Abstract
Rift Valley fever phlebovirus (RVFV) is an emerging, mosquito-borne, zoonotic pathogen. Real time RT-qPCR genotyping (GT) assays were developed to differentiate between two RVFV wild-type strains (128B-15 and SA01–1322) and a vaccine strain (MP-12). The GT assay uses a one-step RT-qPCR mix, with two different RVFV strain-specific primers (either forward or reverse) with long or short G/C tags and a common primer (either forward or reverse) for each of the 3 genomic segments. The GT assay produces PCR amplicons with unique melting temperatures that are resolved in a post PCR melt curve analysis for strain identification. Furthermore, a strain specific RT-qPCR (SS-PCR) assay was developed to allow for specific detection of low titer RVFV strains in mixed RVFV samples. Our data shows that the GT assays are capable of differentiating L, M, and S segments of RVFV strains 128B-15 versus MP-12, and 128B-15 versus SA01–1322. The SS-PCR assay results revealed that it can specifically amplify and detect a low titer MP-12 strain in mixed RVFV samples. Overall, these two novel assays are useful as screening tools for determining reassortment of the segmented RVFV genome during co-infections, and could be adapted and applied for other segmented pathogens of interest.
Keywords: Rift valley fever phlebovirus, reassortment, genotyping assays, strain-specific RT-PCR, melt curve analysis
1. Introduction
Rift Valley fever phlebovirus (RVFV) is a member of the Phenuiviridae family and has a segmented negative- or ambi-sense RNA genome consisting of large (L; 6.4 kb), medium (M; 3.8 kb) and small (S; 1.7 kb) RNA segments. RVFV causes Rift Valley fever (RVF) in various animal species including cattle, sheep, goats, camels, and white-tailed deer (Wilson et al., 2018). RVF is characterized by fever and abortion storms, which can cause devastating economic losses to livestock industries (Peyre et al., 2015). In humans, RVFV can cause mild to severe disease, with few cases resulting in death due to severe hemorrhagic disease (Ikegami and Makino, 2011). Thus, RVFV poses a threat to livestock, humans and wildlife. In areas where RVFV is endemic, inactivated and live attenuated vaccines are used for livestock (Indran and Ikegami, 2012; Faburay et al., 2017). No vaccines are available for humans although some progress has been made (Gerkin et al., 2022). RVFV is capable of exchanging or reassorting genomic segments with other RVFV strains following co-infection of hosts (Gaudreault et al, 2019). In such case of co-infections with different RVFV strains, the genomic segments can reassort and generate novel strains with potentially altered virulence, tropisms and/or transmissibility; this represents a significant safety concern regarding the use of live attenuated RVFV vaccines (Gaudreault et al., 2019). The occurrence of RVFV reassortment in nature and in the laboratory has been previously reported (Sall et al., 1999; Freire et al., 2015; Turell et al., 1990; Gaudreault et al., 2019). Besides playing a role in viral evolution and diversity, reassortment has practical implications for vaccine development. The potential for reassortant viruses to emerge after application of live attenuated RVFV vaccines is a significant animal and public health concern and may limit their use. For control of RVF in animals, three modified live vaccines (MLVs) have been developed and are licensed for use in Africa: the Smithburn vaccine, the MP-12 vaccine, and a vaccine based on Clone-13. Importantly, there has been a report of a RVFV reassortant virus isolated from a human patient while vaccinating sheep with the Smithburn vaccine, as well as evidence of historical reassortment events from a fatal RVF infection in a human (Grobbelaar et al., 2011).
Current RVFV diagnostic assays are based on virus isolation in cell culture (Anderson et al., 1989), RT-qPCR for detection of RVFV RNA (Sall et al., 2002), and detection of anti-RVFV antibodies in serum by ELISA and virus neutralization test (VNT) (Paweska et al., 2003; Williams et al., 2011). However, none of these assays have the ability to identify either reassortant viruses (RAVs) or different RVFV strains in mixed samples, for which usually sequencing is required. A major challenge in developing an assay that differentiates between RVFV strains to identify RAVs is the rather highly conserved virus genome. For example, the genome of the vaccine strain MP-12 has more than 96% nucleotide sequence identity with wild-type strain 128B-15; and WT strain SA01–1322 has more than 98% nucleotide sequence homology with the 128B-15 strain.
Monoclonal antibodies (MAbs) have been used to identify S and M segment reassortants of RVFV under experimental conditions (Turell et al., 1990); however this method could not identify L segment RAVs, and thus is not feasible for diagnostic and epidemiological purposes to detect RAVs in general. Sanger sequencing and Restriction Fragment Length Polymorphism (RFLP) PCR have also been used to identify RAVs from RT-PCR amplified products of various RVFV isolates (Sall et al., 1999; Ly et al., 2017). Whole genome sequencing remains a relatively expensive and time-consuming method for screening large numbers of samples, and performing sequence analyses in a BSL-3 or BSL-3Ag facility is not always feasible. Basic RT-qPCR melt curve analysis has been used successfully for the identification of influenza reassortants, another segmented RNA virus (Kalthoff et al., 2013; Poon et al., 2010). However, a more complex RT-qPCR melt curve assay design was required for genotyping RVFV reassortants because of its highly conserved genome.
The goal for designing these assays was to enable investigation of RVFV reassortment in the mosquito vector and the mammalian host. A rapid, sensitive and specific as well as cost-effective assay is required to screen a large number of RVFV samples. Therefore, these assays described in this manuscript were developed to detect and differentiate between genetically different RVFV strains, and to identify parental and reassortant viruses. This methodology, while tailored specifically to strains used in our studies, can be easily modified and used for the detection of other RVFV viruses, and for the differentiation of vaccine from wild-type RVFV strains. Moreover, the GT assay described here takes advantage of single nucleotide polymorphism (SNP) differences between strains and uses specialized primers to generate distinct melting temperature profiles for differentiation. This makes it broadly applicable for the detection and differentiation of viral isolates with low nucleotide diversity.
2. Materials and methods
2.1. Virus strains and sample source
The 128B-15 strain isolated from Kenya in 2006 (Sang et al., 2010), the SA01–1322 strain from Saudi Arabia 2001 (Miller et al., 2002) and the attenuated MP-12 vaccine strain of RVFV (Caplen et al., 1985) were used. The Kenya 128B-15 and Saudi Arabian SA01–1322 strains are representative of circulating virulent wild-type RVFV strains, which differ only slightly genetically (<2% for the whole genome). The MP-12 strain represents a licensed live attenuated RVFV vaccine which is used in the field. Therefore, these three relevant RVFV strains were chosen to establish assays to detect viral reassortment. These viruses were grown in Vero-MARU cells, originally obtained from the Middle America Research Unit, and maintained in Dulbecco minimum essential medium (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (Atlanta, biologicals, USA) and 1% penicillin-streptomycin solution (Gibco, USA). For the development of the GT and SS-PCR assays, virus-infected cell supernatants were collected for RNA extraction using the QIAmp viral RNA mini kit (Cat No: 52904, Qiagen, Germany) following the manufacturer’s protocol. RNA was eluted in 60 μl of elution buffer and stored at −80°C until further use.
2.2.1. Primer design for the GT assay
The nucleotide sequence of each genomic segment of RVFV strains 128B-15 (accession numbers: KX096938, KX096939, and KX096940) and MP-12 were aligned with Clustal W method, using vector NTI software version 11.5.3 (Invitrogen, USA). Similarly, a separate alignment and analysis was performed for RVFV strains 128B-15 and SA01–1322 genomic sequences (accession numbers: KX096941, KX096942, and KX096943). Primers were designed using an approach described previously with modifications by Wang and colleagues (Wang et al., 2005). For differentiation of these RVFV strains, two separate forward primers were designed to target genomic regions with SNPs specific for each corresponding strain, and a common reverse primer to target a conserved region shared by the two RVFV strains. For the M and S segments of the 128B-15 and SA01–1322 strains, separate reverse primers were designed to target genomic regions with SNPs and a common forward primer to target a conserved region between the two strains. Primer sets were designed for each of the three RVFV genomic segments targeting each pair of strains in the samples analyzed. The primers that performed the best were selected for GT assays (Table 1).
Table 1.
RT-qPCR primer sets used for genotyping L, M, and S segments of RVFV strains. The underlined nucleotides were the SNPs identified. The short and long GC clamp nucleotides are in bold.
| Set of primers | RVFV strain detection & differentiation | Genome segment | Primer sequences (5’ – 3’) | Product size (bps) | Volume of primers 10 μM (μl)/reaction |
|---|---|---|---|---|---|
| #1 | 128B-15 and MP-12 | L | 1-Low Tm forward (128B-15) GCGGGCACAGCTTTTCTCCCCAGATCATA |
81 | 0.1 |
| 2-High Tm forward (MP-12) GCGGGCAGGGCGGCACAGCCTTTCTCCCCAGACCACG |
89 | 0.2 | |||
| 3-Common reverse GCCCACAAGTCACTAGAGCATGCA |
0.2 | ||||
| #2 | 128B-15 and MP-12 | M | 1-High Tm forward (128B-15) GCGGGCAGGGCGGCGGATTACAGCCTTCATCAGATGGGTG (MP-12) |
67 | 0.2 |
| 2-Low Tm forward GCGGGCGGATCACAGCCTTCATCAGATGGATA |
59 | 0.2 | |||
| 3-Common reverse TGCCACTCTGGCAACCATCTTCTT |
0.2 | ||||
| #3 | 128B-15 and MP-12 | S | 1-Low Tm forward (128B-15) GCGGGCTGCAAGATTACCAATGATTTAGAA |
97 | 0.1 |
| 2-High Tm forward (MP-12) GCGGGCAGGGCGGCTGCAAGATCACCAATGATCTAGAG |
105 | 0.2 | |||
| 3-Common reverse GGCACAGGTCAATCCCTCTGAGG |
0.2 | ||||
| #4 | 128B-15 and SA01-1322 | L | 1-High Tm forward (128B-15) GCGGGCAGGGCGGCGATTAAACGACTACTGGGTTCTGGA |
71 | 0.4 |
| 2-Low Tm forward (SA01–1322) GCGGGCAATTAAACGATTACTAGGTTCTGGG |
63 | 0.2 | |||
| 3-Common reverse TTGCTGGAAGTGGGCTTGATTAGC |
0.4 | ||||
| #5 | 128B-15 and SA01-1322 | M | 1-High Tm reverse (128B-15) GCGGGCAGGGCGGCCAAGAACCCTTGCCGAGGGATC |
125 | 0.4 |
| 2-Low Tm reverse (SA01–1322) GCGGGCCAAGAACCCTTGCCGAGGAATT |
117 | 0.2 | |||
| 3-Common forward GACGCAGAGGGCATTTCAGGC |
0.4 | ||||
| #6 | 128B-15 and SA01-1322 | S | 1-Low Tm reverse (128B-15) GCGGGCGTGTAAGCCAACAAAGGAGTCTTCTAA |
170 | 0.1 |
| 2-High Tm reverse (SA01–1322) GCGGGCAGGGCGGCGTGTAAGCCAACAAAGGAGTCCTCTAG |
178 | 0.2 | |||
| 3-Common forward AAGCCATATCCTGGCCTCTTGGAG |
0.2 |
Tm=melting temperature
2.2.2. Genotyping (GT) assay
Each GT assay reaction consist of 3 primers in a single RT-PCR reaction for each segment with one-step RT-qPCR q-script XLT (2x) master mix (Quantabio, USA), and the sample template. The volumes of each primer solution (10 μM) used for the GT assay are shown in Table 1. Each primer set was mixed with 10 μl of q-script XLT (2x) mix, 1 μl of Eva green (20x) dye (VWR, USA), 2.5 μl of RNA template, and nuclease-free water up to a total reaction volume of 20 μl. All the PCR reactions were carried out on the CFX96 Real-Time thermocycler (Bio-Rad, Hercules, CA, USA). The thermocycling conditions for the GT assay are as follows: cDNA synthesis at 50 °C for 15 min followed by denaturation at 95 °C for 10 min. Polymerase chain reaction (PCR) was then initiated with denaturation at 95 °C for 30 seconds followed by annealing at 65 °C for 30 seconds and extension at 72 °C for 30 seconds. The PCR amplification cycle was repeated 40 times and the amplified PCR products denatured again at 95 °C for 30 seconds and extended at 72 °C for 30 seconds. The products were then subjected to melt curve analysis by increasing temperatures from 70 °C to 95 °C at the increment of 0.2 °C change every 10 seconds. The generated melt curves were analyzed using CFX 3.1 software (Bio-Rad, USA). Genotyping assays were repeated more than three times in duplicate, and the average melting temperatures with standard deviation (SD) calculated.
2.3. Restriction enzyme (RE) analysis and Sanger sequencing
To validate GT assays results, RE analysis and Sanger sequencing was performed on the genotyped samples. Unique RE sites were first mapped to the L, M and S segments of the three RVFV strains using vector NTI software version 11.5.3 (Invitrogen, USA). The following regions spanning RVFV genomic nucleotide positions 4,874–5,532, 514–1,201, and 248–1,300 of the L, M and S segments, respectively, were amplified using a one-step RT-PCR kit (Superscript III; Thermo Fischer Scientific, USA). All the thermocycling conditions were carried out on C1000 Touch Thermal cycler (Bio-Rad, USA). Briefly, 5 μl of the extracted viral RNA was mixed with 1 μl of forward primer and 1 μl of reverse primer (Table 2) for each of the L, M and S segments in separate tubes, and heat denatured at 65°C for 5 min. The denatured RNA was snap cooled on ice, and then the RT-PCR mixture was added to each tube. The target regions were amplified by following the manufacturer’s instruction, with an annealing temperature of 58 °C for 30 seconds. The PCR amplification cycle was repeated 40 times. The amplicons were then purified using a QIAquick PCR purification kit (Qiagen, Germany), and 500 ng of amplified products for each segment were digested with 1 μl of the appropriate RE summarized in Table 2. The amplified L products were digested with EcoR V, and the S products digested with Bam HI Res to differentiate the three RVFV strains. The amplified M products were digested with Afl II RE to differentiate the 128B-15 and MP-12 strains and digested with Dra I RE to differentiate the 128B-15 from SA01–1322 strains. Following an hour of digestion, the products were resolved on a 1.5% agarose gel stained with SYBR safe dye (Thermo Fischer Scientific, USA) in 1x TBE for 1 hour and the gel imaged using a gel doc imager (Bio-Rad, USA). As a final confirmation, the amplified products were also submitted to Sanger sequencing.
Table 2.
Primers used for RT-PCR amplification of L, M and S segments of RVFV strains for restriction enzyme (RE) analysis, sequencing and SS-PCR.
| Primer name | Sequence (5’- 3’) | PCR product size (bps) | REs | Expected RE digestion products size (bps) | ||
|---|---|---|---|---|---|---|
| 128B-15 | MP-12 | SA01-1322 | ||||
| L- Fwd | TTATCCGTGACAATTTCTCCCG CATCTCCACCTCTTCCTTTCTCAG |
681 |
EcoR
V |
681 | 388 293 |
388 293 |
| L- Rev | ||||||
| M- Fwd | TCAGAAACAGACCAGGGAAGGG AGCTCCCTCTTGGTCTGACC |
724 | Dra I | 572 152 |
N/A | 724 |
| M- Rev | AflII | 724 | 609 115 |
N/A | ||
| S- Fwd | TTCCCATACCGAGTCGGACTTG TTTGGTCGTCTTGAGTGAGTGGC |
1074 |
Bam
HI |
521 553 |
964 110 |
1074 |
| S- Rev *MP-12 L- Fwd | CCCTCTTCAGGGACTAGATGTG AATTCAGATATGTGATCCACATCG |
333 | N/A | N/A | N/A | N/A |
| *MP-12 L- Rev | ||||||
SS-PCR primers and the underlined nucleotides are the SNPs specific to MP-12; N/A=not applicable
2.4. Strain specific qPCR (SS-PCR) assay
To enhance detection of a low copy viral strain such as MP-12 in mixed samples with a high copy strain such as Kenya 128B-15, we designed this SS-PCR assay. The L segment was chosen as the target segment, since it has more nucleotide variation between MP-12 and Kenya 128B-15. The L segment sequence of strains 128B-15 and MP-12 were aligned using ClustalW on vector NTI software version 11.5.3 (Invitrogen, USA). The alignment was scanned for SNPs and regions with greater nucleotide variation were identified. A pair of primers (Table 2) having SNPs at the 3’end specific to MP-12 L segment, and amplifying a target region spanning nucleotide positions 1,043–1,376, was selected. The SS-PCR assay was performed in two separate steps. First, cDNA synthesis was performed with MP-12 strain-specific forward primer (MP-12 L-Fwd), and second, the cDNA was then used for PCR and the amplified PCR products were subjected to melt curve analysis. The Superscript III first strand synthesis kit (Thermo Fischer Scientific, USA) was used for cDNA synthesis. The reaction volumes for cDNA synthesis consisted of 0.5 μl (10 μM) of primer (MP-12 L- Fwd) mixed with 1.0 μl of dNTP (10 mM), and 8.5 μl of nuclease free water. The extracted RNA (4 μl) was added to the primer-reaction mix and incubated at 65 °C for 5 min, before being snap cooled on ice for 1 minute. Added to the reaction were 2 μl of cDNA buffer (10x), 4 μl of MgCl2 (25 mM), and 0.5 μl of RT, cDNA was synthesized at 50 °C for 50 minutes, and the RT was inactivated at 85 °C for 3 minutes. Quantitative PCR was performed using qScript PCR 2x mix (Quantabio, USA) with the following reaction volumes in the CFX96 Real-Time thermocycler (Bio-Rad, Hercules, CA, USA). Each reaction consisted of 3 μl of synthesized cDNA, 10 μl of qScript PCR buffer, 0.5 μl of MP-12 L-Fwd and MP-12 L-Rev primers (10 μM, Table 2), 1 μl of Eva green (20x) dye and 4 μl nuclease-free water. The thermocycling conditions are described in section 2.2.2 above, with an annealing temperature of 58 °C for 30 seconds. The amplified products were resolved in 2% agarose gel stained with SYBR safe dye (Thermo Fischer Scientific, USA) in 1x TBE for 1 hour and the gel was imaged using a gel doc imager (Bio-Rad, USA). The amplified products were purified by QIAquick PCR purification kit (Qiagen, Germany), and submitted to Genewiz (USA) for Sanger sequencing using MP-12 L-Rev (Table 2).
3. Results
3.1. Establishment of the genotyping (GT) assays
To distinguish between RVFV strains, we first identified regions containing multiple SNPs between each RVFV strain. Then, separate sets of specialized primers were designed to differentiate the L, M and S segments of the RVFV strains 128B-15 and MP12, or 128B-15 and SA01–1322 (Table 1). Our GT assays were able to resolve each strain segment into distinguishable melt curves profiles as shown in Fig. 1A–F. The average peak melting temperatures obtained by the GT assays for each RVFV strain segment were summarized in Table 3. The specificity of these assays (i.e. very low SD of 0.08 to 0.18 °C) allowed for differentiation between strains with only a 1 °C difference between melting temperatures, as demonstrated by the melt temperatures of the M and S segments of RVFV strains 128B-15 and SA01–1322 (Fig. 1E, F). The no template control (NTC) for some of the reactions also generated a melt curve that sometimes overlapped with the melt curves generated for each of the samples, which could be due to the formation of primer-dimers in the absence of template RNA (Fig. 1A–C). To avoid false positive results, only the samples with Ct value below 29, the lowest value of the NTC produced in these assays, were considered. Rarely, two uneven peaks from the amplicons of the RVFV strain 128B-15 M segment were observed, possibly as a result of lower concentration of the 128B-15 template in the reaction (Fig. 1E). Nonetheless, the primary peak for 128B-15 M segment was still clearly distinguishable from the single peak of SA01–1322 M segment amplicons (Fig. 1E).
Fig. 1. Results of the RVFV strains RT-qPCR genotyping assays:
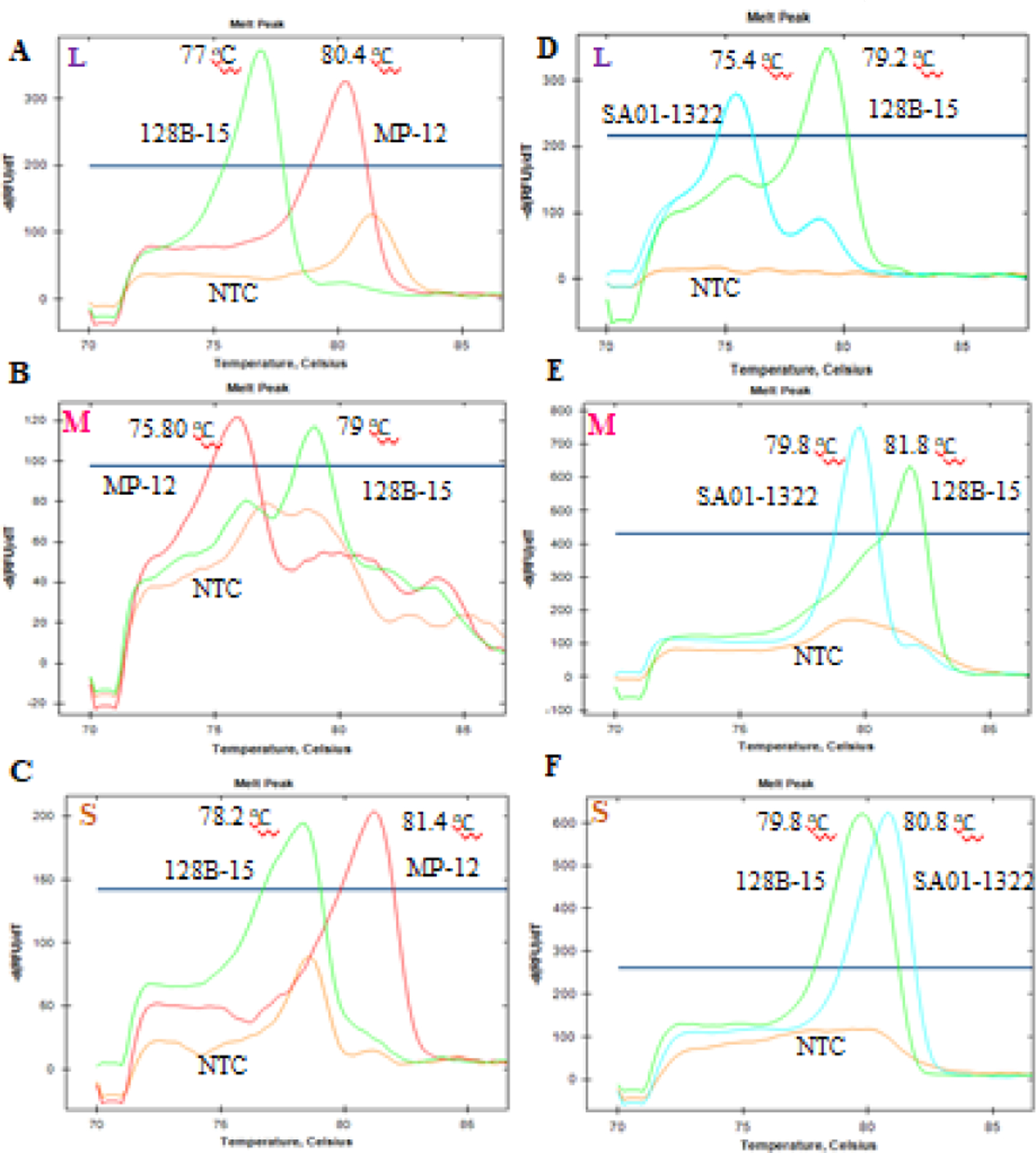
Representative melt curves and peak melting temperatures from the PCR amplicons of L, M and S segments of strains 128B-15, MP-12, and SA01–1322, are indicated here. Each melt curve was generated from a single tube PCR reaction with GT assay primers in the presence of a single strain of RVFV viral RNA. Each plot represents the three melt curves generated from three separate tube reactions with respective RVFV strains RNA and no template control (NTC). The left panel (A, B, C) top to bottom was the L, M and S segment genotyping PCR results from 128B-15 and MP-12 strain, and right panel (D, E, F) top to bottom was the L, M and S segment genotyping PCR results from 128B-15 and SA01–1322 strain. Green tracing indicates strain 128B-15, red tracing for strain MP-12 and light blue for SA01–1322 strain.
Table 3.
RT-qPCR genotyping assays: The peak average melting temperatures and standard deviation (SD) were obtained from all the RT-qPCR genotyping assay.
| Genomic segment | Strain | Average peak melting temperature ± SD (°C) | Strain | Average peak melting temperature ± SD (°C) |
|---|---|---|---|---|
| L | 128B-15 | 76.97±0.08 | 128B-15 | 79.23±0.08 |
| MP-12 | 80.33±0.1 | SA01-1322 | 75.4 | |
| NTC | ND | NTC | ND | |
| M | 128B-15 | 79 | 128B-15 | 81.63±0.15 |
| MP-12 | 75.8 | SA01-1322 | 79.7±0.11 | |
| NTC | ND | NTC | ND | |
| S | 128B-15 | 78.4±0.18 | 128B-15 | 79.67±0.1 |
| MP-12 | 81.33±0.1 | SA01-1322 | 80.77±0.08 | |
| NTC | ND | NTC | ND |
ND=not detected, below the threshold of detection; NTC=no template control.
3.3. Genotype confirmation by RE analysis and Sanger sequencing
Genotypes identified by GT assays were confirmed by RE analysis of the amplified products of L (681 bps), M (724 bps) and S (1074 bps) segments of each RVFV strain. The REs EcoR V, Dra I, Afl II and Bam HI were used based on their ability to produce unique strain-specific RE digestion profiles as shown in Table 2, with the corresponding predicted product sizes. The expected bp size of the RE digested products of 128B-15 and MP-12 co-infected samples for 128B-15 were as follows: L: 681, M: 724 and S: 553 and 521; and for MP-12: L: 388 and 293, M: 572 and 152, S: 964 and 110. Similarly, the expected RE digested products of 128B-15 and SA01–1322 co-infected samples for 128B-15 were as follows: L: 681, M: 572 and 152, S: 553 and 521; and for SA01–1322: L: 388 and 293, M: 724, S: 1074. The RE analysis revealed all predicted RE products were confirmed, except for the small 115 and 110 bp products of the MP-12 strain M and S segments (Fig. 2A) and the 152 bp product of the 128B-15 strain M segment which were not detected (Fig. 2B). Additionally, undigested products for the L and S segments of MP-12 were also observed (Fig. 2A). Importantly, the RE analysis enabled the ability to distinguish each of the RVFV strains. In addition, the strain identity of the amplified products was also confirmed by Sanger sequencing (data not shown).
Fig. 2. Results of the RVFV strains restriction enzyme analysis:
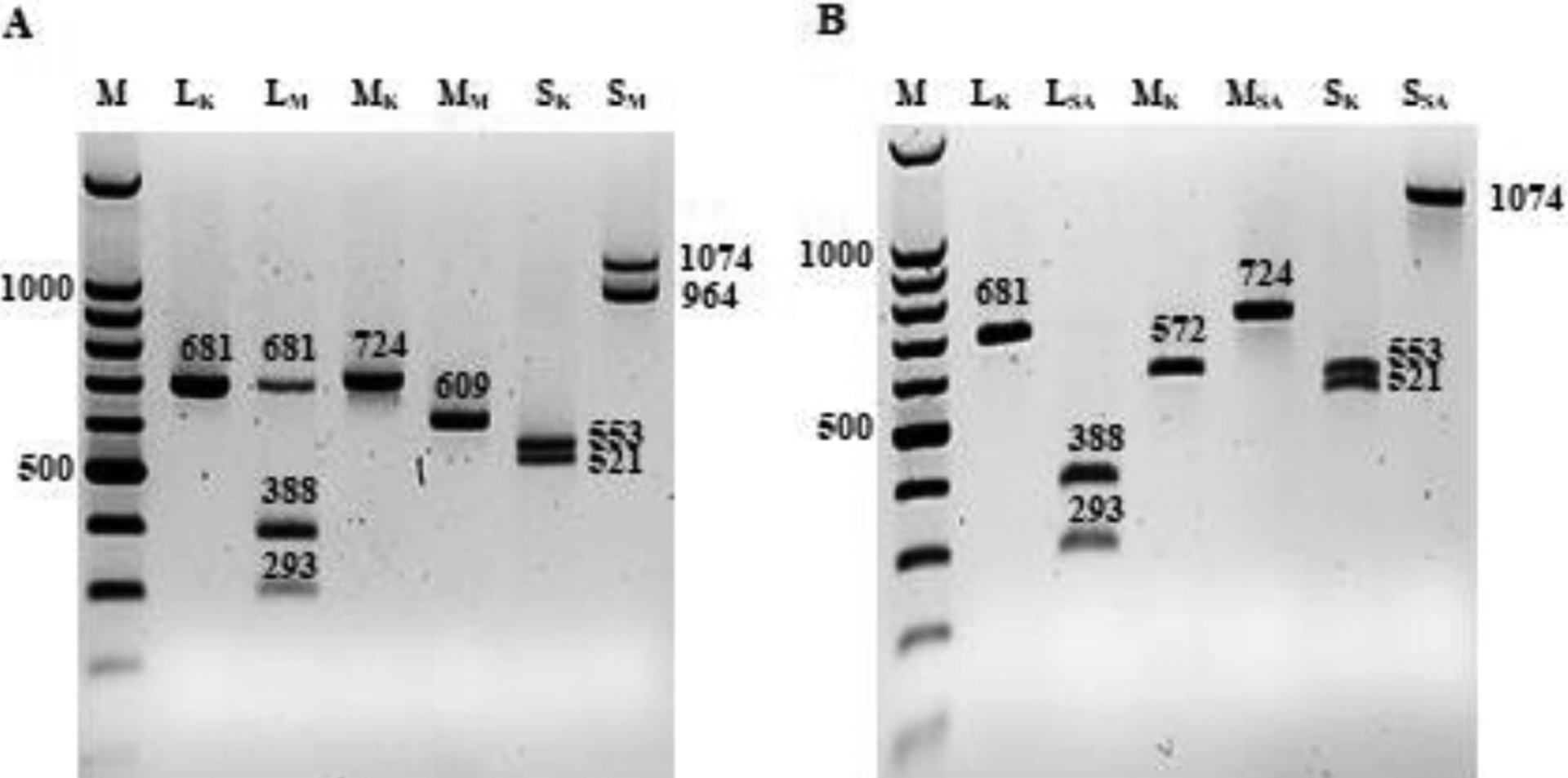
(A) Restriction enzyme (RE) analysis of strains 128B-15 and MP-12, and 128B-15 and SA01–1322 strains L, M and S segments with EcoRV, AflII, or Dra I, and Bam HI, respectively. Lane M is a 1kp plus ladder, lane 1 and 3 shows 128B-15 L (LK) and M (MK) restriction fragments, respectively; lane 2 and 4 shows MP-12 L (LM) and M segment (MM) restriction fragments, respectively. Lane 5 and 6 shows strain 128B-15 S segment (SK) and strain MP-12 S segment (SM) restriction fragments, respectively. (B) Lane 7 shows 128B-15 L segment (LK) restriction fragments, lane 8 shows strain SA01–1322 L segment (LSA) restriction fragments, Lane 9 is 128B-15 M segment (MK) restriction fragments, lane 10 is SA01–1322 M segment (MSA) restriction fragments, lane 11 is 128B-15 S segment (SK) restriction fragments and lane 12 is strain SA01–1322 S segment (SSA) restriction fragments.
3.4. Strain Specific (SS)-PCR assay for detection of a low titer RVFV strain
The SS-PCR assay was designed to detect the low copy MP-12 strain in mixed samples with the high copy Kenya 128B-15 strain. The specificity of the SS-PCR assay was first tested on individual, and then on mixed RNA samples with the 128B-15 and MP-12 strains. When tested with MP-12 RNA, the SS-PCR amplified the MP-12 L segment with a unique melting temperature of 85.4 °C (Fig. 3E). Importantly, poor to no amplification was detected when this assay was tested on the Kenya 128B-15 RNA (Fig. 3E). Next, to evaluate the specificity of SS-PCR on mixed RNA samples, the 128B-15 and MP-12 RNAs (normalized to 21Cq, Fig. 3A) were mixed at ratios of 5:1, 10:1 and 25:1 by volume/volume. When subjected to the GT assay on these mixed samples, the GT assay melt curve results were biased towards 128B-15, which was present at higher copy compared to MP-12 (Fig. 3 B–D). In contrast, testing the SS-PCR assay on the same samples, results clearly revealed the presence of a single melt curve corresponding to MP-12 strain, but no signal of the 128B-15 strain (Fig. 3 F–H), respectively. Identity of amplified products were confirmed by Sanger sequencing (data not shown). Overall, our SS-PCR assay was highly specific for detection of the MP-12 L segment in individual and mixed RNA samples.
Fig. 3. Results of the RVFV strain-specific qPCR assay:
Representative melt curves and melting temperatures from the GT assay (A-D) and SS-PCR (E-H) amplicons of L segment of strains 128B-15 and MP-12, respectively, are indicated here. Left panel top to bottom (A) melt curves were generated from a single tube PCR reaction with GT assay L primers in the presence of RVFV viral RNAs, and (B, C, D) melt curves were generated from a single tube PCR reaction with GT assay L primers in the presence of viral RNA of strains 128B-15 and MP-12 mixed at various titer ratios (5:1, 10:1, 25:1) as indicated in each plot graph. NTC (no template control) represents melt curve generated from a single tube PCR reaction with GT assay L primers but no viral RNA template. Right panel, top to bottom, (E) melt curves were generated from a single tube PCR reaction with SS-PCR primers in the presence of viral RNA from a single strain of RVFV and (F, G, H) melt curves were generated from a single tube PCR reaction with SS-PCR primers in the presence viral RNA from strains 128B-15 and MP-12 mixed at various titer ratios as indicated in each plot graph. Green tracing indicates strain 128B-15 and red tracing indicates strain MP-12.
4. Discussion
To study RVFV reassortment in experimental co-infection models, we developed genotyping (GT) assays, which were able to differentiate between RVFV strains. Our GT assays consistently identified L, M and S segments of RVFV strains 128B-15, MP-12, and SA01–1322, by distinct melt curve analyses. The GT assays have shown 100% specificity, as confirmed by RE analysis and Sanger sequencing. Moreover, the GT assays were specific enough to differentiate target regions with a minimum of 2–4 nucleotide variation. We recently applied these GT assays to identify L, M, and S reassortant RVFV viruses that emerged after experimental co-infections between the RVFV strains 128B-15 and MP-12, and 128B-15 and SA01–1322, in mammalian and insect vector systems (data not shown). The results revealed that our assays were highly specific in identifying all three RVFV strains from these different samples in our ongoing studies. Overall, the developed GT assays are able to detect and differentiate all segments of three highly conserved RVFV strains, are practical for use in BSL-3 containment. Based on our cost-comparison analysis, the GT assay is approximately 5 or 10 times less expensive than Sanger and Next Generation Sequencing methods, respectively, and relatively inexpensive compared to probe-based methods (Paudel et al., 2011).
Additionally, we also developed a strain-specific PCR (SS-PCR) assay to detect RVFV strains that could be present at a low titer in mixed samples. The assay was highly specific in identification of the MP-12 L segment from the mixed RVFV samples, as confirmed by Sanger sequencing. This assay could be applied to samples derived from co-infections, where one strain has more replicative fitness over another. For example, the attenuated MP-12 vaccine strain does not replicate as well as wild type RVFV strains (i.e. 128B-15) in animals, resulting in absence or low viremia and RNAemia in infected animals (Morrill et al., 1997, 1991; Wilson et al., 2014). Furthermore, the SS-PCR assay could also be applied for determining and differentiating infected animals from vaccinated animals by specifically designing primers targeting the L or any other segment of any non-gene deleted vaccine (e.g. MP-12 or Smithburn) and circulating field strain.
Our GT assays have the following limitations: 1) it requires detailed knowledge of the nucleic acid sequences of all targets being investigated for the design of the assay primers, and 2) for studies investigating virus reassortment, samples must first be plaque purified to isolate RNA of individual viruses, which are then subjected to the genotyping analysis.
5. Conclusions
We conclude that the RVFV-specific GT and SS-PCR assays described here are both economical and practical methods for screening large number of samples in a quick and easy 96-well format. Each of these assays can be custom designed for specific virus strains that require differentiation, even if they carry highly conserved genome sequences.
Supplementary Material
Highlights.
Assays were developed to detect genomic segments of parental and reassortant RVFVs
RVFV strains with high genetic homology were clearly distinguished by these assays
A strain-specific RT-PCR assay was developed to detect low-titer RVFV mixed samples
With minor modifications, these assays can identify and differentiate other RVFVs
Acknowledgment
This work is funded in part by the Department of Homeland Security Center of Excellence for Emerging and Zoonotic Animal Diseases (CEEZAD), Grant No. 2010-ST061-AG0001, Kansas State NBAF Transition Funds, USDA, Agricultural Research Service and AMP Core of the CEZID COBRE. We thank Dr. Jayme Souza-Neto and Dr. David A. Meekins for critical reading of the manuscript and providing feedback.
We would like to acknowledge Richard Bowen CSU for wild-type RVFV strains used in these studies and Michael Turell, USAMRIID for MP-12.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The conclusions in this report are those of the authors and do not necessarily represent the views of the USDA. USDA is an equal opportunity provider and employer.
CRediT authorship contribution statement
Velmurugan Balaraman: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Visualization, and Writing. Natasha N Gaudreault: Conceptualization, Methodology, Investigation, Review, and Editing. Jessie D Trujillo: Methodology, Investigation, Review, and Editing. Sabarish V Indran: Methodology, Review, and Editing. William C Wilson and Juergen A Richt: Conceptualization, Investigation, Resources, Review, and Editing, Supervision.
Declaration of Competing Interest
All the authors declare that there is no conflict of interest. J.A.R. is inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections, owned by Kansas State University, KS, or the Icahn School of Medicine at Mount Sinai, New York.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anderson GW, Saluzzo JF, Ksiazek TG, Smith JF, Ennis W, Thureen D, Peters CJ, Digoutte JP, 1989. Comparison of in vitro and in vivo systems for propagation of rift valley fever virus from clinical specimens. Res. Virol 10.1016/S0923-2516(89)80090-1 [DOI] [PubMed] [Google Scholar]
- Faburay B, LaBeaud A, McVey D, Wilson W, Richt J, 2017. Current Status of rift valley fever vaccine development. Vaccines. 10.3390/vaccines5030029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire CCM, Iamarino A, Soumaré POL, Faye O, Sall AA, Zanotto PMA, 2015. Reassortment and distinct evolutionary dynamics of Rift Valley Fever virus genomic segments. Sci. Rep 5, 1–7. 10.1038/srep11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplen H, Peters CJ, Bishop DHL, 1985. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J. Gen. Virol 66, 2271–2277. 10.1099/0022-1317-66-10-2271 [DOI] [PubMed] [Google Scholar]
- Gaudreault NN, Indran SV, Balaraman V, Wilson WC, Richt JA, 2019. Molecular aspects of rift valley fever virus and the emergence of reassortants. Virus Genes. 10.1007/s11262-018-1611-y [DOI] [PubMed] [Google Scholar]
- Gerken KN, et al. (2022). “Paving the way for human vaccination against Rift Valley fever virus: A systematic literature review of RVFV epidemiology from 1999 to 2021.” PLoS Negl Trop Dis 16(1): e0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, Swanepoel R, 2011. Molecular epidemiology of Rift Valley fever virus. Emerg Infect Dis 17:2270–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Makino S, 2011. The pathogenesis of rift valley fever. Viruses 3, 493–519. 10.3390/v3050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indran SV, Ikegami T, 2012. Novel approaches to develop Rift Valley fever vaccines. Front. Cell. Infect. Microbiol 10.3389/fcimb.2012.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D, Beer M, Hoffmann B, 2013. High resolution melting analysis: Rapid and precise characterisation of recombinant influenza A genomes. Virol. J 10.1186/1743-422X-10-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum KJ, Britch SC, Anyamba A, 2016. Rift Valley Fever: An Emerging mosquito-borne Disease. Annu. Rev. Entomol 10.1146/annurev-ento-010715-023819 [DOI] [PubMed] [Google Scholar]
- Ly HJ, Lokugamage N, Nishiyama S, Ikegami T, 2017. Risk analysis of inter-species reassortment through a rift valley fever phlebovirus MP-12 vaccine strain. PLoS One. 10.1371/journal.pone.0185194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Godsey MS, Crabtree MB, Savage HM, Al-Mazrao Y, Al-Jeffri MH, Abdoon AMM, Al-Seghayer SM, Al-Shahrani AM, Ksiazek TG, 2002. Isolation and genetic characterization of rift valley fever virus from Aedes vexans arabiensis, Kingdom of Saudi Arabia. Emerg. Infect. Dis 10.3201/eid0812.020194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JC, Carpenter L, Taylor D, Ramsburg HH, Quance J, Peters CJ, 1991. Further evaluation of a mutagen-attenuated rift valley fever vaccine in sheep. Vaccine 9, 35–41. 10.1016/0264-410X(91)90314-V [DOI] [PubMed] [Google Scholar]
- Morrill JC, Mebus CA, Peters CJ, 1997. Safety of a mutagen-attenuated rift valley fever virus vaccine in fetal and neonatal bovids. Am. J. Vet. Res 58, 1110–1114. [PubMed] [Google Scholar]
- Paudel D, Jarman R, Limkittikul K, Klungthong C, Chamnanchanunt S, Nisalak A, Gibbons R, Chokejindachai W, 2011. Comparison of real-time SYBR green dengue assay with real-time taqman RT-PCR dengue assay and the conventional nested PCR for diagnosis of primary and secondary dengue infection. N. Am. J. Med. Sci 3, 478–485. 10.4297/najms.2011.3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweska JT, Burt FJ, Anthony F, Smith SJ, Grobbelaar AA, Croft JE, Ksiazek TG, Swanepoel R, 2003. IgG-sandwich and IgM-capture enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in domestic ruminants. J. Virol. Methods 10.1016/S0166-0934(03)00228-3 [DOI] [PubMed] [Google Scholar]
- Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Moussiaux N, Thiry E, Roger F, 2015. A Systematic scoping study of the socio-sconomic impact of rift valley fever: Research gaps and needs. Zoonoses Public Health 62, 309–325. 10.1111/zph.12153 [DOI] [PubMed] [Google Scholar]
- Poon LLM, Mak PWY, Li OTW, Chan KH, Cheung CL, Ma E, Yen HL, Vijaykrishna D, Guan Y, Peiris JSM, 2010. Rapid detection of reassortment of pandemic H1N1/2009 influenza virus. Clin. Chem 10.1373/clinchem.2010.149179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sall AA, Macondo EA, Sène OK, Diagne M, Sylla R, Mondo M, Girault L, Marrama L, Spiegel A, Diallo M, Bouloy M, Mathiot C, 2002. Use of reverse transcriptase PCR in early diagnosis of rift valley fever. Clin. Diagn. Lab. Immunol 10.1128/CDLI.9.3.713-715.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sall AA, Zanotto A, Sene OK, Zeller HG, Digoutte JP, Thiongane Y, Bouloy M, 1999. Genetic reassortment of rift valley fever virus in Nature Genetic Reassortment of Rift Valley Fever Virus in Nature. J. Virol 10.1016/j.csm.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang R, Kioko E, Lutomiah J, Warigia M, Ochieng C, O’Guinn M, Lee JS, Koka H, Godsey M, Hoel D, Hanafi H, Miller B, Schnabel D, Breiman RF, Richardson J, 2010. Rift valley fever virus epidemic in Kenya, 2006/2007: The entomologic investigations. Am. J. Trop. Med. Hyg 83, 28–37. 10.4269/ajtmh.2010.09-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, Saluzzo JF, Tammariello RF, Smith JF, 1990. Generation and transmission of Rift Valley fever viral reassortants by the mosquito Culex pipiens. J. Gen. Virol 10.1099/0022-1317-71-10-2307 [DOI] [PubMed] [Google Scholar]
- Wang J, Chuang K, Ahluwalia M, Patel S, Umblas M, Mirel D, Higuchi R, Germer S, 2005. High-throughput SNP genotyping by single-tube PCR with Tm-shift primers. Biotechniques. 10.2144/000112028 [DOI] [PubMed] [Google Scholar]
- Williams R, Ellis CE, Smith SJ, Potgieter CA, Wallace D, Mareledwane VE, Majiwa PAO, 2011. Validation of an IgM antibody capture ELISA based on a recombinant nucleoprotein for identification of domestic ruminants infected with Rift Valley fever virus. J. Virol. Methods 10.1016/j.jviromet.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Wilson WC, Bawa B, Drolet BS, Lehiy C, Faburay B, Jasperson DC, Reister L, Gaudreault NN, Carlson J, Ma W, Morozov I, McVey DS, Richt JA, 2014. Evaluation of lamb and calf responses to Rift Valley fever MP-12 vaccination. Vet. Microbiol 172, 44–50. 10.1016/j.vetmic.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Wilson WC, Kim IJ, Trujillo JD, Sunwoo SY, Noronha LE, Urbaniak K, McVey DS, Drolet BS, Morozov I, Faburay B, Schirtzinger EE, Koopman T, Indran SV, Balaraman V, Richt JA, 2018. Susceptibility of white-tailed deer to Rift valley fever virus. Emerg. Infect. Dis 10.3201/eid2409.180265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


