Significance
In the European Alps, ongoing global change is causing contractions of the elevational range of native plants. In particular, red-listed plants are retracting their lower elevation limit much faster than common ones, posing further concerns on their conservation. These range contractions are the result of the erosion of rear margin populations in the hotter and more disturbed lowlands. While most native plants were not able to track climate warming at the leading edge of their distribution, aliens quickly expanded their range upslope probably due to a combination of temperature warming and disturbance. Our results call attention to an urgent biodiversity crisis in low-elevation areas of temperate mountains where rare native plants are being threatened, while alien plants are favored.
Keywords: alien invasion, climate change, range shift, rarity, land-use change
Abstract
Mountain ecosystems are exposed to multiple anthropogenic pressures that are reshaping the distribution of plant populations. Range dynamics of mountain plants exhibit large variability with species expanding, shifting, or shrinking their elevational range. Using a dataset of more than 1 million records of common and red-listed native and alien plants, we could reconstruct range dynamics of 1,479 species of the European Alps over the last 30 y. Red-listed species were not able to track climate warming at the leading edge of their distribution, and further experienced a strong erosion of rear margins, resulting in an overall rapid range contraction. Common natives also contracted their range, albeit less drastically, through faster upslope shift at the rear than at the leading edge. By contrast, aliens quickly expanded upslope by moving their leading edge at macroclimate change speed, while keeping their rear margins almost still. Most red-listed natives and the large majority of aliens were warm-adapted, but only aliens showed high competitive abilities to thrive under high-resource and disturbed environments. Rapid upward shifts of the rear edge of natives were probably driven by multiple environmental pressures including climate change as well as land-use change and intensification. The high environmental pressure that populations encounter in the lowlands might constrain the ability of expanding species to shift their range into more natural areas at higher elevations. As red-listed natives and aliens mostly co-occurred in the lowlands, where human pressures are at their highest, conservation should prioritize low-elevation areas of the European Alps.
Mountain ecosystems are exposed to multiple anthropogenic pressures that are reshaping the distribution and abundance of plant populations (1, 2). A large body of research has focused on climate warming effects, showing that warm-adapted species have rapidly shifted their elevational range upward, whereas species inhabiting high elevations have often contracted their range and decreased their population size (3, 4). At the same time, current land use changes are expected to reduce habitat availability and connectivity, constraining plant ability to track climate change and threatening, in particular, rare and specialist species (2, 3, 5, 6). By contrast, a growing contingent of alien plants is spreading from the lowlands to higher elevations due to the combination of temperature warming and increased habitat disturbances (1, 3, 7–10). Hence, depending on their origin, competition ability and specialization, mountain plants are expected to exhibit large variability in their response to global change: expanding, shifting or shrinking their elevational range (11).
Despite a growing body of empirical research on plant redistribution dynamics, there has been no attempt to compare simultaneously range shifts of common native, rare native, and alien species along complete elevational gradients. This might be due to methodological constraints of the previous studies that have mainly captured regionally common species while rare species are often underrepresented due to their small population size (4). However, rare and/or declining plant species are expected to be disproportionately affected by global change, because they are more susceptible to a changing climate (12), as well as being more vulnerable to habitat change and anthropic disturbance (13). In addition, most previous research has focused on high-elevation areas and climate change effects, while disturbed low-elevation areas, where human pressures are at their highest, have often been disregarded (14–17). These limitations constrain our ability to evaluate the compounded effect of climate and land-use pressures on a large pool of plant species and, hence, to implement effective conservation measures.
Here, using a unique dataset consisting of over 1 million plant records sampled along a complete elevational gradient from the foothills to the snow line throughout NE Italy, we could reconstruct the range shift dynamics of 1,479 plant species of the European Alps. Our long-term, high-resolution dataset allowed us to consider a large number of native species, including also rare native species, and alien species. First, we tested whether the elevational range of red-listed species shrank, through local extinction at the rear margin, faster than common natives. Species that are included in the International Union for Conservation of Nature (IUCN) Red Lists are mostly rare and/or declining species, with narrow ecological niches and small geographical ranges, and are expected to face the highest risk of extinction (13, 18–20). Considering the increasing spread of alien plants in temperate mountains and the associated potential risks, we also compared range shifts between natives and aliens, assuming that alien species will shift upward faster than natives (9). Second, we used data related to species ecological strategy and temperature preference, to gain indirect insights into the relative effects of climate warming and land use on range shift dynamics. Third, using a hot spot analysis of spatially explicit occurrence data, we could identify high-priority areas for implementing conservation and assess the potential risks posed by the co-occurrence of red-listed and alien species.
Results and Discussion
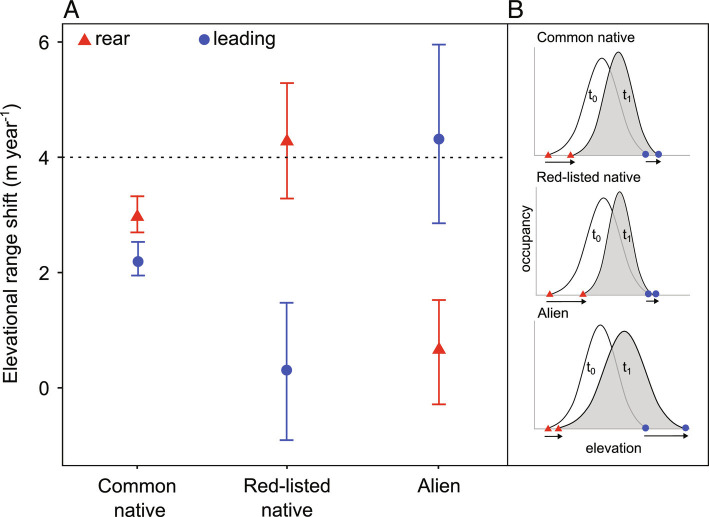
We found that over the last 30 y (1990 to 2019), common native species (i.e., native plant species not included in the IUCN Red list) have shifted upward at both the rear and the leading edge. However, they shifted faster at the rear than at the leading edge, causing an overall range contraction (Fig. 1). Upward shifts were highly consistent for most common species, showing a lower interspecific variability than expected (3, 4). The large majority of plant species moved their elevation range upward on average by 2.9 ± 0.16 m y−1 (mean ± SEM) at the rear edge, and 2.2 m ± 0.17 y−1 (mean ± SEM) at the leading edge according to climate change direction but slower than expected based on changes in temperatures only. In the study area, mean temperatures increased by c. 0.75 °C between 1981 and 2010 (21), and therefore, species should move upward with a speed of c. 4.0 m y−1. However, this speed would be expected under the simplest scenario where plants do not show physiological adaptations to warming, or lags in population dynamics, and where macroclimate change is the only driver of range shifts (4, 22). In particular, the complex topography of mountain terrain may offer microclimatic refugia enabling species to cope with warming without the need of large-scale movements (23, 24). If this microclimate effect is taken into account, the velocity of range expansion needed to catch up the shifting isotherms should be smaller and perhaps more in line with our and previous findings (25, 26). Therefore, reported slower speeds likely indicate the existence of more complex mechanisms behind range shifts than only macroclimate warming (27).
Fig. 1.
Range shifts of common native, red-listed native and alien plant species. (A) Mean shift rate and CIs (95%) at the rear (red) and leading (blue) edge over the last 30 y (1990 to 2019). The mean and 95% CIs around the mean were computed using 1,000 permutations in the EzPlot() function in R. The horizontal dashed line shows the expected shift to track climate change based on the current rate of warming in the study area (c. 4 m y−1). (B) Graphical schematic representation of the historical (t0) and current (t1) density distributions for common native, red-listed native and alien species. The average range change percentage was −0.44, −5.69, and +12.02% for common natives, red-listed natives and aliens, respectively. N = 1,318 native common species, N = 108 native red-listed species, and N = 53 alien species, for which at least 30 records in the historical (1990 to 2004) and 30 records in the current period (2005 to 2019) were available.
Similarly to common natives, red-listed species also retracted their elevational range, with the rear edges moving upslope faster than the leading edges (Fig. 1). However, the magnitude of the range retraction signal was much higher compared to common species. The lack of change at the leading edge can be caused by several mechanisms including dispersal limitation (28), availability of local microclimate refugia (24) or lack of suitable habitats beyond the historical range (2, 29). In our study area, forests at mid-elevations are expanding at the expense of open seminatural areas, reducing suitable open habitats and likely preventing the upward movement of some species (3, 30). As red-listed plants often possess narrow ecological niches (13, 18, 19), finding the suitable environment along the elevational gradient might be more challenging than for more generalist species. Consistent with this hypothesis, amongst red-listed plants, specialists of wetlands and semi-natural dry grasslands were overrepresented in our species pool (SI Appendix, Fig. S1). Second, the observed strong erosion of rear margin populations might have been caused by several processes including exceeding temperatures (31), land-use changes in the more intensive lowlands (3), or competitive replacement by warm adapted species that are fostered by climate change (32, 33). All of these drivers are expected to disproportionately affect red-listed species compared to common ones (12, 13), and this likely led to the faster disappearance of rear margin populations.
On the contrary, alien plants were the only group that expanded upward at the leading edge at the average speed of macroclimate warming, while keeping their rear edge almost still (Fig. 1). This suggests that, under current climate warming, alien species can still tolerate increasing temperatures at the rear margin, while they can effectively track temperature warming at the leading edge (9, 34). This is consistent with the directional ecological filtering hypothesis claiming that elevational distributions of alien species are a result of the sequential filtering of species with progressively broader climatic niches along a gradient of increasing temperature severity (34). As alien introductions often occur in the lowlands (35), species might require several years to extend their range up to their potential cold thermal limit (36, 37). In addition, the reported rapid expansion might have been accelerated by human-assisted dispersal associated with increasing soil disturbance events and the presence of roads, rails, and trails (9, 34, 38, 39). If we consider the potential availability of microclimate refugia, reported velocity of range shifts for alien plant species would potentially even exceed the actual velocity of isotherms’ shifts. This upward movement faster than the rate of microclimate change would suggest that alien distributions are not yet in equilibrium with the climate (9, 37).
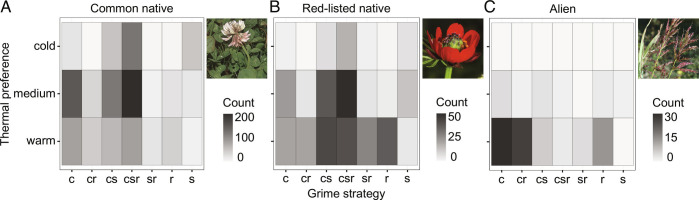
To gain insights into the processes driving certain species to expand or contract their range, we compared ecological traits of common, red-listed native and alien species. First, we focused on climate change, deriving Landolt’s indicator values for temperature classifying species in cold- and warm-adapted species. Second, we applied the Grime’s Competitor-Stress tolerator-Ruderal (CSR) strategy classification that measures species association with different environments (22, 40, 41) (see Methods for details on the classification). Grime’s CSR strategies and temperature preferences differed between common, red-listed native and alien species (Cochran-Mantel-Haenszel M2 = 111.12, df = 4, P < 0.001) (Fig. 2). Common species were mostly associated with intermediate temperatures and were frequently C and CSR-strategist species. As expected, common species often exhibit a good competitive ability well adapted to high-resources conditions (41). Red-listed species were more frequently warm-adapted species with competitor stress-tolerator CS/CSR and R strategy, while pure C- and S-strategies were rarer. The occurrence of R-strategy indicated that several red-listed species were poor competitor ruderal weeds associated with disturbed habitats. Finally, aliens were clearly associated with warm temperatures and mostly exhibited a C/CR-strategy. According to Grime’s classification, species with competitor strategies are able to thrive under high resource conditions, such as fertilized soils, by outcompeting neighboring plants. Due to the combination of several traits such as high relative growth rate, short leaf-life, and high allocation to leaf construction, alien species are able to monopolize and maximize resource acquisition and, therefore, show a strong competitive strategy (42). In addition, with a very few exceptions, all aliens were warm-adapted species, supporting the hypothesis of the directional ecological filtering to explain their rapid range expansion toward higher elevations (34). At low elevations, red-listed and alien species showed the highest number of ruderal species, indicating that they were both associated with highly disturbed environments. Finally, the S-strategy, that measures the ability to thrive in environments poor in resources, was almost completely absent for aliens, indicating that they were not able to tolerate harsh environmental conditions such as shortage of light, water, or nutrients. These trait patterns establish a potential link between intensive land uses, alien success, and the outcompetition of red-listed species (6, 43), suggesting that under highly productive conditions such as in areas heavily disturbed by human activities, competitive interactions might play a fundamental role in determining populations’ success or failure (32, 44). Observed trait patterns also support the hypothesis that the differences in range shifts observed between common and red-listed natives, and aliens are possibly due to multiple drivers including climate warming as well as habitat destruction, land-use changes, and intensification (45–49). As the distribution of traits strongly differed between the three plant groups, we could not directly test the effect of traits on range shift magnitude, i.e., the effect of species status was confounded with a group-specific trait syndrome.
Fig. 2.
Two-way contingency table for thermal preference and Grime CSR strategy. Thermal preference is based on Landolt's ecological indicator value for temperature, varying between 1 (alpine-nival plants) and 5 (very warm-colline species). Cold thermal preference includes thermophilic indexes from 1 to 2, medium from 2.5 to 3.5, warm from 4 to 5. Grime CSR strategy C = competitor, R = ruderal, S = stress tolerator (40). Counts are reported for (A) common native species, in the picture as an example Trifolium repens (Thermophilic index = 3, Grime strategy csr), (B) red-listed native species, as an example Adonis aestivalis (Thermophilic index = 4.5, Grime strategy sr) and (C) alien species, as an example Sorghum halepense (Thermophilic index = 5, Grime strategy c). N = 1,668 species recorded in our dataset with defined thermophilic index and Grime CSR strategy.
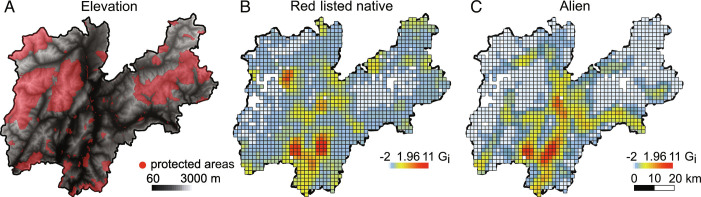
To understand whether red-listed and alien species co-occur and potentially interact in the same geographical areas, we performed a spatially explicit hot spot analysis. Populations of red-listed and alien species were mostly clustered in the same geographical locations (Fig. 3) (Pearson’s correlation: r = 0.65; P < 0.001). Most of the hot spots were associated with highly urbanized, low-elevation areas, while intermediate and high elevations were usually characterized by a low density of both red-listed and alien species (Fig. 4). In particular, alien hot spots sharply disappeared above 1,000 m a.s.l., while red-listed species showed some hot spots above 2,500 m a.s.l. The latter consisted of hot spots of stress tolerator, endemic species adapted to the harsh conditions of Alpine calcareous mountains. Accordingly, the IUCN regional Red List described a decrease in the level of threat with increasing elevation (50). In our study area, elevation can be considered a good proxy for intensive land-uses. In particular, we found a negative correlation between elevation and agricultural land (Pearson’s correlation r = −0.60, P < 0.001), and urban areas (Pearson’s correlation r = −0.51, P < 0.001). We also found that the rate of soil loss (i.e., urbanization) in the last decade was higher in the hot spots than in the remaining areas (Mann–Whitney test P < 0.001 for both hot spots of red-listed and alien species), indicating an ongoing intensifying use of low-elevation areas. The observed spatial overlap between red-listed species occurrence and alien invasions confirms that the drivers threatening native plants such as warming temperatures, intensive land uses, and soil disturbance appear to be similar to those promoting alien invasions. After identifying the hot spots, we overlaid the existing network of protected areas to verify the current degree of habitat protection (Fig. 4). Similarly to several mountain ranges across the globe (51), the large majority of protected areas was established at high elevations, while low-elevation areas lack of habitat protection. Mountain top extinctions have aroused wide interest over the last years. However, plants inhabiting the lowlands showed stronger changes in community composition (52) and higher climate change inertia (53) than species found in harsh environments such as high elevations, that are usually more protected and less exposed to human disturbances (54, 55).
Fig. 3.
Digital elevation model, protected areas and hot spots of red-listed native and alien species. (A) Digital elevation model (25 × 25 m) for the study area (Province of Trento, NE Italy); and hot spot maps (2 × 2 km) for (B) red-listed native and (C) alien species occurrence. Colors show different values of the Getis-Ord Gi statistic from low (light blue) to high values (red). Yellow to red cells represent statistically significant hot spots (P < 0.05). Data on protected areas come from regional land-use maps (Methods). Hot spots are based on 604 red-listed native species and 134 alien species.
Fig. 4.
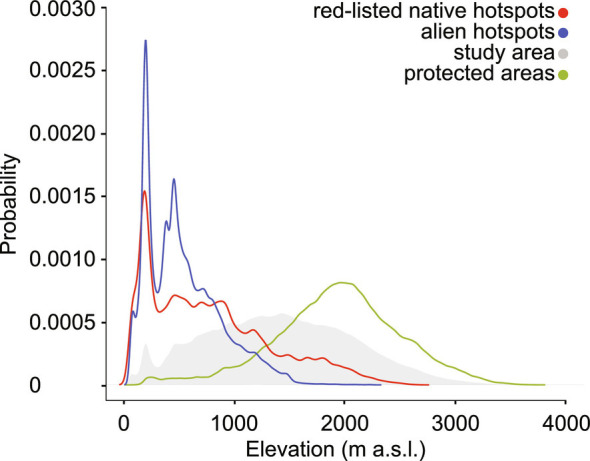
Probability density distribution of elevation for hot spots and protected areas. Elevational distribution of hot spots at the regional scale for red-listed native (red), alien (blue) species, protected areas (green) and the whole study area (shaded background). Probability distributions were estimated using default settings of the density() function in R. Data on protected areas come from regional land-use maps (Methods).
In conclusion, native plants are contracting their elevational range, with red-listed plants retracting from lower elevations at a much faster pace than common plants. This result raises additional concern for the fate of rare and declining plant species that are not able to track climate change. On the contrary, aliens are expanding upslope at climate change speed, still maintaining their rear edge populations. Asynchronous range shifts can have complex consequences on species interactions and community dynamics, leading to biotic interactions between mixes of movers and stayers (56, 57). To predict novel community assemblages, it would be important to comprehend the role of microclimatic refugia in affecting species range shifts (58–60). For instance, colder northern slopes or aspects could serve as effective microclimatic refugia for cold-dwelling species at an early phase of climate warming (59). In accordance with recent studies (46, 47, 52), our results indicated that increased disturbance and eutrophication induced by intensive land-uses might be important drivers of range shifts besides temperature warming. The high environmental pressures that species encounter in the lowlands might constrain the ability of expanding species to shift their range into more natural areas at higher elevations (27), causing a lowland biotic attrition similar to that observed in tropical regions (61). However, in several mountain ranges across the globe (51), the large majority of protected areas are usually established at high elevations because of the conflicts with economic development. Even if in the future endemic high-altitude plants might be increasingly threatened by climate warming (14, 59), most of them do not appear to be at immediate risk and, therefore, we should prioritize the lowlands for implementing more urgent conservation measures. As plants usually exhibit large extinction debts, i.e., negative effects of invasions, climate change and landscape transformations could last for hundreds of years (8, 47, 62, 63), it is crucial to preserve relict populations of rare and declining plants in the lowlands and to implement mitigation measures outside the current network of protected areas to reduce plant extinctions in the future.
Materials and Methods
Study Area.
Plants were sampled throughout the Trento Province, NE Italy (6,207 km 2, elevation range 66 to 3,769 m; SI Appendix, Fig. S2). The region hosts c. half of the total plant species pool of the European Alps including species whose geographic ranges are Alpine, central and northern European and Mediterranean (64). Climate depends primarily on elevation: it is alpine at high elevations and continental in the lowlands. Maximum annual temperature between 1980 and 2010 was 17.5 °C, while minimum annual temperature was 7.8 °C (at 200 m a.s.l.) during that the same period. Between 1981 and 2010, mean annual temperature increased by c. 0.75 °C (21). Therefore, species are expected to shift upward with a speed of c. 4.0 m y−1 based on macroclimate change only. The most recent global analyses on elevational range shifts have reported that terrestrial plant species moved their range upward at an average speed of 1.2 m y−1 (65), more precisely 2.3 m y−1 at the leading edge and 1.2 m y−1 at the rear edge (27). Mean annual precipitation over the past 40 y was c. 1,050 mm. Annual precipitation slightly increased between 1981 and 2010 (+2%), but precipitations decreased during winter time (−6%). The study area has experienced major land-use changes in recent decades. First, agricultural land increased, became more intensive and expanded upward from the lowlands to mid-elevations (up to c. 900 to 1,000 m) (66). Second, forests increased downward at the expense of open seminatural areas at mid-elevations (approximately between 600 and 1,500 m) due to land abandonment (30). Third, human settlements (urban, industrial, and roads) increased, especially in the lowlands (66).
Plant Data.
Plant data were collected from 1990 to 2019 for a total of 1,052,149 plant occurrence records over an elevation gradient spanning from 61 to 3,456 m a.s.l. The sampling campaign was co-ordinated by Filippo Prosser and Alessio Bertolli and carried out by a group of botanists that systematically covered the whole study area. Each study site was visited only once. To aid a systematic sampling of the area, the province was divided into 228 quadrants (c. 7 × 5 km) following the standard central European floristic cartography (Messtischblatt 1: 25,000). The aim of the sampling was to map a detailed point-based distribution atlas of all the species occurring in the study area. The occurrence of each recorded population was localized with a global positioning system (GPS). Using the GPS co-ordinates, we derived the elevation (m a.s.l.) of each record from a high-resolution regional digital elevation model (DEM) (25 × 25 m). After excluding from the original data subspecies, hybrids, aggregates of species with difficult taxonomy, and casual species, we obtained records for a total of 2,245 species.
Ecological Characterization of Plant Species.
Plant status.
We classified plant species in three groups according to their origin and conservation status: common natives (n = 1,507), red-listed natives (n = 604), and aliens (n = 134). Common species were all of the native species not included in the regional IUCN Red List (50). Red-listed natives included species listed in the regional IUCN Red List (50) as near-threatened (NT), vulnerable (VU), endangered (EN), or critically endangered (CR). Data-deficient species were removed from the analyses since they mostly included species with uncertain taxonomy. Alien species comprised established species introduced accidentally or deliberately by humans from a different continent in Europe after 1,500 AD (67, 68).
Habitat preference.
We assigned each species (n = 2,245) to one habitat preference category following the regional flora (50). Habitat categories were nonoverlapping and consisted in: 1) alpine, that is cold-adapted species growing in alpine open areas above the tree-line; 2) semi-natural dry grassland, that is species specialized in open grasslands with shallow, well‐drained soils below the tree line; 3) forest, that is species occurring in shrubland, broadleaf or conifer forests; 4) grassland, that is species growing in pastures, mown meadows, abandoned grasslands, grass margins, from low elevations to alpine habitats; 5) rocky, that is species specialized in rocky soils and cliffs; 6) ruderal, that is species growing in highly disturbed areas such as agricultural fields, road or field margins, railways, urban areas or quarries; and 7) wetland, that is species occurring in fens, mires, ponds and aquatic species.
Landolt’s indicator for temperature.
For each species, we defined the temperature preference using the ordinal scale (1 to 5) from Landolt et al. (69) (from alpine: 1 to very warm: 5). We excluded 64 species with nonassessed temperature preference.
Grime CSR strategy.
Following (70), we classified species in seven categories according to Grime CSR classification (40): competitor (C); competitor ruderal (CR); competitor stress-tolerator (CS); competitor stress-tolerator ruderal (CSR); ruderal (R); stress-tolerator (S) and stress-tolerator ruderal (SR). Competitor species are found in highly productive environments and are primarily composed of plants with high relative growth rate and high allocation to leaf construction. Stress-tolerator species are usually found under extreme environmental conditions with low disturbance. Due to the low resources available, growth and reproduction are usually reduced. Ruderals usually inhabit habitats with intense disturbance regimes (i.e., tillage, mowing) and allocate their resources mainly to seed reproduction. Often, these ruderals are annuals or short-lived perennials. We excluded 564 species because we could not find information on their Grime CSR strategy, for a total of 1,681 species.
Range Shift.
We computed rates of shift in the elevational distribution of species, i.e., changes at the rear (low-elevation) and leading (high-elevation) edge using density distribution of the elevation of each recorded occurrence (3, 71). To quantify the shift between the recent historical (hereafter “historical”) and current range, we split the data into two periods of 15 y (1990 to 2004 and 2005 to 2019). For each species, with at least 30 records per period (1,318 common native species, and 108 red-listed native, and 53 alien species), we estimated a density distribution of the elevation of occurrence for the first and second period, separately. The rear edge was calculated as the 10% quantile of the density distribution, and the leading edge as the 90% quantile of the density distribution. The shift was measured by subtracting historical (1990 to 2004) from current (2005 to 2019) quantiles. We divided the total shift by 15 y to obtain an annual shift rate. We also calculated rear and leading edges as the 5 and 95% quantiles of the density distribution respectively. Shift rates at rear and leading edges using 5 and 95% quantiles were highly correlated with those using 10 and 90% quantiles (Pearson’s correlation r = 0.93, P < 0.001) and were therefore not presented. To account for potential nonrandom sampling effort across the study region (72), we checked the elevational distribution in each period for all records. Both elevational range and average elevation of the records were similar between the two periods (SI Appendix, Fig. S3). As no bias emerged, we used the raw data with no correction in the range shift estimation (3).
Hot Spot Analysis.
To understand where the maximum density of red-listed native species was and whether alien invasions in the last 30 y occurred in the same locations, we ran a spatially explicit hot spot analysis based on occurrence data. We imposed over the study area a regular grid with a 2 × 2 km resolution. We chose this resolution because a smaller grid would have created a very patchy grid with too many empty cells. The total number of records of common species can be considered a good proxy for the sampling effort, therefore, in our analyses, we included only those cells where at least 100 records of common species were reported, for a total of 99% of the surveyed cells. As the occurrence of both red-listed and alien species depends also on the sampling effort, we corrected the occurrence data for red-listed and alien species with the total number of occurrence records of common native species per grid cell (73). The corrected count consisted in the number of observations per grid cell divided by the natural logarithm of the total number of common native species occurrence records for that grid cell. We used the natural logarithm of the number of records because a few cells had been oversampled compared to the others. Hot spots with no sampling effort correction yielded qualitatively similar results.
We performed hot spot analyses using the Getis‐Ord Gi* statistic (74), that detects hot spots while also computing their statistical significance by examining each grid cell within the context of the neighboring cells. We built a neighbor list for all grid cells using the Queen case contiguity (contiguity between each focal cell and the eight neighboring cells around it) and then used the neighbor list to calculate a row‐standardized spatial weights matrix. The matrix informs every grid cell relationship to all other cells in the neighborhood. We used the counts and the spatial weights matrix to calculate the Gi* for each grid cell. Gi* produces a z‐score for each grid cell, where high positive values are statistically significant and indicate the possibility of a local cluster of high species occurrence (i.e., a hot spot) that is unlikely to be due to random chance.
Finally, using the Zonal Statistics tool in QGis (75), for each grid cell used in the hot spot analyses we calculated several environmental metrics: mean elevation based on the regional digital elevation model (25 × 25 m), rate of soil consumption between 2012 and 2019 based on the oldest and most recent available satellite-based maps at the regional scale (10 × 10 m) from Sentinel 1 and 2 images (76) and area covered by urban elements and crops based on the regional land cover map (2003) (1:100,000) (77).
Statistical Analyses.
Range shift.
We tested whether common, red-listed native and alien species differed in their range shift. We used shift rate as response variable and edge type (rear vs. leading), plant status, and their interaction as fixed effects. A significant interaction between edge type and status would indicate that plants belonging to different status are moving at different speeds at the rear vs. leading edge of their distribution. We added species as random effect (random intercepts). Because residuals from standard linear mixed models exhibited strong deviation from normality, the significance of the effects for all models was computed using a nonparametric permutation test with the ezPerm() function of the ez R package (78). The function ezPlot2() with bootstrapping (n = 1,000) was used to estimate 95% CIs around the mean and to visualize the effects. In addition, as several nonparametric tests are now available, we fitted the same model using the aovp() function with default settings in the lmPerm R package (SI Appendix, Table S1) (79). To additionally assess the robustness of the results, we performed a two-way between-/within-subjects ANOVA using bootstrap on trimmed means (80). We specified different amounts of trimming (5, 10, 20, 30%) and used the function bwtrim() in the R package WRS2 (81). Results computed by trimming means were highly consistent with nontrimmed models and are reported in the SI Appendix, Table S2.
In addition, we took into account the potential phylogenetic nonindependence of the data, by testing the presence of a phylogenetic signal in our range shift metrics at the leading and at the rear edge, separately. We used the functions phyloSignal() and lipaMoran() from the R package phylosignal (82) and assumed that trait evolution conformed to a Brownian motion process. We used a tree extracted from the supertree of Daphne (83), including most of our tested species except for 245 species. We estimated five complementary metrics measuring a phylogenetic signal: Abouheif’s Cmean index, Moran’s I index, Blomberg’s K, Blolmberg’s K *and Pagel’s λ. We found little support for a phylogenetic signal in our dataset (SI Appendix, Table S3 and Fig. S4). We did not fit a phylogenetic-corrected model because the assumptions of normality were not met.
Ecological characterization.
To evaluate the association between thermal preference and Grime CSR strategy in each group of plants (i.e., common and red-listed natives and aliens), we first assigned each species to one category of thermal preference according to the thermophilic index, i.e., cold, medium, and warm adapted species. We considered cold adapted plants species showing a thermophilic index from 1 to 2, intermediate species with a thermophilic index from 2.5 to 3.5 and warm adapted plants species with a thermophilic index higher than 3.5. s, we tested the association between thermal preference and Grime CSR strategy for common native, red-listed native and alien species with the Cochran–Mantel–Haenszel chi-squared test using the function mantelhaen.test() in R.
Hot spots.
First, we tested for spatial congruence in the hot spots using Pearson’s correlation between red-listed and alien species Gi* statistics. Second, to visualize a potential spatial overlap in the elevational distribution of hot spots, we clipped the DEM (25 × 25 m) using the significant hot spots of red-listed native and alien species (i.e., cells with Gi*≥ 1.96) and estimated the probability density distribution of the elevation of the clipped rasters. Third, to visualize the degree of habitat protection, we clipped the DEM (25 × 25 m) using the existing protected areas and estimated the probability density distribution of the elevation. Finally, we tested for correlation between mean elevation of each grid cell and the cover of agricultural land, broadleaf forest, conifer forest, alpine grassland, managed grassland, urban area, and wetland (SI Appendix, Fig. S5). All statistical analyses were performed with R 3.6.2 (84) and data are available at (85).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank all the botanists involved in the floristic inventory and Robert J. Wilson for comments on the text. The research was supported by the University of Padua Supporting Talent in ReSearch Consolidator Grant (STARS‐CoG–2017) to L.M. (project BICE—Global Change, Biotic Interactions, and Plants Invasions in Cold Environment).
Author contributions
C.G. and L.M. designed research; A.B. and F.P. performed research; C.G. and L.M. analyzed data; and C.G. and L.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. J.L. is a guest editor invited by the Editorial Board.
Contributor Information
Costanza Geppert, Email: costanzageppert@gmail.com.
Lorenzo Marini, Email: lorenzo.marini@unipd.it.
Data, Materials, and Software Availability
Data and Code data have been deposited in Zenodo (10.5281/zenodo.7598185) (85).
Supporting Information
References
- 1.Nomoto H. A., Alexander J. M., Drivers of local extinction risk in alpine plants under warming climate. Ecol. Lett. 24, 1157–1166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo F., Lenoir J., Bonebrake T. C., Land-use change interacts with climate to determine elevational species redistribution. Nat. Commun. 9, 1315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geppert C., et al. , Consistent population declines but idiosyncratic range shifts in Alpine orchids under global change. Nat. Commun. 11, 5835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumpf S. B., et al. , Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. U.S.A. 115, 1848–1853 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley J. A., et al. , Global consequences of land use. Science 309, 570–574 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Sykes L., Santini L., Etard A., Newbold T., Effects of rarity form on species’ responses to land use. Conserv. Biol. 34, 688–696 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Matthies D., Bräuer I., Maibom W., Tscharntke T., Population size and the risk of local extinction: Empirical evidence from rare plants. Oikos 105, 481–488 (2004). [Google Scholar]
- 8.Dullinger S., et al. , Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2, 619–622 (2012). [Google Scholar]
- 9.Dainese M., et al. , Human disturbance and upward expansion of plants in a warming climate. Nat. Clim. Chang. 7, 577–580 (2017). [Google Scholar]
- 10.McDougall K. L., et al. , Running off the road: Roadside non-native plants invading mountain vegetation. Biol. Invasions 20, 3461–3473 (2018). [Google Scholar]
- 11.Lenoir J., Svenning J.-C., Climate-related range shifts–A global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2015). [Google Scholar]
- 12.Vincent H., Bornand C. N., Kempel A., Fischer M., Rare species perform worse than widespread species under changed climate. Biol. Conserv. 246, 108586. (2020). [Google Scholar]
- 13.Slatyer R. A., Hirst M., Sexton J. P., Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 16, 1104–1114 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Gottfried M., et al. , Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2, 111–115 (2012). [Google Scholar]
- 15.Steinbauer M. J., et al. , Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Hülber K., et al. , Habitat availability disproportionally amplifies climate change risks for lowland compared to alpine species. Glob. Ecol. Conserv. 23, e01113 (2020). [Google Scholar]
- 17.Pauli H., et al. , Recent plant diversity changes on Europe’s mountain summits. Science 336, 353–355 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Clavel J., Julliard R., Devictor V., Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228 (2011). [Google Scholar]
- 19.McKinney M. L., Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516 (1997). [Google Scholar]
- 20.Pimm S. L., Jones H. L., Diamond J., On the risks of extinction. Am. Nat. 132, 757–785 (1988). [Google Scholar]
- 21.Di Piazza E., A. Eccel, Analisi di serie di temperatura e precipitazione in Trentino nel periodo 1958–2010 (Provincia autonoma di Trento, 2012). [Google Scholar]
- 22.Parmesan C., Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (2006). [Google Scholar]
- 23.Greiser C., Ehrlén J., Meineri E., Hylander K., Hiding from the climate: Characterizing microrefugia for boreal forest understory species. Glob. Chang. Biol. 26, 471–483 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldfather M. F., Ackerly D. D., Microclimate and demography interact to shape stable population dynamics across the range of an alpine plant. New Phytol. 222, 193–205 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Lembrechts J. J., Nijs I., Microclimate shifts in a dynamic world. Science 368, 711–712 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Zellweger F., et al. , Forest microclimate dynamics drive plant responses to warming. Science 368, 772–775 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Lenoir J., et al. , Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044–1059 (2020). [DOI] [PubMed] [Google Scholar]
- 28.González-Varo J. P., Onrubia A., Pérez-Méndez N., Tarifa R., Illera J. C., Fruit abundance and trait matching determine diet type and body condition across frugivorous bird populations. Oikos 2022 (2022). [Google Scholar]
- 29.Platts P. J., et al. , Habitat availability explains variation in climate-driven range shifts across multiple taxonomic groups. Sci. Rep. 9, 15039 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tattoni C., Ianni E., Geneletti D., Zatelli P., Ciolli M., Landscape changes, traditional ecological knowledge and future scenarios in the Alps: A holistic ecological approach. Sci. Total Environ. 579, 27–36 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Vilà-Cabrera A., Premoli A. C., Jump A. S., Refining predictions of population decline at species’ rear edges. Glob. Chang. Biol. 25, 1549–1560 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Alexander J. M., Diez J. M., Levine J. M., Novel competitors shape species’ responses to climate change. Nature 525, 515–518 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Pauli H., Gottfried M., Reiter K., Klettner C., Grabherr G., Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Chang. Biol. 13, 147–156 (2007). [Google Scholar]
- 34.Alexander J. M., et al. , Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc. Natl. Acad. Sci. U.S.A. 108, 656–661 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marini L., et al. , Beta-diversity patterns elucidate mechanisms of alien plant invasion in mountains. Glob. Ecol. Biogeogr. 22, 450–460 (2013). [Google Scholar]
- 36.Alexander J. M., Naylor B., Poll M., Edwards P. J., Dietz H., Plant invasions along mountain roads: The altitudinal amplitude of alien Asteraceae forbs in their native and introduced ranges. Ecography 32, 334–344 (2009). [Google Scholar]
- 37.Pyšek P., Jarošík V., Pergl J., Wild J., Colonization of high altitudes by alien plants over the last two centuries. Proc. Natl. Acad. Sci. 108, 439–440 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geppert C., et al. , Contrasting response of native and non-native plants to disturbance and herbivory in mountain environments. J. Biogeogr. 48, 1594–1605 (2021). [Google Scholar]
- 39.Liedtke R., et al. , Hiking trails as conduits for the spread of non-native species in mountain areas. Biol. Invasions 22, 1121–1134 (2020). [Google Scholar]
- 40.Grime J. P., Plant Strategies, Vegetation Processes, and Ecosystem Properties (John Wiley and Sons, Chichester, 1979). [Google Scholar]
- 41.Pierce S., et al. , A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 31, 444–457 (2017). [Google Scholar]
- 42.Van Kleunen M., Weber E., Fischer M., A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Prévosto B., et al. , Impacts of land abandonment on vegetation: Successional pathways in european habitats. Folia Geobot. 46, 303–325 (2011). [Google Scholar]
- 44.Lembrechts J. J., Milbau A., Nijs I., Trade-off between competition and facilitation defines gap colonization in mountains. AoB Plants 7, plv128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sala O. E., Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Díaz S., et al. , Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366, eaax3100 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Le Roux J. J., et al. , Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 29, 2912–2918.e2 (2019). [DOI] [PubMed] [Google Scholar]
- 48.LaForgia M. L., Harrison S. P., Latimer A. M., Invasive species interact with climatic variability to reduce success of natives. Ecology 101, 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Eskelinen A., Harrison S. P., Resource colimitation governs plant community responses to altered precipitation. Proc. Natl. Acad. Sci. U.S.A. 112, 13009–13014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prosser F., Bertolli A., Festi F., Perazza G., Flora del Trentino (Osiride/Fondazione Museo Civico, Rovereto, 2019). [Google Scholar]
- 51.Elsen P. R., Monahan W. B., Dougherty E. R., Merenlender A. M., Keeping pace with climate change in global terrestrial protected areas. Sci. Adv. 6, eaay0814 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virtanen R., et al. , Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Glob. Ecol. Biogeogr. 19, 810–821 (2010). [Google Scholar]
- 53.Bertrand R., et al. , Changes in plant community composition lag behind climate warming in lowland forests. Nature 479, 517–520 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Vittoz P., Randin C., Dutoit A., Bonnet F., Hegg O., Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Glob. Chang. Biol. 15, 209–220 (2009). [Google Scholar]
- 55.Wilson S. D., Nilsson C., Arctic alpine vegetation change over 20 years. Glob. Chang. Biol. 15, 1676–1684 (2009). [Google Scholar]
- 56.Lyu S., Alexander J. M., Competition contributes to both warm and cool range edges. Nat. Commun. 13, 2502 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs R. J., Valentine L. E., Standish R. J., Jackson S. T., Movers and stayers: Novel assemblages in changing environments. Trends Ecol. Evol. 33, 116–128 (2018). [DOI] [PubMed] [Google Scholar]
- 58.De Frenne P., et al. , Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. U.S.A. 110, 18561–18565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rota F., et al. , Topography of the Dolomites modulates range dynamics of narrow endemic plants under climate change. Sci. Rep. 12, 1–12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lenoir J., Hattab T., Pierre G., Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 40, 253–266 (2017). [Google Scholar]
- 61.Colwell R. K., Brehm G., Cardelús C. L., Gilman A. C., Longino J. T., Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Catford J. A., Bode M., Tilman D., Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 9, 1–7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rumpf S. B., et al. , Extinction debts and colonization credits of non-forest plants in the European Alps. Nat. Commun. 10, 4293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aeschimann D., Lauber K., Moser D. M., Theurillat J. P., Flora alpina, atlas des 4500 plantes vasculaires des Alpes (Belin, 2004). [Google Scholar]
- 65.Chen I.-C.I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D., Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Monteiro A. T., Fava F., Hiltbrunner E., Della Marianna G., Bocchi S., Assessment of land cover changes and spatial drivers behind loss of permanent meadows in the lowlands of Italian Alps. Landsc. Urban Plan. 100, 287–294 (2011). [Google Scholar]
- 67.Richardson D. M., et al. , Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 6, 93–107 (2000). [Google Scholar]
- 68.Conti F., Abbate G., Alessandrini A., Blasi C., An Annotated Checklist of the Italian Vascular Flora (Palombi Editori, 2005). [Google Scholar]
- 69.Landolt E., et al. , Flora indicativa: Okologische Zeigerwerte und biologische Kennzeichen zur Flora der Schweiz und der Alpen (Haupt Verlag, 2010). [Google Scholar]
- 70.Klotz S., Durka W., BIOLFLOR–Eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland (Bundesamt fuer Naturschutz, Bonn, 2002). [Google Scholar]
- 71.Rumpf S. B., Hülber K., Zimmermann N. E., Dullinger S., Elevational rear edges shifted at least as much as leading edges over the last century. Glob. Ecol. Biogeogr. 28, 533–543 (2019). [Google Scholar]
- 72.Aikio S., Duncan R. P., Hulme P. E., Herbarium records identify the role of long-distance spread in the spatial distribution of alien plants in New Zealand. J. Biogeogr. 37, 1740–1751 (2010). [Google Scholar]
- 73.Sussman A. L., et al. , A comparative analysis of common methods to identify waterbird hotspots. Methods Ecol. Evol. 10, 1454–1468 (2019). [Google Scholar]
- 74.Getis A., Ord J. K., The analysis of spatial association by the use of distance statistics. Geogr. Anal. 24, 189–206 (1992). [Google Scholar]
- 75.Q. Association, QGIS.org, QGIS geographic information system (QGIS Association, 2022). Available at: http://www.qgis.org. [Google Scholar]
- 76.ISPRA, "Consumo di suolo, dinamiche territoriali e servizi ecosistemici" (Tech. Rep. SNPA 22/21 2021, Edizione, 2021). [Google Scholar]
- 77.Büttner G., “The CORINE land cover 2000 project” in eProceedings of EARSeL (2004), pp. 331–346.
- 78.Lawrence M. A., M. M. A. Lawrence, ez: Easy Analysis and Visualization of Factorial Experiments (R package Version 4.4-0, 2016), https://CRAN.R-project.org/package=ez.
- 79.Wheeler R. E., M. Torchiano, lmPerm: Permutation Tests for Linear Models. (R package version 2.1.0, 2016), https://CRAN.R-project.org/package=lmPerm.
- 80.Wilcox R. R., “Trimmed means” in Wiley StatsRef: Statistics Reference Online (John Wiley & Sons Ltd, 2014). [Google Scholar]
- 81.Mair P., Wilcox R., Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 52, 464–488 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Keck F., Rimet F., Bouchez A., Franc A., Phylosignal: An R package to measure, test, and explore the phylogenetic signal. Ecol. Evol. 6, 2774–2780 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Durka W., Michalski S. G., Daphne: A dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93, 2297–2297 (2012). [Google Scholar]
- 84.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2020). https://www.R-project.org/. [Google Scholar]
- 85.Geppert C., Bertolli A., Prosser F., Marini L., Data and code for: Red-listed plants are contracting their elevational range faster than common plants in the European Alps. Zenodo. 10.5281/zenodo.7598185. Deposited 3 February 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Data and Code data have been deposited in Zenodo (10.5281/zenodo.7598185) (85).