Abstract
Background
COVID-19 is associated with a dysregulated immune response but it is unclear how immune dysfunction contributes to the chronic morbidity persisting in many COVID-19 patients during convalescence (long COVID).
Methods
We assessed phenotypical and functional changes of monocytes in COVID-19 patients during hospitalisation and up to 9 months of convalescence following COVID-19, respiratory syncytial virus or influenza A. Patients with progressive fibrosing interstitial lung disease were included as a positive control for severe, ongoing lung injury.
Results
Monocyte alterations in acute COVID-19 patients included aberrant expression of leukocyte migration molecules, continuing into convalescence (n=142) and corresponding with specific symptoms of long COVID. Long COVID patients with unresolved lung injury, indicated by sustained shortness of breath and abnormal chest radiology, were defined by high monocyte expression of C-X-C motif chemokine receptor 6 (CXCR6) (p<0.0001) and adhesion molecule P-selectin glycoprotein ligand 1 (p<0.01), alongside preferential migration of monocytes towards the CXCR6 ligand C-X-C motif chemokine ligand 16 (CXCL16) (p<0.05), which is abundantly expressed in the lung. Monocyte CXCR6 and lung CXCL16 were heightened in patients with progressive fibrosing interstitial lung disease (p<0.001), confirming a role for the CXCR6–CXCL16 axis in ongoing lung injury. Conversely, monocytes from long COVID patients with ongoing fatigue exhibited a sustained reduction of the prostaglandin-generating enzyme cyclooxygenase 2 (p<0.01) and CXCR2 expression (p<0.05). These monocyte changes were not present in respiratory syncytial virus or influenza A convalescence.
Conclusions
Our data define unique monocyte signatures that define subgroups of long COVID patients, indicating a key role for monocyte migration in COVID-19 pathophysiology. Targeting these pathways may provide novel therapeutic opportunities in COVID-19 patients with persistent morbidity.
Short abstract
Immune dysfunction is a key factor in acute COVID-19 pathophysiology, with monocyte abnormalities sustained during convalescence for at least 9 months following hospital discharge and corresponding to specific long COVID symptoms https://bit.ly/3xEIY0H
Introduction
The COVID-19 pandemic has resulted in over 762 million infections and 6.8 million deaths worldwide, to date [1]. COVID-19 manifests with a wide range of symptoms, from mild influenza-like symptoms to life-threatening consequences of acute respiratory distress syndrome in severe disease [2]. There has been a significant improvement in our understanding of COVID-19 pathology from research efforts detailing changes in host immunity during SARS-CoV-2 infection, with key alterations in myeloid cells of the innate immune system corresponding to disease severity, incidence of intensive care admission and death [3–9].
Monocytes are released into the circulation from bone marrow and recruited to the lung during respiratory infections, in keeping with our previous work demonstrating heightened systemic levels of monocyte-chemoattractant protein 1 (MCP-1) in COVID-19 patients and enhanced Ki67 on monocytes suggestive of rapid release from bone marrow [3]. Other early studies during the first wave of COVID-19 demonstrated enhanced monocyte infiltrates in the lungs, kidney, heart, spleen and muscle from deceased COVID-19 patients [10–12], supporting a role for abnormal monocyte migration to peripheral tissues in COVID-19. Indeed, subsequent studies indicate severe COVID-19-associated variants are linked to chemokine receptor gene control in monocytes and macrophages [13]. A detailed understanding of the migratory properties of monocytes in COVID-19 and how they correspond to disease severity and intensive care admission would support the development of better, targeted clinical interventions.
There is now a wealth of evidence indicating that chronic morbidity persists in many COVID-19 patients during convalescence, with common long-lasting symptoms including extreme fatigue, shortness of breath, myalgia, brain fog, depression, fibrotic lung disease and pulmonary vascular disease (long COVID) [14–18]. Recent evidence demonstrates systemic inflammation is sustained in COVID-19 convalescence, with long COVID symptoms remaining at 1-year follow-up [17] and long-term activation of the innate immune system during convalescence even in patients with non-severe disease [19]. There are currently limited treatment options for long COVID because the development of targeted therapeutic strategies requires an in-depth understanding of the underlying immunological pathophysiology.
We sought to address whether monocyte migratory signatures corresponded to acute COVID-19 disease severity and whether any persistent changes in immune profiles in convalescence were associated with long COVID symptoms. We collected blood samples as part of the Coronavirus Immune Response and Clinical Outcomes (CIRCO) study [3] based at four hospitals in Greater Manchester, UK, and identified key monocyte migratory signatures in acute disease that persisted into convalescence up to 9 months after hospital discharge. Unique monocyte profiles distinguished long COVID patients with shortness of breath and unresolved lung injury from those with ongoing fatigue, and from asymptomatic patients. Our data reveal sustained monocyte dysfunction as a key feature of acute and long COVID.
Methods
Study design
Between July 2020 and January 2021, two cohorts of COVID-19 patients (acute and convalescent) were recruited from four hospitals across two trusts: Manchester University NHS Foundation Trust (MFT) and Salford Royal NHS Foundation Trust (SRFT). Samples were collected through Manchester Allergy, Respiratory and Thoracic Surgery (ManARTS) Biobank (study number M2020-88; MFT) or the Northern Care Alliance Research Collection (NCARC) tissue biobank (study number NCA-009; SRFT). Ethical approval was provided by the National Research Ethics Service (REC reference 15/NW/0409 for ManARTS; 18/WA/0368 for NCARC). Informed consent was provided by all study participants.
Patient inclusion and exclusion criteria were the same as in our previous study [3]; demographics and clinical information are provided in the supplementary material (supplementary figures S1 and S3). Peripheral blood samples were collected within 7 days of hospital admission for acute patients, and at follow-up appointments during outpatient clinics 3–9 months post discharge for convalescent patients. Healthy blood samples were obtained from frontline workers at the University of Manchester and MFT and were examined alongside patient samples. All healthy controls tested negative for anti-Spike 1 receptor binding domain antibodies (age range 35–71 years, median age 50.9 years, 52% male).
Quantitative computed tomography analysis
Computed tomography (CT) thorax scan images from symptomatic convalescent COVID-19 patients (breathless and/or fatigued) taken at follow-up appointments at MFT (ManARTS study number M2022-119) were analysed using Lung Density Analysis and Lung Texture Analysis programs (Imbio Inc., Minneapolis, MN, USA) to generate quantitative CT reports. This generated quantitative scan reports as shown in supplementary figure S5. Further information on this technology can be found in the Imbio LDA+ user manual version 4.1.0 and the lung texture analysis user manual version 2.2.0 (https://www.imbio.com/support-documentation/).
Isolation of peripheral blood mononuclear cells and serum
Peripheral blood mononuclear cells (PBMCs) and serum from whole blood samples were isolated as previously described [3]. PBMCs were stained for flow cytometry, set up for microbial stimulation assays, or stored in 10% DMSO in fetal bovine serum (FBS) at −150°C.
Cell culture
Fresh PBMCs were stimulated with 100 ng·mL−1 lipopolysaccharide (LPS) (Sigma) in the presence of Protein Transport Inhibitor Cocktail (eBioscience) in RPMI containing 10% FBS, L-glutamine, nonessential amino acids, HEPES, streptomycin and penicillin (complete media) for 3 h at 37°C. Following stimulation, cells were washed and stained for flow cytometric analysis to determine intracellular levels of the enzyme cyclooxygenase 2 (COX-2) and the cytokines tumour necrosis factor α (TNF-α) and interleukin 1β (IL-1β).
Transwell migration assay
Frozen PBMCs from healthy controls and from breathless and non-breathless convalescent COVID-19 patients were thawed, washed and resuspended in complete media. PBMCs were incubated at 37°C for 18 h. PBMCs (2×105 cells) were then plated in the upper wells of a 98-well cell migration/chemotaxis plate with Boyden chamber (5 µm membrane; Abcam). The lower wells contained C-X-C motif chemokine ligand 16 (CXCL16) (0.1, 0.5 or 1 µg·mL−1) or media only (negative control). PBMCs were left to migrate for 4 h at 37°C. Migrated cells were counted manually using a haemocytometer.
Flow cytometry
For surface stains (migration markers), samples were fixed with BD Cytofix (BD Biosciences) following antibody staining, prior to acquisition. For intracellular stains (TNF-α, IL-1β, COX-2), the FoxP3/Transcription Factor Staining Buffer Set (eBioscience) was used. Antibodies used are listed in supplementary table S1. All samples were acquired on an LSRFortessa flow cytometer (BD Biosciences) and analysed using FlowJo (Tree Star).
Luminex assay
Leukocyte migration markers were measured in serum using Luminex assays (R&D Systems) according to manufacturer's instructions.
Statistics
Normality tests were performed on all datasets, and statistical analyses were carried out using Prism 9 Software (Graphpad Software Inc.). Information on statistical tests used is detailed in figure legends. In all cases, a p-value of ≤0.05 was considered significant. Where no statistical difference is shown on graphs, there were no significant differences.
Results
Clinical characteristics during acute COVID-19
Between 6 July and 5 November 2020, 71 patients with acute disease were recruited during their inpatient stay for COVID-19 within 7 days of admission. All patients were stratified for maximum disease severity based on supplemental oxygen requirements (<28% inspiratory oxygen fraction (FiO2) for mild disease, 28–60% FiO2 for moderate disease and >60% FiO2 or noninvasive/mechanical ventilation for severe disease). Additionally, the use of acute noninvasive ventilation in non-chronic obstructive pulmonary disease (COPD) patients, invasive ventilation and admission to intensive care automatically led to a classification of severe disease. The most frequent comorbidities were hypertension, ischaemic heart disease, diabetes, asthma and other chronic pulmonary disease, although only diabetes correlated with disease severity (55% of patients with severe COVID-19 were diabetic). 45% of severe cases of COVID-19 resulted in death within 30 days, with 30-day mortality, C-reactive protein and length of hospital stay all positively associated with COVID-19 disease severity (supplementary figure S1).
Abnormal migratory signature in monocytes during acute COVID-19 defines disease severity
Following recruitment to sites of inflammation, monocytes contribute significantly to inflammatory disease directly or via differentiation into macrophages or dendritic cells in peripheral tissues [20, 21]. Monocyte-derived macrophages predominate in the bronchoalveolar lavage (BAL) of patients with severe COVID-19 [8], contributing to the inflammatory environment. We examined circulating monocytes for altered expression of a range of molecules involved in leukocyte migration, and for function based on their capacity to produce inflammatory mediators (summarised in supplementary figure S2a).
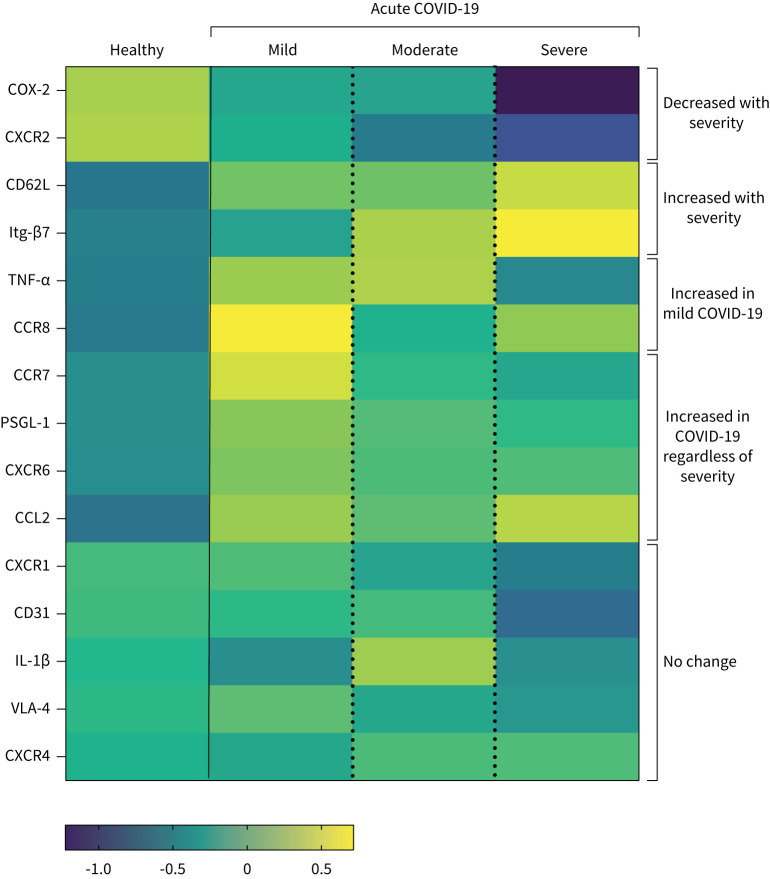
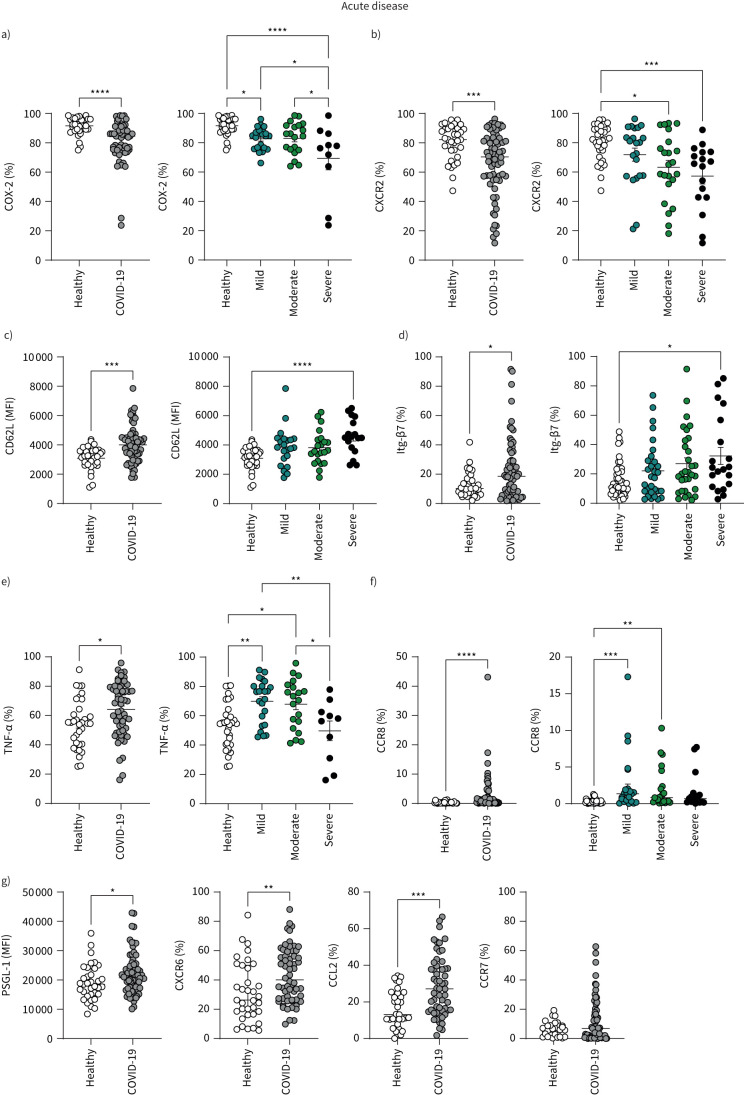
By combining these differential analyses, we generated an overview of severity-specific monocyte signatures (figure 1). Severe COVID-19 was characterised by reduced proportions of CD14+ classical monocytes (gating strategy: supplementary figure S2b) expressing the prostaglandin-generating enzyme COX-2 and the chemokine receptor C-X-C motif chemokine receptor 2 (CXCR2) (figures 1 and 2a, b), alongside enhanced expression of adhesion molecule CD62L (figures 1 and 2c) and the gut-homing integrin β7 (Itg-β7) (figures 1 and 2d). Patients with mild disease and a quicker recovery from COVID-19 displayed a distinctive signature and were characterised by heightened production of TNF-α (figures 1 and 2e) and enhanced expression of chemokine receptor C-C motif chemokine receptor 8 (CCR8) on monocytes (figures 1 and 2f). Chemokine receptor CXCR6, adhesion molecule P-selectin glycoprotein ligand-1 (PSGL-1) and chemokine C-C motif chemokine ligand 2 (CCL2) on monocytes were aberrantly increased in COVID-19 patients overall but did not stratify with disease severity (figures 1 and 2g, supplementary figure S2c).
FIGURE 1.
Summary of distinct monocyte profiles in patients with acute COVID-19. Heatmap of indicated immune parameters by row. Each column represents average (mean) Z-scores for each parameter calculated from individual values of expression by monocytes from healthy individuals or patients with mild, moderate or severe COVID-19 as separate groups. Heatmap was generated as a visual guide, using a subset of individuals from each group to include all individuals per group with results for every immune parameter analysed (i.e. individuals where samples were used for surface staining for all migration molecules, and microbial stimulation assays for cytokine and COX-2 readouts). Healthy individuals, n=25; mild COVID-19, n=13; moderate COVID-19, n=14; severe COVID-19, n=7.
FIGURE 2.
Monocyte profiles of patients with acute COVID-19. Summary graphs showing data from healthy individuals and all COVID-19 patients. Patients with COVID-19 were also stratified into mild, moderate and severe disease groups. a) Frequencies of CD14+ monocytes expressing COX-2 following lipopolysaccharide (LPS) stimulation, from healthy individuals (n=32) and all patients with acute COVID-19 (n=56). Disease stratification: mild (n=23), moderate (n=20) and severe (n=10). b) Frequencies of CD14+ monocytes expressing CXCR2 from healthy individuals (n=34) and all patients with acute COVID-19 (n=66). Disease stratification: mild (n=22), moderate (n=23) and severe (n=17). c) CD14+ monocyte L-selectin (CD62L) expression levels as determined by mean fluorescence intensity (MFI) in healthy individuals (n=33) and all patients with acute COVID-19 (n=65). Disease stratification: mild (n=22), moderate (n=22) and severe (n=17). d) Frequencies of CD14+ monocytes expressing integrin β7 (Itg-β7) from healthy individuals (n=35) and all patients with acute COVID-19 (n=67). Disease stratification: mild (n=23), moderate (n=23) and severe (n=16). e) Frequencies of CD14+ monocytes producing TNF-α following LPS stimulation, from healthy individuals (n=34) and all patients with acute COVID-19 (n=55). Disease stratification: mild (n=23), moderate (n=20) and severe (n=10). f) Frequencies of CD14+ monocytes expressing CCR8 from healthy individuals (n=33) and all patients with acute COVID-19 (n=66). Disease stratification: mild (n=22), moderate (n=23) and severe (n=17). g) CD14+ monocyte expression levels of PSGL-1 and frequencies of monocytes expressing CXCR6, CCL2 and CCR7 in healthy individuals (PSGL-1: n=35; CXCR6: n=35; CCL2: n=33; CCR7: n=31) and all patients with acute COVID-19 (PSGL-1: n=66; CXCR6: n=60; CCL2: n=57; CCR7: n=66). Graphs show individual patient data with bar representing mean±sem (a, c, e) or median±interquartile range (b, d, f, g). Comparison of groups was carried out using unpaired t-test (a, c, e: healthy versus COVID-19), Mann–Whitney test (b, d, f, g: healthy versus COVID-19), one-way ANOVA with Holm–Sidak post hoc test (a, c, e: COVID-19 severity) or Kruskal–Wallis with Dunn's post hoc test (b, d, f: COVID-19 severity). *: p≤0.05; **: p≤0.01; ***: p≤0.001; ****: p≤0.0001.
There was a trend towards increased proportions of monocytes expressing the chemokine receptor associated with lymph node homing, CCR7, in acute COVID-19 but this did not reach statistical significance (figure 2g). There was no change in monocyte expression of chemokine receptor CXCR1 or integrin very late antigen-4 (VLA-4) (used for adhesion to activated endothelium [22, 23]) between COVID-19 patients and healthy controls (supplementary figure S2d). Levels of CD31 (used for migration through the endothelium [24]) and CXCR4 were assessed rather than proportion of monocytes due to 100% of circulating monocytes expressing these molecules; there were no differences between COVID-19 patients and healthy controls. There was also no difference in monocyte production of IL-1β (supplementary figure S2d).
These data indicate abnormal monocyte migration as a key factor in COVID-19 pathophysiology.
Defined soluble mediators involved in leukocyte migration are dysregulated in acute COVID-19
We, and others, have previously observed aberrant expression of soluble inflammatory mediators in serum from patients with acute COVID-19, in particular cytokines IL-6 and IL-10 and chemokines MCP-1 and CXCL10, which were dominant in severe COVID-19 [2, 3, 25–27]. Here, we extended our analyses of serum proteins using a Luminex bead array targeted for leukocyte migration.
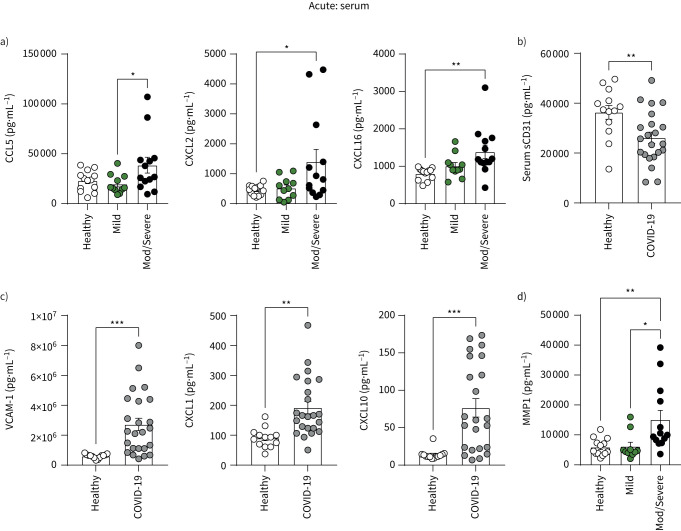
Serum levels of chemokines CCL5 (recruits leukocytes to sites of inflammation), CXCL2 (myeloid cell chemoattractant) and CXCL16 (binding partner for CXCR6 that is increased on monocytes in COVID-19 in figure 2g) were increased in patients with acute moderate/severe COVID-19 (figure 3a). Soluble CD31, which inhibits CD31-mediated transendothelial migration [28], was reduced in COVID-19 patients, further supporting a role for heightened leukocyte migration into peripheral tissues in COVID-19 (figure 3b). Adhesion molecule vascular adhesion molecule 1 (VCAM-1) and chemokines CXCL1 and CXCL10 were also elevated (figure 3c) but did not stratify with disease severity (data not shown). Serum levels of matrix metalloprotease 1 (MMP-1), which mediates interactions between monocytes and vascular endothelial cells [29], were also heightened in patients with moderate/severe COVID-19 (figure 3d).
FIGURE 3.
Serum profiles of patients with acute COVID-19. Levels of systemic a) CCL5, CXCL2, CXCL16; b) soluble CD31 (sCD31); c) VCAM-1, CXCL1, CXCL10; and d) MMP-1 were measured in the serum from healthy individuals (n=13) and COVID-19 patients (n=24) using Luminex assays. a, d) Patients with COVID-19 were also stratified into mild (n=11) and moderate/severe disease (n=13). Graphs show individual patient data with bars representing mean±sem. Comparison of groups was carried out using unpaired t-test (b, c: healthy versus COVID-19) or one-way ANOVA with Holm–Sidak post hoc test (a, d: COVID-19 severity). *: p≤0.05; **: p≤0.01; ***: p≤0.001.
These data further support a role for abnormal leukocyte migration into peripheral tissues in COVID-19, suggesting abnormal interactions of monocytes with blood vessel endothelial cells.
Clinical characteristics during COVID-19 convalescence
Alongside recruitment of patients with acute COVID-19, between 14 July and 3 December 2020, 142 patients attending outpatient follow-up after inpatient admission for COVID-19 were recruited as convalescent COVID-19 patients (follow-up mean: 151 days; range: 63–246 days after discharge). At outpatient review patients were asked whether they were experiencing breathlessness or fatigue, and whether this was new since SARS-CoV-2 infection. If the symptom was pre-existing, patients were asked if it was better, stable or worse since SARS-CoV-2 (quantified using Medical Research Council dyspnoea scale for breathlessness). Further clinical tests, e.g. echocardiograms, sleep studies and blood panels, were performed by reviewing specialist respiratory physicians where indicated for shortness of breath and fatigue to exclude alternate pathologies. If on review patient symptoms were felt to be attributable to other pathologies, they were excluded from the analysis.
At outpatient follow-up, 31% of patients continued to display abnormalities on chest radiology attributed to COVID-19 (supplementary figure S3). Shortness of breath was the most common symptom of long COVID and indicates unresolved lung injury (48% of all convalescent patients; supplementary figure S3). Here, we used the combination of abnormal chest radiology with shortness of breath to confirm unresolved lung injury, and indeed there was a significant association between these two parameters (supplementary figure S3). Fatigue was also a predominant symptom of long COVID (44% of all convalescent patients; supplementary figure S3). Critically, shortness of breath and fatigue in convalescence did not correspond to initial disease severity during acute admission; thus, in this cohort, COVID-19 severity did not explain persisting symptoms during convalescence. This information is important to consider in light of long COVID remaining a major public health issue worldwide at the time of publication, despite the emergence of milder strains of SARS-CoV-2 and vaccination leading to a reduced risk of severe disease and hospitalisation.
There was a general decrease in all lung function parameters measured in convalescent COVID-19 patients overall, compared with predicted values [30] of the healthy population (supplementary figure S4). Reductions in forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1) and diffusion capacity of the lungs for carbon monoxide (DLCO) compared to healthy populations (% predicted) are expected due to comorbidities associated with hospitalisation for COVID-19. For example, high body mass index may reduce lung volumes (FVC) and asthma may affect air flow (FEV1), whilst COPD and emphysema can affect the alveolar capillary membrane and alveolar volume (DLCO).
Breathless convalescent patients had lower FEV1 than non-breathless convalescent patients, which may indicate patients within this group were experiencing greater airflow obstruction secondary to inflammation after SARS-CoV-2 infection (supplementary figure S4). The small reduction in DLCO in all convalescent COVID-19 patients indicates a reduced capacity to transport oxygen into the bloodstream from the lungs. There was a strong trend towards a greater reduction in DLCO (% predicted) in patients with persisting abnormal radiology at follow-up (supplementary figure S4), which may have been due to the contribution of persisting lung injury. This study was not sufficiently powered to explore the relationship between lung function measures. It is therefore unknown whether FEV1 and DLCO findings represent a single or separate mechanisms of breathlessness. The high prevalence of radiological abnormalities in breathless (44.4%) and fatigued (38.2%) compared to asymptomatic (25.2%) patients (supplementary figure S3) is also suggestive of ongoing lung damage or incomplete repair contributing to long COVID. Indeed, recent evidence indicates inflammatory mediators of tissue damage and repair in plasma are associated with the severity of long COVID symptoms [17].
Other studies have indicated enhanced detection of lung abnormalities in COVID-19 using non-standard imaging techniques, e.g. hyperpolarised xenon-129 magnetic resonance imaging [31]. To assess whether we may have missed any ongoing lung abnormalities using conventional radiology approaches, we carried out quantitative CT analysis using Lung Density Analysis and Lung Texture Analysis programs (Imbio Inc.) to generate quantitative CT reports of the lungs of a subset of symptomatic convalescent COVID-19 patients who displayed ongoing symptoms of breathlessness and/or fatigue at follow-up (supplementary figure S5). CT scans were generated and assessed in sagittal, coronal and transverse planes for satisfactory scan quality prior to segmentation and mapping of the lungs (supplementary figure S5a). This automated computer analysis of lung texture provided detailed information regarding different types of lung damage such as hyperlucency, ground glass changes, reticulations and honeycombing (supplementary figure S5b). This analysis also provided detailed information regarding lung damage in the context of lung density analysis, with high density representing inflammation and ground glass changes and very high density representing dense areas of inflammation or infection (supplementary figure S5c).
Whilst this approach is previously untested in COVID-19 convalescence, it has predictive value for severity and outcome in acute COVID-19 [32]. Only 62.5% of our COVID-19 convalescent patients exhibited abnormal radiological findings using standard testing (supplementary figure S6a), but quantitative CT analysis detected lung abnormalities in all of these assessed symptomatic patients, with the most common abnormalities being ground glass changes and reticulations (supplementary figure S6b), which are commonly associated with inflammatory processes. These results indicate that ongoing lung damage may be occurring in the majority of symptomatic COVID-19 patients during convalescence. We next assessed whether innate immune dysfunction corresponded to ongoing lung damage and symptoms of long COVID.
Abnormal monocyte migratory profile persists in COVID-19 convalescence and corresponds to symptoms of long COVID
The mechanisms by which monocytes may be involved in exacerbation versus resolution of lung injury are unclear, despite the key role of monocytes in the development of pulmonary fibrosis arising from failure of lung injury resolution [33]. Indeed, pro-fibrotic monocyte-derived macrophages accumulate in the lungs of patients with severe COVID-19 with the same hallmarks of those in patients with idiopathic pulmonary fibrosis [34]. Here, we focused on shortness of breath alongside abnormal chest radiology indicative of unresolved lung injury and investigated whether sustained monocyte dysregulation corresponds to resolution of lung injury and long COVID.
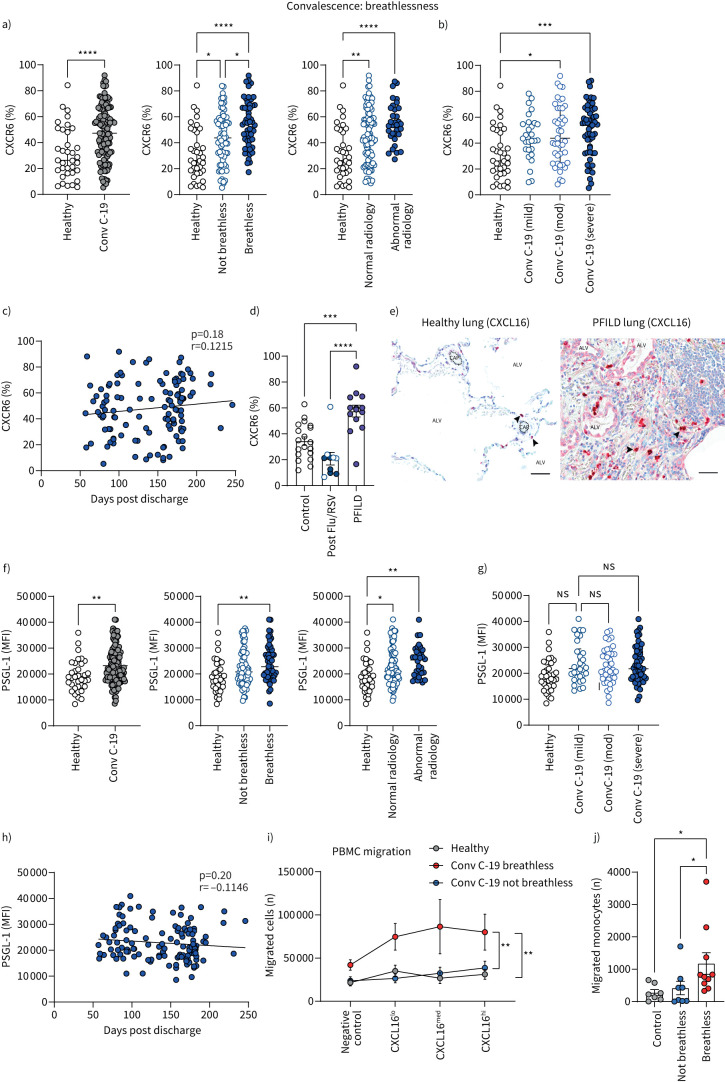
Having shown that monocyte expression of chemokine receptor CXCR6 and adhesion molecule PSGL-1 were increased in acute COVID-19, we show here that these changes are sustained into convalescence, in particular in patients with ongoing symptoms of breathlessness. Furthermore, expression of these molecules was also highest in patients with abnormal chest radiology (assessed by conventional methods; figure 4a, f). Thus, increased CXCR6 and PSGL-1 on monocytes in breathless convalescent patients may be due to active monocyte recruitment in response to ongoing inflammation and injury in the lung. Indeed, CXCR6 directs migration towards chemokine CXCL16, which is expressed in the lung [35], with PSGL-1 mediating tethering and rolling on activated endothelium to facilitate migration into tissues from the bloodstream [36].
FIGURE 4.
Monocyte migratory profiles of patients with long COVID with unresolved lung injury. a) Frequencies of CD14+ monocytes expressing CXCR6 from healthy individuals (n=35) and all convalescent COVID-19 patients (Conv C-19) (n=127). Patients with convalescent COVID-19 were also stratified into breathless (n=52), not breathless (n=71), normal radiology (n=91) and abnormal radiology (n=33). b) Patients with convalescent COVID-19 were also stratified into mild (n=28), moderate (n=43) or severe (n=56) disease referring to their acute COVID-19 severity during previous hospital admission. c) Correlation of CXCR6 (percentage of monocytes expressing CXCR6) with number of days since hospital discharge in all convalescent COVID-19 patients (n=122). d) Frequencies of CD14+ monocytes expressing CXCR6 in a different cohort of healthy individuals (n=19), all convalescent influenza A (flu)/respiratory syncytial virus (RSV) patients (n=10) and all progressive fibrosing interstitial lung disease (PFILD) patients (n=14). Patients with convalescent flu/RSV were stratified into breathless (n=5, filled circles) and not breathless (n=5, open circles) within the same group. e) Human lung tissue sections. Healthy human lung (left): alveoli can be seen with occasional CXCL16-expressing immune cells (black arrows) proximal to capillaries (dashed lines). Right shows human idiopathic pulmonary fibrosis lung, with extracellular matrix replacing normal lung architecture. CXCL16-expressing immune cells are present within blood vessels (black arrows). CXCL16 is also seen in the epithelium of damaged alveoli and stromal cells. Counterstained with toluidine blue. Scale bars: 50 µm. ALV: alveoli; CAP: capillary. f) CD14+ monocyte expression level of PSGL-1 as determined by mean fluorescence intensity (MFI) from healthy individuals (n=35) and all convalescent COVID-19 patients (n=130). Patients with convalescent COVID-19 were also stratified into breathless (n=54), not breathless (n=74), normal radiology (n=95) and abnormal radiology (n=32). g) Patients with convalescent COVID-19 were also stratified into mild (n=30), moderate (n=43) or severe (n=57) disease, referring to their acute COVID-19 severity during previous hospital admission. h) Correlation of PSGL-1 (levels of expression as determined by MFI) with number of days since hospital discharge in all convalescent COVID-19 patients (n=130). i) Numbers of migrated peripheral blood mononuclear cells (PBMCs) as counted in the bottom of a Boyden chamber in media only (negative control), 0.1 µg·mL−1 CXCL16 (CXCL16lo), 0.5 µg·mL−1 CXCL16 (CXCL16med) and 1 µg·mL−1 CXCL16 (CXCL16hi) following 4 h incubation, starting with 2×105 cells in the top chamber in all cases (healthy controls: n=10; breathless convalescent COVID-19: n=10; not breathless convalescent COVID-19: n=10). Graph shows combined patient data with mean±sem of each group, under each condition. j) Numbers of migrated monocytes identified by flow cytometry as CD45+ live CD19−CD3−CD66b−HLA-DR+CD64+CD14+ from migrated cells in the bottom of a Boyden chamber in CXCL16hi conditions (1 µg·mL−1 CXCL16) (healthy controls: n=7; breathless convalescent COVID-19: n=10; not breathless convalescent COVID-19: n=8). All graphs other than c and i show individual patient data with bars representing median±interquartile range. Comparison of groups was carried out using Mann–Whitney test (a, f: healthy versus convalescent COVID-19), Kruskal–Wallis with Dunn's post hoc test (a, b, d, f, g, j), Spearman's rank correlation coefficient test (c, h) or one-way ANOVA with repeated measures and Holm–Sidak post hoc test (i). *: p≤0.05; **: p≤0.01; ***: p≤0.001; ****: p≤0.0001.
CXCR6 expression on monocytes in COVID-19 convalescence remained high, in particular in patients who had severe COVID-19 during acute admission (figure 4b), but initial acute disease severity had no impact on PSGL-1 expression in COVID-19 convalescence (figure 4g). Linear regression analysis comparing CXCR6 and PSGL-1 expression against time (days post hospital discharge) revealed that convalescent COVID-19 patients showed no reduction of monocyte CXCR6 expression back to healthy control levels during the maximum time studied post hospital discharge (9 months; figure 4c). However, there was a trend towards a very gradual decline in PSGL-1 levels over time (figure 4h).
To determine whether enhanced monocyte CXCR6 expression featured in convalescence following hospitalisation with other respiratory viruses, we assessed CXCR6 expression on monocytes isolated from patients at outpatient follow-up after inpatient admission with respiratory syncytial virus (RSV) or influenza A (flu) with comparable mean follow-up times (COVID-19: 151 days; RSV/flu: 155 days). All RSV and flu patients had pneumonitis upon hospital admission and five of 10 (50%) remained breathless at follow-up (supplementary figure S7, showing clinical characteristics and lung function). Compared to age-matched controls, there were no significant differences in post-RSV/flu patients for monocyte expression of CXCR6, nor were there indications of heightened CXCR6 in those who remained short of breath at follow-up (figure 4d). As a positive control for unresolved lung injury, we assessed CXCR6 expression on monocytes from patients with progressive fibrosing interstitial lung disease (PFILD) who had severe ongoing lung inflammation and injury (supplementary figure S7). Monocytes from PFILD patients expressed heightened levels of CXCR6 (figure 4d), akin to convalescent COVID-19 patients with unresolved lung injury. However, PSGL-1 levels on monocytes were unchanged in post flu/RSV patients and PFILD patients (supplementary figure S8a).
To further interrogate the CXCR6–CXCL16 axis during ongoing lung injury, we characterised expression of the CXCR6 ligand CXCL16 on lung tissue sections from PFILD patients. Alongside clear evidence of structural damage and disorder, CXCL16 levels were visibly heightened in PFILD sections compared to healthy lung tissue, with expression localised to PFILD-damaged alveolar epithelial cells and infiltrating immune cells (figure 4e). Thus, heightened monocyte CXCR6 is a feature of ongoing lung injury with clear evidence for dysregulated CXCR6–CXCL16 signalling. Nonetheless, the increased monocyte CXCR6 expression in the context of respiratory infections was only detected in COVID-19 patients.
Next, we ascertained whether the increased expression of CXCR6 on monocytes of convalescent COVID-19 patients with unresolved lung injury was functionally relevant to leukocyte migratory capacity. To do so, we performed a transwell migration assay to reveal the migratory potential of PBMCs towards CXCR6 ligand CXCL16 (expressed at high levels in the lung). PBMCs from convalescent breathless COVID-19 patients demonstrated an enhanced capacity to migrate towards CXCL16 compared to their non-breathless convalescent counterparts and healthy controls (figure 4i). Upon flow cytometric analysis of the cellular components within the migrated cells from the highest CXCL16 concentration, there was heightened monocyte migration from convalescent COVID-19 patients who were breathless (figure 4j).
These data strongly implicate abnormal monocyte migration in long COVID pathology, in particular in those with unresolved lung injury and the most common symptom of long COVID, i.e. shortness of breath.
Defined soluble mediators involved in leukocyte migration are dysregulated in convalescent COVID-19
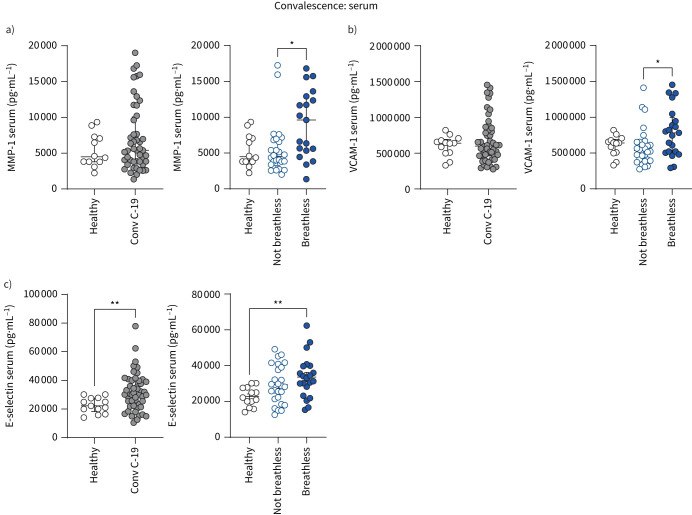
Having shown that several soluble inflammatory mediators were increased in the serum of patients with acute COVID-19, we now show that two of these serum proteins, MMP-1 (which is involved in monocyte–endothelial cell interactions) and adhesion molecule VCAM-1, remain elevated in COVID-19 convalescent patients with breathlessness (figure 5a, b). Furthermore, having demonstrated that PSGL-1 is increased on monocytes in breathless convalescent patients with unresolved lung injury (figure 4d), we found that serum levels of the PSGL-1 binding partner E-selectin, which binds PSGL-1 to facilitate leukocyte–endothelial interactions, were enhanced in breathless COVID-19 convalescent patients (figure 5c).
FIGURE 5.
Serum profiles of patients with long COVID with unresolved lung injury. Levels of systemic a) MMP-1, b) VCAM-1 and c) E-selectin were measured in the serum from healthy individuals (n=12) and convalescent COVID-19 (Conv C-19) patients (n=48) using Luminex assays. Patients with convalescent COVID-19 were also stratified into breathless (MMP-1: n=19; VCAM-1: n=21; E-selectin: n=21) and not breathless (MMP-1: n=26; VCAM-1: n=28; E-selectin: n=26). Graphs show individual patient data with bars representing mean±sem (c) or median±interquartile range (a, b). Comparison of groups was carried out using unpaired t-test (c: healthy versus convalescent COVID-19), Mann–Whitney test (a, b: healthy versus convalescent COVID-19), one-way ANOVA with Holm–Sidak post hoc test (c: breathlessness) or Kruskal–Wallis with Dunn's post hoc test (a, b: breathlessness). *: p≤0.05; **: p≤0.01.
These data reveal that the dysregulated serum levels of specific mediators involved in leukocyte migration in acute COVID-19 remain elevated during COVID-19 convalescence and highlight their role in long COVID, in particular in shortness of breath. Together, these data so far suggest that targeting leukocyte migration and monocyte–endothelial interactions may be of therapeutic value in long COVID.
TNF-α production by monocytes persists in COVID-19 convalescence and corresponds to resolved lung injury
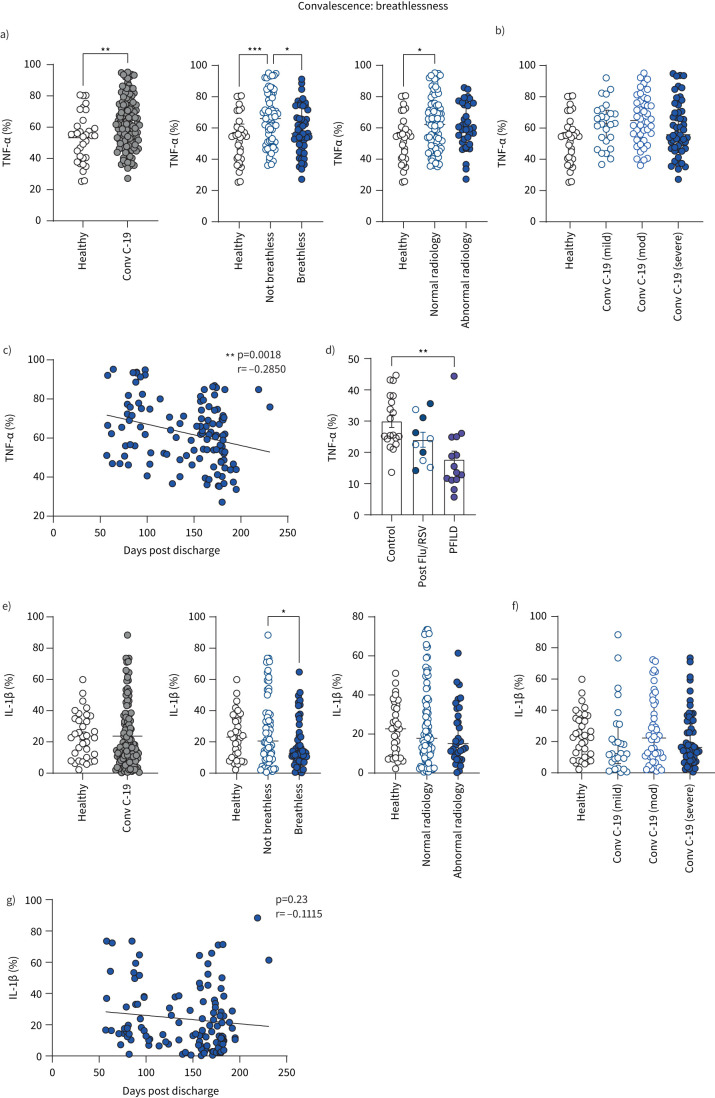
We have demonstrated in previous studies during the first wave of COVID-19 that heightened TNF-α production is prominent during hospitalisation in patients with mild COVID-19 who recovered quickly [2]. Having replicated this finding in this study (samples taken during the second wave of COVID-19 in the UK), we now demonstrate that heightened monocyte production of TNF-α is sustained into COVID-19 convalescence, and again corresponds to good outcome in terms of lung injury. Convalescent COVID-19 patients continued to produce aberrantly high levels of cytokine TNF-α, in particular those patients with no symptoms of shortness of breath (figure 6a). In keeping with increased TNF-α production in patients with resolved lung injury, TNF-α was significantly increased in patients with normal chest radiology during COVID-19 convalescence (figure 6a). TNF-α production in monocytes during convalescence did not correlate with initial disease severity during acute admission (figure 6b), and significantly decreased over time, indicative of a return back to healthy control levels (figure 6c).
FIGURE 6.
Monocyte cytokine profiles of patients with long COVID-19 with unresolved lung injury. a) Frequencies of CD14+ monocytes producing TNF-α from healthy individuals (n=33) and all convalescent (Conv C-19) COVID-19 patients (n=122). Patients with convalescent COVID-19 were also stratified into breathless (n=49), not breathless (n=67), normal radiology (n=87) and abnormal radiology (n=35). b) Patients with convalescent COVID-19 were also stratified into mild (n=26), moderate (n=44) or severe disease (n=52), referring to their acute COVID-19 severity during previous hospital admission. c) Correlation of TNF-α (percentage of monocytes producing TNF-α) with number of days since hospital discharge in all convalescent COVID-19 patients (n=117). d) Frequencies of CD14+ monocytes producing TNF-α in a different cohort of healthy individuals (n=19), all convalescent influenza A (flu)/respiratory syncytial virus (RSV) patients (n=10) and all progressive fibrosing interstitial lung disease (PFILD) patients (n=14). Patients with convalescent flu/RSV were stratified into breathless (n=5, filled circles) and not breathless (n=5, open circles) within the same group. e) Frequencies of CD14+ monocytes producing IL-1β from healthy individuals (n=34) and all convalescent COVID-19 patients (n=122). Patients with convalescent COVID-19 were also stratified into breathless (n=49), not breathless (n=68), normal radiology (n=87) and abnormal radiology (n=34). f) Patients with convalescent COVID-19 were also stratified into mild (n=26), moderate (n=44) or severe (n=52) disease, referring to their acute COVID-19 severity during previous hospital admission. g) Correlation of IL-1β (percentage of monocytes producing IL-1β) with number of days since hospital discharge in all convalescent COVID-19 patients (n=118). All graphs show individual patient data with bars representing mean±sem (a, b) or median±interquartile range (e, f). Comparison of groups was carried out using unpaired t-test (a: healthy versus convalescent COVID-19), Mann–Whitney test (e: healthy versus convalescent COVID-19), one-way ANOVA with Holm–Sidak post hoc test (a, b), Kruskal–Wallis with Dunn's post hoc test (d, e, f), Pearson correlation coefficient test (c) or Spearman's rank correlation coefficient test (g). *: p≤0.05; **: p≤0.01; ***: p≤0.001.
To determine whether altered TNF-α production by monocytes featured in convalescence following hospitalisation with other respiratory viruses, we assessed TNF-α production by monocytes isolated from convalescent flu and RSV patients. There were no significant differences between controls and post-RSV/flu patients in monocyte production of TNF-α (figure 6d). To determine whether differences in TNF-α levels were a general feature of resolved/unresolved lung injury, we assessed TNF-α production from monocytes in PFILD patients with severe ongoing lung inflammation and injury. In keeping with a role for TNF-α in resolving lung injury, monocytes from PFILD patients produced significantly lower levels of TNF-α (figure 6d).
The capacity of monocytes to produce cytokine IL-1β was also significantly higher in non-breathless compared to breathless patients during COVID-19 convalescence, although there was no change compared to healthy controls and no associations between IL-1β production and chest radiology findings (figure 6e). There was no correlation between IL-1β production from monocytes and acute disease severity (figure 6f) and there was a gradual decrease of IL-1β production from monocytes in COVID-19 convalescence over time (figure 6g).
These data indicate that the capacity of monocytes to produce the cytokine TNF-α correspond to good outcome in terms of resolved lung injury (normal chest radiology and no shortness of breath). This information highlights the importance of considering cytokines individually for their use in anti-cytokine-targeted therapies in COVID-19, because not all cytokines traditionally associated with inflammation may be contributing to pathology.
Distinct monocyte signatures during COVID-19 convalescence differentiate long COVID patients with chronic fatigue from those with unresolved lung injury
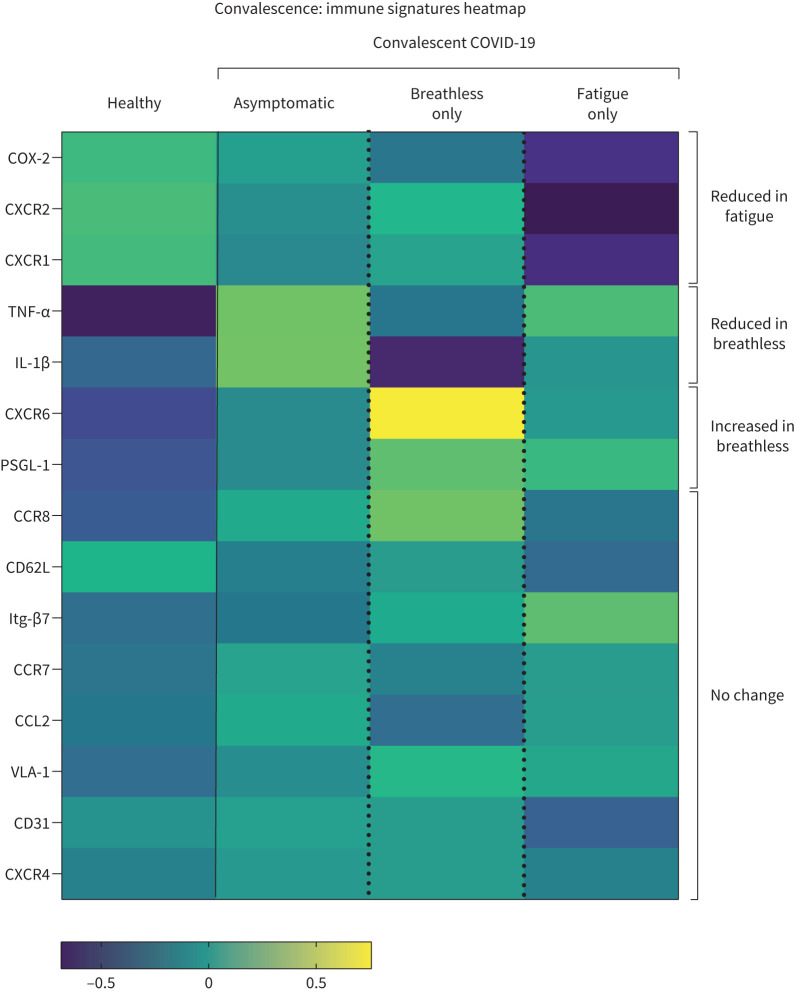
Shortness of breath and fatigue are the most commonly reported symptoms of long COVID, with two distinct clusters of long COVID patients showing respiratory symptoms in one cluster and fatigue in the other [37]. We show here that convalescent COVID-19 patients with ongoing fatigue were distinct from those with shortness of breath, displaying a different immune signature. To define a fatigue-specific immune signature, we removed long COVID patients with overlapping symptoms of both shortness of breath and fatigue from these analyses. Thus, we compared asymptomatic patients with long COVID patients experiencing shortness of breath only (no fatigue) and with patients with ongoing COVID-19-induced fatigue (no shortness of breath) to generate an overview of monocyte signatures corresponding to specific features of long COVID (figure 7).
FIGURE 7.
Summary of distinct monocyte profiles in subsets of patients with long COVID. Heatmap of indicated immune parameters by row. Each column represents average (mean) Z-scores for each parameter calculated from individual values of expression by monocytes from healthy individuals or convalescent COVID-19 patients without breathlessness or fatigue (asymptomatic), with breathlessness but not fatigue (breathless) and with fatigue but not breathlessness (fatigue) as separate groups. Heatmap was generated as a visual guide, using a subset of individuals from each group to include all individuals per group with results for every immune parameter analysed (i.e. individuals where samples were used for surface staining for all migration molecules, and microbial stimulation assays for cytokine and COX-2 readouts). Healthy individuals (n=25), asymptomatic (n=29), breathless (n=13), fatigue (n=16).
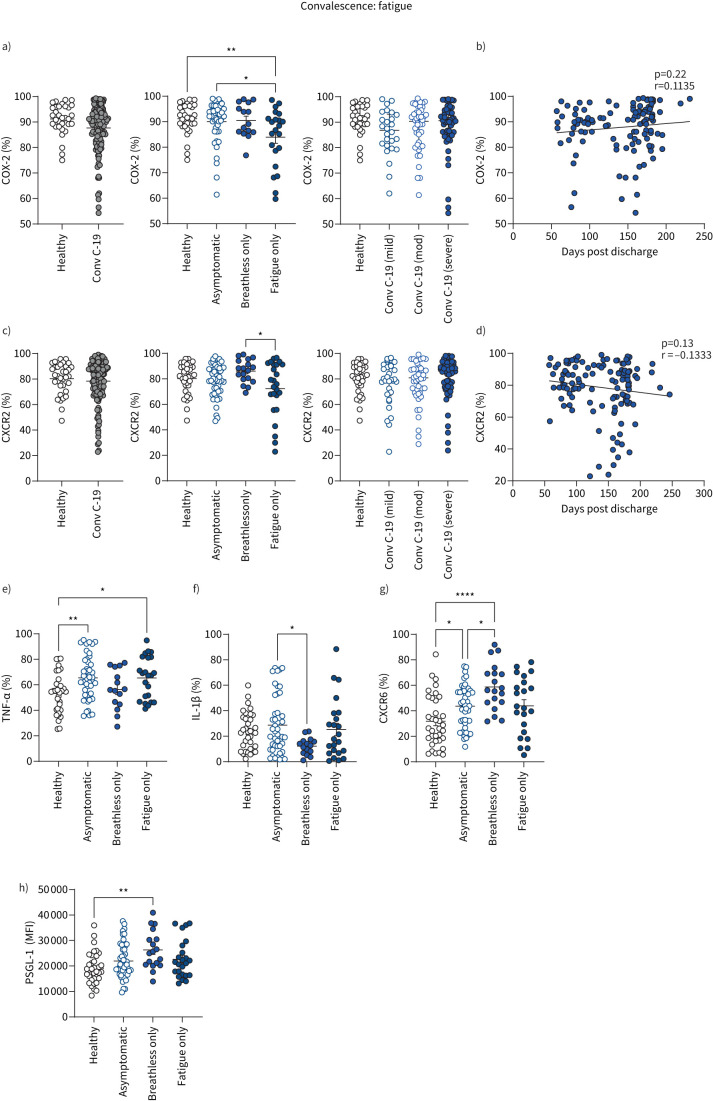
Having shown earlier that reduced monocyte expression of the prostaglandin-generating enzyme COX-2 and chemokine receptor CXCR2 was a feature of severe COVID-19 during acute disease, lowest in those requiring intensive therapy unit care, we show here that these abnormal monocyte features were sustained into convalescence but specifically in patients with ongoing fatigue (figures 7 and 8a–d). Interestingly, there was no correlation of either COX-2 or CXCR2 expression in convalescence with initial acute disease severity (figure 8a–d). COX-2 expression on monocytes gradually increased over time (figure 8b) at a slow rate whilst CXCR2 levels were not restored back to normal levels (figure 8d), suggesting long-term monocyte dysfunction in long COVID patients with fatigue. Despite indications of a trend towards decreased monocyte expression of chemokine receptor CXCR1 in patients with fatigue (figure 7), there were no significant differences in monocyte expression of CXCR1 between healthy controls and convalescent COVID-19 patients (fatigue or no fatigue; supplementary figure S8b).
FIGURE 8.
Monocyte profiles of patients with long COVID with fatigue. Summary graphs showing data from healthy individuals and all convalescent COVID-19 patients. a) Frequencies of CD14+ monocytes expressing COX-2 from healthy individuals (n=32) and all convalescent COVID-19 (Conv C-19) patients (n=121). Patients with convalescent COVID-19 were also stratified into asymptomatic (no breathlessness or fatigue: n=45), breathless only (breathless but not fatigued: n=15) or fatigue only (fatigued but not breathless: n=22) and into mild (n=26), moderate (n=44) or severe (n=52) disease, referring to acute COVID-19 severity during previous hospital admission (for all convalescent patients). b) Correlation of COX-2 (percentage of monocytes expressing COX-2) with number of days since hospital discharge, in all convalescent COVID-19 patients (n=122). c) Frequencies of CD14+ monocytes expressing CXCR2 from healthy individuals (n=34) and all convalescent COVID-19 patients (n=133). Patients with convalescent COVID-19 were also stratified into asymptomatic (n=51), breathless only (n=18) or fatigue only (n=25) and into mild (n=30), moderate (n=44) or severe (n=59) disease, referring to acute COVID-19 severity during previous hospital admission (for all convalescent patients). d) Correlation of CXCR2 (percentage of monocytes expressing CXCR2) with number of days since hospital discharge in all convalescent COVID-19 patients (n=128). e) Frequency of CD14+ monocytes producing TNF-α in healthy individuals (n=33) and convalescent COVID-19 patients stratified into asymptomatic (n=45), breathless only (n=15) or fatigue only (n=23). f) Frequency of CD14+ monocytes producing IL-1β in healthy individuals (n=34) and convalescent COVID-19 patients stratified into asymptomatic (n=45), breathless only (n=15) or fatigue only (n=23). g) Frequency of CD14+ monocytes expressing CXCR6 in healthy individuals (n=35) and convalescent COVID-19 patients stratified into asymptomatic (n=45), breathless only (n=19) or fatigue only (n=22). h) CD14+ monocyte levels of expression of PSGL-1 as determined by mean fluorescence intensity (MFI) in healthy individuals (n=35) and convalescent COVID-19 patients stratified into asymptomatic (n=50), breathless only (n=18) or fatigue only (n=24). All graphs show individual patient data with bars representing mean±sem. Comparison of groups was carried out using unpaired t-test (a, c: healthy versus convalescent COVID-19), one-way ANOVA with Holm–Sidak post hoc test (a, c, e-h: long COVID symptoms, original severity) or Pearson correlation coefficient test (b, d). *: p≤0.05; **: p≤0.01; ***: p≤0.001; ****: p≤0.0001.
This differential analysis of fewer patients based on the specific symptoms of breathlessness only versus fatigue only again confirms that heightened TNF-α production by monocytes in convalescence was restricted to patients with resolved lung injury (i.e. not breathless; figure 8e). Monocyte production of IL-1β was higher in asymptomatic patients than in those who were breathless (without fatigue; figure 8f). These data support earlier data inclusive of all convalescent patients, demonstrating cytokine production by monocytes (TNF-α in particular) is associated with good outcome in terms of resolution of lung injury (figure 6a). However, it is noteworthy that heightened monocyte production of TNF-α in patients with resolved lung injury included those with ongoing fatigue (figure 8e), raising the possibility that heightened TNF-α could be involved in the pathology in long COVID patients with ongoing fatigue. These data also clearly elucidate that heightened CXCR6 and PSGL-1 in convalescent COVID-19 patients with unresolved lung injury (figure 4a, f) is specific to breathlessness rather than fatigue (figures 7 and 8g, h).
In summary, we show for the first time, that dysregulated monocyte migration corresponds to COVID-19 severity, both during the acute phase and convalescence. Features of monocyte dysfunction in acute COVID-19 were sustained into convalescence, with distinct monocyte signatures corresponding to the two most commonly reported symptoms of long COVID, shortness of breath and fatigue (summarised in supplementary figure S8c).
Discussion
The COVID-19 pandemic continues to cause devastating global disease as a result of inflammatory pathology from acute disease and persistent immune dysfunction during convalescence, contributing to long COVID, which remains a worldwide public health issue. It is now widely established that many patients develop long-term chronic symptoms following SARS-CoV-2 infection. Our data implicate dysregulated monocyte migration in COVID-19 pathophysiology and we show that monocyte dysfunction persists into convalescence even up to 9 months following hospital discharge, with differential monocyte signatures corresponding to specific aspects of long COVID (shortness of breath and fatigue).
We show a wide range of molecules involved in leukocyte migration are aberrantly expressed on monocytes in acute and convalescent COVID-19, several of which are associated with transendothelial migration to allow egress from blood vessels into peripheral tissues. We show increased VCAM-1 and E-selectin levels in serum in long COVID patients with breathlessness, amongst other chemokines, further implicating involvement of the pulmonary endothelium and supporting previous studies showing increased levels of VCAM-1 and E-selectin in acute COVID-19 [38].
The heightened expression of monocyte CXCR6 through convalescence is functionally relevant, with monocytes from breathless convalescent patients preferentially migrating towards the CXCR6 ligand CXCL16 in vitro and CXCL16 expressed at high levels in the lung [35]. The enhanced expression of CXCR6 on monocytes from PFILD patients and clear evidence for CXCL16 involvement in these patients is supportive of a key role for the CXCR6–CXCL16 axis in ongoing lung injury. The lack of heightened monocyte CXCR6 expression in RSV or flu convalescence may reflect the difference in immune responses generated by the different respiratory viruses or the different disease aetiology and severity in patients admitted for acute COVID-19, RSV and flu. Further studies with larger cohorts of follow-up flu and RSV patients are necessary to fully define the differences in immune responses generated by these different viruses.
Our data show two distinct immune signatures corresponding to specific features of long COVID-19: breathlessness and fatigue. Whilst heightened monocyte CXCR6 and PSGL-1 expression defined breathless patients, reduced expression of COX-2 and CXCR2 defined those with ongoing fatigue. Our data are in keeping with studies identifying two distinct clusters of long COVID patients in a large UK cohort of 500 000, with fatigue predominating in one and respiratory symptoms in the other [37]. Importantly, changes in prostaglandin E2 levels (generated by COX-2 from conversion of arachidonic acid) have been associated with chronic fatigue syndrome [39] and targeting this eicosanoid pathway may have therapeutic benefit. Our data demonstrating low levels of CXCR2 associated with severe disease during acute COVID-19 and ongoing fatigue during convalescence support a protective role for CXCR2 in inflammation, in keeping with CXCR2 deficiency inducing an exaggerated inflammatory response associated with increased macrophage accumulation at inflamed sites [40].
The heightened production of the monocyte TNF-α in patients with mild acute disease and resolved lung injury during convalescence supports the results of studies indicating a role for TNF-α in a good outcome in mouse models, in which it is thought to resolve lung injury by accelerating resolution of pulmonary fibrosis [41]. Indeed, we show here that TNF-α production was reduced in PFILD patients who have severe, sustained, ongoing lung injury. Furthermore, increased responsiveness to toll-like receptor ligation in monocytes and subsequent inflammatory cytokine production is a feature of patients who have recovered from COVID-19 [42]. This heightened TNF-α production in convalescence appeared specific to COVID-19, with no change in convalescent RSV or flu patients. The heightened TNF-α production in long COVID patients with resolved lung injury, however, included those with ongoing fatigue. Whilst it is unclear how TNF-α may contribute to fatigue pathology, our data support recent studies implicating a role for TNF-α in COVID-19-associated fatigue during convalescence [43].
In summary, we have identified abnormal features of circulating monocytes in COVID-19 with profound implications for our understanding of long COVID. Our data support studies demonstrating prolonged changes in innate immunity during COVID-19 convalescence [19, 42, 44, 45] and indicate abnormal monocyte migration as a key factor in COVID-19 pathophysiology during both acute disease and convalescence. Some of these molecules, e.g. CXCR6, indicated no return back to healthy control levels over time. These findings have direct relevance for the Omicron era due to the persistence of long COVID, despite milder infections and the introduction of vaccination programmes worldwide. Whilst new variants may cause different degrees of antibody neutralisation and memory T-cell responses due to different epitopes, we describe changes in innate rather than adaptive immunity that correspond to ongoing symptoms of long COVID. With the underlying pathophysiology essentially unchanged, our data are highly likely to be relevant for Omicron and any future variants and subvariants. Furthermore, our PFILD data reveal changes to the CXCR6–CXCL16 axis as a broadly applicable mechanism important to the pathophysiology of ongoing lung injury that remains a feature of long COVID patients with breathlessness today. These data provide novel opportunities for therapeutic targeting in COVID-19 patients with persistent morbidity.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures ERJ-02226-2022.Figures (39.7MB, pdf)
Supplementary table S1: list of antibodies ERJ-02226-2022.Table (100.2KB, pdf)
Shareable PDF
Acknowledgements
We thank all participants and their families for their contribution, without which this study would not have been possible. This report is independent research supported by the UK Coronavirus Immunology Consortium (UK-CIC), the North West Lung Centre Charity and the NIHR Manchester Clinical Research Facility at Wythenshawe Hospital. We thank the Manchester Allergy, Respiratory and Thoracic Surgery Biobank, and we thank the Post-Hospitalisation COVID-19 study (PHOSP-COVID) Collaborative Group, and the Northern Care Alliance Research Collection tissue bank for supporting this project. We thank Imbio Inc. (Minneapolis, MN, USA) for the Lung Density Analysis and Lung Texture Analysis programmes used to generate quantitative CT reports. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. A. Simpson, A. Horsley, T. Felton, P. Dark and T. Hussell are supported by the NIHR Manchester Biomedical Research Centre. C. Brightling is supported by the NIHR Leicester Biomedical Research Centre. In addition, we would like to thank the Immunology community within the Lydia Becker Institute of Immunology and Inflammation, the Flow Cytometry Core Facility at the University of Manchester, the Manchester COVID-19 Rapid Response Group and the study participants for their contribution.
Footnotes
CIRCO investigators: R. Ahmed, M. Avery, K. Birchall, R. Cairns, E. Charsley, A. Chenery, C. Chew, R. Clark, E. Connolly, K. Connolly, O. Corner, S. Dawson, L. Durrans, H. Durrington, J. Egan, C. Fox, H. Francis, S. Glasgow, N. Godfre, K.J. Gray, S. Grundy, J. Guerin, M. Iqbal, C. Hayes, E. Hardy, J. Harris, A. John, B. Jolly, S. Krishnan, S. Lui, N. Majeed, J. Mitchell, K. Moore, S. Moss, S. Murtuza Baker, R. Oliver, G. Padden, C. Parkinson, M. Phuycharoen, M. Rattray, A. Saha, B. Salcman, S. Sharma, J. Shaw, T.N. Shaw, E. Shepley, S. Stephan, R. Stephens, G. Tavernier, R. Tudge, A. Uriel, L. Wareing, R. Warren, L. Willmore, R. Wiltshire, M. Younas and S. Zriba.
This article has an editorial commentary: https://doi.org/10.1183/13993003.00409-2023
Conflict of interest: G. Lindergard is co-founder and scientific advisory board member of Gritstone Bio Inc., which is a public company that develops therapeutic vaccines for the treatment of cancer and infectious diseases, including COVID-19. The other authors declare that they have no competing interests.
Support statement: This work was supported by The Wellcome Trust/Royal Society (E.R. Mann, 206206/Z/17/Z); The Kennedy Trust for Rheumatology Research, who provided a Rapid Response Award for costs associated with the laboratory analysis of the immune response in COVID-19 patients to J.R. Grainger; Medical Research Council (L. Pearmain, MR/R00191X/1; K. Piper Hanley, MR/P023541/1); The Wellcome Trust (T. Hussell, 202865/Z/16/Z; 106898/A/15/Z, which helped support some CIRCO members), the Lister Institute (J.E. Konkel); BBSRC (J.E. Konkel, BB/M025977/1); and Innovate UK (K. Piper Hanley, 40896). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . World Health Organization (COVID-19) Dashboard. https://covid19.who.int/ Date last accessed: 13 April 2023. [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann ER, Menon M, Knight SB, et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol 2020; 5: eabd6197. doi: 10.1126/sciimmunol.abd6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melms JC, Biermann J, Huang H, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature 2021; 595: 114–119. doi: 10.1038/s41586-021-03569-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo PA, Dogra P, Gray JI, et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 2021; 54: 797–814. doi: 10.1016/j.immuni.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell 2020; 182: 1419–1440. doi: 10.1016/j.cell.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvin A, Chapuis N, Dunsmore G, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell 2020; 182: 1401–1418. doi: 10.1016/j.cell.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26: 842–844. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 9.COMBAT Consortium . A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell 2022; 185: 916–938. doi: 10.1016/j.cell.2022.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from Northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20: 1135–1140. doi: 10.1016/S1473-3099(20)30434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigmohammadi MT, Jahanbin B, Safaei M, et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol 2021; 29: 135–145. doi: 10.1177/1066896920935195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmukh V, Motwani R, Kumar A, et al. Histopathological observations in COVID-19: a systematic review. J Clin Pathol 2021; 74: 76–83. doi: 10.1136/jclinpath-2020-206995 [DOI] [PubMed] [Google Scholar]
- 13.Stikker BS, Stik G, van Ouwerkerk AF, et al. Severe COVID-19-associated variants linked to chemokine receptor gene control in monocytes and macrophages. Genome Biol 2022; 23: 96. doi: 10.1186/s13059-022-02669-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser E. Long term respiratory complications of COVID-19. BMJ 2020; 370: m3001. doi: 10.1136/bmj.m3001 [DOI] [PubMed] [Google Scholar]
- 15.Williams FMK, Muirhead N, Pariante C. COVID-19 and chronic fatigue. BMJ 2020; 370: m2922. doi: 10.1136/bmj.m2922 [DOI] [PubMed] [Google Scholar]
- 16.Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute COVID-19 in primary care. BMJ 2020; 370: m3026. doi: 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 17.PHOSP-COVID Collaborative Group . Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022; 10: 761–775. doi: 10.1016/S2213-2600(22)00127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23: 210–216. doi: 10.1038/s41590-021-01113-x [DOI] [PubMed] [Google Scholar]
- 20.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity 2018; 49: 595–613. doi: 10.1016/j.immuni.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 21.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020; 20: 355–362. doi: 10.1038/s41577-020-0331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meerschaert J, Furie MB. Monocytes use either CD11/CD18 or VLA-4 to migrate across human endothelium in vitro. J Immunol 1994; 152: 1915–1926. [PubMed] [Google Scholar]
- 23.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol 1995; 154: 4099–4112. [PubMed] [Google Scholar]
- 24.Liao F, Huynh HK, Eiroa A, et al. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med 1995; 182: 1337–1343. doi: 10.1084/jem.182.5.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584: 463–469. doi: 10.1038/s41586-020-2588-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369: 718–724. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46: 846–848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberger A, Middleton KA, Oliver JA, et al. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J Biol Chem 1994; 269: 17183–17191. [PubMed] [Google Scholar]
- 29.Hojo Y, Ikeda U, Takahashi M, et al. Matrix metalloproteinase-1 expression by interaction between monocytes and vascular endothelial cells. J Mol Cell Cardiol 2000; 32: 1459–1468. doi: 10.1006/jmcc.2000.1179 [DOI] [PubMed] [Google Scholar]
- 30.Stanojevic S, Kaminsky DA, Miller M, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022; 60: 2101499. doi: 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
- 31.Grist JT, Chen M, Collier GJ, et al. Hyperpolarized 129Xe MRI abnormalities in dyspneic patients 3 months after COVID-19 pneumonia: preliminary results. Radiology 2021; 301: E353–EE60. doi: 10.1148/radiol.2021210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan X, Yao L, Tan Y, et al. Quantitative and semi-quantitative CT assessments of lung lesion burden in COVID-19 pneumonia. Sci Rep 2021; 11: 5148. doi: 10.1038/s41598-021-84561-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016; 44: 450–462. doi: 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendisch D, Dietrich O, Mari T, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell 2021; 184: 6243–6261.e27. doi: 10.1016/j.cell.2021.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan AJ, Guillen C, Symon FA, et al. Expression of CXCR6 and its ligand CXCL16 in the lung in health and disease. Clin Exp Allergy 2005; 35: 1572–1580. doi: 10.1111/j.1365-2222.2005.02383.x [DOI] [PubMed] [Google Scholar]
- 36.Xia L, Sperandio M, Yago T, et al. p-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest 2002; 109: 939–950. doi: 10.1172/JCI14151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508, 707 people. medRxiv 2021; preprint [ 10.1101/2021.06.28.21259452]. [DOI] [Google Scholar]
- 38.Birnhuber A, Fliesser E, Gorkiewicz G, et al. Between inflammation and thrombosis: endothelial cells in COVID-19. Eur Respir J 2021; 58: 2100377. doi: 10.1183/13993003.00377-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Meirleir KL, Mijatovic T, Subramanian K, et al. Evaluation of four clinical laboratory parameters for the diagnosis of myalgic encephalomyelitis. J Transl Med 2018; 16: 322. doi: 10.1186/s12967-018-1696-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dyer DP, Pallas K, Medina-Ruiz L, et al. CXCR2 deficient mice display macrophage-dependent exaggerated acute inflammatory responses. Sci Rep 2017; 7: 42681. doi: 10.1038/srep42681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redente EF, Keith RC, Janssen W, et al. Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am J Respir Cell Mol Biol 2014; 50: 825–837. doi: 10.1165/rcmb.2013-0386OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brauns E, Azouz A, Grimaldi D, et al. Functional reprogramming of monocytes in acute and convalescent severe COVID-19 patients. JCI Insight 2022; 7: e154183. doi: 10.1172/jci.insight.154183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Son K, Jamil R, Chowdhury A, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J 2023; 61: 2200970. doi: 10.1183/13993003.00970-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You M, Chen L, Zhang D, et al. Single-cell epigenomic landscape of peripheral immune cells reveals establishment of trained immunity in individuals convalescing from COVID-19. Nat Cell Biol 2021; 23: 620–630. doi: 10.1038/s41556f-021-00690-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohnacker S, Hartung F, Henkel F, et al. Mild COVID-19 imprints a long-term inflammatory eicosanoid- and chemokine memory in monocyte-derived macrophages. Mucosal Immunol 2022; 15: 515–524. doi: 10.1038/s41385-021-00482-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figures ERJ-02226-2022.Figures (39.7MB, pdf)
Supplementary table S1: list of antibodies ERJ-02226-2022.Table (100.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02226-2022.Shareable (537.9KB, pdf)