Abstract
Mesenchymal stem/stromal cells (MSCs) are widely distributed in the body and play essential roles in tissue regeneration and homeostasis. MSCs can be isolated from discarded tissues, expanded in vitro and used as therapeutics for autoimmune diseases and other chronic disorders. MSCs promote tissue regeneration and homeostasis by primarily acting on immune cells. At least six different types of MSCs have been isolated from postnatal dental tissues and have remarkable immunomodulatory properties. Dental stem cells (DSCs) have been demonstrated to have therapeutic effects on several systemic inflammatory diseases. Conversely, MSCs derived from nondental tissues such as the umbilical cord exhibit great benefits in the management of periodontitis in preclinical studies. Here, we discuss the main therapeutic uses of MSCs/DSCs, their mechanisms, extrinsic inflammatory cues and the intrinsic metabolic circuitries that govern the immunomodulatory functions of MSCs/DSCs. Increased understanding of the mechanisms underpinning the immunomodulatory functions of MSCs/DSCs is expected to aid in the development of more potent and precise MSC/DSC-based therapeutics.
Keywords: Mesenchymal stem cells, Dental stem cells, Immunoregulation, Inflammation
Subject terms: Inflammatory diseases, Gene regulation in immune cells
Introduction
In almost every vertebrate organ, there are mesenchymal stromal cells (MSCs). Because these cells possess the capacities of self-renewal and are progenitors that can give rise to adipocytes, chondrocytes, osteoblasts and myofibroblasts in response to differentiation or inflammatory cues, they are frequently referred to as mesenchymal stem cells (also abbreviated MSCs). Due to their multilineage differentiation potential, ability to regulate the immune response, easy accessibility to source tissues and excellent propagation capacity in vitro, MSCs have been regarded as an ideal source for therapeutics in tissue regeneration and autoimmune and hyperinflammatory diseases. MSCs are a highly heterogeneous cell type with multiple postnatal tissue origins. These cells originate either from the mesoderm or ectoderm [1–4]. The distinct differences in their lineage, development and tissue distribution can not only determine their functional diversity but also have implications in clinical applications. Because MSCs derived from nondental tissues such as bone marrow, adipose tissues and umbilical cord have been extensively reviewed elsewhere [5–8], this review will focus on MSCs derived from dental tissues, although some general mechanistic aspects of MSC biology will be presented.
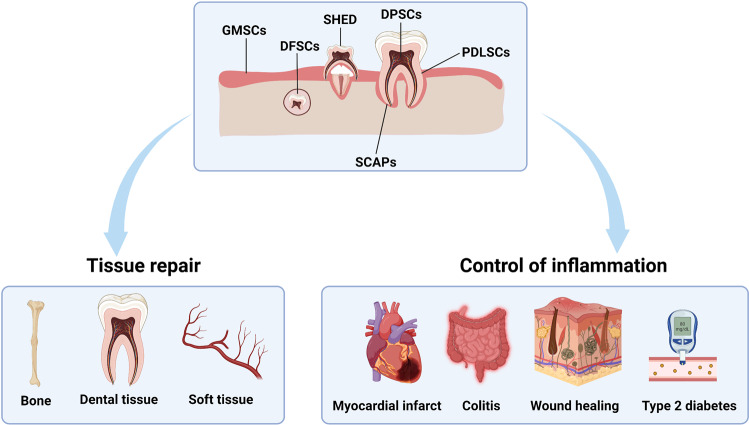
To date, at least six different types of postnatal dental tissue-derived MSCs have been isolated and characterized, including stem cells from dental pulp (DPSCs), periodontal ligament (PDLSCs), deciduous teeth (SHED), apical papilla (SCAPs), dental follicles (DFSCs), and gingiva (GMSCs) (Fig. 1). STRO-1 and CD146 may serve as reliable surface markers for dental stem cells (DSCs). Due to their easy access, excellent propagation capacity in vitro and potent immunoregulatory properties, DSCs have been used in some preclinical studies and clinical trials for treating hyperinflammatory disorders, neurodegenerative diseases, organ injuries, autoimmune diseases, orthopedic disorders and diabetes (Table 1). Conversely, MSCs derived from nondental tissues such as umbilical cord and adipose tissue are being explored for their therapeutic benefits in the treatment of periodontitis and other dental diseases. This review summarizes the main therapeutic uses of MSCs/DSCs, highlights their mechanisms of action in immunoregulation, and discusses the extrinsic inflammatory cues that elicit the immunoregulatory properties and the intrinsic metabolic circuitries in these cells. We also provide some perspectives on possible strategies that can augment the therapeutic effects of MSCs/DSCs.
Fig. 1.
The classification and therapeutic applications of DSCs. Six different types of DSCs have been isolated and characterized, including stem cells from dental pulp (DPSCs), periodontal ligament (PDLSCs), deciduous teeth (SHED), apical papilla (SCAPs), dental follicles (DFSCs), and gingiva (GMSCs). DSCs have therapeutic potential for tissue repair (bone regeneration, dental tissue regeneration and soft tissue reconstruction) and inflammatory diseases (myocardial infarct, colitis, wound healing and type 2 diabetes)
Table 1.
Clinical trials for DSCs (source: https://www.clinicaltrials.gov/)
| Disease | Infusion method | Sample size | Cell mass | Cell source | Study phase | NCT number | Location |
|---|---|---|---|---|---|---|---|
| COVID-19 | Intravenously infusion | 20 | 3 × 10^7 cells | DPSCs | Recruiting | NCT04336254 | China |
| Periodontitis | Local injection | 36 | 1 × 10^6–4 × 10^7 cells | DPSCs | Recruiting | NCT04983225 | China |
| Acute Ischemic Stroke | Intravenously infusion | 79 | 1 or 3 × 10^8 cells | DPSCs | Completed | NCT04608838 | Japan |
| Cleft Lip and Palate | Local implantation | 62 | N/A | DPSCs | Completed | NCT03766217 | Brazil |
| Knee Osteoarthritis | Intraarticular injection | 60 | N/A | DPSCs | Unknown | NCT04130100 | China |
| Periodontal Diseases | Local implantation | 29 | N/A | DPSCs | Completed | NCT03386877 | Italy |
| Periodontal Diseases | Local injection | 40 | 1 × 10^6 cells | DPSCs | Unknown | NCT02523651 | China |
| Periodontal tissue regeneration | Local implantation | 30 | N/A | PDLSCs | Completed | NCT01357785 | China |
| Periodontitis | Local implantation | 80 | N/A | PDLSCs | Completed | NCT01082822 | China |
| Periodontal Intrabony Defect | Local implantation | 20 | N/A | GMSCs | Completed | NCT03638154 | Egypt |
| Periodontitis | Local implantation | 30 | N/A | GMSCs | Unknown | NCT03137979 | China |
| Liver Cirrhosis | Peripheral vein infusion | 40 | 1 × 10^6 cells/kg | SHEDs | Unknown | NCT03957655 | China |
| Cleft Lip and Palate | Local implantation | 5 | N/A | SHEDs | Completed | NCT01932164 | Brazil |
| Type 2 Diabetes | Intravenous drip | 24 | 0.1 IU/kg | SHEDs | Completed | NCT03658655 | China |
| Dental Pulp Necrosis | Local implantation | 80 | N/A | SHEDs | Unknown | NCT01814436 | China |
| Type 1 Diabetes | Intravenously infusion | 24 | 0.11 IU/kg | SHEDs | Unknown | NCT03912480 | China |
Ontogeny and differentiation of DSCs
MSCs residing in most internal organs are primarily derived from the mesoderm. DSCs originate from the neural crest. Despite their distinct origins, MSCs/DSCs all possess general properties such as self-renewal, multipotent differentiation, immunomodulation, and the expression of a set of MSC surface markers [9]. However, DSCs tend to have stronger proliferative and neural differentiation potential [10], which is beneficial in the regeneration of neuronal tissue and the treatment of neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease [11]. Feng et al. showed that NG2+ pericyte-derived DSC precursors contributed to odontoblast differentiation during dental pulp development and tissue regeneration [12, 13]. Genetic lineage-tracing studies showed that DPSCs originated from peripheral nerve-associated glia, and the glia-to-mesenchymal cell transition pattern may be unique to the lineage evolution of DPSCs [14]. Zhao et al. reported that Gli1+ MSCs surrounding the cervical loop of the incisor are the progenitor cells of PDLSCs and DPSCs, and Gli1+ cells within the suture mesenchyme are the major MSCs responsible for craniofacial bone development and homeostasis [15, 16]. CD24a+ SCAPs are multipotent tooth root stem cells that can regenerate tooth roots with functional dentin and neurovascular-like structures [17]. These studies show that DSCs have multiple subsets.
DSCs exhibit increased odontogenic preference but have reduced chondrogenic and adipogenic differentiation potential [18]. The differentiation of DSCs is regulated by multiple signaling pathways, such as the BMP/TGF-β and WNT/β-catenin pathways. BMP/TGF-β signaling activity is required for the odontogenic differentiation of DSCs and root development [19]. Specifically, the loss of BMP/TGF-β signaling in DSCs leads to a delay in tooth eruption, defects in root elongation, and failure of extracellular matrix formation [20]. During tooth development, DSCs give rise to transit amplifying cells, which then differentiate into different cell types, including odontoblasts, cementoblasts and dental pulp cells [21]. The WNT/β-catenin pathway plays an important role in the maintenance of DSCs by transit amplifying cells (TACs), and the loss of Wnt5a results in the loss and impaired differentiation of DSCs and diminished TACs [21].
DSCs can be isolated from different parts of the tooth, and accumulating evidence has shown functional differences among DSCs in terms of proliferation rate, osteo/odonto differentiation rate and angiogenic potential [22]. Therefore, tissue-specific markers are needed, and the functions of the different types of DSCs need to be further explored. The advancement of single-cell lineage tracing technology holds great promise in elucidating the heterogeneity and specific functions among different types of DSCs.
Interactions of DSCs with the tissue microenvironment
Previous studies have shown that the regenerative and immunomodulatory activity of DSCs is dynamically regulated by the stem cell niche and immune microenvironment [23, 24]. PDLSCs isolated from inflamed tissue showed increased proliferation rates but decreased osteogenic potential [25, 26]. Further study suggested that periodontitis could undermine the osteogenic ability of PDLSCs via the UCHL1/BMP2/Smad signaling pathway, accelerating bone resorption [27]. PDLSCs can undergo gasdermin-D (GSDMD)-dependent pyroptosis, leading to periodontitis by increasing IL-1β release, enhancing inflammation, and promoting osteoclastogenesis [28]. These studies suggest that the properties of DSCs vary with their microenvironment.
DSCs exert immunosuppressive effects mostly through the secretion of immunoregulatory cytokines, which modulate innate and adaptive immunity, as well as the complementary system [29]. Thus, the reciprocal interactions between DSCs and immune cells can help maintain tissue homeostasis and avoid excessive inflammatory responses. When tissue injury occurs, DSCs can react rapidly via their immunomodulatory activities and prevent the exacerbation of injury [24, 30]. DPSCs from inflammatory pulpitis (I-DPSCs) were shown to suppress the proinflammatory functions of macrophages through the TNF-α/IDO axis, attenuating the exacerbation of pulpitis [31]. Compared to DPSCs from healthy pulp tissue, I-DPSCs expressed increased levels of IL-6. I-DPSCs and DPSCs treated with IL-6 have impaired neurogenic potential [32]. I-DPSCs were also shown to have diminished immunosuppressive properties [33]. The periodontitis microenvironment compromises the immunomodulatory effects of PDLSCs, leading to the accumulation of inflammatory immune cells in periodontal bone [25]. GMSC-derived exosomes in the inflammatory microenvironment can enhance M2 macrophage polarization and inhibit periodontal bone loss [34]. Therefore, these studies indicate that the regenerative and immunomodulatory activities of DSCs are vital for dental tissue homeostasis.
Current therapeutic applications of harvested MSCs/DSCs in tissue repair and the control of inflammation
Tissue repair
Compared with mesoderm-derived MSCs, neural crest-derived MSCs show increased differentiation potential to neural cells and chondrocytes, as well as increased immunomodulatory and anti-inflammatory effects in vitro and in vivo, suggesting that DSCs have great therapeutic potential for applications in tissue repair, including bone, dental and soft tissues [35].
A previous study demonstrated that PDLSCs showed a stronger tissue regeneration capacity than MSCs derived from bone marrow (BM-MSCs) on critical-size defects in the immunodeficient rat calvarium, suggesting the superiority of using PDLSCs in bone tissue regenerative therapy [36]. The transplantation of SHED can ameliorate secondary osteoporosis and promote bone regeneration in mice with systemic lupus erythematosus [37]. DPSC-derived extracellular matrix enhances artificial bone integration and promotes artificial bone regeneration and repair; thus, DPSC-derived extracellular matrix can be used to decorate various biomaterials in bone tissue engineering [38]. Another study reported that implanted DPSCs could survive in the defect area and accelerate the regeneration of calvarial bone via an endochondral bone ossification-like process [39]. These studies showed that DSCs may promote bone tissue repair through multiple mechanisms.
In dental tissue repair, DSCs can generate dental-related tissue after transplantation in vivo. Gronthos et al. reported that DPSCs could regenerate a dentin-pulp complex, which is composed of mineralized matrix and fibrous tissue [40]. Functional cementum/PDL-like structures can be regenerated after PDLSCs are transplanted into immunocompromised mice, which is vital for alveolar bone regeneration and periodontitis treatment [10]. In general, DSCs have potential in bone tissue regeneration and repair, especially in the oral and maxillofacial areas.
In addition to their applications in bone regeneration, DSCs are also recommended for soft tissue reconstruction, such as in periodontal ligament, dental pulp and nerve tissue [41–43]. Because of their origin from the neural crest and their residence in a neurovascular niche, DSCs have the potential to re-establish neurovascular inductive activity [44]. Extracellular vesicles (EVs) derived from GMSCs were reported to promote the regeneration of periodontal ligament in damaged periodontal tissue [45]. SHEDs and DPSCs possess strong neurogenic and angiogenic abilities and are thus optimal candidates for dental pulp regeneration [46]. SHEDs were administered to minipigs and regenerated physiologic pulp patterns with odontoblasts lining the dentin wall using cell aggregate technology. Similarly, DPSC aggregates form pulp-like tissues with rich blood vessels within the human root canal 6 weeks after implantation [47]. Importantly, DPSCs may serve as a better choice for treating Parkinson’s disease than BM-MSCs due to their predisposition toward neural differentiation and their potential to regenerate neurons [48]. A recent study showed that Nestin+ DPSCs could survive for 1 month after transplantation in the brains of nude mice and form complete blood vessels that integrated into the host cerebrovascular system [49].
Control of inflammation
The immunomodulatory property of DSCs makes them suitable therapeutics for aberrant immune and inflammatory diseases. DSCs can exert their immunomodulatory effects through direct and indirect mechanisms [50, 51]. DPSCs induce apoptosis in activated T cells in vitro and reduce inflammatory tissue damage in mice with colitis, which is related to the expression of Fas ligand (FasL) by DPSCs. Downregulation of FasL expression in DPSCs compromises their immunomodulatory properties [52]. Conditioned medium (CM) from DPSCs effectively improved healing and attenuated the inflammatory response of skin fibroblasts [53]. Systemic infusion of GMSCs significantly ameliorated colonic inflammation, restored injured gastrointestinal mucosal tissues, reversed diarrhea and weight loss, and suppressed overall disease activity in mice with experimental colitis [54]. GMSCs are capable of polarizing macrophages into the M2 phenotype, which is characterized by upregulated expression of CD206, increased secretion of the anti-inflammatory cytokine IL-10 and phagocytotic activity [55]. Unlike murine BM-MSCs, which are impaired in their migration and anti-inflammatory response with aging [56], human GMSCs have been shown to retain stable karyotypes and immunomodulatory characteristics independent of donor age [57, 58]. DFSCs can also reduce inflammation through the paracrine factors TGF-β3 and TSP-1 [59].
The administration of SHED-derived CM reduces myocardial infarct size and inflammatory cytokine levels, such as TNF-α, IL-6, and IL-β [60]. The anti-inflammatory effect of SHED-CM is superior to that of CM derived from BM-MSCs and adipose-derived stem cells (ADSCs) [60]. DPSC-CM alleviates Sjögren’s syndrome by promoting Treg cell differentiation and inhibiting Th17 cell differentiation in the mouse spleen [61]. DPSC-EVs can reduce the levels of inflammatory cytokines and senescence-associated secretory phenotypic factors, thus reversing oxidative stress in submandibular cells and preventing irradiation-induced cellular senescence [62]. DPSC-EVs can improve ischemia‒reperfusion in mice through anti-inflammatory mechanisms mediated by the HMGB1/TLR4/MyD88/NF-κB pathway [63]. Therefore, DSCs have outstanding anti-inflammatory functions that can be harnessed for therapeutic applications in inflammatory diseases.
Clinical Studies
Since the safety and efficacy of DSCs have been extensively verified, DSCs are increasingly being used in clinical studies. Currently, there are more than 15 clinical trials for tissue regeneration and disease repair using DSCs (Table 1), including DPSCs for COVID-19 and SHEDs for dental pulp regeneration. Some clinical studies have yielded exciting outcomes. Chen et al. conducted a randomized trial using autologous PDLSCs for periodontal intrabony defects and validated the safety of PDLSCs in clinical applications, which is the first study of DSCs in a clinical trial [64]. Xuan et al. implanted ex vivo-expanded autologous SHEDs in patients with pulp necrosis [65]. The implanted SHEDs regenerated three-dimensional whole dental pulp with an odontoblast layer, blood vessels, and nerves, which represents the first human organ that was successfully regenerated by DSCs in randomized clinical trials [65]. Li et al. reported that SHED infusion was effective in improving glucose metabolism and islet function in type 2 diabetes mellitus patients, which is the first successful study using DSCs to treat systemic disease [66]. Ferrarotti et al. reported that the delivery of DPSCs into intrabony defects in a collagen scaffold significantly improved the clinical parameters of periodontal regeneration 1 year after treatment [67]. Thus, the clinical benefits of DSCs may be attributed to their regenerative capacity, as well as their ability to restore tissue immune homeostasis.
The immunoregulatory pathways of MSCs/DSCs
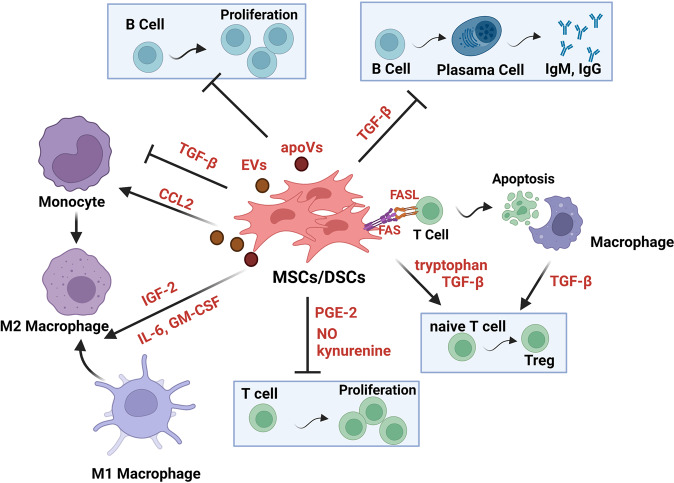
A few studies have demonstrated that in vitro expanded MSCs/DSCs can facilitate the repair and regeneration of damaged tissue through their immunomodulatory actions [7]. MSCs/DSCs possess strong immunomodulatory abilities, which endows MSCs/DSCs with therapeutic potency in various degenerative and inflammatory disorders [68]. The immunomodulatory effects of MSCs/DSCs are exerted via the production of metabolites, cytokines, growth factors, chemokines, EVs, and apoptotic vesicles and T-cell death-mediated immunoregulation (Fig. 2). Below, we highlight some of the main pathways that contribute to the immunomodulation mediated by MSCs/DSCs.
Fig. 2.
The main routes through which MSCs/DSCs exert their immunoregulatory effects. MSCs/DSCs exert their immunomodulatory effects by producing metabolites, cytokines, growth factors, chemokines, extracellular vesicles, and apoptotic vesicles and T-cell death-mediated immunoregulation. MSCs/DSCs can suppress T-cell and B-cell proliferation, promote naive CD4+ T cells to differentiate into Treg cells, instruct macrophages to acquire an immunosuppressive phenotype, or inhibit the production of IgM and IgG. In addition, MSCs/DSCs induce T-cell apoptosis and subsequently trigger macrophages to produce TGFβ, which induces the differentiation of Tregs and immune tolerance
Metabolites: NO, tryptophan metabolites, PGE2
It has been demonstrated that when exposed to an inflammatory environment, MSCs can orchestrate local and systemic innate and adaptive immune responses. Inflammation-primed MSCs express inducible nitric oxide synthase (iNOS) in rodents (such as mice, rats, hamsters and rabbits) or indoelmine-2–3-dixoygenase (IDO) in other mammalian species to suppress T-cell responsiveness [7, 69]. Murine MSCs express high levels of iNOS in response to activation by proinflammatory cytokines and produce NO, which is one of the major mediators of T-cell suppression by MSCs. MSCs from inducible NOS−/− mice had reduced abilities to suppress T-cell proliferation [70]. Moreover, IL-17 dramatically enhanced the immunosuppressive effects of MSCs by promoting iNOS expression in MSCs. This reinforcing effect of IL-17 was mediated by enhancing mRNA stability by downregulating ARE/poly(U)-binding/degradation factor 1 (AUF1), a well-known factor that promotes mRNA decay [71]. However, NO-mediated immunosuppression by MSCs may become immune-enhancing under inadequate stimulation. When iNOS production was inhibited or genetically ablated, MSCs strongly enhanced T-cell proliferation in vitro and the delayed-type hypersensitivity response in vivo. It is likely that in the absence of NO, MSC-produced chemokines can still attract immune cells [72].
The immunosuppressive effects of human MSCs in response to stimulation with IFN-γ plus TNF-α or IL-1 primarily rely on IDO. IDO is a catabolic enzyme that degrades tryptophan to kynurenine. Depletion of local tryptophan by IDO activates the stress-response kinase general control nonderepressible 2 (GCN2), which senses amino acid withdrawal. GCN2 activation inhibits T-cell proliferation and biases naive CD4+ T cells toward differentiation into Treg cells [73, 74]. Moreover, the soluble factors (kynurenine and downstream metabolites) generated by IDO can bind and activate the aryl hydrocarbon receptor, which can also promote Treg cell differentiation [75] and induce dendritic cells to adopt an immunosuppressive phenotype [76].
A recent study showed that local injection of ADSCs significantly reduced alveolar bone loss and the ratio of iNOS+/CD206+ macrophages in a rat model of periodontitis [77]. IDO expression in ADSCs was upregulated by inflammatory stimuli and was required for the therapeutic effects of ADSCs in experimental periodontitis. Mechanistically, ADSCs release kynurenine, which is a tryptophan metabolite catalyzed by IDO, to activate the aryl hydrocarbon receptor and enhance its downstream target NFE2L2 in macrophages. NFE2L2-encoded NRF2 not only functions as a master regulator of antioxidant defense but also represses the expression of inflammatory genes. As expected, NRF2 upregulation in macrophages was inhibited by inhibiting IDO and 1-methyltryptophan (1-MT), and the anti-inflammatory effect of ADSCs on macrophages was blocked when NRF2 expression in macrophages was silenced. Kynurenic acid, another IDO-derived metabolite that shares the same aryl hydrocarbon receptor as kynurenine, can promote TNF-α-stimulated gene-6 (TSG-6) expression and alleviate neutrophil infiltration in injured lungs [78].
In addition to iNOS and IDO, the immunosuppressive properties of MSCs are also mediated through the expression of COX1/COX2 enzymes and the production of prostaglandin E2 (PGE2). While COX1 is constitutively expressed in most mammalian cells, COX2 is an inducible enzyme and cab ne induced by proinflammatory cytokines and growth factors [79]. PGE2 has been reported to mediate the immunoregulatory function of MSCs via the induction of IL-10 secretion by macrophages [80], suppression of NK cell cytotoxic function [81] and suppression of Th17 differentiation [82].
DSCs exert their regulatory effects on inflammation similar to MSCs derived from other tissues. GMSCs specifically suppress peripheral blood lymphocyte proliferation and induce the expression of a wide range of immunosuppressive factors, including IDO, iNOS, and COX-2, in response to inflammatory cytokines [54]. PGE2 plays a crucial role in PDLSC-mediated immunomodulation and periodontal tissue regeneration in a miniature pig periodontitis model [83]. GMSC-induced blockade of de novo synthesis of proinflammatory cytokines by mast cells is also mediated by PGE2 [84].
Growth factors, cytokines and chemokines
MSCs secrete a variety of growth factors, which not only function as effector molecules during tissue repair but also regulate the differentiation of MSCs themselves. During adipose tissue injury and repair, fibroblast growth factor-2 strongly promotes ADSC proliferation and HGF secretion through the c-Jun N-terminal kinase signaling pathway. HGF expression in MSCs contributes to the regeneration of adipose tissue and suppression of fibrogenesis after injury [85]. The secretion of VEGF-C by MSCs was greatly increased by pretreatment with the inflammatory cytokines IFN-γ and TNF-α and played a key role in mediating the wound healing effect of MSCs [86]. In addition, VEGF-C treatment significantly increased the expression of RUNX2 and osteogenic marker genes and the mineralization of MSCs [87]. DPSCs overexpressing hepatocyte growth factor were shown to facilitate the repair of DSS-induced ulcerative colitis [88].
MSCs can produce insulin-like growth factor 2 (IGF-2) to preprogram macrophages to undergo anti-inflammatory polarization during their maturation [89]. When exposed to low concentrations of IGF-2, maturing macrophages are committed to oxidative phosphorylation (OXPHOS) and consequently increase the expression of PD-L1, which is required for the beneficial effect of IGF-2 on experimental autoimmune encephalomyelitis. Further study showed that IGF-2 at low concentrations conferred anti-inflammatory properties to maturing macrophages by binding to IGF2R, which resulted in its nuclear translocation, the activation of GSK3 α/β, and Dnmt3a-mediated repression of v-ATPase. Due to the lack of v-ATPase, protons are rerouted to mitochondria from lysosomes, enabling the increase in OXPHOS that is characteristic of anti-inflammatory macrophages [90].
Similar to BM-MSCs, which are capable of reprogramming macrophages into the M2 phenotype [91], GMSCs can also promote the polarization of PBMC-derived macrophages toward the M2 phenotype. IL-6 and GM-CSF synergistically contribute to the induction of M2 macrophages in coculture with GMSCs [92]. A comparison of DPSCs and DFSCs isolated from the same tooth showed that DFSCs proliferated faster than DPSCs, while the latter produced more transforming growth factor (TGF)-β and suppressed the proliferation of peripheral blood mononuclear cells [93]. DPSCs were also shown to inhibit acute allogeneic immune responses by releasing TGF-β, which inhibits the production of IgM and IgG by allogeneic activation of responder B lymphocytes [94]. Moreover, similar to DPSCs, DPSCs isolated from inflamed pulp can suppress macrophage functions via the TNF-α/IDO axis, thereby providing a physiologically relevant context for their innate immunomodulatory activity in the dental pulp and their capability for pulp repair [31].
The chemokines expressed by MSCs have been reported to regulate the migration and function of immune cells to maintain tissue homeostasis. BM-MSCs can rapidly express MCP1 in response to circulating TLR ligands or bacterial infection and induce monocyte trafficking into the bloodstream [95]. BM-MSC-derived CCL2 inhibits CD4+ T-cell activation by suppressing STAT3 phosphorylation and reversing symptomatic neuroinflammation in experimental autoimmune encephalomyelitis [96]. CCL2 and CXCL12 secreted by BM-MSCs acts as a heterodimer to upregulate IL-10 expression in CCR2+ macrophages in vitro, and CCL2 expression by MSCs is required for IL-10-mediated polarization of intestinal and peritoneal resident macrophages in vivo [97]. We also found that ionizing radiation could activate the cGAS-STING signaling pathway in MSCs and consequently lead to the upregulation of CCL5. CCL5 can then recruit macrophages to the lung to establish a microenvironment that is conducive to tumor metastasis [98].
EVs
MSCs also exert their therapeutic effects through the secretion of EVs. EVs are lipid-bound vesicles that are secreted into the extracellular space. The cargo of EVs consists of proteins, organelles and nucleic acids, which are responsible for the regulatory function of MSCs. The main subtypes of EVs include exosomes (50–100 nm in diameter), microvesicles (MVs; 0.1–1 μm in diameter), and apoptotic bodies (1 μm to 5 μm in diameter). MSCs have been shown to secrete at least 3 types of EVs, which can be differentially isolated based on their affinities for membrane lipid binding ligands and are likely to have different biogenesis pathways and different functions [99].
MSC-derived EVs have been shown to exhibit potent immunoregulatory properties. For example, human adipose MSC-derived exosomes inhibit the differentiation and activation of T cells and reduce T-cell proliferation and IFN-γ release [100]. The exosomes secreted by murine ADSCs can skew macrophages toward M2 polarization, which is mainly dependent on active STAT3 transferred by exosomes. Obese mice treated with ADSC-derived exosomes exhibited reduced adipose tissue inflammation, improved metabolic homeostasis, and resistance to obesity progression [101].
DSCs can also produce EVs to promote immune homeostasis. GMSCs even have a higher yield of small EVs than BM-MSCs and skin MSCs [96]. These small EVs are rich in IL-1RA. Mechanistically, GMSCs use the Fas/Fas-associated phosphatase-1/caveolin-1 complex to activate SNARE-mediated membrane fusion to secrete small EVs. Small EVs can accelerate wound healing in the gingiva [102]. Examination of inflammatory bone loss in a ligature-induced periodontitis mouse model showed that local injection of GMSC-derived exosomes significantly reduced periodontal bone resorption and the number of tartrate-resistant acid phosphatase-positive osteoclasts, and GMSCs treated with TNF-α exhibited further enhanced benefits [34]. Moreover, receptor activator of NF-κB ligand (RANKL) expression was upregulated by Wnt5a in periodontal ligament cells, and miR-1260b in exosomes could suppress the Wnt5a-mediated RANKL pathway and thus inhibit osteoclastogenesis. Cell-conditioned medium and purified EVs from PDLSCs reduced inflammatory damage in animal models of experimental autoimmune encephalomyelitis. The vesicles contained the anti-inflammatory cytokines IL-10 and TGF-β [103]. Exosomes secreted by PDLSCs were also shown to reduce bone loss in periodontitis [104].
MSCs undergo mitophagy in response to oxidative stress and promote the translocation of depolarized mitochondria to the plasma membrane via arrestin domain-containing protein 1-mediated microvesicles. The vesicles are then engulfed and reused by macrophages, resulting in enhanced macrophage bioenergetics and impaired TLR signaling [105]. MSC EV-mediated mitochondrial transfer also promotes an anti-inflammatory and highly phagocytic macrophage phenotype by enhancing macrophage oxidative phosphorylation [106].
Exosomal miR-182 delivery from BM-MSCs to macrophages converted inflammatory macrophages to the M2 phenotype by targeting TLR4, reducing inflammation levels in a mouse model of myocardial ischemia/reperfusion [107].
Apoptotic vesicles (apoVs)
During the treatment of immune diseases with MSCs, researchers found that despite being immunosuppressive, MSCs were undetectable after administration. Galleu. et al. found that MSCs were actively induced to undergo perforin-dependent apoptosis in a murine graft-versus-host disease model and that their apoptosis is essential to initiate MSC-induced immunosuppression. After infusion, recipient phagocytes engulf apoptotic MSCs and produce IDO, which mediates the immunosuppressive function of MSCs [108]. In addition, it was reported that calreticulin exposed on the surface of MSC-derived apoVs acted as a critical ‘eat-me’ signal mediating apoV efferocytosis by macrophages. MSC-derived apoVs can induce macrophage reprogramming at the transcriptional level, leading to the inhibition of macrophage accumulation and the transformation of macrophages toward an anti-inflammatory phenotype in T2D livers [109]. A recent study showed that MSC-derived apoVs could attenuate sepsis by switching neutrophil NETosis to apoptosis. ApoVs were recruited by bone marrow NETs via electrostatic charge interactions. FasL in apoVs could mediate the cell death pattern switch in neutrophils from NETosis to apoptosis and then inhibit the migration of neutrophils from bone marrow to distal organs, alleviating organ injury and sepsis [110].
T-cell death-mediated immunoregulation
It has been reported that BM-MSCs induce T-cell apoptosis via the FasL-dependent Fas pathway. Fas and FasL are members of the tumor necrosis factor (TNF)-receptor and TNF family, respectively. The binding of Fas to FasL results in the activation of a caspase cascade that initiates apoptosis. Apoptotic T cells subsequently trigger macrophages to produce high levels of TGF-β, which in turn leads to an increase in regulatory T cells and immune tolerance [111]. DPSCs can also induce activated T-cell apoptosis by the same mechanism and ameliorate inflammation-related tissue injuries when systemically infused into a murine colitis model [52]. Moreover, when cocultured with normal B cells, human PDLSCs suppressed B-cell proliferation, differentiation and migration. Mechanistically, human PDLSCs suppressed B-cell activation through cell-to-cell contact, which was mostly mediated by PD1 and PD-L1. Furthermore, the transplantation of allogenic human PDLSCs suppresses humoral immunity in a minipig periodontitis model [112].
How MSCs/DSCs acquire and maintain their immunomodulatory properties
Cytokines and other secretory factors
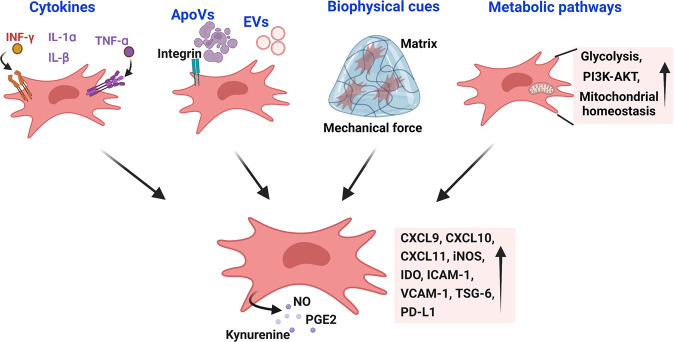
A landmark paper by Ren et al. demonstrated that the immunomodulatory properties of murine MSCs were not innate but were elicited by a set of inflammatory cytokines [69] (Fig. 3). T-cell chemotactic chemokines such as CXCL9, CXCL10, and CXCL11 can be upregulated over one million-fold. Nos2, which encodes iNOS, is upregulated to a similar extent and inhibits T cells. It should be noted that the immunoregulatory effects of MSCs are most efficiently elicited by a combination of a set of cytokines. Among these cytokines, IFN-γ appears to be indispensable for MSCs to acquire maximal immunosuppressive properties, although the concomitant presence of any of three other proinflammatory cytokines, TNF-α, IL-1α or IL-β, is also required [69]. Interestingly, instead of using NO as the effector to inhibit T cells by murine MSCs, human MSCs primarily use IDO to exert their inhibitory effect on T cells [113, 114]. MSCs also require the expression of adhesion molecules to exert their immunoregulatory effects [115]. The expression levels of ICAM-1 and VCAM-1 on MSCs were greatly upregulated in response to IFN-γ and inflammatory cytokines (TNF-α or IL-1). The immunosuppressive effect was significantly attenuated when the adhesion molecules were genetically deleted or functionally blocked, indicating the importance of cell‒cell contact in immunosuppression by MSCs. The inflammatory factor IL-17 was recently shown to synergize with IFN-γ and TNF-α to increase PD-L1 expression in MSCs [116]. Interestingly, the enhanced PD-L1 expression induced by IL-17 appeared to depend on iNOS because the upregulation of PD-L1 was not observed in deficient MSCs or in the presence of an iNOS inhibitor. It was recently reported that endothelial cell-derived IL-6 could activate IL-6R and STAT3 in DPSCs [117]. DPSCs in which STAT3 was knocked down were compromised in their vasculogenic potential when transplanted into immunodeficient mice.
Fig. 3.
The mechanisms by which MSCs/DSCs acquire and maintain their immunomodulatory properties. The immunomodulatory properties of MSCs/DSCs are not constitutive but are induced by proinflammatory cytokines. Cytokines activate PI3K and AKT to initiate glycolysis, which is critically required for MSCs/DSCs to produce high levels of chemokines, adhesion molecules and effector molecules. Apoptotic bodies can endow MSCs/DSCs with enhanced immunomodulatory properties. The extracellular matrix and scaffold also support the immunomodulatory functions of MSCs/DSCs. MSCs/DSCs could also sacrifice themselves to fulfill their mission of immunosuppression
While the aforementioned inflammatory cytokines are critical in inducing the immune modulatory properties of MSCs, the anti-inflammatory cytokine TGF-β has the opposite effect on the immunosuppressive effect of MSCs on anti-CD3-activated splenocytes. TGF-β can inhibit iNOS expression in a SMAD3-dependent manner. TGF-β produced by MSCs can act in an autocrine manner to reduce iNOS [118].
Single-cell transcriptome analysis of human oral mucosa in healthy individuals and patients with periodontitis was recently reported [119]. The transcriptome atlas revealed a complex cellular landscape of oral mucosal tissues consisting of epithelial, endothelial, immune and stromal cells. The study identified distinct epithelial and stromal cell populations with inflammatory signatures that promoted antimicrobial defenses and neutrophil recruitment. Stromal cells are particularly active in recruiting neutrophils in periodontitis. However, the exact stimuli that activate stromal cells to produce chemokines for neutrophils in periodontitis remain to be characterized.
Apoptotic vesicles and metabolites
In the human body, billions of cells undergo apoptosis every day, and a large number of apoptotic bodies need to be cleared to maintain tissue homeostasis [120]. Apoptotic cells release nucleotides as ‘find-me’ signals for clearance by phagocytes [121]. It was recently shown that apoptotic cells release metabolites, such as ATP, spermidine and creatine, in a regulated manner [122]. Uptake of these apoptotic bodies by phagocytes, which is also known as efferocytosis, has long been known to confer anti-inflammatory properties on recipient cells [123–125]. It appears that MSCs also need to feed on apoptotic bodies to maintain their stemness [126]. When the formation of apoptotic bodies was reduced, as in Fas-deficient MRL/lpr and Caspase 3−/− mice, the self-renewal and osteogenic/adipogenic differentiation of MSCs was significantly impaired. The infusion of exogenous apoptotic bodies rescued these impairments and ameliorated the osteopenia phenotype in MRL/lpr, Caspase 3−/− and ovariectomized mice. MSCs could engulf apoptotic bodies via integrin αvβ3 and reuse the apoptotic body-derived ubiquitin ligase RNF146 and miR-328-3p to inhibit Axin1 and thereby activate the Wnt/β-catenin pathway [126]. In addition to maintaining MSC stemness, the apoptotic cells engulfed by MSCs could also increase the immunosuppressive properties of the latter [127]. The apoptotic cells stimulated MSCs to express COX2 and consequently produced more PGE2, which inhibited T-cell responses.
As discussed previously, MSCs produce abundant levels of EVs to influence immune cells and other types of cells. It is likely that MSCs may also take up circulating vesicles and change their function and behaviors accordingly. The metabolites released by vesicles, as well as those produced from other routes, could have an impact on the immunomodulatory functions of MSCs. Future studies should provide more insights into the effects of vesicles on MSCs.
Biophysical cues
Mechanical force plays a critical role in determining the fate and characteristics of PDLSCs. Alveolar bone osteocytes produce sclerostin, a Win inhibitor, to negatively regulate Gli1+ PDLSC activity. Sclerostin expression is inhibited by physiological occlusal force [16]. Force-treated PDLSCs exhibited increased proliferation and reduced differentiation into osteocytes and adipocytes. These cells also highly expressed cytokines and RANKL to promote the generation of osteoclasts and bone remodeling [128]. Mechanical force was also shown to increase the level of exosomal proteins in PDLSCs to facilitate exosome internalization by macrophages and promote osteoclast differentiation [129]. These findings indicate that mechanical force may govern the immunomodulatory property of DSCs. Studies of the effect of the matrix and other biophysical properties on the function of other types of MSCs showed that biophysical cues could determine the immunoregulatory function of MSCs [130]. Many matrix biophysical parameters, such as fiber orientation, stiffness, dimensionality, and viscoelasticity, could impact the immunomodulatory function of MSCs.
A recent study showed that MSCs on a soft matrix produce higher levels of immunomodulatory factors than those on a stiff matrix [131]. The expression of TSG-6, the key mediator of the immunoregulatory effect of MSCs, was mechanosensitively regulated by the MAPK and Hippo signaling pathways and the downstream AP1 complex. Interestingly, when MSCs were exposed to various grades of wall shear stress within a scalable conditioning platform, their immunomodulatory potential was enhanced independent of classical proinflammatory cytokines, as evidenced by increased production of PGE2 and IDO1, as well as the suppression of TNF-α and IFN-γ production by activated immune cells [132]. Another study showed that compared with exosomes generated by MSCs in 2D culture, those produced by MSCs in 3D culture (3D-exos) exhibited enhanced anti-inflammatory effects in a ligature-induced model of periodontitis by restoring the Th17 and Treg balance in inflamed periodontal tissues [133]. 3D-exos were shown to be enriched in in miR-1246, which can suppress the expression of Nfat5, which promotes Th17 cell polarization. Local injection of 3D-exos attenuated experimental colitis. These findings indicate that biophysical conditions can have a great impact on the immunomodulatory properties of MSCs.
Anisotropic silk protein nanofiber-based hydrogels were developed to mimic the physical microenvironment inside the blastocele and were more effective in sustaining the stemness of mouse embryonic stem cells (mESCs) than the classical recipe containing leukemia inhibitory factor and mouse embryonic fibroblasts (MEFs) [134]. The mESCs on hydrogels could achieve pluripotency, developmental capacity, and germline transmission that was superior to those cultured with the standard protocol. Such biomaterials could stimulate the production of autocrine factors that maintain the pluripotency and propagation of ESCs. It is possible that the biophysical niches in which MSCs reside in situ play a critical role in determining their immunomodulatory functions. Future studies are expected to provide more insights into the role of biomechanical factors in the regulation of the immunomodulatory capacity of MSCs.
Metabolic pathways
The metabolic pathways required for MSCs to acquire and sustain their immunoregulatory functions have been studied recently. Inflammatory cytokines cause metabolic shifts in MSCs, including enhancing glycolysis and increasing fatty acid oxidation, but only interference with glycolysis but not fatty acid oxidation impairs IDO upregulation and the immunosuppressive function of MSCs [135, 136]. The ATP synthesis inhibitor oligomycin enhanced the immunosuppressive and therapeutic functions of MSCs [137]. It was shown that enhanced glucose turnover was associated with STAT1 glycosylation. Inhibiting the responsible O-acetylglucosamine (O-GlcNAc) transferase abolishes STAT1 activity and IDO upregulation [135]. It was recently shown that the PI3K-AKT signaling axis was rapidly activated and required for skewing toward glycolysis induced by TNF-α and IFN-γ. Moreover, MSCs expressing dominant-negative AKT were compromised in their therapeutic efficacy on IBD [136].
Maintaining proper mitochondrial turnover and function is critical for cellular homeostasis and many biological processes. It is well recognized that disrupted mitochondrial homeostasis contributes to neural degeneration. Likewise, mitochondrial dysfunction may compromise the functions of MSCs and lead to chronic inflammation-associated bone diseases, such as periodontitis and osteoarthritis. It was recently shown that chronic inflammation leads to excess Ca2+ transfer from the ER to mitochondria, and mitochondrial calcium overload further damages mitochondria. Due to the inhibition of mitophagy under chronic inflammatory conditions, damaged mitochondria continuously accumulate in MSCs [138]. Nanoparticles fabricated to capture Ca2+ around mitochondria reduced mitochondrial calcium flux, physiologically restoring the function of mitochondria and MSCs.
The cell death pathway of MSCs and immunomodulatory functions
While transplanted MSCs have been widely documented to exert potent immunomodulatory effects in various autoimmune diseases and graft-versus-host disease (GvHD), MSCs are rapidly cleared in recipients after their administration. The dynamics of transplanted MSCs in recipients indicate that MSCs do not need to persist very long to exert their therapeutic effects. It appears that their only mission is to deliver signals or messages to the recipients or to awaken host tissues. Using a murine model of GvHD, Dazzi and colleagues observed that MSCs actively underwent perforin-dependent apoptosis, which is essential for MSCs to have immunosuppressive functions [108]. In GvHD patients who received MSCs, only those with high cytotoxic activity against MSCs responded to the MSC infusion. The infusion of apoptotic MSCs generated ex vivo similarly exerted therapeutic effects. After the infusion, recipient phagocytes engulfed apoptotic MSCs and produced IDO1, the effector molecule for immunosuppression. Apoptosis was also shown to be required for DPSCs to have immunosuppressive functions in an allergic airway inflammation model [139]. A recent study showed that ferroptosis in MSCs induced by superparamagnetic iron oxide promoted efferocytosis by macrophages and thus enhanced the protective effect on septic mice, while these benefits were impaired after MSCs were treated with inhibitors of ferroptosis [140]. Whether and how the other fates of MSCs, such as pyroptosis and necroptosis, affect the immunosuppressive functions of MSCs remain to be explored.
Perspectives on strategies for enhancing the therapeutic properties of MSCs/DSCs
With increased understanding of the mechanisms by which MSCs/DSCs exert their immunoregulatory effects and the extrinsic and intrinsic factors that stimulate and sustain the immunoregulatory functions of MSCs, it is possible to manipulate MSCs by various means to achieve enhanced therapeutic efficacies. We discuss some of the strategies that can increase MSC potency.
Like immune cells that need to be activated by intrinsic or extrinsic signals to achieve their mission, MSCs also need to be activated or induced to acquire their immunomodulatory function. Inflammatory cytokines produced by immune cells or other types of cells serve as essential stimuli to induce immunomodulatory functions [69]. Thus, MSCs and immune cells appear to form a negative feedback loop to keep immune responses in check. Interestingly, while TGF-β is generally regarded as an anti-inflammatory factor due to its ability to induce Treg differentiation and tissue fibrosis, it compromises the immunosuppressive function of MSCs. How to make the most use of this MSC-immune cell feedback loop to harness the immunomodulatory function of MSCs/DSCs clearly represents a promising venue for advancing MSC-based medicine.
Glycolysis is critical for the inflammatory function of various immune cells, and blocking glycolysis using 2-deoxy-D-glucose (2-DG) greatly compromises the proinflammatory response of immune cells. However, unlike in immune cells, this metabolic pathway serves to support the immunosuppressive function of MSCs. To behave as immunomodulators, MSCs need to be switched to a massive biosynthesis mode to produce a large number of chemokines and effector molecules in large quantities. Glucose understandably provides the carbon resources that are in great demand. Therefore, any means that can boost glycolysis in MSCs may increase the production of factors by MSCs. Because MSCs and inflammatory immune cells may compete for glucose in the tissue microenvironment, strategies that block glycolysis in immune cells but favor glucose uptake and glycolysis in MSCs will likely enhance their immunomodulatory functions. Metabolites derived from glycolysis, such as lactate, may also participate in the immunomodulatory function of MSCs. Mitochondria, where some crucial metabolic activities take place, may also contribute to the regulation of MSC immunomodulatory functions, and the exact roles may be elucidated in future studies.
Since the functions and fates of MSCs are determined by various biophysical cues they sense, it is possible to amplify the therapeutic functions of MSCs by implanting special MSC-laden scaffolds. MSCs seeded on certain matrices may possess functions that are not present in MSCs in monolayer cultures and last longer in vivo. An emerging field called developmental tissue engineering mimics morphogenetic processes during development to harvest biofunctional tissues and organs [141]. These approaches can be used to generate MSC-based materials that have long-lasting immunomodulatory functions in vivo.
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2021YFA1100600, 2022YFA0807300), the National Natural Science Foundation of China (81972682 and 32150710523), the Jiangsu Province International Joint Laboratory for Regenerative Medicine Fund and the National Center for International Research-Cambridge-Su Genomic Research Center (2017B01012).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Peishan Li, Qianmin Ou.
Contributor Information
Songtao Shi, Email: shisongtao@mail.sysu.edu.cn.
Changshun Shao, Email: shaoc@suda.edu.cn.
References
- 1.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Li P, Zhang S, Xiang J, Yang R, Liu J, et al. Prrx1 marks stem cells for bone, white adipose tissue and dermis in adult mice. Nat Genet. 2022 doi: 10.1038/s41588-022-01227-4. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe PT. Dental mesenchymal stem cells. Development. 2016;143:2273–80. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515–30. doi: 10.1016/j.stem.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Krampera M, Le Blanc K. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell. 2021;28:1708–25. doi: 10.1016/j.stem.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 8.Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–33. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Gronthos S, Shi S. Dental pulp stem cells. Methods Enzymol. 2006;419:99–113. doi: 10.1016/S0076-6879(06)19005-9. [DOI] [PubMed] [Google Scholar]
- 10.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui B, Wu D, Xiang L, Fu Y, Kou X, Shi S. Dental pulp stem cells: from discovery to clinical application. J Endod. 2020;46:S46–S55. doi: 10.1016/j.joen.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 2011;108:6503–8. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Y, Chai Y. Regulatory mechanisms of jaw bone and tooth development. Curr Top Dev Biol. 2019;133:91–118. doi: 10.1016/bs.ctdb.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–4. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–73. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Men Y, Wang Y, Yi Y, Jing D, Luo W, Shen B, et al. Gli1+ periodontium stem cells are regulated by osteocytes and occlusal force. Dev Cell. 2020;54:639–54. doi: 10.1016/j.devcel.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Fu H, Wu X, Duan Y, Zhang S, Hu H, et al. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells. Sci Adv. 2020;6:eaay1514. doi: 10.1126/sciadv.aay1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2019;13:152–9. doi: 10.1007/s11684-018-0628-x. [DOI] [PubMed] [Google Scholar]
- 19.Feng J, Jing J, Li J, Zhao H, Punj V, Zhang T, et al. BMP signaling orchestrates a transcriptional network to control the fate of mesenchymal stem cells in mice. Development. 2017;144:2560–9. doi: 10.1242/dev.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Cox MK, Coricor G, MacDougall M, Serra R. Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Dev Biol. 2013;382:27–37. doi: 10.1016/j.ydbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing J, Feng J, Li J, Zhao H, Ho TV, He J, et al. Reciprocal interaction between mesenchymal stem cells and transit amplifying cells regulates tissue homeostasis. eLife. 2021;10, 10.7554/eLife.59459. [DOI] [PMC free article] [PubMed]
- 22.Okic-Dordevic I, Obradovic H, Kukolj T, Petrovic A, Mojsilovic S, Bugarski D, et al. Dental mesenchymal stromal/stem cells in different microenvironments- implications in regenerative therapy. World J Stem Cells. 2021;13:1863–80. doi: 10.4252/wjsc.v13.i12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aydin S, Sahin F. Stem cells derived from dental tissues. Adv Exp Med Biol. 2019;1144:123–32. doi: 10.1007/5584_2018_333. [DOI] [PubMed] [Google Scholar]
- 24.Zhou LL, Liu W, Wu YM, Sun WL, Dorfer CE, Fawzy El-Sayed KM. Oral mesenchymal stem/progenitor cells: the immunomodulatory masters. Stem Cells Int. 2020;2020:1327405. doi: 10.1155/2020/1327405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang HN, Xia Y, Yu Y, Wu RX, Gao LN, Chen FM. Stem cells derived from “inflamed” and healthy periodontal ligament tissues and their sheet functionalities: a patient-matched comparison. J Clin Periodontol. 2016;43:72–84. doi: 10.1111/jcpe.12501. [DOI] [PubMed] [Google Scholar]
- 26.Tomasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, Campisi G, et al. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 2017;8:179. doi: 10.1186/s13287-017-0633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Li S, Hu S, Yu W, Jiang B, Mao C, et al. UCHL1 impairs periodontal ligament stem cell osteogenesis in periodontitis. J Dent Res. 2022 doi: 10.1177/00220345221116031. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Liu X, Wang D, Zheng J, Chen L, Xie Q, et al. Periodontal inflammation-triggered by periodontal ligament stem cell pyroptosis exacerbates periodontitis. Front Cell Dev Biol. 2021;9:663037. doi: 10.3389/fcell.2021.663037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magalhaes FD, Sarra G, Carvalho GL, Pedroni ACF, Marques MM, Chambrone L, et al. Dental tissue-derived stem cell sheet biotechnology for periodontal tissue regeneration: a systematic review. Arch oral Biol. 2021;129:105182. doi: 10.1016/j.archoralbio.2021.105182. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Deng M, Hao M, Tang J. Periodontal ligament stem cells in the periodontitis niche: inseparable interactions and mechanisms. J Leukoc Biol. 2021;110:565–76. doi: 10.1002/JLB.4MR0421-750R. [DOI] [PubMed] [Google Scholar]
- 31.Lee S, Zhang QZ, Karabucak B, Le AD. DPSCs from inflamed pulp modulate macrophage function via the TNF-alpha/IDO axis. J Dent Res. 2016;95:1274–81. doi: 10.1177/0022034516657817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YT, Lee SM, Kou X, Karabucak B. The role of interleukin 6 in osteogenic and neurogenic differentiation potentials of dental pulp stem cells. J Endod. 2019;45:1342–8. doi: 10.1016/j.joen.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Inostroza C, Vega-Letter AM, Brizuela C, Castrillon L, Saint Jean N, Duran CM, et al. Mesenchymal stem cells derived from human inflamed dental pulp exhibit impaired immunomodulatory capacity in vitro. J Endod. 2020;46:1091–8.e1092. doi: 10.1016/j.joen.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, et al. Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021;122:306–24. doi: 10.1016/j.actbio.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper LF, Ravindran S, Huang CC, Kang M. A role for exosomes in craniofacial tissue engineering and regeneration. Front Physiol. 2019;10:1569. doi: 10.3389/fphys.2019.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu BH, Zhou Q, Wang ZL. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J Biomater Appl. 2014;29:243–53. doi: 10.1177/0885328214521846. [DOI] [PubMed] [Google Scholar]
- 37.Ma L, Aijima R, Hoshino Y, Yamaza H, Tomoda E, Tanaka Y, et al. Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice. Stem Cell Res Ther. 2015;6:104. doi: 10.1186/s13287-015-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alksne M, Kalvaityte M, Simoliunas E, Gendviliene I, Barasa P, Rinkunaite I, et al. Dental pulp stem cell-derived extracellular matrix: autologous tool boosting bone regeneration. Cytotherapy. 2022;24:597–607. doi: 10.1016/j.jcyt.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Collignon AM, Castillo-Dali G, Gomez E, Guilbert T, Lesieur J, Nicoletti A, et al. Mouse Wnt1-CRE-Rosa(Tomato) dental pulp stem cells directly contribute to the calvarial bone regeneration process. Stem Cells. 2019;37:701–11. doi: 10.1002/stem.2973. [DOI] [PubMed] [Google Scholar]
- 40.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Rao N, Jiang H, Dai Y, Yang S, Yang H, et al. Small extracellular vesicles from dental follicle stem cells provide biochemical cues for periodontal tissue regeneration. Stem Cell Res Ther. 2022;13:92. doi: 10.1186/s13287-022-02767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Yang G, Li J, Ding M, Zhou N, Dong H, et al. Stem cell therapies for periodontal tissue regeneration: a network meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:427. doi: 10.1186/s13287-020-01938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed MN, Shi D, Dailey MT, Rothermund K, Drewry MD, Calabrese TC, et al. Dental pulp cell sheets enhance facial nerve regeneration via local neurotrophic factor delivery. Tissue Eng Part A. 2021;27:1128–39. doi: 10.1089/ten.TEA.2020.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu J, Wang X, Zhou H, Zhang C, Wang Y, Huang J, et al. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 2020;11:42. doi: 10.1186/s13287-019-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zarubova J, Hasani-Sadrabadi MM, Dashtimoghadam E, Zhang X, Ansari S, Li S, et al. Engineered delivery of dental stem-cell-derived extracellular vesicles for periodontal tissue regeneration. Adv Health. Mater. 2022;11:e2102593. doi: 10.1002/adhm.202102593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sui B, Chen C, Kou X, Li B, Xuan K, Shi S, et al. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98:27–35. doi: 10.1177/0022034518808754. [DOI] [PubMed] [Google Scholar]
- 47.Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J Dent Res. 2018;97:1137–43. doi: 10.1177/0022034518772260. [DOI] [PubMed] [Google Scholar]
- 48.Sharma Y, Shobha K, Sundeep M, Pinnelli VB, Parveen S, Dhanushkodi A. Neural basis of dental pulp stem cells and its potential application in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2022;21:62–76. doi: 10.2174/1871527320666210311122921. [DOI] [PubMed] [Google Scholar]
- 49.Luzuriaga J, Pastor-Alonso O, Encinas JM, Unda F, Ibarretxe G, Pineda JR. Human dental pulp stem cells grown in neurogenic media differentiate into endothelial cells and promote neovasculogenesis in the mouse brain. Front Physiol. 2019;10:347. doi: 10.3389/fphys.2019.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang X, Li W, Wen X, Zhang Z, Chen W, Yao G, et al. Transplantation of dental tissue-derived mesenchymal stem cells ameliorates nephritis in lupus mice. Ann Transl Med. 2019;7:132. doi: 10.21037/atm.2019.02.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dave JR, Tomar GB. Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Crit Rev Biomed Eng. 2018;46:429–68. doi: 10.1615/CritRevBiomedEng.2018027342. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Wang L, Jin Y, Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res. 2012;91:948–54. doi: 10.1177/0022034512458690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chin YT, Liu CM, Chen TY, Chung YY, Lin CY, Hsiung CN, et al. 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside-stimulated dental pulp stem cells-derived conditioned medium enhances cell activity and anti-inflammation. J Dent Sci. 2021;16:586–98. doi: 10.1016/j.jds.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-derived mesenchymal stem cells: potential application in tissue engineering and regenerative medicine – a comprehensive review. Front Immunol. 2021;12:667221. doi: 10.3389/fimmu.2021.667221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189:787–98. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–83. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 58.Dave JR, Chandekar SS, Behera S, Desai KU, Salve PM, Sapkal NB, et al. Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age. Sci Adv. 2022;8:eabm6504. doi: 10.1126/sciadv.abm6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Yang B, Tian J, Hong H, Du Y, Li K, et al. Dental follicle stem cells ameliorate lipopolysaccharide-induced inflammation by secreting TGF-beta3 and TSP-1 to elicit macrophage M2 polarization. Cell Physiol Biochem. 2018;51:2290–308. doi: 10.1159/000495873. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi S, Shibata R, Yamamoto N, Nishikawa M, Hibi H, Tanigawa T, et al. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. doi: 10.1038/srep16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumura-Kawashima M, Ogata K, Moriyama M, Murakami Y, Kawado T, Nakamura S. Secreted factors from dental pulp stem cells improve Sjogren’s syndrome via regulatory T cell-mediated immunosuppression. Stem Cell Res Ther. 2021;12:182. doi: 10.1186/s13287-021-02236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong J, Sakai K, Koma Y, Watanabe J, Liu K, Maruyama H, et al. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem Biophys Res Commun. 2021;575:28–35. doi: 10.1016/j.bbrc.2021.08.046. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Luo L, He Y, Li R, Xiang Y, Xing Z, et al. Dental pulp stem cell-derived exosomes alleviate cerebral ischaemiareperfusion injury through suppressing inflammatory response. Cell Prolif. 2021;54:e13093. doi: 10.1111/cpr.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. doi: 10.1186/s13287-016-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10, 10.1126/scitranslmed.aaf3227. [DOI] [PubMed]
- 66.Li W, Jiao X, Song J, Sui B, Guo Z, Zhao Y, et al. Therapeutic potential of stem cells from human exfoliated deciduous teeth infusion into patients with type 2 diabetes depends on basal lipid levels and islet function. Stem Cells Transl Med. 2021;10:956–67. doi: 10.1002/sctm.20-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrarotti F, Romano F, Gamba MN, Quirico A, Giraudi M, Audagna M, et al. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: a randomized controlled clinical trial. J Clin Periodontol. 2018;45:841–50. doi: 10.1111/jcpe.12931. [DOI] [PubMed] [Google Scholar]
- 68.Paganelli A, Trubiani O, Diomede F, Pisciotta A, Paganelli R. Immunomodulating profile of dental mesenchymal stromal cells: a comprehensive overview. Front Oral Health. 2021;2:635055. doi: 10.3389/froh.2021.635055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 71.Han X, Yang Q, Lin L, Xu C, Zheng C, Chen X, et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014;21:1758–68. doi: 10.1038/cdd.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–13. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 75.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–73. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Yuan Y, Chen H, Dai H, Li J. Indoleamine 2,3-dioxygenase mediates the therapeutic effects of adipose-derived stromal/stem cells in experimental periodontitis by modulating macrophages through the kynurenine-AhR-NRF2 pathway. Mol Metab. 2022;66:101617. doi: 10.1016/j.molmet.2022.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209–23. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharm Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 80.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galland S, Vuille J, Martin P, Letovanec I, Caignard A, Fregni G, et al. Tumor-derived mesenchymal stem cells use distinct mechanisms to block the activity of natural killer cell subsets. Cell Rep. 2017;20:2891–905. doi: 10.1016/j.celrep.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 82.Tatara R, Ozaki K, Kikuchi Y, Hatanaka K, Oh I, Meguro A, et al. Mesenchymal stromal cells inhibit Th17 but not regulatory T-cell differentiation. Cytotherapy. 2011;13:686–94. doi: 10.3109/14653249.2010.542456. [DOI] [PubMed] [Google Scholar]
- 83.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, et al. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–38. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29:1849–60. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 85.Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, et al. IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells. 2009;27:238–49. doi: 10.1634/stemcells.2008-0261. [DOI] [PubMed] [Google Scholar]
- 86.Zhu M, Chu Y, Shang Q, Zheng Z, Li Y, Cao L, et al. Mesenchymal stromal cells pretreated with pro-inflammatory cytokines promote skin wound healing through VEGFC-mediated angiogenesis. Stem Cells Transl Med. 2020;9:1218–32. doi: 10.1002/sctm.19-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami J, Ishii M, Suehiro F, Ishihata K, Nakamura N, Nishimura M. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem Biophys Res Commun. 2017;484:710–8. doi: 10.1016/j.bbrc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 88.Li N, Zhang Y, Nepal N, Li G, Yang N, Chen H, et al. Dental pulp stem cells overexpressing hepatocyte growth factor facilitate the repair of DSS-induced ulcerative colitis. Stem Cell Res Ther. 2021;12:30. doi: 10.1186/s13287-020-02098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du L, Lin L, Li Q, Liu K, Huang Y, Wang X, et al. IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 2019;29:1363–75 e1368. doi: 10.1016/j.cmet.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Lin L, Lan B, Wang Y, Du L, Chen X, et al. IGF2R-initiated proton rechanneling dictates an anti-inflammatory property in macrophages. Sci Adv. 2020;6, 10.1126/sciadv.abb7389.. [DOI] [PMC free article] [PubMed]
- 91.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G, et al. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by tolllike receptor agonists. Stem Cells Dev. 2011;20:695–708. doi: 10.1089/scd.2010.0145. [DOI] [PubMed] [Google Scholar]
- 94.Kwack KH, Lee JM, Park SH, Lee HW. Human dental pulp stem cells suppress alloantigen-induced immunity by stimulating T cells to release transforming growth factor beta. J Endod. 2017;43:100–8. doi: 10.1016/j.joen.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 95.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 97.Giri J, Das R, Nylen E, Chinnadurai R, Galipeau JCCL2. and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep. 2020;30:1923–34 e1924. doi: 10.1016/j.celrep.2020.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng Z, Jia S, Shao C, Shi Y. Irradiation induces cancer lung metastasis through activation of the cGAS-STING-CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 2020;11:326. doi: 10.1038/s41419-020-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. doi: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–47. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 102.Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10, 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed]
- 103.Rajan TS, Giacoppo S, Diomede F, Ballerini P, Paolantonio M, Marchisio M, et al. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci Rep. 2016;6:38743. doi: 10.1038/srep38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lei F, Li M, Lin T, Zhou H, Wang F, Su X. Treatment of inflammatory bone loss in periodontitis by stem cellderived exosomes. Acta Biomater. 2022;141:333–43. doi: 10.1016/j.actbio.2021.12.035. [DOI] [PubMed] [Google Scholar]
- 105.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–86. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–16. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9, 10.1126/scitranslmed.aam7828. [DOI] [PubMed]
- 109.Zheng C, Sui B, Zhang X, Hu J, Chen J, Liu J, et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021;10:e12109. doi: 10.1002/jev2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ou Q, Tan L, Shao Y, Lei F, Huang W, Yang N, et al. Electrostatic charge-mediated apoptotic vesicle biodistribution attenuates sepsis by switching neutrophil NETosis to apoptosis. Small. 2022;18:e2200306. doi: 10.1002/smll.202200306. [DOI] [PubMed] [Google Scholar]
- 111.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu O, Xu J, Ding G, Liu D, Fan Z, Zhang C, et al. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 2013;31:1371–82. doi: 10.1002/stem.1387. [DOI] [PubMed] [Google Scholar]
- 113.Ren G, Su J, Zhang L, Zhao X, Ling W, L’Huillie A, et al. Species variation in the mechanisms of mesenchymal stem cellmediated immunosuppression. Stem Cells. 2009;27:1954–62. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 114.Su J, Chen X, Huang Y, Li W, Li J, Cao K, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–96. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–8. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang S, Wang G, Zhang L, Li F, Liu K, Wang Y, et al. Interleukin-17 promotes nitric oxide-dependent expression of PDL1 in mesenchymal stem cells. Cell Biosci. 2020;10:73. doi: 10.1186/s13578-020-00431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh M, Zhang Z, Mantesso A, Oklejas AE, Nor JE. Endothelial-initiated crosstalk regulates dental pulp stem cell self-renewal. J Dent Res. 2020;99:1102–11. doi: 10.1177/0022034520925417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu C, Yu P, Han X, Du L, Gan J, Wang Y, et al. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. J Immunol. 2014;192:103–9. doi: 10.4049/jimmunol.1302164. [DOI] [PubMed] [Google Scholar]
- 119.Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184:4090–104. e4015. doi: 10.1016/j.cell.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Henson PM. Cell removal: efferocytosis. Annu Rev Cell Dev Biol. 2017;33:127–44. doi: 10.1146/annurev-cellbio-111315-125315. [DOI] [PubMed] [Google Scholar]
- 121.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580:130–5. doi: 10.1038/s41586-020-2121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. 2020;21:398–414. doi: 10.1038/s41580-020-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doran AC, Yurdagul A, Jr., Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254–67. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. doi: 10.1146/annurev-immunol-042617-053010. [DOI] [PubMed] [Google Scholar]
- 126.Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018;28:918–33. doi: 10.1038/s41422-018-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S, et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine. 2019;45:341–50. doi: 10.1016/j.ebiom.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin SS, He DQ, Wang Y, Zhang T, Yu HJ, Li ZX, et al. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020;53:e12912. doi: 10.1111/cpr.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang HM, Han CS, Cui SJ, Zhou YK, Xin TY, Zhang T, et al. Mechanical force-promoted osteoclastic differentiation via periodontal ligament stem cell exosomal protein ANXA3. Stem cell Rep. 2022;17:1842–58. doi: 10.1016/j.stemcr.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wong SW, Lenzini S, Giovanni R, Knowles K, Shin JW. Matrix biophysical cues direct mesenchymal stromal cell functions in immunity. Acta Biomater. 2021;133:126–38. doi: 10.1016/j.actbio.2021.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhuang Z, Zhang Y, Yang X, Yu T, Zhang Y, Sun K, et al. Matrix stiffness regulates the immunomodulatory effects of mesenchymal stem cells on macrophages via AP1/TSG-6 signaling pathways. Acta Biomater. 2022;149:69–81. doi: 10.1016/j.actbio.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 132.Skibber MA, Olson SD, Prabhakara KS, Gill BS, Cox CS., Jr Enhancing mesenchymal stromal cell potency: inflammatory licensing via mechanotransduction. Front Immunol. 2022;13:874698. doi: 10.3389/fimmu.2022.874698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43. doi: 10.1038/s41368-021-00150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hang Y, Ma X, Liu C, Li S, Zhang S, Feng R, et al. Blastocyst-inspired hydrogels to maintain undifferentiation of mouse embryonic stem cells. ACS Nano. 2021;15:14162–73. doi: 10.1021/acsnano.0c10468. [DOI] [PubMed] [Google Scholar]
- 135.Jitschin R, Bottcher M, Saul D, Lukassen S, Bruns H, Loschinski R, et al. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia. 2019;33:1783–96. doi: 10.1038/s41375-018-0376-6. [DOI] [PubMed] [Google Scholar]
- 136.Xu C, Feng C, Huang P, Li Y, Liu R, Liu C, et al. TNFalpha and IFNgamma rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res Ther. 2022;13:491. doi: 10.1186/s13287-022-03178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Contreras-Lopez R, Elizondo-Vega R, Luque-Campos N, Torres MJ, Pradenas C, Tejedor G, et al. The ATP synthase inhibition induces an AMPKdependent glycolytic switch of mesenchymal stem cells that enhances their immunotherapeutic potential. Theranostics. 2021;11:445–60. doi: 10.7150/thno.51631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhai Q, Chen X, Fei D, Guo X, He X, Zhao W, et al. Nanorepairers rescue inflammation-induced mitochondrial dysfunction in mesenchymal stem cells. Adv Sci. 2022;9:e2103839. doi: 10.1002/advs.202103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laing AG, Riffo-Vasquez Y, Sharif-Paghaleh E, Lombardi G, Sharpe PT. Immune modulation by apoptotic dental pulp stem cells in vivo. Immunotherapy. 2018;10:201–11. doi: 10.2217/imt-2017-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan Y, Li J, Wang J, Jiang Q, Yang J, Dou H, et al. Ferroptotic MSCs protect mice against sepsis via promoting macrophage efferocytosis. Cell Death Dis. 2022;13:825. doi: 10.1038/s41419-022-05264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Contessi Negrini N, Angelova Volponi A, Higgins CA, Sharpe PT, Celiz AD. Scaffold-based developmental tissue engineering strategies for ectodermal organ regeneration. Mater Today Bio. 2021;10:100107. doi: 10.1016/j.mtbio.2021.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]