Abstract
Background:
Low-dose fluconazole is commonly used to treat vulvovaginal candidiasis, a condition occurring frequently during pregnancy. Conflicting information exists on the association between low-dose fluconazole use among pregnant women and risk of major birth defects.
Objective:
We used data from the National Birth Defects Prevention Study to examine this association.
Study Design:
The National Birth Defects Prevention Study is a multisite, population-based, case-control study that includes pregnancies with estimated delivery dates from 1997–2011. Information on early pregnancy fluconazole use was collected by self-report from 31,645 mothers of birth defect cases and 11,612 mothers of unaffected controls. Adjusted odds ratios and 95% confidence intervals were estimated for birth defects with 5 or more exposed cases; crude odds ratios and exact 95% confidence intervals were estimated for birth defects with 3–4 exposed cases.
Results:
Of the 43,257 mothers analyzed, 44 case mothers and 6 control mothers reported using fluconazole. Six exposed infants had cleft lip with cleft palate, four had an atrial septal defect, and each of the following defects had three exposed cases: hypospadias, tetralogy of Fallot, d-transposition of the great arteries, and pulmonary valve stenosis. Fluconazole use was associated with cleft lip with cleft palate (odds ratio=5.53; confidence interval=1.68–18.24) and d-transposition of the great arteries (odds ratio =7.56; confidence interval =1.22–35.45).
Conclusions:
The associations between fluconazole and both cleft lip with cleft palate and d-transposition of the great arteries are consistent with earlier published case reports, but not recent epidemiologic studies. Despite the larger sample size of the National Birth Defects Prevention Study, fluconazole use was rare. Further investigation is needed in large studies, with particular emphasis on oral clefts and conotruncal heart defects.
Keywords: fluconazole, birth defect, congenital malformation
Introduction
Fluconazole is a systemic azole antifungal drug taken orally or intravenously. Low-dose fluconazole (150 mg/day) is often prescribed for the treatment of vulvovaginal candidiasis, a common condition during pregnancy. 1 High-dose fluconazole use (400–800 mg/day) is used to treat systemic fungal infections. Case reports have linked high-dose fluconazole use to a pattern of birth defects marked by the presence of craniofacial, skeletal, and sometimes heart defects.2-6 Animal studies have confirmed the teratogenicity of fluconazole when given at high doses (human equivalent of more than 800 mg per day).7-11
Several epidemiologic studies have examined the risk of birth defects among infants whose mothers took low doses of fluconazole.12-20 The majority of these studies did not report an increased risk of birth defects, but most either were not large enough to examine individual birth defects or were focused on the presence of birth defects overall.12-17,21 In contrast, a recent Danish cohort study reported an association between maternal first-trimester fluconazole use and tetralogy of Fallot in offspring.20 A prior analysis using data from the National Birth Defects Prevention Study (NBDPS) found that early pregnancy use of antifungal medications (including fluconazole) was associated with an increased risk of hypoplastic left heart syndrome.19 Thus, it remains unclear if early pregnancy use of low-dose fluconazole is associated with specific birth defects.
Understanding the teratogenicity of fluconazole at low doses remains important given that pregnant women are at increased risk of vulvovaginal candidiasis.1 In 2002 and 2006, the U.S. Centers for Disease Control and Prevention (CDC) recommended that pregnant women avoid use of fluconazole, suggesting the use of topical azoles during pregnancy instead.22,23 In 2011, the U.S. Food and Drug Administration (FDA) issued a drug safety communication regarding the possible teratogenic risks associated with long-term, high-dose fluconazole use, and changed the FDA pregnancy category for high-dose fluconazole from category C to D (evidence of human fetal risk, but the benefits may warrant use).24
To investigate the potential teratogenicity of low-dose fluconazole, we examined the association between first trimester fluconazole use and the risk of major birth defects using data from the NBDPS.
Materials and Methods
The NBDPS is a large, multisite, population-based, case-control study of birth defects that began collecting data in 1997.25 Infants with one or more of 30 different categories of major structural birth defects (cases), excluding those attributed to a known chromosomal or single-gene abnormality, were ascertained through birth defect surveillance programs in ten states (Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, and Utah). Control infants were live births without birth defects randomly selected from hospital records or birth certificates in the same time period and geographic area as the cases. Estimated delivery dates included in this analysis were October 1, 1997 through December 31, 2011. Each study site obtained Institutional Review Board approval for the NBDPS and case and control mothers provided informed consent.
Case inclusion criteria have been described previously.25 Case information, including clinical information abstracted from case medical records, was obtained from birth defect surveillance programs. The clinical record of each case was reviewed by a clinical geneticist to determine eligibility prior to the interview. Clinical geneticists also reviewed and classified each case infant as having isolated, multiple, or complex birth defects.26 Congenital heart defect (CHD) cases were further categorized as simple, association, or complex.27 CHD cases classified as atrial septal defects (ASD) not otherwise specified were viewed as probably ASD secundum type and were counted as such in the analysis.27 Starting in January 2006, the collection of ventricular septal defects (VSDs) changed; the current analysis excludes VSDs diagnosed after 2005.27 Certain study sites did not ascertain cases during the entire study period for pulmonary valve stenosis (PVS) (California before 2002) and muscular VSDs (after first year for sites participating 1997–1998). When we analyzed those defects, cases and controls were excluded for the study sites and years for which case ascertainment was incomplete.
Non-cardiac cases were grouped according to NBDPS standard classification criteria. Microtia included dysplastic ear pinna and stenosis or atresia of external auditory canal. Infants with intestinal atresia limited to the duodenum were grouped together and counted as duodenal atresias; other intestinal atresias (ileal, jejunal, and multiple intestinal atresias or stenosis) were counted as small intestinal atresias. Infants with esophageal or small intestine atresia that occurred as a component of a VATER/VACTERL association defects (an association of birth defects including vertebral anomalies, anal atresia, cardiac defects, tracheoesophageal fistula/esophageal atresia, renal anomalies, and limb defects) were included and classified as having multiple defects. Only second- and third-degree hypospadias cases were included; the control group was restricted to male infants.
Trained interviewers conducted computer-assisted telephone interviews with the mothers of case and control infants between 6 weeks and 24 months after the estimated date of delivery. Demographics, pregnancy history, various health conditions, and exposures before and during pregnancy were collected. Mothers were asked about all medications taken during the period from three months preconception through the end of pregnancy and information was collected on timing, frequency, and duration of medication use. The Slone Epidemiology Center Drug Dictionary was used to code all reported medications. During the study period, 66.7% of eligible case mothers and 63.7% of eligible control mothers participated in the interview. In total, 44,029 mothers (32,200 case and 11,829 control mothers) completed the NBDPS interview.
Infants were classified as exposed if the mother reported any fluconazole use in the month before pregnancy through the third month of pregnancy. The first three months of pregnancy were chosen as this is the critical period in the development of the fetus associated with most structural birth defects. Given that it is often hard to pinpoint the exact date of conception, the month before pregnancy was also included in the exposure time frame to ensure all exposed infants were identified. Infants of mothers who were missing information on timing of fluconazole use were excluded from analysis, as were infants of mothers who did not answer all the medication questions during the interview.
Covariates included maternal age at delivery (≤24, 25–29, 30–34, ≥35 years), maternal race/ethnicity (non-Hispanic white, other), mother’s state of residence at the time of infant’s birth, cigarette smoking in the month before through the third month of pregnancy (yes/no), and gestational diabetes during the current pregnancy (yes/no). Chi-square tests were used to compare maternal characteristics between those with and without fluconazole exposure. For birth defects with five or more exposed cases, logistic regression models were constructed to estimate the adjusted odds ratio (ORs) and 95% confidence intervals (CIs) for the association between maternal fluconazole exposure and each type of birth defect, while controlling for covariates. For birth defects with three or four exposed cases, crude ORs and exact CIs were estimated. ORs were not estimated for birth defects with fewer than three exposed cases. We examined all cases and then separately examined isolated cases within each birth defect (i.e., cases with only one defect). Analyses were performed using SAS software, version 9.3 (SAS Corporation, Cary, NC).
Results
After excluding 772 infants (555 cases, 217 controls) due to missing information on maternal fluconazole use, 43,257 infants (31,645 cases, 11,612 controls) were analyzed. The average time between the estimated delivery date and interview was 11.5 months among cases and 9.2 months among controls.
Overall, 50 (0.12%) mothers reported using fluconazole during the one month before pregnancy through the third month of pregnancy. Of the 50 exposed infants, 44 were cases and 6 were controls. The majority of the exposed mothers reported taking fluconazole for vulvovaginal candidiasis (36/50; 72%); the remainder did not specify why fluconazole was used. Most mothers (49/50; 98%) reported taking fluconazole for a short period of time (less than one week).
Mothers who reported using fluconazole were similar to mothers who did not in terms of maternal age, race, and smoking status, and varied somewhat by study center and maternal gestational diabetes (Table 1). Two exposed case mothers reported type 1 or 2 diabetes before the index pregnancy. Mothers were less likely to report fluconazole use after 2006; 42 (84%) of the exposed infants were born prior to 2006.
Table 1.
Selected characteristics of mothers, by fluconazole use in the National Birth Defects Prevention Study 1997–2011
| Used fluconazole |
Did not use fluconazole |
P valueb | |
|---|---|---|---|
| n = 50 | n = 43,207 | ||
| n (%)a | n (%)a | ||
| Maternal age | |||
| ≤ 24 | 15 (30) | 14405 (33) | |
| 25–29 | 11 (22) | 11721 (27) | 0.324 |
| 30–34 | 12 (24) | 10665 (25) | |
| 35 + | 12 (24) | 6416 (15) | |
| Maternal race/ethnicity | |||
| Non-Hispanic White | 35 (70) | 25321 (59) | 0.103 |
| Other | 15 (30) | 17873 (41) | |
| Study Center | |||
| Arkansas | 13 (26) | 5664 (13) | |
| California | 5 (10) | 5163 (12) | |
| Iowa | 2 (4) | 4242 (10) | |
| Massachusetts | 6 (12) | 5334 (12) | |
| New Jersey | 2 (4) | 2209 (5) | 0.326 |
| New York | 5 (10) | 3124 (7) | |
| Texas | 4 (8) | 4811 (11) | |
| Atlanta | 4 (8) | 4776 (11) | |
| North Carolina | 5 (10) | 3374 (8) | |
| Utah | 4 (8) | 4510 (10) | |
| Smokingc | |||
| Yes | 9 (18) | 8397 (20) | 0.778 |
| No | 41 (82) | 34473 (80) | |
| Gestational diabetes during pregnancy | |||
| Yes | 5 (11) | 2156 (5) | 0.079 |
| No | 40 (89) | 38855 (95) |
Numbers vary because of missing values.
Chi-square P values.
From one month before pregnancy through the third month of pregnancy.
Mothers who took fluconazole also reported using other medications during pregnancy. Ninety-six percent of case mothers and 83% of control mothers reported using at least one other medication (excluding vitamins) during pregnancy. The most common medications reported were acetaminophen, ibuprofen, amoxicillin, and pseudoephedrine. No mother who used fluconazole also used folic-acid antagonist medications, which have been associated with specific birth defects. Two case mothers who used fluconazole also took valproic acid, an anti-seizure medication associated with increased risk of specific birth defects.28 One infant had simple, isolated d-transposition of the great arteries (dTGA), and the other had isolated hypospadias.
Eight exposed infants had an oral cleft (Table 2): one had a cleft palate, six had a cleft lip with cleft palate (CLP), and one had a cleft lip only. Three exposed infants had hypospadias. There were nine exposed infants with a conotruncal defect, three of whom had dTGA and three of whom had tetralogy of Fallot. Three exposed infants had PVS, and four had ASDs. There were fewer than three exposed infants for all other birth defects. The CHDs among exposed infants were classified as simple with four exceptions: (1) one infant had dTGA with VSD, ASD, and PVS; (2) one infant had a double-outlet right ventricle with transposed great arteries with an ASD; (3) one infant had PVS and VSD; and (4) one infant had heterotaxy.
Table 2.
Counts of fluconazole-exposed and unexposed infants, by birth defects included in the National Birth Defects Prevention Study 1997–2011
| Exposed/ Unexposed | ||
|---|---|---|
| Birth Defect | All Casesa | Isolated Cases |
| Amniotic band sequence | 1/333 | 1/281 |
| Central nervous system defects | ||
| Anencephaly | 1/646 | 1/578 |
| Spina bifida | 2/1275 | 2/1120 |
| Encephalocele | 0/227 | 0/171 |
| Holoprosencephaly | 0/173 | 0/125 |
| Dandy-Walker malformation | 0/186 | 0/114 |
| Hydrocephaly | 1/518 | 0/361 |
| Cerebellar hypoplasia | 0/64 | 0/38 |
| Eye defects | ||
| Anophthalmia/microphtalmia | 0/232 | 0/139 |
| Congenital cataracts | 1/383 | 1/341 |
| Glaucoma | 0/186 | 0/153 |
| Anotia/microtia | 1/690 | 1/475 |
| Orofacial defects | ||
| Cleft palate only | 1/1602 | 1/1284 |
| Cleft lip only | 1/1097 | 1/1021 |
| Cleft lip with palate | 6/2016 | 4/1717 |
| Choanal atresia | 0/166 | 0/87 |
| Gastrointestinal defects | ||
| Esophageal atresia | 2/754 | 0/318 |
| Duodenal atresia/stenosis | 0/237 | 0/148 |
| Small intestine atresia/stenosis | 0/477 | 0/407 |
| Anorectal atresia/stenosis | 2/1075 | 0/465 |
| Biliary atresia | 0/199 | 0/170 |
| Cloacal exstrophy | 0/100 | 0/59 |
| Colonic atresia/stenosis | 0/56 | 0/50 |
| Genitourinary defects | ||
| Hypospadias | 3/2556 | 3/2283 |
| Renal agenesis/hypoplasia | 0/190 | 0/135 |
| Bladder exstrophy | 0/74 | 0/55 |
| Musculoskeletal defects | ||
| Transverse limb deficiency | 0/721 | 0/604 |
| Longitudinal limb deficiency | 0/480 | 0/268 |
| Intercalary limb deficiency | 0/67 | 0/46 |
| Limb NOS | 0/24 | 0/16 |
| Craniosynostosis | 1/1597 | 1/1446 |
| Diaphragmatic hernia | 2/872 | 2/665 |
| Omphalocele | 0/439 | 0/258 |
| Gastroschisis | 0/1412 | 0/1282 |
| Sacral agenesis | 0/109 | 0/12 |
| Congenital heart defects | ||
| Heterotaxyb | 1/344 | -- |
| Conotruncal defects | ||
| Truncus arteriosus | 2/136 | 2/105 |
| IAA type b | 0/50 | 0/35 |
| IAA NOS | 0/8 | 0/7 |
| Tetralogy of Fallot | 3/1207 | 1/964 |
| d-TGA | 3/768 | 3/707 |
| DORV-TGA | 1/191 | 1/158 |
| Other DORV | 0/123 | 0/91 |
| Conoventricular VSD | 0/117 | 0/95 |
| Atrioventricular septal defect | 1/371 | 1/277 |
| Total anomalous pulmonary venous return | 0/303 | 0/278 |
| LVOTO defects | ||
| Hypoplastic left heart syndrome | 1/659 | 1/600 |
| IAA type a | 0/22 | 0/18 |
| Coarctation of the aorta | 2/1169 | 2/1007 |
| Aortic stenosis | 0/513 | 0/474 |
| LVOTO Associationc | 0/534 | 0/441 |
| RVOTO defects | ||
| Pulmonary atresia | 1/264 | 0/245 |
| Pulmonary valve stenosis | 3/1553 | 2/1433 |
| Ebstein anomaly | 0/180 | 0/163 |
| Tricuspid atresia | 1/178 | 1/157 |
| RVOTO Associationd | 1/447 | 0/386 |
| Septal defects | ||
| Perimembranous VSD | 2/1443 | 1/1228 |
| Muscular VSD | 0/161 | 0/145 |
| VSD NOS | 0/17 | 0/13 |
| Multiple VSDs | 0/69 | 0/58 |
| Secundum atrial septal defect | 4/3069 | 1/2491 |
| Atrial septal defect OS | 0/11 | 0/6 |
| Septal associationse | 0/767 | 0/600 |
| Single ventricle defectsb | 0/174 | -- |
NOS=not otherwise specified, IAA=interrupted aortic arch, dTGA=d-transposition of the great arteries, DORV-TGA=double outlet right ventricle and transposition of the great artery, VSD=ventricular septal defect, LVOTO=left ventricular outflow tract obstruction, RVOTO=right ventricular outflow tract obstruction, ASD OS=sinus venosus, coronary sinus, sinoseptal, and caval atrial septal defects.
Includes cases with isolated defects and those with additional defects.
All cases in birth defect group were considered complex.
LVOTO associations include coarctation of the aorta + aortic stenosis, coarctation of the aorta + VSD, coarctation of the aorta + VSD + ASD, and VSD + ASD + aortic stenosis.
RVOTO associations include PVS + VSD, PVS + ASD, and VSD + ASD + PVS.
Septal associations include VSD + ASD.
For analyses that included both isolated and non-isolated cases of each type of birth defect, we found that fluconazole use was significantly associated with CLP (OR=5.53, 95% CI=1.68–18.24; Figure 1). For comparison to studies that have analyzed cleft lip with or without cleft palate as a single group, we estimated the OR for that grouping as well (OR=4.16, 95% CI=1.34–12.98). Fluconazole use was also associated with dTGA (OR=7.56, 95% CI=1.22–35.45; Figure 1). Fluconazole exposure was associated with hypospadias, tetralogy of Fallot, PVS, and ASD, but these increases were not statistically significant.
Figure 1. Association of fluconazole use and select birth defects in the National Birth Defects Prevention Study 1997–2011.
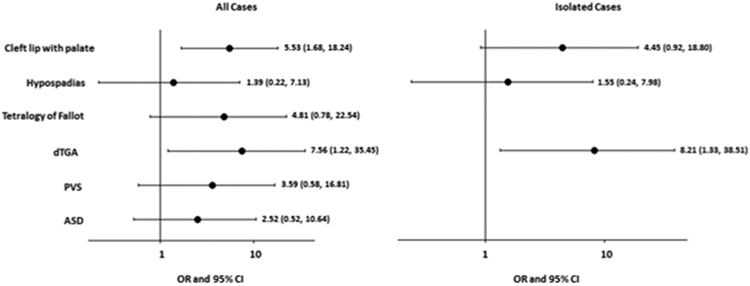
For analyses of all cases (isolated and non-isolated), an adjusted odds ratio (OR) and 95% confidence interval (CI) are presented for cleft lip with palate, adjusted for mother’s state of residence at the time of birth, age, race/ethnicity, smoking, and gestational diabetes. Crude ORs with exact 95% CIs are presented for the remaining defect groups that had 3–4 exposed cases. Among isolated defects, no defect had ≥5 exposed cases; crude ORs and exact 95% CIs are presented for the defect groups with 3–4 exposed cases. Analyses included 6 exposed and 11,606 unexposed control infants, with two exceptions: (1) the analysis for orofacial clefts includes 6 exposed and 11,472 unexposed control infants, and (2) the analysis for hypospadias includes 5 exposed and 5,906 unexposed male controls.
When case groups were restricted to isolated birth defects, the statistically significant increased risk of dTGA associated with fluconazole use persisted (OR=8.21, 95% CI=1.33–38.51; Figure 1). The OR for the association between maternal fluconazole use and both CLP (OR=4.45, 95% CI=0.92–18.80) and hypospadias (OR=1.55, 95% CI=0.24–7.98) remained positive, but did not reach statistical significance. The association for the grouping of cleft lip with or without cleft palate persisted (OR=3.91, 95% CI=1.19–12.88).
Comment
While exposure to fluconazole in the first trimester was not associated with the majority of birth defects examined, it was associated with an increased risk of CLP, hypospadias, dTGA, tetralogy of Fallot, PVS, and ASD, although only the increased risk for CLP and dTGA were statistically significant. If these estimates represent a true increase in risk, based on an estimated prevalence of 6.64 infants with CLP per 10,000 live births, an OR of 5.53 would translate to a potential increase in absolute risk from 1 infant with CLP per 1,506 live births to 1 in 272 live births among women exposed to fluconazole during the first trimester.29 Based on an estimated prevalence of 3 infants with dTGA per 10,000 live births, an OR of 7.56 would translate to a potential increase in absolute risk from 1 infant with dTGA per 3,333 live births to 1 in 441 live births among women exposed to fluconazole during the first trimester.30
While dose information was not collected, we believe that all 50 mothers who reported taking fluconazole during the month before through the third month of pregnancy used low-dose fluconazole because (1) most of these mothers reported taking fluconazole for vaginal candidiasis, and (2) most used fluconazole for a short duration.
Most previous epidemiologic studies reported no increased risk of birth defects after low-dose fluconazole exposure during the first trimester (Table 3). Previous studies were based on prescription-event monitoring, reports of exposure to a teratogen information service, and prescription-database linkage to electronic hospital records, and no study observed more than seven birth defects among the offspring of exposed women.12-15,17 There have been a few studies of specific defects. One such cohort study reported that fluconazole-exposed women had no significant increased risk of craniofacial defects or CHDs.16 Molgaard-Nielsen et al. reported that maternal first-trimester use of fluconazole was not associated with increased risk for 14 of the 15 specific birth defects examined (including cleft lip with or without palate).20 A recent meta-analysis did not find an association between fluconazole and any birth defect or craniofacial defects, specifically, but reported an increased risk for CHDs.21
Table 3.
Epidemiological studies of fluconazole use and birth defects
| Author (Year) |
Design and setting | Subject groups | Dose/duration of fluconazole use (number of exposed mothers) |
Findings |
|---|---|---|---|---|
| Inman (1994)13 | Survey of adverse health outcomes among patients prescribed fluconazole in England from 12/1988–01/1989 | 289 pregnancies among women prescribed fluconazole | Single 150 mg dose (n = 275) Multiple 150 mg doses (n = 11) Multiple 50 mg doses (n = 3) |
7 (2.4%) pregnancies affected by birth defects; all 7 mothers were prescribed a single 150 mg dose between 1 and more than 26 weeks before the last menstrual period |
| Mastroiacovo (1996)15 | Cohort of women who contacted Teratology Information Service centers in Italy from 01/1992–06/1994 | 226 pregnant women who used fluconazole in the first trimester, 452 pregnant women with first-trimester exposure to nonteratogenic agents | Single 150 mg dose (n = 105) Multiple 150 mg doses (n = 81) Single 100 mg dose (n = 5) Multiple 100 mg doses (n = 9) Single 50 mg dose (n = 3) Multiple 50 mg doses (n = 23) |
OR (95% CI) for birth defects overall: 1.07 (0.41, 2.77) |
| Campomori (1997)12 | Survey of birth defects among women who contacted an Italian drug information center | 16 women who used fluconazole in the first trimester | Median dose = 300 mg (range 150–1000 mg); Duration of treatment = 1±0.3 weeks | No birth defects were observed in the offspring |
| Jick (1999)14 | Cohort based on patient records in the United Kingdom General Practice Research Database | 234 women prescribed fluconazole in the first trimester, and 1,629 women with no first-trimester prescriptions for either topical or oral azole antifungals | 92% of the exposed women were prescribed a single 150 mg dose | RR (95% CI) for birth defects overall: 1.1 (0.4, 3.3) |
| Sørensen (1999)17 | Cohort of women who gave birth from 1991–1996 in one region of Denmark | 121 women who filled a prescription for fluconazole in the first trimester, and 13,327 women who did not fill any prescriptions during pregnancy | Not stated. The authors reported fluconazole is usually prescribed as a single 150 mg dose to treat vaginal candidiasis in Denmark | OR (95% CI) for birth defects overall: 0.65 (0.24, 1.77) |
| Nørgaard (2008)16 | Cohort of women who gave birth from 1991–2005 in four regions of Denmark | 1,079 women who filled a prescription for fluconazole in the first trimester, and 170,453 women with no fluconazole prescriptions during pregnancy | Cumulative doses of: 150 mg (n = 797) 300 mg (n = 235) 350 mg (n = 24) 600 mg (n = 23) |
OR (95% CI) for (a) birth defects overall: 1.0 (0.8, 1.4); (b) craniofacial defects: 1.3 (0.6, 2.6); (c) heart defects: 1.3 (0.7, 2.1) |
| Mølgaard-Nielsen (2013)20 | Cohort of live births in Denmark from 01/1996–03/2011 | 7,352 women who filled a prescription for fluconazole in the first trimester, and 968,236 women who did not fill any prescriptions for oral azole antifungals in the first trimester | Cumulative doses of: 150 mg (n = 4,082) 300 mg (n = 2,252) 350 – 6,000 mg (n = 1,018) |
OR (95% CI) for (a) birth defects overall: 1.06 (0.92, 1.21); (b) tetralogy of Fallot: 3.16 (1.49, 6.71) |
| Alsaad (2015)21 | Meta-analysis of studies Mastroiacovo (1996), Jick (1999), Nørgaard (2008), Mølgaard-Nielsen (2013) | 8,842 women exposed to fluconazole in the first trimester, and 1,140,724 women not exposed to fluconazole in the first trimester | As reported for the individual studies | OR (95% CI) for (a) birth defects overall: 1.10 (0.98, 1.25); (b) heart defects: 1.29 (1.05, 1.58); (c) craniofacial defects: 1.25 (0.88, 1.77); (d) limb or musculoskeletal defects: 0.82 (0.59, 1.13) |
CI=confidence interval, OR=odds ratio, RR=relative risk.
We observed an increased risk of CLP among fluconazole-exposed infants. While this has not been reported in other observational studies, case reports have linked high-dose fluconazole exposure during the first trimester to orofacial clefts, as have animal studies.2-10 One such study reported branchial arch defects in rat embryos exposed to fluconazole (125–500 μM),7,8 while another found cleft palate in mice after exposure to 700 mg/kg of fluconazole.9
We found an increased risk for hypospadias associated with fluconazole use, but the increase was not statistically significant. This defect was previously reported in a prescription-event monitoring study, but the authors stated that the association may have been due to the underlying disease rather than fluconazole exposure. 13 No other observational study has reported this association.
Our study found nine conotruncal defects among the infants of exposed mothers, a significantly increased risk for dTGA, and a non-significant increased risk of tetralogy of Fallot. Case reports have highlighted a potential association with conotruncal and septal heart defects.5,6 Molgaard-Nielsen et al. found an increased risk for tetralogy of Fallot, but dTGA was not one of the individual defects examined in their analysis.20
A case-control study using NBDPS data for births through 2003 found an increased risk of hypoplastic left heart syndrome in association with the use of any antifungal medication. While fluconazole was included in the analysis, the most common antifungal was a topical azole.19 That analysis included 216 mothers (132 case mothers, and 84 control mothers) who reported any antifungal medication use. While there was some overlap among subjects in both studies, our study focused specifically on fluconazole use and found only one fluconazole-exposed infant with hypoplastic left heart syndrome.
Fluconazole is a rare exposure among mothers in the NBDPS, which is reassuring given the CDC recommendations and the FDA guidelines. Despite these treatment guidelines, we found that fluconazole was still prescribed during the study timeframe.
Because the NBDPS relies on retrospective reporting of medication use during pregnancy, recall bias is always a concern. However, we observed associations for a very small proportion of the defects studied and had no exposed cases for more than half of the birth defects. We do not expect there to be a difference in recall of fluconazole use between mothers of infants with different birth defects, and so do not believe that recall bias strongly influenced our findings. The average time between birth and interview was 11.5 months among case mothers and 9.2 months among control mothers. This lag may have resulted in inaccurate reporting of fluconazole use due to problems in recalling medication use during pregnancy. If inaccurate reporting of fluconazole use existed, we would expect mothers to underreport use because most mothers are likely to have used it infrequently. If underreporting did not differ by case-control status, our findings may be underestimates of the true risk.
While the NBDPS collects data on duration of medication use, we were limited by the lack of data on dose. Given that high-dose fluconazole use has been associated with a pattern of birth defects in previous studies, including craniofacial, skeletal, and some heart defects,2-6 inclusion of high-dose users may have made our results appear greater in magnitude than for only low-dose users. However, the majority of mothers taking fluconazole reported using it for vaginal candidiasis and reported using fluconazole for a short period of time. We believe it is likely that the mothers included in this analysis used a low dose of fluconazole.
Given that fluconazole use was evaluated in the month before pregnancy through the third month of pregnancy, we may have classified infants whose mothers used fluconazole in the month prior to pregnancy as exposed when they were not. However, this misclassification would be non-differential, and would have biased our estimates toward the null.
Despite the large size of the NBDPS, a small number of mothers were reportedly taking fluconazole in the first trimester, a small number of exposed infants with each birth defect, and only six exposed control infants. Because there were so few exposed cases in several of the birth defect groups it was difficult to calculate stable risk estimates. We were able to calculate a total of eleven ORs for birth defects with three or more exposed cases. Chance alone could explain some of the findings; however, the birth defects for which we found statistically significant increased risks have been previously reported in case reports and animal studies. There may be some uncontrolled confounding by factors not measured, or residual confounding by measured factors. Lastly, we could not rule out that the associations were due to the underlying disease and not fluconazole.
The small numbers of exposed infants upon which our findings are based suggest that the findings should be interpreted cautiously. While our study does not provide definitive evidence that low-dose fluconazole is a teratogen, our findings support the recommendation of the CDC and FDA to avoid fluconazole for the treatment of vulvovaginal candidiasis in pregnancy.22-24 Based on the observations of birth defects in these 50 infants, the potential of low-dose fluconazole to act as a teratogen should be further studied with an emphasis on oral clefts and conotruncal CHDs.
Condensation:
While fluconazole use was rare, associations were found between fluconazole and both cleft lip with cleft palate and d-transposition of the great arteries.
Acknowledgements
This study was supported by a cooperative agreement from the Centers for Disease Control and Prevention (grant number: U01/DD00048702). Coding of drug information in the NBDPS used the Slone Epidemiology Center Drug Dictionary, under license from the Slone Epidemiology Center at Boston University. We would like to thank the participating families, scientists, and staff from all of the NBDPS sites.
Financial Support:
This study was supported by a cooperative agreement from the Centers for Disease Control and Prevention (grant number: U01/DD00048702
Footnotes
Conflict of Interest/Disclosure Statement: The authors report no conflict of interest.
Study Location: The National Birth Defect Prevention Study is conducted in Atlanta, Georgia, and the following states: Arkansas, California, Iowa, Massachusetts, New Jersey, New York, North Carolina, Texas, Utah.
References
- 1.Sobel JD. Vulvovaginal candidosis. Lancet 2007;369:1961–71. [DOI] [PubMed] [Google Scholar]
- 2.Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet 1997;72:253–6. [PubMed] [Google Scholar]
- 3.Lee BE, Feinberg M, Abraham JJ, Murthy AR. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J 1992;11:1062–4. [PubMed] [Google Scholar]
- 4.Lopez-Rangel E, Van Allen MI. Prenatal exposure to fluconazole: an identifiable dysmorphic phenotype. Birth Defects Res A Clin Mol Teratol 2005;73:919–23. [DOI] [PubMed] [Google Scholar]
- 5.Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazole-induced congenital anomalies in three infants. Clin Infect Dis 1996;22:336–40. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez JM, Moya G. Fluconazole teratogenicity. Prenat diagn 1998;18:862–3. [PubMed] [Google Scholar]
- 7.Menegola E, Broccia ML, Di Renzo F, Giavini E. Pathogenic pathways in fluconazole-induced branchial arch malformations. Birth Defects Res A Clin Mol Teratol 2003;67:116–24. [DOI] [PubMed] [Google Scholar]
- 8.Menegola E, Broccia ML, Di Renzo F, Massa V, Giavini E. Relationship between hindbrain segmentation, neural crest cell migration and branchial arch abnormalities in rat embryos exposed to fluconazole and retinoic acid in vitro. Reprod Toxicol 2004;18:121–30. [DOI] [PubMed] [Google Scholar]
- 9.Tiboni GM, Giampietro F. Murine teratology of fluconazole: evaluation of developmental phase specificity and dose dependence. Pediatr Res 2005;58:94–9. [DOI] [PubMed] [Google Scholar]
- 10.Tiboni GM, Marotta F, Carletti E. Fluconazole alters CYP26 gene expression in mouse embryos. Reprod Toxicol 2009;27:199–202. [DOI] [PubMed] [Google Scholar]
- 11.Diflucan (Fluconazole Tablets) [package labeling]. New York, NY: Pfizer Roerig; 2013. (Accessed June 2, 2015, at http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019949s057,019950s061,020090s040lbl.pdf) [Google Scholar]
- 12.Campomori A, Bonati M. Fluconazole treatment for vulvovaginal candidiasis during pregnancy. Ann Pharmacother 1997;31:118–9. [DOI] [PubMed] [Google Scholar]
- 13.Inman W, Pearce G, Wilton L. Safety of fluconazole in the treatment of vaginal candidiasis. A prescription-event monitoring study, with special reference to the outcome of pregnancy. Eur J Clin Pharmacol 1994;46:115–8. [DOI] [PubMed] [Google Scholar]
- 14.Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy 1999;19:221–2. [DOI] [PubMed] [Google Scholar]
- 15.Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol 1996;175:1645–50. [DOI] [PubMed] [Google Scholar]
- 16.Norgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother 2008;62:172–6. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen HT, Nielsen GL, Olesen C, et al. Risk of malformations and other outcomes in children exposed to fluconazole in utero. Br J Clin Pharmacol 1999;48:234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother 2015; 70:14–22. [DOI] [PubMed] [Google Scholar]
- 19.Carter TC, Druschel CM, Romitti PA, Bell EM, Werler MM, Mitchell AA. Antifungal drugs and the risk of selected birth defects. Am J Obstet Gynecol 2008;198:191.e1–7. [DOI] [PubMed] [Google Scholar]
- 20.Molgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med 2013;369:830–9. [DOI] [PubMed] [Google Scholar]
- 21.Alsaad AM, Kaplan YC, Koren G. Exposure to fluconazole and risk of congenital malformations in the offspring: A systematic review and meta-analysis. Reprod Toxicol 2015;52:78–82. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51:1–78. [PubMed] [Google Scholar]
- 23.Workowski KA, Berman SM, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55:1–94. [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. Rockville, MD: 2011. (Accessed October 22, 2014, at http://www.fda.gov/Drugs/DrugSafety/ucm266030.htm.) [Google Scholar]
- 25.Reefhuis J, Gilboa SM, Anderka M, et al. The National Birth Defects Prevention Study: A Review of the Methods. Birth Defects Res A Clin Mol Teratol. 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 2003;67:193–201. [DOI] [PubMed] [Google Scholar]
- 27.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A. Seeking causes: Classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol 2007;79:714–27. [DOI] [PubMed] [Google Scholar]
- 28.Werler MM, Ahrens KA, Bosco JL, et al. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann Epidemiol 2011;21:842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IPDTOC Working Group. Prevalence at birth of cleft lip with or without cleft palate: data from the International Perinatal Database of Typical Oral Clefts (IPDTOC). Cleft Palate Craniofac J 2011;48:66–81. [DOI] [PubMed] [Google Scholar]
- 30.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol 2010;88:1008–16. [DOI] [PubMed] [Google Scholar]


