Summary
Herein, we report a novel strategy for the synthesis of chiral difluoroalkyl-substituted cyclopropanes through enantioselective [2 + 1] cyclopropanation of alkenes and difluoroalkyl-substituted carbenes under rhodium catalysis, wherein the newly designed α, α-difluoro-β-carbonyl ketone N-triftosylhydrazones are used as the difluoroalkyl-substituted carbenes precursors. This approach represents the first asymmetric cyclopropanation of alkenes with difluoroalkyl carbenes, featuring high yield, high enantioselectivity, and broad substrate scope. Gram-scale synthesis and further interconversion of diverse functional groups demonstrate the usefulness of this protocol in the preparation of diverse functionalized chiral difluoroalkyl-substituted cyclopropanes.
Subject areas: Catalysis, Organic synthesis, Organic compound
Graphical abstract

Highlights
-
•
Chiral difluoromethylene cyclopropanes
-
•
Difluoroalkyl-substituted carbenes
-
•
Broad substrate scope
Catalysis; Organic synthesis; Organic compound
Introduction
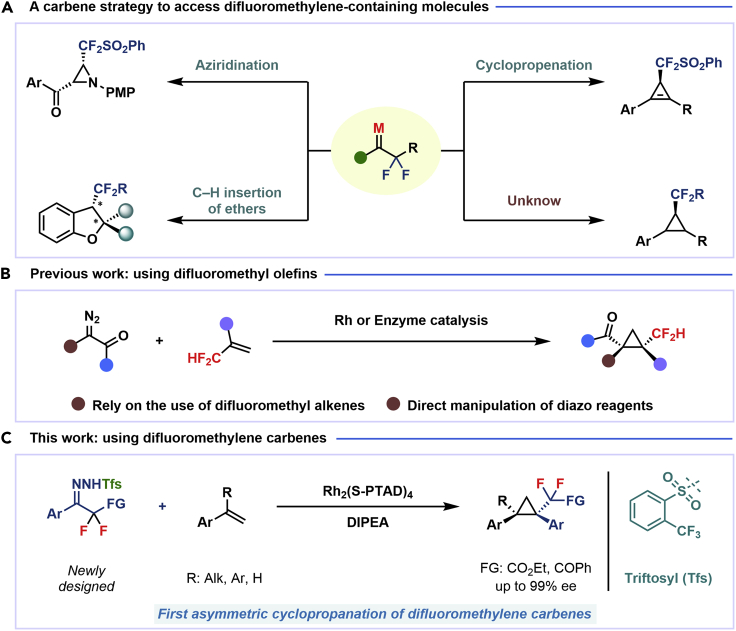
The synthesis of partially fluorinated chiral organic molecules has drawn increasing attention, owing to the applications of such substrates in the design and development of bioactive molecules and drugs.1,2,3,4 Difluoromethylene carbenes, which can considered as a suitable source of partially fluorinated moieties, have been widely used for the introduction of difluoroalkyl units into molecular skeletons.5,6,7,8,9 However, in sharp contrast to the significant advances in the asymmetric reaction of perfluoroalkyl carbenes,5,6,7,8,9,10,11,12,13,14,15,16,17,18 the study on the enantioselective reactions involving difluoromethylene carbenes is still in its very early stage. To date, only three types of reactions are known in the prior art, namely the enantioselective cyclopropenation and aziridination reactions developed by Ma and coworkers19,20 and our report of the asymmetric intramolecular C–H insertion of ethers (Scheme 1A).21 The [2 + 1] cyclopropanation represents one of the most typical reactions of carbenes; however, the analogous asymmetric reaction involving difluoroalkyl-substituted carbenes, which would allow for the synthesis of chiral difluoroalkyl-substituted cyclopropanes that have been found as the key structural unit of HIV inhibitors, remains undeveloped so far.22,23 This may be due to the low reactivity of the available difluoroalkyl-substituted carbenes and to the lack of appropriate carbene precursors. Regarding the synthesis of chiral difluoroalkyl cyclopropanes, only one strategy has been disclosed, which involves the cycloaddition of difluoromethyl olefins with diazo compounds under, respectively, rhodium and enzyme catalysis.24,25 On the other hand, the direct handling of hazardous diazo reagents remains until now the main disadvantage of this strategy. Consequently, an alternative strategy for asymmetric cyclopropanation of alkenes using difluoroalkyl-substituted carbenes is highly desirable.
Scheme 1.
Synthetic strategies for chiral difluoromethylene- or difluoromethyl-substituted molecules
As our continued interest in the chemistry of fluoroalkyl N-triftosylhydrazones,26,27,28,29,30 herein, we report the first enantioselective cyclopropanation of alkenes with difluoroalkyl-substituted carbenes in situ generated from α, α-difluoro-β-carbonyl ketone, which enables the successful application of difluoromethylene carbene in the asymmetric cyclopropanation. The use of N-triftosylhydrazones as difluoroalkyl-substituted carbenes surrogates avoids the need for direct manipulation of hazardous diazo reagents and, thus, reduces safety issues. It is worth mentioning that diverse alkenes, including aryl-alkyl, diaryl, and aryl terminal alkenes, are suitable in this protocol and have been proved to provide the chiral cyclopropanes in high yield and excellent enantioselectivity.31,32,33 More importantly, N-triftosylhydrazones, with the advantage of easy decomposition at relatively low temperature, plays an essential role in the success of this kind of novel asymmetric [2 + 1] cycloaddition reaction.
Results and discussion
We began the study with the screening of chiral dirhodium catalyst for the cyclopropanation of suitable precursors in α-methyl-phenylethylene (2) with N-triftosylhydrazone (1). All the catalysts Rh2(S-DOSP)4, Rh2(S-PTTL)4, and Rh2(S-PTAD)4 afforded the desired difluoroalkyl-substituted cyclopropane in moderate yield at room temperature except with Rh2(S-PTAD)4 showing enantiocontrol up to 91% ee (Table 1, entry 1–3). Notably, the D2 symmetric catalyst Rh2(S-BTPCP)4 was found to be ineffective in this reaction (entry 4), whereas the C4 symmetric catalysts (Rh2(S-DOSP)4, Rh2(S-PTTL)4, and Rh2(S-PTAD)4) were more suitable for this [2 + 1] cycloaddition reaction.34,35 Commonly used bases, such as K2CO3, Cs2CO3, and tBuOK, were not suitable for the cyclopropenation reaction (entries 5–7). Performing the reaction in other solvents, such as toluene, DCM, and DMF, led to lower yield or no reaction (entries 8–10). Subsequently, a decrease in enantioselectivity was found when the reaction temperature was increased, which indicated the advantage of N-triftosylhydrazone decomposable at low temperatures (room temperature or below) in such asymmetric reactions (room temperature or lower) (entry 11 and 12).19,20,28 However, at low catalyst loadings (0.5 mol % of Rh2(S-PTAD)4), no significant changes in both yield and enantioselectivity were found (entry 13). Notably, low yield and enantioselectivity were observed when the reaction was run with 0.25 mol % of catalyst (entry 14). Surprisingly, when α-methyl-4-NO2-phenylethylene was used instead of α-methyl-phenylethylene, the desired product was obtained in 93% yield with 95% ee (entry 15). Based on these results, we speculated that the electronic setup of the substrate might have a significant effect on the yield of this reaction. Finally, the reaction condition as stated in entry 9 [DIPEA (2.0 equiv), Rh2(S-PTAD)4 (0.5 mol %), benzotrifluoride (TFT, 3.0 mL) at room temperature] was identified as the optimal condition for investigating the scope of this reaction.
Table 1.
Optimization of the reaction conditions
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Rh catalysta | Base | Solvent | Temp. | Yield | ee |
| 1 | Rh2(S-DOSP)4 (1 mol %) | DIPEA | TFT | 25°C | 58% | 59% |
| 2 | Rh2(S-PTTL)4 (1 mol %) | DIPEA | TFT | 25°C | 66% | 87% |
| 3 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | TFT | 25°C | 61% | 91% |
| 4 | Rh2(S-BTPCP)4 (1 mol %) | DIPEA | TFT | 25°C | N.D. | – |
| 5 | Rh2(S-PTAD)4 (1 mol %) | K2CO3 | TFT | 25°C | N.R. | – |
| 6 | Rh2(S-PTAD)4 (1 mol %) | CsCO3 | TFT | 25°C | N.R. | – |
| 7 | Rh2(S-PTAD)4 (1 mol %) | tBuOK | TFT | 25°C | N.R. | – |
| 8 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | Toluene | 25°C | 53% | 82% |
| 9 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | DCM | 25°C | 47% | 20% |
| 10 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | DMF | 25°C | Trace | – |
| 11 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | TFT | 40°C | 73% | 87% |
| 12 | Rh2(S-PTAD)4 (1 mol %) | DIPEA | TFT | 0°C | 21% | – |
| 13 | Rh2(S-PTAD)4 (0.5 mol %) | DIPEA | TFT | 25°C | 61% | 92% |
| 14 | Rh2(S-PTAD)4 (0.25 mol %) | DIPEA | TFT | 25°C | 50% | 74% |
| 15b | Rh2(S-PTAD)4 (0.5 mol %) | DIPEA | TFT | 25°C | 93% | 95% |
 | ||||||
N.D., Not detect.
Reaction conditions: N-Triftosylhydrazone 1 (0.2 mmol), 1,1-diphenylethylene 2 (0.1 mmol), [Rh] catalyst (1 mol %), DIPEA (N, N-diisopropylethylamine) (0.2 mmol), TFT (benzotrifluoride, 3.0 mL), 12 h, under N2.
α-Methyl-4-NO2-phenylethylene was used.
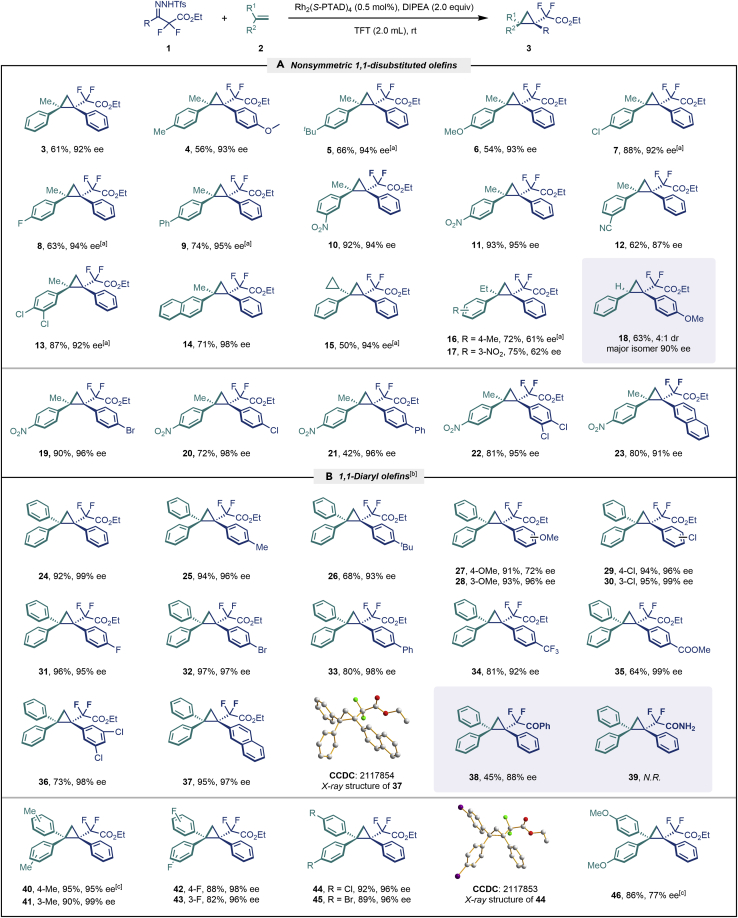
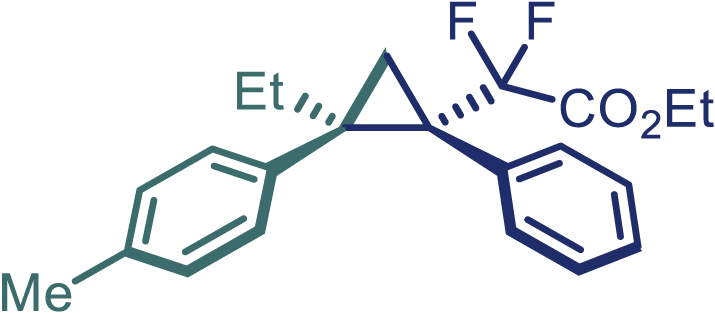
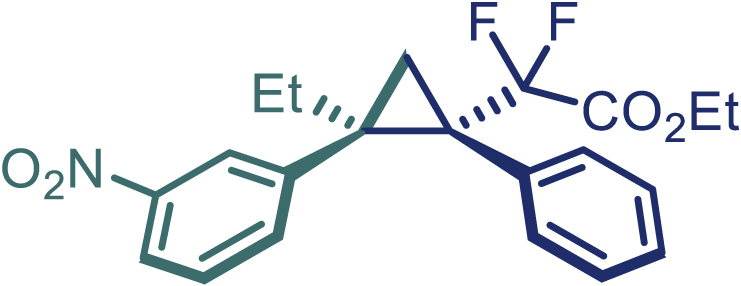
With the optimized condition in hand, initially, we directed our studies toward exploring the scope of α-methyl-phenylethylenes in this asymmetric [2 + 1] cycloaddition reaction. Remarkably, under the reaction condition as shown in Scheme 2A, α-methyl-phenylethylenes bearing a wide range of functional groups, such as methyl (4), tert-butyl (5), methoxy (6), halogen (7; 8), phenyl (9), nitro (10), and cyano (11), were tolerated, leading to the desired chiral difluoroalkyl-substituted cyclopropanes (3–12) in moderate to high yield with excellent enantioselectivity. The substrates having electron-withdrawing and electron-donating substituents have no significant effect on enantioselectivity, albeit moderate yields were observed from the latter (3–6). The 3,4-dichloro phenyl and naphthyl-substituted α-methyl terminal alkenes were also found to be suitable coupling partners to afford the corresponding products in good yield and excellent enantioselectivity (13 and 14). By substituting methyl by cyclopropyl, the chiral difluoromethylene bicyclopropane (15) product was obtained in 94% ee with a moderate yield (50%; Scheme 2). Notably, such a bicyclopropane structural skeleton is present in a variety of protein inhibitors, antibiotic, and antifungal agents.36,37,38,39 Both methyl- and nitro-containing α-ethylstyrene substrates (16 and 17) responded well under these conditions to afford good yields (72%, 75%; Scheme 2), but with a significant decrease in enantioselectivity (61%, 62%). Styrene was also found to be suitable for this transformation and provided the chiral difluoroalkyl-substituted cyclopropane in 63% yield with 4:1 diastereoisomer ratio (18), wherein the major configuration retained high enantioselectivity (90% ee). We subsequently screened several difluoromethylene N-triftosylhydrazones with various substituents such as Br, Cl, phenyl, naphthyl etc., and were pleased to find that all of them reacted with excellent enantioselectivity (19–23).
Scheme 2.
Reaction conditions: 1 (0.4 mmol), Rh2(S-PTAD)4 (0.5 mol %), 2 (0.2 mmol) and DIPEA (0.4 mmol) in TFT (3 mL) at rt
(A) The enantiomer ratio was determined by converting the compound to an alcohol.
(B) Rh2(S-PTAD)4 (0.25 mol %) was used.
(C) DCM (3 mL) used as solvent. Diastereoselective ratio >20:1.
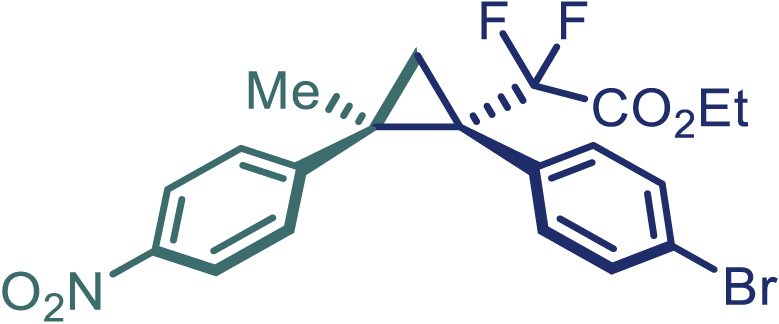
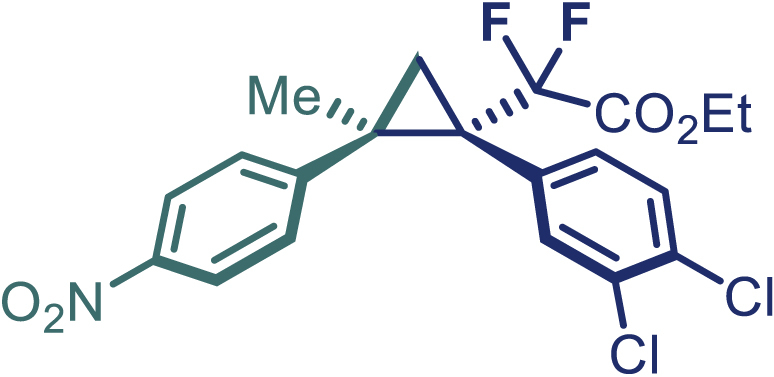
Encouraged by these promising results, we envisioned that this strategy could be extended to the more sterically hindered 1,1-diarylethenes (Scheme 2B). After fine optimization of the reaction condition (for detail see Supplemental information), when the reaction was run under the catalysis of 0.25 mol % Rh2(S-PTAD)4 between 1,1-diphenylethylene and difluoromethylene hydrazone 1, chiral triaryl difluoroalkyl-substituted cyclopropane 24 was obtained with remarkable enantiocontrol and high yield (92% yield, 99% ee). Subsequently, various structurally diverse difluoromethylene hydrazones were proved to be effective reaction components without having affected by any electronic and substituent effects. Substituents such as methyl (25), tert-butyl (26), methoxy (27, 28), halogen (29–32), phenyl (33), trifluoromethyl (34), and ester (35) were well tolerated and the corresponding products were obtained in excellent yield and enantioselectivity. Multi-substituted aryl and naphthyl difluoromethylene hydrazones also underwent asymmetric cyclopropanation smoothly to provide the desired products (36, 37) in 98% and 97% ee, respectively. The absolute configuration of 37 was assigned by X-ray crystallographic analysis. In addition, chiral difluorocarbonyl cyclopropane 38 could also be prepared with high enantioselectivity (88% ee) starting from difluorocarbonyl phenyl N-triftosylhydrazone. However, difluoramide phenyl N-triftosylhydrazone is not compatible with this reaction, mainly due to its low solubility which renders it incapable releasing the diazo compound in the reaction medium (39). This transformation also proved its usefulness, affording excellent yield and high enantioselectivity using various 1,1-diphenylethylene derivatives, which illustrates the potential of this method for the synthesis of diverse optically pure triaryl difluoroalkyl substituted (40–46).
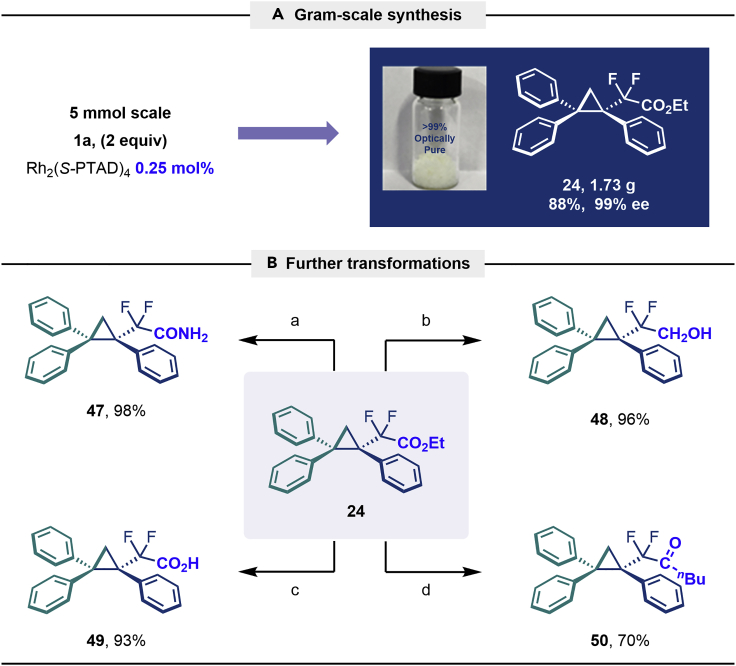
The same synthetic protocol was demonstrated to be robust and applicable also on gram-scale synthesis. As shown in Scheme 3A, the cyclopropanation of 1,1-diphenylethylene can be scaled up to 5 mmol, affording 24 in high yield (1.73 g, 88%) with excellent enantioselectivity (99% ee) even with low catalyst loading Rh2(S-PTAD)4 (0.25 mol %). It is also noteworthy that the use of sulfonyl hydrazones instead of diazo compounds can significantly reduce the safety risk during large-scale synthesis and simplify operational procedures. Taking into account the ester group interconversion, we subsequently carried out the functionality derivatization of the optically pure product 24. As demonstrated in Scheme 3B, diversely functionalized chiral difluoroalkyl-substituted cyclopropane derivatives were readily obtained from 24. The latter was converted into amide 47 (98% yield), alcohol 48 (96% yield), acid 49 (93% yield), and ketone (50, 70% yield) using appropriate reagents.40
Scheme 3.
Gram-scale synthesis and functional-group transformations Reaction conditions
(A) 24 (0.2 mmol), Ammonia (7 M in methanol) (0.5 mL), 25°C, 16 h.
(B) 24 (0.5 mmol), LiAlH4 (0.75 mmol), THF (3 mL), N2, 0–25°C, 4 h.
(C) 24 (0.2 mmol), NaOH (0.4 mmol), EtOH (2 mL), 60°C, 2 h.
(D) 24 (0.2 mmol), nBuLi (1.6 M in hexane, 0.25 mmol), THF (1 mL), N2, -78°C, 2.5 h.
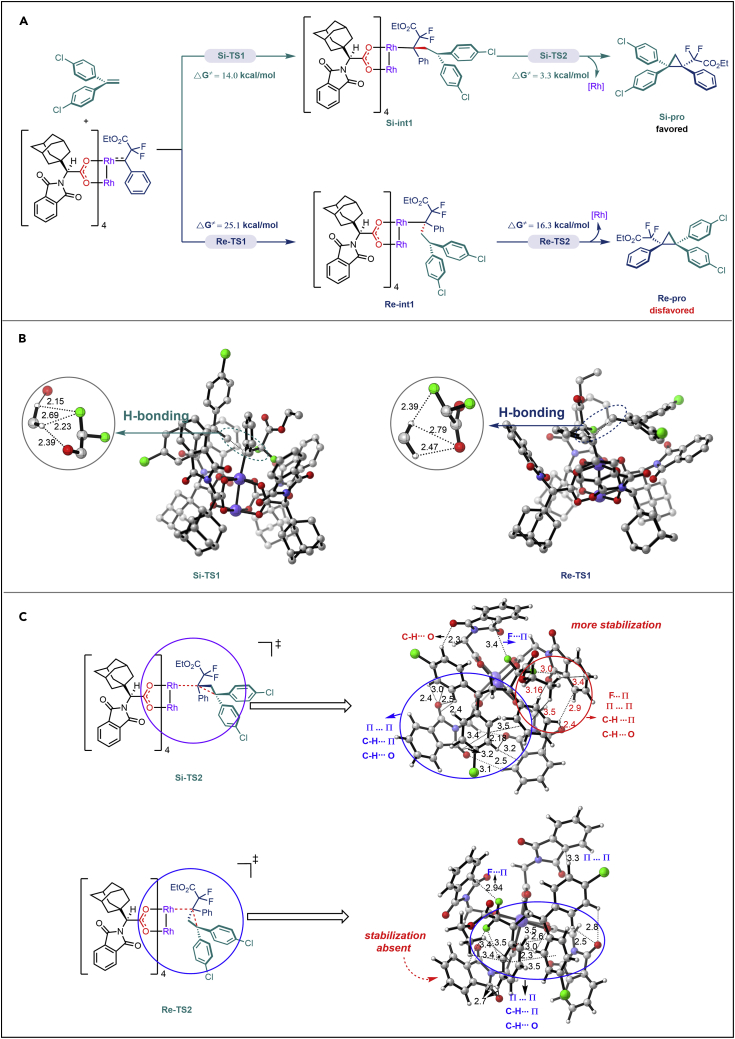
Experimental outcome has shown that the Rh2(S-PTAD)4 catalyst has a highly stereo-selectivity for this asymmetric [2 + 1] cycloaddition process. To better understand the origin for the stereo-selectivity, a plausible mechanism was proposed using the cycloaddition of N-triftosylhydrazone 1, which could be decomposed in situ to form the diazo compound under alkaline conditions,41 with 1,1-diphenylethylene as model and rationalized by density functional theory calculations at M06L/6-31G(d)-SDD(Rh) level of theory (for detailed computational methods, see Supplemental information). As shown in Scheme 4, the cycloaddition process is stepwisely owing to the geometric constraints derived from the body cavity of rhodium carbene. Firstly, the nucleophilic attack of 1,1-diphenylethylene to the carbene carbon can occur separately from the Si- or Re-plane of the carbene carbon, which determines the stereoselectivity of the product (ΔG≠(Si-TS1) = 14.0 kcal/mol; ΔG≠(Re-TS1) = 25.1 kcal/mol) (Scheme 4A). The non-covalent interactions analysis (Scheme 4B) shows that Si-TS1 displayed stronger hydrogen bond interactions (C-H···F, C-H···O) than Re-TS1, resulting in a more stable transition state and thus a lower kinetic energy barrier. Subsequently, the cyclization process from Si-int1 and Re-int1 to form the final enantioselective product via Si-TS2 (ΔG≠ = 3.3 kcal/mol) and Re-TS2 (ΔG≠ = 16.3 kcal/mol) occurred. The lower energy barrier for Si-TS2 may be derived from the more and stronger interactions exist in the configuration (See the part circled in red; Scheme 4C, upside), which renders the transition state more stable. Therefore, the formation of Si-pro is both kinetically and thermodynamically more favorable than Re-pro; this is in consistence with the experimental outcomes.
Scheme 4.
Theoretical research on proposed asymmetric [2 + 1] cycloaddition process at M06L/6-31G (d)-SDD(Rh) level of theory
The computed relative free energies are in kcal/mol and the values in (B) and (C) are in Å.
In summary, we have reported the first asymmetric [2 + 1] cycloaddition reaction of alkenes with difluoroalkyl-substituted carbenes, which were generated in situ from the easily decomposable α,α-difluoro-β-carbonyl ketone N-triftosylhydrazones. This protocol enables the enantioselective cyclopropanation of difluoroalkyl-substituted carbenes with alkenes, thereby providing a facile route to access enantioenriched difluoroalkyl-substituted cyclopropanes in high yield and excellent enantioselectivity. No doubt that the α, α-difluoro-β-carbonyl ketone N-triftosylhydrazones as the difluoroalkyl-substituted carbenes surrogates would find wide applications in asymmetric difluoroalkyl carbene transfer reactions in the near future.
Limitations of the study
This study is limited to the terminal alkenes, and it is still necessary to further develop an asymmetric catalytic system suitable for internally substituted alkenes.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 2-(Trifluoromethyl)benzenesulfonyl chloride | 776-04-5 | Energy Chemical |
| DIPEA | 7087-68-5 | Energy Chemical |
| Rh2(S-PTAD)4 | 909389-99-7 | J&K Scientific |
| TFT(Benzotrifluoride) | 98-08-8 | Energy Chemical |
| Hydrazinium hydroxide solution | 10217-52-4 | Energy Chemical |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xihe Bi (bixh507@nenu.edu.cn).
Materials availability
This study did not generate new unique reagents.
All reagents were purchased from commercial sources (Energy Chemical, Adamas-beta®, J&K Scientific, Sigma-Aldrich) and used without purification unless otherwise mentioned. The products were purified by column chromatography over silica gel (200–400 size). 1H, 13C and 19F nuclear magnetic resonance (NMR) spectra were recorded at 25°C on a Bruker 600 MHz, 150 MHz and 565 MHz or Bruker 500 MHz, 125 MHz and 470 MHz (TMS was used as internal standard). High resolution mass spectra (HRMS) were recorded on Waters Premier GC-TOF MS by using EI method or Bruck microTof by using ESI method. High-pressure liquid chromatography (HPLC) was performed on Agilent 1220 Series chromatographs using a chiral column (25 cm) as noted for each compound. Enantiomer excess was determined by HPLC analysis employing Darcel Chiracel AD-H, OD-H, OJ-H, AS-H or IF column.
Method details
General procedure for the asymmetric cyclopropanation
In a nitrogen-filled glovebox, a flame-dried screw-cap reaction tube equipped with a Teflon-coated magnetic stir bar was charged with sulfonyl hydrazone 1 (0.8 mmol) and anhydrous TFT solvent (1.0 mL). The reaction mixture was stirred until complete dissolve, DIPEA (0.8 mmol) was added to the reaction system. The alkene 2 (0.4 mmol) was dissolved in 1.0 mL of anhydrous TFT and added to the reaction system. Rh2(S-PTAD)4 (0.5 mol%) was dissolved in anhydrous TFT (1 mL) and then added to the reaction system. The mixture was stirred at 25°C for 12 h until the reaction was complete as indicated by 1H NMR. The reaction crude was filtered through a short silica gel eluting with DCM. The filtrate was evaporated under reduced pressure to leave a crude mixture, which was separated by flash column chromatography to afford the pure product.
Note: The yield of the reaction decreases as the dosage of DIPEA decreases, but the enantioselectivity remains unchanged.
Synthesis of racemate: The same procedure uses Rh2(esp)2 instead of Rh2(S-PTAD)4 and performs at room temperature.
General procedure for the synthesis of olefins
A sealed flask was charged with benzaldehyde (10 mmol), then THF (30 mL) was added and stirred at 0°C. Grignard reagent (15 mmol) was slowly added and the mixture was placed at room temperature for 4 h. When the reaction was complete (monitored by TLC), saturated NH4Cl was added. The mixture was extracted with EA (30 mL × 3), then the organic phase was dried with anhydrous Na2SO4, concentrated under reduced pressure to give the pure compound.
Synthesis of benzophenones
A round-bottomed flask was charged with alcohol and dissolved in DCM (40 mL). Then Dess-Martin oxidant (1.5 equiv) was slowly added and stirred at room temperature overnight. After completion of the reaction (monitored by TLC), saturated NaHCO3 and Na2S2O3 solution were slowly added. Extracted with DCM (30 mL × 3) and washed the organic phases with brine, then dried with anhydrous Na2SO4. The residue was purified by flash column chromatography to give the corresponding benzophenone compound.
Synthesis of olefins
A sealed flask was charged with methyltriphenylphosphine bromide (1.5 equiv), THF (30 mL) was added and stirred at 0°C. Potassium tert-butoxide (1.5 equiv) and ketone (1.0 equiv) in THF (20 mL) was slowly added, the mixture was reacted at room temperature for 4 h. After completion of the reaction (monitored by TLC), NH4Cl solution was slowly added. The mixture was extracted with EA (30 mL × 3) and dried with anhydrous Na2SO4. Then concentrated under reduced pressure to give the pure compound.perform flash column chromatography.
General procedure for the synthesis of compound 47
A sealed reaction tube was charged with 24 (0.2 mmol) and ammonia (7 M in methanol) (0.5 mL). The mixture was stirred at 25°C for 16 h. Then the mixture was concentrated under reduced pressure to give the pure compound.
General procedure for the synthesis of compound 48
A sealed reaction tube was charged with LiAlH4 (0.3 mmol) under nitrogen atmosphere. 24 (0.2 mmol) in THF (3.0 mL) was added to the reaction tube under 0°C. Then the mixture was stirred at 25°C for 4 h. After the reaction was complete (monitored by TLC), aqueous NH4Cl was added, and quenched with EA. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the pure compound.
General procedure for the synthesis of compound 49
A bottom flask was charged with 24 (0.2 mmol), NaOH (0.4 mmol) and EtOH (2.0 mL). The mixture was stirred at 60°C for 2 h. After the reaction was complete (monitored by TLC), aqueous NH4Cl was added, and quenched with EA. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the pure compound.
General procedure for the synthesis of compound 50
A sealed reaction tube was replaced nitrogen three times, then 24 (0.2 mmol) in dry THF (3.0 mL) was added. The mixture was placed at −78°C, nBuLi (1.6 M in hexane, 0.25 mmol) was added dropwise and stirred for 2.5 h. After the reaction was complete, aqueous NH4Cl was added, and quenched with CH3CO2Et. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure and purified by column chromatography to give the pure compound.
Characterization data for the products
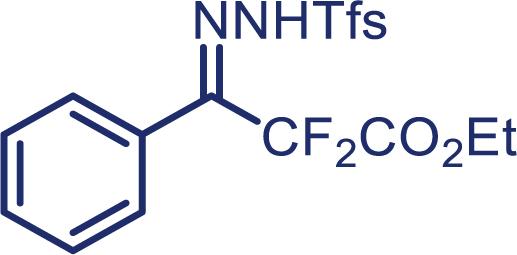
1a, White solid; m.p: 80–81°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.27 (m, 1H), 8.10 (s, 1H), 7.92–7.88 (m, 1H), 7.81–7.77 (m, 2H), 7.58–7.52 (m, 3H), 7.28–7.26 (m, 2H), 4.33 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 162.0 (t, J = 31.0 Hz), 146.8 (t, J = 27.5 Hz), 136.0, 133.9, 133.5, 132.5, 131.5, 129.9, 128.4 (q, J = 6.3 Hz), 128.2, 127.7 (q, J = 32.3 Hz), 124.9, 122.6 (q, J = 281.3 Hz), 111.9 (t, J = 250.9 Hz), 63.2, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.4, −104.0. HRMS (ESI) m/z calculated C18H15F5N2O4S [M]+ 450.0567, found 450.0565.
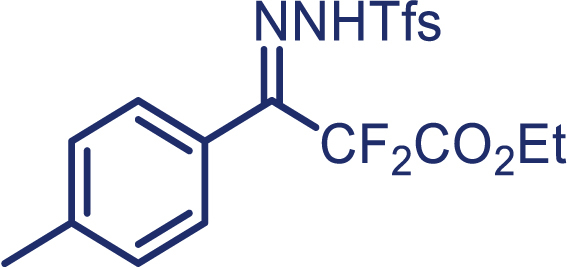
1e, White solid; m.p: 83–84°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.26 (m, 1H), 8.13 (s, 1H), 7.91–7.87 (m, 1H), 7.80–7.76 (m, 2H), 7.34 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.0 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 2.42 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 162.1 (t, J = 30.9 Hz), 147.0 (t, J = 32.3 Hz), 142.0, 136.0, 133.9, 133.5, 132.5, 130.5, 128.4 (q, J = 6.3 Hz), 128.1, 127.7 (q, J = 34.3 Hz), 122.6 (q, J = 271.8 Hz), 121.9, 111.9 (t, J = 248.8 Hz), 63.2, 21.5, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.3, −104.0.
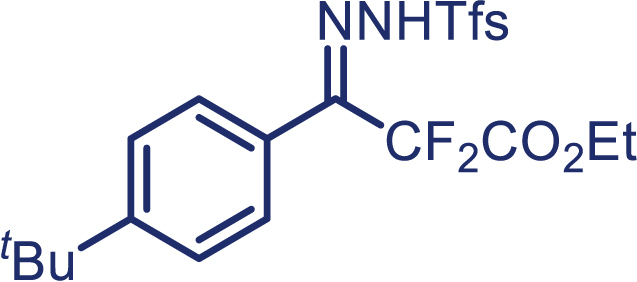
1f, White solid; m.p: 82–83°C; 1H NMR (600 MHz, CDCl3) δ 8.30–8.24 (m, 1H), 8.17 (s, 1H), 7.91–7.89 (m, 1H), 7.80–7.77 (m, 2H), 7.55–7.54 (m, 2H), 7.20 (d, J = 8.3 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 1.36 (s, 9H), 1.34 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 162.1 (t, J = 30.9 Hz), 155.0, 147.0 (t, J = 32.3 Hz), 136.0, 133.9, 133.5, 132.5, 128.4 (q, J = 6.1 Hz), 127.9, 127.7 (q, J = 32.3 Hz), 126.8, 122.4 (q, J = 274.0 Hz), 121.9, 112.0 (t, J = 250.5 Hz), 63.2, 35.0, 31.0, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −104.0. HRMS (ESI) m/z calculated C22H23F5N2O4S [M]+ 506.1199, found 506.1191.

1g, White solid; m.p: 92–93°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.26 (m, 1H), 8.15 (s, 1H), 7.90–7.87 (m, 1H), 7.80–7.76 (m, 2H), 7.23 (d, J = 8.6 Hz, 2H), 7.03 (d, J = 8.6 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 3.87 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 162.1 (t, J = 31.1 Hz), 161.8, 146.9 (t, J = 32.1 Hz), 136.0, 133.9, 133.5, 132.5, 129.9, 128.4 (q, J = 6.3 Hz), 127.7 (q, J = 33.4 Hz), 122.5 (q, J = 270.0 Hz), 116.6, 115.3, 112.1 (t, J = 250.4 Hz), 63.2, 55.4, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.3, −103.9. HRMS (ESI) m/z calculated C19H17F5N2O5S [M]+ 480.0670, found 480.0671.
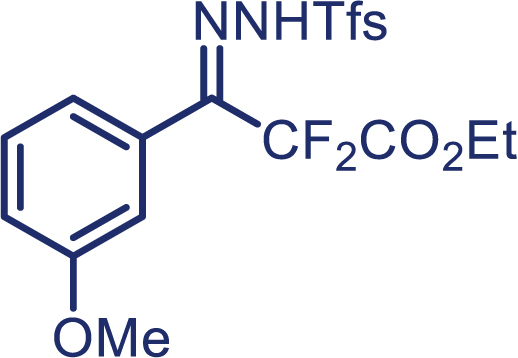
1h, White solid; m.p: 96–97°C; 1H NMR (600 MHz, CDCl3) δ 8.30–8.27 (m, 1H), 8.17 (s, 1H), 7.92–7.89 (m, 1H), 7.81–7.77 (m, 2H), 7.45 (t, J = 7.8 Hz, 1H), 7.09–7.07 (m, 1H), 6.82 (d, J = 7.8 Hz, 1H), 6.77–6.75 (m, 1H), 4.33 (q, J = 7.2 Hz, 2H), 3.83 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 162.0 (t, J = 31.3 Hz), 160.5, 146.5 (t, J = 32.4 Hz), 136.0, 133.9, 133.5, 132.5, 131.1, 128.5 (q, J = 6.3 Hz), 127.7 (q, J = 33.3 Hz), 126.0, 122.5 (q, J = 274.6 Hz), 120.1, 117.5, 113.2, 111.9 (t, J = 250.6 Hz), 63.2, 55.4, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −104.0. HRMS (ESI) m/z calculated C19H17F5N2O5S [M]+ 480.0670, found 480.0671.

1i, White solid; m.p: 116–117°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.26 (m, 1H), 8.08 (s, 1H), 7.91–7.88 (m, 1H), 7.82–7.77 (m, 2H), 7.70 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.5 Hz, 2H), 4.32 (q, J = 7.0 Hz, 2H), 1.35 (t, J = 7.0 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 161.8 (t, J = 30.3 Hz), 145.4 (t, J = 32.7 Hz), 135.9, 134.0, 133.5, 133.3, 132.6, 129.9, 128.5 (q, J = 6.1 Hz), 127.7 (q, J = 30.3 Hz), 126.5, 123.7, 122.5 (q, J = 274.2 Hz), 111.7 (t, J = 250.8 Hz), 63.3, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.8. HRMS (ESI) m/z calculated C18H14BrF5N2O4S [M]+ 527.9684, found 527.9670.
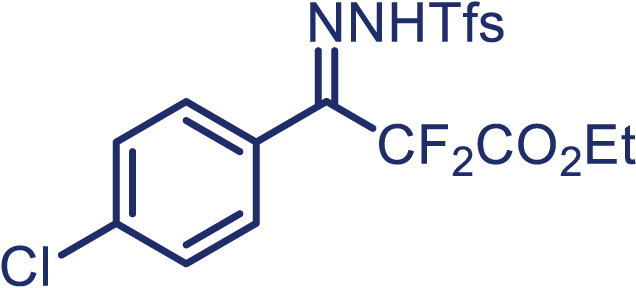
1j, White solid; m.p: 96–97°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.26 (m, 1H), 8.07 (s, 1H), 7.91–7.88 (m, 1H), 7.81–7.78 (m, 2H), 7.55–7.53 (m, 2H), 7.24 (d, J = 8.5 Hz, 2H), 4.32 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 161.8 (t, J = 31.0 Hz), 145.4 (t, J = 32.7 Hz), 138.1, 135.9, 134.0, 133.5, 132.5, 130.3, 129.8, 128.5 (q, J = 6.3 Hz), 127.7 (q, J = 33.6 Hz), 123.2, 122.4 (d, J = 273.5 Hz), 111.8 (t, J = 250.8 Hz), 63.3, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.3, −103.8. HRMS (ESI) m/z calculated C18H14ClF5N2O4S [M]+ 484.0180, found 484.0175.

1k, White solid; m.p: 127–128°C; 1H NMR (600 MHz, CDCl3) δ 8.30–8.26 (m, 1H), 8.11 (s, 1H), 7.92–7.90 (m, 1H), 7.82–7.78 (m, 2H), 7.56–7.54 (m, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.27 (t, J = 1.8 Hz, 1H), 7.18–7.16 (m, 1H), 4.33 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 161.7 (t, J = 31.1 Hz), 144.9 (t, J = 32.8 Hz), 136.1, 135.8, 134.1, 133.5, 132.6, 131.8, 131.2, 128.5 (q, J = 6.6 Hz), 128.4, 127.7 (q, J = 33.3 Hz), 126.6, 126.4, 122.5 (q, J = 274.0 Hz), 111.7 (t, J = 251.0 Hz), 63.4, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.7. HRMS (ESI) m/z calculated C18H14ClF5N2O4S [M]+ 484.0171, found 484.0175.
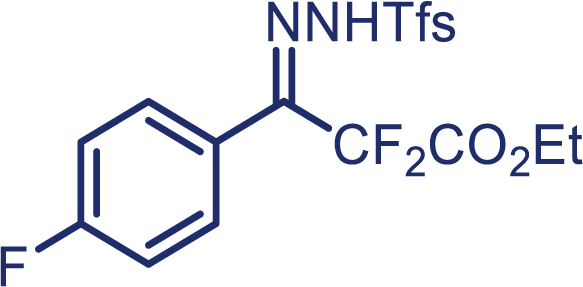
1l, White solid; m.p: 87–88°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.27 (m, 1H), 8.09 (s, 1H), 7.92–7.89 (m, 1H), 7.81–7.78 (m, 2H), 7.32–7.23 (m, 4H), 4.32 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 164.3 (d, J = 253.5 Hz), 161.8 (t, J = 30.9 Hz), 145.6 (t, J = 32.7 Hz), 135.9, 134.0, 133.5, 132.5, 130.7 (d, J = 8.6 Hz), 128.5 (q, J = 6.2 Hz), 127.7 (q, J = 33.0 Hz), 122.4 (q, J = 274.2 Hz), 120.8 (d, J = 3.5 Hz), 117.3 (d, J = 22.0 Hz), 111.8 (t, J = 250.5 Hz), 63.3, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.3, −103.8, (−106.6)-(-106.7) (m). HRMS (ESI) m/z calculated C18H14F6N2O4S [M]+ 468.0471, found 468.0471.
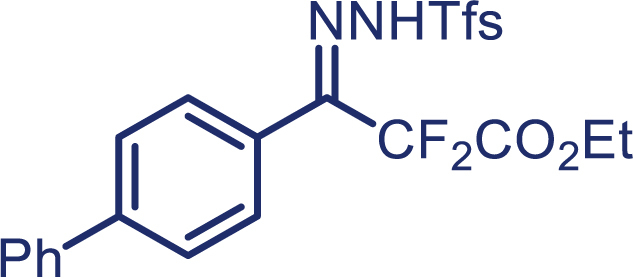
1m, White solid; m.p: 97–98°C; 1H NMR (600 MHz, CDCl3) δ 8.31–8.29 (m, 1H), 8.21 (s, 1H), 7.92–7.89 (m, 1H), 7.81–7.78 (m, 2H), 7.76–7.73 (m, 2H), 7.62–7.60 (m, 2H), 7.50–7.48 (m, 2H), 7.44–7.41 (m, 1H), 7.36 (d, J = 8.2 Hz, 2H), 4.34 (q, J = 7.2 Hz, 2H), 1.36 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 162.0 (t, J = 31.1 Hz), 146.5 (t, J = 32.3 Hz), 144.5, 139.5, 136.0, 133.9, 133.5, 132.5, 129.0, 128.7, 128.5, 128.4 (q, J = 6.1 Hz), 128.3, 127.7 (q, J = 33.2 Hz), 127.2, 123.5, 122.6 (q, J = 272.3 Hz), 112.0 (t, J = 250.7 Hz), 63.3, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.8. HRMS (ESI) m/z calculated C24H19F5N2O4S [M]+ 526.0886, found 526.0878.
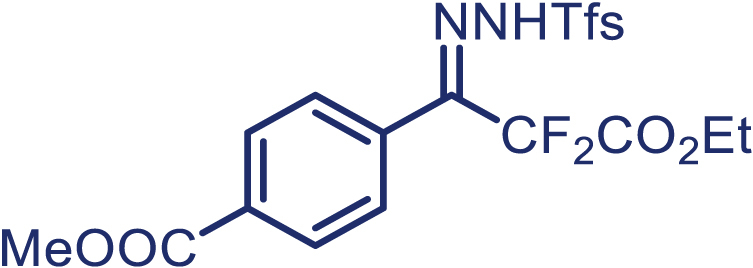
1n, White solid; m.p: 111–112°C; 1H NMR (600 MHz, CDCl3) δ 8.29–8.24 (m, 2H), 8.19–8.16 (m, 2H), 7.92–7.89 (m, 1H), 7.82–7.78 (m, 2H), 7.39 (d, J = 8.1 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 3.94 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 165.6, 161.7 (t, J = 30.7 Hz), 145.3 (t, J = 32.4 Hz), 135.8, 134.0, 133.4, 132.8, 132.5, 130.8, 129.3, 128.5, 128.4 (q, J = 6.2 Hz), 127.6 (q, J = 33.4 Hz), 122.5 (q, J = 274.0 Hz), 111.7 (t, J = 251.0 Hz), 63.3, 52.5, 13.8. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.6.

1o, White solid; m.p: 95–96°C; 1H NMR (500 MHz, CDCl3) δ 8.30–8.28 (m, 1H), 8.06 (s, 1H), 7.93–7.91 (m, 1H), 7.83–7.80 (m, 4H), 7.44 (d, J = 8.0 Hz, 2H), 4.33 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 161.6 (t, J = 30.9 Hz), 144.9 (t, J = 32.9 Hz), 135.8, 134.1, 133.6 (q, J = 32.5 Hz), 133.5, 132.6, 129.1, 128.7, 128.6 (q, J = 6.3 Hz), 127.7 (q, J = 33.2 Hz), 126.9 (q, J = 3.8 Hz), 123.2 (q, J = 271.3 Hz), 122.6 (q, J = 272.5 Hz), 111.7 (t, J = 250.9 Hz), 63.4, 13.9. 19F NMR (470 MHz, CDCl3) δ −58.3, −63.3, −103.6. HRMS (ESI) m/z calculated C19H14F8N2O4S [M]+ 518.0446, found 518.0439.

1p, 1H NMR (500 MHz, CDCl3) δ 8.29–8.27 (m, 1H), 8.12 (s, 1H), 7.94–7.92 (m, 1H), 7.84–7.78 (m, 2H), 7.57 (t, J = 1.9 Hz, 1H), 7.17 (d, J = 1.9 Hz, 2H), 4.33 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 161.5 (t, J = 31.1 Hz), 143.3 (t, J = 32.8 Hz), 137.0, 135.7, 134.2, 133.5, 132.6, 131.9, 128.6 (q, J = 5.6 Hz), 127.8 (q, J = 33.2 Hz), 127.7, 126.7, 122.5 (q, J = 273.5 Hz), 111.5 (t, J = 243.0 Hz), 63.5, 13.9. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.5.

1q, White solid; m.p: 96–97°C; 1H NMR (500 MHz, CDCl3) δ 8.27–8.25 (m, 1H), 8.21 (s, 1H), 7.93–7.90 (m, 1H), 7.83–7.77 (m, 2H), 7.62 (d, J = 8.2 Hz, 1H), 7.40 (d, J = 2.0 Hz, 1H), 7.14 (dd, J = 8.3, 2.0 Hz, 1H), 4.31 (q, J = 7.2 Hz, 2H), 1.33 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 161.6 (t, J = 30.8 Hz), 143.7 (t, J = 32.8 Hz), 136.4, 135.7, 134.6, 134.1, 133.4, 132.6, 132.0, 130.4, 128.6 (q, J = 6.6 Hz), 127.7 (q, J = 32.2 Hz), 127.6, 124.6, 122.5 (q, J = 274.0 Hz), 111.6 (t, J = 251.2 Hz), 63.4, 13.8. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.6. HRMS (ESI) m/z calculated C18H13Cl2F5N2O4S [M]+ 517.9799, found 517.9785.

1r, White solid; m.p: 108–109°C; 1H NMR (600 MHz, CDCl3) δ 8.30–8.28 (m, 1H), 8.24 (s, 1H), 7.99 (d, J = 8.4 Hz, 1H), 7.90–7.87 (m, 3H), 7.81 (s, 1H), 7.80–7.76 (m, 2H), 7.62–7.57 (m, 2H), 7.31 (d, J = 8.4 Hz, 1H), 4.33 (q, J = 7.2 Hz, 2H), 1.35 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 162.0 (t, J = 30.9 Hz), 146.7 (t, J = 32.4 Hz), 135.9, 134.1, 133.9, 133.4, 132.9, 132.5, 130.0, 129.0, 128.5, 128.4 (q, J = 6.5 Hz), 128.3, 127.9, 127.6 (q, J = 33.5 Hz), 127.4, 123.9, 122.5 (q, J = 272.3 Hz), 122.1, 112.1 (t, J = 250.7 Hz), 63.2, 13.8. 19F NMR (564 MHz, CDCl3) δ −58.3, −103.5.

1s, White solid; m.p: 125–126°C; 1H NMR (500 MHz, CDCl3) δ 8.15 (s, 1H), 7.90–7.86 (m, 3H), 7.80 (d, J = 7.8 Hz, 1H), 7.65 (t, J = 7.7 Hz, 1H), 7.60–7.56 (m, 4H), 7.48 (t, J = 7.7 Hz, 1H), 7.41 (t, J = 7.8 Hz, 2H), 7.35–7.34 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 187.1 (t, J = 27.8 Hz), 148.0 (t, J = 31.9 Hz), 135.5, 134.0, 133.71, 133.65, 132.6, 132.4, 131.6, 123.0, 128.5, 128.2, 128.1 (q, J = 6.3 Hz), 127.4 (q, J = 32.5 Hz), 125.4, 122.5 (q, J = 278.8 Hz), 113.9 (t, J = 252.0 Hz). 19F NMR (470 MHz, CDCl3) δ −58.4, −99.5.
3, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.09–7.02 (m, 6H), 7.01–6.94 (m, 4H), 3.97 (q, J = 7.1 Hz, 2H), 2.17–2.14 (m, 1H), 1.87 (d, J = 2.1 Hz, 3H), 1.81–1.78 (m, 1H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.8 (dd, J = 36.1, 34.9 Hz), 142.5, 134.6 (d, J = 6.8 Hz), 131.5, 128.0, 127.7, 127.5, 127.3, 126.0, 117.1 (t, J = 255.7 Hz), 62.3, 39.5 (dd, J = 26, 22.9 Hz), 32.7, 22.0 (d, J = 6.7 Hz), 20.7 (t, J = 5.0 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −97.9 (d, J = 249.6 Hz), (−103.5)-(-105.0) (m). IR (Film): 2963, 1772, 1497, 1450, 1260, 1100 cm–1. [α]25 D = −10.3, (c = 1.00, CH2Cl2).

4, White solid; mp: 76–77°C; 1H NMR (500 MHz, CDCl3) δ 7.01 (d, J = 8.8 Hz, 2H), 6.96–6.94 (m, 2H), 6.86 (d, J = 7.9 Hz, 2H), 6.55–6.53 (m, 2H), 4.03–3.96 (m, 2H), 3.64 (s, 3H), 2.16 (s, 3H), 2.04–2.02 (m, 1H), 1.82–1.80 (m, 3H), 1.75–1.72 (m, 1H), 1.06 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.9 (dd, J = 35.4, 34.4 Hz), 158.5, 139.7, 135.3, 132.7, 128.4, 127.8, 126.8 (d, J = 6.1 Hz), 117.3 (t, J = 256.3 Hz), 112.9, 62.3, 55.0, 38.8 (dd, J = 25.8, 22.0 Hz), 32.3, 22.2 (d, J = 6.8 Hz), 21.0 (t, J = 4.3 Hz), 20.9, 13.7. 19F NMR (470 MHz, CDCl3) δ −97.8 (d, J = 248.2 Hz), −104.7 (d, J = 248.2 Hz). HRMS (ESI) m/z calculated C22H24F2O3 [M]+ 374.1587, found 374.1586. IR (Film): 2926, 2923, 1773, 1600, 1260, 1109, 1021 cm–1. [α]25 D = −12.1, (c = 1.00, CH2Cl2).
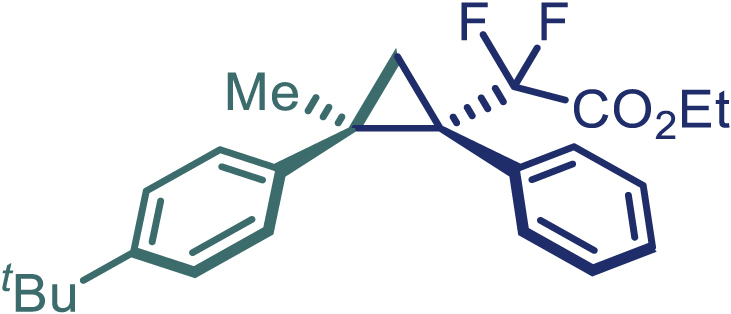
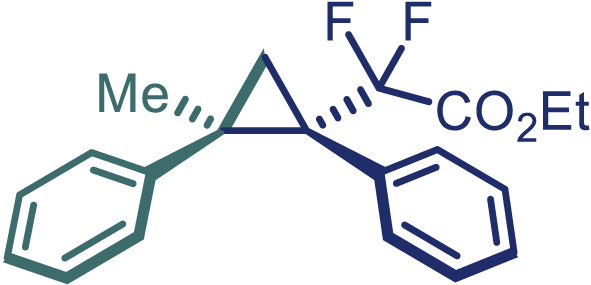
5, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.07–7.02 (m, 4H), 6.98–6.94 (m, 5H), 3.96 (q, J = 7.2 Hz, 2H), 2.11 (t, J = 5.0 Hz, 1H), 1.87 (s, 3H), 1.77 (d, J = 5.9 Hz, 1H), 1.15 (s, 9H), 1.01 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.9 (dd, J = 35.4, 34.4 Hz), 148.7, 139.3, 134.8 (d, J = 6.0 Hz), 131.6, 127.4, 127.1, 124.4, 117.1 (t, J = 256.0 Hz), 62.3, 39.6 (dd, J = 26.4, 22.4 Hz), 34.1, 32.1, 31.2, 21.8 (d, J = 6.6 Hz), 20.9 (t, J = 5.0 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −97.9 (d, J = 248.5 Hz), −104.4 (d, J = 248.5 Hz). HRMS (ESI) m/z calculated C24H28F2O2 [M]+ 386.1948, found 386.1950. IR (Film): 2960, 1772, 1500, 1260, 1100 cm–1. [α]25 D = −3.9, (c = 1.00, CH2Cl2).
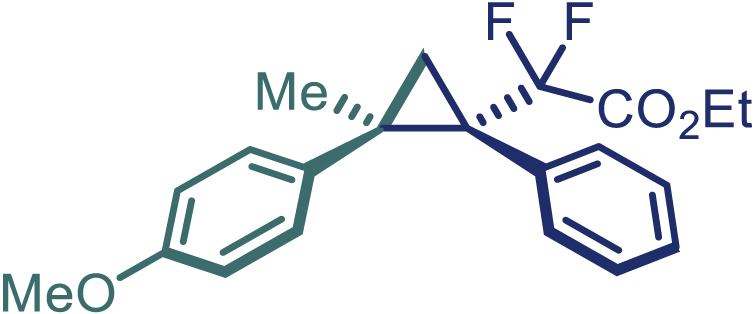
6, White solid; mp: 80–81°C; 1H NMR (500 MHz, CDCl3) δ 7.09 (d, J = 7.2 Hz, 2H), 7.02–6.96 (m, 5H), 6.60–6.56 (m, 2H), 3.95 (q, J = 7.1 Hz, 2H), 3.65 (s, 3H), 2.09–2.07 (m, 1H), 1.83 (d, J = 1.5 Hz, 3H), 1.78–1.76 (m, 1H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.8 (dd, J = 35.5, 33.9 Hz), 157.5, 134.8 (d, J = 6.0 Hz), 134.7, 131.5, 128.9, 127.6, 127.3, 117.1 (d, J = 255.8 Hz), 113.0, 62.3, 55.0, 39.5 (dd, J = 26.5, 22.8 Hz), 32.1, 22.3 (d, J = 6.6 Hz), 20.9 (t, J = 4.9 Hz), 13.5. 19F NMR (470 MHz, CDCl3) δ −97.8 (d, J = 248.6 Hz), −104.2 (d, J = 248.6 Hz). HRMS (ESI) m/z calculated C21H22F2O3 [M]+ 360.1419, found 360.1429. IR (Film): 3021, 2959,1765, 1613, 1516 cm–1 [α]25 D = −15.5, (c = 1.00, CH2Cl2).

7, White solid; mp: 75–76°C; 1H NMR (500 MHz, CDCl3) δ 7.09–7.07 (m, 2H), 7.03–6.98 (m, 7H), 3.96 (q, J = 7.1 Hz, 2H), 2.10 (t, J = 5.4 Hz, 1H), 1.84–1.80 (m, 4H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.6 (t, J = 34.7 Hz), 141.2, 134.3 (d, J = 5.8 Hz), 131.7, 131.2, 129.3, 127.8, 127.7, 127.6, 116.9 (t, J = 256.3 Hz), 62.4, 39.6 (dd, J = 26.1, 22.1 Hz), 32.1, 22.0 (d, J = 6.8 Hz), 20.8 (t, J = 5.2 Hz), 13.5. 19F NMR (564 MHz, CDCl3) δ −97.9 (d, J = 250.7 Hz), −104.4 (d, J = 250.7 Hz). HRMS (ESI) m/z calculated C20H19ClF2O2 [M]+ 364.0927, found 364.0934. IR (Film): 3053, 2989, 2950, 1765, 1493 cm–1. [α]25 D = −20.4, (c = 1.00, CH2Cl2).

8, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.08–7.01 (m, 7H), 6.73 (t, J = 8.7 Hz, 2H), 3.97 (q, J = 7.1 Hz, 2H), 2.10 (t, J = 5.0 Hz, 1H), 1.84 (s, 3H), 1.80 (d, J = 6.1 Hz, 1H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.7 (t, J = 34.2 Hz), 160.9 (d, J = 244.3 Hz), 138.4, 134.5 (d, J = 6.5 Hz), 131.3, 129.5 (d, J = 8.0 Hz), 127.7, 127.4, 117.0 (t, J = 255.8 Hz), 114.5 (d, J = 21.0 Hz), 62.4, 39.5 (dd, J = 25.7, 22.2 Hz), 32.1, 22.2 (d, J = 6.2 Hz), 20.9 (t, J = 5.0 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −97.9 (d, J = 249.8 Hz), −104.3 (d, J = 251.6 Hz), (−114.5)-(-120.9) (m). IR (Film): 2935, 1773, 1560, 1512, 1161 cm–1. [α]25 D = −3.1, (c = 1.00, CH2Cl2).

9, White solid; mp: 65–66°C; 1H NMR (500 MHz, CDCl3) δ 7.46–7.44 (m, 2H), 7.37–7.33 (m, 2H), 7.30–7.26 (m, 3H), 7.13–7.11 (m, 4H), 7.01–6.95 (m, 3H), 3.97 (q, J = 7.1 Hz, 2H), 2.18 (t, J = 5.5, 1H), 1.90 (d, J = 1.4 Hz, 3H), 1.83 (d, J = 6.0 Hz, 1H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.8 (dd, J = 35.4, 33.9 Hz), 141.7, 140.6, 138.5, 134.6 (d, J = 6.1 Hz), 131.5, 128.6, 128.3, 127.6, 127.4, 127.0, 126.8, 126.3, 117.1 (t, J = 257.1 Hz), 62.3, 39.7 (dd, J = 25.9, 22.5 Hz), 32.4, 22.0 (d, J = 5.6 Hz), 20.9 (t, J = 4.8 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −97.9 (d, J = 249.3 Hz), −104.4 (d, J = 249.9 Hz). HRMS (ESI) m/z calculated C26H24F2O2 [M]+ 406.1646, found 406.1637. IR (Film): 3054, 2985, 2306, 1767, 1421, 1264, 1118, 895, 731, 702 cm–1. [α]25 D = −46.1, (c = 1.00, CH2Cl2).
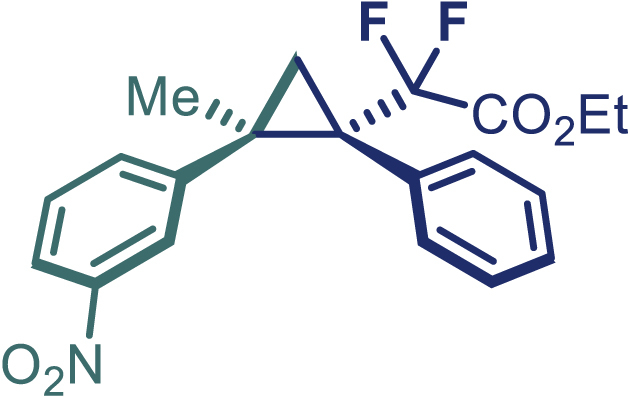
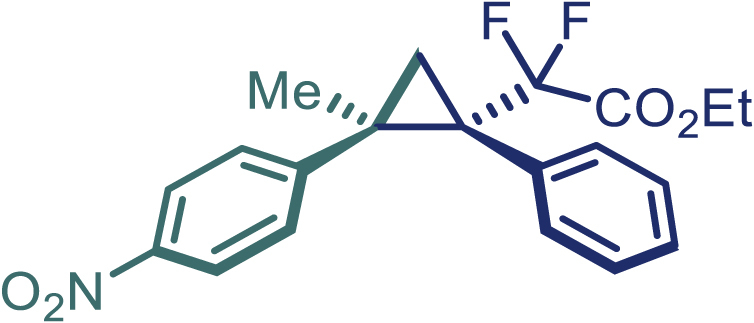
10, White solid; mp: 110–111°C; 1H NMR (600 MHz, CDCl3) δ 7.90 (t, J = 1.9 Hz, 1H), 7.84–7.82 (m, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.22 (t, J = 8.0 Hz, 1H), 7.08 (d, J = 7.2 Hz, 2H), 7.02–6.97 (m, 3H), 4.00–3.97 (m, 2H), 2.22 (t, J = 5.2 Hz, 1H), 1.93–1.89 (m, 4H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (t, J = 34.1 Hz), 147.7, 144.9, 134.4, 133.7 (d, J = 6.3 Hz), 131.1, 128.6, 128.0, 127.8, 122.8, 121.2, 116.7 (t, J = 257.4 Hz), 62.5, 39.8 (dd, J = 25.8, 22.7 Hz), 32.2, 21.6 (d, J = 6.9 Hz), 20.9 (t, J = 4.7 Hz), 13.5. 19F NMR (564 MHz, CDCl3) δ −98.0 (d, J = 251.1 Hz), −104.4 (d, J = 251.1 Hz). IR (Film): 3055, 2936, 2306, 1770, 6530, 1348, 1265, 1112, 909, 730, 704 cm–1. [α]25 D = −19.0, (c = 1.00, CH2Cl2).
11, White solid; mp: 89–90°C; 1H NMR (600 MHz, CDCl3) δ 7.91 (d, J = 8.9 Hz, 2H), 7.23 (d, J = 8.9 Hz, 2H), 7.10–7.06 (m, 2H), 7.04–7.00 (m, 3H), 3.98 (q, J = 7.2 Hz, 2H), 2.23–2.21(m, 1H), 1.93–1.89 (m, 4H), 1.01 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (t, J = 34.0 Hz), 150.3, 146.0, 133.6 (d, J = 5.8 Hz), 131.0, 128.8, 128.0, 127.9, 123.0, 116.7 (t, J = 256.8 Hz), 62.5, 40.0 (t, J = 22.5 Hz), 32.4, 21.5 (d, J = 6.7 Hz), 21.1 (t, J = 4.7 Hz), 13.5. 19F NMR (564 MHz, CDCl3) δ −98.1 (d, J = 251.0 Hz), −104.5 (d, J = 251.0 Hz). HRMS (ESI) m/z calculated C20H19F2NO4 [M]+ 375.1172, found 375.1174. IR (Film): 3060, 2990, 2939, 1771, 1599, 1518, 1345 cm–1. [α]25 D = −32.2, (c = 1.00, CH2Cl2).
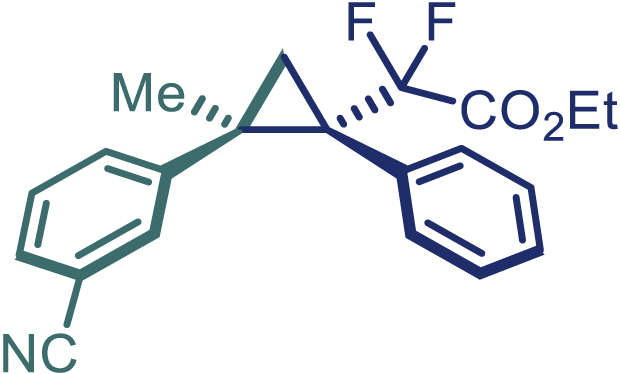
12, White solid; mp: 91–92°C; 1H NMR (600 MHz, CDCl3) δ 7.34–7.31 (m, 2H), 7.26–7.24 (m, 1H), 7.16 (t, J = 8.0 Hz, 1H), 7.09–6.99 (m, 5H), 3.98 (q, J = 7.1 Hz, 2H), 2.15 (t, J = 5.2 Hz, 1H), 1.88–1.85 (m, 4H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (t, J = 35.2 Hz), 144.2, 133.8 (d, J = 6.1 Hz), 132.7, 131.5, 131.1, 129.8, 128.5, 127.9, 127.8, 118.8, 116.7 (t, J = 256.4 Hz), 111.8, 62.5, 39.8 (dd, J = 26.4, 22.4 Hz), 32.1, 21.6 (d, J = 5.4 Hz), 20.6 (t, J = 4.8 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −98.0 (d, J = 251.2 Hz), −104.4 (d, J = 251.2 Hz). HRMS (ESI) m/z calculated C21H19F2NO2 [M]+ 355.1279, found 355.1276. IR (Film): 2940, 2255, 1559, 1502, 1160 cm–1. [α]25 D = −21.1, (c = 1.00, CH2Cl2).
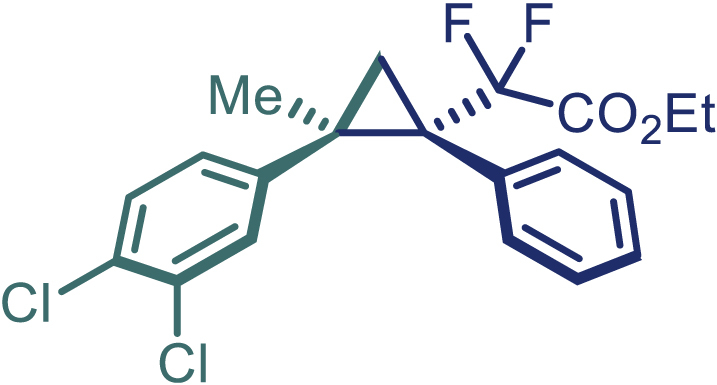
13, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.15–7.05 (m, 7H), 6.89 (d, J = 7.1 Hz, 1H), 3.97 (q, J = 7.1 Hz, 2H), 2.08 (t, J = 5.3 Hz, 1H), 1.86–1.79 (m, 4H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.5 (t, J = 34.6 Hz), 143.1, 133.9 (d, J = 5.9 Hz), 131.7, 130.1, 129.9, 129.6, 128.4 (d, J = 4.1 Hz), 127.9, 127.8, 127.5, 116.8 (t, J = 256.4 Hz), 62.5, 39.7 (dd, J = 25.2, 21.9 Hz), 31.9, 21.8 (d, J = 6.9 Hz), 20.9 (t, J = 4.8 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −98.0 (d, J = 250.7 Hz), −104.4 (d, J = 250.7 Hz). HRMS (ESI) m/z calculated C20H18Cl2F2O2 [M]+ 398.0539, found 398.0544. IR (Film): 3066, 2963, 2937, 1773, 1654, 1102 cm–1. [α]25 D = −10.7, (c = 1.00, CH2Cl2).
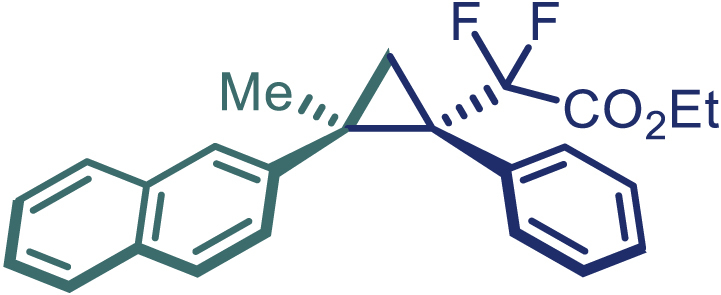
14, White solid; mp: 70–71°C; 1H NMR (500 MHz, CDCl3) δ 7.66–7.62 (m, 2H), 7.55 (d, J = 8.5 Hz, 1H), 7.44 (d, J = 1.9 Hz, 1H), 7.37–7.31 (m, 3H), 7.16 (d, J = 7.6 Hz, 2H), 6.96–6.86 (m, 3H), 3.97 (q, J = 7.2 Hz, 2H), 2.30–2.27 (m, 1H), 1.93 (d, J = 2.0 Hz, 3H), 1.89 (d, J = 6.0 Hz, 1H), 1.01 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.8 (t, J = 34.7 Hz), 140.3, 134.4 (d, J = 6.7 Hz), 133.0, 131.8, 131.3, 127.62, 127.57, 127.4, 127.34, 127.27, 127.1, 126.0, 125.7, 125.4, 117.2 (t, J = 256.0 Hz), 62.4, 39.7 (dd, J = 26.0, 21.9 Hz), 33.1, 22.2 (d, J = 6.7 Hz), 20.9 (t, J = 4.9 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −97.9 (d, J = 249.4 Hz), −104.2 (d, J = 249.4 Hz). HRMS (ESI) m/z calculated C24H22F2O4 [M]+ 380.1471, found 380.1480. IR (Film): 3057, 3020, 2970, 2934, 1768, 1496, 1312,1107 cm–1. [α]25 D = +2.3, (c = 1.00, CH2Cl2).

15, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.15–6.90 (m, 10H), 3.98 (q, J = 7.2 Hz, 2H), 1.98 (t, J = 5.4 Hz, 1H), 1.92–1.85 (m, 1H), 1.79 (d, J = 6.2 Hz, 1H), 1.01 (t, J = 7.2 Hz, 3H), 0.91–0.81 (m, 1H), 0.77–0.71 (m, 1H), 0.62–0.55 (m, 1H), 0.50–0.46 (m, 1H). 13C NMR (125 MHz, CDCl3) δ 164.0 (dd, J = 36.1, 33.8 Hz), 141.5, 134.7 (d, J = 6.1 Hz), 131.4, 128.7, 127.5, 127.4, 127.3, 125.8, 117.0 (t, J = 256.3 Hz), 62.3, 40.3 (t, J = 22.5 Hz), 38.7, 18.2 (d, J = 4.2 Hz), 15.1 (d, J = 3.8 Hz), 13.6, 8.5 (d, J = 7.2 Hz), 6.6 (d, J = 3.3 Hz). 19F NMR (470 MHz, CDCl3) δ −98.8 (d, J = 251.4 Hz), −104.7 (d, J = 251.4 Hz). HRMS (ESI) m/z calculated C22H22F2O2 [M]+ 356.1489, found 356.1480. IR (Film): 1740, 1535, 1503, 1152 cm–1. [α]25 D = −3.7, (c = 1.00, CH2Cl2).
16, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.19–7.06 (m, 1H), 7.01–6.93 (m, 6H), 6.83 (d, J = 7.9 Hz, 2H), 3.93 (q, J = 7.1 Hz, 2H), 2.37–2.30 (m, 1H), 2.14 (s, 3H), 2.06–1.99 (m, 2H), 1.72 (d, J = 5.8 Hz, 1H), 0.98 (t, J = 7.1 Hz, 3H), 0.79 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.8 (dd, J = 35.6, 33.8 Hz), 136.9, 135.4, 134.9 (d, J = 6.5 Hz), 131.2, 129.1, 128.3, 127.5, 127.1, 117.4 (t, J = 257.7 Hz), 62.3, 40.2 (dd, J = 26.4, 21.6 Hz), 39.4, 27.1 (d, J = 7.2 Hz), 20.9, 18.9 (t, J = 5.2 Hz), 13.5, 11.9. 19F NMR (564 MHz, CDCl3) δ −96.2 (d, J = 248.4 Hz), (−102.6)-(-103.1) (m). HRMS (ESI) m/z calculated C22H24F2O2 [M]+ 358.1632, found 358.1637. IR (Film): 1726, 1540, 1513, 1135 cm–1. [α]25 D = −2.0, (c = 1.00, CH2Cl2).
17, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.90 (t, J = 2.0 Hz, 1H), 7.84–7.81 (m, 1H), 7.46–7.43 (m, 1H), 7.26 (s, 1H), 7.23 (t, J = 8.0 Hz, 1H), 7.03–6.95 (m, 4H), 3.96 (q, J = 7.1 Hz, 2H), 2.44–2.36 (m, 1H), 2.19–2.09 (m, 2H), 1.86 (t, J = 6.3 Hz, 1H), 0.99 (t, J = 7.1 Hz, 3H), 0.82 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.4 (dd, J = 35.5, 34.1 Hz), 147.6, 142.7, 135.6, 133.9 (d, J = 6.1 Hz), 132.3, 128.6, 128.0, 127.7, 124.1, 121.3, 116.9 (t, J = 257.2 Hz), 62.5, 40.4 (dd, J = 26.1, 22.3 Hz), 39.2, 26.7 (d, J = 7.3 Hz), 18.9 (d, J = 5.1 Hz), 13.5, 11.9. 19F NMR (470 MHz, CDCl3) δ −96.4 (d, J = 250.5 Hz), −102.9 (d, J = 250.5 Hz). HRMS (ESI) m/z calculated C21H21F2NO4 [M]+ 389.1324, found 389.1331. IR (Film): 1730, 1610, 1513, 1320, 1140 cm–1. [α]25 D = +5.0, (c = 1.00, CH2Cl2).

18, Colorless oil; 1H NMR (600 MHz, CDCl3) δ 7.11–7.05 (m, 3H), 6.94 (d, J = 8.4 Hz, 2H), 6.82–6.79 (m, 2H), 6.63 (d, J = 8.4 Hz, 2H), 4.16 (q, J = 7.1 Hz, 2H), 3.70 (s, 3H), 2.91 (dd, J = 9.6, 6.6 Hz, 1H), 1.93 (dd, J = 9.0, 5.7 Hz, 1H), 1.61–1.57 (m, 1H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.8 (t, J = 35.2 Hz), 159.2, 136.6, 133.6, 127.9, 127.7, 126.1, 124.1, 115.2 (t, J = 254.1 Hz), 113.4, 62.5, 55.0, 36.2 (t, J = 24.6 Hz), 25.1 (t, J = 3.4 Hz), 15.1 (t, J = 3.7 Hz), 13.9. 19F NMR (564 MHz, CDCl3) δ −108.8 (d, J = 244.6 Hz), −110.7 (d, J = 244.6 Hz). HRMS (ESI) m/z calculated C20H20F2O3 [M]+ 346.1268, found 346.1273. IR (Film): 3057, 2253, 1766, 1610, 1515, 1265, 906, 721, 649 cm–1. [α]25 D = +0.9, (c = 1.00, CH2Cl2).
19, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.5 Hz, 2H), 7.23 (d, J = 8.5 Hz, 2H), 7.17 (d, J = 8.3 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 4.07–3.99 (m, 2H), 2.16 (t, J = 5.5 Hz, 1H), 1.92 (d, J = 6.4 Hz, 1H), 1.87 (s, 3H), 1.07 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.2 (dd, J = 37.5, 34.5 Hz), 149.8, 146.2, 132.9 (d, J = 6.0 Hz), 132.7, 131.2, 128.8, 123.2, 122.3, 116.3 (t, J = 257.0 Hz), 62.8, 39.4 (dd, J = 25.8, 22.8 Hz), 32.6, 21.6 (d, J = 6.7 Hz), 21.1 (t, J = 4.9 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −98.2 (d, J = 253.1 Hz), −104.3 (d, J = 253.1 Hz). HRMS (ESI) m/z calculated C20H18BrF2NO4 [M]+ 453.0289, found 453.0279. IR (Film): 3078, 2926, 1766, 1516, 1346, 1104 cm–1. [α]25 D = −40.8, (c = 1.00, CH2Cl2).

20, White solid; mp: 93–94°C; 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.8 Hz, 2H), 7.23 (d, J = 8.8 Hz, 2H), 7.05–7.00 (m, 4H), 4.08–3.98 (m, 2H), 2.16 (t, J = 5.2 Hz, 1H), 1.92 (d, J = 6.4 Hz, 1H), 1.88 (s, 3H), 1.07 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.2 (dd, J = 35.1, 33.0 Hz), 149.9, 146.2, 134.0, 132.5, 132.3 (d, J = 5.9 Hz), 128.8, 128.3, 123.2, 116.3 (t, J = 257.0 Hz), 62.7, 39.3 (dd, J = 25.4, 8.3 Hz), 32.6, 21.6 (d, J = 6.9 Hz), 21.1 (t, J = 4.5 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −98.2 (d, J = 252.7 Hz), −104.3 (d, J = 252.7 Hz). HRMS (ESI) m/z calculated C20H18ClF2NO4 [M]+ 409.0787, found 409.0785. IR (Film): 3078, 2986, 2940, 1769, 1757, 1517, 1370, 1126 cm–1. [α]25 D = −20.3, (c = 1.00, CH2Cl2).

21, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.9 Hz, 2H), 7.44–7.40 (m, 2H), 7.36 (t, J = 7.6 Hz, 2H), 7.31–7.26 (m, 5H), 7.15 (d, J = 8.1 Hz, 2H), 4.04–3.97 (m, 2H), 2.24 (t, J = 5.5 Hz, 1H), 1.96–1.90 (m, 4H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (t, J = 34.4 Hz), 150.3, 146.1, 140.5, 139.9, 132.7 (d, J = 5.8 Hz), 131.6, 128.9, 128.7, 127.5, 126.9, 126.5, 123.1, 116.7 (t, J = 257.1 Hz), 62.6, 39.7 (dd, J = 25.5, 24.1 Hz), 32.6, 21.7 (d, J = 6.7 Hz), 21.2 (t, J = 5.0 Hz), 13.6 (d, J = 8.1 Hz). 19F NMR (564 MHz, CDCl3) δ −102.7 (d, J = 251.2 Hz), −109.2 (d, J = 251.2 Hz). HRMS (ESI) m/z calculated C26H23F2NO4 [M]+ 451.1485, found 451.1487. IR (Film): 3031, 2931, 1771, 1518, 1345, 1111 cm–1. [α]25 D = −10.9, (c = 1.00, CH2Cl2).
22, White solid; mp: 98–99°C; 1H NMR (500 MHz, CDCl3) δ 7.99 (d, J = 8.8 Hz, 2H), 7.26 (d, J = 8.6 Hz, 2H), 7.20 (s, 1H), 7.11 (d, J = 8.4 Hz, 1H), 6.95 (d, J = 8.4 Hz, 1H), 4.09 (q, J = 7.1 Hz, 2H), 2.15 (t, J = 5.5 Hz, 1H), 1.94 (d, J = 6.5 Hz, 1H), 1.87 (s, 3H), 1.11 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.0 (t, J = 34.1 Hz), 149.4, 146.4, 134.2 (d, J = 5.8 Hz), 133.0, 132.4, 132.2, 130.6, 130.0, 128.8, 123.4, 116.0 (t, J = 256.7 Hz), 62.9, 39.0 (dd, J = 26.1, 24.3 Hz), 32.8, 21.6 (d, J = 6.8 Hz), 21.1 (t, J = 4.7 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −98.2 (d, J = 253.8 Hz), −103.9 (d, J = 253.8 Hz). HRMS (ESI) m/z calculated C20H17Cl2F2NO4 [M]+ 443.0385, found 443.0395. IR (Film): 3078, 2984, 2935, 1769, 1598, 1516 1370, 1110 cm–1. [α]25 D = −77.7, (c = 1.00, CH2Cl2).
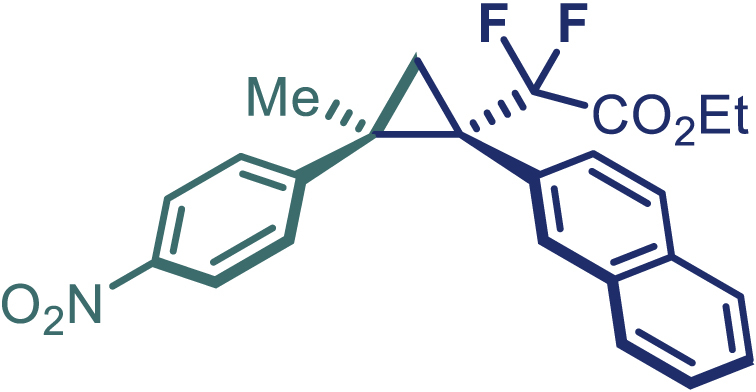
23, Colorless oil; 1H NMR (600 MHz, CDCl3) δ 7.85 (d, J = 8.9 Hz, 2H), 7.64–7.60 (m, 2H), 7.56 (s, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.40–7.36 (m, 2H), 7.29 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.0 Hz, 1H), 3.89 (q, J = 7.1 Hz, 2H), 2.35 (t, J = 5.0 Hz, 1H), 2.00 (d, J = 6.3 Hz, 1H), 1.94 (s, 3H), 0.86 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (dd, J = 35.0, 33.5 Hz), 150.1, 146.0, 132.6, 132.5, 131.3, 128.8, 128.4, 127.7, 127.6, 127.5, 126.5, 126.2, 123.0, 116.8 (t, J = 257.1 Hz), 62.5, 40.1 (dd, J = 25.5, 22.1 Hz), 32.6, 21.7 (d, J = 6.5 Hz), 21.4, 13.5. 19F NMR (564 MHz, CDCl3) δ −98.0 (d, J = 251.4 Hz), −104.2 (d, J = 251.4 Hz). HRMS (ESI) m/z calculated C24H21F2NO4 [M]+ 425.1322, found 425.1331. IR (Film): 3057, 2935, 1770, 1518, 1345, 1114, 749 cm–1. [α]25 D = −36.9, (c = 1.00, CH2Cl2).
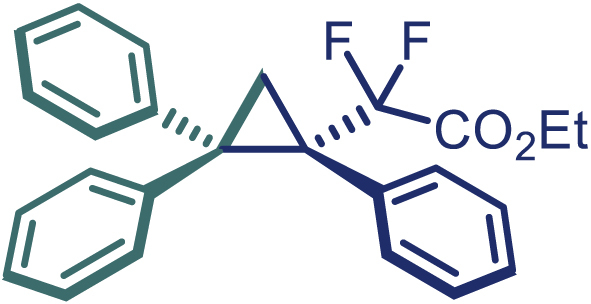
24, White solid; mp: 87–88°C; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 8.0 Hz, 2H), 7.36–7.29 (m, 4H), 7.24–7.19 (m, 1H), 7.15–7.06 (m, 5H), 6.98–6.94 (m, 2H), 6.90–6.86 (m, 1H), 3.96 (q, J = 7.0 Hz, 2H), 2.46–2.43 (m, 1H), 2.41–2.38 (m, 1H), 1.00 (t, J = 7.0 H z, 3H). 13C NMR (150 MHz, CDCl3) δ 163.6 (dd, J = 35.7, 32.3 Hz), 141.6, 140.9, 133.4 (d, J = 5.7 Hz), 131.7, 130.2, 129.0, 128.1, 127.8, 127.74, 127.69, 126.7, 126.0, 116.4 (t, J = 255.5 Hz), 62.3, 42.7, 40.7 (dd, J = 26.4, 21.6 Hz), 20.4 (t, J = 3.8 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.8 (d, J = 249.5 Hz), −105.1 (d, J = 249.5 Hz). HRMS (ESI) m/z calculated C25H22F2O2 [M]+ 392.1485, found 392.1480. IR (Film): 3057, 2253, 1766, 1494, 1304, 1261, 1136, 906, 724, 649 cm–1. [α]25 D = −51.2, (c = 1.00, CH2Cl2).
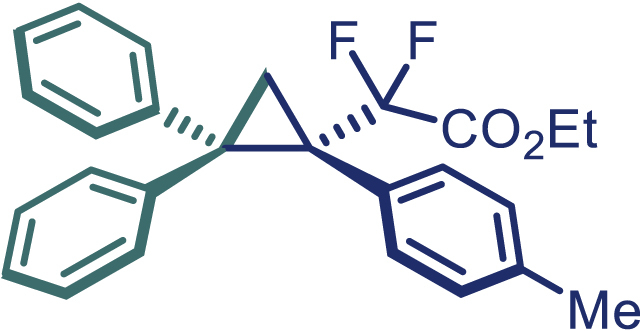
25, White solid; mp: 89–90°C; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 7.5 Hz, 2H), 7.31 (t, J = 7.5 Hz, 2H), 7.23–7.17 (m, 3H), 7.15 (d, J = 8.0 Hz, 2H), 6.98 (t, J = 7.5 Hz, 2H), 6.93–6.87 (m, 3H), 4.00–3.93 (m, 2H), 2.41–2.35 (m, 2H), 2.18 (s, 3H), 1.03 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.6 (dd, J = 32.9, 3.6 Hz), 141.8, 141.1, 137.4, 131.5, 130.20 (d, J = 6.2 Hz), 130.16, 129.0, 128.6, 128.1, 127.7, 126.6, 126.0, 116.5 (t, J = 255.2 Hz), 62.3, 42.7, 40.4 (dd, J = 26.5, 20.9 Hz), 21.0, 20.6 (dd, J = 5.2, 3.6 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.7 (d, J = 248.3 Hz), −105.2 (d, J = 248.3 Hz). HRMS (ESI) m/z calculated C26H24F2O2 [M]+ 406.1642, found 406.1637. IR (Film): 3055, 2253, 1765, 1305, 1264, 1088, 906, 726, 649 cm−1.

26, White solid; mp: 80–81°C; 1H NMR (600 MHz, CDCl3) δ 7.68 (d, J = 7.8 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.23–7.19 (m, 3H), 7.13–7.10 (m, 4H), 6.95 (t, J = 7.8 Hz, 2H), 6.87 (t, J = 7.5 Hz, 1H), 4.00–3.90 (m, 2H), 2.41–2.37 (m, 2H), 1.18 (s, 9H), 0.95 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.7 (dd, J = 36.2, 32.7 Hz), 150.6, 141.7, 141.1, 131.3, 130.22, 130.16 (d, J = 9.8 Hz), 129.0, 128.1, 127.6, 126.6, 125.8, 124.7, 116.5 (t, J = 255.4 Hz), 62.2, 42.6, 40.4 (dd, J = 26.4, 20.8 Hz), 34.3, 31.1, 20.6 (t, J = 4.2 Hz), 13.5. 19F NMR (564 MHz, CDCl3) δ −102.6 (d, J = 247.8 Hz), −105.6 (d, J = 247.8 Hz). HRMS (ESI) m/z calculated C29H30F2O2 [M]+ 448.2112, found 448.2106. IR (Film): 3057, 2957, 2923, 2859, 2358, 1761, 1459, 1378, 1265, 806, 736, 706 cm–1. [α]25 D = −16.9, (c = 1.00, CH2Cl2).

27, White solid; mp: 106–107°C; 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 7.5 Hz, 2H), 7.23–7.19 (m, 3H), 7.15 (d, J = 7.5 Hz, 2H), 6.99 (t, J = 7.8 Hz, 2H), 6.90 (t, J = 7.3 Hz, 1H), 6.65 (d, J = 9.0 Hz, 2H), 3.99 (q, J = 7.3 Hz, 2H), 3.69 (s, 3H), 2.39–2.35 (m, 2H), 1.06 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.6 (dd, J = 36.3, 33.0 Hz), 158.9, 141.7, 141.1, 132.8, 130.2, 129.0, 128.1, 127.7, 126.6, 126.0, 125.3 (d, J = 4.8 Hz), 116.5 (t, J = 256.9 Hz), 113.3, 62.3, 55.0, 42.7, 40.0 (dd, J = 26.4, 20.9 Hz), 20.7, 13.7. 19F NMR (470 MHz, CDCl3) δ −102.6 (d, J = 249.0 Hz), −105.4 (d, J = 249.0 Hz). HRMS (ESI) m/z calculated C26H24F2O3 [M]+ 422.1590, found 422.1586. IR (Film): 3055, 2253, 1766, 1514, 1294, 1261, 905, 725, 649 cm–1. [α]25 D = −32.5, (c = 1.00, CH2Cl2).

28, White solid; mp: 87–88°C; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 7.7 Hz, 2H), 7.32 (t, J = 7.7 Hz, 2H), 7.22 (t, J = 7.4 Hz, 1H), 7.16 (d, J = 7.6 Hz, 2H), 7.04 (t, J = 7.9 Hz, 1H), 6.98 (t, J = 7.7 Hz, 2H), 6.94–6.89 (m, 2H), 6.85 (s, 1H), 6.63 (dd, J = 8.0, 2.4 Hz, 1H), 4.02–3.97 (m, 2H), 3.68 (s, 3H), 2.44–2.41 (m, 1H), 2.39–2.36 (m, 1H), 1.04 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.5 (dd, J = 35.9, 32.2 Hz), 159.0, 141.6, 140.9, 134.9 (d, J = 5.5 Hz), 130.2, 128.9, 128.7, 128.1, 127.7, 126.7, 126.1, 124.1, 117.7, 116.3 (t, J = 257.0 Hz), 113.2, 62.3, 55.1, 42.7, 40.8 (dd, J = 26.0, 21.1 Hz), 20.6 (t, J = 4.5 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.6 (d, J = 249.6 Hz), −104.8 (d, J = 249.6 Hz). HRMS (ESI) m/z calculated C26H24F2O3 [M]+ 422.1590, found 422.1587. IR (Film): 3055, 2923, 2841, 1753, 1580, 1493 cm–1. [α]25 D = −54.9, (c = 1.00, CH2Cl2).
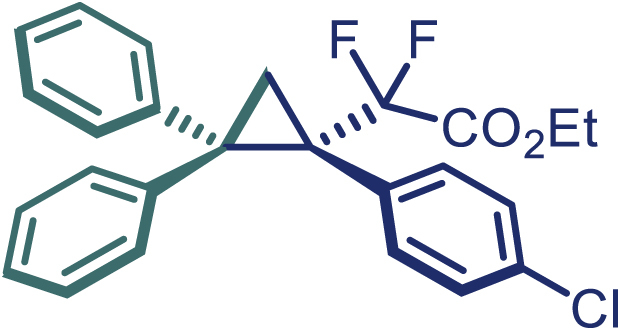
29, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 7.5 Hz, 2H), 7.34–7.24 (m, 4H), 7.24–7.20 (m, 1H), 7.15–7.09 (m, 4H), 7.00 (t, J = 7.5 Hz, 2H), 6.94–6.90 (m, 1H), 4.01–3.96 (m, 2H), 2.43–2.38 (m, 2H), 1.05 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.4 (dd, J = 36.3, 32.5 Hz), 141.3, 140.5, 133.8, 133.0, 132.2 (d, J = 5.0 Hz), 130.1, 128.9, 128.2, 128.1, 127.9, 126.8, 126.3, 116.1 (t, J = 255.6 Hz), 62.5, 42.9, 40.1 (dd, J = 26.3, 21.9 Hz), 20.5 (t, J = 5.0 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.7 (d, J = 250.5 Hz), −104.7 (d, J = 250.5 Hz). HRMS (ESI) m/z calculated C25H21ClF2O2 [M]+ 426.1086, found 426.1090. IR (Film): 3057, 2983, 2253, 1381, 1263, 908, 723, 649 cm–1. [α]25 D = −33.5, (c = 1.00, CH2Cl2).
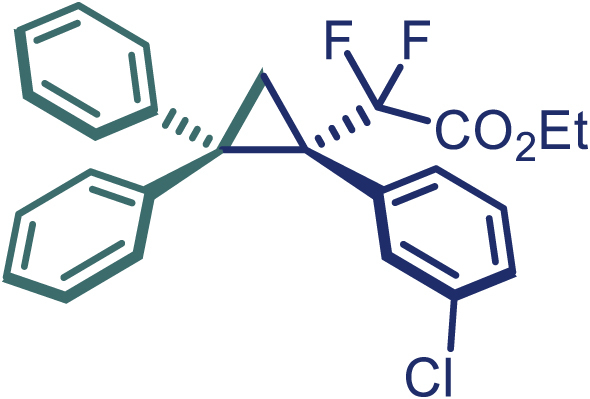
30, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.34–7.30 (m, 3H), 7.25–7.20 (m, 2H), 7.16–7.14 (m, 2H), 7.09–7.04 (m, 2H), 7.04–6.99 (m, 2H), 6.94–6.90 (m, 1H), 4.00 (q, J = 7.0 Hz, 2H), 2.44–2.39 (m, 2H), 1.05 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.3 (dd, J = 35.6, 32.8 Hz), 141.2, 140.4, 135.7 (d, J = 5.5 Hz), 133.6, 131.7, 130.1, 129.1, 128.9, 128.2, 128.0, 127.9, 126.8, 126.3, 116.0 (t, J = 255.3 Hz), 62.5, 42.9, 40.4 (dd, J = 26.2, 21.8 Hz), 20.4 (t, J = 4.2 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.9 (d, J = 251.0 Hz), −104.7 (d, J = 251.0 Hz). HRMS (ESI) m/z calculated C25H21ClF2O2 [M]+ 426.1090, found 426.1090. IR (Film): 3055, 2253, 1767, 1449, 1304, 1265, 1137, 906, 726, 649 cm–1. [α]25 D = −48.3, (c = 1.00, CH2Cl2).
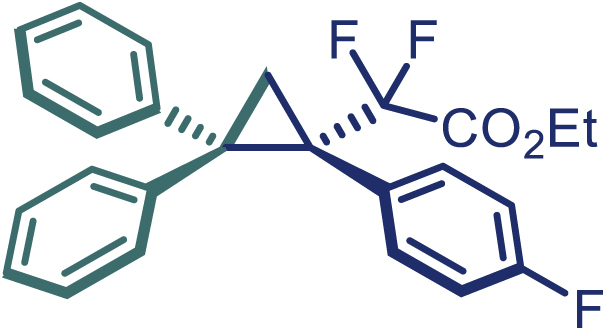
31, White solid; mp: 113–114°C; 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 8.0 Hz, 2H), 7.34–7.29 (m, 4H), 7.22 (t, J = 7.5 Hz, 1H), 7.13 (d, J = 7.5 Hz, 2H), 6.99 (t, J = 7.5 Hz, 2H), 6.91 (t, J = 7.5 Hz, 1H), 6.81 (t, J = 7.5 Hz, 2H), 3.99 (q, J = 7.2 Hz, 2H), 2.43–2.38 (m, 2H), 1.05 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.5 (dd, J = 35.0, 32.5 Hz), 162.1 (d, J = 248.5 Hz), 141.4, 140.7, 133.4, 130.2, 129.4 (dd, J = 5.5, 3.4 Hz),128.9, 128.1, 127.9, 126.8, 126.2, 116.2 (t, J = 255.0 Hz), 114.8 (d, J = 22.5 Hz), 62.4, 42.8, 40.0 (dd, J = 27.5, 21.9 Hz), 20.6 (dd, J = 5.0, 3.8 Hz), 13.7. 19F NMR (470 MHz, CDCl3) δ −102.8 (d, J = 249.1 Hz), −105.1 (d, J = 249.1 Hz), (−113.7)-(-113.8) (m). HRMS (ESI) m/z calculated C25H21F3O2 [M]+ 410.1394, found 410.1386. IR (Film): 3055, 2358, 2253, 1766, 1512, 1303, 1261, 1135, 905, 725, 649 cm–1. [α]25 D = −50.2, (c = 1.00, CH2Cl2).
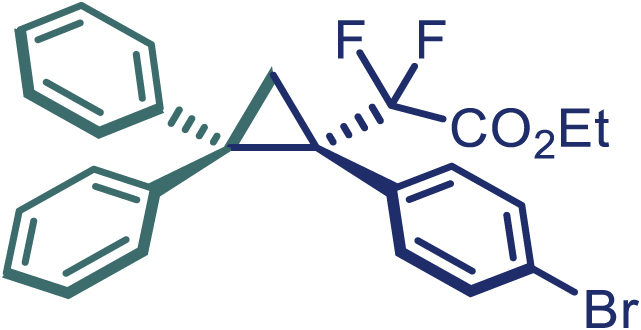
32, White solid; mp: 114–115°C; 1H NMR (500 MHz, CDCl3) δ 7.66 (d, J = 7.5 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H), 7.27–7.19 (m, 5H), 7.13 (d, J = 7.5 Hz, 2H), 7.00 (t, J = 7.5 Hz, 2H), 6.94–6.90 (m, 1H), 4.03–3.93 (m, 2H), 2.42–2.37 (m, 2H), 1.05 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.3 (dd, J = 35.6, 32.8 Hz), 141.3, 140.5, 133.3, 132.7 (d, J = 5.6 Hz), 131.0, 130.1, 128.9, 128.1, 127.9, 126.8, 126.3, 122.1, 116.0 (t, J = 255.4 Hz), 62.5, 42.9, 40.2 (dd, J = 26.4, 21.4 Hz), 20.4 (t, J = 4.3 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.8 (d, J = 251.0 Hz), −104.7 (d, J = 251.0 Hz). HRMS (ESI) m/z calculated C25H21BrF2O2 [M]+ 470.0587, found 470.0585. IR (Film): 2986, 2253, 1766, 1491, 1264, 1136, 906, 726, 649 cm–1. [α]25 D = −51.1, (c = 1.00, CH2Cl2).

33, Colorless oil; 1H NMR (600 MHz, CDCl3) δ 7.70 (d, J = 8.4 Hz, 2H), 7.50–7.47 (m, 2H), 7.40–7.36 (m, 6H), 7.35–7.28 (m, 3H), 7.24–7.21 (m, 1H), 7.19–7.17 (m, 2H), 6.99–6.96 (m, 2H), 6.90–6.87 (m, 1H), 4.01–3.95 (m, 2H), 2.48–2.46 (m, 1H), 2.44–2.42 (m, 1H), 1.01 (t, J = 6.9 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.6 (dd, J = 35.9, 32.0 Hz), 141.6, 140.9, 140.3, 132.5 (d, J = 5.7 Hz), 132.0, 130.2, 129.0, 128.7, 128.1, 127.8, 127.4, 126.9, 126.7, 126.4, 126.1, 116.4 (t, J = 255.5 Hz), 62.4, 42.9, 40.5 (dd, J = 26.4, 20.9 Hz), 20.6, 13.6. 19F NMR (564 MHz, CDCl3) δ −102.6 (d, J = 250.0 Hz), −104.9 (d, J = 250.0 Hz). HRMS (ESI) m/z calculated C31H26F2O2 [M]+ 468.1787, found 468.1793. IR (Film): 3057, 2253, 1766, 1424, 1265, 906, 722, 649 cm–1. [α]25 D = −25.4, (c = 1.00, CH2Cl2).
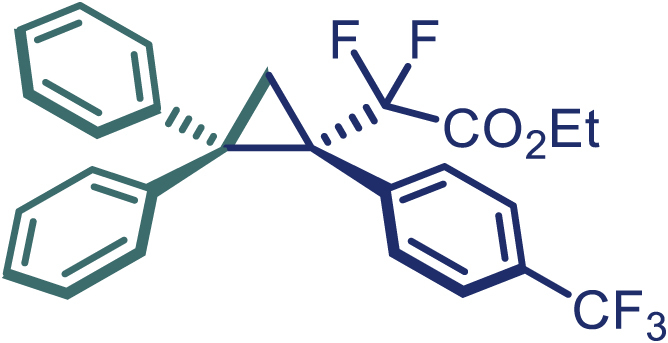
34, White solid; mp: 97–98°C; 1H NMR (500 MHz, CDCl3) δ 7.68 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 7.0 Hz, 2H), 7.38 (d, J = 8.5 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.23 (t, J = 7.3 Hz, 1H), 7.12 (d, J = 8.0 Hz, 2H), 6.98 (t, J = 8.0 Hz, 2H), 6.91 (t, J = 8.0 Hz, 1H), 4.01–3.90 (m, 2H), 2.49–2.44 (m, 2H), 1.00 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.3 (dd, J = 35.4, 32.6 Hz), 141.1, 140.3, 137.8 (d, J = 5.1 Hz), 132.1, 130.0, 129.9 (q, J = 32.5 Hz), 128.8, 128.2, 128.0, 126.9, 126.4, 124.7 (q, J = 3.4 Hz), 123.7 (q, J = 270.6 Hz), 116.0 (t, J = 255.4 Hz), 62.6, 43.0, 40.5 (dd, J = 26.4, 21.9 Hz), 20.4 (t, J = 4.3 Hz), 13.5. 19F NMR (470 MHz, CDCl3) δ −62.8, −102.8 (d, J = 251.5 Hz), −104.3 (d, J = 251.5 Hz). IR (Film): 3055, 2253, 1766, 1449, 1326, 1264, 1129, 907, 726, 649 cm–1. [α]25 D = −27.8, (c = 1.00, CH2Cl2).
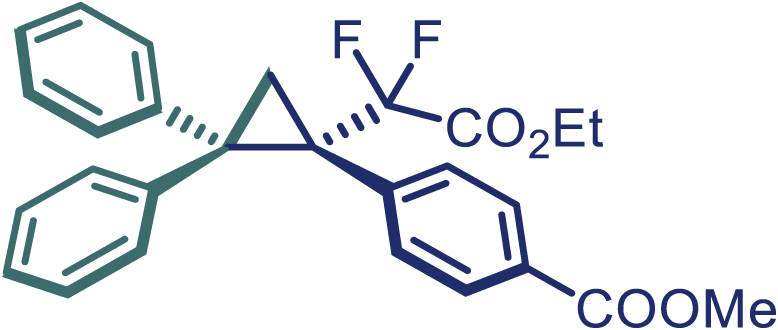
35, Colorless oil; 1H NMR (600 MHz, CDCl3) δ 7.81–7.78 (m, 2H), 7.68 (d, J = 7.8 Hz, 2H), 7.42 (d, J = 7.2 Hz, 2H), 7.33 (t, J = 7.5 Hz, 2H), 7.24–7.21 (m, 1H), 7.15–7.12 (m, 2H), 6.98–6.95 (m, 2H), 6.90–6.87 (m, 1H), 3.96 (q, J = 7.2 Hz, 2H), 3.84 (s, 3H), 2.51–2.48 (m, 1H), 2.45–2.42 (m, 1H), 1.02 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 166.6, 163.3 (dd, J = 35.7, 32.2 Hz), 141.2, 140.4, 138.9 (d, J = 5.0 Hz), 131.6, 130.0, 129.4, 129.1, 128.9, 128.2, 127.9, 126.8, 126.3, 116.0 (t, J = 255.3 Hz), 62.5, 52.1, 43.1, 40.6 (dd, J = 26.6, 21.8 Hz), 20.4 (t, J = 4.5 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.8 (d, J = 251.4 Hz), −104.2 (d, J = 251.4 Hz). HRMS (ESI) m/z calculated C27H24F2O4 [M]+ 450.1538, found 450.1535. IR (Film): 3055, 2985, 1768, 1720, 1264, 1113, 731, 703, 601 cm–1. [α]25 D = −33.4, (c = 1.00, CH2Cl2).
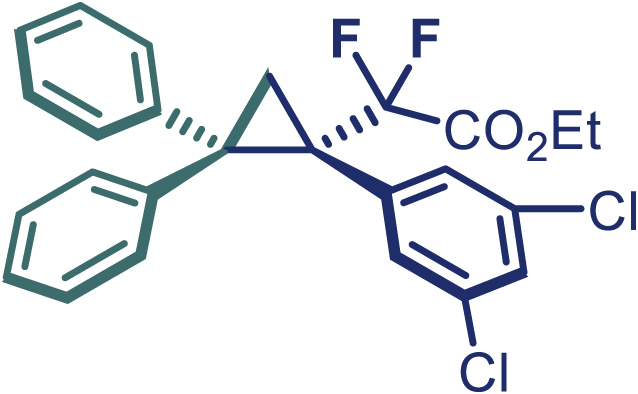
36, White solid; mp: 95–96°C; 1H NMR (600 MHz, CDCl3) δ 7.66 (d, J = 8.4 Hz, 2H), 7.32 (t, J = 8.1 Hz, 2H), 7.24–7.21 (m, 3H), 7.17–7.15 (m, 2H), 7.09 (t, J = 1.8 Hz, 1H), 7.07–7.03 (m, 2H), 6.98–6.94 (m, 1H), 4.08–4.01 (m, 2H), 2.40 (s, 2H), 1.10 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.1 (dd, J = 35.9, 32.9 Hz), 140.8, 139.9, 137.3 (d, J = 5.3 Hz), 134.3, 130.3, 130.0, 128.7, 128.2, 128.1, 128.0, 127.0, 126.6, 115.7 (t, J = 255.5 Hz), 62.7, 43.1, 40.2 (dd, J = 26.7, 22.4 Hz), 20.4 (t, J = 3.9 Hz), 13.7. 19F NMR (564 MHz, CDCl3) δ −102.9 (d, J = 253.0 Hz), −104.2 (d, J = 253.0 Hz). HRMS (ESI) m/z calculated C25H20Cl2F2O2 [M]+ 460.0712, found 460.0701. IR (Film): 3086, 2984, 2253, 1768, 1264, 905, 726, 649 cm–1. [α]25 D = −40.0, (c = 1.00, CH2Cl2).

37, White solid; mp: 122–123°C; 1H NMR (500 MHz, CDCl3) δ 7.77–7.71 (m, 3H), 7.70–7.67 (m, 2H), 7.60 (d, J = 8.5 Hz, 1H), 7.54 (s, 1H), 7.40–7.32 (m, 4H), 7.25–7.19 (m, 3H), 6.90 (t, J = 7.8 Hz, 2H), 6.79 (t, J = 7.3 Hz, 1H), 3.87 (q, J = 7.0 Hz, 2H), 2.58 (t, J = 4.0 Hz, 1H), 2.49 (d, J = 5.5 Hz, 1H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.5 (dd, J = 35.8, 32.8 Hz), 141.7, 140.8, 132.8, 132.6, 131.2 (d, J = 4.8 Hz), 130.9, 130.2, 129.3, 129.0, 128.1, 127.8, 127.7, 127.44, 127.39, 126.7, 126.2, 126.1, 125.9, 116.5 (t, J = 255.7 Hz), 62.3, 42.9, 40.9 (dd, J = 26.4, 21.3 Hz), 20.8 (t, J = 4.3 Hz), 13.5. 19F NMR (470 MHz, CDCl3) δ −102.4 (d, J = 249.1 Hz), −104.7 (d, J = 249.1 Hz). HRMS (ESI) m/z calculated C29H24F2O2 [M]+ 442.1641, found 442.1637. IR (Film): 3055, 2253, 1766, 1378, 1261, 1009, 907, 725, 649 cm–1. [α]25 D = −2.1, (c = 1.00, CH2Cl2).
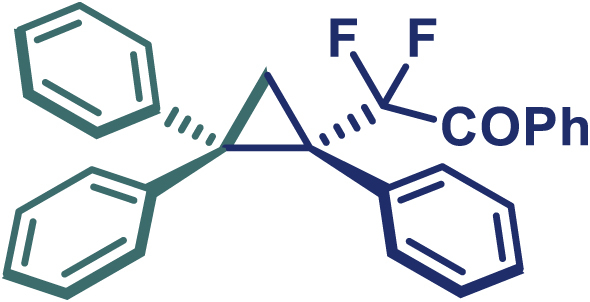
38, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J = 7.7 Hz, 2H), 7.63 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.5 Hz, 1H), 7.37–7.28 (m, 5H), 7.24–7.22 (m, 2H), 7.16–7.13 (m, 2H), 7.02–6.94 (m, 5H), 6.90–6.86 (m, 1H), 2.50–2.44 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 190.5 (dd, J = 33.6, 29.0 Hz), 141.9, 140.9, 133.7, 133.5 (d, J = 5.8 Hz), 133.3, 132.2, 130.4, 129.6, 129.0, 128.11, 128.09, 127.69, 127.65, 127.5, 126.6, 126.0, 119.5 (t, J = 260.1 Hz), 42.7, 41.1 (dd, J = 26.3, 20.9 Hz), 21.1 (d, J = 6.1 Hz). 19F NMR (564 MHz, CDCl3) δ −96.0 (d, J = 264.6 Hz), −99.4 (d, J = 264.6 Hz). HRMS (ESI) m/z calculated C29H22F2O [M]+ 424.1535, found 424.1531. IR (Film): 3087, 2253, 1710, 1449, 1264, 906, 726, 649 cm–1. [α]25 D = −18.9, (c = 1.00, CH2Cl2).

40, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 2H), 7.39–7.29 (m, 2H), 7.15–7.07 (m, 5H), 7.00 (d, J = 8.0 Hz, 2H), 6.76 (d, J = 8.0 Hz, 2H), 3.95 (q, J = 7.1 Hz, 2H), 2.39–2.34 (m, 2H), 2.30 (s, 3H), 2.07 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.6 (dd, J = 36.0, 32.8 Hz), 138.9, 138.3, 136.1, 135.4, 133.7 (d, J = 5.7 Hz), 131.7, 129.9, 128.81, 128.76, 128.4, 127.8, 127.7, 116.5 (t, J = 256.4 Hz), 62.3, 42.1, 40.7 (dd, J = 26.8, 20.9 Hz), 21.1, 20.7, 20.5 (t, J = 4.5 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.7 (d, J = 248.1 Hz), −105.0 (d, J = 248.1 Hz). HRMS (ESI) m/z calculated C27H26F2O2 [M]+ 420.1787, found 420.1789. IR (Film): 3060, 2245, 1705, 1435, 1260 cm–1. [α]25 D = −18.9, (c = 1.00, CH2Cl2).

41, White solid; mp: 73–74°C; 1H NMR (600 MHz, CDCl3) δ 7.51 (d, J = 7.8 Hz, 1H), 7.45 (s, 1H), 7.38–7.28 (m, 2H), 7.21 (t, J = 7.8 Hz, 1H), 7.14–7.06 (m, 3H), 7.02 (d, J = 7.8 Hz, 1H), 6.96 (d, J = 7.8 Hz, 1H), 6.93 (s, 1H), 6.85 (t, J = 7.8 Hz, 1H), 6.68 (d, J = 7.8 Hz, 1H), 3.95 (q, J = 7.2 Hz, 2H), 2.41–2.38 (m, 1H), 2.37–2.34 (m, 4H), 2.09 (s, 3H), 1.00 (t, J = 7.2 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.6 (dd, J = 35.6, 32.7 Hz), 141.6, 141.0, 137.5, 137.0, 133.5 (d, J = 5.6 Hz), 131.6, 130.8, 129.8, 127.8, 127.72, 127.68, 127.5, 127.4, 127.3, 126.7, 126.2, 116.4 (t, J = 255.3 Hz), 62.3, 42.7, 40.6 (dd, J = 26.4, 21.1 Hz), 21.5, 21.3, 20.5 (t, J = 4.4 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.7 (d, J = 248.6 Hz), −105.0 (d, J = 248.6 Hz). IR (Film): 3057, 2924, 2253, 1766, 1603, 1449, 1264, 1132, 906, 726, 649 cm–1. [α]25 D = −19.8, (c = 1.00, CH2Cl2).
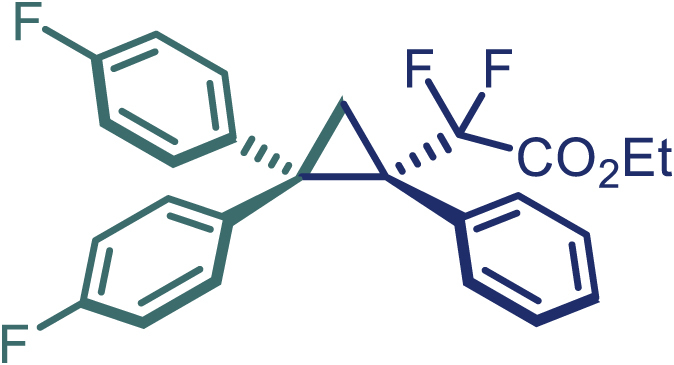
42, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.65–7.60 (m, 2H), 7.34–7.26 (m, 2H), 7.16–7.09 (m, 3H), 7.08–6.99 (m, 4H), 6.69–6.63 (m, 2H), 3.97 (q, J = 7.1 Hz, 2H), 2.41–2.35 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.4 (dd, J = 35.7, 32.5 Hz), 161.7 (d, J = 245.3 Hz), 161.0 (d, J = 245.5 Hz), 137.3, 136.7 (d, J = 3.1 Hz), 133.0 (d, J = 5.6 Hz), 131.6 (d, J = 7.9 Hz), 130.4 (d, J = 8.1 Hz), 128.04, 128.00, 118.0, 116.3 (d, J = 257.3 Hz), 115.1 (d, J = 21.7 Hz), 114.7 (d, J = 21.3 Hz), 62.4, 41.2, 40.9 (dd, J = 26.1, 21.0 Hz), 20.8 (t, J = 4.5 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.7 (d, J = 249.0 Hz), −105.2 (d, J = 249.0 Hz), (−115.7)-(-115.8) (m), (−116.3)-(-116.4) (m). HRMS (ESI) m/z calculated C25H20F4O2 [M]+ 428.1290, found 428.1292. IR (Film): 2926, 2360, 1751, 1511, 1230 cm–1. [α]25 D = −22.6 (c = 1.00, CH2Cl2).
43, White solid; mp: 84–85°C; 1H NMR (600 MHz, CDCl3) δ 7.46 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 10.2 Hz, 1H), 7.33–7.28 (m, 3H), 7.17–7.10 (m, 3H), 6.97–6.92 (m, 2H), 6.90 (d, J = 7.8 Hz, 1H), 6.83–6.81 (m, 1H), 6.63–6.60 (m, 1H), 3.98 (q, J = 7.2 Hz, 2H), 2.43–2.40 (m, 1H), 2.39–2.36 (m, 1H), 1.01 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.3 (dd, J = 35.7, 32.0 Hz), 162.5 (d, J = 245.2 Hz), 162.2 (d, J = 246.3 Hz), 143.4 (d, J = 7.5 Hz), 142.9 (d, J = 7.0 Hz), 132.6 (d, J = 5.1 Hz), 131.5, 129.7 (d, J = 8.5 Hz), 129.2 (d, J = 8.2 Hz), 128.09 (d, J = 6.9 Hz), 128.07, 125.9, 124.6 (d, J = 3.3 Hz), 117.2 (d, J = 21.6 Hz), 116.1 (t, J = 255.5 Hz), 116.0 (d, J = 21.9 Hz), 114.0 (d, J = 20.9 Hz), 113.3 (d, J = 21.3 Hz), 62.5, 41.8, 41.1 (dd, J = 26.3, 20.9 Hz), 20.6 (t, J = 4.6 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.9 (d, J = 249.6 Hz), −105.5 (d, J = 249.6 Hz), (−113.1)-(-113.4) (m). HRMS (ESI) m/z calculated C25H20F4O2 [M]+ 428.1290, found 428.1292. IR (Film): 3065, 2963, 2925, 1753, 1587, 1487, 1261, 1183 cm–1. [α]25 D = −38.1, (c = 1.00, CH2Cl2).

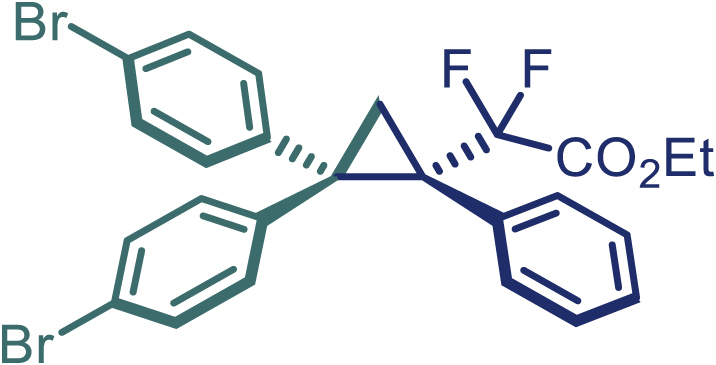
44, White solid; mp: 94–95°C; 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 7.6 Hz, 2H), 7.33–7.27 (m, 4H), 7.17–7.13 (m, 3H), 7.02 (d, J = 8.7 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 3.97 (q, J = 7.1 Hz, 2H), 2.40–2.35 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 163.3 (dd, J = 35.6, 30.5 Hz), 139.7, 139.2, 132.8, 132.7 (d, J = 5.5 Hz), 132.2, 131.4, 130.2, 128.5, 128.2, 128.1, 128.0, 116.2 (t, J = 257.3 Hz), 62.5, 41.4, 41.0 (dd, J = 26.3, 20.9 Hz), 20.6 (t, J = 4.5 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.6 (d, J = 249.5 Hz), −105.3 (d, J = 249.5 Hz). HRMS (ESI) m/z calculated C25H20Cl2F2O2 [M]+ 460.0707, found 460.0701. IR (Film): 3055, 2985, 2253, 1422, 1263, 902, 719, 649 cm–1. [α]25 D = −22.6, (c = 1.00, CH2Cl2).
45, Colorless oil; 1H NMR (600 MHz, CDCl3) δ 7.52 (d, J = 7.8 Hz, 2H), 7.45 (d, J = 8.6 Hz, 2H), 7.34–7.27 (m, 2H), 7.17–7.13 (m, 3H), 7.12–7.08 (m, 2H), 6.97–6.94 (m, 2H), 3.97 (q, J = 7.1 Hz, 2H), 2.39–2.34 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.3 (dd, J = 35.6, 32.7 Hz), 140.2, 139.6, 132.7 (d, J = 5.4 Hz), 131.7, 131.4, 131.0, 130.6, 128.19, 128.17, 121.0, 120.4, 116.2 (t, J = 257.1 Hz), 62.5, 41.5, 41.0 (dd, J = 26.4, 21.0 Hz), 20.6 (t, J = 4.3 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.6 (d, J = 249.5 Hz), −105.3 (d, J = 249.5 Hz). IR (Film): 3052, 2985, 2253, 1767, 1491, 1421, 1264, 907, 728, 703, 649 cm–1. [α]25 D = −27.6, (c = 1.00, CH2Cl2).
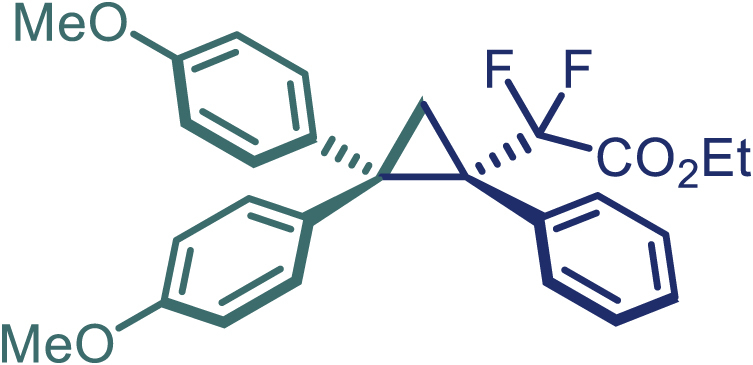
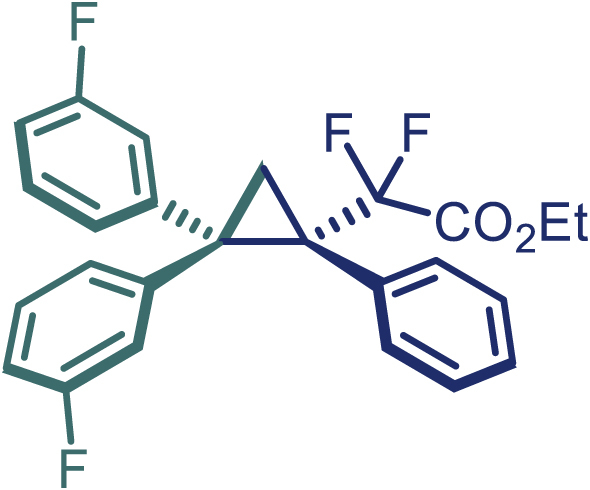
46, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.56 (d, J = 7.9 Hz, 2H), 7.35–7.30 (m, 2H), 7.14–7.07 (m, 3H), 7.00 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 6.50 (d, J = 8.9 Hz, 2H), 3.95 (q, J = 7.1 Hz, 2H), 3.78 (s, 3H), 3.60 (s, 3H), 2.37–2.32 (m, 2H), 1.01 (t, J = 7.1 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 163.7 (dd, J = 36.0, 32.6 Hz), 158.2, 157.5, 134.1, 133.7 (d, J = 5.7 Hz), 133.6, 131.7, 131.0, 129.8, 127.9, 127.7, 116.5 (t, J = 256.5 Hz), 113.5, 113.1, 62.3, 55.2, 55.0, 41.3, 40.9 (dd, J = 26.6, 21.1 Hz), 20.8 (t, J = 4.5 Hz), 13.6. 19F NMR (470 MHz, CDCl3) δ −102.8 (d, J = 248.6 Hz), −104.8 (d, J = 248.6 Hz). HRMS (ESI) m/z calculated C27H26F2O4 [M]+ 452.1688, found 452.1691. IR (Film): 3046, 2253, 1766, 1512, 1264, 905, 726, 649 cm–1. [α]25 D = −9.2 (c = 1.00, CH2Cl2).
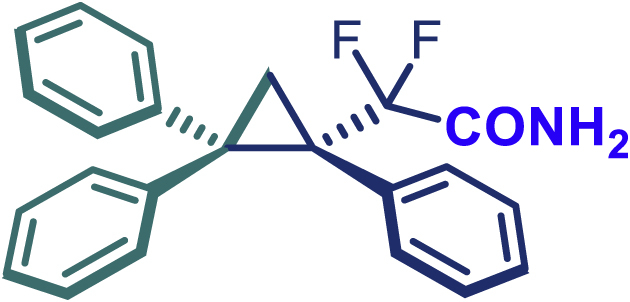
47, White solid; mp: 98–99°C; 1H NMR (500 MHz, CDCl3) δ 7.67 (d, J = 7.6 Hz, 2H), 7.33–7.28 (m, 4H), 7.20 (t, J = 7.4 Hz, 1H), 7.11–7.03 (m, 5H), 6.94 (t, J = 7.6 Hz, 2H), 6.86 (t, J = 7.3 Hz, 1H), 5.73 (s, 1H), 5.64 (s, 1H), 2.47–2.44 (m, 1H), 2.38 (d, J = 6.0 Hz, 1H). 13C NMR (150 MHz, CDCl3) δ 166.0 (dd, J = 33.2, 30.1 Hz), 141.7, 140.7, 133.7 (d, J = 4.9 Hz), 132.0, 130.2, 128.8, 128.0, 127.8, 127.6, 126.6, 125.9, 117.8 (t, J = 258.4 Hz), 41.8, 40.4 (dd, J = 26.7, 21.8 Hz), 20.6 (t, J = 4.2 Hz). 19F NMR (564 MHz, CDCl3) δ −102.8 (d, J = 248.0 Hz), −105.1 (d, J = 248.0 Hz). HRMS (ESI) m/z calculated C23H19F2NO [M]+ 363.1326, found 363.1327. IR (Film): 3035, 2250, 1740, 1501, 1151, 750, 637 cm–1. [α]25 D = −15.3 (c = 1.00, CH2Cl2).

48, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.6 Hz, 2H), 7.38–7.29 (m, 4H), 7.22–7.19 (m, 1H), 7.16–7.06 (m, 5H), 6.95 (t, J = 7.6 Hz, 2H), 6.86 (t, J = 7.3 Hz, 1H), 3.56–3.41 (m, 2H), 2.40 (t, J = 5.1 Hz, 1H), 2.14 (d, J = 5.7 Hz, 1H), 1.65 (s, 1H). 13C NMR (125 MHz, CDCl3) δ 142.5, 141.1, 135.2 (d, J = 6.0 Hz), 131.1, 130.1, 129.0, 128.0, 127.6, 127.4, 126.4, 125.9, 123.0 (dd, J = 249.1, 246.3 Hz), 64.8 (dd, J = 34.4, 27.3 Hz), 41.8, 40.0 (dd, J = 25.9, 21.1 Hz), 19.8 (dd, J = 5.8, 3.7 Hz). 19F NMR (470 MHz, CDCl3) δ −105.9 (dd, J = 247.7, 12.7 Hz), −109.4 (dt, J = 247.5, 16.8 Hz). IR (Film): 2237, 1421, 1209, 1150, 920 cm–1. [α]25 D = −30.2, (c = 1.00, CH2Cl2).
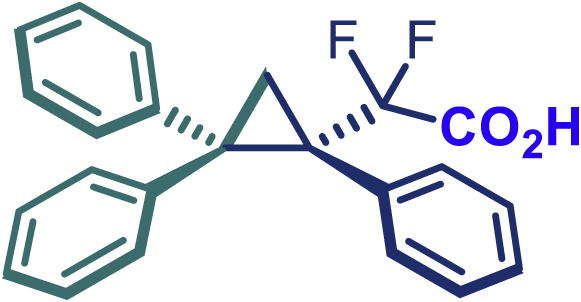
49, White solid; mp: 90–91°C; 1H NMR (500 MHz, CDCl3) δ 8.33–8.10 (m, 1H), 7.67 (d, J = 7.7 Hz, 2H), 7.36–7.27 (m, 4H), 7.22 (t, J = 7.4 Hz, 1H), 7.12–7.09 (m, 5H), 6.97 (t, J = 7.5 Hz, 2H), 6.89 (t, J = 7.3 Hz, 1H), 2.45 (t, J = 5.1 Hz, 1H), 2.36 (d, J = 6.0 Hz, 1H). 13C NMR (125 MHz, CDCl3) δ 167.2 (dd, J = 37.8, 33.5 Hz), 141.3, 140.6, 132.8 (d, J = 5.2 Hz), 131.7, 130.1, 128.9, 128.2, 128.0, 127.9, 127.7, 126.8, 126.1, 116.1 (t, J = 256.7 Hz), 42.8, 40.3 (dd, J = 25.9, 20.9 Hz), 20.5. 19F NMR (470 MHz, CDCl3) δ −103.4 (d, J = 251.4 Hz), −105.4 (d, J = 251.4 Hz). IR (Film): 3023, 2267, 1420, 922 cm–1. [α]25 D = −27.5, (c = 1.00, CH2Cl2).

50, Colorless oil; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.8 Hz, 2H), 7.35–7.20 (m, 5H), 7.14–7.05 (m, 5H), 6.96 (t, J = 7.6 Hz, 2H), 6.88 (t, J = 7.3 Hz, 1H), 2.46–2.42 (m, 1H), 2.39–2.35 (m, 1H), 2.31–2.23 (m, 1H), 1.90–1.82 (m, 1H), 1.31–1.13 (m, 2H), 1.06–0.95 (m, 2H), 0.70 (t, J = 7.3 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 201.6 (dd, J = 35.8, 27.0 Hz), 141.8, 140.7, 133.3 (d, J = 5.8 Hz), 132.1, 130.3, 128.9, 128.1, 128.0, 127.71, 127.67, 126.6, 126.0, 118.2 (t, J = 259.5 Hz), 42.2, 40.3 (dd, J = 27.0, 21.4 Hz), 37.5, 24.3, 21.7, 20.5 (dd, J = 26.1, 22.2 Hz), 13.6. 19F NMR (564 MHz, CDCl3) δ −102.4 (d, J = 250.4 Hz), −108.4 (d, J = 250.4 Hz). IR (Film): 3050, 2250, 1715, 1433, 1251, 922, 630 cm–1. [α]25 D = −22.3, (c = 1.00, CH2Cl2).
Theoretical methodology
The quantum chemical calculations described in this work were carried out with Gaussian16 package.42 Geometry optimizations were conducted in the framework of the density functional theory (DFT) at the M06l43 level. The effective core potential SDD44 basis set was used to represent Rh atom, all the other atoms (C, H, O, F etc.) were described with 6-31G(d) basis set.45,46,47 The nature of the local minima was established with analytical frequencies calculations. Intrinsic reaction coordinate (IRC)48,49 calculations were carried out to ascertain the true nature of the transition states. 3D diagrams of the computed species were generated using CYLview visualization software.50 All structures are optimized under gas phase conditions.
Acknowledgments
Financial support was provided by the NSFC (21871043, 21961130376), the Department of Science and Technology of Jilin Province (20190701012GH, 20190103128JH, 20200801065GH), and the Fundamental Research Funds for the· Central Universities (2412019ZD001, 2412019FZ006).
Author contributions
X.Z., Y.N., and C.T. performed the experimental investigations and theoretical calculations. X.Z. and X.B. conceived the concept, designed the project, analyzed the data, and together with G.Z. discussed the results and prepared this manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 11, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105896.
Supplemental information
Data and code availability
-
•
All original crystal structures have been deposited at CCDC and are publicly available as of the date of publication. Compound 37: CCDC 2117854. Compound 44: CCDC 2117853.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.
References
- 1.Meanwell N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011;54:2529–2591. doi: 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- 2.Zafrani Y., Yeffet D., Sod-Moriah G., Berliner A., Amir D., Marciano D., Gershonov E., Saphier S. Difluoromethyl bioisostere: examining the “lipophilic hydrogen bond donor” concept. J. Med. Chem. 2017;60:797–804. doi: 10.1021/acs.jmedchem.6b01691. [DOI] [PubMed] [Google Scholar]
- 3.Barreiro E.J., Kümmerle A.E., Fraga C.A.M. The methylation effect in medicinal chemistry. Chem. Rev. 2011;111:5215–5246. doi: 10.1021/cr200060g. [DOI] [PubMed] [Google Scholar]
- 4.Sessler C.D., Rahm M., Becker S., Goldberg J.M., Wang F., Lippard S.J. CF2H, a hydrogen bond donor. J. Am. Chem. Soc. 2017;139:9325–9332. doi: 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens L., Koenigs R.M. Fluorinated diazoalkanes – a versatile class of reagents for the synthesis of fluorinated compounds. Org. Biomol. Chem. 2016;14:10547–10556. doi: 10.1039/C6OB01618A. [DOI] [PubMed] [Google Scholar]
- 6.Bos M., Poisson T., Pannecoucke X., Charette A.B., Jubault P. Recent progress toward the synthesis of trifluoromethyl- and difluoromethyl-substituted cyclopropanes. Chem. Eur J. 2017;23:4950–4961. doi: 10.1002/chem.201604564. [DOI] [PubMed] [Google Scholar]
- 7.Zeng J.-L., Chen Z., Zhang F.-G., Ma J.-A. Direct regioselective [3+2] cycloaddition reactions of masked difluorodiazoethane with electron-deficient alkynes and alkenes: synthesis of difluoromethyl-substituted pyrazoles. Org. Lett. 2018;20:4562–4565. doi: 10.1021/acs.orglett.8b01854. [DOI] [PubMed] [Google Scholar]
- 8.Peng X., Xiao M.-Y., Zeng J.-L., Zhang F.-G., Ma J.-A. Construction of difluoromethylated tetrazoles via silver-catalyzed regioselective [3+2] cycloadditions of aryl diazonium salts. Org. Lett. 2019;21:4808–4811. doi: 10.1021/acs.orglett.9b01697. [DOI] [PubMed] [Google Scholar]
- 9.Zeng J.-L., Zhang Y., Zheng M.-M., Zhang Z.-Q., Xue X.-S., Zhang F.-G., Ma J.-A. Chemodivergent and stereoselective construction of gem-difluoroallylic amines from masked difluorodiazo reagents. Org. Lett. 2019;21:8244–8249. doi: 10.1021/acs.orglett.9b02989. [DOI] [PubMed] [Google Scholar]
- 10.Mykhailiuk P.K. 2,2,2-Trifluorodiazoethane (CF3CHN2): a long journey since 1943. Chem. Rev. 2020;120:12718–12755. doi: 10.1021/acs.chemrev.0c00406. [DOI] [PubMed] [Google Scholar]
- 11.Denton J.R., Sukumaran D., Davies H.M.L. Enantioselective synthesis of trifluoromethyl-substituted cyclopropanes. Org. Lett. 2007;9:2625–2628. doi: 10.1021/ol070714f. [DOI] [PubMed] [Google Scholar]
- 12.Morandi B., Mariampillai B., Carreira E.M. Enantioselective cobalt-catalyzed preparation of trifluoromethyl-substituted cyclopropanes. Angew. Chem., Int. Ed. Engl. 2011;50:1101–1104. doi: 10.1002/anie.201004269. [DOI] [PubMed] [Google Scholar]
- 13.Tinoco A., Steck V., Tyagi V., Fasan R. Highly diastereo- and enantioselective synthesis of trifluoromethyl-substituted cyclopropanes via myoglobin-catalyzed transfer of trifluoromethylcarbene. J. Am. Chem. Soc. 2017;139:5293–5296. doi: 10.1021/jacs.7b00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotozaki M., Chanthamath S., Fujii T., Shibatomi K., Iwasa S. Highly enantioselective synthesis of trifluoromethyl cyclopropanes by using Ru(ii)–Pheox catalyst. Chem. Commun. 2018;54:5110–5113. doi: 10.1039/C8CC02286K. [DOI] [PubMed] [Google Scholar]
- 15.Adly F.G., Gardiner M.G., Ghanem A. Design and synthesis of novel chiral dirhodium(II) carboxylate complexes for asymmetric cyclopropanation reaction. Chemistry. 2016;22:3447–3461. doi: 10.1002/chem.201504817. [DOI] [PubMed] [Google Scholar]
- 16.Simonneaux G., Le Maux P., Juillard S. Asymmetric synthesis of trifluoromethylphenyl cyclopropanes catalyzed by chiral metalloporphyrins. Synthesis. 2006;2006:1701–1704. https://DOI/10.1055/s-2006-926451 [Google Scholar]
- 17.Müller P., Grass S., Shahi S.P., Bernardinelli G. Rh(II)-Catalyzed asymmetric carbene transfer with ethyl 3,3,3-trifluoro-2-diazopropionate. Tetrahedron. 2004;60:4755–4763. doi: 10.1016/j.tet.2004.04.015. [DOI] [Google Scholar]
- 18.Pons A., Tognetti V., Joubert L., Poisson T., Pannecoucke X., Charette A.B., Jubault P. Catalytic enantioselective cyclopropanation of α-fluoroacrylates: an experimental and theoretical study. ACS Catal. 2019;9:2594–2598. doi: 10.1021/acscatal.9b00354. [DOI] [Google Scholar]
- 19.Zhang Z.Q., Zheng M.M., Xue X.S., Marek I., Zhang F.G., Ma J.-A. Catalytic enantioselective cyclopropenation of internal alkynes: access to difluoromethylated three-membered carbocycles. Angew. Chem. Int. Ed. Engl. 2019;58:18191–18196. doi: 10.1002/anie.201911701. [DOI] [PubMed] [Google Scholar]
- 20.Tan X.-F., Zhang F.-G., Ma J.-A. Asymmetric synthesis of CF2-functionalized aziridines by combined strong Brønsted acid catalysis. Beilstein J. Org. Chem. 2020;16:638–644. doi: 10.3762/bjoc.16.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Sivaguru P., Zanoni G., Han X., Tong M., Bi X. Catalytic asymmetric C(sp3)–H carbene insertion approach to access enantioenriched 3-fluoroalkyl 2,3-dihydrobenzofurans. ACS Catal. 2021;11:14293–14301. doi: 10.1021/acscatal.1c04523. [DOI] [Google Scholar]
- 22.Bester S.M., Wei G., Zhao H., Adu-Ampratwum D., Iqbal N., Courouble V.V., Francis A.C., Annamalai A.S., Singh P.K., Shkriabai N., et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science. 2020;370:360–364. doi: 10.1126/science.abb4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margot N., Ram R., Rhee M., Callebaut C. Absence of lenacapavir (GS-6207) phenotypic resistance in HIV gag cleavage site mutants and in isolates with resistance to existing drug classes. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02057-20. 020577-20-e2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos M., Huang W.S., Poisson T., Pannecoucke X., Charette A.B., Jubault P. Catalytic enantioselective synthesis of highly functionalized difluoromethylated cyclopropanes. Angew. Chem., Int. Ed. Engl. 2017;56:13319–13323. doi: 10.1002/anie.201707375. [DOI] [PubMed] [Google Scholar]
- 25.Carminati D.M., Decaens J., Couve-Bonnaire S., Jubault P., Fasan R. Biocatalytic strategy for the highly stereoselective synthesis of CHF2-containing trisubstituted cyclopropanes. Angew. Chem., Int. Ed. Engl. 2021;60:7072–7076. doi: 10.1002/anie.202015895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivaguru P., Bi X. Fluoroalkyl N-sulfonyl hydrazones: an efficient reagent for the synthesis of fluoroalkylated compounds. Sci. China Chem. 2021;64:1614–1629. doi: 10.1007/s11426-021-1052-7. [DOI] [Google Scholar]
- 27.Zhang X., Liu Z., Yang X., Dong Y., Virelli M., Zanoni G., Anderson E.A., Bi X. Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications. Nat. Commun. 2019;10:284. doi: 10.1038/s41467-018-08253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning Y., Zhang X., Gai Y., Dong Y., Sivaguru P., Wang Y., Reddy B.R.P., Zanoni G., Bi X. Difluoroacetaldehyde N-triftosylhydrazone (DFHZ-Tfs) as a bench-stable crystalline diazo surrogate for diazoacetaldehyde and difluorodiazoethane. Angew. Chem. Int. Ed. Engl. 2020;59:6473–6481. doi: 10.1002/anie.202000119. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Tian C., Wang Z., Sivaguru P., Nolan S.P., Bi X. Fluoroalkyl N-triftosylhydrazones as easily decomposable diazo surrogates for asymmetric [2 + 1] cycloaddition: synthesis of chiral fluoroalkyl cyclopropenes and cyclopropanes. ACS Catal. 2021;11:8527–8537. doi: 10.1021/acscatal.1c01483. [DOI] [Google Scholar]
- 30.Zhang X., Zhang X., Song Q., Sivaguru P., Wang Z., Zanoni G., Bi X. A carbene strategy for progressive (Deutero)Hydrodefluorination of fluoroalkyl ketones. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202116190. [DOI] [PubMed] [Google Scholar]
- 31.Cindy Lee W.C., Wang D.-S., Zhang C., Xie J., Li B., Zhang X.P. Asymmetric radical cyclopropanation of dehydroaminocarboxylates: stereoselective synthesis of cyclopropyl α-amino acids. Chem. 2021;7:1588–1601. doi: 10.1016/j.chempr.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Wen X., Cui X., Wojtas L., Zhang X.P. Asymmetric radical cyclopropanation of alkenes with in situ-generated donor-substituted diazo reagents via Co (II)-Based metalloradical catalysis. J. Am. Chem. Soc. 2017;139:1049–1052. doi: 10.1021/jacs.6b11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Xie J., Lee W.C.C., Wang D.S., Zhang X.P. Radical differentiation of two ester groups in unsymmetrical diazomalonates for highly asymmetric olefin cyclopropanation. Chem Catal. 2022;2:330–344. doi: 10.1016/j.checat.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies H.M.L., Liao K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019;3:347–360. doi: 10.1038/s41570-019-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao K., Yang Y.-F., Li Y., Sanders J.N., Houk K.N., Musaev D.G., Davies H.M.L. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 2018;10:1048–1055. doi: 10.1038/s41557-018-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao K., Pickel T.C., Boyarskikh V., Bacsa J., Musaev D.G., Davies H.M.L. Site-selective and stereoselective functionalization of non-activated tertiary C–H bonds. Nature. 2017;551:609–613. doi: 10.1038/nature24641. [DOI] [PubMed] [Google Scholar]
- 37.Barrett A.G.M., Doubleday W.W., Tustin G.J., White A.J.P., Williams D.J. Approaches to the assembly of the antifungal agent FR-900848: studies on the asymmetric synthesis of bicyclopropanes and an X-ray crystallographic analysis of (4R,5R)-2-[(1R,3S,4S,6R)-6-phenyl-1-bicyclopropyl]-1,3-dimethyl-4,5-diphenylimidazolidine. J. Chem. Soc., Chem. Commun. 1994:1783–1784. doi: 10.1039/C39940001783. [DOI] [Google Scholar]
- 38.Barrett A.G.M., Kasdorf K., Williams D.J., White A.J.P., Williams D.J. Approaches to the assembly of the antifungal agent FR-900848: studies on double asymmetric cyclopropanation and an X-ray crystallographic study of (1R,2R)-1,2-bis-[(1S,2S)-2-methylcyclopropyl]-1,2-ethanediyl 3,5-dinitrobenzoate. J. Chem. Soc. Chem. Commun. 1994:1781–1782. doi: 10.1039/C39940001781. [DOI] [Google Scholar]
- 39.Kuo M.S., Zielinski R.J., Cialdella J.I., Marschke C.K., Dupuis M.J., Li G.P., Kloosterman D.A., Spilman C.H., Marshall V.P. Discovery, isolation, structure elucidation, and biosynthesis of U-106305, a cholesteryl ester transfer protein inhibitor from UC 11136. J. Am. Chem. Soc. 1995;117:10629–10634. doi: 10.1021/ja00148a004. [DOI] [Google Scholar]
- 40.Hsieh M.-T., Lee K.-H., Kuo S.-C., Lin H.-C. Lewis acid-mediated defluorinative [3+2] cycloaddition/aromatization cascade of 2,2-difluoroethanol systems with nitriles. Adv. Synth. Catal. 2018;360:1605–1610. doi: 10.1002/adsc.201701581. [DOI] [Google Scholar]
- 41.Wu Y., Cao S., Douair I., Maron L., Bi X. Computational insights into different mechanisms for Ag-Cu-and Pd-catalyzed cyclopropanation of alkenes and sulfonyl hydrazones. Chemistry. 2021;27:5999–6006. doi: 10.1002/chem.202005193. [DOI] [PubMed] [Google Scholar]
- 42.Gaussian 16 Revision C.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, et al., Gaussian, Inc. Wallingford CT, 2019.
- 43.Zhao Y., Truhlar D.G., Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. [Google Scholar]
- 44.Dolg M., Wedig U., Stoll H., Preuss H. Energy-adjustedabinitiopseudopotentials for the first row transition elements. J. Chem. Phys. 1987;86:866–872. [Google Scholar]
- 45.Andzelm J., Huzinaga S. Elsevier Science; 1984. Gaussian Basis Sets for Molecular Calculations. [Google Scholar]
- 46.Hehre W.J., Ditchfield R., Pople J.A. Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972;56:2257–2261. [Google Scholar]
- 47.Dill J.D., Pople J.A. Self-consistent molecular orbital methods. XV. Extended Gaussian-type basis sets for lithium, beryllium, and boron. J. Chem. Phys. 1975;62:2921–2923. [Google Scholar]
- 48.Fukui K. Formulation of the reaction coordinate. J. Phys. Chem. A. 1970;74:4161–4163. [Google Scholar]
- 49.Fukui K. The path of chemical reactions - the IRC approach. Acc. Chem. Res. 1981;14:363–368. [Google Scholar]
- 50.Legault C.Y. Université de Sherbrooke; 2009. CYLview, 1.0b.http://www.cylview.org [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All original crystal structures have been deposited at CCDC and are publicly available as of the date of publication. Compound 37: CCDC 2117854. Compound 44: CCDC 2117853.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper can be obtained from the lead contact upon request.