Abstract
The mineral deposits and traces in the Iberian Peninsula are very numerous and of varied mineralogy. This study aimed to analyze the geochemical and environmental changes detected in soil, water, and sediments around the La Sierre mine, and to determine if contamination persists over time. The concentrations of ten trace elements (As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, Zn) were measured in 20 soil samples, 10 water samples, and 6 sediment samples, sampling at the most affected points. Soil and sediment samples were analyzed by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and water samples by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). From the Principal Component Analysis (PCA), soil samples SOI-6, 7, and 20 presented high amounts ranging from 14489.86a±7 to 3031.72b ± 1 mg/kg of Co, Cu, Ni, and As, respectively. Water samples WAT-6, 8, and 10 presented high contents of As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn, being sample WAT-8 with high values of As, Co, Cu, Fe, and Ni with 48.1 ± 0.82, 368 ± 4, 68.3 ± 0.1, 97.5 ± 1.2, and 152 ± 2 μg/L, respectively, exceeding the regulation given by R.D 314/2016 (Water for human consumption). The sediment samples were compared with the IInterim Sediment Quality Guideline (ISQG) and Probable Effect Level (PEL) values given by the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. When presenting a high ISQG value, but low PEL, they partially comply with the regulations, which happens with samples SED-1, 2, and 8 for As and SED-5, 6, and 7 for Pb. On the contrary, Cr and Cu do not comply with what is established in samples SED-8 and SED-1 and 8, respectively, whereas, in samples SED-2 and 5, Cu partially complies with the established regulations. The values found for the trace elements present in the abandoned traditional mining area with abundant epithermal deposits prevail over time in soil, water, and sediments.
Keywords: Geochemistry, Environmental, Assessment, Trace elements, Traditional mining
1. Introduction
Mining is the industrial activity of extracting substances and minerals from the earth's crust. The mining sector provides many of the basic raw materials to the industry in modern society. Potential problems associated with mining activities are land degradation, water pollution, acid mine drainage, air pollution due to the release of gases and dust, noise pollution, mine fires, damage to forest flora and fauna, and health risks, among others [1].
Soil has characteristics that vary depending on different factors, such as topography, climatology, bedrock properties, and biological action. Also considered as a critical environment, it can accumulate pollutants produced by human activity such as industry, agriculture, mining, and, mainly, mineral processing [2]. Due to the latter, the soil is degraded causing environmental damage for a long time after ceasing operations [3]. The presence of metals and metalloids as natural components has great importance in soils where mineral fraction comprises a stock of potentially mobile metal species, such as components of clays, minerals, and iron and manganese oxides which, in turn, have a dramatic influence on soil geochemistry [4]. Cobalt is currently considered a critical metal [5]; it is associated with iron, copper, and nickel [6].
Metals are accumulated in the soil from both natural and anthropogenic sources, making it difficult to identify and determine the origin of the heavy metals present in it, so it can be considered contaminated soil. However, it contains significant levels of As, Cd, Hg, Pb, and Sb in average concentrations of 1.5, 0.2, 0.08, 13, and 0.2 mg/kg, respectively, in the earth's crust [[7], [8], [9]]. The composition of water resources can be affected by different factors, one of which can be acid mine drainage and leachate, suspended solids, soil erosion, mining waste in surface waters [10], dispersion in rivers rainfall, and other hydrological processes [11,12]. Acidic solutions produced by mine drainage and industrial activities can reach the surface water or groundwater according to the hydrogeology of the site, leading to the deterioration of water quality by accumulation and transport of sediment that is detrimental to health [13,14]. Physicochemical parameters such as pH, thermal conductivity, and total dissolved solids in water can track heavy metal contamination [15].
The high concentration of potentially toxic elements in the environment is a worldwide concern and soil geochemistry is used as a tool for environmental monitoring [16].
The study area is located in the Principality of Asturias where mining was one of the main activities developed. Currently, there are regulations regarding the permitted content of metals in the water to be considered for human consumption, and in the soil to be considered contaminated, exceeding the values of metals naturally existing in the soil, according to its mineralization.
The objective of this study was to geochemically and environmentally evaluate the potential impact of trace elements existing in considerable concentrations in soil, water, and sediments. The study was carried out in the area surrounding La Sierre mine, and the geochemical and environmental changes detected made it possible to determine whether contamination persists over time.
2. Material and methods
2.1. Description of the study area
Mining in areas of the Principality of Asturias is one of the most remarkable ones due to the extraordinary variety of metals extracted. The origin of cobalt mining in Asturias is located in Cabrales since the 16th century [17]. The largest exploitation took place in the 19th century at the La Sierre mine, extracting cobalt since 1874 by the Cantabrian, Antonio Diestro. The mine works ended in the second decade of the 20th century [18]. It is important to pay attention to the geochemical characteristics of the chemical elements because it would be a risk to human health, animals, and the ecosystem [2].
The study area consists of tailings and waste from the activities coming from La Sierre mine, also known as the Niñón mine, which is located about 500 m north of Carreña village, belonging to Cabrales council (Fig. 1), in the north of the Iberian Peninsula, the former Santa Amelia concession, through which “La Ría” stream intersects [19]. Unlike other Spanish regions, Asturias has a humid climate characterized by abundant mild rainfall most of the year; and average annual precipitation temperatures in the east (where most of the Cu mines are located) are above 800 mm, and average monthly temperatures range from 7 to 19 °C, with an average of 13 °C per year [19].
Fig. 1.
Location map of the council of Cabrales.
It is located in the Picos de Europa Unit. Cabrales is a council of the autonomous community of the Principality of Asturias, on a sloping hillside. Its distribution can be observed as follows: it is limits to the north with Llanes, to the south with the provinces of Cantabria and Castilla y León, to the east with Peñamellera Alta, and to the west with Onís. Its capital is Carreña, with a total of 2543 inhabitants, according to the last census conducted in 2017, with an approximate area of 237.76 km2 [20].
Two main rivers are running through the Cabrales council, on whose banks most of the council's villages are located: Casaño, which crosses Cabrales and turns it into an intense ravine until it joins the Cares. The Casaño, on the other hand, is the second most important river course, coming from the mountains of the nearby western ouncil of Onís through a channel to where Carreña, the capital of the municipality, begins. The tributary of the Cares flows into it, on the outskirts of the Arenas village. There are very important rivers in Cabrales, short and torrential. Different streams feed the Cares, such as Duje, Poncebos, and the Bulnes or Texu river, which is located a few meters before. The Oscuro and Ribeles rivers do the same with the Casaño, as well as the streams of La Ría, Miñón, Cabrales, and Vaniella, among others [20,21].
The genetic model of mineralization is epithermal, linked to fractures and karstified zones of the Carboniferous limestones. Temperatures of main phase mineral formation have been defined to be between 90 and 130 °C. There are intense supergene alteration processes in the later stages of mineral deposition, giving rise to an extraordinary variety of mineral species [22].
2.2. Sampling
The soil and water samples were taken from the study area and the sediment from the water sample. The sampling network was designed by selecting the measurement points every 25 m; the points described in Fig. 2, were taken as reference points, taking into account the most vulnerable receptors in the area (village).
Fig. 2.
Location map in 1/90000 scale of Carreña village, belonging to Cabrales council, where the sampling points (SP) are distributed. (a) Distribution of sampling points in the Google Earth satellite image of soil, sediment and water samples, UTM 350380x, 4797769y. (b) Pithead of “La Sierre” mine (SOI 7). ArcGIS software version 10.3.
A stratified random sampling was carried out. A prior area survey was conducted to identify the entrances to the mine and areas close to it, as well as any other element of interest for the exploration of contaminants. Based on this criterion, it was determined that the sampling points should delimit the affected area.
The sampled area consisted of approximately 25.200 m2, twenty soil samples were manually taken at a maximum depth of 20 cm, using a Hand Augers. At each point, 3 subsamples were collected to be homogenized, obtaining a representative sample with a weight of approximately 1 kg, collected in plastic bags duly coded (SOI-) (Fig. 2a), using a wooden mark as a sign of the sampling point. Then, the samples were transported to the laboratory and stored at room temperature for further treatment, using the AENOR guideline [23].
A total of 10 water samples were collected, considering the most affected points upstream and downstream, taking as reference the Cares River, in glass containers with their respective coding (WAT) (Fig. 2a). A few drops of concentrated HNO3 were immediately added in situ to acidify and obtain a pH < 2 (making sure that the reagent used does not contain any element of interest in concentrations that could affect the result of the analysis) after 16 h of rest before performing the respective analysis. The measurements of conductivity, temperature, and redox potential were also taken and then transported to the laboratory, using the AENOR guide [24].
Sediment samples were collected manually with a 60 mL disposable syringe with a PTFE tip. The samples were taken from the water sampling points 1, 2, 5, 6, 7, and 8 (Fig. 2a). They were filtered with 0.45 μm pore spacing by removing the larger residues and placed in topaz-colored containers duly coded (SED-); transferred to the laboratory, and refrigerated for 48 h for subsequent treatment, using the AENOR guide [25].
In Fig. 2b, the Pithead of “La Sierre” mine is shown, from where the SOI-7 sample was taken.
2.3. Geochemical analysis
Samples were treated according to the following standards: ISO 5667-1:2006 (Water quality sampling. Guidance on the design of sampling programs and sampling techniques), ISO 11464:2006 (Soil quality-pretreatment of samples for physico-chemical analysis), ISO 11277:2009 (Determination of particle size distribution in mineral soil), ISO 10381-1:2007 (Part 101: Framework for the preparation and application of a soil sampling plan), and ISO 5667-13:2011 (Guidance on sampling of sludges - sediments).
The soil and sediment samples were stored for 7 days at 23 °C. Sampling was carried out by quartering, dividing diagonally into four equal portions, taking two of them and rejecting the other two, and obtaining a representative sample. The sample obtained was dried in an oven at 105 °C for 7 days until moisture was completely eliminated. The dried samples were sieved at 2 mm, to eliminate stones and flora residues, and re-sieved at 100 μm to obtain a sample of fine fractions [26]. Samples were placed in bags, sealed, coded, and stored at 20 °C until the analysis.
Before analysis, the samples were treated by digestion, which was performed by placing 1.5 g of soil in a tube, and 1 mL of type-I water (milli Q) to moisten; then, 10.5 mL of hydrochloric acid at 37% and aqua regia were added and left to act for 24 h. After that time, the soil was filtered and the supernatant was refrigerated until analysis [2,27].
The water samples were analyzed in situ for pH, electrical conductivity, temperature, and redox potential. These samples were immediately packaged in hermetically sealed and properly coded (WAT) transparent glass containers, and stored at 4 °C for 7 days until analysis [28].
The geochemical composition of the samples was performed using the instrumental technique of Atomic Absorption Spectrometry to determine the presence of trace elements. The techniques for the analyses were performed by the Inductively Coupled Plasma Mass Spectrometry (ICP-MS) method [2] for soil and sediment samples, and Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) [29] for water samples. Concentrations are represented in mg/kg units for solid samples (soil and sediment) and liquid samples in μg/L. Soil, water, and sediment sampling points (SP) were environmentally characterized using ArcGis software version 10.3.
2.4. Data treatment
All analyses were performed in triplicate and the results were expressed using the mean ± standard deviation. One-way ANOVA at a 5% significance level was used to detect differences, followed by the Scott-Knott post-hoc test, to determine differences between simples [30].
The Shapiro test was used to verify the residuals normal distribution; the Box-Cox transformation was performed only for variables whit lacked normal distribution; then Box-Cox transformation was employed by calculated the lambda (λ) to achieve the normal distribution [31]. The Pearson correlation coefficient was also determined, which indicated the existence of a relationship between two variables [32]. Positive correlation - the other variable tends to increase as well; Negative correlation - the other variable tends to decrease.
3. Results and discussions
3.1. Geochemical characteristics of the samples
There was statistical differences (p < 0.05) between the ten trace elements (As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, Zn) in the twenty sampling points, finding the highest values at sampling points SOI-7 (pithead), SOI-20 (SOI-6 replicate), and SOI-7 for As, Co, Cr, Cu, Fe, Ni, and Pb at SOI-6 and 20. The concentrations 41940.84a mg/kg, 40578.36b mg/kg, and 39789.39c mg/kg of Fe are highest compared to the other points, which are characteristic of mining soils that present high concentrations of Fe, ranging from 2.1 to 26% [33].
Trace elements depend on complex interactions that influence the genesis and development of the soil. The mineralization paragenesis of the “La Sierre” mine is mainly composed of chalcopyrite, with small amounts of pyrite, cattierite [CoS2], bravoite [(Ni, Co, Fe) S2], cobaltite [CoAsS], and supergene alteration minerals of Cu, Co, Ni, and Fe, highlighting the presence of erythrite, annabergite, and heterogenite, with some calcite, quartz, and microcrystalline silica [22].
As, Co, Cr, Cu, Fe, Ni, and Pb present throughout the mine are accentuated in higher concentrations in the pithead due to the mobility and availability of elements in the different sampling points [33]. Soils in mineralization areas show concentrations of Zn, Cu, Ni, Cr, Pb, and Cd [34], which may be directly related to anthropogenic contamination of the site, especially with Pb, As and Cu that show high concentrations in the soils sampled [35].
Mn presented higher concentrations at points SOI-4, 10, and 5 with values of 5156.01a, 4664.18b, and 4222.92c; and Cr, V, and Zn were the ones that presented lower concentrations with values of 55.28a, 59.19a, and 287.34a, respectively (Table 1).
Table 1.
Ten Trace elements (As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, Zn) in soil samples expressed in mg/kg, obtained from twenty sampling points of Carreña village, belonging to Cabrales council, by the method of Inductively Coupled Plasma Optical Emission Spectrometry ICP-OES.
| Sampling point | As | Co | Cr | Cu | Fe | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| SOI 1 | 387.49f ± 0.2 | 695.50g ± 0.2 | 39.41b ± 0.02 | 990.26f ± 0.5 | 13678.21k ± 6 | 639.20k ± 0.3 | 539.84f ± 0.2 | 52.33i ± 0.02 | 40.07d ± 0.01 | 65.25j ± 0 |
| SOI 2 | 27.30mn ± 0.06 | 12.32l ± 0.03 | 2.66l ± 0 | 33.96l ± 0.05 | 3928.62r ± 2 | 23.97n ± 0.02 | 11.99l ± 0.01 | 12.32o ± 0.03 | 12.98ij ± 0.01 | 10.99m ± 0 |
| SOI 3 | 596.15e ± 0.2 | 1625.26e ± 0.8 | 9.99h ± 0 | 1508.69e ± 1.1 | 15986.15j ± 11 | 2401.25f ± 1.6 | 692.73e ± 0.3 | 68.27f ± 0.07 | 19.32h ± 0.01 | 144.54e ± 0.03 |
| SOI 4 | 291.07g ± 0.21 | 785.04f ± 0.2 | 24.62a ± 0.01 | 921.43g ± 0.4 | 33563.97g ± 10 | 5156.01a ± 1 | 176.30hi ± 0.03 | 58.88h ± 0.06 | 52.23b ± 0.03 | 84.83h ± 0.01 |
| SOI 5 | 169.58i ± 0.18 | 369.09i ± 0.1 | 28.26e ± 0.01 | 465.52h ± 0.1 | 36975.46e ± 15 | 4222.92c ± 2 | 147.97ij ± 0.04 | 51.87i ± 0.07 | 59.19a ± 0.02 | 85.79h ± 0.02 |
| SOI 62 | 3356.60a ± 1 | 12063.81a ± 3 | 7.64k ± 0 | 14489.86a ± 7 | 41940.84a ± 16 | 1831.17h ± 0.4 | 6547.03a ± 3 | 121.97a ± 0.08 | 18.61h ± 0.02 | 178.79d ± 0.08 |
| SOI 71 | 1756.20c ± 0.3 | 5865.10c ± 2 | 14.99g ± 0.01 | 8731.01c ± 1 | 39789.39c ± 10 | 2076.11g ± 0.5 | 3465.74c ± 1 | 80.31d ± 0.05 | 32.66e ± 0.02 | 140.29e ± 0.05 |
| SOI 8 | 772.92d ± 0.4 | 2455.36d ± 0.6 | 4.99k ± 0.01 | 2675.24d ± 1.3 | 10827.56m ± 5 | 749.60j ± 0.4 | 909.52d ± 0.2 | 73.96e ± 0.06 | 10.66jk ± 0.01 | 121.94f ± 0.05 |
| SOI 9 | 226.96h ± 0.18 | 559.89h ± 0.2 | 4.67k ± 0.01 | 603.21i ± 0.3 | 8631.61o ± 5 | 363.26l ± 0.2 | 7237.29gh ± 0.08 | 30.66m ± 0.04 | 12.33ij ± 0.01 | 44.66l ± 0.01 |
| SOI 10 | 106.61k ± 0.18 | 238.21j ± 0.03 | 28.99c ± 0.01 | 259.19j ± 0.04 | 33315.57g ± 18 | 4664.18b ± 3 | 85.95jk ± 0.02 | 62.63gh ± 0.07 | 55.30a ± 0.02 | 85.62h ± 0.01 |
| SOI 11 | 64.27l ± 0.22 | 147.52k ± 0.03 | 18.32f ± 0.01 | 213.45j ± 0.1 | 25940.73h ± 9 | 2440.89f ± 0.8 | 64.94kl ± 0.02 | 52.61i ± 0.03 | 35.63d ± 0.02 | 118.55g ± 0.04 |
| SOI 12 | 71.58l ± 0.22 | 153.47k ± 0.03 | 25.30d ± 0.01 | 244.69j ± 0.11 | 38651.04d ± 16 | 3179.31d ± 1.3 | 83.56k ± 0.01 | 35.62l ± 0.06 | 53.93a ± 0.03 | 69.91i ± 0.01 |
| SOI 13 | 138.65j ± 0.19 | 583.26h ± 0.3 | 21.33e ± 0.01 | 656.58g ± 0.3 | 34928.68f ± 12 | 3152.91d ± 1.1 | 248.97g ± 0.14 | 44.66k ± 0.07 | 47.99c ± 0.03 | 65.99j ± 0.02 |
| SOI 14 | 71.27l ± 0.1 | 208.81jk ± 0.04 | 55.28a ± 0.02 | 343.01i ± 0.2 | 21213.53i ± 10 | 2444.39f ± 1.2 | 93.25jk ± 0.03 | 121.89a ± 0.05 | 27.64f ± 0.02 | 279.74b ± 0.15 |
| SOI 15 | 30.32m ± 0.12 | 56.63l ± 0.01 | 13.33gh ± 0.01 | 359.78i ± 0.1 | 12192.68l ± 4 | 849.49i ± 0.3 | 32.31kl ± 0.01 | 105.27c ± 0.06 | 19.99g ± 0.02 | 211.54c ± 0.06 |
| SOI 16 | 28.97m ± 0.25 | 63.60l ± 0.01 | 13.98gh ± 0.01 | 128.19k ± 0.06 | 15615.64j ± 8 | 2623.69e ± 1.4 | 32.96kl ± 0.01 | 64.59g ± 0.07 | 16.65h ± 0.01 | 287.34a ± 0.12 |
| SOI 17 | 11.53mn ± 0.1 | 22.66l ± 0.01 | 5.33jk ± 0 | 47.66l ± 0.02 | 5332.62q ± 2 | 104.65m ± 0.16 | 16.99l ± 0.02 | 47.33j ± 0.04 | 7.33k ± 0.02 | 41.33l ± 0.02 |
| SOI 18 | 7.49mn ± 0.2 | 6.99l ± 0.01 | 6.32jk ± 0.01 | 23.30l ± 0.02 | 7855.67p ± 3 | 349.51l ± 0.1 | 7.66l ± 0.01 | 26.63n ± 0.07 | 9.99k ± 0.01 | 53.59k ± 0.01 |
| SOI 19 | 7.23n ± 0.3 | 13.99l ± 0.01 | 9.67i ± 0.01 | 32.33l ± 0.03 | 9832.02n ± 5 | 376.62l ± 0.2 | 13.67l ± 0.01 | 33.99l ± 0.04 | 14.33i ± 0.01 | 70.32i ± 0.02 |
| SOI 202 | 3031.72b ± 1 | 9328.36b ± 3 | 9.99i ± 0.7 | 14025.85b ± 8 | 40578.36b ± 20 | 2055.57g ± 1.3 | 5230.54b ± 2 | 110.28b ± 0.06 | 23.99g ± 0.02 | 180.90d ± 0.05 |
| Mean | ||||||||||
| Box Cox | 557.70 | 1762.74 | 17.2535 | 2337.66 | 22538.9175 | 1985.24 | 1281.9605 | 62.80 | 16.788 | 81.57 |
| Λ | – | 0.2626 | – | 0.1818 | 1.6364 | 0.2222 | 0.1414 | – | – | 0.5859 |
As-Arsenic. Co-Cobalt. Cr-Chromium. Cu-Copper. Fe-Iron. Mn-Manganese. Ni-Nickel. Pb-Lead. V-Vanadium. Zn-Zinc. SOI 1-UTM 350244x, 4798180y; SOI 2-UTM 350241x, 4798158y; SOI 3-UTM 350242x, 4798126y; SOI 4-UTM 350243x, 4798105y; SOI 5-UTM 350250x, 4798071y; SOI 6-UTM 350255x, 4798049y; SOI 7-UTM 350257x, 4798031y (Pithead); SOI 8-UTM 350270x, 4798196y; SOI 9-UTM 350278x, 4798133y; SOI 10-UTM 350278x, 4798107y; SOI 11-UTM 350288x, 4798080y; SOI 12-UTM 350288x, 4798052y; SOI 13-UTM 350282x, 4798028y; SOI 14-UTM 350317x, 4798110y; SOI 15-UTM 350330x, 4798033y; SOI 16-UTM 350334x, 4798124y; SOI 17-UTM 350334x, 4798204y; SOI 18-UTM 350334x, 4798222y; SOI 19-UTM 350308x, 4797962y; SOI 20-UTM 350255x, 4798049y (SOI-6 replicate). Values represent mean ± SD (n = 3). Different letters in the same column indicate statistical differences (p < 0.05) between samples by Scott-Knott post-hoc test. The Shapiro-Wilk test was used to determinate the normal distribution of the data; and the Box-Cox transformation factor (λ), for nonparametric data.
The elements with normal distribution were Co, Cu, Fe, Mn, and Ni; this was determined by the Sharipo Wilk test.
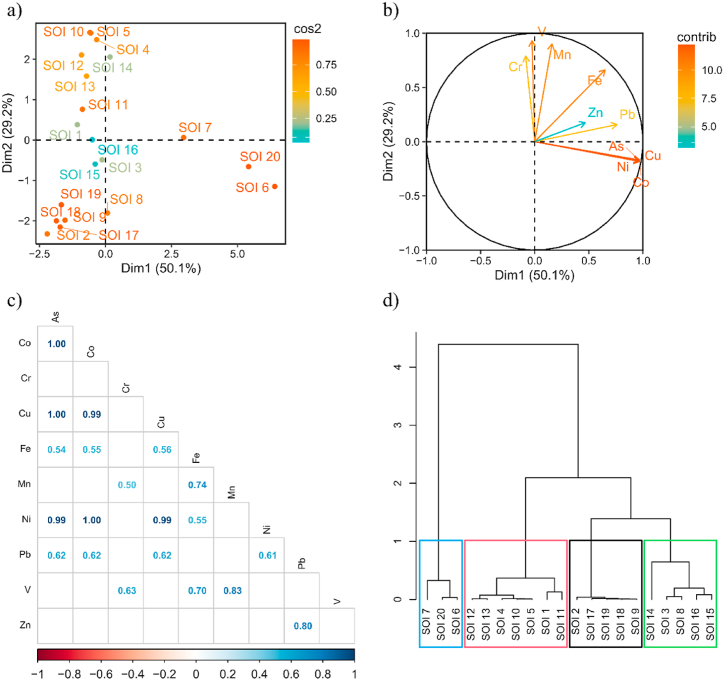
PCA (Fig. 3a and b) explained 79.3% of the total variance (50.1% and 29.2% for the first and second PCs respectively), this percentage of explanation is adequate considering the dimensionality reduction of the PCA technique to explain the whole experiment in a single map. In the loading plots for response variables for the soil samples, SOI 6, 7 and 20 presented the highest amounts of As, Co, Cu, and Ni, and lower amounts of Cr, Mn, V, and Zn. On the other hand, SOI-14, 15, and 16 presented high amounts of Zn and lower amounts of As, Co, Cu, Fe, Mn, Ni, and V.
Fig. 3.
Principal component analysis (PCA) of soil samples. (a) PCA loading plot for response for samples (b) score plot for soil for variables, (c) Pearson correlation coefficients of trace elements such As, Co, Cr, Cu, Fe, Mn, Ni, Ni, Pb, V, Zn in soil (d) hierarchical clustering of principal components of soil samples.
On the other hand, SOI-12 and 13 show high amounts of V and lower values of As, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn. Likewise, in SOI-4, 5 and 10 had the highest values of, Mn and V and lower amounts of As, Co, Cr, Cu, Fe, Ni, Pb, and Zn in minimum values. The presence of Fe, Zn, and Pb can be attributed to a common source such as sulfide minerals already existing in most soils as pyrite (FeS2), sphalerite (ZnS), and galena (PbS). Fe and Mn are mainly associated with minerals present in the soil [36], which determines the long-term stability of trace elements.
The Pearson's correlation coefficients (Fig. 3c) indicated strong correlations (positive p-value close to or equal to 1) between the level of As–Co with 1, As–Cu with 1, As–Ni with 0.99; as well as Co–Cu with 0.99, Co–Ni with 1, and Cu–Ni with 0.99, but with negative significance between As–Cr, As–V; as well as Co–Cr, Co–V, Cr–Cu, Cr–Ni, Cu–V, Mn–Ni, Ni–V, Pb–V, and V–Zn. A positive correlation indicates as the concentration of one element increases in the particular area within the study environment, there is a corresponding increase in the concentration of the other element. On the other in case of existence of a negatively correlated elements, indicate to have an inverse relationship. Therefore, an increase in one element in the study environment results in a corresponding decrease in the other element [37].
Fig. 3d shows four hierarchical clusters where samples SOI-6, 7, and 20 formed a cluster (they also were close each other), which contain higher concentrations of elements than the other clusterings. In general, the groups were formed by point sample which were close, this could demonstrate the influences of the geographic location, in this sense the point close to the mine presented the highest contaminant values.
Considering the elapsed time since mine works ended (second decade of the 20th century), these findings could indicate the mineral extraction procedures in the area are signals that metal concentration prevails over time with contributions from lithogenic factors making significant inputs to the total metal content, taking into account the type of mineralization in the area.
The water (Table 2) were compared with the regulations governing the sanitary criteria for the quality of water for human consumption, being this Royal Decree 314/2016, of July 29, which establishes the sanitary criteria that must be met by water for human consumption and the facilities that allow its supply from the catchment to the consumer dispenser and the control of these, guaranteeing its salubrity, quality, and cleanliness, to protect people's health from the adverse effects derived from any type of water contamination. The study by Ref. [38], carried out around abandoned mines and/or deposits in the Iberian Pyrite Belt (southwestern Spain), determines that the values are significantly higher for elements such as, Cu, Pb, and Zn. However, in this study sample WAT- 8 presented values exceed those given by the regulations for As, Co, Cu, Fe, and Ni with 48.1 ± 0.82, 368 ± 4, 68.3 ± 0.1, 97.5 ± 1.2, and 152 ± 2 μg/L, respectively. This is due to the fact that the sample was taken from the pithead leachate and, therefore, there are high concentrations.
Table 2.
Ten Trace elements (As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, Zn) analyzed in water samples expressed in μg/L obtained at ten sampling points compared with Royal Decree 314/2016, of July 29 by ICP-MS.
| Sampling point | As | Co | Cr | Cu | Fe | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| WAT 01 | 0.71ef ± 0.03 | 0.11d ± 0.004 | 0.27g ± 0.005 | 1.78i ± 0.02 | 91.6g ± 0.4 | 2.09f ± 0.02 | 0.79f ± 0.01 | 1.67e ± 0.05 | 0.3d ± 0.01 | 15.6f ± 0.2 |
| WAT 02 | 0.97c ± 0.02 | 0.37cd ± 0.005 | 0.25g ± 0.005 | 22.7c ± 0.1 | 48.8j ± 1.4 | 2.17ef ± 0.01 | 1.82de ± 0.02 | 1.69e ± 0.04 | 0.26e ± 0 | 19.5e ± 0.3 |
| WAT 03 | 0.71ef ± 0.03 | 0.12d ± 0.003 | 0.21g ± 0.002 | 3.71g ± 0.04 | 68.9h ± 1.6 | 2.4e ± 0.03 | 0.81f ± 0.01 | 0.97h ± 0.03 | 0.26e ± 0.01 | 14.3g ± 0.2 |
| WAT 04 | 0.71ef ± 0.02 | 0.06d ± 0.005 | 0.33f ± 0.004 | 2.92h ± 0.01 | 50.1i ± 3.9 | 1.37h ± 0.03 | 0.73f ± 0.02 | 1.47f ± 0.05 | 0.23e ± 0 | 10.4i ± 0.2 |
| WAT 05 | 0.69f ± 0.01 | 0.11d ± 0.002 | 0.8c ± 0.004 | 5.74f ± 0.03 | 206c ± 2 | 1.58h ± 0.01 | 2.47c ± 0.03 | 1.59ef ± 0.01 | 0.51c ± 0.01 | 15.6f ± 0.1 |
| WAT 06 | 0.73ef ± 0.02 | 0.26d ± 0.007 | 2.32a ± 0.01 | 16.7d ± 0.1 | 133d ± 2 | 4.7a ± 0.02 | 2.58c ± 0.03 | 4.3c ± 0.02 | 0.47c ± 0.01 | 43.7c ± 0.5 |
| WAT 07 | 0.86d ± 0.02 | 0.17d ± 0.009 | 0.69d ± 0.001 | 63.3b ± 0.6 | 269b ± 4 | 3.12d ± 0.01 | 13.6b ± 0.2 | 1.81d ± 0.02 | 0.61b ± 0.02 | 77.7b ± 0.6 |
| WAT 08 | 48.1a ± 0.82 | 368a ± 4 | 0.94b ± 0.01 | 68.3a ± 0.1 | 97.5e ± 1.2 | 3.98b ± 0.03 | 152a ± 2 | 5.84b ± 0.01 | 0.38d ± 0.01 | 113.9a ± 2 |
| WAT 09 | 0.75e ± 0.04 | 1.24b ± 0.01 | 0.59e ± 0.004 | 3.59g ± 0.03 | 96.4f ± 1 | 1.84g ± 0.01 | 2.2cd ± 0.02 | 1.14g ± 0.04 | 0.28e ± 0.01 | 20.3d ± 0.1 |
| WAT 10 | 1.05b ± 0.02 | 0.74c ± 0.01 | 0.71d ± 0.01 | 6.73e ± 0.08 | 363a ± 2 | 3.58c ± 0.03 | 1.59e ± 0.02 | 137a ± 0 | 0.7a ± 0.01 | 10.9h ± 0.1 |
| Water for human consumption (R.D 314/2016) | 10 | – | 50 | 2 | – | – | 20 | 10 | – | – |
WAT 1-UTM 350270x, 4797509y; WAT 2-UTM 350273x, 4797497y; WAT 3-UTM 350265x, 4797501y; WAT 4-UTM 350249x, 4797499y; WAT 5-UTM 350306x, 4797963y; WAT 6-UTM 4797995x, 4798049y; WAT 7-UTM 350346x, 350346y; WAT 8-UTM 350257x, 4798031y; WAT 9-UTM 350348x, 4798038y; WAT 10-UTM 350358x, 4798074y.
Values are the average of 3 repetitions ± standard deviation.
In sample WAT-10 had 137 ± 0 μg Pb/L, which is 13 times higher compared to the regulations (Table 2). Thus, it is evident that anthropogenic activity contributes significantly to contamination in some specific areas near populations, as well as to the influence of the metal load transported by rivers. Ni and Co are an exception to this trend, because they are found as part of the existing soil in the area. According to Ref. [39], the presence of Fe, Mn, Cu, Zn in bodies of water or rivers is evident, in areas where mining activities have been carried out.
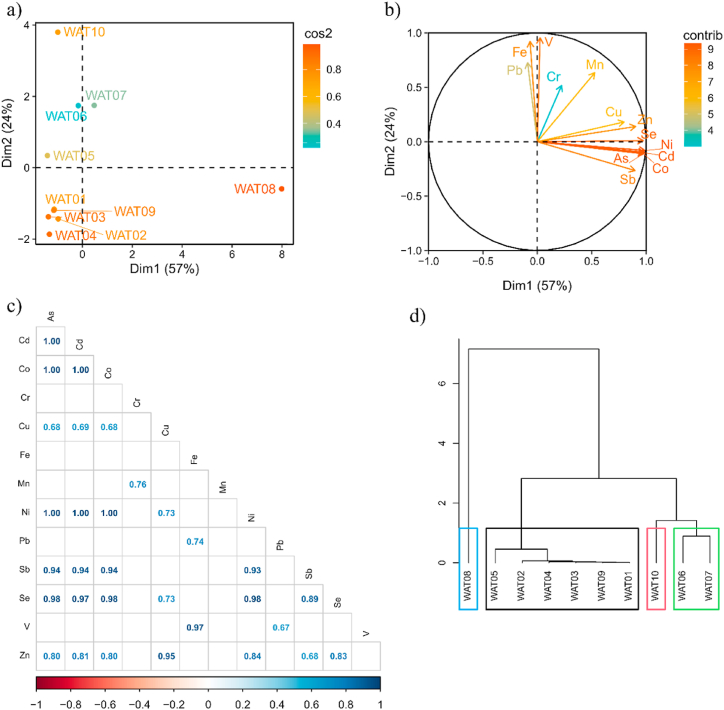
In PCA for water samples explain 81% of total data variance (Fig. 4 a and b), it is observed that, samples WAT-6, 8, and 10 (Fig. 4a) show high contents of As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn (Fig. 4b). On the other hand, sample WAT-5 presents high values of Cr, Fe, Ni, and V; as well as sample WAT-7, of Cu, Fe, Ni, V, and Zn. In contrast. The mobility of metals in water is controlled by the dissolution-precipitation equilibrium. The pH and Eh properties of water control the solubility of the compounds. Generally, medium acidification implies the release of metals, resulting in an increase of metals in dissolution and a very basic state. However, arsenic is an exception to this generalization.
Fig. 4.
Principal component analysis (PCA) of water samples. (a) PCA loading plot for response variables (b) score plot for soil samples, (c) Pearson correlation coefficients of trace elements such as, Co, Cr, Cu, Fe, Mn, Ni, Ni, Pb, V, Zn in soil, (d) hierarchical clustering of principal components of soil samples.
The Pearson correlation coefficients for the water samples indicated strongly positive correlations (p-value close to or equal to 1) between the level of As–Cd with 1, As–Co with 1, As–Ni with 1, As–Se with 0.98; as well as Cd–Co with 1, Cd–Ni with 1, Cd–Se with 0.97, Co–Ni with 1, Co–Se with 0.98, and Ni–Se with 0.98 (Fig. 4d).
Fig. 4c shows four hierarchical clusters where the samples WAT-8 belong to the first cluster, meaning that it contains higher concentrations of metals than the other clusters.
The mean content of trace elements in the Casaño riverbed sediments followed the same order of abundance as in the soil (mg/kg): Fe (11877.448) > Cu (4012.143) > Cr (1311.923), as shown in Table 3. The fine texture of the riverbed sediments may have contributed to the higher retention of metals. The amber color of the Casaño river is attributed to this phenomenon due to the high concentration of Fe.
Table 3.
Ten Trace elements (As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, Zn) analyzed in sediment samples expressed in mg/Kg obtained from six water sampling points by ICP-OES.
| Sampling points | As | Co | Cr | Cu | Fe | Mn | Ni | Pb | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| SED 01 | 30.67b ± 0.2 | 115.66b ± 0.48 | 15.67b ± 0.01 | 198.99b ± 0.45 | 17798.81b ± 7 | 1053.26b ± 0.5 | 73.99b ± 0.13 | 79.66a ± 0.04 | 17.99a ± 0.01 | 215.99a ± 0.04 |
| SED 02 | 19.98b ± 0.1 | 15.97c ± 0.02 | 10.99b ± 0.01 | 60.29c ± 0.03 | 19084.73a ± 8 | 859.31c ± 0.4 | 19.32c ± 0.01 | 39.97b ± 0.04 | 18.65a ± 0.02 | 90.26c ± 0.02 |
| SED 05 | 5.42c ± 0.2 | 12.31c ± 0.01 | 7.32b ± 0.01 | 27.95d ± 0.02 | 10813.15d ± 5 | 342.69e ± 0.1 | 13.64c ± 0.01 | 24.62c ± 0.05 | 10.31b ± 0.02 | 76.19e ± 0.02 |
| SED 06 | 4.70c ± 0.01 | 11.99c ± 0.01 | 6.66b ± 0 | 16.32e ± 0.01 | 9858.78e ± 5 | 349.72e ± 0.2 | 11.99c ± 0.01 | 19.32c ± 0.03 | 8.99c ± 0.01 | 70.28f ± 0.02 |
| SED 07 | 5.00c ± 0.1 | 13.32c ± 0.01 | 7.99b ± 0 | 14.32e ± 0.02 | 12490.84c ± 7 | 409.70d ± 0.2 | 14.32c ± 0.01 | 21.98c ± 0.06 | 11.66b ± 0.02 | 80.61d ± 0.03 |
| SED 08 | 1168.44a ± 0.5 | 4427.43a ± 2 | 7822.90a ± 3 | 23754.99a ± 7.4 | 1218.38f ± 0.4 | 2616.51a ± 1.3 | 2430.09a ± 1.3 | 66.58c ± 0.07 | 16.31a ± 0.01 | 159.45b ± 0.09 |
| Mean values | ||||||||||
| CEQG (1) | 205.63 | 766.11 | 1311.92 | 4012.14 | 11877.45 | 938.53 | 427.23 | 42.02 | 13.99 | 115.46 |
| ISQGA | 7.24 | – | 52.3 | 18.7 | – | – | – | 30.2 | – | 124 |
| PELB | 41.6 | – | 160 | 108 | – | – | – | 112 | – | 271 |
Note: Sediment samples SED (01, 02, 05, 06, 07, 08) belong to water points WAT (01, 02, 05, 06, 07, 08). ±: standard deviation.
CEQG1: Canadian Environmental Quality Guidelines for sediments from inland bodies of water (mg/Kg).
A: ISQG: Interim Sediment Quality Guideline, B: PEL: Probable Effect Level.
Abnormal behavior (positive anomalies) was observed in the rivers for As, Cr, and Cu, with enrichment factors of 51, 7, and 18, respectively, which determines the anthropogenic origin of these elements. High concentrations of As, as well as Cr and Cu, were present. The spatial distribution of trace elements shows that the concentrations of As, Cu, and Hg are diversely distributed along the rivers; i. e., they do not follow any pattern [40]. In this context, along the course of the streams, substantial contamination is evident at the entrance of along of Carreña village, belonging to Cabrales council.
Metal concentrations in sediment samples taken along the Casaño river (As varied between 1168.442 and 4696 mg/kg, Co from 4427.430 to 11.990 mg/kg, Fe ranged 19084.732–1218.375 mg/kg, Mn between 2616.511 and 342.694 mg/kg, Cu from 23754.993 to 14.323, and Ni between 2430.093 and 11.990, among others). This fact may indicate a natural origin for these metals, which were mainly derived from erosion, transport, and sediment processes, as typical in a basin in the Iberian Peninsula [41]. There are natural elements (Fe and Mn) and anthropogenic elements (Cr, Cu, Ni, Pb, and Zn). Most of the Fe and Mn found in sediments are natural, coming from rocks. Industries and port activities generate wastewater and are the main sources of Cr, Cu, Ni, Pb, and Zn. However, the destination, bioavailability, and effects of these elements are not adequately addressed [42]. These processes are controlled by many factors, including the binding capacity with geochemical carriers such as clay particles, organic matter (i.e.humic acids), CaCO3 or sulfides, oxides, and hydroxides. CaCO3 contents are important factors that control not only the destination but also the bioavailability of metals, corroborating the correlations found [29].
Sediments with concentrations in a range acording to Interim Sediment Quality Guideline (ISQG) and the Probable Effect Level (PEL) could pose a potential risk to exposed aquatic organisms. Although biological effects are possible, their frequency, nature, and severity are difficult to predict. Sediments with concentrations at or above the PEL are likely to pose significant and immediate risks to exposed aquatic organisms, and concentrations at or below the ISQG do not represent expected adverse biological effects according to Canadian Environmental Quality Guidelines (CEQG) [43].
As concentrations exceed the ISQG value in samples SED-1, 2, and 8, but the latter does not exceed the PEL value, therefore, it partially complies with the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. As for Cr in sample SED-8, it exceeds the ISQG and PEL parameters, exceeding the maximum permissible limits, therefore, it does not comply with the regulations. The same happens with Cu in samples SED-1 and 8; on the contrary, in samples SED-2 and 5, they partially comply with the established regulations. Samples SED-5, 6, and 7 of Pb do not exceed the ISQG value, but none exceed the PEL value, partially complying with the values established by the Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. Something similar happens with Zinc: none of the samples exceed the PEL value, but they do exceed the ISQG value.
The differences between sampling points could be explained by the greater management of adjacent land than upstream land uses, because the watershed landscape is a mosaic of different land uses, and because the river has a low transport capacity for suspended sediment. In this watershed, the greatest transfer of metals to the river occurs during floods, particularly when combined with times of turbulence in the Waters [40].
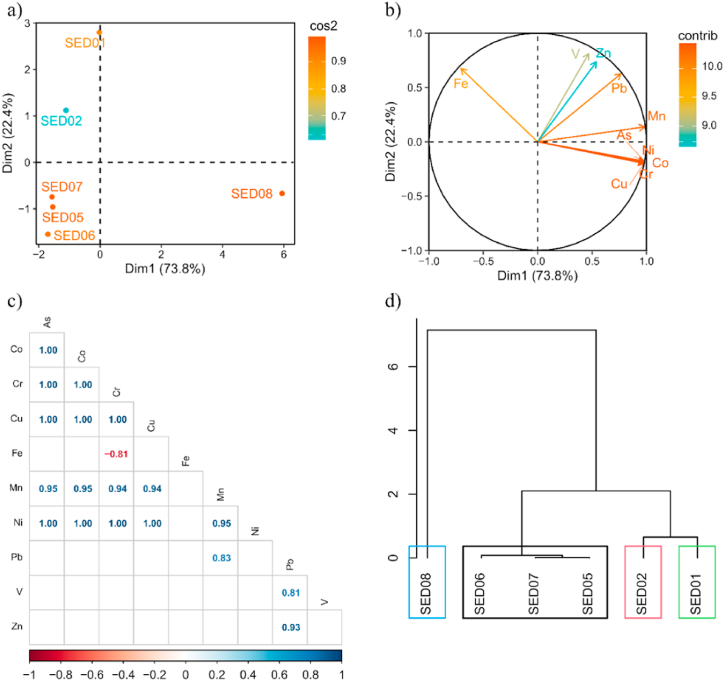
PCA for sediments explained 96.2% of the total data variance, it was observed that samples SED-1, 2, and 8 show highest values of As, Co, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn. As for the SED-5 sample, there is evidence of high levels of As and Pb, unlike the SED-6 sample, which shows high contents of V; as well as in the SED-7 sample, which also shows high levels of Fe (Fig. 5a and b).
Fig. 5.
Principal component analysis (PCA) of sediment samples. (a) PCA loading plot for response variables (b) score plot for soil samples, (c) Pearson correlation coefficients of trace elements such as, Co, Cr, Cu, Fe, Mn, Ni, Ni, Pb, V, Zn in soil, (d) hierarchical clustering of principal components of soil samples.
The values of Pearson correlation coefficients for the water sediments indicated strongly positive correlations between the level of As–Co with 1, As–Cr with 1, As–Cu with 1, As–Ni with 1; as well as Co–Cr with 1, Co–Cu with 1, Co–Ni with 1, Cr–Cu with 1 and Cr–Ni with 1, but with a negative significance between Cr–Fe with −0.81 as shown in Fig. 5c.
Fig. 5d shows four hierarchical clusters where the SED-8 samples belong to the first cluster, which means that it contains higher concentrations of metals than the other clusterings.
4. Conclusions
The geochemical and environmental characterization of the study area shows that mining and mineral processing activities cause soil degradation, and many continue to cause environmental damage long after operations have ceased. Inadequate disposal of mining waste without proper control measures causes pollution over time, with the participation of climatic agents such as rain and wind, affecting soil, water, air, and biota. The remoteness and extension of these areas, coupled with the fact that the responsible mining companies have become extinct, makes environmental recovery expensive and difficult. As a result, there are numerous mining areas that continue to contaminate many years after their closure.
The results indicate that the highest concentrations in the sampled soils are As, Co, Cr, Cu, Fe, Ni, and Pb, the soils affected by mining activities were highly contaminated. The water samples were compared with the values established by the legislation, exceeding the maximum permissible parameters according to the Official Gazette of Asturias, the trace elements that exceed these limits are Cu and Fe content. The levels of trace elements in the sediments indicate a natural origin for these metals derived from erosion and transport processes due to rain, being a seasonal climate in this area causing a percolation of leachate from the mine to the surface waters of the La Ría stream and, therefore, to the Casaño river, evidenced by the amber color of these waters.
The trace elements from soil, water, and sediment were used to identify areas where urgent action is needed which allowed determining how contamination persists over time. The authorities of the Council of Cabrales raise awareness of the population for the non -consumption of the waters of the La Ría stream for the presence of pollutants in it.
Author contribution statement
Claudia Araceli Vásquez Ugaz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Noemí León-Roque; Juan L. Nuñez-León: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Davy W. Hidalgo-Chávez; Jimy Oblitas: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no competing interests.
Acknowledgements
The authors would like to thank the Universidad Politécnica de Madrid for their assistance in sampling and data collection in the field, as well as for permitting the access to and use of their laboratories for the analysis and experimental part of this article. And scholarships received by Claudia A. Vasquez from the National Scholarship and Educational Credit Program (PRONABEC) with resolution No. 142-2017-MINEDU-VMGIPRONABEC- OBPOST - President of the Republic Scholarship, by Davy Chávez from the FAPERJ project No E_26/204,328/2021.
References
- 1.Warhate S.R., Yenkie M.K.N., Pokale W.K. Impacts of mining on water and soil. J. Environ. Sci. Eng. 2007;49(2):143–152. [PubMed] [Google Scholar]
- 2.Bavec Š., Gosar M., Biester H., Grčman y H. Geochemical investigation of mercury and other elements in urban soil of Idrija (Slovenia) J. Geochem. Explor. 2015;154:213–223. [Google Scholar]
- 3.Cánovas C.R., Caro-Moreno D., Jiménez-Cantizano F.A., Macías F., Pérez-López R. Assessing the quality of potentially reclaimed mine soils: environmental implications for the construction of a nearby water reservoir. Chemosphere. 2019;216:19–30. doi: 10.1016/j.chemosphere.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Violante A., Cozzolino V., Perelomov L., Caporale A., Pigna M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010;10(3):268–292. [Google Scholar]
- 5.Baran E.J. Cobalto: un elemento crítico y estratégico. Anales. 2018;70:77–106. [Google Scholar]
- 6.Johnson D.B., Dybowska A., Schofield P.F., Herrington R.J., Smith S.L., Santos y A.L. Bioleaching of arsenic-rich cobalt mineral resources , and evidence for concurrent biomineralisation of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy. 2020;195:1–10. [Google Scholar]
- 7.Masi E.B., Bicocchi G., Catani y F. Soil organic matter relationships with the geotechnical-hydrological parameters, mineralogy and vegetation cover of hillslope deposits in Tuscany (Italy) Bull. Eng. Geol. Environ. 2020;79(8):4005–4020. [Google Scholar]
- 8.Opuene K., Agbozu y.I.E. Relationships between heavy metals in shrimp (Macro brachium felicinum) and metal levels in the water column and sediments of taylor creek. Int. J. Environ. Res. 2008;2(4):343–348. [Google Scholar]
- 9.Resmi G., Thampi S.G., Chandrakaran y S. Brevundimonas vesicularis:A novel bio-sorbent for removal of lead from wastewater. Int. J. Environ. Res. 2010;4(2):281–288. [Google Scholar]
- 10.Alianza Mundial de Derecho Ambiental (Elaw), Guía Para Evaluar EIAs de Proyectos Mineros, Estados Unidos, 2010.
- 11.Oliveira M.L.S., Da Boit K., Schneider I.L., Teixeira E.C., Crissien Borrero T.J., Silva y L.F. O. Study of coal cleaning rejects by FIB and sample preparation for HR-TEM: mineral surface chemistry and nanoparticle-aggregation control for health studies. J. Clean. Prod. 2018;188:662–669. [Google Scholar]
- 12.Sánchez-Peña N.E., et al. Chemical and nano-mineralogical study for determining potential uses of legal Colombian gold mine sludge: experimental evidence. Chemosphere. 2018;191:1048–1055. doi: 10.1016/j.chemosphere.2017.08.127. [DOI] [PubMed] [Google Scholar]
- 13.Guillén M.T., Delgado J., Albanese S., Nieto J.M., Lima A., De Vivo y B. Environmental geochemical mapping of Huelva municipality soils (SW Spain) as a tool to determine background and baseline values. J. Geochem. Explor. 2011;109(1–3):59–69. [Google Scholar]
- 14.Li J., et al. Tracing geochemical pollutants in stream water and soil from mining activity in an alpine catchment. Chemosphere. 2020;242 doi: 10.1016/j.chemosphere.2019.125167. [DOI] [PubMed] [Google Scholar]
- 15.Wellen C., Shatilla N.J., Carey y S.K. The influence of mining on hydrology and solute transport in the Elk Valley, British Columbia, Canada. Environ. Res. Lett. 2018;13(7) [Google Scholar]
- 16.Zuzolo D., et al. Potentially toxic elements in soils of Campania region (Southern Italy): combining raw and compositional data. J. Geochem. Explor. 2020;213 [Google Scholar]
- 17.De Blas M.A. 2008. Minería Prehistórica del Cobre en el Reborde Septentrional de Los Picos de Europa: Las olvidadas. [Google Scholar]
- 18.Rodríguez Terente L., Luque Cabal C., Gutiérrez Claverol y M. Los registros mineros para sustancias metálicas en Asturias. Trab. Geol. 2006;26(26):19–55. [Google Scholar]
- 19.Álvarez R., Ordóñez A., Pérez A., De Miguel E., Charlesworth y S. Mineralogical and environmental features of the Asturian copper mining district (Spain) Eng. Geol. 2018;243:206–217. [Google Scholar]
- 20.Gutierrez Claverol M., Garcia-Ramos y J.C. La geología de Asturias a través de las Topografías Médicas. Trab. Geol. 2018;36(36):203–236. [Google Scholar]
- 21.Hughes R. Concejo de Cabrales. J. Chem. Inf. Model. 2011;53(9):287. [Google Scholar]
- 22.López Castaño R., Lueje García N., Izquerido Ruiz y A. 2011. Mineralizaciones de Cobalto en Asturias. [Google Scholar]
- 23.Asociacion Española de Normalizacion y Certificacion (AENOR), ISO 10381-2:2002 – Parte 2: Directrices Para el Diseño de Los Programas de Muestreo de la Calidad del Suelo, España, 2007.
- 24.Asociacion Española de Normalizacion y Certificacion (AENOR) 2007. Guía Para el Diseño de Los Programas de Muestreo y Técnicas de Muestreo de Calidad de Agua. [Google Scholar]
- 25.Asociacion Española de Normalizacion y Certificacion (AENOR), Guía Para el Muestreo de Lodos. España, 2011.
- 26.Asociacion Española de Normalizacion y Certificacion (AENOR) 2009. Determinación de La Distribución Granulométrica de La Materia de Los Suelos. [Google Scholar]
- 27.International Standard . 2006. Pretratamiento de Muestras Para Análisis Físico – Químico de La Calidad Del Suelo. [Google Scholar]
- 28.Loredo J., Ordóñez A., Álvarez R. Environmental impact of toxic metals and metalloids from the Muñón Cimero mercury-mining area (Asturias, Spain) J. Hazard Mater. 2006;136(3):455–467. doi: 10.1016/j.jhazmat.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 29.de Campos B.G., et al. Integrating multiple lines of evidence of sediment quality in a tropical bay (Guanabara Bay, Brazil) Mar. Pollut. Bull. 2019;146:925–934. doi: 10.1016/j.marpolbul.2019.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Bhering L.L., Cruz C.D., De Vasconcelos E.S., Ferreira A., De Resende M.F.R. Alternative methodology for Scott-Knott test. Crop Breed. Appl. Biotechnol. 2008;8(1):9–16. [Google Scholar]
- 31.Atkinson A.C., Riani M., Corbellini y A. The box–cox transformation: review and extensions. Stat. Sci. 2021;36(2):239–255. [Google Scholar]
- 32.Esezi Isaac O., Eric Chikweru y A. Test for significance of pearson's correlation coefficient (r) Int. J. Innov. Math. Stat. Energy Policy. 2018;1(1):11–23. http://seahipaj.org/journals-ci/mar-2018/IJIMSEP/full/IJIMSEP-M-2-2018.pdf Recuperado de: [Google Scholar]
- 33.Batista M.J., et al. Geochemical characterisation of soil of Beira city, Mozambique: Geogenic origin and relation with land cover. J. Geochem. Explor. 2018;187:184–200. [Google Scholar]
- 34.Sungur A., Vural A., Gundogdu A., Soylak y M. Effect of antimonite mineralization area on heavy metal contents and geochemical fractions of agricultural soils in Gümüşhane Province, Turkey. Catena. 2020:184. [Google Scholar]
- 35.Schreck E., et al. Tillandsia usneoides as biomonitors of trace elements contents in the atmosphere of the mining district of Cartagena-La Unión (Spain): new insights for element transfer and pollution source tracing. Chemosphere. 2020;241 doi: 10.1016/j.chemosphere.2019.124955. [DOI] [PubMed] [Google Scholar]
- 36.Kusin F.M., et al. Geo-ecological evaluation of mineral, major and trace elemental composition in waste rocks, soils and sediments of a gold mining area and potential associated risks. Catena. 2019;183 [Google Scholar]
- 37.Daniel N., Kingsley T., Blestmond B.A. Geochemistry of minor and trace elements in soils of Akuse area, Southeastern Ghana. Geosciences. 2019;9(1):8–17. [Google Scholar]
- 38.Fernández-Caliani J.C., Barba-Brioso C., González I., Galán E. Heavy metal pollution in soils around the abandoned mine sites of the iberian pyrite belt (Southwest Spain) Water Air Soil Pollut. 2009;200(1–4):211–226. [Google Scholar]
- 39.Palleiro L., Patinha C., Rodríguez-Blanco M.L., Taboada-Castro M.M., Taboada-Castro y M.T. Metal fractionation in topsoils and bed sediments in the Mero River rural basin: bioavailability and relationship with soil and sediment properties. Catena. 2016;144:34–44. [Google Scholar]
- 40.Sierra C., Ruíz-Barzola O., Menéndez M., Demey J.R., Vicente-Villardón J.L. Geochemical interactions study in surface river sediments at an artisanal mining area by means of Canonical (MANOVA)-Biplot. J. Geochem. Explor. 2017;175:72–81. [Google Scholar]
- 41.García-Ordiales E., et al. Anthropocene footprint in the Nalón estuarine sediments (northern Spain) Mar. Geol. 2020;424 [Google Scholar]
- 42.Besada V., Bellas J., Sánchez-Marín P., Bernárdez P., Schultze F. Metal and metalloid pollution in shelf sediments from the Gulf of Cádiz (Southwest Spain): long-lasting effects of a historical mining area. Environ. Pollut. November 2021;295:2022. doi: 10.1016/j.envpol.2021.118675. [DOI] [PubMed] [Google Scholar]
- 43.Canadian Council of Ministers of the Environment . vol. 5. Canadian Council of Ministers of the Environment; 2001. (Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Summary Tables). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.