Abstract
Background:
More than four decades after the eradication of smallpox, the ongoing 2022 monkeypox outbreak and increasing transmission events of other orthopoxviruses necessitate a deeper understanding of the global distribution of susceptibility to orthopoxviruses, as shaped by the landscape of smallpox vaccination pre-eradication.
Methods:
We characterize the fine-scale global spatial landscape of smallpox vaccine protection by integrating data on current demography with historical smallpox vaccination program features (coverage and cessation dates) from eradication documents and published literature. We analyze this landscape to identify the factors that are most associated with geographic heterogeneity in current vaccination coverage, and consider how smallpox vaccination history may translate into age-specific susceptibility profiles for orthopoxviruses under different vaccination effectiveness scenarios.
Findings:
We find significant global spatial heterogeneity in the landscape of smallpox vaccination, with vaccination coverage estimated to be 7 to 60% within admin-1 regions globally, with negligible uncertainty (99.6% of regions have a standard deviation less than 5%). We identify that geographic variation in vaccination coverage is driven largely by differences in sub-national demography. Additionally, we highlight that susceptibility for orthopoxviruses is highly age-specific based on age at cessation and age-specific coverage, but that the age profile is consistent across vaccine effectiveness values.
Interpretation:
The legacy of smallpox eradication can be observed in the current landscape of smallpox vaccine protection. The strength and longevity of the smallpox vaccination campaign in each nation shape the epidemiological landscape today and reveal significant geographic variation in orthopoxvirus susceptibility. Our work alerts public health decision-makers to non-endemic regions that may be at greatest risk in the case of widespread and sustained transmission in the 2022 monkeypox outbreak and highlights the importance of demography and fine-scale spatial dynamics in predicting future public health risks from orthopoxviruses.
Funding:
Research reported in this publication was supported by the National Institutes of Health under award number R01GM123007 (SB) and National Science Foundation DEB-1557022 (JOL-S).
Keywords: monkeypox, smallpox, orthopoxvirus, immunity, susceptibility, vaccination, spatial heterogeneity, immune landscape
Introduction
More than four decades after the landmark eradication of smallpox, the ongoing 2022 outbreak of monkeypox in over 70 non-endemic countries threatens global health. If the outbreak is not contained and widespread transmission ensues, the spatial distribution of impacts will be shaped by heterogeneity in the smallpox immune landscape, due to cross-protection conferred by the Vaccinia-based smallpox vaccine. Multiple factors influence this immune landscape, including country-specific timelines of smallpox endemicity and elimination, protocols for routine smallpox vaccination, and demographic and migration patterns post-eradication. However, global estimates of the current population immunized against smallpox are unavailable, limiting our ability to predict large-scale transmission dynamics of the current monkeypox outbreak or of potential future outbreaks of other Orthopoxvirus genus viruses. We seek to characterize the current global spatial heterogeneity in smallpox vaccination history and orthopoxvirus susceptibility as driven by geographic variation in demography, cessation dates for routine smallpox vaccination, and vaccination coverage.
Smallpox afflicted humans for millennia and remains the only endemic human disease to be successfully eradicated. The World Health Organization (WHO) committed to an intensified global smallpox eradication campaign in 1967, particularly focusing on countries with high rates of endemic smallpox across Africa, Asia, and South America.1 Although the WHO initially set a target vaccination rate of 80% worldwide, novel vaccination strategies helped eliminate smallpox country-by-country while also introducing spatial heterogeneity in vaccination coverage based on the vaccination campaign of each nation. The jet injector enabled mass vaccination efforts, the bifurcated needle stretched vaccine stocks further, and the use of targeted ring vaccination allowed for successful elimination at lower vaccination rates.1 Progress towards elimination in each country was tracked via scar surveys for vaccination and pockmark surveys for infection (both considered validated measures of immunity).1,2 In 1977, just ten years into the intensified eradication campaign, the last naturally-occurring case of smallpox occurred in Somalia; the WHO declared smallpox eradicated in 1980.3 There have been no naturally-occurring smallpox cases since, but the use of smallpox as a biological weapon remains a risk.
Monkeypox is an orthopoxvirus closely related to but less severe than smallpox. Unlike smallpox, monkeypox was historically geographically limited to central and western Africa with relatively small outbreaks of zoonotic origin.4 Studies during a period of intensified WHO monkeypox surveillance in the 1980s found that previous smallpox vaccination led to reduced secondary attack rates and reduced symptom severity upon monkeypox infection, estimating 85% effectiveness of the Vaccinia-based smallpox vaccine against monkeypox infection and disease.5–7 More recent studies support these findings, reporting continued evidence that past smallpox vaccination prevented clinical monkeypox infection with >80% effectiveness and reduced infection severity in cases that did arise.8–10
Monkeypox outbreaks have risen in frequency and size since routine smallpox vaccination ended and reports of zoonotic transmission of cowpox and buffalopox, two other members of the Orthopoxvirus genus which infect humans, have also increased.8,11–13 Since the Vaccinia-based smallpox vaccine confers some level of immunity against almost all human orthopoxviruses, we expect these other viruses to benefit from the growth in unvaccinated populations.14,15 Mapping global heterogeneity in smallpox vaccination coverage and cessation is thus important to accurately understand the populations most vulnerable to sustained transmission arising from the 2022 monkeypox outbreak, a smallpox biological attack, or future emerging orthopoxvirus outbreaks. However, considering these processes at large spatial scales obscures important geographical heterogeneity that can drive disease transmission dynamics, including potentially high-risk areas where targeted interventions may be needed, and necessitates a fine-scale approach to characterizing the orthopoxvirus susceptibility landscape.16–18
Here, we characterize the current global spatial landscape of smallpox vaccination history and orthopoxvirus susceptibility based on geographical heterogeneity in demography and past smallpox vaccination efforts. We consider age distributions of populations, smallpox vaccination coverage (by age, where available), and smallpox vaccination cessation dates at the national level. We characterize uncertainties in these estimates, and analyze the role of each demographic and vaccination campaign characteristic. While other factors, like contact patterns, remain critical to predicting monkeypox transmission, our work maps the current global landscape of susceptibility to monkeypox and other orthopoxviruses, providing key information to guide public health decision-making for the evolving 2022 outbreak and beyond.
Methods
Our goal is to generate geographic estimates for smallpox vaccine history and in turn, population susceptibility to orthopoxviruses at the sub-national level globally. To achieve this, we generate a database of routine smallpox vaccination campaign cessation dates, estimated smallpox vaccination coverage before cessation, and age compositions for each admin-1 (administrative-level below country, e.g., state in the U.S.) region globally. From this database, we estimate the proportion of each current-day population that is expected to be vaccinated against smallpox. Additionally, we estimate how this vaccination history may translate to susceptibility to orthopoxviruses such as monkeypox, under different scenarios of cross-protection between smallpox vaccination and orthopoxviruses.
Data
For data on smallpox vaccination near eradication, we use Fenner et al.’s landmark book1 documenting the WHO smallpox eradication campaign and country-specific data during the intensified eradication period in Africa, Asia, and South America. For countries in Europe, Central America, the Middle East, and Oceania where intensified eradication efforts were not concentrated, information was sparse and scattered. Available information for these areas was supplemented via searches of WHO eradication documents, Bulletin of the World Health Organization and Morbidity and Mortality Weekly Report digital archives, and published literature and reports via Google Scholar and PubMed for data on smallpox vaccination coverage rates and cessation dates for each country. Search terms included: “smallpox vaccination cessation”, “end of smallpox vaccination”, “stop smallpox vaccination”, “smallpox vaccination coverage”, “smallpox scar surveys”, and “smallpox serum surveys”, combined with each country name.
Our database containing information on smallpox vaccination, our sources, and the quality of evidence for each country can be accessed in Table S1. We report the data quality distribution below and show the spatial distribution in Figure S1.
Vaccination cessation:
Cessation dates reflect the end of routine smallpox vaccination programs after country-specific smallpox elimination or global smallpox eradication. Global vaccine cessation date information was obtained through a literature review for each country (see dates, sources in Table S1). For 31% of countries (49% of the world population), we found direct evidence of the year of cessation. For another 24% of countries (2% of world population), exact cessation year was unavailable but we could estimate it from an approximate date or range provided in the literature; for overseas territories, we used the cessation date of the governing country at the time. If no information on vaccine cessation date was available, the cessation date was assumed to be 1980 based on WHO recommendations to discontinue vaccination that year (44% of countries, 49% of world population), and the lowest data quality grade was recorded (Table S1).1
Vaccination coverage:
Vaccination coverage data was obtained, whenever possible, via scar surveys conducted in each country (Table S1). We note that scar surveys are based on “take” and thus represent efficacious vaccine coverage but were largely conducted during the WHO intensified eradication campaign starting in 1967, and are thus largely unavailable for countries that achieved elimination previously.1,2 Scar survey information was first obtained in Fenner et al.1 or the WHO Final Report,3 then a search of WHO eradication documents and general literature search. If multiple within-country coverages were given in a scar survey, the population-weighted average was taken as the national coverage. If age-specific coverage rates were available, the 5–14 age group coverage was used for all individuals of that age or younger at the time of the scar survey, or born between the scar survey and the cessation date, while the ≥15 age group coverage, if available, or the overall coverage, was used for all individuals born 15 years or more prior to the scar survey (see Appendix S1). If no age-stratified information was available, the overall population coverage was used, if available. If country-specific coverage information was not available (39% of countries, 16% of world population), we performed spatial imputation of coverage based on countries that were spatially proximate with a comparable sociopolitical profile. If such an imputation was not possible (37% of countries, 14% of world population), coverage was assumed to be 80% based on the WHO target vaccination coverage for the eradication campaign,1 and the lowest data quality grade was recorded (Table S1).
Demography:
To estimate global demography at the admin-1-level, we used the most recent admin-1-level age distribution data from the Gridded Population of the World (GPW) dataset19 in 5-year age classes from 2010, as well as admin-1 population sizes from GPW from 2020. We validate our assumption of constant age distributions from 2010 to 2020 at the national scale (Figure S2).
Population vaccination coverage estimates
To estimate smallpox vaccination history for current populations of each admin-1 level region in all countries, we used the smallpox vaccination cessation dates and historical vaccination coverages from our global database for each country, and demographic information at the admin-1 level.
We first estimated the proportion of each 5-year age group that was vaccinated with the smallpox Vaccinia vaccine based on their age at cessation and coverage at cessation within that country (Figures S3–S21). To estimate overall admin-1-level smallpox vaccine history, we took an age-specific population-weighted mean across historical vaccination coverage estimates.
To validate our estimates, we compared our estimated age-specific smallpox vaccination coverage, projected to the appropriate year, to more recent age-specific smallpox scar survey data8,20–22 and antibody study data23 from five countries (see Appendix S4).
We do not include the effect of natural immunity to smallpox in our estimates of protection because incidence levels in individuals alive today was low during the 1970s even in the last smallpox endemic countries (Figures S22–24).
Estimating uncertainty in population vaccination estimates
To quantify the robustness of our population vaccination estimates under varying data quality, we characterized the uncertainty in smallpox vaccination cessation dates and coverage levels, as well as admin-1-level age distributions. We derived uncertainty intervals for coverage and cessation date for each country by placing bounds on plausible values for those inputs, characterizing the part of the interval with the highest likelihood, and parameterizing probability distributions based on these bounds and skew. Uncertainty intervals for cessation dates were defined based on known information about each country’s smallpox vaccination campaign, and aggregate regional data available from source literature. Intervals for vaccination coverage were derived from within-country or regional spatial variability in coverage from scar surveys. For locations without uncertainty information, generous default uncertainty intervals were assumed (see Appendix S2). Additionally, we include variability in age distributions due to noise in demographic data sources and the unavailability of current fine-scale demographic data (see Appendix S2). To integrate these sources of uncertainty, we performed parametric bootstrapping to estimate population vaccination history with uncertainty.
To estimate the effect of data resolution on our estimates, we also conduct a more detailed analysis of the U.S. using finer-scale age distribution data, additional information on immigration, and spatial heterogeneity in vaccination coverage (see Appendix S8).
Estimating the role of demography and vaccination campaigns
To assess the role of each factor in our vaccination history estimates, we removed heterogeneity in a given demographic or vaccination factor and compared the resulting population vaccination estimates against the original estimates. The three scenarios we considered are: (1) all countries ceased routine smallpox vaccination in 1984, the last date that any country stopped vaccination; (2) all countries achieved 100% vaccination coverage before cessation, removing heterogeneity in coverage; (3) all administrative regions have the same global average age distribution, removing admin-1 level heterogeneity in age.
Population susceptibility estimates
To demonstrate how smallpox vaccination history may translate to orthopoxvirus susceptibility, defined as the complement of the product of age-specific vaccination coverage and vaccine effectiveness, and given the limited information available on vaccine effectiveness, we consider a number of scenarios of plausible values of vaccine effectiveness. We additionally consider the effect of age-specific waning based on time since vaccination.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We develop a high-resolution global map of current populations with smallpox vaccination based on sub-national and age-specific estimates of demography and historical vaccination efforts, and find substantial variation across countries with vaccination coverage varying from 7 to 47% (Figure S25). We characterize the robustness of our estimates to uncertainty in the inputs, and find that coverage estimates have a standard deviation of less than 5% in 99.6% of admin-1 regions (Figure S26). To validate our projected estimates of population vaccine immunity, we compare them to field data from recent smallpox scar surveys or antibody studies from five countries, and find that our model estimates are quantitatively consistent with empirical estimates from larger surveys, and qualitatively consistent with surveys challenged by smaller sample sizes in large heterogeneous countries (Figure S27).
We find high spatial heterogeneity in the landscape of smallpox vaccine history, with admin-1-level population vaccination ranging from 7 to 60% globally, and varying by up to 9% within countries (Figure 1A). Parts of Finland, Bulgaria, Japan, and Sweden have the highest levels of vaccination, while some of the least vaccinated regions are in Yemen, Colombia, Guinea-Bissau, and Ethiopia. Significant subnational heterogeneity can be observed in many areas but is particularly evident in large countries such as India, China, Brazil, and the U.S. Given that central and western Africa are common locations for monkeypox spillover, their low levels of historical vaccination are notable. Overall, we find a high degree of spatial clustering in vaccination history at the admin-1 level (Moran’s I = 0·94).
Figure 1. The global landscape of smallpox vaccination.
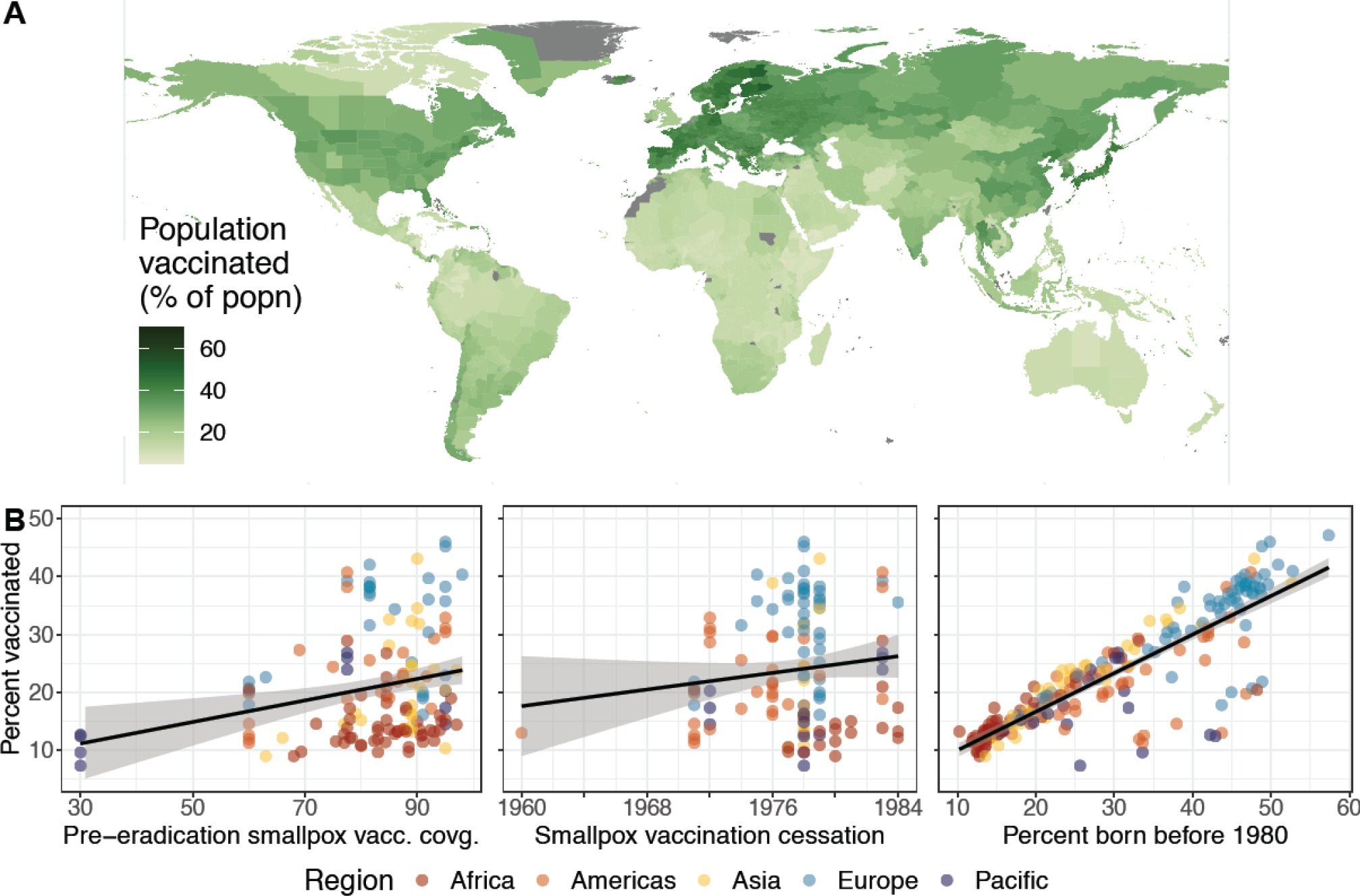
(A) Population smallpox vaccination coverage at the admin-1 scale globally. (B) Relationship between the current average national population smallpox vaccination coverage and (left) average national smallpox vaccination coverage near cessation, (middle) the year that routine smallpox vaccination ceased within the country, and (right) the proportion of the country’s current population that was born before 1980. The black lines are based on linear regressions of the data, and are meant to guide the eye, rather than assert a linear relationship. Outlier countries are Australia and New Zealand (low coverage), Cuba (early cessation), and the United Kingdom (low coverage).
In Figure 1B, we demonstrate the role of demographic and historical vaccination factors in shaping the modern landscape of vaccine immunity. We find that a nation’s current demography (as measured by the proportion of the population born before smallpox eradication in 1980) is highly predictive of current vaccination coverages (Pearson’s correlation of linear association, ⍴ = 0·86, 95% CI = [0·83, 0·89]), while vaccination coverage near cessation is only moderately predictive of current coverage (Pearson’s correlation of linear association, ⍴ = 0·18, 95% CI = [0·046, 0·30]). Additionally, while historical smallpox vaccination coverage is not predictive on its own, it is correlated to current smallpox vaccine protection in countries with older populations (e.g., in Europe and Asia).
To tease out the factors driving the observed spatial heterogeneity in the smallpox vaccination landscape, we compare estimates under three counterfactual scenarios: (a) simultaneous cessation (i.e., all countries share a routine smallpox vaccination cessation date of 1984); (b) universal vaccination efforts (i.e., all countries achieve 100% vaccination coverage before cessation); and (c) homogeneous demography (i.e., all countries and regions share the global average age distribution). In Figure 2, we summarize the impact of each scenario relative to the empirical estimates (summarized in Figure 1A). All three counterfactuals highlight significant differences in population vaccination coverage under different conditions. These results further demonstrate that demography is the largest contributing factor to the smallpox vaccination landscape and allow us to geographically localize these effects nationally and subnationally. The simultaneous cessation scenario (Figure 2A) highlights that later smallpox vaccination cessation would lead to a systematic global increase in population vaccination levels. The effect of later cessation is small in most nations, with some of the largest average increases of over 28% in Cuba and nearly 16% in the United States and its associated territories, countries which stopped routine smallpox vaccination relatively early. The universal vaccination counterfactual (Figure 2B) similarly leads to small increases in most national vaccination levels worldwide, with the exception of New Zealand and Australia which had particularly low coverage. In contrast, the homogeneous demography scenario (Figure 2C) reveals significant changes in the landscape of vaccine protection. These changes are greatest in countries with age structures that deviate substantially from the global average: African, southern Asian, and northern South American nations experience an increase in average age under this counterfactual, leading to higher levels of vaccination today. Countries in North America, Europe, northern Asia, and Australia experience decreases in average age, reducing vaccination levels substantially. At a finer geographical scale, we find that the north/south gradients observed in India and Brazil (in Figure 1A) are driven by differences in age distributions (Figure S28). Results assuming homogeneous demography within countries are shown in Figure S29 to highlight the role of subnational demographic data, and in Figure S30, we highlight that the lack of demographic data at finer scales may be obscuring further spatial heterogeneity that may exist subnationally.
Figure 2. The role of demography and vaccination in shaping the current smallpox vaccination landscape.
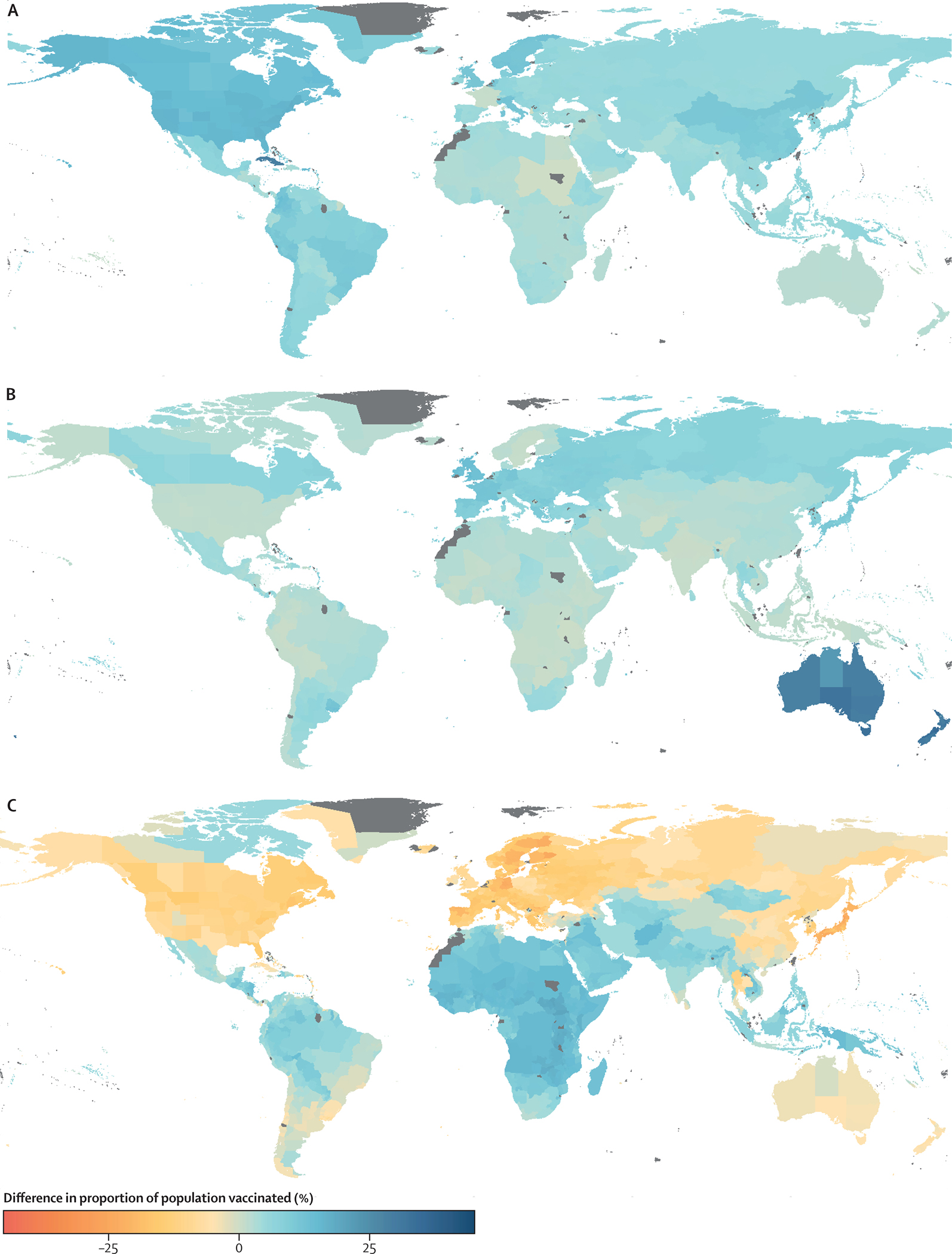
We illustrate differences in current vaccination coverage due to three counterfactual scenarios: (A) All countries ceased routine smallpox vaccination in 1984. (B) All countries achieved 100% vaccination coverage before cessation. (C) All administrative regions have the same global average age distribution.
Finally, we translate these vaccination levels into susceptibility estimates under different scenarios of vaccine effectiveness, to inform current and future global health decisions and to further emphasize the role of demography in shaping the orthopoxvirus immune landscape (Figure 3). In particular, we consider five hypothetical levels of protection arising from past smallpox vaccination (reflecting cross-immunity against different orthopoxviruses and overall immune waning over time since cessation), and illustrate their effects in four countries as case studies (all of which have high quality data). While we emphasize that current-day effectiveness is unknown against any virus, for context we review data on Vaccinia-based smallpox vaccine effectiveness against a range of orthopoxviruses (Figure S31): 91·1% effectiveness against Variola major outbreaks, 74·9% effectiveness against Variola minor, 85% vaccine effectiveness against monkeypox outbreaks in the 1980s, and 80·7% effectiveness during outbreaks in the 2000s. As an alternative scenario, we consider age-specific waning by assuming that everyone was vaccinated once as a young child, with an initial vaccine effectiveness of 85%, which then wanes exponentially by 1·4% a year24 from time of vaccination (assumed to be by 5 years of age or the year 1980, whichever is earlier). (We note that this amounts to a degraded effectiveness of less than 30% in the oldest cohorts, and is not consistent with effectiveness estimates from recent outbreaks.e.g., 8) All scenarios demonstrate that orthopoxvirus susceptibility is highly age-specific with complete susceptibility for younger individuals, and partial susceptibility for older cohorts born before cessation in that country. Relative levels of susceptibility between middle-aged versus older individuals depend on the history and age profile of vaccination campaigns in that country, and become further differentiated among the older cohort in the age-specific waning scenarios. Crucially, the overall age profile of susceptibility is conserved across different scenarios of vaccine effectiveness.
Figure 3. Demography drives susceptibility differences, with magnitude determined by vaccine effectiveness and differences by age group determined by waning.
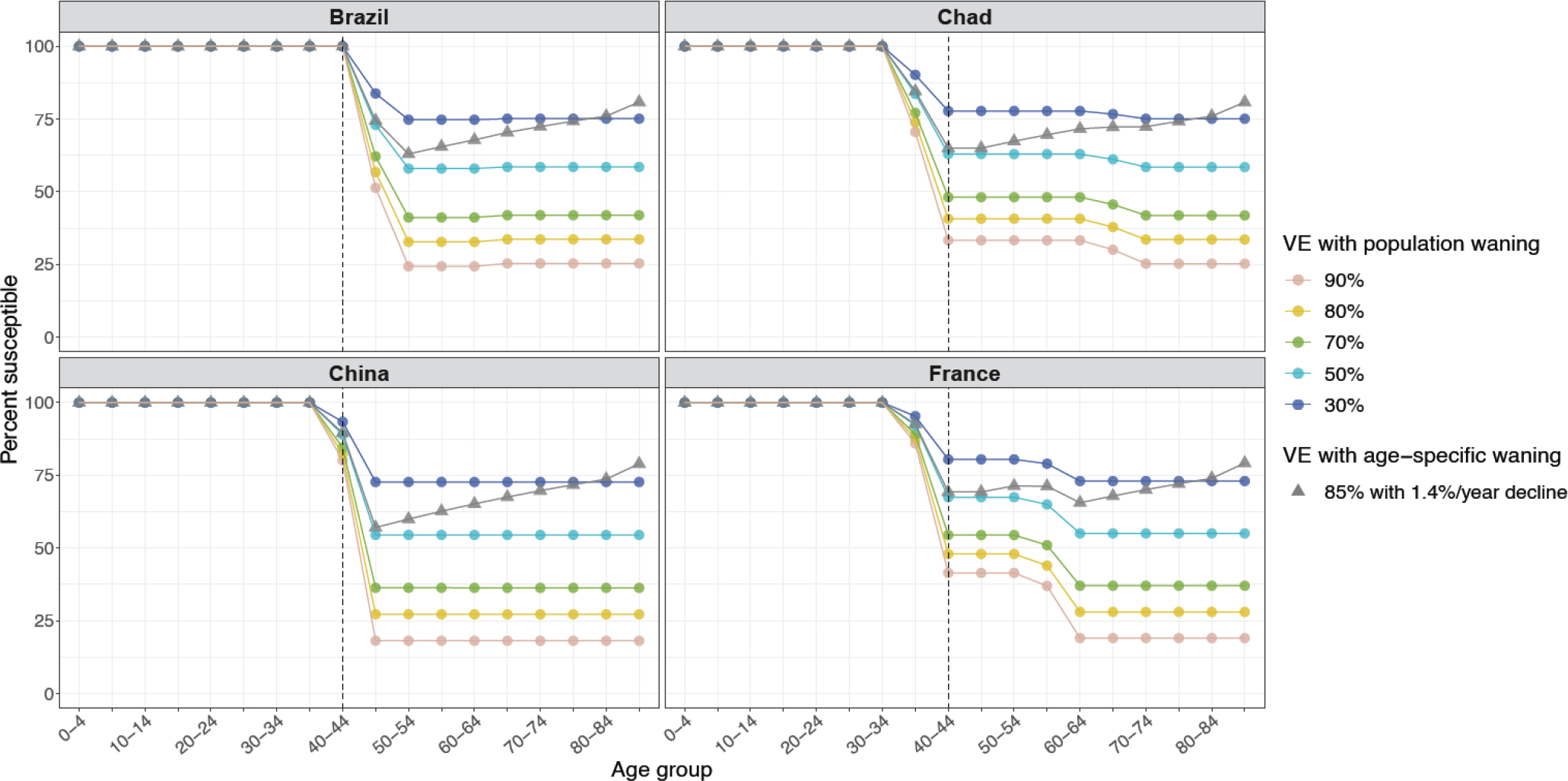
We illustrate susceptibility profiles for countries with varying vaccination cessation dates and reported coverages. Vaccine effectiveness values ranging from 30 to 90% are shown, which overlap with reported estimates in the literature for monkeypox, Variola major, and Variola minor (see Figure S31), under overall population waning. We additionally illustrate an individual-level waning scenario with 1·4% per year since time of vaccination, assuming all eligible individuals were vaccinated by 5 years of age or in 1980, whichever is earlier.
Discussion
Despite the massive success and organizational prowess of the global smallpox eradication campaign, fine-scale vaccination coverage estimates are limited and only focus on the period near eradication four decades ago. Contemporary estimates are needed for public health officials to make informed decisions in the context of increasing orthopoxvirus emergence. We address this gap by creating a comprehensive, high-resolution global map of current population smallpox vaccine history, accounting for demographic changes since smallpox was eradicated. We identify substantial global vaccination coverage against smallpox which may translate to other orthopoxvirus diseases. However, we characterize significant, previously undocumented, spatial heterogeneity in protection, at national and subnational scales, and demonstrate that this is driven chiefly by differences in demographic change since eradication, and less by differences in historical smallpox vaccination efforts.
The 2022 global monkeypox outbreak has highlighted the immediate relevance of mapping this susceptibility landscape, but our work has broader implications for future risks from all orthopoxviruses. As time passes since smallpox eradication, the fraction of human populations with any acquired immunity to orthopoxviruses (from vaccination or infection) dwindles, and other human-infecting orthopoxviruses stand to benefit from the increasing pool of susceptible individuals (known as ‘competitive release’ in ecological terms).15 Indeed, human case incidence of a number of orthopoxviruses appears to be rising.11–13 Our work quantifies the geographic distribution of vulnerability to this ensemble of zoonotic viruses, via construction of national susceptibility profiles. The effectiveness and durability of Vaccinia-based vaccines against other zoonotic orthopoxviruses is unknown (though for some viruses, like buffalopox, their close genetic relationship to Vaccinia makes substantial cross-protection likely), so absolute estimates of susceptibility are not possible. Nevertheless, by characterizing the spatial patterns of smallpox vaccine history, our mapping reveals the landscape of relative risk from any human-infecting orthopoxvirus. Determining the strength and waning rates of cross-protection against all orthopoxviruses remains a key research frontier, and will enable absolute estimates of susceptibility against these emerging threats.
Another dimension of our findings that is robust to unknown vaccine effectiveness is the age structure of protection. We translated our estimates of vaccination history to age-specific susceptibility profiles, by considering a range of hypothetical vaccine effectiveness values. However, our understanding of smallpox vaccine effectiveness against orthopoxviruses remains limited and arises largely from monkeypox and Variola outbreaks from the 1970s to the 2000s, and from public health settings and transmission routes quite distinct from the 2022 monkeypox crisis. These estimates (which range from 80–85% for monkeypox) primarily quantify vulnerability to symptomatic disease, and thus the full implications for infection or onward transmission are not known. Smallpox vaccination also entails a diversity of vaccine types, injector types, and booster schedules, each of which can impact effectiveness and durability.14,25 Our scenario analyses produce estimates of age-specific population susceptibility, which can inform epidemiological risk analyses and public health decision-making but are vulnerable to these unknown programmatic factors. At the individual level, susceptibility is closer to dichotomous, with unvaccinated individuals generally vulnerable to infection and disease, and vaccinated individuals carrying some degree of protection; our core findings on the age structure of vaccination history are centered on this robust distinction. The age-assortative nature of human contact patterns, which can amplify age-specific infection risks, increases the potential impact of this heterogeneity in individual susceptibility.
The process of finding and aggregating country-specific data from frequently incomplete historical records brings inevitable uncertainty to the data and our estimates. We adopted a strategy of finding the best data available for each location, grading its quality explicitly in our public database, and conducting an uncertainty analysis to assess the impact of data gaps on our study outputs. Despite some dismaying gaps in the retrievable evidence base (e.g., for 37% of countries, and 14% of the world population, no estimate of smallpox vaccination coverage could be found), our analysis showed that current-day vaccination history could be estimated to within 5% in 96% of countries. This surprising precision arises from the fact that current-day coverage is driven chiefly by demographic factors, and details of historic vaccination programs have relatively weak influence. Nevertheless, we call on the global health community to assist in filling these gaps, and provide the infrastructure for continuing contributions to our living database. Some additional uncertainty arose from a lack of recent data on demographic age structure at the admin-1 level, and we urge that such data be made available. Looking forward, modern data collection approaches could make invaluable contributions to validating and extending our vaccination estimates. In particular, recent calls for large-scale, routine, and representative serological surveys to characterize population immune histories could provide vital data to ground-truth our model.26
In this study, we compile the first high-resolution global map of smallpox vaccine history using demographic and smallpox vaccination data. Our work reveals the vast spatial heterogeneity of vaccination coverage, highlighting the need to consider spatial clustering of susceptible individuals, whether due to age or other factors, and underscoring the importance of fine-scale spatial analysis in assessing risks of orthopoxvirus emergence and establishment. In response to the 2022 global monkeypox outbreak, we contribute an open database of all our sub-national estimates and uncertainties as an immediate resource for the global health community. We also invite the community to contribute both historical information and future data (e.g., on the distribution of the ACAM2000 and JYNNEOS vaccines) to this living database.29 Until data emerges on vaccine effectiveness from the current outbreak, our scenario-based age-specific susceptibility profiles can guide targeting of high-risk groups for vaccination and treatment. Additionally, they highlight that older age groups (born before cessation of smallpox vaccination) are at significant risk due to the combined effects of imperfect initial coverage, imperfect vaccine effectiveness against monkeypox, and possible waning over the decades since vaccines were administered. This prediction, alongside behavioural research that indicates that contact rates relevant to current transmission patterns are non-negligible in these age groups,27 aligns with evidence of substantial numbers of cases among older adults in the current outbreak.e.g.,28 In addition, there are continuing concerns about the potential for transmission in other social contexts, including congregate living facilities such as retirement homes or prisons. The hope remains that the current outbreak will be contained, but in the event that transmission becomes widespread, our smallpox vaccination landscape can inform public health efforts on assessing risks of geographic introductions, allocation of scarce vaccine supplies, and predicting transmission dynamics in concert with data on contact patterns, mobility, and real-time prevalence.
Supplementary Material
Research in Context.
Evidence before this study:
Prior to this work, smallpox vaccination cessation dates and vaccination coverage pre-eradiction were distributed in different WHO documents and in the landmark book by Fenner et al.1 but no single source provided this historical data for all countries. Additionally, there has been no work since smallpox eradication that provides current fine-scale global estimates of residual smallpox vaccination coverage.
Added value of this study:
We characterize the fine-scale global spatial landscape of smallpox vaccination history based on geographical heterogeneity in demography and past smallpox vaccination program features, including vaccination coverage and cessation dates. We find significant spatial heterogeneity in current smallpox vaccination coverage, driven in large part by age structure, specifically what proportion of the population in a region was born before smallpox vaccination cessation. We contribute an open (and living) database of all subnational vaccination history estimates and uncertainties as an immediate resource for the global health community working on the monkeypox outbreak.
Implications of all of the available evidence:
Our findings highlight the need to consider spatial clustering of unvaccinated individuals and the importance of fine-scale spatial analysis in light of increased risk of orthopoxvirus emergence. In the event that transmission becomes widespread during the 2022 global monkeypox outbreak, our vulnerability map can inform public health efforts on identifying non-endemic regions and age cohorts at greatest risk, allocation of scarce vaccine supplies, and predicting transmission dynamics in concert with data on contact patterns, mobility, and real-time prevalence.
Acknowledgement
Research reported in this publication was supported by the National Institutes of Health under award number R01GM123007 (SB) and National Science Foundation DEB-1557022 (JOL-S).
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
All code to reproduce figures and data to produce our estimates (as of September 20, 2022), as well as the vaccination coverage estimates generated by our study, are available at https://github.com/bansallab/mpx_landscape. Information on how to contribute to this living database is also available at that link.
References
- [1].Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID, World Health Organization, et al. Smallpox and its eradication. World Health Organization; 1988. [Google Scholar]
- [2].Cherry J, McIntosh K, Connor J, Benenson A, Alling D, Rolfe U, et al. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Primary percutaneous vaccination. The Journal of Infectious Diseases. 1977;135(1):145–54. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization. The global eradication of smallpox: Final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979. World Health Organization; 1980. [Google Scholar]
- [4].Khodakevich L, Ježek Z, Messinger D. Monkeypox virus: Ecology and public health significance. Bulletin of the World Health Organization. 1988;66(6):747. [PMC free article] [PubMed] [Google Scholar]
- [5].Ježek Z, Grab B, Szczeniowski M, Paluku K, Mutombo M. Human monkeypox: Secondary attack rates. Bulletin of the World Health Organization. 1988;66(4):465. [PMC free article] [PubMed] [Google Scholar]
- [6].Jezek Z, Marennikova S, Mutumbo M, Nakano J, Paluku K, Szczeniowski M. Human monkeypox: A study of 2,510 contacts of 214 patients. Journal of Infectious Diseases. 1986;154(4):551–5. [DOI] [PubMed] [Google Scholar]
- [7].Fine P, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. International Journal of Epidemiology. 1988;17(3):643–50. [DOI] [PubMed] [Google Scholar]
- [8].Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proceedings of the National Academy of Sciences. 2010;107(37):16262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karem KL, Reynolds M, Hughes C, Braden Z, Nigam P, Crotty S, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clinical and Vaccine Immunology. 2007;14(10):1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nature Medicine. 2005;11(9):1005–11. [DOI] [PubMed] [Google Scholar]
- [11].Eltom KH, Samy AM, Abd El Wahed A, Czerny CP. Buffalopox virus: An emerging virus in livestock and humans. Pathogens. 2020;9(9):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vorou RM, Papavassiliou VG, Pierroutsakos IN. Cowpox virus infection: An emerging health threat. Current Opinion in Infectious Diseases. 2008;21(2):153–6. [DOI] [PubMed] [Google Scholar]
- [13].Leendertz SAJ, Stern D, Theophil D, Anoh E, Mossoun A, Schubert G, et al. A cross-sectional serosurvey of anti-orthopoxvirus antibodies in central and Western Africa. Viruses. 2017;9(10):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson D, Henderson DA, et al. Smallpox vaccination: A review, part I. Background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clinical Infectious Diseases. 2003;37(2):241–50. [DOI] [PubMed] [Google Scholar]
- [15].Lloyd-Smith JO. Vacated niches, competitive release and the community ecology of pathogen eradication. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1623):20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee EC, Arab A, Colizza V, Bansal S. Spatial aggregation choice in the era of digital and administrative surveillance data. PLOS Digital Health. 2022;1(6):e0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Truelove SA, Graham M, Moss WJ, Metcalf CJE, Ferrari MJ, Lessler J. Characterizing the impact of spatial clustering of susceptibility for measles elimination. Vaccine. 2019;37(5):732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Masters NB, Eisenberg MC, Delamater PL, Kay M, Boulton ML, Zelner J. Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data. Proceedings of the National Academy of Sciences. 2020;117(45):28506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Center For International Earth Science Information Network-CIESIN-Columbia University. Gridded Population of the World, Version 4 (GPWv4): Basic Demographic Characteristics, Revision 11. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC); 2018. Accessed May 24, 2022. Available from: https://sedac.ciesin.columbia.edu/data/set/gpw-v4-basic-demographic-characteristics-rev11. [Google Scholar]
- [20].Rieckmann A, Villumsen M, Jensen ML, Ravn H, da Silva ZJ, Sørup S, et al. The effect of smallpox and Bacillus Calmette-Guérin vaccination on the risk of Human Immunodeficiency Virus-1 infection in Guinea-Bissau and Denmark. In: Open Forum Infectious Diseases. vol. 4. Oxford University Press; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Costa GB, Augusto LTS, Leite JA, Ferreira PCP, Bonjardim CA, Abrahão JS, et al. Seroprevalence of Orthopoxvirus in rural Brazil: Insights into anti-OPV immunity status and its implications for emergent zoonotic OPV. Virology Journal. 2016;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Styczynski A, Burgado J, Walteros D, Usme-Ciro J, Laiton K, Farias AP, et al. Seroprevalence and risk factors possibly associated with emerging zoonotic vaccinia virus in a farming community, Colombia. Emerging Infectious Diseases. 2019;25(12):2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pütz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. Journal of General Virology. 2005;86(11):2955–60. [DOI] [PubMed] [Google Scholar]
- [24].Eichner M Analysis of historical data suggests long-lasting protective effects of smallpox vaccination. American Journal of Epidemiology. 2003;158(8):717–23. [DOI] [PubMed] [Google Scholar]
- [25].Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunological Reviews. 2006;211(1):320–37. [DOI] [PubMed] [Google Scholar]
- [26].Mina MJ, Metcalf CJE, McDermott AB, Douek DC, Farrar J, Grenfell BT. A Global lmmunological Observatory to meet a time of pandemics. eLife. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anderson EJ, Weiss KM, Morris MM, Sanchez TH, Prasad P, Jenness SM. HIV and Sexually Transmitted Infection Epidemic Potential of Networks of Men Who Have Sex With Men in Two Cities. Epidemiology. 2021; 32(5): 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Centers for Disease Control and Prevention. Monkeypox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Accessed September 23, 2022. Available from: https://web.archive.org/web/20220923030545/https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html.
- [29].Taube JC, Rest EC, Lloyd-Smith JO, Bansal S. The global landscape of smallpox vaccination history– Data & Code. https://github.com/bansallab/mpx_landscape [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code to reproduce figures and data to produce our estimates (as of September 20, 2022), as well as the vaccination coverage estimates generated by our study, are available at https://github.com/bansallab/mpx_landscape. Information on how to contribute to this living database is also available at that link.


