Significance
Our results provide evidence that functional pre-existing SARS-CoV-2-reactive memory CD4+ T cells are elicited in early childhood and linked to seroconversion with the seasonal coronavirus OC43 but not many other viral infections. Compared to other viruses, the high OC43 seroprevalence at age two indicates that memory responses to coronaviruses develop at a young age. The distinct age-dependent profiles of the responding T cells suggest that cross-reactive T cells can contribute to the different clinical outcomes of COVID-19 in children and the elderly. The present results provide important advances regarding antigen-specific memory CD4+ T cell development and maturation, which can help guide future vaccine and therapeutic interventions relating to specificity, function, and phenotype of memory T cell responses throughout the human life span.
Keywords: T cell specificity, cross-protection, human coronavirus OC43, SARS-CoV-2, age groups
Abstract
Pre-existing SARS-CoV-2-reactive T cells have been identified in SARS-CoV-2-unexposed individuals, potentially modulating COVID-19 and vaccination outcomes. Here, we provide evidence that functional cross-reactive memory CD4+ T cell immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is established in early childhood, mirroring early seroconversion with seasonal human coronavirus OC43. Humoral and cellular immune responses against OC43 and SARS-CoV-2 were assessed in SARS-CoV-2-unexposed children (paired samples at age two and six) and adults (age 26 to 83). Pre-existing SARS-CoV-2-reactive CD4+ T cell responses targeting spike, nucleocapsid, and membrane were closely linked to the frequency of OC43-specific memory CD4+ T cells in childhood. The functional quality of the cross-reactive memory CD4+ T cell responses targeting SARS-CoV-2 spike, but not nucleocapsid, paralleled OC43-specific T cell responses. OC43-specific antibodies were prevalent already at age two. However, they did not increase further with age, contrasting with the antibody magnitudes against HKU1 (β-coronavirus), 229E and NL63 (α-coronaviruses), rhinovirus, Epstein–Barr virus (EBV), and influenza virus, which increased after age two. The quality of the memory CD4+ T cell responses peaked at age six and subsequently declined with age, with diminished expression of interferon (IFN)-γ, interleukin (IL)-2, tumor necrosis factor (TNF), and CD38 in late adulthood. Age-dependent qualitative differences in the pre-existing SARS-CoV-2-reactive T cell responses may reflect the ability of the host to control coronavirus infections and respond to vaccination.
The clinical relevance of pre-existing SARS-CoV-2-reactive T cells has been intensely debated since the discovery of immune reactions against this virus in samples collected before the coronavirus disease 2019 (COVID-19) pandemic (1). It remains to be defined if age influences the functional capacity of such cross-reactive T cells. Similar to SARS-CoV-2-specific memory (m)CD4+ T cells (2–4), cross-reactive mCD4+ T cells and recent infection with seasonal human coronaviruses (HCoVs) have been associated with reduced COVID-19 severity (1, 5–7). Thus, it has been suggested that the lack of cross-reactivity could partly explain the high incidence of severe and fatal COVID-19 among the elderly, in contrast to the mild or asymptomatic disease more typically seen in children. We here hypothesized that the potential beneficial role of pre-existing SARS-CoV-2-reactive T cells might be influenced by their functional quality in relation to age.
Several lines of evidence have shown that pre-existing SARS-CoV-2-reactive T cells are likely induced by previous infections with seasonal HCoVs causing respiratory infections (8). These HCoVs are the α-coronaviruses 229E and NL63, and the lineage A β-coronaviruses OC43 and HKU1 (9). While HCoV infections and reinfections occur throughout life, severe disease disproportionately affects children and the elderly (10). Conserved T cell epitopes have been identified between SARS-CoV-2 and several of the HCoVs located primarily within the structural and functional proteins (5, 6, 11, 12).
In response to SARS-CoV-2 infection, lower magnitudes of SARS-CoV-2-specific T cells have been observed in children and the elderly compared to young/middle-aged adults (13, 14), suggesting age-dependent features in the SARS-CoV-2 immune response. The frequency of pre-existing SARS-CoV-2-reactive memory T cells has been reported to decrease from early to late adulthood (5, 15, 16) and to be higher in children than in adults (17), potentially reflecting the frequent exposure to HCoVs in this population. Similarly, the functional capacity of pre-existing SARS-CoV-2-reactive mCD4+ T cells and the avidity of HCoV-specific mCD4+ T cells have been suggested to decrease with age (5). However, since studies comparing the pre-existing SARS-CoV-2-reactive and HCoV-specific mCD4+ T cell responses in children and the elderly are largely lacking, the relationship between pre-existent immunity and age, as well as their role in COVID-19, remains unclear.
Both pandemic SARS-CoV-2-specific and seasonal HCoV-specific mCD4+ T cells have been shown to predominately produce the T helper 1 (Th1) signature cytokines IFN-γ, TNF, and IL-2 (18, 19). While a similar functional profile has been described for cross-reactive T cells, conflicting results are apparent, either showing limited functional capacity compared to bona fide SARS-CoV-2-specific memory T cells (1, 18, 20) or suggesting that the quality of the cross-reactive memory T cell response is epitope-dependent (21, 22). The SARS-CoV-2 proteome has been screened for immunogenic and cross-reactive T cell epitopes using convalescent samples, T cell proliferation assays, and epitope prediction tools (23). While these approaches have effectively demonstrated the existence and breadth of pre-existing SARS-CoV-2-reactive mCD4+ T cells, they fail to evaluate the unaltered functional repertoire of cross-reactive mCD4+ T cells circulating in SARS-CoV-2-unexposed individuals.
Here, we conducted a comprehensive study of the humoral and cellular HCoV-OC43-specific and pre-existing SARS-CoV-2-reactive immune responses in cohorts comprising samples from SARS-CoV-2-unexposed children, young/middle-aged adults, older adults, and COVID-19 convalescents 12 mo post-infection for comparison. Out of the four seasonal HCoVs, we chose to study OC43, the prototype of the genus β-coronaviruses, to which SARS-CoV-2 belongs (9, 24). The selection was based on the relatively high sequence similarity between the structural proteins of OC43 and SARS-CoV-2 and the expected prevalence of OC43 in the study populations (Scandinavian) and other countries, including China and the United States, with an epidemic spread every second winter (25–28).
To mimic the direct ex vivo characteristics of the memory T cell repertoire, we conducted short-term stimulations, followed by functional and phenotypic characterization of the responding antigen-specific mCD4+ T cell populations identified by the expression of activation-induced markers (AIMs). This approach allowed us to characterize in detail the functional quality of mCD4+ T cell responses against HCoV-OC43 and SARS-CoV-2 spike and nucleocapsid in relation to age.
Results
Close Correlation between SARS-CoV-2 Spike-Reactive and Seasonal OC43 Spike-Specific T Cell Responses.
To investigate the relationship between SARS-CoV-2-reactive and the seasonal OC43-specific T cell responses, we collected samples from SARS-CoV-2 seronegative and unvaccinated blood donors collected pre- and post-pandemic until October 2020 (age 26 to 77 y.o., n = 63, Table 1 and Fig. 1A). T cell responses against the OC43 and SARS-CoV-2 spike (S), membrane (M), and nucleocapsid (N) regions were evaluated using defined peptide megapools (Fig. 1A). OC43-specific T cell responses against S and N were readily detected by FluoroSpot (IFN-γ) and AIM assays, while M-specific responses were of significantly lower magnitudes (Fig. 1B and SI Appendix, Fig. S1A and B). SARS-CoV-2-reactive T cell responses were mainly directed to the S region, with significantly lower magnitudes toward both the M and N regions (Fig. 1B and SI Appendix, Fig. S1B). The proportion of individuals with a positive response to OC43 S (65%) was higher than that against SARS-CoV-2 S (37%) but not significantly different (SI Appendix, Fig. S1B). However, the magnitude, defined by average relative spot volume (RSV), of the SARS-CoV-2 S-reactive T cell response was significantly lower, compared with the OC43 S-specific response (SI Appendix, Fig. S1C). Correlation analysis provided further evidence of a close link between the overall SARS-CoV-2 S-, M-, and N-reactive, and OC43 S-, M-, and N-specific T cell responses (Fig. 1D and SI Appendix, Fig. S1E). Although antibodies toward OC43 S were readily detectable (Fig. 1C), their levels did not correlate with mCD4+ T cell responses against OC43 S (Fig. 1E).
Table 1.
Clinical characteristics of the study cohorts
| Parameter | 2 y.o. | 6 y.o. | Blood donors | Adults <60 y.o. | Adults ≥60 y.o. | Convalescents: Mild** | Convalescents: Severe** |
|---|---|---|---|---|---|---|---|
| n (% Blood donors) | 19* | 29* | 65 [100 %]† | 55 [84 %]‡ | 39 [36 %]‡ | 34 | 24 |
| % pre-pandemic | 100% | 100% | 54% | 53% | 38% | 0% | 0% |
| Sex (% M) | 53%§ | 53%§ | 45% | 44% | 36% | 74% | 88% |
| Age, years: Median (range) | 2 | 6 | 50 (26 to 77) | 45 (26 to 58) | 66 (60 to 83) | 55 (45 to 74) | 58 (35 to 70) |
| Plasma availability | 19/19 | 29/29 | 58/65¶ | 44/55¶ | 30/39¶ | 34/34 | 24/24 |
| PBMC availability | 17/19 | 16/29 | 65/65 | 55/55 | 39/39 | 34/34 | 24/24 |
| Sampling period | 2002 to 2005 | 2007 to 2008 | 2016 to 2020¶ | 2016 to 2020¶ | 2017 to 2020¶ | March 2021 | March 2021 |
| Co-morbidities# | N/A | N/A | N/A | N/A | N/A | 13/34 | 13/24 |
| COVID-19 vaccine | N/A | N/A | 0/65 | 0/55 | 0/39 | 1/32|| | 1/24 |
*Includes 17 paired plasma samples and 16 paired PBMC samples at ages two and six.
†Includes two SARS-CoV-2 seropositive individuals, excluded from all analyses except for validation of SARS-CoV-2 epitope mapping results (SI Appendix, Fig. S1G).
‡Adults <60 y and ≥60 y of age, including samples from the blood donors.
§Sex distribution of donors with available PBMC samples.
¶Plasma available for all samples collected post-pandemic.
#Co-morbidities included hypertension, cancer, rheumatoid arthritis, diabetes, lung disease, cardiovascular disease, kidney disease, and stroke.
||Vaccination information unavailable for two individuals.
**Severity score defined in the Materials and Methods section. y.o., years old. Convalescents, 12 mo COVID-19 convalescents. PBMC, peripheral blood mononuclear cell. N/A, not applicable or not available.
Fig. 1.
Study outline and mapping of T cell responses against OC43 and SARS-CoV-2. (A) Outline of the study design, cohorts, and methodology created with BioRender.com. Cells were stimulated with 20-mer peptides spanning the spike (S), membrane (M), and nucleocapsid (N) proteins from OC43 and SARS-CoV-2, followed by analysis using IFN-γ FluoroSpot and flow cytometry by gating on antigen-specific CD69+ CD40L+ memory (m)CD4+ T cells. Control peptides included optimal MHC-I- and MCH-II-restricted peptides from SARS-CoV-2 (SARS-CoV-2 Opt) and CMV, and a pool combining peptides from CMV, EBV, and flu (CEF). See Materials and Methods for details. (B) Fold change of average spot forming units (average SFU) relative to negative control in blood donors (n = 60) stimulated with OC43 or SARS-CoV-2 peptide megapools. Kruskal–Wallis test with Dunn’s multiple comparison post hoc test. (C) Plasma levels of IgG in blood donors (n = 52) targeting the spike regions 1 (S1) and 2 (S2) and hemagglutinin (HA) of OC43, as well as S1 of 229E, NL63, and HKU1. Kruskal–Wallis test with Dunn’s multiple comparison post hoc test. (D and E) Spearman correlation of CD69+ CD40L+ mCD4+ T cells provided as the stimulation index against OC43 S versus SARS-CoV-2 S (D; n = 60) or anti-OC43-S IgG plasma levels (E; n = 49). The threshold for a positive antigen-specific response, set at 1.5 (dotted line), is indicated. (F) Immunogenic peptides identified by FluoroSpot in blood donors after stimulation with peptide matrix pools and single peptides derived from OC43. Presented as fold change of average SFU compared to negative control, with peptide name and region along the x axis (RBD; receptor-binding domain).*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The threshold for a positive response, set at 2, is shown (dotted line). The median (dark red) is depicted when applicable.
Through the simultaneous mapping of individual T cell responses against OC43 and SARS-CoV-2 (Fig. 1A), we identified 56 unique immunogenic peptides not previously described within the S (n = 38), N (n = 17), and M (n = 1) proteins of OC43 (SI Appendix, Table S1 and Fig. 1F). Several OC43-derived peptides were highly immunogenic, inducing a positive T cell response in five or more donors, including peptides S-110, N-19, N-27, N-33, N-35, and N-36 (Fig. 1F and SI Appendix, Table S1). Nine SARS-CoV-2-derived peptides generated positive SARS-CoV-2-reactive responses in at least one SARS-CoV-2 seronegative donor, including S-81, S-82, M-11, and N-11 (SI Appendix, Fig. S1F and Table S1). Two of these cross-reactive epitopes, S-22 and S-26, are affected by amino acid variations (N211I, Δ212, V213G, ins215EPE, and G257S) first identified in circulating SARS-CoV-2 variants of concern (VOCs) Omicron BA.2 and BA.2.75. Several highly immunogenic OC43 epitopes, particularly in the nucleocapsid, did not induce a SARS-CoV-2-reactive response. To confirm our ability to detect SARS-CoV-2 reactivity, epitope mapping was performed on two SARS-CoV-2 seropositive blood donors, in which 40 immunogenic epitopes were identified (SI Appendix, Fig. S1G). Over time, both the OC43-specific and SARS-CoV-2-reactive T cell responses against S and N remained stable at the population level (SI Appendix, Fig. S1H).
Functional and Phenotypic Qualities of SARS-CoV-2 S-Reactive mCD4+ T Cell Responses Mimic T Cell Responses against OC43 S.
Based on the initial results suggesting a link between the frequencies of OC43-specific and SARS-CoV-2-reactive T cell responses, we next investigated if the antigen-specific mCD4+ T cell responses possessed similar functional profiles. Due to the low frequency of responses against the M protein, subsequent analyses focused on the responses against the S and N proteins. The mCD4+ T cell characteristics were investigated by measuring the expression of five functional markers: IFN-γ, IL-2, TNF, CD107a, and granzyme B (GzmB) (Fig. 2A and SI Appendix, Fig. S2A). Initial data revealed that the functional qualities of control cytomegalovirus (CMV)-specific responses were clearly distinguishable from both OC43-specific and SARS-CoV-2-reactive mCD4+ T cells, which displayed no expression of CD107a or GzmB (Fig. 2A and SI Appendix, Fig. S2B). There was no significant difference in the polyfunctional profiles of the OC43 S-specific and SARS-CoV-2 S-reactive mCD4+ T cell populations (Fig. 2A and SI Appendix, Fig. S2B). However, when analyzing the proportion of cells producing a single cytokine (Fig. 2B), there was a significant difference identified in the magnitude of IFN-γ produced by the two cell populations, when paired by donor (Fig. 2C). Several combinations of mono- and polyfunctional T cell subsets separated OC43 N-specific mCD4+ T cells from SARS-CoV-2 N-reactive mCD4+ T cells, suggesting a reduced functional capacity of the cross-reactive responses targeting this protein (Fig. 2 A and B and SI Appendix, Fig. S2 B and C).
Fig. 2.
Qualitative aspects of OC43 and cross-reactive SARS-CoV-2 T cell immunity. (A) Functional profiles of CD69+ CD40L+ mCD4+ T cells. OC43 S, n = 39; SARS-CoV-2 S, n = 22; OC43 N, n = 45; SARS-CoV-2 N, n = 14; SARS-CoV-2 Opt, n = 25; CMV, n = 52. Permutation test. Black represents functional combinations that appear in response to CMV only. (B) IFN-γ+, IL-2+, and TNF+ antigen-specific mCD4+ T cells. Kruskal–Wallis test with Dunn’s multiple comparison post hoc test. (C) Paired comparisons of IFN-γ+, IL-2+, and TNF+ OC43-specific and pre-existing SARS-CoV-2 S-reactive mCD4+ T cells, n = 21. Wilcoxon matched-pairs signed-rank test. (D) Fold change of average spot forming units (average SFU), relative to negative control, acquired by IFN-γ FluoroSpot assay after stimulation with decreasing concentrations of peptides. The black line represents the mean; gray lines represent individual donor responses. The OC43 and SARS-CoV-2 peptide sequences and the reference sequence for each of the other HCoVs are provided. –, represents sequence homology; ., indicates a gap. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The median (dark red) is depicted when applicable.
Next, we evaluated whether the mCD4+ T cell reactivity induced by the peptide pools reflected individual epitope-specific responses. Compared with individual peptides, OC43 S and OC43 N peptide megapools induced higher frequencies of IFN-γ+ and IL-2+ mCD4+ T cells (SI Appendix, Fig. S2D), suggesting polyclonal T cell reactivity. Another important functional measure is the amount of antigen needed to induce a T cell response, which can be evaluated as functional avidity. We observed an association between the functional avidity of the cross-reactive T cell responses and the degree of sequence similarity between the tested SARS-CoV-2 and OC43 S and N peptide sequences (Fig. 2D), where a higher sequence similarity was associated with a lower amount of antigen needed to induce a response.
The functional ability of T cells is closely interconnected with the combined expression patterns of activation, stimulatory, and inhibitory immune checkpoint markers defining the responding T cell subsets. While the permutation test revealed no significant phenotypic differences between OC43 S-specific and SARS-CoV-2 S-reactive mCD4+ T cell responses, significant differences were observed between the N-specific T cell populations (SI Appendix, Fig. S2E). However, when comparing responses separately for each marker, and paired by donor, SARS-CoV-2 S-reactive mCD4+ T cells were found to express modestly, but significantly, higher levels of TIGIT compared to their OC43 S-specific counterparts (SI Appendix, Fig. S2F). The phenotypic difference between OC43 N-specific and the SARS-CoV-2 N-reactive mCD4+ T cells was seemingly driven by a moderate decrease in the expression of inhibitory molecule PD-1 by the SARS-CoV-2-reactive cells (SI Appendix, Fig. S2F). In conclusion, the phenotype of SARS-CoV-2 S-reactive mCD4+ T cell responses resembles the response against OC43 S.
Increased Functional Capacity of the OC43-Specific mCD4+ T Cells after SARS-CoV-2 Infection.
To investigate the impact of SARS-CoV-2 infection on the quality of the mCD4+ T cell responses against OC43, we analyzed samples from 58 COVID-19 convalescents 12 mo post-infection in comparison to SARS-CoV-2 seronegative blood donors (Table 1). SARS-CoV-2 infection did not induce any significant difference in the magnitudes of OC43-specific T cell responses (Fig. 3A and SI Appendix, Fig. S3A) or for the control antigens CMV and CEF, i.e., CMV, Epstein–Barr virus (EBV), and flu (influenza virus) (Fig. 3A). However, significant differences in the polyfunctional profiles of the mCD4+ T cell responses to OC43 S and N regions were observed between blood donors and convalescents (Fig. 3B and SI Appendix, Fig. S3B). T cells directed against OC43 S were more prone to produce IL-2 and TNF in convalescents, compared with seronegative blood donors (SI Appendix, Fig. S3C). The newly induced SARS-CoV-2-specific mCD4+ T cell responses in the convalescents were highly polyfunctional, particularly against the S region, and displayed clear significant differences compared to responses against OC43 S and N (Fig. 3B and SI Appendix, Fig. S3 B–D). SARS-CoV-2 S-specific mCD4+ T cells were more polyfunctional (IFN-γ, IL-2, and TNF) and of higher magnitudes than OC43 S-specific cells (SI Appendix, Fig. S3 B and E). In contrast, SARS-CoV-2 N-specific responses were less polyfunctional than OC43 N-specific responses and produced lower amounts of IFN-γ and TNF but not IL-2 (SI Appendix, Fig. S3B and E). Still, like our observations in blood donors (Fig. 2D), COVID-19 convalescents displayed comparable functional avidity between matched epitopes of SARS-CoV-2 and OC43 from both S and N (SI Appendix, Fig. S3F). In all, SARS-CoV-2 S-, but not SARS-CoV-2 N-, specific T cells displayed elevated functional capacity compared to OC43, after SARS-CoV-2 infection.
Fig. 3.
Cellular and humoral immunity against OC43 and SARS-CoV-2 in 12 mo post-COVID-19 convalescents. (A) Stimulation index of CD69+ CD40L+ mCD4+ T cells in SARS-CoV-2 seronegative blood donors (n = 60) and 12 mo post-COVID-19 convalescents (n = 58, except for OC43 M, n = 9, and SARS-CoV-2 M, n = 14). The dotted line depicts the threshold, set at 1.5, for a positive antigen-specific response. Mann–Whitney test. (B) Functional profiles of identified OC43-specific and SARS-CoV-2-reactive mCD4+ T cells against S in blood donors and 12 mo convalescents. OC43 S (blood donors), n = 39; OC43 S (convalescents), n = 43; SARS-CoV-2 S (blood donors), n = 22; SARS-CoV-2 S (convalescents), n = 56. Permutation test. (C) Plasma levels of IgG targeting SARS-CoV-2 and HCoV-OC43 in mild (n = 34) or severe (n = 24) 12 mo convalescents (S-810, amino acid position 810 in S). Mann–Whitney test. (D) Stimulation index of CD69+ CD40L+ mCD4+ T cells in mild (n = 34) and severe (n = 24) 12 mo convalescents. SARS-CoV-2 S-82 and corresponding OC43 peptide S-92 are immunogenic single peptides identified during epitope mapping. Mann–Whitney test. (E) Spearman correlation between the stimulation index of CD69+ CD40L+ OC43- and SARS-CoV-2-specific mCD4+ T cells against the S region in mild and severe 12 mo convalescents. (F) Function of detected SARS-CoV-2 S-specific mCD4+ T cells in mild (n = 32) and severe (n = 24) 12 mo convalescents. Permutation test. (G) Comparison of IFN-γ+, IL-2+, and TNF+ between SARS-CoV-2 S-specific mCD4+ T cells from mild (n = 32) and severe (n = 24) 12 mo convalescents. Mann–Whitney test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The median (dark red) is depicted when applicable.
Next, we analyzed whether mild versus severe COVID-19 severity had an impact on humoral or cellular immunity. No differences were detected in the antibody response against OC43 (Fig. 3C) or any other HCoVs (SI Appendix, Fig. S3G), and the frequencies of OC43 S- or N-specific mCD4+ T cell responses were similar between the convalescent groups (Fig. 3D and SI Appendix, Fig. S3H). The mild COVID-19 convalescent group had significantly higher frequency of mCD4+ T cells recognizing OC43 S-92 which, alongside its corresponding SARS-CoV-2 peptide S-82 (5, 22), was identified as immunogenic in the epitope mapping (Fig. 1F and SI Appendix, Fig. S1F and Table S1). As expected, convalescents who had suffered from severe disease, compared to mild disease, had higher frequencies of SARS-CoV-2-specific mCD4+ T cells (Fig. 3D and SI Appendix, Fig. S3H). The magnitudes of the SARS-CoV-2-specific T cell responses correlated with the OC43 S- and N-specific mCD4+ T cells, with the correlation being driven by the responses in subjects who had experienced a mild SARS-CoV-2 infection (Fig. 3E and SI Appendix, Fig. S3I). Detailed evaluation of the functional capacity of the responding T cells showed that the mild COVID-19 convalescent group had higher frequencies of polyfunctional SARS-CoV-2-specific mCD4+ T cell responses, and higher magnitude of IFN-γ-producing cells, than the severe group (Fig. 3 F and G and SI Appendix, Fig. S3 J and K). In summary, convalescents who had experienced a milder COVID-19 disease had more functionally active SARS-CoV-2-specific mCD4+ T cells that were linked to their responses against OC43.
Diminished T Cell Reactivity against OC43 and SARS-CoV-2 Associated with Older Age.
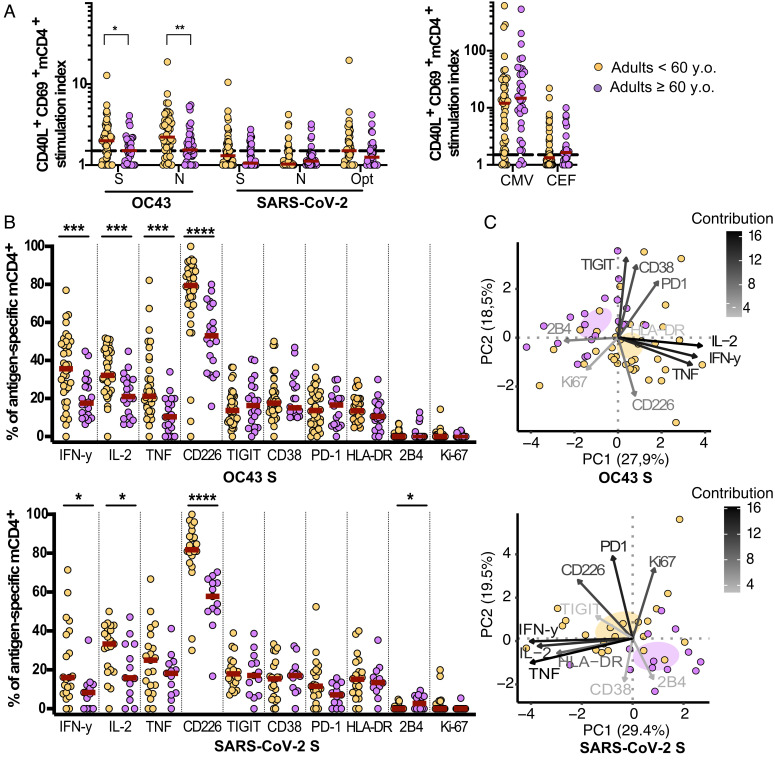
To investigate the role of age on the quantity and quality of the OC43-specific and SARS-CoV-2-reactive mCD4+ T cells, we stratified the SARS-CoV-2 seronegative adults based on age into two groups: adults <60 and ≥60 y.o. (Table 1). All subjects had overall similar antibody levels against OC43, other HCoVs, rhinovirus, EBV, and influenza virus (SI Appendix, Fig. S4A), as well as T cell responses against the CMV and CEF peptide pools (Fig. 4A). The magnitudes of OC43-specific mCD4+ T cells against the S and N regions were significantly diminished in the adults ≥60 y.o., while the lower frequency of SARS-CoV-2-reactive mCD4+ T cells did not reach significance (Fig. 4A and SI Appendix, Fig. S4B). Detailed functional and phenotypic analysis revealed substantial differences in the T cell responses against OC43 S, OC43 N, and SARS-CoV-2 S (Fig. 4B and SI Appendix, Fig. S4C), confirmed by principal component analysis (PCA), showing a clear segregation of mCD4+ T cells between the two age groups (Fig. 4C and SI Appendix, Fig. S4D). Differences in the OC43 S-specific mCD4+ T cell responses between the groups were driven by significantly decreased levels of IFN-γ, IL-2, and TNF in adults ≥60 y.o. (Fig. 4B). The phenotype of the responding cells remained unchanged, except for a decrease in CD226 expression in adults ≥60 y.o. The same patterns observed in the OC43 S-specific mCD4+ T cells were displayed by the OC43 N-specific and SARS-CoV-2 S-reactive mCD4+ T cells in the young/middle-aged versus older adults, with the addition of an increase in 2B4 expression in the older population. Despite the lower frequencies of OC43-specific mCD4+ in adults ≥60 y.o., the correlation between mCD4+ T cell reactivity against OC43 and SARS-CoV-2 was maintained in this group (SI Appendix, Fig. S4E). In all, we demonstrated diminished functional qualities of the OC43-specific T cell responses, which are mimicked by the cross-reactive T cell responses against SARS-CoV-2 in adults older than 60 y.
Fig. 4.
Impact of older age on OC43-specific and pre-existing SARS-CoV-2-reactive T cell immunity. (A) Stimulation index of CD69+ CD40L+ mCD4+ T cells in SARS-CoV-2 seronegative adults <60 y.o. (OC43 S, SARS-CoV-2 S, n = 55; OC43 N, n= 53; SARS-CoV-2 N, n = 54; SARS-CoV-2 Opt, n = 46; CMV, n = 49; CEF, n = 48) and adults ≥60 y.o. (OC43 S, SARS-CoV-2 N, n = 39; OC43 N, SARS-CoV-2 S, n = 38; SARS-CoV-2 Opt, n = 33; CMV, n = 32; CEF, n = 27). The dotted line depicts the threshold for a positive response. Mann–Whitney test. (B) Function and phenotype of identified OC43 S-specific and pre-existing SARS-CoV-2 S-reactive mCD4+ T cells. Mann–Whitney test. (C) Principal component (PC) analysis of functional and phenotypic markers among antigen-specific mCD4+ T cells. OC43 S (<60 y.o.), n = 38; OC43 S (≥60 y.o.), n = 20; SARS-CoV-2 S (<60 y.o.), n = 21; SARS-CoV-2 S (≥60 y.o.), n = 13. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The median (dark red) is depicted when applicable.
SARS-CoV-2-Reactive and OC43-Specific T Cell Responses Are Tightly Linked in Children.
Since children generally experience mild symptoms from SARS-CoV-2 infection, we next evaluated the humoral and cellular immunity in a pre-pandemic cohort of children consisting of paired samples at ages two and six, compared to adults <60 y.o. (Table 1). Already at age two, most children had developed antibodies against OC43, and no statistically significant differences were observed between the magnitudes of the OC43 humoral response at age two, age six, and in adulthood (SI Appendix, Fig. S5A). The magnitudes of the humoral responses against β-coronavirus HKU1 and rhinovirus significantly increased from age two to adulthood, while the responses against the α-coronaviruses (229E and NL63) increased significantly between ages two and six, and between age six and adulthood, as did the humoral responses to the control antigens EBV and influenza (SI Appendix, Fig. S5 A and B).
In contrast to the OC43 humoral response, increased magnitudes as detected by the stimulation index of OC43-specific mCD4+ T cells, against both the S and N regions, were observed in children at age six compared to adults <60 y.o. (Fig. 5A). Moreover, the frequency, but not the stimulation index, of OC43 N-specific mCD4+ T cells increased during childhood (Fig. 5A). No differences were observed in the frequencies of the responding OC43 S-specific or SARS-CoV-2 S- and N-reactive T cell populations between ages two and six (Fig. 5A). The OC43-specific mCD4+ T cell responses were mimicked by the SARS-CoV-2-reactive T cell responses, as shown by the close correlation between the responses directed toward these antigens (Fig. 5B). The correlation was strengthened between ages two and six for both S and N peptide pools, as well as for SARS-CoV-2 individual peptide S-82 and its corresponding OC43 peptide S-92 (Fig. 5B), although the magnitudes of each antigen-specific response remained stable (SI Appendix, Fig. S5C). Overall, these results indicate a maturation of the mCD4+ T cell responses against OC43, linked to the cross-reactive responsiveness to SARS-CoV-2 S and N, in children between ages two and six.
Fig. 5.
Functional and phenotypic markers distinguish T cell responses at ages two and six. (A) Stimulation index of CD69+ CD40L+ mCD4+ T cells in 2 y.o. (n = 17), 6 y.o. (OC43 S, SARS-CoV-2 S, SARS-CoV-2 N, n = 16; OC43 N, n = 15), and adults <60 y.o. (OC43 S, SARS-CoV-2 S, n = 55; OC43 N, n= 53; SARS-CoV-2 N, n = 54). Kruskal–Wallis test with Dunn’s multiple comparison post hoc test. (B) Spearman correlation between the stimulation index of antigen-specific mCD4+ T cells in children. Left panels (blue), 2 y.o.; Right panel (orange), 6 y.o.; OC43 S and SARS-CoV-2 S, OC43 epitope S-92, and corresponding SARS-CoV-2 epitope S-82, OC43 N, and SARS-CoV-2 N. (C) Function and phenotype of OC43 S-specific and SARS-CoV-2 S-reactive mCD4+ T cells in children at ages two and six. OC43 S (2 y.o.), n = 15; OC43 S (6 y.o.), n = 15; SARS-CoV-2 S (2 y.o.), n = 6; SARS-CoV-2 S (6 y.o.), n = 10. Mann–Whitney test. (D) Principal component (PC) analysis of functional and phenotypic markers of OC43-specific and pre-existing SARS-CoV-2-reactive mCD4+ T cells in 2- and 6-y.o. children. OC43 S (2 y.o.), n = 15; OC43 S (6 y.o.), n = 15; SARS-CoV-2 S (2 y.o.), n = 6; SARS-CoV-2 S (6 y.o.), n = 10. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The median (dark red) is depicted when applicable.
To shed light on the functional and phenotypic profiles of the responding T cell pool in children at ages two and six, we analyzed the expression level of 10 functional and phenotypic markers (Fig. 5C and SI Appendix, Fig. S5D). Between ages two and six, OC43 S-specific mCD4+ T cells showed a significant increase in their ability to produce IFN-γ, IL-2, and TNF (Fig. 5C). At the same time, the expression of CD38 was decreased from the high levels observed at age two. The median expression levels of CD38 at age six were still more than twice that of the adult population in response to OC43 S, OC43 N, and SARS-CoV-2 S (SI Appendix, Fig. S5F). Additional significant differences included increased expression of the activating receptor CD226, while its inhibitory counterpart, TIGIT, decreased between childhood and adulthood (Fig. 5C and SI Appendix, Fig. S5D). A PCA confirmed the segregation of the OC43-specific mCD4+ T cell populations at ages two and six, weighing the functional markers high in the separation and highlighting the role of the phenotypic markers Ki-67 and HLA-DR (Fig. 5D). Similar observations were made in response to OC43 N (SI Appendix, Fig. S5 D and E). The cross-reactive mCD4+ T cell responses against SARS-CoV-2 S displayed an increased ability to produce IFN-γ between ages two and six, although the values were not significant (Fig. 5C). The visually increased expression of CD226 and decreased expression of CD38 were not significant between children at ages two and six (Fig. 5C), but significant in comparison to the adult group (SI Appendix, Fig. S5F), thus showing that the function and phenotype of SARS-CoV-2-reactive mCD4+ T cells closely resemble that of OC43-specific mCD4+ T cells.
OC43-Specific and Cross-Reactive mCD4+ T Cell Responses from Childhood to Late Adulthood.
Finally, we investigated in detail how the functional and phenotypic qualities of the responding mCD4+ T cell changed with age from childhood (6 y.o.) until late adulthood (until 83 y.o.). The proportion of donors with mCD4+ T cells responding to OC43 and SARS-CoV-2 S and/or N decreased significantly from age six to adults ≥60 y.o. (SI Appendix, Fig. S6A). A significant decrease was also observed for responses against SARS-CoV-2 from age six to adults <60 y.o. Including all samples from age six throughout adulthood, we observed an inverse correlation between age and the frequency of OC43 S- and N-specific mCD4+ T cells (Fig. 6A and SI Appendix, Fig. S6B). The decrease in OC43-specific T cells was also reflected by the SARS-CoV-2 S-reactive mCD4+ T cells (Fig. 6A), but not by the low frequency of N-specific mCD4+ T cells (SI Appendix, Fig. S6B).
Fig. 6.
Superior function of OC43 S-specific and pre-existing SARS-CoV-2 S-reactive CD4+ T cells in children compared to adulthood. (A) Spearman correlation between age and the stimulation index of antigen-specific CD69+ CD40L+ mCD4+ T cells. OC43 S, n = 110; SARS-CoV-2 S, n = 109. (B) Contour UMAP plot of bulk and antigen-specific mCD4+ T cells from four representative donors from each age group. 6 y.o. (n = 16), adults age < 60 (n = 46), and adults age ≥ 60 (n = 39). (C) UMAP plots of the distribution of antigen-specific mCD4+ T cell populations (color) overlayed over the bulk mCD4+ T cell population (gray). (D) UMAP plots showing the expression of measured markers. (E) Visualization of cell clusters identified by PhenoGraph. (F) Hierarchical clustering heatmap of relative marker expression across each cell cluster from (E). (G) Proportion of cells from each age group and antigen-specific population residing in each cluster from (E).
We next performed a Uniform Manifold Approximation and Projection (UMAP) dimensionality reduction to investigate the range of phenotypes in the present OC43-specific and SARS-CoV-2-reactive populations across age groups (Fig. 6 B and C). Notably, we identified a considerable accumulation in the upper region of the UMAP structure, which corresponded to cells with a polyfunctional cytokine-producing phenotype (TNF+, IL-2+, IFN-γ+) (Fig. 6D). The distribution of cells was also strongly influenced by the expression of CD38. There was a tendency for the six y.o. children to have more CD38+ mCD4+ T cells in comparison to the elderly group (Fig. 6 C and D). Next, we used PhenoGraph to identify clusters defined by patterns of marker co-expression (Fig. 6 E and F) and quantified the cluster distribution of OC43-specific and SARS-CoV-2-reactive mCD4+ T cells from each group (Fig. 6G). As revealed from this analysis, the cytokine-enriched cluster 13 contained the most antigen-specific mCD4+ T cells and was the least frequent in adults ≥60 y.o. (Fig. 6G). Furthermore, clusters 1 and 7 were characterized by higher expression of CD38 (Fig. 6F) and contained high fractions of mCD4+ T cells from six y.o. children (Fig. 6G), while cluster 4 was defined by low CD38 expression and comparatively higher 2B4+/HLA-DR+ expression (Fig. 6F), which was only observed among elderly donors (Fig. 6G). Cluster 6, defined by higher expression of PD-1 and moderate expression of CD226 (Fig. 6F), was rarely detected in children at age six (Fig. 6G). The cluster distribution of OC43-specific and SARS-CoV-2-reactive mCD4+ T cells from each group was similar (Fig. 6G). Overall, our analyses demonstrate that polyfunctional responses are generated across age groups, with the elderly group having the comparatively lowest distribution of mCD4+ T cells in highly functional clusters.
Discussion
COVID-19 manifests a clinical profile that is generally mild in children and more likely to cause severe disease in the elderly. Previously induced CD4+ T cell responses against seasonal HCoVs (11, 22, 29, 30) might provide effective cross-reactive T cell responses (1, 5). The suggested beneficial effect of cross-reactive T cell responses refers to their association with SARS-CoV-2 infection (1, 5–7) and vaccination outcome (5, 22, 31). The quality of pre-existing cross-reactive T cells in association with HCoVs and age has not been evaluated. We performed a comprehensive epitope mapping of HCoV-OC43-specific and SARS-CoV-2-reactive mCD4+ T cells and have identified immunogenic epitopes. Subsequently, by thoroughly evaluating pre-existing SARS-CoV-2-reactive mCD4+ T cells in relation to seasonal OC43-specific T cell responses, from early childhood (2 y.o.) to late adulthood (83 y.o.), we shed further light on the nature of these responses in relation to age. Pre-existing SARS-CoV-2-reactive CD4+ T cell responses targeting the structural proteins spike, nucleocapsid, and membrane were linked to the frequency of T cell responses, but not antibody titers, against OC43. The functional profile of pre-existing SARS-CoV-2-reactive mCD4+ T cells targeting spike, but not nucleocapsid, closely mirrored the OC43-specific T cell immunity. The pre-existing SARS-CoV-2-reactive mCD4+ T cell immunity was inversely correlated with age, with a peak at age six and declining functionality with older age. Collectively, our results suggest that pre-existing SARS-CoV-2-reactive T cells induced by previous encounters with OC43, and possibly the other seasonal HCoVs, are influenced by age and might have an impact on early SARS-CoV-2 control and response to vaccination.
By simultaneously identifying SARS-CoV-2-reactive and OC43-specific T cell responses to the structural proteins S, M, and N, we demonstrated a close relationship between the mCD4+ T cell populations. In adults, our data confirmed that the OC43-specific and SARS-CoV-2-reactive T cell responses remain stable over time (32). While most adults had responses against OC43 S and N, the cross-reactive responses more frequently targeted the S rather than the N region, as displayed at both protein and epitope levels. Our results are supported by a recent finding in silico, suggesting that the structural proteins S, followed by N, from OC43 have the highest sequence identity to SARS-CoV-2, compared with all other HCoVs (33). Overall, the frequencies of both SARS-CoV-2 S-reactive and OC43 S-specific mCD4+ T cells were inversely correlated with age, corroborating findings by Loyal et. al. (5). These data support our hypothesis that the T cell response against OC43 may impact the response against SARS-CoV-2 from childhood until late adulthood. This notion is strengthened by the polyfunctional and phenotypic profiles of the SARS-CoV-2 S-reactive mCD4+ T cells that are not different from that of OC43 S-specific T cells. In contrast, SARS-CoV-2 N-reactive mCD4+ T cells had an impaired ability to produce IFN-γ, IL-2, and TNF, compared to the OC43 N-specific mCD4+ T cells. The disparate functional capacity of cross-reactive mCD4+ T cells targeting different proteins or epitopes provides insights, in addition to technical considerations discussed below, into why some studies detected a link between pre-existing SARS-CoV-2-reactive T cells and disease outcome after SARS-CoV-2 infection (1, 5, 6), while others did not (20). Finally, by evaluating T cell reactivity in a COVID-19-convalescent control cohort, we also observed that the group who had experienced a milder disease, compared to severe disease, displayed SARS-CoV-2-specific mCD4+ T cells that were more polyfunctional and closely correlated with their OC43-specific responses.
At an epitope level, in SARS-CoV-2-unexposed blood donors, we identified numerous OC43 epitopes and corroborated the cross-reactive potential of SARS-CoV-2 epitopes S-81 (aa 801 to 820) and S-82 (aa 811 to 830) (5, 21, 22), an epitope also targeted by neutralizing antibodies (34, 35). Limited cross-reactivity was observed between the N regions, but previously identified N-11 (aa 101 to 120) was among the most immunogenic cross-reactive peptides in our blood donor cohort (29, 36). When evaluating the functional avidity of the most immunogenic peptides in relation to the amino acid sequence, we observed an association between OC43 and SARS-CoV-2 sequence similarity and the potential for T cells to cross-recognize epitopes between OC43 and SARS-CoV-2. Furthermore, we confirmed that the majority of the defined immunogenic cross-reactive epitopes including, S-81, S-82 and N-11, were not affected by amino acid variations identified in circulating SARS-CoV-2 VOCs. Thus, such SARS-CoV-2-reactive T cells might provide immunological aid, a hypothesis that will need to be evaluated in future studies. It is worth mentioning that a part of the cross-reactive responses is not likely to be of coronavirus origin, e.g., commensal bacteria (1, 37). Additional factors, such as sequence similarity, peptide processing and presentation, and the frequency of the presenting HLA molecule in the population, will also affect cross-reactivity.
OC43-specific antibodies were readily detected in children at age two, with comparable magnitudes to those at age six and adults. This corroborates OC43 as a childhood infection and common seasonal HCoV in Scandinavia (26). Conversely, the humoral immune responses against β-coronavirus HKU1, as well as α-coronaviruses 229E and NL63, significantly increased between childhood and adulthood, reflecting their less commonly spread in young children (25–27). While the frequency of OC43-specific mCD4+ T cell responses remained stable between ages two and six, their functional capacity was enhanced at age six. Importantly, we showed that the functional and phenotypic profiles of the OC43-specific mCD4+ T cell population were shared by the pre-existing SARS-CoV-2-reactive mCD4+ T cells. Considering that the mortality rate in children is the lowest from ages five to nine, and higher in younger children (38), our results imply that cross-reactive mCD4+ T cells may have a role in the control of SARS-CoV-2 infection in children, alongside other hypothesized factors, including their lower prevalence of comorbidities, distinct expression patterns of viral receptor ACE2, and enhanced antiviral capabilities of innate and adaptive immunity at mucosal sites, compared with adults and the elderly (39–41).
When investigating T cell function in relation to activating and inhibitory molecules expressed by OC43-specific and SARS-CoV-2-reactive T cell populations throughout life, we discovered that age, rather than specificity, was the delineating factor. Clustering analysis highlighted that the vast majority of the OC43-specific and SARS-CoV-2-reactive mCD4+ T cells were contained within a cluster of cytokine-producing cells, expressing moderate levels of the inhibitory molecule PD-1 and activating molecule CD226. The cytokine-producing T cell population was substantially diminished in older adults (≥60 y.o.). In children, the most common population without the ability to produce cytokines was defined by high expression of CD38 and moderate levels of the inhibitory molecules PD-1 and TIGIT. This finding is in line with the high frequency of central mCD4+ T cells expressing CD38 in children compared to adults (42, 43). In addition to its role in activation and proliferation upon infection, CD38 may also facilitate mobilization or tissue migration (44, 45). Based on the limited expression of additional activating markers, such as HLA-DR and CD226, our results imply that these cells are likely to be recently activated (46) or undergoing homeostatic proliferation (47). In the adult cohorts, a population cluster expressing PD-1 and CD226, without the ability to produce cytokines, was prevalent. Expression of PD-1 might imply an age-dependent transition toward senescence (48). Finally, a T cell population expressing high levels of 2B4 and moderate levels of CD226 was common in late adulthood but rarely seen in children. Inducible expression of 2B4 (also called CD244 or SLAMf4) on CD4+ T cells has been shown to mediate co-inhibition (49), and its expression has been related to the functional capacity of T cells during infection (50, 51). Although differences existed in the expression of functional markers and activating and inhibitory receptors between T cell subsets, the phenotype and cytokine responsiveness of OC43 S-specific and pre-existing SARS-CoV-2 S-reactive mCD4+ T cells were similar and strongly associated with age, where cross-reactive T cell responses were most prominent in children and diminished in late adulthood.
We are aware of the fact that techniques and experimental approaches used to define and characterize SARS-CoV-2-reactive T cells may give different results; e.g., the prevalence of cross-reactive T cells ranging from 0 to 80% of unexposed individuals has been reported (2, 4, 18, 22, 29, 36, 52–54). In some studies, enrichment (4, 5, 20, 36) and/or expansion for several days (5, 6, 11, 12, 20, 22, 29, 36) were performed before responses were identified. Here, we opted for FluoroSpot for the mapping of T cell responses, and for a single high-parameter flow cytometry panel to identify antigen-specific T cells via an AIM assay, while simultaneously characterizing their phenotype and function via an intracellular cytokine staining (ICS) assay. To investigate the unaltered SARS-CoV-2-reactive mCD4+ T cell population, the analysis was conducted ex vivo directly following a short (10 h) cell stimulation without prior enrichment and/or expansion. The present study characterizes circulating OC43-specific and SARS-CoV-2-reactive mCD4+ T cells from peripheral blood, showing differences in relation to age. Whether age-dependent differences influence pre-existing SARS-CoV-2-reactive T cells in clinically relevant tissues, e.g., in airway-resident tonsil tissue (41) or bronchoalveolar lavages (55), was not assessed. In addition, the results are based on the enumeration of Th1 cytokines established to be produced by SARS-CoV-2-specific T cells, although other T cell profiles (e.g., production of IL-10) may also contribute to the age-dependent manifestation of COVID-19 (56). We observed enhanced OC43-specific and SARS-CoV-2 S-reactive mCD4+ T cell responses at age six, compared to age two, but lacked samples from older children and younger adults, which will be necessary for further investigation.
In summary, we show that many children at age two have highly functional mCD4+ T cells that cross-recognize SARS-CoV-2. These cells are characterized by the expression of high levels of CD38 but no co-inhibitory molecules. From age six, the frequencies and functional capacity of this pre-existing T cell immunity wane, particularly in older adults. In all age groups, the functional capacity of the cross-reactive SARS-CoV-2 immunity against the Sregion was closely linked to the simultaneously characterized OC43-specific mCD4+ T cell responses. These findings are of importance not only for COVID-19 and vaccination outcomes but also translate to other diseases caused by existing and emerging pathogens. The distinct age-dependent functional and phenotypic profiles of the responding mCD4+ T cells provide general insights into the immune system development in childhood and subsequent waning during late adulthood. Additional studies are needed to define the contribution of cross-reactive T cells to the distinct clinical outcomes of COVID-19 in children and the elderly.
Materials and Methods
Human Subjects.
The characteristics of the study cohorts are provided in detail in Table 1. Blood samples, peripheral blood mononuclear cells (PBMCs), and plasma samples were obtained pre-pandemic from healthy children sampled longitudinally in 2002 to 2008 at ages two and six (57). This birth cohort study was approved by the Ethical Review Board in Linköping (Dr 99184 and complementary Dr M98-06), Sweden. From each of the 65 blood donors collected pre-pandemic (54%) or during 2020 (until October 2020), we obtained >100 million PBMCs to map OC43-specific and pre-existing SARS-CoV-2-reactive mCD4+ T cell responses, including longitudinal pre-pandemic samples from 10 donors. Samples from these blood donors were obtained from the Department of Clinical Immunology and Transfusion Medicine, Karolinska Institutet, as approved by the Ethical Review Board in Stockholm (Dnr 2016/1415-32, 2006/229-31/3), Sweden. When screening for SARS-CoV-2 antibodies, two blood donors, whose samples were collected post-pandemic, were confirmed seropositive and excluded from further analysis but were used as controls for SARS-CoV-2 epitope mapping (SI Appendix, Fig. S1G). In addition to the blood donors, we obtained PBMCs from healthy adults (n = 37) recruited as “age-matched, healthy volunteers” in a study of immune function in end of life, performed in Stockholm, Sweden, during 2017 to 2020 (on or before October 2020) (Dnr 2017/203-31/4). Samples obtained in 2020 were screened for SARS-CoV-2 antibodies, among which three volunteers were confirmed seropositive and excluded from further analyses. The adult cohorts were subsequently divided into two age groups <60 y.o (n = 55) and ≥60 y.o. (n = 39). It was confirmed that the magnitudes of the detected T cell responses were indistinguishable in samples obtained pre versus post-pandemic (SI Appendix, Fig. S1D). All samples were collected before access to a COVID-19 vaccine. Additional samples were acquired from COVID-19 convalescents 12 mo after RT-PCR—verified infection with SARS-CoV-2, leading to mild (n = 34) or severe (n = 24) disease, in March–April 2020, before the emergence of VOCs (Dnr 2020-06133, 2016/1415-42). Disease severity was graded based on the NIH Ordinal Scale and Sequential Organ Failure Assessment (SOFA), as previously reported (18). Severe disease was defined by the need for hospitalization in the intensive care unit, with low- or high-flow oxygen support or invasive mechanical ventilation.
Study Outline.
The goal of the study was to map and investigate the humoral and cellular immunological memory to the seasonal HCoV-OC43 in relation to pre-existing SARS-CoV-2-reactive T cell immunity and age (Fig. 1A). The study cohorts consisted of children (ages two and six), adults < 60 y.o., adults ≥60 y.o., and 12 mo post-COVID-19 convalescents (Table 1 and SI Appendix, Table S2). Antibody screening for SARS-CoV-2, seasonal human coronaviruses (HCoVs), EBV, influenza, and rhinovirus was conducted. Peptide megapools spanning the spike (S), membrane (M), and nucleocapsid (N) proteins from HCoV-OC43 and SARS-CoV-2, and peptide matrix pools, were constructed using 20-mer individual peptides. Epitope screening and epitope avidity analysis were performed by FluoroSpot assay (IFN-γ detection) using PBMCs from blood donors. A single flow cytometry panel combining AIM and ICS assays enabled the characterization of antigen-specific (CD69+ CD40L+) mCD4+ T cells. Peptide megapools (OC43 and SARS-CoV-2), optimal peptide pools (SARS-CoV-2; CMV; and CEF, CMV + EBV + influenza virus), and individual peptides were used (SI Appendix, Table S2). For further details, see SI Appendix, Supporting Information Text and Tables S1 and S2.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to warmly thank R. Neher for sequence alignments and expertise regarding genetic diversity of human coronaviruses, P. Bergman, R. Sultana Rekha, C. Klasson, and M. Helde Frankling for skillful work in the clinical study of healthy elderly and preparation and handling of samples, C. Janefjord for the handling of children samples, A. Perez-Potti for reconstituting the SARS-CoV-2 peptides and sharing his expertise on peptide handling, and all donors and healthy volunteers participating in the clinical studies. J.N. was supported by the European Molecular Biology Organization (EMBO) Postdoctoral Fellowship (ALTF 1062-2020) and the Svenska Sällskapet för Medicinsk Forskning (SSMF) Postdoctoral Fellowship. U.N. was supported by the Swedish Research Council grants 2021-00993 and 2021-01756. F.L.-J. was supported by the South-Eastern Norway Regional Health Authority. L.B.-B. was supported by the Stockholm County Council (FoUI-953862), Swedish Cancer Society (CAN2018/316), and Centrum for Innovative Medicine (CIMED). H.-G.L. was supported by the Knut and Alice Wallenberg Foundation (2020.0299 and 2020.0185) and Nordstjernan. M.B. was supported by the Swedish Research Council (2020-06121 and 2018-02330), the European Research Council (101057129 and 101041484), the Knut and Alice Wallenberg Foundation (2021.0136), the Karolinska Institutet (2019-00969), the Swedish Cancer Society (20 0176 Pj), the Swedish Childhood Cancer Fund (PR2020-0072), Åke Wibergs Stiftelse (M20-0190), the Jonas Söderquist Stiftelse, and the Sven and Ebba-Christina Hagbergs stiftelse. A.C.K. was supported by the Swedish Research Council (Dnr 2020-02033), CIMED project grant, senior (Dnr: 20190495), and Karolinska Institutet (Dnr: 2019-00931 and 2020-01599). E.B.H. was supported by the Swiss National Science Foundation through grant number 31CA30 196046.
Author contributions
M.H., M.C.J., H-G.L., M.B., and A.C.K. designed research; M.H., A.O., D.W., J.N., E.B.H., Y.G., E.S., R.D., F.L.-J. performed research; U.N., J.A., F.L.-J., K.-J.M., S.A., L.B.-B., M.C.J. contributed new reagents or analytic tools; M.H., A.O., D.W., J.N., E.B.H., C.C., R.D., F.M., F.L.-J., M.C.J., H.-G.L., M.B., A.C.K. analyzed data; C.C., E.S., E.B.H., R.D., F.M., U.N., J.A., F.L.-J., L.B.-B., M.C.J. contributed writing; M.H., A.O., H.-G.L., M.B., and A.C.K. wrote the paper.
Competing interests
The authors have organizational affiliations to disclose, K.-J.M. is a consultant with ownership interests at Fate Therapeutics and Vycellix and has research funding from Fate Therapeutics, not related to this work. He has a Royalty agreement with FATE Therapeutics through licensing of intellectual property (IP), not related to this work. K.-J.M. has received honoraria from Oncopeptides, Cytovia and has research funding from Oncopeptides and Merck, not related to this work. E.S. is a paid consultant at Fate Therapeutics, not related to this work. S.A. has received honoraria for lectures and educational events, not related to this work, from Gilead, AbbVie, Merck Sharp & Dohme (MSD), and Biogen, and reports grants from Gilead and AbbVie.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All other data are included in the manuscript and/or SI Appendix. Key resources, incuding antibodies, peptides, proteins, peptide sequences, software, and algorithms, are included in SI Appendix, Tables S1 and S2.
Supporting Information
References
- 1.Murray S. M., et al. , The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 1–13 (2022), 10.1038/s41577-022-00809-x. [DOI] [PMC free article] [PubMed]
- 2.Grifoni A., et al. , Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e1415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rydyznski Moderbacher C., et al. , Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996–1012.e1019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meckiff B. J., et al. , Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4(+) T cells in COVID-19. Cell 183, 1340–1353.e1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loyal L., et al. , Cross-reactive CD4(+) T cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science 374, eabh1823 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swadling L., et al. , Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 601, 110–117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillmore N. R., et al. , Recent common human coronavirus infection protects against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A Veterans Affairs cohort study. Proc. Natl. Acad. Sci. U.S.A. 119, e2213783119. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monto A. S., Medical reviews. Coronaviruses. Yale J. Biol. Med. 47, 234–251 (1974). [PMC free article] [PubMed] [Google Scholar]
- 9.Su S., et al. , Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 24, 490–502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Channappanavar R., Perlman S., Age-related susceptibility to coronavirus infections: role of impaired and dysregulated host immunity. J. Clin. Invest. 130, 6204–6213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low J. S., et al. , Clonal analysis of immunodominance and cross-reactivity of the CD4 T cell response to SARS-CoV-2. Science 372, 1336–1341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y., et al. , An immunodominant NP105-113-B*07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease. Nat. Immunol. 23, 50–61 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen C. A., et al. , SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 12, 4678 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul K., et al. , Specific CD4+ T cell responses to ancestral SARS-CoV-2 in children increase with age and show cross-reactivity to beta variant. Front. Immunol. 13, 867577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saletti G., et al. , Older adults lack SARS CoV-2 cross-reactive T lymphocytes directed to human coronaviruses OC43 and NL63. Sci. Rep. 10, 21447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taira N., et al. , Altered pre-existing SARS-CoV-2-specific T cell responses in elderly individuals. Clin. Immunol. Commun. 2, 6–11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowell A. C., et al. , Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 23, 40–49 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekine T., et al. , Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183, 158–168.e114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner S., et al. , HCoV- and SARS-CoV-2 cross-reactive T cells in CVID patients. Front. Immunol. 11, 607918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacher P., et al. , Low-Avidity CD4(+) T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 53, 1258–1271.e1255 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becerra-Artiles A., et al. , Broadly recognized, cross-reactive SARS-CoV-2 CD4 T cell epitopes are highly conserved across human coronaviruses and presented by common HLA alleles. Cell Rep. 39, 110952 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateus J., et al. , Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 370, 89–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grifoni A., et al. , SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host. Microbe. 29, 1076–1092 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogimi C., et al. , What’s new with the old coronaviruses? J. Pediatr. Infect. Dis. Soc. 9, 210–217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyrdak R., Hodcroft E. B., Wahlund M., Neher R. A., Albert J., Interactions between seasonal human coronaviruses and implications for the SARS-CoV-2 pandemic: A retrospective study in Stockholm, Sweden, 2009–2020. J. Clin. Virol. 136, 104754 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimdal I., et al. , Human Coronavirus in Hospitalized Children With Respiratory Tract Infections: A 9-Year Population-Based Study From Norway. J. Infect Dis. 219, 1198–1206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W., Wang W., Wang H., Lu R., Tan W., First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect Dis. 13, 433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killerby M. E., et al. , Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 101, 52–56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelde A., et al. , SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 22, 74–85 (2021). [DOI] [PubMed] [Google Scholar]
- 30.da Silva Antunes R., et al. , Differential T-Cell Reactivity to Endemic Coronaviruses and SARS-CoV-2 in Community and Health Care Workers. J Infect Dis. 224, 70–80 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dangi T., et al. , Cross-protective immunity following coronavirus vaccination and coronavirus infection. J. Clin. Invest. 131, e151969 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu E. D., et al. , Immunological memory to common cold coronaviruses assessed longitudinally over a three-year period pre-COVID19 pandemic. Cell Host. Microbe. 30, 1269–1278 (2022), 10.1016/j.chom.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cicaloni V., et al. , A bioinformatics approach to investigate structural and non-structural proteins in human coronaviruses. Front. Genet. 13, 891418 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dacon C., et al. , Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 377, 728–735 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low J. S., et al. , ACE2-binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies. Science 377, 735–742 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bert N., et al. , SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 584, 457–462 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Tan C. C. S., et al. , Pre-existing T cell-mediated cross-reactivity to SARS-CoV-2 cannot solely be explained by prior exposure to endemic human coronaviruses. Infect. Genet. Evol. 95, 105075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Driscoll M., et al. , Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590, 140–145 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Amodio D., Cotugno N., Palma P., COVID-19 in children: From afterthought to unknown. Cell Rep. Med. 3, 100558 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J., et al. , Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging. Cell 19, e13168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niessl J., et al. , Identification of resident memory CD8(+) T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci. Immunol. 6, eabk0894 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Martino M., et al. , Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years Pediatr. Res. 43, 752–758 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Song C. B., et al. , CD4(+)CD38(+) central memory T cells contribute to HIV persistence in HIV-infected individuals on long-term ART J. Transl. Med. 18, 95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henrick B. M., et al. , Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e3811 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Dianzani U., et al. , Interaction between endothelium and CD4+CD45RA+ lymphocytes. Role of the human CD38 molecule. J. Immunol. 153, 952–959 (1994). [PubMed] [Google Scholar]
- 46.Funaro A., et al. , Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J. Immunol. 145, 2390–2396 (1990). [PubMed] [Google Scholar]
- 47.Surh C. D., Sprent J., Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192, F9–F14 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe A. H., Wherry E. J., Ahmed R., Freeman G. J., The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8, 239–245 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Brown D. R., et al. , Cutting edge: an NK cell-independent role for Slamf4 in controlling humoral autoimmunity. J. Immunol. 187, 21–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fromentin R., et al. , CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART PLoS Pathog. 12, e1005761. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porichis F., et al. , Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 118, 965–974 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun J., et al. , SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587, 270–274 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Weiskopf D., et al. , Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng Y., et al. , Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19 Nat. Immunol. 21, 1336–1345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diniz M. O., et al. , Airway-resident T cells from unexposed individuals cross-recognize SARS-CoV-2. Nat. Immunol. 23, 1324–1329 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Bert N., et al. , Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 218, e20202617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abelius M. S., et al. , Th2-like chemokine levels are increased in allergic children and influenced by maternal immunity during pregnancy. Pediatr. Allergy Immunol. 25, 387–393 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All other data are included in the manuscript and/or SI Appendix. Key resources, incuding antibodies, peptides, proteins, peptide sequences, software, and algorithms, are included in SI Appendix, Tables S1 and S2.