Abstract
Posttranslational modifications (PTMs) are covalent modifications of proteins that modulate the structure and functions of proteins and regulate biological processes. The development of various mass spectrometry-based proteomics workflows has facilitated the identification of hundreds of PTMs and aided the understanding of biological significance in a high throughput manner. Improvements in sample preparation and PTM enrichment techniques, instrumentation for liquid chromatography-tandem mass spectrometry (LC-MS/MS), and advanced data analysis tools enhance the specificity and sensitivity of PTM identification. Highly prevalent PTMs like phosphorylation, glycosylation, acetylation, ubiquitinylation, and methylation are extensively studied. However, the functions and impact of less abundant PTMs are not as well understood and underscore the need for analytical methods that aim to characterize these PTMs. This review focuses on the advancement and analytical challenges associated with the characterization of three less common but biologically relevant PTMs, specifically, adenosine diphosphate-ribosylation, tyrosine sulfation, and tyrosine nitration. The advantages and disadvantages of various enrichment, separation, and MS/MS techniques utilized to identify and localize these PTMs are described.
Keywords: ADP-ribosylation, ADP-ribosylome, nitroproteome, proteomics, sulfoproteome, tandem mass spectrometry, tyrosine nitration, tyrosine sulfation
1 |. INTRODUCTION
Bottom-up proteomics using liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the most common strategy to identify proteins in complex mixtures by matching the experimental MS/MS spectra of proteolytic peptides to the in silico fragmentation patterns in a database and reconstructing the protein sequences. Beyond just the determination of protein sequences, sophisticated MS/MS methods and computational algorithms are essential for aiding the identification and localization of posttranslational modifications (PTMs). PTMs are covalent modifications of amino acid sidechains that play critical roles in mediating protein structure, function, and activity. Decades of development and optimization have cemented the LC-MS/MS workflow as the method of choice for high throughput proteomics, allowing the identification of hundreds or thousands of proteins in each run with impressive sensitivity, accuracy, and reproducibility. Characterization of PTMs is a demanding facet of proteomics, typically requiring robust enrichment methods to target specific types of low-level modifications relative to far more abundant unmodified counterparts. There have been tremendous inroads in the evolution of strategies for enrichment and identification of the most prevalent PTMs, such as phosphorylation (Urban, 2022) and glycosylation (Gabriele et al., 2021; Gutierrez-Reyes et al., 2022). Approaches for targeting structurally complicated or less common PTMs, such as ADP-ribosylation, nitration, and sulfation, among other PTMs, are analytically challenging and continue to evolve. This review focuses on the enrichment, separation, and MS/MS methods employed to identify and localize less prevalent PTMs, specifically ADP-ribosylation, tyrosine sulfation, and tyrosine nitration. Table 1 summarizes the distribution of the five most common PTMs along with three less prevalent PTMs discussed in this review. Although these numbers are not direct evidence of the biological abundance of these PTMs, they represent how these PTMs are under-characterized and are less prevalent in the literature.
TABLE 1.
Summary of the number of reviewed proteins listed in the UniprotKB database searched by a unique keyword (KW)a
| Code | Keyword | # Reviewed proteins | # Human proteins |
|---|---|---|---|
|
| |||
| KW-9991 | All PTMs | 122,905 | 13,951 |
| KW-0597 | Phosphoprotein | 48,652 | 8245 |
| KW-0325 | Glycoprotein | 32,275 | 4686 |
| KW-0007 | Acetylation | 18,417 | 3419 |
| KW-0832 | Ubiquitinylation | 10,675 | 2517 |
| KW-0488 | Methylation | 8552 | 1001 |
| KW-0013 | ADP-ribosylation | 568 | 105 |
| KW-0765 | Tyrosine sulfation | 548 | 47 |
| KW-0944 | Tyrosine nitration | 243 | 48 |
Note: Each PTM has a unique code assigned. Accessed on 9–1-2022.
More than one PTM can comodify a single protein.
2 |. ADENOSINE DIPHOSPHATE (ADP)-RIBOSYLATION
ADP-ribosylation is a highly conserved PTM in which ADP-ribosyltransferases (ARTs) transfer one (mono-ADP-ribosylation or MARylation) or more (poly-ADP-ribosylation or PARylation) ADP-ribose (ADPr) groups from nicotinamide adenine dinucleotide (NAD+) to substrate proteins (Cohen & Chang, 2018; Lüscher et al., 2018). ADP-ribosylation was first discovered in bacteria and later found in different organisms (Cohen & Chang, 2018; Palazzo et al., 2017; Perina et al., 2014). There are two families of ARTs in humans: (1) diphtheria toxin-like ARTs (ARTDs), commonly known as poly (ADP-ribose) polymerases (PARPs), and (2) clostridial toxin-like ARTs (ARTCs). ARTDs or PARPs are the most extensively studied family of ARTs and consist of 17 members (PARP 1–17) with a common catalytic domain, whereas there are only four members in the ARTC family (discussed in more detail in another review; Lüscher et al., 2018). ARTCs and many ARTDs can only MARylate, while some ARTDs (ARTD-1,2,5,6) can PARylate their substrates (Lüscher et al., 2018; Perina et al., 2014).
Although ADPr groups can modify the sidechains of many different amino-acid residues (Asp/Glu/Ser/Arg/Lys/Asn/Thr/Tyr/His/Cys) in the substrate proteins, the addition of ADPr at Asp/Glu/Ser (O−type glycosidic linkage) and the addition of ADPr at Arg/Lys (N-type glycosidic linkage) are more common (Bartlett et al., 2018; Daniels et al., 2015; Feijs & Žaja, 2022; Hendriks et al., 2021; Larsen et al., 2018; Leslie Pedrioli et al., 2018; Leung, 2017; Rosenthal & Hottiger, 2014). Each ADPr group carries two negative charges at physiological pH, and up to 200 ADPr groups can be added in linear or branched chains via glycosidic linkage making it one of the bulkier types of PTMs as illustrated in Figure 1 (Sukhanova et al., 2020). Therefore, PARylated proteins are highly negatively charged that, along with the additional steric hindrance of the PAR groups, can alter their structure, function, localization, and interaction with other proteins or DNA (Hengel & Goodlett, 2012). While PARylation of proteins disrupts the existing protein-protein interactions, it helps form a scaffold to recruit other proteins at the site of DNA damage for DNA repair or to recruit ubiquitin ligases (ubiquitinylation) where proteasomal degradation is required (Gibson & Kraus, 2012; Leung, 2017). PARPs are known to regulate DNA damage repair, stress response, mitochondrial functions, RNA metabolism, transcription, cell cycle regulation, apoptosis, necrosis, and chromatin regulation, among other functions (Gibson & Kraus, 2012; Lüscher et al., 2018; Palazzo et al., 2015; Perina et al., 2014).
FIGURE 1.
Structure of poly(ADP)-ribose highlighting the different chain architecture and the bulkiness of this PTM. Branching may occur every 20–50 ADP-ribose units and the chain length can be up to 200 ADP-ribose units (Sukhanova et al., 2020).
PARP1, the most thoroughly studied member among the 17 PARPs in humans, plays a significant role in DNA damage repair and is activated upon binding to DNA strand breaks (Ida et al., 2016). Therefore, PARP1 inhibition is a compelling strategy to selectively target cancer cells with defective DNA repair pathways that rely on PARP1 for DNA repair (Leung, 2017). To date, four PARP1 inhibitors are used to treat ovarian and breast cancer (Sachdev et al., 2019; Slade, 2020). Understanding the roles of PARP in DNA damage repair is a critical step in finding potential therapeutic targets for diseases that display dysfunctional PARP signaling pathways. However, many other functional roles of PARPs are not yet fully deciphered in part because of the analytical challenges associated with the global profiling of PAR-modified substrates.
ADP-ribosylation (PARylation or MARylation) is a dynamic and transient PTM, rapidly removed by ADP-ribosyl hydrolases (ARH) and macrodomain (MacroD1, MacroD2, and TARG1) families of proteins (Rack et al., 2020). PAR glycohydrolase (PARG) hydrolyzes the ribose-ribose ether bonds but not the protein-ribose bond, thus converting PARylated substrates to MARylated forms (Abplanalp et al., 2017; Lüscher et al., 2018; Slade et al., 2011). MacroD1, MacroD2, TARG1, and ARH1 remove the MARs by hydrolyzing the glycosidic ester bond between the modified residue and ADPr groups, whereas ARH3 can hydrolyze both PAR and MAR (Abplanalp et al., 2017; Lüscher et al., 2018; Vivelo & Leung, 2015). PAR hydrolases are less specific and act on a broad range of PARylated substrates regardless of the attachment sites, whereas MAR hydrolases exhibit higher site specificity. For example, ARH1 specifically hydrolyzes the ADPr group from modified arginine; ARH3 instead hydrolyzes PAR or MAR group from modified serine (Abplanalp et al., 2017; Rack et al., 2020).
2.1 |. Associated challenges
The rapid synthesis and breakdown of PAR by the action of PARPs and hydrolases make it challenging to determine the levels of PARylation in cells (Ida et al., 2016; Vivelo & Leung, 2015). PARG knockdown cells have been used to overcome the hydrolysis of PAR by PARG; however, this practice is not widely adopted to map the ADP-ribosylated proteome because it alters the physiological relevance of the PTM (Larsen et al., 2017). These experiments require precise timing to capture the PTMs at the most physiological relevant levels (Ida et al., 2016; Vivelo & Leung, 2015). In addition, the stress during cell lysis induces artificial levels of PARylation, whereas the PARG present in cell lysate induces PAR hydrolysis. Therefore, the nondenaturing lysis buffer must be supplemented both with PARP inhibitor to abolish artificially induced PARylation and PARG inhibitor to halt the hydrolysis of PAR (Vivelo & Leung, 2015). Alternatively, denaturing lysis buffer (supplemented with 6 M guanidine-HCl or urea) can be used for more efficient inactivation of the enzymes responsible for PAR hydrolysis or PARylation (Leidecker et al., 2016; Leutert et al., 2018). Because ADPr can modify many possible amino acids that are acidic or basic, developing a universal and unbiased sample preparation and detection method remains an ongoing analytical challenge (Daniels et al., 2015; Gagné et al., 2018; Hendriks et al., 2021; Lüthi et al., 2021; Nowak et al., 2020).
Owing to the low levels of ADP-ribosylation in cells, enrichment is the first step toward characterizing the ADP-ribosylated proteome. Most of the studies are based on damage-induced cells that increase the levels of ADP-ribosylation at the cost of altered physiological relevance. Another obstacle associated with MS-based proteomics of ADP-ribosylation is the labile nature of this PTM that results in complete or partial loss of the ADPr group with traditional ion activation methods like collision-induced dissociation (CID) (Hendriks et al., 2019). This yields CID mass spectra dominated by product ions resulting from the fragmentation of the modification itself. A typical workflow for the analysis of ADP-ribosylation is shown in Figure 2, combining multiple steps, including lysis, digestion, enrichment, and MS/MS analysis, all critical for successfully profiling ADP-ribosylation sites.
FIGURE 2.
Overview of the most widely applicable proteomics method for site-specific characterization of ADP ribosylation using Af1521 macrodomain enrichment. Adapted from Buch-Larsen et al. (2020).
The heterogeneity in attachment sites (Asp/Glu/Ser/Thr/Tyr/His/Lys/Asn/Cys) (Hendriks et al., 2021) also adds a layer of complexity to MS/MS analysis as it requires attaining near-complete sequence coverage for confident localization of the ADPr acceptor site. Electron-based methods are frequently employed to achieve site-specific characterization of ADPr-modified peptides. Finally, the lack of a single unique mass signature for PARylation due to its heterogeneous length (2–200 ADPr subunits) makes it incompatible with many search engines utilized in proteomics which is further exacerbated by the exponentially large search space and the extensive time required to identify one of the many possible PAR attachment sites. Several approaches overcome these hurdles by converting PARylated substrates to MARylated forms or other forms with distinctive mass signatures before MS/MS analysis. A diverse array of methods streamlines the analysis of ADP-ribosylated proteins by modifying the ADPr group to enhance the enrichment process or facilitate MS/MS characterization, as summarized in Figure 3. These approaches enable the determination of MAR/PAR attachment sites at the cost of elucidation of chain length or architecture (linear or branched). In the following sections, various inroads in the development of enrichment, separation, and alternative MS/MS strategies for characterization of the ADPr-modified proteome are described.
FIGURE 3.
Schematic showing PAR modified protein and truncation of ADPr group using different strategies to make PAR/MARylated proteins amenable for MS/MS-based site localization: release of ADPr by hydroxylamine (NH2OH) to generate a +15. 0109 Da tag, treatment with hydrofluoric acid (HF) to generate a ribose tag (+132.0423 Da), digestion with the phosphodiesterase (PDE) to generate phosphoribose tag (+212.0086 Da), and hydrolysis of PAR by PARG/ARH3 to generate an intact MAR (+541.0611 Da, highlighted in grey). Adapted from Vivelo and Leung (2015) with an additional truncation site from Gagné et al. (2018).
2.2 |. ADP-ribosylation: Enrichment strategies
Methods to enrich PAR/MAR-modified substrates are discussed in a recent review by Hopp and Hottiger, 2021 as well as in earlier reviews by Daniels et al. (2015), Rosenthal and Hottiger (2014); Vivelo and Leung (2015). Protein-level enrichment can be divided into three main categories: (1) those that use anti-ADPr or PAR-binding antibodies, (2) those that use ADPr-binding domains, and (3) those that use chemical labeling of NAD+ or ADPr. Alternatively, peptide-level enrichment can be utilized for site-specific characterization of ADP-ribosylation using different strategies including, (1) boronate enrichment, (2) phosphodiesterase (PDE) digestion, (3) hydrofluoric acid derivatization, and (4) Af1521 macrodomain enrichment following PAR hydrolysis (Daniels et al., 2015; Hopp & Hottiger, 2021; Rosenthal & Hottiger, 2014; Vivelo & Leung, 2015).
2.3 |. Protein-level enrichment
2.3.1 |. Anti-ADP-ribose or PAR-binding antibodies
Anti-polyADPr mouse monoclonal antibody 10H was first developed 40 years ago (Kawamitsu et al., 1984) and is routinely used to enrich PARylated substrates, typically via immunoprecipitation strategies. The 10H antibody demonstrates high-affinity binding to long PAR chains (at least 10 ADPr units) but exhibits no affinity for MARylated proteins (Grimaldi et al., 2018; Vivelo & Leung, 2015). The demanding synthesis of MARylated peptide immunogens that requires the formation of pyrophosphate linkages impedes the development of suitable MAR-binding antibodies (Liu et al., 2019). Additionally, producing antibodies that specifically bind MAR but not PAR or other PTMs derived from adenosine has proven formidable (Hopp & Hottiger, 2021). In recent years, some MAR-binding antibodies have become commercially available because of the advancement in ADPr-peptide synthesis (Hopp & Hottiger, 2021; Lu et al., 2019; Zhu et al., 2020). For example, Matic and coworkers synthesized peptides with ADPr-serine using a phospho-guided chemoenzymatic approach, employing the strategy to develop five antibodies that recognize MARylated substrates, including two with site-specificity, two with broad specificity, and one with anti-pan-ADPr properties (Bonfiglio et al., 2020; Hopp & Hottiger, 2021).
2.3.2 |. ADP-ribose-binding macrodomains
Macrodomains are protein modules that interact with monomeric and polymeric forms of ADPr and perform critical cellular functions (Hopp & Hottiger, 2021; Lüscher et al., 2018). One such example is the interaction of the human histone macrodomain (H2A.1) with PAR chains, which happens in response to DNA damage and initiates chromatin reorganization (Timinszky et al., 2009). The varying affinities of macrodomains towards MAR/PAR facilitate selective enrichment of MARylated/PARylated substrates in cellular lysates. Three examples include (1) the WWE and histone H2A.1 macrodomains that exclusively bind PARylated substrates, (2) the macro2/macro3 domains from murine PARP14/ARTD8 that bind MARylated PARP10, and (3) the Af1521 macrodomain that binds both MARylated and PARylated substrates with similar selectivity (Bütepage et al., 2018; Forst et al., 2013; Hopp & Hottiger, 2021; Kalisch et al., 2012; Timinszky et al., 2009). The Af1521 macrodomain from Archaebacteria is the most common macrodomain employed to enrich MARylated/PARylated proteins. The first study that used the Af1521 macrodomain in a proteomics workflow was published in 2009 (Dani et al., 2009) to capture MARylated proteins and is still frequently used to enrich MAR/PAR-modified substrates in large-scale proteomics studies (Buch-Larsen et al., 2020; Kamata et al., 2019; Larsen et al., 2017, 2018; Martello et al., 2016). Recently, Hottiger and coworkers deployed random mutagenesis to increase the affinity of the Af1521 macrodomain towards ADPr by 1000X fold, enabling impressive enrichment and improved coverage of ADPr proteome using LC-MS/MS analysis (Nowak et al., 2020). The lack of specificity of the Af1521 macrodomain towards MAR/PAR remains a persistent problem as it leads to the loss of information about the MARylation/PARylation state of substrate proteins because the enriched proteins are truncated to MARylated forms before MS/MS analysis. Therefore, the development of macrodomains with a greater degree of selectivity towards MAR/PAR is crucial to dissecting the physiological roles of MARylation and PARylation. Kraus and coworkers prepared antibody-like ADPr binding proteins in which macrodomains from Af1521, ARTD8, and histone H2A.1 were fused to the Fc region of rabbit immunoglobulin, thus endowing them with selective binding affinities towards MAR/PAR when compared to the unmodified form of macrodomains (Gibson et al., 2017).
2.3.3 |. Chemical labeling of NAD+ or ADP-ribose
Chemical modification of NAD+ or ADPr groups is another general strategy that facilitates the detection of ADPr-modified substrates (Ando et al., 2019; Carter-O’Connell et al., 2014, 2016; Hopp & Hottiger, 2021; Kalesh et al., 2019; Morgan & Cohen, 2017; van der Heden van Noort, 2020; Vivelo & Leung, 2015). NAD+ analogs cannot cross cell membranes, rendering them unsuitable for determining physiologically relevant ADP-ribosylation states (Vivelo & Leung, 2015). Although brief permeabilization of cells before labeling with biotinylated NAD+ permits the incorporation of biotinylated-NAD+ in the PARP substrate proteins, the biological impact is perturbed by permeabilizing the cells (Farrar et al., 2010). Moreover, chemical modification of NAD+ may disrupt the affinity of ARTs towards NAD+ or interfere with the PAR chain formation or branching, diminishing the biological significance of the findings (Hopp & Hottiger, 2021).
Enzymatic or metabolic labeling using different reagents can overcome the impermeability of NAD+. For example, Ando et al. (2019) used 2′−5′-oligoadenylate synthetase (OAS1) to tag ADPr-modified peptides with N6-(N-azido) hexyl-deoxyadenosine triphosphate (dATP), conjugated them to dibenzocyclooctyne-agarose, treated them with PDE to convert ADPr-modified peptides to phosphoribose-modified peptides by cleaving the pyrophosphate bonds, before LC-MS/MS analysis (Ando et al., 2019; Daniels et al., 2017). The enzymatic labeling of terminal ADPr (ELTA) based enrichment was successfully applied on H2O2-treated HeLa cell lysate, resulting in the identification of 175 endogenous ADP-ribosylated peptides from 123 proteins (Ando et al., 2019). In another study, Hang and coworkers metabolically labeled HeLa cells with N6-propargyl adenosine (N6pA) to facilitate the detection of the ADP-ribosylated proteins (Westcott et al., 2017). N6pA-labeled proteins were conjugated with azide-biotin, then affinity purified using streptavidin beads, before on-bead protease digestion and LC-MS/MS analysis. They reported that 240 and 259 N6pA-labeled proteins were enriched above the DMSO controls in H2O2-treated and untreated samples, respectively. Additionally, they reported that oxidative stress induces ADP-ribosylation of several GTPases which inhibited downstream kinase signaling pathways, and demonstrated PARP inhibitors as potential targets to rescue the signaling pathways in oxidatively stressed cells (Westcott et al., 2017).
The Cohen group engineered different PARP-modified NAD+ analogs (5-ethyl-6-alkyne-NAD+, 5-benzyl-6-alkyne-NAD+, and 5-benzyl-2-alkyne-NAD+) by introducing a hydrophobic pocket near the active site of the PARP to accommodate NAD+ analog to label ADP-ribosylated substrates in complex cellular lysates (Carter-O’Connell & Cohen, 2015; Carter-O’Connell et al., 2014, 2016, 2018; Rodriguez et al., 2021). They used 5-benzyl-6-alkyne-NAD+ to label HEK293T cell lysate, conjugated the resulting alkyne-modified species with biotin-PEG3-azide, enriched the biotin-tagged products using NeutrAvidin agarose, proteolyzed, and analyzed the resulting peptides using LC-MS/MS. They identified 114 substrates of PARP-14 MARylation (Carter-O’Connell et al., 2018). They further improved the detection of MARylated substrates by utilizing 5-benzyl-2-alkyne-NAD+ leading to the identification of 250 direct targets of PARP-7 (Rodriguez et al., 2021).
DiMaggio and coworkers developed a dual metabolic labeling method featuring adenosine analogs with a terminal alkyne functionality: 2-alkyne adenosine (2YnAd) and 6-alkyne adenosine (6YnAd) for global enrichment of ADP-ribosylated proteins. Following the incorporation of 2YnAd and 6YnAd into live cells, the ADP-ribosylated proteome was tagged using copper-catalyzed azide-alkyne click chemistry (CuAAC), enriched using NeutrAvidin, and subjected to on-bead digestion before LC-MS/MS analysis. By integrating this strategy for TMT10plex-based quantitation (Figure 4), they profiled the global changes in the cellular ADP-ribosylome and reported a significant improvement in proteomic coverage (identified 1600 target proteins) compared to other studies (Kalesh et al., 2019).
FIGURE 4.
Schematic illustrating the workflow involved in the TMT10plex-based quantitation of global changes in the cellular ADP-ribosylome. Reprinted with permission from Kalesh et al. (2019).
More recently, Marx and coworkers developed affinity-conjugated (for enrichment) or fluorophore-conjugated (for visualization of ADPr in live cells) NAD+ derivatives. They incorporated desthiobiotin (DTB)-conjugated NAD+ analog into live cells to metabolically label ADPr groups without affecting cell viability. DTB-NAD+ labeling facilitated sensitive enrichment of ADP-ribosylated proteins using streptavidin-agarose beads. The proteins were eluted using excess biotin, treated with PARG, followed by gel electrophoresis (GE) and Western blot (WB) before in-gel trypsin digestion and LC-CID-MS/MS. The results outperformed concentration-dependent CuAAC reactions that are inefficient for capturing less abundant proteins. In an independent experiment, metabolic incorporation of a fluorophore tagged NAD+, tetramethylrhodamine (TMR)-NAD+, facilitated fluorescence imaging of ADPr-modified proteins in the nucleus of live cells (Lehner et al., 2022).
2.4 |. Peptide-level enrichment
While protein-level enrichment is a widely used strategy in the field of proteomics, it does not effectively reduce the sample complexity to achieve the detection of low-level PTMs because the chromatograms are still dominated by a large abundance of unmodified peptides. Therefore, PTM-specific enrichment at the peptide level is a robust strategy to selectively capture the low abundance PTMs and reduce the sample complexity. Several methods are employed to capture the ADPr-modified peptides and are discussed below.
2.4.1 |. Boronate enrichment
Boronate affinity chromatography uses a boronic acid resin to capture any cis-diol-containing compounds by forming diester bonds between hydroxylated boron and the cis-diol moiety under basic conditions before elution under acidic conditions (B.-Y. Huang et al., 2014). Boronate affinity is commonly used for the enrichment of O- and N-linked glycopeptides and glycoproteins (J. Chen et al., 2017; Y. Li et al., 2001) and can be easily applied to ADP-ribosylated peptides (Rosenthal et al., 2011; Vivelo & Leung, 2015; Y. Zhang et al., 2013). ADPr-modified peptides are captured at basic pH (pH 8.5) and eluted at acidic pH (hydroxylamine [NH2OH or HA]); Karch et al., 2017; Y. Zhang et al., 2013) or acid (Hendriks et al., 2021; P. Li et al., 2019; Rosenthal et al., 2011), desalted and analyzed using an LC-MS/MS workflow. HA elution results in the derivatization of ADPr to hydroxamic acid (resulting in a mass shift of +15.0109 Da), whereas acid elution maintains the ADPr modification intact (Hendriks et al., 2021). Hydroxylamine releases the peptides by breaking the ester bond between the first ADPr unit of MAR/PAR and the sidechain carboxyl group of the modified aspartic/glutamic acid residue (Y. Zhang et al., 2013). While the fragmentation of conventional ADPr-modified peptides frequently results in complete loss of the labile ADPr modification, the alternative hydroxamic acid analogs are very stable, thus making it feasible to localize this modification (+15.0109 Da) by collision-based activation methods (Gagné et al., 2015). Using boronic affinity and hydroxamic-based approach, the Yonghao group unambiguously localized 1048 unique Asp- and Glu-ADP-ribosylation sites on 340 proteins in human colorectal carcinoma cells (Y. Zhang et al., 2013). More recently, they performed a global characterization of the PARP1-dependent Asp/Glu-ADP-ribosylation occurring in breast cancer cell lines using the boronate affinity and HA elution approach and identified 503 unique ADPr acceptor sites on 322 proteins (Zhen et al., 2017).
A recent investigation re-evaluating published tandem mass tags (TMT) data discovered a highly prevalent aberrant mass shift of 15.0109 Da when HA was involved in sample preparation (Geiszler et al., 2021). HA elution can broadly convert carboxylic acids to hydroxamic acids during sample preparation, leading to erroneous identification of ADPr at Asp/Glu (Geiszler et al., 2021; Hendriks et al., 2021). The Nielsen group tested this possibility based on the evaluation of BSA, a protein that is not a target of MARylation/PARylation, by incubating BSA digest with HA and analyzing the resulting peptides by CID. They reported that almost half of all identified Asp/Glu residues (52% sequence coverage for BSA) exhibited a mass shift of 15.0109 Da, an outcome not found for the BSA digest in the absence of HA (Hendriks et al., 2021). They also reanalyzed previously published boronate affinity and HA elution data acquired for analysis of breast cancer cell lines (Zhen et al., 2017) and found that peptides modified by hydroxamic acid contributed ~20% of the total signal. This collection of findings conveys that HA promotes the 15.0108 Da mass shift of unmodified Asp/Glu residues, rendering it unsuitable for any strategy focused on profiling ADP-ribosylation at Asp/Glu residues (Hendriks et al., 2021). Therefore, an acidic solution is recommended for the elution of peptides retaining intact ADPr group after boronate enrichment to evade the problematic mass shift artifacts arising from HA elution (Hendriks et al., 2021).
2.4.2 |. Phosphodiesterase digestion (PDE)
Snake venom phosphodiesterase (SVP) cleaves pyrophosphate bonds and thus can be used to generate phosphoribose tags at ADPr-modified sites. The resulting mass shift (+212.0086 Da) alleviates the PAR chain complexity while simultaneously making the ADPr peptides compatible with highly effective phosphoproteomics enrichment protocols like immobilized metal affinity chromatography (IMAC) (Chapman et al., 2013; Daniels et al., 2014; Daniels et al., 2015; Haag & Buck, 2015; Vivelo & Leung, 2015; Zhen & Yu, 2018). Using this approach, Goodlett and coworkers identified nine unique PARP1 auto-modification sites (encompassing 6 Glu, 3 Asp, and 1 Lys) using CID after SVP digestion, IMAC enrichment, and ammonium hydroxide elution of human PARP1 (Chapman et al., 2013). However, highly basic ammonium hydroxide can result in the loss of phosphoribose groups from acidic amino acids (Asp/Glu) (Daniels et al., 2014). Therefore, Daniels et al. used a neutral phosphate buffer to elute the IMAC-enriched phosphoribosyl-modified peptides to preserve the labile phosphoribose groups, ultimately enhancing the number of identified ADPr modification sites. They identified 20 unique PARP1 auto-modification sites (12 Glu, 4 Asp, 3 Lys, and 1 Arg) using CID and higher energy collision dissociation (HCD) for MS/MS analysis (Daniels et al., 2014). Generally, the PDE strategy facilitates global enrichment of ADPr peptides modified at both acidic (Asp/Glu) and basic (Arg/Lys) residues, otherwise restricted to the detection of ADPr-Asp/Glu when using boronate enrichment and HA elution (Daniels et al., 2014). Although this method does not require macrodomain enrichment or PARG treatment, the purification of SVP from snake venom is complicated leading to high lot-to-lot variability (Daniels et al., 2015), and the resulting phosphoribose tag is labile upon CID, impeding its site localization (Daniels et al., 2017). Additionally, coenrichment of phosphopeptides and identification of incomplete ADPr moieties are other limitations of this approach (Daniels et al., 2014).
Nucleoside diphosphate-linked moiety X (Nudix) hydrolases are another class of enzymes that act on a range of pyrophosphate bonds. Human Nudix hydrolase (Nudix16) and an analogous bacterial Nudix hydrolase (EcRppH) are two of the few Nudix hydrolases that target MAR/PAR-conjugated proteins to generate ribose-5′-phosphate-modified substrates. These Nudix hydrolases serve as alternatives to PDE reactions but exhibit lower catalytic activities than SVP (Daniels et al., 2015; Palazzo et al., 2015). The performance attributes of bacterial EcRppH and human Nudix16 were compared to SVP in the PDE-based workflow, identifying 14 phosphoribose-modified peptides, among which only two were common among the three enzymes (Daniels et al., 2015). Nudix hydrolase digestion and IMAC enrichment coupled with HCD-triggered electron transfer dissociation (ETD) identified 12 unique ADP-ribosylation sites in human cancer cells, including a novel ADP-ribosylated Ser (Leidecker et al., 2016). Leung and coworkers designed Nudix16 (Δ17, F36A, and F61S) mutants to improve their efficiency. Although these mutants exhibited enhanced hydrolysis of pyrophosphates in PARylated PARP1, they showed decreased activity for MARylated substrates and free ADPr (Thirawatananond et al., 2019). These findings demonstrated that engineered Nudix hydrolase mutants with higher catalytic activity in both PAR/MAR are essential to increase the coverage of the ADPr proteome when used with SVP in phosphodiesterase-based proteomics investigations (Daniels et al., 2015).
2.4.3 |. PARG treatment and peptide level purification strategy for PAR analysis
The length and the number of negative charges of PAR chains can vary considerably, contributing to significant heterogeneity in the resulting mass shifts of the PTM. Therefore, PARylated substrates are routinely hydrolyzed to MARylated forms using PARG, followed by Af1521 macrodomain enrichment of the resulting ADPr species before MS analysis (Larsen et al., 2018; Martello et al., 2016). PARG imparts a unique mass shift (+541.0611 Da) to all ADPr-modified peptides, streamlining the detection and increasing the ionization efficiency of ADPr-modified peptides in positive mode, owing to the removal of excessive negative charges (Daniels et al., 2015; Larsen et al., 2017; Martello et al., 2016; Rosenthal & Hottiger, 2014; Vivelo & Leung, 2015; Zhen & Yu, 2018). Additionally, a unique mass shift generated by PAR hydrolysis reduces the search space and makes it easier for integration with various search engines. Nielsen and coworkers used the Af1521 macrodomain to enrich PARG hydrolysis products (i.e., MARylated peptides) and identified over 900 ADPr acceptor sites in the mammalian cells and liver samples using LC-MS/MS (with HCD and ETD). This study achieved unbiased enrichment of peptides containing both ADPr-modified acidic and basic sites by utilizing the Af1521 macrodomain method, permitting confirmation of lysine residues as the primary ADPr acceptor site during oxidative stress and arginine residues as the key ADPr acceptor site in the liver (Martello et al., 2016). Although the mass shift of +541.0611 Da was only searched at Asp/Glu/Lys/Arg, this study demonstrated the utility of Af1521 macrodomain for the enrichment of both N- and O-linked ADPr modifications. The Nielsen group implemented peptide level enrichment using the Af1521 macrodomain after PARG treatment in several proteomics studies to uncover thousands of unique ADP-ribosylation sites (Buch-Larsen et al., 2020; Hendriks et al., 2017, 2019; Larsen et al., 2018). Because PARG treatment degrades the PAR chain length, differentiation of MARylated or PARylated forms is not possible using this general strategy.
Leutert and coworkers generated tryptic peptides from PARG digested proteins using filter-aided sample preparation (FASP), enriched the resulting MARylated peptides using magnetic Ti4+-IMAC microspheres, and desalted the eluted peptides using C18-stage tips before LC-MS/MS analysis (Leutert et al., 2017). This protocol was only applied for the identification of in vitro ADPr, but it seems like a promising alternative to macrodomain enrichment (Leutert et al., 2017).
To further enhance the depth and width of proteome coverage, offline fractionation is often undertaken before separation using reversed-phase C18 chromatography to reduce the complexity of samples (Batth et al., 2014). ADP-ribosylated peptides are hydrophilic and can benefit from offline fractionation based on hydrophobic interactions (Batth et al., 2014). In particular, reversed-phase fractionation at high pH (20 mM ammonium hydroxide, pH ~10.8) offers impressive resolution for the separation of hydrophilic peptides and can be easily integrated with an existing C18 chromatography setup (Hendriks et al., 2019). The Nielsen group has demonstrated the significant benefits of high pH fractionation of ADPr-modified peptides using C18 StageTips to streamline sample complexity and improve the identification of ADPr sites when analyzed by LC-MS/MS (Buch-Larsen et al., 2020; Hendriks et al., 2017, 2019; Larsen et al., 2018).
Although peptide-level enrichment provides a more robust and targeted approach for the identification of ADPr acceptor sites, parallel or serial enrichment is required to discover PTM crosstalk (Leutert et al., 2021). In contrast, protein-level enrichment allows the discovery of other PTMs that comodify ADP-ribosylated proteins with fewer sample preparation steps.
2.4.4 |. Hydrofluoric acid-based derivatization strategy for site-specific characterization
More recently, Gagné et al. utilized hydrogen fluoride to truncate ADPr to a ribose tag (+132 Da adduct), allowing the localization of ribose adduct on several Asp, Glu, Ser, Lys, and Arg residues of automodified PARP1 using MS/MS methods (Gagné et al., 2018). Although HF is a harsh chemical, the authors noted that undesirable side products were not formed upon overnight treatment of an ADPr-modified peptide with 48% ice-cold HF. A key advantage of HF-based derivatization compared to the HA-based methods described earlier is that it enables global profiling of ADP-ribosylation in contrast to the more limited identification of only ADPr-Asp/Glu sites for the HA method (Gagné et al., 2015, 2018). Additionally, the HF method does not convert carboxylic acids to hydroxamic acids during sample processing, an artifact noted earlier that contributed to the erroneous identification of ADPr at Asp/Glu residues (Hendriks et al., 2021). However, this method does not identify intact ADPr-modified peptides and still requires an enrichment strategy either at the protein or peptide level before it can be applied to identify ADPr acceptor sites in complex biological samples.
2.5 |. Mass spectrometry of ADP-ribosylated peptides
Liquid chromatography coupled with tandem mass spectrometry (typically CID) is the most common strategy used for the large-scale characterization of numerous types of PTMs, including ADP-ribosylation (Jiang et al., 2013). However, initial efforts to localize ADPr modifications on specific amino-acid residues were unsuccessful because collisional activation methods preferentially cleave the most labile bonds of peptides, frequently resulting in the complete or partial loss of labile PTMs (Hengel & Goodlett, 2012). The fragmentation of ADPr-modified peptides using CID results in MS/MS spectra dominated by ADPr fragments and lacking sequence-specific b/y ions, while HCD results in complete or partial loss of the ADPr from the peptides, generating b/y ions devoid of the PTM (Bonfiglio et al., 2017; Hengel & Goodlett, 2012; Hengel et al., 2009; Zhen & Yu, 2018). An example of a representative HCD spectrum obtained for a triply charged ADP-ribosylated peptide is shown in Figure 5, illustrating the numerous cleavages of the ADPr group and production of low mass fragment ions (Leutert et al., 2017).
FIGURE 5.
(A) Annotated HCD spectrum (NCE 35%) for a triply charged ADPr-modified peptide. ADPr fragment ions are shown in red font. (B) Nomenclature for ADPr fragments is shown in the inset. Reprinted with permission from Leutert et al. (2017).
In contrast, electron-based activation (generally grouped as “ExD” methods) causes energization and dissociation via electron transfer reactions, resulting in backbone cleavages that produce c/z-type sequence ions without significantly disrupting labile PTMs (Hengel et al., 2009; Molina et al., 2007). While ETD or electron capture dissociation (ECD) provides valuable information to sequence peptides and localize ADPr modifications, as shown in several studies, these ExD methods are generally less successful for peptides in low charge states (1+ and 2+) owing to the reduction of the exothermicity of the electron transfer reactions (Gunawardena et al., 2005; Hendriks et al., 2019; Hengel et al., 2009; Larsen et al., 2018; Rosenthal et al., 2015; Wiesner et al., 2008). The effectiveness of ExD methods may be hampered by the acquisition of noninformative MS/MS spectra of peptides in low-charge states.
A strategy combining HCD and ETD named the “marker ion approach” was proposed to increase the efficiency of ExD methods and the number of true ADPr-modified peptides sampled in a mixture (Hengel & Goodlett, 2012; Rosenthal et al., 2015). This method relies on the detection of at least one (or two) of the four low mass marker ions generated by the fragmentation of the ADPr moiety attached to a peptide: adenine+ (m/z 136.06), (adenosine −H2O)+ (m/z 250.09), AMP+ (m/z 348.07), and ADP+ (m/z 428.03), providing a unique signature to confirm that the peptide of interest is ADP-ribosylated. ExD is then triggered to activate the same precursor ion only if one (or more) of these marker ions are detected in the HCD mass spectrum, thus allocating more run time for the analysis of ADP-modified peptides (Rosenthal et al., 2015). This technique combines the utility of both (HCD and ETD), increasing the sequence coverage of the identified proteins using HCD while localizing the ADPr groups on precise residues using sequence-specific c/z ions generated by ETD/ECD (Rosenthal et al., 2015). The efficiency of this method can be improved by excluding the selection of doubly charged precursors for ETD/ECD activation. Additionally, alternate proteases (e.g., Lys C instead of trypsin) that generate larger peptides that can accommodate more charges can improve the fragmentation efficiency of ExD methods.
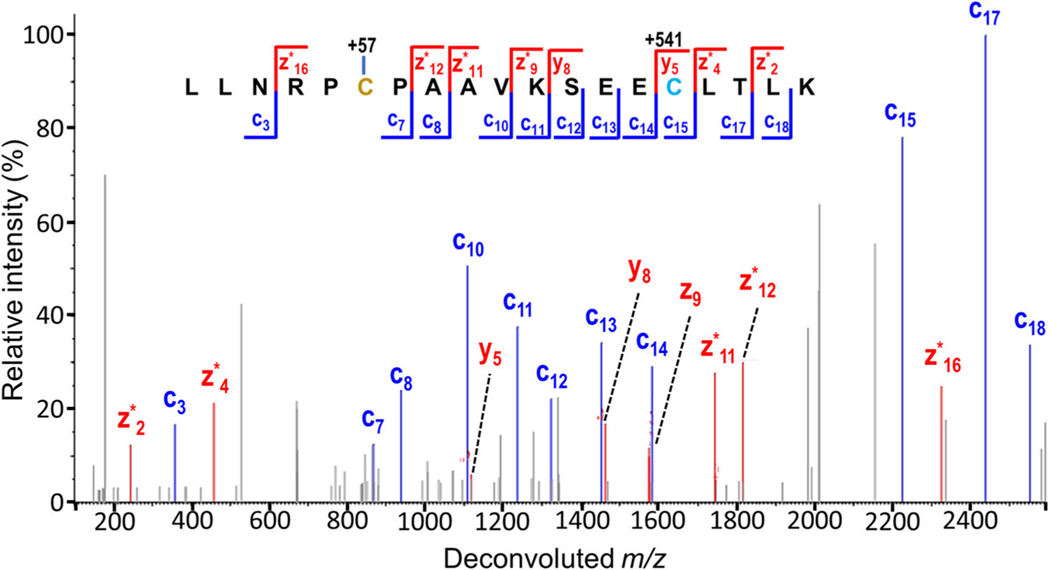
A hybrid MS/MS method developed by Frese et al. combining electron transfer and higher energy collision dissociation (EThcD) offered significant gains in peptide fragmentation efficiency and production of more diagnostic fragment ions while still preserving labile PTMs (Frese et al., 2012). During EThcD, the population of ions generated by ETD is subjected to supplemental activation using HCD to facilitate the fragmentation of nondissociated and charge-reduced precursor ions into b/y and additional c/z ions (Frese et al., 2012). Hendriks et al. capitalized on this concept to improve the characterization of ADPr-modified peptides as demonstrated to comprehensively profile ADP-ribosylation induced by DNA damage in HeLa cells (Hendriks et al., 2019). By utilizing an alternate protease to generate longer and more highly charged peptides suitable for ETD, an improved sample preparation strategy including offline high pH fractionation and EThcD fragmentation, more than 7000 unique ADP-ribosylation sites were identified and localized (Hendriks et al., 2019). An example of the high information content obtained from EThcD and the ability to localize the site of the ADP-ribosylation is shown for a representative peptide in Figure 6 (Hendriks et al., 2019).
FIGURE 6.
Annotated EThcD MS/MS spectrum, demonstrating confident localization of one ADP-ribosylation and two phosphorylation events. Reprinted with permission from Hendriks et al. (2019).
By integrating EThcD with the marker ion approach previously demonstrated for ETD (Rosenthal et al., 2015), Bilan et al. further optimized the identification of ADPr-modified sites via a method termed HCD product-dependent EThcD (HCD-PD-EThcD) (Bilan et al., 2017). In HCD-PD-EThcD, detection of at least one ADPr marker ion during the HCD scan triggers the acquisition of an EThcD scan of the same precursor. HCD-PD-EThcD proved to be faster than EThcD alone because unmodified peptides are subjected to HCD (a significantly faster method than EThcD), while only modified peptides were fragmented using EThcD. HCD-PD-EThcD substantially improves the detection of ADPr-modified peptides from unenriched and complex samples while also improving the identification of ADPr attachment sites from enriched samples by avoiding the sampling of coenriched unmodified peptides. To further improve the duty cycle and collect as many scans as possible, they also implemented a clever HCD product-preview EThcD method (HCD-PP-EThcD) in which a fast lower resolution (30K) HCD preview scan is performed before triggering higher resolution (60 K) HCD and EThcD scans of the same precursor upon detection of at least two of the ADPr marker ions (Bonfiglio et al., 2017). This HCD-PP-HCD/EThcD workflow identified an additional 42% unique ADP-ribosylated peptides in an oxidatively stressed HeLa cell lysate enriched using Af1521 macrodomain compared to the number of ADPr-modified peptides uncovered by HCD-PD-EThcD (Bilan et al., 2017; Martello et al., 2016). Although applying supplemental collisional activation to dissociate the charge-reduced precursors generated during ETD enhances the fragmentation efficiency of peptides when subjected to EThcD, the undissociated charge-reduced ions were still prevalent in MS/MS spectra acquired for ADPr-modified peptides (Buch-Larsen et al., 2020; Hendriks et al., 2019). To further increase the efficiency of ETD, the Coon group developed a general strategy to irradiate peptides with infrared (IR) photons during ETD, in a process called activated ion ETD (AI-ETD) (Ledvina et al., 2010). The supplemental IR heating disrupted the noncovalent interactions that prevented fragment ions from separating, thus releasing more peptide fragment ions and boosting their abundances. Since AI-ETD showed impressive performance for mapping other types of peptides containing labile PTMs like glycosylation and phosphorylation, they applied the same strategy for profiling Af1521 macrodomain-enriched ADPr samples (Buch-Larsen et al., 2020). The extensive series of c/z ions generated by AI-ETD is invaluable for the localization of ADPr modifications, as illustrated in a representative MS/MS spectrum in Figure 7 (Buch-Larsen et al., 2020). Utilizing this strategy, they confidently localized more than 5000 unique ADPr sites, including 1363 novel ADPr sites from oxidative stressed HeLa cells (Buch-Larsen et al., 2020), using a considerably lower sample quantity compared to a prior study (Hendriks et al., 2019). The identified 1363 novel ADPr acceptor sites included 58% serine, 17% arginine, 8.7% histidine, 4.8% tyrosine, 4.7% lysine, 3.2% cysteine, 2.1% threonine, 1.1% aspartate, and 0.7% glutamate residues, underscoring the unbiased detection potential of AI-ETD. In addition, AI-ETD improved the detection of ADPr-modified proteins from untreated HeLa cells, identifying 450 unique ADPr acceptor sites, including 286 novel sites, 79% more compared to an earlier study by Nielsen and coworkers in 2018 (Larsen et al., 2018).
FIGURE 7.
Annotated AI-ETD spectrum of an ADPr-modified PARP8 peptide, demonstrating confident localization of ADPr at Cys376. Reprinted with permission from Buch-Larsen et al. (2020).
2.6 |. Proteomic studies
Owing to the importance of ADP-ribosylation and the notable technical advances in mapping this challenging PTM, there have been a growing number of studies aimed at site localization and profiling of ADPr. Nielsen and coworkers utilized macrodomain Af1521 to enrich ADPr-modified substrates in oxidatively stressed HeLa cells depleted of histone PARylation factor 1 (HPF1) and ADP-ribosyl hydrolase 3 (ARH3), both of which are critical regulators of PARP1 activity (Hendriks et al., 2021). In addition to identifying ~1600 ADPr sites (searched for ADPr at Asp, Glu, His, Lys, Arg, Ser, and Tyr), they found that HPF1 knockout in cells resulted in the depletion of ADPr, whereas ARH3 knockout caused a 100-fold increase in accumulation of ADPr when compared to control cells. Oxidative stress (H2O2) induced ADPr in control cells more significantly than in ARH3 knockout cells but had a negligible impact in HPF1 knockout cells (Hendriks et al., 2021). These results suggest that HPF1 and ARH3 inversely regulate serine ADP-ribosylation on a proteome-wide scale. They also compared the performance of the Af1521 macrodomain, two independent antibodies raised against PAR/MAR, and boronate affinity for the enrichment of ADPr in oxidatively stressed HeLa cell lysate. All three enrichment strategies identified Ser as the main ADPr target, while the Af1521 macrodomain identified the most modification sites (Hendriks et al., 2021).
Both human and mouse ARTC1 (clostridium-like ecto-ADP-ribosyltransferase) are known to MARylate arginine residues in skeletal and heart muscle. Hottiger and coworkers investigated ARTC1-specific ADP-ribosylation in murine skeletal and heart muscle tissues and C2C12 myotubes using peptide level Af1521 enrichment (Martello et al., 2016) and LC-MS/MS analysis (HCD-PP-EThcD) (Leutert et al., 2018). Using this approach, they identified 80 ADPr-modified proteins from NAD+ treated C2C12 cells and 7 ADPr-modified proteins from the untreated cells, establishing Arg as the primary acceptor of ADPr only upon NAD+ treatment (searched for ADPr at Ser, Arg, Lys, Asp, and Glu). Additionally, they identified 558 ADPr-modified skeletal muscle proteins (78% proteins with a single ADPr acceptor site) from wild-type (WT) mice and 29 from ARTC1 deficient mice and 127 ADPr-modified heart muscle proteins from WT mice and 23 from ARTC1 deficient mice (71% proteins with a single ADPr acceptor site). These results suggest that skeletal and heart ADP-ribosylation is driven primarily by ARTC1 and targets extracellular matrix, plasma membrane, endoplasmic reticulum, and mitochondrial proteins as revealed by Gene Ontology analysis. Although at a lower intensity when compared to other ADPr marker ions (m/z 136, 250, 348, and 428), the Arg-specific ADPr-carbodiimide marker ion of m/z 584.0902 confirmed the presence of Arg-ADPr and can be included in the list of marker ions for product-dependent MS/MS methods (Leutert et al., 2018).
Matic and coworkers identified 12 unique ADPr serines in histones derived from human osteosarcoma cells (U2OS) (searched for ADPr at Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr, Asn, and Gln) in response to oxidative stress-induced DNA damage (Leidecker et al., 2016). They reported an in vivo proteomics approach to identify ADPr sites in histone proteins in which the histones were extracted, subjected to partial FASP, and then PARylated/MARylated peptides were hydrolyzed to phosphoribose-modified peptides. The latter were enriched using IMAC and analyzed using the HCD-triggered ETD method (Leidecker et al., 2016).
Lipopolysaccharide (LPS), a highly abundant component of the Gram-negative cell wall, stimulates the immune cells to produce proinflammatory cytokines in response to bacterial infections. LPS-treated pig blood serves as a model for human systemic inflammatory response syndrome (SIRS) or sepsis. Hottiger and coworkers utilized the Af1521 macrodomain to enrich ADPr-modified peptides and established an MS-based workflow to identify in vivo ADP-ribosylated proteins from whole blood and plasma samples of LPS-treated pigs. These proteins served as potential biomarkers for the detection of SIRS/sepsis by comparing against healthy pig blood and plasma (Lüthi et al., 2021). They used HCD/EThcD analysis to identify 60 ADP-ribosylated proteins, including 17 plasma proteins, and revealing histidine (searched for ADPr at Asp, Glu, His, Lys, Arg, Ser, and Tyr) as the primary ADPr acceptor site in blood and plasma proteins. Although no significant differences were noted between the control and LPS-treated pigs, the authors provided a foundation for the development of a more targeted proteomics study using a larger sample size to statistically validate and discover unique ADP-ribosylated proteins between the control and sepsis samples.
Vermeulen and coworkers synthesized biotinylated mono-, di-, and tri- ADPr probes and used them for affinity purification of ADP-ribosylated substrates in mammalian cell lysates (Kliza et al., 2021). The enriched proteins were digested, proteolyzed, and the resulting tryptic digest was analyzed using label-free quantitative mass spectrometry. They identified several known and novel MAR and PAR substrates and used them to build a proteome-wide ADPr interactome (Kliza et al., 2021).
Nielsen and coworkers performed high pH fractionation following Af1521 macrodomain enrichment of ADPr-modified peptides derived from oxidatively stressed HeLa cells and analyzed using LC-MS/MS (ETD) (Larsen et al., 2018). Under oxidative stress, they identified 3090 serine ADPr sites from among 1283 proteins mainly localized in the nucleus. Furthermore, they performed phosphorylation analysis under similar conditions and discovered that 483 serine ADPr sites were phosphorylated, suggesting a significant crosstalk between the two PTMs (Larsen et al., 2018).
In another study, Nielsen and coworkers used the Af1521 macrodomain for unbiased enrichment of ADP-ribosylated substrates and high pH fractionation of modified peptides before MS/MS analysis (EThcD) to identify and quantify the modification sites in response to DNA damage (Buch-Larsen et al., 2021). They detected 1681 ADP-ribosylation sites on 716 proteins in U2OS cells exposed to genotoxic stress at different time points. Additionally, they identified serine as the primary ADPr acceptor site for various genotoxic stress inducers across all time points evaluated. Differences in abundances of ADPr modification were observed upon exposure to H2O2 or methyl methoxy sulfonate (MMS) at different time points. For example, exposure to MMS (which causes alkylating stress) induced the highest level of ADP-ribosylation after 30 min, while exposure to H2O2 (which causes oxidative stress) induced the highest level of ADP-ribosylation after 60 min, mainly modifying proteins involved in DNA damage pathways (Buch-Larsen et al., 2021).
Cohen and coworkers transfected HEK293T cells with GFP-I631G-PARP-7, incubated the cell lysate with a clickable NAD+ analog (5-Bn-2-e-NAD+), conjugated labeled proteins with biotin-azide, enriched using NeutrAvidin agarose, and analyzed the proteolytic digest using LCMS/MS. Using this chemical genetics (CG) approach they identified 250 direct substrates of PARP-7 and revealed PARP-13 as the primary target of PARP-7. They also identified 1712 ADPr sites (including Cys, Glu, Asp, His, Lys, Arg, Ser, Thr, and Tyr) in the proteome of PARP-13.2, PARP-7, and PARP-7+PARP-13.2 overexpressing HEK293T cells using peptide level Af1521 enrichment and EThcD analysis. PARP-13 was exclusively MARylated at cysteine in PARP-7 overexpressing cells (374 MARylated proteins) whereas at Ser/Arg (primarily Ser) in the absence of PARP-7. However, the two methods exhibited very little overlap in the identification of Cys-ADP-ribosylated proteins (only 48 common) from PARP-7 overexpressing cells, suggesting that the strategies can be used as complementary tools to study ADP-ribosylation (Rodriguez et al., 2021).
Some of the most recent or extensive proteomics studies are summarized in Table 2. These studies either highlight the usage of advanced activation methods or unique enrichment techniques.
TABLE 2.
Summary of recent large-scale proteomics studies highlighting enrichment types and data analysis software used for the identification or site-specific localization of ADP-ribosylation
| Enrichment type | Samples analyzed | Activation | Data analysis | Sites searched | # Sites identified | # Proteins | References |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Peptide level Afl521 | H2O2 treated HeLa cells and mouse liver digest | HCD | MaxQuant (+541.0611) | Lys, Arg, Glu, Asp | >900 | >500 | Martello et al. (2016) |
| Boronate, HA elution | Breast cancer cells | CID | SEQUEST (+15.0109) | Asp, Glu | 503 | 322 | Zhen et al. (2017) |
| Peptide level Afl521 | Mouse C2C12 cell line (myotubes), Mouse skeletal muscle (SM), Mouse heart muscle (HM) | HCD-PP- EThcD, HCD | Mascot (+541.0611) | Ser, Arg, Lys, Asp, Glu | Mostly Arg in WT; Ser, Lys, Asp, Glu in ARTC1KO | NAD+: 80C2C12 Control: 7C2C12 WT: 558SM ARTC1KO:29SM WT: 127HM ARTC1KO:23HM | Leutert et al. (2018) |
| Peptide level Afl521 | H2O2 treated HeLa cells | ETD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | 3450 3090 at Ser | 1370 1283 at Ser | Larsen et al. (2018) |
| Peptide level Afl521 | H2O2 treated HeLa cells | ETD, EThcD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | 7040 sites | 2149 | Hendriks et al. (2019) |
| Protein level Afl521 IP | PAPR7 overexpressed lung cancer cells | CID | Scaffold | Not specified | Not reported | 954 | Lu et al. (2019) |
| Peptide level; WT and eAfl521 | H2O2 treated and control HeLa cells | HCD-PP-EThcD | Mascot (+541.0611) | Ser, Arg, Lys, Asp, Glu, Tyr | Control: 5WT Treated: 118WT Control: 24e Treated: 327e | Control: 3WT Treated: 89WT Control: 19e Treated: 245e | Nowak et al. (2020) |
| Peptide level Afl521 | H2O2 treated and control HeLa cells | AI-ETD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | Control: 450; Treated: 5206 | Control: 295; Treated: 1800 | Buch-Larsen et al. (2020) |
| Peptide level Afl521 | H2O2 and methyl methanesulfonate treated U20S cells | EThcD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | Control: 268 Total: 1681 | Control: 168 Total: 716 | Buch-Larsen et al. (2021) |
| Peptide level Afl521; high pH fractionation | WT, HPF1 KO, and ARH3 KO U20S cells with or without H2O2 | EThcD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | 1596 | 799 | Hendriks et al. (2021, p. 3) |
| Peptide level Afl521 | Whole blood and plasma | HCD, EThcD | Mascot (+541.0611) | Asp, Glu, His, Lys, Arg, Ser, Tyr | Not reported | 60 in blood 17 in plasma | Lüthi et al. (2021) |
| Peptide level Afl521 | Spleen T-cells treated with NAD+ | HCD | Mascot (+541.0611) | Ser, Arg, Lys, Asp, Glu, Thr, Tyr | 36 (Arg, Ser) | 67 | Leutert, Duan, et al. (2021) |
| Peptide level Afl521 | HEK 293T cell lysate | EThcD | MaxQuant (+541.0611) | Cys, Asp, Glu, His, Lys, Arg, Ser, Thr, Tyr | 939 sites; 471 sites at Cys | 490; 374 Cys modified | Rodriguez et al. (2021) |
| Metabolic labeling (DTB-NAD+ analog) | HeLa cells treated with H2O2 and Olaparib | HCD | MaxQuant | Not specified | Not reported | Control: 35 H2O2: 120 Olaparib: 259 Both: 206 | Lehner et al. (2022) |
Abbreviations: DTB, desthiobiotin; e, engineered; HA, hydroxylamine; KO, knockout; Olaparib, PARP inhibitor; WT, wild type.
3 |. POSTTRANSLATIONAL MODIFICATIONS OF TYROSINE
Tyrosine is posttranslationally modified via enzymatic (ADP-ribosylation, phosphorylation, and sulfation) or nonenzymatic (nitration) processes (Cao et al., 2018; Kanan et al., 2012). In this part of the review, we will discuss sulfation and nitration as two PTMs that occur on tyrosine (Tyr) residues and have received less attention than other more common PTMs.
3.1 |. Tyr sulfation
Tyr O-sulfation is the transfer of a sulfate group from 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to the hydroxyl group of a Tyr residue forming a Tyr O-sulfate ester (Figure 8) (Baeuerle & Huttner, 1987; Ludeman & Stone, 2014). In eukaryotes, Tyr sulfation is catalyzed by one of the two membrane-bound enzymes called tyrosylprotein sulfotransferases (TPST-1 and TPST-2). TPSTs are present in the trans-Golgi region, where the secretory and transmembrane proteins are sulfated as they transit this region (Moore, 2009; Seibert & Sakmar, 2008). Although there is no consensus sequence for TPST recognition, acceptor Tyr residues are more likely to be in the intrinsically disordered regions of proteins and have acidic amino acids within a few residues on either side of the Tyr (Bundgaard et al., 1997; Maxwell & Payne, 2020).
FIGURE 8.
PAPS-dependent sulfate incorporation into a Tyr residue of a substrate by TPST enzyme. Adapted from Byrne et al. (2018).
The regulatory roles of Tyr sulfation have begun to be deciphered with the introduction of [35S]-labeling and other biochemical tools designed to probe sulfotyrosine, and it is evident that sulfation mediates critical cellular functions (Huttner, 1984; Klement et al., 2016; Seibert & Sakmar, 2008). The introduction of negative charges by Tyr sulfation in the target proteins leads to the changes in the dynamic conformation and modulates extracellular protein-protein interactions that play crucial roles in leukocyte adhesion, hemostasis, chemokine signaling, and inflammatory response (Colvin et al., 2006; Kehoe & Bertozzi, 2000). Sulfation of Tyr is a common PTM found in the proteins of the secretory system (Ripoll-Rozada et al., 2022). Tyr-sulfation in chemokine receptors modulates the binding of chemokines and plays a vital role in the host immune response (Maxwell & Payne, 2020). For example, sulfation of N-terminal Tyr residues in chemokine receptor CCR5 contributes to HIV-1 infection by facilitating the entry of HIV-1 to the cells, whereas in contrast mutating the sTyr to phenylalanine inhibits HIV-1 infection by 50–75% in cultured cells (Farzan et al., 1999; L. Huang et al., 2017). Tyr-sulfation of ocular proteins is essential for proper visual functions, and sTyr has been identified in various optical tissues, including lens, cornea, iris, sclera, vitreous and aqueous humor, and retina (Kanan et al., 2012, 2014). Tyr sulfated proteins are also present in mouse atherosclerotic lesions, and mice with TPST deficient hematopoietic cells exhibited a 68% reduction in atherosclerosis lesions suggesting that TPST is critical for the progression of atherosclerosis (Westmuckett & Moore, 2009). Therefore, TPST inhibitors are a potential therapeutic target for atherosclerosis and other inflammatory diseases (Westmuckett & Moore, 2009). Tyr-sulfation in the disordered region of glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1) increases its lipoprotein lipase binding affinity, aiding the hydrolysis of stored triglycerides for use by the surrounding cells (Kristensen et al., 2018). Sulfotyrosine (sTyr) is often colocalized with O-glycosylation suggesting crosstalk between the two PTMs. For example, P-selectin glycoprotein ligand-1 (PSGL1), a glycoprotein found on white blood cells, requires sulfation of several Tyr residues to facilitate high-affinity binding to P-selectin (an adhesion molecule in platelets) (Mehta et al., 2020). TPST2 deficient mice exhibit sperm motility defects, infertility, and altered sperm-egg interactions indicating a potential link between Tyr sulfation and male fertility (Marcello et al., 2011). Tyr-sulfation has also been identified in secreted peptides in plants, and a Tyr-sulfated glycopeptide in Arabidopsis thaliana promotes cellular proliferation and expansion (Amano et al., 2005).
3.2 |. Associated challenges
Although sTyr was first identified in a peptide from human fibrinogen by Bettelheim in 1954 [Color figure can be viewed at wileyonlinelibrary.com] (Bettelheim, 1954) and predicted to occur on 1% of eukaryotic proteins, only about 160 Tyr-sulfated proteins are experimentally reported (L. Huang et al., 2017; Önnerfjord et al., 2004). Tyr-sulfation is one of the most under-characterized PTM, a finding well echoed in the UniprotKB database (https://www.uniprot.org, accessed 9–1-2022), where only 548 Tyr sulfated proteins (reviewed) are reported in striking contrast to 48,652 phosphoproteins and 32,275 glycoproteins based on direct evidence or sequence similarity.
The large discrepancy in the comprehensive mapping of the sulfoproteome is mainly due to the analytical challenges associated with preserving the sulfate group during analysis. Two very acidic modifications with similar chromatographic properties can modify Tyr residues: phosphorylation (+79.9663 Da) or sulfation (+79.9568 Da). These modifications differ only by a monoisotopic mass of 9.5 mDa requiring high-accuracy mass spectrometers for confident identification (Medzihradszky et al., 2007). The acid labile Tyr sulfate ester moiety undergoes neutral loss of sulfur trioxide (SO3) when exposed to low pH used during phosphopeptide enrichment requiring alternative strategies for the isolation of sulfopeptides (Balsved et al., 2007; Maxwell & Payne, 2020). The majority of PTMs are identified on a large-scale via mass spectrometry-based workflows that use acidic conditions during sample preparation or analysis, resulting in the removal of sulfation and further confounding profiling of the sulfoproteome (Maxwell & Payne, 2020). The sulfate ester is also sensitive to thermal degradation and undergoes in-source dissociation during heated electrospray ionization (HESI) or matrix-assisted laser desorption ionization (MALDI) (Nemeth-Cawley et al., 2001; Robinson et al., 2014). In positive mode ESI, partial loss of SO3 is observed during initial mass analysis (MS1) due to in-source dissociation (Itkonen et al., 2008; Nemeth-Cawley et al., 2001), whereas using negative mode ionization preserves this modification to a certain extent (Önnerfjord et al., 2004). Even if successfully ionized as intact sulfopeptides, neutral loss of SO3 (−80 Da) is often a dominant fragmentation pathway during CID, generating fragment ions devoid of SO3 signature and making it difficult to localize the modification (Robinson et al., 2014). In contrast, CID of phosphopeptides results in the characteristic loss of phosphoric acid (−98 Da) via beta elimination, allowing easier identification (Medzihradszky et al., 2004). Moreover, the proteomics databases can misassign sulfopeptides as phosphopeptides introducing another layer of complexity and ambiguity. For instance, Proteome Discoverer identified the same peptide (same sequence and retention time) as sulfated and phosphorylated (Capriotti et al., 2020).
3.2.1 |. Tyrosine sulfation: Enrichment strategies
Characterization of Tyr sulfation requires robust enrichment strategies to overcome the incredibly low abundance of this modification. Several efficient enrichment strategies like IMAC, metal oxide affinity chromatography (MOAC), and strong cation exchange chromatography (SCX) are well optimized for phosphopeptide enrichment. Despite the similar chemical properties of sulfopeptides and phosphopeptides, these strategies are not suited for sulfopeptide enrichment because they use acidic solutions (pH 2) to avoid coisolation of unmodified acidic peptides. Low pH leads to the protonation of the carboxy groups of the peptides, mitigating their enrichment, but simultaneously promotes the hydrolysis of the acid labile sulfate groups (Maxwell & Payne, 2020). Therefore, alternative strategies are required to enrich sulfoproteins/sulfopeptides and are reviewed elsewhere (Klement et al., 2016). Most of these studies utilize different approaches to isolate model sulfopeptides from spiked protein digests, and only a few of them have the potential to be used in a large-scale proteomics study. The evolution of enrichment strategies and recent developments are discussed to highlight the potential or shortcomings of each approach.
The first report of sulfopeptide enrichment utilized weak anion exchange (WAX) chromatography to enrich sulfopeptides from bovine fibrinogen (Amano et al., 2005). In this approach, C-terminal Lys and Arg were removed from tryptic peptides using carboxypeptidase B to enhance the binding of negatively charged SO3 groups to the WAX sorbent and eluted using high concentrations of ammonium acetate to preserve the SO3 moiety before LC-MS/MS analysis (Amano et al., 2005). Although model sulfopeptides spiked into BSA digest and one sulfopeptide from fibrinogen (a standard sulfoprotein) were enriched and identified using this approach, removal of the basic residues (Arg/Lys) from acidic sulfopeptides is not desirable for the analysis of complex samples as it further reduces the charge state and diminishes the ionization efficiency of the acidic sulfopeptides. In another study, the primary amines were carbamylated before WAX enrichment to enhance the binding affinity of Tyr-sulfated peptides to the WAX sorbent, enabling the identification of a sulfopeptide from bovine coagulation factor V protein using LC-MS/UVPD analysis in negative mode (Robinson & Brodbelt, 2016). Carbamylation favored the generation of more negative charge states without diminishing the ionization or UVPD efficiency of sulfopeptides, thus demonstrating the potential of this method for the analysis of more complex samples (Robinson & Brodbelt, 2016). However, this approach relies on the complete derivatization of primary amines and is challenging to implement in complex samples.
Antibody-based affinity enrichment is extensively used for the large-scale proteomics analysis of many different PTMs. While there are several antibodies with high-affinity and selectivity towards pTyr, there are a limited number of commercially available anti-sTyr antibodies (Lawrie et al., 2021). Moore and coworkers developed an anti-sTyr antibody (PSG2) to bind sTyr in peptides and proteins with high affinity and specificity, independently of the flanking peptide sequence (Hoffhines et al., 2006). They used PSG2 affinity enrichment and successfully identified four sulfoproteins from mouse epididymis, an outcome confirmed with WB before in-gel digestion and LC-MS/MS (Hoffhines et al., 2006). Although the antibody-based affinity enrichment strategy has shown promising results for the isolation of sulfoproteins, it has not been widely adopted for large-scale analysis, possibly owing to a high cost of production while only demonstrating moderate selectivity towards sTyr over pTyr (Lawrie et al., 2021).
Src homology 2 (SH2) domain is a structurally conserved protein domain and the largest class of naturally occurring pTyr-recognition domains (Machida & Mayer, 2005). Although smaller, cheaper, and more accessible than sTyr antibodies, the SH2 domain contains a highly evolvable binding pocket that can be engineered to switch its specificity from pTyr to sTyr (Ju et al., 2016; Lawrie et al., 2021). Ju et al. (2016) engineered the binding pocket of an SH2 domain to bind sTyr with a 1700-fold higher affinity than the wild-type SH2 domain using phase display technology (Ju et al., 2016). When compared to the anti-sTyr antibodies, this approach improved the cost and binding affinity but lacked specificity towards sTyr and instead coenriched pTyr (Ju et al., 2016). Guo and coworkers isolated two different SH2 mutants using hyperphage technology with improved specificity towards sTyr (Lawrie et al., 2021). These SH2 mutants were used to selectively enrich a sulfoprotein from a 100-fold excess of BSA containing chemically phosphorylated BSA and from HEK293 cell lysate (Lawrie et al., 2021). The use of the engineered sTyr recognizing domain demonstrates some potential for the characterization of sulfoproteome.
Ga(III)-IMAC, typically used for phosphopeptide enrichment, was used to enrich sulfopeptides from a complex peptide mixture isolated from the skin secretions of a frog (Balderrama et al., 2011). LC-MS/MS analysis following Ga-IMAC enrichment allowed the identification of four sulfopeptides and several other coenriched nonsulfated acidic peptides. IMAC suffers from poor selectivity because it relies on the affinity of negative charges on phosphate/sulfate groups towards the positively charged metal cation resulting in coisolation of unmodified acidic peptides. Therefore, the pH of loading and elution buffers needs to be optimized to reduce the coisolation of acidic peptides while improving the retention and recovery of sulfopeptides. Additionally, the coenrichment of acidic peptides can be prevented by converting carboxylic acid groups to methyl esters before IMAC enrichment as adopted for phosphopeptide enrichment (Ficarro et al., 2002).
In a more recent study, Piovesana and coworkers compared the performance metrics of two WAX mixed-mode sorbents (Strata X-AW and Oasis WAX) and three commercial phosphopeptide enrichment kits (Fe-IMAC, TiO2 kit, and polydopamine-based magnetic Ti4+-IMAC), to enrich sulfopeptide standards spiked into bovine serum albumin (BSA) digest (Capriotti et al., 2020). Samples were analyzed in negative mode to overcome the poor ionization efficiency in the positive mode. Although Strata X-AW and Fe-IMAC offered >80% recovery of sulfopeptides from BSA digest, Fe-IMAC exhibited better selectivity with substantially fewer coisolated peptides (195 vs. 877). To further evaluate the capability of the Fe-IMAC protocol to enrich sulfopeptides from a complex mixture and its adaptability to conventional proteomics workflow, sulfopeptides spiked into a serum sample (1:1000, sulfopeptide/protein) were analyzed in positive mode. Spiked serum was dephosphorylated before trypsin digestion and Fe-IMAC enrichment to avoid coenrichment of phosphopeptides. When compared to the same serum processed identically but spiked after the enrichment, Fe-IMAC offered a 20% recovery of the sulfopeptides. Lower recovery of sulfopeptides from the serum sample could be attributed to increased sample complexity and poor ionization efficiency in the positive mode when compared to the recovery of sulfopeptides from the BSA digest analyzed in the negative mode. Moreover, indigenous serum sulfopeptides were searched using Max-Quant and Proteome Discoverer but were not confidently identified, possibly due to ultra-low concentration and poor ionization in the positive mode. The Fe-IMAC workflow can benefit by using negative mode nano-ESI to enhance the ionization efficiency, improving the detection and identification of indigenous serum sulfopeptides (Capriotti et al., 2020).
3.2.2 |. Mass spectrometry of sulfopeptides
CID is primarily used in conventional proteomics studies and is readily available. CID results in the neutral loss of SO3 (−80 Da) from sulfopeptides due to the labile nature of the sulfo-ester moiety, generating MS/MS spectra that allow peptide sequencing but not localization of the sulfo-modifications (Robinson et al., 2014). Therefore, efforts have been made to develop alternative MS/MS methods that preserve this PTM and pinpoint the sTyr sites.
Although electron-based activation (ExD) methods preserve labile PTMs like phosphorylation and glycosylation, ECD of sulfopeptides (sulfated at Ser, Thr, and Tyr) with a single basic residue resulted in fragment ions devoid of the PTM, impeding its site localization (Medzihradszky et al., 2007). The stability of sulfate moiety was improved by adding metal cations (Na+ and K+) that resulted in the reduction of neutral loss of SO3 from the precursor or product ions, presumably by forming a salt bridge (Medzihradszky et al., 2007). ExD has not been thoroughly investigated for the analysis of sulfopeptides because sulfopeptides naturally occur in lower charge states owing to negatively charged SO3 groups making them less suitable for ExD. Based on the findings from several other studies, it is generally well-known that alternative proteases can increase the precursor charge-states by generating longer peptides with more basic sites that may also enhance the efficiency of ETD fragmentation. For instance, ETD fragmentation of a triply protonated sulfo-Ser containing peptide (GRLGsSRAGR) resulted in no detectable loss of SO3 from the precursor or product ions (Mikesh et al., 2006). This could be due to enhanced stability resulting from the formation of salt bridges between the negatively charged sulfate moiety and the three positively charged Arg residues (Medzihradszky et al., 2007; Yagami et al., 2000). However, further investigation is required to draw conclusive evidence for the stability of more basic Tyr-sulfated peptides upon ETD fragmentation.
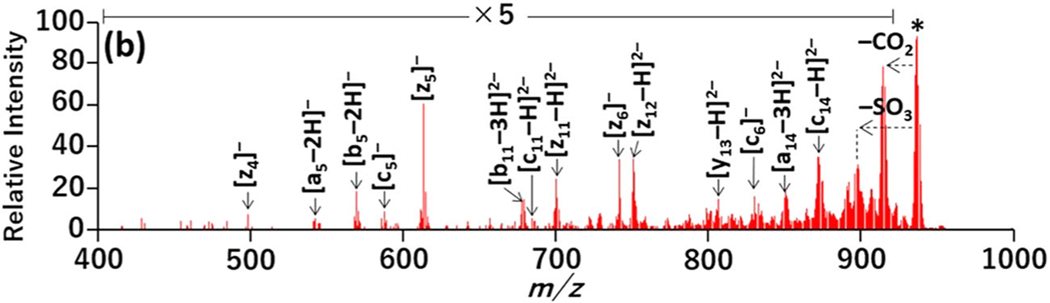
Several studies have investigated fragmentation techniques uniquely geared for anions to capitalize on the efficient ionization of sulfopeptides in the negative mode (Table 3). Hersberger et al. surveyed several activation methods for fragmentation of sulfopeptide anions: CID, electron detachment dissociation (EDD), negative ETD (NETD), and negative ion ECD (niECD) (Hersberger & Håkansson, 2012). Among them, niECD afforded complete sulfate retention in the product ions, minimum neutral loss products, and extensive sequence coverage, ultimately allowing the most successful localization of sTyr. Despite the promising performance, the long timescale of the niECD reaction period (1 s) and the number of scans averaged (32 scans) to generate high-quality spectra offset the feasibility for large-scale proteomics experiments in LC-MS/MS workflow. Robinson et al. used 193 nm ultraviolet photodissociation (UVPD), a high-energy activation technique, to characterize mono-sulfated peptide anions and successfully identified sulfopeptides from bovine fibrinogen digest in an LC-MS/MS workflow (Robinson et al., 2014). Although the neutral loss of SO3 from the precursor and charge-reduced precursor ions were dominant spectral features, UVPD also produced diagnostic sequence ions that retained the sulfate groups and allowed site localization (Figure 9) (Robinson et al., 2014).
TABLE 3.
Different activation techniques applied for MS/MS-based characterization of sulfopeptides
| Activation method | Polarity | Samples analyzed | Advantage | Disadvantage | Reference |
|---|---|---|---|---|---|
|
| |||||
| MAD | Pos Neg | Model sulfopeptides | Retention and localization of SO3 | Low-efficiency long reaction period (~100 ms) biased cleavages next to acidic residues | Cook and Jackson (2011b) |
| niECD | Neg | Model sulfopeptides | Complete SO3 retention, good sequence coverage, minimal neutral loss products | Low efficiency due to long reaction time (1 s) | Hersberger and Håkansson (2012) |
| 193 nm UVPD | Neg | Model sulfopeptides | Retention and localization of SO3 | Dominant neutral loss of SO3 from the precursor and charge-reduced precursor ions | Robinson et al. (2014) |
| FRIPS | Neg | Model sulfopeptides | Retention and localization of SO3 | Not applied to complex samples | Borotto et al. (2018) |
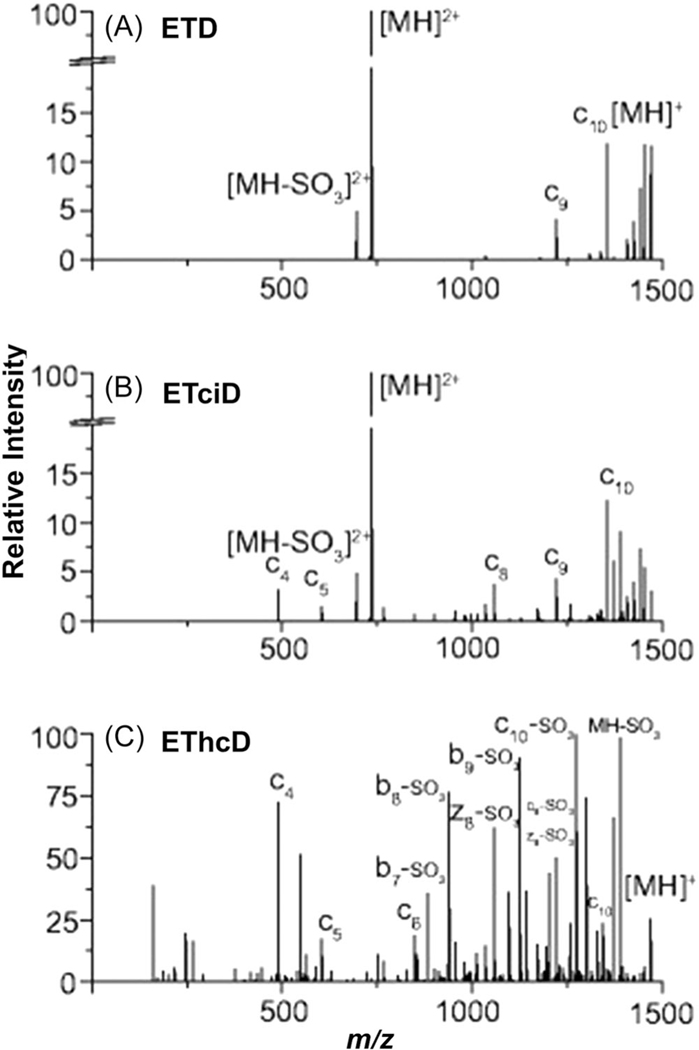
| ETciD | Pos | Model sulfopeptides spiked into serum digest at 1:200, 1:1200, 1:2000 (w/w) | Positive mode is adaptable to existing workflows, retention and localization of SO3 | Poor sensitivity of very acidic sulfopeptides in positive mode | G. Chen et al. (2018) |
| HAD | Neg | Model sulfopeptides and bovine fibrinogen digest | Identified a sulfopeptide from fibrinogen digest using LC-MS/MS No charge reduced precursor | Low efficiency long reaction/accumulation time (1 min) | Asakawa et al. (2019) |
FIGURE 9.
UVPD spectra for (A) noncarbamylated and (B) carbamylated cholecystokinin in the 2− charge state using two pulses at 2 mJ. The carbamylation site is designated as +43 at the N-terminus of the peptide sequence. Reprinted with permission from Robinson and Brodbelt (2016).
In another negative polarity study, Borotto et al. used amine-reactive TEMPO-based free radical initiated peptide sequencing (FRIPS) to analyze two sulfopeptides (and two phosphopeptides) (Borotto et al., 2018). FRIPS is a radical-driven MS/MS technique that generates informative sequence ions with minimal PTM loss and exhibits little dependence of performance on peptide charge state (Borotto et al., 2018). Although the FRIPS method demonstrated promising results for the characterization of two standard sulfopeptides, hirudin and cholecystokinin, its utility for more complex samples is yet to be investigated. In a more recent study, Asakawa et al. utilized hydrogen attachment/abstraction dissociation (HAD) to analyze highly acidic sulfopeptides and sulfoprotein digest ionized in the negative mode (Asakawa et al., 2019). HAD uses the interaction between a peptide and a hydrogen atom generated by homolytic cleavage of H2 when exposed to a heated filament and can be combined with matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) (Asakawa et al., 2019). HAD of a singly deprotonated sulfopeptide from MALDI resulted in fragment ions retaining the sulfate, whereas HAD of doubly deprotonated sulfopeptides resulted in both sulfate-free and sulfate-containing fragment ions. Additionally, they used an integrated LC-ESI-HAD method to separate and identify a doubly deprotonated sulfopeptide in a bovine fibrinogen digest (Figure 10). However, the suitability of HAD for large-scale proteomics is uncertain because MS/MS spectra were acquired after reacting the precursor ions with hydrogen atoms for 1 min, making it incompatible with the LC-MS/MS time scale.
FIGURE 10.
HAD-MS/MS spectrum of a doubly deprotonated sulfopeptide (GlpFPTDsYDEGQDDRPK). Reprinted with permission from Asakawa et al. (2019).
Dann and coworkers used the high mass accuracy and resolution (500,000 at 200m/z) of an Orbitrap Fusion Lumos mass spectrometer to distinguish sTyr from pTyr and localize the PTMs using ETD-based methods (G. Chen et al., 2018). Unlike previous studies, ETD product ions retained the sulfate moiety even in the absence of sulfate stabilizing basic residues, suggesting the sulfate loss upon ETD was instrument-dependent. They also investigated two hybrid activation methods to analyze doubly protonated precursors of eight model sulfopeptides (containing 3–7 Asp/Glu, only two containing a single Lys/Arg), including electron-transfer/collision-induced dissociation (ETciD) and EThcD. They reported that only ETciD preserved the sulfate moiety, whereas EThcD resulted in the loss of sulfate even using low supplemental collision energies. Representative ETD, ETciD, and EThcD spectra of Tyr sulfated peptide are shown in Figure 11, demonstrating the retention of SO3 upon ETCiD. To extend the performance evaluation of ETciD to characterize sTyr in a complex mixture, the sulfopeptides were spiked into trypsin-digested proteins extracted from human serum at ratios of 1:200, 1:1200, and 1:2000 (w/w) and analyzed by reversed-phase LC-MS/MS. For the three ratios analyzed, the targeted MS/MS strategy identified eight, six, and three sulfopeptides, whereas the nontargeted MS/MS strategy identified six, two, and two sulfopeptides, respectively. A sulfopeptide containing three Asp/Glu and a Lys residue near the C-terminus (MGSGDsYDSMKE) was detected using targeted and nontargeted methods across all three ratios analyzed. However, the sulfopeptide containing three Asp/Glu and an Arg residue near the N-terminus (ISDRDsYMGWMD) was not identified at the lowest concentration suggesting that a tryptic sulfopeptide would demonstrate a better sensitivity. Moreover, the peptides with 5–7 Asp/Glu and no Arg/Lys were detected only by the targeted approach at the highest concentration due to poor ionization efficiency and may benefit from negative mode analysis. While ETciD proved to be an effective MS/MS method for sulfopeptide characterization in positive mode, extending to a high throughput workflow for complex biological samples will require a robust enrichment strategy, a prefractionation step, and the use of trypsin/LysC protease for digestion to improve the sensitivity and ionization efficiency (G. Chen et al., 2018).
FIGURE 11.
(A) ETD, (B) ETciD, and (C) EThcD spectrum of a sulfopeptide from Cholecystokinin at 2+ charge state (ISDRDsYMGWMD). Reprinted with permission from G. Chen et al. (2018).
Although significant advances in MS-based proteomics have driven numerous large-scale proteomics studies to uncover the biological significance of many PTMs, there are no large-scale proteomic studies that aim to characterize the sulfoproteome. The sulfoproteome remains largely undiscovered mainly due to the labile nature of this PTM during MS analysis and the lack of robust enrichment methods. Therefore, several improvements are essential to overcome the challenges associated with the precise mapping of sTyr that will ultimately aid in the efforts to unravel the biological significance. The design of a highly selective and efficient method for enrichment is the first step towards a large-scale sulfopeptide characterization. Incorporating a protein-level fractionation step like size exclusion chromatography before peptide-level enrichment affords the discovery of low abundance PTMs by reducing sample complexity and may provide a deeper sulfoproteome coverage. Alternatively, protein-level immunoaffinity enrichment used in conjunction with peptide-level enrichment can improve specificity, while optimization of ionization efficiency and the MS/MS methods will enhance sensitivity, facilitating site-specific characterization of sulfopeptides. One way to improve ionization efficiency is by using alternative proteases to generate larger peptides with more basic sites to stabilize SO3 by forming salt bridges. Since sulfopeptides demonstrate poor ionization efficiency and may undergo insource or gas-phase dissociation during MS1 events, negative mode ionization is better suited for sulfopeptide analysis. Reversed-phase nanoLC-ESI-MS/MS in negative mode has been used to analyze sulfated glycans employing 0.1% formic acid in the solvents and may be compatible for sulfopeptide analysis in either polarity (C.-W. Cheng et al., 2015). Moreover, robust software designed to analyze negative mode MS/MS data will allow easier identification and mapping of sulfopeptides.
3.3 |. Tyrosine nitration
Covalent addition of nitro groups to cysteine (Cys), methionine (Met), tryptophan (Trp), and tyrosine (Tyr) residues in response to oxidative stress induced by reactive nitrogen species (RNS) is called protein nitration (Y. Cheng et al., 2022). In particular, Tyr nitration is the addition of a nitro group at the 3-hydroxyl position to generate 3-nitrotyrosine (3-nTyr/nTyr) (Zhan et al., 2015). In cells, nTyr can be formed by the oxidation of Tyr by peroxynitrite (ONOO−) or by the enzymatic reaction of peroxidases with nitrite and hydrogen peroxide (Bandookwala & Sengupta, 2020). Nitric oxide reacts with superoxide to generate peroxynitrite in response to an increased amount of reactive oxygen species (ROS). Compared to nitrosylation of cysteine and methionine (S-nitrosylation), Tyr nitration (nitration) is under-characterized because the frequency of Tyr nitration is much lower partly due to the comparative relative ease of S-nitrosylation reactions (Batthyány et al., 2017). Nitration of Tyr lowers its pKa (pKa of 3-nTyr is ~7.1 and pKa of free Tyr is ~10) while increasing its size and hydrophobicity (Batthyány et al., 2017). This leads to structural changes, disrupts hydrogen bonding interactions, alters protein-protein interactions, inactivates enzymes and receptors, and impedes the phosphorylation of Tyr residues (Bandookwala & Sengupta, 2020; Zhan et al., 2015; Q. Zhang et al., 2007). There is no consensus motif for Tyr nitration, but several studies have shown that nTyrs are present in flexible loops and turns and in more solvent-accessible regions (Bartesaghi et al., 2007; Darie-Ion et al., 2022; Ion et al., 2020; Sacksteder et al., 2006). The presence of a nearby charged residue also favors Tyr nitration as it enables the formation of hydrogen bonds and salt bridges (Batthyány et al., 2017). There are contradictory reports regarding the inhibition of Tyr nitration by the nearby Cys or Met residues in vitro (Ion et al., 2020; Smallwood et al., 2003; H. Zhang et al., 2005), but in vivo nitration is not inhibited by the neighboring Cys or Met residues (Sacksteder et al., 2006). Additionally, the subcellular localization and structure of the target proteins, nitrating agent, and reaction conditions also contribute to the site-specificity and degree of nitration (Abello et al., 2009; Y. Zhang et al., 2011). For example, preferential nitration of proteins occurs in the subcellular compartments or mitochondrial proteins where nitrating agents are generated in response to oxidative stress (Abello et al., 2009; Radi, 2013).
Nitration plays a critical role in many physiological processes involved in neurotransmission and redox signaling (Teixeira et al., 2016; Zhan & Desiderio, 2016). An elevated level of nTyr is noted with aging and in numerous nitro-oxidative stress-induced pathological conditions: diabetes, cancer, asthma, and cardiac and inflammatory diseases (Teixeira et al., 2016; Zhan & Desiderio, 2016). Additionally, an increased level of 3-nTyr is noted in neurodegenerative diseases induced by nitro-oxidative stress: amyotrophic lateral sclerosis, Alzheimer’s, Parkinson’s, Huntington’s, and Prion disease (Bandookwala & Sengupta, 2020). Antioxidant therapy can decrease the levels of 3-nTyr in these diseases, demonstrating some therapeutic potential (Bandookwala & Sengupta, 2020). Additionally, nTyr levels are elevated in ripening peppers (Chaki et al., 2015), wounded pumpkin leaves (Gaupels et al., 2016), or salt-stressed sunflower seedlings, exhibiting their role in plant growth or stress (David et al., 2015). The functional roles of Tyr nitration in plants are discussed in another review (Kolbert et al., 2017). Several bioanalytical techniques are emerging to quantify free 3-nTyr due to increased levels of 3-nTyr in several diseases and are discussed in a recent review (Bandookwala et al., 2020). However, MS-based methods that facilitate the identification of nitration sites are required to understand the functional roles of nTyr (Bandookwala & Sengupta, 2020).
Site-specific characterization of Tyr nitration is essential for deciphering the biological and physiological significance. PTM attachment sites are identified in a high throughput manner using MS-based proteomics strategies. However, ultra-low amounts of nitration in biological samples and chemical artifacts introduced during chemical derivatization (discussed later) or artificial nitration induced by use of acid hydrolysis (Bandookwala et al., 2020) impede the characterization of nTyr. Therefore, various efforts have been made to improve the identification and localization of Tyr nitration in a high throughput fashion. Several reviews have focused on the enrichment, chemical derivatization, proteomics, and bioanalytical approaches employed to detect 3-nTyr and the biological and physiological roles of Tyr nitration (Bandookwala & Sengupta, 2020; Batthyány et al., 2017; Evans & Robinson, 2013; Tsikas & Duncan, 2014; Yeo et al., 2015; Zhan et al., 2015). In this section, we will discuss the latest developments in enrichment and advances in the MS/MS workflow to characterize the nitroproteome.
3.3.1 |. Tyrosine nitration: Enrichment strategies
About 3% of amino-acid residues in a protein are Tyr residues and among them, only one or two Tyr are physiologically nitrated (Abello et al., 2009; Bartesaghi et al., 2007; Yeo et al., 2015). The short half-life of nitrating agents limits nitration to nearby proteins in response to oxidative damage. Nitration of Tyr is less common than sulfation; only 243 Tyr-nitrated proteins, compared to 548 Tyr sulfated proteins, are reported in the UniprotKB database (https://www.uniprot.org, accessed 9–1-2022) based on direct evidence or sequence similarity. Therefore, the design of a robust enrichment method is an essential first step toward the characterization of nitroproteome (Bartesaghi et al., 2007; Y. Cheng et al., 2022). Immunoprecipitation using anti-3-nTyr antibodies and chemical derivatization are two commonly employed techniques to enrich Tyr-nitrated proteins or peptides (Batthyány et al., 2017; Zhan et al., 2015).
Affinity-based enrichment using anti-tyrosine antibodies Anti-3-nTyr antibodies enable affinity-based enrichment of nitroproteins or nitropeptides. The traditional approach to identifying nitroproteins entails separation using two-dimensional gel electrophoresis (2DGE), detection using anti-3-nTyr immunoblot (WB), in-gel digestion, and identification via LC-MS/MS (Peng et al., 2015; Sultana et al., 2009; Ulrich et al., 2008; Zhan & Desiderio, 2004). Protein-level enrichment using gel-based methods suffers from poor sensitivity due to sample loss during in-gel digestion and interference from unmodified tryptic peptides. For example, Zhan and coworkers selectively detected nitroproteins from human astrocytoma tissue using 2DGE-based anti-3-nTyr immunoblot and analyzed them using in-gel digestion and LC-MS/MS analysis. They identified 20 in vivo Tyr nitration sites from 18 nitroproteins involved in processes like drug resistance, signal transduction, and cell proliferation, suggesting their roles in the spread and treatment of astrocytoma (Peng et al., 2015). They reported a very high interference or coenrichment of 870 nonnitrated proteins, likely obscuring the detection of low abundance nitroproteins (Peng et al., 2015). Immunoaffinity enrichment using anti-3-nTyr antibody and in-solution digestion can mitigate the poor sensitivity of the gel-based approach. Kobeissy et al. used anti-3-nTyr immunoaffinity to enrich nitroproteins from the brain lysate of rats exposed to secondhand smoke (SHS). In-solution digestion and LC-MS/MS analysis identified 29 differentially expressed proteins, two of which were unique to SHS exposure (Kobeissy et al., 2017). The results (validated using WB), support that SHS can lead to oxidative stress and induce Tyr nitration due to the overproduction of ROS. Network analysis revealed the association of these proteins in oxidative stress, ROS generation, and cell-death-related pathways that link SHS exposure to neurodegenerative diseases (Kobeissy et al., 2017).
Brasier and coworkers developed a peptide-level affinity enrichment (Figure 12) using a highly selective anti-3-nTyr antibody to reduce the interference from nonnitrated tryptic peptides and applied it to a sample of whole cell lysate (Zhao et al., 2017). High-accuracy LC-MS/MS analysis unambiguously identified 1881 nitration sites in 907 nitroproteins (Zhao et al., 2017). Despite selective enrichment of N-terminal nitropeptides, this method was integrated with a label-free approach to characterize mouse lung nitroproteome after polyinosinic-polycytidylic acid-induced inflammation. LC-MS/MS analysis identified 147 NT peptides and 237 NT sites, among which 14 NT sites were significantly upregulated after inducing inflammation, demonstrating its utility for large-scale proteomics applications (Zhao et al., 2017). In an earlier study, an anti-3-nTyr antibody exhibited a higher affinity toward synthetic nitropeptides containing basic residues N-terminal to nTyr (Drǎguşanu et al., 2011). Additionally, anti-3-nTyr antibodies demonstrate high-affinity binding to both nitro and hydroxy-Tyr containing synthetic peptides lowering the specificity towards nTyr (Darie-Ion et al., 2022). These studies exhibit the need for a high affinity pan-specific anti-3-nTyr antibody regardless of the peptide sequence to permit unbiased profiling of nitroproteome (Drǎguşanu et al., 2011; Zhao et al., 2017).
FIGURE 12.
Peptide level affinity capture using an anti-3-nTyr antibody. Reprinted with permission from Zhao et al. (2017).
Anti-3-nTyr antibody-based enrichment of Tyr nitrated proteins in plants identified six nitroproteins in wounded pumpkin leaves (Gaupels et al., 2016) and nine nitrated proteins in red and green pepper fruits (including four catalase variants) (Chaki et al., 2015). Similarly, Tyr nitration was also identified in the heme cavity of leghemoglobin found in the root nodules of beans and soybeans using an anti-3-nTyr antibody, in-gel digestion, and targeted LC-MS/MS analysis (Sainz et al., 2015).
Since it is challenging to synthesize peptide immunogens used to generate highly selective pan-specific antibodies, Cheng and coworkers used phage-display technology to create a 3-nTyr affinity ligand (NT-1) to bind Tyr nitrated peptides with high affinity without any sequence bias (Y. Cheng et al., 2022). Endogenous nitropeptides were enriched from BSA digest (1000-fold excess) using an immobilized NT-1 ligand (Y. Cheng et al., 2022). The NT-1 ligand exhibited improved performance compared to a commercial anti-3-nTyr antibody, demonstrating the potential for peptide-level enrichment of low levels of 3-nTyr from complex mixtures. Other alternatives to anti-3-nTyr antibodies are also emerging to aid the enrichment of nitroproteins. Nanobodies (Nbs) are single-domain antibody fragments (~15 kDa) derived from the heavy chain antibodies of camelids (Van Fossen et al., 2022; Zare et al., 2021). Due to their small size, cost-effective production, and good tissue permeability, nanobodies are emerging as a treatment alternative for viral diseases (Van Fossen et al., 2022; Zare et al., 2021). Mehl and coworkers recently developed an anti-NT Nb library from the serum of an animal immunized with five nitrated immunogens (Van Fossen et al., 2022). A protein-specific Nb, selected using phage-display technology, was used to enrich nitrated 14–3-3 signaling protein. However, high affinity and broad specificity nanobodies are essential for the selective enrichment of nitroproteins in a complex sample.
Chemical derivatization
Chemical derivatization of the nitro group is an attractive strategy to incorporate enrichment tags. This approach involves three main steps: (1) blocking of primary amines by acetylation, (2) reduction of 3-nTyr to 3-aminotyrosine (3-AT) using dithionite, and (3) chemical derivatization of 3-AT modified peptides using different enrichment tags before downstream LC-MS/MS analysis (further details and applications are discussed by Zhan et al., 2015). The earliest report of this method incorporated a cleavable biotin tag at the modified 3-AT to purify the biotinylated tryptic peptides using a streptavidin affinity column before LC-MS/MS analysis (Nikov et al., 2003). Alternatively, 3-AT-modified peptides were derivatized using different reagents: sulfhydryl group, bis-pyridine-Tyr, perfluoro tag, or a fluorogenic reagent to make them amenable to various enrichment methods (Dremina et al., 2011; Kim et al., 2011; Lee et al., 2009; Q. Zhang et al., 2007). For example, Kim and coworkers tagged 3-AT modified peptides with N-succinimidyl-3-perfluorobutyl propionate, captured them on fluorinated carbon-linked silica beads, eluted them with 100% methanol, and identified 28 Tyr nitrated peptides (28 proteins) from human hepatoma cell lysate (Kim et al., 2011). The Kim group also used fluorogenic enrichment and LC-MS/MS analysis to localize 14 nitropeptides in 14 nitroproteins involved in yeast mating signal transduction (Kang et al., 2015). This was the first report of unicellular nitroproteome that demonstrated the applicability of the fluorogenic enrichment method to characterize endogenous Tyr nitration (Kang et al., 2015).
Using acetylation to block the primary amines decreases the ionization efficiency of modified peptides by removing the protonation sites and can be mitigated by using methylation (Guo et al., 2012; Prokai et al., 2014; Prokai-Tatrai et al., 2011). Additionally, reduction of 3-nTyr to 3-AT using dithionite leads to the formation of a more favorable and acidic side-product (sulfated 3-aminotyrosine) that cannot be further derivatized with enrichment tags, resulting in fewer identifications of nitropeptides (Ghesquière et al., 2009). In a more recent study, 3-nTyr was reduced to 3-AT at a high temperature using dithiothreitol (DTT) and hemin after acetylation of primary amines. The 3-AT modified peptides were captured on a stationary phase functionalized with a base-cleavable NHS-ester and eluted by acid-catalyzed hydrolysis at room temperature before LC-MS/MS analysis (Yang, 2017). This sample preparation strategy minimized the number of derivatization and desalting steps while mitigating the acidic side-product. To evaluate the performance of this strategy, it was used to enrich 3-AT modified angiotensin II from BSA human serum digest at different ratios. The author reported 50% recovery from BSA digest (spiked at 1:100 ratio) and 41% recovery from human plasma digest (spiked at 1:10,000 ratio), exhibiting minimal sample loss and potential application for large-scale nitroproteome characterization (Yang, 2017). One limitation of this method is the potential for acid-catalyzed hydrolysis which is known to induce artificial levels of nitration (Bandookwala et al., 2020; Delatour et al., 2002).
Gross and coworkers developed an improved solidphase chemical-capture approach to detect aromatic nitration sites (Trp and Tyr) by addressing several problems in the existing methods (Nuriel et al., 2016). Different reagents were used to optimize the derivatization steps: (1) primary amines were blocked by dimethylation using formaldehyde and dimethylamine borane instead of acetylation to preserve the positive charges on the primary amines, (2) 3-nTyr was reduced to 3-AT with hemin/DTT at high temperature instead of dithionite to avoid the formation of acidic side products, and (3) aminoaromatic peptides were captured on aldehyde-agarose beads to overcome the nonspecific interactions on NHS-ester beads. The captured peptides were eluted at acidic pH to release amino-aromatic peptides (previously nitrated) and detected using LC-MS/MS analysis. This method identified 244 nitrated sites (including two at Trp) from 145 proteins in 1 mM ONOO− treated rat brain digest, 19 Tyr nitrated peptides from 13 proteins in 250 μM ONOO− treated rat brain digest, and 3 Tyr nitrated peptides from three proteins in an untreated sample of rat brain digest (Ando et al., 2019). Additionally, primary amines of nitrated and non-nitrated rat brain digest were blocked using heavy and light isotopes (C13D2O and CH2O) to allow relative quantitation (Nuriel et al., 2016). Overall, this proved to be a promising approach for sensitive and relative quantitation of nitration sites in complex biological samples.
Although considerable improvement is being made to avoid unwanted artifacts (artificial nitration) and side-products (sulfated 3-aminotyrosine) of these multistep derivatization strategies, each step requires careful optimization and may suffer from reproducibility issues due to incomplete chemical reactions making it challenging for routine large-scale nitroproteome characterization.
3.3.2 |. Mass spectrometry of nitropeptides
CID is the most common ion activation technique employed for the site-specific localization of 3-nTyr because it is readily available and is the most established method. Upon CID, nitropeptides produce a characteristic 3-nTyr immonium ion of m/z 181.06 that can be used in a precursor ion scanning method to accurately identify nitropeptides in complex samples (Petersson et al., 2001). A representative CID MS/MS spectrum of a synthetic nitropeptide along with the immonium ion is shown in Figure 13.
FIGURE 13.
Annotated MS/MS spectra of a synthetic histone H4 peptide nitrated at Tyr51. Diagnostic nTyr immonium ion at 181.0601 Da. Reprinted with permission from Kriss et al. (2021).
Although 3-nTyr is not a labile PTM, it can be difficult to obtain complete sequence coverage using CID, hindering precise localization of the modification in larger peptides containing multiple Tyr residues. Therefore, it is desirable to investigate alternative activation methods. ExD-based methods have been transformative for the localization of labile PTMs like phosphorylation and glycosylation, but the characterization of nitropeptides using ExD is hindered by the electron capturing NO2-group that results in the formation of a stable radical intermediate which impedes the formation of sequence ions (Guo & Prokai, 2012; Jones et al., 2010). To overcome this limitation, nitro groups have been converted to amino groups to improve sequence coverage, both at the protein and peptide levels (Guo & Prokai, 2012). Additionally, 3-AT can be converted to other functional groups with distinct fragmentation patterns to improve ECD fragmentation (Prokai-Tatrai et al., 2011). Another exploratory attempt to characterize Tyr-nitrated peptides utilized metastable atom-activated dissociation (MAD)-MS. During MAD-MS, different charge states of isolated precursor ions are interacted with a high kinetic energy beam of helium metastable atoms, resulting in the extensive fragmentation of peptide backbone (a/b/c/x/y/z type ions) (Cook & Jackson, 2011a). MAD offered confident localization of the nitration sites by combining spectra from different charge states (Cook & Jackson, 2011a). However, MAD-MS is not readily available and requires relatively long reaction times (~100 ms), limiting its application for large-scale proteomics studies.
In a more focused study, Luo and coworkers analyzed in vitro nitrated fibrinogen γ chains (20 Tyr residues) using precursor-ion scanning LC-MS/MS and identified 5 Tyr residues susceptible to nitration (Luo et al., 2015). More recently, Medeiros and coworkers used ESI-QTOF-MS to identify 20 nitrated Tyr in fibrinogen by searching for a 45 Da mass increase and validated the results by comparing the MS/MS spectra of modified and unmodified peptides and de novo sequencing (Medeiros et al., 2021). They also used selected reaction monitoring (SRM) to detect endogenous nitration in plasma fibrinogen. SRM exhibited improved selectivity and sensitivity, demonstrating the potential of this approach when there are no robust enrichment methods available.
3.3.3 |. Proteomics studies
There are a limited number of proteomics studies aimed at characterizing the sites of Tyr nitration and understanding the roles of nitroproteins in disease conditions or the basal levels, which are mostly reported before 2015 (J. Li & Zhan, 2021). A few of the more recent studies using different methods to identify 3-nTyr are high-lighted in Table 4. Laminin is a major protein of the extracellular matrix (ECM) present in the artery walls that undergoes ONOO− induced nitration upon inflammation (Lorentzen et al., 2019). To understand the roles of laminin nitration in cardiovascular diseases, Davies and coworkers nitrated lamin-111 by exposing it to ONOO− (1–500X excess), deglycosylated, and digested using the FASP protocol on 10 kDa molecular weight cut-off filters before LC-MS/MS analysis (Lorentzen et al., 2019). First, they designed a protocol to detect Tyr and Trp nitration using a high molar excess of ONOO−, identifying 148 mono-nitration (126 Tyr and 22 Trp) in addition to 14 dinitration (10 Tyr and 4 Trp) sites. They performed feature detection using Skyline (Skylinedaily 3.6.1.10556) to establish a database for the targeted detection of nitration sites in laminin-111 exposed to lower concentrations of ONOO−. The targeted approach enhanced the detection sensitivity, identifying 34 nitration sites from the laminin-111 sample treated with equimolar ONOO− (Lorentzen et al., 2019).
TABLE 4.
A summary of different approaches used to characterize nTyr using LC-MS/MS analysis
| Methods used | Samples analyzed | # Sites | # Proteins | References |
|---|---|---|---|---|
|
| ||||
| WB 3-nTyr antibody | Human astrocytoma tissue | 20 | 18 | Peng et al. (2015) |
| Precursor ion scanning | In vitro nitrated Bovine fibrinogen | 5 | - | Luo et al. (2015) |
| Fluorogenic enrichment | Yeast lysate (in vivo) | 14 | 14 | Kang et al. (2015) |
| Solid-phase chemical capture of aromatic amines | Peroxynitrite treated rat brain lysate | 244 | 144 | Nuriel et al. (2016) |
| Protein-level IP anti-3-nTyr antibody | Secondhand smoke exposed rat’s brain lysate | - | 29 | Kobeissy et al. (2017) |
| Peptide-level IP using anti-3-nTyr antibody | In vitro nitrated whole-cell lysate poly(I:C) treated mouse lung tissue | Cell lysate: 18811 Lung: 237 | Cell lysate: 907 Lung: 147 peptides | Zhao et al. (2017) |
| 2DGE, 3-nTyr WB, in-gel digestion, LC-MS/MS | In vitro nitrated beef heart mitochondria | 6 | 5 | Kohutiar et al. (2018) |
| Filter-aided sample preparation (FASP) 1 targeted detection using Skyline | Peroxynitrite treated laminin | 148 | - | Lorentzen et al. (2019) |
| 2DGE and MALDI-TOF/TOF | Ischemia reperfused rat heart | - | 8 | Tatarkova et al. (2019) |
| Anti-3-nTyr affinity, in-gel digestion, and LC-MS/MS analysis | In vitro nitrated S. meliloti proteins | 2 | 2 | Cazalé et al. (2020) |
| 3-nTyr antibody WB, in-gel digestion, and LC-MS/MS, PRM analysis using Skyline | Sodium nitroprusside treated CDKA;1 from maize embryo | 2 | - | Méndez et al. (2020) |
| Untargeted LC-MS/MS analysis for treated SRM analysis for in vivo | Peroxynitrite treated and in vivo ischemic fibrinogen | Treated: 20 In vivo SRM: 9 | - | Medeiros et al. (2021) |
| Result validation by comparing MS/MS spectra to synthetic nitropeptides | Hepatocyte nuclei from control and ethanol-exposed mice liver | 6 | 4 | Kriss et al. (2021) |
Abbreviation: CDKA, cyclin-dependent kinase A.
Traumatic brain injury (TBI) is a critical public health problem that induces nitrosative and oxidative stress. TBI may be associated with long-term neurodegenerative disorders like Alzheimer’s disease (Ramos-Cejudo et al., 2018). Gamma-glutamyl cysteine ethyl ester (GCEE), a known precursor to glutathione (GSH), alleviates oxidative stress by activating the antioxidant defense system (Henderson et al., 2016). Reed and coworkers analyzed the brain proteins of control (no TBI), saline-treated (post-TBI), or GCEE-treated (post-TBI) rats to understand the roles of nitration and oxidative damage in the brain (Henderson et al., 2016). WB analysis revealed significantly elevated levels of nitroproteins in saline-treated TBI rats compared to control rats. However, no changes were observed in GCEE-treated rats, suggesting GCEE as a potential neuroprotective agent for TBI treatment. In-gel digestion and LC-MS/MS analysis of anti-3-nTyr immunoblot positive-spots upregulated in saline-treated TBI rats identified seven nitroproteins. These nitroproteins elevated in TBI rats may serve as potential biomarkers to determine the pathogenesis and progression of neurodegenerative diseases.
Mitochondria is an essential source of ROS/RNS like superoxide, nitric oxide, and peroxynitrite. Although ONOO− induces oxidative damage to mitochondrial proteins, it maintains the balance between ROS/RONS (reactive oxygen and nitrogen species) and the antioxidant capacity of cells and plays a vital role in the signal transduction system (Kohutiar et al., 2018). To investigate the ONOO− induced oxidative damage and understand the roles of nitration in cellular metabolism, Kohutiar and coworkers isolated beef heart mitochondria and exposed them to ONOO− (Kohutiar et al., 2018). Water-soluble and lipophilic fractions of nitrated proteins were analyzed using 2DGE, WB using an anti-3-nTyr antibody, in-gel digestion, and LC-MS/MS. Among the many 3-nTyr-positive spots detected, sites of Tyr nitration in five of the proteins were confirmed by MS/MS: BSA (Tyr355), ATP synthase subunit beta (Tyr395), pyruvate dehydrogenase E1 component subunit beta (Tyr63 and Tyr67), citrate synthase (Tyr381), and acetyl-CoA acetyltransferase (Tyr326). Most nitroproteins were identified only in the soluble fraction, whereas ATP synthase was identified in both the soluble and lipophilic fractions. This set of results suggested that ONOO− can break the peptide bond by free radical mechanism and release the membrane proteins into the solution, exposing Tyr residues for nitration (Kohutiar et al., 2018). Similarly, Kaplan and coworkers analyzed mitochondrial proteins after ischemia-reperfusion using 2DGE and MALDI-TOF/TOF MS and identified eight mitochondrial nitroproteins vital in electron transport, fatty acid oxidation, tricarboxylic acid cycle, ATP synthesis, and control of high-energy phosphates (Tatarkova et al., 2019).
A nontargeted LC-MS/MS study aimed to characterize the proteomic signature of exudates from acutely healing surgical trauma wounds identified 15 unique 3-nTyr sites corresponding to 15 proteins, along with highly prevalent Cys and Met oxidation (Bekeschus et al., 2018). Although this study did not focus on nitroproteomics analysis, it showed that oxidative-stress-induced Tyr nitration is prevalent during wound healing.
Ischemic stroke leads to the reduction of blood supply to the brain due to fibrin clot that induces a surge of ROS (NO and OO−), leading to the production of ONOO− (Medeiros et al., 2021). Fibrinogen is a plasma protein that gets converted into fibrins by thrombin during blood coagulation and therefore is a potential target for oxidative-stress induced PTM. Medeiros and coworkers used an untargeted LC-MS/MS to identify 20 nTyr (out of 49 Tyr residues) in fibrinogen samples exposed to 200X molar excess of ONOO− based on the mass increase of 45 Da at Tyr residues (Medeiros et al., 2021). They identified appropriate fragment ions from nine unique Tyr positions to design an SRM method and applied it to detect in vivo nitration sites in ischemic fibrinogen (Medeiros et al., 2021). SRM improved the overall selectivity and sensitivity of detection. Among the nine Tyr positions analyzed using SRM, three were more elevated in ischemic fibrinogen than in the control group, suggesting nitrated fibrinogen as a potential diagnostic marker for ischemic stroke. The authors note that in vitro experiments do not reflect the physiological nitration state of proteins because nitration is highly specific in vivo.
Betula verrucosa (Bet v 1a) is a major allergen from birch pollen whose allergenicity is enhanced upon nitration. Gusenkov and Stutz treated Bet v 1a with ONOO− to induce Tyr nitration and analyzed using top-down and bottom-up HCD-MS/MS after separation using capillary zone electrophoresis (CZE) (Gusenkov & Stutz, 2018). CZE separated the intact protein based on the location of nitration sites and the degree of nitration. The bottom-up MS/MS analysis identified six nTyr (Tyr5, 66, 81, 83, 150, and 158), while the top-down MS/MS analysis of three singly nitrated species identified one nTyr (Tyr5). Both approaches identified Tyr5 as the most favorable nitration site, likely because it is more surface exposed. Either the absence of +45 Da mass shifted diagnostic fragment ions bracketing the nTyr or the lack of C-terminal coverage using HCD impeded the localization of nTyr present in the protein core (Tyr66, 81, and 83) and those present in the C-terminal of the sequence (Tyr120, 150, and 158). Top-down MS/MS of doubly nitrated species did not provide site-specific information, underscoring the lack of desirable MS/MS techniques for nitration analysis.
Cazalé and coworkers studied the effects of NO exposure on Rhizobia bacteria (Sinorhizobium meliloti) that live in soil or root nodules legumes (Cazalé et al., 2020). Nitric oxide is present in both environments, indicating the role of nitration in bacterial signaling. Nitrating agents (spermine NONOate or ONOO−) were used to induce nitration of bacterial cells or proteins. Nitroproteins were enriched using an anti-3-nTyr affinity sorbent and separated on an SDS gel before in-gel digestion and LC-CID-MS/MS analysis. This approach identified two nitroproteins (two flagellins A and B) and suffered from the interference from unmodified peptides (3010 peptides from 406 proteins), demonstrating the need for peptide level enrichment before MS analysis.
Cyclin-dependent kinase A (CDKA) controls cell cycle progression in plants and may be a potential substrate of nitration under oxidative stress. Gallego and coworkers used gel electrophoresis, anti-3-nTyr antibody WB, in-gel digestion, and LC-MS/MS analysis to identify two nTyr (Tyr15 and Tyr19) at the ATP binding site of CDKA;1 from Zea mays (ZmCDKA;1). The fluorometric assay demonstrated that nitration results in a decreased affinity of ZmCDKA;1 toward ATP binding and suggested that nitration could act as a modulator for cell cycle progression and growth (Méndez et al., 2020).
In a more recent study, Stevens and coworkers used LC-MS/MS to identify the sites of Tyr nitration in hepatic nuclei samples from male mice upon chronic ethanol exposure (28 days) (Kriss et al., 2021). High-resolution MS/MS identified six novel nitration sites on four nitrated histone proteins without prefractionation or enrichment steps. In vivo nitration sites were validated by MS/MS analysis of synthetic nitropeptides with a similar sequence to each in vivo nTyr site. Additionally, the molecular dynamic simulation suggested that Tyr nitration is involved in chromatin remodeling of histone proteins upon alcohol-induced oxidative stress (Kriss et al., 2021).
The ultra-low levels of Tyr nitration and lack of robust enrichment methods impede the site-specific characterization of Tyr nitration. Therefore, fractionation of proteins using techniques such as size exclusion chromatography before digestion and enrichment or use of gas-phase fractionation will facilitate the identification of nitroproteins. Additionally, utilizing a more targeted approach like SRM and PRM will improve the sensitivity and enable a more confident identification of nTyr.
4 |. CONCLUSION AND PERSPECTIVES
Recent advances in high-mass accuracy, high-resolution, and hybrid mass spectrometers have delivered tremendous enhancements to acquisition speed and sensitivity, expanding the depth and scope of proteomic coverage for many applications (Hebert et al., 2018). The evolution of separation methods, such as two-dimensional liquid chromatography (2DLC), alongside offline prefractionation and robust enrichment techniques, have further improved specificity to discover new types and sites of PTMs (Z. Li et al., 2022). While these strategies have established the foundation for characterizing less common PTMs, the incorporation of newer technologies such as ion mobility in the proteomics workflow adds another dimension, enhancing the detection of lower abundance PTMs and expanding the depth of coverage (Hebert et al., 2018; Meier et al., 2018). Data independent acquisition (DIA) is another emerging method in which multiple precursor ions in a predefined m/z range is activated simultaneously and analyzed in a high-resolution mass analyzer, and the process is repeated to cover the entire range of interest (J. Li et al., 2021). The DIA method circumvents the preferential intensity-dependent one-at-a-time precursor sampling mode of the conventional data-dependent acquisition (DDA) approach. By offering a high dynamic range and high throughput (J. Li et al., 2021), DIA provides the opportunity to uncover low abundant disease biomarkers from serum and plasma proteins that might otherwise remain obscured using DDA methods (Bekker-Jensen et al., 2020; Lin et al., 2018). DIA has been used to obtain deeper coverage and reproducible results for global analysis of phosphoproteome (Kitata et al., 2021; Srinivasan et al., 2022) and glycoproteomes (Yang et al., 2021; Ye & Vakhrushev, 2021). DIA affords comparable proteomic coverage to a fractionation-based method in a single-shot analysis (Kitata et al., 2021). The high dynamic range of DIA might be beneficial for the detection of low abundance PTMs like ADP-ribosylation and specifically, tyrosine nitration or sulfation, both of which lack robust enrichment methods. However, site-specific localization of PTMs using DIA is still a challenge, especially when advanced activation methods are required (Ye & Vakhrushev, 2021). DIA and DDA are unfocused approaches that aim to maximize the number of peptides/PTMs during an LC-MS/MS run and are valuable tools for discovery-based proteomics. However, both methods are biased toward more abundant peptides in the mixture and may lack the reproducibility required for the identification of low abundant peptides of interest (van Bentum & Selbach, 2021). A more targeted approach can be employed for highly sensitive and reproducible detection of a peptide/PTM of interest using a targeted approach like selected reaction monitoring (SRM) or multiple peptides using multiple reaction monitoring (MRM). SRM/MRM method are less commonly employed for higher throughput applications as they are typically applied to small sets of peptides. Another option, parallel reaction monitoring (PRM), offers high sensitivity like MRM while exhibiting higher throughput and high resolution in quadrupole-Orbitrap type hybrid instruments and has been utilized to discover low abundance phosphorylation sites in tau proteins of the brain and cerebrospinal fluid (Barthélemy et al., 2019). Therefore, the use of PRM for a more targeted analysis of these low abundant PTMs shows some promise for the detection and quantitation of disease biomarkers.
Ion-mobility spectrometry (IMS) has long been used for the structural characterization of proteins and protein complexes. IMS separates molecules based on their collision cross-sections in the gas phase. With the availability of advanced data analysis tools, IMS has been utilized to add another dimension to LC-MS/MS-based proteomics workflow, thus allowing higher proteomic coverage (Prianichnikov et al., 2020). Very recently, gas phase fractionation via trapped IMS (TIMS) followed by parallel accumulation-serial fragmentation (PASEF) using a quadrupole time-of-flight instrument was used to obtain enhanced proteomic coverage using a small sample amount of 10–200 ng (Guergues et al., 2022; Meier et al., 2018). TIMS fractionation coupled to DDA-PASEF improved protein identifications by ~20% and peptide identifications by ~30% using a 90-min LC gradient compared to DDA-PASEF alone (Guergues et al., 2022). Therefore, gas-phase fractionation using a TIMS device offers a new concept for the detection of very low abundant PTMs from complex biological mixtures.
Overall, the site-specific localization of less common PTMs has been aided by advanced instrumentation, streamlined proteomics workflow, and sophisticated bioinformatics tools that facilitate the analysis and interpretation of large amounts of proteomics data (Popov et al., 2009). Moreover, top-down proteomics can be used in conjunction with bottom-up proteomics to obtain complementary information and more complete pictures of proteoforms, and thus combining top-down and bottom-up methods offers a compelling strategy to extend PTM mapping. Top-down analysis in discovery-based proteomics is challenging because it is difficult to achieve baseline separation of intact proteoforms from complex samples, and it is formidable to achieve high sequence coverage on a chromatographic timescale to confidently localize PTMs. Prefractionation of proteins before LC-MS/MS analysis is one way to lower the sample complexity and is discussed in more detail in a recent review (Toby et al., 2016). Capillary electrophoresis (CE) has also shown promise for the separation of intact proteins (Y. Li et al., 2014). Robust enrichment of low abundant phosphoproteins from a complex tissue sample and top-down analysis of intact proteoforms using online LC-MS/MS have previously been reported for the identification of phosphorylation sites (B. Chen et al., 2017). Utilizing multiple proteomics approaches is essential for unraveling the biological significance of the most analytically challenging PTMs.
ACKNOWLEDGMENTS
Funding from NSF (CHE-2203602), NIH (R35GM139658), and the Robert A. Welch Foundation (F-1155) is gratefully acknowledged.
Abbreviations:
- ADP-ribosylation
adenosine diphosphate-ribosylation
- ARTC
clostridial toxin-like ARTs
- ARTD
diphtheria toxin-like ARTs
- ARTs
ADP-ribosyltransferases
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MAR
mono (ADP-ribose)
- PAR
poly (ADP-ribose)
- PARG
PAR glycohydrolase
- PARP
poly (ADP-ribose) polymerase
- PDE
phosphodiesterase digestion
- PTM
posttranslation modification.
Biographies
AUTHOR BIOGRAPHIES
 Aarti Bashyal earned an MS degree in chemistry from the University of Texas at Arlington in 2013. She is currently pursuing a Ph.D. in analytical chemistry in Professor Jennifer Brodbelt’s lab. The primary focus of her research is the characterization of protein post-translational modifications using liquid chromatography in tandem with ultraviolet photodissociation mass spectrometry.
Aarti Bashyal earned an MS degree in chemistry from the University of Texas at Arlington in 2013. She is currently pursuing a Ph.D. in analytical chemistry in Professor Jennifer Brodbelt’s lab. The primary focus of her research is the characterization of protein post-translational modifications using liquid chromatography in tandem with ultraviolet photodissociation mass spectrometry.
 Jennifer S. Brodbelt earned her B.S in chemistry from the University of Virginia and her Ph.D. from Purdue University. After a postdoctoral stint at the University of California at Santa Barbara, she joined the faculty at the University of Texas at Austin where she is currently a professor and the Rowland Pettit Centennial Chair in Chemistry. She serves as an associate editor for Journal of the American Society for Mass Spectrometry. Her research focus is on the development of ultraviolet photodissociation and ion trap mass spectrometry for characterization of peptides, proteins, protein complexes, and lipids.
Jennifer S. Brodbelt earned her B.S in chemistry from the University of Virginia and her Ph.D. from Purdue University. After a postdoctoral stint at the University of California at Santa Barbara, she joined the faculty at the University of Texas at Austin where she is currently a professor and the Rowland Pettit Centennial Chair in Chemistry. She serves as an associate editor for Journal of the American Society for Mass Spectrometry. Her research focus is on the development of ultraviolet photodissociation and ion trap mass spectrometry for characterization of peptides, proteins, protein complexes, and lipids.
REFERENCES
- Abello N, Kerstjens HAM, Postma DS, & Bischoff R.(2009). Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. Journal of Proteome Research, 8(7), 3222–3238. [DOI] [PubMed] [Google Scholar]
- Abplanalp J, Leutert M, Frugier E, Nowak K, Feurer R, Kato J, Kistemaker HVA, Filippov DV, Moss J, Caflisch A, & Hottiger MO (2017). Proteomic analyses identify ARH3 as a serine mono-ADP-ribosylhydrolase. Nature Communications, 8(1), 2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano Y, Shinohara H, Sakagami Y, & Matsubayashi Y.(2005). Ion-selective enrichment of tyrosine-sulfated peptides from complex protein digests. Analytical Biochemistry, 346(1), 124–131. [DOI] [PubMed] [Google Scholar]
- Ando Y, Elkayam E, McPherson RL, Dasovich M, Cheng S-J, Voorneveld J, Filippov DV, Ong S-E, Joshua-Tor L, & Leung AKL (2019). ELTA: enzymatic labeling of terminal ADP-ribose. Molecular Cell, 73(4), 845–856.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa D, Takahashi H, Sekiya S, Iwamoto S, & Tanaka K.(2019). Sequencing of sulfopeptides using negative-ion tandem mass spectrometry with hydrogen attachment/abstraction dissociation. Analytical Chemistry, 91(16), 10549–10556. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, & Huttner WB (1987). Tyrosine sulfation is atrans-Golgi-specific protein modification. Journal of Cell Biology, 105(6), 2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderrama GD, Meneses EP, Orihuela LH, Hernández OV, Franco RC, Robles VP, & Batista CVF (2011). Analysis of sulfated peptides from the skin secretion of the Pachymedusa dacnicolor frog using IMAC-Ga enrichment and high-resolution mass spectrometry. Rapid Communications in Mass Spectrometry, 25(8), 1017–1027. [DOI] [PubMed] [Google Scholar]
- Balsved D, Bundgaard JR, & Sen JW (2007). Stability of tyrosine sulfate in acidic solutions. Analytical Biochemistry, 363(1), 70–76. [DOI] [PubMed] [Google Scholar]
- Bandookwala M, & Sengupta P.(2020). 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. International Journal of Neuroscience, 130(10), 1047–1062. [DOI] [PubMed] [Google Scholar]
- Bandookwala M., Thakkar D., & Sengupta P. (2020). Advancements in the analytical quantification of nitroxidative stress biomarker 3-nitrotyrosine in biological matrices. Critical Reviews in Analytical Chemistry, 50(3), 265–289. [DOI] [PubMed] [Google Scholar]
- Bartesaghi S, Ferrer-Sueta G, Peluffo G, Valez V, Zhang H,Kalyanaraman B, & Radi R.(2007). Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids, 32(4), 501–515. [DOI] [PubMed] [Google Scholar]
- Barthélemy NR, Mallipeddi N, Moiseyev P, Sato C, & Bateman RJ (2019). Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Frontiers in Aging Neuroscience, 11, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett E, Bonfiglio JJ, Prokhorova E, Colby T, Zobel F, Ahel I, & Matic I.(2018). Interplay of histone marks with serine ADP-ribosylation. Cell Reports, 24(13), 3488–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batth TS, Francavilla C, & Olsen JV (2014). Off-line high-ph reversed-phase fractionation for in-depth phosphoproteomics.Journal of Proteome Research, 13(12), 6176–6186. [DOI] [PubMed] [Google Scholar]
- Batthyány C, Bartesaghi S, Mastrogiovanni M, Lima A, Demicheli V, & Radi R.(2017). Tyrosine-nitrated proteins: proteomic and bioanalytical aspects. Antioxidants & Redox Signaling, 26(7), 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekeschus S, Lackmann J-W, Gümbel D, Napp M,Schmidt A, & Wende K.(2018). A neutrophil proteomic signature in surgical trauma wounds. International Journal of Molecular Sciences, 19(3), 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker-Jensen DB, Bernhardt OM, Hogrebe A, Martinez-Val A, Verbeke L, Gandhi T, Kelstrup CD, Reiter L, & Olsen JV (2020). Rapid and site-specific deep phosphoproteome profiling by data-independent acquisition without the need for spectral libraries. Nature Communications, 11(1), 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim FR (1954). Tyrosine-O-sulfate in a peptide from fibrinogen. Journal of the American Chemical Society, 76(10), 2838–2839. [Google Scholar]
- Bilan V, Leutert M, Nanni P, Panse C, & Hottiger MO (2017). Combining higher-energy collision dissociation and electron-transfer/higher-energy collision dissociation fragmentation in a product-dependent manner. Analytical Chemistry, 89(3), 1523–1530. [DOI] [PubMed] [Google Scholar]
- Bonfiglio JJ, Colby T, & Matic I.(2017). Mass spectrometry for serine ADP-ribosylation? Think o-glycosylation! Nucleic Acids Research, 45(11), 6259–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio JJ, Leidecker O, Dauben H, Longarini EJ, Colby T, San Segundo-Acosta P, Perez KA, & Matic I.(2020). An HPF1/PARP1-based chemical biology strategy for exploring ADP-Ribosylation. Cell, 183(4), 1086–1102.e23. [DOI] [PubMed] [Google Scholar]
- Borotto NB, Ileka KM, Tom CATMB, Martin BR, & Håkansson K.(2018). Free radical initiated peptide sequencing for direct site localization of sulfation and phosphorylation with negative ion mode mass spectrometry. Analytical Chemistry, 90(16), 9682–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch-Larsen SC, Hendriks IA, Lodge JM, Rykær M, Furtwängler B, Shishkova E, Westphall MS, Coon JJ, & Nielsen ML (2020). Mapping physiological ADP-ribosylation using activated ion electron transfer dissociation.Cell Reports 32(12), 108176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch-Larsen SC, Rebak AKLFS, Hendriks IA, & Nielsen ML (2021). Temporal and site-specific ADP-ribosylation dynamics upon different genotoxic stresses. Cells, 10(11), 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard JR, Vuust J, & Rehfeld JF (1997). New consensus features for tyrosine O-sulfation determined by mutational analysis*. Journal of Biological Chemistry, 272(35), 21700–21705. [DOI] [PubMed] [Google Scholar]
- Bütepage M, Krieg S, Eckei L, Li J, Rossetti G, Verheugd P,& Lüscher B.(2018). Assessment of intracellular auto-modification levels of ARTD10 using mono-ADP-ribose-specific macrodomains 2 and 3 of murine Artd8. In Chang P.(Ed.), ADP-Ribosylation and NAD+ Utilizing Enzymes: Methods and Protocols (pp. 41–63). Springer. [DOI] [PubMed] [Google Scholar]
- Cao M, Chen G, Wang L, Wen P, & Shi S.(2018). Computational prediction and analysis for tyrosine post-translational modifications via elastic net. Journal of Chemical Information and Modeling, 58(6), 1272–1281. [DOI] [PubMed] [Google Scholar]
- Capriotti AL, Cerrato A, Laganà A, Montone CM, Piovesana S, Zenezini Chiozzi R, & Cavaliere C.(2020). Development of a sample-preparation workflow for sulfopeptide enrichment: from target analysis to challenges in shotgun sulfoproteomics. Analytical Chemistry, 92(11), 7964–7971. [DOI] [PubMed] [Google Scholar]
- Carter-O’Connell I, & Cohen MS (2015). Identifying direct protein targets of poly-ADP-ribose polymerases (PARPs) using engineered parp variants-orthogonal nicotinamide adenine dinucleotide (NAD+) analog pairs: identifying targets of poly-ADP-ribose polymerases. Current Protocols in Chemical Biology, 7(2), 121–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-O’Connell I, Jin H, Morgan RK, David LL, & Cohen MS (2014). Engineering the substrate specificity of ADP-ribosyltransferases for identifying direct protein targets. Journal of the American Chemical Society, 136(14), 5201–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-O’Connell I, Jin H, Morgan RK, Zaja R, David LL, Ahel I, & Cohen MS (2016). Identifying family-member-specific targets of mono-ARTDs by using a chemical genetics approach. Cell Reports, 14(3), 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-O’Connell I, Vermehren-Schmaedick A, Jin H, Morgan RK, David LL, & Cohen MS (2018). Combining chemical genetics with proximity-dependent labeling reveals cellular targets of Poly(ADP-ribose) polymerase 14 (PARP14). ACS Chemical Biology, 13(10), 2841–2848. [DOI] [PubMed] [Google Scholar]
- Cazalé A-C, Blanquet P, Henry C, Pouzet C, Bruand C, & Meilhoc E.(2020). Tyrosine nitration of flagellins: a response of sinorhizobium meliloti to nitrosative stress. Applied and Environmental Microbiology, 87(1), e02210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki M, Álvarez de Morales P, Ruiz C, Begara-Morales JC, Barroso JB, Corpas FJ, & Palma JM (2015). Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Annals of Botany, 116(4), 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JD, Gagné J-P, Poirier GG, & Goodlett DR (2013). Mapping PARP-1 auto-ADP-ribosylation sites by liquid chromatography–tandem mass spectrometry. Journal of Proteome Research, 12(4), 1868–1880. [DOI] [PubMed] [Google Scholar]
- Chen B, Hwang L, Ochowicz W, Lin Z, Guardado-Alvarez TM, Cai W, Xiu L, Dani K, Colah C, Jin S, & Ge Y.(2017). Coupling functionalized cobalt ferrite nanoparticle enrichment with online LC/MS/MS for top-down phosphoproteomics. Chemical Science, 8(6), 4306–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhang Y, Trinidad JC, & Dann C.(2018). Distinguishing sulfotyrosine containing peptides from their phosphotyrosine counterparts using mass spectrometry. Journal of the American Society for Mass Spectrometry, 29(3), 455–462. [DOI] [PubMed] [Google Scholar]
- Chen J, Li X, Feng M, Luo K, Yang J, & Zhang B.(2017). Novel boronate material affords efficient enrichment of glycopeptides by synergized hydrophilic and affinity interactions. Analytical and Bioanalytical Chemistry, 409(2), 519–528. [DOI] [PubMed] [Google Scholar]
- Cheng C-W, Chou C-C, Hsieh H-W, Tu Z, Lin C-H, Nycholat C, Fukuda M, & Khoo K-H (2015). Efficient mapping of sulfated glycotopes by negative ion mode nanoLC–MS/MS-based sulfoglycomic analysis of permethylated glycans. Analytical Chemistry, 87(12), 6380–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qi J, Liu K, Zhou C, Wang W, Zuo Y, Zhang P, Liang S, Wang Y, Chen P, Tang C, & Liu Z.(2022). Characterization of a novel affinity binding ligand for tyrosine nitrated peptides from a phage-displayed peptide library. Talanta, 241, 123225. [DOI] [PubMed] [Google Scholar]
- Cohen MS, & Chang P.(2018). Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nature Chemical Biology, 14(3), 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Campanella GSV, Manice LA, & Luster AD (2006). CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Molecular and Cellular Biology. 26,5838–5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SL, & Jackson GP (2011a). Characterization of tyrosine nitration and cysteine nitrosylation modifications by metastable atom-activation dissociation mass spectrometry. Journal of the American Society for Mass Spectrometry, 22(2), 221–232. [DOI] [PubMed] [Google Scholar]
- Cook SL, & Jackson GP (2011b). Metastable atom-activated dissociation mass spectrometry of phosphorylated and sulfonated peptides in negative ion mode. Journal of the American Society for Mass Spectrometry, 22(6), 1088–1099. [DOI] [PubMed] [Google Scholar]
- Dani N, Stilla A, Marchegiani A, Tamburro A, Till S, Ladurner AG, Corda D, & Girolamo MD (2009). Combining affinity purification by ADP-ribose-binding macro domains with mass spectrometry to define the mammalian ADP-ribosyl proteome. Proceedings of the National Academy of Sciences of the United States of America, 106(11), 4243–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Ong S-E, & Leung AKL (2014). Phosphoproteomic approach to characterize protein mono- and poly(ADP-ribosyl)ation sites from cells. Journal of Proteome Research, 13(8), 3510–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Ong S-E, & Leung AKL (2015). The promise of proteomics for the study of ADP-ribosylation. Molecular Cell, 58(6), 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Ong S-E, & Leung AKL (2017). ADP-ribosylated peptide enrichment and site identification: the phosphodiesterase-based method. In Tulin AV (Ed.), Poly (ADP-Ribose) Polymerase: Methods and Protocols (pp. 79–93). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels CM, Thirawatananond P, Ong S-E, Gabelli SB, & Leung AKL (2015). Nudix hydrolases degrade protein-conjugated ADP-ribose. Scientific Reports, 5(1), 18271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darie-Ion L, Neamtu A, Iliescu R, & Petre BA (2022). Immuno-affinity study of oxidative tyrosine containing peptides. International Journal of Peptide Research and Therapeutics, 28(1), 41. [Google Scholar]
- David A, Yadav S, Baluška F, & Bhatla SC (2015). Nitric oxide accumulation and protein tyrosine nitration as a rapid and long distance signalling response to salt stress in sunflower seedlings. Nitric Oxide, 50, 28–37. [DOI] [PubMed] [Google Scholar]
- Delatour T, Richoz J, Vuichoud J, & Stadler RH (2002). Artifactual nitration controlled measurement of protein-bound 3-nitro-l-tyrosine in biological fluids and tissues by isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry. Chemical Research in Toxicology, 15(10), 1209–1217. [DOI] [PubMed] [Google Scholar]
- Drǎguşanu M, Petre B-A, & Przybylski M.(2011). Epitope motif of an anti-nitrotyrosine antibody specific for tyrosine-nitrated peptides revealed by a combination of affinity approaches and mass spectrometry. Journal of Peptide Science, 17(3), 184–191. [DOI] [PubMed] [Google Scholar]
- Dremina ES, Li X, Galeva NA, Sharov VS, Stobaugh JF,& Schöneich C.(2011). A methodology for simultaneous fluorogenic derivatization and boronate affinity enrichment of 3-nitrotyrosine-containing peptides. Analytical Biochemistry, 418(2), 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, & Robinson RAS (2013). Proteomics quantification of protein nitration. Reviews in Analytical Chemistry, 32(3), 173–187. [Google Scholar]
- Farrar D, Rai S, Chernukhin I, Jagodic M, Ito Y, Yammine S, Ohlsson R, Murrell A, & Klenova E.(2010). Mutational analysis of the poly(ADP-Ribosyl)ation sites of the transcription factor CTCF provides an insight into the mechanism of its regulation by poly(ADP-Ribosyl)ation. Molecular and Cellular Biology, 30, 1199–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, & Choe H.(1999). Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 Entry. Cell, 96(5), 667–676. [DOI] [PubMed] [Google Scholar]
- Feijs KLH, & Žaja R.(2022). Are PARPs promiscuous? Bioscience Reports, 42(5), BSR20212489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, & White FM (2002). Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nature Biotechnology, 20(3), 301–305. [DOI] [PubMed] [Google Scholar]
- Forst AH, Karlberg T, Herzog N, Thorsell A-G, Gross A, Feijs KLH, Verheugd P, Kursula P, Nijmeijer B, Kremmer E, Kleine H, Ladurner AG, Schüler H, & Lüscher B.(2013). Recognition of mono-ADP-ribosylated ARTD10 substrates by ARTD8 macrodomains. Structure, 21(3), 462–475. [DOI] [PubMed] [Google Scholar]
- Frese CK, Altelaar AFM, van den Toorn H, Nolting D, Griep-Raming J, Heck AJR, & Mohammed S.(2012). Toward full peptide sequence coverage by dual fragmentation combining electron-transfer and higher-energy collision dissociation tandem mass spectrometry. Analytical Chemistry, 84(22), 9668–9673. [DOI] [PubMed] [Google Scholar]
- Gabriele C, Prestagiacomo LE, Cuda G, & Gaspari M.(2021). Mass spectrometry-based glycoproteomics and prostate cancer. International Journal of Molecular Sciences, 22(10), 5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné J-P., Ethier C., Defoy D., Bourassa S., Langelier M-F., Riccio AA., Pascal JM., Moon K-M., Foster LJ., Ning Z., Figeys D., Droit A., & Poirier GG. (2015). Quantitative site-specific ADP-ribosylation profiling of DNA-dependent PARPs. DNA Repair, 30, 68–79. [DOI] [PubMed] [Google Scholar]
- Gagné J-P, Langelier M-F, Pascal JM, & Poirier GG (2018). Hydrofluoric acid-based derivatization strategy to profile PARP-1 ADP-ribosylation by LC–MS/MS. Journal of Proteome Research, 17(7), 2542–2551. [DOI] [PubMed] [Google Scholar]
- Gaupels F, Furch ACU, Zimmermann MR, Chen F, Kaever V, Buhtz A, Kehr J, Sarioglu H, Kogel K-H, & Durner J.(2016). Systemic induction of NO-, redox-, and cGMP signaling in the pumpkin extrafascicular phloem upon local leaf wounding. Frontiers in Plant Science, 7, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszler DJ, Kong AT, Avtonomov DM, Yu F, Leprevost F.da V., & Nesvizhskii, A. I. (2021). PTM-shepherd: analysis and summarization of post-translational and chemical modifications from open search results. Molecular & Cellular Proteomics, 20, 100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesquière B, Colaert N, Helsens K, Dejager L, Vanhaute C, Verleysen K, Kas K, Timmerman E, Goethals M, Libert C, Vandekerckhove J, & Gevaert K.(2009). In vitro and in vivo protein-bound tyrosine nitration characterized by diagonal chromatography*. Molecular & Cellular Proteomics, 8(12), 2642–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BA, Conrad LB, Huang D, & Kraus WL (2017). Generation and characterization of recombinant antibody-like ADP-ribose binding proteins. Biochemistry, 56(48), 6305–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BA, & Kraus WL (2012). New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature Reviews Molecular Cell Biology, 13(7), 411–424. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Catara G, Valente C, & Corda D.(2018). In vitro techniques for ADP-ribosylated substrate identification. In Chang P.(Ed.), ADP-ribosylation and NAD+ Utilizing Enzymes: Methods and Protocols (pp. 25–40). Springer. [DOI] [PubMed] [Google Scholar]
- Guergues J, Wohlfahrt J, & Stevens SM (2022). Enhancement of proteome coverage by ion mobility fractionation coupled to PASEF on a TIMS-QTOF instrument. Journal of Proteome Research, 21(8), 2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena HP, He M, Chrisman PA, Pitteri SJ, Hogan JM, Hodges BDM, & McLuckey SA (2005). Electron transfer versus proton transfer in gas-phase ion/ion reactions of polyprotonated peptides. Journal of the American Chemical Society, 127(36), 12627–12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, & Prokai L.(2012). Conversion of 3-nitrotyrosine to 3-aminotyrosine residues facilitates mapping of tyrosine nitration in proteins by electrospray ionization-tandem mass spectrometry using electron capture dissociation. Journal of Mass Spectrometry, 47(12), 1601–1611. [DOI] [PubMed] [Google Scholar]
- Guo J, Prokai-Tatrai K, & Prokai L.(2012). Relative quantitation of protein nitration by liquid chromatography-mass spectrometry using isotope-coded dimethyl labeling and chemoprecipitation. Journal of Chromatography A, 1232, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusenkov S, & Stutz H.(2018). Top-down and bottom-up characterization of nitrated birch pollen allergen Bet v 1a with CZE hyphenated to an Orbitrap mass spectrometer. Electrophoresis, 39(9–10), 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Reyes CD, Jiang P, Atashi M, Bennett A, Yu A, Peng W, Zhong J, & Mechref Y.(2022). Advances in mass spectrometry-based glycoproteomics: an update covering the period 2017–2021. Electrophoresis, 43(1–2), 370–387. [DOI] [PubMed] [Google Scholar]
- Haag F, & Buck F.(2015). Identification and analysis of ADP-ribosylated proteins. In Koch-Nolte F.(Ed.), Endogenous ADP-Ribosylation (pp. 33–50). Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Hebert AS, Prasad S, Belford MW, Bailey DJ, McAlister GC, Abbatiello SE, Huguet R, Wouters ER, Dunyach J-J, Brademan DR, Westphall MS, & Coon JJ (2018). Comprehensive single-shot proteomics with FAIMS on a hybrid Orbitrap mass spectrometer. Analytical Chemistry, 90(15), 9529–9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M, Rice B, Sebastian A, Sullivan PG, King C, Robinson RAS, & Reed TT (2016). Neuroproteomic study of nitrated proteins in moderate traumatic brain injured rats treated with gamma glutamyl cysteine ethyl ester administration post injury: insight into the role of glutathione elevation in nitrosative stress. Proteomics—Clinical Applications, 10(12), 1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks IA, Buch-Larsen SC, Prokhorova E, Elsborg JD, Rebak AKLFS, Zhu K, Ahel D, Lukas C, Ahel I, & Nielsen ML (2021). The regulatory landscape of the human HPF1- and ARH3-dependent ADP-ribosylome. Nature Communications, 12(1), 5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks IA, Larsen SC, & Nielsen ML (2019). An advanced strategy for comprehensive profiling of ADP-ribosylation sites using mass spectrometry-based proteomics. Molecular & Cellular Proteomics, 18(5), 1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks IA, Lyon D, Young C, Jensen LJ, Vertegaal ACO, & Nielsen ML (2017). Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nature Structural & Molecular Biology, 24(3), 325–336. [DOI] [PubMed] [Google Scholar]
- Hengel SM, & Goodlett DR (2012). A review of tandem mass spectrometry characterization of adenosine diphosphate-ribosylated peptides. International Journal of Mass Spectrometry, 312, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengel SM, Shaffer SA, Nunn BL, & Goodlett DR (2009). Tandem mass spectrometry investigation of ADP-ribosylated kemptide. Journal of the American Society for Mass Spectrometry, 20(3), 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersberger KE, & Håkansson K.(2012). Characterization of O-sulfopeptides by negative ion mode tandem mass spectrometry: superior performance of negative ion electron capture dissociation. Analytical Chemistry, 84(15), 6370–6377. [DOI] [PubMed] [Google Scholar]
- Hoffhines AJ, Damoc E, Bridges KG, Leary JA, & Moore KL (2006). Detection and purification of tyrosine-sulfated proteins using a novel anti-sulfotyrosine monoclonal antibody. Journal of Biological Chemistry, 281(49), 37877–37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp A-K, & Hottiger MO (2021). Uncovering the invisible: mono-ADP-ribosylation moved into the spotlight. Cells, 10(3), 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B-Y, Yang C-K, Liu C-P, & Liu C-Y (2014). Stationary phases for the enrichment of glycoproteins and glycopeptides. Electrophoresis, 35(15), 2091–2107. [DOI] [PubMed] [Google Scholar]
- Huang L, Wang N, Mitchell CE, Brownlee T, Maple SR, & De Felippis MR (2017). A novel sample preparation for shotgun proteomics characterization of HCPs in antibodies. Analytical Chemistry, 89(10), 5436–5444. [DOI] [PubMed] [Google Scholar]
- Huttner WB. (1984). Determination and occurrence of tyrosine O-sulfate in proteins. In Methods in Enzymology (Vol. 107, pp. 200–223). Academic Press. [DOI] [PubMed] [Google Scholar]
- Ida C, Yamashita S, Tsukada M, Sato T, Eguchi T, Tanaka M, Ogata S, Fujii T, Nishi Y, Ikegami S, Moss J, & Miwa M.(2016). An enzyme-linked immuno-sorbent assay-based system for determining the physiological level of poly(ADP-ribose) in cultured cells. Analytical Biochemistry, 494, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ion L, Lupaescu A-V, Neamtu A, Drochioiu G, & Petre B-A (2020). Binding affinities studies of nitrated model peptides to monoclonal anti-3-nitrotyrosine antibody. Revista de Chimie, 71(1), 259–265. [Google Scholar]
- Itkonen O, Helin J, Saarinen J, Kalkkinen N, Ivanov KI, Stenman U-H, & Valmu L.(2008). Mass spectrometric detection of tyrosine sulfation in human pancreatic trypsinogens, but not in tumor-associated trypsinogen. The FEBS Journal, 275(2), 289–301. [DOI] [PubMed] [Google Scholar]
- Jiang H, Sherwood R, Zhang S, Zhu X, Liu Q, Graeff R, Kriksunov IA, Lee HC, Hao Q, & Lin H.(2013). Identification of ADP-ribosylation sites of CD38 mutants by precursor ion scanning mass spectrometry. Analytical Biochemistry, 433(2), 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Mikhailov VA, Iniesta J, & Cooper HJ (2010). Electron capture dissociation mass spectrometry of tyrosine nitrated peptides. Journal of the American Society for Mass Spectrometry, 21(2), 268–277. [DOI] [PubMed] [Google Scholar]
- Ju T, Niu W, & Guo J.(2016). Evolution of Src homology 2 (SH2) domain to recognize sulfotyrosine. ACS Chemical Biology, 11(9), 2551–2557. [DOI] [PubMed] [Google Scholar]
- Kalesh K, Lukauskas S, Borg AJ, Snijders AP, Ayyappan V, Leung AKL, Haskard DO, & DiMaggio PA (2019). An integrated chemical proteomics approach for quantitative profiling of intracellular ADP-ribosylation. Scientific Reports, 9(1), 6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Amé J-C, Dantzer F, & Schreiber V.(2012). New readers and interpretations of poly(ADP-ribosyl)ation. Trends in Biochemical Sciences, 37(9), 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T, Yang C-S, Jividen K, Spencer A, Dworak N, Oostdyk LT, & Paschal BM (2019). Detection of ADP-ribosylation of the androgen receptor using the recombinant macrodomain AF1521 from Archaeoglobus fulgidus. In Badr MZ (Ed.), Nuclear Receptors: Methods and Experimental Protocols (pp. 107–124). Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Hamilton RA, Sherry DM, & Al-Ubaidi MR (2012). Sulfotyrosine. Experimental Eye Research, 105, 85–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Siefert JC, Kinter M, & Al-Ubaidi MR (2014). Complement factor H, vitronectin, and opticin are tyrosine-sulfated proteins of the retinal pigment epithelium. PLOS One, 9(8), e105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JW, Lee NY, Cho K-C, Lee MY, Choi D-Y, Park S-H, & Kim KP (2015). Analysis of nitrated proteins in Saccharomyces cerevisiae involved in mating signal transduction. Proteomics, 15(2–3), 580–590. [DOI] [PubMed] [Google Scholar]
- Karch KR, Langelier M-F, Pascal JM, & Garcia BA (2017). The nucleosomal surface is the main target of histone ADP-ribosylation in response to DNA damage. Molecular BioSystems, 13(12), 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamitsu H, Hoshino H, Okada H, Miwa M, Momoi H, & Sugimura T.(1984). Monoclonal antibodies to poly(adenosine diphosphate ribose) recognize different structures. Biochemistry, 23(16), 3771–3777. [DOI] [PubMed] [Google Scholar]
- Kehoe JW, & Bertozzi CR (2000). Tyrosine sulfation: a modulator of extracellular protein-protein interactions. Chemistry & Biology, 7(3), R57–R61. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee JR, Kang JW, Lee SJ, Shin GC, Yeo W-S, Kim K-H, Park HS, & Kim KP (2011). Selective enrichment and mass spectrometric identification of nitrated peptides using fluorinated carbon tags. Analytical Chemistry, 83(1), 157–163. [DOI] [PubMed] [Google Scholar]
- Kitata RB, Choong W-K, Tsai C-F, Lin P-Y, Chen B-S, Chang Y-C, Nesvizhskii AI, Sung T-Y, & Chen Y-J (2021). A data-independent acquisition-based global phosphoproteomics system enables deep profiling. Nature Communications, 12(1), 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement É, Hunyadi-Gulyás É, & Medzihradszky KF (2016). Biological significance and analysis of tyrosine sulfation. In Analysis of Protein Post-Translational Modifications by Mass Spectrometry (pp. 333–349). John Wiley & Sons, Ltd. [Google Scholar]
- Kliza KW, Liu Q, Roosenboom LWM, Jansen PWTC, Filippov DV, & Vermeulen M.(2021). Reading ADP-ribosylation signaling using chemical biology and interaction proteomics. Molecular Cell, 81(21), 4552–4567. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, Guingab-Cagmat J, Bruijnzeel AW, Gold MS, & Wang K.(2017). Effect of second-hand tobacco smoke on the nitration of brain proteins: a systems biology and bioinformatics approach. In Kobeissy FH & Stevens Stanley M.(Eds.), Neuroproteomics: Methods and Protocols (pp. 353–372). Springer. [DOI] [PubMed] [Google Scholar]
- Kohutiar M, Eckhardt A, Mikšík I, Šantorová P, & Wilhelm J.(2018). Proteomic analysis of peroxynitrite-induced protein nitration in isolated beef heart mitochondria. Physiological Research, 67, 239–250. [DOI] [PubMed] [Google Scholar]
- Kolbert Z, Feigl G, Bordé Á, Molnár Á, & Erdei L.(2017). Protein tyrosine nitration in plants: present knowledge, computational prediction and future perspectives. Plant Physiology and Biochemistry, 113, 56–63. [DOI] [PubMed] [Google Scholar]
- Kriss CL, Duro N, Nadeau OW, Guergues J, Chavez-Chiang O, Culver-Cochran AE, Chaput D, Varma S, & Stevens SM (2021). Site-specific identification and validation of hepatic histone nitration in vivo: implications for alcohol-induced liver injury. Journal of Mass Spectrometry, 56(6), e4713. [DOI] [PubMed] [Google Scholar]
- Kristensen KK, Midtgaard SR, Mysling S, Kovrov O, Hansen LB, Skar-Gislinge N, Beigneux AP, Kragelund BB, Olivecrona G, Young SG, Jørgensen TJD, Fong LG, & Ploug M.(2018). A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase. Proceedings of the National Academy of Sciences of the United States of America, 115(26), E6020–E6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SC, Hendriks IA, Lyon D, Jensen LJ, & Nielsen ML (2018). Systems-wide analysis of serine ADP-ribosylation reveals widespread occurrence and site-specific overlap with phosphorylation. Cell Reports, 24(9), 2493–2505. [DOI] [PubMed] [Google Scholar]
- Larsen SC, Leutert M, Bilan V, Martello R, Jungmichel S, Young C, Hottiger MO, & Nielsen ML (2017). Proteome-wide identification of in vivo ADP-ribose acceptor sites by liquid chromatography-tandem mass spectrometry. In Tulin AV (Ed.), Poly(ADP-Ribose) Polymerase: Methods and Protocols (pp. 149–162). Springer. [DOI] [PubMed] [Google Scholar]
- Lawrie J, Waldrop S, Morozov A, Niu W, & Guo J.(2021). Engineering of a small protein scaffold to recognize sulfotyrosine with high specificity. ACS Chemical Biology, 16(8), 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledvina AR, Beauchene NA, McAlister GC, Syka JEP, [Google Scholar]
- Schwartz JC, Griep-Raming J, Westphall MS, & Coon JJ (2010). Activated-ion electron transfer dissociation improves the ability of electron transfer dissociation to identify peptides in a complex mixture. Analytical Chemistry, 82(24), 10068–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JR, Lee SJ, Kim TW, Kim JK, Park HS, Kim D-E, Kim KP, & Yeo W-S (2009). Chemical approach for specific enrichment and mass analysis of nitrated peptides. Analytical Chemistry, 81(16), 6620–6626. [DOI] [PubMed] [Google Scholar]
- Lehner M, Rieth S, Höllmüller E, Spliesgar D, Mertes B, Stengel F, & Marx A.(2022). Profiling of the ADP-ribosylome in living cells. Angewandte Chemie International Edition, 61(18), e202200977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidecker O, Bonfiglio JJ, Colby T, Zhang Q, Atanassov I, Zaja R, Palazzo L, Stockum A, Ahel I, & Matic I.(2016). Serine is a new target residue for endogenous ADP-ribosylation on histones. Nature Chemical Biology, 12(12), 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie Pedrioli DM, Leutert M, Bilan V, Nowak K, Gunasekera K, Ferrari E, Imhof R, Malmström L, & Hottiger MO (2018). Comprehensive ADP-ribosylome analysis identifies tyrosine as an ADP-ribose acceptor site. EMBO Reports, 19(8), e45310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AKL (2017). PARPs. Current Biology, 27(23), R1256–R1258. [DOI] [PubMed] [Google Scholar]
- Leutert M, Bilan V, Gehrig P, & Hottiger MO (2017). Identification of ADP-ribose acceptor sites on in vitro modified proteins by liquid chromatography-tandem mass spectrometry. In Tulin AV (Ed.), Poly(ADP-Ribose) Polymerase: Methods and Protocols (pp. 137–148). Springer. [DOI] [PubMed] [Google Scholar]
- Leutert M, Entwisle SW, & Villén J.(2021). Decoding post-translational modification crosstalk with proteomics. Molecular & Cellular Proteomics, 20, 100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutert M, Menzel S, Braren R, Rissiek B, Hopp A-K, Nowak K, Bisceglie L, Gehrig P, Li H, Zolkiewska A, Koch-Nolte F, & Hottiger MO (2018). Proteomic characterization of the heart and skeletal muscle reveals widespread arginine ADP-ribosylation by the ARTC1 ectoenzyme. Cell Reports, 24(7), 1916–1929.. [DOI] [PubMed] [Google Scholar]
- Li J, Smith LS, & Zhu H-J (2021). Data-independent acquisition (DIA): an emerging proteomics technology for analysis of drug-metabolizing enzymes and transporters. Drug Discovery Today: Technologies, 39, 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, & Zhan X.(2021). Mass spectrometry-based proteomics analyses of post-translational modifications and proteoforms in human pituitary adenomas. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1869(3), 140584. [DOI] [PubMed] [Google Scholar]
- Li P, Zhen Y, & Yu Y.(2019). Site-specific analysis of the Asp- and Glu-ADP-ribosylated proteome by quantitative mass spectrometry. In Methods in Enzymology (Vol. 626, pp. 301–321). Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Compton PD, Tran JC, Ntai I, & Kelleher NL (2014). Optimizing capillary electrophoresis for top-down proteomics of 30–80 kDa proteins. Proteomics, 14(10), 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Pfüller U, Linné Larsson E, Jungvid H, Galaev IY, & Mattiasson B.(2001). Separation of mistletoe lectins based on the degree of glycosylation using boronate affinity chromatography. Journal of Chromatography A, 925(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Li Z, Li S, Luo M, Jhong J-H, Li W, Yao L, Pang Y, Wang Z, Wang R, Ma R, Yu J, Huang Y, Zhu X, Cheng Q, Feng H, Zhang J, Wang C, Hsu JB-K, Chang W-C, … Lee T-Y (2022). dbPTM in 2022: an updated database for exploring regulatory networks and functional associations of protein post-translational modifications. Nucleic Acids Research, 50(D1), D471–D479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Zheng J, Yu Q, Chen W, Xing J, Chen C, & Tian R.(2018). High throughput and accurate serum proteome profiling by integrated sample preparation technology and single-run data independent mass spectrometry analysis. Journal of Proteomics, 174, 9–16. [DOI] [PubMed] [Google Scholar]
- Liu Q, van der Marel GA, & Filippov DV (2019). Chemical ADP-ribosylation: mono-ADPr-peptides and oligo-ADP-ribose. Organic & Biomolecular Chemistry, 17(22), 5460–5474. [DOI] [PubMed] [Google Scholar]
- Lorentzen LG, Chuang CY, Rogowska-Wrzesinska A, & Davies MJ (2019). Identification and quantification of sites of nitration and oxidation in the key matrix protein laminin and the structural consequences of these modifications. Redox Biology, 24, 101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AZ, Abo R, Ren Y, Gui B, Mo J-R, Blackwell D, Wigle T, Keilhack H, & Niepel M.(2019). Enabling drug discovery for the PARP protein family through the detection of mono-ADP-ribosylation. Biochemical Pharmacology, 167, 97–106. [DOI] [PubMed] [Google Scholar]
- Ludeman JP, & Stone MJ (2014). The structural role of receptor tyrosine sulfation in chemokine recognition. British Journal of Pharmacology, 171(5), 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Shi J, & Li J.(2015). Peroxynitrite induced fibrinogen site identification. Bio-Medical Materials and Engineering, 26(s1), S2241–S2248. [DOI] [PubMed] [Google Scholar]
- Lüscher B, Bütepage M, Eckei L, Krieg S, Verheugd P, & Shilton BH (2018). ADP-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chemical Reviews, 118(3), 1092–1136. [DOI] [PubMed] [Google Scholar]
- Lüthi SC, Howald A, Nowak K, Graage R, Bartolomei G, Neupert C, Sidler X, Leslie Pedrioli D, & Hottiger MO (2021). Establishment of a mass-spectrometry-based method for the identification of the in vivo whole blood and plasma ADP-ribosylomes. Journal of Proteome Research, 20(6), 3090–3101. [DOI] [PubMed] [Google Scholar]
- Machida K, & Mayer BJ (2005). The SH2 domain: versatile signaling module and pharmaceutical target. Biochimica Et Biophysica Acta, 1747(1), 1–25. [DOI] [PubMed] [Google Scholar]
- Marcello MR, Jia W, Leary JA, Moore KL, & Evans JP (2011). Lack of tyrosylprotein sulfotransferase-2 activity results in altered sperm-egg interactions and loss of ADAM3 and ADAM6 in epididymal sperm*. Journal of Biological Chemistry, 286(15), 13060–13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martello R., Leutert M., Jungmichel S., Bilan V., Larsen SC., Young C., Hottiger MO., & Nielsen ML. (2016). Proteome-wide identification of the endogenous ADP-ribosylome of mammalian cells and tissue. Nature Communications, 7, 12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JWC, & Payne RJ (2020). Revealing the functional roles of tyrosine sulfation using synthetic sulfopeptides and sulfoproteins. Current Opinion in Chemical Biology, 58, 72–85. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Sousa B, Rossi S, Afonso C, Bonino L, Pitt A, López E, Spickett C, & Borthagaray G.(2021). Identification and relative quantification of 3-nitrotyrosine residues in fibrinogen nitrated in vitro and fibrinogen from ischemic stroke patient plasma using LC-MS/MS. Free Radical Biology and Medicine, 165, 334–347. [DOI] [PubMed] [Google Scholar]
- Medzihradszky KF, Darula Z, Perlson E, Fainzilber M, Chalkley RJ, Ball H, Greenbaum D, Bogyo M, Tyson DR, Bradshaw RA, & Burlingame AL (2004). O-sulfonation of serine and threonine: mass spectrometric detection and characterization of a new posttranslational modification in diverse proteins throughout the eukaryotes. Molecular & Cellular Proteomics, 3(5), 429–440. [DOI] [PubMed] [Google Scholar]
- Medzihradszky KF, Guan S, Maltby DA, & Burlingame AL (2007). Sulfopeptide fragmentation in electron-capture and electron-transfer dissociation. Journal of the American Society for Mass Spectrometry, 18(9), 1617–1624. [DOI] [PubMed] [Google Scholar]
- Mehta AY, Heimburg-Molinaro J, Cummings RD, & Goth CK (2020). Emerging patterns of tyrosine sulfation and O-glycosylation crosstalk and co-localization. Current Opinion in Structural Biology, 62, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier F, Brunner A-D, Koch S, Koch H, Lubeck M, Krause M, Goedecke N, Decker J, Kosinski T, Park MA, Bache N, Hoerning O, Cox J, Räther O, & Mann M.(2018). Online parallel accumulation–serial fragmentation (PASEF) with a novel trapped ion mobility mass spectrometer*. Molecular & Cellular Proteomics, 17(12), 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez AAE, Mangialavori IC, Cabrera AV, Benavides MP, Vázquez-Ramos JM, & Gallego SM (2020). Tyr-nitration in maize CDKA;1 results in lower affinity for ATP binding. Biochimica et Biophysica Acta (BBA)— Proteins and Proteomics, 1868(10), 140479. [DOI] [PubMed] [Google Scholar]
- Mikesh LM, Ueberheide B, Chi A, Coon JJ, Syka JEP, Shabanowitz J, & Hunt DF (2006). The utility of ETD mass spectrometry in proteomic analysis. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1764(12), 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H, Horn DM, Tang N, Mathivanan S, & Pandey A.(2007). Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America, 104(7), 2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KL (2009). Protein tyrosine sulfation: a critical posttranslation modification in plants and animals. Proceedings of the National Academy of Sciences of the United States of America, 106(35), 14741–14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RK, & Cohen MS (2017). Detecting Protein ADP-Ribosylation Using a Clickable Aminooxy Probe. In Tulin AV(Ed.), Poly(ADP-Ribose) Polymerase (Vol. 1608, pp. 71–77). Springer; New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth-Cawley JF, Karnik S, & Rouse JC (2001). Analysis of sulfated peptides using positive electrospray ionization tandem mass spectrometry. Journal of Mass Spectrometry, 36(12), 1301–1311. [DOI] [PubMed] [Google Scholar]
- Nikov G, Bhat V, Wishnok JS, & Tannenbaum SR (2003). Analysis of nitrated proteins by nitrotyrosine-specific affinity probes and mass spectrometry. Analytical Biochemistry, 320(2), 214–222. [DOI] [PubMed] [Google Scholar]
- Nowak K, Rosenthal F, Karlberg T, Bütepage M, Thorsell A-G, Dreier B, Grossmann J, Sobek J, Imhof R, Lüscher B, Schüler H, Plückthun A, Leslie Pedrioli DM, & Hottiger MO (2020). Engineering Af1521 improves ADP-ribose binding and identification of ADP-ribosylated proteins. Nature Communications, 11(1), 5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel T, Whitehouse J, Ma Y, Mercer EJ, Brown N, & Gross SS (2016). ANSID: a solid-phase proteomic approach for identification and relative quantification of aromatic nitration sites. Frontiers in Chemistry, 3, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önnerfjord P, Heathfield TF, & Heinegård D.(2004). Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry*. Journal of Biological Chemistry, 279(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Palazzo L, Mikoč A, & Ahel I.(2017). ADP-ribosylation: new facets of an ancient modification. The FEBS Journal, 284(18), 2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo L, Thomas B, Jemth A-S, Colby T, Leidecker O, Feijs KLH, Zaja R, Loseva O, Puigvert JC, Matic I, Helleday T, & Ahel I.(2015). Processing of protein ADP-ribosylation by Nudix hydrolases. Biochemical Journal, 468(2), 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Li J, Guo T, Yang H, Li M, Sang S, Li X, Desiderio DM, & Zhan X.(2015). Nitroproteins in human astrocytomas discovered by gel electrophoresis and tandem mass spectrometry. Journal of the American Society for Mass Spectrometry, 26(12), 2062–2076. [DOI] [PubMed] [Google Scholar]
- Perina D, Mikoč A, Ahel J, Ćetković H, Žaja R, & Ahel I.(2014). Distribution of protein poly(ADP-ribosyl)ation systems across all domains of life. DNA Repair, 23, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson A-S, Steen H, Kalume DE, Caidahl K, & Roepstorff P.(2001). Investigation of tyrosine nitration in proteins by mass spectrometry. Journal of Mass Spectrometry, 36(6), 616–625. [DOI] [PubMed] [Google Scholar]
- Popov I, Nenov A, Petrov P, & Vassilev D.(2009). Bioinformatics in proteomics: a review on methods and algorithms. Biotechnology & Biotechnological Equipment, 23(1), 1115–1120. [Google Scholar]
- Prianichnikov N, Koch H, Koch S, Lubeck M, Heilig R, Brehmer S, Fischer R, & Cox J.(2020). MaxQuant software for ion mobility enhanced shotgun proteomics. Molecular & Cellular Proteomics, 19(6), 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai L, Guo J, & Prokai-Tatrai K.(2014). Selective chemoprecipitation to enrich nitropeptides from complex proteomes for mass-spectrometric analysis. Nature Protocols, 9(4), 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokai-Tatrai K, Guo J, & Prokai L.(2011). Selective chemoprecipitation and subsequent release of tagged species for the analysis of nitropeptides by liquid chromatography-tandem mass spectrometry*. Molecular & Cellular Proteomics, 10(8), M110.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack JGM., Palazzo L., & Ahel I. (2020). (ADP-ribosyl) hydrolases: structure, function, and biology. Genes & Development, 34(5–6), 263–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R.(2013). Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Accounts of Chemical Research, 46(2), 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Cejudo J, Wisniewski T, Marmar C, Zetterberg H, Blennow K, de Leon MJ, & Fossati S.(2018). Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMedicine, 28, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll-Rozada J, Maxwell JWC, Payne RJ, & Barbosa Pereira PJ (2022). Tyrosine-O-sulfation is a widespread affinity enhancer among thrombin interactors. Biochemical Society Transactions, 50, 387–401. [DOI] [PubMed] [Google Scholar]
- Robinson MR, & Brodbelt JS (2016). Integrating weak anion exchange and ultraviolet photodissociation mass spectrometry with strategic modulation of peptide basicity for the enrichment of sulfopeptides. Analytical Chemistry, 88(22), 11037–11045. [DOI] [PubMed] [Google Scholar]
- Robinson MR, Moore KL, & Brodbelt JS (2014). Direct identification of tyrosine sulfation by using ultraviolet photodissociation mass spectrometry. Journal of The American Society for Mass Spectrometry, 25(8), 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KM, Buch-Larsen SC, Kirby IT, Siordia I, Hutin D, Rasmussen M, Grant DM, David LL, Matthews J, Nielsen ML, & Cohen MS (2021). Chemical genetics and proteome-wide site mapping reveal cysteine MARylation by PARP-7 on immune-relevant protein targets. ELife, 10, e60480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal F, & Hottiger MO (2014). Identification of ADP-ribosylated peptides and ADP-ribose acceptor sites. Frontiers in Bioscience (Landmark Edition), 19, 1041–1056. [DOI] [PubMed] [Google Scholar]
- Rosenthal F, Messner S, Roschitzki B, Gehrig P, Nanni P, & Hottiger MO (2011). Identification of distinct amino acids as adp-ribose acceptor sites by mass spectrometry. In Tulin AV (Ed.), Poly(ADP-ribose) Polymerase: Methods and Protocols (pp. 57–66). Humana Press. [DOI] [PubMed] [Google Scholar]
- Rosenthal F, Nanni P, Barkow-Oesterreicher S, & Hottiger MO (2015). Optimization of LTQ-Orbitrap mass spectrometer parameters for the identification of ADP-ribosylation sites. Journal of Proteome Research, 14(9), 4072–4079. [DOI] [PubMed] [Google Scholar]
- Sachdev E, Tabatabai R, Roy V, Rimel BJ, & Mita MM (2019). PARP inhibition in cancer: an update on clinical development. Targeted Oncology, 14(6), 657–679. [DOI] [PubMed] [Google Scholar]
- Sacksteder CA, Qian W-J, Knyushko TV, Wang H, Chin MH, Lacan G, Melega WP, Camp DG, Smith RD, Smith DJ, Squier TC, & Bigelow DJ (2006). Endogenously nitrated proteins in mouse brain: links to neurodegenerative disease. Biochemistry, 45(26), 8009–8022. [DOI] [PubMed] [Google Scholar]
- Sainz M, Calvo-Begueria L, Pérez-Rontomé C, Wienkoop S, Abián J, Staudinger C, Bartesaghi S, Radi R, & Becana M.(2015). Leghemoglobin is nitrated in functional legume nodules in a tyrosine residue within the heme cavity by a nitrite/peroxide-dependent mechanism. The Plant Journal, 81(5), 723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert C, & Sakmar TP (2008). Toward a framework for sulfoproteomics: synthesis and characterization of sulfotyrosine-containing peptides. Peptide Science, 90(3), 459–477. [DOI] [PubMed] [Google Scholar]
- Slade D.(2020). PARP and PARG inhibitors in cancer treatment. Genes & Development, 34(5–6), 360–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade D, Dunstan MS, Barkauskaite E, Weston R, Lafite P, Dixon N, Ahel M, Leys D, & Ahel I.(2011). The structure and catalytic mechanism of a poly(ADP-ribose) glycohydrolase. Nature, 477(7366), 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood HS, Galeva NA, Bartlett RK, Urbauer RJB, Williams TD, Urbauer JL, & Squier TC (2003). Selective nitration of Tyr99 in calmodulin as a marker of cellular conditions of oxidative stress. Chemical Research in Toxicology, 16(1), 95–102. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Sing JC, Gingras A-C, & Röst HL (2022). Improving phosphoproteomics profiling using data-independent mass spectrometry. Journal of Proteome Research, 21(8), 1789–1799. [DOI] [PubMed] [Google Scholar]
- Sukhanova MV, Singatulina AS, Pastré D, & Lavrik OI (2020). Fused in sarcoma (FUS) in DNA repair: tango with poly(ADP-ribose) polymerase 1 and compartmentalisation of damaged DNA. International Journal of Molecular Sciences, 21(19), 7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Reed T, & Butterfield DA (2009). Detection of 4-hydroxy-2-nonenal- and 3-nitrotyrosine-modified proteins using a proteomics approach. In Tyther R& Sheehan D(Eds.), Two-Dimensional Electrophoresis Protocols (pp. 351–361). Humana Press. [DOI] [PubMed] [Google Scholar]
- Tatarkova Z, Kovalska M, Sivonova MK, Racay P, Lehotsky J, & Kaplan P.(2019). Tyrosine nitration of mitochondrial proteins during myocardial ischemia and reperfusion. Journal of Physiology and Biochemistry, 75(2), 217–227. [DOI] [PubMed] [Google Scholar]
- Teixeira D, Fernandes R, Prudêncio C, & Vieira M.(2016). 3-Nitrotyrosine quantification methods: current concepts and future challenges. Biochimie, 125, 1–11. [DOI] [PubMed] [Google Scholar]
- Thirawatananond P, McPherson RL, Malhi J, Nathan S, Lambrecht MJ, Brichacek M, Hergenrother PJ, Leung AKL, & Gabelli SB (2019). Structural analyses of NudT16-ADP-ribose complexes direct rational design of mutants with improved processing of poly(ADP-ribosyl)ated proteins. Scientific Reports, 9(1), 5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timinszky G, Till S, Hassa PO, Hothorn M, Kustatscher G, Nijmeijer B, Colombelli J, Altmeyer M, Stelzer EHK, Scheffzek K, Hottiger MO, & Ladurner AG (2009). A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nature Structural & Molecular Biology, 16(9), 923–929. [DOI] [PubMed] [Google Scholar]
- Toby TK, Fornelli L, & Kelleher NL (2016). Progress in top-down proteomics and the analysis of proteoforms. Annual Review of Analytical Chemistry, 9(1), 499–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikas D, & Duncan MW (2014). Mass spectrometry and 3-nitrotyrosine: strategies, controversies, and our current perspective. Mass Spectrometry Reviews, 33(4), 237–276. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Petre A, Youhnovski N, Prömm F, Schirle M, Schumm M, Pero RS, Doyle A, Checkel J, Kita H, Thiyagarajan N, Acharya KR, Schmid-Grendelmeier P, Simon H-U, Schwarz H, Tsutsui M, Shimokawa H, Bellon G, Lee JJ, … Döring G.(2008). Post-translational tyrosine nitration of eosinophil granule toxins mediated by eosinophil peroxidase*. Journal of Biological Chemistry, 283(42), 28629–28640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.(2022). A review on recent trends in the phosphoproteomics workflow. From sample preparation to data analysis. Analytica Chimica Acta, 1199, 338857. [DOI] [PubMed] [Google Scholar]
- van Bentum M, & Selbach M.(2021). An introduction to advanced targeted acquisition methods. Molecular & Cellular Proteomics, 20, 100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heden van Noort GJ. (2020). Chemical tools to study protein ADP-ribosylation. ACS Omega, 5(4), 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Fossen EM, Grutzius S, Ruby CE, Mourich DV, Cebra C, Bracha S, Karplus PA, Cooley RB, & Mehl RA (2022). Creating a selective nanobody against 3-nitrotyrosine containing proteins. Frontiers in Chemistry, 10, 835229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivelo CA, & Leung AKL (2015). Proteomics approaches to identify mono-(ADP-ribosyl)ated and poly(ADP-ribosyl)ated proteins. Proteomics, 15(2–3), 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott NP, Fernandez JP, Molina H, & Hang HC (2017). Chemical proteomics reveals ADP-ribosylation of small GTPases during oxidative stress. Nature Chemical Biology, 13(3), 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmuckett AD, & Moore KL (2009). Lack of tyrosylprotein sulfotransferase activity in hematopoietic cells drastically attenuates atherosclerosis in Ldlr−/− mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 29(11), 1730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner J, Premsler T, & Sickmann A.(2008). Application of electron transfer dissociation (ETD) for the analysis of posttranslational modifications. Proteomics, 8(21), 4466–4483. [DOI] [PubMed] [Google Scholar]
- Yagami T, Kitagawa K, Aida C, Fujiwara H, & Futaki S.(2000). Stabilization of a tyrosine O-sulfate residue by a cationic functional group: formation of a conjugate acid-base pair. The Journal of Peptide Research, 56(4), 239–249. [DOI] [PubMed] [Google Scholar]
- Yang Y.(2017). Specific enrichment of a targeted nitrotyrosine-containing peptide from complex matrices and relative quantification for liquid chromatography-mass spectrometry analysis. Journal of Chromatography A, 1485, 90–100. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yan G, Kong S, Wu M, Yang P, Cao W, & Qiao L.(2021). GproDIA enables data-independent acquisition glycoproteomics with comprehensive statistical control. Nature Communications, 12, 6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, & Vakhrushev SY (2021). The role of data-independent acquisition for glycoproteomics. Molecular & Cellular Proteomics, 20, 100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo W-S, Kim YJ, Kabir MH, Kang JW, & Kim KP (2015). Mass spectrometric analysis of protein tyrosine nitration in aging and neurodegenerative diseases. Mass Spectrometry Reviews, 34(2), 166–183. [DOI] [PubMed] [Google Scholar]
- Zare H, Aghamollaei H, Hosseindokht M, Heiat M, Razei A, & Bakherad H.(2021). Nanobodies, the potent agents to detect and treat the Coronavirus infections: a systematic review. Molecular and Cellular Probes, 55, 101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, & Desiderio DM (2004). The human pituitary nitroproteome: detection of nitrotyrosyl-proteins with two-dimensional Western blotting, and amino acid sequence determination with mass spectrometry. Biochemical and Biophysical Research Communications, 325(4), 1180–1186. [DOI] [PubMed] [Google Scholar]
- Zhan X, & Desiderio DM (2016). Mass spectroscopy measurements of nitrotyrosine-containing proteins. In Handbook of Measurement in Science and Engineering (pp. 2431–2473). John Wiley & Sons, Ltd. [Google Scholar]
- Zhan X, Wang X, & Desiderio DM (2015). Mass spectrometry analysis of nitrotyrosine-containing proteins. Mass Spectrometry Reviews, 34(4), 423–448. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu Y, Joseph J, & Kalyanaraman B.(2005). Intramolecular electron transfer between tyrosyl radical and cysteine residue inhibits tyrosine nitration and induces thiyl radical formation in model peptides treated with myeloperoxidase, H2O2, and NO2-: EPR spin trapping studies*. Journal of Biological Chemistry, 280(49), 40684–40698. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Qian W-J, Knyushko TV, Clauss TRW, Purvine SO, Moore RJ, Sacksteder CA, Chin MH, Smith DJ, Camp DG, Bigelow DJ, & Smith RD (2007). A method for selective enrichment and analysis of nitrotyrosine-containing peptides in complex proteome samples. Journal of Proteome Research, 6(6), 2257–2268. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Ding M, & Yu Y.(2013). Site-specific characterization of the Asp- and Glu-ADP-ribosylated proteome. Nature Methods, 10(10), 981–984. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang H, & Pöschl U.(2011). Analysis of nitrated proteins and tryptic peptides by HPLC-chip-MS/MS: site-specific quantification, nitration degree, and reactivity of tyrosine residues. Analytical and Bioanalytical Chemistry, 399(1), 459–471. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang Y, Sun H, Maroto R, & Brasier AR (2017). Selective affinity enrichment of nitrotyrosine-containing peptides for quantitative analysis in complex samples. Journal of Proteome Research, 16(8), 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, & Yu Y.(2018). Proteomic analysis of the downstream signaling network of PARP1. Biochemistry, 57(4), 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Zhang Y, & Yu Y.(2017). A cell-line-specific atlas of PARP-mediated protein Asp/Glu-ADP-ribosylation in breast cancer. Cell Reports, 21(8), 2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Li X, Bai L, Zhu G, Guo Y, Lin J, Cui Y, Tian G, Zhang L, Wang J, Li XD, & Li L.(2020). Biomimetic α-selective ribosylation enables two-step modular synthesis of biologically important ADP-ribosylated peptides. Nature Communications, 11(1), 5600. [DOI] [PMC free article] [PubMed] [Google Scholar]