Highlights
-
•
Sonochemistry as an advanced oxidation process.
-
•
Mechanism of remediation/disinfection of microbial hazards in polluted water.
-
•
Types and comparison of sonochemical reactors for remediation.
-
•
Energy and economic aspects.
-
•
Challenges and recommendations for future studies.
Keywords: Advanced oxidation process, Bacteria, Cavitation, Disinfection, Water and wastewater, Ultrasound
Abstract
Water is one of the major sources that spread human diseases through contamination with bacteria and other pathogenic microorganisms. This review focuses on microbial hazards as they are often present in water and wastewater and cause various human diseases. Among the currently used disinfection methods, sonochemical reactors (SCRs) that produce free radicals combined with advanced oxidation processes (AOPs) have received significant attention from the scientific community. Also, this review discussed various types of cavitation reactors, such as acoustic cavitation reactors (ACRs) utilizing ultrasonic energy (UE), which had been widely employed, involving AOPs for treating contaminated waters. Besides ACRs, hydrodynamic cavitation reactors (HCRs) also effectively destroy and deactivate microorganisms to varying degrees. Cavitation is the fundamental phenomenon responsible for initiating many sonochemical reactions in liquids. Bacterial degradation occurs mainly due to the thinning of microbial membranes, local warming, and the generation of free radicals due to cavitation. Over the years, although extensive investigations have focused on the antimicrobial effects of UE (ultrasonic energy), the primary mechanism underlying the cavitation effects in the disinfection process, inactivation of microbes, and chemical reactions involved are still poorly understood. Therefore, studies under different conditions often lead to inconsistent results. This review investigates and compares other mechanisms and performances from greener and environmentally friendly sonochemical techniques to the remediation of microbial hazards associated with water and wastewater. Finally, the energy aspects, challenges, and recommendations for future perspectives have been provided.
1. Introduction
The microbial hazards associated with safe drinking water and wastewater are due to the presence of harmful and pathogenic microorganisms that often lead to epidemic situations [1], [2]. A wide range of bacteria, viruses, parasites, helminths, algae, fungi, and protozoa endanger water quality directly or indirectly. Bacteria such as Escherichia coli release toxic compounds into the water. When contaminated, water can transmit various dangerous diseases, such as typhoid, cholera, dysentery, infectious hepatitis, gastroenteritis, shigellosis, etc., to the human body (Table 1) [1], [2], [3]. Global studies have shown that 80 % of diseases worldwide are caused by water-containing pathogenic microbes [3].
Table 1.
Common bacteria found in water/raw domestic wastewater.
| Bacteria | Disease | Symptoms in brief | Incubation period | Reservoir/Common vehicle |
|---|---|---|---|---|
| Campylobacter jejuni | Campylobacter enteritis | Watery diarrhoea | 1–10 days (2–5 days on average) | Contaminated water |
| Vibrio cholera, Vibrio comma | Cholera | Extremely heavy diarrhoea, dehydration, rice-water stools |
9–72 h | Human faeces, contaminated water |
|
Escherichia coli (Enteropathogenic) |
E. coli Infection (Gastroenteritis) | Diarrhoea | 12–72 h | Contaminated water, human faeces |
| Genus, Shigella (flexneri, sonnei, boydii, dysenteriae) | Shigellosis (Bacillary dysentery) | Acute onset with diarrhoea | 1–7 days | contaminated Water, Human faeces |
| Typhoid fever, Salmonella typhosa | Typhoid fever | High fever, diarrhoea disturbances | 7–21 days | Contaminated water, Human faeces and urine, Milk and milk products |
| Salmonella paratyphi A | Paratyphoid fever | Diarrhoea disturbances | 1–10 days for gastroenteritis and 1–3 weeks for enteric fever | Drinking water contaminated with bacteria Faeces and urine of carrier or patient, milk and milk products |
According to the World Health Organization (WHO), about 1.1 billion people worldwide lack access to safe drinking water. The most significant microbial risk is the consumption of water contaminated with human or animal faece. Discharging sewage into freshwater and coastal waters is a significant source of the entry of faecal microorganisms and pathogens into water. In developing countries, 50 % of the people are exposed to contaminated water sources that lead to diseases. These conditions result in the prevalence of diarrhoea in 4 billion people every year. Also, it is estimated that this contamination causes the deaths of 2.2 million people, most of whom are children under five years of age [1], [3]. Thus, microorganism removal from wastewater has received significant attention during the last half-century to ensure that it is safe for drinking.
For this reason, several traditional technologies are used to disinfect water from waterborne microorganisms, including ozonation, UV irradiation, chlorination, hydrogen peroxide, sunlight, membrane technology, and photo-Fenton oxidation. These are classified as light-based and non-light-based technologies [4]. However, those traditional technologies have some practical issues, such as by-product formation, incomplete disinfection, and toxicity issues, that remain unclear and complex in practical applications. For instance, although chlorination is a well-known disinfection technique, incapable of treating some microorganisms, the formation of disinfection by-products and its significant impact on some kinds of water filters, such as cellulose membranes, are some of the disadvantages [5]. Membrane technology is widely used for wastewater treatment, including microorganism removal [6]; however, accumulating a dynamic layer and high turbidity by disinfected microorganisms leads to fouling porous membranes is the main disadvantage [7]. Although hydrogen peroxide disinfection is an alternative to the chlorination to avoid the formation of chlorine-disinfected by-products, and the formation of a toxic compound, iodoacetic acid, may threaten human health [8]. Ozone is a powerful oxidant widely used to disinfect microorganisms [9], [10]. However, toxicity concerns remain unclear due to its power and disinfection by-products [7]. Further, sunlight-mediated wastewater disinfection has complicated sequences, mechanisms and products [11].
Advanced oxidation processes (AOPs) are well acknowledged as the heart of pathogen elimination treatment methods. They generate a large number of free radicals, which increases their efficiency [7]. However, the formation of radicals can be increased in the presence of activators such as light, electricity, sonochemical reactors, and catalysts [12]. Numerous reports conclude that AOPs combined with hydrodynamic and acoustic cavitation can lead to a higher quality water treatment [7]. For instance, the sonochemical process combined with AOPs is widely used to disinfect microorganisms from water [13]. The disinfection process is enhanced by forming radicals via the formation of gas bubbles by ultrasound, leading to sonochemical reactions in the water showing promising results [12], [13].
Radical reactions are the primary mode of oxidation. Different types of ions in the solution can help or inhibit direct interactions between pollutants and hydroxyl radicals when they are produced. An analysis of concentration changes of monitored pollutants, particularly in the case of nitric acid, reveals that these by-products also degrade via AOP studied. However, at a much slower rate than primary pollutants, their final concentration is reported as increased compared to the primary components for the studied treatment period. Two additional strategies can be used in a real-world setting. First, extending the treatment period ought to cause the byproducts to degrade even more. The application of microorganisms in biological treatment plant-activated sludge must be evaluated for ultimate effluent purification in the second choice. Due to the significantly cheaper costs of biological treatment compared to AOPs, this approach should be considered based on their usefulness [7], [14]. In environmental protection, using cavitation in AOPs to treat acidic effluents and process water has emerged as a promising trend [12], [14].
One of the crucial factors in the optimization process is the effluents' pH value, which is frequently acidified using an inorganic acid [14]. The effectiveness of hydrodynamic cavitation (HC) in removing Brilliant Cresyl Blue (BCB) dye from effluents has been proved. Hydrodynamic cavitation and ozone (HC/O3) may be particularly efficient for degrading BCB dye effluents [15]. ZnO nanostructures were combined with nano-cellulose (NC) for the efficient degradation of tetracycline (TC) antibiotics under ultrasonic irradiation [16]. ZnO nanostructures electrochemically synthesized were successfully immobilized on powdered waste from the stone cutting industry and effectively used for the catalytic destruction of a model anti-inflammatory pharmaceutical compound. In the sonocatalysis of the pharmaceutical pollutant, the immobilized form of ZnO nanostructures was more efficient than the non-immobilized form [17]. The effectiveness of acoustic cavitation combined with ozonation or oxidation with hydrogen peroxide for preliminary treatment of effluents from the production of bitumens to reduce the total organic load to oxidize persistent organic contaminants, allowing effective biological treatment by activated sludge in the following stage of effluent treatment [18].
The effectiveness and reaction rate constants of the oxidation of sulfide ions and organic sulfides in real industrial effluents from bitumen production were examined using hydrodynamic and acoustic cavitation [19]. Compared to conventional cyanidation, the combination of PS (Persulfates) and iodide present advantages in leaching time and extraction rate for Au in a wide pH range. More significantly, PS is non-toxic and non-polluting, proving that the system is a viable, environmentally friendly way to leach gold [20]. Sono-cavitation effectively converts PS to radical species, demonstrating a synergistic effect by increasing the reaction rate and decreasing the required energy for activation. It is a viable and widely used alternative to direct thermal activation. A single and two-stage injection of PS was compared to eliminate self-scavenging effects caused by an excess of oxidant in the system. GC–MS analysis was used to determine the dioxane degradation products, and a degradation mechanism was proposed. The studies revealed that adding PS at a molar ratio of oxidant to pollutant with a two-stage injection significantly improved degradation [21]. Under ultrasound (US) irradiation, the simultaneous degradation of benzene, toluene, ethylbenzene, and o-xylene (BTEX) by persulfate (PS) and peroxymonosulfate (PMS) were activated by asphaltenes (Asph). Asphaltenes are an appealing carbonaceous material for heterogeneous catalysis due to high thermal stability, low production cost, and widespread availability [22]. Hydrodynamic cavitation (HC) is a new water treatment technology that is gaining popularity for its ability to eliminate a wide range of organic pollutants [19].
The energy released during the cavitation phenomenon can be used for various purposes, including the activation of persulfate (PS) and peroxymonosulfate (PMS). In the current study, hybrid techniques for the degradation of BTEX in water were tested, including HC combined with persulfates - HC-PS and HC-PMS [23]. There has never been a bigger need for gold in electronics, and the extraction procedure could be carried out even without iodine. The benefits and drawbacks of NaPS, KPS, APS, and PMS. NaPS and KPS are frequently used when discussing PS-based AOPs in the literature. The widespread use of PMS for sterilization makes it more appropriate for the microbial breakdown of contaminants. APS is not a favoured option in other PS applications since it may disintegrate to produce ammonia, although it has more advantages in material modification or metal extraction [24].
It is believed that sonochemical reactors (SCRs) are one of the most appropriate for the remediation and disinfection of water and wastewater. Easy operation and non-production of byproducts are the key advantages. Therefore, this review briefly assesses and compares different mechanisms, performances, and energy assessments of cavitation reactors as a green and environment-friendly route for destroying/inactivating waterborne bacteria. Besides, the challenges and recommendations for future studies have been discussed.
2. Theory and mechanism of ultrasonic waves
Oscillations with frequencies higher than 20 kHz are not commonly perceived by humans and are referred to as ultrasonic frequencies. Depending on the frequency range, it is subdivided into (i) Low frequency, high power ultrasound; (ii) High frequency, medium power ultrasound and (iii) High frequency, low power ultrasound. Ultrasonic reactions generally occur in the 20–100 kHz range, causing the desired chemical and physical transformations [25]. Various applications are briefed with their corresponding ultrasonic frequency ranges specified in other literature, as shown in Fig. 1 .
Fig. 1.
Applications with their corresponding ultrasonic frequency ranges [26].
Sonochemical processes are commonly preferred to control environmental pollutants due to the features such as (i) source of clean energy, (ii) accelerating chemical or biological processes in water and wastewater, (iii) causing real chemical changes in solutions without any additional substances (iv) providing faster and safer degradation [27], [28].
Ultrasonic effects at high frequencies (in the MHz range) allow better mass transfer from solid surfaces to the liquid medium. At low frequencies, the induced cavitation has strong mechanical and sonochemical effects that degrade large molecules into small ones and destroy the suspended particles in water and wastewater. Moreover, the rate of reactions in biological processes increases [28], [29]. Hence, the use of ultrasonic techniques results in better biodegradation processes. Fig. 2 shows the various ultrasonic wave energy applications.
Fig. 2.
Current applications of ultrasound in various areas.
3. Types and comparison of sonochemical reactors
ACRs and HCRs are widely used to disinfect water and wastewater with microbes. ACRs have been used successfully to destroy and deactivate microbial populations on a laboratory scale. However, a few drawbacks limit the application of these reactors in large-scale industries, as their cavitational intensity decreases rapidly with increasing distance from the source of ultrasound dissipation and disappears at a distance of about 5 cm; thus, they have low thermal efficiency and a high cost of installation [30], [31], [32]. In contrast, HCRs are more economical and efficient than ACRs due to their simple structure, low cost, and high thermal efficiency, and thus have good potential for use in large-scale industries [33], [34]. Among these, ultrasonic horn, ultrasonic bath, venturi, orifice, rotating and vortex-based types have been widely used for water and wastewater treatments [35], [36], [37], [38]. Based on the geometry, flow, and operation mode, sonochemical reactors can be divided into a batch and continuous reactors [13].
The batch reactors are further classified as ultrasonic batch reactors, ultrasonic sonotrode-based batch reactors, and ultrasonic cup-horn reactors. Due to their simplicity and cost-effectiveness, laboratories widely use ultrasonic baths. The ultrasonic baths are usually equipped with ultrasonic transducers attached at the bottom or/and on the rectangular cross-section stainless steel tank walls or mounted in an external enclosure [13]. In this, the sample can be sonicated either directly or indirectly. In these reactors, ultrasonication frequency can be from 20 to 132 kHz using quartz or piezo-ceramic transducers as ultrasound sources [39]. The ultrasonic bath reactors are widely used for water treatment because they consume relatively low energy, simple operation and installation [40]. Ultrasonic sonotrode-based batch reactors are used to directly contact the sample, with high ultrasonic power for high cavitation. Ultrasonic sonotrode-based batch reactors usually provide 1000 times higher ultrasonic energy than ultrasonic baths. Thus, they are widely used for food processing, nanoparticle synthesis, extraction, liquid degassing, welding, moulding and bonding [13]. One of the key disadvantages is horn erosion, which not only adds to the expense of replacement but can also pollute the processing liquids, resulting in inaccurate findings [13], [41]. Ultrasonic cup horn reactors are made up of a reaction vessel and an ultrasonic horn installed inside. The ultrasonic emitter is a sonotrode that transmits ultrasonic waves with 50 times higher energy than ultrasonic baths and is frequently used for indirect sonication [13]. This type of reactor is usually used for trace metal extraction from a complex mixture [42], [43] and has less corrosion than ultrasonic sonotrode-based batch reactors [44].
In the large-scale treatment process, flow arrangement facilitates greater advantages for the design of reactors. If treatment allows, continuous reactors are preferred over batch reactors. The continuous reactors consist of an ultrasonic treatment chamber with a sonication horn connected to a stainless-steel cylindrical feed reactor. The continuous sonochemical reactors are further classified as continuous with emitting walls, continuous ultrasonic microreactors, and continuous reactors with incorporated sonotrodes [13]. The continuous flow reactors can also be combined with microwaves/ultrasound waves, which received significant interest in both scientific and industrial applications [13], [45]. Continuous ultrasonic microreactors are widely used for industrial scale because of the easy control of conditions, better heat and mass and heat transfer compared to batch reactors [46], [47], [48], [49].
4. ACRs: Mechanism and performance
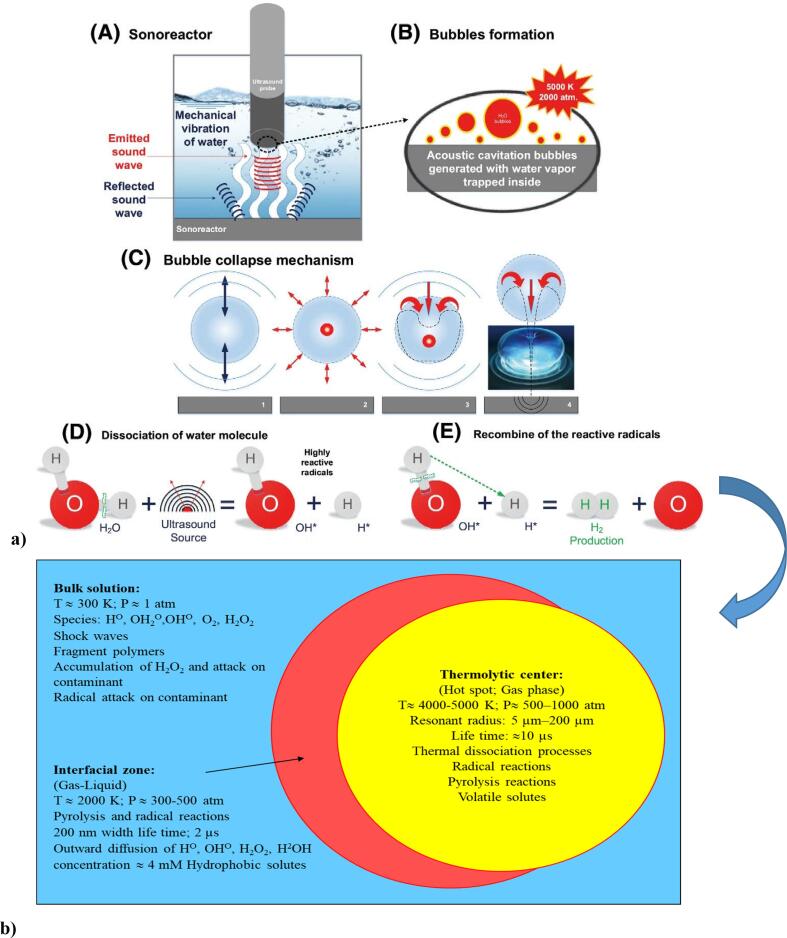
The word cavitation is derived from the Latin word cavus. Ultrasonic energy (UE) progression in an aqueous medium causes molecules to oscillate. This progression creates cycles of contraction and expansion. In the contraction cycle, the average distance between molecules decreases, whereas, in the expansion cycle, this distance increases. If the negative pressure is applied to the liquid medium, the average distance between molecules exceeds the critical molecular distance required to maintain the liquid intact. This way, the liquid breaks down, and cavities are formed [50], [51], [52]. The formation of cavitation bubbles is shown in Fig. 3a. The creation of bubbles occurs due to the difference in acoustic pressure. When bubbles are formed, they absorb energy while growing. The bubbles expand slightly during each contraction and can expand to a critical size that cannot absorb more energy, leading to collapse. The gases and vapours in the bubbles are compressed, resulting in a very high-temperature hot spot. To generate these bubbles, a very high negative pressure is required so that the tensile strength of the liquid can be overcome. Computationally, the sound pressure required for cavitation in water is approximately 1500 atm. UE generated due to pressure gradients, and cavitation and vibration of bubbles can cause mechanical, thermal, and chemical effects on the environment. Phenomena such as ionization in the volume of gas due to severe oscillations and highly variable gas pressure inside the bubbles are also observed. This causes the creation of free radicals and increases the density of radicals in the aquatic environment. These radicals can lead to a series of chemical reactions. This type is referred to stable cavitation. At the same time, another mode is transient cavitation, which occurs only at very high energy intensities [50], [51], [52], [53], [54]. Fig. 3b shows the reaction zones in the cavitation process.
Fig. 3.
Stages of formation, growth, and implosion of the bubble in an aqueous medium (a), three different sonochemical reaction zones (b) [115], [116], [117].
The type of reactor and physicochemical properties of the liquid phase affect the efficiency of sonochemical processes. The effects depend on the nucleus size, cavity lifetime, threshold conditions, and amount of nucleation. As the liquid properties affect the cavitation phenomenon, evaluating the optimum conditions in the sonochemical process is essential. Liquid viscosity and the nature of dissolved gases have significant effects on the cavitation intensity. Vapour pressure combined with temperature is the most crucial tool for controlling and optimizing the sonochemical processes [30], [31], [32], [33], [34], [35].
On the contrary, high-frequency and low-intensity (up to an optimum value) UE is the most suitable for sonochemical processes. Comparing the chemistry of UE efficiency at different frequencies is a complicated task because this process is associated with cavitation bubbles. The formation and behaviour of a bubble are closely related to sound pressure, which depends on the reactor design and ultrasonic parameters (frequency and intensity) and surface area. Therefore, sound frequency changes the number of bubbles formed in a reactor with a specific shape and design that can affect the reaction efficiency [54], [55]. Physical factors affecting the cavitation and bubble collapse process are illustrated in Table 2.
Table 2.
Factors affecting the cavitation and bubble collapse process in SCRs.
| Factors | Remarks | Ref. |
|---|---|---|
| Frequency | Cavitation activity in water takes place at different frequencies. This usually occurs in the range of 5 kHz − 5 MHz. The cavitation activity depends on the sound pressure. At a constant sound pressure amplitude, as the frequency increases, the cavitation intensity decreases while the number of holes at a resonant frequency increases. Therefore, if the sound pressure produced by the transducers remains constant, the bubble size decreases with the frequencies, resulting in a less intense explosion. Studies with different ultrasonic frequencies show that the number of cavitation sites is proportional to the frequency. At 40 kHz, the cavitation threshold is at 1 atm and for 850 kHz it is more than 100 atm. However, some studies show a threshold of 10 atm at this frequency. | [121] |
| Intensity/ Power | Increasing the intensity can increase the acoustic amplitude and cause the cavitation bubble to burst. The ultrasonic intensity (I) can be calculated using Eq. (5). (5)The ultrasonic power (P) can be measured using the calorimetric technique (Eq. (6)). P = mCp (dT/dt) (6) Cp = 4.18 J/(g K), is the specific heat capacity of water m is the mass of sonicated water dT is the change in the temperature dt is the applied sonication time Ultrasonic power is one of the important factors as it has a significant influence on the number of cavitation bubbles as well as their collapse intensity. |
[121], [122], [123], [124], [125], [126] |
| Temperature | It is one of the most important parameters to control the cavitation phenomenon. Temperature affects the viscosity, the solubility of the gas, the diffusion rate of the gas dissolved in the liquid, and the vapour pressure. In pure water, cavitation around 70 °C is the maximum. The viscosity in a given fluid should be minimal for cavitation to occur easily. High viscosity results in more energy loss. Viscous liquids do not quickly form cavitation bubbles and do not lead to severe internal explosions. In most liquids, the viscosity decreases with increasing temperature. Although low vapour pressure results in cavitation bubbles with relatively large collapse intensity, fewer bubbles are produced, which facilitates increasing the cavitation threshold. In this, the cavitation bubbles are filled with cavernous liquid vapour. When outlet gases run out of the liquid, the cavitation of steam is compressed into bubble-shaped steam bubbles, which explode readily. This type of cavitation is the most effective in water clarification. However, as the liquid reaches its boiling point, cavitation intensity at the cavitation sites gradually decreases. | [122], [127], [128], [129] |
| External pressure | It can reduce the fluid vapour pressure and increase the intensity required to induce cavitation. | [121] |
| Vapour pressure | Cavitation is difficult in solvents with low vapour pressure. A more volatile solvent will form cavitation at lower acoustic energy. | [130] |
| Solvents characteristics | Solvents with high vapour pressure and low viscosity and surface tension will easily form bubbles. | [122], [131] |
| Soluble gases properties | The presence of dissolved gases forms a large number of cavitation cores. However, the higher gas solubility causes more gas molecules to penetrate the cavitation bubble. This also makes the bubbles explode weakly. | [121], [122], [132] |
| Surfactants | They are commonly used to reduce surface tension. To achieve optimum cavitation, surface tension must be at a moderate level. A high surface tension produces bubbles of low elasticity, which disintegrate more strongly. Although a low surface tension allows the bubbles to grow larger, they reduce the intensity of cavitation. | [131] |
| Sonication time | Usually, as the time of sonication increases, the temperature and the reactivity in the optimum range increase. | [132] |
| Mass transfer | Mass transfer coefficient increases with the power intensity of sonoreactors (horn). The mass transfer coefficient is highly dependent on the operating pressure as well as the reactor temperature. |
[133], [134], [135] |
| Mixing time | Mixing time decreases with an increase in the ultrasonic intensity as well as the Reynolds number. Mixing time may also depend on ultrasonic power, which decreases mixing time with increasing power density. |
[136], [137], [138], [139], [140], [141], [142], [143] |
| Flow velocity | The average flow velocity decreases linearly with water height and increases with liquid viscosity. It has been shown that vessel diameter and power intensity must also be determined to produce a uniform mixing in a reactor. In rectangular reactors, the average current speed increased with increasing electric power. The efficiency of some of the reactors is reduced due to the absence of active bubbles per unit volume. However, the reactor efficiency could be increased by adding a stirrer and combining the mechanical flow and acoustic streaming. |
[133], [144] |
| Location and number of transducers | The location and number of transducers should be based on the fluid's height and the SCR's diameter. The position of the transducers decides the resultant maximum cavitational activity. In rectangular or hexagonal types, the distribution of the cavitational activity is usually good, and these SCRs have been proposed for large-scale applications. The mixing pattern and hydrodynamic behaviour of these SCRs can also be affected by the location and number of transducers. |
[145], [146], [147] |
| Pressure drop | Pressure drop is one of the basic parameters, and its optimization is crucial for achieving the complete inactivation of bacteria. Increasing the pressure to a certain level increases the disinfection efficiency. However, high pressure leads to a decrease in the production of hydroxyl radicals and, consequently, a decrease in disinfection efficiency. | [136], [148], [149] |
Studies on the abundance of cavitation at different ultrasonic frequencies have shown that the number of cavitation sites is directly proportional to the ultrasonic frequency. For example, 60–70 % of cavitation sites per unit volume of the liquid are produced at 68 kHz rather than 40 kHz. In the case of ultrasonic particle removal, higher frequencies are likely to have better effects on removing some pollutants. Further, the removal efficiency of one-micron and sub-micron particles in deionized water is increased at a higher frequency. The removal efficiency for one-micron particles at 65 kHz is about 95 %, whereas, at 40 kHz, it is 88 %. The same capability was observed for particles with diameters of 0.7 and 0.5 µm. At 65 kHz and 862 kHz, the particle removal efficiency was 95 %, and for particles with diameters of 0.7 and 0.5 µm, it was 87–90 % and 84–85 %, respectively. At 20 kHz, bubbles demonstrate a diameter of about 170 µm. The minimum energy required to produce cavities at different frequencies should be above the ultrasonic threshold. The minimum energy needed above the ultrasonic threshold for water at ambient temperature is about 0.5 and 0.3 W/cm2 for 40 kHz and 20 kHz, respectively. At 25 kHz, the vacuum bubbles or vapour particles have a diameter between 50 and 150 µm. At 20 kHz, the pressure is about 35–70 kPa, the local temperature is about 5000 °C, and the wave velocity is approximately 400 km/h [56], [57], [58], [59], [60].
In addition to the parameters mentioned, the shape of the emitted wave also affects the cavitation intensity. At low frequencies (20–30 kHz), a relatively small number of cavitations are produced with larger sizes and more energy. At higher frequencies (60–100 kHz), denser cavitation is produced at medium or lower energies. Lower frequencies are more suitable for larger cleaning components. High frequencies of 60–80 kHz are recommended for cleaning contaminated surfaces, whereas 35–45 kHz are suitable for industrial materials and components. A frequency of 68 kHz induced enough cavitation to remove the detergent layer and sub-micron particles [121], [122], [123], [124], [125], [126].
As indicated, cavitation is the growth and implosion of microbubbles filled with gas or vapour, which produces energy in the liquid that induces chemical, mechanical, or physical effects. It also causes high temperatures (5000 K) and pressure (1500 atm). This leads to the production of hydroxyl (OH•), hydrogen (H•), and hydroperoxyl (HO2•) radicals, as well as hydrogen peroxide (H2O2), which create an oxidative environment. Reactions related to these free radicals can occur in the collapsing bubble and the surrounding liquid. The production of •OH and H2O2 during sonolysis is influenced by the implosion temperature and pressure as well as the lifetime of the bubbles. In solutions in the presence of oxygen, the production of free radicals by UE is described by the following Eqs. 1–16 [56].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
The rate of hydrogen peroxide production during the sonolysis of water is also estimated using Eq. (17) [57].
| (17) |
Where kOH, H2O2 = 2.7 × 107 M−1 s−1; kHO2, HO2 = 8.3 × 105; kOH, OH = 5.5 × 109 M−1 s−1 in a solution at 25 °C and 3 × 10-12 and 1. 5 × 10–11.cm3.molecule-1. s−1 in the gaseous phase. When ultrasound passes through a liquid medium, time-dependent changes in the pressure range occur, which can be described by Eq. (18) [58]:
| (18) |
Where P0 is the ambient pressure, Pa is the amplitude of the pressure, and f represents the frequency.
The pressure amplitude is calculated using Eq. (19) [59]:
| (19) |
Where q is the density of the liquid medium (kg/m3), and c is the ambient sound velocity (m/s).
Bubble temperature is another essential feature of the cavitation phenomenon. Based on a simple thermodynamic model for bubble collapse and assuming adiabatic compression, the maximum temperature (Tmax) inside the bubble is theoretically calculated using Eq. (20) [58]:
| (20) |
Where T0 is the liquid temperature, Pm is the pressure of the liquid (total hydrostatic and acoustic pressure), γ = is the specific heat ratio of the vapour/gas mixture, and Pv is the pressure in a bubble at its maximum size.
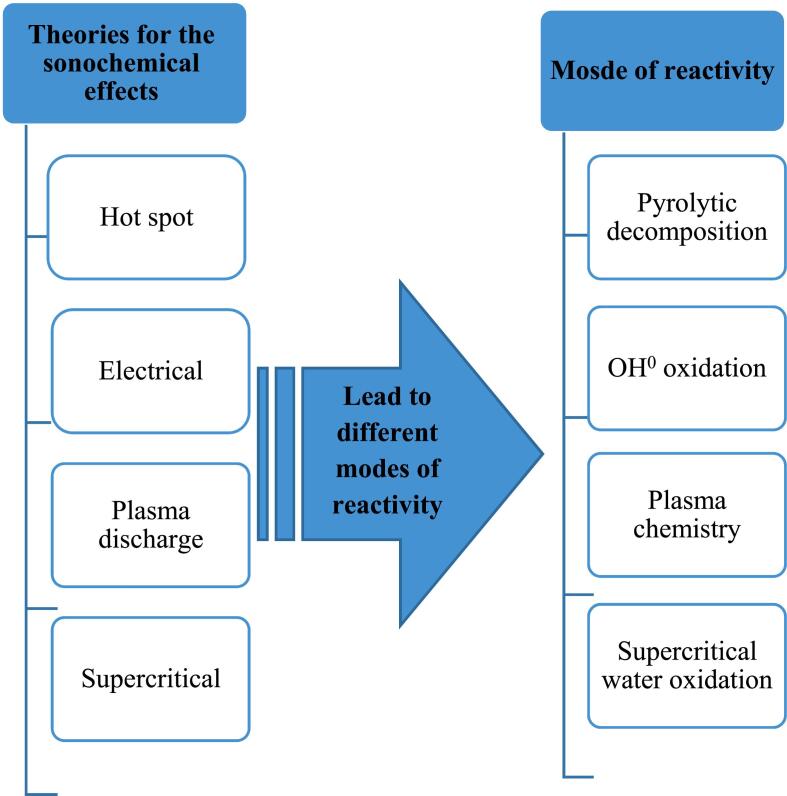
It is usually assumed that this pressure is equal to the vapour pressure of the liquid. However, gas molecules that are initially soluble in liquids are also known [58]. Four theories describe the ultrasonic effects [Fig. 4]. The hot spot theory is mostly used in environmental chemistry [58]. Based on this theory, each fine bubble is a microreactor that generates OH+, H+, O+, and OOH+ and heat during its collapse. According to this theory and the compound's physical and chemical properties, sonochemical reactions can usually occur in three regions [58]. Table 3 provides about these reaction zones.
Fig. 4.
Schematic of different sonochemical theories that lead to different modes of reactivity.
Table 3.
| Reaction region | Remarks |
|---|---|
| Thermolytic centre (hot spot) | Substances that reach the cavitation bubbles are decomposed by pyrolysis, and oxidation reactions occur in the presence of oxygen. |
| Interface region |
Higher variations in temperature and pressure have been demonstrated. This region has higher concentrations of hydroxyl radicals, and the conditions are very close to inside the cavitation bubble. |
| Bulk liquid region |
Under extreme conditions (high temperatures and pressures), water breaks down into OH radicals and hydrogen atoms in the cavitation bubbles. Radicals reach the insulating phase and can decompose soluble pollutants by oxidation. Also, the collapse of the cavitation bubbles releases the hydrodynamic shear forces. These forces can break down macromolecules and polymers into simpler compounds. |
5. Application and mechanism of UE for removing bacterial contaminants of water and wastewater
In recent years, UE has been widely used as an AOP for water and wastewater treatment. UE is an effective way to synthesise organic matter into less toxic compounds and, in certain cases, can completely decompose compounds. UE can accelerate chemical or biological processes in water and wastewater treatment. Ultrasonic effects at high frequencies in the MHz range allow better mass transfer from solid surfaces to the liquid medium. Cavitation at low frequencies displays strong mechanical and sonochemical effects that affect large molecules and suspended particles in water and wastewater. The size of large molecules reduces, and the particles are destroyed; hence, better biodegradation is observed. It also increases the rate of reactions in biological processes, and increased mass transfer is a function of ultrasonic power [61], [62].
Recently, water and wastewater disinfection using UE has been extensively studied to inactivate microorganisms. The propagation of UE through water causes cavitation and a wide range of physical effects, which cause chemical and mechanical effects. Studies have shown that the effects of bactericide with UE are due to the mechanical and sonochemical effects of AC. In other words, the mechanism of microbial death is mainly due to the thinning of microbial membranes, local warming, and cavitation-induced free radical production. The effect of cavitation on the biological system is through the production of local temperature, mechanical pressure, or free radicals, all of which begin with a non-thermal mechanism. As described earlier, cavitation is classified into stable and transition. In the transition cavitation, at a high temperature, the pressure is formed in the solution for a few seconds. Studies have shown that low-frequency UE can partially eliminate pathogenic factors in water. Transition cavitation is, therefore, a physical process responsible for altering the structure of microorganisms. The higher the intensity of the UE, the more stable the cavitation phenomenon [58], [59], [60], [61], [62].
In summary, the antibacterial activity of cavitation is divided into four stages [63], [64]:
-
i.
Resonance of the bacterial cell surface, pressure, and pressure gradient caused by the implosion of gas bubbles entering the bacterial suspension.
-
ii.
The shear forces from micro-flows occur inside the bacterial cells.
-
iii.
The effects of free radicals from gases in the aquatic environment.
-
iv.
The formation of a strong bactericidal agent, such as hydrogen peroxide (H2O2), results from water's sonochemical decomposition.
The susceptibility of the bacteria to the sonication process depends on the composition, viscosity, sound transmission, penetration of radiation in the solution, as well as the size and shape of the bacteria. Large bacteria are generally more susceptible to sonication than small ones as more surface is exposed to ultrasound. Cocci/spherical bacteria are more resistant to UE than bacilli/rod-shaped bacteria [65]. However, other soluble components, such as organic solvents, slowly decompose during sonication and have little contribution to the sonochemical reactions. Table 4 shows how these factors influence the disinfection process by UE.
Table 4.
Factors and conditions affecting the disinfection efficiency using UE.
| Parameters | Effects | Ref. |
|---|---|---|
| Cell shape | Larger cells are more susceptible to cavitation impacts than smaller cells. | [153] |
| Species | Larger-sized cells, as well as rod-shaped bacteria, are generally more susceptible to UE than coccal forms. Gram-positive bacteria are more resistant to ultrasound than gram-negative bacteria. Aerobic species than anaerobic species, and spores of bacteria are more resistant to UE than vegetative cells. | [154], [155], [156], [157] |
| Cell size | With an increase in cell size, the cavitation resistance decreases. | [[158], [159], [160]] |
| Cell development | The dividing cells are more sensitive than the cells in the fixed phase. | [161] |
| Hydrostatic pressure | The inactivation of spores and vegetative cells increases with the hydrostatic pressure of the ultrasonic medium. The bacterial inactivation increases gradually in the (0 to 200–300 kPa) range. At higher pressures, bacterial inactivation hardly occurs. | [156], [157], [158], [159], [160], [161], [162], [163], [164] |
| Amplitude | The inactivation of vegetative cells and spores of bacteria increases with the amplitude of ultrasound. The inactivation rate of most bacteria on larger slopes could be due to an increase in the number of bubbles over time or an increase in the fluid volume where cavitation occurs. | [156], [157], [158], [159], [160], [161], [162], [163], [164] |
| pH | Microbial heat resistance is usually reduced in acidic environments. However, studies have shown that the resistance of microorganisms to UE depends on the acidic environment. | [156], [165], [166] |
The effectiveness of ultrasound on microorganism killing has been examined and described here. Ultrasonic waves are highly effective at addressing bacterial and algal contamination in water. The cavitation bubbles reach temperatures and pressures in thousands of degrees and atmospheres, respectively. The resultant implosion and subsequent shock wave, stress, and dramatic temperature shift kill the bacteria. The results of the study demonstrate that ultrasound may be utilized efficiently for water disinfection and has various advantages. It greatly reduces the number of bacteria present in water tests when combined with chlorine. In addition, ultrasound minimizes the amount of chlorine necessary for disinfection. Increasing the power of ultrasound results in more efficient bacterial cell death. High-frequency ultrasound is more effective in water disinfection than low-frequency ultrasound [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]. Table 5 summarizes some recent reports on ultrasonic-assisted disinfection of waterborne microorganisms.
Table 5.
Summary of some recent reports on ultrasonic assisted disinfection of waterborne microorganisms.
| Microorganism (introduced dose in CFU/mL) | Experimental conditions (Utrasonication frequency and power density) and other hybrid systems | Media of sample and volume | Remarks | Ref. |
|---|---|---|---|---|
| B.subtilis spores (106), Escherichia coli (250) | 20 KHz frequency, 38.5, 50, 285.7 W/cm2 power intensity | 120 mL distilled water |
B. subtilis was resistant, but E. coli was inactivated by 2.97 ± 0.58-logs in 5 min at 817 kHz |
[167] |
| Microcystis aeruginosa (1.3 × 106) | 20–1137 KHz frequency, 0.45 W/cm3 power density | 300 mL sample | Higher ultrasonic frequencies (60 % inactive) more effective than lower frequencies (30 % inactive) | [168] |
| Bacillus subtilis (104) | Dual-frequency (70 + 33 KHz and 70 + 100 KHz) and 0.025 W/cm3 power densisty | 30 L/h flow-through pilot scale | It is efficient treatment with 70 + 100 KHz after pretreatment with NaOCl and 17 + 33 KHz | [169] |
| Microcystis aeruginosa (106) | 120 KHz to 1120 KHz frequency with 0.01–0.09 power density | Ultrapure water sample | Cells are more inactivate at 430 and 740 KHz than other frequencies with 80–90 % turbidity removal | [170] |
| Escherichia coli (1 × 109) | 2 MHz frequency | 0.1 mL of 3 % tryptic soy broth |
2-log disinfection is achieved within 10 min | [171] |
| E. coli (105) | 20 KHz frequency with 11 W/cm2 power intensity + 2.25 mW/cm2 UV intensity | 70 mL/min flow through 0.9 % saline solution | A synergistic ehnacement is observed under utrasonication and UV light inactivation | [172] |
|
Fecal coliform bacteria (3.67 × 102 −9.48 × 104) |
28 KHz frequency + 254 nm UV light | 900–2100 L/h continuous flow baffled secondary effluents | A synergistic effect observed. 4.24 log reduction observed at specific energy, 0.219 kWh/m3 and 1200 L/h | [173] |
| E. coli (108) | 33 KHz frequency + 0.035 mW/cm2 UV intensity | 2L (utrasonification) 30 mL for UV treatment in batch mode. Deionized water, Kaoline suspension, secondary effluents |
Utrasonication pre-treatment increased the inactivation rates and reduced photo-ractivation | [174] |
|
Fecal coliform (3.7 × 102 ± 2.0 × 102), E. coli (2.2 × 102 ± 78) and fecal streptococci (1.0 × 102 ± 33) |
28 KHz + 254 nm UV light | 1200 L/h continuous flow baffled secondary effluents | High long-term disinfection efficiency for fecal coliforms (87 % < 100 CFU/L). No algae growth and fouling of the lamps | [175] |
| Natural bacteria (7.03 ± 0.34) | 26 KHz + 254 nm UV light | 2L Simulated lettuce wash water with 3 L/min flow | 3.57 ± 0.39 log CFU/mL inactivated. Colour (43.31 %), suspended particles (30 %) and COD (79 %) reduction with the energy consumption of 0.114 kW/h |
[176] |
The recent literature survey shows that a high sonochemical frequency range of 100–500 kHz is the best outcome [66]. Also, concluded that hybrid systems such as ultrasonication with UV light and other oxidation processes cannot only enhance but also synergistic increment in the disinfection/inactivation of microorganisms was observed. For instance, Mukherjee and Ahn [67] reported ultrasonic removal of muti-drug bacteria (Enterobacter sp., Citrobacter freundii, and Klebsiella pneumonia) load of 6.4 log CFU/mL was 100 % killed in 25 min using Terpinolene as an enhancer. This finding implies that adding a bio-additive to the ultrasonic-activated disinfection process can efficiently eradicate pathogenic multi-drug-resistant bacteria while substantially reduce operating time and cost, making it appropriate to replace conventional wastewater disinfection. However, the overall studies found that UV light disinfection in the presence of ultrasonication was more effective than other hybrid systems [68].
6. Effects of UE on bacterial suspensions
Harvey-Loomis reported the first degradation of microorganisms by UE in the 1920's. This study was performed to reduce the amount of Bacillus fischeri in seawater at 375 kHz and 19 °C. They reported that heat is one of the parameters that can damage a bacterial colony. However, UE has a more significant effect. In the 1950's and 1960's, research focused on understanding the mechanism of ultrasonic reactions with microbial cells. It has been shown that cavitation followed by cell wall disintegration, local heat, and free radical formation contribute to microbes' degradation. Recent studies have shown that exposure to UE weakens the cell wall and releases the cytoplasmic membrane from the cell wall [69], [70].
Various physical, chemical and heat impacts can cause the inactivation of bacteria. However, mechanical impacts play a major role in the destruction of bacteria [70]. Bacterial degradation using low frequency and high power UE is mainly due to the mechanical effects of AC [70]. On the mechanism of the effect of high-frequency UE, sonochemical effects [71] and the physical and chemical impacts have been proposed as the actual process for bacterial elimination. The process is done by determining the performance of H2O2. Accordingly, the initial sonochemistry processes in a water environment include the production of OH, H+ and H2O2 radicals [31], [70]. Cell degradation can also be due to chemical inconstancy in the bacterial cell wall, the fast influence of waves into the bacteria and the alkaline pH of the environment· H2O2 alone does not cause the oxidation of cell components, but rather reactive species derived from it cause oxidation. Because of its small size, H2O2 can pass via the bacterial membrane through diffusion and become an OH radical by intracellular Haber-Weiss reaction. Whether or not this reaction occurs depends on the influence of intracellular Fe2+ and superoxide ions [72]. Other studies have also shown that the main target of the ROS (reactive oxygen species) attack is the outer bacterial layer called peptidoglycan. It has been proposed that OH radicals invade the cell wall, eventually leading to the complete degradation of the extracellular layer [73]. OH radicals formed during the ultrasonic process are responsible for the initial oxidation in the lipid of the cell membrane of some bacteria such as E. coli [70], [73].
Table 6 summarizes the studies that focus on the effect and mechanism of UE on the inactivation of water and wastewater-borne bacteria. It can be noticed that the effect of UE on bacteria under different conditions varies. Also, Fig. 5 shows the degradation of different bacteria before and after sonication.
Table 6.
Disinfection efficacy of UE for the inactivation of bacteria under different conditions.
| Bacteria | Frequency | Intensity/ Density/ Power |
Sonication time | Removal Percentage | Ref. |
|---|---|---|---|---|---|
|
E. coli, S. aureus, B. subtilis, P. aeruginosa |
26 kHz | 0.2 to 0.5 W.cm−2 | Varies | – | [177] |
|
P. aeruginosa, E. coli, S. epidermidis, S. aureus |
67 kHz | 0.3 W.cm−2 | – | – | [178] |
| E. coli | 800 kHz, 20 kHz | 5 W.cm−2 30 W.cm−2 |
15 min |
80 |
[179] |
|
E. coli (ATCC 10798), S. epidermidis |
28 kHz, 48 kHz |
100 mW.cm−2,300 mW.cm−2 |
24 h | 99–99.9 | [180] |
| E. coli | 20 kHz | 4.6 to 74 W.cm−2 | 60 min | – | [181] |
| E. coli | 70 kHz 500 kHz |
2 to 200 mW.cm−2 | 2 h | 82–99 | [182] |
| Coliform | 15 min | 30–44 | [183] | ||
|
S. epidermidis (RP62A, ATCC 35984), P. aeruginosa (ATCC 27853), E. coli (ATCC 10798) |
70 kHz | 2 W.cm−2 | – | – | [184] |
| B. Subtilis | 27 kHz | 300 W | 60 min | 96 | [185] |
| B. Subtilis | 38 kHz 20 kHz 512 kHz 850 kHz |
180 W.L-1 240 W.L-1 71 W.L-1 64 W.L-1 |
15 min | – | [186] |
| E. coli | 40 min | 3 log | [187] | ||
|
E. coli. S. epidermidis (ATCC 35984) |
28.48 kHz | 500 mW.cm−2 | 24 or 48 h |
90–99 |
[188] |
|
E. coli (XL1-Blue) |
27.5 kHz | 42 W.mL−1 | 180 s | 99 | [189] |
| Total coliforms, Fecal coliforms, Fecal streptococci | 22 kHz and 20.5 kHz |
240 W and 120 W | 15 min |
– |
[190] |
| E. coli (ATCC 10798) | 70 kHz | 500 m.Wcm−2 | 24–72 h | 58 and 69 | [191] |
| E. coli | 24–80 kHz | 150–450 W | 120 min | 92.3 | [192] |
| E. coli | 24 kHz 80 kHz |
450 W | 20–30 min |
99.9 |
[193] |
|
E. coli B. subtilis |
– | – | – | 100 | [194] |
| E. coli | – | – | 2 h | 70–100 | [195] |
|
E. coli, P. aeruginosa, E. faecalis, S. aureus, S. epidermidis |
40 kHz | 350 W | 60 min | 100 | [196] |
|
Total coliforms, Faecal coliforms, P. spp. Cl. Perfringens, F.streptococci |
24 kHz | 1500 W.L-1 | 15, 30, 60 min (respectively) |
94, 97.3, 99.5 88.4, 98.3, 99.2 90.1, 99.3, 99.7 59.1, 64.4, 65.8 34.5, 60.5, 84 |
[197] |
| E. coli | 490 W.L-1 | 3 min | 75 | [198] | |
|
E. coli LMG 2092 T |
20 kHz | 680 W 810 W |
120 and 180 min |
90–99 |
[199] |
|
E. coli MC 4100 Klebsiella |
– | – | 60–270 min 60 min 90 min 20 min |
4–7 log 5 log 3 log 6 log |
[200] |
|
E. coli ATCC 10536, S. Typhimurium ATCC 14028, L. monocytogenes |
35 kHz, 130 kHz | 250 W 50.95 W.cm−2 2500 W.L-1 |
5, 10, 20, 30 min | – | [201] |
|
E. coli ATCC 25922, S. aureUS ATCC 25923 |
30 kHz | 100 W | 30 min | 99–99.9 | [202] |
|
E. aerogenes B. subtilis |
20 kHz | 12.7 W | 20 min | 99.99 | [203] |
|
V. cholera (ATCC 15748), E. coli (ATCC 11775), E. avium (ATCC14025) , C. marina (ATCC 35142) B. subtilis var. niger |
19–20 kHz | 80–1240 J/ml | 1–22 min | 90 | [204] |
|
E. coli, L. rhamnosUS |
20 kHz | 17.6 W | 22 min |
99 |
[205] |
|
E. coli (IAM 12058) S. mutans (JCM 5175) |
20 kHz and 500 kHz | 1.7–12.8 W 1.4 – 10.6 W |
10–12 min | – | [206] |
|
E. coli, L. pneumophila, B. subtilis |
1 Hz to 170 kHz | – | 60 min 120 min 120 min |
99.95 99.98 99.98 |
[207] |
|
E. coli ATCC11229, B.subtilis ATCC6633 |
20 kHz | 600 W.L-1 | 45 min | 99 | [208] |
|
E. coli K12 TEAG 1133, Listeria innocua NCTC 11288 |
20 kHz | 96 W | 60 min |
99.9999 99.99 |
[209] |
| Total coliform | 20 kHz | 400 W | 5, 15 min | 40, 70 | [210] |
| E. coli | – | – | 30 min | 98–100 | [211] |
| E. coli | – | – | 30, 60, 90, 120 min | 42, 98, 99.5, 99.99 | [212] |
| E. coli | 2 kHz | 1 MHz | 5 min 20 min |
50–75 95–99 |
[213] |
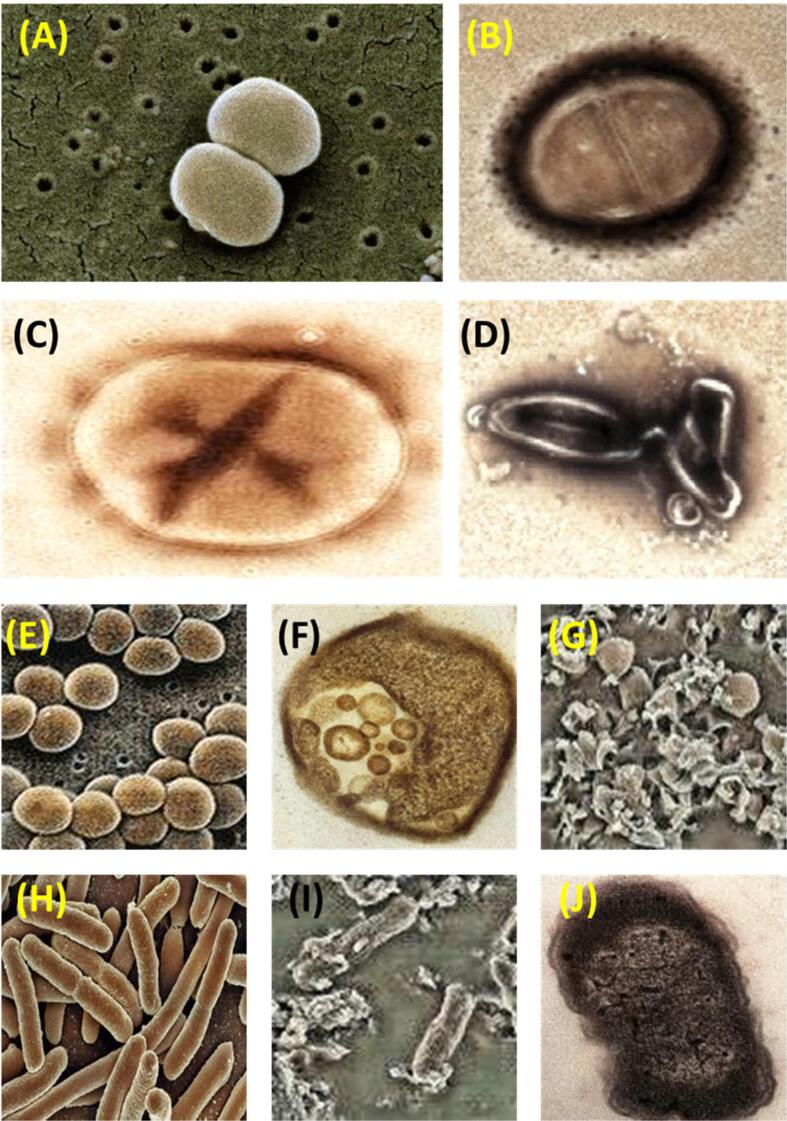
Fig. 5.
Degradation of bacteria types before and after sonication: Staphylococcus epidermidis cells (A) before sonication, (B) after sonication; Enterobacter aerogenes cells (C) before sonication, (D) after sonication; Staphylococcus aureus cells cells (E) before sonication, (F & G) after sonication; E.Coli cells (H) before sonication, (I & J) after sonication.
In the following, more details of the studies, as well as more similar and contradictory results, have been presented. Various researchers around the world have extensively studied the effect of UE cavitation on different bacterial species under different bacterial conditions, several bacterial samples, different culture media, application of different types of cavitation (AC or hydrodynamic cavitation (HC)), cavitation number, geometry, cavitation operation, frequency, intensity, density, power and sonication time [74]. However, comparing and generalizing the results of these studies can be challenging. For example, few researchers have not reported a positive effect on the inactivation of Enterococcus avium at low frequencies but observed positive effects on the inactivation of E. coli and Vibrio cholera bacteria. Most researchers have observed different results at low frequency, high frequency, both frequencies and the increasing power of bacterial inactivation on the inactivation of bacteria. A study found that high-frequency UE resulted in more inactivation of E. coli and S. mutants than low-frequency UE. A positive correlation was also observed between the high frequency and degradation of S. epidermidis. The inactivation of Mycobacterium sp., E. coli, and Kl. Pneumonia by UE at higher frequency was found to be lower. No differences were observed in the bacterial degradation based on the type of gram stain, shape, or size of the bacteria. Experimental investigations with L. pneumophila showed that the degradation of these bacteria against AC and HC was low, but inactivation increased in the super cavitation [34], [75]. Contrasting results have been reported for S. aureus. Monsen et al. [76] observed an inactivation rate of only 40 %, whereas Li et al. [77] noted about 82 % in a shorter time. Many authors have observed the effects of UE using TEM and SEM and found that UE damages cell walls and alters cell morphology, fragmentation, and cellular contraction [71], [78].
Few authors reported that most of the cells of E. aerogenes and S. mutans were deformed at high frequencies, but no significant effect on cell wall degradation was observed. However, inactivation rates were high, and only a small number of cells were damaged [70]. Similar results were observed for the degradation of E. coli at low frequencies [79]. B. subtilis (gram-positive bacteria) are rod-shaped; their size is 0.7–0.8 µm × 2.0–3.0 µm. It has been noted that in the first 5 min of sonication, both 20 kHz and 38 kHz and high frequency caused an immediate increase in the concentration of microorganisms (CFU) and then a uniform decrease. However, its concentration was higher than the initial concentration after 15 min of sonication, revealing that inactivation is lower at high frequency. At the same time, most bacteria were killed at low frequencies (higher power) [80]. The effect of high-frequency UE (850 kHz) on E. aerogenes, B. subtilis and S. epidermis was investigated in the two phases of constant growth and logarithmic growth [70]. It has been found that the inactivation of these bacteria increased with increasing ultrasonic power in both phases. The reduction values for these three bacteria under ultrasound (62 W, 20 min) were Log 4.2, Log 2.5 and Log 4.4, respectively. On the other hand, low-power UE (9 W or 15 W) = illustrated no significant effect on bacterial activity. It is probably due to the low amount of hydroxyl and H202 radicals generated at low power [70]. Other researchers reported that the hydroxyl radicals and H2O2 produced directly invade the bacterial cells [70].
The efficiency of the ultrasonic process for inactivating E. coli was investigated at 20 kHz as well as in the ranges of 205–1071 kHz and 80–140 W, where bacterial inactivation was noted to be high at higher power intensity. The effect of sonication at 20 kHz (0.012 W.cm−3), 40 kHz (0.013 W.cm−3) and 580 kHz (0.013 W.cm−3) on the inactivation and declumping of E. coli and K. pneumonia were evaluated. The plate counting method showed that E. coli and K. Pneumonia exposed to ultrasound in the range of 20–40 kHz resulted in an immediate and continuous decrease in CFU. At 580 kHz with similar acoustic intensity, a declumping effect on the above bacteria was observed in the first 5 min. However, after 15 min, there was a slight decrease in CFU. Cell loss at 580 kHz was significantly lower than at low frequencies (20 kHz and 40 kHz). At frequencies below 20 and 40 kHz, a significant decrease in the number of bacterial cells was observed. The most effective ultrasonic frequency for E. coli was reported at 205 kHz. They also examined the effect of UE on the inactivation of E. coli in the range of 4.6–74 W.cm−2, where the inactivation rate was very high at 74 W.cm−2. After 180 sec at 20 kHz and 74 W.cm−2, the inactivation of E. coli was reported to be 1.6 log10. However, the inactivation rates at 4.6 W.cm−2 and 18.5 W.cm−2 were about 1.3 Log10 and 1 Log10, respectively. The impact of various factors on the degradation of E. coli ATCC 25922 at 24 kHz and 85 W.cm−2 has been reported, where it has been shown that ultrasonic disinfection is successful with a 10 log10 reduction after 10 min [179], [184], [191], [192], [193], [194], [195], [207].
The bactericidal effect of ultrasound increases with increasing sonication time and intensity. The changes in the sonication time on bacterial inactivation were also examined. Few studies have found a significant gap in the bacterial cell wall after subjecting to sonication, and notably, as the sonication time increased, the cell wall completely disintegrated. When the sonication time increased from 2 to 30 min (3 Wcm−2), the population of P. aeruginosa from 68 to 72 %, B. subtilis from 52 to 76 % and S. aureus from 42 to 43 % were increased. After 15 min of sonication (1–3 W.cm−2), the bacterial population from 31 to 78 % for P. aeruginosa, from 11 to 100 % for B. subtilis and from 22 to 39 % for S. aureus were found. The efficiency of ultrasound at 24 kHz and 1–3 W.cm−2 for aqueous suspensions containing different bacteria (E. coli, S. aureus, B. subtilis and P. aeruginosa) showed that the bacterial population of E. coli is significantly reduced with increasing sonication time. The outcome of these studies demonstrates that ultrasound in the low-frequency (kHz) range can inactivate a degree of residual pathogens in water. Also, the mechanism for bacterial inactivation appears to be transient cavitation [177], [178], [202], [203], [204], [205], [206], [207].
Few studies have addressed the stimulation of bacterial metabolism by UE under certain conditions and also in analyzing the effect of UE on bacteria in the form of planktonic or biofilm. However, exactly it is unclear what mechanisms are responsible in the presence of an ultrasonic field. It is noteworthy that P. aeruginosa is a gram-negative and rod-shaped bacterium widely found in soils and causes human disease . The size of these bacteria is 0.5–1.0 μm × 1–5 μm. E. coli are gram-negative and motile bacilli without sholes, and most of them are not pathogenic in the intestine, but some types can cause disease. Enterotoxigenic E. coli (ETEC) causes food poisoning in travellers by producing toxins (traveller’s diarrhoea); Enteropathogenic E. coli (EPEC) causes diarrhoea in children with direct damage to the intestinal tissue; Enterohemorrhagic E. coli (EHEC) causes bloody diarrhoea with toxin secretion; Enteroinvasive E. coli (EIEC) like Shigella damages the intestinal cells; Uropathogenic E. coli (UPEC) results in urinary tract infection. S. epidermidis adheres to the surface of water pipes and causes severe microbial contamination. These bacteria are gram-positive and have a diameter of 0.8–1.0 μm. This study showed that their growth is improved when bacterial biofilm is exposed to low-intensity, low-frequency ultrasound. Also, under these conditions, the growth of planktonic cultures is enhanced. This could be due to increased nutrients and waste transport during biofilm formation. Other studies have also confirmed the effect of UE on the biofilm of E. coli. These studies investigated the bacterium's survival after biofilm degradation using UE of 1000 kHz. In another study, the effect of UE on the activation of E. coli at 27.5 kHz (42 W m−1) in squeeze film was investigated. After 180 sec of sonication, 99 % of the bacteria were destroyed. Also, the use of UE at 42 kHz to remove E. coli, faecal coliform and total coliform from aqueous suspensions showed that sonication at 120 W.lit−1 inactivated 99.5–99.8 % of this group of bacteria within 90 min. In most studies, the low frequency was employed, which exhibited the best performance against the inactivation of microorganisms compared to the high frequency [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207], [208], [209].
Few studies have shown that gram-positive bacteria such as S. aureus are more resistant to UE at 20 kHz than gram-negative bacteria such as EE. coli. . coli.aureus S. aureus is a gram-positive and facultative anaerobic coccyx in the clustered form. It causes food poisoning by producing enterotoxin. It can also survive on dry surfaces for weeks to months. These bacteria produce other toxins, such as alpha, beta, and gamma, which decompose the membrane of many body cells [177], [178], [196], [202], [203].
Studies also show that UE eliminates bacterial accumulation and consequently increases the planktonic bacteria in the suspension. The higher bacterial accumulation increased the resistance of the bacteria to UE, resulting in a reduced bacterial death rate. The reason for the higher population of S. aureus at the end of sonication is associated with the type of cell wall structure and formation of clumps in these bacteria. Staphylococcus species in cluster form larger clusters under these conditions [81]. In a study, the survival curves of E. coli and S. aureus derived by ultrasonic disinfection were compared based on specific time and energy. Accordingly, when the population of bacteria decreases due to inactivation, specific energy increases. Besides, the efficiency of the disinfection process is determined by UE based on the number of bacteria. In other words, UE is more effective when the bacterial density is low [82]. The bacterial accumulation degradation rate of E. coli and S. aureus after disinfection within 20 min were 98 % and 93 %, respectively. This confirmed that the bacterial inactivation rate was independent of the initial bacterial count. The effect of initial cell concentration on the bacterial inactivation was also examined, which demonstrated that the initial cell concentration showed no significant effect on the performance of UE. However, the mechanical and chemical energy produced by ultrasound can have different effects on the high-density bacteria. As the initial number of bacteria increases, the resistance of the bacteria to degradation by ultrasound also increases [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81].
Studies have also shown that the effect of UE on bacterial inactivation does not depend solely on bacterial size, gram-status, bacterial type, or bacterial hydrophobicity. Some gram-positive and gram-negative bacteria' cell wall properties and resistance to UE were investigated [70]. Gram-positive bacteria have a thicker and more integrated layer of peptidoglycan in their cell wall than gram-negative bacteria. The damage to the inner membrane of the cytoplasm due to UE has been reported. B. subtilis is more sensitive to the mechanical impacts of UE than E. aerogenes and S. epidermidis. B. subtilis is composed of rod-shaped cells more sensitive to UE than coccus forms [76,124,215]. S. epidermidis also has a thick layer that protects it from the mechanical impact of UE. In addition to the properties mentioned, capsule thickness is another physical property of bacteria that can react against UE. The bacterial capsule is a layer located outside the plasma membrane, which plays a role in maintaining cellular integrity. It contains proteins and homogeneous polysaccharides. The resistance of S. epidermidis to UE depends on the thickness or softness of the capsule [83]. Liao et al. [74] investigated the impact of UE on gram-negative (E. coli ATCC 25922) and gram-positive bacteria (S. aureus ATCC 25923). This study showed that these bacteria are inactivated within 12 min by 98 % and 81 %, respectively. Other studies have shown that UE at 400 W/L for 1 h decrease the E. coli content by 2.9 Log10. In these studies, S. fecalis was also tested. These bacteria are a better indicator for presenting faecal contamination since they have a longer lifetime in water. In addition, they are more resistant to environmental factors. On the other hand, these bacteria are less vulnerable to UE exposure since they are gram-positive, and their cell wall thickness is 20 nm (the wall thickness of gram-negative Enterobacteria is 10–15 nm). E. coli and S. aureus at 90 kHz and 350 W after 60 min of exposure to ultrasound were eliminated by 90 % and 40 %, respectively [84]. Other studies have shown that the high resistance of S. aureus to UE is due to the thickness and stiffness of the gram-positive cell wall. In addition to the cell wall, cell shape also plays an important role in bacterial resistance to UE [74], [84]. Also, the resistance of cocci bacteria to UE is greater than rod bacteria. Similar observations have been noted in other studies [74], [84]. This may be due to the thicker and stronger peptidoglycan layer in gram-positive cells. It is believed that the outer membrane, the cell wall, the cytoplasmic membrane, and the internal structure have a target for UE. Alternatively, UE disrupts the cell membrane, inactivates enzymatic activity, inhibits some bacteria's metabolic function, and does not relate to the initial bacterial count. Importantly, the outer membrane for gram-negative bacteria and the cytoplasmic membrane for gram-positive bacteria are probably the primary target of UE [74], [84]. Yusof et al. [85] found that free radicals destroy cell nuclei without affecting the cellular membrane. During sonication, intracellular components are damaged by the reactive oxygen species (ROS) [74], [85]. Some studies have found that bacteria degrade immediately if the cell membrane is damaged. On the other hand, when these bacteria come into contact with ultrasound, ATP levels are reduced. However, the reduction rate of ATP in these bacteria varies with UE. For example, ATP depletion rates in E. coli and S. aureus occur within 3 and 5 min, respectively [74], [85].
In addition to the parameters mentioned above, ultrasonic power is another parameter that affects the inactivation of bacteria. Studies have shown that the effect of ultrasonic power on bacterial survival is a combination of decoupling and inactivation. However, the overall impact of UE depends on the frequency used in the disinfection process. When the power is too high and the sonication time is prolonged, the logarithm of the survival rate of bacteria (such as E. coli) declines abruptly [80], [81], [82], [83], [84], [85], [86], [101], [102], [103], [104], [105], [106], [107], [108].
The inactivation of bacteria such as E. coli follows pseudo-first-order behaviour and depends on total power and power intensity. It has been shown that the sonochemical reaction rate constantly increases at high frequencies above 200 kHz. Based on these studies, the inactivation of E. coli is due to a combination of physical and chemical mechanisms that occur during AC at higher intensities. The correlation of the chemical reaction rate with the ultrasonic intensity has also been demonstrated [86]. Various researchers also studied the bacterial inactivation rate constant in the ultrasonic process. Although the cell structure of E. coli and S. mutans is not similar, the rate constant of E. coli is approximately similar to S. mutans. On the other hand, at high frequency to express the role of OH radicals in the inactivation of E. coli and S. mutans, the rate constant of the sonicated bacteria with or without radical scavenger was compared. In this study, ku in the ultrasonic process has been calculated using Eq. (21):
| (21) |
Where ku is the rate constant for the inactivation of bacteria, t is time, N0 is the initial microbial population, and N is the number of CFU before and after sonication of the bacteria.
The rate constant for E. coli and S. mutans decreased with the addition of OH scavengers. The result of the scavenger-free sonication showed that the rate constants are strongly affected by the chemical effects of OH radicals. By adding a scavenger, the rate constant is significantly reduced due to the reduction of OH radicals in the solution. This study showed that the physical effects at 500 kHz were minimal, and the chemical effects of ultrasound presented the greatest a key role in inactivating the bacteria [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79].
Various studies have also been performed to understand the effect of UE on wastewater microbiology. In a study, when sonication was applied to synthetic wastewater after 15 and 90 min at 24 kHz (90 W and 450 W), almost all E. coli XL-1 blue bacteria were inactivated. After 24 h, the samples were re-analyzed, and no bacterial growth was observed. All E. coli XL-1 blue bacteria in the septic tank were inactivated after 20–30 min of sonication at 24 kHz and 450 W. The effect of sonication on domestic wastewater also showed that the disappearance of F. coliforms at 20 kHz (1250 70–50 W/L) was 52 % due to heat, 36 % due to mechanical stress caused by cavitation and 12 % due to uncertain synergistic effects. The effects of sonication (20 kHz, 0.3–1.5 kWL-1) on E. coli and E. fecalis bacteria in wastewater treatment plants showed that UE was very low because of the sensitivity of E. coli, which also destroys many bacterial cells. E. fecalis bacterium is more resistant than E. coli, even at high levels of UE. A study performed on E. coli-included wastewater samples at 35 kHz, and 130 kHz for 5 to 30 min revealed that the bacterial population increased significantly with increasing frequency (130 kHz) and sonication time (30 min) [87]. The ultrasonic effects on the sediment particles in wastewater samples were also investigated. The mean particle size of the sample was 40 μm which was reduced to 0.5 μm after 8 min of ultrasound. Using UE in biological processes reduced the sludge anaerobic digestion time four times without reducing biogas production. UE also has the potential to reduce fermentation time; thus, the volume of new anaerobic digesters can be optimized. UE is also useful for sludge dewatering. Changes in the sludge structure affect the effectiveness of the dewatering process. One of the methods of dewatering is the use of polyelectrolytes. Recently, studies focused on the structure and properties of sludge that modifies polyelectrolytes in an ultrasonic field. UE changes the structure of polyelectrolytes, and the ultrasonic time and dose of polyelectrolyte significantly affect sludge recovery. UE at 20 kHz changes the internal structure of the polyelectrolytes. These changes increase the activity of polyelectrolytes on sewage sludge, and the ultrasonication time is the key parameter for success. UE can also prevent the growth of filamentous microorganisms that lead to bulking in the activated sludge. For example, UE at 25–30 kHz for 2 sec in 2 days (5 WL-1) significantly reduced the number of filamentous microorganisms. In this case, foam production and buckling could greatly reduce the operation problems of the activated sludge system. The results from these studies demonstrate that parameters such as suspended solids, dissolved gases, alkalinity, and radical scavengers significantly impact the ultrasonic process's efficiency. On the other hand, other parameters such as ultrasonic time, frequency, intensity as well as concentration and composition of the wastewater could also affect the sonication process [190], [191], [192], [193], [197], [198], [199], [201], [208].
7. HCRs: Mechanism and performance
To understand the mechanism and efficiency of HCRs in the destruction of various pollutants, the behaviour and nature of the contaminants and the properties of the reactors must be well studied. In HCR, parameters such as high temperature and pressure and high-velocity liquid jets can assist in degrading various pollutants, thereby oxidising the produced hydroxyl radicals [88]. The produced radicals have a higher oxidation potential than other oxidants in water pollutants' degradation. The reactor geometry and inlet pressure, the cavitation number, and the physicochemical properties of water, such as density, viscosity and surface tension, significantly affect the degradation of pollutants [88]. Hydrophobic and volatile molecules are more likely to react with active radicals generated inside the bubbles [88], [89].
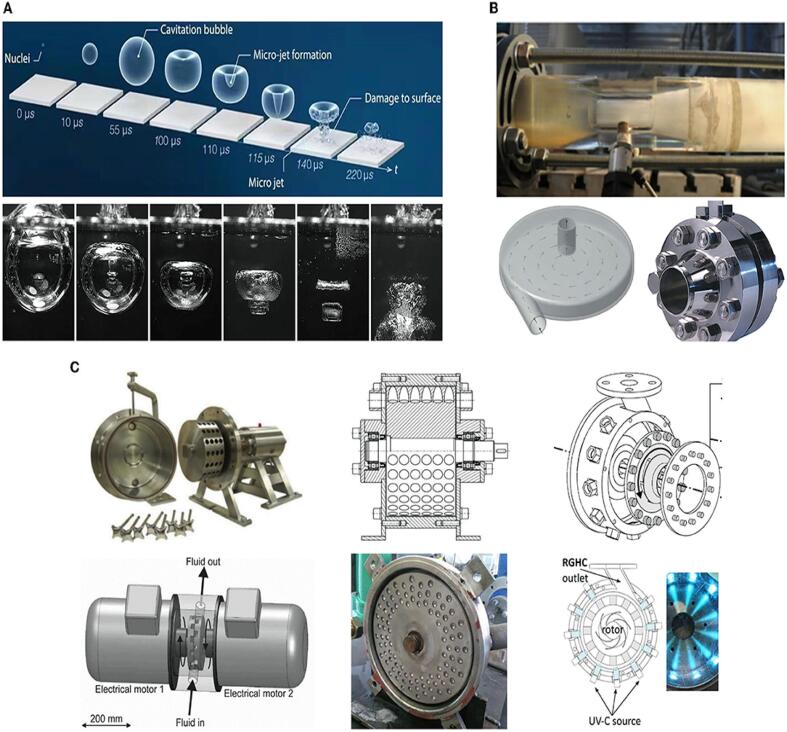
In contrast, hydrophilic molecules can only react with residues of non-reactive active radicals in the bulk liquid medium surrounding the bubbles [89]. Studies have also shown that the geometry of reactors under different conditions affects bacterial degradation and inactivation rate differently. Thus, the efficiency of hydrodynamic cavitation (HC) depends on the geometry of the reactor and the inlet pressure and the number of cavitation and can be controlled by calculating the parameters such as cavitation intensity and associated chemical effects. The number of cavitation events in the reactor medium and the cavity collapse intensity depends on the reactor geometry [36], [38], [89], [90]. Fig. 6 shows the life cycle of a cavitation bubble (A), conventional non-rotational hydrodynamic cavitation reactor (NRHCRs) (B), and representative advanced rotational HCRs (ARHCRs) (C) .
Fig. 6.
Life cycle of a cavitation bubble (A), conventional non-rotational hydrodynamic cavitation reactor (NRHCRs) (B), and representative advanced rotational HCRs (ARHCRs) (C) [118].
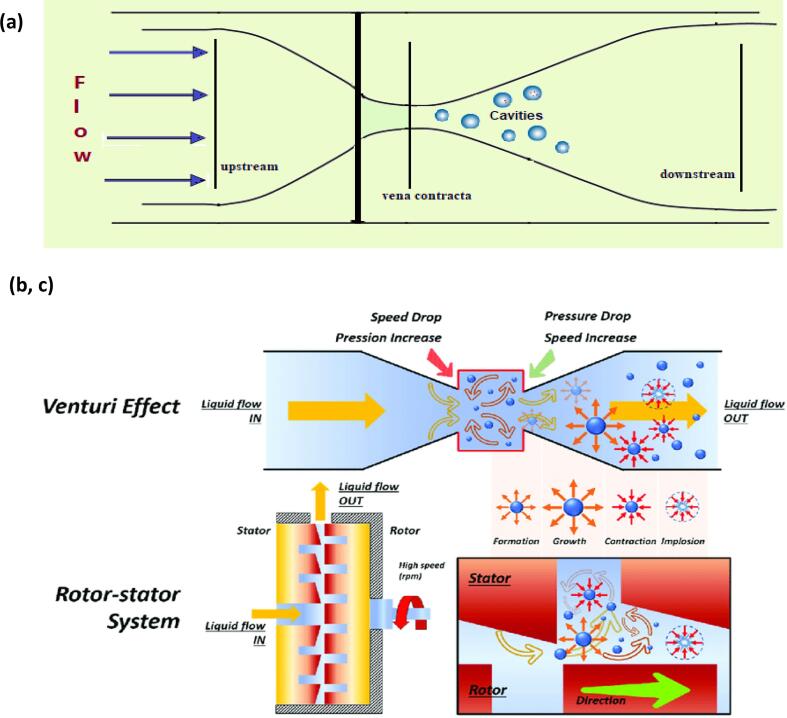
The HC generated by venturi tubes and orifice plates (Non-rotational HCRs) has been studied intensively [37], [89], [91]. Cavitation in the orifice reactor (Fig. 7a) is transient but often stable in the venturi (Fig. 7b, c). Multiple-hole orifice plates are often used to control the intensity and number of cavitation processes inside the SCR.
Fig. 7.
Hydrodynamic cavitation process in a fluid: (a) orifice plates and (b, c) venturi tube and rotor–stator [90].
A combination of mechanical, chemical, and thermal effects in HC degrades bacteria such as E. coli. The mechanical and chemical effects of disinfection by HC occur in several stages: (a) in the first step, the mechanical effects cause the formation of holes in the cell's outer wall and release protein and periplasmic cells. (b) in the second step, the mechanical effects combined with the chemical effects of cavitation damage the cytoplasmic membrane wall and release some cytoplasmic products. (c) in the third stage, as the outer wall of the cell is more in contact with cavitation than the inner membrane, the bacterial cell wall in the cavitation zone is affected by the bubbles more frequently. The exact mechanism seems to be complex, as cavitation occurs very quickly [90], [92], [95], [96].
Various studies have proposed that Eq. (22) determines the extent of cellular degradation based on the cell wall strength as well as other geometric and operating parameters of the cavitation reactor. This equation helps to identify the amount of stress needed to break the cell wall. It also shows that the destruction of bacterial cells is affected by cavitation. Cell degradation (X) is subject to the following parameters [89].
| (22) |
where Scell is the cell wall strength, Cv is the cavitation number, Ccn is the choked cavitation number, d0 is the orifice diameter, Ph is the hole perimeter, and Ap is the area of the pipe.
The effects of the orifice, venturi reactors and cavitation number on bacterial inactivation were investigated [77], [92]. Most studies employed orifice and venturi types of cavitation reactors owing to several benefits (higher product yields, easy scale-up, and cost-effectiveness) [89]. More cellular damage usually occurs using a geometry with a higher perimeter and lower cavitation number. Thus, the extent of cellular degradation can be controlled by varying the operating pressure, cavitation number, and reactor geometry. The cavitation number is an important parameter that describes the cavitation intensity [38], [89], [93]. This cavitation number is used to determine the conditions in the reactor and the degree of cavitation, which is defined by the following Eq. (23) [37], [93], [94].
| (23) |
Where p2 is the downstream pressure, pv is the vapour pressure of water, and v0 is the average velocity at the throat of the cavitating constriction.
It has been illustrated that a reactor employing geometry with the largest hole size and the smallest diameter has the highest efficiency in disinfecting bacteria such as E. coli. Orifice plates with multiple circular holes produce more periplasm than non-circular holes. In the case of orifice plates with smaller holes, more jet streams and cavities are created; as a result, more cells are destroyed [95]. More severe cavity collapses occur in the venturi due to sudden pressure. Due to the high concentration of bacteria in the orifice, disinfection efficiency is low [96].
Venturi and orifice have been used to inactivate E. coli [95]. Nozzle velocity, flow rate, and cavitation intensity affect the bacterial inactivation rate. Venturi is more effective for inactivating bacteria compared to orifice [96]. In a multiple-hole orifice, an increased discharge pressure led to a more significant bacterial inactivation effect, whereas the inactivation rate is lower in a venturi. Inactivation also increases with increasing inlet pressure, whereas inactivation gradually decreases with a further increase in the inlet pressure [33], [92]. The use of orifice plates also showed that the initial concentration of E. coli (CFU/mL) reached near zero after 20 min, whereas 30 min was required with the venturi injector [91], [97]. This study showed that the orifice plate is faster and more effective for disinfection [98]. The results of other studies displayed that bacteria are reduced by approximately 98 % within 60 min by using a multiple-hole orifice plate [99].
In contrast, a venturi slit reactor is more energy efficient than multiple-hole orifice plates and cylindrical venturi [92]. The effect of different HCRs on seawater microbial degradation was examined. Based on these studies, it has been noted that the slit-type reactor performs better than the cylindrical venturi and orifice plate in the process of microbial degradation and the amount of energy consumed [92].
The effect of changes in pressure on the reduction of bacteria in HCRs has also been studied. In high-speed and high-pressure homogenization reactors using bore well water at 1.72 bar, 3.3 bar and 5.17 bar, the largest reduction of bacteria (about 44 %) was noted at 5.17 bar. The effect of the disinfection rate of DynaJets reactors on the inactivation of E. coli at different concentrations (5 × 108 –2 × 109 CFU/mL) and different pressures of 4.13 bar, 5.17 bar and 10.3 bar was investigated. It has been observed that the bacterial reduction rate during 30 min at 10.3 bar was 5 Log10 and during 20–30 min at 4.13 bar and 5.17 bar was 3 Log10. However, with these pressures at a concentration of 107 CFU/mL and within 20–30 min, the rate of reduction of bacteria was reported to be 3 Log10 and 5 Log10, respectively. This study did not state the cavitation number and geometry of the cavitation reactor. The cavitating jet reactor was used to study the inactivation of B. subtilis (JCM1465T) and E. coli (JCM1649T). In this reactor, the discharge pressure was 1050 bar, the cavitation number was between 0.037 and 0.487, and the upstream nozzle velocity was between 176 m/s and 384 m/s. In another study which employed jet pressure reactors for 15 min, the reduction rate of E. coli using synthetic contaminated water was 90 % at 100 bar. After 30 min of contact at 80 bar, 100 bar, and 120 bar, 98 %, 99.96 %, and 100 % bacteria were inactivated, respectively. However, 98.89 % and 97.3 % of bacteria in naturally contaminated water were inactivated after 30 min exposure at 100 bar [100], [101].
E. coli and K. pneumonia at a concentration of 107 CFU/mL were reduced by 99.99 % using a DynaSwirl single orifice reactor over 60 min at 1.2 bar. Further, the StratoJet 8-orifice reactor at 16.5 bar reduced E. coli at 5 Log10 by using over 120 min, P. aeruginosa for 90 min at 3 Log10, and P. syringae by using for 20 min at 6 Log10. The disinfection efficiency of the DynaSwirl reactor at a pressure drop of 3.45 bar, 2.1 bar, and 1 bar with the cavitation numbers of 1, 0.5, and 0.33, respectively, was examined. The best disinfection efficiency was achieved at 2.1 bar. At this pressure drop, the concentrations of B. subtilis and E. coli bacteria were decreased at 4.5 Log10 and 7 Log10, respectively. 98.60 % of L. pneumophila was also inactivated using these reactors [33]. However, other studies reported 100 % inactivation of E. coli [33], [34]. The differences observed in these results can be attributed to the difference in the turbulence flow occurring in these two reactors. It can also be due to the differences in the cell wall thickness of gram-positive and gram-negative bacteria [33], [34], [100], [101], [102]. Other hydrodynamic reactors utilizing orifice (1.25–1.8 bar) and vortex diode (0.3–0.5 bar) have also been used to inactivate bacteria. In one study, E. coli and Streptococcus. aureus was tested. In this study, 99 % of E. coli (ATCC-8739) was removed using a vortex diode with a pressure drop of 0.5 bar for 1 h. Subsequently, by increasing the pressure drop to 2 bar, the removal rate increased significantly, and the disinfection time was reduced. However, the degradation rate of S. aureus (ATCC6538) using the vortex diode reactor was about 60 % compared to E. coli, which reached 98 % with 1 bar and 2 bar pressure drops within 1 h. However, in the orifice reactor, E. coli degradation was reported to be 99 %. The studies with S. aureus also showed that at 2 bar, 5 bar and 10 bar, the degradation rate of bacteria was 68 %, 88 %, and 98 %, respectively [35].
The differences in the observed results are due to the different flow patterns produced in the reactors. The cavitation number cannot be easily estimated in the vortex diode reactor's vortex flow compared to the linear flow of the orifice reactor [35], [37]. Further, the resultant pressure from cavity collapse and the reactors' geometrical structure cause the reactors' different performance in degrading bacteria [33], [34], [37]. Few studies have suggested that in the vortex diode reactor, the obtained higher efficiency and lower pressure drop for bacterial disinfection can be attributed to the vortex flow, which probably results in an increased number and quality of the cavity collapse compared to the orifice [37], [38]. On the contrary, the disinfection due to mechanical effects were estimated by the Reynolds Number (Re). For example, Re is about 9000 for a venturi injector, which is less than that for an orifice plate. Hence, the effect of reduction of E. coli with a venturi injector is less than that with the orifice plate as there is less interference with the bacterial wall when the eddies flow is produced smaller [33], [34].
The influence of various parameters such as cavitation time, throat length-to-radius ratio, velocity, and cavitation number on E. coli inactivation has also been studied [103]. These studies indicate that bacterial degradation in HCR occurs due to microjets and shock waves. The bacterial degradation rate is high when these reactors have low cavitation numbers. In contrast, when the flow rate is low, the bacterial degradation rate gradually increases with an increase in throat length-to-radius ratio or cavitation time and reaches 100 % [104]. However, the disinfection increases with the jet velocity regardless of the cavitation number. Therefore, the reduction rate of microbial cells is highly dependent on the nozzle velocity and high static pressure. These studies also showed that disinfection rates are lower when the initial microbial density is high [103], [104].
Further, bacterial degradation occurs due to mechanical stress. This could be due to shearing stress caused by the shear flow in the nozzle and the shock wave produced by the cavitation bubble collapse. The effect of shear flow prevails when the nozzle speed is greater than 300 m/s. When the velocity is above 355 m/s and the cavitation number is 0.154, E. coli is eliminated [76], [100], [101]. Other studies have shown that the flow velocity does not affect the disinfection process and is inversely proportional to electrical energy consumption. Therefore, the disinfection process can be economical by adjusting the flow velocity or disinfection rate. Increased pump pressure and rotational speed increase flow velocity and generate heat with power consumption [32], [104]. The effect of velocity (V) on the microbial reduction rate could be calculated using the following Eq. (24).
| (24) |
As indicated earlier, the thermal, mechanical, and chemical effects play a key role in the bacterial disinfection process in the reactor. Concentrations of bacteria (such as E. coli) are often constant at different flow velocities. However, the bacterial concentration decreases gradually when the temperature reaches 54 °C. Therefore, temperature variation in the disinfection process is an effective criterion for degrading bacteria such as E. coli [32]. It has also been shown that HCR could be used along with other disinfection techniques to reduce the time of water disinfection. For example, HCR coupled with thermal disinfection at 60 °C increases the microbial degradation rate by four times. HCR with chlorination also increases the disinfection time compared to the chlorination process [92]. The disinfection efficiency of HCR alone and combined with chlorine compounds for bacteria in bore well water also showed that the disinfection efficiency in the case of the combination was greater compared to using it alone. Further, smaller holes and smaller diameters led to the higher efficiency of the reactor [95].
The concentration of incoming cells on the performance of HCR has been studied, and different results were noted [105], [106]. It has been shown that reactor efficiency decreases in the inactivation of bacteria when the initial cell concentration is high [33]. However, in a few other studies, it has been observed that the initial cell concentration does not significantly affect the inactivation over a long time [107]. The effect of the initial cell concentration on the efficiency of the orifice reactor is higher than the venturi reactor [30], [33]. Also, it has been noted that cavitation affects a larger number of cells when the cell density is high. However, cavitation's effect is limited due to the increased accumulation of cells [30], [33].
8. Energy efficiency aspects
Energy consumption is a determining parameter for using UE in water purification. To determine the sonochemical efficiency, the chemical yield of UE is usually calculated using the calorimetric power method [108]. Hence, this calorimetric method is beneficial to determine the energy efficiency of the device. In this method, the temperature rise of the water in an insulated vessel is measured over a specified period [100], [108], [177].
Energy efficiency represents the amount of energy effectively dissipated in the reactor, part of which is used to generate cavities. In all sonoreactors, electrical energy is the main form of energy considered as input. This energy is converted to various forms (i.e., pressure, velocity, vibration, etc.) before being utilized for cavitation. Generally, electrical energy is converted to mechanical energy (for example, the vibrational motion of the transducer in ACRs and the high-pressure liquids in HCRs). This energy is used to produce cavitation and eventually causes chemical reactions in the reactor. However, during energy conversion, there is continuous energy loss. Therefore, comparing and a better understanding of the energy consumption of cavitation equipment will be useful in achieving optimal cavitational activity [97], [100], [104], [108].
Sufficient knowledge about the properties and characteristics of SCRs facilitates the proper design and optimal operating parameters that significantly affect process efficiency. The correct selection of the transducer type with appropriate frequency and power intensity, the number of transducers, and their position in the reactor can be beneficial in optimizing the cavitational activity and acoustic flow and increasing the efficiency of SCRs [37], [105], [108].
Among ACRs, the triple-frequency flow cell reactor and the dual-frequency flow cell reactor have the highest energy efficiency due to uniform energy distribution via multiple transducers (75 % and 55 %, respectively). In contrast, the batch reactor (with three transducers) and horn reactor have the lowest energy efficiency (18.5 % and 7.6 %, respectively). The energy efficiency in HCRs such as high-speed homogenizers, high-pressure homogenizers, orifice, shock wave power reactors, and cavitation heat generators with various capacities is reported to be 23.6 %, 54.4 %, 60 %, 84 %, and 90.5 %, respectively. Therefore, energy efficiency in HCRs is higher than ACRs (excluding multiple-frequency flow cells). Further, the cavitational yield in the triple-frequency flow cell reactor is higher than in other SCRs. The cavitational yield in the dual-frequency flow cell reactor is about 20 % higher than a batch reactor and is twice as low in ultrasonic reactors. Therefore, reactors with multiple transducers have a higher cavitational yield than reactors with a single transducer. Studies have shown that HCRs are almost similar in energy efficiency (50–70 %) compared to a multi-transducer reactor [32], [37], [97], [109], [129], [142], [147], [177].
Many studies have been performed to compare the energy efficiency of hydrodynamic and ultrasonic cavitation processes for degrading organic pollutants. However, studies examining the cavitation yields among HCRs are limited. Reports on the influence of venturi tube and orifice plate design parameters can be used for optimizing HCRs in water and wastewater treatment processes. The pressure drop in the orifice plate is higher than that in the venturi tube; hence, the cavitation intensity in the orifice plate is higher than in the venturi tube. Accordingly, a few essential solutions have been proposed for the design of HCR. It is difficult to accurately evaluate the cavitation yield of different cavitation reactors because of the varying operating and geometric parameters. However, the hydraulic and physicochemical properties are still considered the most important parameters for selecting the optimal cavitation reactors [142], [143], [144], [145], [146], [147].
In HCRs, pumping cost is an important economic aspect. In these reactors, there is a significant energy loss in the pumping of process liquids. The energy loss depends on the flow rate as well as pressure. For example, a small-diameter orifice causes a higher pressure to drop, but there is also greater turbulence downstream, which releases more energy during cavity implosion. For example, studies have shown that energy efficiency among high-speed homogenizers, high-pressure homogenizers, and orifice reactors with capacities of 1.5, 2.0, and 50 L is 43 %, 54 %, and 60 %, respectively. HCRs are more energy-efficient than other ACRs (except for multiple frequency flow cells) [37], [97].
Energy consumption in the water disinfection process is the best way to compare the efficiency of different types of SCRs, including ultrasonic horn, high-speed homogenizer, high-pressure homogenizer, and HCR. Studies have shown that the percentage of disinfection (and the amount of energy consumed) in these SCRs are 82 (126 J/mL), 92 (41 J/mL), 79 (1023 J/mL), and 30 (23 J/mL), respectively [37], [97]. Further, high-speed homogenizer has demonstrated the highest efficiency of eliminating E. coli after 15 min compared to other SCRs. HCR exhibits the highest efficiency in energy used in water disinfection compared to other reactors. The ultrasonic horn has the highest energy consumption compared to the above reactors. However, the homogeniser is the most efficient in terms of dissipated energy. Therefore, hydrodynamic cavitation is one of the most attractive and effective methods for large-scale water disinfection. Depending on the operating conditions, the energy transfer efficiency of HCRs is 70–60 %, and for ultrasonic reactors, it is about 10–40 % [109].
Another important issue with cavitation is thermal performance, which has been largely overlooked in most studies. The main effect of cavitation on HCR is the thermal effect, which needs to be studied in detail for an improved understanding and proper use. The rotational speed and pressure regulation of the pump significantly influence the thermal efficiency of HCR. It should be used at a rotational speed of 3600 rpm (1.5 bar) to achieve the highest disinfection. Under such conditions, the heat production rate is 48.15 MJ/h, and the thermal efficiency is 82 %. The thermal performance of HCR can be evaluated by increasing the instantaneous temperature, heat generation rate, and thermal efficiency. The heat generation rate and thermal efficiency indirectly display cavitation's impact and cavitation intensity. However, the thermal efficiency and the rate of electricity consumption that reflects the process's economic efficiency is a major concerns for utilizing these reactors at the industrial scale [32], [37], [97], [100], [109], [104]. Increasing the pressure of the pump increases the flow rate, heat production rate, electrical energy consumption, and thermal efficiency. Increasing the rotation speed significantly increases the flow rate and instantly increases the temperature, heat generation rate, electrical consumption, and thermal efficiency [32].
The thermal effect of HCR is very effective in the disinfection of water containing E. coli. Heat treatment with HCR at 60 °C can increase the disinfection rate by four times compared to conventional heat treatment. In addition to the thermal effect, cavitation's mechanical and chemical effects play a major role in disinfection. Since the disinfection rate or flow rate is inversely proportional to cost, it is possible to make the disinfection process economical by fine-tuning them [32], [35], [95].
9. Economic assessment and optimization
Cost plays a vital role in the choice of the disinfection process and can affect the economy of a water treatment plant. An ideal disinfection method is a technique that can be economical in addition to optimizing the bacterial population. For example, when large-scale physical water treatment is desired, hybrid techniques in terms of energy consumption will be more economical than ultrasonic techniques alone. To reduce the disinfection time and costs, cavitation can be combined with other methods such as thermal processes, chlorination, hydrogen peroxide, ozonation, ultraviolet, etc. [92]. Chlorination with cavitation increases the disinfection rate compared to conventional chlorination. It also reduces the number of by-products produced during traditional chlorination [92]. Hydrogen peroxide, combined with others, can increase the disinfection rate and energy efficiency. However, the disadvantages associated with chemical disinfection, such as the formation of toxic by-products, can be reduced or eliminated by the combined methods. Further, the efficiency could be increased if HC is combined with AOPs (O3/H2O2, UV/H2O2, and O3) or other techniques. Integrating these methods with ultrasonic techniques is highly economical [37], [92], [110].
In addition, H2O2 commonly used in bleaching and organic synthesis, has been extensively used in treating municipal disinfection wastewater and industrial effluents. Nonetheless, H2O2 is commonly used to prepare organic peroxides, phenol oxidation, and wood delignification. Along with a relatively high oxidation potential (1.78 V), the application of H2O2 has several technological advantages, such as high solubility in water, a broad range of operating temperatures, simplicity in hardware design, and environmental friendliness· H2O2 is sufficient towards the degradation of chlorinated alkanes, carboxylic acids and polycyclic aromatic hydrocarbons [111].
Furthermore, hybrid methods based on combining cavitation with AOPs possessed enhanced oxidation capacity. Compared to the individual utilization of cavitation and AOPs (e.g., O3, H2O2, Fenton's process), hybrid processes can degrade even highly persistent contaminants and shorten the operation time, reducing the overall energy consumption and oxidants. The synergistic effect that occurs between integrated technologies is attributed to the improved performance of hybrid methods, as expressed by the synergistic index [112].
Economic aspects are an essential factor for the employment of cavitation reactors for industrial-scale applications. To use a proper water disinfection technique, electricity consumption and the cost of disinfection and water treatment must be evaluated carefully. In a study which measured the amount of electrical energy consumed for different disinfection techniques, energy consumption was found to be increased with an increase in the flow rate. Thus, when the flow rate was increased to 3 L/min, electricity consumption increased by about 10 %. Further, when the flow rate was about 14 L/min and 11 L/min, the water disinfection rate was 100 % after 14 min. Therefore, the estimated cost in the normal flow is US $ 0.052/kWh and US $ 3.019/m3, respectively. To improve economic performance, excess heat generated during sonication can be recovered using a heat exchanger or other appropriate techniques [32]. Studies have shown that the sonication of bacteria consumes about 12.5 kWh/m3 after 45 min at 20 W. Ultraviolet disinfection consumes about 0.5 and 10 kWh/m3 and ozonation consumes about 20 and 100 kWh /m3. The cost of using ultrasound could be 1.25 to 25 times higher than UV disinfection. However, the presence of suspended solids can reduce the efficiency of UV. Also, a UV lamp needs to be replaced every 0.7 years of usage, whereas ultrasonic devices need servicing only every-six months [113].
10. Challenges and problems
-
•
Most research has emphasized ACRs, but the potential of HCRs for the degradation of microorganisms has recently received more attention. The biggest problem and challenge are that most researchers only rely on previous assumptions about cavitation mechanisms, and no new opportunities have been explored. However, it is agreed that these reactors remove most microorganisms, as they do not produce any hazardous by-products. However, the precise mechanism for eliminating or inactivating microorganisms in the event of a collision in SCRs needs to be identified precisely and optimized for the efficient operation and application of these reactors.
-
•
Cavitation is a complex process for reactive species; however, studies indicate that optimising the disinfection process occurring by cavitation is challenging. Hence, it is not easy to compare and generalize the results.
-
•
Although SCRs have promising potential for water disinfection, they are not widely used at the industrial scale due to the lack of accurate information on their design, operation, and performance characteristics. No proper design strategies link the existing theoretical knowledge to the experimental results. The information available is mostly on a laboratory scale, so the results may not be sufficiently valid.
-
•
Studies have shown that SCRs have the potential to be used in environmental remediation. However, they have not attracted experts' attention as an alternative technology to large-scale chemical processes because SCRs alone may not be economically feasible. The challenge is to scale up the sonochemical processes to meet industrial needs in terms of volumetric flow rate, reaction energy rate, and overall cost.
11. Recommendations and future perspectives
-
•
Chlorination, H2O2, UV radiation, O3, sunlight-mediated wastewater disinfection, and advanced oxidation are among the disinfection processes that must be enhanced and strengthened. It is vital to note that the virus's protein envelope may self-renew following disinfection. As a result, combining these methods could be considered to improve virus treatment efficiencies.
-
•
The increasing tendency to use HCR as a water disinfection method has grown rapidly in academics and industries in recent years. Existing studies have shown that HCR is a clean and flexible technique that can be used alone or in series with other methods. However, reliable design tools that can move these reactors from the laboratory to the field and industry are still unknown. Therefore, using these two techniques together or with other methods is recommended for better performance and energy conservation. Further, reaction mechanisms, intermediate products, routes and processes of mineralization, and modelling need to be studied when coupling SCRs with other disinfection techniques.
-
•
The design and construction of different cavitation reactors under different flow, turbulence and reactor geometry conditions should be examined further.
-
•
A detailed theoretical analysis of the bubble dynamics phenomena under different operating conditions is necessary to predict the cavitation intensity and its effect on the rate of physical or chemical processes.
-
•
Based on different types of reactors, cavitation images need to be prepared if possible, and pressure changes need to be measured under different conditions. The exact location of the pressure measurement, flow velocity, velocity measurement position and average temperature must be determined.
-
•
The cavitation number unclearly explains cavitation; hence, most authors misinterpret the results or draw contradictory conclusions. It is important to know that cavitation behaviour depends on many operational parameters. Therefore, changing one of these parameters will affect the other parameters.
-
•
Many studies have examined the problem empirically. It is recommended that future research efforts should be directed towards fundamental studies aimed at understanding the effect of liquid stress on bacteria. These studies should not rely solely on equations, empirical formulas, or theory.
-
•
Based on the performed simulations, several strategies have been proposed for optimizing the HCRs: (i) An orifice is only suitable for severe chemical reactions. Venturi is suitable for milder processes (typically between 10 and 25 times). In the case of an orifice, the most convenient way to control cavitation intensity is to control the orifice-to-pipe diameter ratio. Increasing the size of the pipe downstream of the orifice increases the cavitation effect. Although using larger pipes will allow for greater flow volume, it will increase the fixed cost of the process. (ii) In the case of the venturi, the most economical way to increase the cavitation intensity is to reduce the venturi length. However, for high-volume flows, there might be limitations. Cavitation intensity can be increased by decreasing the ratio of the venturi throat to the tube diameter.
-
•
The following should also be considered in further studies: (i) Validation of empirical equations for a range of operational aspects; (ii) Quantification of produced free radicals during the reaction; (iii) Measurement of bubble activity under different conditions; (iv) Evaluation of the size of the cavity.
-
•
In addition to understanding the properties of reaction mixtures and kinetics, an effective scale-up method is essential. It requires all parameters that impact cavitation efficiency need to be thoroughly studied and properly measured and optimized.
-
•
The hybrid systems and ultrasonication-assisted AOPs can overcome the issues associated by using AOPs, and other oxidations processes alone, as they produce secondary contaminants and incomplete disinfection of microorganisms. However, suitable transducer usage in ultrasonication is needed to avoid erosion of horn tips that may cause contamination of the sample.
-
•
Various studies consider acoustic cavitation an extremely effective water disinfection method; acoustic cavitation becomes even more effective when combined with other biocides. Acoustic cavitation is quite effective at the lab scale, but it is still difficult to apply it to large scale water disinfection.
-
•
The cost of employing an ultrasonic horn alone for water disinfection is substantially higher than the cost of using ozone/hydrogen peroxide to achieve the same intensity of disinfection. Even though the cost of employing an ultrasonic horn with ozone is lower, it is still greater than using ozone alone for the same disinfection.
-
•
Further research is needed to improve the energy conversion efficiency and achieve maximum and minimum energy input.
12. Conclusions
-
•
Ultrasonic disinfection, as an advanced oxidation technology, is considered a novel, green, and environment-friendly method to remove pollutants. Four different theories are commonly used to explain the sonochemical effects. However, the hot spot theory is typically used to describe the process in which microbubbles are created to generate heat and various reactive species. Cavitation can produce a variety of reactive radicals such as OH., H., O., and OOH. that can oxidize almost all the pollutants in the environment.
-
•
Studies have shown that bacterial inactivation can be caused by various physical (acoustic streaming, micro-jet streaming and shock waves), chemical (formation of hydroxyl radicals at high frequency) and heat impact (production of local hot spots with extreme temperatures). However, studies have shown that mechanical impact plays a significant role in the destruction of bacteria. The velocity constant is low when inactivation is performed using UE in solutions with the scavenger. Therefore, physical effects also play a major role in inactivating bacteria at high frequencies.
-
•
Therefore, the most likely mechanism for bacterial inactivation at high frequency is as follows: implosion of the bubbles in the solution causes local damage to the bacterial cell wall and chemical activities of OH radicals, causing more significant and progressive decomposition of the bacteria from the exterior to internal structures. The bacteria and cell surface are attacked by the radicals produced in the solution, and the intracellular contents leak out of the localized damage sites. Under high-intensity sonication, cells are partially or entirely deformed and cannot maintain their original shape.
-
•
UE may not be beneficial for inactivating some bacteria. However, several studies have examined the bacterial characteristics responsible for this inefficiency. For example, gram-positive bacteria are more resistant to UE than gram-negative bacteria. The size and shape of the bacteria also affect the sonication efficiency. In some cases, the sensitivity of bacteria to UE depends only on the bacterial species. It has been shown that radicals formed during cavitation decrease the solution pH, resulting in bacterial inactivation. These considerations are not addressed by most of the studies.
-
•
Given that bacteria can withstand a certain degree of oxidative stress, the reactive species and the extent to which they are produced need to be determined to understand whether the formed species are responsible for bacterial inactivation. Notably, bacteria can only defend against reactive species to a certain extent. When their defence mechanisms disappear, oxidative stress decreases. For example, the nutrients in the growth environment of bacteria can affect their defence mechanisms. Laboratory strains are also more resistant to oxidative stress than environmental strains.
-
•
The effect of UE on bacteria varies under different conditions and can lead to the formation of different amounts of reactive species. The impact of UE on different bacterial species under different bacterial conditions, such as the number of bacterial samples, different culture media, application of different types of cavitation (AC or HC), cavitation number, reactor geometry (orifice and venturi), throat velocity, throat length-to-radius ratio, cavitation operation, frequency, intensity, density, power, and sonication time, were investigated and different results were observed. Simulation studies illustrate that bacterial cell degradation in HCR is far higher than in ACR. Cellular degradation depends on the number and density of cavities, concentration and nature of microbes, reactor geometry, pressure drop, and presence of other contaminants. In HCR, the bubble number densities are higher, and the investment cost is lower, but the collapse intensity of bubbles is relatively low.
CRediT authorship contribution statement
Mohammad Hadi Dehghani: Conceptualization, Methodology, Visualization, Investigation, Project administration, Writing – review & editing. Rama Rao Karri: Conceptualization, Methodology, Resources, Writing – original draft. Janardhan Reddy Koduru: Conceptualization, Methodology, Resources, Writing – original draft. Sivakumar Manickam: Writing – review & editing. Inderjeet Tyagi: Visualization, Investigation. Nabisab Mujawar Mubarak: Writing – review & editing. Suhas: Visualization, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research has been supported by The Tehran University of Medical Sciences.
Data availability statements
Data sharing does not apply to this article as no datasets were generated or analysed during the current study.
Funding
None.
Ethical approval
Not required.
Data availability
Data will be made available on request.
References
- 1.Vortmann M., Balsari S., Holman S.R., Greenough P.G. Water, sanitation, and hygiene at the world’s largest mass gathering. Curr. Infect. Dis. Rep. 2015;17(2):5. doi: 10.1007/s11908-015-0461-1. [DOI] [PubMed] [Google Scholar]
- 2.Cabral J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int J Environ Res Public Health. 2010;7:3657–3703. doi: 10.3390/ijerph7103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou H., Wang L. The disinfection effect of a novel continuous-flow water sterilizing system coupling dual-frequency ultrasound with sodium hypochlorite in pilot scale. Ultrason. Sonochem. 2017;36:246–252. doi: 10.1016/j.ultsonch.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y., Sun T., Li G., An T. Traditional and emerging water disinfection technologies challenging the control of antibiotic-resistant bacteria and antibiotic resistance genes. ACS ES&T Eng. 2021;1(7):1046–1064. [Google Scholar]
- 5.Shokri Z., Seidi F., Saeb M.R., Jin Y., Li C., Xiao H. Elucidating the impact of enzymatic modifications on the structure, properties, and applications of cellulose, chitosan, starch and their derivatives: a review. Mater. Today Chem. 2022;24 [Google Scholar]
- 6.Liu J., Shen L., Lin H., Huang Z., Hong H., Chen C. Preparation of Ni@ UiO-66 incorporated polyethersulfone (PES) membrane by magnetic field assisted strategy to improve permeability and photocatalytic self-cleaning ability. J. Colloid Interface Sci. 2022;618:483–495. doi: 10.1016/j.jcis.2022.03.106. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hazmi H.E., Shokrani H., Shokrani A., Jabbour K., Abida O., Khadem S.S.M., Habibzadeh S., Sonawane S.H., Saeb M.R., Bonilla-Petriciolet A. Recent advances in aqueous virus removal technologies. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.135441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piazzoli A., Breider F., Aquillon C.G., Antonelli M., Von Gunten U. Specific and total N-nitrosamines formation potentials of nitrogenous micropollutants during chloramination. Water Res. 2018;135:311–321. doi: 10.1016/j.watres.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Bui X.-T., Chen S.-S., Nguyen P.-D., Nguyen T.-T., Nguyen T.-B. Hospital wastewater treatment by sponge membrane bioreactor coupled with ozonation process. Chemosphere. 2019;230:377–383. doi: 10.1016/j.chemosphere.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Chen H. Catalytic ozonation for water and wastewater treatment: recent advances and perspective. Sci. Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135249. [DOI] [PubMed] [Google Scholar]
- 11.Wilczewska P., Pancielejko A., Łuczak J., Kroczewska M., Lisowski W., Siedlecka E.M. The influence of ILs on TiO2 microspheres activity towards 5-FU removal under artificial sunlight irradiation. Appl. Surf. Sci. 2022;573 [Google Scholar]
- 12.Agarwal S., Tyagi I., Gupta V.K., Dehghani M.H., Bagheri M., Yetilmezsoy K., Amrane A., Heibati B., Rodriguez-Couto S. Degradation of azinphos-methyl and chlorpyrifos from aqueous solutions by ultrasound treatment, Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Journal of Molecular Liquids. 2016;221:1237–1242. [Google Scholar]
- 13.Dehghani M.H., Najafpoor A.A., Azam K. Using sonochemical reactor for degradation of LAS from effluent of wastewater treatment plant. Desalination. 2010;250(1):82–86. [Google Scholar]
- 14.Gągol M., Cako E., Fedorov K., Soltani R.D.C., Przyjazny A., Boczkaj G. Hydrodynamic cavitation based advanced oxidation processes: Studies on specific effects of inorganic acids on the degradation effectiveness of organic pollutants. J. Mol. Liq. 2020;307 [Google Scholar]
- 15.Cako E., Gunasekaran K.D., Cheshmeh Soltani R.D., Boczkaj G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs) Water Resour. Ind. 2020;24 [Google Scholar]
- 16.Soltani R.D.C., Mashayekhi M., Naderi M., Boczkaj G., Jorfi S., Safari M. Sonocatalytic degradation of tetracycline antibiotic using zinc oxide nanostructures loaded on nano-cellulose from waste straw as nanosonocatalyst. Ultrason. Sonochem. 2019;55:117–124. doi: 10.1016/j.ultsonch.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Soltani R.D.C., Miraftabi Z., Mahmoudi M., Jorfi S., Boczkaj G., Khataee A. Stone cutting industry waste-supported zinc oxide nanostructures for ultrasonic assisted decomposition of an anti-inflammatory non-steroidal pharmaceutical compound. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104669. [DOI] [PubMed] [Google Scholar]
- 18.Gągol M., Przyjazny A., Boczkaj G. Effective method of treatment of industrial effluents under basic pH conditions using acoustic cavitation – A comprehensive comparison with hydrodynamic cavitation processes. Chem. Eng. Process. - Process Intesif. 2018;128:103–113. [Google Scholar]
- 19.Gągol M., Soltani R.D.C., Przyjazny A., Boczkaj G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs) Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Lin D., Fu Y., Li X., Wang L., Hou M., Hu D., Li Q., Zhang Z., Xu C., Qiu S., Wang Z., Boczkaj G. Application of persulfate-based oxidation processes to address diverse sustainability challenges: A critical review. J. Hazard. Mater. 2022;440 doi: 10.1016/j.jhazmat.2022.129722. [DOI] [PubMed] [Google Scholar]
- 21.Sonawane S., Fedorov K., Rayaroth M.P., Boczkaj G. Degradation of 1,4-dioxane by sono-activated persulfates for water and wastewater treatment applications. Water Resour. Ind. 2022;28 [Google Scholar]
- 22.Fedorov K., Plata-Gryl M., Khan J.A., Boczkaj G. Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. J. Hazard. Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122804. [DOI] [PubMed] [Google Scholar]
- 23.Fedorov K., Sun X., Boczkaj G. Combination of hydrodynamic cavitation and SR-AOPs for simultaneous degradation of BTEX in water. Chem. Eng. J. 2021;417 [Google Scholar]
- 24.Natarajan P., Priya, and D. Chuskit, Persulfate-activated charcoal mixture: an efficient oxidant for the synthesis of sulfonated benzo[d][1,3]oxazines from N-(2-vinylphenyl)amides and thiols in aqueous solution. RSC. Advances. 2021;11(26):15573–15580. doi: 10.1039/d1ra02377b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng K., Qin F.G., Jiang R., Qu W., Wang Q. Production and dispersion of free radicals from transient cavitation Bubbles: An integrated numerical scheme and applications. Ultrason. Sonochem. 2022;88 doi: 10.1016/j.ultsonch.2022.106067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashwan S.S., Dincer I., Mohany A., Pollet B.G. The Sono-Hydro-Gen process (Ultrasound induced hydrogen production): Challenges and opportunities. International Journal of Hydrogen Energy, 44. 2019:14500–14526. [Google Scholar]
- 27.Thanekar P., Gogate P.R. Improved processes involving hydrodynamic cavitation and oxidants for treatment of real industrial effluent. Sep. Purif. Technol. 2020;239 [Google Scholar]
- 28.Dehane A., Merouani S., Hamdaoui O., Ashokkumar M. An alternative technique for determining the number density of acoustic cavitation bubbles in sonochemical reactors. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerboua K., Hamdaoui O. Sonochemistry for Water Remediation: Toward an Up-Scaled Continuous Technology. Applied Water Science: Remediation Technologies. 2021;2:437–467. [Google Scholar]
- 30.Zhou Q., Wang Y., Xiao J., Fan H. Adsorption and removal of bisphenol A, α-naphthol and β-naphthol from aqueous solution by Fe3O4@ polyaniline core–shell nanomaterials. Synth. Met. 2016;212:113–122. [Google Scholar]
- 31.Ji R., Pflieger R., Virot M., Nikitenko S.I. Multibubble Sonochemistry and Sonoluminescence at 100 kHz: The Missing Link between Low-and High-Frequency Ultrasound. J. Phys. Chem. B. 2018;122(27):6989–6994. doi: 10.1021/acs.jpcb.8b04267. [DOI] [PubMed] [Google Scholar]
- 32.Sun X., Park J.J., Kim H.S., Lee S.H., Seong S.J., Om A.S., Yoon J.Y. Experimental investigation of the thermal and disinfection performances of a novel hydrodynamic cavitation reactor. Ultrason. Sonochem. 2018;49:13–23. doi: 10.1016/j.ultsonch.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 33.Loraine G., Chahine G., Hsiao C.-T., Choi J.-K., Aley P. Disinfection of gram-negative and gram-positive bacteria using DynaJets® hydrodynamic cavitating jets. Ultrason. Sonochem. 2012;19(3):710–717. doi: 10.1016/j.ultsonch.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Šarc A., Oder M., Dular M. Can rapid pressure decrease induced by supercavitation efficiently eradicate Legionella pneumophila bacteria? Desalin. Water Treat. 2016;57(5):2184–2194. [Google Scholar]
- 35.Jain P., Bhandari V.M., Balapure K., Jena J., Ranade V.V., Killedar D.J. Hydrodynamic cavitation using vortex diode: An efficient approach for elimination of pathogenic bacteria from water. J. Environ. Manage. 2019;242:210–219. doi: 10.1016/j.jenvman.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 36.Šarc A., Stepišnik-Perdih T., Petkovšek M., Dular M. The issue of cavitation number value in studies of water treatment by hydrodynamic cavitation. Ultrason. Sonochem. 2017;34:51–59. doi: 10.1016/j.ultsonch.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Tao Y., Cai J., Huai X., Liu B., Guo Z. Application of hydrodynamic cavitation to wastewater treatment. Chem. Eng. Technol. 2016;39(8):1363–1376. [Google Scholar]
- 38.Sarvothaman V.P., Simpson A.T., Ranade V.V. Modelling of vortex based hydrodynamic cavitation reactors. Chem. Eng. J. 2019;377 [Google Scholar]
- 39.Mason T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016;29:519–523. doi: 10.1016/j.ultsonch.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z., Yang Y., Li X., Zhang Y., Guo X. Characterization of drinking water treatment sludge after ultrasound treatment. Ultrason. Sonochem. 2015;24:19–26. doi: 10.1016/j.ultsonch.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Demaerel J., Govaerts S., Paul R.R., Van Gerven T., De Borggraeve W.M. Non-innocent probes in direct sonication: Metal assistance in oxidative radical CH functionalization. Ultrason. Sonochem. 2018;41:134–142. doi: 10.1016/j.ultsonch.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 42.De La Calle I., Cabaleiro N., Lavilla I., Bendicho C. Analytical evaluation of a cup-horn sonoreactor used for ultrasound-assisted extraction of trace metals from troublesome matrices. Spectrochim. Acta B At. Spectrosc. 2009;64(9):874–883. [Google Scholar]
- 43.Bizzi C.A., Zanatta R.C., Santos D., Giacobe K., Dallago R.M., Mello P.A., Flores E.M. Ultrasound-assisted extraction of chromium from residual tanned leather: An innovative strategy for the reuse of waste in tanning industry. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104682. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira L.S., Vieira H.P., Windmöller C.C., Nascentes C.C. Fast determination of trace elements in organic fertilizers using a cup-horn reactor for ultrasound-assisted extraction and fast sequential flame atomic absorption spectrometry. Talanta. 2014;119:232–239. doi: 10.1016/j.talanta.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Chatel G., Varma R.S. Ultrasound and microwave irradiation: contributions of alternative physicochemical activation methods to Green Chemistry. Green Chem. 2019;21(22):6043–6050. [Google Scholar]
- 46.Dong Z., Zhao S., Zhang Y., Yao C., Yuan Q., Chen G. Mixing and residence time distribution in ultrasonic microreactors. AIChE J. 2017;63(4):1404–1418. [Google Scholar]
- 47.Rivas D.F., Kuhn S. Synergy of microfluidics and ultrasound. Sonochemistry. 2017:225–254. [Google Scholar]
- 48.Dong Z., Yao C., Zhang X., Xu J., Chen G., Zhao Y., Yuan Q. A high-power ultrasonic microreactor and its application in gas–liquid mass transfer intensification. Lab Chip. 2015;15(4):1145–1152. doi: 10.1039/c4lc01431f. [DOI] [PubMed] [Google Scholar]
- 49.Zhao S., Yao C., Dong Z., Liu Y., Chen G., Yuan Q. Intensification of liquid-liquid two-phase mass transfer by oscillating bubbles in ultrasonic microreactor. Chem. Eng. Sci. 2018;186:122–134. [Google Scholar]
- 50.Kalmár C., Turányi T., Zsély I.G., Papp M., Hegedűs F. The importance of chemical mechanisms in sonochemical modelling. Ultrason. Sonochem. 2022;83:105925. doi: 10.1016/j.ultsonch.2022.105925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasui K. Springer; 2018. Acoustic cavitation and bubble dynamics. [Google Scholar]
- 52.Izadifar Z., Babyn P., Chapman D. Ultrasound cavitation/microbubble detection and medical applications. Journal of Medical and Biological Engineering. 2019;39(3):259–276. [Google Scholar]
- 53.Kosel J., Gutiérrez-Aguirre I., Rački N., Dreo T., Ravnikar M., Dular M. Efficient inactivation of MS-2 virus in water by hydrodynamic cavitation. Water Res. 2017;124:465–471. doi: 10.1016/j.watres.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 54.de Andrade M.O., Haqshenas R., Pahk K.J., Saffari N. Mechanisms of nuclei growth in ultrasound bubble nucleation. Ultrason. Sonochem. 2022;88:106091. doi: 10.1016/j.ultsonch.2022.106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalmár C., Klapcsik K., Hegedűs F. Relationship between the radial dynamics and the chemical production of a harmonically driven spherical bubble. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104989. [DOI] [PubMed] [Google Scholar]
- 56.Pang Y.L., Abdullah A.Z., Bhatia S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination. 2011;277(1–3):1–14. [Google Scholar]
- 57.Adewuyi Y.G. Sonochemistry in environmental remediation. 2. Heterogeneous sonophotocatalytic oxidation processes for the treatment of pollutants in water. Environ. Sci. Tech. 2005;39(22):8557–8570. doi: 10.1021/es0509127. [DOI] [PubMed] [Google Scholar]
- 58.Leighton T. Academic press; 2012. The acoustic bubble. [Google Scholar]
- 59.Mason T.J., Lorimer J.P. Applied sonochemistry: the uses of power ultrasound in chemistry and processing. Synthesis. 2002;61(3) [Google Scholar]
- 60.Peng K., Qin F.G., Tian S., Zhang Y. An inverse method to fast-track the calculation of phase diagrams for sonoluminescing bubbles. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serna-Galvis E.A., Porras J., Torres-Palma R.A. A critical review on the sonochemical degradation of organic pollutants in urine, seawater, and mineral water. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glienke J., Schillberg W., Stelter M., Braeutigam P. Prediction of degradability of micropollutants by sonolysis in water with QSPR-a case study on phenol derivates. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collis J., Manasseh R., Liovic P., Tho P., Ooi A., Petkovic-Duran K., Zhu Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 2010;50(2):273–279. doi: 10.1016/j.ultras.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Tan C., Yan B., Han T., Alfred C., Qin P. Improving temporal stability of stable cavitation activity of circulating microbubbles using a closed-loop controller based on pulse-length regulation. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erriu M., Blus C., Szmukler-Moncler S., Buogo S., Levi R., Barbato G., Madonnaripa D., Denotti G., Piras V., Orrù G. Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason. Sonochem. 2014;21(1):15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Bengtsson-Palme J., Milakovic M., Švecová H., Ganjto M., Jonsson V., Grabic R., Udikovic-Kolic N. Industrial wastewater treatment plant enriches antibiotic resistance genes and alters the structure of microbial communities. Water Res. 2019;162:437–445. doi: 10.1016/j.watres.2019.06.073. [DOI] [PubMed] [Google Scholar]
- 67.Mukherjee A., Ahn Y.-H. Terpinolene as an enhancer for ultrasonic disinfection of multi-drug-resistant bacteria in hospital wastewater. Environ. Sci. Pollut. Res. 2022;29(23):34500–34514. doi: 10.1007/s11356-022-18611-6. [DOI] [PubMed] [Google Scholar]
- 68.Matafonova G., Batoev V. Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019;166 doi: 10.1016/j.watres.2019.115085. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y., Wang J., Abe A., Wang Y., Du T., Huang C. A theoretical model to estimate inactivation effects of OH radicals on marine Vibrio sp. in bubble-shock interaction. Ultrason. Sonochem. 2019;55:359–368. doi: 10.1016/j.ultsonch.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Gao S., Hemar Y., Ashokkumar M., Paturel S., Lewis G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014;60:93–104. doi: 10.1016/j.watres.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 71.Koda S., Miyamoto M., Toma M., Matsuoka T., Maebayashi M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500 kHz. Ultrason. Sonochem. 2009;16(5):655–659. doi: 10.1016/j.ultsonch.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Adolfsen K.J., Brynildsen M.P. A kinetic platform to determine the fate of hydrogen peroxide in Escherichia coli. PLoS Comput. Biol. 2015;11(11) doi: 10.1371/journal.pcbi.1004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flores M.J., Brandi R.J., Cassano A.E., Labas M.D. Chemical disinfection with H2O2− The proposal of a reaction kinetic model. Chem. Eng. J. 2012;198:388–396. [Google Scholar]
- 74.Liao X., Li J., Suo Y., Chen S., Ye X., Liu D., Ding T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Human Wellness. 2018;7(1):102–109. [Google Scholar]
- 75.Šarc A., Kosel J., Stopar D., Oder M., Dular M. Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super) cavitation. Ultrason. Sonochem. 2018;42:228–236. doi: 10.1016/j.ultsonch.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Monsen T., Lövgren E., Widerström M., Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 2009;47(8):2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shokri A.R., Babadagli T. Field scale modeling of CHOPS and solvent/thermal based post CHOPS EOR applications considering non-equilibrium foamy oil behavior and realistic representation of wormholes. J. Pet. Sci. Eng. 2016;137:144–156. [Google Scholar]
- 78.Lee H., Zhou B., Liang W., Feng H., Martin S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: microbial responses and kinetics modeling. J. Food Eng. 2009;93(3):354–364. doi: 10.1111/j.1750-3841.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- 79.Li J., Ahn J., Liu D., Chen S., Ye X., Ding T. Evaluation of ultrasound-induced damage to Escherichia coli and Staphylococcus aureus by flow cytometry and transmission electron microscopy. Appl. Environ. Microbiol. 2016;82(6):1828–1837. doi: 10.1128/AEM.03080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naddeo V., Cesaro A., Mantzavinos D., Fatta-Kassinos D. Belgiorno; Water and wastewater disinfection by ultrasound irradiation-a critical review: 2014. and V. [Google Scholar]
- 81.Herceg Z., Režek Jambrak A., Lelas V., Mededovic Thagard S. The effect of high intensity ultrasound treatment on the amount of Staphylococcus aureus and Escherichia coli in milk. Food Technol. Biotechnol. 2012;50(1):46–52. [Google Scholar]
- 82.Sesal N.C., Kekeç Ö. Inactivation of escherichia coli and staphylococcus aureus by ultrasound. J. Ultrasound Med. 2014;33(9):1663–1668. doi: 10.7863/ultra.33.9.1663. [DOI] [PubMed] [Google Scholar]
- 83.Gao S., Lewis G.D., Ashokkumar M., Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014;21(1):446–453. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 85.Yusof N.S.M., Babgi B., Alghamdi Y., Aksu M., Madhavan J., Ashokkumar M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016;29:568–576. doi: 10.1016/j.ultsonch.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Joyce E., Al-Hashimi A., Mason T.J. Assessing the effect of different ultrasonic frequencies on bacterial viability using flow cytometry. J. Appl. Microbiol. 2011;110(4):862–870. doi: 10.1111/j.1365-2672.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 87.Kumar R., Yadav N., Rawat L., Goyal M. Effect of two waves of ultrasonic on waste water treatment. Journal of Chemical Engineering & Process Technology. 2014;5(3):1. [Google Scholar]
- 88.Saharan V.K., Rizwani M.A., Malani A.A., Pandit A.B. Effect of geometry of hydrodynamically cavitating device on degradation of orange-G. Ultrason. Sonochem. 2013;20(1):345–353. doi: 10.1016/j.ultsonch.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 89.Carpenter J., Badve M., Rajoriya S., George S., Saharan V.K., Pandit A.B. Hydrodynamic cavitation: an emerging technology for the intensification of various chemical and physical processes in a chemical process industry. Rev. Chem. Eng. 2017;33(5):433–468. [Google Scholar]
- 90.Verdini F., Calcio Gaudino E., Grillo G., Tabasso S., Cravotto G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021;11(10):4693. [Google Scholar]
- 91.Ferrarese G., Messa G.V., Rossi M.M., Malavasi S. New method for predicting the incipient cavitation index by means of single-phase computational fluid dynamics model. Adv. Mech. Eng. 2015;7(3) 1687814015575974. [Google Scholar]
- 92.Badve M.P., Bhagat M.N., Pandit A.B. Microbial disinfection of seawater using hydrodynamic cavitation. Sep. Purif. Technol. 2015;151:31–38. [Google Scholar]
- 93.Burzio E., Bersani F., Caridi G., Vesipa R., Ridolfi L., Manes C. Water disinfection by orifice-induced hydrodynamic cavitation. Ultrason. Sonochem. 2019 doi: 10.1016/j.ultsonch.2019.104740. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y., Tian Y., Zhang Z., Lin S. Experimental and numerical study of cavitating flow with suction in a mixing reactor for water treatment. Chem. Eng. J. 2018;353:796–804. [Google Scholar]
- 95.Wang Y., Jia A., Wu Y., Wu C., Chen L. Disinfection of bore well water with chlorine dioxide/sodium hypochlorite and hydrodynamic cavitation. Environ. Technol. 2015;36(4):479–486. doi: 10.1080/09593330.2014.952345. [DOI] [PubMed] [Google Scholar]
- 96.Balasundaram B., Harrison S. Optimising orifice geometry for selective release of periplasmic products during cell disruption by hydrodynamic cavitation. Biochem. Eng. J. 2011;54(3):207–209. [Google Scholar]
- 97.Dindar E. An Overview of the Application of Hydrodinamic Cavitation for the Intensification of Wastewater Treatment Applications: A Review. Innov. Energy Res. 2016;5(137):1–7. [Google Scholar]
- 98.Karnik B., Davies S., Baumann M., Masten S. The effects of combined ozonation and filtration on disinfection by-product formation. Water Res. 2005;39(13):2839–2850. doi: 10.1016/j.watres.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 99.Liu Z., Zhu M., Deng C., Su H., Chen P., Wang Z. Pollutant and microorganism removal from water by hydrodynamic cavitation. The Open. Biotechnol. J. 2016;10(1) [Google Scholar]
- 100.Kalumuck, K., G. Chahine, C. Hsiao, and J. Choi. Remediation and disinfection of water using jet generated cavitation. in Fifth International Symposium on Cavitation. November. 2003.
- 101.Azuma Y., Kato H., Usami R., Fukushima T. Bacterial sterilization using cavitating jet. J. Fluid Sci. Technol. 2007;2(1):270–281. [Google Scholar]
- 102.Jyoti K., Pandit A.B. Water disinfection by acoustic and hydrodynamic cavitation. Biochem. Eng. J. 2001;7(3):201–212. [Google Scholar]
- 103.Dalfré Filho J.G., Assis M.P., Genovez A.I.B. Bacterial inactivation in artificially and naturally contaminated water using a cavitating jet apparatus. J. Hydro Environ. Res. 2015;9(2):259–267. [Google Scholar]
- 104.Dong Z., Qin Z. Experimental Study of Pathogenic Microorganisms Inactivated by Venturi-Type Hydrodynamic Cavitation with Different Throat Lengths. in Journal of the Civil Engineering. Forum. 2018 [Google Scholar]
- 105.Zhang X., Li W., Blatchley E.R., III, Wang X., Ren P. UV/chlorine process for ammonia removal and disinfection by-product reduction: comparison with chlorination. Water Res. 2015;68:804–811. doi: 10.1016/j.watres.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 106.Chen D., Sharma S.K., Mudhoo A. CRC Press; 2011. Handbook on applications of ultrasound: sonochemistry for sustainability. [Google Scholar]
- 107.Reddy P., Kim K.-H., Kim Y.-H. A review of photocatalytic treatment for various air pollutants. Asian J. Atmos. Environ. 2011;5(3):181–188. [Google Scholar]
- 108.Al-Juboori R.A., Aravinthan V., Yusaf T. Impact of pulsed ultrasound on bacteria reduction of natural waters. Ultrason. Sonochem. 2015;27:137–147. doi: 10.1016/j.ultsonch.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Langone M., Ferrentino R., Trombino G., de Puiseau D.W., Andreottola G., Rada E.C., Ragazzi M. Application of a novel hydrodynamic cavitation system in wastewater treatment plants. UPB Scientific Bulletin, Series D. 2015;77(1):225–234. [Google Scholar]
- 110.M'Arimi M., Mecha C., Kiprop A., Ramkat R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production. Renew. Sustain. Energy Rev. 2020;121 [Google Scholar]
- 111.Fedorov K., Dinesh K., Sun X., R. Darvishi Cheshmeh Soltani, Z. Wang, S. Sonawane, and G. Boczkaj, Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon – A review. Chem. Eng. J. 2022;432 [Google Scholar]
- 112.Gągol M., Przyjazny A., Boczkaj G. Wastewater treatment by means of advanced oxidation processes based on cavitation – A review. Chem. Eng. J. 2018;338:599–627. [Google Scholar]
- 113.Petkovšek M., Mlakar M., Levstek M., Stražar M., Širok B., Dular M. A novel rotation generator of hydrodynamic cavitation for waste-activated sludge disintegration. Ultrason. Sonochem. 2015;26:408–414. doi: 10.1016/j.ultsonch.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 115.Rashwan S.S., Dincer I., Mohany A. Sonication to hydrogenization: Sono‐hydro‐gen, Int. J. Energy Res. 2019;43:1045–1048. [Google Scholar]
- 116.Joseph C.G., Puma G.L., Bono A., Krishnaiah D. Sonophotocatalysis in advanced oxidation process: a short review. Ultrason. Sonochem. 2009;16(5):583–589. doi: 10.1016/j.ultsonch.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Chowdhury P., Viraraghavan T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes–a review. Science Total Environ. 2009;407(8):2474–2492. doi: 10.1016/j.scitotenv.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 118.Sun X., You W., Wu Y., Tao Y., Yoon J.Y., Zhang X., Xuan X. Hydrodynamic Cavitation: A Novel Non-Thermal Liquid Food Processing Technology. Front Nut.r. 2022;9:843808. doi: 10.3389/fnut.2022.843808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Vajnhandl S., Majcen Le Marechal A. Ultrasound in textile dyeing and the decolouration/mineralization of textile dyes. Dyes and Pigments. 2005;65(2):89–101. [Google Scholar]
- 122.Adewuyi Yusuf G. Sonochemistry Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001;40:4681–4715. [Google Scholar]
- 123.Gao Shengpu, Yacine Hemar, Muthupandian Ashokkumar, Sara Paturel, Gillian D. Lewis. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014;60:93–104. doi: 10.1016/j.watres.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 124.Zhou Qingxiang, Wang Yuqin, Xiao Junping, Huili Fan. Adsorption and removal of bisphenol A, α-naphthol and β-naphthol from aqueous solution by Fe3O4@polyaniline core–shell nanomaterials. Synthetic Metals. 2016;212:113–122. [Google Scholar]
- 125.Raut-Jadhav, Sunita, Dipak V. Pinjari, Daulat R. Saini, Shirish H. Sonawane, and Aniruddha B. Pandit. 2016. 'Intensification of degradation of methomyl (carbamate group pesticide) by using the combination of ultrasonic cavitation and process intensifying additives', Ultrason. Sonochem., 31: 135-42. [DOI] [PubMed]
- 126.Yi Chunhai, Qianqian Lu, Wang Yun, Wang Yixuan, Bolun Yang. Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ultrason. Sonochem. 2018;43:156–165. doi: 10.1016/j.ultsonch.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 127.Storey, Brian D., Andrew J. Szeri. Water vapour, sonoluminescence and sonochemistry. Proceedings of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences. 2000;456:1685–1709. [Google Scholar]
- 128.Colussi, A. J., Linda K. Weavers, and Michael R. Hoffmann. 1998. 'Chemical Bubble Dynamics and Quantitative Sonochemistry', The Journal of Physical Chemistry A, 102: 6927-34.
- 129.Gogate, Parag R., Anne Marie Wilhelm, Aniruddha B. Pandit. Some aspects of the design of sonochemical reactors. Ultrason. Sonochem. 2003;10:325–330. doi: 10.1016/S1350-4177(03)00103-2. [DOI] [PubMed] [Google Scholar]
- 130.Chen, Dong, Sanjay K Sharma, and Ackmez Mudhoo. 2011. Handbook on applications of ultrasound: sonochemistry for sustainability (CRC press).
- 131.Peters Dietmar. Ultrasound in materials chemistry. Journal of Materials Chemistry. 1996;6:1605. [Google Scholar]
- 132.Wu, Ta Yeong, Ningqun Guo, Chee Yang Teh, and Jacqueline Xiao Wen Hay. 2012. "Applications of Ultrasound Technology in Environmental Remediation." In SpringerBriefs in Molecular Science, 13-93. Springer Netherlands.
- 133.Asgharzadehahmadi Seyedali, Abdul Aziz Abdul Raman, Rajarathinam Parthasarathy, Baharak Sajjadi. Sonochemical reactors: Review on features, advantages and limitations. Renewable and Sustainable Energy Reviews. 2016;63:302–314. [Google Scholar]
- 134.Kumar, Ajay, Parag R. Gogate, Aniruddha B. Pandit, Anne Marie Wilhelm, and Henri Delmas. 2005. 'Investigation of induction of air due to ultrasound source in the sonochemical reactors', Ultrasonics Sonochemistry, 12: 453-60. [DOI] [PubMed]
- 135.Laugier F., Andriantsiferana C., Wilhelm A.M. Ultrasound in gas–liquid systems: Effects on solubility and mass transfer. Ultrason. Sonochem. 2008;15:965–972. doi: 10.1016/j.ultsonch.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 136.Monnier H., Wilhelm A.M. The influence of ultrasound on micromixing in a semi-batch reactor. Chem. Eng. Sci. 1999;54:2953–2961. [Google Scholar]
- 137.Kumar, Ajay, T. Kumaresan, Aniruddha B. Pandit, and Jyeshtharaj B. Joshi. 2006. 'Characterization of flow phenomena induced by ultrasonic horn', Chem. Eng. Sci., 61: 7410-20.
- 138.Monnier H., Wilhelm A.-M., Delmas H. Influence of ultrasound on mixing on the molecular scale for water and viscous liquids. Ultrason. Sonochem. 1999;6(1–2):67–74. doi: 10.1016/s1350-4177(98)00034-0. [DOI] [PubMed] [Google Scholar]
- 139.Baḱldyga J., Podgórska W. Mixing-precipitation model with application to double feed semibatch precipitation. Chem. Eng. Sci. 1995;50:1281–1300. [Google Scholar]
- 140.Baldyga J., Bourne J.R. The influence of viscosity on mixing in jet reactors. Chem. Eng. Sci. 1995;50:1877–1880. [Google Scholar]
- 141.Monnier H., Wilhelm A.M. Effects of ultrasound on micromixing in flow cell. Chem. Eng. Sci. 2000;55:4009–4020. [Google Scholar]
- 142.Vichare, Nilesh P., Parag R. Gogate, Vishwas Y. Dindore, and Aniruddha B. Pandit. 2001. 'Mixing time analysis of a sonochemical reactor', Ultrason. Sonochem., 8: 23-33. [DOI] [PubMed]
- 143.Yusaf, Talal F., David R. Buttsworth. Characterisation of mixing rate due to high power ultrasound. Ultrason. Sonochem. 2007;14:266–274. doi: 10.1016/j.ultsonch.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 144.Kojima Yoshihiro, Yoshiyuki Asakura, Genki Sugiyama, Shinobu Koda. The effects of acoustic flow and mechanical flow on the sonochemical efficiency in a rectangular sonochemical reactor. Ultrason. Sonochem. 2010;17:978–984. doi: 10.1016/j.ultsonch.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 145.Sutkar, Vinayak S., Parag R. Gogate. Design aspects of sonochemical reactors: Techniques for understanding cavitational activity distribution and effect of operating parameters. Chem. Eng. J. 2009;155:26–36. [Google Scholar]
- 146.Gonze E., Gonthier Y., Boldo P. Standing waves in a high frequency sonoreactor: Visualization and effects. Chem. Eng. Sci. 1998;53:523–532. [Google Scholar]
- 147.Gogate, Parag R., Vinayak S. Sutkar, and Aniruddha B. Pandit. 2011. 'Sonochemical reactors: Important design and scale up considerations with a special emphasis on heterogeneous systems', Chem. Eng. J., 166: 1066-82.
- 148.Mane, Maya B., Vinay M. Bhandari, Kshama Balapure, and Vivek V. Ranade. 2020. 'A novel hybrid cavitation process for enhancing and altering rate of disinfection by use of natural oils derived from plants', Ultrason. Sonochem., 61: 104820. [DOI] [PubMed]
- 149.Badve, Mandar P., Mihir N. Bhagat, and Aniruddha B. Pandit. 2015. 'Microbial disinfection of seawater using hydrodynamic cavitation', Separation and Purification Technology, 151: 31-38.
- 150.Leighton T.G. The Acoustic Bubble. Elsevier; 1994. The Forced Bubble; pp. 287–438. [Google Scholar]
- 151.Peng, Kewen, Frank G. F. Qin, Shouceng Tian, and Yiqun Zhang. 2021. 'An inverse method to fast-track the calculation of phase diagrams for sonoluminescing bubbles', Ultrason. Sonochem., 73: 105534-34. [DOI] [PMC free article] [PubMed]
- 152.Serna-Galvis, Efraím A., Jazmín Porras, Ricardo A. Torres-Palma. A critical review on the sonochemical degradation of organic pollutants in urine, seawater, and mineral water. Ultrason. Sonochem. 2022;82:105861. doi: 10.1016/j.ultsonch.2021.105861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mason T.J., Joyce E., Phull S.S. Potential uses of ultrasound in the biological decontamination of water. Ultrason. Sonochem. 2003;10:319–323. doi: 10.1016/S1350-4177(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 154.Soria, Ana Cristina, and Mar Villamiel. 2010. 'Effect of ultrasound on the technological properties and bioactivity of food: a review', Trends in Food Science & Technology, 21: 323-31.
- 155.Ahmed F.I.K. Synergism between Ultrasonic Waves and Hydrogen Peroxide in the Killing of Micro-organisms. J. Appl. Bacteriol. 1975;39:31–40. doi: 10.1111/j.1365-2672.1975.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 156.Pagán R., Mañas P., Raso J. Bacterial resistance to ultrasonic waves under pressure at nonlethal (manosonication) and lethal (manothermosonication) temperatures. Applied and environmental microbiology. 1999;65:297–300. doi: 10.1128/aem.65.1.297-300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanz, B., Palacios, P., Lopez, P., and Ordonez, J. 1985. Effect of ultrasonic waves on the heat resistance of Bacillus stearothermophilus spores. in FEMS symposium-Federation of European Microbiological Societies (USA).
- 158.Mahvi A.H., Dehghani M.H. Ultrasonic Technology Effectiveness in Total Coliforms Disinfection of Water. J. Appl. Sci. 2005;5:856–858. [Google Scholar]
- 159.Crum Lawrence A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995;2:S147–S152. [Google Scholar]
- 160.Erriu Matteo, Cornelio Blus, Szmukler-Moncler Serge, Silvano Buogo, Raffaello Levi, et al. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014;21:15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 161.O’Donnell C.P., Tiwari B.K., Bourke P. Effect of ultrasonic processing on food enzymes of industrial importance. Trends in Food Science & Technology. 2010;21:358–367. [Google Scholar]
- 162.Raso J., Palop A., Pagán R. Inactivation of Bacillus subtilis spores by combining ultrasonic waves under pressure and mild heat treatment. J. Appl. Microbiol. 1998;85:849–854. doi: 10.1046/j.1365-2672.1998.00593.x. [DOI] [PubMed] [Google Scholar]
- 163.Whillock G.O.H. Ultrasonically enhanced corrosion of 304L stainless steel I: The effect of temperature and hydrostatic pressure. Ultrason. Sonochem. 1997;4:23–31. doi: 10.1016/s1350-4177(96)00014-4. [DOI] [PubMed] [Google Scholar]
- 164.Suslick K.S. Sonochemistry. science. 1990;247(4949):1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 165.Pagán R., Mañas P., Alvarez I. Resistance ofListeria monocytogenesto ultrasonic waves under pressure at sublethal (manosonication) and lethal (manothermosonication) temperatures. Food Microbiol. 1999;16:139–148. doi: 10.1128/aem.65.1.297-300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kinsloe H., Ackerman E. Exposure of microorganisms to measured sound fields. J. Bacteriol. 1954;68:373–380. doi: 10.1128/jb.68.3.373-380.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vajnhandl Simona, Željko Tina, Alenka Majcen Le Marechal, Julija Volmajer Valh. Feasibility study of ultrasound as water disinfection technology. Desalination and Water Treatment. 2014:1–7. [Google Scholar]
- 168.Park C.B., Baik S., Kim S., Choi J.W., Lee S. ., et al. The use of ultrasonic frequencies to control the bloom formation, regrowth, and eco-toxicity in Microcystis aeruginosa. International Journal of Environmental Science and Technology. 2017;14:923–932. [Google Scholar]
- 169.Zou Huasheng, Lifang Wang. The disinfection effect of a novel continuous-flow water sterilizing system coupling dual-frequency ultrasound with sodium hypochlorite in pilot scale. Ultrason. Sonochem. 2017;36:246–252. doi: 10.1016/j.ultsonch.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 170.Li Yitao, Shi Xingdong, Zhang Zhi, Yazhou Peng. Enhanced coagulation by high-frequency ultrasound in Microcystis aeruginosa-laden water: Strategies and mechanisms. Ultrason. Sonochem. 2019;55:232–242. doi: 10.1016/j.ultsonch.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 171.Brayman, Andrew A., Brian E. MacConaghy, Yak-Nam Wang, Keith T. Chan, Wayne L. Monsky, et al. 2017. 'Inactivation of Planktonic Escherichia coli by Focused 2-MHz Ultrasound', Ultrasound in medicine & biology, 43: 1476-85. [DOI] [PMC free article] [PubMed]
- 172.Sato Chikashi, Verona Veturia Nicolae, Bharath Ramalingam, Malcolm Shields, Hiroaki Tanaka. Inactivation of E. coli Using Flow-Through Ultrasound and Ultraviolet Light Reactors in Series. Journal of Environmental Engineering. 2015;141 [Google Scholar]
- 173.Zhou Xiaoqin, Guo Hao, Li Zifu, Zhao Junyuan, Yupan Yun. Experimental study on the disinfection efficiencies of a continuous-flow ultrasound/ultraviolet baffled reactor. Ultrason. Sonochem. 2015;27:81–86. doi: 10.1016/j.ultsonch.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 174.Zhou, Xiaoqin, Zifu Li, Juanru Lan, Yichang Yan, and Nan Zhu. 2017. 'Kinetics of inactivation and photoreactivation of Escherichia coli using ultrasound-enhanced UV-C light-emitting diodes disinfection', Ultrason. Sonochem., 35: 471-77. [DOI] [PubMed]
- 175.Zhou Xiaoqin, Yichang Yan, Li Zifu. Disinfection effect of a continuous-flow ultrasound/ultraviolet baffled reactor at a pilot scale. Ultrasonics Sonochemistry. 2017;37:114–119. doi: 10.1016/j.ultsonch.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 176.Millan-Sango, David, Ana Allende, David Spiteri, Jan F. Van Impe, and Vasilis P. Valdramidis. 2017. 'Treatment of fresh produce water effluents by non-thermal technologies', Journal of Food Engineering, 199: 77-81.
- 177.Scherba, G., R. M. Weigel, and W. D. O'Brien, Jr. 1991. 'Quantitative assessment of the germicidal efficacy of ultrasonic energy', Applied and environmental microbiology, 57: 2079-84. [DOI] [PMC free article] [PubMed]
- 178.Pitt W.G., McBride M.O., Lunceford J.K., Roper R.J. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrobial Agents and Chemotherapy. 1994;38:2577–2582. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Phull S.S., Newman A.P., Lorimer J.P., Pollet B. The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason. Sonochem. 1997;4:157–164. doi: 10.1016/s1350-4177(97)00029-1. [DOI] [PubMed] [Google Scholar]
- 180.Rediske A.M., Roeder B.L., Brown M.K., Nelson J.L., Robison R.L., et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrobial Agents and Chemotherapy. 1999;43:1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hua I. Inactivation of Escherichia coli by sonication at discrete ultrasonic frequencies. Water Res. 2000;34:3888–3893. [Google Scholar]
- 182.Peterson, R. Vaughn, and William G. Pitt. 2000. 'The effect of frequency and power density on the ultrasonically-enhanced killing of biofilm-sequestered Escherichia coli', Colloids and Surfaces B: Biointerfaces, 17: 219-27.
- 183.Jyoti K.K. Water disinfection by acoustic and hydrodynamic cavitation. Biochemical Engineering Journal. 2001;7:201–212. [Google Scholar]
- 184.Pitt, William G., and S. Aaron Ross. 2003. 'Ultrasound increases the rate of bacterial cell growth', Biotechnology Progress, 19: 1038-44. [DOI] [PMC free article] [PubMed]
- 185.Mason Timothy J. Sonochemistry and the environment – Providing a “green” link between chemistry, physics and engineering. Ultrason. Sonochem. 2007;14:476–483. doi: 10.1016/j.ultsonch.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 186.Joyce E., Phull S.S., Lorimer J.P. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003;10:315–318. doi: 10.1016/S1350-4177(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 187.Kalumuck, K., Chahine, G., Hsiao, C., and Choi, J. 2003. Remediation and disinfection of water using jet generated cavitation. in Fifth International Symposium on Cavitation. November.
- 188.Carmen J.C., Roeder B.L., Nelson J.L., Beckstead B.L., Runyan C.M., et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. Journal of Biomaterials Applications. 2004;18:237–245. doi: 10.1177/0885328204040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Furuta M., Yamaguchi M., Tsukamoto T., Yim B., Stavarache C.E., et al. Inactivation of Escherichia coli by ultrasonic irradiation. Ultrasonics Sonochemistry. 2004;11:57–60. doi: 10.1016/S1350-4177(03)00136-6. [DOI] [PubMed] [Google Scholar]
- 190.Jyoti K., Pandit A. Ozone and cavitation for water disinfection. Biochemical Engineering Journal. 2004;18(1):9–19. [Google Scholar]
- 191.Ensing G.T., Roeder B.L., Nelson J.L., van Horn J.R., van der Mei H.C., et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. Journal of Applied Microbiology. 2005;99:443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paleologou, Andreas, Haridimos Marakas, Nikolaos P. Xekoukoulotakis, Armando Moya, Yolanda Vergara, et al. 2007. 'Disinfection of water and wastewater by TiO2 photocatalysis, sonolysis and UV-C irradiation', Catalysis Today, 129: 136-42.
- 193.Antoniadis Apostolos, Ioannis Poulios, Eleni Nikolakaki, Dionissios Mantzavinos. Sonochemical disinfection of municipal wastewater. Journal of Hazardous materials. 2007;146:492–495. doi: 10.1016/j.jhazmat.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 194.Azuma Yohei, Kato Hiroharu, Usami Ron, Tadamasa Fukushima. Bacterial Sterilization Using Cavitating Jet. Journal of Fluid Science and Technology. 2007;2:270–281. [Google Scholar]
- 195.Arrojo, S., Y. Benito, and A. Martínez Tarifa. 2008. 'A parametrical study of disinfection with hydrodynamic cavitation', Ultrason. Sonochem., 15: 903-08. [DOI] [PubMed]
- 196.Monsen Tor, Lövgren Elisabeth, Micael Widerström, Lars Wallinder. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. Journal of Clinical Microbiology. 2009;47:2496–2501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Drakopoulou S., Terzakis S., Fountoulakis M.S., Mantzavinos D. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochem. 2009;16:629–634. doi: 10.1016/j.ultsonch.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 198.Mezule L., Tsyfansky S., Yakushevich V. A simple technique for water disinfection with hydrodynamic cavitation: Effect on survival of Escherichia coli. Desalination. 2009;248:152–159. [Google Scholar]
- 199.Hulsmans Ann, Koen Joris, Nico Lambert, Rediers Hans, Priscilla Declerck, et al. Evaluation of process parameters of ultrasonic treatment of bacterial suspensions in a pilot scale water disinfection system. Ultrason. Sonochem. 2010;17:1004–1009. doi: 10.1016/j.ultsonch.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 200.Loraine Gregory, Georges Chahine, Chao-Tsung Hsiao, Choi Jin-Keun, Patrick Aley. Disinfection of gram-negative and gram-positive bacteria using DynaJets® hydrodynamic cavitating jets. Ultrason. Sonochem. 2012;19:710–717. doi: 10.1016/j.ultsonch.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 201.Kumar R., Yadav N., Rawat L., Goyal M. Effect of two waves of ultrasonic on waste water treatment. Journal of Chemical Engineering & Process Technology. 2014;5(3):1. [Google Scholar]
- 202.Sesal, Nüzhet Cenk, and Özge Kekeç. 2014. 'Inactivation of Escherichia coli and Staphylococcus aureus by Ultrasound', Journal of Ultrasound in Medicine, 33: 1663-68. [DOI] [PubMed]
- 203.Gao, Shengpu, Gillian D. Lewis, Muthupandian Ashokkumar, and Yacine Hemar. 2014. 'Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria', Ultrason. Sonochem., 21: 446-53. [DOI] [PubMed]
- 204.Holm, Eric R., David M. Stamper, Robert A. Brizzolara, Laurie Barnes, Nora Deamer, et al. 2008. 'Sonication of bacteria, phytoplankton and zooplankton: Application to treatment of ballast water', Marine Pollution Bulletin, 56: 1201-08. [DOI] [PubMed]
- 205.Ananta E., Voigt D., Zenker M., Heinz V. Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosus to high-intensity ultrasound. Journal of Applied Microbiology. 2005;99:271–278. doi: 10.1111/j.1365-2672.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- 206.Koda Shinobu, Masaki Miyamoto, Maricela Toma, Tatsuro Matsuoka, Masahiro Maebayashi. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500 kHz. Ultrason. Sonochem. 2009;16:655–659. doi: 10.1016/j.ultsonch.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 207.Šarc Andrej, Janez Kosel, Stopar David, Oder Martina, Matevž Dular. Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super)cavitation. Ultrason. Sonochem. 2018;42:228–236. doi: 10.1016/j.ultsonch.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 208.Amabilis-Sosa Leonel, Monserrat Vázquez-López, Juan Rojas, Adriana Roé-Sosa, Gabriela Moeller-Chávez. Efficient Bacteria Inactivation by Ultrasound in Municipal Wastewater. Environments. 2018;5:47. [Google Scholar]
- 209.Inguglia, Elena S., Brijesh K. Tiwari, Joseph P. Kerry, and Catherine M. Burgess. 2018. 'Effects of high intensity ultrasound on the inactivation profiles of Escherichia coli K12 and Listeria innocua with salt and salt replacers', Ultrasonics Sonochemistry, 48: 492-98. [DOI] [PubMed]
- 210.Al-Juboori, Raed A., Vasantha Aravinthan, Talal Yusaf. Impact of pulsed ultrasound on bacteria reduction of natural waters. Ultrason. Sonochem. 2015;27:137–147. doi: 10.1016/j.ultsonch.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 211.Dalfré Filho, José Gilberto, Maiara Pereira Assis, and Ana Inés Borri Genovez. 2015. 'Bacterial inactivation in artificially and naturally contaminated water using a cavitating jet apparatus', Journal of Hydro-environment Research, 9: 259-67.
- 212.Liu Zhimeng, Zhu Mengfu, Cheng Deng, Su Hongbo, Chen Ping, et al. Pollutant and Microorganism Removal From Water by Hydrodynamic Cavitation. The Open Biotechnology Journal. 2016;10:258–264. [Google Scholar]
- 213.Brayman, Andrew A., Brian E. MacConaghy, Yak-Nam Wang, Keith T. Chan, Wayne L. Monsky, et al. 2018. 'Inactivation of Planktonic Escherichia coli by Focused 1-MHz Ultrasound Pulses with Shocks: Efficacy and Kinetics Upon Volume Scale-Up', Ultrasound in medicine & biology, 44: 1996-2008. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
Data sharing does not apply to this article as no datasets were generated or analysed during the current study.