Abstract
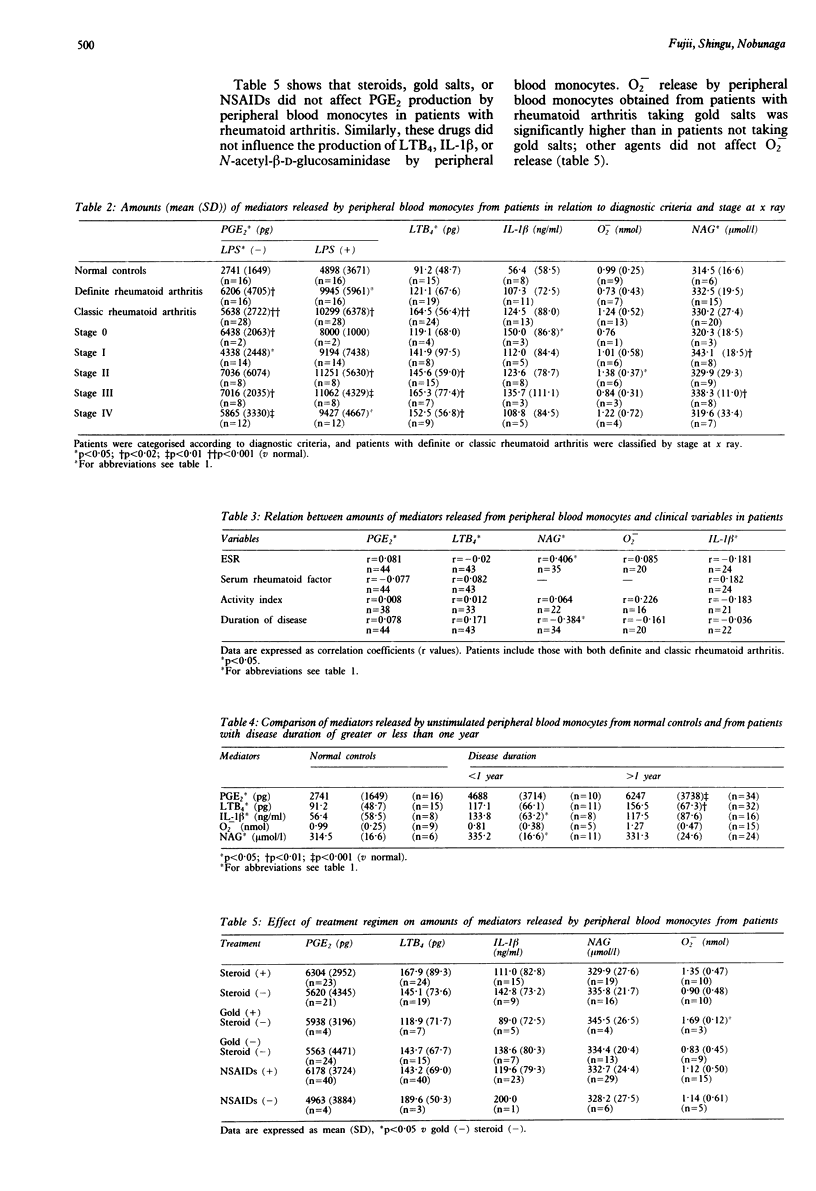
Monocytes from peripheral blood and synovial fluid of patients with definite and classic rheumatoid arthritis spontaneously produced significantly greater amounts of prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and interleukin-1 beta (IL-1 beta) than samples of peripheral blood from normal controls. Peripheral blood monocytes from patients with rheumatoid arthritis produced significantly greater amounts of PGE2 than control samples when stimulated with lipopolysaccharide. There were no significant differences in the spontaneous release of superoxide or N-acetyl-beta-D-glucosaminidase by peripheral blood monocytes between patients and healthy controls. Both stimulated and unstimulated peripheral blood monocytes from patients with definite or classic rheumatoid arthritis produced significantly greater amounts of PGE2 than samples from normal controls. This was true, regardless of the stage of disease and the presence or absence of roentgenological joint abnormalities. Amounts of N-acetyl-beta-D-glucosaminidase released by peripheral blood monocytes from patients correlated positively with the erythrocyte sedimentation rate (ESR) and negatively with duration of disease. Amounts of IL-1 beta and N-acetyl-beta-D-glucosaminidase released from the peripheral blood monocytes of patients who had had their disease for less than one year were significantly higher than those of normal controls. There were no significant correlations between the types of treatment and the amounts of PGE2, LTB4, IL-1 beta or N-acetyl-beta-D-glucosaminidase released by peripheral blood monocytes in patients with rheumatoid arthritis. The findings suggest that monocytes are activated in patients with rheumatoid arthritis both at the onset of disease and during its chronic phase, and that they produce large amounts of mediators which may have a role in the induction and extension of the inflammatory process which leads to tissue damage.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Clarris B. J., Fraser J. R., Ash P., Leizer T., Hamilton J. A. Interleukin-1 beta and interleukin-1 alpha stimulate the N-acetyl-beta-glucosaminidase activity of human synovial cells. Rheumatol Int. 1987;7(6):271–275. doi: 10.1007/BF00270528. [DOI] [PubMed] [Google Scholar]
- Clarris B. J., Hamilton J. A. Peripheral blood mononuclear cells stimulate N-acetyl-beta-glucosaminidase levels of human synovial fibroblast-like cells. Rheumatol Int. 1985;5(2):55–60. doi: 10.1007/BF00270297. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Stephenson M. L., Schmidt E., Karge W., Krane S. M. Purification of a factor from human blood monocyte-macrophages which stimulates the production of collagenase and prostaglandin E2 by cells cultured from rheumatoid synovial tissues. FEBS Lett. 1981 Feb 23;124(2):253–253. doi: 10.1016/0014-5793(81)80149-4. [DOI] [PubMed] [Google Scholar]
- Dingle J. T. The secretion of enzymes into the pericellular environment. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):315–324. doi: 10.1098/rstb.1975.0055. [DOI] [PubMed] [Google Scholar]
- Dominguez J. H., Mundy G. R. Monocytes mediate osteoclastic bone resorption by prostaglandin production. Calcif Tissue Int. 1980;31(1):29–34. doi: 10.1007/BF02407164. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. I. A cytofluorographic study of monocyte differentiation antigens and class II antigens and their regulation by gamma-interferon. Arthritis Rheum. 1987 Aug;30(8):857–863. doi: 10.1002/art.1780300803. [DOI] [PubMed] [Google Scholar]
- French J. K., Hurst N. P., McColl S. R., Cleland L. Effects of piroxicam on superoxide generation, phospholipid methylation and leukotriene production by human blood mononuclear cells. J Rheumatol. 1987 Oct;14(5):1018–1021. [PubMed] [Google Scholar]
- Hamilton J. A., Leizer T., Lingelbach S. R. The stimulation of human synovial fibroblast plasminogen activator activity. Involvement of cyclic AMP and cyclooxygenase products. Biochim Biophys Acta. 1986 Apr 29;886(2):195–202. doi: 10.1016/0167-4889(86)90137-0. [DOI] [PubMed] [Google Scholar]
- Haynes D. R., Garrett I. R., Whitehouse M. W., Vernon-Roberts B. Do gold drugs inhibit interleukin-1? Evidence from an in vitro lymphocyte activating factor assay. J Rheumatol. 1988;15(5):775–778. [PubMed] [Google Scholar]
- Hurst N. P., Bell A. L., Nuki G. Studies of the effect of D-penicillamine and sodium aurothiomalate therapy on superoxide anion production by monocytes from patients with rheumatoid arthritis: evidence for in vivo stimulation of monocytes. Ann Rheum Dis. 1986 Jan;45(1):37–43. doi: 10.1136/ard.45.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst N. P., Bessac B., Nuki G. Monocyte superoxide anion production in rheumatoid arthritis: preliminary evidence for enhanced rates of superoxide anion production by monocytes from patients receiving penicillamine, sodium aurothiomalate and corticosteroids. Ann Rheum Dis. 1984 Feb;43(1):28–33. doi: 10.1136/ard.43.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ziff M. Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum. 1976 Jan-Feb;19(1):1–14. doi: 10.1002/art.1780190101. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- Kay N. E., Douglas S. D. Monocyte metabolic activation in patients with rheumatoid arthritis. Proc Soc Exp Biol Med. 1979 Jul;161(3):303–306. doi: 10.3181/00379727-161-40541. [DOI] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Daniele R. P., Nowell P. C. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J Clin Invest. 1988 Jan;81(1):237–244. doi: 10.1172/JCI113301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Shapleigh C., Goetzl E. J. Lipoxygenation of arachidonic acid as a source of polymorphonuclear leukocyte chemotactic factors in synovial fluid and tissue in rheumatoid arthritis and spondyloarthritis. J Clin Invest. 1980 Nov;66(5):1166–1170. doi: 10.1172/JCI109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANSBURY J. Quantitation of the activity of rheumatoid arthritis. 5. A method for summation of the systemic indices of rheumatoid activity. Am J Med Sci. 1956 Sep;232(3):300–310. [PubMed] [Google Scholar]
- MacKinnon S. K., Starkebaum G. Monocyte Fc receptor function in rheumatoid arthritis. Enhanced cell-binding of IgG induced by rheumatoid factors. Arthritis Rheum. 1987 May;30(5):498–506. doi: 10.1002/art.1780300503. [DOI] [PubMed] [Google Scholar]
- Marshall J. L., Fraser J. R., Muirden K. D. Lysosomal activation by neutral saccharides in cell cultures of synovium. Ann Rheum Dis. 1977 Apr;36(2):130–138. doi: 10.1136/ard.36.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- Mochan E., Uhl J., Newton R. Evidence that interleukin-1 induction of synovial cell plasminogen activator is mediated via prostaglandin E2 and cAMP. Arthritis Rheum. 1986 Sep;29(9):1078–1084. doi: 10.1002/art.1780290904. [DOI] [PubMed] [Google Scholar]
- Muirden K. D. Lysosomal enzymes in synovial membrane in rheumatoid arthritis. Relationship to joint damage. Ann Rheum Dis. 1972 Jul;31(4):265–271. doi: 10.1136/ard.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Nishino N., Takano M., Kawai K., Bando K., Masui Y., Nakai S., Hirai Y. cDNA cloning of IL-1 alpha and IL-1 beta from mRNA of U937 cell line. Biochem Biophys Res Commun. 1987 Feb 27;143(1):345–352. doi: 10.1016/0006-291x(87)90671-1. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Payne T., Dinarello C. A. Human monocyte or recombinant interleukin 1's are specific for the secretion of a metalloproteinase from chondrocytes. J Immunol. 1987 Jan 15;138(2):496–503. [PubMed] [Google Scholar]
- Seitz M., Hunstein W. Enhanced prostanoid release from monocytes of patients with rheumatoid arthritis and active systemic lupus erythematosus. Ann Rheum Dis. 1985 Jul;44(7):438–445. doi: 10.1136/ard.44.7.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A., Jaglal S., Keystone E. C. Enhanced interleukin 1 generation by monocytes in vitro is temporally linked to an early event in the onset or exacerbation of rheumatoid arthritis. Clin Exp Immunol. 1986 Aug;65(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Tanaka K., Ishikawa E., Ohmoto Y., Hirai Y. In vitro production of human interleukin 1 alpha and interleukin 1 beta by peripheral blood mononuclear cells examined by sensitive sandwich enzyme immunoassay. Eur J Immunol. 1987 Oct;17(10):1527–1530. doi: 10.1002/eji.1830171024. [DOI] [PubMed] [Google Scholar]
- Toyoda K., Saito S., Naito S., Konomi K., Yamamoto H. HLA antigens in classical and malignant rheumatoid arthritis in Japanese population. Tissue Antigens. 1977 Jul;10(1):56–59. doi: 10.1111/j.1399-0039.1977.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Wegelius O., Klockars M., Vainio K. Acid phosphatase activity in rheumatoid synovia. Acta Med Scand. 1968 Jun;183(6):549–551. doi: 10.1111/j.0954-6820.1968.tb10521.x. [DOI] [PubMed] [Google Scholar]
- Whicher J. T., Gilbert A. M., Westacott C., Hutton C., Dieppe P. A. Defective production of leucocytic endogenous mediator (interleukin 1) by peripheral blood leucocytes of patients with systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis and mixed connective tissue disease. Clin Exp Immunol. 1986 Jul;65(1):80–89. [PMC free article] [PubMed] [Google Scholar]



