ABSTRACT
Background and Objective:
Many biological activities and pharmacological usages have been revealed for the Cuscuta species. The present study aimed to assess the beneficial effects of Cuscuta on removing skin dark spots in healthy individuals, which is an important cosmetic concern, especially in women.
Materials and Methods:
This prospective, interventional before–after trial was conducted in 70 healthy individuals without any evidence of skin or systemic disorders who presented for consultation on removing skin darkening. The Cuscuta extract was prepared and then assessed for plant quality control and lack of microbial contamination. The content of melanin at baseline and at 1 and 3 months after intervention was assessed using Dermacatch, an accurate skin colorimetric measurement tool.
Results:
Comparison of the melanin content of the lesions and treated area to the surrounding normal area at baseline and at 1 month after treatment showed a significant reduction in melanin content from 519.61 ± 45.09 to 498.50 ± 39.35 (P < 0.001). This reducing trend remained significant from the first month to the third month after treatment (from 498.50 ± 39.35 to 483.53 ± 40.99, P < 0.001). This decreasing trend was persistent even after adjusting baseline characteristics including gender, age, and duration of skin lesions. Both patients and investigators had high satisfaction level with the anti-melanogenesis effect of Cuscuta extract.
Conclusion:
Cuscuta extract is useful for removing hyperpigmented lesions and for skin lightening in healthy individuals.
Keywords: Constant Darkness, Cuscuta, herbal extra, herbal medicine, hyperpigmentation, melasma, skin care, skin darkness, skin lightening, trial
Introduction
Skin hyperpigmentation, whether natural or in the form of abnormal condition, can cause potential mental or even social problems affecting individuals or their social activities or even leading to depression.[1,2] Hence, recognizing the causes of hyperpigmentation and its proper treatment is highly demanded, while improper treatment could increase patient’s problem.[3] The treatment should be selected according to the cause of hyperpigmentation, the severity of the disease, and the occupational and social conditions of patients.[4,5]
The natural color of the skin depends on three factors including the concentrations of hemoglobin, carotenoid, and melanin pigments, and the third factor is the main determinant of skin color affected by the racial and geographic factors.[6,7] Various other factors including cellular metabolism disorders, hormonal or inflammatory diseases, local skin infections, chemical agents and drugs, nutritional causes including vitamin deficiencies and malnutrition, and skin malignancies affect the quality and quantity of pigmentation, which could change the color of the skin and cause hyperpigmented spots.[8,9,10,11] However, in most individuals, no evidence of a particular disease could be found.
Recently, different drugs and lotions have been used to remove skin dark spots, such as sunscreen lotions, vitamin supplements, and bleaching creams. In addition, many herbal extracts have been examined to remove darkening of the skin.[12,13,14] Cuscuta (dodder) is a parasitic plant with slender yellow, orange, or red stems that is particularly grown in tropical regions of the world.[15,16,17] This plant belongs to Cuscutaceae family and has about 100–170 different species. Cuscuta chinensis has been traditionally used as a therapeutic herb to treat systemic inflammatory diseases, melasma, and freckles. However, reports of its anti-melanogenesis effect is controversial.[18,19,20,21] We recently ran the first controlled clinical trial showing the efficacy of Cuscuta in the treatment of melasma in humans. Here, we aimed to extend our finding by examining the ability of Cuscuta in removing hyperpigmented lesions and its skin lightening effects in healthy individuals as a before–after clinical trial, since this is an important cosmetic concern, especially in women.
Materials and Methods
Study population
The current prospective, interventional before–after trial was conducted in 70 healthy individuals (66 females and 4 males) who were consecutively selected from the patients referred to the Skin and Stem Cell Research Center, Tehran University of Medical Sciences, Tehran, Iran, for consultation on removing skin dark spots. The mean age of the participants was 41.5 ± 8.6 years, ranging from 18 to 65 years, and they had no evidence of any skin or systemic disorder.
However, subjects with any skin pathological involvement or familial history of skin disorders were excluded. Additionally, taking oral contraceptives at the time of study, having history of hemorrhoids, pulmonary problems, hormonal disorders, or any intolerance to the desired drug, getting sunburn within last 3 months, performing lasers within last 3 months, using anti-staining drugs within last 3 months, pregnancy, and lactation were the other exclusion criteria for the study.
The study was registered in the Iranian Registry of Clinical Trials (IRCT 2016030826967N1) after obtaining approval of the research and ethics committees at the university. Written informed consent was also obtained from all the participants before their enrollment. Also, the patients signed a “photo consent/release form” to have their photos published.
Plant extraction
The dried C. chinensis Lam. (Cuscutaceae) was purchased from the local markets (Jan 2016, Tehran, Iran). The expert botanists at the Traditional Medicine and Material Medica Research Center (TMRC) of the Shahid Beheshti University of Medical Sciences, Tehran, Iran, confirmed the scientific identity. The aerial parts of C. chinensis (77 kg) were immersed in 90°C of distilled water and boiled under reflux for 150 min. The resultant extract was centrifuged (2000 × g) for 20 min at 4°C and then filtered through a 0.2-mm filter. The filtered extract was then evaporated to dryness by the spray drying technique (Soha Jissa factory, Salmanshahr, Iran). Afterward, the dried extract (4.8 kg) was packed in the containers to be used in the clinical trial.
Quality control evaluation
We used the thin layer chromatographic (TLC) evaluation on a silica-coated gel 60 F254 plate with a solvent system of ethyl acetate, formic acid, acetic acid, and water (100 + 11 + 11 + 27) for the evaluation of extraction’s components. The relative Retardation factors were compared to those reported in the reference.
The extract was probed for microbial contamination according to the World Health Organization (WHO) quality control methods for medicinal plant materials. The total aerobic microbial count, total fungal count, and total Enterobacteriaceae count and the presence of Escherichia coli, Staphylococcus aureus, Shigella, Pseudomonas aeruginosa, and Salmonella spp were determined using plate count and multiple dilution methods.
In the next step, the plant material was burnt, and the total amount of material left after burning that included ash-derived residual from the plant was measured as total and acid-insoluble ash of the extract. The total phenolic content in the aerial parts and extract of the dried C. chinensis was also determined using spectrophotometric techniques with Folin–Ciocalteu reagent.
Clinical assessment
On the first admission, patients’ demographic characteristics, the time when the skin dark spots appeared, drug history, and previous treatments for the skin lesions were recorded. At baseline, 1 month, and 3 months after the treatment, one investigator performed clinical evaluation of the primary outcome, the mean of skin melanin content, which determines the severity of skin darkness. Dermacatch was our colorimetric measuring instrument for determining the mean melanin content of the lesion and the surrounding normal area.[22] Standard facial images were gleaned using cannon s95 10 MP camera.
Finally, the individuals’ and the investigators’ viewpoints on the efficacy of the treatment were subjectively asked. They had to select an item on the list that best described the effect of the treatment, which included 1) no effect (no visible changes of pigmentation), 2) mild (visible decrease of pigmentation, but still some visible border), 3) moderate (marked decrease of visible pigmentation, but still some visible border), and 4) excellent (complete loss of visible abnormal pigmentation). The Investigator’s Global Assessment (IGA) was used in accordance with Lee’s scoring system.
Statistical analysis
For statistical analysis, the results were presented as mean ± standard deviation (SD) for quantitative variables and were summarized by absolute frequencies and percentages for categorical variables. Normality of the data was analyzed using the Kolmogorov–Smirnov test. The change in mean the melasma area and severity index (MASI) score was examined by the paired t test or the Wilcoxon test. For statistical analysis, the statistical software Statistical Package for the Social Sciences (SPSS) version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used. P values of 0.05 or less were considered statistically significant.
Results
Quality control of the extract
Results of assessing the quality control of the herb extract showed the evaluated parameters to be in acceptable ranges. The Rfs from the TLC evaluation were similar to those of the references. The microbial assessments did not also signify any contamination of oral or any other hazardous pathogen. The total ash (8.20%), acid-insoluble ash (6.35%), alcohol-soluble extractive (13.16%), water-soluble extract (16.00%), total phenol (pyrogallol equivalent per 100 g of plant material, 462.16 ± 18.20 mg), total phenol (pyrogallol equivalent per 100 g of plant material, 462.16 ± 18.20 mg), and total phenol (pyrogallol equivalent per 100 g of plant material, 5.36 ± 0.18 g) were in acceptable limits [Table 1].
Table 1.
The quality control assessment of the plant material and extract
| Assay | Results | Acceptable limit |
|---|---|---|
| Total ash | 8.20% | Not more than 10% |
| Acid insoluble ash | 6.35% | Not more than 9% |
| Alcohol soluble extractive | 13.16% | Not less than 9% |
| Water soluble extractive | 16.00% | Not less than 16% |
| Total phenol (pyrogallol equivalent per 100g plant material) | 462.16±18.20 mg | -- |
| Total phenol (pyrogallol equivalent per 100g extract | 5.36±0.18g | -- |
Patient characteristics
The follow-up rate was 80.0% (56 out of 70 included subjects were followed up for the outcomes). The flow diagram of the contributors in the trial based on the consolidated standards of reporting trials (CONSORT) guidelines is shown in Figure 1. The mean age of the patients was 39.5 ± 7.4 years, ranging from 18 to 56 years. The percentage of participating females and males was 92.8% and 7.2%, respectively [Table 2].
Figure 1.

Flow chart of study
Table 2.
Demographics of the patients
| Variable | Amount (%) |
|---|---|
| Age (mean±SD) | 39.5±7.4 |
| Gender, n (%) | |
| Female | 52 (92.8%) |
| Male | 4 (7.2%) |
SD=standard deviation
The change in melanin content
Assessment of the mean differences in the melanin content of the lesions and the surrounding normal area with Dermacatch in response to C. chinensis revealed a significant decline during the 3 months of follow-up (P < 0.001) [Figure 2]. This decreasing trend was persistent even after adjusting the baseline characteristics including gender, age, and duration of skin involvement.
Figure 2.
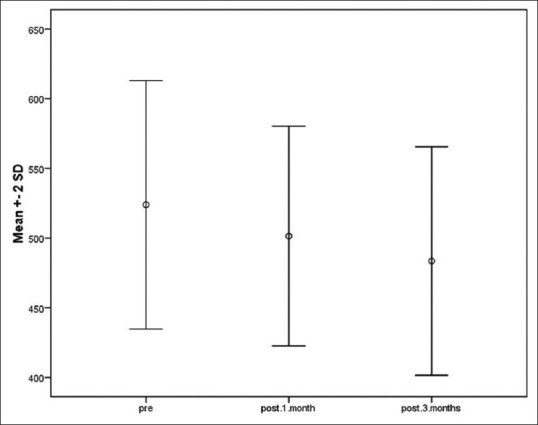
The content of melanin at baseline and at 1 and 3 months after administration of Cuscuta extract
A one-way repeated measure analysis of variance (ANOVA) was conducted that examined the effect of treatment and time (3 months) on the mean differences in the melanin content of the lesions and the surrounding normal areas. While time was shown to be independently effective in score changes (P < 0.05), there was a statistically significant interaction of the treatment group and time with melanin content (P < 0.05).
Multiple comparisons using Tukey post hoc test indicated that the difference in the mean melanin content of the lesion and the surrounding area at 1 month (498.50 ± 39.35, P < 0.001) and 3 months after treatment (483.53 ± 40.99, P < 0.001) was significantly less than that of the value at baseline (519.61 ± 45.09). Additionally, this score in the Cuscuta-receiving group was significantly reduced at the third month compared with the first month of follow-up (P < 0.001).
Patients’ and investigators’ assessment of hypopigmentation rate
Regarding individuals’ satisfaction of the intervention, only 1.5% expressed to be unsatisfied from the intervention, while mild, moderate, and excellent satisfaction were reported by 25.0%, 54.0%, and 19.55% of the subjects, respectively. In addition, viewpoint of the investigators showed that 3.0% were dissatisfied with the intervention, whereas mild, moderate, and excellent satisfaction were reported by 34.0%, 61.5%, and 1.5%, respectively [Table 3].
Table 3.
Patients’ and investigators’ viewpoint on hypopigmentation rate after treatment
| Hypopigmentation grading, n (%) | IGA | Patients’ viewpoints |
|---|---|---|
| No effect | 2 (3%) | 1 (1.5%) |
| Mild | 24 (34%) | 17 (25%) |
| Moderate | 43 (61.5%) | 38 (54%) |
| Excellent | 1 (1.5%) | 14 (19.5%) |
IGA=Investigator’s Global Assessment
In some participants, there was also melasmatic component in their face, but the aim of study was assessment of skin lightening as a whole. In Figures 3– 5, you can see the before and after therapy pictures of the participants.
Figure 3.

Skin lightening after treatment with Cuscuta extract (there is also significant lightening of basic melasma)
Figure 5.

Skin lightening after treatment with Cuscuta extract (there is also significant lightening of basic melasma)
Figure 4.
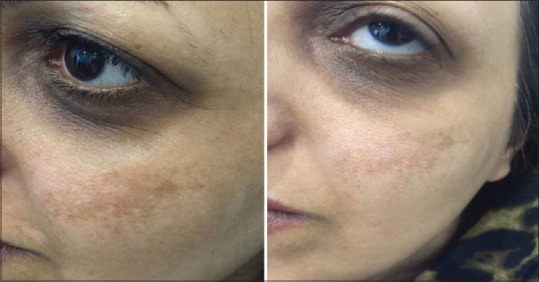
Skin lightening after treatment with Cuscuta extract (there is also significant lightening of basic melasma)
Discussion
Cuscuta extract is frequently used to treat systemic inflammatory disorders, while its cosmetic effects are not commonly known. As shown in the present study, the use of C. chinensis extract significantly reduced the content of melanin in hyperpigmented areas. Compared to previous agents, such as different types of noninvasive procedures (various creams or lotions) or invasive treatments (laser therapy or plasma-rich platelet injection), our regimen seems to have a considerably good effect in removing skin hyperpigmentation.
Jung-Chun Liao et al. reported the anti-nociceptive and anti-inflammatory effect of C. chinensis for the first time The mechanism of its action is through decreasing in nitric oxide and Muscular Dystrophy Association (MDA) in the edema paw, and consequent stimulation of superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GRd) activity in the liver of mice.[23] It has also been revealed that suppressing the release of inflammatory cytokines and reducing recruitment of inflammatory cells could be the mechanisms underlying the anti-inflammatory actions of the Cuscuta extract. Suresh et al.[18] have shown that Cuscuta reflexa inhibits tumor necrosis factor (TNF)-α, cyclooxygenase (COX)-2, and nuclear factor (NF)-κB in lipopolysaccharide (LPS)-induced inflammation of RAW264.7 cells. It also upregulates p53 and BAX, while it downregulates Bcl-2 in Hep3B cells, which leads to induction of apoptosis. Moreover, previous studies have established the antioxidant, antibacterial, and antiviral properties of C. reflexa extract.[24] In addition, Zhan et al.[25] have reported the beneficial effect of C. chinensis extract on strengthening the immune system. They showed that charcoal-herb Extractum Complex (CHC-1) isolated and purified from C. chinensis promotes the proliferation of T cells and B cells in vitro. A recent survey has revealed that C. chinensis extract could prevent development of 7,12-dimethylbenz[a] anthracene (DMBA)-induced skin papilloma in Swiss albino mice.[26] In a study by Patel et al.,[27] administration of C. reflexa led to a significant hair regrowth and proliferation of active follicles in the animal model of cyclophosphamide-induced hair fall.
Taken together, various therapeutic properties have been reported with Cuscuta extract. It also has traditionally been used to treat freckles and melasma; however, its anti-melanogenesis effect has not been investigated in humans. Wang et al. showed that in vitro, the aqueous fraction of Cuscuta japonica markedly inhibited p38 mitogen-activated protein kinase (MAPK) signaling by downregulation of alpha-melanocyte-stimulating hormone (α-MSH)-induced cyclic adenosine monophosphate (cAMP), which resulted in significant reduction of melanin in B16F10 cells.[28] Later, they reported that the water fraction of C. chinensis, despite its ethanol fraction, inhibits 3-isobutyl-1-methylxanthine (IBMX)-stimulated synthesis, melanin synthesis, and tyrosine activity in vivo and in vitro.[29] Since melasma is one of the most hyperpigmented cosmetic concerns in dermatology, there are many articles about its probable associations and etiologies[30,11] and also newer emerging therapeutic options.[31,32,33,34] Recently, we, for the first time, reported the therapeutic effect of C. chinensis extract with milk in patients with melisma.[35] Here, we extended our findings with C. chinensis by showing its inhibitory effect on the skin content of melanin and treating patients with skin hyperpigmented spots.
It is really worth to try new and safe therapies for common dermatologic disorders,[2,5,7,11,14,17] even some special concerns of recent pandemic,[20,21] which we tried to approach in this study.
Conclusion
Cuscuta extract is an effective skin whitening medicine. Treatment with Cuscuta extract inhibits skin melanin synthesis and removes hyperpigmented lesions in healthy individuals. In addition, using this herbal agent could result in high satisfaction level in both individuals and investigators.
Declaration of patient consent
Written informed consent was obtained from the patients for publication of any accompanying images in this manuscript. Following the ethical principles, names of the patients have not been mentioned in the paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to express their gratitude to the staff of the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) specially Mrs Farahnaz Nikkhah for their technical and editorial assistance.
References
- 1.França K, Keri J. Psychosocial impact of acne and postinflammatory hyperpigmentation. An Bras Dermatol. 2017;92:505–9. doi: 10.1590/abd1806-4841.20175645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrangi E, Goodarzi A, Roohaninasab M, Sadeghzadeh-Bazargan A, Nobari NN, Ghassemi M. A review of scar treatment related to acne and burn. J Crit Rev. 2020;7:714–22. [Google Scholar]
- 3.Davis EC, Callender VD. Postinflammatory hyperpigmentation:A review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesth Dermatol. 2010;3:20–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Desai SR. Hyperpigmentation therapy:A review. J Clin Aesth Dermatol. 2014;7:13–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Elham B, Somayeh S, Afsaneh S-B, Azadeh G, Mohammadreza G, Saba S, et al. The effect of metformin in the treatment of intractable and late onset acne:A comparison with oral isotretinoin. Iran J Dermatol. 2019;22:47–52. [Google Scholar]
- 6.Cestari TF, Dantas LP, Boza JC. Acquired hyperpigmentations. An Bras Dermatol. 2014;89:11–25. doi: 10.1590/abd1806-4841.20142353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodarzi A. Non-medical treatments for inflammatory acne vulgaris:A comprehensive review on laser, radiofrequency and microneedling. Iran J Dermatol. 2019;22:97–106. [Google Scholar]
- 8.Baxter LL, Pavan WJ. The etiology and molecular genetics of human pigmentation disorders. Wiley Interdiscip Rev Devel Biol. 2013;2:379–92. doi: 10.1002/wdev.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner M, Hearing VJ. Modifying skin pigmentation–approaches through intrinsic biochemistry and exogenous agents. Drug Discov Today Dis Mech. 2008;5:e189–99. doi: 10.1016/j.ddmec.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees JL. Genetics of hair and skin color. Ann Rev Genet. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- 11.Goodarzi A, Mozafarpoor S, Bodaghabadi M, Mohamadi M. The potential of probiotics for treating acne vulgaris:A review of literature on acne and microbiota. Dermatol Ther. 2020;33:e13279. doi: 10.1111/dth.13279. [DOI] [PubMed] [Google Scholar]
- 12.Kanlayavattanakul M, Lourith N. Skin hyperpigmentation treatment using herbs:A review of clinical evidences. J Cosmet Laser Ther. 2018;20:123–31. doi: 10.1080/14764172.2017.1368666. [DOI] [PubMed] [Google Scholar]
- 13.Tabassum N, Hamdani M. Plants used to treat skin diseases. Pharmacogn Rev. 2014;8:52–60. doi: 10.4103/0973-7847.125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodarzi A, Behrangi E, Ghassemi M, Nobari NN, Sadeghzadeh-Bazargan A, Roohaninasab M. Acne scar;a review of classification and treatment. J Crit Rev. 2020;7:815–23. [Google Scholar]
- 15.Gupta M, Mazumder UK, Pal D, Bhattacharya S, Chakrabarty S. Studies on brain biogenic amines in methanolic extract of Cuscuta reflexa Roxb and Corchorus olitorius Linn. seed treated mice. Acta Pol Pharm. 2003;60:207–10. [PubMed] [Google Scholar]
- 16.Pal D, Panda C, Sinhababu S, Dutta A, Bhattacharya S. Evaluation of psychopharmacological effects of petroleum ether extract of Cuscuta reflexa Roxb, stem in mice. Acta Pol Pharm. 2003;60:481–6. [PubMed] [Google Scholar]
- 17.Mehran G, Sepasgozar S, Rohaninasab M, Goodarzi A, Ghassemi M, Fotooei M, et al. Comparison between the therapeutic effect of microneedling versus tretinoin in patients with comedonal acne:A randomized clinical trial. Iran J Dermatol. 2019;22:87–91. [Google Scholar]
- 18.Suresh V, Sruthi V, Padmaja B, Asha V. In vitro anti-inflammatory and anti-cancer activities of Cuscuta reflexa Roxb. J Ethnopharmacol. 2011;134:872–7. doi: 10.1016/j.jep.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Pandit S, Chauhan NS, Dixit V. Effect of Cuscuta reflexa Roxb on androgen-induced alopecia. J Cosmet Dermatol. 2008;7:199–204. doi: 10.1111/j.1473-2165.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 20.Seirafianpour F, Mozafarpoor S, Fattahi N, Sadeghzadeh-Bazargan A, Hanifiha M, Goodarzi A. Treatment of COVID-19 with pentoxifylline:Could it be a potential adjuvant therapy?Dermatol Ther. 2020;33:e13733. doi: 10.1111/dth.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atefi N, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N-acetylcysteine and coronavirus disease. 2019:May it work as a beneficial preventive and adjuvant therapy?A comprehensive review study. J Res Med Sci. 2020;25:109. doi: 10.4103/jrms.JRMS_777_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farshi S, Mansouri P, Kasraee B. Efficacy of cysteamine cream in the treatment of epidermal melasma, evaluating by Dermacatch as a new measurement method:A randomized double blind placebo controlled study. J Dermatol Treat. 2018;29:182–9. doi: 10.1080/09546634.2017.1351608. [DOI] [PubMed] [Google Scholar]
- 23.Liao J-C, Chang W-T, Lee M-S, Chiu Y-J, Chao W-K, Lin Y-C, et al. Antinociceptive and anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am J Chin Med. 2014;42:223–42. doi: 10.1142/S0192415X14500153. [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Sharma V, Chauhan NS, Dixit VK. An updated review on the parasitic herb of Cuscuta reflexa Roxb. Zhong Xi Yi Jie He Xue Bao. 2012;10:249–55. doi: 10.3736/jcim20120302. [DOI] [PubMed] [Google Scholar]
- 25.Zhan W, Ji-Nian F, Dong-Ling G, Xiao-Yu L. Chemical characterization and immunological activities of an acidic polysaccharide isolated from the seeds of Cuscuta chinensis Lam. Acta Pharmacol Sin. 2000;21:1136–40. [PubMed] [Google Scholar]
- 26.Nisa M, Akbar S, Tariq M, Hussain Z. Effect of Cuscuta chinensis water extract on 7, 12-dimethylbenz [a] anthracene-induced skin papillomas and carcinomas in mice. J Ethnopharmacol. 1986;18:21–31. doi: 10.1016/0378-8741(86)90040-1. [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Sharma V, Chauhan NS, Dixit VK. A study on the extracts of Cuscuta reflexa Roxb, in treatment of cyclophosphamide induced alopecia. Daru. 2014;22:7. doi: 10.1186/2008-2231-22-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang JY, Kim HN, Kim YR, Choi YH, Kim BW, Shin HK, et al. Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells. J Ethnopharmacol. 2012;141:338–44. doi: 10.1016/j.jep.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 29.Wang T-J, An J, Chen X-H, Deng Q-D, Yang L. Assessment of Cuscuta chinensis seeds? effect on melanogenesis:Comparison of water and ethanol fractions in vitro and in vivo. J Ethnopharmacol. 2014;154:240–8. doi: 10.1016/j.jep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Behrangi E, Baniasadi F, Esmaeeli S, Hedayat K, Goodarzi A, Azizian Z. Serum iron level, ferritin and total iron binding capacity level among nonpregnant women with and without melasma. J Res Med Sci. 2015;20:281–3. [PMC free article] [PubMed] [Google Scholar]
- 31.Ehsani A, Noormohammadpour P, Goodarzi A, Mirshams Shahshahani M, Hejazi SP, Hosseini E, et al. Comparison of long-pulsed alexandrite laser and topical tretinoin-ammonium lactate in axillary acanthosis nigricans:A case series of patients in a before-after trial. Caspian J Intern Med. 2016;7:290–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Roohaninasab M, Goodarzi A, Ghassemi M, Sadeghzadeh-Bazargan A, Behrangi E, Najar Nobari N. Systematic review of platelet-rich plasma in treating alopecia:Focusing on efficacy, safety, and therapeutic durability. Dermatol Ther. 2021;34:e14768. doi: 10.1111/dth.14768. [DOI] [PubMed] [Google Scholar]
- 33.Lajevardi V, Ghayoumi A, Abedini R, Hosseini H, Goodarzi A, Akbari Z, et al. Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. topical hydroquinone 4% alone in melasma:A parallel-group, assessor-and analyst-blinded, randomized controlled trial with a short-term follow-up. J Cosmet Dermatol. 2017;16:235–42. doi: 10.1111/jocd.12291. [DOI] [PubMed] [Google Scholar]
- 34.Ghassemi M, Hosseinchi S, Seirafianpour F, Dodangeh M, Goodarzi A. Non-alcoholic fatty liver and lipid profile status in patients with melasma:A case-control study. J Cosmet Dermatol. 2021;20:3656–60. doi: 10.1111/jocd.14014. [DOI] [PubMed] [Google Scholar]
- 35.Mojtabaee M, Mokaberinejad R, Hamzeloo-Moghadam M, Nasab MR, Adhami S, Farshi S, et al. The effect of the traditional medicine product“Milk-Cuscuta”on skin hyperpigmentation in patients with Melasma. Middle East J Family Med. 2018;7:204. [Google Scholar]


