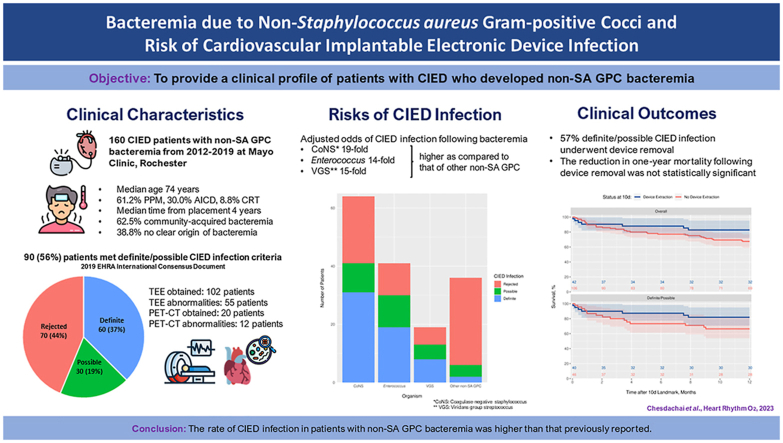
Abstract
Background
Cardiovascular implantable electronic device (CIED) infection carries significant morbidity and mortality with bacteremia being a possible marker of device infection. A clinical profile of non–Staphylococcus aureus gram-positive cocci (non-SA GPC) bacteremia in patients with CIED has been limited.
Objective
To examine characteristics of patients with CIED who developed non-SA GPC bacteremia and risk of CIED infection.
Methods
We reviewed all patients with CIED who developed non-SA GPC bacteremia at the Mayo Clinic between 2012 and 2019. The 2019 European Heart Rhythm Association Consensus Document was used to define CIED infection.
Results
A total of 160 patients with CIED developed non-SA GPC bacteremia. CIED infection was present in 90 (56.3%) patients, in whom 60 (37.5%) were classified as definite and 30 (18.8%) as possible. This included 41 (45.6%) cases of coagulase-negative Staphylococcus (CoNS), 30 (33.3%) cases of Enterococcus, 13 (14.4%) cases of viridans group streptococci (VGS), and 6 (6.7%) cases of other organisms. The adjusted odds of CIED infection in cases due to CoNS, Enterococcus, and VGS bacteremia were 19-, 14-, and 15-fold higher, respectively, as compared with other non-SA GPC. In patients with CIED infection, the reduction in risk of 1-year mortality associated with device removal was not statistically significant (hazard ratio 0.59; 95% confidence interval 0.26–1.33; P = .198).
Conclusions
The prevalence of CIED infection in non-SA GPC bacteremia was higher than previously reported, particularly in cases due to CoNS, Enterococcus species, and VGS. However, a larger cohort is needed to demonstrate the benefit of CIED extraction in patients with infected CIED due to non-SA GPC.
Keywords: Bacteremia, Cardiovascular implantable electronic device, Consensus document, Infection, Definition, Non–Staphylococcus aureus gram-positive cocci, Outcomes
Graphical abstract
Key Findings.
-
▪
The rate of cardiovascular implantable electronic device (CIED) infection following non–Staphylococcus aureus gram-positive cocci was higher than previously reported.
-
▪
Patients with coagulase-negative staphylococcal, enterococcal, and viridans group streptococcal bacteremia are increased risk of CIED infection as compared with other non–S. aureus gram-positive cocci.
-
▪
While 72% of patients with definite CIED infection underwent device removal, only 27% of patients with possible CIED infection underwent device removal.
Introduction
Over the past 2 decades, the number of people living with cardiovascular implantable electronic devices (CIEDs) has rapidly increased due to the aging population and novel indications for CIED usage.1 The growth rate of CIED implantation has also led to a surge in the incidence of CIED infection, which has significantly impacted healthcare utility, financial burden, quality of life, and mortality.2, 3, 4 Bacteremia from certain organisms in patients with CIED is a serious concern because it could be a manifestation of CIED infection or predispose to hematogenous seeding of the device.5,6 Staphylococcus aureus bacteremia (SAB) is associated with a 20% to 80% risk of CIED infection,7, 8, 9, 10, 11 while the rate of CIED infection following gram-negative bacteremia is only 6% to 17%.9,12,13 In contrast to SAB and gram-negative bacteremia, the data on the risk of CIED infection following non–S. aureus gram-positive cocci (non-SA GPC) bacteremia are both limited and dated. A previous study from our institution14 demonstrated that coagulase-negative Staphylococcus (CoNS) bacteremia posed the highest risk of CIED infection compared with other non-SA GPC bacteremia. Given the complexity of devices and updated practice guideline for diagnosis and management of CIED infection, the prevalence and risk of CIED infection following non-SA GPC bacteremia may have changed over the past decade. Therefore, the goal of this study was to provide a contemporary clinical characterization of patients with CIED who developed non-SA GPC bacteremia and the rate of CIED infection based on pathogen.
Methods
Participants and data collection
A retrospective cohort study included all adult patients (18 years of age or older) with CIED who developed first episode of non-SA GPC bacteremia from January 1, 2012, to December 31, 2019, at the Mayo Clinic in Rochester, Minnesota. Exclusion criteria included: (1) patients with a left ventricular assist device, (2) patients who developed polymicrobial bacteremia, and (3) patients who declined Minnesota research authorization to use their medical record for research purposes. Cardiovascular Devices Database and Mayo Data Explorer software were used to identify the cases. Mayo Data Explorer retrieves data from multiple Mayo Clinic clinical databases that contain over 30 years of electronic medical record systems including microbiology data. Clinical variables including demographics, hospitalization, microbiology, treatment, and outcomes were manually abstracted from electronic medical records. All data were collected and managed using REDCap electronic data capture tools15,16 hosted at the Mayo Clinic. The research in this study was conducted according to the Helsinki Declaration guidelines. The study was reviewed by the Mayo Clinic Institutional Review Board and granted an exemption due to the use of de-identified and retrospective data (number 20-009376). The written informed consent was also waived due to the same reasons.
Definitions and objectives
Non-SA GPC included aerobic GPC in the following genera: Abiotrophia, Aerococcus, Enterococcus, Gemella, Granulicatella, Micrococcus, Staphylococcus except S. aureus, and Streptococcus. A bacteremia episode was defined as a positive blood culture with non-SA GPC. Contaminated blood culture with non-SA GPC was previously defined.14,17 These included (1) discordant growth in only 1 bottle out of multiple bottles without prior antimicrobial exposure; (2) culture from a central or arterial catheter in the absence of positive culture from peripheral blood; (3) incompatible clinical syndromes (or asymptomatic) without clinical deterioration in the absence of targeted antimicrobial therapy; and (4) that the primary care team pursued a more plausible alternative diagnosis. Patients with contaminated blood cultures were excluded from our study. Follow-up blood cultures were obtained daily in all patients until clearance of bacteremia. The definitions for type of bacteremia (community acquired, healthcare associated, and nosocomial), time to positivity, and duration of bacteremia are detailed in previous studies.7,18
CIEDs include automated implantable cardioverter-defibrillator, cardiac resynchronization therapy (CRT), and permanent pacemaker. The definition of CIED infection was based on the 2019 European Heart Rhythm Association (EHRA) International Consensus document.19 Patients were categorized into definite, possible, and rejected CIED infection. Definite CIED infection criteria were met if (1) there was evidence of clinical signs of pocket or generator infection or (2) 2 major criteria or 1 major criterion plus 3 minor criteria were met. Possible CIED infection criteria needed either 1 major criterion plus 1 minor criterion or 3 minor criteria. Rejected CIED infection was defined as patients who did not meet the definite and possible criteria. Major and minor criteria were adopted from modified Duke criteria and European Society of Cardiology 2015 guidelines criteria.20 For purposes of this study, the definite and possible CIED infections were combined into a single CIED infection group. Complete CIED extraction was defined as removal of the generator, all leads, and lead material from the pocket site and the cardiovascular space.21 A new episode of bacteremia with the same organism within 12 weeks of initial bacteremic episode was defined as relapse of bacteremia.
The primary objective was to determine the rate of CIED infection following non-SA GPC bacteremia episode. Secondary objectives were to assess the following: (1) risk of CIED infection based on type of non-SA GPC, (2) all-cause mortality at 1 year after bacteremia, and (3) effect of CIED extraction and mortality.
Statistical analysis
Descriptive statistics on baseline data are reported as median (interquartile range [IQR]) for continuous variables and number and percentage for categorical variables. Differences between the groups with and without CIED infection were determined using Wilcoxon rank sum tests and Pearson chi-square tests, as appropriate. A multivariable logistic regression model for CIED infection was constructed to estimate the odds of CIED infection based on pathogen designation, adjusting for type of device, duration of bacteremia, and type of bacteremia. Survival was estimated over 1-year follow-up using the Kaplan-Meier method. The association of device extraction with 1-year mortality was analyzed using extended Cox proportional hazards regression, treating device removal as a time-dependent covariate, and stratifying on the 2-group classification for CIED infection. Within each stratum, the treatment effect was assessed by a likelihood ratio chi-square test. All analyses were performed using R statistical software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics
A total of 160 patients with CIED developed non-SA GPC bacteremia from 2012 to 2019. Median age was 73.8 (IQR 62.5–83.6) years; 116 (72.5%) were male, and 151 (94.4%) were White. Valvular abnormalities included 47 (29.4%) prosthetic heart valves, 8 (5.0%) annuloplasties or other valve repairs, and 22 (13.8%) other valvular abnormalities without repair. The median Charlson Comorbidity Index was 6 (IQR 4–7). Ninety-eight (61.3%) patients had permanent pacemaker, 48 (30.0%) had an automated implantable cardioverter-defibrillator, and 14 (8.8%) had CRT. The median time from the device placement to first episode of non-SA GPC bacteremia was 3.7 (IQR 1.3–8.0) years. Types of bacteremia were community acquired (n = 100 [62.5%]), healthcare associated (n = 35 [21.9%]), and nosocomial (n = 25 [15.6%]). Sixty-two (38.8%) patients had no clear origin of bacteremia. Among those with known origin of bacteremia, the most common source was central venous catheter–related bacteremia (n = 32 [32.7%]) followed by skin and soft tissue infection (n = 12 [12.2%]). The clinical characteristics stratified by diagnostic criteria can be found in Table 1. The microorganisms causing bacteremia were CoNS (n = 64 [40.0%]), Enterococcus (n = 41 [25.6%]), viridans group streptococci (VGS) (n = 19 [11.9%]), non–group A and B beta-hemolytic streptococci (n = 10 [6.2%]), Streptococcus pneumoniae (n = 9 [5.6%]), S. agalactiae (n = 8 [5.0%]), S. pyogenes (n = 6 [3.8%]), Granulicatella (n = 2 [1.3%]), and Micrococcus (n = 1 [0.6%]). The detailed species of CoNS, Enterococcus, VGS, and non–group A and B beta-hemolytic streptococci are highlighted in Table 2. Median time to positivity of the first set of blood cultures was 14 (IQR 11–20) hours and median duration of bacteremia was 2 (IQR 1–3) days.
Table 1.
Clinical characteristics of patients with CIEDs who developed non-SA GPC bacteremia from 2012 to 2019
| Characteristic | Rejected CIED infection (n = 70) | Definite/possible CIED infection (n = 90) | P value |
|---|---|---|---|
| Male | 48 (68.6) | 68 (75.6) | .326∗ |
| Age, y | 75.5 (63.0–85.4) | 72.8 (61.8–82.4) | .196† |
| BMI, kg/m2 | 28.9 (24.4–35.0) | 26.2 (24.0–32.5) | .159† |
| Comorbidities | |||
| Charlson Comorbidity Index | 7 (5–8) | 6 (4–7) | .037† |
| Diabetes mellitus | 21 (30.0) | 37 (41.1) | .147∗ |
| ESRD with dialysis | 7 (10.0) | 6 (6.7) | .444∗ |
| Moderate or severe liver disease | 2 (2.9) | 1 (1.1) | .419∗ |
| Malignancy | 15 (21.4) | 11 (12.2) | .117∗ |
| Prosthetic heart valve | 3 (4.3) | 44 (48.9) | <.001∗ |
| Annuloplasty or other valve repairs | 1 (1.4) | 7 (7.8) | .068∗ |
| Other valvular abnormality | 8 (11.4) | 14 (15.6) | .452∗ |
| Congenital heart diseases | 0 (0.0) | 6 (6.7) | .028∗ |
| Type of current device | .121∗ | ||
| PPM | 42 (60.0) | 56 (62.2) | |
| AICD | 25 (35.7) | 23 (25.6) | |
| CRT | 3 (4.3) | 11 (12.2) | |
| Duration of bacteremia, d | 1 (1–2) | 3 (2–4) | <.001† |
| Type of bacteremia | .002∗ | ||
| Community acquired | 34 (48.6) | 66 (73.3) | |
| Healthcare associated | 24 (34.3) | 11 (12.2) | |
| Nosocomial | 12 (17.1) | 13 (14.4) | |
Values are n (%) or median (interquartile range)
AICD = automatic implantable cardioverter-defibrillator; BMI = body mass index; CIED = cardiovascular implantable electronic device; CRT = cardiac resynchronization therapy; ESRD = end-stage renal disease; GPC = gram-positive cocci; PPM = permanent pacemaker; SA = Staphylococcus aureus.
Pearson chi-square test.
Wilcoxon rank sum test.
Table 2.
Species of organisms
| Coagulase-negative staphylococci (n = 64) | Enterococci (n = 41) | Viridans group streptococci (n = 19) | Other non-SA GPC (n = 36) |
||
|---|---|---|---|---|---|
| Group A and B beta-hemolytic streptococci (n = 23) | Non–group A and B beta-hemolytic streptococci (n = 10) | Other (n = 3) | |||
| Staphylococcus epidermidis (n = 44, 68.8%) | Enterococcus faecalis (n = 36, 87.8%) | Streptococcus mitis (n = 9, 47.4%) | Streptococcus pneumoniae (n = 9, 39.1%) | Streptococcus dysgalactiae (n = 6, 60.0%) | Granulicatella adiacens (n = 2, 66.7%) |
| Staphylococcus lugdunensis (n = 4, 6.3%) | Enterococcus faecium (n = 4, 9.8%) | Streptococcus anginosus (n = 3, 15.8%) | Streptococcus agalactiae (n = 8, 34.8%) | Group C Streptococcus (n = 2, 20.0%) | Micrococcus (n = 1, 33.3%) |
| S. capitis (n = 2, 3.1%) | Enterococcus gallinarum (n = 1, 2.4%) | Streptococcus bovis (n = 3, 15.8%) | Streptococcus pyogenes (n = 6, 26.1%) | Streptococcus canis (n = 1, 10.0%) | |
| Staphylococcus caprae (n = 1, 1.6%) | Streptococcus mutans (n = 2, 10.5%) | Group G Streptococcus (n = 1, 10.0%) | |||
| Staphylococcus hominis (n = 1, 1.6%) | No identification to the species level (n = 2, 10.5%) | ||||
| No identification to the species level (n = 12, 18.8%) | |||||
GPC = gram-positive cocci; SA = Staphylococcus aureus
Clinical courses
All 160 patients were hospitalized for a median length of stay of 11 (IQR 7–17) days. Eighty-eight (55.0%) patients required intensive care support during their hospitalization. Seventy-one (44.4%) patients had fever on admission. Only 4 (2.5%) patients had signs of pocket infection. Transthoracic echocardiography and transesophageal echocardiography (TEE) were obtained in 78 (48.8%) and 102 (63.8%) patients, respectively. Positive transthoracic echocardiography for vegetations was demonstrated in 4 (5.1%) patients: 3 with vegetations seen on the aortic valve and 1 with vegetations seen on the mitral valve. TEE abnormalities were demonstrated in 55 (53.9%) patients. These finding included vegetations on the device lead (n = 31), vegetations on the aortic valve (n = 23), vegetations on the mitral valve (n = 7), perivalvular abscess (n = 5), unclear findings (n = 3), and vegetations on the tricuspid valve (n = 2). Positron emission tomography–computed tomography (PET-CT) was obtained in 20 (12.5%) patients, of whom 12 (60.0%) had findings suggestive of infection of either the valves (n = 6), CIED pockets (n = 3), or leads (n = 3).
According to the 2019 EHRA International Consensus classification, 90 (56.3%) patients had CIED infection. Sixty (37.5%) patients were classified as definite CIED infection and 30 (18.8%) patients with possible CIED infection (Supplemental Figure 1). A significantly higher proportion of patients in the CIED infection group had prosthetic heart valve (48.9% vs 4.3%; P < .001) and congenital heart disease (6.7% vs 0%; P = .028). When compared with the rejected CIED infection group, those in the CIED infection group were more likely to present with community-acquired bacteremia (73.3% vs 48.6%; P = .002) and had a longer duration of bacteremia (median 3 [IQR 2–4] days vs 1 [IQR 1–2] days; P < .001).
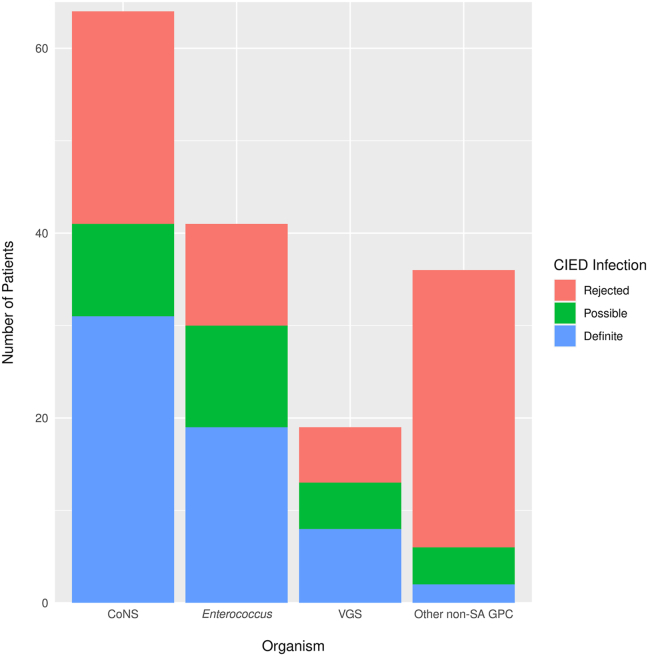
When stratified by organism, the proportion of patients with CIED infection was 41 (64.1%) of 64 for CoNS, 30 (73.2%) of 41 for Enterococcus species, 13 (68.4%) of 19 VGS, and 6 (16.6%) of 36 for other non-SA GPC (these 6 cases were Granulicatella species, n = 2; S. agalactiae, n = 2; S. pneumoniae, n = 1; non–group A and B beta-hemolytic streptococci, n = 1). (Figure 1). After adjustment for type of device, duration of bacteremia, and type of bacteremia, the odds of CIED infection for those with CoNS, Enterococcus, and VGS bacteremia were 19-fold (95% confidence interval [CI] 4–81), 14-fold (95% CI 4–52), and 15-fold (95% CI 3–64) higher, respectively, as compared with that with other non-SA GPC (P < .001) (Table 3).
Figure 1.
Proportion of organism causing cardiovascular implantable electronic device (CIED) infection following bacteremia. The proportion of CIED infection was high in the patients with coagulase-negative Staphylococcus (CoNS), enterococci, and viridans group streptococci (VGS) bacteremia compared with other non–Staphylococcus aureus gram-positive cocci (non-SA GPC).
Table 3.
Odds of CIED infection from prespecified risk factors
| Predictor | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Type of current device | .041 | |
| PPM | 1.0 (reference) | — |
| AICD | 0.4 (0.1–1.0) | — |
| CRT | 2.3 (0.5–11.0) | — |
| Organism of bacteremia | <.001 | |
| CoNS | 19.0 (4.5–80.7) | — |
| Enterococci | 13.8 (3.7–52.0) | — |
| VGS | 14.7 (3.4–63.9) | — |
| Other non-SA GPC | 1.0 (reference) | — |
| Duration of bacteremia (per 1 d of bacteremia) | 2.1 (1.3–3.2) | .001 |
| Type of bacteremia | <.001 | |
| Community acquired | 1.0 (reference) | — |
| Healthcare associated | 0.1 (0.04–0.4) | — |
| Nosocomial | 0.2 (0.1–0.7) | — |
Results obtained from a multivariable logistic regression model for CIED infection based on all the variables listed in the table.
AICD = automatic implantable cardioverter-defibrillator; CI = confidence interval; CIED = cardiovascular implantable electronic device; CoNS = coagulase-negative Staphylococcus; CRT = cardiac resynchronization therapy; ESRD = end-stage renal disease; GPC = gram-positive cocci; SA = Staphylococcus aureus; VGS = viridans group streptococci.
Management and outcomes
Among those with either definite or possible CIED infection, 51 (56.7%) patients underwent complete CIED extraction after a median of 6.0 (IQR 3.5–10.0) days from the time of bacteremia. The clinical characteristic of patient who underwent extraction vs who did not was demonstrated in Supplemental Table 1. Forty-seven (92.2%) extracted devices were sent for bacterial culture, of which 31 (66.0%) grew similar organisms as in the blood culture and 16 (34.0%) showed no growth. Nineteen patients of those who did not undergo device removal received long-term antibiotic suppression. The clinical course of the patients who did not undergo device extraction can be found in Supplemental Figure 2. A total of 6 patients had relapsing bacteremia within 3 months after initial episode of bacteremia; of these, the index CIED infection was definite in 2, possible in 3, and rejected in 1. All 6 were diagnosed as a definite CIED infection at the time of relapse (Supplemental Table 2).
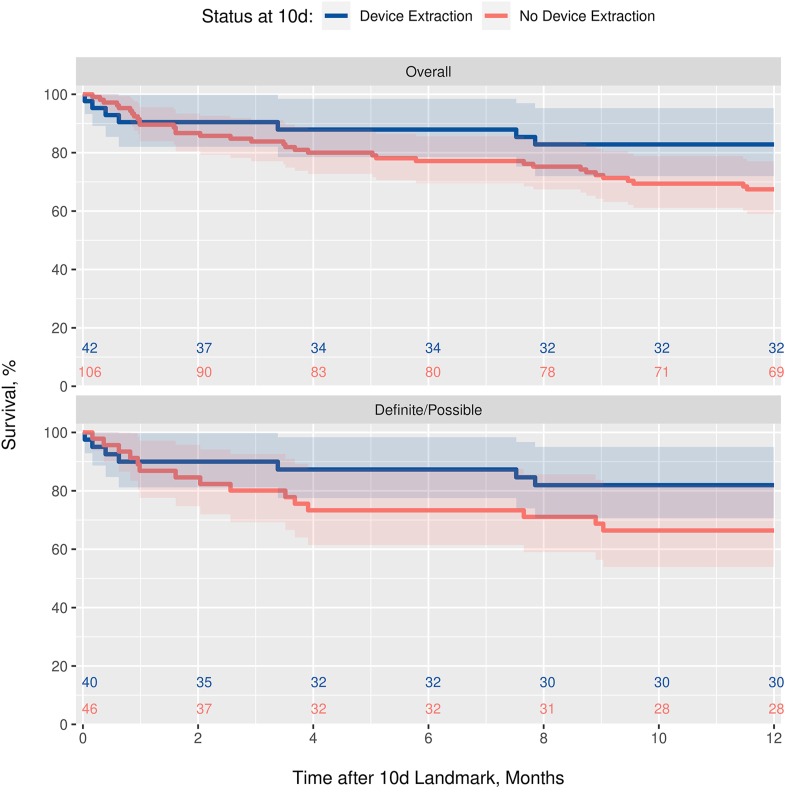
Overall, 1-year survival was 66.6%, with no difference observed in the patients with CIED infection vs those without (P = .354). A landmark analysis of 10-day survivors (n = 148) was used to graphically depict 1-year survival curves according to “early” device removal (within the first 10 days), both overall and in those with CIED infection (Figure 2). The treatment effect was formally assessed within each diagnostic group in unadjusted Cox regression analysis with device removal incorporated as a time-dependent covariate. The reduction in risk of 1-year mortality associated with device removal was not statistically significant among the patients with possible or definite CIED infection (P = .198; hazard ratio 0.59; 95% CI 0.26–1.33) or among patients for whom infection was rejected (P = .101; because few of the patients in the rejected group had their device removed, estimates of the hazard ratio of device extraction could not be computed).
Figure 2.
Survival curves by device removal status via 10-day landmark analysis. Based on a landmark analysis of 10-day survivors, the figure shows Kaplan-Meier curves of survival to 12 months according to device extraction status at day 10, both overall and in the subgroup with cardiovascular implantable electronic device infection. The plot for the subgroup without cardiovascular implantable electronic device infection was suppressed because only 2 of the patients in the rejected group had their device removed by day 10.
Discussion
The finding of over half of the patients with non-SA GPC bacteremia in our cohort had CIED infection was somewhat unanticipated. Only 4 patients had clinical sign of pocket infection. Risk factors associated with CIED infection included presence of a prosthetic valve, CRT devices, community-acquired bacteremia, and prolonged bacteremia without a defined origin. Regarding the pathogens causing bacteremia, the highest rate of CIED infection was due to CoNS, followed by enterococci and VGS. Only half of patients with CIED infection underwent complete device removal, while about 49% of patients who did not undergo device removal received chronic oral antibiotic suppressive therapy. The impact of CIED removal on 1-year mortality was not demonstrated in our study.
Madhavan and colleagues14 investigated 74 patients with CIED who developed non-SA GPC bacteremia at the Mayo Clinic from 2001 to 2007. There are distinct observations in the current study as compared with that of the prior investigation. First, the prevalence of CIED infection following non-SA GPC bacteremia was higher (56%) than previously reported (30%). This is likely due to differences of CIED diagnostic criteria of the 2019 EHRA International Consensus document. It included the category of possible CIED infection, which had not been used in the previous study. Interestingly, most of the patients in our cohort automatically fulfilled 1 major criterion (“blood culture positive for typical organisms for CIED infection or infective endocarditis”) to begin with, and they needed only an additional minor criterion such as fever to be categorized as a possible CIED infection. Hence, it appears that criteria for possible CIED infection increase diagnostic sensitivity of CIED infection yet are not specific. The overdiagnosis of the possible CIED infection group was evident in our previous study in the setting of gram-negative bacilli bacteremia.13 Nevertheless, when we compare only the number of definite CIED infections in our cohort with the previous study, the prevalence of CIED infection is similar.
Second, in addition to the type and duration of bacteremia, the species of non-SA GPC bacteremia that pose a sizable risk for CIED infection have not changed during the past decade. Our study reiterated that CIED infection is highly likely in the setting of CoNS, enterococcal, and VGS bacteremia. The pattern is comparable to a proclivity for development of infective endocarditis following an episode of bacteremia due to these organisms. In a prospective multicenter study of 344 with E. faecalis bacteremia,22 the prevalence of definite infective endocarditis was approximately 26%. Seventy percent of enterococcal bacteremia patients in our cohort met the criteria for CIED infection. Interestingly, this included 4 of 5 non-faecalis enterococcal species (3 E. faecium and 1 E. gallinarum), which were not previously described as high-risk organisms associated with infective endocarditis. VGS causing bacteremia in our cohort included S. mitis, S. mutans, S. bovis, and S. anginosus. These organisms have been categorized in the high to very high-risk groups (as high as 50% in prevalence) for infective endocarditis in the setting of bacteremia.23 Therefore, the provider should be vigilant for CIED infection when encountered bacteremia from these organisms.
Third, the use of PET-CT has increased. Twenty patients in our cohort underwent PET-CT and 12 of them had positive results, while no patients underwent PET-CT in the earlier investigation. A systematic review and meta-analysis of 18 observational studies demonstrated that PET-CT had high diagnostic sensitivity and specificity for CIED infection, especially in the setting of infected device lead.24 PET-CT was recently included in endocarditis or CIED guidelines as an additive imaging modality.19, 20, 21
Most patients with TEE abnormality in our study had vegetation on the device lead, which would meet the definite CIED infection criteria without an additional PET-CT scan. Interestingly, a recent study from our institution investigated 25 consecutive cases of CIED lead infection and found that TEE is not a reliable tool to distinguish between infectious and noninfectious thrombi.25 According to this finding, the diagnosis of definite CIED infection for those who has only lead vegetation (without vegetation seen on the valve) may have been overestimated. Hence, it is tempting to speculate that CIED infection could be broken down into 2 separate entities: (1) device infection and (2) cardiac valve infection. On the one hand, PET-CT may be more useful in the diagnosis of device infection and in some cases where a prosthetic valve is also present with the concern for prosthetic valve endocarditis; in contrast, TEE may be more useful in the diagnosis of native valve endocarditis. Thus, the need for both procedures to enhance the likelihood of securing a correct diagnosis should be further investigated.
Fourth, the device extraction rate was not different from the previous study. Approximately 54% of CIED-infected patients in the Madhavan and colleagues study14 underwent device extraction, compared with 56% in our study. Based on multiple guidelines,19,21,26 all patients with CIED infection should undergo complete device removal. However, there are factors, such as poor surgical candidacy, that influence the likelihood of complete device removal. Our recent study demonstrated that CIED extraction provided a survival benefit among patients with SAB with possible or definite CIED infection after SAB episode.7 However, the survival benefit was not observed with gram-negative bacteremia13 and the current study. There are at least 2 possibilities to explain the observed lack of survival benefit. First, successful chronic oral antibiotic suppressive therapy may provide some survival benefit. Second, there is marked heterogeneity of virulence among non-SA GPC. Finally, there may have been an overdiagnosis of CIED infection in patients who met the possible CIED infection criteria.
Our study has several limitations. First, the 1-year mortality rate did not permit a detailed multivariable survival analysis to rigorously assess the treatment benefit of device extraction, especially when stratifying on CIED infection status. It was also challenging to differentiate the risks of CIED infection between different species within CoNS, enterococci, and VGS due to limit in number and lack of species identification be done in the laboratory in the early years of the investigation. Second, the number of patients who underwent PET-CT was low, which likely influenced the ability to diagnosis true CIED infection. Third, there was a variability in the amount of evaluation such as TEE on the patients with CoNS, enterococci, or VGS as opposed to other pathogens, which could result in a detection bias. Fourth, both selection bias and lack of generalizability were unavoidable.
Conclusion
Our study provides an insight into an important clinical question: what do we do in the setting of non-SA GPC bacteremia in the patients with CIED? We found that patients with CoNS, Enterococcus, and VGS bacteremia, compared with those with other organisms, are at higher risk for CIED infection regardless of pocket finding from clinical exam. Additional diagnostic modalities such as TEE or PET-CT should be obtained to look for device infection in these patients.
Acknowledgments
Funding Sources
This research was made possible by the Clinical and Translational Science Awards (CTSA) Grant UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS).
Disclosures
Supavit Chesdachai – No conflict of interest. Larry M. Baddour – UpToDate, Inc. (royalty payments – authorship duties), Boston Scientific (consultant duties). M. Rizwan Sohail – Medtronic (research funding, honoraria/consulting fees), Boston Scientific (honoraria/consulting fee), Philips (honoraria/consulting fee). Bharath Raj Palraj – Armor Health (consulting fee). Malini Madhavan – Convatec (consulting), Biotronik Inc (consulting), Biosense Webster (consulting), Boston Scientific (research funding). Hussam Tabaja – No conflict of interest. Madiha Fida – No conflict of interest. Brian D. Lahr – No conflict of interest. Daniel C. DeSimone – No conflict of interest.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Consent was waived due to the use of de-identified and retrospective data.
Ethics Statement
The research in this study was conducted according to the Helsinki Declaration guidelines. The study was reviewed by the Mayo Clinic Institutional Review Board and granted an exemption due to the use of de-identified and retrospective data (Study IRB number 20-009376).
Appendix. Supplementary data
References
- 1.Dai M., Cai C., Vaibhav V., et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population-based study. J Am Coll Cardiol EP. 2019;5:1071–1080. doi: 10.1016/j.jacep.2019.06.016. 2019/09/01/ [DOI] [PubMed] [Google Scholar]
- 2.Voigt A., Shalaby A., Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33:414–419. doi: 10.1111/j.1540-8159.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 3.Sohail M.R., Henrikson C.A., Braid-Forbes M.J., Forbes K.F., Lerner D.J. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171:1821–1828. doi: 10.1001/archinternmed.2011.441. Nov 14. [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff B.L., Boriani G., Mittal S., et al. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esquer Garrigos Z., George M.P., Khalil S., et al. Predictors of bloodstream infection in patients presenting with cardiovascular implantable electronic device pocket infection. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz084. ofz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSimone D.C., Sohail M.R. Management of bacteremia in patients living with cardiovascular implantable electronic devices. Heart Rhythm. 2016;13:2247–2252. doi: 10.1016/j.hrthm.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Chesdachai S., Baddour L.M., Sohail M.R., et al. Evaluation of European Heart Rhythm Association consensus in patients with cardiovascular implantable electronic devices and Staphylococcus aureus bacteremia. Heart Rhythm. 2022;19:570–577. doi: 10.1016/j.hrthm.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Sohail M.R., Palraj B.R., Khalid S., et al. Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (PREDICT-SAB) Circ Arrhythm Electrophysiol. 2015;8:137–144. doi: 10.1161/CIRCEP.114.002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maskarinec S.A., Thaden J.T., Cyr D.D., et al. The risk of cardiac device-related infection in bacteremic patients is species specific: results of a 12-year prospective cohort. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofx132. ofx132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uslan D.Z., Dowsley T.F., Sohail M.R., et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol. 2010;33:407–413. doi: 10.1111/j.1540-8159.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 11.Chamis A.L., Peterson G.E., Cabell C.H., et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104:1029–1033. doi: 10.1161/hc3401.095097. [DOI] [PubMed] [Google Scholar]
- 12.Uslan D.Z., Sohail M.R., Friedman P.A., et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis. 2006;43:731–736. doi: 10.1086/506942. [DOI] [PubMed] [Google Scholar]
- 13.Chesdachai S., Baddour L.M., Sohail M.R., et al. Risk of cardiovascular implantable electronic device infection in patients presenting with gram-negative bacteremia. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac444. ofac444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavan M., Sohail M.R., Friedman P.A., et al. Outcomes in patients with cardiovascular implantable electronic devices and bacteremia caused by gram-positive cocci other than Staphylococcus aureus. Circ Arrhythm Electrophysiol Dec. 2010;3:639–645. doi: 10.1161/CIRCEP.110.957514. [DOI] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chesdachai S., Yetmar Z.A., Tabaja H., et al. Contemporary experience of Abiotrophia, Granulicatella and Gemella bacteremia. J Infect. 2022;84:511–517. doi: 10.1016/j.jinf.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Friedman N.D., Kaye K.S., Stout J.E., et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 19.Blomström-Lundqvist C., Traykov V., Erba P.A., et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Europace. 2020;22:515–549. doi: 10.1093/europace/euz246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habib G., Lancellotti P., Antunes M.J., et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 21.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Dahl A., Iversen K., Tonder N., et al. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol. 2019;74:193–201. doi: 10.1016/j.jacc.2019.04.059. [DOI] [PubMed] [Google Scholar]
- 23.Chamat-Hedemand S., Dahl A., Østergaard L., et al. Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal species. Circulation. 2020;142:720–730. doi: 10.1161/CIRCULATIONAHA.120.046723. [DOI] [PubMed] [Google Scholar]
- 24.Holcman K., Rubiś P., Stępień A., et al. The diagnostic value of 99mTc-HMPAO-labelled white blood cell scintigraphy and 18F-FDG PET/CT in cardiac device-related infective endocarditis-a systematic review. J Pers Med. 2021;11:1016. doi: 10.3390/jpm11101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George M.P., Esquer Garrigos Z., Vijayvargiya P., et al. Discriminative ability and reliability of transesophageal echocardiography in characterizing cases of cardiac device lead vegetations versus noninfectious echodensities. Clin Infect Dis. 2021;72:1938–1943. doi: 10.1093/cid/ciaa472. [DOI] [PubMed] [Google Scholar]
- 26.Baddour L.M., Epstein A.E., Erickson C.C., et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.