Abstract
Continuous exposure of young women to calabash chalk, especially at child-bearing age, necessitated this study to analyze the chemical constituents of calabash chalk and to evaluate its effect on locomotor activities and behavior in Swiss albino mice. Dried cubes of calabash chalk were purchased and analyzed by atomic and flame atomic absorption spectrophotometer. Twenty-four Swiss-albino mice were taken and divided into four groups: control (1 ml distilled water) and three treated groups with the doses of 200 mg/kg, 400 mg/kg, and 600 mg/kg of calabash chalk suspension, respectively, by oral gavage. Hole Cross, Hole Board, and Open Field tests were performed to evaluate the locomotor activities, behavior, and anxiety along with measurement of body weight. The data were analyzed by SPSS-software. Chemical analysis of calabash chalk showed the presence of trace elements along with some heavy metals such as lead (19.26 ppm), Chromium (34.73 ppm), and arsenic (4.57 ppm). After 21 days of oral administration of calabash chalk, the study showed a significant decrease in body weight in the treated groups of mice (p < 0.01). Decreased locomotor activities were also observed in all three experiments. Significantly decreased locomotion and behaviors were also observed in the hole crossing, line crossing, head dipping, grooming, rearing, stretch attend, central square entry, central square duration, defecation, and urination, in a dose-dependent manner (p < 0.01). These effects also prove the anxiogenic behavior of calabash chalk in albino mice. Heavy metals are believed to be harmful to the brain and responsible for cognitive dysfunction and elevated anxiety. In this study, decreased body weight in mice may occur due to disorders in hunger and thirst centers of the brain by heavy metals. Therefore, heavy metals may be responsible for muscle weakness, decreased locomotor activities, and axiogenic effects of mice.
Keywords: Calabash chalk, Heavy metals, Locomotor activity, Behavioral tests, Anxiogenic effect
1. Introduction
Calabash chalk or soil is a naturally occurring mineral, similar to that of pica [1,2] which is common among the rural and tribal people of Sub-Saharan African nations such as Nigeria, Tanzania, Rwanda, Kenya, Ghana, and South Africa. The practice of eating calabash chalk is known as geophagy. Pregnant women usually consume earth materials to alleviate the symptoms of morning sickness or vomiting. However, geophagy is not restricted by age group, sex, race, geographic region, or period, and is present in almost every country of the world [1,3]. Cultural transfer of geophagy is taking place around the globe by the immigrants. That is why, geophagy has been noticed in the UK, USA, and Canada and this is mainly occurring among the West African and South Asian immigrants. Calabash chalk is imported from Nigeria and South Asia. Importantly, these can be ordered online and marketed in ethnic stores [1,3,4]. The reasons behind the consumption of this soil/chalk can be attributed to medicinal usage, regular diet, nutritional needs, religious belief, feeling of misery, homesickness, depression, and alienation [5].
Calabash chalk, a geophagic substance, has distinct names such as Calabar rock in English, Ndom/Nzu by the Nigerian tribe Efik/Ibibio/Igbo, Argile/La Craie by French, and Sikor in Bangladesh, India, and Pakistan. Ebumba, Poto, and Ulo are also used elsewhere [3]. Naturally occurring calabash chalk is composed of fossilized shells. Artificial calabash chalk can be made by clay, mud and sometimes by adding components like wood ash and occasionally salt. It can be available as dust, molded shapes and blocks. A combination of molding and heating is done to produce the finished item. Among pregnant women, a perpetual desire, in addition to attraction to the scent and taste of the soil, has also been reported [6]. The daily consumption of calabash chalk varies among women from 5 to 10 g [1].
Scientists found aluminum Silicate Hydroxide Al₂Si₂O₅(OH)₄ in calabash chalk, a common representative of the kaolin clay group, along with several other metallic components such as iron, manganese, potassium, aluminum, copper, zinc, barium, chromium, nickel, titanium, rubidium, lead, tin and arsenic. Other organic pollutants, for example, alpha lindane, endosulfan II, and p, endrin, and pI-dichloro diphenyl dichloroethane (DDD) are also present in varying amounts [2,5,7,8,9,10,11].
Studies of calabash chalk in a rat model showed depletion of red blood cells, which was responsible for the changes in hemoglobin level and erythrocyte sedimentation rate [4]. It showed edema and hemorrhages in the mucosa of the stomach. According to their findings, it was also associated with gastrointestinal disorders which may increase anxiety and the perception of pain infrequent intake [3,12]. Moreover, since lead, one of the components in calabash chalk, can cross the placental barrier during pregnancy, it may also affect fetal brain development and behavior as well [2,13].
Moreover, animal [2] and human [13] studies showed that some heavy metals in calabash chalk (e.g. lead, Arsenic) can cross the blood-brain barrier and trigger various consequences including neurotoxicity which may lead to increased anxiety, and behavioral dysfunctions [2,13], decreased IQ, and overall cognitive development [4]. Moreover, the hypothalamic-pituitary-gonadal axis, hunger and thirst centers are reportedly affected by the consumption of calabash chalk [3,14].
Given the context that the chemical constituents of Calabash chalk have a number of health hazards, chemical analysis has been undertaken in different parts of the world [10,15,16]. It has, however, never been performed in the South Asian region, let alone Bangladesh. This study, therefore, intends to analyze the chemical constituents of calabash chalk and to evaluate its effect on locomotor activities, and behavior in Swiss albino mice.
2. Materials and methods
2.1. Ethical approval
Ethical approval has been granted by the Biosafety, Biosecurity & Ethical Committee, Faculty of Biological Sciences, Jahangirnagar University, Savar, Dhaka, Bangladesh (Ref No: BBEC, JU/M 2020 (9)3). All animals were treated humanely throughout the experimental period and maximum care was taken in case of handling following the internationally accepted guide for the care and use of laboratory animals, published by the US National Institutes of Health (NIH Publication No. 85-23, Revised in 1985).
2.2. Sample collection and chemical analysis
Cube-shaped dried calabash chalk was purchased from the local market of Sylhet, Bangladesh. The chemical analysis of the chalk samples was performed at the Bangladesh Council of Scientific and Industrial Research (BCSIR). The samples were oven-dried (electronic oven) at 105°C for 24 h prior to a microwave digestion (Application note: HPR-EN-13, Model: Start D, Milestone, Italy). Chemical constituents of the chalk were analyzed by Atomic Absorption Spectrophotometer (AAS; Model: AA 7000, Shimadzu, Japan) with a direct Air-Acetylene Flame Method for Mg, Ca, Mn, Cu, Zn, Cd, Fe, Al, Cr, and Pb. Potassium (K) was analyzed by Flame Photometer (Model: PFP 7, Jenway, UK). Arsenic (As) was analyzed by Continuous Hydride Generation Atomic Absorption Spectroscopy (Model: AA 7000, Shimadzu, Japan) with HVG (Hydride Vapor Generator, Model: HVG-1, Shimadzu, Japan) Unit; and the analysis of mercury (Hg) was done by Cold-Vapor Atomic Absorption Spectrometric Method (Model: AA 7000, Shimadzu, Japan) with MVU (Mercury Vaporizer Unit, Model: MVU-1A, Shimadzu, Japan). Methods by the Soil Science Society of America (SSSA) were followed for all the tests [17,18,19,20,21,22].
2.3. Materials used
Calabash chalk was chopped and ground into a fine powder with the help of an operated grinder. A stock solution was prepared by dissolving 40 gm of calabash chalk powder in 1000 ml of distilled water in a glass jar [23]. Since this chalk is partially miscible with water, the suspension was appropriately stirred before the oral administration in the mice.
2.4. Animals
Twenty-four female Swiss albino mice aged 26–28 days, with nearly the same time of the estrous cycle (±1 day) [24], weighing between 25 and 30 g were selected for this experiment from the animal house of the Pharmacy Department, Jahangirnagar University, Bangladesh. Throughout the experiment, the mice were nurtured and housed in polypropylene cages. It helps to avoid extraneous trace element contamination. The room temperature was maintained 25 °C ± 1 °C and relative humidity was 45–55%, with a 12:12 h light/dark cycle. Animals were given free access to commercial food pellets, and water, and these were allowed ad libitum.
2.5. Study design
After 26–28 days of weaning, the experimental mice were acclimatized for 21 days before starting the oral administration of calabash chalk. The doses of calabash chalk suspension were started when the mice were 47–49 days old. Four experimental groups consisting of 6 mice were created for this study. The mice of the control group (Group 1) were given 1 ml of distilled water. The mice of treated groups (Group 2, 3 and 4) were administered with 1, 2 and 3 ml i.e 200, 400 and 600 mg/kg doses of calabash chalk suspension, respectively. Calabash chalk was administered once a day by oral gavage between the hours of 10:00 a.m.–12:00 noon. At the same time, the bodyweight of each mouse was recorded for the next 21 days before conducting the experiments. Locomotor activities and behavioral tests of the experimental mice were performed between 68 and 70 days of their age i.e. between 21 and 23 days after administering the doses of calabash chalk. The tests were performed in the most active phase of the animals i.e. between 10:00 a.m. and 2:00 p.m. [25,26]. The following figure (Fig. 1) shows the study design with the timing of weaning and acclimation. It also showed the grouping of mice with the dose administered and experiments performed in the study.
Fig. 1.
Schematic diagram showing the Study design.
2.6. Experimental method
According to the research aim, the following tests were performed on the experimental mice after oral gavage of calabash chalk.
2.6.1. Hole cross test (HCT)
In a 30 cm × 20 cm × 14 cm sized wooden box, a partition was fixed in the center. A hole of 3 cm diameter was created in the middle of the box from a height of 7.5 cm from the floor. The mouse was placed in the middle of the cage on one side of the box. The spontaneous movement of the mice from one chamber to another through the hole was observed for over 3 min. The number of passages through the hole was recorded at 0, 30, 60, 90, and 120 min [27,28].
2.6.2. Hole board test (HBT)
The hole-board is made of wooden apparatus measuring 40 cm by 40 cm with 16 evenly spaced holes (3 cm in diameter) and 25 cm in height. A mouse was allowed to explore the apparatus for 5 min by placing it at the middle of the device. Exploratory features of mice like several areas crossed and head dipping were observed and recorded in addition to the frequency of defecation. The head dip was a success since both eyes reached the bottom of the hole. The board along with floors were cleansed with 70% alcohol between each trial [2,29].
2.6.3. Open field test (OFT)
The open field apparatus was constructed with white plywood (72 cm × 72 cm × 36 cm), where the floor was made of cardboard and divided into 16 equal black and white-colored squares (18 cm × 18 cm) for assessing the locomotion and behavior of mice. The experiment began by placing the mouse in the center for 3 min, and factors such as horizontal locomotion (Square crossing, stretching, central square entry, central square duration), rate of vertical operation (rearing), and grooming were assessed along with the frequency of urination and defecation started at 0, 30, 60, 90 and 120 min after oral administration of calabash chalk. During the given time, the exploratory behavior of the mice was determined manually by an experienced observer. Both the walls and the floors were cleaned by using 70% ethyl alcohol between the trials [3,29].
2.7. Statistical analysis
Statistical analyses were done by the Statistical Package for Social Science Software (SPSS) ver 24 (IBM, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was applied where experimental data were collected at several time intervals (e.g. HCT and OFT). Significant observations were further analyzed using Bonferroni's post hoc tests [29]. A two-tailed p-value of <0.05 was considered statistically significant.
3. Results
Table 1 shows the concentration of trace elements and heavy metals, in parts per million (i.e. mg/kg), in calabash chalk obtained by the chemical analysis.
Table 1.
Concentration of some trace elements and heavy metals in calabash chalk obtained by chemical analysis.
| Sample | Sl.No | Elements analyzed | Concentration of elements (ppm ± SD) |
|---|---|---|---|
| Calabash Chalk Soil Sample |
01. | Magnesium (Mg) | 31.47 ± 0.42 |
| 02. | Potassium (K) | 0.20% ± 0.01% | |
| 03. | Calcium (Ca) | 26.52 ± 0.37 | |
| 04. | Manganese (Mn) | 37.29 ± 0.36 | |
| 05. | Copper (Cu) | 19.39 ± 0.25 | |
| 06. | Zinc (Zn) | 55.36 ± 0.41 | |
| 07. | Iron (Fe) | 2.53% ± 0.08 | |
| 08. | Aluminum (Al) | 223.30 ± 0.24 | |
| 09. | Chromium (Cr) | 34.45 ± 0.30 | |
| 10. | Lead (Pb) | 19.52 ± 0.32 | |
| 11. | Cadmium (Cd) | BDLa | |
| 12. | Arsenic (As) | 4.60 ± 0.18 | |
| 13. | Mercury (Hg) | BDLa |
BDL= Below Detection Limit (Method detection limit = 0.10 ppm).
3.1. Measurement of body weight
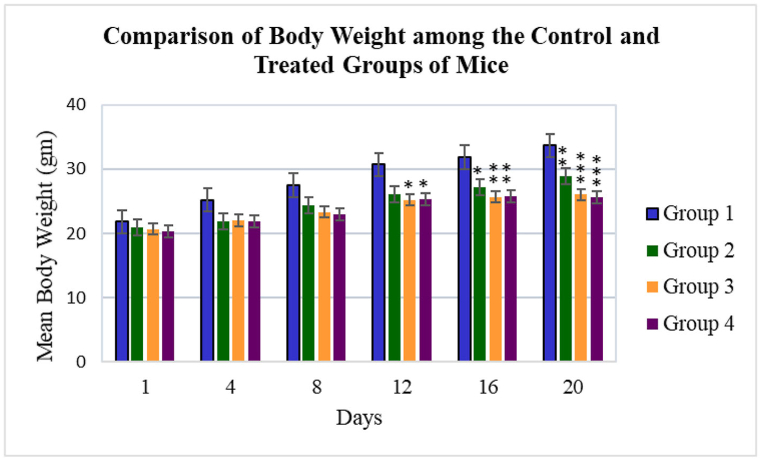
Significantly decreased body weight was found among the treated groups when compared with the control group (p = 0.000). No statistically significant differences were observed among the treated groups (p > 0.05). However, on the 20th day of the dose administration, significantly decreased body weight was recorded (p = 0.053) in groups 3 and 4 as compared to group 2 (Fig. 2).
Fig. 2.
Comparison of body weight among the control and the treated groups of mice.
3.1.1. Hole cross test (HCT)
The F-statistic from the analysis of variance has appeared to be highly significant (F = 12.33; p = 0.000), showing statistically significant variation across the groups in the hole cross test. This observational study showed a significant decrease in the number of holes crossed by the mice (p < 0.01) from the third (60 min) observation period and continued up to the fifth (120 min) observation period when compared with the control group (Fig. 3). Moreover, during the same observational period, Bonferroni's post hoc tests showed a significantly decreased outcomes in groups 3 and 4 as compared to group 2. However, no statistically significant differences were observed between groups 3 and 4 during each observation time (p = 1.000).
Fig. 3.
Evaluation of behavioral effects of calabash chalk by Hole Cross Test (Mean ± SE; n = 6). Bonferroni's post hoc analyses as compared to control: *p < 0.05, **p < 0.01 and ***p < 0.001.
3.1.2. Hole board test (HBT)
In the hole board test, the Analysis of Variance showed highly significant difference in all the parameters including area crossed (F = 13.72, p = 0.000), head dipping (F = 21.74, p = 0.000) and defecation (F = 5.285, p = 0.008). The post hoc test confirmed that, the treated groups, especially groups 3 and 4, showed a significantly lower number of area crossing (Fig. 4A) and head dipping (Fig. 4B) compared to the control group (p = 0.000).
Fig. 4.
Evaluation of behavioral effects of calabash chalk by Hole Board Test (Mean ± SE; n = 6). A) Area crossed, B) Head Dipping. Bonferroni's post hoc analyses as compared to control: ***p < 0.001.
3.1.3. Open field test (OFT)
From the ANOVA tests, the experimental parameters of OFT such as square crossing (F = 23.15), grooming (F = 62.43), rearing (F = 12.69), stretching (F = 4.34), central square entry (F = 12.20), and central square duration (F = 16.14) were decreased significantly (p < 0.001) among the treated groups in comparison with the control group (Fig. 5A-D). The data between central square entries versus central square duration also showed the same observation (F = 21.34, p < 0.000). They remained close to the walls of the field. In all the parameters of the experiments, the decreased effects started from 30 min or 60 min observational period and continued until 120 min as compared to the control group. All the observational parameters showed a gradual decrease in locomotion and behavior with increased doses. However, among the treated groups, the post hos test showed a significantly decreased effect of calabash chalk in groups 3 and 4, once compared with group 2 (p < 0.05). Moreover, in all the parameters of the OFT, no significant differences were observed when group 3 was compared with group 4 (p > 0.05). A significant decrease in the frequency of defecation was observed in the treated group 4 when compared with the control group (p = 0.000). The frequency of urination was not significantly different among the treated groups (F = 3.12, p > 0.05).
Fig. 5.
Evaluation of locomotors and behavioral effects of calabash chalk by Open Field Test. A) number of squares travelled, B) grooming, C) rearing and D) stretch attend, (Mean ± SE, n = 6). Bonferroni's post hoc analyses as compared to control: *p < 0.05, **p < 0.01 and ***p < 0.001.
4. Discussion
Since the chemical constituents of calabash chalk have a number of health hazards, few studies have done the chemical analysis of the chalk, however, it has never been performed in the South Asian region of the world. This study, therefore, intends to analyze the chemical composition of calabash chalk obtained from Sylhet, the north-eastern region of Bangladesh. Some trace elements and heavy metals were found in the chalk. Chemical analysis of the chalk revealed the following minerals: iron, magnesium, potassium, manganese, calcium, copper, zinc, among others. These minerals are known to be beneficial for both plants and animals. On the other hand, the presence of metals such as aluminum, lead, arsenic, chromium, and cadmium in the chalk may be harmful for the body depending on its dose and bioavailability [3,4,12,13]. Thus, the potential toxic effects of the chalk may supersede its beneficial effects.
The Food Standard Agency UK, stated that along with other metallic constituents the amount of lead in Calabash chalk ranges from 8.2 mg/kg to 16.1 mg/kg [1]. However, scientists found a significantly high amount of lead in calabash chalk at a mean concentration of 40 mg/kg [1,3]. In this study, the concentration of lead in calabash chalk is 19.52 ppm i.e. 19.52 mg/kg. It also contains aluminum and arsenic at a concentration of 223.30 ppm and 4.60 ppm, respectively. According to the CDC, blood lead concentration in humans should be less than 5 μg/dl [8,9]. Prolonged exposure to these metals is reported as a cumulative toxicant and affects almost every organ in the body including the central and peripheral nervous system, hematopoietic system, cardiovascular system, reproductive system, kidney, and bones [2,12,14,30,31].
This study also examined the effect of calabash chalk on body weight, locomotor activities, (fear-related) behavior, and anxiety of Swiss albino mice. The data were analyzed by ANOVA tests since there were more than two groups to observe in every time-intervals. Since this study includes multiple pair-wise tests between different groups, in addition to the comparison across the groups captured by ANOVA, Bonferroni's post hoc tests have been performed to reduce the chances of obtaining false-positive results (Type I errors). A significant decrease in the body weight gain was observed in the treated groups of mice than in the control group. Decreased locomotor activities were also observed significantly in the measurement of hole cross, hole board, and open field tests. Other significantly decreased activities in head dipping, frequency of hole crossing, line crossing, grooming, rearing, stretch attends, frequency of central square entry, and duration suggest increased anxiety in the test animals. These results demonstrated that calabash chalk shows decreased locomotion and an anxiogenic effect on the CNS [2], [3], [4], [12], [13], [14].
The significant decrease in body weight among the treated groups of mice might have occurred due to the presence of toxic substances in the chalk [4,10,30]. These effects may be responsible for gastritis, nausea, vomiting, constipation, stomach upset, colicky pain, and anorexia [32,33,34,35], which may lead to loss of appetite and reduced body weight. Moreover, the presence of calabash chalk in gastrointestinal mucosa reduces the absorption of nutrients which might play a vital role in this regard [35]. Furthermore, the reduction of body weight in the treated groups might be due to the disturbance in the hunger and thirst centers because of the consumption of calabash chalk [3,14].
In the areas of biomedical, pharmacological, and psychological research, a series of behavioral tests have been used to investigate the effects of environmental and genetic factors on the physiological and psycho-social status of experimental subjects [3]. In behavioral pharmacology, the exploratory behavior of laboratory rodents is of interest in several areas. However, which animal model is the best test to measure exploratory behavior in rodents remains a contentious issue [29]. To evaluate the changes in the emotional state of mice, scientists evaluate the changes in exploratory activity. Therefore, this study focused on the three most frequently used behavioral tests in animal research, such as hole cross, hole board, and open field tests.
In the hole cross test, significantly decreased locomotor activity (number of crossing the hole) was observed among the mice in the treated groups (groups 3 and 4) when compared with the control group (p = 0.000). This indicates a reduction in the inquisitiveness of the animal to a new environment which is deemed as a sedative [27,28]. Sedative activity is accompanied by behavioral effects and this includes a decreased number of hole crosses (Fig. 3). However, calabash chalk is not reported to cause sedation in animals. Furthermore, neuromuscular toxicity is reported in some studies [3,36]. Therefore, decreased locomotion in the mice could arise from muscle weakness caused by calabash chalk which ultimately limits the performance of the animals. The muscular impairment could be issued from the presence of lead in the calabash chalk in high amounts since lead can increase oxidative stress and influence central pattern generators present in the spinal cords which are responsible for rhythmic movements [3].
The methodological simplicity of the hole-board model makes it useful for measuring the animal's response to an unfamiliar or a new environment that can detect anxiogenic and/or anxiolytic effects. Several animal behavioral responses to new environment are possible to observe and quantify by this method. A strong relationship has been observed between the head-dipping behavior of the animals and their emotional state [37]. The decreased number of head dips observed in this study suggested anxiogenic effects of calabash chalk (Fig. 4B). However, since the clay is a mixture of compounds with both organic and inorganic pollutants, it is very difficult to pinpoint the elements which are responsible for increased anxiety in the subjects. Nevertheless, lead and arsenic had previously been reported to increase anxiety and perception of pain [3,12].
Inhibitory networks of γ-aminobutyric acid-ergic (GABAergic) neurons play a vital role in the modulation of anxiety responses Changes in the GABAA receptor subunit may cause neuronal inhibition downregulated in anxiety states. In this way, neurosteroids are synthesized in the brain and are regulated by stress and anxiogenic stimuli [29]. After oral administration of calabash chalk, changes in neurosteroid synthesis and GABAA receptor axis may be changed and responsible for the modulation of anxiety.
Open field test potentially confound general locomotor activity with exploration [29]. It is a robust behavioral test to determine the vertical and horizontal locomotion of test subjects. The locomotion pattern is used to estimate the subjects’ anxiety. A subject with increased anxiety will have decreased overall locomotion and will stay closer to the walls of the apparatus. This study observed a significant decrease in the frequency of square crossing, grooming, rearing, stretch attends, frequency of central square entry, and duration which suggests increased anxiety in the test subjects. The linear pattern of decreased locomotion is suggestive of a dose-dependent anxiogenic effect of calabash chalk. Moreover, neurotoxicity can also be an undermining factor for the decreased locomotion due to the presence of heavy metals in calabash chalk [3]. A low frequency of rearing indicates decreased locomotion and exploration and/or a higher level of anxiety. Consequently, increased levels of anxiety lead to decreased exploratory behavior and vice versa. This study found less locomotion among the treated groups of mice and a desire to stay close to the walls of the field which indicates increased anxiety due to the consumption of calabash chalk [37,38].
The most established indicators of emotional behavior in the open field test are ambulation [37,38]. It has been proposed that the fear or anxiety response of the animal exposed to a new environment is accompanied by low ambulation, especially in the central zone [37,38,39,40,41]. This study showed a decreased effect on both the central square entry and duration, which may happen due to the consumption of calabash chalk [3,37]. On the other hand, a significant decrease in the frequency of defecation in the treated group 4 might arise from decreased bowel movement as some of the constituents of the calabash chalk has the potential to fortify intestinal mucosa and slow down digestion to increase intestinal absorption of elements [30].
5. Conclusion
The study findings of the behavioral tests indicate that a decrease in locomotor activity and anxiogenic effects are prominent in swiss albino mice by the consumption of calabash chalk. Lower locomotor activity and higher anxiety may occur due to the presence of heavy metals in calabash chalk and may be an alteration of GABAA-ergic receptor activity. An elevated level of anxiety might result from trace metals like lead and arsenic in the chalk. Studies showed that these metals can also cause neurotoxicity. Moreover, since lead can cross the placental barrier during pregnancy, it may also affect the unborn fetus [2]. Therefore, it is predictable that long-term consumption of a calabash chalk diet by a human may impair locomotor and social behavior. As a result, people should be discouraged from the consumption of calabash chalk by highlighting the health issues.
6. Recommendations
Chemical analysis of calabash chalk showed several minerals and metals, some of which are beneficial, and some are harmful for the human body. In acute usage, calabash chalk may be relatively non-toxic, however, chronic consumption may be toxic [2]. Moreover, their microbial sterility is questionable and needs to be investigated [1]. Therefore, consumption of calabash chalk is discouraged to the habituated consumers by the scientists.
Funding
The author(s) received no specific funding for this work.
Acknowledgments
The contribution of Luluin Maknun Shova for performing the laboratory experiments, Syeda Fahria Hoque Mimmi and Iffat Islam Mayesha for referencing are highly acknowledged. Laboratory support from the Department of Pharmacy, Jahangirnagar University are gratefully appreciated.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14463.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Abrahams P.W., Davies T.C., Solomon A.O., Trow A.J., Wragg J. Human geophagia, calabash chalk and undongo: mineral element nutritional implications. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekong M., Peter A., kanem T., Eluwa M., Mbadugha C., Osim E. Calabsh chalk geophagy affects gestating rats behavior and the histomorphology of the cerebral cortex. J. Behav. Brain Sci. 2014;2014 doi: 10.1155/2014/394847. article ID 394847 http/dx/doi/org/10.1155/2014/394847. [DOI] [Google Scholar]
- 3.Owhorji B., Okon U., Nwankwo A., Osim E. Chronic consumption of calabash chalk diet impairs locomotor activities and social behaviour in Swiss white Cd-1 mice. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moses B.E., Emma E.J., Christopher C.M., Enobong I B., Theresa B.E. Effect of calabash chalk on the histomorphology of the gastro-oesophageal tract of growing wistar rats. Malays. J. Med. Sci. MJMS. 2012;19:30–35. [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Rmalli S.W., Jenkins R.O., Watts M.J., Haris P.I. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ. Health. 2010;9:79. doi: 10.1186/1476-069X-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyanza E.C., Joseph M., Premji S.S., Thomas D.S., Mannion C. Geophagy practices and the content of chemical elements in the soil eaten by pregnant women in artisanal and small scale gold mining communities in Tanzania. BMC Pregnancy Childbirth. 2014;14:144. doi: 10.1186/1471-2393-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabash chalk may pose health risk for pregnant and breastfeeding women. Nat. Health Care Can. 2022 https://naturalhealthcare.ca/health_canada_warnings.phtml?id=135 accessed September 7. [Google Scholar]
- 8.CDC Updates Blood Lead Reference Value | Lead. CDC; 2022. https://www.cdc.gov/nceh/lead/news/cdc-updates-blood-lead-reference-value.html accessed. [Google Scholar]
- 9.CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention. 2012. p. 16. [Google Scholar]
- 10.Dean J.R., Deary M.E., Gbefa B.K., Scott W.C. Characterisation and analysis of persistent organic pollutants and major, minor and trace elements in Calabash chalk. Chemosphere. 2004;57:21–25. doi: 10.1016/j.chemosphere.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Dooley E.E. The beat. Environ. Health Perspect. 2011;119:A246–A247. doi: 10.1289/ehp.119-a246b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owhorji B., Okon U., Osim E. Calabash chalk chronic diet consumption elevates anxiety and pain perception. World J. Pharmaceut. Res. 2018;7 doi: 10.20959/wjpr201812-12652. [DOI] [Google Scholar]
- 13.Cleveland Lisa M., Minier Monica L., Cobb Kathleen.A., Scott Anthony.A., German Victor.F. Lead hazards for pregnant women and children: part 2: more can still be done to reduce the chance of exposure to lead in at-risk populations. Am. J. Nurs. 2008;108 doi: 10.1097/01.NAJ.0000339156.09233.de. [DOI] [PubMed] [Google Scholar]
- 14.Oyewopo A., Obasi K., Anumudu K., Yawson E. Histological and hormonal studies of calabash chalk on ovarian function in adult female wistar rats. J. Morphol. Sci. 2017;34:173–177. doi: 10.4322/jms.114317. [DOI] [Google Scholar]
- 15.Ekanem T.B., Ekong M.B., Eluwa M.A., Igiri A.O., Osim E.E. Maternal geophagy of calabash chalk on foetal cerebral cortex histomorphology. Malays. J. Med. Sci. MJMS. 2015;22:17–22. [PMC free article] [PubMed] [Google Scholar]
- 16.D'Elia L., Barba G., Cappuccio F.P., Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J. Am. Coll. Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Ganje T.j., Rains D.W. Methods Soil Anal. John Wiley & Sons, Ltd; 1983. Arsenic; pp. 385–402. [DOI] [Google Scholar]
- 18.Gambrell R.P., Patrick W.H., Jr. Methods Soil Anal. John Wiley & Sons, Ltd; 1983. Manganese; pp. 313–322. [DOI] [Google Scholar]
- 19.Baker D.E., Amacher M.C. Methods Soil Anal. John Wiley & Sons, Ltd; 1983. Nickel, copper, zinc, and cadmium; pp. 323–336. [DOI] [Google Scholar]
- 20.Reisenauer H.M. Methods Soil Anal. John Wiley & Sons, Ltd; 1983. Chromium; pp. 337–346. [DOI] [Google Scholar]
- 21.Burau R.g. Methods Soil Anal. John Wiley & Sons, Ltd; 1983. Lead; pp. 347–365. [DOI] [Google Scholar]
- 22.Olson R.V., Ellis R., Jr. John Wiley & Sons, Ltd; 1983. Iron, in: Methods Soil Anal; pp. 301–312. [DOI] [Google Scholar]
- 23.Akpantah A.O., Ibok O.S., Ekong M.B., Eluwa M.A., Ekanem T.B. The effect of calabash chalk on some hematological parameters in female adult Wistar rats. Turk. J. Haematol. 2010;27:177–181. doi: 10.5152/tjh.2010.25. [DOI] [PubMed] [Google Scholar]
- 24.Champlin A.K., Dorr D.L., Gates A.H. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol. Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 25.Labots M., Van Lith H.A., Ohl F., Arndt S.S. The modified hole board - measuring behavior, cognition and social interaction in mice and rats. J. Vis. Exp. 2015 doi: 10.3791/52529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomons A.R., van Luijk J.a.K.R., Reinders N.R., Kirchhoff S., Arndt S.S., Ohl F. Identifying emotional adaptation: behavioural habituation to novelty and immediate early gene expression in two inbred mouse strains. Gene Brain Behav. 2010;9:1–10. doi: 10.1111/j.1601-183X.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 27.Apu A.S., Hossain F., Rizwan F., Bhuyan S.H., Matin M., Jamaluddin A.T.M. Study of pharmacological activities of methanol extract of Jatropha gossypifolia fruits. J. Basic Clin. Pharm. 2012;4:20–24. doi: 10.4103/0976-0105.109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moniruzzaman M., Sharoti Bhattacharjee P., Rahman Pretty M., Sarwar Hossain M. Sedative and anxiolytic-like actions of ethanol extract of leaves of Glinus oppositifolius (linn.) aug. DC, evid.-based complement. Altern. Med. ECAM. 2016 doi: 10.1155/2016/8541017. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown G.R., Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav. Process. 2008;78:442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry J.M., Cring D., Geophagy . Soils Hum. Health; 2012. An Anthropological Perspective; pp. 179–198. [DOI] [Google Scholar]
- 31.Kawai K., Saathoff E., Antelman G., Msamanga G., Fawzi W.W. Geophagy (Soil-eating) in relation to Anemia and Helminth infection among HIV-infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009;80:36–43. [PMC free article] [PubMed] [Google Scholar]
- 32.Aluminum Hydroxide: MedlinePlus drug information. https://medlineplus.gov/druginfo/meds/a699048.html (n.d.) accessed.
- 33.Ganrot P.O. Metabolism and possible health effects of aluminum. Environ. Health Perspect. 1986;65:363–441. doi: 10.1289/ehp.8665363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata K., Iwata T., Dakeishi M., Karita K. Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J. Occup. Health. 2009;51:1–12. doi: 10.1539/joh.k8003. [DOI] [PubMed] [Google Scholar]
- 35.Opara Julia K., Nwagbaraocha Eileen C. The effect of calabash chalk on the uterus of adult female Wistar rats. GSC Biol. Pharm. Sci. 2018;5 doi: 10.30574/gscbps.2018.5.2.0109. [DOI] [Google Scholar]
- 36.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarrar B., Al‐Doaiss A., Shati A., Al‐Kahtani M., Jarrar Q. Behavioural alterations induced by chronic exposure to 10 nm silicon dioxide nanoparticles. IET Nanobiotechnol. 2021;15:221–235. doi: 10.1049/nbt2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anchan D., Clark S., Pollard K., Vasudevan N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav. 2014;4:51–59. doi: 10.1002/brb3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X., Ding Z., Fan T., Wang K., Li S., Zhao J., Zhu W. Childhood social isolation causes anxiety-like behaviors via the damage of blood-brain barrier in amygdala in female mice. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.943067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laaziz A., El Mostafi H., Elhessni A., Touil T., Doumar H., Mesfioui A. Chronic clomipramine treatment reverses depressogenic-like effects of a chronic treatment with dexamethasone in rats. IBRO Neurosci. Rep. 2022;13:147–155. doi: 10.1016/j.ibneur.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Accrombessi G., Galineau L., Tauber C., Serrière S., Moyer E., Brizard B., Le Guisquet A.-M., Surget A., Belzung C. An ecological animal model of subthreshold depression in adolescence: behavioral and resting state 18F-FDG PET imaging characterization. Transl. Psychiatry. 2022;12:356. doi: 10.1038/s41398-022-02119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.