Microvillus inclusion disease (MVID) is a congenital disorder that presents with severe secretory diarrhea, typically within the first few hours of life. Inactivating mutations of myosin VB (MYO5B) cause MVID, which usually requires lifetime total parenteral nutrition or small-bowel transplantation.1 A recent analysis of 114 MYO5B mutations identified in MVID patients demonstrated that most mutations were compound heterozygous.2 Although previously developed homozygous MYO5B knockout animal models showed success in recapitulating MVID,3 there is a lack of research into the phenotypes caused by compound heterozygous mutations of MYO5B.
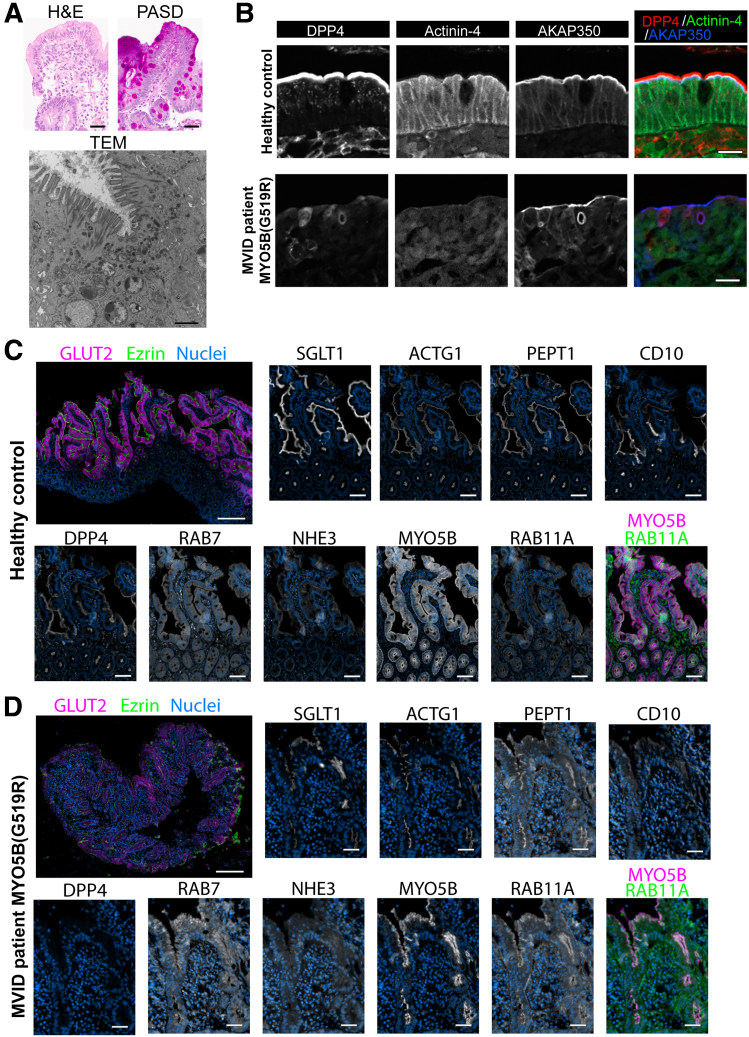
Targeted exome sequencing in an MVID patient and both parents identified 2 variants in the MYO5B gene: c.1821delG (p.S608HfsX14), likely a de novo mutation that leads to early truncation, and c.1555G>A (p.G519R) (Supplementary Figure 1A), which was maternally inherited. This patient mutation is referred to as MYO5B(G519R) in this study. The patient had copious diarrhea from birth with non-ion gap acidosis and intermittent feeding intolerance. An esophagogastroduodenoscopy and flexible sigmoidoscopy at 3 weeks of age were grossly normal. Biopsy specimens showed villus blunting and abnormal accumulation of periodic acid–Schiff (PAS)–positive vesicles in the enterocytes (Figure 1A). Transmission electron microscopy showed disorganized and shortened microvilli, an abnormal accumulation of subapical vesicles, and multivesicular bodies. Brush-border dipeptidyl peptidase 4 (DPP4) and terminal web α-actinin-4 were obscure in the MVID patient tissues (Figure 1B). Intriguingly, A-kinase anchoring protein (AKAP) 350 expression was found around the inclusion-like structure, suggesting that the A-kinase anchoring protein–mediated scaffolding pathway is involved in microvillus inclusion formations.
Figure 1.
Impaired enterocyte structures and mislocalization of apical proteins in anMVID patient with a point mutation at MYO5B(G519R). (A) Histology of an MVID patient with a MYO5B(G519R) point mutation. Scale bars: 20 μm (upperpanels). Transmission electron microscopy (TEM) image of the patient biopsy specimen shows disorganized microvilli, an abnormal accumulation of subapical vesicles, and large lysosomes. Scale bar: 1 μm (lower panel). (B) Immunostaining for the brush-border marker dipeptidyl peptidase 4 (DPP4) (red), a terminal web marker, α-actinin-4 (green), and A-kinase anchoring protein (AKAP)350 (blue) in the patient biopsy specimens. Scale bars: 20 μm. Multiplexed immunofluorescence staining for various epithelial proteins in (C) control pediatric patient biopsy specimens and the (D) MVID patient with a MYO5B(G519R) mutation. MYO5B and RAB11A signals are virtually merged using nuclei signals as a guide. Scale bars: 100 μm in whole biopsy images, 50 μm in cropped images.
Multiplexed immunofluorescence staining (MxIF) showed MVID characteristics on a single slide of duodenum biopsy specimens using 15 markers, including previously known and unexplored proteins in MVID tissues (Figure 1C and D). Basolateral nutrient transporters other than Na–K–adenosine triphosphatase have not been characterized in MVID tissues.4 The present MxIF demonstrates a remarkable decrease in GLUT2-expressing enterocytes in the MVID patient with MYO5B(G519R), implicating a deficit in enterocyte maturation that could contribute to malabsorption.5,6 Consistent with reports from MVID patient tissues with other MYO5B mutations,5,6 apical nutrient transporters, such as sodium-dependent glucose transporter 1 (SGLT1) and sodium hydrogen antiporter 3 (NHE3), were internalized, and brush-border markers CD10 and Ezrin were scarcely detected in the enterocytes from the patient with the MYO5B(G519R) mutation. MYO5B and Ras-associated binding (RAB)11A staining overlapped and was found primarily in the subapical region of enterocytes of the MYO5B(G519R) patient. The subapical RAB11A staining pattern seen in this patient differs from previous MVID patients with MYO5B(P660L)4 or MYO5B-deficient mouse models,7,8 in which RAB11A was diffuse in the cytoplasm. The present MxIF panel is useful for documenting deficits in brush-border transporters in congenital diarrheal diseases using single sections of limited biopsy specimens from infants, and this technique will facilitate an accurate diagnosis and provide novel insights into molecular mechanisms of diseases.
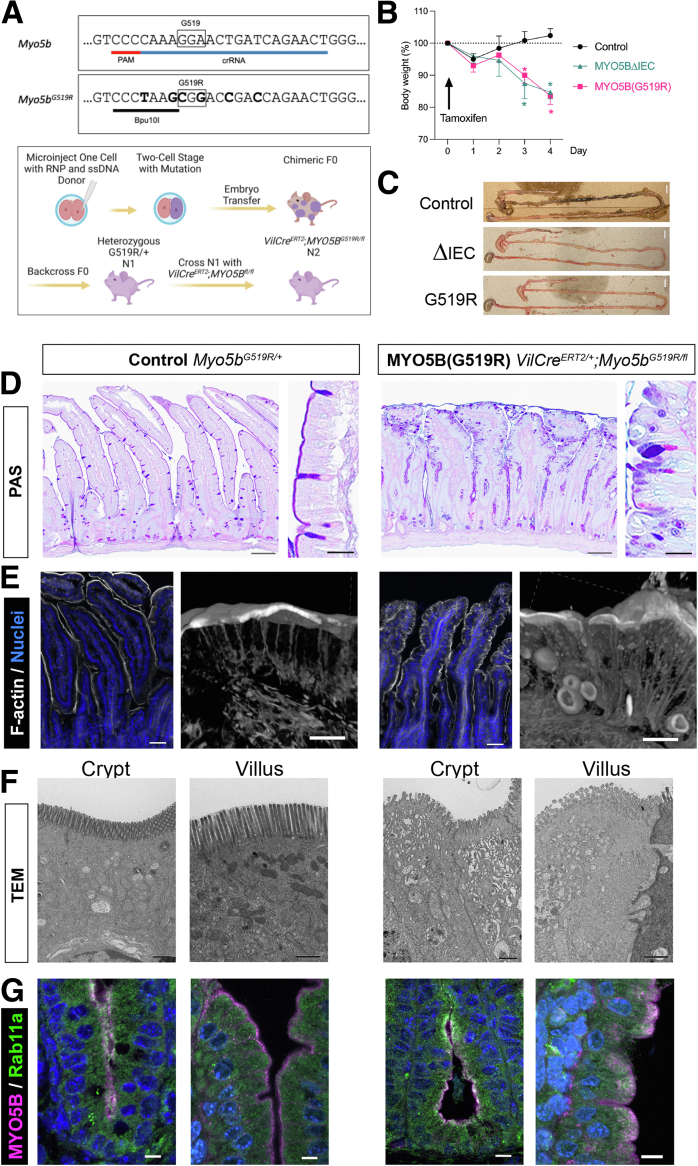
To understand the molecular basis of MVID pathology resulting from compound heterozygous mutations in MYO5B in this patient, we developed a mouse model mimicking the patient’s genotype. The 1-step 2-cell embryo microinjection technique9 was used to avoid early lethality in homozygous Myo5bG519R/G519R mice (Figure 2A and Supplementary Figure 1B). Next, we cross-bred the Myo5bG519R/+ mouse with a Villin-CreERT2;Myo5bflox/flox mouse to generate tamoxifen-inducible, patient-mimicking VilCreERT2;Myo5bG519R/flox mice. In this study, the tamoxifen-injected VilCreERT2;Myo5bG519R/flox mice are referred to as Myo5b(G519R). After a single tamoxifen injection, Myo5b(G519R) mice showed an average 19% body weight decrease by day 4, similar to that in VilCreERT2;Myo5bflox/flox (Myo5bΔIEC) mice (Figure 2B). The Myo5b(G519R) mouse ileum and colon indicated a severe watery diarrhea phenotype (Figure 2C). Tamoxifen-treated, Cre-lacking Myo5bG519R/+ mice, which represent the patient’s mother’s genotype, as well as treated VilCreERT2;Myo5bflox/+, displayed healthy intestinal phenotypes (Figure 2B and C) and were used as controls. Myo5b(G519R) intestines showed PAS–positive vesicles, disordered brush-border structures, microvillus inclusions, and subapical accumulation of MYO5B together with Rab11a, recapitulating the MVID patient’s phenotype (Figure 2D–G). These observations indicate that the Myo5b(G519R) strain phenocopies the MVID patient.
Figure 2.
Myo5b(G519R) mice recapitulate the MVIDpatient'sphenotype. (A) Genomic editing design to create the G519R mutation in MYO5B and breeding strategy for patient-mimicking mouse model using the combination of 1-step 2-cell embryo microinjection and Cre/loxp techniques. (B) Significant body weight loss after a tamoxifen injection in Myo5bΔIEC and Myo5b(G519R) mice. ∗P < .0001 vs control by 2-way analysis of variance with Bonferroni multiple comparisons. n = 5–6 mice in each group. (C) Diarrhea phenotype in MVID mouse models. No solid feces were observed in the Myo5b(G519R) mouse colon. Scale bars: 1 cm. (D) Elongated crypts and blunted villi are present in the Myo5b(G519R) mouse duodenum in Alcian Blue/periodic acid–Schiff (PAS) staining. Scale bars: 50 μm; and 10 μm (insets). (E) Phalloidin staining in the duodenum. Confocal volume images (right panels for each mouse) reveal mislocalized microvilli in the inclusions and lateral domain of Myo5b(G519R) enterocytes. Scale bars: 50 μm (left) and 10 μm (right). (F) Transmission electron microscopy (TEM) images of crypt and villus cells of the jejunum. In Myo5b(G519R) mice, both crypt and villus enterocytes possess blunted and disorganized microvilli and contain numerous subapical vesicles. Scale bars: 1 μm. (G) Immunostaining for MYO5B and Rab11a (sc-166912) in the crypts and villi of small intestine. Scale bars: 10 μm. PAM, rotospacer-adjacent motif; RNP, ribonucleoprotein; ssDNA, single stranded DNA.
Important apical sodium transporters, SGLT1, apical sodium-dependent bile acid transporter (ASBT), and NHE3, were internalized away from the apical membrane in Myo5b(G519R) enterocytes (Supplementary Figure 2A–C), consistent with limited absorption of sodium and water, resulting in watery diarrhea. The actinin-4+ terminal web structure was disrupted (Supplementary Figure 2F), and the expanded SGLT1 localization with lysosome-associated membrane protein 1 (LAMP1)-expressing lysosomes in the Myo5b(G519R) enterocytes (Supplementary Figure 2A) suggests degradation of mislocalized proteins, similar to the expanded RAB7+ vesicles in the MVID patient tissues.
The localization and function of crypt cystic fibrosis transmembrane conductance regulator (CFTR) were intact in Myo5b(G519R) duodenum (Supplementary Figure 2C and D). Üssing chamber experiments showed that CFTR–dependent short-circuit current (Isc), which represents chloride secretion, was up-regulated in steady-state Myo5b(G519R) mouse duodenum. However, bicarbonate secretion, which is likely predominate in villi, showed no significant differences at baseline, after NHE3 inhibition, or forskolin-stimulated conditions (Supplementary Figure 2E).
The Myo5b(G519R) mouse intestine expanded proliferating cell nuclear antigen (PCNA)+ crypts and sustained subapical olfactomedin 4 (OLFM4) signals in upper crypt and lower villus, suggesting an expansion of immature cells (Supplementary Figure 3A and B). EdU (5-ethynyl-2'-deoxyuridine)+ cells reached 90% of the total epithelial height within 24 hours, suggesting more rapid shedding of villus enterocytes in Myo5b(G519R) mice than in healthy controls (Supplementary Figure 3C and D). Indeed, scanning electron microscope images showed immature and disorganized microvilli in Myo5b(G519R) mouse enterocytes (Supplementary Figure 3E). These findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation.
In summary, we showed that the compound heterozygous MYO5B(G519R) mutation in an MVID patient and a patient genotype-mimicking, genetically engineered mouse model causes severe diarrhea and disrupts epithelial microvillus structure. This study reveals a methodology for the rapid establishment of a novel MVID mouse model, Myo5b(G519R), to study the effects of compound heterozygous MYO5B mutations in vivo, and suggests that the combination of 1-step 2-cell embryo microinjection and Cre/loxp systems is a useful tool for modeling patient-specific monogenic congenital disorders.
Acknowledgments
The authors thank Dr. Lane Clarke at the University of Missouri for his expertise in bicarbonate secretion measurements. The authors thank the Vanderbilt Digital Histology (VA Shared Equipment grant 1IS1BX003097) and Vanderbilt Cell Imaging Shared Resources (CA68485, DK20593, DK58404, DK59637, and EY08126) for excellent imaging techniques. Generation of Myo5bG519R mice by the Vanderbilt Genome Editing Resource (RRID: SCR_018826) was supported by Cancer Center Support grant CA68485, the Vanderbilt Diabetes Research and Training Center grant DK020593, and the Center for Stem Cell Biology. Paraffin sectioning and histological staining were performed by the Vanderbilt Translational Pathology Shared Resource (National Cancer Institute/National Institutes of Health Cancer Center Support grant P30 CA68485).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by National Institutes of Health (NIH) R01 DK128190, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant P30 058404, and the American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award to I.K., and NIH RC2 DK118640, R01 DK48370, and a gift from the Christine Volpe Fund to J.R.G.. A.B. was supported by National Foundation of Science Graduate Research Fellowship Program.
Data Availability Statement The Myo5b(G519R) mouse strain will be available from the Vanderbilt Cryopreservation Repository.
Supplementary Material
References
- 1.Thiagarajah J.R., et al. Gastroenterology. 2018;154:2045–2059.e6. doi: 10.1053/j.gastro.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrian D., et al. J Clin Med. 2021;10:481. [Google Scholar]
- 3.Bowman D.M., et al. Cell Mol Gastroenterol Hepatol. 2022;14:553–565. doi: 10.1016/j.jcmgh.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowles B.C., et al. J Clin Invest. 2014;124:2947–2962. doi: 10.1172/JCI71651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kravtsov D.V., et al. Am J Physiol Gastrointest Liver Physiol. 2016;311:G142–G155. doi: 10.1152/ajpgi.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engevik A.C., et al. Gastroenterology. 2018;155:1883–1897.e10. doi: 10.1053/j.gastro.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeberger K., et al. Proc Natl Acad Sci U S A. 2015;112:12408–12413. doi: 10.1073/pnas.1516672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weis V.G., et al. Cell Mol Gastroenterol Hepatol. 2016;2:131–157. doi: 10.1016/j.jcmgh.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., et al. Nat Commun. 2019;10:2883. doi: 10.1038/s41467-019-10748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.