Abstract
To cope with life in the mountains, populations of the same species can exhibit substantial variability in their altitudinal migration patterns and phenotypes in response to local weather conditions. Studying such variability can provide valuable insights into how local populations respond to environmental challenges, and this information can be useful for conservation efforts in mountain ecosystems. Here, we used δ2H values of feathers and blood to evaluate latitudinal variation in altitudinal migration patterns and its possible links with body size, oxidative status, and exploratory behavior in 72 individuals of rufous‐collared sparrow (Zonotrichia capensis) that breed at low and high elevations in the center (~33°) and south (~38°) of Chile. Our results show that both altitudinal migration patterns and oxidative status were significantly influenced by the latitude of breeding sites, while exploratory behavior was associated with elevation. Notably, we found that fast‐explorer birds inhabiting low elevations in central Chile displayed higher levels of oxidative damage than slow‐explorer birds. These outcomes underscore the possibility of local adaptations in response to diverse local environmental conditions in the Andes. We discuss the implications of latitude, elevation, and environmental temperature in shaping the observed patterns and highlight the significance of identifying local adaptations in mountain birds for better predicting their response to climate change and other challenges stemming from anthropogenic activities.
Keywords: altitudinal migration, exploratory behavior, local adaptations, mountains birds, oxidative stress
Individuals of the same species can substantially vary their altitudinal migration pattern and phenotype according to local weather conditions. Here, we evaluate latitudinal variation in altitudinal migration patterns and its possible links with body size, oxidative status, and exploratory behavior in a neotropical bird.

1. INTRODUCTION
Mountains cover approximately one fifth of the planet's land surface with a complex topography and environmental gradients changing over short distances as a result of elevation (Körner, 2007). This has favored the evolution of a wide variety of ecosystems and generated high biodiversity, with close to 25% of all terrestrial species dwelling at high elevations (Spehn et al., 2019). In the mountains, approximately 1240 species of birds survive and reproduce with morphological, physiological, and behavioral mechanisms that have been rarely explored in neotropical birds (Barçante et al., 2017; Tsai et al., 2021).
Various bird species perform altitudinal migration, defined as short‐distance seasonal movements between breeding and wintering sites located at different elevations (Boyle, 2017; Faaborq et al., 2010; Rappole, 2013). The benefits of altitudinal migration include high resource availability and suitable sites for both reproduction and wintering (Boyle, 2017; Bridge et al., 2010; Gillis et al., 2008). In contrast, the costs associate to migration may include higher rates of energy expenditure, an increase in the probability of being predated in route and a shorter time for reproduction (Boyle et al., 2016; Hsiung et al., 2018). The main hypothesis explaining the trigger of altitudinal migration is the ‘climatic constraint’ hypothesis, which posits that birds migrate, because there are physiological constraints that limit their abilities to cope with adverse weather conditions (Cox, 1985). Nevertheless, altitudinal migration may also be influenced by a range of ecological factors, including fluctuations in food availability, predator abundance, and inter‐individual variations in dominance (see Hsiung et al., 2018 for detail of these alternative hypotheses). Such drivers may be particularly significant in tropical environments or when the distance between breeding and wintering elevation is short (Rappole, 2013; Tsai et al., 2021).
Comparative studies about morphology and physiology of mountain birds show that at high elevations, birds often show large body size (Meiri & Dayan, 2003) and down‐regulate their adrenocortical stress response during parental care (Bears et al., 2003; Breuner & Hahn, 2003; Poblete et al., 2020), increases its metabolism (Lindsay et al., 2009; Soobramoney et al., 2003; Weathers et al., 2002), develop a large thermal neutral zone (Martin et al., 1993) and show high‐hematocrit values (Borras et al., 2010) to cope with elevation and weather extremes. As a result of the increased rate of aerobic metabolism at high elevation, it is also likely that birds produce more reactive oxygen species (ROS), which should be counteracted by an increase in antioxidant levels, because an imbalance in intracellular homeostasis between ROS, and antioxidants can lead to oxidative stress (Metcalfe & Alonso‐Alvarez, 2010; Monaghan et al., 2009). Under this condition, organisms suffer cumulative molecular damage, which triggers various diseases and can even cause death (Costantini et al., 2010; van de Crommenacker et al., 2010). A recent study of passerines of the genus Cinclodes showed that at high elevations (2.000–5.000 m), C. fuscus and C. oustaleti had significantly higher total antioxidant capacity (TAC) than C. nigrofumosus inhabiting the marine coastal zones (Tapia‐Monsalve et al., 2018). This suggests that altitudinal migrant birds are exposed to higher ROS levels, which are compensated by a higher production of antioxidants. In addition, studies of long‐distance migrants have shown that the physical wear generated by flight produces an increase in ROS and an increase in plasma antioxidant levels (Costantini et al., 2007; Eikenaar et al., 2020). Together, these studies suggest that altitudinal migrant birds should have specific mechanisms to increase antioxidant levels in response to an increase in ROS.
Evidence of behavioral strategies to cope with high‐elevation conditions have also been found. For instance, dark‐eyed junco (Junco hyemalis) builds subterranean nests and match the duration of their reproductive period with the period of favorable weather and available food (Bears et al., 2009), and several bird species provide greater parental care to their offspring (Badyaev & Ghalambor, 2001). Under these conditions, birds should also be able to quickly detect the environmental fluctuations to decide when is better seeking food and refuge, migrate, and/or breed (Mettke‐Hofmann et al., 2009; Poblete et al., 2018). Exploratory behavior allows individuals to obtain information about different aspects of the environment (Mettke‐Hofmann et al., 2002). This behavior is usually characterized by the mean activity levels displayed by individuals in response to unfamiliar objects or novel environments (Wilson et al., 1994). Thus, individuals who visit a high number of novel sites, approach closer to unfamiliar objects and show a high movement rate during tests are identified as fast‐exploring, while those showing lower scores in these variables are identified as slow‐exploring (e.g., Dingemanse et al., 2002). Comparative studies on exploratory behavior and migration suggest that long‐distance migrant bird species are more prone to explore novel environments than resident bird species (Mettke‐Hofmann et al., 2009; Mettke‐Hofmann & Gwinner, 2004). Nonetheless, evidence of the relationship between exploratory behavior and altitudinal migration in species with both resident and migrant populations remains scarce and inconclusive. For instance, Kozlovsky et al. (2014) found that Poecile gambeli individuals breeding at high elevations visited fewer substrates during a novel environmental test than those living at lower elevations. This suggests that slow explorers could be better able to deal with the more unpredictable environment found at high altitudes since in this situation, trustworthy knowledge and greater flexibility would be needed to make decisions at minimal cost. In contrast, Poblete et al. (2018) found the opposite result in Zonotrichia capensis, suggesting the ability to rapidly obtain environmental information via exploration is likely important for funding food or shelter under high‐elevation conditions (see Mettke‐Hofmann et al., 2006).
It is worth noting that both migratory birds and fast‐exploring individuals frequently exhibit higher metabolic rates compared to their resident and/or slow‐exploring counterparts, attributed to their increased activity levels (e.g., Careau et al., 2010; Careau & Garland, 2012; Eikenaar et al., 2020; Huntingford et al., 2010). This is consistent with the ‘Extended Pace‐of‐Life Syndrome’ hypothesis (POLS; Careau et al., 2008; Réale et al., 2010), which proposes that there is a group of interrelated traits that are associated with fast life‐history strategies, such as high‐metabolic rates, short lifespan, high‐reproductive effort, and behaviors like aggression and exploratory behavior. Thus, it is conceivable that both phenotypes are linked as part of a set of local adaptations to life in high‐altitude environments.
Consequently, fast‐exploring individuals may show higher levels of oxidative damage due to their higher metabolic rate than slow‐exploring individuals (Arnold et al., 2015). This is consistent with the idea that higher levels of physical activity, which are often associated with fast‐exploring behavior, can increase the formation of ROS, leading to oxidative damage (e.g., Larcombe et al., 2010). However, the relationship between exploratory behavior and oxidative status is not entirely clear, as a limited number of studies have assessed this relationship and produced mixed results. For example, in mice, less active individuals demonstrated greater antioxidant capacity than more active individuals (Costantini et al., 2008), whereas in birds, low‐activity levels were associated with lower antioxidant capacity and higher ROS levels (Arnold et al., 2015; Herborn et al., 2011).
Collectively, these findings suggest that the variation across environmental gradients can drive selection for diverse life‐history strategies, shaping relationships between phenotypic traits to cope local environmental conditions. In fact, latitudinal temperature and rainfall variation are among the most influential abiotic factors on the ecological conditions experienced by individuals (Hochachka & Somero, 2014). For instance, in areas with highly seasonal climates, birds adjust their timing of breeding and migration to coincide with resource availability (Both & Visser, 2001). Conversely, in temperate areas, birds may time their breeding to coincide with the peak availability of insect prey during the summer months (Visser et al., 2006). Physiological traits such as metabolic rate have also been tested for their effects on latitude, exhibiting a positive association with latitude (e.g., Cavieres & Sabat, 2008; Maldonado, Bozinovic, et al., 2012). Additionally, several studies have reported intraspecific latitudinal variation in behavioral traits, such as sexual behavior (e.g., Botero‐delgadillo, Quirici, Poblete, Ippi, et al., 2020; Petrie & Kempenaers, 1998), competitiveness (e.g., Fujimoto et al., 2015), and nest defense (e.g., Ippi et al., 2013). Thus, assessing the phenotypical variation associated with altitudinal migration between latitudes is probably one of the most appropriate methods to understand altitudinal migration, its subjacent mechanisms, and triggers from an evolutionary perspective (Foster, 1999).
Here, we characterized altitudinal migration, body size, ROS levels, TAC, oxidative status (ROS/TAC ratio), and exploratory behavior of Rufous‐collared sparrows (Z. capensis; Figure 1) breeding at high and low elevations in two regions that differ in weather conditions in the center and south of Chile (Figure 2; Mediterranean weather 33°S and Temperate weather ~38°S, respectively). Considering the range of environmental conditions that exists across elevations in the Andes, it is reasonable to hypothesize that breeding birds have developed a range of adaptations that enable them to thrive in their respective local environments. Thus, we predicted that birds breeding at high elevations migrate toward lower elevations during winter, exhibit larger body sizes, have a greater propensity for exploration, and possess higher levels of ROS and TAC than birds that breed at low elevations. Furthermore, we hypothesized that there is a positive relationship between exploratory behavior and oxidative status, as ROS levels and TAC are likely to be influenced by metabolic rate and activity levels. Accordingly, our research objectives were (1) assessing altitudinal migration patterns, (2) comparing the body size, oxidative markers, and exploratory behavior between migrant and resident populations and investigating any differences between study sites, breeding elevation, and latitude, and (3) evaluating the relationship between oxidative status and exploratory behavior among study sites.
FIGURE 1.

Zonotrichia capensis. Photography by Alvaro Huerta.
FIGURE 2.
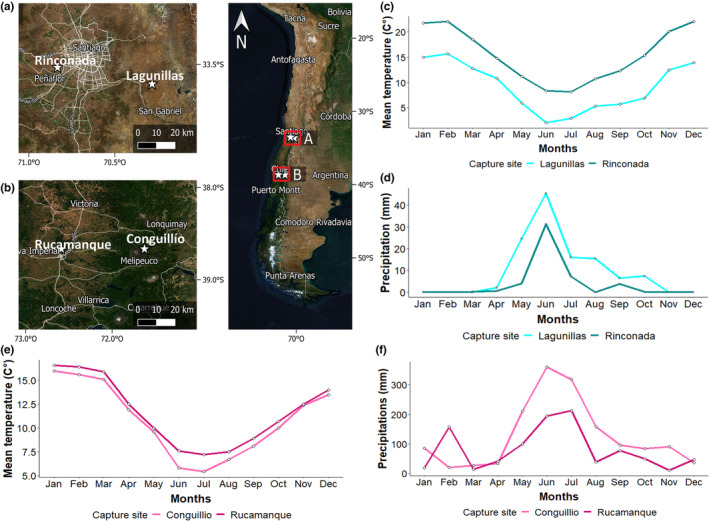
Figure showing the study sites and its weather conditions. The left map shows the four sampled sites: Rinconada (low) and Lagunillas (high) in the center (a) and Rucamanque (low) and Conguillío (high) in the south (b) of Chile (white stars). The right map shows extent of rufous‐collared sparrow's (Zonotrichia capensis) distribution in the country (geographical extent of the left panel). Graphics show mean temperature and accumulated precipitation patterns during the study years in the center (2019; c, d) and south (2020; e, f) of Chile.
2. MATERIALS AND METHODS
2.1. Study species
Rufous‐collared sparrows is well‐suited species to evaluate differences in body size, oxidative status, and exploratory behavior associated with altitudinal migration because they occupy in a huge variety of environments from southern Mexico (10°N) to the southern tip of South America (55°S) and from sea level to >4000 m of elevation (Chapman, 1940). Variations in the behavior and physiology of rufous‐collared sparrows along latitudinal (Maldonado, Bozinovic, et al., 2012; Sabat et al., 2009; van Dongen et al., 2010) and altitudinal gradients (Poblete et al., 2018, 2020; Ruiz et al., 1995) account for their adaptability to different environmental conditions. Rufous‐collared sparrows are a generalist, omnivorous species that feed mainly on fruits, seeds, and insects (Lopez‐Calleja, 1995). These birds establish their breeding territories during the Austral spring, from September to December at high elevation (see Poblete et al., 2018) and until February at low elevation. The post‐breeding period, from ~January to March, is employed to molt and is followed by the non‐breeding period, from April to August, where some populations moving at low elevations. However, it is important to note that rufous‐collared sparrow may have different timing and duration of their life‐history stages depending on the local environmental conditions (Chapman, 1940).
2.2. Study sites
We captured 72 breeding adult individuals of rufous‐collared sparrows using mist nets from four locations that differ in elevation (high >1500; low <500 m) and latitude (central ~33°; south ~38°) in the Southern Hemisphere (Figure 2). At the beginning of breeding season, in September 2019, we sampled in Lagunillas (33°21′S, 70°17′W 2300–2700 m; n = 19) and Rinconada de Maipú (33°31′ S, 70°50′ W ~ 450 m; n = 21), which are located in the Metropolitan Region (center of Chile; Figure 2a). This area has a Mediterranean climate characterized by dry and hot summers and cold and rainy winters according to the Köppen climate classification (Figure 2c,d; Peel et al., 2007). Historical weather data (reviewed in Center for Climate and Resilience Research – CR2; https://explorador.cr2.cl/) reveal that the mean annual precipitation in the lowlands, situated at approximately 500 m.a.s.l., is 219 mm, whereas the high elevations, located at around 2500 m.a.s.l., receive an average annual precipitation of 547 mm. Furthermore, the annual mean temperature at high‐elevation sites is approximately 8.7°C, whereas that in the lowlands is 14.7°C. Natural vegetation is dominated by shrubs and woods of evergreen sclerophyllous species (Donoso, 1982).
In September 2020, we conducted sampling in Conguillío National Park (38°40′S, 71°38′W 1000–1800 m; n = 15) and Rucamanque (38°40′S, 72°36′W ~ 400 m; n = 17), which are both sites located in the Araucanía Region (south of Chile; Figure 2b). Here, the climate is temperate and characterized by cold and rainy winters, with summer precipitation (Figure 2e,f). Historical weather data (Reviewed in Center for Climate and Resilience Research – CR2; https://explorador.cr2.cl/) shows that the lowlands (~400 m.a.s.l.) in this study region receive a mean annual precipitation of approximately 1170.4 mm, while high elevations (~1500 m.a.s.l.) receive 2077 mm. Moreover, the annual mean temperature at high‐elevation sites is found to be approximately 10.7°C, which is only slightly lower than the temperature recorded in the lowlands at 11.2°C. Natural vegetation is dominated by deciduous Nothofagus species (e.g., N. obliqua, N. alessandrii, and N. glauca, Nothofagaceae; di Castri & Hajek, 1976).
2.3. Bird sampling
For each bird captured, we measured their bill, tarsus, wing, and tail length with digital calipers to the nearest 0.1 cm, and we measured body mass with a 60 g balance scale (±0.1 g). These variables were used to obtain a covariate of body size (see below). Before releasing the individuals, we collected a small blood sample (c. 50–100 μL) from the brachial vein using heparinized tubes, which were stored on ice (4°C) for a maximum of 5 h before reaching the laboratory. We also stored a blood sample (c. 20 μL) in FTA cards (Whatman, Buckinghamshire, UK) for molecular sexing by amplifying the CHD locus using the primers 2550F (5′‐GTTACTGATTCGTCTACGAGA‐3′) and 2718R (5′‐ATTGAAATGATCCAGTGCTTG‐3′) (Fridolfsson & Ellegren, 1999; see Appendix S1 for details). Finally, a second set of aliquots of blood were dried on two glass microscope slides and a primary feather (p9) was collected from seven individuals randomly picked in each study site to analyze the proportions of stable hydrogen isotopes (δ2H).
2.4. Stable isotope analysis describing altitudinal migration
In mountainous regions, the relationship between δ2H and elevation can vary depending on factors such as topography, seasonality, and proximity to moisture sources. However, it is generally accepted that there is a decrease of approximately 0.5‰ in δ2H values for every 100 m increase in elevation (Gonfiantini, 1986; Newsome et al., 2015). Here, we compared δ2H present in primary feathers (p9) and blood to detect altitudinal migration during the annual cycle of the birds (Newsome et al., 2015; Poblete et al., 2018; Villegas et al., 2016). The primary feather (p9) is produced annually after the breeding stage and prior to migration in the study species (see Poblete et al., 2018), providing a record of the bird's breeding sites. Furthermore, this feather is a convenient sampling material for isotopic analysis, owing to its ease of collection without causing harm to the bird and its substantial quantity of material for analysis. We also used whole blood, which has a renovation rate of ~1 months, and hence, the δ2H values of the blood samples collected in September (onset spring) represent the elevations occupied by the birds during the winter months (July and August; Poblete et al., 2018).
Primary feathers were cut into small (<1 mm) pieces, air‐dried and placed in a 2:1 chloroform:methanol solvent mixture to remove surface contaminants. Then, we scraped the whole dried blood samples off the slides, put them into microcentrifuge tubes, and homogenized them by mixing. δ2H values were measured with a Thermo Scientific high‐temperature conversion elemental analyzer coupled to a Thermo Scientific Delta V isotope ratio mass spectrometer at the Center for Stable Isotopes at the University of New Mexico. Isotopic results are expressed as d values, δ2H = 1000 × [(Rsample − Rstandard/Rstandard)], where Rsample and Rstandard are the 2H/1H of the sample and the standard, respectively. Vienna Standard Mean Ocean Water (V‐SMOW) is the internationally accepted standard for ™2H analysis, and the units are parts per thousand or per mil (‰). Precision for δ2H was determined by analysis of the three exchangeable internal reference materials for each tissue (blood or feather); the within‐run variation (SD) in δ2H values of these reference materials was 3–4‰.
2.5. Oxidative status
We centrifuged the blood samples collected in heparinized tubes at 7871 g and then separated the plasma and froze it at −80°C until measurement of ROS and TAC (Sabat et al., 2017; see below). We used the TAC as an indicator of non‐enzymatic molecular antioxidants and thiobarbituric acid‐reactive substances (TBARS) as an indicator of oxidative damage (Fernández et al., 2021; Sabat et al., 2017), as it has been done in other bird species (e.g., Gutiérrez et al., 2019; Tapia‐Monsalve et al., 2018). Plasma TAC levels were determined using the antioxidant capacity reduction method (Apak et al., 2006; Ribeiro et al., 2011). The assay evaluates the reduction of the copper (II)‐neocuproine complex to the copper (I)‐neocuproine complex by antioxidants present in the plasma. This reaction can be measured by colorimetry at 450 nm. Finally, the sample value was compared with a Trolox standard curve (Ribeiro et al., 2011). The level of TBARS was estimated using the thiobarbituric acid (TBA) concentration based on a reaction that evaluates the 1:2 adduct formed by malondialdehyde (MDA; a product of lipid peroxidation) and TBA. The MDA‐TBA adduct was determined to have 532 nm colorimetry (Ohkawa et al., 1979). Finally, the oxidative status of individuals was estimated using the TBARS/TAC ratio, with higher values indicating higher oxidative stress (Gutiérrez et al., 2019).
2.6. Exploratory behavior
To evaluate exploratory behavior, we conducted novel environmental tests shortly after capturing the birds, following the protocol described by Verbeek et al. (1996). To this end, we used a field‐portable aviary (270 cm long × 150 cm wide × 150 cm high) made of removable poles and semitransparent black cloth (see Poblete et al., 2018, 2021; Botero‐delgadillo, Quirici, Poblete, Poulin, et al., 2020 for aviary details). The aviary had 14 possible perching locations, including perches and walls. Before the trial, the birds had a five‐minute acclimatization period in a small cage (30 cm long × 25 cm wide × 39 cm high) placed in a corner of the aviary and covered with a black cloth. About 1 min before starting the trial, we removed this cloth, and the door of the acclimatization cage was opened for each subject who was free to explore. We used a digital camera (Sony DCR‐68) for recording the behavior of each bird for 10 min (Botero‐delgadillo et al., 2019; Poblete et al., 2018, 2021).
We analyzed the footage using J‐Watcher software. The frequency of visits in each of the 14 areas was used to calculate the Brillouin diversity index, which was used as an indicator of ‘exploratory diversity’ (Blumstein & Daniel, 2007; Botero‐delgadillo et al., 2019; Poblete et al., 2018).
2.7. Statistical tests
To mitigate the risk of overparameterization arising from our limited isotopic sample size and the numerous variables involved, we employed distinct models for each of our three objectives. First, to evaluate the altitudinal migration patterns, we used repeated‐measures ANOVA with elevation (high and low) as factor and δ2H values of each tissue as repeated measures within each individual, to compare feather (representing summer) and blood (representing winter) δ2H values among birds breeding at different elevations in the center and south of Chile and used a post hoc Tukey test to assess for specific differences among means in δ2H values of both tissues between seasons and elevations. To this end, we corrected the blood δ2H values using the δ2Hfeather‐blood offset of +24‰ observed in Cinclodes nigrofumosus, a passerine bird dwelling in coastal sites all year‐round in Chile, to represent tissue specific differences in 2H discrimination between blood and feather (P. Sabat unpublished data). A similar, but of larger magnitude, δ2Hfeather‐blood offset was observed in quails (+38‰, Wolf et al., 2012).
Second, we used nested ANOVA and post hoc Tukey test to compare the body size, oxidative markers, and exploratory behavior between migrant and resident populations and to assess differences between study sites, breeding elevation, and latitude. Here, we fitted separate models that included body size, physiological (TAC, TBARS levels, and ROS/TAC ratio), and behavioral (exploratory diversity) data as the response variables and population status (migrant or resident) and study site nested in elevation (‘high’ or ‘low’) and latitude (‘center’ or ‘south’) as predictors. Given that both body size and sex affect the physiology (e.g., Markó et al., 2011) and behavior of adult birds (e.g., Kelleher et al., 2017; van Overveld et al., 2014), we preliminarily fitted linear models for each response variable and included sex and body size index (BSI) (i.e., the first principal component obtained from principal component analysis (PCA) between morphological variables; see Table S1 for PCA details; Grant & Grant, 2008; Weeks et al., 2020) as predictors (see Table S2 for models). BSI and sex were included as covariates and cofactors in the predictive models, only if their effects on the response variable were statistically significant to avoid overparameterizing the models.
Finally, we used Linear Mixed Model (LMM) with a Gaussian error distribution and log‐link function to evaluate the relationship between oxidative status and exploratory behavior. To this end, we fitted separate models that included TAC, TBARS levels, and ROS/TAC ratio as response variables, exploratory behavior nested in study sites as predictor variables and elevation nested in latitude as a random effect. For all analyses, we checked and validated the model assumptions. Box Cox transformation (x λ −1)/(λ) with λ = −1.595 used to fit thess TBARS levels and ROS/TAC ratio to a normal distribution. We considered an effect to be statistically supported when p < .05 and the 95% CI around the estimate did not overlap zero. All analyses were performed in the R Studio statistical environment v.1.4.17.17 using the Base (version v.1.4.17.17), stats (version 3.6.2) and lme4 (version 1.1.‐27.1) packages.
3. RESULTS
In the center of Chile, the δ2H values of blood representing winter collected from high elevation (−84 ± 2‰) were significantly lower to δ2H values of blood collected at the same time from sparrows at low elevation (−67 ± 2‰; Tukey: p < .001; Figure 3a). The δ2H values of feathers representing summer collected from high elevations (−95 ± 2‰) had significantly lower δ2H values than feathers collected at the same time from sparrows at low elevations (−67 ± 2‰; Tukey: p < .001; Figure 3a). The δ2H values of blood samples representing winter (−86 ± 2‰) collected from high elevation were significantly lower than δ2H values of feather representing summer collected at the same elevation (−95 ± 2‰; F = 9.96; p > .05; Figure 3a). The δ2H values of blood samples representing winter (−67 ± 2‰) collected from low elevation were similar to δ2H values of feather representing summer collected at the same elevation (−67 ± 2‰; F = 9.96; p > .05; Figure 3a).
FIGURE 3.
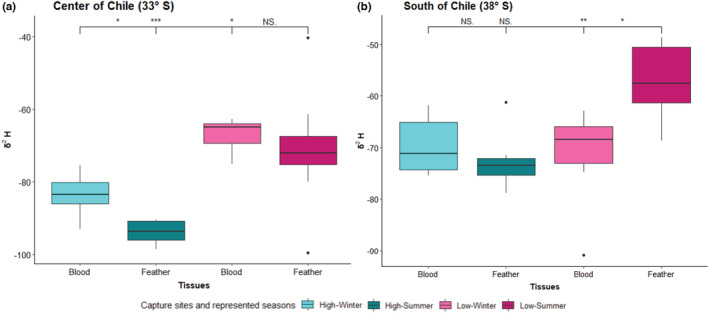
Whole blood and feather δ2 H (mean ± SE) values in Z. capensis in birds that breeding at high and low elevations in the Center (a) and South (b) of Chile. The dates show significant difference between feather δ2H values (representing summer) between birds that breeding at high and low elevation in each latitude. Comparison of blood δ2H values (representing winter) between elevations show significant differences only in central zone. Significant difference between blood δ2H values (representing winter) and feather δ2H values (representing summer) were found at high elevation in the central zone and at low elevation in south zone. The bold horizontal line inside the box represents the median δ2H values. Vertical lines indicate standard error and asterisks indicate significant differences among groups.
In the south of Chile, we found that δ2H values of blood samples representing winter (−70 ± 2‰) collected from high elevation were similar to δ2H values of blood collected at the same time from sparrows at low elevation (−72 ± 2‰; p > .05; Figure 3b). The δ2H values of feathers representing summer collected from high elevations (−73 ± 2‰) had significantly lower δ2H values than feathers collected at the same time from sparrows at low elevations (−57 ± 2‰; Tukey: p < .01; Figure 3b). The δ2H values of blood samples representing winter (−70 ± 2‰) collected from high elevation were similar to δ2H values of feather representing summer collected at the same elevation (−73 ± 2‰; p > .05; Figure 3b). The δ2H values of blood samples representing winter (−72 ± 2‰) collected from low elevation were significantly lower than δ2H values of feather representing summer collected at the same elevation (−57 ± 2‰; F = 9.96; p < .05; Figure 3b).
Regardless of differences observed in altitudinal migration patterns, we did not find significant differences in body mass, TAC, TBARS levels, oxidative status, or exploratory behavior associated with migrant or resident population status (Table 1). However, we found significant differences in these variables among breeding elevations, study sites, and latitudes (Table 1; Figure 4). Specifically, in the center of Chile, the birds that breed at high elevation were significantly larger than those that breed at low elevations (Figure 4a), but in the south of Chile, we did not find significant differences in body size associated with the elevation of the breeding sites (Figure 4a; see Table S3 for pairwise comparisons).
TABLE 1.
Results from nested ANOVAs for testing relationship between variation in Body size, TAC, TBARS, oxidative status and exploratory behavior in Rufous‐collared sparrow (n = 72).
| df | F value | p | |
|---|---|---|---|
| Effect on BSI | |||
| Population status (migrant or resident) | 1 | 1.859 | .176 |
| Population: Elevation: Latitude | 2 | 5.099 | .008** |
| Residuals | 84 | ||
| Effect on TAC | |||
| Population status (migrant or resident) | 1 | 0.122 | .728 |
| Population:Elevation:Latitude | 2 | 23.652 | 2.04e‐08*** |
| Residuals | 64 | ||
| Effect on TBARS | |||
| Population status (migrant or resident) | 1 | 1.83 | .237 |
| Population:Elevation:Latitude | 2 | 26.61 | 4.91e10*** |
| Sex | 1 | 11.37 | .001** |
| Residuals | 64 | ||
| Effect on TBARS/TAC ratio | |||
| Population status (migrant or resident) | 1 | 3.506 | .066 |
| Population:Elevation:Latitude | 2 | 6.624 | .002** |
| Residuals | 64 | ||
| Effect on exploratory diversity | |||
| Population status (migrant or resident) | 1 | 0.16 | .699 |
| Population:Elevation:Latitude | 2 | 4.78 | .011* |
| BSI | 1 | 12.36 | .001** |
| Residuals | 64 | ||
Note: Aesthetics indicate significant effects.
*p < .005, **p < .01 and ***p < .001, respectively.
FIGURE 4.
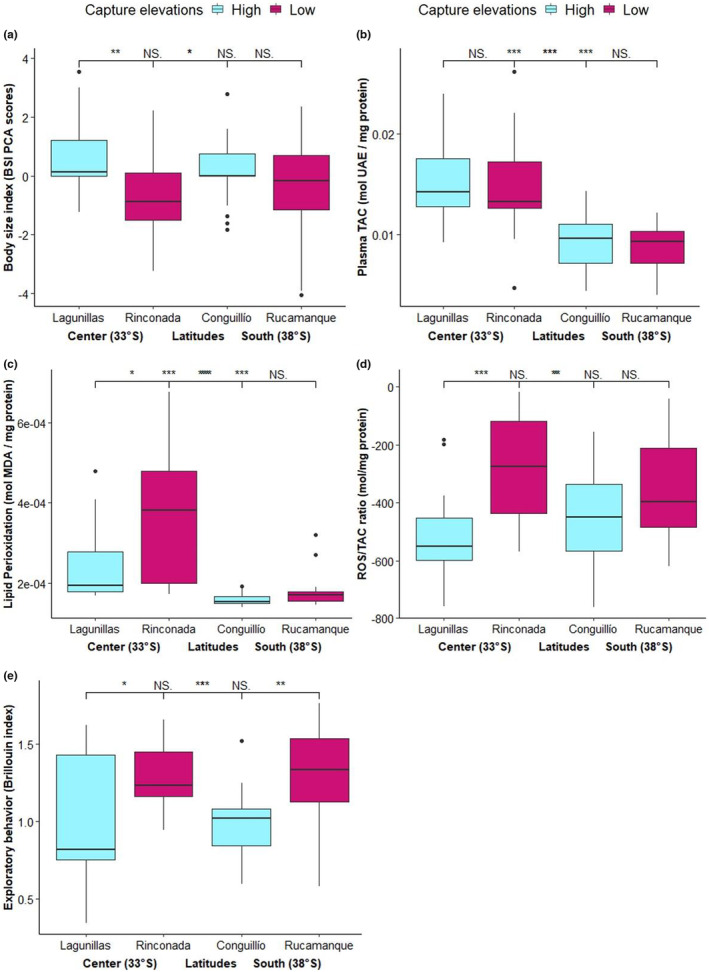
Differences in body size, TAC, TBARS, oxidative status, and exploratory behavior between populations of Rufous‐collared sparrow located at high and low elevations in the center and south of Chile. The bold horizontal line inside the box represents the median score for body size (a) TAC (b), TBARS (c), oxidative status (d), and exploratory behavior (e), respectively. Vertical lines indicate standard error and asterisks indicate significant differences among groups.
Plasma levels of TAC and TBARS were significantly higher in birds from central Chile than from the south (Table 1; Figure 4). In the center of Chile, TAC values were similar between birds from different elevations (Figure 4b), but birds that breed at low elevation showed TBARS levels higher than those that breed at high elevation (Figure 4c). In the south of Chile, we did not observe any differences in TAC and TBARS levels between elevations (Figure 4b,c; see Table S3 for pairwise comparisons). Regarding the ROS/TAC ratio, we found that birds that breed at low elevations in the center of Chile showed significantly higher signal of oxidative damage (Figure 4d see Table S3 for pairwise comparisons).
The exploratory diversity index was significantly higher in the birds breeding at low elevations, regardless of latitude and study site (Table 1; Figure 4e; see Table S3 for pairwise comparisons). Finally, we found a positive and significant relationship between exploratory diversity with TAC levels in Conguillío (Figure 5a) and with ROS/TAC ratio in Rinconada (Table 2; Figure 5c). We did not find significant relationships between TBARS levels and exploratory behavior (Table 2; Figure 5b).
FIGURE 5.
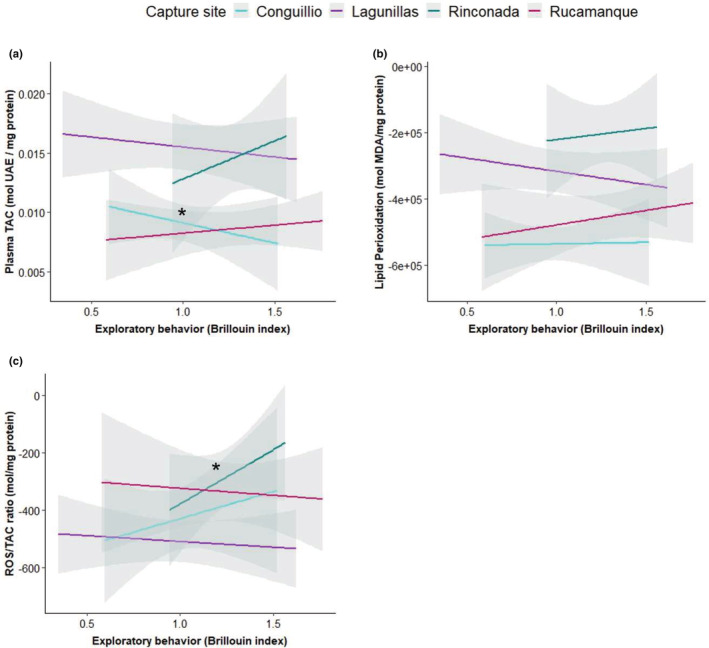
Relationships between TAC (a), TBAR (b) and oxidative status (c) with exploratory behavior in four populations of Rufous‐collared sparrow. Shown model predictions with 95% confidence intervals (lines and shaded area). Asterisks indicate intervals that did not include zero.
TABLE 2.
Mixed‐effects linear models showing the relationships between TAC, TBARS and oxidative status levels with exploratory behavior in Rufous‐collared sparrow (n = 72).
| Estimate | SE | L 95% CI | U 95% CI | |
|---|---|---|---|---|
| Effect on TAC | ||||
| Intercept | 0.011 | 0.005 | 2.018 e‐03 | 2.070 e‐02 |
| Exploratory diversity: Conguillío | −0.006 | 0.002 | −1.069 e‐02 | −2.099 e‐03 |
| Exploratory diversity: Lagunillas | −0.004 | 0.002 | −4.043e‐03 | 3.738e‐03 |
| Exploratory diversity: Rinconada | 0.005 | 0.003 | −2.32e‐04 | 1.059e‐02 |
| Exploratory diversity: Rucamanque | 0.001 | 0.003 | −4.944e‐03 | 5.733e‐03 |
| Random effect | σ 2 | |||
| Latitude: Elevation | 1.633e‐15 | |||
| Effect on TBARS | ||||
| Intercept | −550,505 | 138,153 | −828685.4 | −270373.8 |
| Exploratory diversity: Conguillío | −102,428 | 168,959 | −468138.0 | 240738.2 |
| Exploratory diversity: Lagunillas | −61,169 | 98,533 | −253321.5 | 141509.6 |
| Exploratory diversity: Rinconada | 180,163 | 153,059 | −177430.1 | 526933.4 |
| Exploratory diversity: Rucamanque | 39,293 | 116,069 | −219309.5 | 279116.7 |
| Random effect | σ 2 | |||
| Latitude: Elevation | 3.755e+10 | |||
| Effect on oxidative status | ||||
| Intercept | −469.89 | 81.66 | −625.887 | −313.676 |
| Exploratory diversity: Conguillío | 45.30 | 91.82 | −130.116 | 220.661 |
| Exploratory diversity: Lagunillas | −38.87 | 80.40 | −192.462 | 114.725 |
| Exploratory diversity: Rinconada | 153.80 | 73.17 | 14.005 | 293.488 |
| Exploratory diversity: Rucamanque | 94.10 | 68.02 | −35.991 | 224.062 |
| Random effect | σ 2 | |||
| Latitude: Elevation | 0.000 | |||
Note: Bold numbers indicate intervals that did not include zero.
Abbreviations: L/U 95% CI = lower/upper bound; SE, standard error.
4. DISCUSSION
In this study, we observed variations in altitudinal migration, body size, oxidative status, and exploratory behavior of Zonotrichia capensis across different elevations and latitudes of the study sites. Our stable isotope analysis revealed that Z. capensis from central Chile, bred at high elevations (~2500 m) and migrated downslope during the winter months, while those breeding at low elevations (~500 m) remained at the same elevation throughout the year (Figure 3a). Similar observations have been reported in the rufous‐collared‐sparrow, which breeds at high and low elevations in sites nearby our study areas (Poblete et al., 2018). In the south of Chile, on the other hand, we found that birds that breed at high elevations remained at high elevations year‐round (~1500 m), while birds that breed at low elevations (~400 m) moved upslope during the winter months.
The ‘climatic constraint’ hypothesis predicts that altitudinal migration is a behavior forced by harsh weather conditions that occur at high elevations during winter (Cox, 1985). Our results support this hypothesis and may explain the pattern observed in the center of Chile because climatic variability between seasons increased with elevation (Figure 2c). Based on data of The Center for Climate and Resilience, during the winter months the breeding site at high elevations was cool and rainy, while in the summer, the temperature increased, and precipitation considerably decreased (Figure 2c,d). Interesting, the wintering sites located at ~1000 m.a.s.l. in the center of Chile show precipitation and temperature conditions similar to those at high‐elevation site during breeding season (~1000 m.a.s.l. winter: mean temperature: 11.8°C; mean accumulated precipitation: 13.0 mm) suggesting that the birds move between elevations to maintain year‐round under certain weather conditions. At low‐elevation sites (~500 m), where birds are resident, the mean temperature was between 10.9°C and 21.0°C and precipitation did not exceed 4.6 mm year‐round (Figure 2d). Some bird species can reside in an area throughout the year when the environmental factors of that area meet their requirements. For instance, if a bird species is adapted to the climate and food resources of a particular location and those resources are available year‐round, then there may be no need for the birds to migrate (Rappole, 2013). A previous study performed in this area shown that 83% of raptor species are resident birds that breed and wintering in central Chile (Jaksic et al., 2001) and ebird.org data show that several passerine bird species such as Troglodytes aedon, Diuca diuca, Spinus barbatus, Scytalopus fuscus can be found year‐around in this location. These findings suggest that the temperature and precipitation range in this area may not be the primary drivers of the evolution of altitudinal migration in this area.
In the south of Chile, on the other hand, birds breeding at high elevations appear to exhibit minor seasonal movement by moving slightly downslope during winter. This short altitudinal movement between seasons, however, did not have statistical significance. Instead, the birds that breed at low elevations move upslope during winter. This pattern does not support the ‘climatic constraint’ hypothesis, possibly because during the winter and summer months, the mean temperatures between elevations are similar (Winter: high = 7.5°C, low = 8.9°C; Summer: high = 14.1°C, low = 13.8°C), and both sites are rainy year‐round (Winter: high = 211.37 mm, low = 100.66 mm; Summer: high = 84.8 mm, low = 30.4 mm). Thus, it is possible that weather variation in temperature and precipitation in this region is not sufficient to drive altitudinal migration. Some birds also migrate to higher elevations to avoid competition or predation from other species or to find more suitable habitats for their specific needs (Rappole, 2013). The microclimates of high‐elevation habitats can also provide more stable temperatures and shelter from harsh winter conditions (Hsiung et al., 2018). In additon, it is possible that some birds move downslope for breeding as a strategy to reduce intraspecific competition for territories and sites appropriated for nesting. The ‘dominance’ hypothesis predicts that migrating individuals would be subdominant and hence excluded during the competition for high‐quality habitats with better nesting sites (Gauthreaux, 1978; Marra, 2000). Usually, subdominant individuals are smaller than dominant individuals (e.g., French & Smith, 2005). Here, we did not find significant differences in BSI between birds that breed at high and low elevations in this region, but we observed that at low elevations, the birds tended to be smaller than those that breed and remain at high elevations during winter (see Figure S1 for comparison between all morphometric measures). This result suggests that the dominance hypothesis might partially explain the pattern observed.
Our results also show that in the center of Chile, birds at high elevations were significantly larger than those breeding at low elevations. This result is similar to that shown in a previous study comparing morphological measures between birds breeding at high and low elevations in this region (Poblete et al., 2018). However, as stated above, in the south of Chile, the difference in BSI between birds that breed at high and low elevations was not significant. According to Bergman's rule, it is expected that at high elevations, individuals will have a larger body size because it allows them to face the low environmental temperatures present at high elevations better due to a higher mass/surface area ratio (Meiri & Dayan, 2003). Moreover, it has also been suggested that larger individuals might cope with altitudinal migration better because they would be more resistant to the harsh weather conditions and lower food abundance present at high elevations (Ketterson & Nolan, 1976). In line with this, our results suggest that the difference in temperature between elevations may influence the body size variation in the center of Chile but not in the south of Chile. Moreover, because in the south the birds that migrated were not larger than those that were at high elevation year‐round, our data cannot support the body size hypothesis.
Our results also showed that ROS and TAC levels were significantly higher in the center than south of Chile. Diverse studies agree that high environmental temperatures increase ROS production (Gonzalez‐Rivas et al., 2020; Mujahid et al., 2007), so it is expected that at lower latitudes ROS and TAC levels will be higher than high latitude. Although, the most birds showed a balance between ROS levels and TAC, the birds that breed at low elevations in the Center of Chile showed significantly higher unbalance ROS/TAC indicating oxidative stress. This unbalance ROS/TAC might be result of the long exposure to extremely high environmental temperatures and the absence of water product of a long drought (>10 years) as a consequence of climatic change (Burger et al., 2018), which may be environmental conditions that overcome the physiological tolerance limits of these birds. In line with this, a recent laboratory study performed in individuals of Z. capensis from this location, found lower TAC values in birds acclimated to 27°C in comparison to those acclimated to 17°C (Sabat et al., 2019). This suggests that despite the high resistance to oxidative stress observed in birds, it is possible that in this location they have reached a state that is beyond their capacity to counter oxidative damage (Bozinovic & Pörtner, 2015). The climatic variability hypothesis predicts that individuals experiencing a low variation in ambient temperatures should be more affected by both increased and extreme ambient temperatures because their thermal tolerance variation would be smaller than in those individuals living under more variable temperature conditions (Janzen, 1967). This hypothesis may explain why at high elevations, the birds did not show high ROS levels, similar to individuals breeding at low elevations in the center of Chile. However, evidence also shows that high metal levels are directly linked to high ROS levels (Ercal et al., 2001; Koivula & Eeva, 2010). Indirectly, metals may also affect the antioxidant richness present in the food of birds, altering ROS levels (Eeva et al., 2005, 2008). Thus, it is important to assess metal presence and food quality at this study site to clearly understand the cause of the higher oxidative damage in these birds.
Our findings also shown that birds breeding at high elevations exhibited lower exploratory diversity (i.e., slow exploring) compared to those breeding at low elevations (i.e., fast exploring).
The slow exploration behaviors have been linked to higher accuracy in obtaining information (Sih & del Giudice, 2012) and high neophobia toward novel objects (Verbeek et al., 1994). Moreover, slow‐exploring individuals seem to be more flexible in their foraging routine (Marchetti & Drent, 2000), and they might learn better in unpredictable environments than fast‐exploring individuals (Dougherty & Guillette, 2018; Guillette et al., 2010; Kozlovsky et al., 2015). This suggests that slow‐exploring individuals might cope better with the more unpredictable environment present at high elevations because under this condition, reliable information, and greater flexibility would be required to make decisions at low cost. In line with this, a previous study performed in Poecile gambeli reporting similar results to those presented here suggested that differences in spatial memory and cognition between birds that breed at high and low elevations may be linked to differences in exploratory behavior (Kozlovsky et al., 2014). However, it is important to note that a previous study of Z. capensis performed in the center of Chile with data collected during 2013 showed a patter inverse to those reported here, suggesting that exploratory behavior is a context‐dependent trait and may vary from year to year according to the local availability of resources (Dingemanse et al., 2004; Dingemanse & Réale, 2005).
Furthermore, we found that TAC values and exploratory diversity were negatively associated only in birds that breed at high altitudes in southern Chile, suggesting that a decrease in exploratory behavior may favor the ROS/TAC balance, as a result of lower activity levels (Costantini, 2008). However, the fact that this association is lacking in other populations suggests, the links between TAC and exploratory behavior may be context dependent (Costantini & Verhulst, 2009). Interestingly, we also found a direct relationship between oxidative stress and exploratory diversity only in birds breeding at low elevations in the Center of Chile, where the environmental mean and maximum temperatures are higher than those of the other breeding sites. In line with this, a previous study about physiological and behavioral traits in Z. capensis showed higher exploratory behavior scores and a positive relation between exploratory behavior and metabolic rate, in birds from a similar Mediterranean site (Maldonado, van Dongen, et al., 2012). The ‘Extended’ pace of life hypothesis predicts that individuals that fast‐exploring should show high‐metabolic rate (POLS; Careau et al., 2008; Réale et al., 2010), and hence, metabolic rate might be a possible mechanism behind link between exploratory behavior and oxidative stress in this study population (Arnold et al., 2015). Alternatively, it has been suggested that chronic levels of corticosterone (CORT) increase oxidative damage in birds (Lin et al., 2004). However, a recent study that compared baseline CORT levels of breeding rufous‐collared sparrows present at low elevation (same study site as that of this study) with breeding individuals present at high elevation in the center of Chile showed that both populations have similar basal CORT levels, suggesting that these birds do not show signals of chronic stress (Poblete et al., 2020).
Finally, although there is a considerable debate regarding the effects of environmental temperature on oxidative stress in endothermic animals, recent studies revealed that dehydration and temperature may influence oxidative status, yielding an elevated antioxidant response, and/or oxidative damage (Jacobs et al., 2020; Navarrete et al., 2021; Sabat et al., 2019). Thus, we propose that the links between exploratory behavior and oxidative stress might arise in populations exposed to extreme environmental temperatures and/or dehydration, because under these conditions the capacity to counter the oxidative damage resulting from high‐activity levels might be affected by energetic constraints.
In summary, our results indicate that both altitudinal migration patterns and oxidative status of mountain birds were significantly influenced by the latitude of breeding sites, whereas exploratory behavior exhibited a significant association with elevation. Notably, we observed that fast‐explorer birds inhabiting low elevations in the center of Chile displayed higher levels of oxidative damage in comparison to their slow‐explorer counterparts. These outcomes underscore the possibility of local adaptations in the studied populations, which may have evolved in response to diverse local environmental conditions. Understanding how populations in mountain ecosystems have adapted to their unique environmental conditions, can help us predict how they will respond to challenges stemming from anthropogenic activities, such as climate change, habitat fragmentation, or invasive species. This knowledge is key to designing effective conservation strategies for maintaining the biodiversity and ecological function of mountain ecosystems.
AUTHOR CONTRIBUTIONS
Carolina Contreras: Methodology (equal); writing – review and editing (equal). Carolina Fernández: Methodology (equal). Cristian R. Flores: Methodology (equal). Patricia Vega: Methodology (equal). Miguel Ávila: Methodology (equal). Pablo Sabat: Formal analysis (supporting); resources (supporting); supervision (lead); writing – review and editing (equal). Yanina Poblete: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (equal); project administration (lead); resources (lead); writing – original draft (lead); writing – review and editing (equal).
FUNDING INFORMATION
Funding was provided by grants from ANID‐FONDECYT 3190111 to YP. We thank funding ANID PIA/BASAL FB0002.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank Javiera Pantoja, Iñigo Bidegain, Lucas Navarrete, Ernesto Gutierrez and Gabriel Quezada for their collaboration during fieldwork. We thank Seth Newsome for support with the isotope analysis. We thank Gerardo Soto for production of the map in the Figure 2. We thank to Gareth Jenkins and Alex Jahn for revising an earlier version of the manuscript. Birds were captured under a permit issued by the Servicio Agricola Ganadero. Fieldwork in protected areas was possible thanks to people from Parque Nacional Conguillío and Estación experimental Rucamanque.
Poblete, Y. , Contreras, C. , Fernández, C. , Flores, C. R. , Vega, P. , Ávila, M. , & Sabat, P. (2023). Geographic variation in the altitudinal migration patterns, body size, oxidative status and exploratory behavior in a neotropical bird. Ecology and Evolution, 13, e9941. 10.1002/ece3.9941
DATA AVAILABILITY STATEMENT
The datasets produced and evaluated during the present study have been archived in the Dryad repository and can be accessed through the following DOI: https://doi.org/10.5061/dryad.0rxwdbs4t.
REFERENCES
- Apak, R. , Güçlü, K. , Özyürek, M. , Esin Karademir, S. , & Erçağ, E. (2006). The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. International Journal of Food Sciences and Nutrition, 57, 292–304. 10.1080/09637480600798132 [DOI] [PubMed] [Google Scholar]
- Arnold, K. E. , Herborn, K. A. , Adam, A. , & Alexander, L. (2015). Individual variation in the oxidative costs of personality traits. Functional Ecology, 29, 522–530. 10.1111/1365-2435.12375 [DOI] [Google Scholar]
- Badyaev, A. V. , & Ghalambor, C. K. (2001). Evolution of life histories along elevational gradients: Trade‐off between parental care and fecundity. Ecology, 82, 2948–2960. 10.1890/0012-9658(2001)082 [DOI] [Google Scholar]
- Barçante, L. , Vale, M. M. , & Maria, M. A. (2017). Altitudinal migration by birds: A review of the literature and a comprehensive list of species. Journal of Field Ornithology, 88, 321–335. 10.1111/jofo.12234 [DOI] [Google Scholar]
- Bears, H. , Martin, K. , & White, G. C. (2009). Breeding in high‐elevation habitat results in shift to slower life‐history strategy within a single species. The Journal of Animal Ecology, 78, 365–375. 10.1111/j.1365-2656.2008.01491.x [DOI] [PubMed] [Google Scholar]
- Bears, H. , Smith, J. N. M. , & Wingfield, J. C. (2003). Adrenocortical sensitivity to stress in dark‐eyed juncos (Junco hyemalis oregonus) breeding in low and high elevation habitat. Ecoscience, 10, 127–133. 10.1080/11956860.2003.11682758 [DOI] [Google Scholar]
- Blumstein, D. T. , & Daniel, J. C. (2007). Quantifying behavior the JWatcher way. Sinauer Associates, Inc. [Google Scholar]
- Borras, A. , Cabrera, J. , & Senar, J. C. (2010). Hematocrit variation in response to altitude changes in wild birds: A repeated‐measures design. Condor, 112, 622–626. 10.1525/cond.2010.090113 [DOI] [Google Scholar]
- Botero‐delgadillo, E. , Quirici, V. , Poblete, Y. , Ippi, S. , Kempenaers, B. , & Vásquez, R. A. (2020). Extrapair paternity in two populations of the socially monogamous thorn‐tailed Rayadito Aphrastura spinicauda (Passeriformes: Furnariidae). Ecology and Evolution, 10, 1–8. 10.1002/ece3.6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botero‐delgadillo, E. , Quirici, V. , Poblete, Y. , Poulin, E. , Kempenaers, B. , & Vásquez, R. A. (2020). Exploratory behavior, but not aggressiveness, is correlated with breeding dispersal propensity in the highly philopatric thorn‐tailed rayadito. Journal of Avian Biology, 51, 1–12. 10.1111/jav.02262 [DOI] [Google Scholar]
- Botero‐delgadillo, E. , Quirici, V. , Poblete, Y. , Cuevas, É. , Kuhn, S. , Girg, A. , Poulin, E. , Kempenaers, B. , & Vásquez, R. A. (2019). Ecological and social correlates of natal dispersal in female and male Thorn‐tailed Rayadito (Aphrastura spinicauda) in a naturally isolated and fragmented habitat. The Auk, 136, 1–14. 10.1093/auk/ukz016 [DOI] [Google Scholar]
- Both, C. , & Visser, M. E. (2001). Adjustment to climate change is constrained by arrival date in a long‐distance migrant bird. Nature, 411, 296–298. 10.1038/35077063 [DOI] [PubMed] [Google Scholar]
- Boyle, A. , Sandercock, B. K. , & Martin, K. (2016). Patterns and drivers of intraspecific variation in avian life history along elevational gradients: A meta‐analysis. Biological Reviews, 91, 469–482. 10.1111/brv.12180 [DOI] [PubMed] [Google Scholar]
- Boyle, W. A. (2017). Altitudinal bird migration in North America. Auk, 134, 443–465. 10.1642/auk-16-228.1 [DOI] [Google Scholar]
- Bozinovic, F. , & Pörtner, H. O. (2015). Physiological ecology meets climate change. Ecology and Evolution, 5, 1025–1030. 10.1002/ece3.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner, C. W. , & Hahn, T. P. (2003). Integrating stress physiology, environmental change, and behavior in free‐living sparrows. Hormones and Behavior, 43, 115–123. 10.1016/S0018-506X(02)00020-X [DOI] [PubMed] [Google Scholar]
- Bridge, E. S. , Kelly, J. F. , Bjornen, P. E. , Curry, C. M. , Crawford, P. H. C. , & Paritte, J. M. (2010). Effects of nutritional condition on spring migration: Do migrants use resource availability to keep pace with a changing world? The Journal of Experimental Biology, 213, 2424–2429. 10.1242/jeb.041277 [DOI] [PubMed] [Google Scholar]
- Burger, F. , Brock, B. , & Montecinos, A. (2018). Seasonal and elevational contrasts in temperature trends in Central Chile between 1979 and 2015. Global and Planetary Change, 162, 136–147. 10.1016/j.gloplacha.2018.01.005 [DOI] [Google Scholar]
- Careau, V. , & Garland, T. (2012). Performance, personality, and energetics: Correlation, causation, and mechanism. Physiological and Biochemical Zoology, 85, 543–571. 10.1086/666970 [DOI] [PubMed] [Google Scholar]
- Careau, V. , Réale, D. , Humphries, M. M. , & Thomas, D. W. (2010). The pace of life under artificial selection: Personality, energy expenditure, and longevity are correlated in domestic dogs. The American Naturalist, 175, 753–758. 10.1086/652435 [DOI] [PubMed] [Google Scholar]
- Careau, V. , Thomas, D. , Humphries, M. M. , & Réale, D. (2008). Energy metabolism and animal personality. Oikos, 117, 641–653. 10.1111/j.0030-1299.2008.16513.x [DOI] [Google Scholar]
- Cavieres, G. , & Sabat, P. (2008). Geographic variation in the response to thermal acclimation in rufous‐collared sparrows: Are physiological flexibility and environmental heterogeneity correlated? Functional Ecology, 22, 509–515. 10.1111/j.1365-2435.2008.01382.x [DOI] [Google Scholar]
- Chapman, F. M. (1940). The post‐glacial history of Zonotrichia capensis . Bulletin of the American Museum of Natural History, 78, 381–438. [Google Scholar]
- Costantini, D. (2008). Oxidative stress in ecology and evolution: Lessons from avian studies. Ecology Letters, 11, 1238–1251. 10.1111/j.1461-0248.2008.01246.x [DOI] [PubMed] [Google Scholar]
- Costantini, D. , Cardinale, M. , & Carere, C. (2007). Oxidative damage and anti‐oxidant capacity in two migratory bird species at a stop‐over site. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology, 144, 363–371. 10.1016/j.cbpc.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Costantini, D. , Carere, C. , Caramaschi, D. , & Koolhaas, J. M. (2008). Aggressive and non‐aggressive personalities differ in oxidative status in selected lines of mice (Mus musculus). Biology Letters, 4, 119–122. 10.1098/rsbl.2007.0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini, D. , Rowe, M. , Butler, M. W. , & McGraw, K. J. (2010). From molecules to living systems: Historical and contemporary issues in oxidative stress and antioxidant ecology. Functional Ecology, 24, 950–959. 10.1111/j.1365-2435.2010.01746.x [DOI] [Google Scholar]
- Costantini, D. , & Verhulst, S. (2009). Does high antioxidant capacity indicate low oxidative stress? Functional Ecology, 23, 506–509. 10.1111/j.1365-2435.2009.01546.x [DOI] [Google Scholar]
- Cox, G. W. (1985). The evolution of avian migration systems between temperate and tropical regions of the New World. The American Naturalist, 126, 451–474. 10.1086/284432 [DOI] [Google Scholar]
- di Castri, F. , & Hajek, E. R. (1976). Bioclimatología de Chile. Vicerrectoria Académica de la Universidad Católica de Chile Santiago. [Google Scholar]
- Dingemanse, N. J. , Both, C. , Drent, P. J. , & Tinbergen, J. M. (2004). Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society B: Biological Sciences, 271, 847–852. 10.1098/rspb.2004.2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse, N. J. , Both, C. , Drent, P. J. , van Oers, K. , & van Noordwijk, A. J. (2002). Repeatability and heritability of exploratory behaviour in great tits from the wild. Animal Behaviour, 64, 929–938. 10.1006/anbe.2002.2006 [DOI] [Google Scholar]
- Dingemanse, N. J. , & Réale, D. (2005). Natural selection and animal personality. Behaviour, 142, 1159–1184. 10.1163/156853905774539445 [DOI] [Google Scholar]
- Donoso, C. (1982). Reseña Ecológica de los Bosques Mediterráneos de Chile. Bosque, 4, 117–146. 10.4206/bosque.1982.v4n2-04 [DOI] [Google Scholar]
- Dougherty, L. R. , & Guillette, L. M. (2018). Linking personality and cognition: A meta‐analysis. Philosophical Transactions of the Royal Society B: Biological Science, 373, 20170282. 10.1098/rstb.2017.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeva, T. , Ryömä, M. , & Riihimäki, J. (2005). Pollution‐related changes in diets of two insectivorous passerines. Oecologia, 145, 629–639. 10.1007/s00442-005-0145-x [DOI] [PubMed] [Google Scholar]
- Eeva, T. , Sillanpää, S. , Salminen, J. P. , Nikkinen, L. , Tuominen, A. , Toivonen, E. , Pihlaja, K. , & Lehikoinen, E. (2008). Environmental pollution affects the plumage color of great tit nestlings through carotenoid availability. EcoHealth, 5, 328–337. 10.1007/s10393-008-0184-y [DOI] [PubMed] [Google Scholar]
- Eikenaar, C. , Hegemann, A. , Packmor, F. , Kleudgen, I. , & Isaksson, C. (2020). Not just fuel: Energy stores are correlated with immune function and oxidative damage in a long‐distance migrant. Current Zoology, 66, 21–28. 10.1093/cz/zoz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercal, N. , Gurer‐Orhan, H. , & Aykin‐Burns, N. (2001). Toxic metals and oxidative stress part I: Mechanisms involved in metal induced oxidative damage. Current Topics in Medicinal Chemistry, 1, 529–539. 10.2174/1568026013394831 [DOI] [PubMed] [Google Scholar]
- Faaborq, J. , Holmes, R. T. , Anders, A. D. , Bildstein, K. L. , Dugger, K. M. , Gauthreaux, S. A. , Gauthreaux, S. A., Jr. , Heglund, P. , Hobson, K. A. , Jahn, A. E. , Johnson, D. H. , & Latta, S. C. (2010). Recent advances in understanding migration systems of New World land birds. Ecological Monographs, 80, 3–48. 10.1890/09-0395.1 [DOI] [Google Scholar]
- Fernández, C. , Villaseñor, N. R. , Contreras, C. , Ávila, M. , Sabat, P. , & Poblete, Y. (2021). Do Green space attributes influence avian health? Evidence from the Nutritional and Oxidative Status of a Neotropical Bird. SSRN Electronic Journal. 10.2139/ssrn.3989508 [DOI] [Google Scholar]
- Foster, S. A. (1999). The geography of behaviour: An evolutionary perspective. Trends in Ecology and Evolution, 14, 190–195. 10.1016/S0169-5347(98)01577-8 [DOI] [PubMed] [Google Scholar]
- French, A. R. , & Smith, T. B. (2005). Importance of body size in determining dominance hierarchies among diverse tropical Frugivores1. Biotropica, 37, 96–101. 10.1111/j.1744-7429.2005.04051.x [DOI] [Google Scholar]
- Fridolfsson, A. K. , & Ellegren, H. (1999). A simple and universal method for molecular sexing of non‐ratite birds. Journal of Avian Biology, 30, 116–121. [Google Scholar]
- Fujimoto, S. , Miyake, T. , & Yamahira, K. (2015). Latitudinal variation in male competitiveness and female choosiness in a fish: Are sexual selection pressures stronger at lower latitudes? Evolutionary Biology, 42, 75–87. 10.1007/s11692-014-9300-9 [DOI] [Google Scholar]
- Gauthreaux, S. A. (1978). The ecological significance of behavioral dominance. In Bateson P. P. G. & Klopfer P. H. (Eds.), Perspectives in ethology (pp. 17–54). Springer US. 10.1007/978-1-4684-2901-5_2 [DOI] [Google Scholar]
- Gillis, E. A. , Green, D. J. , Middleton, H. A. , & Morrissey, C. A. (2008). Life history correlates of alternative migratory strategies in American dippers. Ecology, 89, 1687–1695. 10.1890/07-1122.1 [DOI] [PubMed] [Google Scholar]
- Gonfiantini, R. (1986). Environmental Isotopes in Lake Studies. In Fritz P., & Fontes J.‐Ch. (Eds.), Handbook of Environmental Isotope Geochemistry (pp. 113–168). Elsevier. 10.1016/B978-0-444-42225-5.50008-5 [DOI] [Google Scholar]
- Gonzalez‐Rivas, P. A. , Chauhan, S. S. , Ha, M. , Fegan, N. , Dunshea, F. R. , & Warner, R. D. (2020). Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Science, 162, 108025. 10.1016/j.meatsci.2019.108025 [DOI] [PubMed] [Google Scholar]
- Grant, P. R. , & Grant, B. R. (2008). Pedigrees, assortative mating and speciation in Darwin's finches. Proceedings of the Royal Society B: Biological Sciences, 275, 661–668. 10.1098/rspb.2007.0898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette, L. M. , Farrell, T. M. , Hoeschele, M. , Nickerson, C. M. , Dawson, M. R. W. , & Sturdy, C. B. (2010). Mechanisms of call note‐type perception in black‐capped chickadees (Poecile atricapillus): Peak shift in a note‐type continuum. Journal of Comparative Psychology, 124, 109–115. 10.1037/a0017741 [DOI] [PubMed] [Google Scholar]
- Gutiérrez, J. S. , Sabat, P. , Castañeda, L. E. , Contreras, C. , Navarrete, L. , Peña‐Villalobos, I. , & Navedo, J. G. (2019). Oxidative status and metabolic profile in a long‐lived bird preparing for extreme endurance migration. Scientific Reports, 9, 1–11. 10.1038/s41598-019-54057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborn, K. A. , Coffey, J. , Larcombe, S. D. , Alexander, L. , & Arnold, K. E. (2011). Oxidative profile varies with personality in European greenfinches. The Journal of Experimental Biology, 214, 1732–1739. 10.1242/jeb.051383 [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W. , & Somero, G. N. (2014). Biochemical adaptation. Princeton University Press. 10.1515/9781400855414 [DOI] [Google Scholar]
- Hsiung, A. C. , Boyle, W. A. , Cooper, R. J. , & Chandler, R. B. (2018). Altitudinal migration: Ecological drivers, knowledge gaps, and conservation implications. Biological Reviews, 93, 2049–2070. 10.1111/brv.12435 [DOI] [PubMed] [Google Scholar]
- Huntingford, F. A. , Andrew, G. , Mackenzie, S. , Morera, D. , Coyle, S. M. , Pilarczyk, M. , & Kadri, S. (2010). Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio . Journal of Fish Biology, 76, 1576–1591. 10.1111/j.1095-8649.2010.02582.x [DOI] [PubMed] [Google Scholar]
- Ippi, S. , van Dongen, W. F. D. , Lazzoni, I. , Venegas, C. I. , & Vásquez, R. A. (2013). Interpopulation comparisons of antipredator defense behavior of the thorn‐tailed rayadito (Aphrastura spinicauda). Ethology, 119, 1107–1117. 10.1111/eth.12173 [DOI] [Google Scholar]
- Jacobs, P. J. , Oosthuizen, M. K. , Mitchell, C. , Blount, J. D. , & Bennett, N. C. (2020). Heat and dehydration induced oxidative damage and antioxidant defenses following incubator heat stress and a simulated heat wave in wild caught four‐striped field mice Rhabdomys dilectus . PLoS One, 15, 1–17. 10.1371/journal.pone.0242279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaksic, F. , Pavez, E. , Jimenez, J. , & Torres‐Murra, J. (2001). The conservation status of raptors in the metropolitan region, Chile. Journal of Raptor Research, 35, 151–158. [Google Scholar]
- Janzen, D. H. (1967). Why mountain passes are higher in the tropics. The American Naturalist, 101, 233–249. 10.1086/282487 [DOI] [Google Scholar]
- Kelleher, S. R. , Silla, A. J. , Dingemanse, N. J. , & Byrne, P. G. (2017). Body size predicts between‐individual differences in exploration behaviour in the southern corroboree frog. Animal Behaviour, 129, 161–170. 10.1016/j.anbehav.2017.05.013 [DOI] [Google Scholar]
- Ketterson, E. D. , & Nolan, V. (1976). Geographic variation and its climatic correlates in the sex ratio of eastern‐wintering dark‐eyed juncos (Junco hyemalis hyemalis). Ecology, 57, 679–693. 10.2307/1936182 [DOI] [Google Scholar]
- Koivula, M. J. , & Eeva, T. (2010). Metal‐related oxidative stress in birds. Environmental Pollution, 158, 2359–2370. 10.1016/j.envpol.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Körner, C. (2007). The use of “altitude” in ecological research. Trends in Ecology & Evolution, 22, 569–574. 10.1016/j.tree.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Kozlovsky, D. , Branch, C. , Freas, C. A. , & Pravosudov, V. V. (2014). Elevation‐related differences in novel environment exploration and social dominance in food‐caching mountain chickadees. Behavioral Ecology and Sociobiology, 68, 1871–1881. 10.1007/s00265-014-1796-6 [DOI] [Google Scholar]
- Kozlovsky, D. Y. , Branch, C. L. , & Pravosudov, V. V. (2015). Problem‐solving ability and response to novelty in mountain chickadees (Poecile gambeli) from different elevations. Behavioral Ecology and Sociobiology, 69, 635–643. 10.1007/s00265-015-1874-4 [DOI] [Google Scholar]
- Larcombe, S. D. , Coffey, J. S. , Bann, D. , Alexander, L. , & Arnold, K. E. (2010). Impacts of dietary antioxidants and flight training on post‐exercise oxidative damage in adult parrots. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 155, 49–53. 10.1016/j.cbpb.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Lin, H. , Decuypere, E. , & Buyse, J. (2004). Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 1. Chronic exposure. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 139, 737–744. 10.1016/j.cbpc.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Lindsay, C. V. , Downs, C. T. , & Brown, M. (2009). Physiological variation in amethyst sunbirds (Chalcomitra amethystina) over an altitudinal gradient in winter. The Journal of Experimental Biology, 212, 483–493. 10.1242/jeb.025262 [DOI] [PubMed] [Google Scholar]
- Lopez‐Calleja, M. V. (1995). Dieta de Zonotrichia capensis (Emberizidae) Y Diuca diuca (Fringillidae): efecto de la variación estacional de los recursos tróficos y la riqueza de aves granívoras en Chile central. Revista Chilena de Historia Natural, 68, 321–331. [Google Scholar]
- Maldonado, K. , Bozinovic, F. , Cavieres, G. , Fuentes, C. A. , Cortés, A. , & Sabat, P. (2012). Phenotypic flexibility in basal metabolic rate is associated with rainfall variability among populations of rufous‐collared sparrow. Zoology, 115, 128–133. 10.1016/j.zool.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Maldonado, K. , van Dongen, W. F. D. , Vásquez, R. , & Sabat, P. (2012). Geographic variation in the association between exploratory behavior and physiology in rufous‐collared sparrows. Physiological and Biochemical Zoology, 85, 618–624. 10.1086/667406 [DOI] [PubMed] [Google Scholar]
- Marchetti, C. , & Drent, P. J. (2000). Individual differences in the use of social information in foraging by captive great tits. Animal Behaviour, 60, 131–140. 10.1006/anbe.2000.1443 [DOI] [PubMed] [Google Scholar]
- Markó, G. , Costantini, D. , Michl, G. , & Török, J. (2011). Oxidative damage and plasma antioxidant capacity in relation to body size, age, male sexual traits and female reproductive performance in the collared flycatcher (Ficedula albicollis). Journal of Comparative Physiology. B, 181, 73–81. 10.1007/s00360-010-0502-x [DOI] [PubMed] [Google Scholar]
- Marra, P. P. (2000). The role of behavioral dominance in structuring patterns of habitat occupancy in a migrant bird during the nonbreeding season. Behavioral Ecology, 11, 299–308. 10.1093/beheco/11.3.299 [DOI] [Google Scholar]
- Martin, K. , Holt, R. F. , & Thomas, D. W. (1993). Getting by on high: Ecological energetics of arctic and alpine grouse. In Life in the Cold (pp. 33–41). CRC Press. [Google Scholar]
- Meiri, S. , & Dayan, T. (2003). On the validity of Bergmann's rule. Journal of Biogeography, 30, 331–351. 10.1046/j.1365-2699.2003.00837.x [DOI] [Google Scholar]
- Metcalfe, N. B. , & Alonso‐Alvarez, C. (2010). Oxidative stress as a life‐history constraint: The role of reactive oxygen species in shaping phenotypes from conception to death. Functional Ecology, 24, 984–996. 10.1111/j.1365-2435.2010.01750.x [DOI] [Google Scholar]
- Mettke‐Hofmann, C. , & Gwinner, E. (2004). Differential assessment of environmental information in a migratory and a nonmigratory passerine. Animal Behaviour, 68, 1079–1086. 10.1016/j.anbehav.2004.02.012 [DOI] [Google Scholar]
- Mettke‐Hofmann, C. , Lorentzen, S. , Schlicht, E. , Schneider, J. , & Werner, F. (2009). Spatial neophilia and spatial neophobia in resident and migratory warblers (Sylvia). Ethology, 115, 482–492. 10.1111/j.1439-0310.2009.01632.x [DOI] [Google Scholar]
- Mettke‐Hofmann, C. , Rowe, K. C. , Hayden, T. J. , & Canoine, V. (2006). Effects of experience and object complexity on exploration in garden warblers (Sylvia borin). Journal of Zoology, 268, 405–413. 10.1111/j.1469-7998.2005.00037.x [DOI] [Google Scholar]
- Mettke‐Hofmann, C. , Winkler, H. , & Leisler, B. (2002). The significance of ecological factors for exploration and neophobia in parrots. Ethology, 108, 249–272. 10.1046/j.1439-0310.2002.00773.x [DOI] [Google Scholar]
- Monaghan, P. , Metcalfe, N. B. , & Torres, R. (2009). Oxidative stress as a mediator of life history trade‐offs: Mechanisms, measurements and interpretation. Ecology Letters, 12, 75–92. 10.1111/j.1461-0248.2008.01258.x [DOI] [PubMed] [Google Scholar]
- Mujahid, A. , Pumford, N. R. , Bottje, W. , Nakagawa, K. , Miyazawa, T. , Akiba, Y. , & Toyomizu, M. (2007). Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. The Journal of Poultry Science, 44, 439–445. 10.2141/jpsa.44.439 [DOI] [Google Scholar]
- Navarrete, L. , Bozinovic, F. , Peña‐Villalobos, I. , Contreras‐Ramos, C. , Sanchez‐Hernandez, J. C. , Newsome, S. D. , Nespolo, R. F. , & Sabat, P. (2021). Integrative physiological responses to acute dehydration in the rufous‐collared sparrow: Metabolic, enzymatic, and oxidative traits. Frontiers in Ecology and Evolution, 9, 1–12. 10.3389/fevo.2021.767280 [DOI] [Google Scholar]
- Newsome, S. D. , Sabat, P. , Wolf, N. , Rader, J. A. , del Rio, C. M. , & Peters, D. P. C. (2015). Multi‐tissue δ2H analysis reveals altitudinal migration and tissue‐specific discrimination patterns in Cinclodes. Ecosphere, 6, art213. 10.1890/ES15-00086.1 [DOI] [Google Scholar]
- Ohkawa, H. , Ohishi, N. , & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358. 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- Peel, M. C. , Finlayson, B. L. , & McMahon, T. A. (2007). Updated world map of the K¨oppen‐Geiger climate classificatio. Hydrology and Earth System Sciences, 11, 1633–1644. 10.5194/hess-11-1633-2007 [DOI] [Google Scholar]
- Petrie, M. , & Kempenaers, B. (1998). Extra‐pair paternity in birds: Explaining variation between species and populations. Trends in Ecology & Evolution, 13, 52–58. 10.1016/S0169-5347(97)01232-9 [DOI] [PubMed] [Google Scholar]
- Poblete, Y. , Botero‐Delgadillo, E. , Espíndola‐Hernández, P. , & Vásquez, R. A. (2021). Risk‐taking behaviour relates to timing of breeding in a sub‐Antarctic rainforest bird. Ibis, 163, 812–823. 10.1111/ibi.12941 [DOI] [Google Scholar]
- Poblete, Y. , Gutiérrez, V. , Cid, V. , Newsome, S. D. , Sabat, P. , & Vasquez, R. A. (2018). Intraspecific variation in exploratory behavior and elevational affinity in a widely distributed songbird. Oecologia, 186, 931–938. 10.1007/s00442-018-4076-8 [DOI] [PubMed] [Google Scholar]
- Poblete, Y. , Gutierrez, V. , González, P. L. , Wingfield, J. C. , & Vásquez, R. A. (2020). Differences in circulating corticosterone levels associated with elevation of breeding sites in rufous ‐ collared sparrows Zonotrichia capensis . Journal für Ornithologie, 162, 487–496. 10.1007/s10336-020-01846-w [DOI] [Google Scholar]
- Rappole, J. H. (2013). Adaptations for migration. In The avian migrant: The biology of bird migration (pp. 21–24). Columbia University Press. [Google Scholar]
- Réale, D. , Garant, D. , Humphries, M. , Bergeron, P. , Careau, V. , & Montiglio, P. O. (2010). Personality and the emergence of the pace‐of‐ life syndrome concept at the population level personality and the emergence of the pace‐of‐life syndrome concept at the population level. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 4051–4063. 10.1098/rstb.2010.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, J. P. N. , Magalhães, L. M. , Reis, S. , Lima, J. L. F. C. , & Segundo, M. A. (2011). High‐throughput Total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate‐based methods. Analytical Sciences, 27, 483–488. 10.2116/analsci.27.483 [DOI] [PubMed] [Google Scholar]
- Ruiz, G. , Rosenmann, M. , & Novoa, F. (1995). Seasonal changes of blood values in rufous‐collared sparrows from high and low altitude. International Journal of Biometeorology, 39, 103–107. [Google Scholar]
- Sabat, P. , Bozinovic, F. , Contreras‐Ramos, C. , Nespolo, R. F. , Newsome, S. D. , Quirici, V. , Maldonado, K. , Peña‐Villalobos, I. , Ramirez‐Otarola, N. , & Sanchez‐Hernandez, J. C. (2019). The interplay between ambient temperature and salt intake affects oxidative status and immune responses in a ubiquitous neotropical passerine, the rufous‐collared sparrow. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 234, 50–59. 10.1016/j.cbpa.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Sabat, P. , Cavieres, G. , Veloso, C. , Canals, M. , & Bozinovic, F. (2009). Intraspecific basal metabolic rate varies with trophic level in rufous‐collared sparrows. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, 154, 502–507. 10.1016/j.cbpa.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Sabat, P. , Narváez, C. , Peña‐Villalobos, I. , Contreras, C. , Maldonado, K. , Sanchez‐Hernandez, J. C. , Newsome, S. D. , Nespolo, R. , & Bozinovic, F. (2017). Coping with salt water habitats: Metabolic and oxidative responses to salt intake in the rufous‐collared sparrow. Frontiers in Physiology, 8, 1–11. 10.3389/fphys.2017.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih, A. , & del Giudice, M. (2012). Linking behavioural syndromes and cognition: A behavioural ecology perspective. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 2762–2772. 10.1098/rstb.2012.0216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soobramoney, S. , Downs, C. T. , & Adams, N. J. (2003). Physiological variability in the fiscal shrike Lanius collaris along an altitudinal gradient in South Africa. Journal of Thermal Biology, 28, 581–594. 10.1016/j.jtherbio.2003.08.004 [DOI] [Google Scholar]
- Spehn, E. M. , Messerli, B. , & Körner, C. (2019). A global assessment of mountain biodiversity: Synthesis. Mountain Biodiversity, 1, 325–330. 10.4324/9780429342585-28 [DOI] [Google Scholar]
- Tapia‐Monsalve, R. , Newsome, S. D. , Sanchez‐Hernandez, J. C. , Bozinovic, F. , Nespolo, R. , & Sabat, P. (2018). Terrestrial birds in coastal environments: Metabolic rate and oxidative status varies with the use of marine resources. Oecologia, 188, 65–73. 10.1007/s00442-018-4181-8 [DOI] [PubMed] [Google Scholar]
- Tsai, P. Y. , Ko, C. J. , Chia, S. Y. , Lu, Y. J. , & Tuanmu, M. N. (2021). New insights into the patterns and drivers of avian altitudinal migration from a growing crowdsourcing data source. Ecography, 44, 75–86. 10.1111/ecog.05196 [DOI] [Google Scholar]
- van de Crommenacker, J. , Horrocks, N. P. C. , Versteegh, M. A. , Komdeur, J. , Tieleman, B. I. , & Matson, K. D. (2010). Effects of immune supplementation and immune challenge on oxidative status and physiology in a model bird: Implications for ecologists. The Journal of Experimental Biology, 213, 3527–3535. 10.1242/jeb.045591 [DOI] [PubMed] [Google Scholar]
- van Dongen, W. F. D. , Maldonado, K. , Sabat, P. , & Va, R. A. (2010). Geographic variation in the repeatability of a personality trait. Behavioral Ecology, 21, 1243–1250. 10.1093/beheco/arq145 [DOI] [Google Scholar]
- van Overveld, T. , Careau, V. , Adriaensen, F. , & Matthysen, E. (2014). Seasonal‐ and sex‐specific correlations between dispersal and exploratory behaviour in the great tit. Oecologia, 174, 109–120. 10.1007/s00442-013-2762-0 [DOI] [PubMed] [Google Scholar]
- Verbeek, M. , Drent, P. J. , & Piet, W. (1994). Consistent individual differences in early exploratory behavior of male great tits. Animal Behaviour, 48, 1113–1121. [Google Scholar]
- Verbeek, M. E. M. , Boon, A. , & Drent, P. J. (1996). Exploration, agressive behavior and dominance in pair‐wise confrontations of juvenile male great titis. Behaviour, 133, 945–963. [Google Scholar]
- Villegas, M. , Newsome, S. D. , & Blake, J. G. (2016). Seasonal patterns in δ2H values of multiple tissues from Andean birds provide insights into elevational migration. Ecological Applications, 26, 2381–2387. 10.1002/eap.1456 [DOI] [PubMed] [Google Scholar]
- Visser, M. E. , Holleman, L. J. M. , & Gienapp, P. (2006). Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia, 147, 164–172. 10.1007/s00442-005-0299-6 [DOI] [PubMed] [Google Scholar]
- Weathers, W. W. , Davidson, C. L. , Olson, C. R. , Morton, M. L. , Nur, N. , & Famula, T. R. (2002). Altitudinal variation in parental energy expenditure by white‐crowned sparrows. The Journal of Experimental Biology, 205, 2915–2924. 10.1242/jeb.205.18.2915 [DOI] [PubMed] [Google Scholar]
- Weeks, B. C. , Willard, D. E. , Zimova, M. , Ellis, A. A. , Witynski, M. L. , Hennen, M. , & Winger, B. M. (2020). Shared morphological consequences of global warming in north American migratory birds. Ecology Letters, 23, 316–325. 10.1111/ele.13434 [DOI] [PubMed] [Google Scholar]
- Wilson, D. , Clark, A. B. , Coleman, K. , & Dearstyne, T. (1994). Shyness and boldness in humans and other animals. Trends in Ecology and Evolution, 9, 442–446. 10.1016/0169-5347(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Wolf, N. , Newsome, S. D. , Fogel, M. L. , & del Rio, C. M. (2012). An experimental exploration of the incorporation of hydrogen isotopes from dietary sources into avian tissues. Journal of Experimental Biology, 215, 1915–1922. 10.1242/jeb.065219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets produced and evaluated during the present study have been archived in the Dryad repository and can be accessed through the following DOI: https://doi.org/10.5061/dryad.0rxwdbs4t.


