Abstract
Brain health requires circuits, cells and molecular pathways to adapt when challenged and to promptly reset once the challenge has resolved. Neurodegeneration occurs when adaptability becomes confined, causing challenges to overwhelm neural circuitry. Studies of rare and common neurodegenerative diseases suggest that the accumulation of lipids can compromise circuit adaptability. Using microglia as an example, we review data that suggests increased lipid concentrations cause dysfunctional inflammatory responses to immune challenges, leading to Alzheimer’s disease, Parkinson’s disease and dementia. We highlight current approaches to treat lipid metabolic and clearance pathways and identify knowledge gaps towards restoring adaptive homeostasis in individuals who are at-risk of losing cognition.
Keywords: Cholesterol, sphingolipid, inflammation, neurodegeneration, aging
Graphical Abstract
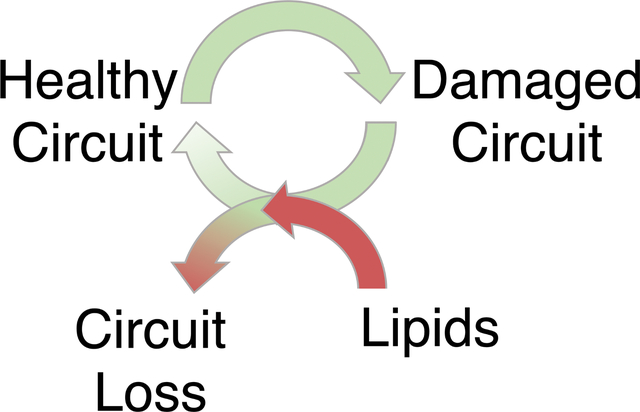
Brain health requires circuits, cells and molecular pathways to adapt when challenged and to promptly reset once the challenge has resolved. Neurodegeneration occurs when this cycle of adaptability becomes confined, causing challenges to overwhelm neural circuitry. Here, we discuss how abnormal lipids compromise circuit adaptability through microglia, potentially causing Alzheimer’s disease, Parkinson’s disease and dementia.
Lipid diversity enables the adaptive homeostasis of the human brain
The term adaptive homeostasis describes the responsiveness of molecular pathways, cells and organs to maintain an individual’s health [1, 2]. A diverse repertoire of homeostatic responses can even be considered a hallmark of organismal health. In the case of the human brain, adaptive homeostasis coordinates several processes that include metabolism, vesicular trafficking, intercellular signals, synaptic plasticity and inflammation, which maintain the high functional thresholds of neuronal circuits controlling cognition and motor function [3]. When adaptive homeostasis fails, high functional threshold systems erode and vulnerable neuronal circuits degenerate (Figure 1).
Figure 1. Multiple challenges to cell type specific circuitry cause flux between functional states (healthy aging) or cumulative synaptic loss and circuit failure (neurodegeneration) at rates dependent upon the circuit’s lipid diversity and genomic sequence.
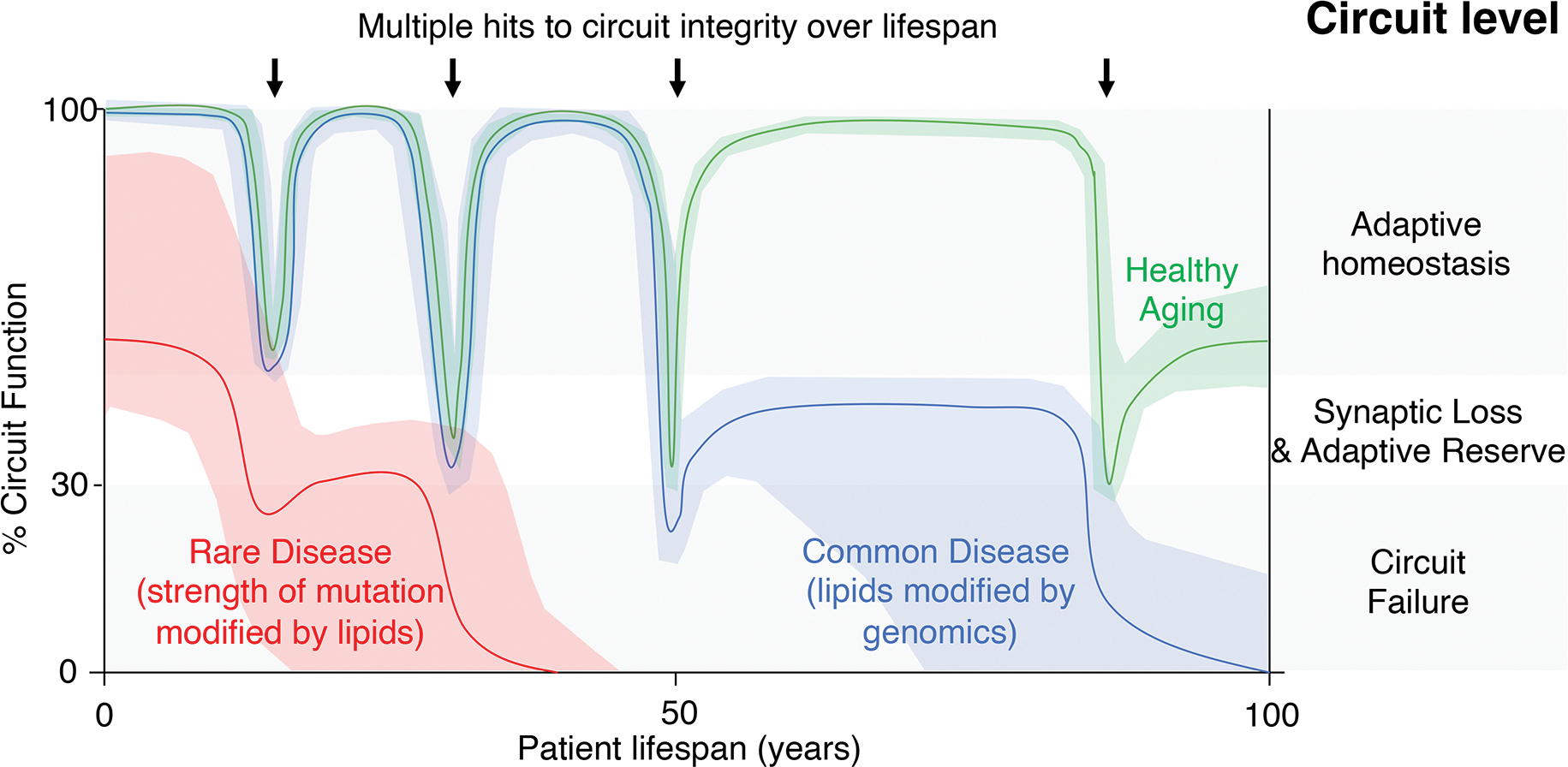
Healthy aging of the human brain is enabled by the adaptive homeostasis of neuronal circuits (green line). Periodic challenges to the integrity of vulnerable neuronal circuits, for example infections and acute toxicity, lead to transient synaptic loss. Metabolic reprogramming confers an adaptive reserve that supports the regrowth of synapses and the return of the circuit to adaptive homeostasis although cumulative hits reset the baseline circuit function. Rare neurodegenerative diseases cause a range of circuit dysfunction that is influenced by the strength of the genetic mutation, the vulnerability of the neuronal circuit and modified by lipid transport status (red line). Such a dominant strain on the neuronal circuit causes early onset failure. Common neurodegenerative diseases such as PD and AD withstand early challenges to circuit integrity but cumulative damage leads to an inability to return circuitry to a homeostatic rate (for example, in the case of unresolved neuroinflammation linked to lipid accumulation), leading to late-onset circuit failure (blue line, e.g. PD or AD).
Since lipids are the dominant structural component of the brain and each type of lipid is likely to have specific functions, parsimony suggests that the adaptive homeostasis of human brain circuits requires a tightly regulated and diverse lipidome. Consistent with this argument, the healthy human brain has two phases of lipid diversity. First, the range of lipid molecules increases from infancy to adulthood during a period that includes myelination and significant synaptic growth and plasticity [4]. Second, the developed lipid diversity is retained during healthy aging of the adult brain [4]. However, genetic mutations can cause specific lipids to accumulate, simplifying lipid diversity and leading to rare neurodegenerative diseases (Figure 1). The causes for more common neurodegenerative diseases remain unclear but emerging data links multiple challenges, such as neuroinflammatory events, to lipid accumulation and neurodegeneration [5–7]. Indeed, biochemistry has linked protein aggregation to lipid hydrocarbon chain length but the upstream intracellular causes of lipid changes and accumulation remain elusive [8]. Furthermore, patient analyses have used a range of brain regions to evaluate bulk lipid profiles during neurodegeneration but the results have been quite varied, indicating the importance of cellular resolution to evaluate lipid distribution processes between cell types in vulnerable brain regions (Table 1). Here we consider lipid diversity as an upstream regulator of adaptive homeostasis and inflammation in the human brain that is lost in Alzheimer’s disease (AD), Parkinson’s disease (PD) and dementias.
Table 1.
Analysis of published lipid profiles of brain tissue from FTD, PD and AD patients.
| Disease (Reference) | Region | PE | LPE | PC | LPC | PS | PI | PA | CL | MAG | DAG | TAG | Chol | CE | SE | Cer | HexCer | GlcCer | GlcSph | SM | GM1 | GM2 | GM3 | GD3 | GD2 | GD1 | GT3 | GT1 | BMP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTD GRN [63] | FL | ||||||||||||||||||||||||||||
| FTD GRN [63] | OL | ||||||||||||||||||||||||||||
| FTD GRN [64] | MFGC | ||||||||||||||||||||||||||||
| PD [145] | SN | ||||||||||||||||||||||||||||
| PD [146] | SN | ||||||||||||||||||||||||||||
| PD [146] | Putamen | ||||||||||||||||||||||||||||
| PD [147] | Putamen | ||||||||||||||||||||||||||||
| PD [147] | Cerebellum | ||||||||||||||||||||||||||||
| PD [148] | Caudate | ||||||||||||||||||||||||||||
| PD [148] | GP | ||||||||||||||||||||||||||||
| PD [148] | Putamen | ||||||||||||||||||||||||||||
| PD [149] | ACG | ||||||||||||||||||||||||||||
| PD [149] | Amygdala | ||||||||||||||||||||||||||||
| PD [149] | Visual cortex | ||||||||||||||||||||||||||||
| Early pathology, no dementia [150] | TL | ||||||||||||||||||||||||||||
| Early AD [150] | TL | ||||||||||||||||||||||||||||
| Mild AD (Braak stage 3–4) [151] | Cortex | ||||||||||||||||||||||||||||
| Late AD [150] | TL | ||||||||||||||||||||||||||||
| Severe AD (Braak stage 5–6) [151] | Cortex | ||||||||||||||||||||||||||||
| AD [64] | MFGC | ||||||||||||||||||||||||||||
| AD [152] | PFC | ||||||||||||||||||||||||||||
| AD [152] | EC | ||||||||||||||||||||||||||||
| AD APOE e2 [119] | RIPL | ||||||||||||||||||||||||||||
| AD APOE e4 [119] | RIPL |
Orange cells = significantly enriched lipid. grey cells = no significant change from healthy subjects. Green cells = significantly depleted. FL, frontal lobe, OL, occipital lobe, MFGC, mid frontal gyrus cortex, ACC, anterior cingulate cortex, SN, substantia nigra, GP, globus pallidus, TL, temporal lobe, EC, entorhinal cortex, PFC, prefrontal cortex, RIPL, right inferior parietal lobule.
Homeostatic cholesterol and sphingolipid metabolic pathways resolve initial inflammatory challenges without significant lipid accumulation or neurodegeneration
Thousands of different lipids form the human brain, cerebrospinal fluid and plasma and the concentration of these lipids changes during aging and disease [4, 9]. Different lipids regulate a range of organ functions from neuronal conductance to protein trafficking between subcellular compartments and receptor-mediated signal transduction [10, 11]. Although the human liver is a significant source of lipids, lipid transporters and related-regulatory signals for the entire body, the blood-brain barrier segregates circulating lipoprotein bound lipids from the brain, placing an emphasis on the brain to synthesize and tightly regulate the levels of its own range of lipids. Of particular interest for brain health are two groups of lipids: cholesterol and sphingolipids whose synthesis, utilization and clearance are linked to adaptive homeostasis and cell type specific functions.
Different organs rely on characteristic combinations of biochemical pathways to synthesize cholesterol. The brain is cholesterol-rich but produces low levels of cholesterol (2% of the total cholesterol produced by the liver) via a modified Kandutsch-Russell (K-R) pathway while the liver mainly uses the Bloch pathway to produce cholesterol [12–14]. A constitutively active K-R pathway is proposed to maintain myelin and plasma membrane pools of cholesterol in the brain, leaving the microglial Bloch pathway to adapt during chronic inflammation to sense phagocytosed lipids by activating Liver X Receptor (LXR) target gene expression without producing cholesterol [12, 15, 16]. Once synthesized, human brain cholesterol has a half-life of up to five years depending upon the pool [17]. Cholesterol in the brain can be cleared to the blood either directly by reverse transport using glial apolipoprotein E (APOE) or after metabolic conversion to 24S-hydroxycholesterol predominantly by neuronal cytochrome P450 family 46 subfamily A group 1 (CYP46A1) enzyme activity [17]. In a similar manner to cholesterol, each brain cell type synthesizes and uses patterns of intracellular sphingolipids and these patterns can change with age [5, 18–21]. Microglia in particular alter the expression of enzymes that metabolize sphingolipids during different inflammatory states while not synthesizing cholesterol. Such inflammatory stimuli can include viral infections [6, 7, 22, 23]. Singular, weak inflammatory stimuli can be readily handled by the brain’s immune system and efficiently resolved, although brain structure may remain altered for several months [24]. However, multiple stimuli over a lifespan, perhaps due to repeated viral infections, combined with genetic or environmental factors that increase lipid concentrations may lead to early microglial senescence and neural dysfunction, especially since the population of human microglia renews slowly (Figure 1)[25–27].
Lipopolysaccharide (LPS) is broadly used as a strong microglial stimulant that increases the expression of both anabolic and catabolic sphingolipid enzymes, immune signals and the transient accumulation of lipid droplets, an adaptive reserve of energy to support a high metabolic state (Figure 1) [28–31]. LPS-stimulated microglia can upregulate a range of lipids that include sphingolipids, phosphatidylcholine, lysophosphatidylcholine, diacylglycerols and triacylglycerols [32]. Autofluorescent, dysfunctional microglia upregulate the expression of catabolic sphingolipid enzymes and lipid transfer proteins [33]. Aged microglia do not alter the expression of sphingolipid metabolic enzymes but upregulate the expression of both immune signals and lipid transfer proteins [34]. The cholesterol and sphingolipids produced by these synthetic pathways may also interact within the membranes of different cell types. For example, sphingolipids interact with cholesterol at the neuronal synapse and regulate Toll-like receptor activity at the phagocytic plasma membrane [35–39].
Lipid accumulation uncouples adaptive homeostasis, leading to aberrant neuroinflammation and the degeneration of vulnerable brain circuits.
Rare neurodegenerative diseases provide direct evidence that genetic mutations can cause lipids to accumulate, ending homeostatic control over neuroinflammation and leading to neurodegeneration. Niemann-Pick disease type C1 (NPC1) is primarily caused by loss-of-function genetic mutations in NPC1, which encodes an intracellular cholesterol transporter. NPC1 patients accumulate endolysosomal cholesterol and monosialotetrahexosyl (GM1) ganglioside across many organs but experience symptoms mainly due to brain and liver dysfunction, suggesting organ specific vulnerabilities to lipid accumulation. In the NPC1 patients’ brain, the age at onset of cognitive decline correlates with the extent of Lewy bodies (LBs) and neurofibrillary tangles (NFTs) [40, 41]. These data suggest that lipid accumulation is sufficient to cause the hallmarks of common neurodegenerative diseases even though NPC1 mutations are not associated with AD or PD. From the perspective of therapeutics, inhibiting brain cholesterol synthesis is not therapeutic. Statins are a clinically validated approach to lowering cholesterol levels by inhibiting the rate limiting enzyme for cholesterol synthesis, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, reducing the incidence of major vascular events [42]. While epidemiological links between statin use and neurodegeneration remain unclear, lipophilic statins that can readily enter the brain have been investigated as treatments for neurodegenerative disease. Recently, Lovastatin, which is a lipophilic statin, slowed the deterioration of 18F-dopa uptake but did not treat the clinical rating scale of 77 patients with early stage PD over two years [43]. Instead, preclinical studies in NPC1 have demonstrated neuron and oligodendrocyte contributions to neurodegeneration and clinical signals link APOE status to human disease severity, implicating cholesterol clearance as a rational therapeutic approach for neurodegenerative disease [44–46].
In a similar manner to NPC1, Gaucher’s disease (GD) is a rare disease that causes lipids to accumulate in the liver and brain. Instead of the cholesterol and GM1 accumulation observed in NPC1 patients, GD patients accumulate sphingolipids in myeloid cells (Gaucher cells, foamy macrophages). Two copies of glucosylceramidase beta 1 (GBA1) mutations cause GD, and a single copy increases the risk of developing PD and cognitive decline (Figure 2) [36, 47–50]. The lost sphingolipid diversity leads to neurodegeneration and neuroinflammation. In the brain tissue of PD patients, a loss of glucocerebrosidase activity is associated with an accumulation of insoluble alpha-synuclein [51] and triglyceride concentrations correlate with a potential biomarker of inflammation [52, 53]. The administration of a small molecule inhibitor of glucocerebrosidase to mice reproduces the findings in patients [54]. Similar increases in high molecular weight alpha-synuclein protein are found in the brains of patients with Sandhoff’s disease and transgenic mice [55, 56]. Enzyme replacement therapy has successfully treated GD patients but does not enter the human brain in meaningful quantities. Miglustat is an inhibitor of glucosylceramide synthase (GCS), which lowers glucosylceramide levels and is an approved treatment for GD, reducing the risk associated with the loss of glucocerebrosidase activity but with low brain penetrance. Miglustat also has limited approval for treating NPC1, targeting the sphingolipids that accumulate alongside cholesterol, stabilizing the disease in patients. Miglustat is administered to nearly all NPC1 patients, either on- or off-label. Venglustat is another small molecule inhibitor of GCS with improved brain penetrance but fails to improve the symptoms of PD patients (NCT02906020). An alternate modality considered for improving glucocerebrosidase activity in the brain is gene therapy. Preclinical studies demonstrate that increased neuronal expression of GBA1 using an adeno-associated virus can reduce the accumulation of alpha-synuclein [57, 58]. Similar approaches are being tested in patients (NCT04127578).
Figure 2. Multiple challenges to microglial function within cell type specific circuitry cause flux between functional states (healthy aging) or failure to support synapse health (neurodegeneration) at rates dependent upon the circuit’s lipid diversity and genomic sequence.
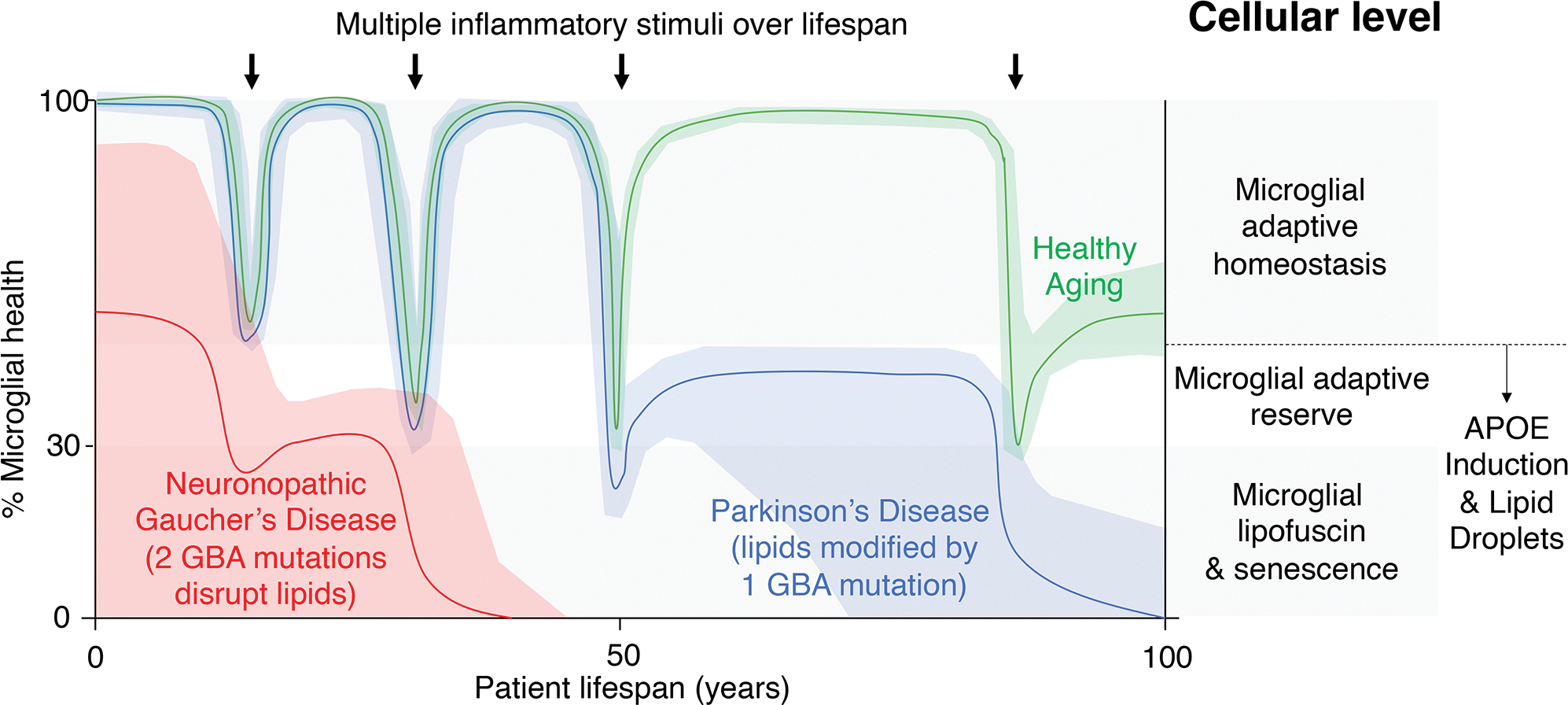
Healthy aging of the human brain is enabled by the adaptive homeostasis of microglia as a cellular component of brain circuitry (green line). Periodic immune challenges to the integrity of vulnerable neuronal circuits, for example viral infections, lead to transient dysfunctional synaptic surveillance and synaptic loss. Lipid droplets support the reprogramming of microglial metabolism to stabilize the neuronal circuitry and the return of the circuit to adaptive homeostasis although cumulative hits reset the baseline circuit function. Neuronopathic forms of GD are caused by 2 rare mutations in GBA that disrupt lipid metabolism and inflammation (red line). Such a dominant strain on the neuronal circuit causes early onset failure. Idiopathic PD is influenced by lipid levels and genetic variation such as single copies of GBA mutations. Early challenges to microglial function are withstood but cumulative damage leads to an inability to return circuitry to a homeostatic rate (for example, in the case of unresolved neuroinflammation linked to lipid accumulation), leading to late-onset circuit failure (blue line, PD).
Frontotemporal lobar dementia (FTD) is another rare disease that is linked to dysfunctional neuroinflammation and lipid accumulation. As with GD, the number of genetic mutations in a specific gene causes different types of neurodegenerative disease. Homozygous mutations in granulin precursor (GRN) cause neuronal ceroid lipofuscinosis (NCL) in childhood while FTD can be caused by heterozygous GRN mutations and presents in adulthood. GRN FTD patients accumulate lipids in the brain and the cerebrospinal fluid (CSF) and serum contains inflammatory biomarkers [59–64]. Similar results linking lipid accumulation to inflammation have been observed in experimental models (Figure 3). Loss of Grn accelerates atherosclerosis and increases levels of polyunsaturated triacylglycerides [64, 65]. Microglia in mouse brains without Grn accumulate high levels of lipid droplets [29]. Brain aging and FTD are both marked by the accumulation of lipofuscin, a substance rich in lipids and proteins that is resistant to intracellular degradation in a manner similar to other pathological hallmarks of neurodegenerative disease. Indeed, lipofuscin levels can be used to discern young tissue from old tissue based on autofluorescence [66]. Lipofuscin accumulates in the neuronal subtypes that are vulnerable to degeneration in PD, AD and dementia, marks senescent cells, including microglia, is a pathological hallmark of NCL and GRN-FTD as well the brains of Grn knockout and aged mice [33, 67–71].
Figure 3. Molecular level influences of microglial lipids function.
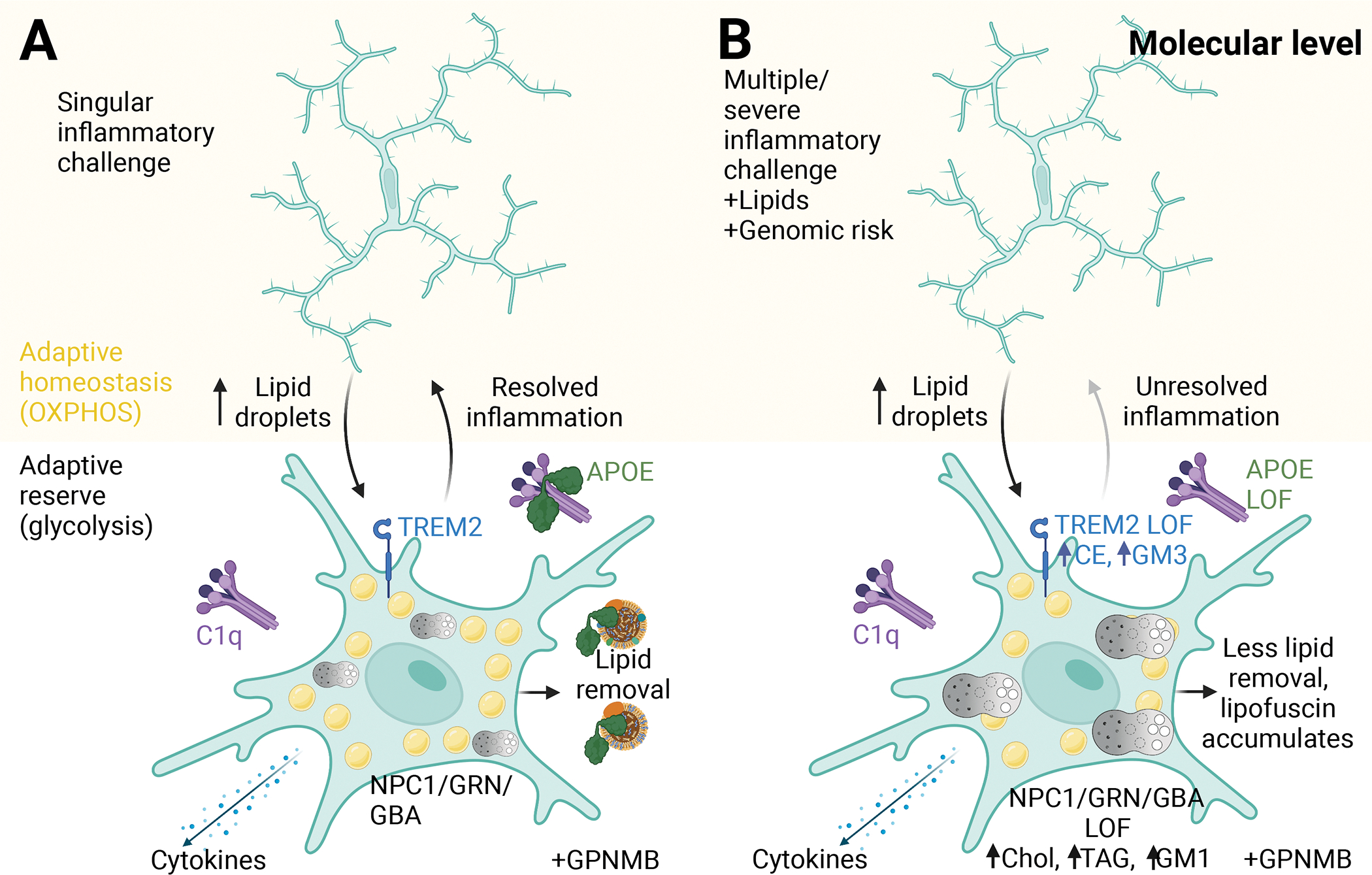
(A) Microglia in a surveillance state (adaptive homeostasis) respond to a singular inflammatory challenge by upregulating lipid droplet synthesis that supports glycolysis (adaptive reserve), activated TREM2 at the plasma membrane leads to an upregulation of C1Q and APOE levels. APOE contributes to the clearance of excess microglial lipids. Lysosomal function is increased through NPC1, GRN and GBA. The increased presence of lipids is detected by astrocytic GPNMB. The inflammatory status of the microglia is efficiently stopped by APOE interactions with C1Q and metabolism returns to a homeostatic state (OXPHOS). (B) In response to sequential inflammatory stimuli, increased lipid levels and genomic sequence changes, microglia are slower to end the inflammatory state, leading to an acquired lipid profile dependent upon the combination of age, challenge, lipid levels and genomic risk. Created with Biorender.com.
In addition to lipid accumulation, the expression of Triggering receptor expressed on myeloid cells 2 (Trem2) and complement pathway components are upregulated in the brains of mice lacking either Npc1 or Grn, functionally linking lipid accumulation to neuroinflammation [64]. Therapeutic strategies to increase progranulin levels (the protein encoded by GRN) in at-risk individuals and FTD patients are being evaluated. Progranulin protein engineered for improved brain distribution can improve sphingolipid levels, lipofuscin levels, lysosomal function and inflammation in mice lacking Grn [72]. Clinical studies of the engineered progranulin protein have now started (NCT05262023). A different approach uses monoclonal antibodies raised against sortilin, a progranulin-interacting protein, to upregulate progranulin levels in preclinical models [73]. A monoclonal antibody raised against sortilin is under investigation to increase progranulin levels in individuals carrying heterozygous GRN mutations (NCT04374136). A third approach for progranulin proposes to use gene therapy to drive the expression of GRN. Preclinical studies of gene therapy using an adeno-associated virus to express Grn have shown reduced lipofuscin levels and improved lysosomal biochemistry in mice lacking Grn although the novel protein was immunogenic [74, 75]. Gene therapy for GRN is now being evaluated in early symptomatic FTD patients with GRN mutations (NCT04747431).
Lipid clearance supports adaptive homeostasis, limiting inflammation and maintaining brain health
Lipid removal pathways strongly influence the pathobiology of cognitive decline in patients and represent a rational group of therapeutic targets. While the concentrations of cholesterol in the cerebrospinal fluid remain unclear, increased plasma concentrations of cholesterol are found in patients with AD and related dementia, PD and amyotrophic lateral sclerosis (ALS) [76–83]. In addition to plasma concentrations of cholesterol, other lipid profiles are linked to common neurodegenerative diseases. Lipid levels in AD patient brains correlate with the extent of neurofibrillary tangles and the genomic signatures of AD are enriched in the enhancers of microglia, linking inflammation to lipids and cognitive decline [84, 85]. With respect to PD, neuromelanin colocalizes with cholesterol in healthy aged brains but PD patients have less nigral cholesterol and lipid droplets become associated with alpha-synuclein fibrils in a process consistent with protein fibrillization in other melanized lysosome-related organelles [52, 86–88].
When lipids accumulate within the cell, several mechanisms are employed to clear and transport the lipids for recycling or excretion. LXRs are nuclear receptors that form heterodimers with retinoid X receptors (RXRs) and activate target gene expression to remove intracellular lipids [89]. LXR/RXR activation can increase the expression of ATP binding cassette subfamily A member 1 (ABCA1), subfamily 6 (ABCG1) and APOE to reduce intracellular lipid concentrations [90, 91]. ABCA1 and ABCG1 are cholesterol transporters that work together to transfer cellular cholesterol to apolipoprotein A-1 and form extracellular high-density lipoprotein [92–95]. Recently generated structures of ABCG1 suggest that sphingomyelin may contribute to the cholesterol efflux by ABCG1 at the membrane [96]. In addition to lipid clearance, LXR, RXR and peroxisome proliferator-activated receptor (PPAR) complexes can regulate Complement Component 1q (C1q) expression and block inflammatory gene expression, directly linking intracellular lipid levels to inflammation [97]. From the perspective of therapeutics, Bexarotene is an RXR agonist that works with ApoE to remove intracellular lipids and clear soluble amyloid beta from mouse models of Alzheimer’s disease and improve memory and cognition [98]. However in the clinic, Bexarotene may reduce brain amyloid in patients with moderate AD without APOE e4 alleles but did not improve patient symptoms and the increased circulating levels of triglycerides posed a risk for cardiovascular disease in the patients (NCT01782742) [99]. It remains unclear whether Bexarotene is sufficiently beneficial to warrant continued investigation in the clinic.
Of these LXR targets, APOE levels appear to be a particularly important link between lipids, inflammation and neurodegeneration (Figure 3). Consistent with clinical data linking lipids and inflammation to neurodegenerative disease, APOE is an intercellular lipid transporter whose genomic sequence variation influences the onset and progression of cognitive decline in dementias, PD and NPC1 [49, 50, 100–106]. Recent data demonstrates that sequence variation in GBA further accelerates cognitive decline in PD patients carrying at least one copy of the APOE disease allele, inferring a significant consequence of concomitant sphingolipid and cholesterol accumulation in the human brain [103].
The molecular mechanisms of APOE-mediated risk of neurodegeneration remain unclear. APOE is produced by multiple cell types in the body, including the brain, liver, blood and vasculature and incorporates into lipoprotein particles that are composed of varying concentrations of cholesterol, triglycerides, proteins and other lipids. The liver uses receptor-mediated uptake of APOE to maintain organismal health by collecting lipids from the blood for metabolism and excretion through the biliary system. However, the mechanism of lipid clearance from the brain has been difficult to demonstrate. Data suggests that the pools of APOE in the plasma and cerebrospinal fluid are not functionally linked during neurodegeneration and liver-derived APOE does not cross the blood-brain barrier [107–110]. However, plasma APOE levels and glucose concentrations in the brain are correlated, suggesting other signals cross the blood-brain barrier to support brain health [109, 111]. These data become important when considering that brain glucose metabolism is reduced before patients’ present with symptoms of dementia, suggesting an indirect influence of plasma APOE on risk of neurodegenerative disease [112–116]. Furthermore, the strength of inflammatory challenges, such as COVID-19 infections (Figure 2), and lipid concentrations in the brain are linked to APOE status, underlining the importance of the linkage between lipids and inflammation for human health [117–119].
In the human brain, astrocytes express APOE to collect and transport locally produced lipids. APOE expression levels are also linked to immune activation and vulnerable populations of human neurons can upregulate APOE expression during early cognitive decline [120]. To examine the links between APOE levels and Tau pathology, a hallmark of AD and other dementias, translational studies have shown that transgenic overexpression of the low-density lipoprotein receptor (LDLR), the ApoE receptor, reduces ApoE concentrations in the cerebrospinal fluid, reduces Tau phosphorylation, and protects against hippocampal atrophy in a mouse model of tauopathy [121]. Furthermore, the study demonstrates the inverse relationship between ApoE and LDLR in microglial metabolism and myelin quality [121]. Reduced APOE function in human microglia leads to the accumulation of lipid droplets and neuronal network dysfunction in vitro [122]. The link between APOE and inflammation has been further investigated in the context of complement mediated inflammation. Complement activity in the brain regulates synaptic pruning and dysregulation is associated with cognitive decline [77, 123–126]. Increased lipid levels, whether from a high fat diet or loss of APOE function, upregulate interferon related gene expression in the mouse brain [84]. This neuroinflammatory signature can be reduced by inhibiting the downstream component of complement signaling, C5. Furthermore, APOE can bind to the upstream component of complement signaling, C1q and regulates the activity of classical complement pathway [84]. The expression of components of the complement signaling pathway are linked to the neuroprotective effect afforded by the neuroprotective APOE allele [127]. Indeed, efforts are underway to compensate for APOE e4 associated loss of lipid trafficking by overexpressing the protective APOE e2 variant using adeno-associated virus in the brains of at-risk individuals (NCT03634007), based on preclinical data that includes non-human primates [128].
Inflammatory signals link lipid accumulation to neurodegeneration
Other disease-associated inflammatory mechanisms are linked to lipid levels. Genome-wide association studies have linked glycoprotein nonmetastatic melanoma protein B (GPNMB) to a reduced risk of PD and more protein is found in PD-related regions of patient’s brains and plasma [53, 76, 129]. Interestingly, lipid accumulation rather than alpha-synuclein overexpression was sufficient to reproduce the patient’s biochemistry in mice, suggesting that GPNMB levels may report lipid levels in the brain [52, 53]. At the cellular level, microglia clear the brain of damaged synapses and dead cells. Infections and repetitive tissue damage cause microglia to activate beyond their homeostatic range, upregulate lipid droplets and fundamentally change metabolism and function before returning to their homeostatic range once the inflammatory trigger is resolved [6, 29, 130]. Repeated episodes of activation alter microglial function leading to altered gene expression patterns, the accumulation of lipofuscin, senescence, and myeloid recruitment of non-microglial cells to the brain, changing the immune status of the brain [29]. Furthermore, macrophages accumulate lipids in GD and NPC1, consistent with myeloid cell dysfunction linked to more common neurodegenerative diseases [131]. TREM2 is a receptor at the plasma membrane that is strongly expressed by myeloid cells, genomic sequence variation increases the risk of AD and can bind to apolipoproteins including APOE [132–134]. Deletion of TREM2 reduces the responsiveness of mouse microglia to extend beyond adaptive homeostasis, altering ApoE and lipoprotein lipase expression, and microglial cholesteryl esters and monosialodihexosylganglioside (GM3) accumulate [135–137]. Mice without either Trem2 or Grn also show reduced brain glucose metabolism, consistent with prodromal states of human neurodegenerative disease [138, 139]. Furthermore, a range of lipids can activate a nuclear factor of activated T cells (NFAT) reporter in vitro and the AD-associated risk allele, TREM2 R47H eliminates such lipid-related immune activity [140]. Similar TREM2 dependent changes in lipid metabolic genes are found in a population of mouse macrophages that expand in adipose tissue during a high fat diet [141]. As a therapeutic approach to stabilize TREM2, monoclonal antibodies against TREM2 can improve microglial activity and reduce plaque burden in preclinical models of AD although the potential therapeutic effects of the antibody on lipid metabolism remains unclear [142–144]. The efficacy and safety of a TREM2 antibody is currently being investigated in patients with early AD (NCT04592874).
Summary
In summary, we review the mechanisms of adaptive homeostasis linked to lipid metabolism and neurodegeneration. Genetics can clearly simplify lipid types and cause neurodegeneration. Our immediate challenge is to translate our findings from rare disease to common disease using genomic and environmental risks as a guide. The clearest example of this translational effort is GBA. Two copies of a GBA mutation are required to cause GD at an early age and one copy increases the risk of PD at a later age. Similar associations between gene copy number and the severity of neurodegenerative disease are found with APOE alleles. From the clinical perspective, biomarkers are needed to identify individuals who are at risk of neurodegeneration so that biochemical interventions can improve and maintain function for a lifetime.
Acknowledgements
Our work was supported by the following sources NIH/NIA R01AG060195, DoD W81XWH2010368, DoD W81XWH2010371, the Orchard Foundation, the Harold and Ronna Cooper Family, and the Consolidated Anti-Aging Foundation.
Abbreviations
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- K-R
Kandutsch-Russell
- LXR
Liver X Receptor
- APOE
Apolipoprotein E
- CYP46A1
cytochrome P450 family 46 subfamily A group 1
- LPS
lipopolysaccharide
- NPC1
Niemann-Pick disease type C1
- GM1
monosialotetrahexosylganglioside
- LB
Lewy body
- NFT
neurofibrillary tangle
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-coenzyme A
- GD
Gaucher’s disease
- GBA1
glucosylceramidase beta 1
- GCS
glucosylceramide synthase
- FTD
frontotemporal lobar dementia
- GRN
granulin precursor
- NCL
neuronal ceroid lipofuscinosis
- CSF
cerebrospinal fluid
- TREM2
Triggering receptor expressed on myeloid cells 2
- ALS
amyotrophic lateral sclerosis
- RXR
retinoid X receptors
- ABCA1
ATP binding cassette subfamily A member 1
- ABCG1
ATP binding cassette subfamily G member 1
- PPAR
peroxisome proliferator-activated receptor
- C1q
Complement Component 1q
- LDLR
low density lipoprotein receptor
- GPNMB
glycoprotein nonmetastatic melanoma protein B
- NFAT
nuclear factor of activated T cells
- GM3
monosialodihexosylganglioside
Footnotes
Conflicts of Interests
The authors declare no conflicts of interests.
References
- 1.Davies KJ (2016) Adaptive homeostasis, Mol Aspects Med. 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Otin C & Kroemer G (2020) Hallmarks of Health, Cell. [DOI] [PubMed] [Google Scholar]
- 3.Engelender S & Isacson O (2017) The Threshold Theory for Parkinson’s Disease, Trends Neurosci. 40, 4–14. [DOI] [PubMed] [Google Scholar]
- 4.Yu Q, He Z, Zubkov D, Huang S, Kurochkin I, Yang X, Halene T, Willmitzer L, Giavalisco P, Akbarian S & Khaitovich P (2020) Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders, Mol Psychiatry. 25, 2952–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isacson O, Brekk OR & Hallett PJ (2019) Novel Results and Concepts Emerging From Lipid Cell Biology Relevant to Degenerative Brain Aging and Disease, Front Neurol. 10, 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deleidi M & Isacson O (2012) Viral and inflammatory triggers of neurodegenerative diseases, Sci Transl Med. 4, 121ps3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isacson O (2020) The Consequences of Coronavirus-Induced Cytokine Storm Are Associated With Neurological Diseases, Which May Be Preventable, Front Neurol. 11, 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sciacca MF, Lolicato F, Tempra C, Scollo F, Sahoo BR, Watson MD, Garcia-Vinuales S, Milardi D, Raudino A, Lee JC, Ramamoorthy A & La Rosa C (2020) Lipid-Chaperone Hypothesis: A Common Molecular Mechanism of Membrane Disruption by Intrinsically Disordered Proteins, ACS Chem Neurosci. 11, 4336–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito K, Hattori K, Hidese S, Sasayama D, Miyakawa T, Matsumura R, Tatsumi M, Yokota Y, Ota M, Hori H & Kunugi H (2021) Profiling of Cerebrospinal Fluid Lipids and Their Relationship with Plasma Lipids in Healthy Humans, Metabolites. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piomelli D, Astarita G & Rapaka R (2007) A neuroscientist’s guide to lipidomics, Nat Rev Neurosci. 8, 743–54. [DOI] [PubMed] [Google Scholar]
- 11.Simons K & Ikonen E (1997) Functional rafts in cell membranes, Nature. 387, 569–72. [DOI] [PubMed] [Google Scholar]
- 12.Mitsche MA, McDonald JG, Hobbs HH & Cohen JC (2015) Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways, Elife. 4, e07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch K (1965) The biological synthesis of cholesterol, Science. 150, 19–28. [DOI] [PubMed] [Google Scholar]
- 14.Kandutsch AA & Russell AE (1960) Preputial gland tumor sterols. 3. A metabolic pathway from lanosterol to cholesterol, J Biol Chem. 235, 2256–61. [PubMed] [Google Scholar]
- 15.Bjorkhem I & Meaney S (2004) Brain cholesterol: long secret life behind a barrier, Arterioscler Thromb Vasc Biol. 24, 806–15. [DOI] [PubMed] [Google Scholar]
- 16.Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, Duking T, Winchenbach J, Neuber J, Ewers D, Scholz P, van der Meer F, Cantuti-Castelvetri L, Sasmita AO, Meschkat M, Ruhwedel T, Mobius W, Sankowski R, Prinz M, Huitinga I, Sereda MW, Odoardi F, Ischebeck T, Simons M, Stadelmann-Nessler C, Edgar JM, Nave KA & Saher G (2021) Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis, Nat Neurosci. 24, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G & Wahren J (1998) Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation, J Lipid Res. 39, 1594–600. [PubMed] [Google Scholar]
- 18.Fitzner D, Bader JM, Penkert H, Bergner CG, Su M, Weil MT, Surma MA, Mann M, Klose C & Simons M (2020) Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome, Cell Rep. 32, 108132. [DOI] [PubMed] [Google Scholar]
- 19.Network BICC (2021) A multimodal cell census and atlas of the mammalian primary motor cortex, Nature. 598, 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallett PJ, Huebecker M, Brekk OR, Moloney EB, Rocha EM, Priestman DA, Platt FM & Isacson O (2018) Glycosphingolipid levels and glucocerebrosidase activity are altered in normal aging of the mouse brain, Neurobiol Aging. 67, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallom KL, Fernandez-Suarez ME, Priestman DA, Te Vruchte D, Huebecker M, Hallett PJ, Isacson O & Platt FM (2021) Glycosphingolipid metabolism and its role in ageing and Parkinson’s disease, Glycoconj J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T, Schielke E, Straube E & German Association of Neuro, A. u. N.-I. (2017) HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment, J Neurol. 264, 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crook H, Raza S, Nowell J, Young M & Edison P (2021) Long covid-mechanisms, risk factors, and management, BMJ. 374, n1648. [DOI] [PubMed] [Google Scholar]
- 24.Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE & Smith SM (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank, Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H & Frisen J (2017) The Lifespan and Turnover of Microglia in the Human Brain, Cell Rep. 20, 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnar Z, Cragg MS, Garaschuk O, Perry VH & Gomez-Nicola D (2017) Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain, Cell Rep. 18, 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menassa DA, Muntslag TAO, Martin-Estebane M, Barry-Carroll L, Chapman MA, Adorjan I, Tyler T, Turnbull B, Rose-Zerilli MJJ, Nicoll JAR, Krsnik Z, Kostovic I & Gomez-Nicola D (2022) The spatiotemporal dynamics of microglia across the human lifespan, Dev Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyoneva S, Hosur R, Gosselin D, Zhang B, Ouyang Z, Cotleur AC, Peterson M, Allaire N, Challa R, Cullen P, Roberts C, Miao K, Reynolds TL, Glass CK, Burkly L & Ransohoff RM (2019) Cx3cr1-deficient microglia exhibit a premature aging transcriptome, Life Sci Alliance. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L & Wyss-Coray T (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain, Nat Neurosci. 23, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olzmann JA & Carvalho P (2019) Dynamics and functions of lipid droplets, Nat Rev Mol Cell Biol. 20, 137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauro C & Limatola C (2020) Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response, Front Immunol. 11, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blank M, Enzlein T & Hopf C (2022) LPS-induced lipid alterations in microglia revealed by MALDI mass spectrometry-based cell fingerprinting in neuroinflammation studies, Sci Rep. 12, 2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns JC, Cotleur B, Walther DM, Bajrami B, Rubino SJ, Wei R, Franchimont N, Cotman SL, Ransohoff RM & Mingueneau M (2020) Differential accumulation of storage bodies with aging defines discrete subsets of microglia in the healthy brain, Elife. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M & Barres BA (2018) Normal aging induces A1-like astrocyte reactivity, Proc Natl Acad Sci U S A. 115, E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borgmeyer M, Coman C, Has C, Schott HF, Li T, Westhoff P, Cheung YFH, Hoffmann N, Yuanxiang P, Behnisch T, Gomes GM, Dumenieu M, Schweizer M, Chocholouskova M, Holcapek M, Mikhaylova M, Kreutz MR & Ahrends R (2021) Multiomics of synaptic junctions reveals altered lipid metabolism and signaling following environmental enrichment, Cell Rep. 37, 109797. [DOI] [PubMed] [Google Scholar]
- 36.Hallett PJ, Engelender S & Isacson O (2019) Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease, J Neuroinflammation. 16, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, Gavin AC & Superti-Furga G (2015) A Conserved Circular Network of Coregulated Lipids Modulates Innate Immune Responses, Cell. 162, 170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruysschaert JM & Lonez C (2015) Role of lipid microdomains in TLR-mediated signalling, Biochim Biophys Acta. 1848, 1860–7. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL & Tall AR (2009) Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K, Circ Res. 104, 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiba Y, Komori H, Takei S, Hasegawa-Ishii S, Kawamura N, Adachi K, Nanba E, Hosokawa M, Enokido Y, Kouchi Z, Yoshida F & Shimada A (2014) Niemann-Pick disease type C1 predominantly involving the frontotemporal region, with cortical and brainstem Lewy bodies: an autopsy case, Neuropathology. 34, 49–57. [DOI] [PubMed] [Google Scholar]
- 41.Villemagne VL, Velakoulis D, Dore V, Bozinoski S, Masters CL, Rowe CC & Walterfang M (2019) Imaging of tau deposits in adults with Niemann-Pick type C disease: a case-control study, Eur J Nucl Med Mol Imaging. 46, 1132–1138. [DOI] [PubMed] [Google Scholar]
- 42.Cholesterol Treatment Trialists C (2019) Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials, Lancet. 393, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CH, Chang CH, Tai CH, Cheng MF, Chen YC, Chao YT, Huang TL, Yen RF & Wu RM (2021) A Double-Blind, Randomized, Controlled Trial of Lovastatin in Early-Stage Parkinson’s Disease, Mov Disord. 36, 1229–1237. [DOI] [PubMed] [Google Scholar]
- 44.Yu T & Lieberman AP (2013) Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin, PLoS Genet. 9, e1003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu T, Shakkottai VG, Chung C & Lieberman AP (2011) Temporal and cell-specific deletion establishes that neuronal Npc1 deficiency is sufficient to mediate neurodegeneration, Hum Mol Genet. 20, 4440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elrick MJ, Pacheco CD, Yu T, Dadgar N, Shakkottai VG, Ware C, Paulson HL & Lieberman AP (2010) Conditional Niemann-Pick C mice demonstrate cell autonomous Purkinje cell neurodegeneration, Hum Mol Genet. 19, 837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mistry PK, Lopez G, Schiffmann R, Barton NW, Weinreb NJ & Sidransky E (2017) Gaucher disease: Progress and ongoing challenges, Mol Genet Metab. 120, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidransky E & Lopez G (2012) The link between the GBA gene and parkinsonism, Lancet Neurol. 11, 986–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, Peng J, Liao Z, Locascio JJ, Corvol JC, Zhu F, Dong X, Maple-Grodem J, Campbell MC, Elbaz A, Lesage S, Brice A, Mangone G, Growdon JH, Hung AY, Schwarzschild MA, Hayes MT, Wills AM, Herrington TM, Ravina B, Shoulson I, Taba P, Koks S, Beach TG, Cormier-Dequaire F, Alves G, Tysnes OB, Perlmutter JS, Heutink P, Amr SS, van Hilten JJ, Kasten M, Mollenhauer B, Trenkwalder C, Klein C, Barker RA, Williams-Gray CH, Marinus J, International Genetics of Parkinson Disease Progression, C. & Scherzer, C. R. (2021) Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson’s disease, Nat Genet. 53, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan MMX, Lawton MA, Jabbari E, Reynolds RH, Iwaki H, Blauwendraat C, Kanavou S, Pollard MI, Hubbard L, Malek N, Grosset KA, Marrinan SL, Bajaj N, Barker RA, Burn DJ, Bresner C, Foltynie T, Wood NW, Williams-Gray CH, Hardy J, Nalls MA, Singleton AB, Williams NM, Ben-Shlomo Y, Hu MTM, Grosset DG, Shoai M & Morris HR (2021) Genome-Wide Association Studies of Cognitive and Motor Progression in Parkinson’s Disease, Mov Disord. 36, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brekk OR, Moskites A, Isacson O & Hallett PJ (2018) Lipid-dependent deposition of alpha-synuclein and Tau on neuronal Secretogranin II-positive vesicular membranes with age, Sci Rep. 8, 15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brekk OR, Honey JR, Lee S, Hallett PJ & Isacson O (2020) Cell type-specific lipid storage changes in Parkinson’s disease patient brains are recapitulated by experimental glycolipid disturbance, Proc Natl Acad Sci U S A. 117, 27646–27654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moloney EB, Moskites A, Ferrari EJ, Isacson O & Hallett PJ (2018) The glycoprotein GPNMB is selectively elevated in the substantia nigra of Parkinson’s disease patients and increases after lysosomal stress, Neurobiol Dis. 120, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocha EM, Smith GA, Park E, Cao H, Graham AR, Brown E, McLean JR, Hayes MA, Beagan J, Izen SC, Perez-Torres E, Hallett PJ & Isacson O (2015) Sustained Systemic Glucocerebrosidase Inhibition Induces Brain alpha-Synuclein Aggregation, Microglia and Complement C1q Activation in Mice, Antioxid Redox Signal. 23, 550–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brekk OR, Korecka JA, Crapart CC, Huebecker M, MacBain ZK, Rosenthal SA, Sena-Esteves M, Priestman DA, Platt FM, Isacson O & Hallett PJ (2020) Upregulating beta-hexosaminidase activity in rodents prevents alpha-synuclein lipid associations and protects dopaminergic neurons from alpha-synuclein-mediated neurotoxicity, Acta Neuropathol Commun. 8, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki K, Iseki E, Togo T, Yamaguchi A, Katsuse O, Katsuyama K, Kanzaki S, Shiozaki K, Kawanishi C, Yamashita S, Tanaka Y, Yamanaka S & Hirayasu Y (2007) Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses, Acta Neuropathol. 114, 481–9. [DOI] [PubMed] [Google Scholar]
- 57.Rocha EM, Smith GA, Park E, Cao H, Brown E, Hayes MA, Beagan J, McLean JR, Izen SC, Perez-Torres E, Hallett PJ & Isacson O (2015) Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons, Neurobiol Dis. 82, 495–503. [DOI] [PubMed] [Google Scholar]
- 58.Sucunza D, Rico AJ, Roda E, Collantes M, Gonzalez-Aseguinolaza G, Rodriguez-Perez AI, Penuelas I, Vazquez A, Labandeira-Garcia JL, Broccoli V & Lanciego JL (2021) Glucocerebrosidase Gene Therapy Induces Alpha-Synuclein Clearance and Neuroprotection of Midbrain Dopaminergic Neurons in Mice and Macaques, Int J Mol Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H & Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17, Nature. 442, 916–9. [DOI] [PubMed] [Google Scholar]
- 60.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S & Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21, Nature. 442, 920–4. [DOI] [PubMed] [Google Scholar]
- 61.Bossu P, Salani F, Alberici A, Archetti S, Bellelli G, Galimberti D, Scarpini E, Spalletta G, Caltagirone C, Padovani A & Borroni B (2011) Loss of function mutations in the progranulin gene are related to pro-inflammatory cytokine dysregulation in frontotemporal lobar degeneration patients, J Neuroinflammation. 8, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galimberti D, Bonsi R, Fenoglio C, Serpente M, Cioffi SM, Fumagalli G, Arighi A, Ghezzi L, Arcaro M, Mercurio M, Rotondo E & Scarpini E (2015) Inflammatory molecules in Frontotemporal Dementia: cerebrospinal fluid signature of progranulin mutation carriers, Brain Behav Immun. 49, 182–7. [DOI] [PubMed] [Google Scholar]
- 63.Boland S, Swarup S, Ambaw YA, Richards RC, Fischer AW, Singh S, Aggarwal G, Spina S, Nana AL, Grinberg LT, Seeley WW, Surma MA, Klose C, Paulo JA, Nguyen AD, Harper JW, Walther TC & Farese RV (2021) Progranulin deficiency results in reduced bis(monoacylglycero)phosphate (BMP) levels and gangliosidosis., bioRxiv. [Google Scholar]
- 64.Evers BM, Rodriguez-Navas C, Tesla RJ, Prange-Kiel J, Wasser CR, Yoo KS, McDonald J, Cenik B, Ravenscroft TA, Plattner F, Rademakers R, Yu G, White CL 3rd & Herz J (2017) Lipidomic and Transcriptomic Basis of Lysosomal Dysfunction in Progranulin Deficiency, Cell Rep. 20, 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen AD, Nguyen TA, Singh RK, Eberle D, Zhang J, Abate JP, Robles A, Koliwad S, Huang EJ, Maxfield FR, Walther TC & Farese RV Jr. (2018) Progranulin in the hematopoietic compartment protects mice from atherosclerosis, Atherosclerosis. 277, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ & Isacson O (2008) Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years, Nat Med. 14, 507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW & Lewis J (2010) Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging, Am J Pathol. 177, 311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray DA & Woulfe J (2005) Lipofuscin and aging: a matter of toxic waste, Sci Aging Knowledge Environ. 2005, re1. [DOI] [PubMed] [Google Scholar]
- 69.Moreno-Garcia A, Kun A, Calero O, Medina M & Calero M (2018) An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration, Front Neurosci. 12, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ward ME, Chen R, Huang HY, Ludwig C, Telpoukhovskaia M, Taubes A, Boudin H, Minami SS, Reichert M, Albrecht P, Gelfand JM, Cruz-Herranz A, Cordano C, Alavi MV, Leslie S, Seeley WW, Miller BL, Bigio E, Mesulam MM, Bogyo MS, Mackenzie IR, Staropoli JF, Cotman SL, Huang EJ, Gan L & Green AJ (2017) Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis, Sci Transl Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kohli J, Wang B, Brandenburg SM, Basisty N, Evangelou K, Varela-Eirin M, Campisi J, Schilling B, Gorgoulis V & Demaria M (2021) Algorithmic assessment of cellular senescence in experimental and clinical specimens, Nat Protoc. 16, 2471–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logan T, Simon MJ, Rana A, Cherf GM, Srivastava A, Davis SS, Low RLY, Chiu CL, Fang M, Huang F, Bhalla A, Llapashtica C, Prorok R, Pizzo ME, Calvert MEK, Sun EW, Hsiao-Nakamoto J, Rajendra Y, Lexa KW, Srivastava DB, van Lengerich B, Wang J, Robles-Colmenares Y, Kim DJ, Duque J, Lenser M, Earr TK, Nguyen H, Chau R, Tsogtbaatar B, Ravi R, Skuja LL, Solanoy H, Rosen HJ, Boeve BF, Boxer AL, Heuer HW, Dennis MS, Kariolis MS, Monroe KM, Przybyla L, Sanchez PE, Meisner R, Diaz D, Henne KR, Watts RJ, Henry AG, Gunasekaran K, Astarita G, Suh JH, Lewcock JW, DeVos SL & Di Paolo G (2021) Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic, Cell. 184, 4651–4668 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyakawa S, Sakuma H, Warude D, Asanuma S, Arimura N, Yoshihara T, Tavares D, Hata A, Ida K, Hori Y, Okuzono Y, Yamamoto S, Iida K, Shimizu H, Kondo S & Sato S (2020) Anti-sortilin1 Antibody Up-Regulates Progranulin via Sortilin1 Down-Regulation, Front Neurosci. 14, 586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arrant AE, Onyilo VC, Unger DE & Roberson ED (2018) Progranulin Gene Therapy Improves Lysosomal Dysfunction and Microglial Pathology Associated with Frontotemporal Dementia and Neuronal Ceroid Lipofuscinosis, J Neurosci. 38, 2341–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amado DA, Rieders JM, Diatta F, Hernandez-Con P, Singer A, Mak JT, Zhang J, Lancaster E, Davidson BL & Chen-Plotkin AS (2019) AAV-Mediated Progranulin Delivery to a Mouse Model of Progranulin Deficiency Causes T Cell-Mediated Toxicity, Mol Ther. 27, 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nalls MA, Blauwendraat C, Vallerga CL, Heilbron K, Bandres-Ciga S, Chang D, Tan M, Kia DA, Noyce AJ, Xue A, Bras J, Young E, von Coelln R, Simon-Sanchez J, Schulte C, Sharma M, Krohn L, Pihlstrom L, Siitonen A, Iwaki H, Leonard H, Faghri F, Gibbs JR, Hernandez DG, Scholz SW, Botia JA, Martinez M, Corvol JC, Lesage S, Jankovic J, Shulman LM, Sutherland M, Tienari P, Majamaa K, Toft M, Andreassen OA, Bangale T, Brice A, Yang J, Gan-Or Z, Gasser T, Heutink P, Shulman JM, Wood NW, Hinds DA, Hardy JA, Morris HR, Gratten J, Visscher PM, Graham RR, Singleton AB, andMe Research, T., System Genomics of Parkinson’s Disease, C. & International Parkinson’s Disease Genomics, C. (2019) Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies, Lancet Neurol. 18, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwartzentruber J, Cooper S, Liu JZ, Barrio-Hernandez I, Bello E, Kumasaka N, Young AMH, Franklin RJM, Johnson T, Estrada K, Gaffney DJ, Beltrao P & Bassett A (2021) Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes, Nat Genet. 53, 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klemann C, Martens GJM, Sharma M, Martens MB, Isacson O, Gasser T, Visser JE & Poelmans G (2017) Integrated molecular landscape of Parkinson’s disease, NPJ Parkinsons Dis. 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Linden RJ, Reus LM, De Witte W, Tijms BM, Olde Rikkert M, Visser PJ & Poelmans G (2021) Genetic overlap between Alzheimer’s disease and blood lipid levels, Neurobiol Aging. 108, 189–195. [DOI] [PubMed] [Google Scholar]
- 80.van Rheenen, van der Spek W, Bakker RAA, van Vugt MK, Hop J, Zwamborn PJ, de Klein RAJ, Westra N, Bakker HJ, Deelen OB, Shireby P, Hannon G, Moisse E, Baird M, Restuadi D, Dolzhenko R, Dekker E, Gawor AM, Westeneng K, Tazelaar HJ, van Eijk GHP, Kooyman KR, Byrne M, Doherty RP, Heverin M, Al Khleifat M, Iacoangeli A, Shatunov A, Ticozzi A, Cooper-Knock N, Smith J, Gromicho BN, Chandran M, Pal S, Morrison S, Shaw KE, Hardy PJ, Orrell J, Sendtner RW, Meyer M, Basak T, van der Kooi N, Ratti AJ, Fogh A, Gellera I, Lauria C, Corti G, Cereda S, Sproviero C, D’Alfonso D, Soraru S, Siciliano G, Filosto G, Padovani M, Chio A, Calvo A, Moglia A, Brunetti C, Canosa M, Grassano A, Beghi M, Pupillo E, Logroscino E, Nefussy G, Osmanovic B, Nordin A, Lerner A, Zabari Y, Gotkine M, Baloh M, Bell RH, Vourc’h S, Corcia P, Couratier P, Millecamps P, Meininger S, Salachas V, Mora Pardina F, Assialioui JS, Rojas-Garcia A, Dion R, Ross PA, Ludolph JP, Weishaupt AC, Brenner JH, Freischmidt D, Bensimon A, Brice G, Durr A, Payan A, Saker-Delye CAM, Wood S, Topp NW, Rademakers S, Tittmann R, Lieb L, Franke W, Ripke A, Braun S, Kraft A, J., et al. (2021) Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology, Nat Genet. 53, 1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Brien JT & Thomas A (2015) Vascular dementia, Lancet. 386, 1698–706. [DOI] [PubMed] [Google Scholar]
- 82.Iadecola C (2013) The pathobiology of vascular dementia, Neuron. 80, 844–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwagami M, Qizilbash N, Gregson J, Douglas I, Johnson M, Pearce N, Evans S & Pocock S (2021) Blood cholesterol and risk of dementia in more than 1.8 million people over two decades: a retrospective cohort study., The Lancet Healthy Longevity. 2, E498–E506. [DOI] [PubMed] [Google Scholar]
- 84.Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, Peng L, Hu D, Bontha SV, Srikakulapu P, Beer M, Megens RTA, Steffens S, Hildner M, Halder LD, Eckstein HH, Pelisek J, Herms J, Roeber S, Arzberger T, Borodovsky A, Habenicht L, Binder CJ, Weber C, Zipfel PF, Skerka C & Habenicht AJR (2019) ApoE attenuates unresolvable inflammation by complex formation with activated C1q, Nat Med. 25, 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nott A, Holtman IR, Coufal NG, Schlachetzki JCM, Yu M, Hu R, Han CZ, Pena M, Xiao J, Wu Y, Keulen Z, Pasillas MP, O’Connor C, Nickl CK, Schafer ST, Shen Z, Rissman RA, Brewer JB, Gosselin D, Gonda DD, Levy ML, Rosenfeld MG, McVicker G, Gage FH, Ren B & Glass CK (2019) Brain cell type-specific enhancer-promoter interactome maps and disease-risk association, Science. 366, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raposo G & Marks MS (2007) Melanosomes--dark organelles enlighten endosomal membrane transport, Nat Rev Mol Cell Biol. 8, 786–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delevoye C, Marks MS & Raposo G (2019) Lysosome-related organelles as functional adaptations of the endolysosomal system, Curr Opin Cell Biol. 59, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, Cartwright MI, Griffiths FM, Shepherd CE & Double KL (2005) Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease, Brain. 128, 2654–64. [DOI] [PubMed] [Google Scholar]
- 89.Zhao C & Dahlman-Wright K (2010) Liver X receptor in cholesterol metabolism, J Endocrinol. 204, 233–40. [DOI] [PubMed] [Google Scholar]
- 90.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ & Tontonoz P (2001) LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes, Proc Natl Acad Sci U S A. 98, 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mallick S, Marshall PA, Wagner CE, Heck MC, Sabir ZL, Sabir MS, Dussik CM, Grozic A, Kaneko I & Jurutka PW (2021) Evaluating Novel RXR Agonists That Induce ApoE and Tyrosine Hydroxylase in Cultured Human Glioblastoma Cells, ACS Chem Neurosci. 12, 857–871. [DOI] [PubMed] [Google Scholar]
- 92.Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G & Bernini F (2009) Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1, Biochemistry. 48, 11067–74. [DOI] [PubMed] [Google Scholar]
- 93.Vaughan AM & Oram JF (2005) ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins, J Biol Chem. 280, 30150–7. [DOI] [PubMed] [Google Scholar]
- 94.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L & Jessup W (2006) ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I, Arterioscler Thromb Vasc Biol. 26, 534–40. [DOI] [PubMed] [Google Scholar]
- 95.Vaughan AM & Oram JF (2006) ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL, J Lipid Res. 47, 2433–43. [DOI] [PubMed] [Google Scholar]
- 96.Xu D, Li Y, Yang F, Sun CR, Pan J, Wang L, Chen ZP, Fang SC, Yao X, Hou WT, Zhou CZ & Chen Y (2022) Structure and transport mechanism of the human cholesterol transporter ABCG1, Cell Rep. 38, 110298. [DOI] [PubMed] [Google Scholar]
- 97.Kidani Y & Bensinger SJ (2012) Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity, Immunol Rev. 249, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA & Landreth GE (2012) ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models, Science. 335, 1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A, Pontecorvo M, Devous M, Tang A & Bena J (2016) Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease, Alzheimers Res Ther. 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davis AA, Inman CE, Wargel ZM, Dube U, Freeberg BM, Galluppi A, Haines JN, Dhavale DD, Miller R, Choudhury FA, Sullivan PM, Cruchaga C, Perlmutter JS, Ulrich JD, Benitez BA, Kotzbauer PT & Holtzman DM (2020) APOE genotype regulates pathology and disease progression in synucleinopathy, Sci Transl Med. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS & Roses AD (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease, Proc Natl Acad Sci U S A. 90, 1977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL & Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families, Science. 261, 921–3. [DOI] [PubMed] [Google Scholar]
- 103.Szwedo AA, Dalen I, Pedersen KF, Camacho M, Backstrom D, Forsgren L, Tzoulis C, Winder-Rhodes S, Hudson G, Liu G, Scherzer CR, Lawson RA, Yarnall AJ, Williams-Gray CH, Macleod AD, Counsell CE, Tysnes OB, Alves G, Maple-Grodem J & Parkinson’s Incidence Cohorts C (2022) GBA and APOE Impact Cognitive Decline in Parkinson’s Disease: A 10-Year Population-Based Study, Mov Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosas I, Martinez C, Coto E, Clarimon J, Lleo A, Illan-Gala I, Dols-Icardo O, Borroni B, Almeida MR, van der Zee J, Van Broeckhoven C, Consortium EE, Bruni AC, Anfossi M, Bernardi L, Maletta R, Serpente M, Galimberti D, Scarpini E, Rossi G, Caroppo P, Benussi L, Ghidoni R, Binetti G, Nacmias B, Sorbi S, Piaceri I, Bagnoli S, Antonell A, Sanchez-Valle R, De la Casa-Fages B, Grandas F, Diez-Fairen M, Pastor P, Ferrari R, Ifgc, Queimalinos-Perez D, Perez-Oliveira S, Alvarez V & Menendez-Gonzalez M (2021) Genetic variation in APOE, GRN, and TP53 are phenotype modifiers in frontotemporal dementia, Neurobiol Aging. 99, 99 e15–99 e22. [DOI] [PubMed] [Google Scholar]
- 105.Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM & Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated, Brain. 141, 2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu R, Yanjanin NM, Elrick MJ, Ware C, Lieberman AP & Porter FD (2012) Apolipoprotein E genotype and neurological disease onset in Niemann-Pick disease, type C1, Am J Med Genet A. 158A, 2775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koch M, Furtado JD, Falk K, Leypoldt F, Mukamal KJ & Jensen MK (2017) Apolipoproteins and their subspecies in human cerebrospinal fluid and plasma, Alzheimers Dement (Amst). 6, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toledo JB, Da X, Weiner MW, Wolk DA, Xie SX, Arnold SE, Davatzikos C, Shaw LM, Trojanowski JQ & Alzheimer’s Disease Neuroimaging I (2014) CSF Apo-E levels associate with cognitive decline and MRI changes, Acta Neuropathol. 127, 621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP & Nielsen HM (2014) Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls, Acta Neuropathol. 127, 633–43. [DOI] [PubMed] [Google Scholar]
- 110.Linton MF, Gish R, Hubl ST, Butler E, Esquivel C, Bry WI, Boyles JK, Wardell MR & Young SG (1991) Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation, J Clin Invest. 88, 270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Edlund AK, Chen K, Lee W, Protas H, Su Y, Reiman E, Caselli R & Nielsen HM (2021) Plasma Apolipoprotein E3 and Glucose Levels Are Associated in APOE varepsilon3/varepsilon4 Carriers, J Alzheimers Dis. 81, 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arbizu J, Festari C, Altomare D, Walker Z, Bouwman F, Rivolta J, Orini S, Barthel H, Agosta F, Drzezga A, Nestor P, Boccardi M, Frisoni GB, Nobili F & Disorders E.-E. T. F. f. t. P. o. F.-P. f. D. N. (2018) Clinical utility of FDG-PET for the clinical diagnosis in MCI, Eur J Nucl Med Mol Imaging. 45, 1497–1508. [DOI] [PubMed] [Google Scholar]
- 113.Greaves CV & Rohrer JD (2019) An update on genetic frontotemporal dementia, J Neurol. 266, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA & Kuhl DE (2011) Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study, J Nucl Med. 52, 848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meles SK, Teune LK, de Jong BM, Dierckx RA & Leenders KL (2017) Metabolic Imaging in Parkinson Disease, J Nucl Med. 58, 23–28. [DOI] [PubMed] [Google Scholar]
- 116.Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S & et al. (1994) The metabolic topography of parkinsonism, J Cereb Blood Flow Metab. 14, 783–801. [DOI] [PubMed] [Google Scholar]
- 117.Kuo CL, Pilling LC, Atkins JL, Masoli JAH, Delgado J, Kuchel GA & Melzer D (2020) APOE e4 Genotype Predicts Severe COVID-19 in the UK Biobank Community Cohort, J Gerontol A Biol Sci Med Sci 75, 2231–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurki SN, Kantonen J, Kaivola K, Hokkanen L, Mayranpaa MI, Puttonen H, FinnGen, Martola J, Poyhonen M, Kero M, Tuimala J, Carpen O, Kantele A, Vapalahti O, Tiainen M, Tienari PJ, Kaila K, Hastbacka J & Myllykangas L (2021) APOE epsilon4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a Finnish biobank, autopsy and clinical study, Acta Neuropathol Commun. 9, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lefterov I, Wolfe CM, Fitz NF, Nam KN, Letronne F, Biedrzycki RJ, Kofler J, Han X, Wang J, Schug J & Koldamova R (2019) APOE2 orchestrated differences in transcriptomic and lipidomic profiles of postmortem AD brain, Alzheimers Res Ther. 11, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zalocusky KA, Najm R, Taubes AL, Hao Y, Yoon SY, Koutsodendris N, Nelson MR, Rao A, Bennett DA, Bant J, Amornkul DJ, Xu Q, An A, Cisne-Thomson O & Huang Y (2021) Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease, Nat Neurosci. 24, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shi Y, Andhey PS, Ising C, Wang K, Snipes LL, Boyer K, Lawson S, Yamada K, Qin W, Manis M, Serrano JR, Benitez BA, Schmidt RE, Artyomov M, Ulrich JD & Holtzman DM (2021) Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms, Neuron. 109, 2413–2426 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Victor MB, Leary N, Luna X, Meharena HS, Bozzelli PL, Samaan G, Murdock MH, von Maydell D, Effenberger AH, Cerit O, Wen H-L, Liu L, Welch G, Bonner M & Tsai L-H (2022) Lipid accumulation induced by APOE impairs microglial surveillance of neuronal-network activity., bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW & Barres BA (2007) The classical complement cascade mediates CNS synapse elimination, Cell. 131, 1164–78. [DOI] [PubMed] [Google Scholar]
- 124.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA & Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner, Neuron. 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ & Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models, Science. 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi Q, Chowdhury S, Ma R, Le KX, Hong S, Caldarone BJ, Stevens B & Lemere CA (2017) Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice, Sci Transl Med. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Panitch R, Hu J, Chung J, Zhu C, Meng G, Xia W, Bennett DA, Lunetta KL, Ikezu T, Au R, Stein TD, Farrer LA & Jun GR (2021) Integrative brain transcriptome analysis links complement component 4 and HSPA2 to the APOE epsilon2 protective effect in Alzheimer disease, Mol Psychiatry. 26, 6054–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosenberg JB, Kaplitt MG, De BP, Chen A, Flagiello T, Salami C, Pey E, Zhao L, Ricart Arbona RJ, Monette S, Dyke JP, Ballon DJ, Kaminsky SM, Sondhi D, Petsko GA, Paul SM & Crystal RG (2018) AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer’s Disease, Hum Gene Ther Clin Dev. 29, 24–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diaz-Ortiz ME, Seo Y, Posavi M, Carceles Cordon M, Clark E, Jain N, Charan R, Gallagher MD, Unger TL, Amari N, Skrinak RT, Davila-Rivera R, Brody EM, Han N, Zack R, Van Deerlin VM, Tropea TF, Luk KC, Lee EB, Weintraub D & Chen-Plotkin AS (2022) GPNMB confers risk for Parkinson’s disease through interaction with alpha-synuclein, Science. 377, eabk0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Korner A, Zhou E, Muller C, Mohammed Y, Herceg S, Bracher F, Rensen PCN, Wang Y, Mirakaj V & Giera M (2019) Inhibition of Delta24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution, Proc Natl Acad Sci U S A. 116, 20623–20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Eijk M & Aerts J (2021) The Unique Phenotype of Lipid-Laden Macrophages, Int J Mol Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Atagi Y, Liu CC, Painter MM, Chen XF, Verbeeck C, Zheng H, Li X, Rademakers R, Kang SS, Xu H, Younkin S, Das P, Fryer JD & Bu G (2015) Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2), J Biol Chem. 290, 26043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bailey CC, DeVaux LB & Farzan M (2015) The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E, J Biol Chem. 290, 26033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yeh FL, Wang Y, Tom I, Gonzalez LC & Sheng M (2016) TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia, Neuron. 91, 328–40. [DOI] [PubMed] [Google Scholar]
- 135.Nugent AA, Lin K, van Lengerich B, Lianoglou S, Przybyla L, Davis SS, Llapashtica C, Wang J, Kim DJ, Xia D, Lucas A, Baskaran S, Haddick PCG, Lenser M, Earr TK, Shi J, Dugas JC, Andreone BJ, Logan T, Solanoy HO, Chen H, Srivastava A, Poda SB, Sanchez PE, Watts RJ, Sandmann T, Astarita G, Lewcock JW, Monroe KM & Di Paolo G (2020) TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge, Neuron. 105, 837–854 e9. [DOI] [PubMed] [Google Scholar]
- 136.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M & Amit I (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease, Cell. 169, 1276–1290 e17. [DOI] [PubMed] [Google Scholar]
- 137.Griciuc A, Patel S, Federico AN, Choi SH, Innes BJ, Oram MK, Cereghetti G, McGinty D, Anselmo A, Sadreyev RI, Hickman SE, El Khoury J, Colonna M & Tanzi RE (2019) TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease, Neuron. 103, 820–835 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reifschneider A, Robinson S, van Lengerich B, Gnorich J, Logan T, Heindl S, Vogt MA, Weidinger E, Riedl L, Wind K, Zatcepin A, Pesamaa I, Haberl S, Nuscher B, Kleinberger G, Klimmt J, Gotzl JK, Liesz A, Burger K, Brendel M, Levin J, Diehl-Schmid J, Suh J, Di Paolo G, Lewcock JW, Monroe KM, Paquet D, Capell A & Haass C (2022) Loss of TREM2 rescues hyperactivation of microglia, but not lysosomal deficits and neurotoxicity in models of progranulin deficiency, EMBO J. 41, e109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gotzl JK, Brendel M, Werner G, Parhizkar S, Sebastian Monasor L, Kleinberger G, Colombo AV, Deussing M, Wagner M, Winkelmann J, Diehl-Schmid J, Levin J, Fellerer K, Reifschneider A, Bultmann S, Bartenstein P, Rominger A, Tahirovic S, Smith ST, Madore C, Butovsky O, Capell A & Haass C (2019) Opposite microglial activation stages upon loss of PGRN or TREM2 result in reduced cerebral glucose metabolism, EMBO Mol Med. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR & Colonna M (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model, Cell. 160, 1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, Lundgren P, Bleriot C, Liu Z, Deczkowska A, Keren-Shaul H, David E, Zmora N, Eldar SM, Lubezky N, Shibolet O, Hill DA, Lazar MA, Colonna M, Ginhoux F, Shapiro H, Elinav E & Amit I (2019) Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner, Cell. 178, 686–698 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S, Mustafa M, Yuede CM, Salazar SV, Kong P, Long H, Ward M, Siddiqui O, Paul R, Gilfillan S, Ibrahim A, Rhinn H, Tassi I, Rosenthal A, Schwabe T & Colonna M (2020) Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model, J Exp Med. 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schlepckow K, Monroe KM, Kleinberger G, Cantuti-Castelvetri L, Parhizkar S, Xia D, Willem M, Werner G, Pettkus N, Brunner B, Sulzen A, Nuscher B, Hampel H, Xiang X, Feederle R, Tahirovic S, Park JI, Prorok R, Mahon C, Liang CC, Shi J, Kim DJ, Sabelstrom H, Huang F, Di Paolo G, Simons M, Lewcock JW & Haass C (2020) Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region, EMBO Mol Med. 12, e11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Price BR, Sudduth TL, Weekman EM, Johnson S, Hawthorne D, Woolums A & Wilcock DM (2020) Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition, J Neuroinflammation. 17, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huebecker M, Moloney EB, van der Spoel AC, Priestman DA, Isacson O, Hallett PJ & Platt FM (2019) Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease, Mol Neurodegener. 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xicoy H, Brouwers JF, Wieringa B & Martens GJM (2020) Explorative Combined Lipid and Transcriptomic Profiling of Substantia Nigra and Putamen in Parkinson’s Disease, Cells. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gegg ME, Sweet L, Wang BH, Shihabuddin LS, Sardi SP & Schapira AH (2015) No evidence for substrate accumulation in Parkinson brains with GBA mutations, Mov Disord. 30, 1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beger AW, Dudzik B, Woltjer RL & Wood PL (2022) Human Brain Lipidomics: Pilot Analysis of the Basal Ganglia Sphingolipidome in Parkinson’s Disease and Lewy Body Disease, Metabolites. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, Kim WS, McCann H, Wenk MR, Halliday GM & Garner B (2011) Lipid pathway alterations in Parkinson’s disease primary visual cortex, PLoS One. 6, e17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barbash S, Garfinkel BP, Maoz R, Simchovitz A, Nadorp B, Guffanti A, Bennett ER, Nadeau C, Turk A, Paul L, Reda T, Li Y, Buchman AS, Greenberg DS, Seitz A, Bennett DA, Giavalisco P & Soreq H (2017) Alzheimer’s brains show inter-related changes in RNA and lipid metabolism, Neurobiol Dis. 106, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Akyol S, Ugur Z, Yilmaz A, Ustun I, Gorti SKK, Oh K, McGuinness B, Passmore P, Kehoe PG, Maddens ME, Green BD & Graham SF (2021) Lipid Profiling of Alzheimer’s Disease Brain Highlights Enrichment in Glycerol(phospho)lipid, and Sphingolipid Metabolism, Cells. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, Wenk MR, Shui G & Di Paolo G (2012) Comparative lipidomic analysis of mouse and human brain with Alzheimer disease, J Biol Chem. 287, 2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


