Abstract
Background
Antimicrobial resistance threatens adequate healthcare provision against infectious diseases. Antibiograms, combined with patient clinical history, enable clinicians and pharmacists to select the best empirical treatments prior to culture results.
Objectives
To develop a local antibiogram for the Ho Teaching Hospital.
Methods
This was a retrospective cross-sectional study, using data collected on bacterial isolates from January–December 2021. Samples from urine, stool, sputum, blood, and cerebrospinal fluid (CSF) were considered as well as, aspirates and swabs from wound, ears and vagina of patients. Bacteria were cultured on both enrichment and selective media including blood agar supplemented with 5% sheep blood and MacConkey agar, and identified by both the VITEK 2 system and routine biochemical tests. Data on routine culture and sensitivity tests performed on bacterial isolates from patient samples were retrieved from the hospital’s health information system. Data were then entered into and analysed using WHONET.
Results
In all, 891 pathogenic microorganisms were isolated from 835 patients who had positive culture tests. Gram-negative isolates accounted for about 77% of the total bacterial species. Escherichia coli (246), Pseudomonas spp. (180), Klebsiella spp. (168), Citrobacter spp. (101) and Staphylococcus spp. (78) were the five most isolated pathogens. Most of the bacterial isolates showed high resistance (>70%) to ampicillin, piperacillin, ceftazidime, ceftriaxone, cefotaxime, penicillin G, amoxicillin, amoxicillin/clavulanic acid, ticarcillin/clavulanic acid and trimethoprim/sulfamethoxazole.
Conclusions
The isolates from the various samples were not susceptible to most of the antibiotics used in the study. The study reveals the resistance patterns of E. coli and Klebsiella spp. to some antibiotics on the WHO ‘Watch’ and ‘Reserve’ lists. Using antibiograms as part of antimicrobial stewardship programmes would optimize antibiotic use and preserve their efficacy.
Introduction
Antimicrobial resistance (AMR) is a global problem with many causes including inappropriate prescribing of antimicrobials.1,2 A key strategy to address AMR is by employing a targeted approach to treatment, to reduce indiscriminate prescribing, thereby conserving the efficacy of antimicrobials. This presents a challenge, especially in the developing world due to limited availability of efficient diagnostic measures, making it difficult to know the true burden of AMR.3 In Ghana, antimicrobial therapy constitutes a major form of treatment in all healthcare facilities.4,5 However, treatment is mainly empirical due to a relative lack of appropriate laboratory and diagnostic facilities for culture and sensitivity testing of bacteria in most healthcare facilities. Even where laboratory facilities are available, culture and sensitivity tests performed present additional medical costs to the patient and may often not be recommended.6
An antibiogram, a periodic summary of antimicrobial susceptibilities of bacterial isolates submitted by a hospital’s clinical microbiology laboratory, can serve as the primary source of validated data to be used by clinicians to assess local antimicrobial susceptibility patterns of pathogens and guide empirical therapy or selection of antimicrobials.7–9 Local, regional and national antibiogram data generated from healthcare facilities are key in the monitoring of trends in AMR and guiding the selection of effective antibiotics for empirical therapy.10,11 The development of local hospital antibiograms can therefore serve as the foundation for AMR surveillance, clinical decision support for rational antimicrobial use, and identify areas for intervention by antimicrobial stewardship (AMS) programmes.12
Data on the AMR profiles of clinically relevant pathogenic bacterial isolates like Neisseria gonorrhoeae and Shigella spp. have been reported from some hospitals in Ghana.13,14 However, there are limited comprehensive institutional data on susceptibility patterns of common pathogens for most hospitals in Ghana and sub-Saharan Africa.3,15 Furthermore, there are no documented data on the bacterial isolates and antibiotic resistance profiles at the Ho Teaching Hospital (HTH). This could lead to the irrational selection of antimicrobials for empirical therapy, which will further compound the problem of AMR within the facility. The objective of this study was therefore to develop a local antibiogram for HTH through retrospective analysis of laboratory data.
Methods
Study design and study site
This was a 12–month retrospective cross-sectional study conducted in HTH, a tertiary healthcare facility located in Ho, Ghana. The hospital has a bed capacity of about 300 and 14 wards. It is the main referral facility in the Volta Region and has five clinical departments, namely, internal medicine, surgical, obstetrics & gynaecology, child health and public health. The facility also has a microbiology laboratory and an AMS committee.
Laboratory techniques
As part of routine care and practice, culture and susceptibility tests were performed on bacterial isolates from urine, stool, sputum and blood samples obtained from both outpatients and inpatients who visited the hospital within the study period. Swabs from wound, ear and vagina of patients were also subjected to these tests. Specimens that had been collected, processed and analysed in the microbiology unit of the HTH employing HTH guidelines for culture and microbial identification were considered. Bacteria were cultured on both enrichment and selective media including blood agar supplemented with 5% sheep blood for Gram-positive cocci and MacConkey agar for Gram-negative bacilli. Isolates were identified by both the VITEK 2 system and routine biochemical tests including catalase and coagulase tests for Gram-positive cocci. Antimicrobial susceptibility was performed using the Kirby–Bauer disc diffusion technique following CLSI 2021 (31st edition) standards.
All the culture-positive samples were included in the study, and repeat isolates from the same person were excluded in order to avoid duplication. The bacterial isolates identified in these samples and their sensitivity or resistance to antimicrobials were recorded in the hospital’s information management system, Lightwave Health Information Management System (LHIMS), as susceptible, intermediate or resistant.
Data collection and analysis
Routinely collected data on all isolates reported on the LHIMS from January 2021 to December 2021 were extracted/entered and organized into a Microsoft Excel 2022 datasheet. This was then exported and analysed using WHONET (version 5.6), a Windows-based database software package for the management of microbiology laboratory data and the analysis of antimicrobial susceptibility test results.
Antibiogram development
The aggregated data from WHONET produced susceptibility percentages for every organism. Organisms with fewer than 30 isolates were initially excluded, given the potential for diminished accuracy. The list was then reviewed to include other clinically important pathogenic microorganisms, ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp.) that did not have 30 isolates.16 The antibiotics included in the antibiogram were narrowed to antibiotics in the WHO Watch and Reserve categories that were tested in the hospital.17
Ethics
Ethical clearance (UHAS-REC A.5[3]21-22) was obtained from the Research Ethics Committee of the University of Health and Allied Sciences, Ho. Permission was also sought from the hospital to carry out the research.
Results
A total of 891 pathogenic microorganisms were isolated from 835 patient samples with positive culture tests (Table 1). Gram-negative isolates accounted for about 77% of the total bacterial species. Escherichia coli (246), Pseudomonas spp. (180), Klebsiella spp. (168), Citrobacter spp. (101) and Staphylococcus spp. (78) were the top five commonly isolated pathogens. Of the total Pseudomonas spp. isolated, 114 were identified to be P. aeruginosa while 54 Klebsiella oxytoca and 13 K. pneumoniae isolates were identified out of the total Klebsiella spp. isolated. 34 Citrobacter koseri isolates and 56 S. aureus isolates were identified from the total Citrobacter spp. and Staphylococcus spp., respectively.
Table 1.
Prevalence of isolates per microorganism
| Microorganism | Number of isolates (n) | Percentage (%) |
|---|---|---|
| E. coli | 246 | 27.61 |
| Pseudomonas spp. | 180 | 20.20 |
| Klebsiella spp. | 168 | 18.86 |
| Citrobacter spp. | 101 | 11.34 |
| Staphylococcus spp. | 78 | 8.75 |
| Enterobacter spp. | 21 | 2.36 |
| Acinetobacter spp. | 20 | 2.24 |
| Enterococcus spp. | 15 | 1.68 |
| Proteus vulgaris | 13 | 1.46 |
| P. mirabilis | 12 | 1.35 |
| Providencia spp. | 10 | 1.12 |
| Serratia marcescens | 7 | 0.79 |
| Morganella morganii ssp. morganii | 5 | 0.56 |
| Moraxella catarrhalis | 4 | 0.45 |
| Salmonella spp. | 4 | 0.45 |
| Shigella spp. | 3 | 0.34 |
| Francisella tularensis ssp. tularensis | 1 | 0.11 |
| Micrococcus luteus | 1 | 0.11 |
| Neisseria meningitidis | 1 | 0.11 |
| Streptococcus pyogenes | 1 | 0.11 |
Urinary tract infections (UTIs; 477), wound (184) and ear (115) infections (Table S1) were the most commonly reported during the study period. E. coli (204) and Klebsiella spp. (62) were responsible for most of the UTIs while Pseudomonas spp. (77) accounted for most of the ear infections. For the wound infections, however, there was a near equal number of isolates for E. coli (26) and S. aureus (23) while 46 Pseudomonas spp. were isolated. A complex diversity of microbial pathogens was associated with urine (21), wound (21), ear (16), blood (15) and vaginal (13) samples obtained from patients (Table S1).
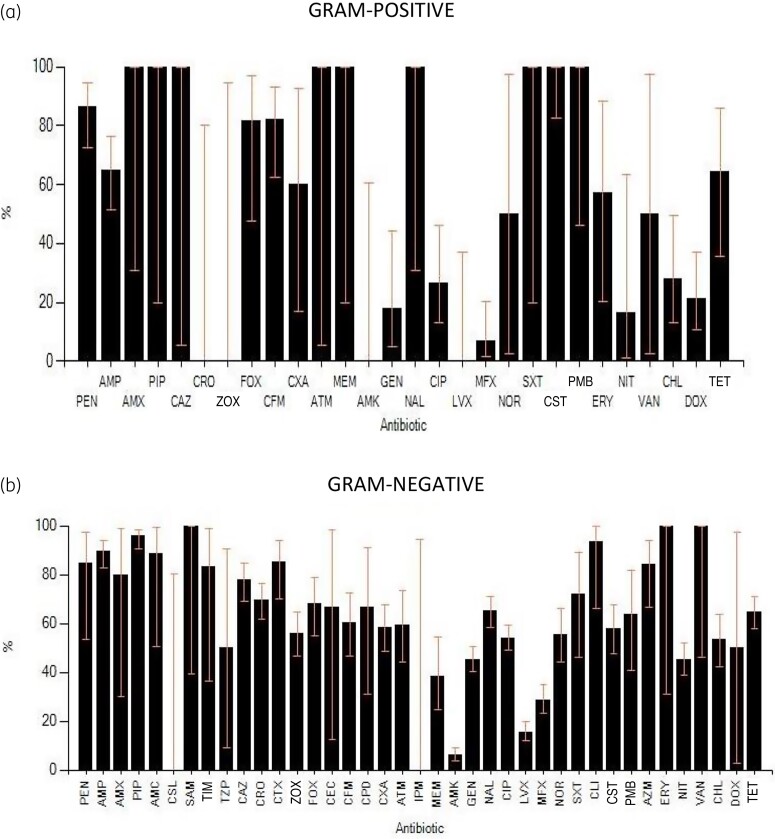
The resistance of bacterial isolates to the antibiotics tested were expressed as percentages (Figure 1). A total of 44 different antibiotics were tested. However, not all the antibiotics were tested on all the microbes by the Infectious Diseases Laboratory at HTH. This decision was informed mainly by the availability of antibiotic discs and the inventory of antibiotics at the hospital. The bacterial isolates showed high resistance (>70%) to ampicillin, piperacillin, ceftazidime, ceftriaxone, cefotaxime, penicillin G, amoxicillin, co-amoxiclav, ampicillin/sulbactam, ticarcillin/clavulanic acid, cefpirome and trimethoprim/sulfamethoxazole. With the exception of ampicillin, piperacillin, ceftazidime, and ceftriaxone, which were tested against more than 100 isolates, all other antibiotics were tested against less than 20 isolates and cefotaxime was tested against 32 isolates. Inference was therefore made considering the number of isolates tested against an antibiotic of interest. Amikacin, levofloxacin and moxifloxacin showed the highest activity against all bacterial isolates.
Figure 1.
Resistance pattern of (a) Gram-positive and (b) Gram-negative bacteria. AMC, amoxicillin/clavulanic acid, 20 μg; AMK, amikacin 30 μg; AMP, ampicillin 10 μg; AMX, amoxicillin 30 μg; ATM, aztreonam 30 μg; AZM, azithromycin 15 μg; CAZ, ceftazidime 30 μg; CEC, cefaclor 30 μg; CFM, cefixime 5 μg; CHL, chloramphenicol 30 μg; CIP, ciprofloxacin 5 μg; CLI, clindamycin 2 μg; CST, colistin 10 μg; CPD, cefpodoxime 10 μg; CPO, cefpirome 30 μg; CRO, ceftriaxone 30 μg; CSL, cefoperazone/sulbactam 30 μg; CTX, cefotaxime 30 μg; CXM, cefuroxime 30 μg; ZOX, ceftizoxime 30 μg; DOX, doxycycline 30 μg; ERY, erythromycin 15 μg; FOX, cefoxitin 30 μg; GEN, gentamicin 10 μg; IPM, imipenem 10 μg; LVX, levofloxacin 5 μg; MEM, meropenem 10 μg; MFX, moxifloxacin 5 μg; NAL, nalidixic acid 30 μg; NIT, nitrofurantoin 300 μg; NOR, norfloxacin 10 μg; PEN, penicillin G 10 μg; PIP, piperacillin 100 μg; PMB, polymyxin B 300 μg; SAM, ampicillin/sulbactam 10 μg; SXT, trimethoprim/sulfamethoxazole 1.2 μg; TCC, ticarcillin/clavulanic acid 75 μg; TET, tetracycline 30 μg; TMP, trimethoprim 5μg; TZP, piperacillin/tazobactam 100 μg; VAN, vancomycin 30 μg.
An antibiogram was generated using the percentage susceptibility of the pathogens indicated in Table 1. The susceptibility of these organisms to selected antibiotics on the WHO Watch and Reserve list are shown in Table S2. Microorganisms with susceptibility greater than 70% for a specific test antibiotic were regarded as highly susceptible while those showing susceptibilities between 40% and 69%, and less than 40% were regarded as intermediate and resistant, respectively.
E. coli, the most abundant Gram-negative isolate, showed very poor susceptibility to piperacillin, piperacillin/tazobactam, ceftriaxone, ceftazidime, ceftizoxime, cefotaxime, aztreonam and ciprofloxacin. However, levofloxacin showed a markedly pronounced activity (78%) against most E. coli isolates. Pseudomonas spp. and P. aeruginosa were susceptible to azithromycin (100% and 83%, respectively) and moxifloxacin (73% and 86%, respectively). S. aureus and Enterococcus spp. were more susceptible to levofloxacin (100% for both microbes) and moxifloxacin (82% and 80%, respectively). Generally, piperacillin, ceftriaxone, cefuroxime, ceftazidime, ceftizoxime, cefixime, cefoxitin, cefpodoxime, cefpirome, cefotaxime, cefaclor and aztreonam showed very poor activity against all the microbial isolates tested.
Discussion
The use of antibiograms to guide the selection of empirical antibiotic therapy for a suspected microbial infection is a well-established practice.9,15,18 To the best of our knowledge, this is the first comprehensive study that describes antibiogram data in HTH. Out of the total pathogenic isolates, E. coli (246), Pseudomonas spp. (180) and Klebsiella spp. (168) were the most commonly isolated microbes from routine tests conducted in HTH from the different patient samples during the study period. Other pathogens like Citrobacter spp. and S. aureus were isolated more than 30 times. Most of these pathogenic microbes were obtained as a result of UTIs (468) and wound (182) and ear (115) infections. UTIs and wounds have been reported to be among the commonest sources typically presenting with pathogens.19–21
The highly diversified nature of microbial pathogens associated with urine, vagina, wound, ear and blood samples suggests a polymicrobial complexity to associated bacteraemia, UTIs, and wound and ear infections. The high number of cultures showing E. coli and Klebsiella spp. did not come as surprise due to the equally high number of UTIs reported during the period. Out of the 477 UTIs, E. coli and Klebsiella spp. were isolated in 204 and 106 instances, respectively. These findings were similar to other reports from referral hospitals, where a high prevalence of these two pathogens were obtained for UTIs.22–24E. coli is known to be the commonest cause of UTI, with other Enterobacteriaceae like Klebsiella spp. also implicated in most of these infections.20,25
Wound infections, the second highest source, had E. coli (26), Pseudomonas spp. (46) and S. aureus (23) isolated often (Table S1). Globally, bacterial infections of wounds are among the leading causes of morbidity and mortality and are regarded as one of the commonest nosocomial infections.26S. aureus, P. aeruginosa and other coliforms (23%) have been reported to be predominant in acute wounds, while chronic wounds usually had Proteus mirabilis, Enterococcus spp. and E. coli.21,23,27 In this work, the nature of the wound was not taken into consideration during data extraction.
The prevalence of ear infections has been reported to be on the rise in developing countries, with bacteria being major causes of these. Although primarily a disease of infants and young children, adults can also be affected by ear infections.28 Complications like meningitis and brain abscess could arise if infection is not properly managed as a result of the causative organisms being resistant to treatment.29 In this study Pseudomonas spp. were responsible for almost 70% of ear infections; S. aureus was identified in about 10% of the cases. Similar findings have been reported where S. aureus and P. aeruginosa were predominant causes of ear infections.29,30 Generally, ear infections are mainly caused by microbes found on the skin of the external ear that gain access to the middle ear through perforation.28
Antibiotic susceptibility testing of the pathogens to the different antibiotics revealed percentage resistance above 70% for most of the antibiotics tested. Taking into consideration the total number of samples tested per antibiotic, ampicillin, piperacillin, ceftriaxone and ceftazidime showed the least activity against the pathogens. It is interesting to note that all these are β-lactam antibiotics, indicating the need to intensify stewardship activities in this regard. Globally, the increase in acquired resistance to β-lactams and ESBL-producing bacteria is one of great concern.21
Further emphasis was placed on obtaining the resistance profile of the commonly isolated organisms (more than 30 isolates) as well as commonly isolated clinically relevant microbes (i.e. other microbes of the ESKAPE group with less than 30 isolates). Other ESKAPE pathogens were considered since these microbes are considered the six most common MDR pathogens globally.16 All these pathogens were Gram-negative organisms except S. aureus and Enterococcus spp.
The epidemiologically significant Gram-negative pathogens indicated high resistance to most of the β-lactams tested (piperacillin, ceftriaxone, cefuroxime, ceftazidime and ceftizoxime) . Only Acinetobacter spp. were susceptible to ceftriaxone, but there was just one isolate; hence it was difficult to draw any meaningful inference. The high resistance of the Gram-negative isolates, particularly in the Enterobacteriaceae genera, to the third-generation cephalosporins suggests notable alert levels of possible circulating ESBL-producing Enterobacteriaceae in the tertiary care facility (Figure 1). Resistance of Gram-negative rods to these β-lactam antibiotics is a recent phenomenon that has been reported.15,20,31–33 Studies from sub-Saharan Africa reveal high rates of ESBL production or resistance, especially to third-generation cephalosporins.22,23,31,34,35 Similarly, a study done at Korle-Bu Teaching Hospital, Ghana found high levels of ESBL-producing enterobacteria as a significant cause of infections and resistance at the hospital.36
Although available literature supports low susceptibility of E. coli and K. pneumoniae to cephalosporins,22,37 these alarming findings are worthy of intensive stewardship activities. A noteworthy observation was the high resistance by the Gram-negative rods to ceftazidime, a drug on the WHO Watch list. E. coli for instance had a resistance of 75% to ceftriaxone, an antibiotic also on the WHO Watch list. Third-generation cephalosporins are commonly used antibiotics in UTIs; however, with the growing high resistance to these antibiotics, prudent use of these may be needed.38,39 Resistance of Pseudomonas spp. to ceftazidime and other third-generation cephalosporins has also been reported at other tertiary healthcare facilities in Rwanda and Tanzania.23,32
Colistin, the most tested polymyxin, had resistance developed to it, especially by S. aureus and P. aeruginosa. (Table S3, available as Supplementary data at JAC-AMR Online). As one of the antibiotics on the WHO Reserve list, these microbes exhibiting high resistance to this antibiotic are a great concern.17 A recent study in Egypt found colistin to be one of their most effective antibiotics, with a susceptibility of at least 90% for Gram-negative rods like E. coli, Klebsiella spp. and A. baumannii. Interestingly, they reported 79.4% susceptibility for P. aeruginosa in their work.37 This indicates that this drug is still an effective treatment option in Africa and efforts to ensure its continued efficacy in HTH facility is warranted.
The quinolones frequently tested during the period were ciprofloxacin, levofloxacin and moxifloxacin. Levofloxacin showed good activity against the pathogens indicated. Moxifloxacin was also effective in most of the microbes with the exception of C. koseri (25%). Ciprofloxacin also had good susceptibility except for C. koseri, E. coli and K. oxytoca, where less than 40% susceptibility was obtained. Increasing resistance to fluoroquinolones has also been reported, especially in E. coli and K. pneumoniae.15,32 From our work, the quinolones remain the class of antibiotic with high efficacy against pathogens in the facility, and effort needs to be put in place to protect these.
A limitation in our study was the fact that the number of isolates of some pathogens or antibiotics tested on some isolates was small. Such organisms were not commented on, even though these could also be pathogens or antibiotics that awareness and stewardship activities should target.
Conclusions
The general antibiotic susceptibility pattern of the study isolates shows an overall high drug resistance to many routinely tested antibiotics. Resistance patterns obtained from the data revealed a trend of some antibiotics on the WHO Watch and Reserve lists gradually losing efficacy towards some of the commonly isolated pathogens, especially E. coli and Klebsiella spp. These findings thus emphasize the need for a robust AMS programme that can implement interventions to improve antibiotic use and preserve the efficacy of antibiotics.
Based on the findings of this study, ceftazidime, ceftriaxone and colistin should be key targets of AMS in HTH. Considering these antibiotics are on the WHO Watch and Reserve lists of antibiotics, they should be used with caution or as last-resort options.
Supplementary Material
Contributor Information
Cornelius C Dodoo, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Hayford Odoi, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Adelaide Mensah, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Karikari Asafo-Adjei, Laboratory Department, Ho Teaching Hospital, Ho, Ghana.
Ruth Ampomah, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Lydia Obeng, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Jonathan Jato, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Araba Hutton-Nyameaye, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Thelma A Aku, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Samuel O Somuah, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Emmanuel Sarkodie, Pharmacy Department, Kwame Nkrumah University of Science and Technology Teaching Hospital, Kumasi, Ghana.
Emmanuel Orman, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Kwadwo A Mfoafo, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Inemesit O Ben, School of Pharmacy, University of Health and Allied Sciences, Ho, Ghana.
Eneyi E Kpokiri, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK.
Fatima Abba, Health Protection Operations Division, UK Health Security Agency, London, UK; Centre for Medicines Optimisation Research and Education, University College London Hospitals NHS Trust & UCL School of Pharmacy, London, UK.
Yogini H Jani, Centre for Medicines Optimisation Research and Education, University College London Hospitals NHS Trust & UCL School of Pharmacy, London, UK.
Funding
This project was funded through the Commonwealth Partnerships for Antimicrobial Stewardship Scheme, which is funded by the UK Department of Health and Social Care through the Fleming Fund (grant number CwE.B02).
Transparency declarations
None to declare.
Supplementary data
Tables S1–S3 are available as Supplementary data at JAC-AMR Online.
References
- 1. Manga MM, Mohammed Y, Suleiman Set al. Antibiotic prescribing habits among primary healthcare workers in Northern Nigeria: a concern for patient safety in the era of global antimicrobial resistance. PAMJ 2021; 5: 19. 10.11604/pamj-oh.2021.5.19.30847 [DOI] [Google Scholar]
- 2. Collignon P, Beggs JJ. Socioeconomic enablers for contagion: factors impelling the antimicrobial resistance epidemic. Antibiotics (Basel) 2019; 8: 86. https://doi.org/ 10.3390/antibiotics8030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray CJL, Ikuta KS, Sharara Fet al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodoo CC, Orman E, Alalbila Tet al. Antimicrobial prescription pattern in Ho Teaching Hospital, Ghana: seasonal determination using a point prevalence survey. Antibiotics (Basel) 2021; 10; 199. 10.3390/antibiotics10020199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman MJ, Frimpong E, Donkor ESet al. Resistance to antimicrobial drugs in Ghana. Infect Drug Resist 2011; 4: 215. https://doi.org/ 10.2147/IDR.S21769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Vello P, Gonzalez-Zorn B, Setsoafia Saba CK. Antibiotic resistance patterns in human, animal, food and environmental isolates in Ghana: a review. Pan Afr Med J 2020; 35: 37. 10.11604/pamj.2020.35.37.18323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Pharmacotherapy 2007; 27: 1306–12. 10.1592/phco.27.9.1306 [DOI] [PubMed] [Google Scholar]
- 8. Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007; 44: 867–73. 10.1086/511864 [DOI] [PubMed] [Google Scholar]
- 9. Joy SC, Sunny A, Nair MRet al. Antibiogram and antimicrobial susceptibility pattern of bacterial isolates from a tertiary care hospital in Kerala. J Evolution Med Dental Sci 2020; 9: 3787–93. 10.14260/jemds/2020/831 [DOI] [Google Scholar]
- 10. WHO . The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2019. https://apps.who.int/iris/handle/10665/327957.
- 11. Guarascio AJ, Brickett LM, Porter TJet al. Development of a statewide antibiogram to assess regional trends in antibiotic-resistant ESKAPE organisms. J Pharm Pract 2019; 32: 19–27. 10.1177/0897190017735425 [DOI] [PubMed] [Google Scholar]
- 12. Kaye KS, Pogue JM. Infections caused by resistant gram-negative bacteria: epidemiology and management. Pharmacotherapy 2015; 35: 949–62. 10.1002/phar.1636 [DOI] [PubMed] [Google Scholar]
- 13. Attram N, Agbodzi B, Dela Het al. Antimicrobial resistance (AMR) and molecular characterization of Neisseria gonorrhoeae in Ghana, 2012–2015. PLoS One 2019; 14: e0223598. 10.1371/journal.pone.0223598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Opintan JA, Newman MJ. Distribution of serogroups and serotypes of multiple drug resistant Shigella isolates. Ghana Med J 2007; 41: 8–29. [PMC free article] [PubMed] [Google Scholar]
- 15. Roth BM, Laps A, Yamba Ket al. Antibiogram development in the setting of a high frequency of multi-drug resistant organisms at University Teaching Hospital, Lusaka, Zambia. Antibiotics (Basel) 2021; 10: 782. 10.3390/antibiotics10070782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zilahi G, Artigas A, Martin-Loeches I. What’s new in multidrug-resistant pathogens in the ICU? Ann Intensive Care 2016; 6: 96. 10.1186/s13613-016-0199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO . 2021 AWaRe classification. 2021. https://www.who.int/publications/i/item/2021-aware-classification.
- 18. Leeman HM, Chan BP, Zimmermann CRet al. Creation of state antibiogram and subsequent launch of public health-coordinated antibiotic stewardship in New Hampshire: small state, big collaboration. Public Health Rep 2022; 137: 72–80. 10.1177/0033354921995778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chanda W, Manyepa M, Chikwanda Eet al. Evaluation of antibiotic susceptibility patterns of pathogens isolated from routine laboratory specimens at Ndola teaching hospital: a retrospective study. PLoS One 2019; 14: e0226676. 10.1371/journal.pone.0226676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melaku S, Kibret M, Abera Bet al. Antibiogram of nosocomial urinary tract infections in Felege Hiwot referral hospital, Ethiopia. Afr Health Sci 2012; 12: 134–9. 10.4314/ahs.v12i2.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kassam NA, Damian DJ, Kajeguka Det al. Spectrum and antibiogram of bacteria isolated from patients presenting with infected wounds in a tertiary hospital, northern Tanzania. BMC Res Notes 2017; 10: 757. 10.1186/s13104-017-3092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maina D, Omuse G, Revathi Get al. Spectrum of microbial diseases and resistance patterns at a private teaching hospital in Kenya: implications for clinical practice. PLoS One 2016; 11: e0147659. 10.1371/journal.pone.0147659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moremi N, Claus H, Mshana SE. Antimicrobial resistance pattern: a report of microbiological cultures at a tertiary hospital in Tanzania. BMC Infect Dis 2016; 16: 756. 10.1186/s12879-016-2082-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biadglegne F, Abera B. Antimicrobial resistance of bacterial isolates from urinary tract infections at Felge Hiwot Referral Hospital, Ethiopia. Ethiop J Health Dev 2009; 23: 236–8. https://doi.org/ 10.4314/ejhd.v23i3.53248 [DOI] [Google Scholar]
- 25. Koripella R, Kanakadurgamba T, Vasanthi Ket al. Bacteriological profile & antibiogram of urinary tract infections in a tertiary care hospital. Int J Res Rev 2020; 7: 429–33. [Google Scholar]
- 26. WHO . Prevention of hospital-acquired infections: a practical guide. 2002. https://apps.who.int/iris/handle/10665/67350.
- 27. Mohammed A, Adeshina GO, Ibrahim YK. Incidence and antibiotic susceptibility pattern of bacterial isolates from wound infections in a tertiary hospital in Nigeria. Trop J Pharmaceut Res 2013; 12: 617–21 . https://doi.org/ 10.4314/tjpr.v12i4.26 [DOI] [Google Scholar]
- 28. Muluye D, Wondimeneh Y, Ferede Get al. Bacterial isolates and drug susceptibility patterns of ear discharge from patients with ear infection at Gondar University Hospital, Northwest Ethiopia. BMC Ear Nose Throat Disord 2013; 13: 10. 10.1186/1472-6815-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hailu D, Mekonnen D, Derbie Aet al. Pathogenic bacteria profile and antimicrobial susceptibility patterns of ear infection at Bahir Dar Regional Health Research Laboratory Center, Ethiopia. SpringerPlus 2016; 5: 466. 10.1186/s40064-016-2123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Javed M. Pathogenic bacteria profile and antimicrobial susceptibility patterns of ear infection at Ayub Medical Complex Abbottabad, Pakistan. Pure Appl Biol 2020; 9: 714–9. 10.19045/bspab.2020.90077 [DOI] [Google Scholar]
- 31. Kumburu HH, Sonda T, Mmbaga BTet al. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Health 2017; 22: 454–64. 10.1111/tmi.12836 [DOI] [PubMed] [Google Scholar]
- 32. Ntirenganya C, Manzi O, Muvunyi CMet al. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg 2015; 92: 865–70. 10.4269/ajtmh.14-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wekesa YN, Namusoke F, Sekikubo Met al. Ceftriaxone- and ceftazidime-resistant Klebsiella species, Escherichia coli, and methicillin-resistant Staphylococcus aureus dominate caesarean surgical site infections at Mulago Hospital, Kampala, Uganda. SAGE Open Med 2020; 8: 2050312120970719. 10.1177/2050312120970719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carroll M, Rangaiahagari A, Musabeyezu Eet al. Five-year antimicrobial susceptibility trends among bacterial isolates from a tertiary health-care facility in Kigali, Rwanda. Am J Trop Med Hyg 2016; 95: 1277–83. 10.4269/ajtmh.16-0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obeng-Nkrumah N, Labi AK, Addison NOet al. Trends in paediatric and adult bloodstream infections at a Ghanaian referral hospital: a retrospective study. Ann Clin Microbiol Antimicrob 2016; 15: 49. 10.1186/s12941-016-0163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obeng-Nkrumah N, Twum-Danso K, Krogfelt KAet al. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg 2013; 89: 960–4. 10.4269/ajtmh.12-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Negm EM, Mowafy SMS, Mohammed AAet al. Antibiograms of intensive care units at an Egyptian tertiary care hospital. Egyptian J Bronchol 2021; 15: 15. 10.1186/s43168-021-00059-w [DOI] [Google Scholar]
- 38. Obeng-Nkrumah N, Labi AK, Acquah MEet al. Bloodstream infections in patients with malignancies: implications for antibiotic treatment in a Ghanaian tertiary setting. BMC Res Notes 2015; 8: 742. 10.1186/s13104-015-1701-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang SS, Ratliff PD, Judd WR. Retrospective review of ceftriaxone versus levofloxacin for treatment of E. coli urinary tract infections. Int J Clin Pharm 2018; 40: 143–9. 10.1007/s11096-017-0560-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.