Abstract
Despite the escalating burden of antimicrobial resistance (AMR), the global response has not sufficiently matched the scale and scope of the issue, especially in low- and middle-income countries (LMICs). While many countries have adopted national action plans to combat AMR, their implementation has lagged due to resource constraints, dysfunctional multisectoral coordination mechanisms and, importantly, an under-recognized lack of technical capacity to adapt evidence-based AMR mitigation interventions to local contexts. AMR interventions should be tailored, context-specific, cost-effective and sustainable. The implementation and subsequent scale-up of these interventions require multidisciplinary intervention-implementation research (IIR). IIR involves both quantitative and qualitative approaches, occurs across a three-phase continuum (proof of concept, proof of implementation and informing scale-up), and across four context domains (inner setting, outer setting, stakeholders and the implementation process). We describe the theoretical underpinnings of implementation research (IR), its various components, and how to construct different IR strategies to facilitate sustainable uptake of AMR interventions. Additionally, we provide real-world examples of AMR strategies and interventions to demonstrate these principles in practice. IR provides a practical framework to implement evidence-based and sustainable AMR mitigation interventions.
Antimicrobial resistance: the silent pandemic
Antimicrobial resistance (AMR) is ranked among the top 10 threats to global health by the WHO.1,2 It is potentially the greatest public health threat of our time, surpassing COVID-19 because of its continuing and progressive nature with extensive adverse effects on the health of humans, animals, crops and the environment.3–7 From a human health perspective, a world without effective antimicrobial medicines would severely compromise healthcare as we know it, limiting our ability to perform major surgeries, conduct organ transplantations, treat premature babies and administer cancer chemotherapies.8 AMR further affects animal health and welfare, food security and food safety. In 2019 alone, 1.27 million deaths were estimated to be directly attributable to AMR globally.9 The Independent O’Neill Review estimates an annual mortality rate of up to 10 million by 2050 due to AMR, with up to 9 million deaths disproportionately occurring in low- and middle-income countries (LMICs) unless immediate and effective action is taken.10 Furthermore, the World Bank estimates that AMR could result in an additional 28 million people living in severe poverty, a 7.5% decline in global livestock production, a 3.8% reduction in global exports and 1 trillion USD in additional healthcare costs by 2050.11 Yet the negative impact of AMR has not engendered adequate and sustainable action, politically or otherwise, especially in LMICs, as AMR is somewhat intangible and frequently described as a silent pandemic, despite the high burden.10 The COVID-19 crisis provides a foretaste of what AMR can mean to the world without appropriate interventions and the human capital to implement them, making pandemic preparedness for AMR imperative.12–14
National action plans on AMR
In 2015, collaboration between the Tripartite consisting of the WHO, the Food and Agriculture Organization (FAO) of the United Nations, and the World Organisation for Animal Health (OIE) resulted in the Global Action Plan (GAP) on AMR.15 The World Health Assembly Resolution 68.7 (WHA68.7) urged member states to have in place national action plans (NAPs) on AMR aligned to the GAP by the 70th World Health Assembly in May 2017,16 and in September 2016, the United Nations General Assembly signed the Political Declaration on Antimicrobial Resistance (AMR) that endorsed WHA68.7.17,18
In its April 2019 final report, the UN Inter-Agency Coordination Group (IACG) on AMR strongly recommended that accelerated implementation of NAPs ‘must be at the heart of the global response to AMR’.19 The report, however, acknowledged that significant challenges remain in the implementation of the NAPs, with few countries having set up functional multisectoral coordination mechanisms and even fewer countries financing their NAPs.19
According to the latest Tripartite AMR country self-assessment survey (TrACSS), 149 countries have developed NAPs on AMR.20 However, the translation of policy to action has not sufficiently matched the scale and scope of the issue. Implementation of NAPs is particularly challenging in LMICs that require substantial development assistance and the whole-of-government ownership to implement their NAPs at scale. Long-term ownership and sustainability of these investments at national level when development funding ceases is a further challenge. LMICs lag behind high-income countries (HICs) in all indicators on the implementation and financing of the NAPs as evident from the country self-assessment reports based on the Tripartite monitoring tool.21
One crucial step to start implementation of NAPs is for countries to develop, test and/or adapt interventions to mitigate AMR. Although there is a growing body of evidence on effective AMR mitigation interventions,22–24 this evidence has been largely developed in high-resource settings and HICs and cannot be directly translated to LMICs or often even between HICs. Mitigating AMR in LMICs requires tailored, context-specific, cost-effective and sustainable interventions.25 This paper proposes the use of implementation research (IR) to provide proof of concept of AMR mitigation interventions in local contexts with the aim of sustainable scale-up.
What is IR?
IR is defined as ‘the scientific inquiry into questions concerning implementation—the act of carrying an intention into effect, which in health research can be policies, programmes, or individual practices (collectively called interventions)’.23 When adapted for AMR, IR may involve the quantitative and/or qualitative scientific validation of processes that will facilitate the systematic and sustainable uptake of evidence-based AMR interventions into routine practice. The ultimate goal of IR in AMR is to improve the capacities of human, animal, agricultural and environmental health systems to mitigate AMR individually and collectively in a coordinated One Health approach.26,27 The achievement of this goal requires human capital development in IR in addition to financial resources.
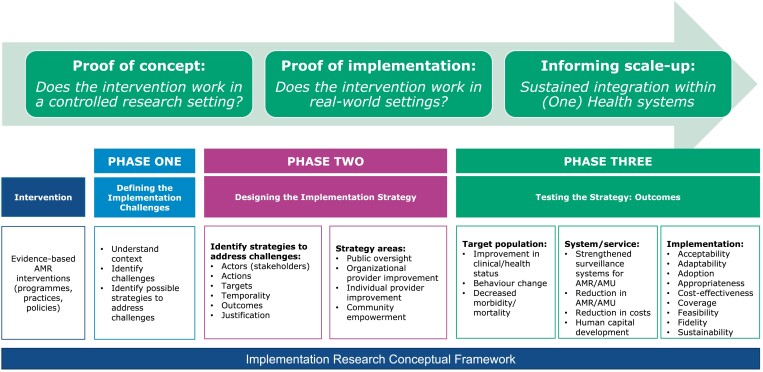
IR occurs across a three-phase continuum (Figure 1): proof of concept, proof of implementation and informing scale-up; and four context domains: inner setting, outer setting, stakeholders involved and the implementation process, all of which influence the implementation of intervention(s).27,28 The IR strategy involves three distinct steps: defining the IR challenge, designing the implementation strategy and testing the implementation strategy. The strategy defines the actors, actions, targets and temporality, and determines outcomes at three levels: target population level outcomes, system/service level outcomes and implementation outcomes.29,30 Each of these components is described below with illustrative examples.
Figure 1.
IR conceptual framework.
IR continuum
Phase one of the continuum provides proof of concept, i.e. does the intervention work in a controlled research setting? Proof of concept is usually associated with basic science, product development, Phase I and II clinical trials, or qualitative studies such as perceptions of illness or quality of health/veterinary services. Research is undertaken in a fully controlled setting such as a laboratory or amongst a defined population where implementation strategies and variables are not relevant. Phase two explores proof of implementation, i.e. does the intervention work in real-world settings in different contexts? Proof of implementation determines the effectiveness of an intervention using effectiveness-implementation trials, observational studies, or participatory research. Research is undertaken in different real-world settings and populations and is partially controlled. Here, implementation strategies and variables are important research components since interventions may work in one setting but not in others. Phase three focuses on informing scale-up, i.e. sustained integration within systems. Scale-up is generally informed by mixed methods, quasi-experimental studies, or observational studies to determine the enablers of and barriers to sustained scale-up. Research is undertaken in a real-world setting and population and implementation strategies and variables are the main or only research focus.30
The discovery and path to the clinical use of penicillin illustrates this continuum. In 1928, Alexander Fleming, following his return from vacation, serendipitously observed a zone on an agar plate around an invading fungus without any staphylococcal growth.31 Fleming isolated the mould and identified it as belonging to the Penicillium genus, naming its active agent penicillin. While he published his findings in 1929,32 he was unable to further purify the compound for therapeutic use.31 In the late 1930s, Ernst Chain, Howard Florey and Norman Heatley of the University of Oxford successfully isolated, purified and produced penicillin based on Fleming’s original work.33 They then proceeded to test the compound on mice infected with Streptococcus isolates and found that the compound had a bactericidal effect, publishing their findings in 1940.31,34 This process reflects phase one of the IR continuum, providing a proof of concept for penicillin in a controlled setting, similar to Phase I and II clinical trials known today. In 1941, a local policeman with a severe infection was one of the first human subjects to receive treatment with penicillin.35 While his condition initially improved, it worsened as the limited supply of penicillin ran out. Following this initial demonstration of effect, other patients were subsequently successfully treated with the compound,36 demonstrating the effectiveness of the compound in a clinical setting and representing proof of implementation for penicillin. However, the mass production and use of penicillin remained a challenge. This required scale-up, representing phase three of the IR continuum, for which Florey and Heatley travelled to the USA.31 Together with scientists from the US Department of Agriculture, and later the US government, production methods were quickly improved, expanding penicillin supplies exponentially. By September 1943, the stock of penicillin was sufficient to cover the needs of the Allied Armed Forces.37 The development of penicillin is thus a prime example of the three main phases of the IR continuum: proof of concept, proof of implementation and informing scale-up.
Context domains
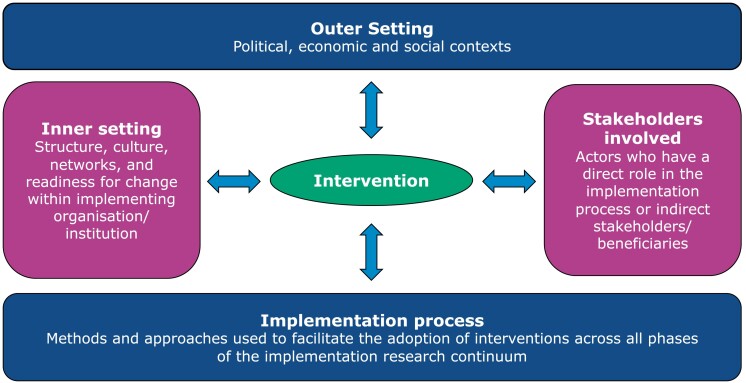
Two key constructs of IR are context and stakeholder inclusion to facilitate implementation and sustainable integration of successful interventions into existing systems. Interventions are implemented within and between four context domains: the outer setting, the inner setting, the stakeholders involved and the implementation process (Figure 2). The economic, political and social contexts in which an intervention is carried out constitute the outer setting, which usually cannot be controlled by the implementing organization/institution/system. The structure, culture, networks and readiness for change within the implementing organization/institution/system is the inner setting. All stakeholders involved in any part of the IR continuum constitute a critical context as their knowledge, attitudes and perceptions to the intervention and its implementation will influence its success and impact. The implementation process is the core context domain and incorporates all the strategies used in facilitating the adaptation and adoption of the intervention across all phases of the continuum, including those explicitly planned as well as the unintended ones that emerge during implementation. The inter-related context domains highlight the complexity of real-life environments requiring an understanding of how to navigate social, economic, political, system and organizational contexts with a diversity of stakeholders at multiple levels.38
Figure 2.
Context domains of IR (adapted from the WHO Implementation Research Toolkit).
The second key construct relates to the deliberate inclusion of all stakeholders that have a direct or indirect role in the implementation process and/or are potential direct/indirect beneficiaries from project inception. They include but are not limited to government ministries, policymakers, administrators, human, animal and environmental health practitioners/providers, patients, farmers and civil society,39 requiring a concurrent top-down and bottom-up approach to IR from inception to scale-up.40
Early engagement and collaboration with stakeholders at all levels is key to implementing and integrating interventions into existing systems, ensuring that contextual considerations are integrated from inception. Stakeholders and researchers co-develop an in-depth understanding of local challenges and bottlenecks, jointly identify relevant research questions and frame interventions within local contexts and available resources, ensuring ownership and commitment to scale-up.39
A project developed by AMR researchers from a Tanzanian university in partnership with the Tanzanian Ministry of Livestock and Fisheries illustrates the importance of context and stakeholder engagement. Given the increasing demand for poultry products in Tanzania, the intensive poultry industry is experiencing steady growth.41,42 Farmers rely on prophylactic and metaphylactic antimicrobials to maintain flock health and increase productivity in the absence of adequate and effective biosecurity and vaccination practices.43,44 The most frequently used antimicrobials are sulphonamides and tetracyclines, with the consequent risk of AMR.45 A project focusing on disease prevention using poultry vaccination and biosecurity interventions (Table S1, available as Supplementary data at JAC-AMR Online) was developed with due consideration of context and stakeholder engagement (Table 1).
Table 1.
Context domains and stakeholders of the Tanzanian intensive poultry industry
| Context | Description |
|---|---|
| Outer setting | Policy context: Government priorities for AMR mitigation are set by the Tanzanian National Action Plan on AMR, which outlined 10 action packages to combat AMR.46 While progress has been made, there remains an important implementation gap in action packages that address the root cause of AMR in agriculture and poultry production. National policies, regulations and guidelines that may influence the poultry production industry include, but are not limited to, the National One Health Strategic Plan,47,48 Tanzania Livestock Master Plan (2017/2018–2021/2022),49 National Livestock Research Agenda 2020–2025,50 National Livestock Policy (2006),51 The Animal Diseases (Hatcheries and Breeding flock farms) Regulations (2019)52 and The Grazing-Land and Animal Feed Resources Act.53 Economic context: The growing demand for poultry meat and eggs as a healthier and cheaper alternative to other meat products has led to growing economic opportunities for the poultry industry.41,42 Policy interventions in Tanzania are also expected to stimulate further growth in the private sector.49 |
| Inner setting | The inner setting is intensive poultry production farms, who are willing to explore strategies to reduce AMU by implementing vaccination and other biosecurity programmes. |
| Stakeholders | Individual actors:
|
| Implementation process |
|
IR strategy
Step one of the IR strategy involves defining the IR challenges with relevant stakeholders in the specific practice/systems context in which an evidence-based intervention is to be implemented, as described under ‘Context Domains’ above. Steps two and three, respectively, involve designing and testing the strategy (Figure 1).
The strategy design identifies actors (stakeholders) both top-down and bottom-up, actions (steps or processes to sustainably implement the intervention), targets (beneficiaries and/or implementers of the intervention) and temporality (chronology of the implementation process). The design also includes the identification of enablers of and barriers to implementation.54 Broad strategy areas include but are not limited to public oversight, organizational provider improvement, individual provider improvement, and household and community empowerment.26
Common research methods are pragmatic trials, effectiveness-implementation hybrid trials, quality improvement studies, participatory action research and mixed methods. Randomized controlled trials (RCTs) typically evaluate the efficacy of an intervention in an ‘ideal’ or controlled setting with narrowly defined inclusion and exclusion criteria and focus on clinical outcomes. Pragmatic or practical trials are RCTs that evaluate the effectiveness of an intervention in the real-world setting with all the relevant stakeholders. Effectiveness-implementation hybrid trials assess the effectiveness of the intervention and implementation strategy in tandem. There are three hybrid research designs: type 1 assesses the effects of an intervention on relevant target or system outcomes while observing and gathering information on implementation in terms of the feasibility and acceptability of the implementation approach through qualitative, process-oriented or mixed-methods study designs; type 2 involves testing of health interventions and implementation strategies equally; and type 3 primarily evaluates the implementation strategy while observing and gathering information on the impact of the intervention on the relevant target or system outcomes.
Quality improvement studies usually take the form of the structured and iterative plan-do-study-act cycle that develops (plan) and implements (do) a plan, as well as analyses and interprets the results (study) to inform next steps (act). Participatory action research (PAR) ensures that implementation occurs with and by the relevant stakeholders at all levels such that stakeholders have power and control over the implementation process. PAR is usually qualitative in nature, but quantitative and mixed-methods techniques are increasingly being used. Mixed methods involve both qualitative and quantitative methods of data collection and analysis in the same study. Mixed methods are particularly suitable for IR because they provide practical ways to understand several perspectives, diverse causal pathways and multiple types of outcomes.26
The strategy is tested against predetermined implementation outcomes such as one or more of acceptability, adaptability, adoption, appropriateness, costs, coverage, feasibility, fidelity (the extent to which an intervention was implemented as described in the IR protocol) and sustainability.26 The IR strategy may additionally be tested against target-level and system/service outcomes. The former may include improvements in health status, behaviour change, a decrease in morbidity or improvement in knowledge, attitudes and practices.55 The latter may include strengthened and/or integrated One Health surveillance systems for AMR and antimicrobial use (AMU), a reduction in AMR, AMU and hospital-acquired infections (HAIs), improved biosecurity, hygiene and sanitation, and human capital development in AMR mitigation (Figure 1). Measuring implementation outcomes improves the understanding of implementation processes, allows comparison of the effectiveness of different implementation strategies and differentiates between intervention failure and implementation failure.29,56 Examples of implementation strategies are illustrated in Table 2 with additional examples in Tables S1 and S2.
Table 2.
Illustrative examples of implementation strategies, adapted from project proposals supported by the International Centre for Antimicrobial Resistance Solutions
| IR strategy concepts | Human health | Animal health | Environmental health |
|---|---|---|---|
| Project title | Facilitating appropriate antibiotic use in respiratory tract infections in children in Kyrgyzstan | Reducing post-weaning diarrhoea and antimicrobial use through improved provision of colostrum and use of vaccines in weaning pigs in Colombia | Mitigating the spread of antimicrobial residues and resistant microbes through the treatment of manure |
| Defining the AMR challenge | |||
|
|
|
|
| Designing the strategy | |||
| Strategy area |
|
|
|
| Research methodology |
|
|
|
| Actors | Individual actors:
|
Individual actors:
|
Individual actors:
|
| Actions |
|
|
|
| Targets |
|
|
|
| Testing the strategy | |||
| IR outcomes |
|
|
|
| System-level outcomes |
|
|
|
| Target-level outcomes |
|
|
|
These examples illustrate the different characteristics of IR research that require researchers and implementers to have a strong understanding of contextual realities at the user and policy levels, highlighting the importance of the outer setting, inner setting, the stakeholders and the actual implementation process, all of which will have an impact on the (un)successful implementation of evidence-based AMR interventions. The examples also highlight that no single aspect of the context exists in isolation, and that successful scale-up requires a bottom-up and top-down approach that is grounded in local realities.
Conclusions
The implementation of AMR mitigation interventions is undoubtedly affected by resource constraints—particularly in LMICs. Implementation is also constrained by the under-recognized lack of technical capacity to adapt and adopt evidence-based AMR mitigation policies, programmes and practices to local country contexts. Incentivizing stakeholders to implement and sustainably integrate evidence-based AMR interventions may be advanced by ‘small tests of change’ in the form of pilot projects where the implementing organization/system has a preview of the outcomes, specifically feasibility and cost-effectiveness, before organization/system-wide implementation or scale-up. Investments in human capital development in IR is critical to ensuring that projects can be adapted to changes in local contexts and sustained in the long term.
IR thus provides a practical framework to address AMR across unique settings. IR highlights the interface between theory and practice, addressing the ‘know-do’ gap. It is context-specific, demand-driven and works at a multidisciplinary level. IR is undertaken in the real world in real time, inclusive of all stakeholders—using research designs and methodologies that are fit for purpose and include both process and outcome indicators.60
Supplementary Material
Acknowledgements
We would like to acknowledge the following individuals for their invaluable efforts during the concept note and/or proposal writing phases: Benard Hangombe, Switihine Kabilika, Bruno Phiri, Chisoni Mumba, Ricky Charzya, Victor Chishimba, Otridah Kapona, Gilbert Nchima, Lê Thị Huệ, Ngo Thi Kim Cuc, Nguyen Anh Phong, Tran Diem Lan, Maamed Mademilov, Azamat Akylbekov and Rune Munck Aabenhus. We would also like to thank the anonymous reviewers for their constructive feedback on the manuscript.
Contributor Information
Mark P Khurana, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark; Section of Epidemiology, Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Sabiha Essack, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark; Antimicrobial Research Unit, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
Ghada Zoubiane, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark.
Nandini Sreenivasan, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark.
Gloria Cristina Cordoba, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark.
Erica Westwood, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark.
Anders Dalsgaard, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark; Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Frederiksberg C, Denmark.
Robinson H Mdegela, Department of Veterinary Medicine and Public Health, Sokoine University of Agriculture, Morogoro, Tanzania.
Mirfin Mpundu, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark; ReAct Africa, Lusaka, Zambia.
Rodrigo Scotini, World Diabetes Foundation, Bagsværd 2880, Denmark.
Augustine B Matondo, Department of Veterinary Medicine and Public Health, Sokoine University of Agriculture, Morogoro, Tanzania.
Alexanda Mzula, Department of Veterinary Medicine and Public Health, Sokoine University of Agriculture, Morogoro, Tanzania.
Nina Chanishvili, George Eliava Institute of Bacteriophage Microbiology and Virology, Gotua Street 3, Tbilisi 0160, Georgia.
Dimitri Gogebashvili, LTD Invet Group, 84a, Vakhushti Bagrationi Street, Tbilisi 0154, Georgia.
Maia Beruashvili, Ministry of Environmental Protection and Agriculture of Georgia, Marshal Gelovani 6, Tbilisi 0159, Georgia; The Faculty of Veterinary Medicine, European University, Tbilisi, Georgia.
Marika Tsereteli, Department of Communicable Diseases, National Center for Disease Control and Public Health, Kakheti Highway 99, Tbilisi 0198, Georgia.
Talant Sooronbaev, National Center of Cardiology and Internal Medicine named after academician M. Mirrakhimov, Togolok Moldo Str, 3, Bishkek 720040, Kyrgyzstan.
Jesper Kjærgaard, Department of Children and Adolescents, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, Copenhagen 2100, Denmark.
Joakim Bloch, Department of Children and Adolescents, Copenhagen University Hospital, Rigshospitalet, Blegdamsvej 9, Copenhagen 2100, Denmark.
Elvira Isaeva, National Center of Maternity and Childhood Care, Akhunbaev Str, 190, Bishkek 720038, Kyrgyzstan; Department of Public Health, The Research Unit for General Practice and Section of General Practice, University of Copenhagen, Øster Farimagsgade 5, Copenhagen 1354, Denmark.
Geoffrey Mainda, Department of Veterinary Services, Ministry of Fisheries and Livestock, PO Box 50060, Lusaka, Zambia.
Geoffrey Muuka, Department of Veterinary Services, Ministry of Fisheries and Livestock, PO Box 50060, Lusaka, Zambia.
Ntombi B Mudenda, School of Veterinary Medicine, University of Zambia, PO Box 32379, Lusaka, Zambia.
Fusya Y Goma, Department of Veterinary Services, Ministry of Fisheries and Livestock, PO Box 50060, Lusaka, Zambia.
Duc-Huy Chu, Department of Animal Health, Ministry of Agriculture and Rural Development, Ha Noi 115-19, Viet Nam.
Duncan Chanda, University Teaching Hospital, Box 17, UTH Post Office, Nationalist Rd., Lusaka, Zambia; Ministry of Health, Ndeke House, Haile Selassie Avenue, PO Box 30205, Lusaka, Zambia.
Uchizi Chirwa, University Teaching Hospital, Box 17, UTH Post Office, Nationalist Rd., Lusaka, Zambia; Ministry of Health, Ndeke House, Haile Selassie Avenue, PO Box 30205, Lusaka, Zambia.
Kaunda Yamba, School of Veterinary Medicine, University of Zambia, PO Box 32379, Lusaka, Zambia; University Teaching Hospital, Box 17, UTH Post Office, Nationalist Rd., Lusaka, Zambia.
Kenneth Kapolowe, University Teaching Hospital, Box 17, UTH Post Office, Nationalist Rd., Lusaka, Zambia.
Sombo Fwoloshi, University Teaching Hospital, Box 17, UTH Post Office, Nationalist Rd., Lusaka, Zambia; Ministry of Health, Ndeke House, Haile Selassie Avenue, PO Box 30205, Lusaka, Zambia.
Lawrence Mwenge, Zambart, Health Economics Unit, Ridgeway, Zambia.
Robert Skov, ICARS, International Centre for Antimicrobial Resistance Solutions, Ørestads Boulevard 5, Copenhagen 2300, Denmark.
Funding
This study was carried out as part of our routine work.
Transparency declarations
R.H.M., A.B.M., A.M., N.C., D.G., M.B., M.T., T.S., J.K., J.B., E.I., G.Ma., G.Mu., N.B.M., F.Y.G., D.H.C., D.C., U.C., K.Y., K.K., S.F. and L.M. have received research funding from the International Centre for Antimicrobial Resistance Solutions (ICARS).
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC-AMR Online.
References
- 1. WHO . Ten threats to global health in 2019. 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 2. WHO . 10 global health issues to track in 2021. 2020. https://www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021.
- 3. Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis 2019; 19: 4–6. 10.1016/S1473-3099(18)30648-0 [DOI] [PubMed] [Google Scholar]
- 4. Naylor NR, Atun R, Zhu Net al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control 2018; 7: 58. 10.1186/s13756-018-0336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect 2014; 20: 973–80. 10.1111/1469-0691.12798 [DOI] [PubMed] [Google Scholar]
- 6. Friedrich MJ. WHO’s top health threats for 2019. JAMA 2019; 321: 1041. 10.1001/jama.2019.1934 [DOI] [PubMed] [Google Scholar]
- 7. Robinson TP, Bu DP, Carrique-Mas Jet al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg 2016; 110: 377–80. 10.1093/trstmh/trw048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laxminarayan R, Duse A, Wattal Cet al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013; 13: 1057–98. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 9. Murray CJ, Ikuta KS, Sharara Fet al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf.
- 11. The World Bank . Drug-resistant infections: a threat to our economic future. 2017. https://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf.
- 12. Strathdee SA, Davies SC, Marcelin JR. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet 2020; 396: 1050–3. 10.1016/S0140-6736(20)32063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murray AK. The novel coronavirus COVID-19 outbreak: global implications for antimicrobial resistance. Front Microbiol 2020; 11: 1020. 10.3389/fmicb.2020.01020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Global Leaders Group on Antimicrobial Resistance . Why AMR must be a substantive element of the international instrument on pandemic prevention, preparedness and response. 2022. https://www.amrleaders.org/resources/why-amr-must-be-a-substantive-element-of-the-international-instrument-on-pandemic-prevention-preparedness-and-response.
- 15. WHO . Global Action Plan on Antimicrobial Resistance. 2015. https://www.who.int/publications/i/item/9789241509763.
- 16. WHO . Global Action Plan on Antimicrobial Resistance. 2016. https://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R7-en.pdf.
- 17. Munkholm L, Rubin O. The global governance of antimicrobial resistance: a cross-country study of alignment between the global action plan and national action plans. Global Health 2020; 16: 109. 10.1186/s12992-020-00639-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United Nations General Assembly . Political declaration of the high-level meeting of the general assembly on antimicrobial resistance. 2016. https://digitallibrary.un.org/record/842813/files/A_71_L-2-EN.pdf.
- 19. Interagency Coordination Group (IACG) on Antimicrobial Resistance . No time to wait: securing the future from drug-resistant infections. 2019. https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf? sfvrsn=5b424d7_6.
- 20. Food and Agriculture Organization of the United Nations, UN Environment Programme, WHO, World Organisation for Animal Health . Global database for tracking antimicrobial resistance (AMR) country self-assessment survey (TrACSS). 2022. https://amrcountryprogress.org/#/map-view.
- 21. WHO, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health . Monitoring Global Progress on Antimicrobial Resistance: Tripartite AMR Country Self-Assessment Survey (TrACSS) 2019–2020: Global Analysis Report. 2021. https://apps.who.int/iris/handle/10665/340236.
- 22. Van Katwyk SR, Hoffman SJ, Mendelson Met al. Strengthening the science of addressing antimicrobial resistance: a framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res Policy Syst 2020; 18: 60. 10.1186/s12961-020-00549-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pokharel S, Raut S, Adhikari B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health 2019; 4: e002104. 10.1136/bmjgh-2019-002104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Editorial . The antimicrobial crisis: enough advocacy, more action. Lancet 2020; 395: 247. 10.1016/S0140-6736(20)30119-7 [DOI] [PubMed] [Google Scholar]
- 25. Ahmad R, Zhu NJ, Leather AJMet al. Strengthening strategic management approaches to address antimicrobial resistance in global human health: a scoping review. BMJ Glob Health 2019; 4: e001730. 10.1136/bmjgh-2019-001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters DH, Adam T, Alonge Oet al. Implementation research: what it is and how to do it. BMJ 2013; 347: f6753. 10.1136/bmj.f6753 [DOI] [PubMed] [Google Scholar]
- 27. Bauer MS, Damschroder L, Hagedorn Het al. An introduction to implementation science for the non-specialist. BMC Psychol 2015; 3: 32. 10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenhalgh T, Robert G, Macfarlane Fet al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004; 82: 581–629. 10.1111/j.0887-378X.2004.00325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proctor E, Silmere H, Raghavan Ret al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011; 38: 65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters DH, Tran NT, Taghreed A. Implementation Research in Health: A Practical Guide. 2013. https://apps.who.int/iris/bitstream/handle/10665/91758/9789241506212_eng.pdf.
- 31. Gaynes R. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis 2017; 23: 849–53. 10.3201/eid2305.161556 [DOI] [Google Scholar]
- 32. Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bull World Health Organ 2001; 79: 780–90. [PMC free article] [PubMed] [Google Scholar]
- 33. Adams J. Antibiotics. Cavendish Square Publishing, LLC, 2017. [Google Scholar]
- 34. Chain E, Florey HW, Gardner ADet al. Penicillin as a chemotherapeutic. Lancet 1940; 236: 226–8. 10.1016/S0140-6736(01)08728-1 [DOI] [Google Scholar]
- 35. Abraham EP, Chain E, Fletcher CMet al. Further observations on penicillin. Lancet 1941; 238: 177–89. 10.1016/S0140-6736(00)72122-2 [DOI] [Google Scholar]
- 36. Wood J. Penicillin: the oxford story. 2010. https://www.ox.ac.uk/news/science-blog/penicillin-oxford-story.
- 37. American Chemical Society. Discovery and Development of Penicillin: International Historic Chemical Landmark. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html.
- 38. WHO . Implementation Research Toolkit. 2014.https://apps.who.int/iris/handle/10665/110523.
- 39. Remme JHF, Adam T, Becerra-Posada Fet al. Defining research to improve health systems. PLoS Med 2010; 7: e1001000. 10.1371/journal.pmed.1001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van de Klundert J, de Korne D, Yuan Set al. ‘Hybrid’ top down bottom up health system innovation in rural China: a qualitative analysis. PLoS One 2020; 15: e0239307. 10.1371/journal.pone.0239307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naggujja J, Njiru N, Msoffe Pet al. Tanzania and Ghana poultry sector policy review. 2020. https://hdl.handle.net/10568/110529.
- 42. Wilson WC, Slingerland M, Oosting Set al. The diversity of smallholder chicken farming in the Southern Highlands of Tanzania reveals a range of underlying production constraints. Poult Sci 2022; 101: 102062. 10.1016/j.psj.2022.102062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mdegela RH, Mwakapeje ER, Rubegwa B, et al. Antimicrobial use, residues, resistance and governance in the food and agriculture sectors, Tanzania. Antibiotics 2021; 10: 454. 10.3390/antibiotics10040454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kimera ZI, Frumence G, Mboera Let al. Assessment of drivers of antimicrobial use and resistance in poultry and domestic pig farming in the Msimbazi River Basin in Tanzania. Antibiotics 2020; 9: 838. 10.3390/antibiotics9120838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nonga HE, Simon C, Karimuribo EDet al. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro Municipality, Tanzania. Zoonoses Public Health 2009; 57: 339–44. 10.1111/j.1863-2378.2008.01226.x [DOI] [PubMed] [Google Scholar]
- 46. WHO, United Republic of Tanzania . United Republic of Tanzania: The National Action Plan on Antimicrobial Resistance 2017–2022. 2017. https://www.flemingfund.org/wp-content/uploads/8b8fc897c422e11504c8c2ba126fac02.pdf.
- 47. Kitua AY, Scribner S, Rasmuson Met al. Building a functional national One Health platform: the case of Tanzania. One Health Outlook 2019; 1: 3. 10.1186/s42522-019-0003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. WHO Africa . Tanzania commits to embrace the One Health approach. 2018. https://www.afro.who.int/news/tanzania-commits-embrace-one-health-approach.
- 49. Michael S, Mbwambo N, Mruttu Het al. Tanzania livestock master plan. 2018. https://faolex.fao.org/docs/pdf/tan185023.pdf.
- 50. The United Republic of Tanzania Ministry of Livestock and Fisheries . National Livestock Research Agenda 2020–2025. 2019. https://www.mifugouvuvi.go.tz/uploads/publications/sw1602244069-NATIONAL%20LIVESTOCK%20RESEACH%20AGENDA%20%202019.pdf.
- 51. The United Republic of Tanzania Ministry of Livestock Development . National Livestock Policy. 2006. https://www.tnrf.org/files/E-INFO_National_Livetock_Policy_Final_as_per_Cabinet_Dec-2006.pdf.
- 52. United Republic of Tanzania Government . The Animal Diseases (Hatcheries and Breeding flock farms) Regulations (2019). 2019. https://www.mifugouvuvi.go.tz/uploads/publications/sw1619688208-Hatcheries%20regulations.pdf.
- 53. United Republic of Tanzania Government . The Grazing-Land and Animal Feed Resources Act. 2021. https://www.mifugouvuvi.go.tz/uploads/publications/sw1626332410-GN.327%20REGISTRATION%20OF%20ANIMAL%20FEED%20RESOURCES%20AND%20PRODUCTS.pdf.
- 54. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013; 8: 139. 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011; 6: 42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Proctor EK, Landsverk J, Aarons Get al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health 2009; 36: 24–34. 10.1007/s10488-008-0197-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Østergaard MS, Kjærgaard J, Kristensen MMet al. Author correction: recurrent lower respiratory illnesses among young children in rural Kyrgyzstan: overuse of antibiotics and possible under-diagnosis of asthma. A qualitative FRESH AIR study. NPJ Prim Care Respir Med 2018; 28: 25. 10.1038/s41533-018-0082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Massé D, Cata Saady NM, Gilbert Y. Potential of biological processes to eliminate antibiotics in livestock manure: an overview. Animals (Basel) 2014; 4: 146–63. 10.3390/ani4020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006; 65: 725–59. 10.1016/j.chemosphere.2006.03.026 [DOI] [PubMed] [Google Scholar]
- 60. Theobald S, Brandes N, Gyapong Met al. Implementation research: new imperatives and opportunities in global health. Lancet 2018; 392: 2214–28. 10.1016/S0140-6736(18)32205-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.