1 |. INTRODUCTION
Promoting healing of damaged tissues via organs with living cells and growth factors has progressively raised a great deal of interest within the scientific community.1–3 At the same time, the perspective of receiving advanced and minimally invasive regenerative treatment for severe or chronic conditions has also been promising for patients.4,5 Tissue engineering is the interdisciplinary combination of the principles of engineering to life sciences for the development or biological substitutes that can restore, maintain, or improve lost or damaged biological tissues and organs.6,7 The progressive scientific advances in biomaterials, cell therapy, and growth factors in recent decades have brought new therapeutic options for injured tissues/organs with limited healing potential.8–11 Nowadays, several tissue-engineered organs—including, but not limited to, blood vessels, skin grafts, tracheas, and esophagus—have been successfully utilized in a clinical setting and may become the standard of care in the future, overcoming the disadvantages of transplantation.12–20
In the oral cavity, a large variety of soft- and/or hard-tissue deformities potentially impairing periodontal and peri-implant health, as well as a patient’s quality of life, are commonly observed.10,21–26 Pathologies, traumas, and congenital conditions can result in severe defects requiring challenging and “invasive” bone and/or soft-tissue reconstruction procedures.3,24,27–29 Tissue engineering strategies involve the enrichment of scaffolds with living cells or growth factors, aiming at mimicking the cascades of wound healing events and the clinical outcomes of conventional autogenous grafts, but in a minimally invasive manner.1,10 Indeed, the recent decades have witnessed an increased attention on patient perspective, quality of life, and satisfaction related to the treatment, at a point that patient-reported outcome measures have become as important as clinical outcomes, limiting the use of autogenous grafts.22,25,30–35 Several studies have shown that patient preference was seldom in line with the clinical—and professional—outcome, and more often towards the less invasive procedure.36–40 Tissue engineering strategies also have the potential of promoting an accelerated healing and recovery, together with periodontal regeneration, owing to the beneficial effect of these living cells and growth factors/biologic agents.41–45
1.1 |. Limitations of conventional techniques
Tissue engineering would probably not be an emerging field if the conventional techniques for periodontal and peri-implant reconstruction were flawless. The main drawback of conventional treatment with autogenous grafts is patient morbidity,3 which largely depends on the size of the defect, and therefore the amount of graft harvested. Intraoral autogenous bone harvesting is often insufficient to completely occupy the defect in presence of severe atrophies.3,24,26 Utilizing autogenous grafts also increases the surgical time, which can exacerbate postoperative swelling and pain. Owing to the second surgical site required for autogenous tissue harvesting, the risk for intra- and postoperative complications is also higher. Major complications described following intraoral bone harvesting include temporary and permanent neurosensory disturbances, altered sensation, loss of tooth vitality, soft-tissue dehiscence, and infection have been described as common complications after autogenous bone harvesting from the mandibular ramus and the chin.46,47 Similarly, bone harvesting from the iliac crest can result in minor complications, such as superficial infections or hematomas, or more serious adverse events, including vascular injuries, neurological injuries, iliac spine fracture, deep infection, and deep hematoma formation requiring surgical intervention, among others.48,49
On the other hand, complications of autogenous soft tissue grafting—which is still the gold standard and most commonly performed procedure around natural teeth and dental implants22,50—mainly include injuries to the greater palatine artery, prolonged intra- and postoperative bleeding, patient morbidity, change of feeding habits, impairment of the patient’s quality of life within the first 1–2 weeks, and sensory disfunction.51–55
Therefore, the rationale of tissue engineering strategies in periodontal and peri-implant reconstruction is to search for a viable and minimally invasive alternative to conventional autogenous graft-based procedures that can enhance the healing potential of the defect, promoting regeneration of the lost tissues. Tissue engineering strategies may also have the potential of enhance the outcomes of current biomaterials, which are often characterized by early resorption or persistence, limited capacity to reconstruct severe defects, and which usually provide inferior outcomes compared with the gold standard autogenous grafts.56,57
2 |. PRINCIPLES OF TISSUE ENGINEERING IN PERIODONTAL AND PERI-IMPLANT RECONSTRUCTION
Tissue engineering approaches involve the combination of cells, signaling molecules, and scaffolds together with a vascular supply from the surgical site (Figure 1).
FIGURE 1.
The critical components of tissue engineering
2.1 |. Cells
Cell therapy aims to enhance the regenerative potential of conventional approaches by bringing living cells to the surgical area that can orchestrate the release of several growth factors crucial for the healing process.3,24,58 Cell therapy can overcome the limitations of autogenous graft substitutes by providing a source of living autogenous cells in the defect for regeneration3,24 (Figure 2). Somatic cells are characterized by lack of self-renewal capability and limited potency, as opposed to stem cells that can perpetuate through mitotic cell division and which can differentiate into several cell populations.10,24 Fibroblasts and keratinocytes are the somatic cells that have been employed for oral soft-tissue reconstruction.24,38,50,59–61 These cells are usually obtained from neonatal foreskin or from the actual patients with a punch biopsy. Autogenous cultured and expanded fibroblasts have also been used for root coverage procedures, widening of attached gingiva, and papilla augmentation.60,62–65
FIGURE 2.
The cell-based therapy workflow, from initiation at harvesting to its clinical implementation
Stem cells retain the ability to renew themselves through cell division and differentiate into a variety of specialized cell types.24 The bone marrow stroma contains hematopoietic stem cells, which can differentiate into blood cells of all lineages, and mesenchymal stem cells, which can give rise to osteoblasts, chondroblasts, adipocytes, myocytes, and fibroblasts, depending on the cell-to-cell interaction at the defect site.10,24 Historically, mesenchymal stem cells were first isolated and expanded from bone marrow aspirate from the iliac crest,66,67 but other sites can also be used to obtain them, including gingival tissue and periodontal ligament.24,68–75 A common method for increasing the concentration of stem and progenitor cells from the bone marrow aspirate is based on density gradient separation utilizing centrifuge-based systems.2,76–78 This approach is simple, fast, and cost-effective,79–81 and it is performed when fresh uncultured (“minimally manipulated”) stem cells are applied right after the bone marrow aspirate, without undergoing cell expansion.68,79,81–83 Nevertheless, this method does not distinguish between peripheral blood cells and stem cells, and therefore it may only increase the concentration of peripheral blood cells rather than stem cells.2 On the other hand, cell cultivation and expansion have allowed billions of stem cells to be obtained from just 1 mL of bone marrow aspirate.83,84 This process has been shown to be an effective method to highly characterize and enrich (up to 100-fold) specific cell populations67 (Figure 3). However, the main drawback of mesenchymal stem cells expansion is the waiting period (up to 2 weeks) between the harvesting from the bone marrow aspirate and their clinical application, together with the risk of contamination and the costs associated with this procedure.79,83,85 It has been advocated that mesenchymal stem cells can also be obtained from allograft tissues.86–89 Advancements in processing techniques have allowed mesenchymal stem cells to be obtained from allograft tissue, by selectively depleting immunogenetic cells while retaining native mesenchymal stem cells and osteoprogenitors.86,87,89
FIGURE 3.
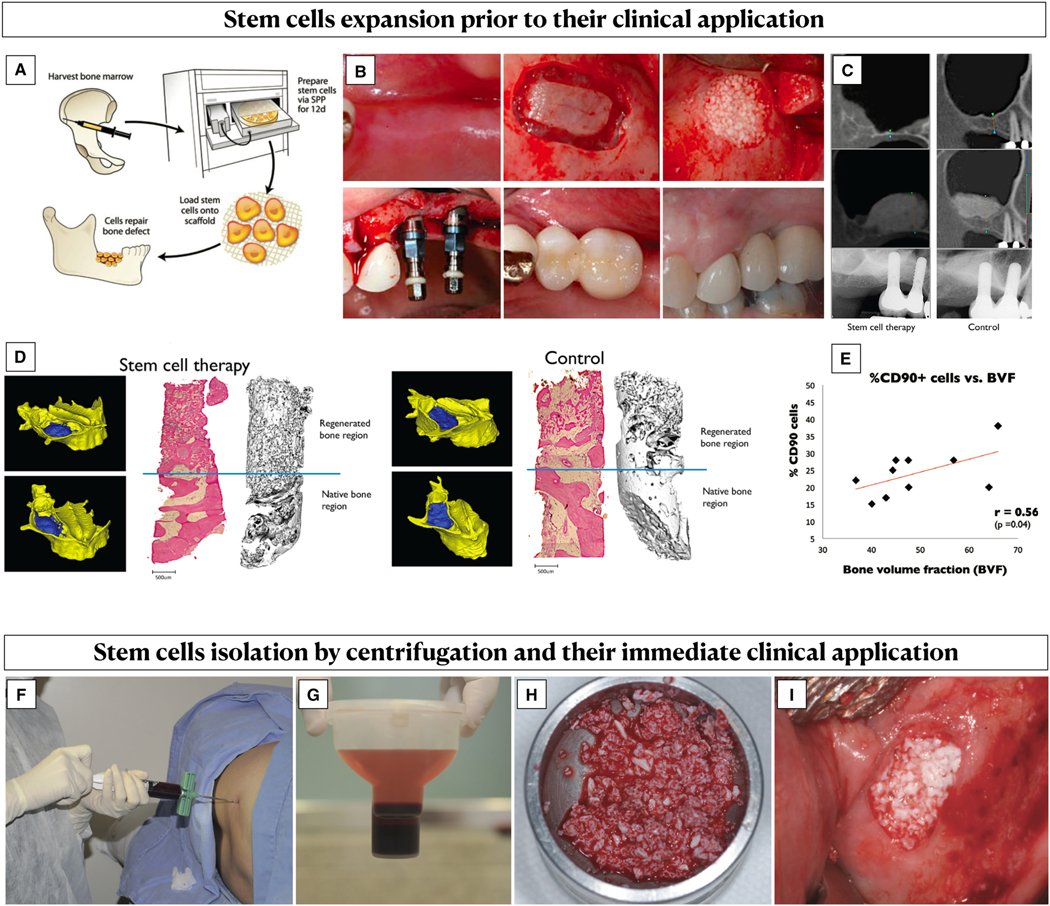
Expanded vs “minimally manipulated” stem cells. A-E, Stem cell expansion involved their harvesting from a donor source, which is usually the iliac crest bone marrow, and their cultivation and expansion using bioreactors. A, After their expansion, the cells are loaded into a scaffold and clinically apply on the target defect (reproduced with permission from SAGE journals26). B, Example of clinical application of bone marrow–derived expanded stem cells into a bone scaffold for sinus floor augmentation. C, Radiographic imaging showing bone gain following sinus floor elevation with and without stem cell therapy. D, Three-dimensional reconstruction from the cone-beam computed tomography, histological and microcomputed tomography analysis of bone biopsies in two samples representing the two groups. A significantly higher bone volume fraction was found in sinus augmented with cell therapy compared with the control group. E, A positive correlation between the degree of CD90+ stem cell enrichment and bone volume fraction was observed (Reproduced with permission from CCSE67). F-I, Clinical application of “minimally manipulated” stem cells from the iliac crest bone marrow. F, Bone marrow aspiration. G, Anticoagulant added to the syringe containing bone marrow, which was then placed in a centrifuge system for 14 minutes. Two phases were obtained, with the plasma that was removed, and the cell concentrate resuspended. H, Enrichment of a xenograft scaffold with the bone marrow concentrate. I, Clinical application for sinus floor augmentation (reproduced with permission from Hindawi Publishing Corporation79)
2.2 |. Signaling molecules
Growth factors are a collective group of highly active signaling molecules able to promote cell chemotaxis, proliferation, and differentiation.41,84,90 These biological mediators have the potential to induce intracellular signaling pathways, activating genes that change the activity and the phenotype of the targeted cell.90,91 Advancements in cellular and molecular biology have allowed for a better understanding of the role of the different growth factors and cytokines on the wound healing dynamics (Figure 4), which is the basis of tissue engineering strategies utilizing recombinant human growth factors or biologic agents.90,92,93 The goal of growth factor therapy is regenerating damaged tissue by mimicking the processes occurring during embryonic and postnatal development.90,94 Although several signal molecules play a role during wound healing, it may be assumed that using a single recombinant growth factor can induce molecular and biochemical cascades that will eventually promote regeneration.90,93,95 Recombinant human platelet–derived growth factor-BB, recombinant human bone morphogenetic protein-2, -4, -7, and -12, recombinant human growth and differentiation factor, and recombinant human fibroblast–growth factor are among the most investigated growth factors for oral regeneration. Enamel matrix derivative is an alternative signal molecule that has been largely used in periodontal regeneration and in several other scenarios as a wound healing enhancer.41,96–101
FIGURE 4.
Cell populations involved in the different phases of wound healing. Four different and partially overlapping wound healing phases have been identified: coagulation, inflammatory, proliferation, and maturation/resolution. Following blood clot formation, degranulating platelets release platelet-derived growth factor that is responsible for stimulating chemotaxis and/or mitogenicity of neutrophils, monocytes, macrophages, and fibroblasts, which play a key role on the initiation of the inflammatory response. Macrophages are the main actors of the subsequent wound healing phases, contributing to the wound debridement and secreting several growth factors, such as platelet-derived growth factor, transforming growth factor beta, epidermal growth factor, fibroblast growth factor-2, and vascular endothelial growth factor. The phase of proliferation and maturation involves several cell populations, based on the injured tissue. At later stages, platelet-derived growth factor stimulates mesenchymal progenitor cell migration and, together with transforming growth factor beta, promotes fibroblast differentiation into myofibroblasts, which is a crucial step for wound healing contraction and closure. The apoptosis of endothelial cells and fibroblasts is orchestrated by transforming growth factor beta, whereas vascular endothelial growth factor promotes angiogenesis and anti-apoptotic effects on other cells
2.3 |. Scaffolds
Scaffolds utilized for tissue engineering strategies can be classified based on their origin (natural vs organic), source (autogenous, allogeneic, xenogeneic, or alloplastic) or main therapeutic goal (promoting bone and/or periodontal regeneration or soft-tissue reconstruction).
2.3.1 |. Scaffolds for bone and periodontal reconstruction
Allogeneic, xenogeneic, and alloplastic bone substitutes are the most widely adopted scaffolds for oral bone regeneration.24,26,56 Though the primary function of bone grafts is promoting new bone regeneration within the bony defects through osteogenesis and osteoinduction, they also play a significant role also as scaffolds by preventing the collapse of the flap/membrane into the defect, therefore maintaining the biologic space necessary for the regeneration (osteoconduction).102 Osteogenesis involves the osteodifferentiation and new bone formation by donor cells from the host or graft, and it is a prerogative of autogenous bone graft only. The osteoinduction capacity of allograft and xenograft largely depends on the processing methods for eliminating antigenic components and preventing disease transmission. Owing to their osteoinductive and osteoconductive properties, allogeneic, xenogeneic, and alloplastic bone grafts have often been combined with growth factors or living cells for enhancing new bone formation.8,83,90
Nevertheless, limitations of these naturally derived scaffolds include the inability to tailor their degradation time, poor processability into porous structure, and inability to maintain the desired volume under mechanical stimuli.24,103 In order to overcome these drawbacks, several natural and synthetic polymers have been evaluated as a scaffold for tissue engineering strategies, including cellulose, chitosan, collagen, hyaluronic acid, polylactic acid, polylacticpolycaprolactone, and so on.24,103 At the same time, advances in technologies have oriented researchers towards the development of customized, image-based, three-dimensional scaffolds.56,104 Additive manufacturing allows production of multilayer scaffolds, with a different array of materials, and with a specific surface topography that can facilitate cell adhesion, migration, and differentiation.56,104 The concept of a fiber-guiding scaffold has been extensively evaluated for periodontal regeneration, with the goal of promoting the growth of periodontal ligament cells and Sharpey fibers in the desired, oriented, direction104,105 (Figures 5 and 6). In 2015, Rasperini et al described the first clinical application of a three-dimensional printed bioresorbable scaffold for periodontal regeneration106 (Figure 6).
FIGURE 5.
Three-dimensional customized printed scaffolds with multi-tissue interfaces. A, Three-dimensional designed hybrid scaffold with perpendicularly oriented internal channel-structures within the periodontal ligament (PDL) portion and a bone-specific compartment (reproduced with permission from Elsevier265). B, Planification of a customized multicompartments scaffold from the microcomputed tomography scan (reproduced with permission from Sage Publications106)
FIGURE 6.

A, Baseline peri-apical X-ray. B, C, Clinical view of the tooth with periodontal infrabony defect. D, Flap elevation. E, Application of 24% ethylenediaminetetraacetic acid for 2 minutes. F, Polycaprolactone scaffold. G, Scaffold soaked with recombinant human platelet–derived growth factor-BB (GEM21; Lynch Biologics, Franklin, TN, USA). H, Scaffold fixation to the alveolar bone. I, Flap closure. J, Healing after 2 weeks. K, One year post-op (reproduced with permission from Sage Publications106)
2.3.2 |. Scaffolds for soft-tissue reconstruction
Scaffolds for soft-tissue reconstructions are usually dermal or collagen matrices that act as “empty” structures promoting the migration and colonization of host cells from the adjacent sites.24,42,107
Human allogeneic acellular dermal matrix was one of the first extracellular matrices to be utilized for the treatment of chronic and burn cutaneous wounds, as well as in periodontal soft-tissue augmentation.107–110 Acellular dermal matrix mimics the extracellular matrix of human dermis, with preserved natural porosity, vessel channels, and basement membrane, which makes the scaffold suitable for epithelial cells and fibroblasts42,111 (Figure 7). After removal of the epidermis, the graft undergoes a decellularization process to make it immunologically inert. Interestingly, it has been shown that the method of decellularization and processing of the acellular dermal matrix has an impact on the characteristics of the matrix, with consequences also on cells migration and proliferation.42,112,113
FIGURE 7.
Human and porcine-derived acellular dermal matrices before and after rehydration. The drawing illustrates the structure of these matrices, with the presence of fibrillar collagen and collagen VI that provide stability to the scaffold and of elastin fibers that contribute to the elasticity of the matrix. Other components of these scaffolds include hyaluronan, proteoglycans, fibronectin, and vascular channels, which play a crucial role for the revascularization of the graft. BM: basement membrane; DM: dermal side of the acellular dermal matrix. The solvent-dehydrated matrix is Puros Dermis (Zimmer Dental, Zimmer Biomet, Warsaw, IN, USA), the freeze-dried matrix is AlloDerm (BioHorizons, Birmingham, AL, USA), and the porcine-derived matrix is NovoMatrix (BioHorizons, USA)
Overall, acellular dermal matrix is a popular matrix for tissue engineering strategies given its preserved extracellular properties, good durability, and reduced antigenicity.114,115 In the periodontal field, different types of acellular dermal matrices have been introduced and utilized in a clinical setting, mainly derived from human skin—and therefore not available in several countries—or obtained from a porcine source.34,109,110,116
The first generation of xenogeneic collagen matrix involves a non-cross-linked bilayered porcine-derived collagen matrix, mainly composed of collagen types I and III42,117,118 (Figure 8). Collagen type I is more resistant and may have angiogenic potential, whereas collagen type III contributes to the mechanical stability of the matrix but degrades quickly. This xenogeneic collagen matrix is composed of a thin (0.4 mm in thickness) occlusive compact layer (derived from peritoneum) that acts as a barrier while providing mechanical stability to the scaffold and by a thicker (1.3 mm) occlusive compact layer that promotes blood clot stabilization and promotion of cells ingrowth.42,117 A second generation of xenogeneic collagen matrix has been more recently introduced.119–123 This novel matrix undergoes a cross-linking process, aiming for a slow degradation process and volume stability of the matrix;42,124 it is characterized by a single porous layer, made of type I and type III collagen and a small amount of elastin fibers.42,120,124
FIGURE 8.
Different types of xenogeneic collagen matrix before and after hydration. The bilayered xenogeneic collagen matrix is Mucograft (Geistlich Pharma, Wolhusen, Switzerland); CL: compact layer; SL: spongy layer. The porous cross-linked xenogeneic collagen matrix is Fibrogide (Geistlich Pharma, Wolhusen, Switzerland). The multilayered cross-linked xenogeneic collagen matrix is Ossix Volumax (Dentsply Sirona, Charlotte, NC, USA)
Dermal and collagen matrices—as scaffolds alone—have often been compared in clinical trials with autogenous grafts, in an attempt to minimize patient morbidity.125–130 Nevertheless, an autogenous graft is still the optimal approach for periodontal and peri-implant reconstruction.35,131,132 Therefore, it is not surprising that these scaffolds have been enriched with signaling molecules, or living cells, to further enhance their properties and outcomes, with the hope to find a patient-oriented, minimally invasive, approach able to mimic the characteristics of autogenous grafts.50,60,65,133–135
2.4 |. Tissue engineering strategies
Tissue engineering strategies can be classified as follows:
cell-based tissue engineering strategies, involving living cells (eg, mesenchymal cells from bone marrow, somatic cells, cells from the periodontal ligament) seeded in a scaffold material (Figure 9); or
signaling molecule–based tissue engineering strategies, involving the application of scaffolds loaded/soaked with signaling molecules (Figures 10–12).
FIGURE 9.
Non–root coverage gingiva augmentation using a cell-based tissue engineering strategy. A living cellular construct, characterized by allogeneic keratinocytes and fibroblasts from newborn foreskin seeded into a collagen membrane (Apligraft; Organogenesis Inc., Canton, MA, USA), was used in this case that was part of a previously published clinical trial.38 A, Baseline. B, Flap elevation. C, Living cellular construct stabilized apically to the canine and premolars. D, An additional layer of the living cellular construct was applied over the graft. E, One week post-op. F, One month post-op. G, Six-month follow-up. H, Outcome after 13 years. Note that Lugol’s solution was used to discriminate the alveolar mucosa from the gingiva
FIGURE 10.
Periodontal regeneration using a signaling molecule–based tissue engineering strategy, involving the use of enamel matrix derivative (Straumann, Basel, Switzerland), in combination with a xenogeneic bone graft scaffold (Geistlich Pharma, Wolhusen, Switzerland). A, Baseline peri-apical X-ray. B, Baseline clinical presentation. C-E, A minimally invasive flap preserving the integrity of the papilla was elevated. After the debridement of the infrabony defect, mechanical and chemical root planing was performed. F, Enamel matrix derivative extraorally combined with demineralized bovine bone matrix. G, H, Application of the tissue engineering strategy into the defect. Note that the suture was already prepared but not tightened. I, J, Flap closure. K, L, Clinical and radiographic outcomes at the 11-year follow-up
FIGURE 12.

Tissue engineering strategy for soft tissue reconstruction at a dental implant previously treated with a resective approach for peri-implantitis. (A) Clinical view of the dental implant at baseline. (B) Flap design. A trapezoidal coronally advanced flap was performed. (C) Split thickness flap elevation. Note that the healthy connective tissue that was adherent to the implant surface was left in place to facilitate the adaptation and nutrition of the soft tissue graft. (D) The level of the buccal bone – underneath the connective tissue – is identified with a periodontal probe. (E-F) Tissue engineering graft consisting of a xenogeneic collagen scaffold (Fibrogide, Geistlich Pharma, Wolhusen, Switzerland) loaded with rhPDGF-BB (GEM21, Lynch Biologics, Franklin, TN, USA). (G) Stabilization of the tissue engineering graft to the de-epitheliliazed anatomical papillae and to the periosteum. (H) Flap advancement and closure. (I) Ultrasonographic (US) scan of the midfacial aspect of the dental implant at baseline (BL). Note that the implant-supported crown is identified as “Cr”, “St” pinpoints the soft tissue component, which is also highlighted in blue and the implant fixture is shown as “Impl”. The scan on the left area of the panel is showing a frame of the blood flow cine loop when recorded as color velocity. (J) Clinical view of the implant at baseline, with the dotted white line showing the region of interest where the ultrasound scan was taken. (K) Clinical view of the implant at 1 year, with the dotted white line showing the region of interest where the ultrasound scan was obtained. (L) Ultrasonographic scan of the midfacial aspect of the dental implant 1 year after the soft tissue augmentation procedure. The ultrasonographic scan on the right area of the panel is showing a frame from the cine loop recording of the tissue perfusion around the dental implant in terms of color velocity. It is possible to appreciate a consistent gain in mucosal thickness compared to baseline and a reduction in the amount of color velocity, that may be interpreted as a resolution of the subclinical inflammation observed prior to the augmentation procedure
For both cell-based and signaling molecule–based tissue engineering strategies, the goal is mimicking the healing events promoted by autogenous grafts and cells of the hosts. Though it can be speculated that cell-based tissue engineering strategies can better simulate the healing cascade typical of autogenous grafts, there are no doubts that signaling molecule–tissue engineering strategies incorporating commercially available biologic agents are easier, less expensive, and more time-efficient procedures.
2.5 |. Aim of the search strategy
The goal of this review was the appraisal of the different tissue engineering strategies utilized for periodontal and peri-implant reconstruction, together with the evaluation of their safety, invasiveness, efficacy, and patient-reported outcomes.
3 |. SYSTEMATIC SEARCH AND METHODOLOGY
3.1 |. Protocol registration and reporting format
The protocol of this study was prepared and registered prior to it starting and allocated the identification number CRD42022309170 on the PROSPERO International Prospective Register of Systematic reviews database.136 This review has been prepared following the Cochrane Collaboration guidelines137 and is reported in accordance with the Preferred Reporting Items for Systematic Reviews (Figure 13).
FIGURE 13.
The PRISMA flowchart illustrating the process of the studies selected
3.2 |. Focus questions
The focused questions of this review can be summarized as follows:
What are the tissue engineering strategies that have been performed for periodontal and peri-implant reconstruction and implant site development? Are tissue engineering strategies safe, minimally invasive, and predictable alternative options to conventional treatments?
3.3 |. Population, intervention, comparison, outcome, time questions
The following population, intervention, comparison, outcome, time framework was used to guide the inclusion and exclusion of studies for our focused questions138:
Population (P):
Patients undergoing surgical intervention for periodontal or peri-implant reconstruction or implant site development.
Intervention (I):
Surgical treatment for infrabony/furcation defects, root coverage procedures, non–root coverage gingival phenotype modification, alveolar ridge preservation, ridge augmentation, sinus floor augmentation, or peri-implant reconstruction utilizing tissue engineering strategies.
Comparison (C):
All possible comparisons among the interventions included were explored, including nonintervention or treatment with the use of a scaffold alone/placebo.
Outcome (O):
Clinical, radiographic, patient-reported outcome measures (including discomfort, painkillers intake, satisfaction, preference and esthetic assessment, invasiveness-related surgical outcomes), chair time, complications, and costs.
Time (T):
Any study duration or follow-up after the surgical intervention of at least 6 months for root coverage, gingival phenotype modification, periodontal regeneration, ridge augmentation, and sinus floor augmentation and 3 months for alveolar ridge preservation. Data at every follow-up time point were recorded.
3.4 |. Eligibility criteria, search strategy, and study selection
Only randomized controlled trials with a well-defined clinical protocol were considered for this study. A detailed computerized systematic search was conducted in the literature to identify eligible randomized controlled trials, followed by additional manual searching in relevant journals, past reviews,1,3,8,11,56,83,131–133,139–145 and cross-reference checks in the articles retrieved. The search strategies were entered and modeled for MEDLINE (via PubMed), EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Scopus, and Web of Science (Appendix S1).
Two pre-calibrated review authors (LT, SB) performed the selection process of the randomized controlled trials, first by titles and abstracts and then followed by a full read through of the studies that remained for careful assessment and alignment with the set inclusion criteria.
3.5 |. Quality assessment and risk of bias
All the studies included were evaluated according to the Cochrane collaboration group146 independently and in duplicate by two examiners (LT, SB).
4 |. RESULTS FROM THE SYSTEMATIC SEARCH
The PRISMA flowchart is shown in Figure 13. Following the removal of duplicates, 1875 records were identified based on titles and abstracts. A full-text assessment was performed for 283 articles. Based on our predetermined inclusion criteria, 128 randomized controlled trials utilizing tissue engineering strategies were included in the qualitative assessment36,38,71,72,79,82,87,101,135,147–253,31–33,59–61,63–65,67–69 (Tables 1–14). Among them, 59 trials evaluated the efficacy of tissue engineering strategies for periodontal regeneration in infrabony and furcation defects71,72,148,49,158,159,162,165,167,177,178,193,198,223,224,227,229,240,242,245,247,248,251,151–155,169–172,180–182,184–187,201–206,212–216,220–223,234–238 (Tables 1 and 2), 16 randomized controlled trials focused on tissue engineering strategies for root coverage procedures43,44,60,61,64,65,135,157,163,168,222,228,238,241,249,250 (Tables 3 and 4), and four trials assessed the outcomes of tissue engineering strategies for non–root coverage gingival phenotype modification therapies36,38,59,63 (Tables 5 and 6). Forty-five randomized controlled trials had utilized tissue engineering strategies for implant site development, with 14 studies evaluating the outcomes of tissue engineering strategies for alveolar ridge preservation160,173,176,183,188,191,196,199,200,208,209,215,217,237 (Tables 7 and 8), eight trials on the outcomes of tissue engineering strategies for staged bone augmentation procedures164,207,216,230,243,31–33 (Tables 9 and 10), and 22 randomized controlled trial tissue engineering strategies for sinus floor augmentation79,82,87,101,156,161,166,174,175,179,192,194,195,197,225,226,239,244,246,67–69 (Tables 11 and 12). Tissue engineering strategies for peri-implant bone reconstruction were described in five randomized controlled trials147,150,189,190,231 (Tables 13 and 14).
TABLE 1.
Characteristics of the assessed clinical trials utilizing tissue engineering strategies for periodontal regeneration
| Cell-based tissue engineering | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reference | Study design | Cell type | Origin | Cells culture medium | Scaffold | Control group |
| Abdal-Wahab et al148 | Parallel-arm randomized controlled trial | Human autogenous fibroblasts | Gingiva or retromolar pad | α-Minimum essential medium with antibiotics | β-Tricalcium phosphate (covered with a collagen membrane) | Guided tissue regeneration (β-tricalcium phosphate + collagen membrane) |
| Apatzidou et al152 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Alveolar bone marrow | α-Minimum essential medium with antimicrobial/antifungal agents | Collagen scaffold enriched with autologous fibrin | Group 1: collagen scaffold enriched with autologous fibrin Group 2: flap alone |
| Chen et al158 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Periodontal ligament | α-Miαα-Minimum essential medium with 10% fetal bovine serum with antibiotics | Demineralized bovine bone matrix | Guided tissue regeneration (demineralized bovine bone matrix + collagen membrane) |
| Dhote et al170 | Parallel-arm randomized controlled trial | Human allogeneic mesenchymal stem cells | Umbilical cord | Serum-free medium specifically formulated for mesenchymal stem cells | β-Tricalcium phosphate (+ recombinant human platelet-derived growth factor-BB) | Flap alone |
| Ferrarotti et al71 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Dental pulp | Cells not expanded | Collagen sponge | Collagen sponge |
| Sánchez et al72 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Periodontal ligament | Serum-free Dulbecco’s modified Eagle’s medium with antibiotics | Xenogeneic bone substitute containing hydroxyapati and collagen | Xenogeneic bone substitute containing hydroxyapatite and collagen |
| Shalini and Vandana236 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Periodontal ligament | Cells not expanded | Gelatin sponge | Flap alone |
| Yamamiya et al248 | Parallel-arm randomized controlled trial | Human autogenous mesenchymal stem cells | Periosteum | Medium containing 10% fetal bovine serum and antibiotics | Hydroxyapatite | Platelet-rich plasma + hydroxyapatite |
| Signaling molecule-based tissue engineering | |||||
|---|---|---|---|---|---|
|
| |||||
| Reference | Study design | Biologic | Scaffold | Combination biologic and scaffold | Control group |
| Abu-Ta’a149 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Not reported | Enamel matrix derivative + demineralized freeze-dried bone allograft |
| Aoki et al151 | Parallel-arm randomized controlled trial | Recombinant fibroblast-human fibroblast-growth factor-2 | Demineralized bovine bone matrix | Extraoral | Recombinant human fibroblast-growth factor-2 |
| Aslan et al153 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Not reported | Flap alone |
| Asprìello et al154 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Intraoral | Demineralized freeze-dried bone allograft |
| Bokan et al155 | Parallel-arm randomized controlled trial | Enamel matrix derivative | β-Tricalcium phosphate | Not reported | Group 1: enamel matrix derivative Group 2: flap alone |
| Cochran et al159 | Parallel-arm randomized controlled trial | Recombinant human fibroblast-growth factor-2 | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| Cortei lini and Tonetti162 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Not reported | Group 1: enamel matrix derivative Group 2: flap alone |
| De Leonardis and Paolantonio165 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Hydroxyapatite/β-tricalcium phosphate | Intraoral | Group 1: enamel matrix derivative Group 2: flap alone |
| Barcellos de Santana and Miller Mattos de Santana167 | Split-mouth randomized controlled trial | Recombinant human fibroblast-growth factor-2 | Hyaluronic acid | Extraoral | Flap alone |
| Devi and Dixit169 | Parallel-arm randomized controlled trial | Recombinant human vascular endothelial growth factor + recombinant human insulin growth factor-1 | β-Tricalcium phosphate | Not reported | Guided tissue regeneration (β-tricalcium phosphate + collagen membrane) |
| Recombinant human vascular endothelial growth factor | β-Tricalcium phosphate | Not reported | Guided tissue regeneration (β-tricalcium phosphate + collagen membrane) | ||
| Recombinant human insulin growth factor-1 | β-Tricalcium phosphate | Not reported | Guided tissue regeneration (β-tricalcium phosphate + collagen membrane) | ||
| Dori et al172 | Parallel-arm randomized controlled trial | Enamel matrix derivative (+ platelet-rich plasma) | Demineralized bovine bone matrix | Intraoral | Enamel matrix derivative + demineralized bovine bone matrix |
| Dori et al171 | Parallel-arm randomized controlled trial | Enamel matrix derivative (+ platelet-rich plasma) | Demineralized bovine bone matrix | Intraoral | Enamel matrix derivative + demineralized bovine bone matrix |
| Ghezzi et al177 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Not reported | Guided tissue regeneration (demineralized bovine bone matrix + collagen membrane) |
| Gurinsky et al178 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Extraoral | Enamel matrix derivative |
| Hoffmann et al180 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Biphasic calcium phosphate | Intraoral | Enamel matrix derivative |
| Hoidal et al181 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Intraoral | Demineralized freeze-dried bone allograft |
| Howell et al 1997182 | Split-mouth randomized controlled trial | 50μg/mL recombinant human platelet-derived growth factor-BB + recombinant human insulin growth factor-1 | Gel vehicle | Extraoral | Flap alone |
| 150μg/mL recombinant human pi ate let-de rived growth factor-BB + recombinant human insulin growth factor-1 | Gel vehicle | Extraoral | Flap alone | ||
| Iorio-Siciliano et al184 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Extraoral | Guided tissue regeneration (demineralized bovine bone matrix + collagen membrane) |
| Jaiswal and Deo185,a | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Intraoral | Group 1: guided tissue regeneration (demineralized freeze-dried bone allograft + bioresorbable membrane containing polyglycolide and poly-L-lactide) |
| Group 2: flap alone | |||||
| Jayakumar et al186 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| Jepsen et al187 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Biphasic calcium phosphate | Intraoral | Enamel matrix derivative |
| Kavyamala et al193 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| Kuru et al198 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Bioactive glass | Extraoral | Enamel matrix derivative |
| Lee et al202 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | Recombinant human platelet-derived growth factor-BB + equine-derived bone matrix |
| Lee et al201 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized porcine bone matrix | Extraoral | Demineralized porcine bone matrix |
| Lekovic et al203 | Split-mouth randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Intraoral | Enamel matrix derivative |
| Lekovic et al204 | Split-mouth randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Intraoral | Autologous fibrinogen/fibronectin system + demineralized bovine bone matrix |
| Losada et al205 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Biphasic calcium phosphate | Intraoral | Enamel matrix derivative |
| Maroo and Murthy206 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| Meyle et al210 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Biphasic calcium phosphate | Extraoral | Enamel matrix derivative |
| Mishra et al 211 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | Hyaluronic acid | Extraoral | Flap alone |
| Moreno Rodriguez and Ortiz Ruiz212 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Natural bovine bone substitute | Extraoral | Enamel matrix derivative |
| Nevins et al 213 | Parallel-arm randomized controlled trial | 0.3 mg/mL recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| 1 mg/mL recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate | ||
| Nevins et al214 | Parallel-arm randomized controlled trial | 0.3 mg/mL recombinant human pi ate let-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate |
| 1 mg/mL recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | β-Tricalcium phosphate | ||
| Ogihara and Wang219 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized freeze-dried bone allograft | Extraoral | Orthodontic therapy + enamel matrix derivative + demineralized freeze-dried bone allograft |
| Ogihara and Tarnow218 | Parallel-arm randomized controlled trial | Enamel matrix derivative Enamel matrix derivative | Freeze-dried bone allograft Demineralized freeze-dried bone allograft | Extraoral Extraoral | Enamel matrix derivative Enamel matrix derivative |
| Peres et al220,a | Parallel-arm randomized controlled trial | Enamel matrix derivative | Hydroxyapatite/β-tricalcium phosphate | Extraoral | Hydroxyapatite/β-tricalcium phosphate |
| Pietruska et al221 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Biphasic calcium phosphate | Extraoral | Enamel matrix derivative |
| Queiroz et al223,a | Parallel-arm randomized controlled trial | Enamel matrix derivative | Hydroxyapatite/β-tricalcium phosphate | Extraoral | Group 1: enamel matrix derivative Group 2: hydroxyapatite/β-tricaldum phosphate |
| Raslan et al224 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | Group 1: platelet-rich fibrin Group 2: flap alone |
| Ridgway et al227 | Split-mouth randomized controlled trial | 0.3mg/mL recombinant human platelet-derived growth factor-BB vs 1 mg/mL recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | No control group |
| Saito et al229 | Parallel-arm randomized controlled trial | Recombinant human fibroblast-growth factor-2 | Demineralized bovine bone matrix | Extraoral mixing recombinant human fibroblast-growth factor-2 and demineralized bovine bone matrix | Recombinant human fibroblast-growth factor-2 |
| Scheyer et al232 | Split-mouth randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Extraoral | Demineralized bovine bone matrix |
| Schincaglia et al233 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB with single flap approach or double flap approach | β-Tricalcium phosphate | Extraoral | No control group |
| Sculean et al234 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Bioactive glass | Extraoral | Bioactive glass |
| Sculean et al235 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Bioactive glass | Extraoral | Enamel matrix derivative |
| Stavropoulos et al240 | Parallel-arm randomized controlled trial | Recombinant human growth and differentiation factor-5 | β-Tricalcium phosphate | Extraoral | Flap alone |
| Thakare and Deo242 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraoral | Hydroxyapatite + β-tricalcium phosphate |
| Velasquez-Plata et al245 | Split-mouth randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Extraoral | Enamel matrix derivative |
| Windisch et al247 | Parallel-arm randomized controlled trial | Recombinant human growth and differentiation factor-5 | β-Tricalcium phosphate | Extraoral | Flap alone |
| Zucchelli et al251 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Demineralized bovine bone matrix | Extraoral | Enamel matrix derivative |
Trials investigating the outcomes of furcation defects.
TABLE 14.
Safety, patient-reported outcomes, and clinical and histomorphometric results of the clinical trials utilizing tissue-engineering strategies for peri-implant bone augmentation
| Reference | Comparison | Safety | Complications | Patient-reported outcome measures | Clinical and histomorphometric outcomes |
|---|---|---|---|---|---|
| Amorfini et al150 | Tissue engineering strategy vs guided bone regeneration | Yes | 2 patients with early exposure of the membrane in the control group (no statistically significant difference, P > 0.05) | Not reported | Tissue engineering strategy: higher bone volume preservation*
Not reported |
| Santana et al231 | Immediate implant placement + tissue engineering strategy vs conventional implant placement | Yes | No | Not reported | Immediate implant placement + guided bone regeneration with tissue engineering strategy showed outcomes similar to conventional implant placement Not reported |
| Jung et al 2003189 | Tissue engineering strategy vs guided bone regeneration | Yes | No statistically significant difference (P > 0.05) | Not reported | Not statistically significant difference (P > 0.05) Tissue engineering strategy: higher fraction of lamellar bone, higher surface of bone substitute particles in direct contact with the newly formed bone (statistically significantly different, P < 0.05)) |
| Jung et al 2009190 | Tissue engineering strategy vs guided bone regeneration | Yes | No statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) Not reported |
| Jung et al 2022147 | Tissue engineering strategy vs guided bone regeneration | Yes | No statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) Not reported |
P-value of 0.05.
TABLE 2.
Comparison of the clinical and radiographic outcomes of tissue engineering strategies vs conventional approaches for the treatment of infrabony defects
| Comparison | Outcome favors tissue engineering strategies | Similar outcomes | Outcome favors conventional therapies |
|---|---|---|---|
| Tissue engineering strategies vs bone graft scaffold alone | Aspriello et al 2011, Cochran et al 2016, Jayakumar et al 2011, Kavyamala et al 2019, Maroo and Murthy 2014, Nevins et al 2005, Nevins et al 2013, Thakare and Deo, Yamamiya et al 2008154,159,186,193,206,213,214,242,248 | Hoidal et al 2008, Lee et al 2020, Lekovic et al 2001, Sánchez et al 2020, Scheyer et al 2002, Sculean et al 200272,181,202,204,232,234 | None |
| Tissue engineering strategies vs guided tissue regeneration | AbdalAbdal-Wahab et al 2020, Devi and Dixit 2016148,169 | Chen et al 2016, Ghezzi et al 2016, lorio-Siciliano et al 2014158,177,184 | None |
| Tissue engineering strategies vs flap alone | Apatzidou et al 2021, Bokan et al 2006, De Leonardis and Paolantonio 2013, de Santana and de Santana 2015, Dhote et al 2015, Ferrarotti et al 2018, Howell et al 1997, Shalini and Vandana 201 871,152,155,165,167,170,182,236 | Aslan et al 2020, Cortellini and Tonetti 2011, Mishra et al 2013, Raslan et al 2021, Stavropoulos et al 2011153,162,211,224,240 | None |
| Tissue engineering strategies vs biologic agent alone | Aoki et al 2021, De Leonardis and Paolantonio 2013, Gurinsky et al 2004, Kuru et al 2006, Lekovic et al 2000, Ogihara and Tarnow 2014, Saito et al 2019, Velasquez-Plata, et al 2002, Zucchelli et al 200 3151,165,178,198,203,218,229,245,251 | Bokan et al 2006, Cortellini and Tonetti 2011, Hoffmann et al 2016, Jepsen et al 2008, Losada et al 2017, Meyle et al 2011, Moreno Rodriguez and Ortiz Ruiz, Pietruska et al 2012, Sculean et al 2005155,162,180,187,205,210,212,221,235 | None |
TABLE 3.
Characteristics of the clinical trials utilizing tissue-engineering strategies for root coverage procedures
| Cell-based tissue engineering | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reference | Study design | Cell type | Origin | Cells culture medium | Scaffold | Control group |
| Jhaveri et al64 | Split-mouth randomized controlled trial | Human autogenous fibroblasts | Attached gingiva | α-Minimum essential medium containing fetal bovine serum and antibiotics | Acellular dermal matrix | Connective tissue graft |
| Koseglu et al60 | Split-mouth randomized controlled trial | Human autogenous fibroblasts | Palatal mucosa | DulDuDulbecco’s modified Eagle’s medium containing fetal bovine serum and antibiotics | Collagen membrane | Collagen membrane alone |
| Milinkovic et al65 | Split-mouth randomized controlled trial | Human autogenous fibroblasts | Palatal mucosa | Nutritional medium | Collagen membrane | Connective tissue graft |
| Wilson et al61 | Split-mouth randomized controlled trial | Human allogeneic fibroblast | Newborn foreskin | Not reported | Bioabsorbable polyglactin mesh |
Connective tissue graft |
| Zanwar et al250 | Parallel-arm randomized controlled trial | Human allogeneic mesenchymal stem cells | Umbilical cord | Serum-free medium specifically formulated for mesenchymal stem cells | Polylactic acid/polyglycolic acid | Connective tissue graft |
| Zanwar et al249 | Parallel-arm randomized controlled trial | Human allogeneic mesenchymal stem cells | Umbilical cord | Serum-free medium specifically formulated for mesenchymal stem cells | Polylactic acid/polyglycolic acid | Polylactic acid/polyglycolic acid alone |
| Signaling molecule–based tissue engineering | |||||
|---|---|---|---|---|---|
|
| |||||
| Reference | Study design | Biologic | Scaffold | ComCombination biologic and scaffold | Control group |
| Carney et al157 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | Acellular dermal matrix | Acellular dermal matrix trimmed and then extraorally hydrated in 2 mL of recombinant human platelet-derived growth factor for at least 3 min | Acellular dermal matrix alone |
| Dandu and Murthy163 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | Xenogeneic collagen matrix | Xenogeneic collagen matrix trimmed and then extraorally saturated with 0.3mg/mL recombinant human platelet-derived growth factor-BB for at least 10 min | Periosteal pedicle graft |
| Deshpande et al168 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | β-Tricalcium phosphate extraorally saturated with recombinant human platelet-derived growth factor-BB | Flap alone, connective tissue graft |
| McGuire et al43 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | β-Tricalcium phosphate extraorally saturated with recombinant human platelet-derived growth factor-BB | Connective tissue graft |
| McGuire et al44 | Split-mouth randomized controlled trial | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | β-Tricalcium phosphate extraorally saturated with recombinant human platelet-derived growth factor-BB | Connective tissue graft |
| Pourabbas et al222 |
Split-mouth/parallel-arm randomized controlled trial | Enamel matrix derivative | Acellular dermal matrix | Intraorally combination of acellular dermal matrix with enamel matrix derivative | Acellular dermal matrix alone |
| Rocha Dos Santos et al228 and Sangiorgio et aI135 | Parallel-arm randomized controlled trial | Enamel matrix derivative | Xenogeneic collagen matrix | IntrazIntraorally combination of xenogeneic collagen matrix with enamel matrix derivative | Flap alone, xenogeneic collagen matrix alone, enamel matrix derivative alone |
| Shin et al238 | Split-mouth randomized controlled trial | Enamel matrix derivative | Acellular dermal matrix | Intraorally combination of acellular dermal matrix with enamel matrix derivative | Acellular dermal matrix alone |
| Tavelli et al241 | Parallel-arm randomized controlled trial | Recombinant human platelet-derived growth factor-BB | XenoXenogeneic cross-linked collagen matrix | Xenogeneic collagen matrix trimmed and then saturated with recombinant human platelet-derived growth factor-BB for at least 15min | Xenogeneic collagen matrix alone |
TABLE 4.
Safety, patient-reported outcomes, and clinical results of the clinical trials utilizing tissue engineering strategies for root coverage procedures
| Reference | ComComparison | Safety; complications | Patient-reported outcome measures | CliniClinical outcomes |
|---|---|---|---|---|
| Carney et al157 | TissuTissue engineering strategy vs scaffold alone | Yes; not reported | Not reported | No stNo statistically significant difference (P > 0.05) |
| Dandu and Murthy163 | TissuTissue engineering strategy vs periosteal pedicle graft | Yes; no | No statistically significant difference (P > 0.05) for pain | TissuTissue engineering strategy: higher mean root coverage, keratinized tissue width gain, and clinical attachment level gain* |
| Deshpande et al168 | TissuTissue engineering strategy vs connective tissue graft vs coronally advanced flap alone | Yes; no | Not reported | Mean root coverage not statistically significantly different (P > 0.05) between tissue engineering strategy and connective tissue graft Tissue engineering strategy: higher mean root coverage than coronally advanced flap alone* Greater keratinized tissue width gain in connective tissue graft group than tissue engineering strategy* |
| Jhaveri et al64 | Tissue engineering strategy vs connective tissue graft | Yes; not reported | Not reported | No stNo statistically significant difference (P > 0.05) |
| Koseglu et al60 | Tissue engineering strategy vs scaffold alone | Yes; no | Not reported | Tissue engineering strategy: greater mean root coverage No statistically significant difference (P > 0.05) for the other clinical parameters |
| McGuire et al43 | Tissue engineering strategy vs connective tissue graft | Yes; no | No statistically significant difference (P > 0.05) for pain and satisfaction/esthetics. Tissue engineering strategy had higher patient preference in case of retreatment | Tissue engineering strategy: lower recession reduction, mean root coverage than connective tissue graft* Tissue engineering strategy: higher probing depth reduction than connective tissue graft* No statistically significant difference (P > 0.05) for the other clinical outcomes |
| McGuire et al44 | Tissue engineering strategy vs connective tissue graft | Yes; no | No statistically significant difference (P > 0.05) for satisfaction/esthetics | Tissue engineering strategy: lower recession reduction, mean root coverage, keratinized tissue width gain, and clinical attachment level gain than connective tissue graft* EstheEsthetics outcomes not statistically significantly different (P > 0.05) |
| Milinkovic et al65 | Tissue engineering strategy vs connective tissue graft | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) for mean root coverage, clinical attachment level, and professional esthetic outcomes (root coverage esthetic score) TissuTissue engineering strategy: less keratinized tissue width gain than connective tissue graft* |
| Pourabbas et al222 | Tissue engineering strategy vs scaffold alone | Yes; no | Not reported | No statistically significant difference (P > 0.05) |
| Rocha Dos Santos et al228 and Sangiorgio et al135 | Tissue engineering strategy vs scaffold alone vs coronally advanced flap vs coronally advanced flap + enamel matrix derivative | Yes; not reported | No statistically significant difference (P > 0.05) in reduction of hypersensitivity and esthetic outcomes Tissue engineering strategy: better impact on oral health-related quality of life than coronally advanced flap,* but not statistically significantly different (P > 0.05) with scaffold alone and coronally advanced flap + enamel matrix derivative |
TissuTissue engineering strategy: higher mean root coverage than coronally advanced flap*
No statistically significant difference (P > 0.05) mean non-root coverage soft tissue augmentation engineering strategy and scaffold alone No statistically significant difference (P > 0.05) in esthetic outcomes Tissue engineering strategy: higher keratinized tissue width gain than coronally advanced flap and coronally advanced flap + enamel matrix derivative* Not statistically significant difference (P > 0.05) keratinized tissue width gain between tissue engineering strategy and scaffold alone |
| Shin et al238 | Tissue engineering strategy vs scaffold alone | Yes; no | No statistically significant difference (P > 0.05) for pain | Tissue engineering strategy: higher keratinized tissue width gain than scaffold alone*
No statistically significant difference (P > 0.05) for the other clinical outcomes |
| Tavelli et al241 | Tissue engineering strategy vs scaffold alone | Yes; no | Tissue engineering strategy: lower morbidity and time to recovery than scaffold alone No statistically significant difference (P > 0.05) for esthetic outcomes, satisfaction, and reduction of hypersensitivity |
Tissue engineering strategy: higher mean root coverage, complete root coverage, gingival thickness gain than scaffold alone and professional esthetic outcomes (root coverage esthetic score) than scaffold alone* |
| Wilson et al61 | Tissue engineering strategy vs connective tissue graft | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) between tissue engineering strategy and connective tissue graft |
| Zanwar et al250 | Tissue engineering strategy vs connective tissue graft | Yes; no | Not reported | No statistically significant difference (P > 0.05) between tissue engineering strategy and connective tissue graft for mean root coverage and complete root coverage Tissue engineering strategy: lower keratinized tissue width gain* |
| Zanwar et al249 | Tissue engineering strategy vs scaffold alone | Yes; no | Not reported | Tissue engineering strategy: higher mean root coverage than scaffold alone*
No statistically significant difference (P > 0.05) for the other clinical outcomes |
Statistically significant difference (P < 0.05).
TABLE 5.
Characteristics of the clinical trials utilizing (cell-based) tissue engineering strategies for non-root coverage soft-tissue augmentation
| Reference | Study design | Cell type | Origin | Cells culture medium | Scaffold | Control group |
|---|---|---|---|---|---|---|
| McGuire and Nunn59 | Split-mouth randomized controlled trial | Allogeneic fibroblast | Newborn foreskin | Not reported | Bioabsorbable Polyglactin mesh | Free gingival graft |
| McGuire et al36 | Split-mouth randomized controlled trial | Allogeneic fibroblast and keratinocytes | Newborn foreskin | Not reported | Collagen membrane | Free gingival graft |
| McGuire et al38 | Split-mouth randomized controlled trial | Allogeneic fibroblast and keratinocytes | Newborn foreskin | Agarose-rich nutrient medium | Collagen membrane | Free gingival graft |
| Mohammadi et al63 | Split-mouth randomized controlled trial | Autogenous fibroblasts | Attached gingiva | NutritNutritional medium containing AB human serum and antibiotics (penicillin and streptomycin) | Collagen scaffold | Periosteal fenestration technique |
TABLE 6.
Safety, patient-reported outcomes, and clinical results of the clinical trials utilizing tissue engineering strategies for non-root coverage soft-tissue augmentation
| Reference | Comparison | Safety; complications | Patient-reported outcome measures | Clinical outcomes |
|---|---|---|---|---|
| McGuire and Nunn59 | Free gingival graft | Yes; no | No statistically significant difference (P > 0.05) | Tissue engineering strategy: lower keratinized tissue width gain than free gingival graft* Tissue engineering strategy: better color match and tissue texture* |
| McGuire et al36 | Free gingival graft | Yes; no | Tissue engineering strategy: less pain and sensitivity of treatment than free gingival graft* Tissue engineering strategy: higher patient preference than free gingival graft* |
Tissue engineering strategy: lower keratinized tissue width gain than free gingival graft* Tissue engineering strategy: better color match and tissue texture* |
| McGuire et al38 | Free gingival graft | Yes; no | No statistically significant difference (P > 0.05) for pain Tissue engineering strategy: higher patient preference and esthetic outcomes than free gingival graft* |
Tissue engineering strategy: lower keratinized tissue width gain than free gingival graft* Tissue engineering strategy: better color match and tissue texture* |
| Mohammadi et al63 | Periosteal fenestration technique | Yes; no | Not reported | Tissue engineering strategy: higher keratinized tissue width gain* |
Statistically significantly difference (P < 0.05).
TABLE 7.
Characteristics of the clinical trials utilizing tissue-engineering strategies for alveolar ridge preservation
| Cell-based tissue engineering | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reference | Defect type | Cell type | Origin | Scaffold | Control group | Flap, complete closure |
| Kaigler et al191 | Not reported | Autogenous stem and progenitor cells | Bone marrow from iliac crest | Gelatin sponge (+ collagen membrane) | Gelatin sponge (+ collagen membrane) | Flaps raised, primary closure |
| Signaling molecule-based tissue engineering | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reference | Defect type | Biologic | Scaffold | Membrane | Control group | Flap, complete closure |
| Coomes et al160 | Buccal bone dehiscence ≥50% | Recombinant human bone morphogenetic protein-2 | Collagen sponge | No | Collagen sponge | FlaplFlapless, no primary closure |
| Fiorellini et al173 | Buccal bone dehiscence ≥50% | Recombinant human bone morphogenetic protein-2 | Collagen sponge | No | Group 1: collagen sponge Group 2: spontaneous healing |
Flaps raised, primary closure |
| Geurs et al176 and Ntounis et al217 | Not reported | Recombinant human platelet-derived growth factor-BB | Freeze-dried bone allograft/β-tricalcium phosphate | No | Group 1: collagen plug Group 2: freeze-dried bone allograft/β-tricalcium phosphate + collagen plug Group 3: freeze-dried bone allograft/β-tricalcium phosphate + platelet-rich plasma + collagen plug | FlaplFlapless, no primary closure |
| Huh et al183 | Teeth to be extracted with <50% alveolar vertical bone loss | Recombinant human bone morphogenetic protein-2 | Hydroxyapatite/β-tricalcium phosphate | No | Hydroxyapatite/β-tricalcium phosphate | Not reported, not reported |
| Jo et al188 | Teeth to be extracted with >50% alveolar bone height | Recombinant human bone morphogenetic protein-2 | Collagen sponge | Bioabsorbable collagen membrane | No control group | FlapsFlaps raised, no primary closure |
| Recombinant human bone morphogenetic protein-2 | Hydroxyapatite/β-tricalcium phosphate | Bioabsorbable collagen membrane | No control group | |||
| Kim et al196 | Residual socket with <50% bone loss | Recombinant human bone morphogenetic protein-2 | Demineralized bone matrix gel | Bioabsorbable collagen membrane | Demineralized bone matrix gel | FlapsFlaps raised, no primary closure |
| Lee and Jeong199 | Residual socket with <50% bone loss | Enamel matrix derivative | Demineralized bovine bone matrix | Bioabsorbable collagen membrane | Group 1: demineralized bovine bone matrix + bioabsorbable collagen membrane Group 2: spontaneous healing | FlaplFFlapless, no primary closure |
| Lee et al200 | Buccal bone dehiscence ≥50% | Enamel matrix derivative | Xenogeneic bone mineral containing collagen | Bioabsorbable collagen membrane | Xenogeneic bone mineral containing collagen + bioabsorbable collagen membrane | Flapless, primary closure |
| McAllister et al208 | Not reported | Recombinant human platelet-derived growth factor-BB | Xenogeneic bone mineral containing collagen | No | No control group | Flaps raised, pediculated palatal connective tissue graft used for primary closure |
| Recombinant human platelet-derived growth factor-BB | β-Tricβ-Tricalcium phosphate | No | No control group | |||
| Mercado et al209 | Buccal bone dehiscence ≤1 mm at the time of extraction, no palatal defect | Enamel matrix derivative | Xenogeneic bone mineral containing collagen | No | Xenogeneic bone mineral containing collagen | Flapless, free gingival graft used for primary closure |
| Nevins et al215 | Not reported | Recombinant human platelet-derived growth factor-BB | Xenogeneic bone mineral containing collagen | No | Xenogeneic bone mineral containing collagen | Flaps Flaps raised, primary closure |
| Enamel matrix derivative | Xenogeneic bone mineral containing collagen | No | Xenogeneic bone mineral containing collagen | |||
| Enamel matrix derivative | BoneBone ceramic | No | Xenogeneic bone mineral containing collagen | |||
| Shim et al237 | Not reported | Recombinant human bone morphogenetic protein-2 | HydroHydroxyapatite | No | Demineralized bovine bone matrix | FlapsFlaps raised, primary closure |
TABLE 8.
Safety, patient-reported outcomes, and clinical, radiographic, and histomorphometric results of the clinical trials utilizing tissue engineering strategies for alveolar ridge preservation
| Reference | Comparison | Safety; complications | Patient-reported outcome measures | Clinical, radiographic, and histomorphometric outcomes |
|---|---|---|---|---|
| Coomes et al160 | Recombinant human bone morphogenetic protein-2 + collagen sponge vs collagen sponge | Yes; mild erythema and localized swelling in the tissue engineering strategy group | Not reported | Tissue engineering strategy: higher buccal plate regeneration, clinical ridge width, and radiographic ridge width (statistically significant difference, P < 0.05) Tissue engineering strategy: less buccal bone dehiscence (statistically significant difference, P <0.05) Tissue engineering strategy: less implants needed additional bone augmentation (statistically significant difference, P <0.05) |
| Fiorellini et al173 | 0.75 or 1.5mg/mL recombinant human bone morphogenetic protein-2 + collagen sponge vs collagen sponge vs spontaneous healing | Yes; more cases with edema and erythema in the tissue engineering strategy groups | Pain, no statistically significant different (P > 0.05) | Tissue engineering strategy: greater bone augmentation than control groups (statistically significant difference, P <0.05) Tissue engineering strategy: less sites requiring augmentation than control (statistically significant difference, P <0.05) No evidence of inflammation or residual collagen matrix from the absorbable sponge carrier |
| Geurs et al176 and Ntounis et al217 | Recombinant human platelet-derived growth factor-BB + freeze-dried bone allograft/β-tricalcium phosphate vs freeze-dried bone allograft/β-tricalcium phosphate vs freeze-dried bone allograft/β-tricalcium phosphate + platelet-rich plasma vs collagen plug | Yes, not reported | Not reported | Not reported Tissue engineering strategy showed the least amount of residual bone particles (statistically significant difference, P < 0.05) Tissue engineering strategy: more organic matrix than bone graft alone (statistically significant difference, P < 0.05) |
| Huh et al183 | Recombinant human bone morphogenetic protein-2 + hydroxyapatite/β-tricalcium phosphate vs hydroxyapatite/β-tricalcium phosphate | Yes; not reported | Not reported | Tissue engineering strategy: less bone remodeling (height and width) than control (statistically significant difference, P <0.05) |
| Jo et l188 | Recombinant human bone morphogenetic protein-2 + collagen sponge + bioabsorbable collagen membrane vs recombinant human bone morphogenetic protein-2 + hydroxyapatite/β-tricalcium phosphate + bioabsorbable collagen membrane |
Yes; no statistically significant difference (P > 0.05) | Pain, no statistically significant difference (P > 0.05) | No statistically significant difference (P >0.05) |
| Kaigler et al191 | Autogenous mesenchymal stem cells + gelatin sponge + bioabsorbable collagen membrane vs gelatin sponge + bioabsorbable collagen membrane | Yes; no statistically significant difference (P > 0.05) | Not reported | Tissue engineering strategy: greater radiographic bone fill at 6weeks (statistically significant difference, P < 0.05) Tissue engineering strategy: sixfold decrease in implant bony dehiscence (statistically significant difference, P < 0.05) and less sites needed additional bone grafting at implant placement (statistically significant difference, P < 0.05) Tissue engineering strategy: higher vascularity. No statistically significant difference (P > 0.05) for bone volume fraction and bone mineral density |
| Kim et al196 | Recombinant human bone morphogenetic protein-2 + demineralized bone matrix gel + bioabsorbable collagen membrane vs demineralized bone matrix gel | Yes; no statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) in terms of radiographic outcomes |
| Lee and Jeong199 | enamel matrix derivative + demineralized bovine bone matrix vs demineralized bovine bone matrix + bioabsorbable collagen membrane vs spontaneous healing |
Yes; no statistically significant difference (P > 0.05) | Pain, no statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) in tissue engineering strategy and bone graft groups |
| Lee et al200 | Enamel matrix derivative + xenogeneic bone mineral containing collagen + bioabsorbable collagen membrane vs xenogeneic bone mineral containing collagen + bioabsorbable collagen membrane | Yes; not reported | Pain, no statistically significant difference (P > 0.05) Tissue engineering strategy: lower duration of pain and swelling (statistically significant difference, P < 0.05) |
No statistically significant difference (P > 0.05) in clinical and radiographic outcomes |
| McAllister et al208 | Recombinant human platelet-derived growth factor-BB + xenogeneic bone mineral containing collagen vs recombinant human platelet-derived growth factor-BB +β-tricalcium phosphate | Not reported | Not reported | No statistically significant difference (P > 0.05) |
| Mercado et al209 | Enamel matrix derivative + xenogeneic bone mineral containing collagen vs xenogeneic bone mineral containing collagen | Yes; no statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) for clinical and radiographic outcomes Tissue engineering strategy: higher new bone formation and lower residual graft particles than control group (statistically significant difference, P < 0.05) Control group showed higher soft tissue and marrow space than tissue engineering strategy (statistically significant difference, P < 0.05) |
| Nevins et al215 | Recombinant human platelet-derived growth factor-BB + xenogeneic bone mineral containing collagen vs enamel matrix derivative + xenogeneic bone mineral containing collagen vs enamel matrix derivative + bone ceramic vs xenogeneic bone mineral containing collagen | Yes; no | Not reported | Not reported Tissue engineering strategy: highest amount of new bone formation but no statistically significant difference (P > 0.05) |
| Shim et al237 | Recombinant human bone morphogenetic protein-2 + hydroxyapatite vs demineralized bovine bone matrix |
Yes; no | Not reported | Tissue engineering strategy: superior outcomes in alveolar bone width changes (statistically significant difference, P < 0.05) Tissue engineering strategy: higher percentage of new bone formation (statistically significant difference, P < 0.05) |
TABLE 9.
Characteristics of the clinical trials utilizing tissue engineering strategies for staged bone augmentation
| Cell-based tissue engineering | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Reference | Ridge Defect type | Cell type | Origin | Cells culture medium | Scaffold | Control group |
| Bajestan et al31 | Horizontal | Autogenous stem cells | Bone marrow from iliac crest | Iscove’s modified Dulbecco’s medium with 10% fetal bovine serum, 10% horse serum, and hydrocortisone | β-Tricalcium phosphate | Autogenous block graft from ramus or symphysis |
| Signaling molecule-based tissue engineering | |||||
|---|---|---|---|---|---|
|
| |||||
| Reference | Ridge Defect | Biologic | Scaffold | Combination biologic and scaffold | Control group |
| De Freitas et al32 De Freitas et al164 | Horizontal | Recombinant human bone morphogenetic protein-2 | Absorbable collagen sponge (+ titanium mesh) | Extraorally | Particulated autogenous bone (+ titanium mesh) |
| Marx et al207 | Horizontal and vertical | Recombinant human bone morphogenetic protein-2 | Absorbable collagen sponge + freeze-dried bone allograft + platelet-rich plasma (+ titanium mesh) | Extraorally | Autogenous bone graft (+ titanium mesh) |
| Nevins et al216 | Horizontal and vertical | Recombinant human platelet-derived growth factor-BB | Demineralized bovine bone matrix (+ bioabsorbable collagen membrane) | Extraorally | No control group |
| Recombinant human platelet-derived growth factor-BB | Equine bone matrix (+ bioabsorbable collagen membrane) | Extraorally | |||
| Santana and Santana230 | Horizontal | Recombinant human platelet-derived growth factor-BB | Hydroxyapatite/β-tricalcium phosphate (+ nonresorbable membrane) | Extraorally | Autogenous bone block |
| Thoma et al 33 Thoma et al243 |
Horizontal | Recombinant human bone morphogenetic protein-2 | Xenogeneic bone block (+ bioabsorbable collagen membrane) | Extraorally | Autogenous bone block + demineralized bovine bone matrix (+ bioabsorbable collagen membrane) |
TABLE 10.
Safety, patient-reported outcomes, and clinical and histomorphometric results of the clinical trials utilizing tissue engineering strategies for staged bone augmentation
| Reference | Comparison | Safety; complications | Patient-reported outcome measures | Clinical and histomorphometric outcomes |
|---|---|---|---|---|
| Bajestan et al31 | Stem cells + β-tricalcium phosphate vs autogenous block graft | Yes; similar | Two patients of each group reported significant discomfort and other two from both groups reported interference with their daily activities* Similar satisfaction and willingness of retreatment* |
Tissue engineering strategy: lower bone width gain*
and less sites allowing for implant placement compared with control group* Not reported |
| De Freitas et al32 De Freitas et al164 | Recombinant human bone morphogenetic protein-2/acellular collagen sponge vs autogenous bone graft | Yes; more swelling and erythema in the tissue engineering strategy group (no statistically significant difference, P > 0.05) | Temporary discomfort and pain from the donor site (control group) | Tissue engineering strategy: higher radiographic horizontal bone gain, not statistically significant difference (P > 0.05) in the other clinical and radiographic parameters Tissue engineering strategy: bone marrow rich in capillaries, undifferentiated cells and bone lining cells (statistically significant difference, P < 0.05). Control group showed higher amount of non-vital bone particles trapped in lamellar vital bone (statistically significant difference, P < 0.05) |
| Marx et al207 | Recombinant human bone morphogenetic protein-2/acellular collagen sponge + freeze-dried bone allograft + platelet-rich plasma vs autogenous bone graft |
Yes; similar | Tissue engineering strategy: lower days of analgesics (P = 0.05), higher post-op edema at days 3, 8, and 15 (statistically significant difference, P < 0.05) Tissue engineering strategy: less operative time (statistically significant difference, P < 0.05) |
No statistically significant difference (P > 0.05) in terms of presence of adequate bone for implant placement and percentage of implant osseointegration Tissue engineering strategy: higher vascular density blood vessels than autogenous graft (P = 0.05) No statistically significant difference (P > 0.05) for the other outcomes |
| Nevins et al216 | Recombinant human platelet-derived growth factor-BB + demineralized bovine bone matrix + collagen membrane vs recombinant human platelet-derived growth factor-BB + EBM + collagen membrane | Yes; no | Not reported | Not reported New bone formation in close association with graft particles. No signs of inflammatory cell infiltration or foreign body reaction |
| Santana and Santana230 | Recombinant human platelet-derived growth factor-BB + hydroxyapatite/β-tricalcium phosphate + nonresorbable membrane vs autogenous block graft | Yes; similar | Not reported | No statistically significant difference (P > 0.05) Not reported |
| Thoma et al 2018,33 Thoma et al 2019243 | Recombinant human bone morphogenetic protein-2 + xenogeneic bone block vs autogenous bone block | Yes; 1 patient had exposure of the autogenous block (no statistically significant difference, P > 0.05) | Tissue engineering strategy: less pain during the surgery (statistically significant difference, P < 0.05). No statistically significant difference (P > 0.05) for the other patient-reported outcome measures | No statistically significant difference (P > 0.05) Similar radiographic (cone-beam computed tomography) and three-dimensional volumetric changes (no statistically significant difference, P > 0.05) Tissue engineering strategy: lower mineralized tissue compared with the control group (statistically significant difference, P < 0.05) |
Only descriptive statistics were performed.
TABLE 11.
Characteristics of the clinical trials utilizing tissue engineering strategies for sinus floor augmentation
| Cell-based tissue engineering | |||||
|---|---|---|---|---|---|
|
| |||||
| Reference | Cells type | Origin | Stem cells isolation and expansion | Scaffold | Control group |
| De Oliveira et al166 | Autogenous stem cells | Bone marrow from iliac crest | Cells obtained from bone marrow aspirate using a centrifugation system. Cells not expanded | Demineralized bovine bone matrix | Demineralized bovine bone matrix |
| Gonshor et al87 | Allogeneic stem cells | Cadavers (recovered within 24 h of death) | Not reported | Allograft cellular bone matrix | Allogeneic bone graft |
| Hermund et al179 | Autogenous bone cells | Maxillary tuberosity | Cell cultivated and expanded for 1 month | Autogenous bone + demineralized bovine bone matrix | Autogenous bone + demineralized bovine bone matrix |
| Kaigler et al67 | Autogenous stem cells | Bone marrow from iliac crest | Cell cultivated and expanded for 12days |
β-Tricalcium phosphate | β-Tricalcium phosphate |
| Pasquali et al79 | Autogenous stem cells | Bone marrow from iliac crest | Cells obtained from bone marrow aspirate using a centrifugation system. Cells not expanded | Demineralized bovine bone matrix | Demineralized bovine bone matrix |
| Payer et al68 | Autogenous stem cells | Bone marrow from tibia | Cells not expanded. Bone marrow aspirate immediately loaded into the scaffold | Demineralized bovine bone matrix | Demineralized bovine bone matrix |
| Rickert and coworkers225,226 |
Autogenous stem cells | Bone marrow from iliac crest | Cells obtained from bone marrow aspirate using a centrifugation system. Cells not expanded | Demineralized bovine bone matrix | Autogenous bone + demineralized bovine bone matrix |
| Sauerbier et al69 | Autogenous stem cells | Bone marrow from pelvic bone | Cells obtained from bone marrow aspirate using a centrifugation system. Cells not expanded | Demineralized bovine bone matrix | Autogenous bone + demineralized bovine bone matrix |
| Whitt et al246 | Allogeneic stem cells | Cadavers | Not reported | Allograft cellular bone matrix | Allogeneic bone graft |
| Wildburger et al82 | Autogenous stem cells | Bone marrow from iliac crest | Cells obtained from bone marrow aspirate using a centrifugation system. Cells not expanded | Demineralized bovine bone matrix | Demineralized bovine bone matrix |
| Signaling molecule-based tissue engineering | ||||
|---|---|---|---|---|
|
| ||||
| Reference | Biologic | Scaffold | Combination biologic and scaffold | Control group |
| Boyne et al156 | Recombinant human bone morphogenetic protein-2 | Collagen sponge | Extraorally | Autogenous bone graft with or without allogeneic bone graft |
| Corinaldesi et al161 | Recombinant human bone morphogenetic protein-7 | Demineralized bovine bone matrix | Extraorally | Demineralized bovine bone matrix |
| Froum et al174 | Recombinant human bone morphogenetic protein-2 | Collagen sponge + allogeneic bone graft | Extraorally | Allogeneic bone graft |
| Froum et al175 | Recombinant human platelet-derived growth factor-BB | Demineralized bovine bone matrix | Extraorally | Demineralized bovine bone matrix |
| Kao et al192 | Recombinant human bone morphogenetic protein-2 | Collagen sponge + demineralized bovine bone matrix | Extraorally | Demineralized bovine bone matrix |
| Kim et al195 | Recombinant human bone morphogenetic protein-2 | Biphasic calcium phosphate | Extraorally | Demineralized bovine bone matrix |
| Kim et al194 | Recombinant human bone morphogenetic protein-2 | Hydroxyapatite | Extraorally | Demineralized bovine bone matrix |
| Koch et al,197 Stavropoulos et al239 | Recombinant human growth and differentiation factor-5 | β-Tricalcium phosphate | Extraorally | Autogenous bone + β-tricalcium phosphate |
| Triplett et al244 | Recombinant human bone morphogenetic protein-2 | Collagen sponge | Extraorally | Autogenous bone |
| Vincent-Bugnas et al101 | Enamel matrix derivative | Demineralized bovine bone matrix | Extraorally | Demineralized bovine bone matrix |
TABLE 12.
Safety, patient-reported outcomes, clinical and histomorphometric results of the clinical trials utilizing tissue-engineering strategies for sinus floor augmentation
| Reference | Comparison | Safety; complications | Patient-reported outcome measures | Clinical and histomorphometric outcomes |
|---|---|---|---|---|
| Boyne et al156 | 0.75 or 1.5 mg/mL recombinant human bone morphogenetic protein-2 + collagen sponge vs autograft with or without allograft | Yes; more edema and rash in the control group (statistically significant difference, P < 0.05) | More patients in pain in the control group (statistically significant difference, P < 0.05) | Tissue engineering strategy: lower bone width gain than control group (statistically significant difference, P < 0.05) Unambiguous bone induction by recombinant human bone morphogenetic protein-2 No differences among the groups |
| Corinaldesi et al161 | Recombinant human bone morphogenetic protein-7 + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) More new bone on the control side than the tissue engineering strategy side (statistically significant difference, P < 0.05) |
| De Oliveira et al166 | Autogenous mesenchymal stem cells + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) No statistically significant difference (P > 0.05) |
| Froum et al174 | Recombinant human bone morphogenetic protein-2 + allograft vs allograft | Yes; not reported | Not reported | Not reported No statistically significant difference (P > 0.05) |
| Froum et al175 | Recombinant human platelet-derived growth factor-BB + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | Not reported Tissue engineering strategy: higher amount of vital bone (statistically significant difference, P < 0.05) at 4–5 months. Higher residual graft in the control group than tissue engineering strategy (statistically significant difference, P < 0.05) |
| Gonshor et al87 | Allogeneic mesenchymal stem cells + allograft vs allograft | Yes; not reported | Not reported | Not reported Tissue engineering strategy: higher vital bone content and lower residual graft content (statistically significant difference, P < 0.05) |
| Hermund et al179 | Autogenous bone cells + autograft + demineralized bovine bone matrix vs autograft + demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) No statistically significant difference (P > 0.05) |
| Kaigler et al67 | Autogenous mesenchymal stem cells + β-tricalcium phosphate vs β-tricalcium phosphate | Yes; no statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) in pain, quality of life assessment, and satisfaction | No statistically significant difference (P > 0.05) in clinical outcomes Tissue engineering strategy: higher bone density (statistically significant difference, P < 0.05) Tissue engineering strategy: in the most severe deficiencies, greater bone volume fraction than control (statistically significant difference, P < 0.05) |
| Kao et al192 | Recombinant human bone morphogenetic protein-2 + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | Not reported Less new bone formation and less residual bone graft particles in the tissue engineering strategy sites than control sites (statistically significant difference, P < 0.05) |
| Kim et al195 | Recombinant human bone morphogenetic protein-2 + biphasic calcium phosphate vs demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) | Not reported | No statistically significant difference (P > 0.05) for clinical, radiographic, and volumetric outcomes No statistically significant difference (P > 0.05) |
| Kim et al194 | Recombinant human bone morphogenetic protein-2 + hydroxyapatite vs demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) | Not reported | Not reported Tissue engineering strategy: Higher new bone formation (statistically significant difference, P < 0.05) |
| Koch et al 2010,197 Stavropoulos et al 2011239 | Recombinant human growth and differentiation factor-5 + β-tricalcium phosphate vs autograft + β-tricalcium phosphate | Yes; no statistically significant difference (P > 0.05) | Additional pain and swelling in the control group due to autogenous bone harvesting | Not reported No statistically significant difference (P > 0.05) |
| Pasquali et al79 | Autogenous mesenchymal stem cells + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) Tissue engineering strategy: higher amount of vital mineralized tissue and higher level of bone particles resorption (statistically significant difference, P < 0.05) |
| Payer et al68 | Autogenous mesenchymal stem cells + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | No statistically significant difference (P > 0.05) in pain, painkiller consumption, satisfaction | No statistically significant difference (P > 0.05) No statistically significant difference (P > 0.05) |
| Rickert et al 2011225 and Rickert et al 2014226 | Autogenous mesenchymal stem cells + demineralized bovine bone matrix vs Autograft + demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) | No statistically significant difference (P > 0.05) Tissue engineering strategy: significantly more bone formation (statistically significant difference, P < 0.05) |
| Sauerbier et al69 | AutogAutogenous mesenchymal stem cells + demineralized bovine bone matrix vs autograft + demineralized bovine bone matrix | Yes; no statistically significant difference (P > 0.05) Two patients of the control group developed infection of the donor site |
No statistically significant difference (P > 0.05) | Tissue engineering strategy: higher radiological gain and persistence of augmented bone height (statistically significant difference, P < 0.05) No statistically significant difference (P > 0.05) for new bone formation Tissue engineering strategy: higher fraction of residual graft (statistically significant difference, P < 0.05) |
| Triplett et aI244 | Recombinant human bone morphogenetic protein-2 + collagen sponge vs autograft | Yes; implant failure for control group twice that of tissue engineering strategy (no statistically significant difference, P > 0.05) Tissue engineering strategy: more facial edema than control group (statistically significant difference, P < 0.05). Control group: 17% sensory loss from donor site at 6months. Also pain and gait disturbance in the long term at the donor site |
Not reported | No statistically significant difference (P > 0.05) for bone gain, but control group showed higher bone density (statistically significant difference, P < 0.05) No marked differences between the groups |
| Vincent-Bugnas et al101 | Enamel matrix derivative + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) Tissue engineering strategy: higher amount of newly formed bone than control sites (statistically significant difference, P < 0.05) |
| Whitt et al246 | Allogeneic mesenchymal stem cells + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | No statistically significant difference (P > 0.05) Tissue engineering strategy: higher vital bone than control group (statistically significant difference, P < 0.05) |
| Wildburger et al82 | Autogenous mesenchymal stem cells + demineralized bovine bone matrix vs demineralized bovine bone matrix | Yes; not reported | Not reported | Not reported No statistically significant difference (P > 0.05) |
TABLE 13.
Characteristics of the clinical trials utilizing tissue engineering strategies for peri-implant bone augmentation
| Reference | Clinical condition | Biologic | Scaffold | Combination biologic and scaffold | Control group |
|---|---|---|---|---|---|
| Amorfini et al150 | Implant placement in healed ridge requiring horizontal bone augmentation | Recombinant human platelet-derived growth factor-BB | Guided bone regeneration (autogenous bone chips mixed with demineralized bovine bone matrix + collagen membrane) | Extraorally | Guided bone regeneration (autogenous bone chips mixed with demineralized bovine bone matrix + collagen membrane) without the growth factor |
| Recombinant human platelet-derived growth factor-BB | Corticocancellous allograft block (+ collagen membrane) | Extraorally | Corticocancellous allograft block (+ collagen membrane) without the growth factor | ||
| Santana et al231 | Immediate implant with buccal bone defects | Recombinant human platelet-derived growth factor-BB | β-Tricalcium phosphate | Extraorally | Implants placed in healed ridges |
| Jung et al 2003,189 Jung et al 2009,190 Jung et al 2022147 | Implant placement in healed ridge requiring bone augmentation | Recombinant human bone morphogenetic protein-2 | Demineralized bovine bone matrix (+ collagen membrane) | Extraorally | Demineralized bovine bone matrix + collagen membrane |
4.1 |. Risk of bias assessment
Sixty-seven randomized controlled trials were considered having a low risk of bias,32,36,38,43,44,59,60,65,72,82,135,147,162,164,165,170,173,176,177,180,182,197,199,200,206,207,220,225,226,228,229,232,233,244,245,247,48,251,151–153,158–160,186–19,1,193–195,212–218,240–243,67–69 54 trials were as signed an unclear risk of bias, 31,33,61,63,64,71,79,87,101,161,163,172,174,175,178,179,181,183,185,192,198,208,209,227,230,231,234,235,242,243,246,148–150,154–157,166–169,201–205,219–221,223–226 and the remaining seven studies showed a high risk of bias.171,184,196,236,237,249,250
4.2 |. Tissue engineering strategies for periodontal reconstruction
4.2.1 |. Tissue engineering strategies for infrabony and furcation defects
4.2.1.1 |. Characteristics of the studies included
Tissue engineering strategies for the regenerative treatment of periodontal infrabony and furcation defects are summarized in Table 1. Among the 59 trials included, 56 reported the surgical outcomes of infrabony defects71,72,148,149,158,159,162,165,167,177,178,184,186,187,193,198,218,219,221,224,227,229,240,242,245,247,248,251,151–155,169–172,180–182,201–206,212–216,234–238 and three randomized controlled trials included furcation defects only.185,220,223 Cell-based tissue engineering strategies for the treatment of infrabony defects were employed in eight trials.71,72,148,152,158,170,236,248 Forty-eight randomized controlled trials utilized signaling molecule–based tissue engineering strategies for the regenerative therapy of periodontal infrabony defects.149,151,159,162,165,167,169,171,172,177,178,184,186,187,193,198,218,219,221,224,227,229,240,242,245,247,251,153–155,180–182,201–206,212–216,234–237 Enamel matrix derivative + bone graft was the most frequently performed approach (30 study arms), followed by recombinant human platelet–derived growth factor-BB + bone graft or a carrier (18 study arms) and recombinant human fibroblast–growth factor-2 + bone graft or a carrier (four study arms). Signaling molecule–based tissue engineering strategies for the treatment of furcation defects were reported in three randomized controlled trials where enamel matrix derivative was utilized in combination with bone graft as a scaffold.185,220,223 The characteristics of the aforementioned randomized controlled trials are presented in detail in Table 1 and Appendix S1.
4.2.1.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies.
Few of the randomized controlled trials included reported postoperative morbidity following periodontal regeneration.72,149,153,162,182,186,187,201,205,213,233 Among them, only Lee et al demonstrated a statistically significant difference between tissue engineering strategies and conventional treatments, with reductions in swelling, pain severity, and duration observed in the tissue engineering strategy group.201 The other studies did not observe a significant difference between tissue engineering strategies and conventional approaches for postoperative pain, analgesic consumption, or self-reported swelling. Surgical time was found to be significantly shorter for the control group over tissue engineering strategies.153,162 No studies observed significant differences in complications, adverse events, or wound healing outcomes between tissue engineering strategies and conventional treatments.72,153,158,162,172,177,178,182,186,201,205,213,221,240 Quality of life and treatment satisfaction were shown to be comparable between tissue engineering strategies and conventional regenerative therapies.72,229
4.2.1.3 |. Clinical outcomes
Overall, no studies reported superior results for conventional therapies over tissue engineering strategies, which were found to promote either comparable or even superior outcomes than conventional techniques for the treatment of infrabony defects.
Nine randomized controlled trials showed clinical and radiographic outcomes statistically significantly in favor of tissue engineering strategies over conventional approaches involving bone graft alone.154,159,186,193,206,213,214,242,248 Among them, six studies utilized recombinant human platelet–derived growth factor-BB in combination with a bone scaffold as a tissue engineering strategy, that was found to be significantly superior to bone graft alone186,193,206,213,214,242 (Table 2).
On the other hand, six studies observed similar outcomes between tissue engineering strategy and bone graft alone.72,181,201,204,232,234 Interestingly, five of them involved the use of enamel matrix derivative as a biologic agent,181,201,204,232,234 and one study employed a cell-based tissue engineering strategy with autologous mesenchymal stem cells from the periodontal ligament.72 When tissue engineering strategies were compared with guided tissue regeneration procedures, two studies observed greater benefits in favor of tissue engineering strategies,148,169 and three randomized controlled trials obtain similar outcomes between the two groups.158,177,184
Eight trials demonstrated that tissue engineering strategies led to greater advantages than flap alone,71,152,155,165,167,170,182,236 but five studies did not support this statement.153,162,211,224,240
When tissue engineering strategies were tested against biologic agents alone for the treatment of infrabony defects, nine studies exhibited significantly higher outcomes in the tissue engineering strategy group, and another nine trials showed similar results.155,162,180,187,205,210,212,221,235
Regarding furcation defects, two studies did not observe benefits in favor of tissue engineering strategies compared with conventional treatments,220,223 but one trial obtained a statistically significantly superior clinical attachment level gain and pocket depth reduction after 1 year for mandibular furcations treated with tissue engineering strategies compared with guided tissue regeneration and flap alone.185 The tissue engineering strategy group was also the treatment with the highest number of cases presenting complete closure of the furcation defect at 1 year.185
4.2.1.4 |. Clinical recommendations
Tissue engineering strategies are safe treatment options for the treatment of periodontal infrabony and furcation defects. When compared with conventional therapies, tissue engineering strategies have demonstrated either similar or superior clinical and radiographic outcomes. In particular, it appears that tissue engineering strategies involving the use of recombinant human platelet–derived growth factor-BB have the greatest probability in providing significantly enhanced outcomes compared with bone grafts alone. There is no evidence supporting the efficacy of tissue engineering strategies in reducing complications/adverse events and postoperative morbidity, and overall, in improving patient-reported outcome measures.
4.2.2 |. Tissue engineering strategies for root coverage procedures
4.2.2.1 |. Characteristics of the studies included
Six trials utilized cell-based tissue engineering approaches for treating gingival recession defects.60,61,64,65,249,250 Human allogeneic fibroblasts, obtained either from the attached gingiva or from the palatal mucosa, were harvested and cultured prior to implantation on a scaffold in three randomized controlled trials.60,64,65 The other three studies utilized human allogeneic cells, either fibroblasts from newborn foreskin or stem cells from the umbilical cord.61,249,250 Signaling molecule–based tissue engineering strategies for root coverage purposes were described in 10 randomized controlled trials.43,44,135,157,163,168,222,228,238,241 The biologic agents utilized were recombinant human platelet–derived growth factor and enamel matrix derivative, that were combined with either acellular dermal matrix, xenogeneic collagen matrix, or β-tricalcium phosphate. The characteristics of the aforementioned randomized controlled trials are presented in detail in Table 3 and Appendix S1.
4.2.2.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies (Table 4).
Four trials evaluated postoperative morbidity using a visual analogue scale.43,163,238,241 Three studies did not observe significant differences among tissue engineering strategies and standard treatments, but one randomized controlled trial showed that subjects allocated to xenogeneic collagen matrix + recombinant human platelet–derived growth factor-BB consistently reported lower pain in the first five postoperative days and a quicker time to recovery (approximately 8–9 days vs 11–12 days) compared with xenogeneic collagen matrix alone241 (Table 4). Tissue engineering strategies did not result in higher patient-reported satisfaction, esthetics, or reduction of dental hypersensitivity, compared with the autogenous connective tissue graft, scaffold alone, or conventional treatments.43,44,228,241 McGuire et al observed a higher patient preference for tissue engineering strategy over connective tissue graft when an additional corrective surgery was needed.43 Another study demonstrated that tissue engineering strategy had a significant positive impact on oral health–related quality of life compared with flap alone, despite the lack of differences between tissue engineering strategy, scaffold alone, and flap + enamel matrix derivative228 (Table 4).
4.2.3 |. Clinical outcomes
4.2.3.1 |. Tissue engineering strategy vs scaffold alone
Eight randomized controlled trials evaluated the outcomes of tissue engineering strategies compared with scaffold alone for root coverage procedures.60,135,157,222,228,238,241,249 Two studies did not find benefits in adding recombinant human platelet–derived growth factor-BB or enamel matrix derivative to acellular dermal matrix compared with acellular dermal matrix alone,157,222 whereas Shin et al observed a significantly higher gain in keratinized tissue for the sites that received acellular dermal matrix in combination with enamel matrix derivative.238 Three randomized controlled trials demonstrated that loading xenogeneic collagen or polylactic acid/polyglycolic acid scaffolds with cells or recombinant human platelet–derived growth factor-BB can result in higher mean root coverage compared with using the scaffold alone.60,241,249 A recent clinical trial also showed that xenogeneic collagen matrix + recombinant human platelet–derived growth factor-BB exhibited higher gingival thickness, as well as an overall volumetric change, compared with xenogeneic collagen matrix alone.241 On the other hand, Sangiorgio et al did not report significant differences in terms of mean and complete root coverage between xenogeneic collagen matrix + enamel matrix derivative and enamel matrix derivative alone.135
4.2.3.2 |. Tissue engineering strategy vs connective tissue graft
Seven randomized controlled trials compared tissue engineering strategies with connective tissue graft.43,44,61,64,65,168,250 The majority of these studies did not find a statistically significant difference in mean root coverage between tissue engineering strategies and connective tissue graft,61,64,65,168,250 whereas McGuire and coworkers observed significantly higher root coverage outcomes at 6 and 60 months for connective tissue graft over bone graft enriched with recombinant human platelet–derived growth factor-BB.43,44 Three studies reported that connective tissue graft obtained significantly more keratinized tissue width than tissue engineering strategies,65,168,250 whereas the others did not observe a statistically significant difference between the two groups.
4.2.3.3 |. Tissue engineering strategy vs other conventional treatments
Three studies assessed the outcomes of tissue engineering strategies compared with other conventional treatments.135,163,168 Overall, it was demonstrated that a tissue engineering strategy obtained higher mean root coverage and gain in keratinized tissue than coronally advanced flap alone135,163,168 (Table 4).
4.2.3.4 |. Clinical recommendations
Tissue engineering strategies are safe treatment options for root coverage procedures. Although they are considered overall less invasive than autogenous grafts (donor site not required), and they may result in less postoperative morbidity and better patient preference, their superiority over conventional treatments in terms of patient-reported outcome measures is weak and needs better exploration. There is evidence that tissue engineering strategies can clinically perform better than flap or scaffolds alone, in terms of mean root coverage, gain in keratinized tissue width, and gain in gingival thickness. Lastly, there are several studies reporting that tissue engineering strategies have the potential to match the mean root coverage of connective tissue graft, although the only long-term trial described opposite findings. More randomized controlled trials reporting patient-reported outcome measures following the use of tissue engineering strategies for root coverage procedures are needed.
4.2.4 |. Tissue engineering strategies for non–root coverage gingival phenotype modification
4.2.4.1 |. Characteristics of the studies included
Four split-mouth randomized controlled trials investigated the outcomes of tissue engineering strategies for non–root coverage gingival phenotype modification36,38,59,63 (Table 5). In three trials, a commercially available tissue-engineering graft consisting of living allogeneic cells from newborn foreskin (fibroblasts alone or in combination with keratinocytes) seeded on a scaffold were utilized,36,38,59 and one study investigated the outcomes of autogenous fibroblasts from the attached gingiva seeded into a collagen scaffold.63 Characteristics of the aforementioned randomized controlled trials are presented in detail in Table 5 and Appendix S1.
4.2.4.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies (Table 6).
Two studies investigated postoperative morbidity.36,38 Whereas the trial of McGuire et al in 2008 showed a significantly lower postoperative pain for the tissue engineering strategy than for an autogenous free gingival graft, this result was not statistically significant for a following trial from the same group in 2011.38 The authors speculated that the split-mouth study design may not have allowed to accurately locate and report the postoperative morbidity.38 Two studies incorporated patient-reported outcome measures when comparing tissue engineering strategies with free gingival graft, showing significantly higher patient preference and esthetic outcomes for the tissue engineering grafts36,38 (Table 6).
4.2.4.3 |. Clinical outcomes
The series of studies from the group of McGuire and coworkers utilized free gingival graft as a control group,36,38,59 which—when pooling the outcomes of the three trials together—showed a mean weighted gingiva gain of 3.11 mm and a mean weighted attached gingiva gain of 3.08 mm. The reported gain in gingiva and attached gingiva for the tissue engineering strategy involving allogeneic fibroblasts was 1.26 mm, on average, for both parameters,59 whereas the living cellular construct combining fibroblasts and keratinocytes showed a mean weighted gingiva gain and attached gingiva gain of 1.69 mm and 1.65 mm, respectively. It is important to mention that McGuire et al concluded that, in 95% of the subjects, this tissue engineering strategy was able to regenerate at least 2 mm of gingiva,38 which is considered a sufficient amount for maintaining periodontal health.38,50,131 Mohammadi et al described a mean gain of 2.8 mm for both gingiva and attached gingiva when using a living cellular construct seeded with autogenous fibroblast.63 These tissue engineering strategies showed minimal changes in pocket depth, recession depth, and clinical attachment level, which is in line with the outcomes reported for free gingival grafts.36,38,59 Tissue engineering strategies were constantly associated with a significantly higher color match and better texture of the soft tissue than with a free gingival graft36,38,59 (Table 6).
4.2.4.4 |. Clinical recommendations
There is evidence that tissue engineering strategies are safe approaches for non–root coverage gingival phenotype modification procedures. They involve a single surgical site only, and they have been related to lower postoperative morbidity and higher patient preference than with autogenous free gingival graft. Professional and patient-reported esthetic evaluation favored tissue engineering strategies over autogenous free gingival graft. Although a significantly lower gain in keratinized tissue width should be expected when utilizing tissue engineering strategies instead of free gingival graft, these alternative approaches are very often able to provide an adequate (2 mm or more) band of gingiva. More randomized controlled trials investigating the use of tissue engineering strategies for non–root coverage gingival phenotype modification are still needed.
4.3 |. Tissue engineering strategies for implant site development
4.3.1 |. Tissue engineering strategies for alveolar ridge preservation
4.3.1.1 |. Characteristics of the studies included
Fourteen randomized controlled trials employed tissue engineering strategies for alveolar ridge preservation.160,173,176,183,188,191,196,199,200,208,209,215,217,237 Inclusion criteria of extraction sockets, as well as surgical intervention (flap vs flapless and primary vs secondary intention healing), was heterogenous among the studies included. Kaigler and coworkers investigated the efficacy of a tissue engineering strategy involving expanded autogenous stem and progenitor cells from iliac crest bone marrow. The other randomized controlled trials utilized signaling molecule–based tissue engineering strategies with recombinant human bone morphogenetic protein-2,160,173,183,188,196,237 recombinant human platelet–derived growth factor-BB,176,208,215,217 or enamel matrix derivative199,200,209 (Table 7).
4.3.1.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies. A higher number of participants presenting mild erythema and localized swelling were observed in the tissue engineering strategy group in two trials160,173 (Table 8).
Four trials investigated morbidity following alveolar ridge preservation.173,188,199,200 These studies did not observe a significant difference between the groups for the severity of postoperative pain.173,188,199,200 However, in one study, the tissue engineering strategy was found to be statistically significantly related to a reduced duration of pain and swelling200 (Table 8).
4.3.1.3 |. Clinical outcomes
Five studies demonstrated statistically significant superior bone preservation for the tissue engineering strategies over conventional alveolar ridge preservation approaches.160,173,183,191,237 In particular, sites treated with tissue engineering strategies required significantly less often additional bone augmentation at the time of implant placement compared with the control groups.160,173,191 Kaigler et al observed a sixfold greater percent of implant exposure necessitating additional bone grafting procedure in sites treated with conventional alveolar ridge preservation compared with sites allocated to the tissue engineering strategy.191 On the other hand, six trials did not find clinical or radiographic differences between alveolar ridge preservation performed with tissue engineering strategies or conventional approaches188,199,200,208,209 (Table 8).
4.3.1.4 |. Clinical recommendations
Tissue engineering strategies are safe treatment options for alveolar ridge preservation. The impact of tissue engineering strategies on the invasiveness of the procedure and patient-reported outcome measures has been poorly addressed at the present time. Tissue engineering strategies were found to be either as effective or more effective than conventional alveolar ridge preservation therapies in maintaining the volume of the ridge, with several studies demonstrating a significantly lower number of sites requiring additional bone grafting at the time of implant placement following tissue engineering strategies compared with standard treatment. More randomized controlled trials reporting patient-reported outcome measures following the use of tissue engineering strategies for alveolar ridge preservation are needed.
4.3.2 |. Tissue engineering strategies for staged bone augmentation
4.3.2.1 |. Characteristics of the studies included
Eight randomized controlled trials reported the outcomes of tissue engineering strategies for staged bone augmentation. 164,207,216,230,243,31–33 One trial employed expanded autogenous stem cells from bone marrow,31 whereas the other studies utilized either recombinant human bone morphogenetic protein-2 or recombinant human platelet–derived growth factor-BB.32,33,164,207,216,230,243 The characteristics of the aforementioned randomized controlled trials are presented in detail in Table 9 and Appendix S1.
4.3.2.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies (Table 10).
Four trials investigated patient-reported morbidity.207,31–33 De Freitas et al highlighted that the subjects allocated to the control group (autogenous bone graft harvested from the mandibular retromolar area) frequently described temporary discomfort and pain from the donor site.32 Bajestan et al31 mentioned that two patients per group experienced significant postoperative discomfort and the other two patients for each group reported that the procedure interfered with daily activities. Marx et al207 observed a marginally significant lower number of days in which analgesics were consumed in subjects that received the tissue engineering strategy, compared with autogenous bone graft. The time of the surgical procedure was significantly reduced when the tissue engineering strategy was performed compared with conventional bone augmentation. Nevertheless, the tissue engineering strategy had significantly higher postoperative edema during the first 15 days.207 Thoma et al reported statistically significant differences in the self-reported pain (visual analogue scale) perceived at the recipient site during the surgical procedure, with the subjects allocated to the control group showing approximately three times more pain than those following a tissue engineering strategy.33 No significant differences were observed between tissue engineering strategies and conventional bone augmentation with autogenous grafts in terms of treatment satisfaction and willingness of retreatment31,33 (Table 10).
4.3.2.3 |. Clinical outcomes
Except for one study that found lower bone width gain and less sites allowing for implant placement following tissue engineering strategy compared with autogenous-based bone augmentation,31 the other studies did not observe statistically significant differences between the two treatment modalities in the clinical outcomes (bone gain, number of sites requiring additional bone augmentation for implant placement, and percentage of osseointegrated implants). De Freitas et al32 reported higher radiographic gain for the tissue engineering strategy over autogenous bone graft, and Thoma and coworkers found similar radiographic and volumetric outcomes among the two interventions33,243 (Table 10).
4.3.2.4 |. Clinical recommendations
Tissue engineering strategies are safe treatment options for staged bone augmentation. These approaches have the potential to reduce the invasiveness of the procedure compared with bone augmentation involving autogenous bone grafting with a secondary surgical site (donor site). Lower pain, reduced chances of complications, but also prolonged edema, should be expected following staged bone augmentation using tissue engineering strategies compared with conventional techniques. Clinical, volumetric and radiographic outcomes seem to indicate an overall similar efficacy of tissue engineering strategies compared with autogenous grafts–based bone regeneration and conventional augmentation techniques. More randomized controlled trials reporting patient-reported outcome measures following the use of tissue engineering strategies for staged bone augmentation are needed.
4.3.3 |. Tissue engineering strategies for sinus floor augmentation
4.3.3.1 |. Characteristics of the studies included
Twenty-two randomized controlled trials applied tissue engineering strategies for sinus floor augmentation. Eleven of them utilized bone grafts loaded with stem cells,79,82,87,166,179,225,226,246,67–69 whereas in the remaining 11 studies the bone graft scaffold was soaked with recombinant human growth factors or biologic age nts.101,156,161,174,175,192,194,195,197,239,244 Among the trials utilizing signaling molecule–based tissue engineering strategies, six enriched bone graft scaffolds with recombinant human bone morphogenetic protein-2,156,174,192,194,195,244 two applied recombinant human growth and differentiation factor recombinant human growth and differentiation factor,197,239 one utilized recombinant human platelet–derived growth factor-BB,175 and one study involved the use of enamel matrix derivative.101 The characteristics of the aforementioned randomized controlled trials are presented in detail in Table 11 and Appendix S1.
4.3.3.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies. One study described infection of the donor site in the control group when autogenous bone grafting was compared with tissue engineering strategies69 (Table 12).
Four studies evaluated postoperative morbidity.67,68,156,197 Two of them did not observe significant differences between the tissue engineering strategies and bone graft substitutes.67,68 However, when compared with autogenous bone grafting, tissue engineering strategies were found to be related to a statistically significant lower invasiveness of the surgical procedure.156,197 Boyne et al showed that participants allocated to the control group experienced significantly higher post-operative pain than subjects treated with the tissue engineering strategy.156 Similarly, Koch et al mentioned that patients in the control group showed additional pain and swelling due to the autogenous bone harvesting.197 No significant differences were observed for other patient-reported outcome measures, including assessment of quality of life and treatment satisfaction9,79,139,148 (Table 12).
4.3.3.3 |. Clinical outcomes
Most of the studies described similar clinical and radiographic outcomes between tissue engineering strategies and conventional sinus augmentation. Boyne et al obtained significantly lower bone width gain for the tissue engineering strategy compared with the control group.156 Another study showed lower bone density for the tissue engineering strategy compared with autograft.244 On the other hand, Kaigler et al67 demonstrated significantly higher bone density for the tissue engineering strategy than for bone graft alone, whereas no differences were observed between the two treatments for other clinical and radiographic outcomes. Sauerbier et al reported that the tissue engineering strategy resulted in a significantly superior radiological gain and persistence of augmented bone height compared with conventional sinus augmentation69 (Table 12).
4.3.3.4 |. Clinical recommendations
Tissue engineering strategies are safe approaches for sinus floor augmentation. Though there is limited evidence supporting a positive effect of tissue engineering strategies on postoperative morbidity and overall patient-reported outcome measures, it appears that that tissue engineering strategies can reduce the invasiveness of the sinus floor elevation compared with autogenous bone grafting, but not to bone graft substitutes. Similar clinical and radiographic outcomes between tissue engineering strategies and conventional sinus floor augmentation techniques should be expected. The main advantage of using tissue engineering strategies for sinus floor augmentation could be the higher new bone formation and less residual bone graft particles compared with conventional therapies. Nevertheless, the clinical significance of these findings has still to be determined. Further evidence in the form of randomized controlled trials reporting patient-reported outcome measures following the use of tissue engineering strategies for sinus floor augmentation are needed.
4.4 |. Tissue engineering strategies for peri-implant bone reconstruction
4.4.1 |. Characteristics of the studies included
Tissue engineering strategies for peri-implant bone augmentation were described in five studies,147,150,189,190,231 with three of them reporting data from the same patient population with different follow-up intervals.147,189,190 These trials utilized recombinant human bone morphogenetic protein-2 or recombinant human platelet–derived growth factor-BB in combination with different bone graft materials.147,150,189,190,231 The characteristics of the aforementioned randomized controlled trials are presented in detail in Table 13 and Appendix S1.
4.4.2 |. Safety and invasiveness
The tissue engineering strategies utilized in the aforementioned trials were shown to be safe, with no serious complications or adverse events related to those strategies (Table 14).
Postoperative morbidity was not assessed in the aforementioned studies147,150,189,190,231 (Table 14). Two articles about the same cohort reported patient-reported outcome measures.147,189 Subjects were asked to fill out a questionnaire evaluating satisfaction, pain, swelling, color, inflammation, uncomfortable sensation, and differing sensation between the left and right sides. The last follow-up study also included the evaluation of the internationally standardized Oral Health Impact Profile. No significant differences were observed between sites that were augmented with or without recombinant human bone morphogenetic protein-2 at any time points147,189 (Table 14).
4.4.3 |. Clinical outcomes
The series of studies from Jung and coworkers showed similar vertical defect fill at the re-entry and similar implant survival rate, peri-implant soft tissue health and stability, radiographic marginal bone levels, and bone thickness over time between implants that received guided bone regeneration with or without recombinant human bone morphogenetic protein-2.147,189,190
Santana et al231 showed that immediate implant with buccal bone defects augmented with a tissue engineering strategy can achieve the same results as conventional implant therapy. Amorfini et al observed that sites that received recombinant human platelet–derived growth factor-BB for lateral bone augmentation preserved the volume of the regenerated bone better than augmented sites without the growth factor did. This difference was at the limit of significance (P = 0.052)150 (Table 14).
4.4.4 |. Clinical recommendations
Tissue engineering strategies are safe treatment options for peri-implant bone reconstruction. There is limited evidence on the benefits of using tissue engineering strategies for peri-implant bone reconstruction at the present. Bearing in mind that adding growth factors to conventional bone augmentation does not seem to negatively affect the outcomes of bone augmentation without biologics, the additional cost may not be justified. More randomized controlled trials are needed for drawing conclusions on the efficacy of tissue engineering strategies for peri-implant bone reconstruction.
5 |. DISCUSSION
This study aimed at systematically appraising the literature on the efficacy, invasiveness, and patient-reported outcome measures of tissue engineering strategies when utilized for oral reconstructions. The ultimate goal of tissue engineering is the development of biological substitutes that can obtain clinical performances comparable to those of autogenous grafts and superior to those of alternative conventional treatments, in a safe and minimally invasive manner.
All in all, from the 128 randomized controlled trials included, we can confirm that tissue engineering strategies are safe treatment approaches for oral reconstructions, with no serious adverse events, or presenting with higher incidence of complications than conventional approaches. Some studies did, however, observe more cases with mild erythema and swelling in subjects treated with tissue engineering strategies incorporating recombinant human bone morphogenetic protein-2. Despite tissue engineering strategies using signaling molecules having generally been considered safe,41,145,252 most concerns have been raised for cell-based tissue engineering strategies involving the use of mesenchymal stem cells.253–256 There is the theoretical possibility that embryonic stem cells may acquire neoplastic mutations.253–256 Nevertheless, as pointed out in a recent study, “the actual incidence of oncogenic polymorphism from naive pluripotent stem cell is close to zero.”255 Therefore, following good manufacturing practice and performing quality control are vital aspects of cell therapies to ensure their safety,67,68,257 but at the same time they inevitably increase the overall timing and costs of these approaches, which probably explains why the majority of the randomized controlled trials included utilized signaling molecule–based tissue engineering strategies with commercially available scaffolds and signaling molecules.
An important component of contemporary clinical research is the assessment of patient-reported outcome measures.40,258,259 Among the aspects that can affect a patient’s experience of the surgical treatment, the invasiveness of the procedure is probably one of the most important. A study demonstrated that patients can still remember the postoperative pain following palatal harvesting 10–15 years after the surgery and that this memory plays a key role in the willingness of retreatment, if necessary.130 Overall, tissue engineering strategies appeared to have a modest effect in reducing the invasiveness of the intervention following periodontal reconstructions. Nevertheless, several points need to be mentioned on this finding. Clinical conditions like single infrabony defects have rarely been correlated with moderate or severe postoperative pain, and therefore it is not surprising that—except for one study200—the other randomized controlled trials included did not find significant differences in postoperative morbidity between tissue engineering strategies and conventional approaches. Similarly, it can be assumed that patient morbidity following the treatment of isolated gingival recessions is usually mild/moderate and easily manageable with painkillers. The effects of tissue engineering strategies on patient morbidity may be more evident in the case of multiple gingival recessions. A recent trial from our group investigating the root coverage outcomes of multiple gingival recessions treated with a collagen scaffold alone or with recombinant human platelet–derived growth factor-BB demonstrated that the subjects treated with the tissue engineering strategy reported significantly less postoperative pain during the first 5 days compared with the control group.241 This is in line with the documented property of recombinant human platelet–derived growth factor of encouraging the migration of neutrophils and macrophages to the wound sites, which may have resulted in a shorter inflammatory phase and quicker healing.260–264
As suggested by McGuire et al,38 another factor that may limit the detection of significant difference in morbidity is the study design, with split-mouth studies (especially if also involving palatal harvesting) not allowing participants to accurately locate and describe postoperative pain. Though the authors in a pilot study demonstrated lower patient morbidity at sites treated with the tissue-engineered graft compared with an autogenous free gingival graft,36 their subsequent multicenter clinical trial comparing the same grafts did not support this finding.38
There are limited data on the impact of tissue engineering strategies on the invasiveness of alveolar ridge preservation, guided bone regeneration, sinus floor elevation, and peri-implant bone reconstruction. One of the possible advantages that has been speculated for tissue engineering strategies is the promotion of wound healing cascades ultimately leading to clinical outcomes comparable to those of autogenous grafts, but without the need for a secondary surgical site or for extending the surgical flap in order to harvest autogenous grafts.1,10 For bone regenerative procedures, avoiding autogenous bone harvesting eliminates the risk for nerve injury or other complications at the harvesting site, which are not rare events.46,47
A substantially lower patient morbidity, especially from the donor site, was reported for autogenous bone grafting compared with tissue engineering strategies in the randomized controlled trials assessing patient-reported outcome measures following staged bone augmentation.207,31–33 The reduced surgical time for tissue engineering strategies and the less invasive procedure involving one surgical site alone could be the main reasons for these results.207 According to Thoma et al,33 subjects allocated to conventional bone augmentation with autogenous block perceived the surgical procedure significantly more invasive and painful than subjects treated with the tissue engineering strategy. Similar findings were also reported following sinus floor elevation with tissue engineering strategies or autogenous grafts,156,197 with patients that underwent conventional sinus augmentation reporting additional pain, swelling, and sometimes even infection at the donor site.69,156,197 It is reasonable to assume that tissue engineering strategies are able to reduce the invasiveness of the procedure when utilized as alternatives to autogenous grafts, whereas they may have limited (or no) benefits in terms of reduced morbidity when compared with scaffolds alone.
Regarding treatment outcomes and efficacy, tissue engineering strategies demonstrated either similar or superior clinical and radiographic outcomes compared with conventional therapies in the treatment of infrabony and furcation defects. In particular, tissue engineering strategies involving the use of recombinant human platelet–derived growth factor-BB consistently showed enhanced outcomes compared with bone graft alone. When it comes to root coverage procedures, it seems that tissue engineering strategies perform better than flap alone and also have the potential of boosting the outcomes of scaffold materials, in terms of mean root coverage, gain in keratinized tissue width, and gain in gingival thickness. It has been suggested that signaling molecules can accelerate cell migration and colonization of the scaffolds, promoting a faster healing and a higher volume stability of the graft compared with scaffolds utilized without biologics.241 More studies are needed to determine the real potential of tissue engineering strategies in matching the clinical outcomes of the gold standard autogenous connective tissue graft. It has to be mentioned that there are several factors that can affect the root coverage outcomes of tissue engineering strategies. Among them, proper flap management, graft stabilization, flap release, and suturing263,264 are crucial to assure a healing of the tissue engineering graft by primary closure, which maximizes the benefits of living cells or signaling molecules within the graft. For these reasons, grafting procedures involving healing by secondary intention may not be the best candidate for tissue engineering strategies. Few studies have explored the effects of tissue engineering strategies on non–root coverage gingival phenotype modification. Although it was shown that living cellular constructs could often provide an adequate band of attached gingiva, they were not able to obtain the same results as for autogenous free gingival graft.36,38,59
Tissue engineering strategies for alveolar ridge preservation seemed to be either as effective or more effective than conventional therapies in maintaining the volume of the ridge, and it was often reported that sites treated with tissue engineering strategies had a lower chance of requiring additional bone augmentation at the time of implant placement than conventional therapies did. The potential of tissue engineering strategies to accelerate the healing of the ridge following extraction and ridge preservation was also demonstrated with histology, where sites allocated to tissue engineering strategies often demonstrated a higher percentage of new bone formation than conventional treatment did.209,215,237 Clinicians are therefore advised that tissue engineering strategies may have the potential to accelerate the healing of extraction sockets and for alveolar ridge preservation.
On the other hand, it appeared that tissue engineering strategies result in comparable clinical and radiographic outcomes to those of conventional therapies in bone augmentation, sinus floor elevation, and peri-implant bone regeneration. The advantage of tissue engineering strategies for sinus floor elevation could be in avoiding autogenous bone harvesting and promoting a quicker healing, with higher new bone formation and less residual bone graft particles compared with conventional therapies,67,79,87,101,161,175,194,225,246 which may allow an earlier re-entry surgical procedure with implant placement or earlier implant loading. Nevertheless, these speculations should be taken with caution, since there is the need for properly designed randomized controlled trials addressing this question.
Cost effectiveness of tissue engineering strategies has not been reported in the studies included. This aspect is crucial for clinicians and patients in determining the approach to utilize for the specific case scenario. Taking into consideration that tissue engineering is a rapidly evolving field and that certain products that are expensive nowadays would probably become more accessible in the future, it can be assumed that tissue engineering strategies can be recommended for the treatment of large sites/defects that require a significant autogenous graft harvesting. Other case scenarios that could benefit from the use of tissue engineering strategies include patients that had a previous “bad” experience with autogenous grafts and refuse to undergo a similar procedure again.130 Loading scaffolds with biologics may be the method of choice in these challenging situations.
6 |. CURRENT LIMITATIONS AND RECOMMENDATIONS FOR FUTURE STUDIES
Limited evidence is available in the literature at present on patient-reported outcome measures following tissue engineering strategies vs conventional therapies. More studies investigating patient-reported outcome measures with more a homogeneous and uniform questionnaire, outcomes, and methods for their assessment40 are encouraged.
The tissue engineering strategies included are heterogeneous in terms of cells, scaffolds, and signaling molecules; therefore, a quantitative analysis is not feasible at present. Future reviews incorporating more randomized controlled trials may have the possibility of performing network meta-analyses comparing different tissue engineering strategies among one another, and vs conventional therapies, together with the evaluation of factors (eg, types of cells, types of scaffolds, defects characteristics) potentially affecting the final outcomes.
Future randomized controlled trials evaluating tissue engineering strategies should involve (a) clinical outcomes, (b) patient-reported outcome measures, (c) volumetric outcomes with three-dimensional digital technologies and/or ultrasonography, (d) radiographic outcomes (when appropriate), and (e) cost-effective analyses.
7 |. CONCLUSIONS
Based on the available evidence, and within the limitations of this study, the following conclusions can be drawn:
Tissue engineering strategies are safe treatment approaches for periodontal and peri-implant reconstructions and implant site development.
Currently used tissue engineering strategies involve the use of scaffolds loaded with mesenchymal or somatic cells or signaling molecules, such as enamel matrix derivatives, recombinant human bone morphogenetic proteins, recombinant human platelet–derived growth factor-BB, or fibroblast growth factor-2, among others.
Tissue engineering strategies for the treatment of infrabony and furcation defects provide either similar or superior clinical and radiographic outcomes compared with conventional approaches, with tissue engineering strategies involving the use of recombinant human platelet–derived growth factor-BB having the greatest probability of obtaining enhanced outcomes compared with bone graft alone. No benefits in reduced morbidity, complications, or overall patient-reported outcome measures were observed.
Tissue engineering strategies for root-coverage procedures may have a positive effect on patient morbidity, preference, and quality of life. There is evidence suggesting that tissue engineering strategies can promote higher root coverage, keratinized tissue width, and gingival thickness gain than scaffolds alone. Tissue engineering strategies may also obtain comparable mean root coverage with the autogenous connective tissue graft.
Few studies investigated the outcomes of tissue engineering strategies for non–root coverage gingival phenotype modification. Though autogenous free gingival graft demonstrated superior keratinized mucosa gain, tissue engineering strategies were shown to be effective in regenerating an adequate band of keratinized mucosa, together with reduced morbidity, superior esthetics, and patient preference.
Tissue engineering strategies for alveolar ridge preservation were found to be either as effective or more effective than conventional alveolar ridge preservation therapies in maintaining ridge dimensions, with higher chances of having less sites requiring additional augmentation at the time of implant placement.
Tissue engineering strategies for staged bone augmentation resulted in comparable clinical outcomes with conventional approaches, though with lower morbidity and complications than autogenous bone grafting.
Tissue engineering strategies and conventional approaches for sinus floor augmentation showed comparable clinical and radiographic outcomes.
Few studies utilized tissue engineering strategies for peri-implant bone reconstruction, and there is limited evidence supporting their use for this indication at present.
Supplementary Material
FIGURE 11.
Signaling molecule–based tissue engineering strategy involving a xenogeneic collagen scaffold (Fibrogide; Geistlich Pharma, Wolhusen, Switzerland) soaked with recombinant human platelet–derived growth factor-BB (GEM21; Lynch Biologics, Franklin, TN, USA). A, Baseline. B, C, Flap design and elevation. D, Xenogeneic collagen matrix. E, Trimming of the scaffold. F, The scaffold was loaded with recombinant human platelet–derived growth factor-BB and left in the dappen dish for 15 minutes. G, Preparation of the recipient side, involving flap release, removal of muscle fibers, and de-epithelialization of the anatomical papillae. H, After mechanical root planing, 24% ethylenediaminetetraacetic acid was applied for 2 minutes. I, Stabilization of the tissue engineering graft to the recipient site. J, Flap closure. K, Two weeks post-op. L, Six-month outcome
ACKNOWLEDGMENTS
We would like to express their gratitude to Dr Teresa Heck, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, MI, USA, for the illustrations presented in Figures 1, 2, and 4. Open access funding enabled and organized by ProjektDEAL.
Funding information
This research was supported by the National Institute of Health/National Institute of Dental and Craniofacial Research (U24 DE026915) and the Delta Dental Foundation (AWS015480)
CONFLICT OF INTEREST
Dr Lorenzo Tavelli has provided lectures sponsored by Geistlich Pharma AG, Wolhusen, Switzerland, and Lynch Biologics, Franklin, TN, USA. Dr Giulio Rasperini has provided lectures sponsored by Geistlich Pharma AG, Wolhusen, Switzerland, and Straumann, Basel, Switzerland. Dr William V. Giannobile has previously consulted for and received grants from Biomimetic Therapeutics, Franklin, TN, USA, and Straumann, Basel, Switzerland.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Data sharing not applicable - no new data generated.
REFERENCES
- 1.Avila-Ortiz G, Bartold PM, Giannobile W, et al. Biologics and cell therapy tissue engineering approaches for the management of the edentulous maxilla: a systematic review. Int J Oral Maxillofac Implants. 2016;31(Suppl):s121–s164. [DOI] [PubMed] [Google Scholar]
- 2.Scarpone M, Kuebler D, Chambers A, et al. Isolation of clinically relevant concentrations of bone marrow mesenchymal stem cells without centrifugation. J Transl Med. 2019;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanbhag S, Suliman S, Pandis N, Stavropoulos A, Sanz M, Mustafa K. Cell therapy for orofacial bone regeneration: a systematic review and meta-analysis. J Clin Periodontol. 2019;46(Suppl 21):162–182. [DOI] [PubMed] [Google Scholar]
- 4.Ashammakhi N, Ahadian S, Darabi MA, et al. Minimally invasive and regenerative therapeutics. Adv Mater. 2019;31:e1804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocchieri R, van de Wetering B, Stijnen M, Riezebos R, de Mol B. The impact of biomedical engineering on the development of minimally invasive cardio-thoracic surgery. J Clin Med. 2021;10:3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. [DOI] [PubMed] [Google Scholar]
- 7.Larsson L, Decker AM, Nibali L, Pilipchuk SP, Berglundh T, Giannobile WV. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donos N, Dereka X, Calciolari E. The use of bioactive factors to enhance bone regeneration: a narrative review. J Clin Periodontol. 2019;46(Suppl 21):124–161. [DOI] [PubMed] [Google Scholar]
- 9.Giannobile WV, Chai Y, Chen Y, et al. Dental, oral, and craniofacial regenerative medicine: transforming biotechnologies for innovating patient care. J Dent Res. 2018;97:361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polymeri A, Giannobile WV, Kaigler D. Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm Metab Res. 2016;48:700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egido-Moreno S, Valls-Roca-Umbert J, Cespedes-Sanchez JM, Lopez-Lopez J, Velasco-Ortega E. Clinical efficacy of mesenchymal stem cells in bone regeneration in oral implantology. Systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann Thorac Surg. 2002;74:1438–1442. [DOI] [PubMed] [Google Scholar]
- 13.Pereira Rodrigues IC, Kaasi A, Maciel Filho R, Jardini AL, Gabriel LP. Cardiac tissue engineering: current state-of-the-art materials, cells and tissue formation. Einstein (Sao Paulo) 2018;16:eRB4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis MW, Russell B. Cardiac tissue engineering. J Cardiovasc Nurs. 2009;24:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. [DOI] [PubMed] [Google Scholar]
- 16.Nilforoushzadeh MA, Sisakht MM, Amirkhani MA, et al. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: a clinical study for diabetic wound healing. J Tissue Eng Regen Med. 2020;14:424–440. [DOI] [PubMed] [Google Scholar]
- 17.McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. [DOI] [PubMed] [Google Scholar]
- 18.Chiang T, Pepper V, Best C, Onwuka E, Breuer CK. Clinical translation of tissue engineered trachea grafts. Ann Otol Rhinol Laryngol. 2016;125:873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott MJ, De Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirsner RS, Sabolinski ML, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015;23:737–744. [DOI] [PubMed] [Google Scholar]
- 21.Cortellini P, Bissada NF. Mucogingival conditions in the natural dentition: narrative review, case definitions, and diagnostic considerations. J Clin Periodontol. 2018;45(Suppl 20):S190–S198. [DOI] [PubMed] [Google Scholar]
- 22.Zucchelli G, Tavelli L, McGuire MK, et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol. 2020;91:9–16. [DOI] [PubMed] [Google Scholar]
- 23.Zucchelli G, Tavelli L, Stefanini M, et al. Classification of facial peri-implant soft tissue dehiscence/deficiencies at single implant sites in the esthetic zone. J Periodontol. 2019;90:1116–1124. [DOI] [PubMed] [Google Scholar]
- 24.Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV. Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev. 2012;64:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang F, Leland H, Jedrzejewski B, et al. Alternatives to autologous bone graft in alveolar cleft reconstruction: the state of alveolar tissue engineering. J Craniofac Surg. 2018;29:584–593. [DOI] [PubMed] [Google Scholar]
- 26.Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16:2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiapasco M, Casentini P. Horizontal bone-augmentation procedures in implant dentistry: prosthetically guided regeneration. Periodontol 2000. 2018;77:213–240. [DOI] [PubMed] [Google Scholar]
- 28.Chiapasco M, Tommasato G, Palombo D, Scarno D, Zaniboni M, Del Fabbro M. Dental implants placed in severely atrophic jaws reconstructed with autogenous calvarium, bovine bone mineral, and collagen membranes: a 3- to 19-year retrospective follow-up study. Clin Oral Implants Res. 2018;29:725–740. [DOI] [PubMed] [Google Scholar]
- 29.Chanchareonsook N, Junker R, Jongpaiboonkit L, Jansen JA. Tissue-engineered mandibular bone reconstruction for continuity defects: a systematic approach to the literature. Tissue Eng Part B Rev. 2014;20:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawashdeh MA, Telfah H. Secondary alveolar bone grafting: the dilemma of donor site selection and morbidity. Br J Oral Maxillofac Surg. 2008;46:665–670. [DOI] [PubMed] [Google Scholar]
- 31.Bajestan MN, Rajan A, Edwards SP, et al. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: a randomized controlled, clinical trial. Clin Implant Dent Relat Res. 2017;19:793–801. [DOI] [PubMed] [Google Scholar]
- 32.De Freitas RM, Susin C, Spin-Neto R, et al. Horizontal ridge augmentation of the atrophic anterior maxilla using rhBMP-2/ACS or autogenous bone grafts: a proof-of-concept randomized clinical trial. J Clin Periodontol. 2013;40:968–975. [DOI] [PubMed] [Google Scholar]
- 33.Thoma DS, Payer M, Jakse N, et al. Randomized, controlled clinical two-centre study using xenogeneic block grafts loaded with recombinant human bone morphogenetic protein-2 or autogenous bone blocks for lateral ridge augmentation. J Clin Periodontol. 2018;45:265–276. [DOI] [PubMed] [Google Scholar]
- 34.Barootchi S, Tavelli L, Di Gianfilippo R, et al. Gingival phenotype modification as a result of root coverage procedure with two human dermal matrices: long-term assessment of a randomized clinical trial. Int J Periodontics Restorative Dent. 2021;41: 719–726. [DOI] [PubMed] [Google Scholar]
- 35.Tavelli L, Barootchi S, Avila-Ortiz G, Urban IA, Giannobile WV, Wang HL. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol. 2021;92:21–44. [DOI] [PubMed] [Google Scholar]
- 36.McGuire MK, Scheyer ET, Nunn ME, Lavin PT. A pilot study to evaluate a tissue-engineered bilayered cell therapy as an alternative to tissue from the palate. J Periodontol. 2008;79:1847–1856. [DOI] [PubMed] [Google Scholar]
- 37.McGuire MK, Scheyer ET. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J Periodontol. 2010;81:1108–1117. [DOI] [PubMed] [Google Scholar]
- 38.McGuire MK, Scheyer ET, Nevins ML, et al. Living cellular construct for increasing the width of keratinized gingiva: results from a randomized, within-patient, controlled trial. J Periodontol. 2011;82:1414–1423. [DOI] [PubMed] [Google Scholar]
- 39.Cairo F, Barbato L, Tonelli P, Batalocco G, Pagavino G, Nieri M. Xenogeneic collagen matrix versus connective tissue graft for buccal soft tissue augmentation at implant site. A randomized, controlled clinical trial. J Clin Periodontol. 2017;44:769–776. [DOI] [PubMed] [Google Scholar]
- 40.Stefanini M, Tavelli L, Barootchi S, Sangiorgi M, Zucchelli G. Patient-reported outcome measures following soft-tissue grafting at implant sites: a systematic review. Clin Oral Implants Res. 2021;32(Suppl 21):157–173. [DOI] [PubMed] [Google Scholar]
- 41.Tavelli L, McGuire MK, Zucchelli G, et al. Biologics-based regenerative technologies for periodontal soft tissue engineering. J Periodontol. 2020;91:147–154. [DOI] [PubMed] [Google Scholar]
- 42.Tavelli L, McGuire MK, Zucchelli G, et al. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J Periodontol. 2020;91:17–25. [DOI] [PubMed] [Google Scholar]
- 43.McGuire MK, Scheyer ET, Schupbach P. Growth factor–mediated treatment of recession defects: a randomized controlled trial and histologic and microcomputed tomography examination. J Periodontol. 2009;80:550–564. [DOI] [PubMed] [Google Scholar]
- 44.McGuire MK, Scheyer ET, Snyder MB. Evaluation of recession defects treated with coronally advanced flaps and either recombinant human platelet–derived growth factor-BB plus β-tricalcium phosphate or connective tissue: comparison of clinical parameters at 5 years. J Periodontol. 2014;85:1361–1370. [DOI] [PubMed] [Google Scholar]
- 45.McGuire MK, Scheyer T, Nevins M, Schupbach P. Evaluation of human recession defects treated with coronally advanced flaps and either purified recombinant human platelet–derived growth factor-BB with beta tricalcium phosphate or connective tissue: a histologic and microcomputed tomographic examination. Int J Periodontics Restorative Dent. 2009;29:7–21. [PubMed] [Google Scholar]
- 46.Starch-Jensen T, Deluiz D, Deb S, Bruun NH, Tinoco EMB. Harvesting of autogenous bone graft from the ascending mandibular ramus compared with the chin region: a systematic review and meta-analysis focusing on complications and donor site morbidity. J Oral Maxillofac Res. 2020;11:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva FM, Cortez AL, Moreira RW, Mazzonetto R. Complications of intraoral donor site for bone grafting prior to implant placement. Implant Dent. 2006;15:420–426. [DOI] [PubMed] [Google Scholar]
- 48.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300–309. [DOI] [PubMed] [Google Scholar]
- 49.Almaiman M, Al-Bargi HH, Manson P. Complication of anterior iliac bone graft harvesting in 372 adult patients from May 2006 to May 2011 and a literature review. Craniomaxillofac Trauma Reconstr. 2013;6:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuire MK, Tavelli L, Feinberg SE, et al. Living cell-based regenerative medicine technologies for periodontal soft tissue augmentation. J Periodontol. 2020;91:155–164. [DOI] [PubMed] [Google Scholar]
- 51.Tavelli L, Barootchi S, Ravida A, Oh TJ, Wang HL. What is the safety zone for palatal soft tissue graft harvesting based on the locations of the greater palatine artery and foramen? A systematic review. J Oral Maxillofac Surg. 2019;77(2):271.e1–271.e9. [DOI] [PubMed] [Google Scholar]
- 52.Buff LR, Burklin T, Eickholz P, Monting JS, Ratka-Kruger P. Does harvesting connective tissue grafts from the palate cause persistent sensory dysfunction? A pilot study. Quintessence Int. 2009;40:479–489. [PubMed] [Google Scholar]
- 53.Del Pizzo M, Modica F, Bethaz N, Priotto P, Romagnoli R. The connective tissue graft: a comparative clinical evaluation of wound healing at the palatal donor site. A preliminary study. J Clin Periodontol. 2002;29:848–854. [DOI] [PubMed] [Google Scholar]
- 54.Tavelli L, Ravida A, Saleh MHA, et al. Pain perception following epithelialized gingival graft harvesting: a randomized clinical trial. Clin Oral Investig. 2019;23:459–468. [DOI] [PubMed] [Google Scholar]
- 55.Tavelli L, Asa'ad F, Acunzo R, Pagni G, Consonni D, Rasperini G. Minimizing patient morbidity following palatal gingival harvesting: a randomized controlled clinical study. Int J Periodontics Restorative Dent. 2018;38:e127–e134. [DOI] [PubMed] [Google Scholar]
- 56.Latimer JM, Maekawa S, Yao Y, Wu DT, Chen M, Giannobile WV. Regenerative medicine technologies to treat dental, oral, and craniofacial defects. Front Bioeng Biotechnol. 2021;9:704048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannobile WV, Berglundh T, Al-Nawas B, et al. Biological factors involved in alveolar bone regeneration: consensus report of Working Group 1 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol. 2019;46(Suppl 21):6–11. [DOI] [PubMed] [Google Scholar]
- 58.Taba M Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. I. Comparison to the gingival autograft: a randomized controlled pilot study. J Periodontol. 2005;76:867–880. [DOI] [PubMed] [Google Scholar]
- 60.Köseoǧlu S, Duran I, Saǧlam M, Bozkurt SB, Kirtiloǧlu OS, Hakki SS. Efficacy of collagen membrane seeded with autologous gingival fibroblasts in gingival recession treatment: a randomized, controlled pilot study. J Periodontol. 2013;84:1416–1424. [DOI] [PubMed] [Google Scholar]
- 61.Wilson TG Jr, McGuire MK, Nunn ME. Evaluation of the safety and efficacy of periodontal applications of a living tissue-engineered human fibroblast-derived dermal substitute. II. Comparison to the subepithelial connective tissue graft: a randomized controlled feasibility study. J Periodontol. 2005;76:881–889. [DOI] [PubMed] [Google Scholar]
- 62.McGuire MK, Scheyer ET. A randomized, double-blind, placebo-controlled study to determine the safety and efficacy of cultured and expanded autologous fibroblast injections for the treatment of interdental papillary insufficiency associated with the papilla priming procedure. J Periodontol. 2007;78:4–17. [DOI] [PubMed] [Google Scholar]
- 63.Mohammadi M, Shokrgozar MA, Mofid R. Culture of human gingival fibroblasts on a biodegradable scaffold and evaluation of its effect on attached gingiva: a randomized, controlled pilot study. J Periodontol. 2007;78:1897–1903. [DOI] [PubMed] [Google Scholar]
- 64.Jhaveri HM, Chavan MS, Tomar GB, Deshmukh VL, Wani MR, Miller PD Jr. Acellular dermal matrix seeded with autologous gingival fibroblasts for the treatment of gingival recession: a proof-of-concept study. J Periodontol. 2010;81:616–625. [DOI] [PubMed] [Google Scholar]
- 65.Milinkovic I, Aleksic Z, Jankovic S, et al. Clinical application of autologous fibroblast cell culture in gingival recession treatment. J Periodontal Res. 2015;50:363–370. [DOI] [PubMed] [Google Scholar]
- 66.Friedenstein AJ, Ivanov-Smolenski AA, Chajlakjan RK, et al. Origin of bone marrow stromal mechanocytes in radiochimeras and heterotopic transplants. Exp Hematol. 1978;6:440–444. [PubMed] [Google Scholar]
- 67.Kaigler D, Avila-Ortiz G, Travan S, et al. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res. 2015;30:1206–1216. [DOI] [PubMed] [Google Scholar]
- 68.Payer M, Lohberger B, Strunk D, Reich KM, Acham S, Jakse N. Effects of directly autotransplanted tibial bone marrow aspirates on bone regeneration and osseointegration of dental implants. Clin Oral Implants Res. 2014;25:468–474. [DOI] [PubMed] [Google Scholar]
- 69.Sauerbier S, Rickert D, Gutwald R, et al. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial—per-protocol analysis. Tissue Eng Part A. 2011;17:2187–2197. [DOI] [PubMed] [Google Scholar]
- 70.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. [DOI] [PubMed] [Google Scholar]
- 71.Ferrarotti F, Romano F, Gamba MN, et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J Clin Periodontol. 2018;45:841–850. [DOI] [PubMed] [Google Scholar]
- 72.Sánchez N, Fierravanti L, Núñez J, et al. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: a 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol. 2020;47:1391–1402. [DOI] [PubMed] [Google Scholar]
- 73.Mason S, Tarle SA, Osibin W, Kinfu Y, Kaigler D. Standardization and safety of alveolar bone-derived stem cell isolation. J Dent Res. 2014;93:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao M, Jin Q, Berry JE, Nociti FH Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santamaria S, Sanchez N, Sanz M, Garcia-Sanz JA. Comparison of periodontal ligament and gingiva-derived mesenchymal stem cells for regenerative therapies. Clin Oral Investig. 2017;21:1095–1102. [DOI] [PubMed] [Google Scholar]
- 76.Hegde V, Shonuga O, Ellis S, et al. A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J Orthop Trauma. 2014;28:591–598. [DOI] [PubMed] [Google Scholar]
- 77.Imam MA, Mahmoud SSS, Holton J, Abouelmaati D, Elsherbini Y, Snow M. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in orthopaedics. SICOT J. 2017;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy MB, Terrazas JA, Buford DA. Bone marrow concentrate and platelet-rich plasma acquisition and preparation: why technique matters. Tech Reg Anesth Pain Manag. 2015;2015:19–25. [Google Scholar]
- 79.Pasquali PJ, Teixeira ML, de Oliveira TA, de Macedo LG, Aloise AC, Pelegrine AA. Maxillary sinus augmentation combining Bio-Oss with the bone marrow aspirate concentrate: a histomorphometric study in humans. Int J Biomater. 2015;2015:121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakai S, Mishima H, Ishii T, et al. Concentration of bone marrow aspirate for osteogenic repair using simple centrifugal methods. Acta Orthop. 2008;79:445–448. [DOI] [PubMed] [Google Scholar]
- 81.Wise JK, Alford AI, Goldstein SA, Stegemann JP. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng Part A. 2014;20:210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wildburger A, Payer M, Jakse N, Strunk D, Etchard-Liechtenstein N, Sauerbier S. Impact of autogenous concentrated bone marrow aspirate on bone regeneration after sinus floor augmentation with a bovine bone substitute—a split-mouth pilot study. Clin Oral Implants Res. 2014;25:1175–1181. [DOI] [PubMed] [Google Scholar]
- 83.Moreno Sancho F, Leira Y, Orlandi M, Buti J, Giannobile WV, D'Aiuto F. Cell-based therapies for alveolar bone and periodontal regeneration: concise review. Stem Cells Transl Med. 2019;8:1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartold M, Gronthos S, Haynes D, Ivanovski S. Mesenchymal stem cells and biologic factors leading to bone formation. J Clin Periodontol. 2019;46(Suppl 21):12–32. [DOI] [PubMed] [Google Scholar]
- 85.Lucarelli E, Donati D, Cenacchi A, Fornasari PM. Bone reconstruction of large defects using bone marrow derived autologous stem cells. Transfus Apher Sci. 2004;30:169–174. [DOI] [PubMed] [Google Scholar]
- 86.Baboolal TG, Boxall SA, El-Sherbiny YM, et al. Multipotential stromal cell abundance in cellular bone allograft: comparison with fresh age-matched iliac crest bone and bone marrow aspirate. Regen Med. 2014;9:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26:123–131. [PubMed] [Google Scholar]
- 88.Hong SY, Teng SW, Lin W, Wang CY, Lin HI. Allogeneic human umbilical cord-derived mesenchymal stem cells reduce lipopolysaccharide-induced inflammation and acute lung injury. Am J Transl Res. 2020;12:6740–6750. [PMC free article] [PubMed] [Google Scholar]
- 89.Palma MB, Luzzani C, Andrini LB, et al. Wound healing by allogeneic transplantation of specific subpopulation from human umbilical cord mesenchymal stem cells. Cell Transplant. 2021;30:963689721993774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3: 129–139. [DOI] [PubMed] [Google Scholar]
- 92.Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giannobile WV, Hollister SJ, Ma PX. Future prospects for periodontal bioengineering using growth factors. Clin Adv Periodontics. 2011;1:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–484. [DOI] [PubMed] [Google Scholar]
- 95.Ripamonti U, Herbst NN, Ramoshebi LN. Bone morphogenetic proteins in craniofacial and periodontal tissue engineering: experimental studies in the non-human primate Papio ursinus. Cytokine Growth Factor Rev. 2005;16:357–368. [DOI] [PubMed] [Google Scholar]
- 96.Lindskog S, Hammarström L. Formation of intermediate cementum. III: 3H-tryptophan and 3H-proline uptake into the epithelial root sheath of Hertwig in vitro. J Craniofac Genet Dev Biol. 1982;2:171–177. [PubMed] [Google Scholar]
- 97.Slavkin HC, Bessem C, Fincham AG, et al. Human and mouse cementum proteins immunologically related to enamel proteins. Biochim Biophys Acta. 1989;991:12–18. [DOI] [PubMed] [Google Scholar]
- 98.Tonetti MS, Fourmousis I, Suvan J, et al. Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J Clin Periodontol. 2004;31:1092–1098. [DOI] [PubMed] [Google Scholar]
- 99.Miron RJ, Chandad F, Buser D, Sculean A, Cochran DL, Zhang Y. Effect of enamel matrix derivative liquid on osteoblast and periodontal ligament cell proliferation and differentiation. J Periodontol. 2016;87:91–99. [DOI] [PubMed] [Google Scholar]
- 100.Graziani F, Gennai S, Petrini M, Bettini L, Tonetti M. Enamel matrix derivative stabilizes blood clot and improves clinical healing in deep pockets after flapless periodontal therapy: a randomized clinical trial. J Clin Periodontol. 2019;46:231–240. [DOI] [PubMed] [Google Scholar]
- 101.Vincent-Bugnas S, Charbit Y, Charbit M, Dard M, Pippenger B. Maxillary sinus floor elevation surgery with BioOss mixed with enamel matrix derivative: a human randomized controlled clinical and histologic study. J Oral Implantol. 2020;46:507–513. [DOI] [PubMed] [Google Scholar]
- 102.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu DT, Munguia-Lopez JG, Cho YW, et al. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules. 2021;26:7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu N, Nguyen T, Cho YD, Kavanagh NM, Ghassib I, Giannobile WV. Personalized scaffolding technologies for alveolar bone regenerative medicine. Orthod Craniofac Res. 2019;22(Suppl 1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park CH, Rios HF, Taut AD, et al. Image-based, fiber guiding scaffolds: a platform for regenerating tissue interfaces. Tissue Eng Part C Methods. 2014;20:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rasperini G, Pilipchuk SP, Flanagan CL, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. 2015;94:153S–157S. [DOI] [PubMed] [Google Scholar]
- 107.van Zuijlen P, Gardien K, Jaspers M, et al. Tissue engineering in burn scar reconstruction. Burns Trauma. 2015;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicholas MN, Yeung J. Current status and future of skin substitutes for chronic wound healing. J Cutan Med Surg. 2017;21:23–30. [DOI] [PubMed] [Google Scholar]
- 109.Barootchi S, Tavelli L, Gianfilippo RD, et al. Acellular dermal matrix for root coverage procedures: 9-year assessment of treated isolated gingival recessions and their adjacent untreated sites. J Periodontol. 2021;92:254–262. [DOI] [PubMed] [Google Scholar]
- 110.Tavelli L, Barootchi S, Di Gianfilippo R, et al. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12-year follow-up from a randomized clinical trial. J Clin Periodontol. 2019;46:937–948. [DOI] [PubMed] [Google Scholar]
- 111.Livesey SA, Herndon DN, Hollyoak MA, Atkinson YH, Nag A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation. 1995;60:1–9. [PubMed] [Google Scholar]
- 112.Rodina AV, Tenchurin TK, Saprykin VP, et al. Proliferative and differentiation potential of multipotent mesenchymal stem cells cultured on biocompatible polymer scaffolds with various physicochemical characteristics. Bull Exp Biol Med. 2017;162:488–495. [DOI] [PubMed] [Google Scholar]
- 113.Kuo S, Kim HM, Wang Z, et al. Comparison of two decellularized dermal equivalents. J Tissue Eng Regen Med. 2018;12:983–990. [DOI] [PubMed] [Google Scholar]
- 114.Moharamzadeh K, Colley H, Murdoch C, et al. Tissue-engineered oral mucosa. J Dent Res. 2012;91:642–650. [DOI] [PubMed] [Google Scholar]
- 115.Moharamzadeh K, Brook IM, Van Noort R, Scutt AM, Thornhill MH. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res. 2007;86:115–124. [DOI] [PubMed] [Google Scholar]
- 116.Stefanini M, Rendon A, Zucchelli G. Porcine-d erived acellular dermal matrix for buccal soft tissue augmentation at single implant sites: a 1-year follow-up case series. Int J Periodontics Restorative Dent. 2020;40:121–128. [DOI] [PubMed] [Google Scholar]
- 117.Ghanaati S, Schlee M, Webber MJ, et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 2011;6:015010. [DOI] [PubMed] [Google Scholar]
- 118.Jung RE, Hurzeler MB, Thoma DS, Khraisat A, Hämmerle CH. Local tolerance and efficiency of two prototype collagen matrices to increase the width of keratinized tissue. J Clin Periodontol. 2011;38:173–179. [DOI] [PubMed] [Google Scholar]
- 119.Thoma DS, Zeltner M, Hilbe M, Hämmerle CH, Husler J, Jung RE. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J Clin Periodontol. 2016;43:874–885. [DOI] [PubMed] [Google Scholar]
- 120.Thoma DS, Villar CC, Cochran DL, Hämmerle CH, Jung RE. Tissue integration of collagen-based matrices: an experimental study in mice. Clin Oral Implants Res. 2012;23:1333–1339. [DOI] [PubMed] [Google Scholar]
- 121.Thoma DS, Naenni N, Benic GI, Hämmerle CH, Jung RE. Soft tissue volume augmentation at dental implant sites using a volume stable three-dimensional collagen matrix—histological outcomes of a preclinical study. J Clin Periodontol. 2017;44:185–194. [DOI] [PubMed] [Google Scholar]
- 122.Cosyn J, Eeckhout C, Christiaens V, et al. A multi-centre randomized controlled trial comparing connective tissue graft with collagen matrix to increase soft tissue thickness at the buccal aspect of single implants: 3-month results. J Clin Periodontol. 2021;48:1502–1515. [DOI] [PubMed] [Google Scholar]
- 123.Stefanini M, Mounssif I, Barootchi S, Tavelli L, Wang HL, Zucchelli G. An exploratory clinical study evaluating safety and performance of a volume-stable collagen matrix with coronally advanced flap for single gingival recession treatment. Clin Oral Investig. 2020;24:3181–3191. [DOI] [PubMed] [Google Scholar]
- 124.Agis H, Collins A, Taut AD, et al. Cell population kinetics of collagen scaffolds in ex vivo oral wound repair. PLoS One. 2014;9:e112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suzuki KT, de Jesus Hernandez Martinez C, Suemi MI, et al. Root coverage using coronally advanced flap with porcine-derived acellular dermal matrix or subepithelial connective tissue graft: a randomized controlled clinical trial. Clinical Oral Investigations. 2020;24:4077–4087. [DOI] [PubMed] [Google Scholar]
- 126.Maluta R, Monteiro MF, Peruzzo DC, Joly JC. Root coverage of multiple gingival recessions treated with coronally advanced flap associated with xenogeneic acellular dermal matrix or connective tissue graft: a 6-month split-mouth controlled and randomized clinical trial. Clin Oral Investig. 2021;25:5765–5773. [DOI] [PubMed] [Google Scholar]
- 127.Gargallo-Albiol J, Barootchi S, Tavelli L, Wang HL. Efficacy of xenogeneic collagen matrix to augment peri-implant soft tissue thickness compared to autogenous connective tissue graft: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2019;34:1059–1069. [DOI] [PubMed] [Google Scholar]
- 128.McGuire MK, Janakievski J, Scheyer ET, et al. Efficacy of a harvest graft substitute for recession coverage and soft tissue volume augmentation: a randomized controlled trial. J Periodontol. 2021;93:333–342. [DOI] [PubMed] [Google Scholar]
- 129.Hutton CG, Johnson GK, Barwacz CA, Allareddy V, Avila-Ortiz G. Comparison of two different surgical approaches to increase peri-implant mucosal thickness: a randomized controlled clinical trial. J Periodontol. 2018;89:807–814. [DOI] [PubMed] [Google Scholar]
- 130.Tavelli L, Barootchi S, Di Gianfilippo R, et al. Patient experience of autogenous soft tissue grafting has an implication for future treatment: a 10- to 15-year cross-sectional study. J Periodontol. 2021;92:637–647. [DOI] [PubMed] [Google Scholar]
- 131.Barootchi S, Tavelli L, Zucchelli G, Giannobile WV, Wang HL. Gingival phenotype modification therapies on natural teeth: a network meta-analysis. J Periodontol. 2020;91:1386–1399. [DOI] [PubMed] [Google Scholar]
- 132.Tavelli L, Barootchi S, Cairo F, Rasperini G, Shedden K, Wang HL. The effect of time on root coverage outcomes: a network meta-analysis. J Dent Res. 2019;98:1195–1203. [DOI] [PubMed] [Google Scholar]
- 133.Mosquera-Perez R, Fernandez-Olavarria A, Diaz-Sanchez RM, Gutierrez-Perez JL, Serrera-Figallo MA, Torres-Lagares D. Stem cells and oral surgery: a systematic review. J Clin Exp Dent. 2019;11:e1181–e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Izumi K, Neiva RF, Feinberg SE. Intraoral grafting of tissue-engineered human oral mucosa. Int J Oral Maxillofac Implants. 2013;28:e295–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sangiorgio JPM, Neves F, Rocha Dos Santos M, et al. Xenogenous collagen matrix and/or enamel matrix derivative for treatment of localized gingival recessions: a randomized clinical trial. Part I: clinical outcomes. J Periodontol. 2017;88:1309–1318. [DOI] [PubMed] [Google Scholar]
- 136.Available at: www.crd.york.ac.uk/PROSPERO. [Google Scholar]
- 137.Higgins JPT GS. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Blackwell. 2011. [Google Scholar]
- 138.Stillwell SB, Fineout-Overholt E, Melnyk BM, Williamson KM. Evidence-based practice, step by step: asking the clinical question: a key step in evidence-based practice. Am J Nurs. 2010;110:58–61. [DOI] [PubMed] [Google Scholar]
- 139.Nino-Sandoval TC, Vasconcelos B, Moraes SL, Lemos CAA, Pellizzer EP. Efficacy of stem cells in maxillary sinus floor augmentation: systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2019;48:1355–1366. [DOI] [PubMed] [Google Scholar]
- 140.Kelly MP, Vaughn OL, Anderson PA. Systematic review and meta-analysis of recombinant human bone morphogenetic protein-2 in localized alveolar ridge and maxillary sinus augmentation. J Oral Maxillofac Surg. 2016;74:928–939. [DOI] [PubMed] [Google Scholar]
- 141.Schliephake H. Clinical efficacy of growth factors to enhance tissue repair in oral and maxillofacial reconstruction: a systematic review. Clin Implant Dent Relat Res. 2015;17:247–273. [DOI] [PubMed] [Google Scholar]
- 142.Freitas RM, Spin-Neto R, Marcantonio Junior E, Pereira LA, Wikesjo UM, Susin C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: a systematic review. Clin Implant Dent Relat Res. 2015;17(Suppl 1):e192–e201. [DOI] [PubMed] [Google Scholar]
- 143.Al-Moraissi EA, Oginni FO, Mahyoub Holkom MA, Mohamed AAS, Al-Sharani HM. Tissue-engineered bone using mesenchymal stem cells versus conventional bone grafts in the regeneration of maxillary alveolar bone: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2020;35:79–90. [DOI] [PubMed] [Google Scholar]
- 144.Tu YK, Needleman I, Chambrone L, Lu HK, Faggion CM Jr. A Bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J Clin Periodontol. 2012;39:303–314. [DOI] [PubMed] [Google Scholar]
- 145.Tavelli L, Ravida A, Barootchi S, Chambrone L, Giannobile WV. Recombinant human platelet–derived growth factor: a systematic review of clinical findings in oral regenerative procedures. JDR Clin Trans Res. 2021;6:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jung RE, Kovacs MN, Thoma DS, Hämmerle CHF. Informative title: guided bone regeneration with and without rhBMP-2: 17-year results of a randomized controlled clinical trial. Clin Oral Implants Res. 2022;33:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Abdal-Wahab M, Abdel Ghaffar KA, Ezzatt OM, Hassan AAA, El Ansary MMS, Gamal AY. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J Periodontal Res. 2020;55:441–452. [DOI] [PubMed] [Google Scholar]
- 149.Abu-Ta'a M. Adjunctive systemic antimicrobial therapy vs asepsis in conjunction with guided tissue regeneration: a randomized, controlled clinical trial. J Contemp Dent Pract. 2016;17:3–6. [DOI] [PubMed] [Google Scholar]
- 150.Amorfini L, Migliorati M, Signori A, Silvestrini-Biavati A, Benedicenti S. Block allograft technique versus standard guided bone regeneration: a randomized clinical trial. Clin Implant Dent Relat Res. 2014;16:655–667. [DOI] [PubMed] [Google Scholar]
- 151.Aoki H, Bizenjima T, Seshima F, et al. Periodontal surgery using rhFGF-2 with deproteinized bovine bone mineral or rhFGF-2 alone: 2-year follow-up of a randomized controlled trial. J Clin Periodontol. 2021;48:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Apatzidou DA, Bakopoulou AA, Kouzi-Koliakou K, Karagiannis V, Konstantinidis A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J Clin Periodontol. 2021;48:1111–1125. [DOI] [PubMed] [Google Scholar]
- 153.Aslan S, Buduneli N, Cortellini P. Clinical outcomes of the entire papilla preservation technique with and without biomaterials in the treatment of isolated intrabony defects: a randomized controlled clinical trial. J Clin Periodontol. 2020;47:470–478. [DOI] [PubMed] [Google Scholar]
- 154.Aspriello SD, Ferrante L, Rubini C, Piemontese M. Comparative study of DFDBA in combination with enamel matrix derivative versus DFDBA alone for treatment of periodontal intrabony defects at 12 months post-surgery. Clin Oral Investig. 2011;15:225–232. [DOI] [PubMed] [Google Scholar]
- 155.Bokan I, Bill JS, Schlagenhauf U. Primary flap closure combined with Emdogain alone or Emdogain and Cerasorb in the treatment of intra-bony defects. J Clin Periodontol. 2006;33:885–893. [DOI] [PubMed] [Google Scholar]
- 156.Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein- 2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693–1707. [DOI] [PubMed] [Google Scholar]
- 157.Carney CM, Rossmann JA, Kerns DG, et al. A comparative study of root defect coverage using an acellular dermal matrix with and without a recombinant human platelet–derived growth factor. J Periodontol. 2012;83:893–901. [DOI] [PubMed] [Google Scholar]
- 158.Chen FM, Gao LN, Tian BM, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cochran DL, Oh TJ, Mills MP, et al. A randomized clinical trial evaluating rh-FGF-2/β-TCP in periodontal defects. J Dent Res. 2016;95:523–530. [DOI] [PubMed] [Google Scholar]
- 160.Coomes AM, Mealey BL, Huynh-Ba G, Barboza-Arguello C, Moore WS, Cochran DL. Buccal bone formation after flapless extraction: a randomized, controlled clinical trial comparing recombinant human bone morphogenetic protein 2/absorbable collagen carrier and collagen sponge alone. J Periodontol. 2014;85: 525–535. [DOI] [PubMed] [Google Scholar]
- 161.Corinaldesi G, Piersanti L, Piattelli A, Iezzi G, Pieri F, Marchetti C. Augmentation of the floor of the maxillary sinus with recombinant human bone morphogenetic protein-7: a pilot radiological and histological study in humans. Br J Oral Maxillofac Surg. 2013;51:247–252. [DOI] [PubMed] [Google Scholar]
- 162.Cortellini P, Tonetti MS. Clinical and radiographic outcomes of the modified minimally invasive surgical technique with and without regenerative materials: a randomized-controlled trial in intra-bony defects. J Clin Periodontol. 2011;38:365–373. [DOI] [PubMed] [Google Scholar]
- 163.Dandu SR, Murthy KR. Multiple gingival recession defects treated with coronally advanced flap and either the VISTA technique enhanced with GEM 21S or periosteal pedicle graft: a 9-month clinical study. Int J Periodontics Restorative Dent. 2016;36:231–237. [DOI] [PubMed] [Google Scholar]
- 164.de Freitas RM, Susin C, Tamashiro WM, et al. Histological analysis and gene expression profile following augmentation of the anterior maxilla using rhBMP-2/ACS versus autogenous bone graft. J Clin Periodontol. 2016;43:1200–1207. [DOI] [PubMed] [Google Scholar]
- 165.De Leonardis D, Paolantonio M. Enamel matrix derivative, alone or associated with a synthetic bone substitute, in the treatment of 1- to 2-wall periodontal defects. J Periodontol. 2013;84:444–455. [DOI] [PubMed] [Google Scholar]
- 166.de Oliveira TA, Aloise AC, Orosz JE, de Mello EOR, de Carvalho P, Pelegrine AA. Double centrifugation versus single centrifugation of bone marrow aspirate concentrate in sinus floor elevation: a pilot study. Int J Oral Maxillofac Implants. 2016;31:216–222. [DOI] [PubMed] [Google Scholar]
- 167.Barcellos de Santana R, Miller Mattos de Santana C. Human intrabony defect regeneration with rhFGF-2 and hyaluronic acid—a randomized controlled clinical trial. J Clin Periodontol 2015;42:658–665. [DOI] [PubMed] [Google Scholar]
- 168.Deshpande A, Koudale SB, Bhongade ML. A comparative evaluation of rhPDGF-BB + β-TCP and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in humans. Int J Periodontics Restorative Dent. 2014;34:241–249. [DOI] [PubMed] [Google Scholar]
- 169.Devi R, Dixit J. Clinical evaluation of insulin like growth factor-I and vascular endothelial growth factor with alloplastic bone graft material in the management of human two wall intra-osseous defects. J Clin Diagn Res. 2016;10:ZC41-ZC46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dhote R, Charde P, Bhongade M, Rao J. Stem cells cultured on beta tricalcium phosphate (β-TCP) in combination with recombinant human platelet–derived growth factor – BB (rh-PDGF-BB) for the treatment of human infrabony defects. J Stem Cells. 2015;10:243–254. [PubMed] [Google Scholar]
- 171.Döri F, Arweiler N, Húszár T, Gera I, Miron RJ, Sculean A. Five-year results evaluating the effects of platelet-rich plasma on the healing of intrabony defects treated with enamel matrix derivative and natural bone mineral. J Periodontol. 2013;84:1546–1555. [DOI] [PubMed] [Google Scholar]
- 172.Dori F, Nikolidakis D, Huszar T, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an enamel matrix protein derivative and a natural bone mineral. J Clin Periodontol. 2008;35:44–50. [DOI] [PubMed] [Google Scholar]
- 173.Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76:605–613. [DOI] [PubMed] [Google Scholar]
- 174.Froum SJ, Wallace S, Cho SC, et al. Histomorphometric comparison of different concentrations of recombinant human bone morphogenetic protein with allogeneic bone compared to the use of 100% mineralized cancellous bone allograft in maxillary sinus grafting. Int J Periodontics Restorative Dent. 2013;33:721–730. [DOI] [PubMed] [Google Scholar]
- 175.Froum SJ, Wallace S, Cho SC, et al. A histomorphometric comparison of Bio-Oss alone versus Bio-Oss and platelet-derived growth factor for sinus augmentation: a postsurgical assessment. Int J Periodontics Restorative Dent. 2013;33:269–279. [DOI] [PubMed] [Google Scholar]
- 176.Geurs N, Ntounis A, Vassilopoulos P, Van der Velden U, Loos BG, Reddy M. Using growth factors in human extraction sockets: a histologic and histomorphometric evaluation of short-term healing. Int J Oral Maxillofac Implants. 2014;29:485–496. [DOI] [PubMed] [Google Scholar]
- 177.Ghezzi C, Ferrantino L, Bernardini L, Lencioni M, Masiero S. Minimally invasive surgical technique in periodontal regeneration: a randomized controlled clinical trial pilot study. Int J Periodontics Restorative Dent. 2016;36:475–482. [DOI] [PubMed] [Google Scholar]
- 178.Gurinsky BS, Mills MP, Mellonig JT. Clinical evaluation of demineralized freeze-dried bone allograft and enamel matrix derivative versus enamel matrix derivative alone for the treatment of periodontal osseous defects in humans. J Periodontol. 2004;75:1309–1318. [DOI] [PubMed] [Google Scholar]
- 179.Hermund NU, Stavropoulos A, Donatsky O, et al. Reimplantation of cultivated human bone cells from the posterior maxilla for sinus floor augmentation. Histological results from a randomized controlled clinical trial. Clin Oral Implants Res. 2012;23:1031–1037. [DOI] [PubMed] [Google Scholar]
- 180.Hoffmann T, Al-Machot E, Meyle J, Jervoe-Storm P-M, Jepsen S. Three-year results following regenerative periodontal surgery of advanced intrabony defects with enamel matrix derivative alone or combined with a synthetic bone graft. Clin Oral Investig. 2016;20:357–364. [DOI] [PubMed] [Google Scholar]
- 181.Hoidal MJ, Grimard BA, Mills MP, Schoolfield JD, Mellonig JT, Mealey BL. Clinical evaluation of demineralized freeze-dried bone allograft with and without enamel matrix derivative for the treatment of periodontal osseous defects in humans. J Periodontol. 2008;79:2273–2280. [DOI] [PubMed] [Google Scholar]
- 182.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet–derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186–1193. [DOI] [PubMed] [Google Scholar]
- 183.Huh JB, Lee HJ, Jang JW, Kim MJ, Yun PY, Kim SH. Randomized clinical trial on the efficacy of Escherichia coli-derived rhBMP-2 with β-TCP/HA in extraction socket. J Adv Prosthodont. 2011;3:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Iorio-Siciliano V, Andreuccetti G, Blasi A, Matarasso M, Sculean A, Salvi GE. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either enamel matrix derivative or collagen membrane. J Periodontol. 2014;85:1342–1350. [DOI] [PubMed] [Google Scholar]
- 185.Jaiswal R, Deo V. Evaluation of the effectiveness of enamel matrix derivative, bone grafts, and membrane in the treatment of mandibular Class II furcation defects. Int J Periodontics Restorative Dent. 2013;33:e58–e64. [DOI] [PubMed] [Google Scholar]
- 186.Jayakumar A, Rajababu P, Rohini S, Butchibabu K, Naveen A, Reddy PK. Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet–derived growth factor with β-tricalcium phosphate in human intra-osseous periodontal defects. J Clin Periodontol. 2011;38:163–172. [DOI] [PubMed] [Google Scholar]
- 187.Jepsen S, Topoll H, Rengers H, et al. Clinical outcomes after treatment of intra-bony defects with an EMD/synthetic bone graft or EMD alone: a multicentre randomized-controlled clinical trial. J Clin Periodontol. 2008;35:420–428. [DOI] [PubMed] [Google Scholar]
- 188.Jo DW, Cho YD, Seol YJ, Lee YM, Lee HJ, Kim YK. A randomized controlled clinical trial evaluating efficacy and adverse events of different types of recombinant human bone morphogenetic protein-2 delivery systems for alveolar ridge preservation. Clin Oral Implants Res. 2019;30:396–409. [DOI] [PubMed] [Google Scholar]
- 189.Jung RE, Glauser R, Schärer P, Hämmerle CHF, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans: a randomized, controlled clinical and histomorphometric study. Clin Oral Implants Res. 2003;14:556–568. [DOI] [PubMed] [Google Scholar]
- 190.Jung RE, Windisch SI, Eggenschwiler AM, Thoma DS, Weber FE, Hämmerle CHF. A randomized-controlled clinical trial evaluating clinical and radiological outcomes after 3 and 5 years of dental implants placed in bone regenerated by means of GBR techniques with or without the addition of BMP-2. Clin Oral Implants Res. 2009;20:660–666. [DOI] [PubMed] [Google Scholar]
- 191.Kaigler D, Pagni G, Park CH, et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 2013;22:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kao DW, Kubota A, Nevins M, Fiorellini JP. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int J Periodontics Restorative Dent. 2012;32:61–67. [PubMed] [Google Scholar]
- 193.Kavyamala D, Sruthima NVS, Dwarakanath GCD, Anudeep M. Evaluation of the efficacy of a 1:1 mixture of β-TCP and rhPDGF-BB in the surgical management of two- and three-wall intraosseous defects: a prospective clinical trial. Int J Periodontics Restorative Dent. 2019;39:107–113. [DOI] [PubMed] [Google Scholar]
- 194.Kim HJ, Chung JH, Shin SY, et al. Efficacy of rhBMP-2/hydroxyapatite on sinus floor augmentation: a multicenter, randomized controlled clinical trial. J Dent Res. 2015;94:158S–165S. [DOI] [PubMed] [Google Scholar]
- 195.Kim MS, Lee JS, Shin HK, Kim JS, Yun JH, Cho KS. Prospective randomized, controlled trial of sinus grafting using Escherichia-coli-produced rhBMP-2 with a biphasic calcium phosphate carrier compared to deproteinized bovine bone. Clin Oral Implants Res. 2015;26:1361–1368. [DOI] [PubMed] [Google Scholar]
- 196.Kim YJ, Lee JY, Kim JE, Park JC, Shin SW, Cho KS. Ridge preservation using demineralized bone matrix gel with recombinant human bone morphogenetic protein-2 after tooth extraction: a randomized controlled clinical trial. J Oral Maxillofac Surg. 2014;72:1281–1290. [DOI] [PubMed] [Google Scholar]
- 197.Koch FP, Becker J, Terheyden H, Capsius B, Wagner W. A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor-5 coated onto β-tricalcium phosphate for sinus lift augmentation. Clin Oral Implants Res. 2010;21:1301–1308. [DOI] [PubMed] [Google Scholar]
- 198.Kuru B, Yilmaz S, Argin K, Noyan Ü. Enamel matrix derivative alone or in combination with a bioactive glass in wide intrabony defects. Clin Oral Investig. 2006;10:227–234. [DOI] [PubMed] [Google Scholar]
- 199.Lee J-H, Jeong S-N. Effect of enamel matrix derivative on alveolar ridge preservation in the posterior maxilla: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2020;22:622–630. [DOI] [PubMed] [Google Scholar]
- 200.Lee J-H, Kim D-H, Jeong S-N. Comparative assessment of anterior maxillary alveolar ridge preservation with and without adjunctive use of enamel matrix derivative: a randomized clinical trial. Clin Oral Implants Res. 2020;31:1–9. [DOI] [PubMed] [Google Scholar]
- 201.Lee JH, Kim DH, Jeong SN. Adjunctive use of enamel matrix derivatives to porcine-derived xenograft for the treatment of one-wall intrabony defects: two-year longitudinal results of a randomized controlled clinical trial. J Periodontol. 2020;91:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee JY, Na HJ, Kim HM, et al. Comparative study of rhPDGF-BB plus equine-derived bone matrix versus rhPDGF-BB plus β-TCP in the treatment of periodontal defects. Int J Periodontics Restorative Dent. 2017;37:825–832. [DOI] [PubMed] [Google Scholar]
- 203.Lekovic V, Camargo PM, Weinlaender M, Nedic M, Aleksic Z, Kenney EB. A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol. 2000;71:1110–1116. [DOI] [PubMed] [Google Scholar]
- 204.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Djordjevic M, Kenney EB. The use of bovine porous bone mineral in combination with enamel matrix proteins or with an autologous fibrinogen/fibronectin system in the treatment of intrabony periodontal defects in humans. J Periodontol. 2001;72:1157–1163. [DOI] [PubMed] [Google Scholar]
- 205.Losada M, González R, Garcia À, Santos A, Nart J. Treatment of non-contained infrabony defects with enamel matrix derivative alone or in combination with biphasic calcium phosphate bone graft: a 12-month randomized controlled clinical trial. J Periodontol. 2017;88:426–435. [DOI] [PubMed] [Google Scholar]
- 206.Maroo S, Murthy KR. Treatment of periodontal intrabony defects using β-TCP alone or in combination with rhPDGF-BB: a randomized controlled clinical and radiographic study. Int J Periodontics Restorative Dent. 2014;34:841–847. [DOI] [PubMed] [Google Scholar]
- 207.Marx RE, Armentano L, Olavarria A, Samaniego J. rhBMP-2/ACS grafts versus autogenous cancellous marrow grafts in large vertical defects of the maxilla: an unsponsored randomized open-label clinical trial. Int J Oral Maxillofac Implants. 2013;28:e243–e251. [DOI] [PubMed] [Google Scholar]
- 208.McAllister BS, Haghighat K, Prasad HS, Rohrer MD. Histologic evaluation of recombinant human platelet–derived growth factor-BB after use in extraction socket defects: a case series. Int J Periodontics Restorative Dent. 2010;30:365–373. [PubMed] [Google Scholar]
- 209.Mercado F, Vaquette C, Hamlet S, Ivanovski S. Enamel matrix derivative promotes new bone formation in xenograft assisted maxillary anterior ridge preservation—a randomized controlled clinical trial. Clin Oral Implants Res. 2021;32:732–744. [DOI] [PubMed] [Google Scholar]
- 210.Meyle J, Hoffmann T, Topoll H, et al. A multi-centre randomized controlled clinical trial on the treatment of intra-bony defects with enamel matrix derivatives/synthetic bone graft or enamel matrix derivatives alone: results after 12 months. J Clin Periodontol. 2011;38:652–660. [DOI] [PubMed] [Google Scholar]
- 211.Mishra A, Avula H, Pathakota KR, Avula J. Efficacy of modified minimally invasive surgical technique in the treatment of human intrabony defects with or without use of rhPDGF-BB gel—a randomized controlled trial. J Clin Periodontol. 2013;40:172–179. [DOI] [PubMed] [Google Scholar]
- 212.Moreno Rodriguez JA, Ortiz Ruiz AJ. Apical approach in periodontal reconstructive surgery with enamel matrix derivate and enamel matrix derivate plus bone substitutes: a randomized, controlled clinical trial. Clin Oral Investig. 2021;26:2793–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–2215. [DOI] [PubMed] [Google Scholar]
- 214.Nevins M, Kao RT, McGuire MK, et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 2013;84:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nevins ML, Camelo M, Schupbach P, Nevins M, Kim SW, Kim DM. Human buccal plate extraction socket regeneration with recombinant human platelet–derived growth factor BB or enamel matrix derivative. Int J Periodontics Restorative Dent. 2011;31:481–492. [PubMed] [Google Scholar]
- 216.Nevins ML, Reynolds MA, Camelo M, Schupbach P, Kim DM, Nevins M. Recombinant human platelet–derived growth factor BB for reconstruction of human large extraction site defects. Int J Periodontics Restorative Dent. 2014;34:156–163. [DOI] [PubMed] [Google Scholar]
- 217.Ntounis A, Geurs N, Vassilopoulos P, Reddy M. Clinical assessment of bone quality of human extraction sockets after conversion with growth factors. Int J Oral Maxillofac Implants. 2015;30:196–201. [DOI] [PubMed] [Google Scholar]
- 218.Ogihara S, Tarnow DP. Efficacy of enamel matrix derivative with freeze-dried bone allograft or demineralized freeze-d ried bone allograft in intrabony defects: a randomized trial. J Periodontol. 2014;85:1351–1360. [DOI] [PubMed] [Google Scholar]
- 219.Ogihara S, Wang HL. Periodontal regeneration with or without limited orthodontics for the treatment of 2- or 3-wall infrabony defects. J Periodontol. 2010;81:1734–1742. [DOI] [PubMed] [Google Scholar]
- 220.Peres MF, Ribeiro ED, Casarin RC, et al. Hydroxyapatite/β-tricalcium phosphate and enamel matrix derivative for treatment of proximal class II furcation defects: a randomized clinical trial. J Clin Periodontol. 2013;40:252–259. [DOI] [PubMed] [Google Scholar]
- 221.Pietruska M, Pietruski J, Nagy K, Brecx M, Arweiler NB, Sculean A. Four-year results following treatment of intrabony periodontal defects with an enamel matrix derivative alone or combined with a biphasic calcium phosphate. Clin Oral Investig. 2012;16:1191–1197. [DOI] [PubMed] [Google Scholar]
- 222.Pourabbas R, Chitsazi MT, Kosarieh E, Olyaee P. Coronally advanced flap in combination with acellular dermal matrix with or without enamel matrix derivatives for root coverage. Indian J Dent Res. 2009;20:320–325. [DOI] [PubMed] [Google Scholar]
- 223.Queiroz LA, Santamaria MP, Casati MZ, et al. Enamel matrix protein derivative and/or synthetic bone substitute for the treatment of mandibular class II buccal furcation defects. A 12-month randomized clinical trial. Clin Oral Investig. 2016;20:1597–1606. [DOI] [PubMed] [Google Scholar]
- 224.Raslan NSE, Nassar MM, Afifi OH, Shoukheba MYM. Intrabony defects management using growth factor enhanced matrix versus platelet rich fibrin utilizing minimally invasive surgical technique: a randomized control study. J Res Med Dent Sci. 2021;9:99–108. [Google Scholar]
- 225.Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011;22:251–258. [DOI] [PubMed] [Google Scholar]
- 226.Rickert D, Vissink A, Slot WJ, Sauerbier S, Meijer HJ, Raghoebar GM. Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. Int J Oral Maxillofac Surg. 2014;43:243–247. [DOI] [PubMed] [Google Scholar]
- 227.Ridgway HK, Mellonig JT, Cochran DL. Human histologic and clinical evaluation of recombinant human platelet–derived growth factor and beta-tricalcium phosphate for the treatment of periodontal intraosseous defects. Int J Periodontics Restorative Dent. 2008;28:171–179. [PubMed] [Google Scholar]
- 228.Rocha Dos Santos M, Menck Sangiorgio JP, da Silva Neves FL, et al. Xenogenous collagen matrix and/or enamel matrix derivative for treatment of localized gingival recessions: a randomized clinical trial. Part II: Patient-reported outcomes. J Periodontol 2017;88:1319–1328. [DOI] [PubMed] [Google Scholar]
- 229.Saito A, Bizenjima T, Takeuchi T, et al. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone mineral or rhFGF-2 alone: a 6-month randomized controlled trial. J Clin Periodontol. 2019;46:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Santana RB, Santana CM. A clinical comparison of guided bone regeneration with platelet-derived growth factor–enhanced bone ceramic versus autogenous bone block grafting. Int J Oral Maxillofac Implants. 2015;30:700–706. [DOI] [PubMed] [Google Scholar]
- 231.Santana RB, Santana CM, Dibart S. Platelet-derived growth factor–mediated guided bone regeneration in immediate implant placement in molar sites with buccal bone defects. Int J Periodontics Restorative Dent. 2015;35:825–833. [DOI] [PubMed] [Google Scholar]
- 232.Scheyer ET, Velasquez-Plata D, Brunsvold MA, Lasho DJ, Mellonig JT. A clinical comparison of a bovine-derived xenograft used alone and in combination with enamel matrix derivative for the treatment of periodontal osseous defects in humans. J Periodontol. 2002;73:423–432. [DOI] [PubMed] [Google Scholar]
- 233.Schincaglia GP, Hebert E, Farina R, Simonelli A, Trombelli L. Single versus double flap approach in periodontal regenerative treatment. J Clin Periodontol. 2015;42:557–566. [DOI] [PubMed] [Google Scholar]
- 234.Sculean A, Barbé G, Chiantella GC, Arweiler NB, Berakdar M, Brecx M. Clinical evaluation of an enamel matrix protein derivative combined with a bioactive glass for the treatment of intrabony periodontal defects in humans. J Periodontol. 2002;73:401–408. [DOI] [PubMed] [Google Scholar]
- 235.Sculean A, Pietruska M, Schwarz F, Willershausen B, Arweiler NB, Auschill TM. Healing of human intrabony defects following regenerative periodontal therapy with an enamel matrix protein derivative alone or combined with a bioactive glass. A controlled clinical study. J Clin Periodontol. 2005;32:111–117. [DOI] [PubMed] [Google Scholar]
- 236.Shalini HS, Vandana KL. Direct application of autologous periodontal ligament stem cell niche in treatment of periodontal osseous defects: a randomized controlled trial. J Indian Soc Periodontol. 2018;22:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shim JY, Lee Y, Lim JH, et al. Comparative evaluation of recombinant human bone morphogenetic protein-2/hydroxyapatite and bovine bone for new bone formation in alveolar ridge preservation. Implant Dent. 2018;27:623–629. [DOI] [PubMed] [Google Scholar]
- 238.Shin SH, Cueva MA, Kerns DG, Hallmon WW, Rivera-Hidalgo F, Nunn ME. A comparative study of root coverage using acellular dermal matrix with and without enamel matrix derivative. J Periodontol. 2007;78:411–421. [DOI] [PubMed] [Google Scholar]
- 239.Stavropoulos A, Becker J, Capsius B, Açil Y, Wagner W, Terheyden H. Histological evaluation of maxillary sinus floor augmentation with recombinant human growth and differentiation factor-5–coated β-tricalcium phosphate: results of a multicenter randomized clinical trial. J Clin Periodontol. 2011;38:966–974. [DOI] [PubMed] [Google Scholar]
- 240.Stavropoulos A, Windisch P, Gera I, Capsius B, Sculean A, Wikesjö UME. A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF-5/β-TCP for periodontal regeneration. J Clin Periodontol. 2011;38:1044–1054. [DOI] [PubMed] [Google Scholar]
- 241.Tavelli L, Barootchi S, Vera Rodriguez M, et al. rhPDGF improves root coverage of a collagen matrix for multiple adjacent gingival recessions: a triple-blinded, randomized, placebo-controlled trial. J Clin Periodontol. Accepted for publication. https://clinicaltrials.gov/ct2/show/NCT04462237. Accessed March 31, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Thakare K, Deo V. Randomized controlled clinical study of rhPDGF-BB + β-TCP versus HA + β-TCP for the treatment of infrabony periodontal defects: clinical and radiographic results. Int J Periodontics Restorative Dent. 2012;32:689–696. [PubMed] [Google Scholar]
- 243.Thoma DS, Bienz SP, Payer M, et al. Randomized clinical study using xenograft blocks loaded with bone morphogenetic protein-2 or autogenous bone blocks for ridge augmentation—a three-dimensional analysis. Clin Oral Implants Res. 2019;30:872–881. [DOI] [PubMed] [Google Scholar]
- 244.Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67:1947–1960. [DOI] [PubMed] [Google Scholar]
- 245.Velasquez-Plata D, Scheyer ET, Mellonig JT. Clinical comparison of an enamel matrix derivative used alone or in combination with a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Periodontol. 2002;73:433–440. [DOI] [PubMed] [Google Scholar]
- 246.Whitt J, Al-Sabbagh M, Dawson D, et al. Efficacy of stem cell allograft in maxillary sinus bone regeneration: a randomized controlled clinical and blinded histomorphometric study. Int J Implant Dent. 2020;6:25. doi: 10.1186/s40729-020-00222-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Windisch P, Stavropoulos A, Molnár B, et al. A phase IIa randomized controlled pilot study evaluating the safety and clinical outcomes following the use of rhGDF-5/β-TCP in regenerative periodontal therapy. Clin Oral Investig. 2012;16:1181–1189. [DOI] [PubMed] [Google Scholar]
- 248.Yamamiya K, Okuda K, Kawase T, Hata K, Wolff LF, Yoshie H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol. 2008;79:811–818. [DOI] [PubMed] [Google Scholar]
- 249.Zanwar K, Kumar Ganji K, Bhongade ML. Efficacy of human umbilical stem cells cultured on polylactic/polyglycolic acid membrane in the treatment of multiple gingival recession defects: a randomized controlled clinical study. J Dent (Shiraz). 2017;18:95–103. [PMC free article] [PubMed] [Google Scholar]
- 250.Zanwar K, Laxmanrao Bhongade M, Kumar Ganji K, Koudale SB, Gowda P. Comparative evaluation of efficacy of stem cells in combination with PLA/PGA membrane versus sub-epithelial connective tissue for the treatment of multiple gingival recession defects: a clinical study. J Stem Cells. 2014;9:253–267. [PubMed] [Google Scholar]
- 251.Zucchelli G, Amore C, Montebugnoli L, De Sanctis M. Enamel matrix proteins and bovine porous bone mineral in the treatment of intrabony defects: a comparative controlled clinical trial. J Periodontol. 2003;74:1725–1735. [DOI] [PubMed] [Google Scholar]
- 252.Hämmerle CHF, Giannobile WV, Working Group 1 of the European Workshop on Periodontology. Biology of soft tissue wound healing and regeneration—consensus report of Group 1 of the 10th European Workshop on Periodontology. J Clin Periodontol. 2014;41(Suppl 15):S1–S5. [DOI] [PubMed] [Google Scholar]
- 253.Dong C, Fischer LA, Theunissen TW. Recent insights into the naive state of human pluripotency and its applications. Exp Cell Res. 2019;385:111645. [DOI] [PubMed] [Google Scholar]
- 254.Guo G, von Meyenn F, Rostovskaya M, et al. Epigenetic resetting of human pluripotency. Development. 2017;144:2748–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stirparo GG, Smith A, Guo G. Cancer-related mutations are not enriched in naive human pluripotent stem cells. Cell Stem Cell. 2021;28:164–169.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Avior Y, Lezmi E, Eggan K, Benvenisty N. Cancer-related mutations identified in primed human pluripotent stem cells. Cell Stem Cell. 2021;28:10–11. [DOI] [PubMed] [Google Scholar]
- 257.Campbell JDM, Fraser AR. Flow cytometric assays for identity, safety and potency of cellular therapies. Cytometry B Clin Cytom. 2018;94:569–579. [DOI] [PubMed] [Google Scholar]
- 258.McGuire MK, Scheyer ET, Gwaltney C. Commentary: incorporating patient-reported outcomes in periodontal clinical trials. J Periodontol. 2014;85:1313–1319. [DOI] [PubMed] [Google Scholar]
- 259.Stefanini M, Jepsen K, de Sanctis M, et al. Patient-reported outcomes and aesthetic evaluation of root coverage procedures: a 12-month follow-up of a randomized controlled clinical trial. J Clin Periodontol. 2016;43:1132–1141. [DOI] [PubMed] [Google Scholar]
- 260.Kim N, Choi KU, Lee E, et al. Therapeutic effects of platelet derived growth factor overexpressed-mesenchymal stromal cells and sheets in canine skin wound healing model. Histol Histopathol. 2020;35:751–767. [DOI] [PubMed] [Google Scholar]
- 261.Steed DL. Clinical evaluation of recombinant human platelet–derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117:143S–149S; discussion 150S-151S. [DOI] [PubMed] [Google Scholar]
- 262.Kaltalioglu K, Coskun-Cevher S. A bioactive molecule in a complex wound healing process: platelet-derived growth factor. Int J Dermatol. 2015;54:972–977. [DOI] [PubMed] [Google Scholar]
- 263.de Sanctis M, Clementini M. Flap approaches in plastic periodontal and implant surgery: critical elements in design and execution. J Clin Periodontol. 2014;41(Suppl 15):S108–S122. [DOI] [PubMed] [Google Scholar]
- 264.Zucchelli G, Tavelli L, Stefanini M, Barootchi S, Wang HL. The coronally advanced flap technique revisited: treatment of peri-implant soft tissue dehiscences. Int J Oral Implantol (Berl). 2021;14:351–365. [PubMed] [Google Scholar]
- 265.Park CH, Rios HF, Jin Q, et al. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials. 2010;31:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable - no new data generated.











