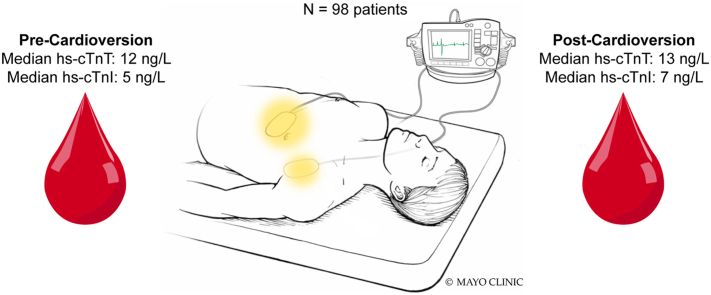
Abstract
Background
Direct current (DC) cardioversion is used to terminate cardiac arrhythmias. Current guidelines list cardioversion as a cause of myocardial injury.
Objective
This study determined whether external DC cardioversion results in myocardial injury measured by serial changes in high-sensitivity cardiac troponin T (hs-cTnT) and high-sensitivity cardiac troponin I (hs-cTnI).
Methods
This was a prospective study of patients undergoing elective external DC cardioversion for atrial fibrillation. hs-cTnT and hs-cTnI were measured precardioversion and at least 6 hours postcardioversion. Myocardial injury was present when there were significant changes in both hs-cTnT and hs-cTnI.
Results
Ninety-eight subjects were analyzed. Median cumulative energy delivered was 121.9 (interquartile range [IQR] 102.2–302.7) J. Multiple cases 23 (23.5%) required 300 J or more. Maximum cumulative energy delivered was 2455.1 J. There were small significant changes in both hs-cTnT (median precardioversion 12 [IQR 7–19) ng/L], median postcardioversion 13 [IQR 8–21] ng/L; P < .001) and hs-cTnI (median precardioversion 5 [IQR 3–10) ng/L], median postcardioversion 7 [IQR 3.6–11) ng/L; P < .001). Results were similar in patients with high-energy shocks and did not vary based on precardioversion values. Only 2 (2%) cases met criteria for myocardial injury.
Conclusion
DC cardioversion resulted in a small but statistically significant changes in hs-cTnT and hs-cTnI in 2% of patients studied irrespective of shock energy. Patients with marked troponin elevations after elective cardioversion should be assessed for other causes of myocardial injury. It should not be assumed the myocardial injury was from the cardioversion.
Keywords: Atrial fibrillation, Cardioversion, Troponin, High-sensitivity cardiac troponin, Myocardial injury
Graphical abstract
Key Findings.
-
▪
Elective external cardioversion in stable patients with atrial fibrillation results in a small, statistically significant but unlikely clinically significant change in median high-sensitivity cardiac troponin T and troponin I values (1 ng/L and 2 ng/L, respectively).
-
▪
The changes do not appear related to shock energy and do not vary based on precardioversion baseline values.
-
▪
Patients who have marked troponin elevations after elective cardioversion should be assessed for other causes of myocardial injury. They should not be assumed to have myocardial injury from the cardioversion alone.
Introduction
Direct current cardioversion (DCCV) with the delivery of electric shock to the heart through electrodes placed on the chest is often used to restore normal cardiac rhythm.
It has been reported that DCCV results in myocardial injury.1, 2, 3, 4, 5, 6 This concept was initially driven by animal studies.7, 8, 9 For that reason, the Fourth Universal Definition of Myocardial Infarction1 lists cardioversion as a cause of myocardial injury. Present guidelines also suggest using lower energies and escalating the energy in a stepwise manner for defibrillation presumably in part to reduce the extent of myocardial injury.10 However, the observations that led to these recommendations were based on studies with monophasic defibrillators, older conductive gels and pads, and conventional (older), less sensitive troponin assays.3, 4, 5, 6 There are mixed data in regard to frequency of myocardial injury with the newer biphasic defibrillators.4,6,11
The aim of this study was to determine more definitively whether external DCCV results in myocardial injury as measured by serial changes in high-sensitivity cardiac troponin T (hs-cTnT) and high-sensitivity cardiac troponin I (hs-cTnI).
Methods
We prospectively recruited patients at the Mayo Clinic in Rochester, Minnesota, undergoing elective DCCV for atrial fibrillation (AF) or atrial flutter (AFL) from July 2019 to July 2020. Written informed consent was obtained. Patients under 18 years of age, who were pregnant, or who were receiving renal replacement therapy were not eligible. Patients with myocardial infarction, coronary artery bypass grafting, or any invasive cardiac procedure in the previous 6 weeks also were excluded. Patients with transesophageal echocardiogram–guided DCCVs were included.
DCCV procedure
A transesophageal echocardiogram was performed if clinically indicated to exclude left atrial appendage thrombus. Intravenous midazolam and fentanyl were given for the transesophageal portion of the procedure. For the DCCV portion of the procedure, patients were sedated with intravenous propofol, dose adjusted according to physician preference.
DCCV was performed using the Zoll R Series Defibrillator (Zoll Medical Corporation, Burlington, MA), which delivers biphasic rectilinear shocks. Medi-Trace Cadence Adult defibrillation electrodes (Product code: 22770R; Cardinal Health, Dublin, OH) were used for all DCCVs. Data regarding delivered energy, delivered current, and impedance were obtained from the defibrillator. These parameters are provided routinely by most defibrillators in the United States. DCCVs were performed using a standardized protocol (Supplemental Material).
Laboratory analyses
hs-cTn levels were measured pre-DCCV and at least 6 hours post-DCCV (Supplemental Material). hs-cTnT was measured using the Troponin T Gen 5 STAT assay on the Cobas e601 analyzer (Roche Diagnostics, Indianapolis, IN). hs-cTnI was measured using the ARCHITECT STAT High Sensitivity Troponin-I assay (Abbott Laboratories, Abbott Park, IL). The 99th percentile for the hs-cTnT assay is 15 ng/L in men and 10 ng/L in women.12 The lowest reportable value or limit of quantitation (LOQ) (the lowest value with a coefficient of variation [CV] ≤20%) for hs-cTnT is 6 ng/L.13 For the hs-cTnI assay, the 99th percentiles are 34 ng/L for men and 16 ng/L for women.14 The LOQ is 3.5 ng/L.14 We also report analyses using the lower 99th percentile values for the hs-cTnI assay from the Universal Sample Bank (USB) study of 20 ng/L for men and 13 ng/L for women.15 All hs-cTn are reported as whole values, except hs-cTnI from 3.5 to 4.0 ng/L, in which the values are reported with 1 decimal place.
Statistical analyses
Using an alpha of 0.01, power of 90%, effect size of 1, and the change in standard deviation from a previous study,11 a sample size of 88 was required for this study. To accommodate a 10% “dropout” rate, we recruited 98 patients with permission of the Institutional Review Board. Statistical analysis was done using IBM SPSS Statistics for Windows, Version 28 (IBM Corp, Armonk, NY). Descriptive statistics are presented as mean ± SD for normally distributed continuous variables, median and range or interquartile range (IQR) for non–Gaussian-distributed continuous variables, or number and percentage for categorical variables. The comparison between groups were done using nonparametric paired testing—Wilcoxon signed rank test. The change in values (postprocedure value – preprocedure value) of hs-cTnT and hs-cTnI, which we call “delta change” throughout the article, was analyzed using nonparametric paired testing—Wilcoxon signed rank test.
Significant changes in hs-cTn were defined as a >50% change, which is the reference change value (RCV),16, 17, 18 when the baseline (pre-DCCV) value was ≤99th percentile sex-specific upper reference limit (URL). A >20% change was used when the baseline value was >99th percentile sex-specific URL to preserve the balance between sensitivity and specificity reported for hs-cTn assays.19
When values were <LOQ, we analyzed the data using a value just below that (5.5 ng/L for hs-cTnT and 3 ng/L for hs-cTnI). As a sensitivity analysis, we also analyzed the data in which the value <LOQ was assigned a value near zero (0.1 ng/L designated as ‘LOQ low’ as opposed to ‘LOQ high’). These analyses are presented separately (Supplemental Material). To increase specificity, we only considered myocardial injury to be present if there were significant changes in both hs-cTnT and hs-cTnI.
Ethics approval was obtained from the Mayo Clinic Institutional Review Board, and the study was registered in ClinicalTrials.gov (NCT04151966). This study was conducted in accordance with the Declaration of Helsinki guidelines.
Results
Demographics of the study sample
Ninety-nine subjects were recruited. One was excluded because of hemolysis (H index > 100) in the pre-DCCV sample, which alters hs-cTn values.13,14 No other interfering substances were noted. The median time to the post-DCCV blood sample collection was 9 (IQR 7–11) hours (Table 1). Echocardiographic data are presented in Supplemental Table 1.
Table 1.
Baseline characteristics (N = 98)
| Age, y | 69 (63–77) |
| Male | 69 (70.4) |
| BMI, kg/m2 | 32 (27.4–37.7) |
| History of CAD | 15 (15.3) |
| CHA2DS2-VASc score | 3 (2–4) |
| Rhythm | |
| Atrial fibrillation | 61 (62.2) |
| Atrial flutter | 37 (37.8) |
| TEE guided | 34 (34.7) |
| Successful | 92 (93.9) |
| Propofol dose, mg | 100 (68–130) |
| Midazolam dose (n = 34) | 4 (3–5) |
| Fentanyl dose (n = 34) | 75 (50–100) |
Values are median (interquartile range) or n (%).
BMI = body mass index; CAD = coronary artery disease; CHA2DS2-VASc = congestive heart failure, hypertension, age 65–74, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, sex category; TEE = transesophageal echocardiogram.
DCCV details
In total, 92 (93.9%) patients had successful restoration of sinus rhythm, with 57 (58.2%) after only 1 shock. Twenty-six (26.5%) subjects received 3 or more shocks. A total of 23 (23.5%) received cumulative energies of more than 300 J. Four (4.1%) subjects received dual simultaneous defibrillator shocks. Median impedance was 103 Ω for the first shock, 103 Ω for the second shock, 109 Ω for the third shock, and 108 Ω for the fourth shock (Table 2). There were no statistically significant changes in impedance between consecutive shocks (Supplemental Table 2).
Table 2.
Cardioversion procedure details including delivered energy, current, and impedance values
| No. of shocks | Cumulative energy delivered (J) | Highest energy delivered per shock (J) | Cumulative current delivered (A) | Highest current delivered per shock (A) | Highest impedance (Ω) | |
|---|---|---|---|---|---|---|
| Median (interquartile range) | 1 (1–3) | 121.9 (102.2–302.7) | 121.1 (62.3–154.8) | 14.3 (10.3–29.7) | 12.9 (9.9–15) | 91.5 (80.8–109.3) |
| Range | 1–8 | 59.1–2455.1 | 59.1–509.2 | 6.7–202.2 | 6.7–42.3 | 57–160 |
hs-cTn measurements
Pre-DCCV hs-cTnT ranged from below 6 ng/L to 120 ng/L. Post-DCCV hs-cTnT ranged from below 6 ng/L to 128 ng/L. The delta hs-cTnT change ranged from –21 ng/L to +64 ng/L.
Pre-DCCV hs-cTnI ranged from below 3.5 ng/L to 211 ng/L. Post-DCCV values ranged from below 3.5 ng/L to 216 ng/L. The delta hs-cTnI change ranged from –16 ng/L to +103 ng/L.
Table 3 shows the distribution of the pre- and post-DCCV differences for hs-cTnT and hs-cTnI. There was a statistically significant difference between the pre- and post-DCCV values for both. This finding was also present using the ‘LOQ low’ value (Supplemental Table 3). However, the median change was only 1 ng/L for hs-cTnT, with 25% of the study population having a change of 0 ng/L and 75% of the population having a change of up to 2 ng/L. Similarly, the median change for hs-cTnI was only 0.7 ng/L, with 25% of the study population having a change of 0 ng/L and 75% of the population having a change of up to 2 ng/L.
Table 3.
High-sensitivity cardiac troponin values before and after cardioversion
| Precardioversion | Postcardioversion | Median difference (post – pre) | P value | |
|---|---|---|---|---|
| hs-cTnT | 12 (7–19) | 13 (8–21) | 1 (0–2) | <.001 |
| hs-cTnI | 5 (3–10) | 7 (3.6–11) | 0.7 (0–2) | <.001 |
Values are median (interquartile range). P values were calculated using the Wilcoxon signed rank test.
hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T.
Supplemental Figures 1 and 2 show the distribution of the pre-DCCV vs the post-DCCV hs-cTnT and hs-cTnI values. Most values were similar.
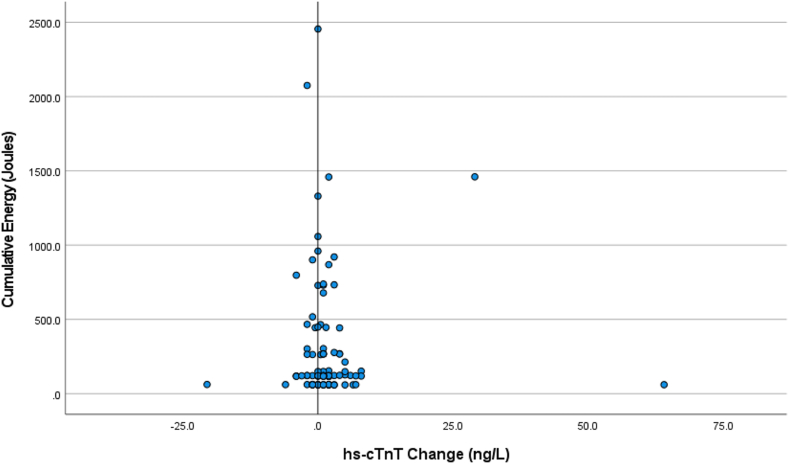
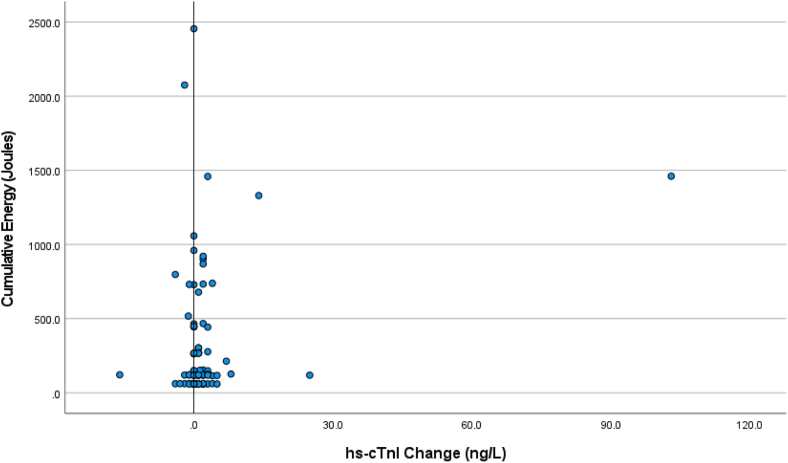
Figures 1 and 2 show the distribution of the change in hs-cTn values (post-DCCV – pre-DCCV) plotted against the cumulative energy delivered. The differences were not related to the cumulative energy delivered.
Figure 1.
Scatter plot showing the change in high-sensitivity cardiac troponin T (hs-cTnT) values (postcardioversion – precardioversion) plotted against the cumulative energy delivered.
Figure 2.
Scatter plot showing the change in high-sensitivity cardiac troponin I (hs-cTnI) values (postcardioversion – precardioversion) plotted against the cumulative energy delivered.
Cases with significant changes in hs-cTn Values
Table 4 shows the patients with significant changes for hs-cTnT and hs-cTnI using standard cutoffs. Data using the USB Abbott cutoff values are shown in Supplemental Table 4. There were 2 patients (1 male and 1 female) who fulfilled our primary endpoint (both hs-cTnT and hs-cTnI changes above RCV occurred).
Table 4.
Distribution of changes for hs-cTnT, and hs-cTnI using standard cutoffs
| Men (n = 69) | Women (n = 29) | |
|---|---|---|
| hs-cTnT | ||
| Pre-DCCV > URL that increased by > 20% post-DCCV | 5 (7.2) | 2 (6.9) |
| Pre-DCCV ≤ URL that increased by > 50% post-DCCV | 1 (1.4) | 4 (13.8) |
| Patients that had changes above RCV (20% OR 50%) | 6 (8.7) | 6 (20.7) |
| hs-cTnI (standard cutoff: 16 ng/L for women and 34 ng/L for men) | ||
| Pre-DCCV > URL that increased by > 20% post-DCCV | 1 (1.4) | 0 |
| Pre-DCCV ≤ URL that increased by > 50% post-DCCV | 6 (8.7) | 4 (13.8) |
| Patients that had changed above RCV (20% OR 50%) | 7 (10.1) | 4 (13.8) |
| Patients that had change above RCV for both hs-cTnT AND hs-cTnI | 1 (1.4) | 1 (3.4) |
Values are n (%).
DCCV = direct current cardioversion; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T; RCV = reference change value; URL = upper reference limit.
When using the lower 99th percentile USB cutoffs for hs-cTnI, there was an increase in cases (3 males and 1 female) with significant changes in which pre-DCCV values were >URL and a decrease in cases (5 males and 4 females) with significant changes where pre-DCCV values were ≤URL (Supplemental Table 4). This resulted in an additional case (1 female) who had fulfilled our primary endpoint.
Using ‘LOQ low’ values accentuated the number of cases with significant changes when the pre-DCCV value was ≤URL (9 males and 8 females for hs-cTnT; 8 males and 8 females for hs-cTnI) (Supplemental Table 4). This resulted in an additional 5 cases (all females) who had fulfilled our primary endpoint. For hs-cTnI using USB cutoffs, there was also accentuation of the number of cases (6 males and 8 females). This resulted in an additional 4 cases (all females) who had fulfilled our primary endpoint (Supplemental Table 4).
The number of cases in which there was a significant change in both hs-cTnT and hs-cTnI were small. Using the USB Abbott cutoff values did not appear to have a large influence on the number of cases (1 male and 2 females) (Supplemental Table 4).
Two cases met our primary endpoint when standard LOQ cutoffs (‘LOQ high’) were used (Table 5). Supplemental Table 5 shows the cases that met our primary endpoint when ‘LOQ low’ cutoffs were used. There were no significant differences in age, sex, body mass index, left ventricular ejection fraction, left ventricular mass index, or CHA2DS2-VASc (congestive heart failure, hypertension, age 65–74 years, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, sex category) score in the patients with significant changes compared with those without.
Table 5.
Cases in which subjects had a significant rise in both hs-cTnT and hs-cTnI using standard LOQ cutoffs
| Patient ID | Sex | LVEF (%) | Number of shocks | Cumulative energy delivered (J) | Maximum energy delivered per shock (J) | Pre-DCCV hs-cTnT | Post-DCCV hs-cTnT | Pre-DCCV hs-cTnI | Post-DCCV hs-cTnI |
|---|---|---|---|---|---|---|---|---|---|
| 47 | F | 31 | 1 | 123 | 123 | 10 | 16 | <3.5 | 6 |
| 109 | M | 65 | 6 | 1460.2 | 509.2 | 30 | 59 | 33 | 136 |
DCCV = direct current cardioversion; F = female; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T; LOQ = limit of quantitation; LVEF = left ventricular ejection fraction; M = male.
Despite larger amounts of energies delivered with dual defibrillation, only 1 case (case 109) had both a substantial hs-cTnT and hs-cTnI rise, and it was not the case that received the highest amount of energy (Table 6).
Table 6.
Cases in which subjects were delivered dual defibrillator simultaneous shocks
| Patient ID | Sex | LVEF (%) | Number of shocks | Number of dual simultaneous shocks | Cumulative energy delivered (J) | Maximum energy delivered per shock (J) | Pre-DCCV hs-cTnT | Post-DCCV hs-cTnT | Pre-DCCV hs-cTnI | Post-DCCV hs-cTnI |
|---|---|---|---|---|---|---|---|---|---|---|
| 98 | M | 62 | 8 | 2 | 2075 | 507.2 | 10 | 8 | 10 | 8 |
| 101 | F | 58 | 8 | 3 | 2455.1 | 509.1 | 10 | 10 | <3.5 | <3.5 |
| 103 | M | 64 | 6 | 1 | 1458.8 | 508 | 24 | 26 | <3.5 | 6 |
| 109 | M | 65 | 6 | 1 | 1460.2 | 509.2 | 30 | 59 | 33 | 136 |
DCCV = direct current cardioversion; F = female; hs-cTnI = high-sensitivity cardiac troponin I; hs-cTnT = high-sensitivity cardiac troponin T; LVEF = left ventricular ejection fraction; M = male.
Discussion
Our data elucidate several important findings. First, there was a statistically significant but small change in median hs-cTnT and hs-cTnI values (1 ng/L and 2 ng/L, respectively) after DCCV for AF or AFL. Because of the small magnitude of the changes, the biological importance of them is unlikely to be profound. Second, of the 98 cases, only 2 (2%) met our criteria for significant myocardial injury; in one, a modest change occurred (case 47), and in the other, a much larger change occurred (case 109). Our data do not suggest that the increases were related to the amount of energy delivered or number of shocks. There also were no discernible differences in clinical characteristics in those patients with significant increases that might explain their differences from other patients. We also noted that patients with a pre-DCCV value that was <99th percentile (no pre-existing myocardial injury) tended to have more frequent significant changes compared with those patients with a pre-DCCV value >99th percentile (pre-existing myocardial injury) with hs-cTnI but not with hs-cTnT (see Supplemental Material for further details).
As mentioned previously, the initial perception that external DCCV caused myocardial injury came from animal studies.7, 8, 9 These studies measured hemodynamic parameters post-cardioversion, rather than measuring biomarkers of myocardial injury. The reduction in hemodynamic function may be simply a marker of postresuscitation myocardial dysfunction, which is a temporary phenomenon,20 rather than of myocardial injury. It is also unclear whether the energy levels used in these animal studies correspond to the energy levels used in humans (not only in terms of weight and body composition, but also in terms of anatomy).
The current study challenges the accepted norm that elective DCCV results in myocardial injury. Thus, patients in this setting who have marked troponin increases in hs-cTnT or hs-cTnI should be assessed for other causes of myocardial injury. It may be that some of these differences are related at least in part to the modern-day use of biphasic defibrillators. Most studies have suggested they result in the lower risk of postshock myocardial injury because the energy requirements are lower.4,21 Monophasic defibrillators deliver higher peak voltages and higher peak current.22 These data are consistent with earlier studies with biphasic devices, albeit with less sensitive cardiac troponin assays that suggested exactly this. Biphasic waveforms have greater shock efficacy, likely secondary to the ability of the second phase to achieve cardioversion more easily by creating a homogeneous distribution of postshock transmembrane voltage and the removal of excess charge left on the myocardial cell membranes at the end of the first phase (charge balancing).23 These effects may be contributory to the lack of injury with biphasic shocks. Importantly, there was no evidence that energy levels were determinative.
Our experience mirrors the findings described by Neal and colleagues24 and has potential clinical implications. Even though we had a 93.9% success rate in restoring sinus rhythm, 41.8% of patients required more than 1 shock. Our practice is to use 100 J in AF for the first biphasic shock and increase if needed to 150 J and 200 J. Whether the second shock using higher energy is successful due to the increased energy is unclear. However, if that were to be the case, one could potentially start cardioversions with higher energy levels because they do not appear to increase the extent of myocardial injury.25 This might reduce the energy required and the time spent under sedation, allowing procedures to be done more rapidly. There are multiple older reports that using higher initial energy levels result in higher conversion rates.26,27 Similarly, the recent Comparison of High vs. Escalating Shocks (CHESS) trial28 showed higher success rates when a maximum fixed (360 J, 360 J, 360 J) biphasic shock protocol was used compared with a low escalating (125 J, 150 J, 200 J) protocol (88% vs 66% respectively). While it should be noted that both the studies used a defibrillator that delivered biphasic truncated exponential waveform shocks as opposed to the biphasic rectilinear waveform that we used in our study, the first shock success rate was comparable to our first shock success rate of 58.2%. The CHESS group reported their cardiac troponin data in the Supplement, and it appeared as if there also was not a signal indicative of myocardial injury.28 However, each waveform has its own unique operating characteristics.23 Assuming that the results with one waveform will mirror those of another approach may not reflect reality. In addition, there may be differences in both the pads and gels used and their relative impedance. These interesting issues require additional studies. There are no data that would allow our observations to be extrapolated to the circumstances in which defibrillation is used, which is, by definition, an ischemically mediated milieu, but perhaps future studies might wish to develop protocols that would allow such questions to be answered.
It is thought that sequential shocks (monophasic or biphasic) result in reduced chest wall impedance (due to tissue electrical injury resulting in edema and hyperemia) and thus subsequent increased current flow to the myocardium.29 We did not observe that phenomenon (Supplemental Table 2). Our data question the validity of those prior data with the device we used. It could be related to the different biphasic waveforms used (biphasic rectilinear vs biphasic truncated exponential), although a previously published randomized trial comparing them showed a progressive reduction in transthoracic impedance in both groups.30
This study has several limitations. First, it is a single-center study and thus is subject to the limitations of a single-center study including the population undergoing DCCV and the specific techniques deployed in terms, not only of the procedure, but also of the support, such as anesthesia. Second, because of logistical difficulties in obtaining the second blood sample, we obtained samples 6 to 24 hours after the procedure (median time = 9 hours). Perhaps we missed some additional late rises. It is possible that some patients with underlying coronary artery disease may not have as marked hs-cTn rises in samples obtained early post-DCCV because of the slower washout of hs-cTn than those with normal coronary arteries.31 Third, the echocardiographic data were obtained from transthoracic echocardiograms that were done over a wide time period, from 6 months before to 6 months after the DCCV, and not all echocardiography parameters were measured. Thus, we did not use the echocardiography data as a primary endpoint of our study. Lastly, while increases in hs-cTn are associated with increased mortality from procedures such as noncardiac surgery as in the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,32 this may not necessarily translate to cardioversions in which the changes were extremely small and, though present, may not be of biological importance. We doubt that we will be able to see differences during follow-up.
Conclusion
Elective external DCCV in stable patients with AF or AFL results in small but statistically significant changes in median hs-cTnT and hs-cTnI values (1 ng/L and 2 ng/L, respectively). The changes did not appear related to shock energy and did not vary based on pre-DCCV baseline values. Patients who have marked troponin elevations after elective cardioversion should be assessed for other causes of myocardial injury. They should not be assumed to have myocardial injury from the cardioversion alone.
Acknowledgments
Funding Sources
This publication was supported by Clinical and Translational Science Award (CTSA) Grant Number UL1 TR002377 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosures
Ronstan Lobo, Roger D. White, Leslie J. Donato, Amy M. Wockenfus, Brandon R. Kelley, and Rowlens M. Melduni: no conflicts. Allan S. Jaffe presently or in the past has consulted for most of the major diagnostic companies.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained.
Ethics Statement
Ethics approval was obtained from the Mayo Clinic IRB and the study was registered in ClinicalTrials.gov (number, NCT04151966). This study was conducted in accordance with the Declaration of Helsinki guidelines.
Appendix. Supplementary data
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., et al. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K., Mair J., Katus H., et al. Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 3.Kosior D., Chwyczko T., Stawicki S., Tadeusiak W., Rabczenko D., Opolski G. [Myoglobin and troponin I as markers of myocardial damage during cardioversion of atrial fibrillation] Pol Arch Med Wewn. 2003;100:827–836. [PubMed] [Google Scholar]
- 4.Kosior D.A., Opolski G., Tadeusiak W., et al. Serum troponin I and myoglobin after monophasic versus biphasic transthoracic shocks for cardioversion of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:S128–S132. doi: 10.1111/j.1540-8159.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 5.Lund M., French J.K., Johnson R.N., Williams B.F., White H.D. Serum troponins T and I after elective cardioversion. Eur Heart J. 2000;21:245–253. doi: 10.1053/euhj.1999.1745. [DOI] [PubMed] [Google Scholar]
- 6.Skulec R., Belohlavek J., Kovarnik T., et al. Serum cardiac markers response to biphasic and monophasic electrical cardioversion for supraventricular tachyarrhythmia – a randomised study. Resuscitation. 2006;70:423–431. doi: 10.1016/j.resuscitation.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Killingsworth C.R., Melnick S.B., Chapman F.W., et al. Defibrillation threshold and cardiac responses using an external biphasic defibrillator with pediatric and adult adhesive patches in pediatric-sized piglets. Resuscitation. 2002;55:177–185. doi: 10.1016/s0300-9572(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 8.Tang W., Weil M.H., Sun S., et al. The effects of biphasic waveform design on post-resuscitation myocardial function. J Am Coll Cardiol. 2004;43:1228–1235. doi: 10.1016/j.jacc.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Xie J., Weil M.H., Sun S., et al. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation. 1997;96:683–688. doi: 10.1161/01.cir.96.2.683. [DOI] [PubMed] [Google Scholar]
- 10.Link M.S., Berkow L.C., Kudenchuk P.J., et al. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 11.Lobo R., Jaffe A.S., Cahill C., et al. Significance of high-sensitivity troponin T after elective external direct current cardioversion for atrial fibrillation or atrial flutter. Am J Cardiol. 2018;121:188–192. doi: 10.1016/j.amjcard.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Sandoval Y., Jaffe A.S. Using high-sensitivity cardiac troponin T for acute cardiac care. Am J Med. 2017;130:1358–1365. doi: 10.1016/j.amjmed.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Elecsys Troponin T Gen 5 STAT [package insert] Roche Diagnostics; Indianapolis: 2018. [Google Scholar]
- 14.ARCHITECT STAT High Sensitivity Troponin-I [package insert] Abbott Laboratories; Abbott Park, IL: 2019. [Google Scholar]
- 15.Apple F.S., Wu A.H.B., Sandoval Y., et al. Sex-specific 99th percentile upper reference limits for high sensitivity cardiac troponin assays derived using a Universal Sample Bank. Clin Chem. 2020;66:434–444. doi: 10.1093/clinchem/hvz029. [DOI] [PubMed] [Google Scholar]
- 16.Wu A.H., Lu Q.A., Todd J., Moecks J., Wians F. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55:52–58. doi: 10.1373/clinchem.2008.107391. [DOI] [PubMed] [Google Scholar]
- 17.Vasile V.C., Saenger A.K., Kroning J.M., Klee G.G., Jaffe A.S. Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57:1080–1081. doi: 10.1373/clinchem.2011.162545. [DOI] [PubMed] [Google Scholar]
- 18.Frankenstein L., Wu A.H.B., Hallermayer K., Wians F.H., Jr., Giannitsis E., Katus H.A. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin Chem. 2011;57:1068–1071. doi: 10.1373/clinchem.2010.158964. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K., Mair J., Giannitsis E., et al. Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 20.Kern K.B., Hilwig R.W., Rhee K.H., Berg R.A. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Faddy S.C., Powell J., Craig J.C. Biphasic and monophasic shocks for transthoracic defibrillation: a meta analysis of randomised controlled trials. Resuscitation. 2003;58:9–16. doi: 10.1016/s0300-9572(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 22.Gliner B.E., Lyster T.E., Dillion S.M., Bardy G.H. Transthoracic defibrillation of swine with monophasic and biphasic waveforms. Circulation. 1995;92:1634–1643. doi: 10.1161/01.cir.92.6.1634. [DOI] [PubMed] [Google Scholar]
- 23.White R.D., Kerber R.E. In: The Textbook of Emergency Cardiovascular Care and CPR. Field J.M., Kudenchuk P.J., O’Connor R., VandenHoek, editors. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. Ventricular fibrillation and defibrillation: experimental and clinical experience with waveforms and energy; pp. 222–231. [Google Scholar]
- 24.Neal S., Ngarmukos T., Lessard D., Rosenthal L. Comparison of the efficacy and safety of two biphasic defibrillator waveforms for the conversion of atrial fibrillation to sinus rhythm. Am J Cardiol. 2003;92:810–814. doi: 10.1016/s0002-9149(03)00888-9. [DOI] [PubMed] [Google Scholar]
- 25.Knight B.P. Cardioversion for specific arrhythmias. UpToDate. https://www.uptodate.com/contents/cardioversion-for-specific-arrhythmias#! Available at:
- 26.Joglar J.A., Hamdan M.H., Ramaswamy K., et al. Initial energy for elective external cardioversion of persistent atrial fibrillation. Am J Cardiol. 2000;86:348–350. doi: 10.1016/s0002-9149(00)00932-2. [DOI] [PubMed] [Google Scholar]
- 27.Glover B.M., Walsh S.J., McCann C.J., et al. Biphasic energy selection for transthoracic cardioversion of atrial fibrillation. The BEST AF Trial. Heart. 2008;94:884–887. doi: 10.1136/hrt.2007.120782. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A.S., Lauridsen K.G., Torp P., Bach L.F., Rickers H., Løfgren B. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur Heart J. 2020;41:626–631. doi: 10.1093/eurheartj/ehz585. [DOI] [PubMed] [Google Scholar]
- 29.Deakin C.D., Ambler J.J., Shaw S. Changes in transthoracic impedance during sequential biphasic defibrillation. Resuscitation. 2008;78:141–145. doi: 10.1016/j.resuscitation.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Deakin C.D., Connelly S., Wharton R., Yuen H.M. A comparison of rectilinear and truncated exponential biphasic waveforms in elective cardioversion of atrial fibrillation: a prospective randomized controlled trial. Resuscitation. 2013;84:286–291. doi: 10.1016/j.resuscitation.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Katus H.A., Remppis A., Scheffold T., Diederich K.W., Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991;67:1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 32.Writing Committee for the VISION Study Investigators. Devereaux P.J., Biccard B.M., Sigamani A., et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642–1651. doi: 10.1001/jama.2017.4360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.