Abstract
Introduction
Neuroendocrine carcinoma of the cervix (NECC) is a rare variant of cervical cancer with poor prognosis and high mortality. Recurrence is seen with multi-organ metastasis including liver.
Case presentation
A 65 year old female presented with vaginal bleeding for the past one year. Cervical cancer screening and biopsy demonstrated poorly differentiated squamous carcinoma. Immunohistochemistry showed positive expression of chromogranin, synaptophysin, pancytokeratin, TTP1, and CEA and negative expression of p40 and estrogen receptors. An adenocarcinoma with neuroendocrine tumor was suggested. Hysterectomy with bilateral salpingo-oophrectomy was performed. This was followed by carboplatin and etoposide therapy to have clinical remission for a year. Then recurrence was observed to start same drugs again resulting in to partial improvement. It was followed by radiotherapy. The patient succumbed to death approximately after three months.
Conclusion
A metastatic lesion in liver may be a case of Neuroendocrine tumor of cervix, a rare condition that can be easily missed on histopathological examination. More studies are required to establish a standard therapeutic protocol.
Keywords: Neuroendocrine neoplasia, Immunohistochemistry, Carcinoma cervix
Highlights
-
•
Neuroendocrine tumor of cervix is a rare condition that can be easily missed on histopathological examination.
-
•
Due to aggressive nature of cervical neuroendocrine tumor, patients must be kept under careful observation.
-
•
Metastasis in such cases, particularly to the liver, lungs, and rib cage, must be kept in mind.
-
•
Pregnancy associated neuroendocrine tumor needs a personalized therapeutic approach.
-
•
More case studies are required to establish a standard therapeutic protocol.
1. Background
Neuroendocrine neoplasms (NENs) are described as malignant growth arising from neuroendocrine cells with a common occurrence in the gastrointestinal tract, pancreas, and lung [1]. These cells belong to the embryonic neuroectodermal origin and demonstrate an immunohistochemical profile similar to that of endocrine cells. Owing to their endocrine nature, NENS can have the ability to secrete hormones, thereby labeled as functional, whereas non-secreting NENs are considered non-functional [2]. Well-differentiated NENs are categorized as low-grade tumors which are further classified as Grade 1 (typical carcinoid) and Grade 2 (atypical carcinoid) based on cell proliferation and mitotic index with poorly differentiated NENs being designated as high-grade tumors of grade 3 which manifest mostly as small cell carcinoma but also occur as large cell carcinoma [3]. The terminology for different NENs includes carcinoid, neuroendocrine tumor (NET), and well-differentiated neuroendocrine carcinoma (NEC) for well-differentiated NENs whereas small cell and large cell NEC for poorly differentiated NENs [4].
NEN of the cervix was first reported in the 1970s and presently constitutes approximately 0.9–1.5 % of all cervical malignancies with small cell NEC being predominant [5], [6]. It is more inclined towards early nodal and distant metastasis and often has a poor prognosis [7]. These cervical NECs are often associated with Human Papilloma Virus infection, which is also proposed to mediate the development of cervical NEC [8]. The mean recurrence-free survival and the mean overall survival have been estimated up to 16 and 48 months respectively [9]. Cervical NENs have also been occasionally found admixed with adenocarcinoma. The diagnosis and subsequent therapeutic intervention in such cases are complicated due to limited incidence. To improve the quality of surgical case reports, The SCARE Guidelines were formulated in 2016, revised in 2018 and now the same have been updated in DELPHI consensus exercise [10]. In this study we are presenting a unique case of mixed malignant tumor of cervix consisting of two components, neuroendocrine carcinoma and adenocarcinoma, which was managed successfully for one year, following which widespread metastatic lesions resulted into death of the patient.
2. Case presentation
A postmenopausal woman in her 60's presented with two episodes of vaginal bleeding for the past years. Cervical screening demonstrated changes consistent with high-grade (poorly differentiated) dyskaryosis/invasive squamous carcinoma. On biopsy, histomorphology showed features suggestive of poorly differentiated invasive keratinising squamous (basaloid) cell carcinoma. Immunohistochemical staining of the tumor tissue showed positive expression of chromogranin, synaptophysin, pancytokeratin, TTP1, and CEA and negative expression of p40 and estrogen receptors. A poorly differentiated adenocarcinoma with neuroendocrine tumor was suggested. The tumor was determined to be of stage pT1b1pN0, and FIGO stage IB1.
A mass lesion detected on Computed Tomography (CT) scan was also observed on MRI to be located in the lower uterine body and cervix measuring 2.7 × 1.8 × 3.2 cm. An ulceroproliferative growth of 1.8 × 1.5 × 1.2 cm was identified on the posterior lip of the cervix. Parametrium and pelvic walls were normal. An umbilical and infra umbilical hernia containing omental fat and bowel loops was also seen. The tumor had a depth of invasion of 1.8 cm with evidence of lymphovascular emboli (Fig. 1, Fig. 2).
Fig. 1.
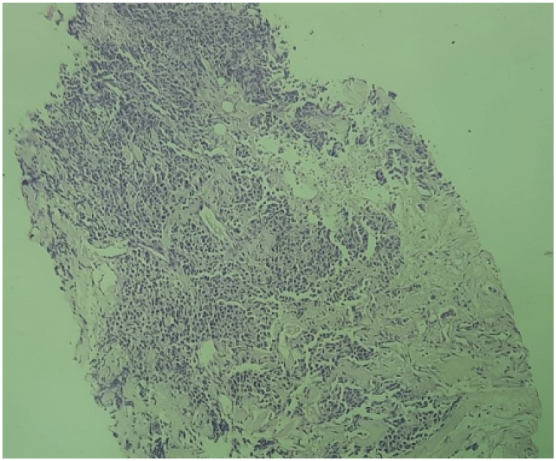
Histopathology of tumor tissue from cervix showing keratinizing squamous cell carcinoma, Hematoxylin & Eosin stain, 4×.
Fig. 2.
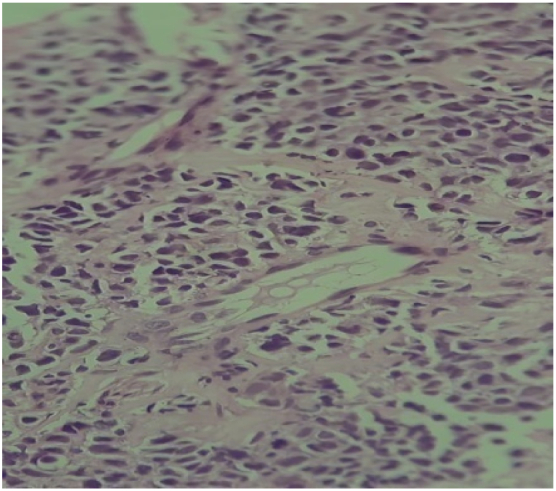
Histopathology of tumor tissue from cervix showing keratinizing squamous cell carcinoma, Hematoxylin & Eosin stain, 40×.
Type 3 Radical Hysterectomy with bilateral salpingo-oophrectomy and umbilical hernia repair was performed. On gross Examination, the hysterectomy specimen consisted of uterus and cervix and attached portion of vagina measuring 1.2 cm in length. An ulcero-proliferative growth was seen on post lip of cervix measuring 1.8 × 1.5 × 1.2 cm. The tumor was lying at 1.0 cm from the resected cut end of vagina, 1.3 cm and 1.0 cm from the cut ends of right and left parametrium respectively. The diagnosis made on biopsy tissue was again confirmed of repeat histopathological examination.
Now, Treatment with adjuvant radiotherapy (EBRT + IVBT) was planned but the patient missed the appointment due to COVID 19 restrictions. Later, the patient received three cycles of carboplatin (400 mg) and etoposide (150 mg). Clinical remission was achieved and she remained disease free for a year.
The patient had recurrence after one year which was revealed on a PET (Positron Emission Tomography) - CT scan revealing a large mass in the region of the uterus and cervix, which was infiltrating into the posterior wall of the urinary bladder, anterior wall of the rectum and extending into the upper 2/3 of vagina. The patient also had demonstrated metastasis to several organs - multiple sub-pleural and centrilobular nodules in both lung parenchyma, lytic lesion in the right posterior second rib, moth-eaten lytic destruction of the right 6th rib at its angle and multiple hypointense lesions in both lobe of the liver.
Ultrasound guided biopsy from the liver lesions displayed features of metastatic poorly differentiated neuroendocrine carcinoma. The patient was given repeated three cycles of carboplatin (240 mg) and etoposide (100 mg). Partial improvement with reduction in the sizes of the uterine and hepatic lesions was seen. The patient was also administered palliative radiotherapy, with 28 cycles of EBRT (external beam radiation therapy), and 3 cycles of ICRT (intracavitary radiation therapy). The patient succumbed to death approximately after three months of completion of palliative radiotherapy.
3. Discussion
Neuroendocrine tumors (NET) originate from the embryonic neuroectoderm. Their immunohistochemical profile is similar to cells of endocrine gland [5]. Peptide hormones may not be secreted from these tumors. Common sites of involvement are the gastrointestinal tract, pancreas, and lungs and are subdivided in well-differentiated (typical & atypical carcinoid, grade 1 & 2 respectively) to poorly differentiated variants (grade 3). Grade 3 is further divided in to small and large cell types [11].
Among the cases of NEN of the cervix, only a few have been reported with the presence of adenocarcinoma. Such tumors with the presence of both adenocarcinoma and neuroendocrine carcinoma have been termed as mixed adenoneuroendocrine carcionma (MANEC) with, each component comprising >30 % of the tumor [12]. Small cell NET is the prominent form of the tumor and is found mostly in pure form, but 4 % of the cases are associated with adenocarcinoma [9], [13].
NEN of the cervix clinically presents as postmenopausal vaginal bleeding, discharge, post-coital spotting and cervical mass [14]. In our case, the patient suffered from postmenopausal vaginal bleeding, vaginal bleeding indicating cervical cancer. Cervical examination revealed an invasive form of carcinoma which was confirmed by histopathology as poorly differentiated non-keratinizing squamous cell carcinoma. In cervical neuroendocrine tumors, markers may be negative. It needs to be differentiated from metastatic foci from extracervical NEC or local spread from NET to surrounding organs like urinary bladder, rectum, etc. Other differential diagnoses of NEC are lymphoma, poorly differentiated squamous cell carcinomas, sarcomas or melanomas [5].The diagnosis becomes challenging when both neuroendocrine and non-neuroendocrine tumors are present together. NETs are positive for synaptophysin (SYN), chromogranin (CHG), CD56 (N-CAM), and neuron-specific enolase (NSE). The diagnosis of NET requires at least two of four markers, being SYN and CD56 the most sensitive. Studies have shown that 60 % cases stain negative for synaptophysin and chromogranin, whereas 33 % stain negative for neuron-specific enolase [15]. Our case exhibited positive staining for both synaptophysin and chromogranin confirming small cell neuroendocrine carcinoma. Lymphovascular invasion consistent with small cell NEC was also seen in our case [16]. Moreover, p40 protein, which is a marker for squamous cell carcinoma was also absent in this case [17]. Immunohistochemical analysis also showed positive staining for CEA and negative staining for estrogen receptor that indicated the presence of non-neuroendocrine component of tumor, that is, endocervical adenocarcinoma [18]. Imaging techniques CT and MRI also divulged a mass in the lower uterine body and cervix verifying the diagnosis.
NETs of the cervix accounted for 1 %–2 % of all cervical cancers [19]. Small cell or oat-cell carcinoma is the most common neuroendocrine carcinoma of the cervix (NECC). It is also the most aggressive form accounting 5-year survival rates up to 35 % in comparison to other histological variants [9]. Recurrences are also seen most commonly within 2 years of diagnosis.
Lu JL et al. 2022 have studied 19 cases of neuroendocrine carcinoma of cervix. They showed that it is associated with HPV 18. It is very aggressive and has very poor prognosis. Its diagnosis needs a panel of immunohistochemical stains as mentioned above. A integrated therapeutic approach involving all three modelaties, chemotherapy, surgery and radiotherapy may improve the survival. A genetic studies of such cases is warranted for targeted therapy of such cases, that may only be the accurate therapeutic approach. For this more studies are required with higher number of cases [20].
4. Conclusion
Due to the aggressive nature of cervical neuroendocrine tumor patients must be kept under careful observation with regular follow up. Cells characteristic of neuroendocrine tumor of cervix are present only in a few nests, and could initially be missed. Metastasis in such cases particularly to the liver, lungs, and rib cage must be kept in mind. Pregnancy associated neuroendocrine tumor needs a personalized therapeutic approach. More case studies are required to establish a standard therapeutic protocol.
Ethical approval and patient (or parent's) consent
This is certified that the Patient's consent has been taken for this manuscript. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consentis available for review by the Editor-in-Chief of this journal on request.
Ethical clearance from Institute Ethics Committee has been taken with Ref No. IEC 591/05.01.2017, RP 10/2017.
Sources of funding
This is certified that this case is a part of research project, that was funded by Indian Council of Medical Research, New Delhi.
Research registration (for case reports detailing a new surgical technique or new equipment/technology)
Not applicable.
Funding
This case has been retrieved from an ongoing project funded by Indian Council of Medical Research, New Delhi. The Institute Ethical Clearance has been taken along with the consent of the patient.
This is certified that there is no COI related to this paper.
Ethical approval
Ethical Approval was provided by the authors' institution.
Consent
Written informed consent was obtained from the patient's relative for this case report and accompanying images. A copy of the written consent is available for review by Editor-in-Chief of this journal on request.
Guarantor
Corresponding author: Dr. Amar Ranjan
Additional Professor, R. No. 422, IRCH, AIIMS, Ansari Nagar, New Delhi – 110029
Mob: +91-9968328620
Email: dr.amarranjan@rediffmail.com
CRediT authorship contribution statement
| Name | Contributions |
|---|---|
| Saransh Verma | Wrote the paper |
| Harshita Dubey | Retrieved data, summarized and extended the paper |
| Anil Sharma | extended and edited the paper |
| Swati Gupta | extended and edited the paper |
| Amar Ranjan | Retrieved data, summarized and extended the paper |
| Harsh Goel | extended and edited the paper |
Declaration of competing interest
This is certified that there is no conflict of interest related to this paper.
Acknowledgments
The authors acknowledge Dr. Anita Chopra and MansiModi for reviewing the manuscript.
Contributor Information
Saransh Verma, Email: saranshverma987@gmail.com.
Harshita Dubey, Email: harshitakd96@gmail.com.
Swati Gupta, Email: g.swati@outlook.com.
Amar Ranjan, Email: dr.amarranjan@rediffmail.com.
Harsh Goel, Email: goel.harsh271@gmail.com.
Anil Sharma, Email: aneelmed@gmail.com.
References
- 1.Hallet J., Law C.H.L., Cukier M., Saskin R., Liu N., Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 2.Pape U.F., Berndt U., Müller-Nordhorn J., Böhmig M., Roll S., Koch M., et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer. 2008 Dec;15(4):1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 3.Raphael M.J., Chan D.L., Law C., Singh S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ. 2017 Mar 13;189(10):E398–E404. doi: 10.1503/cmaj.160771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klöppel G. Neuroendocrine neoplasms: dichotomy, origin and classifications. Visc. Med. 2017;33(5):324–330. doi: 10.1159/000481390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadducci A., Carinelli S., Aletti G. Neuroendrocrine tumors of the uterine cervix: a therapeutic challenge for gynecologic oncologists. Gynecol. Oncol. 2017 Mar;144(3):637–646. doi: 10.1016/j.ygyno.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Albores-Saavedra J., Larraza O., Poucell S., Rodríguez Martínez H.A. Carcinoid of the uterine cervix: additional observations on a new tumor entity. Cancer. 1976 Dec;38(6):2328–2342. doi: 10.1002/1097-0142(197612)38:6<2328::aid-cncr2820380620>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Teoman G., Ersoz S. Mixed endocervical adenocarcinoma and high-grade neuroendocrine carcinoma of the cervix: a case report. Indian J. Pathol. Microbiol. 2021 Jan 1;64(1):174. doi: 10.4103/IJPM.IJPM_1006_19. [DOI] [PubMed] [Google Scholar]
- 8.Alejo M., Alemany L., Clavero O., Quiros B., Vighi S., Seoud M., et al. Contribution of human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018 Jun;1(5):134–142. doi: 10.1016/j.pvr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tempfer C.B., Tischoff I., Dogan A., Hilal Z., Schultheis B., Kern P., et al. Neuroendocrine carcinoma of the cervix: a systematic review of the literature. BMC Cancer. 2018 May;4(18):530. doi: 10.1186/s12885-018-4447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec 1;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. https://www.sciencedirect.com/science/article/pii/S1743919120307718 Available from: [DOI] [PubMed] [Google Scholar]
- 11.Kim J.Y., Hong S.M., Ro J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017 Aug;29:11–16. doi: 10.1016/j.anndiagpath.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kitajima T., Kaida S., Lee S., Haruta S., Shinohara H., Ueno M., et al. Mixed adeno(neuro)endocrine carcinoma arising from the ectopic gastric mucosa of the upper thoracic esophagus. World J. Surg. Oncol. 2013 Sep;4(11):218. doi: 10.1186/1477-7819-11-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner G.J., Reidy-Lagunes D., Gehrig P.A. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol. Oncol. 2011 Jul;122(1):190–198. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Kumar T., Nigam J.S., Kumari M., Swati Pandey J. Cervical neuroendocrine carcinoma: a rare case report. Cureus. 2021 Jun 8;13(6) doi: 10.7759/cureus.15532. https://www.cureus.com/articles/61155-cervical-neuroendocrine-carcinoma-a-rare-case-report Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albores-Saavedra J., Gersell D., Gilks C.B., Henson D.E., Lindberg G., Santiago H., et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch. Pathol. Lab. Med. 1997 Jan;121(1):34–39. [PubMed] [Google Scholar]
- 16.Giordano G., D’Adda T., Pizzi S., Campanini N., Gambino G., Berretta R. Neuroendocrine small cell carcinoma of the cervix: a case report. Mol. Clin. Oncol. 2021 May 1;14(5):1–10. doi: 10.3892/mco.2021.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inzani F., Santoro A., Angelico G., Feraco A., Spadola S., Arciuolo D., et al. Neuroendocrine carcinoma of the uterine cervix: a clinicopathologic and immunohistochemical study with focus on novel markers (Sst2–Sst5) Cancers. 2020 May 12;12(5):1211. doi: 10.3390/cancers12051211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma K.R., Dabbs D.J. Cervical carcinoma with divergent neuroendocrine and gastrointestinal differentiation. Int. J. Gynecol. Pathol. 2018 Sep;37(5):488–491. doi: 10.1097/PGP.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 19.Balderston K.D., Tewari K., Gregory W.T., Berman M.L., Kucera P.R. Neuroendocrine small cell uterine cervix cancer in pregnancy: long-term survival following combined therapy. Gynecol. Oncol. 1998 Oct;71(1):128–132. doi: 10.1006/gyno.1998.5104. [DOI] [PubMed] [Google Scholar]
- 20.Lu J., Li Y., Wang J. Small cell (neuroendocrine) carcinoma of the cervix: an analysis for 19 cases and literature review. Front. Cell. Infect. Microbiol. 2022 Jul;13(12) doi: 10.3389/fcimb.2022.916506. [DOI] [PMC free article] [PubMed] [Google Scholar]


