijcard.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 12.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. Apr 2 2013;127(13):1369–76. doi: 10.1161/circulationaha.113.001559 [DOI] [PubMed] [Google Scholar]
- 13.Chen FF, Chang SY, Hou DQ, et al. [Characteristics of cardiovascular health of children and adolescents aged 6–16 years in Beijing during 2017–2018]. Zhonghua Yu Fang Yi Xue Za Zhi. Nov 6 2018;52(11):1124–1129. doi: 10.3760/cma.j.issn.0253-9624.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Yan Y, Liu J, et al. Alarming trends in ideal cardiovascular health among children and adolescents in Beijing, China, 2004 to 2014. International journal of cardiology. Mar 15 2017;231:264–270. doi: 10.1016/j.ijcard.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 15.Fyfe-Johnson AL, Ryder JR, Alonso A, et al. Ideal Cardiovascular Health and Adiposity: Implications in Youth. Journal of the American Heart Association. Apr 13 2018;7(8)doi: 10.1161/jaha.117.007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning H, Labarthe DR, Shay CM, et al. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. Mar 2015;8(2):164–71. doi: 10.1161/circoutcomes.114.001274 [DOI] [PubMed] [Google Scholar]
- 17.Yan Y, Liu J, Zhao X, et al. Cardiovascular health in urban Chinese children and adolescents. Ann Med. Feb 2019;51(1):88–96. doi: 10.1080/07853890.2019.1580383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henriksson P, Henriksson H, Labayen I, et al. Correlates of ideal cardiovascular health in European adolescents: The HELENA study. Nutrition, metabolism, and cardiovascular diseases : NMCD. Feb 2018;28(2):187–194. doi: 10.1016/j.numecd.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 19.Perng W, Francis EC, Schuldt C, Barbosa G, Dabelea D, Sauder KA. Pre- and Perinatal Correlates of Ideal Cardiovascular Health during Early Childhood: A Prospective Analysis in the Healthy Start Study. The Journal of pediatrics. Jul 2021;234:187–194. doi: 10.1016/j.jpeds.2021.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022/08/02 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Ning H, Labarthe D, et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association’s New “Life’s Essential 8” Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 Through 2018. Circulation. Sep 13 2022;146(11):822–835. doi: 10.1161/circulationaha.122.060911 [DOI] [PubMed] [Google Scholar]
- 22.Laubach ZM, Murray EJ, Hoke KL, Safran RJ, Perng W. A biologist’s guide to model selection and causal inference. Proceedings of the Royal Society B: Biological Sciences. 2021/01/27 2021;288(1943):20202815. doi: 10.1098/rspb.2020.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrod CS, Fingerlin TE, Chasan-Taber L, Reynolds RM, Glueck DH, Dabelea D. Exposure to prenatal smoking and early-life body composition: the healthy start study. Obesity (Silver Spring, Md). Jan 2015;23(1):234–41. doi: 10.1002/oby.20924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. Feb 2015;101(2):302–9. doi: 10.3945/ajcn.114.094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organizaton. WHO child growth standards: height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index for-age: methods and development. 2006. [Google Scholar]
- 26.de Onis M, Garza C, Victora C, Bhan M, Norum K. The WHO Multicentre Growth Reference Study (MGRS): rationale, planning, and implementaton. Food Nutr Bull. 2004;25(Suppl1):S1–S89. [Google Scholar]
- 27.U.S. Department of Health and Human Services. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. 2005. [Google Scholar]
- 28.Butte NF, Wong WW, Lee JS, Adolph AL, Puyau MR, Zakeri IF. Prediction of energy expenditure and physical activity in preschoolers. Med Sci Sports Exerc. 2014;46(6):1216–1226. doi: 10.1249/MSS.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro ALB, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond). 2016;40(7):1056–1062. doi: 10.1038/ijo.2016.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. The Journal of nutrition. Mar 2014;144(3):399–407. doi: 10.3945/jn.113.183079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis EC, Dabelea D, Shankar K, Perng W. Maternal diet quality during pregnancy is associated with biomarkers of metabolic risk among male offspring. Diabetologia. Nov 2021;64(11):2478–2490. doi: 10.1007/s00125-021-05533-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensley JG, De Matteo R, Harding R, Black MJ. The effects of preterm birth and its antecedents on the cardiovascular system. 10.1111/aogs.12880. Acta Obstetricia et Gynecologica Scandinavica. 2016/06/01 2016;95(6):652–663. doi: 10.1111/aogs.12880 [DOI] [PubMed] [Google Scholar]
- 33.Knop MR, Geng TT, Gorny AW, et al. Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies. Journal of the American Heart Association. Dec 4 2018;7(23):e008870. doi: 10.1161/jaha.118.008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai C, Hu Y, He D, et al. U-shaped relationship between birth weight and childhood blood pressure in China. BMC Pediatr. Jul 31 2019;19(1):264. doi: 10.1186/s12887-019-1638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crume TL, Ogden L, Maligie M, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes care. Mar 2011;34(3):641–5. doi: 10.2337/dc10-1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perak AM, Benuck I. Preserving Optimal Cardiovascular Health in Children. Pediatric annals. Dec 1 2018;47(12):e479–e486. doi: 10.3928/19382359-20181115-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breton CV, Marsit CJ, Faustman E, et al. Small-Magnitude Effect Sizes in Epigenetic End Points are Important in Children’s Environmental Health Studies: The Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environ Health Perspect. 2017;125(4):511–526. doi: 10.1289/EHP595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pahkala K, Hietalampi H, Laitinen TT, et al. Ideal Cardiovascular Health in Adolescence. Circulation. 2013;127(21):2088–2096. doi:doi: 10.1161/CIRCULATIONAHA.112.000761 [DOI] [PubMed] [Google Scholar]
- 39.Truesdale KP, Matheson DM, JaKa MM, McAleer S, Sommer EC, Pratt CA. Baseline diet quality of predominantly minority children and adolescents from households characterized by low socioeconomic status in the Childhood Obesity Prevention and Treatment Research (COPTR) Consortium. BMC Nutrition. 2019/09/09 2019;5(1):38. doi: 10.1186/s40795-019-0302-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurley KM, Oberlander SE, Merry BC, Wrobleski MM, Klassen AC, Black MM. The healthy eating index and youth healthy eating index are unique, nonredundant measures of diet quality among low-income, African American adolescents. The Journal of nutrition. Feb 2009;139(2):359–64. doi: 10.3945/jn.108.097113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. Journal of the Academy of Nutrition and Dietetics. Feb 2013;113(2):297–306. doi: 10.1016/j.jand.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 42.Perng W, Oken E, Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia. Oct 2019;62(10):1779–1788. doi: 10.1007/s00125-019-4914-1 [DOI] [PubMed] [Google Scholar]
- 43.Shapiro ALB, Kaar JL, Crume TL, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. International Journal of Obesity. 2016/07/01 2016;40(7):1056–1062. doi: 10.1038/ijo.2016.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rakotonirainy NH, Razafindratovo V, Remonja CR, et al. Dietary diversity of 6- to 59-month-old children in rural areas of Moramanga and Morondava districts, Madagascar. PloS one. 2018;13(7):e0200235. doi: 10.1371/journal.pone.0200235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. Jun 24 2008;117(25):3171–80. doi: 10.1161/circulationaha.107.730366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. American journal of epidemiology. May 1 1991;133(9):884–99. doi: 10.1093/oxfordjournals.aje.a115968 [DOI] [PubMed] [Google Scholar]
- 47.Rundle AG, Factor-Litvak P, Suglia SF, et al. Tracking of Obesity in Childhood into Adulthood: Effects on Body Mass Index and Fat Mass Index at Age 50. Childhood obesity (Print). Apr 2020;16(3):226–233. doi: 10.1089/chi.2019.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.