Summary
BRIDE OF DOUBLETIME (BDBT) interacts with the circadian kinase DOUBLETIME (DBT) and accumulates in eye foci during the dark of a light:dark cycle. BDBT foci are shown here to be broadly expressed in constant dark and low in constant light. Analysis of circadian photoreceptor cry and visual photoreceptor ninaE mutants showed that disappearance of eye BDBT foci requires both the CRYPTOCHROME and the RHODOPSIN-1 pathways. The arr1 and arr2 mutants, which affect rhodopsin quenching, eliminated BDBT foci under dark conditions. arr1 and arr2 mutants also caused increased nuclear PER protein. The changes in BDBT foci do not result from altered BDBT levels in the eye but from changes in its immunodetection. Knockdown of BDBT specifically in the eye produced constitutively nuclear PER and constitutively cytosolic DBT. The results show that BDBT is necessary for co-transport of DBT and PER into the nucleus and suggest that this process is regulated by a light-dependent mechanism.
Subject areas: Genetics, Sensory neuroscience, Cell biology
Graphical abstract
Highlights
-
•
Light regulates BDBT foci in the eye via both circadian and visual photoreceptors
-
•
Arr mutants suppress while Rh1 mutations enhance BDBT foci formation
-
•
Changes in BDBT foci are produced by changed antigenicity and not changes in levels
-
•
BDBT is needed for nuclear localization of DBT but not PER in the eye
Genetics; Sensory neuroscience; Cell biology
Introduction
Circadian rhythms are affected by environmental cues such as light and temperature, but in their absence, an approximately 24 h rhythm persists.1 These cues synchronize (or entrain) oscillations of negative feedback loops between regulatory proteins and transcription factors.2 During the evening PERIOD (PER) and TIMELESS (TIM), proteins accumulate in the cytosol where they dimerize, promoting their nuclear translocation. In the nucleus, PER acts as circadian transcriptional regulator, repressing its own transcription along with other genes controlled by the CLOCK/CYCLE (CLK/CYC) heterodimer. This repression is relieved during the day as CRYPTOCHROME (CRY), a photoreceptor (PR) that dimerizes with TIM, leads to degradation of TIM/CRY in response to light.3,4 Newly synthesized PER is phosphorylated by DOUBLETIME (DBT) and degraded until another round of night and PER/TIM accumulation.5,6 Many of these regulatory circadian proteins in Drosophila are conserved in mammals, making the Drosophila circadian clock a useful model for the more complex mammalian clock.7
An interactor of DBT has been identified as CG17282 or BRIDE OF DOUBLETIME (BDBT).8 BDBT is essential for normal cycles of PER nuclear accumulation in brain circadian neurons and for circadian behavior via elevation of DBT activity toward PER. Immunofluorescent microscopy analysis indicated that BDBT accumulates in cytosolic foci. During the evening from ZT13 and ZT19 (1 h and 7 h after lights out at ZT12, respectively), BDBT cytosolic foci accumulate to a high point in which they are broadly expressed throughout the photoreceptors before again becoming sequestered in foci at ZT1 and ZT7 in the outer part of the retina.8 per0 and UAS-dcr2;timGAL4>/+; UAS-dbt RNAi:/+ flies did not accumulate BDBT puncta formation in the Drosophila photoreceptors at night, indicating foci accumulation is dependent upon DBT and PER.8 Presented here are the effects of light on the formation of BDBT foci and a genetic approach to observe the effects of circadian (cry) mutants and visual mutants of the RHODOPSIN1 (ninaE)-linked heterotrimeric G-protein-coupled signaling pathway on BDBT foci accumulation. The studies reveal a role for dark-mediated Arrestin-dependent generation in the formation of foci and a role for BDBT in circadian co-transport of DBT and PER to nuclei in the eye.
Results
BDBT foci are constitutively high in the dark and low in constant light
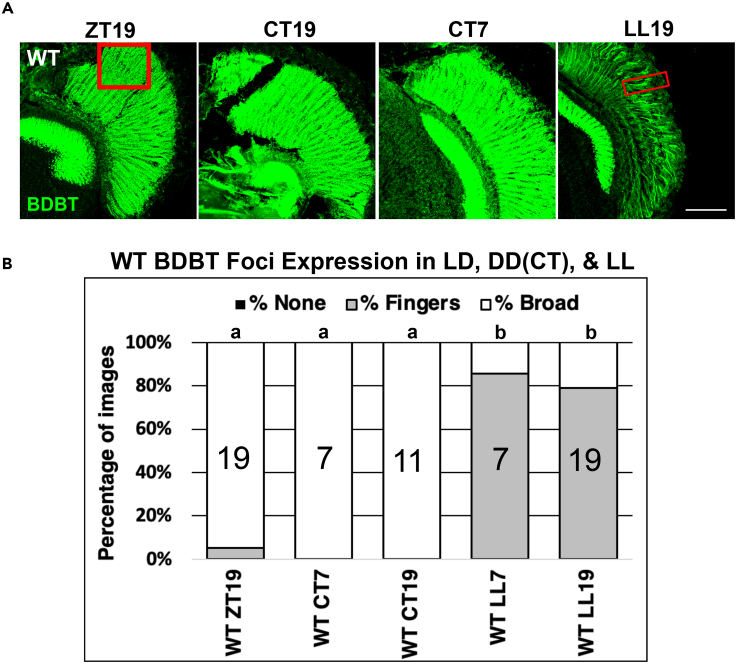
Canton S wild-type (WT) flies were raised in constant darkness under circadian time (CT) to analyze the subcellular localization of BDBT foci in photoreceptor cells, where PER protein is also highly expressed. These CT flies were entrained to 12 h-lights-on/12 h-lights-off cycles and then moved to constant darkness and collected under dark conditions at CT7 (7 h after lights were illuminated in the previous cycle) and CT19 on the second day after termination of LD. This allows us to determine if BDBT foci formation is a light-driven process. Eye sections were collected under illumination with a dim red light that the flies cannot detect and then embedded in random orientations in medium for cryostat sectioning. This results in fly sections occurring through different planes of the eye, producing eye sections of different sizes. Eye sections were prepared for immunofluorescent detection using an antibody against BDBT with confocal microscopy. Despite the variation in eye size, the changes in BDBT that occur in response to light or dark are dramatic and easily scored (Figure 1B). Interestingly, under constant darkness, BDBT foci accumulation remained consistently high and broad at both CT7 and CT19 (Figures 1A and 1B). Despite the broad distribution, BDBT foci persist as intense dots in the image where the overall intensity is higher (see red rectangle in Figure 1A in the ZT19 image), and these are more obvious in single optical sections (Figure S1A); although the expression is broad, it is not uniform. Flies were also collected in LL-7 h and 19 h, whereby they were maintained in constant light for 7 h and 19 h, respectively, after the termination of the final dark period of LD starting at ZT0 (LL-7 is equivalent to ZT7 conditions shown in the next figure). In LL conditions, the abundance of foci was restricted to streaks or “finger” projections in the retina and remained relatively low at all times of day (Figures 1A and 1B; small rectangle around one finger). Some of these fingers colocalize with UV-sensitive Rh3, which is expressed in approximately one-third of R7 neurons (Figure S1B). Therefore, the fingers in constant light are found in R7 neurons, in which BDBT is not eliminated by the wavelengths of our constant light. There are some images with some cross-sections that move toward more tangential orientations, and in these, BDBT can track the Rh3 signal but is slightly displaced from it (Figure S1C). This argues that BDBT foci are in the Rh3 cells at ZT7 but are adjacent to the rhabdomeres, where Rh3 is found. Moreover, even in the typical tangential sections like the one shown in Figure S1B, there are areas in which the Rh3 fluorescence or BDBT fluorescence (either one) comes out in isolation (see yellow rectangle in Figure S1B), presumably because the sectioning has stripped off an underlying or overlying signal. Therefore, we argue that the BDBT foci are mostly not in the rhabdomeres but in the Rh3 cell cytosol adjacent to the rhabdomeres. Expression in the optic lobe lamina (on the left of each image) remains high no matter what the lighting condition is and serves as an internal control in these images. The restricted expression of foci under lighted conditions and broadly expressed foci throughout the cytosol of the photoreceptors in constant darkness show that BDBT foci accumulation is dependent upon darkness and prevented by light. To determine the general nature of this light:dark-dependent effect, BDBT foci were again analyzed through a set of fly lines mutant for circadian (CRY) and visual (Rh1, ARR1, ARR2) proteins.
Figure 1.
Constant Light Suppresses and Constant Darkness Enhances BDBT Foci after LD Entrainment
(A) Wild-type Canton S flies were harvested at the indicated times in LD (ZT, lights on from 0-12), during the second day of constant darkness (CT times indicate the previous time in LD), or during the first day of constant light (LL times indicate the previous time in LD). Heads were sectioned and probed with anti-BDBT antibody. The rectangle in the ZT19 image shows an area where foci are still visible above the general signal; foci are seen throughout the area in single optical sections (Figure S1A). In the LL samples, foci were often concentrated in fingers on the outside of the retina (a red rectangle surrounds a finger in the LL19 sample). These are produced in R7 cells, some of which coexpress Rh3 adjacent to the BDBT fingers (Figures S1B and S1C).
(B) The location of BDBT (broadly throughout the eye or in fingers on the outside; e.g., LL19) or its lack of expression (none) were tabulated for multiple sections after detection by confocal microscopy. A Kruskal-Wallis nonparametric analysis (H (4, N = 63) = 40.5) followed by multiple comparisons of mean ranks for all groups showed that sections of flies from darkness (a) differed significantly from those of flies from light (p ≤ 0.05), while there were no statistically significant differences within the a or b groups. Scale bar represents 50 microns, and the number of sections analyzed for each bar on the plot is indicated inside the bar.
The response of BDBT foci to light is reduced in circadian cry and visual ninaE mutants
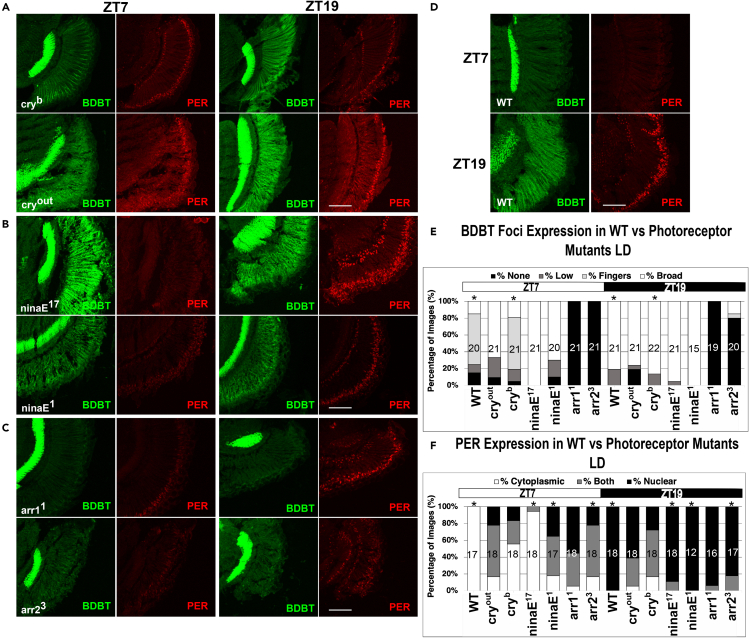
As CRY serves as the circadian clock photoreceptor,9,10 two CRY visual mutants were raised and collected under LD conditions. Cryout consists of a 1490-bp deletion resulting in a loss of function allele,11 and cryb is also a strong allele consisting of the point mutation D410N, which is highly conserved among class I photolyases and produces a missense mutation at a conserved chromophore flavin-binding residue, leading to a lack of response to light.10 All sections were treated for BDBT and imaged and underwent scoring by observers blinded to sample identity as described in methods, with sections scored as those with no foci, with broad but weak foci, with fingers (like those in Figure 1A, LL19), or with broad and strong foci. The strong cryout mutant did not exhibit light-dependent decreases in BDBT foci at ZT7. However, the cryb mutant did exhibit some loss of BDBT foci (Figures 2A and 2E). These results and the results obtained in Figure 5 are inconclusive in determining whether there are strong differences between the cryout and cryb mutants. However, it is clear that cry mutants reduce the sensitivity of the foci to light.
Figure 2.
Effects of Photoreceptor Mutants on BDBT Foci and PER Localization in LD Cycles
Wild-type Canton S flies and mutant lines were harvested at the indicated times in LD (ZT, lights on from 0-12). Heads sections were treated with anti-BDBT and anti-PER antibodies, and the location of BDBT (broadly throughout the eye at high levels, fingers on the outside, broad low expression, or no expression) and PER (nuclear, cytosolic, or both nuclear and cytosolic) were tabulated after detection by confocal microscopy.
(A) Effects of cry mutants on BDBT and PER at ZT7 and ZT19.
(B) Effects of ninaE (Rh1) mutants on BDBT and PER localization at ZT7 and ZT19.
(C) Effects of arrestin mutants on BDBT and PER at ZT7 and ZT19.
(D) BDBT and PER localization in wild-type Canton S flies.
(E) The expression pattern of BDBT foci in eye sections was scored by observers blinded to sample identity for multiple images. The relative percentages showing no foci (none), low levels of broad expression, foci localized to the outer area of the retina (fingers), or high broad expression are shown. The samples labeled with “∗” showed statistically significant differences with the same genotype at the other time point (ZT7 vs ZT19) with p < 0.001, by a Mann Whitney U test (with continuity correction). (F) PER localization in eye sections was scored by observers blinded to sample identity. The relative percentages showing cytosolic, cytosolic and nuclear (both), or nuclear are shown. The samples labeled with “∗” showed statistically significant differences with the same genotype at the other time point (ZT7 vs ZT19) with p < 0.03, by a Mann Whitney U test (with continuity correction). The scale bars indicate 50 μm, and the number of sections analyzed for each bar on the plot is indicated inside the bar.
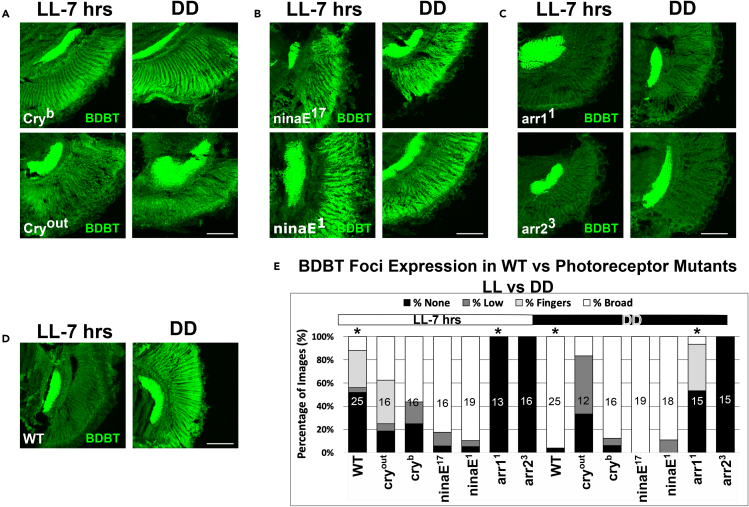
Figure 5.
Effects of Photoreceptor Mutants on BDBT Foci Accumulation in LL-7h and DD
Heads were harvested from flies raised in constant darkness or raised in constant darkness and subjected to 7 h of light. Heads sections were treated with anti-BDBT antibodies, and the location of BDBT was tabulated as in Figure 2.
(A) BDBT detected in cry mutants.
(B) BDBT detected in Rh1 mutants (ninaE).
(C) BDBT detected in arr mutants.
(D) BDBT detected in wild-type flies.
(E) Scores are plotted here. Asterisks indicate statistically significant difference between dark-reared and light-pulsed flies of the same genotype with p < 0.05, by a Mann-Whitney U test (with continuity correction). The scale bars indicate 50 μm, and the number of sections analyzed for each bar on the plot is indicated inside the bar.
To look at the effects of RHODOPSIN-1 mutants (ninaE; the major Drosophila RHODOPSIN expressed in R1-R6) on BDBT foci accumulation, two different ninaE mutants were employed. NinaE1 and ninaE17are both loss of function alleles containing a Q251Stop mutation12 and a large deletion of the 5′ region, producing no detectable ninaE transcripts, respectively.13 Both the ninaE1 and ninaE17 mutants led to a significant increase of BDBT foci formation during light, when BDBT foci are normally low, while also producing normally elevated levels of foci accumulation in DD (Figures 2B and 2E). In summary, these results indicate that both the circadian and visual photoreceptor proteins are needed for reduced BDBT foci during light in an LD cycle.
arr mutants eliminate BDBT foci formation
Next, we looked at the effect of an additional interactor in the ninaE signaling pathway. The visual ARRESTINS, ARR1 and ARR2, mediate endocytosis of Rh1 from the rhabdomere into the cytosol of the photoreceptors and quench Rh1 signaling.14,15 We wanted to determine how absence of normal quenching of Rhodopsin in response to light would affect BDBT foci formation. arr11 contains a DNA insertion resulting in approximately ten percent of WT ARR1 protein levels.15 In the arr11 mutant, BDBT foci formation was completely abolished during LD in both light and dark, producing a phenotype opposite that of the ninaE mutants (Figures 2C and 2E). The lack of BDBT foci accumulation is similar to the results seen in earlier studies in which arr1 mutants lead to no RHODOPSIN-1-immunopositive vesicles budding from the rhabdomere,14 suggesting a role for endocytosis that involves both circadian and visual transduction pathways.
This experiment was then repeated with the arr2 line (arr23) to investigate whether BDBT foci also require ARR2. arr23 contains a single amino acid change (V52D) producing less than one percent of WT ARR2 protein.15 When the arr23 mutants were subjected to an LD cycle, broadly expressed BDBT foci mostly failed to form in the dark (Figures 2C and 2E). These data suggest that an ARRESTIN-dependent mechanism is necessary for the proper formation of BDBT foci, but it appears to affect the dark phase of the LD cycle rather than the light phase (the opposite of the light transduction phase where ARRESTINS show effects on RHODOPSIN-1). However, since BDBT foci are low during the day in WT flies, we cannot rule out an effect on the light phase as well.
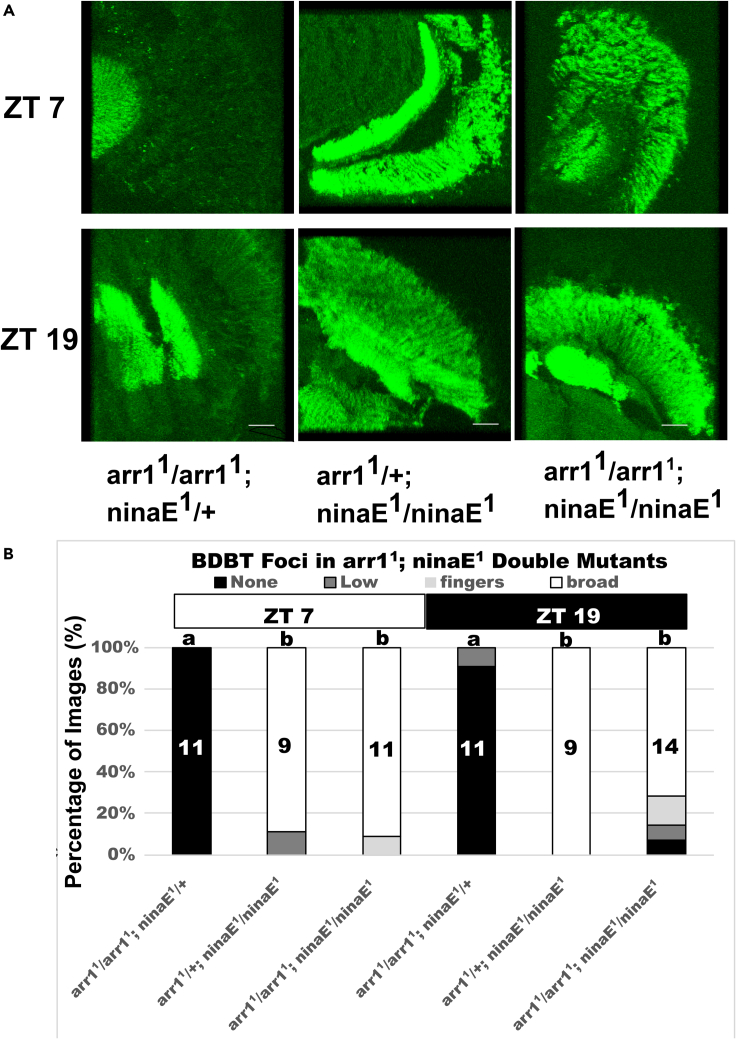
Because the phenotypes of ninaE and arr mutants are opposite to each other, they could exhibit epistasis, i.e., one mutant phenotype could mask the phenotype of the other. In order to address this possibility, we constructed double-mutant flies carrying both the arr11 and ninaE1 mutations. Epistasis was observed because the ninaE mutant phenotype (broad BDBT in both light and dark) was manifest in flies that were double homozygotes for both, while the arr11 mutant phenotype was still manifest in the flies that were homozygous for arr11 but heterozygous for ninaE1 (Figure 3). As covered in more detail in the discussion, this result suggests that signaling through Rhodopsin 1 is needed to repress BDBT formation during the day rather than lack of Rhodopsin 1 interactions with ARR1.
Figure 3.
Analysis of BDBT in fly eyes of arr1; ninaE double mutants
Genetic crosses were used to generate flies with the following genotypes: arr11/arr11; ninaE1/+, arr11/+; ninaE1/ninaE1, and arr11/arr11; ninaE1/ninaE1. Representative images are shown in panel (A) (white lines indicate 50 μm), and tabulation of scoring by an observer blinded to sample identity is shown in panel (B) (numbers in bars indicate the number of sections scored). A Kruskal-Wallis ANOVA (H(6, M = 65) = 53.66) showed statistically significant differences between the samples in groups a and b (Multiple comparisons p values were less than 0.003 for all a-b genotype comparisons with no statistically significant differences for any a to a or b to b comparisons). These results show that the ninaE phenotype overrides the arr1 phenotype in epistasis tests.
arr1/arr2 mutant affects PER localization
Because PER is necessary for BDBT foci accumulation, we next asked if the absence of BDBT foci formation caused by ARRESTIN mutants in turn is associated with the circadian regulator PER. In some of our eye sections in which we detected BDBT, we also detected PER with a different fluor (e.g., the representative examples shown in Figures 2A through 2D). In WT flies, PER remains expressed throughout the cytosol at ZT7 during the day and then at ZT19 PER localizes to the nuclei of the photoreceptors (Figure 2D). As previously shown,10,16 the cry mutants blunt the oscillations of PER, with moderate and equivalent nuclear localization in photoreceptors at both ZT7 and ZT19 (Figures 2A and 2F; 7 types of photoreceptor nuclei on the outside of the eye and one on the inside). By contrast and as previously shown, the ninaE mutants retain robust oscillations of PER localization, with high levels of nuclear PER during the night and low levels during the day (Figures 2B and 2F) as RHODOPSIN-mediated light signaling does not entrain eye PER.17 However, in the arr11 mutant, PER was mostly localized to the nuclei of the photoreceptors during ZT7 and ZT19 (Figures 2C and 2F). This change to PER subcellular localization in arr11 mutant eyes does not alter rhythmicity as activity assays show these flies remain rhythmic (not shown) and PER oscillations in the head (most of which come from the eye) remain rhythmic (Figure S2A). PER was also found to be more nuclear at ZT7 in arr23 mutants than in our WT controls, but the change observed from ZT7 to ZT19 for the arr23 mutant was nevertheless significant (Figure 2F). These results demonstrate that not only are BDBT foci dependent upon visual ARRESTIN proteins but also, in the absence of functional ARR1, the absence of these foci is associated with increased PER nuclear localization, thereby disrupting oscillations of circadian clock proteins in the fly eye.
The daily and genotype changes in BDBT eye immunofluorescence are not caused by changes in protein level
We then investigated whether the changes in immunofluorescent BDBT detection in the eye are due to changes in BDBT protein levels in the eye. There is no robust daily change of BDBT levels in head extracts of WT flies, nor is there robust difference in BDBT levels between WT Canton S and arr mutant heads (Figure S2B). However, detection of BDBT by immunofluorescence in the brain is extensive (data not shown) and does not oscillate in WT flies. This lack of oscillation could mask BDBT oscillations in the eye. Therefore, we dissected eyes from WT and arr mutant flies and analyzed the levels by immunoblot (Figure 4). With this more tissue-specific method, the levels of BDBT in WT flies did not oscillate and were indistinguishable from those in arr mutants. So there is no evidence that BDBT levels oscillate in the eye. In addition, the changes are not likely to be produced by movement of BDBT into the axon termini of R1-6 during the day. These termini could contribute to the high signal detected in the optic lobe lamina since R1-6 terminate in the optic lobe lamina, but the optic lobe was not included in our eye dissection, and its omission should have produced oscillations in the immunoblot analysis if the immunofluorescence changes are produced by changes in BDBT localization.
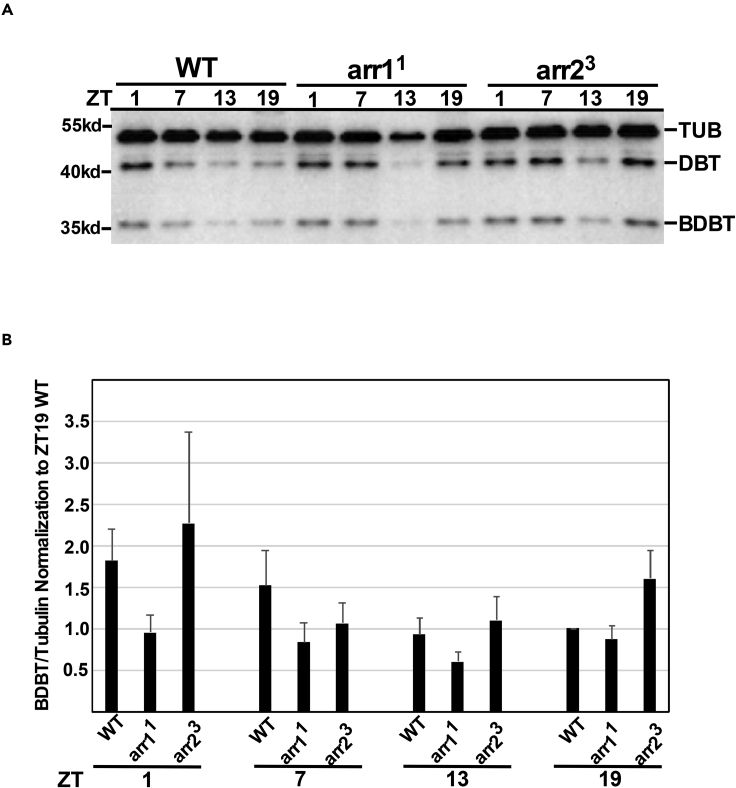
Figure 4.
BDBT does not exhibit detectable daily oscillations in level in the eyes of wild-type flies and is not notably lower in the eyes of arr mutants than in wild-type eyes
Heads were isolated at the indicated times of day from wild-type Canton S and arr11 or arr23 mutants, and extracts of the heads were assayed by immunoblot analysis for PER and BDBT (Figure S2). Here, heads were dehydrated in acetone and dissected after drying for analysis of BDBT, DBT, and tubulin levels by immunoblot analysis. One representative analysis is shown in panel A. The molecular weight of markers is indicated to the left. 4–5 separate analyses were analyzed with scans of the chemiluminescent signals. Each BDBT signal was normalized to the signal for tubulin from the same gel lane, and this normalized signal was further normalized to the tubulin-normalized signal for Canton S wild-type flies at ZT19 (which therefore had a signal of 1 for each analysis). The averages are plotted here with the standard error of the mean shown as a bracket above the bar. Statistical analysis (ANOVA(11,42) = 1.59, p = 138.16) with a Tukey HSD test showed no statistically significant differences between normalized BDBT levels in any of the samples, and likewise statistical analysis of each genotype time course alone showed no statistically significant differences. The variations in BDBT immunofluorescence seen over the course of the day in wild-type flies are suggested to be caused by antigen-masking effects because high levels of BDBT immunofluorescence are detected at ZT7 in wild-type flies when the sections are subjected to 60°C temperatures prior to antibody application (Figure S3).
The lack of BDBT oscillation in WT fly eyes via immunoblot analysis suggests that the oscillations in BDBT foci are likely to arise from changes in nature of BDBT interactions or folding. The immunofluorescence signal passes the usual test of specificity for the anti-BDBT antibody. It is absent if only the secondary antibodies are employed without the primary antibodies (not shown), and it is reduced with RNAi knockdown of BDBT (see Figures 6 and S4). Epitope masking during immunofluorescence is a common observation,18 and masking can be reduced by heat treatment or microwave pulses during immunofluorescence. We have tried both heat treatment and microwave pulses during the procedure and found that both elevate the signal in WT flies at ZT7. Heat treatment after fixation and before antibody treatment was most effective in elevating signal during the day in WT flies (Figure S3). Heat treatment during antibody incubation eliminated detection with the antibody (not shown). However, heat treatment of the sections from the arr11 and arr23 mutant eyes did not elevate BDBT signal (Figure S3). These results suggest that BDBT is in complexes or conformations that mask its detection by the antibody during the day in WT flies and that this state differs from that of the arr mutants.
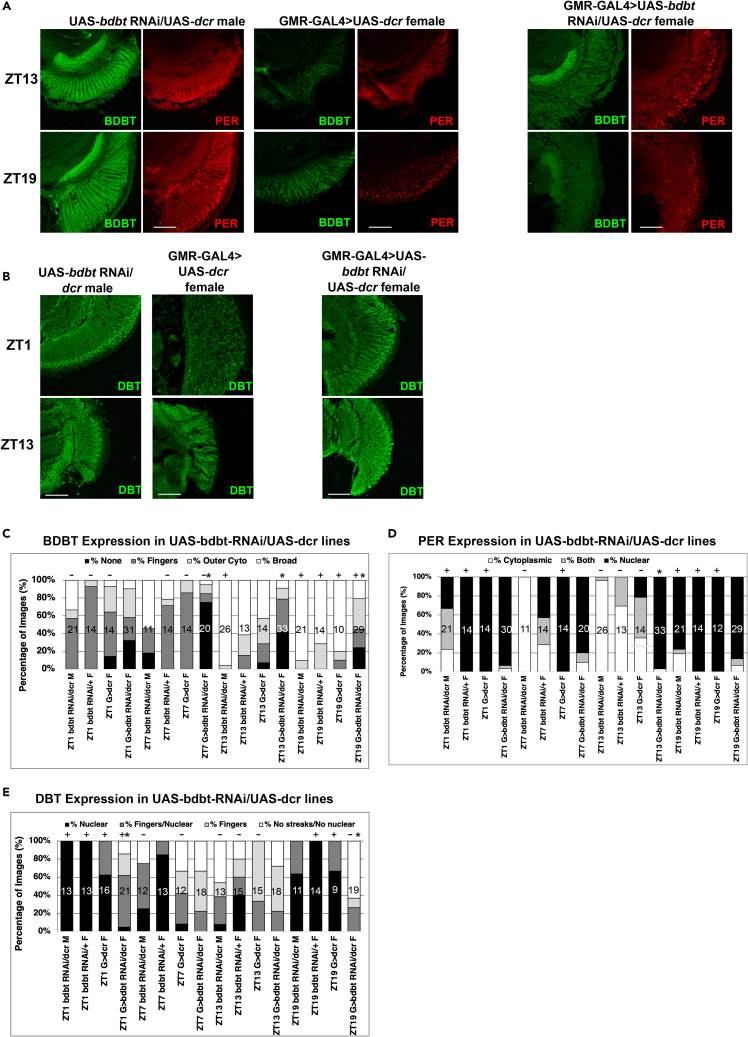
Figure 6.
Effects of BDBT RNAi on PER and DBT Localization in the Fly Eye
Fly heads were collected at ZT1, ZT7, ZT13, and ZT19, and cryosections were prepared for immunofluorescent detection of BDBT, DBT, and PER.
(A) Representative confocal Z stacks of GMR-GAL4/+>UAS-bdbt-RNAi/UAS-dcr female (knockdown), UAS-bdbt RNAi/UAS-dcr male, and GMR-GAL4>UAS-dcr female control photoreceptors at ZT13 and ZT19 for PER and BDBT localization. BDBT knockdown is supported here and also in Figure S4.
(B) Representative DBT subcellular localization in UAS-bdbt RNAi/UAS-dcr control flies, GMR-GAL4>UAS-dcr control flies, and GMR-GAL4>UAS-bdbt-RNAi/UAS-dcr flies.
(C) Graph illustrating the percentage of images scored by observers blinded to sample identity for BDBT foci expression, (D) PER localization, and (E) DBT localization. A star over a sample indicates a statistically significant different BDBT, PER, or DBT localization for GMR-GAL4>UAS-bdbt RNAi/UAS-dcr from that of any of the three control genotypes at that time point (p < 0.05 by multiple comparisons of mean ranks after Kruskal-Wallis nonparametric ANOVA analysis of each time point), while time points marked with a “+” exhibited a peak for nuclear localization (for PER or DBT) or broad expression (for BDBT) relative to those marked with a “-“ for the same genotype with statistical significance (p < 0.04 by multiple comparisons of mean ranks after Kruskal-Wallis nonparametric ANOVA analysis of each genotype). Scale bars indicate 50 μm, and the number of sections analyzed for each bar on the plot is indicated inside the bar.
BDBT foci in genotypes raised in constant darkness or constant darkness followed by 7 h of light are similar to those of flies during darkness or light of an LD cycle
Several of the mutants with affected RHODOPSIN-1-mediated signal transduction lead to long-term light-dependent neurodegeneration in the eye—in particular those affecting ARR219—and so it was important to rule out light-dependent neurodegeneration as a cause of their effects on BDBT foci formation. Therefore, we raised flies entirely in the dark or in the dark followed by 7 h of light and then assessed BDBT foci. The effects of DD and LL in this paradigm (Figure 5) were essentially identical to those in LD, with cry and ninaE mutants suppressing the light-mediated disappearance of BDBT and arr mutants suppressing its appearance in constant darkness (although there was some accumulation of BDBT fingers in the arr1 mutant). Therefore, the absence of effects of light on BDBT foci in photoreceptor mutants and the absence of broadly distributed foci in arr mutants were found under both LD conditions as well as transient-light vs. DD conditions. These results suggest that the effects of visual mutants on BDBT foci formation are not due to light-dependent neurodegeneration but are due to disruptions in the signaling mechanism for light. However, the ninaE mutations drive developmental changes in rhabdomere structure, and arr1 mutations produce progressive neurodegeneration in the eye after eclosion.19 While the collections were performed on young flies to minimize progressive neurodegeneration, we cannot rule out that some of these effects on BDBT foci are produced by neurodegenerative effects of the mutants.
bdbt RNAi in the eye leads to constitutively nuclear PER and cytosolic DBT localization
As previously mentioned, BDBT foci formation has been shown to require circadian regulators like DBT and PER protein.8 If our arr mutants can disrupt the formation of BDBT foci and lead to changes in PER spatial localization, we hypothesized that DBT and PER localization might be affected by knockdown of BDBT. To achieve strong knockdown, we used the UAS-GAL4 system to drive expression of UAS-bdbt-RNAi through the glass multiple reporter (GMR) enhancer, which is strongly expressed in the Drosophila eye, along with dcr (DICER, which cleaves the dsRNAi into short segments that enhance the RNAi effect) to knock down expression of BDBT in the fly eye. Male flies from the cross of GMR-GAL4; UAS-dcr males to UAS-bdbt RNAi females that did not inherit the GMR-GAL4 driver (UAS-dcr/UAS-bdbt RNAi M), female flies from the cross or UAS-bdbt RNAi to Canton S wild type (UAS-bdbt RNAi/+ F), and female flies from the cross or GMR-GAL4; UAS-dcr and Canton S wild type (GMR-GAL4; UAS-dcr) were used as our WT controls, and female flies with the genotype GMR-GAL4>UAS-dcr/UAS-bdbt RNAi were the bdbt-RNAi knockdowns. Sections were treated with anti-DBT (Figure 6B) or with anti-BDBT and anti-PER (Figure 6A). BDBT showed a weakened oscillation in some eyes in the knockdown, but the numbers of eyes with broad/high level expression were lower at three time points than in wild type, with any higher levels of expression typically not extending to the inner regions of the retina, showing persistent knockdown in levels (Figures 4A and 4C and Figure S4A). Likewise, immunoblot analysis of the eyes showed specific knockdown of BDBT in the eyes relative to tubulin and DBT (Figure S2B). Other mutant eyes showed complete loss of BDBT foci at night (Figures 6A and 6C). GMR-GAL4 is expressed in some cells outside the eye, including brain neurons.20 However, the effect on BDBT knockdown with this driver is most likely mediated directly within the eye rather than within the brain lateral neurons as locomotor activity rhythms produced by the brain lateral neurons were not affected by knockdown of BDBT with this driver. In addition, the locomotor activity rhythms were not affected by expression with the GMR-GAL4 driver of a number of transgenic DBT proteins that have produced strong effects on locomotor period lengths in previous work with drivers expressed in the brain lateral neurons (Table 1). As we previously observed in the lateral neurons,8 this knockdown produced high levels of nuclear PER at all times of day rather than the WT oscillation in the controls, and the levels of nuclear PER were significantly higher than those in all three controls at ZT13 (Figures 4A and 4D).
Table 1.
No effect of GMR-GAL4 with circadian UAS responders on circadian activity rhythms
| Genotype | Avg Period (h) ± SD (SEM) | % Rhythmic (n) |
|---|---|---|
| Canton S wild type | 23.4 ± 0.2 (0.06) | 76 (17) |
| GMR-GAL4 | 23.3 ± 0.5 (0.2) | 78 (9) |
| UAS-bdbt RNAi/UAS-dcr | 23.8 ± 0.1 (0.04) | 100 (11) |
| timGAL4>+ | 24.6 (0.3) | 63 (8)8 |
| GMR-GAL4> UAS-bdbt RNAi/UAS-dcr | 23.8 ± 0.3 (0.09) | 93 (15) |
| UAS-dcr: tim-GAL4>/UAS-bdbt RNAi | 30.8 (0.8) | 1 (154)8 |
| tim-GAL4>UAS-bdbt RNAi | 25.5 (0.5) | 93 (27)8 |
| GMR-GAL4>UAS-dbtWT 45F2B | 23.4 ± 0.2 (0.08) | 100 (10) |
| tim-GAL4> UAS-dbtWT 45F2B | 24.9 ± 0.9 (0.2) | 49 (57)21 |
| GMR-GAL4>UAS-dbtK/R 1 MA | 23.5 ± 0.3 (0.2) | 57 (7) |
| timGAL4>UAS-dbtK/R 1 MA | 31.9 ± 2.9 (0.5) | 48 (58)22 |
| GMR-GAL4>UAS-dbtwt NLS1 A9 | 23.2 ± 0.3 (0.1) | 100 (10) |
| tim-GAL4> UAS-dbtwt NLS1 A9 | 22.4 ± 0.2 | 93 (14)23 |
| GMR GAL4>UAS-dbtwt NLS2 B1 | 23.0 ± 0.3 (0.2) | 80 (5) |
| tim-GAL4> UAS-dbtwt NLS2 B1 | 16.6 ± 0.05 | 100 (15)23 |
Lines containing the indicated UAS-bdt RNAi or UAS-DBT insertion were crossed to flies containing the GMR-GAL4 driver, and progeny hemizygous for both the driver and responder or controls lines with only the driver or responder were assayed in DD for locomotor activity. A Canton S wild-type line was also assayed. Each UAS-DBT line (except the UAS-dbtWT line) contained an independent insertion of the responder UAS-DBT gene, generated at the AttP2 locus (at 68A4) by phiC31-mediated integration. The circadian period was determined by chi-square periodogram analysis. Rhythmic flies produced single strong peaks in the periodograms and rhythmicity that was obvious by inspection of actograms. The mean period ±SD (SEM) and the mean percentage of rhythmicity for each genotype class are tabulated for each line. ANOVA showed no significant effect of genotype on period [F(5,30) = 1.9772, p = 0.09181], all of which were wild type and similar to the Canton S, UAS-dcr/UAS-bdbt RNAi or GMR-GAL4 controls. By contrast, previous analysis with a tim-GAL4 driver produced significantly longer and shorter periods (the reference for these numbers is given in the superscript; these are not included in the statistical analysis).
Finally, we observed exactly the opposite effect on DBT nuclear localization. In the GMR-GAL4>/+; UAS-dcr2/UAS-bdbt RNAi lines DBT protein failed to localize significantly to the nuclei in photoreceptor cells at any of the four time points (ZT1, 7, 13, and 19), while it was predominantly nuclear along with PER at ZT1 and ZT 19 in the WT controls (Figures 6B and 6E). Instead, BDBT was often observed in fingers expressed throughout the eye or around the nuclei at all time points in the knockdown flies (Figures 6B and 6E). Similar streaks were observed in WT controls at ZT7 and ZT13.
Discussion
The formation of BDBT foci in the eye is clearly regulated by both the visual and circadian photoreceptor proteins, and normal levels of BDBT foci are associated with concomitant nuclear localization of DBT and PER. With BDBT downregulation, PER is constitutively nuclear and DBT is constitutively cytosolic. We discuss the implications of these findings for 1) possible mechanisms for regulation by visual pathways, 2) possible mechanisms for regulation by circadian photoreceptor protein pathways and whether this regulation is independent of the visual pathway, 3) regulation of DBT and PER nuclear localization, and 4) the basis for BDBT foci formation.
Regulation of BDBT foci by visual pathways
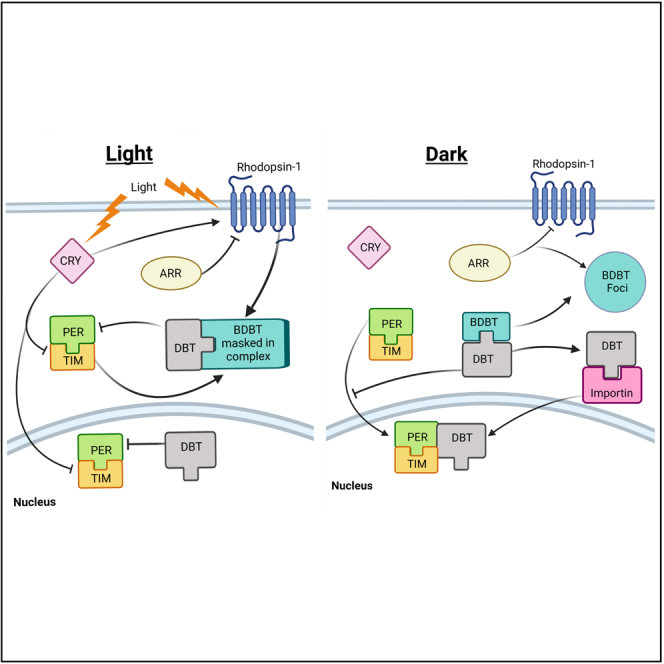
Our finding that WT BDBT foci formation requires ARRESTINS and dark conditions is of interest since the other well-studied ARRESTIN function involving RHODOPSIN—inactivation of photoreceptor signals via interactions with RHODOPSIN—is a light-dependent rather than a dark-dependent process.15 However, several proteins involved with phototransduction (e.g., Gα and TRPL) are regulated through mechanisms involving the translocation into and out of the rhabdomeres of photoreceptors in response to dark and light, and this movement is thought to be involved in the adaptations of eyes to light or dark by altering light sensitivity and involves the Arrestins.24,25,26,27 In dark conditions, both ARR1 and ARR2 are primarily located in the cytosol at times at which BDBT foci are also accumulating.14,28 When RHODOPSIN is converted to M-RHODOPSIN in response to blue light, ARR2 is recruited to the rhabdomere to deactivate further RHODOPSIN/Gα protein signaling.14,29 Release of ARR2 requires phosphorylation of ARR2 by Ca2+/calmodulin-dependent protein kinase II,28,30 and ARR1 may also play a role in deactivating ARR2 signal quenching. If ARR2 requires ARR1 to be released from RHODOPSIN and must be released to form BDBT foci, this codependence could explain why BDBT foci fail to form without either ARR1 or ARR2.
In the ninaE mutants assayed here, which produce no functional transcripts of rhodopsin-1, we saw increased BDBT foci during light periods of LL/DD and LD cycles. A possible explanation could be that, without RHODOPSIN (ninaE mutants) for ARR2 and ARR1 to deactivate, both ARRESTINS are more freely available to generate BDBT foci in both light and dark—thereby explaining the ninaE phenotype of high BDBT foci in both light and dark. This model would explain the dark-dependent role for the ARRESTINS in BDBT foci formation since they would act to deactivate RHODOPSIN under lighted conditions and then serve a role in the formation of BDBT foci under dark conditions. However, this model is not supported by our arr11; ninaE1 double-mutant data because a formative role for both ARRESTINS would predict that BDBT foci should not form in this double mutant but instead they do form in both light and dark (Figure 3). Clearly ARR1 is not needed to form the BDBT foci. Although ARR2 may potentially be required, clearly ARR1 is not required, and the single arr11 mutant eliminates their formation. The persistence of the ninaE1 phenotype in the double-mutant epistasis test instead suggests that a light-dependent signal mediated by Rhodopsin 1 is needed to repress BDBT formation and that this signal is elevated in the arr mutants even in the dark, leading to repression of the BDBT foci.
Is regulation of BDBT foci by the circadian photoreceptor CRY independent of the visual pathway?
Many of the proteins involved in the eye visual response (including Rh1 and TRP channels) are regulated by interactions in a large multimeric signaling complex mediated by inactivation-no-afterpotential D (INAD), a scaffolding protein. Some of these associations are disrupted by light.19 Moreover, INAD can mediate an interaction of the circadian and visual pathways because it also binds to fly CRY in a light-dependent manner, and cry mutants exhibit impaired visual behavior.31 Alternatively, CRY may signal through the circadian pathway (eg, via TIM) to downregulate BDBT. Interaction with TIM and triggering the downregulation of TIM in response to light are the known circadian functions of CRY.3,4 The formation of BDBT foci in the ninaE17 mutant, which produced degeneration of rhabdomeric structures,32 and our results shown in Figure S1 suggests that their formation occurs outside the rhabdomere.
Regulation of DBT and PER nuclear localization by BDBT
Reduced BDBT foci in the eye have a significant effect on the subcellular localization of the circadian regulators DBT and PER. Our finding that BDBT binds to the DBT-NLS, needed for nuclear localization of DBT,22,23 may explain the effects on DBT. It is possible that BDBT foci accumulation recruits BDBT away from DBT, thereby exposing the DBT-NLS, and in WT flies this dissociation is followed by nuclear localization of DBT. BDBT foci peak at time when nuclear PER and DBT are highest, and if these foci release BDBT from the DBT-NLS, they may allow DBT not bound to BDBT and therefore with less activity toward PER to translocate to the nucleus via interactions at the NLS with nuclear importins.33 Both the high levels and nuclear localization of PER are thought to be produced by low DBT activity,21,34 suggesting that the BDBT foci may also downregulate DBT activity at these times by eliminating the BDBT/DBT interaction. The effect of the foci on nuclear localization of DBT could also explain why we also see cytosolic DBT in the absence of BDBT foci such as in BDBT eye-specific knockdown. Independent movement to nuclei of two other circadian components (PER and TIM) that likewise associate in a complex has also been shown.35
Structurally, BDBT is an FK506-binding protein,8 and these proteins typically serve as factors in the assembly of large macromolecular complexes. Several of these complexes are involved in regulation of subcellular localization. BDBT has recently been observed to contribute to different subcellular localization of planar polarity components via DBT.36 Other FKBPs (FKBP51, FKBP52, and FKBPL) have been shown to regulate the nuclear localization of the glucocorticoid receptor (GR)37,38 and to interact with several of the mammalian orthologs of DBT.39 A speculative model postulates that, after BDBT dissociates in foci from DBT, DBT can be moved to the nuclei via interactions with importin and microtubules. This dissociation produces a BDBT state that can be detected by our antibody since BDBT is present at other times in the eye when foci are not detectable (Figure 4).
This work has led to a model for light-dependent regulation of BDBT foci that impact DBT and PER localization in the eye. Light works through both the circadian and visual photoreceptor proteins to downregulate BDBT foci. The circadian photoreceptor CRY may operate through standard circadian pathways involving TIMELESS (TIM), or it may interact with the visual scaffold INAD to mediate its effects. RHODOPSIN-1 is likely to signal through PHOSPHOLIPASE C’s, or it may compete during the day for interactions with ARRESTINS needed for BDBT foci formation (but our double-mutant data argue against this model). The accumulation of foci is needed for DBT localization to photoreceptor nuclei, perhaps by modulating an interaction of the DBT NLS with importins. We have not observed accumulation of BDBT foci during the dark and not in the light but instead continuous expression in the brain neurons that regulate circadian behavior, so this process may be specific to photoreceptors and regulate visual physiology.
Dark-dependent BDBT foci formation in photoreceptors is not due to changes in level or localization of BDBT
The results of Figure 4 show that BDBT levels do not change in response to light as assessed by immunoblot analysis of isolated eyes. Moreover, elevated temperatures prior to antibody application reveal broad immunofluorescence for BDBT in WT eyes (figure S3), suggesting that the elevated temperatures reduce BDBT interactions with other proteins or folding of BDBT, thereby unmasking epitopes that are otherwise masked. However, heat does not unmask the BDBT epitopes in the arr mutants, which have comparable levels of BDBT to WT flies by immunoblot analysis (Figure 4). It is possible that a different conformation or assembled masking state exists in the arr mutants, and this is not dissociated by 60°C treatments or reassembles when the temperature is reduced to room temperature for the antibody treatment. Moreover, the BDBT foci persist in light in the R7s. It is possible that Rh3 and Rh4 are not sensitive enough to the wavelengths in our fluorescent lights (cool-white light, which has little UV emission) to be activated by them. These R7s could also use a modified pathway for light-dependent and circadian regulation of BDBT-dependent processes than the one that occurs in R1-6. The changing BDBT foci might be involved in this process only in R1-6, which express Rh1. The nature of the changes that occur during light and in the arr mutants is not resolved and will be the focus of our future research efforts.
Limitations of the study
Because we have not detected oscillations of BDBT levels in this study or in our previous work, the processes that contribute to foci formation via microscopy are not certain. In addition, we cannot rule out some effects of neurodegeneration in our visual mutants on BDBT foci—particularly in the ninaE mutants.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-tubulin | Developmental Studies Hybridoma Bank | Cat# E7; RRID:AB_528499 |
| guinea pig anti-BDBT #589 | Fan et al., 20138 | N/A |
| rabbit anti-PER | Muskus et al. 200721 | N/A |
| Rabbit anti-DBT-C | Muskus et al., 200721 | N/A |
| Rabbit anti-Rh3 | Britt lab (from C. Zuker); Feiler et al., 199240 | N/A |
| Anti-rabbit HRP | American Qualex | Cat# A102PN |
| Anti-mouse HRP | American Qualex | Cat# A108PN |
| Anti-guinea pig HRP | American Qualex | Cat# A109PS |
| goat anti-guinea pig IgG Alexa Fluor 488 | Invitrogen | Cat#A11073; RPID:AB_2534117 |
| goat anti-rabbit IgG Alexa Fluor 488 | Invitrogen | Cat#: A11073; RRID:AB_2534117 |
| goat anti-rabbit IgG Alexa Fluor 568 | Invitrogen | Cat# A11036; RRID: AB_10563566 |
| Experimental models: Organisms/strains | ||
| Drosophila/Canton S wild type flies | Michael Young’s lab | N/A |
| Drosophila/yw; UAS-dbtWT45F2B | Muskus et al., 200721 | N/A |
| Drosophila/w; UAS-dbtK/R 1 MA | Venkatesan et al., 201522 | N/A |
| Drosophila/w; UAS-dbtWT NLS1 A9 | Venkatesan et al., 201923 | N/A |
| Drosophila/w; UAS-dbtWT NLS2 B1 | Venkatesan et al., 201923 | N/A |
| Drosophila/cryout | Yoshii et al., 200811 | N/A |
| Drosophila/cryb | Stanewsky et al., 199810 | N/A |
| Drosophila/GMR-GAL4 (x) | Takahashi et al., 199941 | N/A |
| Drosophila/w; ort1ninaE1 | Bloomington Drosophila Stock Center | RRID:BDSC_1946 |
| Drosophila/w; sr1ninaE17es | Bloomington Drosophila Stock Center | RRID:BDSC_5701 |
| Drosophila/Arr11cn1bw1 | Bloomington Drosophila Stock Center | RRID:BDSC_42252 |
| Drosophila/w; Arr23 | Bloomington Drosophila Stock Center | RRID:BDSC_42255 |
| Drosophila/w1118; UAS-Dcr-2; Df(3L)Ly, sens[Ly-1]/TM3, Sb1 | Bloomington Drosophila Stock Center | RRID:BDSC_24645 |
| Drosophila/UAS-bdbt RNAi | Vienna Drosophila RNAi center | Cat# 100028 |
| Software and algorithms | ||
| Statistica 13.5 | TIBCO Software | N/A |
| ClockLab | Actimetrics | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents may be directed to and will be fulfilled by the Lead Contact, Dr. Jeffrey Price (pricejL@umkc.edu).
Materials availability
Fly strains and antibodies generated in this study are available from the lead contact upon request.
Experimental model and subject details
Fly stocks
The following fly lines were used for this study: Wild type (WT) Canton S flies, yw; UAS-dbtWT45F2B,21 w; UAS-dbtK/R 1 MA,22 w; UAS-dbtWT NLS1 A9,23 w; UAS-dbtWT NLS2 B1,23 cryout 11, cryb,10 GMR-GAL4 on the X chromosome,41 w; ort1 ninaE1(Bloomington Drosophila Stock Center line 1946). w; sr1 ninaE17 es (Bloomington Drosophila Stock Center line 5701), Arr11cn1 bw1 (Bloomington Drosophila Stock Center line 42252), w; arr23 (Bloomington Drosophila Stock Center line 42255), w1118; UAS-Dcr-2; Df(3L)Ly, sens[Ly-1]/TM3, Sb1(Bloomington Drosophila Stock Center line 24645), UAS-bdbt RNAi (Vienna Drosophila RNAi Center line 100028). arr11(on chromosome II); ninaE (on chromosome III) double mutants were constructed by genetic crosses.
Method details
Fly lines and rearing conditions (LD to DD, LD to LL, LD, DD, DD to LL)
Our fly food is composed of agar (0.52%), yeast (1.1%), corn meal (5.3%), molasses (6.8%), and Tegosept (0.1%) as an anti-fungal agent. Mutants of the indicated type were harvested at the indicated times in LD cycles (ZT, lights on from 0-12), the second day of constant darkness (DD times are subjective day), the first day of constant light (LL times indicate previous time in LD), after rearing in constant darkness, or after rearing in constant darkness followed by 7 h of light. For demonstration of the effects of bdbt RNAi on foci formation, GMR-GAL4; UAS-dcr male flies were crossed to UAS-bdbt RNAi females from the Vienna Drosophila Resource Center, allowing for eye-specific BDBT knockdown in females or a UAS-bdbt RNAi/UAS-dcr male control. Other controls were generated by crossing UAS-bdbt RNAi males to Canton S females or Canton S males to GMR-GAL4: UAS-dcr females.
Locomotor activity analysis
Activity was scored for at least 5 days in constant darkness at 25°C, and rhythmicity was scored by chi-square periodogram analysis as previously described.8
Immunoblot analysis
For analysis of changes to BDBT, PER, and TUBULIN protein levels, heads were prepared from flies collected during LD at ZT 1, 7, 13 and 19, homogenized in 1.1x Laemmli SDS loading buffer (7 μL per head), and then heated for 5 min at 100°C and stored at −80°C. For the eyes analyzed in Figures 4 and S4B, heads were collected at the indicated times, immersed in -70oC acetone, and eyes subsequently dissected from dried heads dissected as previously described42 prior to homogenization in 1.1X Laemmli SDS loading buffer. For immunoblot assays, head extracts were subjected to SDS-PAGE, transferred to nitrocellulose, and antigens detected with the appropriate antibodies as described.8 Extracts were analyzed on either 5.7% (for PER) or 10% (for tubulin and BDBT) SDS-PAGE gels with the ECL procedure (GE Healthcare). The antibodies used were mouse anti-tubulin from the Developmental Studies Hybridoma Bank (Iowa City, Iowa), guinea pig anti-BDBT #589 8, and rabbit anti-PER.21 HRP-coupled secondary antibodies came from American Qualex. Immunoblots of 3-5 independent experiments were performed for BDBT, and the signals were detected and quantified on a BioRad imager. Each BDBT signal was normalized to tubulin for each sample, and these normalized signals were then normalized to the tubulin-normalized signal for Canton S at ZT19.
Immunofluorescence laser scanning confocal microscopy
For detection of BDBT, DBT, Rh3 and PER in the eyes, fly heads were collected under their respective rearing conditions and embedded at -80°C in OCT (Ted Pella). Sections prepared with a cryostat were processed for immunofluorescent detection of BDBT, detected with guinea pig anti-BDBT 589 (1:5000) and goat anti-guinea pig IgG Alexa Fluor 488 (1:1000; Invitrogen) or goat anti-guinea pig IgG Alexa Fluor 647 (for Figures S1B and S1C), detection of DBT, detected with rabbit anti-DBT C (21;1:2000) and goat anti-rabbit IgG Alexa Fluor 488 (1:1000; Invitrogen), detection of PER, detected with rabbit anti-PER (1/10,000) and goat anti-rabbit IgG Alexa Fluor 568 (1/1000; Invitrogen), or detection of Rh3, detected with rabbit anti-Rh3 (1/100 from Steve Britt, UT-Austin;40) and goat anti-rabbit IgG Alexa Fluor 488 (1/1000; Invitrogen) by confocal microscopy (Zeiss LSM5 or an Olympus Fluoview) using a 40x water immersion lens, as previously described.8 For the samples examined in Figure S3, the washing and blocking incubations in PBS, PBT and PBTN after paraformaldehyde fixation and prior to incubation with the primary antibody were conducted at 60oC; all other incubations were conducted at 25oC. The expression pattern of BDBT (low and broad, fingers (as in Figures 1A-LL19), high and broad) or its lack of expression (none), DBT localization (neither fingers nor nuclear, fingers, fingers around nuclei, or nuclear), or PER localization (cytoplasmic, both nuclear and cytoplasmic, or nuclear) was tabulated by two observers blinded to the identity of the samples. Both observers produced equivalent results.
Quantification and statistical analysis
For analysis of the BDBT, DBT and PER expression patterns determined by immunofluorescence, experimental data from blinded scores of at least three experiments by one observer were pooled and subjected to either a Kruskal-Wallis nonparametric ANOVA with multiple comparisons of mean ranks for all groups or a Manny-Whitney U test (with continuity correction). The Statistica software package was used for this analysis. Statistical analysis of immunoblots was a standard ANOVA with a Tukey HSD test.
Acknowledgments
This research was supported by the National Institute on Aging (R15AG053879 grant to JLP). Drosophila lines were provided by Ralf Stanewsky (the cry lines), George Jackson (the GMR-GAL4 line), the Bloomington Drosophila Stock Center, and the Vienna Drosophila RNAi Center. The anti-Rh3 antibody was contributed by Steve Britt from the University of Texas-Austin.
Author contributions
J.L.P., J-Y. F., and R.B.N. designed the study. J-Y. F. made the initial observations of the light regulation and arr mutant effects, while the undergraduates (including initially R.B.N.) extended these findings with extensive confocal microscopy immunofluorescent analysis of all the mutants. R.B.N. (then a Ph.D. student) and J.L.P. formalized this analysis with scoring and developed the bdbt RNAi approach. R.B.N., J-Y. F., and J.L.P. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community.
Published: March 5, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106343.
Contributor Information
Jin-Yuan Fan, Email: priceji@umkc.edu.
Jeffrey L. Price, Email: pricejl@umkc.edu.
Supplemental information
Data and code availability
All relevant data supporting the findings of this study are available from the lead contact upon request.
This paper does not report original code.
References
- 1.Pittendrigh C.S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb. Symp. Quant. Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Patke A., Young M.W., Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 3.Ceriani M.F., Darlington T.K., Staknis D., Más P., Petti A.A., Weitz C.J., Kay S.A. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 4.Koh K., Zheng X., Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price J.L., Blau J., Rothenfluh A., Abodeely M., Kloss B., Young M.W. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 6.Kloss B., Price J.L., Saez L., Blau J., Rothenfluh A., Wesley C.S., Young M.W. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 7.Allada R., Emery P., Takahashi J.S., Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 8.Fan J.Y., Agyekum B., Venkatesan A., Hall D.R., Keightley A., Bjes E.S., Bouyain S., Price J.L. Noncanonical FK506-binding protein BDBT binds DBT to enhance its circadian function and forms foci at night. Neuron. 2013;80:984–996. doi: 10.1016/j.neuron.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emery P., So W.V., Kaneko M., Hall J.C., Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 10.Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., Kay S.A., Rosbash M., Hall J.C. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 11.Yoshii T., Todo T., Wülbeck C., Stanewsky R., Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J. Comp. Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 12.Washburn T., O'Tousa J.E. Molecular defects in Drosophila rhodopsin mutants. J. Biol. Chem. 1989;264:15464–15466. [PubMed] [Google Scholar]
- 13.O'Tousa J.E., Baehr W., Martin R.L., Hirsh J., Pak W.L., Applebury M.L. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 14.Satoh A.K., Ready D.F. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 15.Dolph P.J., Ranganathan R., Colley N.J., Hardy R.W., Socolich M., Zuker C.S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- 16.Dolezelova E., Dolezel D., Hall J.C. Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerr D.M., Hall J.C., Rosbash M., Siwicki K.K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J. Neurosci. 1990;10:2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S.R., Shi Y., Taylor C.R. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J. Histochem. Cytochem. 2011;59:13–32. doi: 10.1369/jhc.2010.957191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T., Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 20.Li W.Z., Li S.L., Zheng H.Y., Zhang S.P., Xue L. A broad expression profile of the GMR-GAL4 driver in Drosophila melanogaster. Genet. Mol. Res. 2012;11:1997–2002. doi: 10.4238/2012.August.6.4. [DOI] [PubMed] [Google Scholar]
- 21.Muskus M.J., Preuss F., Fan J.Y., Bjes E.S., Price J.L. Drosophila DBT lacking protein kinase activity produces long-period and arrhythmic circadian behavioral and molecular rhythms. Mol. Cell Biol. 2007;27:8049–8064. doi: 10.1128/MCB.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan A., Fan J.Y., Nauman C., Price J.L. A doubletime nuclear localization signal mediates an interaction with bride of doubletime to promote circadian function. J. Biol. Rhythms. 2015;30:302–317. doi: 10.1177/0748730415588189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatesan A., Fan J.Y., Bouyain S., Price J.L. The circadian tau mutation in casein kinase 1 is part of a larger domain that can be mutated to shorten circadian period. Int. J. Mol. Sci. 2019;20:813. doi: 10.3390/ijms20040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosloff M., Elia N., Joel-Almagor T., Timberg R., Zars T.D., Hyde D.R., Minke B., Selinger Z. Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronin M.A., Diao F., Tsunoda S. Light-dependent subcellular translocation of Gqalpha in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J. Cell Sci. 2004;117:4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- 26.Bähner M., Frechter S., Da Silva N., Minke B., Paulsen R., Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 27.Meyer N.E., Joel-Almagor T., Frechter S., Minke B., Huber A. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J. Cell Sci. 2006;119:2592–2603. doi: 10.1242/jcs.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alloway P.G., Dolph P.J. A role for the light-dependent phosphorylation of visual arrestin. Proc. Natl. Acad. Sci. USA. 1999;96:6072–6077. doi: 10.1073/pnas.96.11.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranganathan R., Stevens C.F. Arrestin binding determines the rate of inactivation of the G protein-coupled receptor rhodopsin in vivo. Cell. 1995;81:841–848. doi: 10.1016/0092-8674(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 30.Kahn E.S., Matsumoto H. Calcium/calmodulin-dependent kinase II phosphorylates Drosophila visual arrestin. J. Neurochem. 1997;68:169–175. doi: 10.1046/j.1471-4159.1997.68010169.x. [DOI] [PubMed] [Google Scholar]
- 31.Mazzotta G., Rossi A., Leonardi E., Mason M., Bertolucci C., Caccin L., Spolaore B., Martin A.J.M., Schlichting M., Grebler R., et al. Fly cryptochrome and the visual system. Proc. Natl. Acad. Sci. USA. 2013;110:6163–6168. doi: 10.1073/pnas.1212317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinal N., Pichaud F. Dynamin- and Rab5-dependent endocytosis is required to prevent Drosophila photoreceptor degeneration. J. Cell Sci. 2011;124:1564–1570. doi: 10.1242/jcs.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloss B., Rothenfluh A., Young M.W., Saez L. Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron. 2001;30:699–706. doi: 10.1016/s0896-6273(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 34.Cyran S.A., Yiannoulos G., Buchsbaum A.M., Saez L., Young M.W., Blau J. The double-time protein kinase regulates the subcellular localization of the Drosophila clock protein period. J. Neurosci. 2005;25:5430–5437. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Syed S., Saez L., Young M.W. Kinetics of doubletime kinase-dependent degradation of the Drosophila period protein. J. Biol. Chem. 2011;286:27654–27662. doi: 10.1074/jbc.M111.243618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strutt H., Strutt D. DAnkrd49 and Bdbt act via Casein kinase Iepsilon to regulate planar polarity in Drosophila. PLoS Genet. 2020;16:e1008820. doi: 10.1371/journal.pgen.1008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fries G.R., Gassen N.C., Rein T. The FKBP51 glucocorticoid receptor Co-Chaperone: regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2017;18:2614. doi: 10.3390/ijms18122614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKeen H.D., McAlpine K., Valentine A., Quinn D.J., McClelland K., Byrne C., O'Rourke M., Young S., Scott C.J., McCarthy H.O., et al. A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology. 2008;149:5724–5734. doi: 10.1210/en.2008-0168. [DOI] [PubMed] [Google Scholar]
- 39.Kategaya L.S., Hilliard A., Zhang L., Asara J.M., Ptáček L.J., Fu Y.H. Casein kinase 1 proteomics reveal prohibitin 2 function in molecular clock. PLoS One. 2012;7:e31987. doi: 10.1371/journal.pone.0031987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feiler R., Bjornson R., Kirschfeld K., Mismer D., Rubin G.M., Smith D.P., Socolich M., Zuker C.S. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J. Neurosci. 1992;12:3862–3868. doi: 10.1523/JNEUROSCI.12-10-03862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi Y., Hirose F., Matsukage A., Yamaguchi M. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res. 1999;27:510–516. doi: 10.1093/nar/27.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng H., Hardin P.E., Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994;13:3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data supporting the findings of this study are available from the lead contact upon request.
This paper does not report original code.