Highlights
-
•
Juvenile granulosa cell tumor is a rare malignancy diagnosed in adult women.
-
•
Juvenile granulosa cell tumor is a rare malignancy diagnosed during pregnancy.
-
•
Large adnexal masses should be removed during the second or third trimesters of pregnancy.
-
•
Shared decision making should be used when deciding to give chemotherapy during pregnancy.
Keywords: Juvenile granulosa cell tumor, Adult, Ovarian malignancy, Pregnancy
Abstract
Objective
To report a case of stage IIIB juvenile granulosa cell tumor (JGCT) complicating pregnancy in a 33 year-old (y.o.) woman.
Methods
Retrospective review of the clinical data, imaging studies, and pathology reports of a case of JGCT diagnosed during pregnancy. Patient consent was obtained for review and presentation of the case. A literature review was conducted.
Results
A 33 y.o., gravida 3, para 1 was incidentally found to have an 8 cm left ovarian mass on an anatomy scan at 22 weeks gestation. Four days later, she presented to labor and delivery triage with abdominal pain. An ultrasound revealed an 11 cm heterogeneous, solid mass in the left adnexa and free fluid at this level. The diagnosis of degenerating fibroid was made based on her clinical presentation and she was discharged. A follow up outpatient MRI revealed a 15 cm left ovarian mass consistent with a primary malignant ovarian neoplasm with moderate ascites and omental, left cul de sac, and probable paracolic gutter implantation. She re-presented 2 weeks later with an acute abdomen and was admitted for a gynecologic oncology consult. Pre-op tumor markers showed an elevated inhibin B. She underwent an exploratory laparotomy, left salpingo-oophorectomy, omental biopsy, and small bowel resection at 25 weeks gestation. Intra-op findings included a ruptured tumor and metastases. Tumor reductive surgery was completed to R0. Pathology revealed a JGCT, FIGO stage IIIB. The pathology and management were reviewed in collaboration with an outside institution. Chemotherapy was delayed until after delivery with monthly MRI surveillance. She underwent induction of labor at 37 weeks followed by an uncomplicated vaginal delivery. She received 3 cycles of bleomycin, etoposide, and cisplatin starting six weeks postpartum. Last known contact was over five years after the initial diagnosis with no evidence of recurrent disease.
Conclusion
JGCTs account for 5% of granulosa cell tumors and 3% are diagnosed after age 30. JGCT is an uncommon neoplasm in pregnancy. 90% are stage I at diagnosis, but advanced stage tumors are aggressive often resulting in recurrence or death within 3 years of diagnosis. We present a surgically treated case with delay in chemotherapy until after delivery with a good outcome after 5 years of follow up.
1. Introduction
Juvenile granulosa cell tumors (JGCTs) are rare, accounting for 5% of all granulosa cell tumors (GCTs), which in total, make up 5% of all gynecologic malignancies. The average age of diagnosis of JGCTs is 13 years old, and only 3% are diagnosed in women over 30 (Inada et al., 2018). Presentation varies by age and can include sexual pseudo precocity in prepubertal girls, menstrual irregularities in reproductive age women, and postmenopausal bleeding in postmenopausal women. Other presentations include findings of an adnexal mass or an acute abdomen with hemoperitoneum if the tumor has ruptured, which occurs in approximately 15% of cases (Disaia et al., 2017).
Ovarian malignancies are rarely diagnosed during pregnancy with a reported incidence of 0.05 to 0.07 per 1,000 pregnancies. Only 10% of GCTs present during pregnancy, accounting for less than 1% of ovarian neoplasms diagnosed in pregnancy (Young et al., 1984, Hasiakos et al., 2006). Rupture occurs in approximately 14% of ovarian tumors found in pregnancy, most commonly during labor or immediately postpartum (Young et al., 1984).
Approximately 90% of JGCTs are stage I and confined to one ovary at the time of diagnosis (Berek and Hacker, 2021). Stage I JGCTs are much less likely to recur than adult granulosa cell tumors (AGCT), which are usually indolent with late recurrence. However, advanced stage JGCTs are typically aggressive resulting in recurrence and death within 3 years of initial diagnosis (Disaia et al., 2017).
In contrast to AGCTs, JGCTs microscopically have rare Call- Exner bodies and large hyperchromatic rounded nuclei without coffee bean appearance with minimal to severe nuclear atypia. JGCTs also typically have a higher mitotic index than adult types. JGCTs may secrete inhibin A and inhibin B, but inhibin B is more sensitive with equal specificity as inhibin A and is more reflective of disease status (Disaia et al., 2017).
Our objective to present a rare case of surgically treated advanced stage JGCT in a 33-year-old pregnant woman with delay in chemotherapy until after delivery with long term survival.
2. Case presentation
A 33-year-old gravida 3 para 1was incidentally found to have an 8 cm left adnexal mass on an anatomy ultrasound performed at 22 weeks gestation. This mass was not visualized on a prior ultrasound performed at 14 weeks gestation. Four days later, she presented to labor and delivery triage with abdominal pain. An ultrasound revealed an 11 cm heterogeneous solid mass in the left adnexa with internal vascularity and free fluid up to the level of the mass. The patient was diagnosed with a presumed degenerating uterine fibroid based on her clinical presentation and discharged home.
A follow up outpatient MRI, seen in Fig. 1, revealed a 15 cm left ovarian mass consistent with a primary malignant ovarian neoplasm, small to moderate ascites, omental implantation, left cul de sac implantation and probable paracolic gutter implantation.
Fig. 1.
Pre-operative MRI: 14.8 × 9.8 × 14.0 cm left ovarian mass, small to moderate ascites, omental implantation, left cul de sac implantation and probably paracolic gutter implantation. A. Sagittal View. B. Axial View. C. Coronal view in relation to the gravid uterus.
She re-presented 2 weeks later at 24 weeks gestation with an acute abdomen. She was admitted to the antepartum unit and gynecologic oncology was consulted. Pre-op tumor markers were notable for markedly elevated inhibin A (426 pg/mL) and inhibin B (2,593.8 pg/mL), elevated AFP (74.5 ng/mL) and CA-125 (269.6 U/mL), and normal levels of LDH (261 IU/L), CEA (<0.5 ng/mL), and CA 19–9 (<2 U/mL).
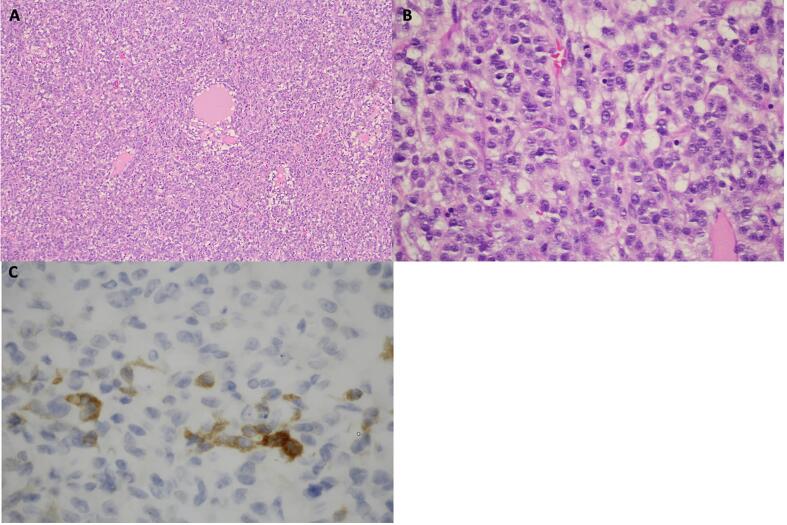
The following day she underwent exploratory laparotomy, left salpingo-oophorectomy, omental biopsy, and small bowel resection. The tumor had ruptured pre-operatively and 600 mL of bloody ascites was present. A 15 cm left ovarian mass with a solid yellow component was adherent to the small bowel and transverse colon, with obvious metastatic disease on both. Tumor reductive surgery was completed to R0. Pathology, seen in Fig. 2, revealed a JGCT with a diameter of 15 cm, capsular rupture with surface tumor involvement, metastases to small bowel and transverse colon, absence of metastases on omental biopsy, and nonmalignant ascites. She was discharged home on post-operative day 7 without complication. Given the findings of JGCT in an adult woman over 30, outside pathology consult was obtained, which confirmed be FIGO stage IIIB JGCT.
Fig. 2.
A. Sheets of granulosa cells surrounding follicle like cystic spaces (H&E stain, 40x magnification). B. Hyperchromatic nuclei lacking the nuclear grooves seen in adult granulosa cell tumors, with numerous mitotic figures (H&E stain, 400x magnification). C. Focal positive staining with immunohistochemical stain for inhibin (IHC stain, 800x magnification).
In collaboration with an outside institution, bleomycin, etoposide, and cisplatin (BEP) was chosen for adjuvant chemotherapy. Due to concern for development of leukemia in the fetus given the toxicity of BEP, chemotherapy was delayed until after delivery with monthly MRI surveillance for the remainder of her pregnancy. It is typical practice in ovarian cancer to initiate chemotherapy within six weeks of surgery, but through collaboration and shared decision making, adjuvant treatment was delayed.
She remained without evidence of disease progression throughout the remainder of her pregnancy. She underwent an induction of labor at 37 weeks gestation followed by an uncomplicated spontaneous vaginal delivery. She received adjuvant chemotherapy with three cycles of BEP starting 6 weeks postpartum. Inhibin A and B were followed at regular intervals and have remained within normal limits to date. The patient’s menses also resumed. Last known contact was over 5 years after initial presentation, with no evidence of recurrent disease.
3. Discussion
JGCTs are most commonly diagnosed in the first 3 decades of life. They are most common in children and adolescents with 80% diagnosed in the first 2 decades of life. They are rare tumors even in children and adolescents, accounting for less than 5% of ovarian malignancies diagnosed in this age group (Berek and Hacker, 2021).
In the largest study of JGCTs to date performed by Young et al. in 1984, only 4 (3.2%) of 125 patients were over 30-years-old. Age ranged from newborn to 67-years-old with an average age of diagnosis of 13-years-old. 44% were diagnosed during the first decade of life, 34% during the second decade, and 18% during the third decade (Young et al., 1984). This was followed by a study of 13 cases of JGCT performed by Biscotti et al. that cited only one patient over the age of 30, with ages ranging from 6 months to 56-years-old (Biscotti and Hart, 1989). A study of 33 cases of JGCT performed by Calaminus et al. did not include any patients over the age of 30 with an age range of 6 months to 17.5-years-old and a median age of diagnosis of 7.6-years-old (Calaminus et al., 1997). Most recently, Karalök et al. performed a single institution retrospective study of 10 cases of JGCT that included only one patient over the age of 30 with a range of 13 to 36-years-old (Karalök et al., 2015).
Histologically, AGCTs are characterized by the presence of Call-Exner bodies, which are small, eosinophilic fluid-filled spaces between granulosa cells resembling ovarian follicles, and “coffee-bean” nuclei, which are grooved, pale nuclei with a low mitotic rate (Zheng et al., 2019). In contrast, JGCTs have rare Call-Exner bodies and cells contain abundant eosinophilic or vacuolated cytoplasm with non-grooved, larger, hyperchromatic nuclei with mild to severe nuclear atypia and higher mitotic rate than adult types (Zheng et al., 2019).
Pathology of this patient’s tumor lacked Call-exner bodies and nuclei were hyperchromatic without nuclear grooves, consistent with diagnosis of JGCT. However, given this patient’s age of 33 and rarity of JGCTs in this age group, outside pathology consultation was obtained, which confirmed the diagnosis.
Both AGCTs and JGCTs are typically low-grade malignancies and most commonly stage I at time of diagnosis. 90% of JGCTs are stage I at the time of diagnosis. AGCTs are usually indolent and recur late, while advanced stage JGCTs are aggressive and recur much earlier (Disaia et al., 2017). In the series of 125 cases by Young et al., 94 were stage IAI, 15 stage IAII, 2 stage IB, 10 stage IC, 2 stage IIB, and 1 stage IIC. Of the 95 patients available for follow up, 6 out of 7 (86%) deaths occurred in patients stage IAII and greater with a 0% one year survival of stage IIB and IIC disease (Young et al., 1984).
Ovarian malignancies are rarely diagnosed in in pregnancy and are most commonly confined to one ovary (Behtash et al., 2008). Management of ovarian malignancies can be controversial during pregnancy and depends on several factors including patient presentation and fetal maturity. It is best to follow the standard of care for treatment of the ovarian malignancy as close as possible, while preserving the pregnancy (Xu et al., 2011). Treatment strategies include conservative management until after delivery or surgical management. Conservative management has been suggested in asymptomatic adnexal masses less than 6 cm, but can result in adverse pregnancy outcomes including rupture, torsion, or hemorrhage. In patients with persistent adnexal masses greater than 6 cm, surgical removal should be delayed until the second trimester if possible due to theoretical risk of spontaneous abortion in the first trimester with anesthesia. In patients with asymptomatic adnexal masses without ascites or evidence of advance disease, it is reasonable to wait until fetal viability to operate. In symptomatic patients, immediate surgical intervention is indicated, regardless of gestational age (Hasiakos et al., 2006).
Tumor markers can be helpful in making the diagnosis of ovarian malignancy during pregnancy. In GCTs, inhibin A and B are typically elevated. Inhibin A can be elevated in pregnancy, especially in preeclampsia, but inhibin B levels are typically within the reference range, so an elevated inhibin B is suspicious for a GCT (Korenaga and Tewari, 2020 Jun).
There are several case reports of management of JGCT in pregnancy to date. Hasiakos et al. reported a 31-year-old with an asymptomatic right ovarian mass diagnosed at 34 weeks gestation. Given the patient was asymptomatic and the mass was confined to one ovary, the patient underwent an elective cesarean section and right salpingo-oophorectomy at 38 weeks gestation. Disease was stage I and last known follow up was 3 months post surgery with no evidence of disease (NED) (Hasiakos et al., 2006).
Xu et al. reported a case of a 24-year-old at 12 weeks gestation with a persistent adnexal mass for 2 months. The patient underwent exploratory laparotomy and left salpingo-oophorectomy resulting in diagnosis of JGCT. Last known follow up was 9 months after diagnosis with NED (Xu et al., 2011). Powell et al. reported a case of a 13-year-old at 26 weeks gestation with a known large complex right sided adnexal mass who presented with abdominal pain. The patient underwent exploratory laparotomy, right oophorectomy with staging. Tumor rupture with associated hemoperitoneum was noted at the time of surgery and greater than 2 cm residual disease remained. Tumor was FIGO stage IIIB. She declined any chemotherapy during pregnancy and delivered at 38 weeks gestation. She underwent a restaging procedure 6 weeks postpartum and received adjuvant chemotherapy. She was NED at last known follow up, 7 years from diagnosis (Powell et al., 1993).
The mainstay of treatment of JGCT in women who desire future childbearing is unilateral salpingo-oophorectomy (Young et al., 1984) In patients who do not desire future childbearing or are older, hysterectomy and bilateral salpingo-oophorectomy can be performed. In our review of the literature, patients over age 30 are most commonly treated with hysterectomy and bilateral salpingo-oophorectomy (Inada et al., 2018, Biscotti and Hart, 1989, Karalök et al., 2015). In Biscotti et al’s study, only the 56-year-old patient underwent hysterectomy and bilateral salpingo-oophorectomy (Biscotti and Hart, 1989). In Karalök et al’s study, only the 36-year-old patient underwent hysterectomy and bilateral salping-oophorectomy (Karalök et al., 2015).
Delaying chemotherapy until after delivery is also controversial. Chemotherapy should be avoided during the first trimester when possible due to teratogenicity and risk of spontaneous abortion. It can be safely administered in the second and third trimesters, but there are associated non teratogenic risks including fetal growth restriction, development of fetal leukemia, or effects on the developing central nervous system (Behtash et al., 2008). It should be avoided after 34 weeks or within 3 weeks of delivery to avoid the hematologic nadir period of both the mother and fetus (Korenaga and Tewari, 2020 Jun). In high risk ovarian cancer, chemotherapy delayed beyond 6 weeks from surgery may result in worse overall survival (Timmermans et al., 2018 Sep). However, this is not well studied in JGCT specifically. Waiting to initiate chemotherapy until the postpartum period resulted in 18 weeks between surgery and start of chemotherapy. The risks and benefits of delay in chemotherapy until after delivery were reviewed with an outside institution. The patient was induced at 37 weeks gestation to minimize the delay in chemotherapy without compromising fetal well- being resulting in a spontaneous vaginal delivery of a healthy infant without any apparent adverse feral outcomes.
4. Conclusion
JGCT is uncommonly diagnosed in patients over 30-years-old and is a rare malignancy in pregnancy. It is most commonly stage I at the time of diagnosis and confined to one ovary. Advanced stage tumors have a poor prognosis. Mainstay of treatment is surgery with addition of adjuvant chemotherapy for advanced stage disease. This is a rare case of an adult pregnant woman with surgically treated advanced stage JGCT with delay in chemotherapy until 6 weeks postpartum with long term survival of more than 4 years.
5. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author Contribution
The manuscript was researched and written by Mackenzie Cummings, MD, with supervision by Joel Sorosky, MD, the patient’s primary gynecologic oncologist. Pamela Edmonds, MD, was one of the pathologists who reviewed the original slides and provided the images for Fig. 2. Mark Shahin, MD, was a collaborating gynecologic oncologist at the time of initial treatment and a reviewer of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Behtash N., Karimi Zarchi M., Modares Gilani M., Ghaemmaghami F., Mousavi A., Ghotbizadeh F. Ovarian carcinoma associated with pregnancy: A clinicopathologic analysis of 23 cases and review of the literature. BMC Pregnancy Childbirth. 2008;8:1–7. doi: 10.1186/1471-2393-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek, J.S., Hacker, N.F., 2021. Berek & Hacker’s gynecologic oncology. Seventh ed. Berek JS, Hacker NF, editors. Philadelphia: Wolters Kluwer.
- Biscotti C.V., Hart W.R. Juvenile granulosa cell tumors of the ovary. Arch. Pathol. Lab Med. 1989;113(1):40–46. [PubMed] [Google Scholar]
- Calaminus G., Wessalowski R., Harms D., Göbel U. Juvenile granulosa cell tumors of the ovary in children and adolescents: Results from 33 patients registered in a prospective cooperative study. Gynecol. Oncol. 1997;65(3):447–452. doi: 10.1006/gyno.1997.4695. [DOI] [PubMed] [Google Scholar]
- Disaia, P., Creasman, W., Mannell, R., McMeekin, S., Mutch, D., 2017. Clinical Gynecologic Oncology, 9th editio. Elsevier, 305–309 p.
- Hasiakos D., Papakonstantinou K., Goula K., Karvouni E., Fotiou S. Juvenile granulosa cell tumor associated with pregnancy: Report of a case and review of the literature. Gynecol. Oncol. 2006;100(2):426–429. doi: 10.1016/j.ygyno.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Inada, Y., Nakai, G., Yamamoto, K., Yamada, T., Hirose, Y., Terai, Y., et al., 2018. Rapidly growing juvenile granulosa cell tumor of the ovary arising in adult: A case report and review of the literature. J. Ovarian Res. 11(1), 10–14. [DOI] [PMC free article] [PubMed]
- Karalök A., Taşçı T., Üreyen I., Türkmen O., Öçalan R., Şahin G., et al. Juvenile granulosa cell ovarian tumor: Clinicopathological evaluation of ten patients. J. Turkish Ger Gynecol Assoc. 2015;16(1):32–34. doi: 10.5152/jtgga.2015.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga T.R.K., Tewari K.S. Gynecologic cancer in pregnancy. Gynecol. Oncol. 2020 Jun;157(3):799–809. doi: 10.1016/j.ygyno.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.L., Johnson N.A., Bailey C.L., Otis C.N. Management of advanced juvenile granulosa cell tumor of the ovary. Gynecol. Oncol. 1993;48(1):119–123. doi: 10.1006/gyno.1993.1019. [DOI] [PubMed] [Google Scholar]
- Timmermans M., van der Aa M.A., Lalisang R.I., Witteveen P.O., Van de Vijver K.K., Kruitwagen R.F., et al. Interval between debulking surgery and adjuvant chemotherapy is associated with overall survival in patients with advanced ovarian cancer. Gynecol. Oncol. 2018 Sep;150(3):446–450. doi: 10.1016/j.ygyno.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Xu H., Shu C., Li N., Xia M., Li T., Zhong Y., et al. Early pregnancy complicated with juvenile granulosa cell tumor. Am. J. Med. Sci. 2011;342(5):435–437. doi: 10.1097/MAJ.0b013e318229992c. [DOI] [PubMed] [Google Scholar]
- Young R.H., Dickersin G.R., Scully R.E. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am. J. Surg. Pathol. 1984;8:575–596. doi: 10.1097/00000478-198408000-00002. [DOI] [PubMed] [Google Scholar]
- Young R.H., Dudley A.G., Scully R.E. Granulosa cell, Sertoli-Leydig cell, and unclassified sex cord-stromal tumors associated with pregnancy: A clinicopathological analysis of thirty-six cases. Gynecol. Oncol. 1984;18(2):181–205. doi: 10.1016/0090-8258(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Zheng, W., Fadare, O., Quick, C.M., Shen, D., Guo, D., 2019. Gynecologic and Obstetric Pathology, Volume 2. 1st ed. 2019. Zheng W, Fadare O, Quick CM, Shen D, Guo D, editors. Singapore: Springer Singapore.