Abstract
The purpose of this review was to evaluate data generated by animal models of intervertebral disc (IVD) degeneration published in the last decade and show how this has made invaluable contributions to the identification of molecular events occurring in and contributing to pain generation. IVD degeneration and associated spinal pain is a complex multifactorial process, its complexity poses difficulties in the selection of the most appropriate therapeutic target to focus on of many potential candidates in the formulation of strategies to alleviate pain perception and to effect disc repair and regeneration and the prevention of associated neuropathic and nociceptive pain. Nerve ingrowth and increased numbers of nociceptors and mechanoreceptors in the degenerate IVD are mechanically stimulated in the biomechanically incompetent abnormally loaded degenerate IVD leading to increased generation of low back pain. Maintenance of a healthy IVD is, thus, an important preventative measure that warrants further investigation to preclude the generation of low back pain. Recent studies with growth and differentiation factor 6 in IVD puncture and multi‐level IVD degeneration models and a rat xenograft radiculopathy pain model have shown it has considerable potential in the prevention of further deterioration in degenerate IVDs, has regenerative properties that promote recovery of normal IVD architectural functional organization and inhibits the generation of inflammatory mediators that lead to disc degeneration and the generation of low back pain. Human clinical trials are warranted and eagerly anticipated with this compound to assess its efficacy in the treatment of IVD degeneration and the prevention of the generation of low back pain.
Keywords: artificial intelligence, facial recognition, nociceptive pain, intervertebral disc degeneration, low back pain, neuropathic pain, discogenic pain, GDF6
Data generated by animal models over the last decade has made invaluable contributions to the identification of molecular events occurring in and contributing to intervertebral disc (IVD) degeneration and pain generation and has identified several potential therapeutic targets. IVD degeneration and associated spinal pain is a complex multifactorial event, its complexity poses difficulties in the selection of the most appropriate therapeutic target of many potential candidates to focus on in the formulation of strategies to effect disc repair and regeneration and the alleviation of associated neuropathic and nociceptive pain. Application of artificial intelligence in facial recognition has improved the assessment of pain in animal models. Deep machine learning and neural networking have also been applied in the analysis of data generated from molecular signaling pathways involved in disc degeneration and pain generation. This methodology has predictive capability, can be automated and offers a superior unbiased assessment of data compared to observer‐based classifications and clinical prognosis data.
1. INTRODUCTION
The aim of this review was to evaluate data generated in the last decade by animal models of intervertebral disc (IVD) degeneration (IVDD) relevant to the interpretation of how low back pain (LBP) is generated and the identification of potential therapeutic targets to treat this condition.
The World Health Organization (WHO) has defined LBP as “pain and discomfort below the costal margin above the inferior gluteal folds, with or without referred leg pain.” This may be experienced as aching, burning, stabbing, sharp or dull, well‐defined, or vague pain of mild to severe intensity. 1 Many recent studies show that IVDD is a major contributor to LBP due to neural ingrowth into the degenerate IVD and a significant increase in mechano‐and nociceptive receptor numbers in the degenerate IVD which produce pain responses due to abnormal loading in the incompetent degenerate IVD. Several other anatomical structures in the spine besides the IVD can also generate pain responses (Table 1). These include, the vertebral body, paradiscal and myotendinous tissues and the osteoarthritic facet joint capsule and articular cartilage. The focus of this review is the IVD since it is a major contributor to the weight bearing and flexibility properties of the spine and when the IVD degenerates a significant contributor to the generation of LBP.
TABLE 1.
Multiple spinal centers of low back pain generation
| Source of pain | Tissue changes potentially leading to pain generation | Mechanism of pain generation |
|---|---|---|
| IVD | Disruption of normal IVD structure, elevation in inflammatory mediator levels and neuroreceptors, neurotrophic factors conducive to neuroproliferation | Increased nociceptive and mechanoreceptor levels in degenerate IVD lead to increased pain perception |
| AF | AF fissure, AF bulging, AF prolapse, disruption in annular cohesiveness, altered IVD mechanics, activation of mechanoreceptors and perception of LBP | Mechanoreceptor activation, increased perception of pain by nociceptors in the mechanically incompetent degenerate IVD |
| NP | NP extrusion, IVD prolapse, exposure of DRGs to NP tissue, hyper‐sensitization of DRGs, pain signaling to dorsal horn of spinal cord | NP neovascularization, elevation in inflammatory mediators and cytokines, neovascularization of NP |
| DRGs | DRG compression, sensitization to extruded NP tissue leading to neuropathic pain, spinal canal stenosis | Pain signaling to sensory dorsal horn of spinal cord due to pain receptor activation by inflammatory mediators and cytokines |
| Vertebral body | Altered spinal biomechanics due to IVDD alters vertebral body loading | Mechanoreceptor activation and elevated pain perception due to nerve compression |
| Facet Joint | Degenerative OA‐like changes in facet joint cartilage and sub‐chondral bone and in synovial joint capsular tissues, increased levels of inflammatory mediators and neurotransmitter peptides | Reduced mechanical support to spinal triad and joint instability leads to activation of mechanoreceptors and perception of pain by nociceptors |
| PLL and ALL | Ligament inflammation, secretion of pro‐inflammatory mediators and cytokines and neurotransmitters | Spinal stenosis, nerve root compression, nociceptive nerve activation |
| Myotendinous tissues, semispinalis, rotatores, multifidis, transversospinales muscle group | Changes in fast and slow twitch muscle fibers, M1 and M2 macrophage polarization, fatty infiltration, elevation in IL‐1, TNFα, cell death, muscle atrophy, impaired spinal flexibility and neuromuscular control and co‐ordination of spinal movement, muscle spasms | Muscle pain receptor activation, muscular spasms, generation of muscle pain due to impaired spinal flexibility and co‐ordination of spinal movements |
2. THE INCIDENCE AND SOCIOECONOMIC IMPACT OF LBP
A 10‐year global study of 291 major human diseases acknowledged that LBP was the most consequential musculoskeletal condition in terms of the resultant years lived with disability and a series of studies of this data has generated further confirmation of the status of LBP as the number one disabling musculoskeletal condition. 2 , 3 , 4 It is generally accepted that ~80% of the general population will be affected by LBP some time in their life‐time and that this will be of sufficient severity to warrant intervention by a physician 5 resulting in a loss of productive work days. 4 UK costings for LBP of £12.3 billion, 6 and $9.17 billion for Australia have been published. 7 The American Academy of Pain Medicine published annual costs in 2006 for chronic pain of $560 to 635 billion, and noted that 53% of all chronic pain patients in the USA were affected by LBP with 31 million people estimated to have LBP at any one time. 8 In 2015, the global point prevalence of activity limiting LBP of 7.3% indicated that 540 million people were affected globally. 9 With the increased incidence of LBP in the 5th and 6th decades 10 and the advancing age of the global general population 11 as shown by data collected by the World Bank 12 and United Nations (UN), 13 the global point prevalence of activity limiting LBP will increase. 14 This is consistent with the recognition of LBP as the most impactful of any muskuloskeletal condition on human well‐being. 2 , 4 The importance of LBP on human health is also reflected in the comprehensive guidelines and bulletins regularly published by the WHO, 5 International Association for the Study of Pain, 15 National Institutes of Health, 16 World Bank, 17 Australian Institute of Health and Welfare, 18 and UN. 19
3. PATHOMECHANICS AND EPIDEMIOLOGY OF LBP
LBP is a common disorder 3 that can be elicited by painful stimuli emanating from the spinal muscles, nerves, vertebral body, and para‐discal tissues such as the ALL, PLL, facet joint cartilage and associated synovial capsular tissues. 20 LBP can vary in intensity from a dull constant ache to a sudden sharp pain 21 and is classified by its duration time as acute (pain duration <6 weeks), sub‐chronic (pain duration 6–12 weeks), or chronic (pain duration >12 weeks). 22 , 23 , 24 LBP may be further classified by its underlying cause as mechanical, non‐mechanical, or referred pain. 22 , 25 Neuropathic pain is caused by inflammation, irritation or excessive compression of neural tissue, whereas nociceptive pain is the body's reaction to painful stimuli such as a damaged back muscle but is not responsible for nerve damage in itself. 22 After an accident or traumatic damage to the PNS/CNS, nerves may become weakened or dysfunctional, causing hypersensitivity to pain and even when the wound has healed, the nerves may continue to give false signals of pain (neuropathic pain). About 40% of the worlds human population suffer from LBP some time in their lifetime 26 and this may be as high as a value frequently quoted for Western societies of 80%. 4 It is conservatively estimated that 9%–12% of the global general population (632 million) have LBP at any one time.
It is conservatively estimated that 9%–12% of the global general population (632 million) have LBP at any one time. Of the numerous causes of back pain, a landmark study estimated that in the 632 million, a meta‐analysis reveals 403 Million people (5.5% of world population) have symptomatic disc degeneration. 9 Hence, it is an imperative to understand the mechanisms underpinning pain related to disc degeneration.
4. INNERVATION OF THE IVD
The IVD is innervated by branches of the sinuvertebral nerve, by nerves derived from the ventral rami of spinal nerves or by nerves derived from gray rami communicantes. 27 In the normal IVD, innervation is restricted to the outermost lamella of the AF (Figures 1 and 2). These consist of small nerve fibers and some large fibers that act as mechanoreceptors. In the degenerated IVD, greater numbers of nerve fibers are present that enter the inner AF and the NP. Dorsal root ganglia (DRG) contain thin myelinated and unmyelinated fibers arising from small niciceptive neurons projecting from the IVD lamina I and II and also extend to the dorsal horn of the spinal cord. Most of the IVD sensory nerve fibers originate from small peptidergic neurons expressing the tyrosine kinase receptors (TrkA/TrkB), non‐peptidergic neurons express the common signaling receptor for glial cell‐derived neurotrophic factor family (Ret). 28
FIGURE 1.
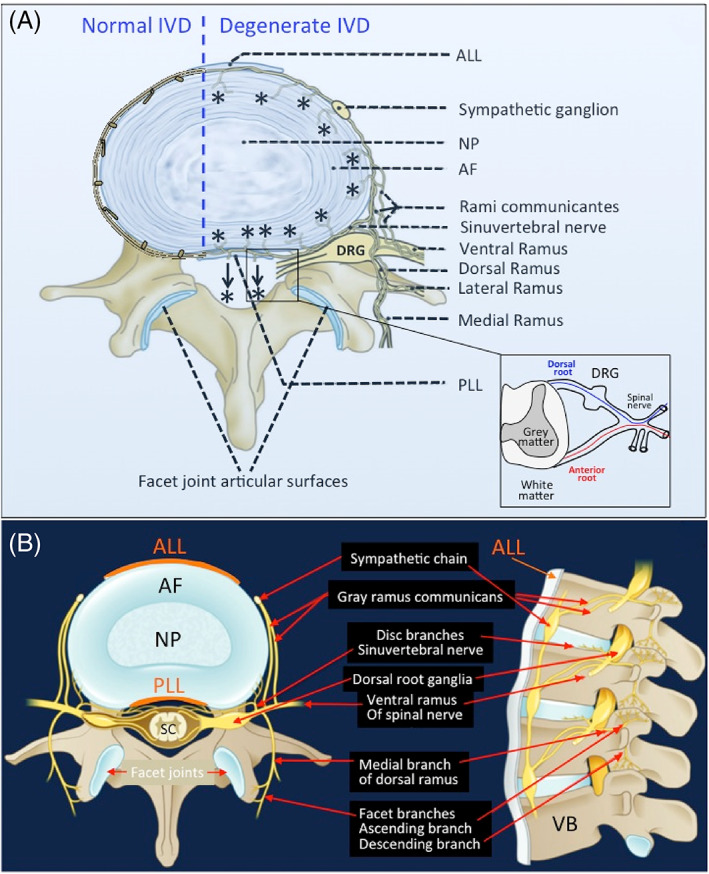
Schematic showing the innervation of the normal and degenerate intervertebral disc (IVD) (A). The normal IVD is the largest predominately aneural structure in the human body where nerves are confined to the outer annular lamellae. With IVD degeneration and depletion of space‐filling aggrecan from the IVD a significant reduction in internal hydrostatic pressure in the IVD and the production of inflammatory mediators and neurotrophic factors provides conditions that are conducive to the ingrowth of nociceptive nerves (*) from the sinu‐intervertebral nerve into the AF of the IVD and significant increases of peripheral mechanoreceptors in the IVD. Thus, there is an increased perception of pain in the mechanically incompetent degenerate IVD. Peripheral IVD neural structures such as the sympathetic ganglion and dorsal root ganglion and the rami communicantes are highly innervated structures. The DRG communicates with the sensory dorsal horn of the spinal cord. There are also connections to the dural nerve plexus (arrows, *) from the sinu‐vertebral nerve. The facet joint capsule and associated subchondral bone and vertebral bodies adjacent to the IVDs and CEPs are all innervated. The spinal nerve has dorsal and anterior connections to the spinal cord (inset). Neural organization of a lumbar spinal segment (B). AF, annulus fibrosus; ALL, anterior longitudinal ligament; DRG, dorsal root ganglion; NP, nucleus pulposus; PLL, posterior longitudinal ligament; SC, spinal cord. Figure modified from Kallewaard et al. 53 with permission
FIGURE 2.
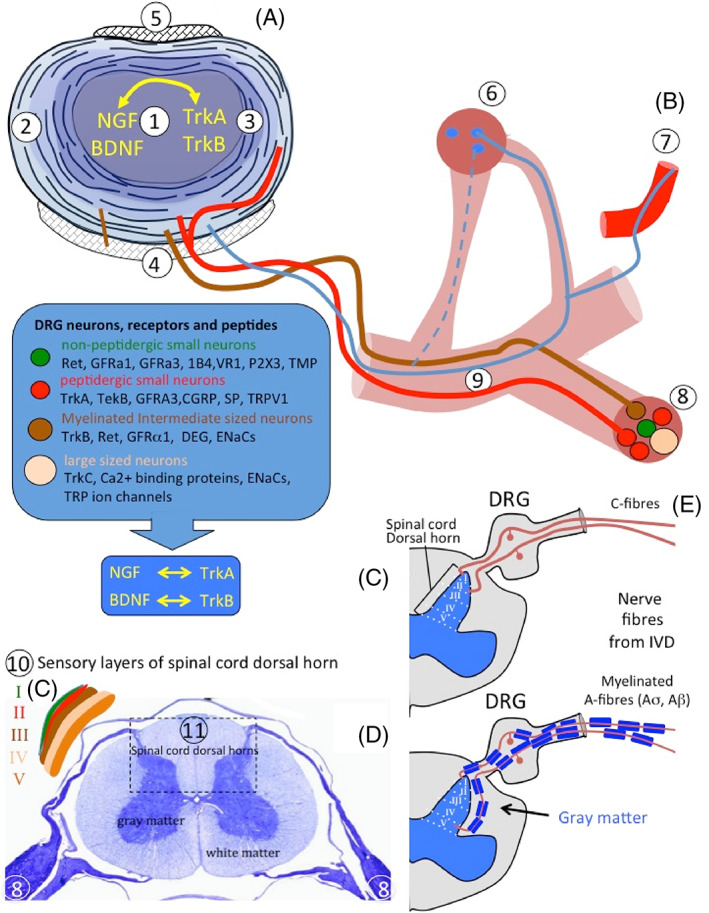
Neural growth factors, neurotransmitters, and neural receptors involved in the ingrowth of nerves into the intervertebral disc (IVD) and transmission of sensory signals to the CNS. Composite schematic figure depicting a horizontally bisected IVD (A), sinuvertebral nerve and its branches to the sympathetic and sensory DRG and sensory nerves that communicate with the sensory layers of the dorsal horns of the spinal cord. 1. Nucleus pulposus, 2. Outer AF, 3. Inner AF, 4. Posterior longitudinal ligament, 5. Anterior longitudinal ligament, 6. Sympathetic root ganglion and its pre‐ and post‐ganglionic white and gray ramus communicantes, 7. Postganglionic nerve fibers, 8. DRG and its nerve fibers, pain receptors, neurotrophins and neurotransmitter associated peptides, 9. Sinuvertebral nerve (B) that communicate with the sensory layers (10) (C) of the spinal cord dorsal horn (11) (D). (i) TrkA/TrkB (Tropomyosin receptor kinase A, B; receptor for nerve growth factor [NGF]/brain‐derived neurotrophic factor, BDNF). (ii) Degenerin/epithelial sodium channels (DEG/ENaCs) (ENaCa, b, and c). (iii) Acid‐sensing ion channels (ASIC1, ASIC2, and ASIC3). (iv) TRP family (TRPA1, TRPC1, TRPC6, and TRPV1‐4). (v) Neuron associated neurotransmitter peptides CGRP (calcitonin gene‐related peptide), GFRα1 and GFRα3 (glial cell‐line‐derived neurotrophic receptor subtypes α1 and α3), P2X3 (ATP‐gated ion channel subtype P2X3), SP (substance P), TMP (thiamine monophosphatase), VR1 (vanilloid receptor subtype 1). BDNF, brain derived nerve factor; NGF, nerve growth factor. The horizontally sectioned spinal cord (d) is stained with Nissl stain to show the white and gray matter and DRG. Figure modified from Takahashi et al. 28 with permission
Neurons in DRGs can be differentiated on the basis of the pattern of receptors for neurotrophic factors they interact with, including ion channels such as degenerin/epithelial sodium channels (DEG/ENaCs) (ENaCa, b and c); acid‐sensing ion channels (ASIC 1–3) and transient receptor potential (TRP) families (TRPA1, TRPC1, TRPC6 and TRPV1‐4).
Expression of CGRP (calcitonin gene‐related peptide); GFRa1 and GFRa3 (glial cell‐line‐derived neurotrophic receptor subtypes α1 and α3); P2X3 (ATP‐gated ion channel subtype P2X3); SP (substance P); TMP (thiamine monophosphatase); VR1 (vanilloid receptor subtype 1) also differentiates DRG neurons. The Mu receptor interacts with opioid peptides to promote analgesic anti‐inflammatory actions to protect cartilage from posttraumatic degeneration and have also been evaluated for the treatment of chronic LBP. 29 , 30 , 31 , 32
5. LBP ARISING FROM CHANGES TO SPINAL MUSCLES FOLLOWING IVDD IN A LUMBAR SPINAL SEGMENT
Acute LBP can result from injury to spinal muscle groups such as the multifidis leading to reduced spinal flexibility, this impacts on neuromuscular control, results in alterations in spinal biomechanics, spinal gait and flexibility effecting spinal ligament, facet and sacroiliac joints and weight loading on the vertebral bodies. Alterations in the expression of connective tissue and collagenous components of the muscle spindle capsule in paraspinal muscles of spinal segments containing degenerate IVDs effects their mechanical properties. 33 Changes in muscle capsule stiffness impacts the transmission properties of muscles and transduction of sensory information accounting for the proprioceptive deficits and neuromuscular dysfunction associated with IVDD leading to muscle spasms and generation of LBP. The Multifidus is a group of short, triangular muscles that along with the semispinalis and rotatores muscles comprises the transversospinal group of deep back muscles. 34 Instability of the spine/trunk leads to poor spinal posture and over‐compensation of secondary stabilizer muscles causing LBP. The lumbar multifidus muscle is the largest and most medial of the lumbar back muscles and is continuously active in the maintenance of the upright posture. The trasversospinales muscle group runs laterally from the transverse process and attaches medially to the spinous processes, filling the groove on either side of the spinous process. 34 The multifidus muscle spanning the whole length of the vertebral column is most developed in the lumbar area 34 acting as a local core stabilizer providing static and dynamic spinal stability. Weakness in the multifidus muscle is associated with LBP. 35 , 36 Lumbar IVDD in an ovine large annular lesion destabilization model 37 , 38 , 39 leads to structural changes in the multifidis with macrophage polarization contributing to local inflammation and structural changes 40 including remodeling of Multifidis muscle, adipose and connective tissue, but not muscle atrophy. 41 Proinflammatory cytokine gene expression contributes to multifidus muscle fiber changes as a result of IVDD. 42 Repair of lesion affected IVDs using bone marrow derived mesenchymal stem cells 38 , 39 prevents fatty infiltration and fibrosis of the multifidus muscle group, but not cytokine and muscle fiber changes. 43 The effect of IVDD induction on the dynamic spinal stiffness, 44 vertebral motion responses 45 and spinal flexibility has also been examined in this model and how controlled IVDD impacts on spinal neurophysiological responses during dorsoventral mechanical excitation of the ovine lumbar spine. 46 This has also allowed evaluation of how spinal manipulation forces and duration affect vertebral movement and neuromuscular responses. 47 , 48 , 49 The controlled outer annular lesion is a manipulable spinal lesion that can also be used to quantitate pathological biomechanical muscle changes in the ovine lumbar spine induced by IVDD. 50 The neurodynamic propagation of electrical impulses in neural networks depends on the elasticity of normal nervous tissue. A reduction in spinal flexibility due to IVDD detrimentally influences such processes contributing to the propogation of LBP. Spinal manipulation influences spinal pain processes and can potentially treat neuropathic and chronic mechanical LBP. 51 , 52 Spinal manipulation is the basis of therapeutic physiotherapy practice to alleviate LBP. IVDD results in changes to the connective tissue and collagen expression in muscle spindle capsules in the multifidis, impacting it's mechanical properties and the transduction of sensory signals required for normal neuromuscular control of spinal muscles. 33 This change in muscle spindle structure may explain the proprioceptive deficits identified with LBP and effects on normal spinal flexibility and spinal movement that affect abnormal loading of the degenerate IVD and simulation of nociceptors and mechanoreceptors leading to the generation of LBP. 33
6. THE PERPLEXING PROBLEM OF ASYMPTOMATIC DEGENERATE IVDS AND SYMPTOMATIC INTACT IVDS
Cases have been reported where degenerate IVDs in patients present with no symptoms of LBP. 54 It has been proposed that persistent inflammation and innervation are key factors which determine whether IVDs are symptomatic and those that are not. 54 IVDD is considered a forerunner to the ingrowth of nociceptor nerves into the IVD and an increase in IVD mechanoreceptor numbers that can generate mechanically induced LBP. As already discussed, spinal pain can be generated by centers other than the IVD such as the OA facet joint, the bone of the vertebral body, and myotendinous tissues. A symptomatic patient with normally structured IVDs can thus be potentially be explained by pain generated in these other centers. Neurotracing studies have shown that sympathetic DRGs serve IVDs several levels below them. 55 , 56 , 57 Trauma to these DRGs can generate noxious neuropeptides that transmit a painful stimulus to IVD nerves but with no apparent derangement in IVD structure. The referred pain that occurs appears to emanate from specific IVD levels but the damage that generates these signals may has another spinal location. 58
7. THE INVOLVEMENT OF SEX HORMONES IN IVD PATHOBIOLOGY
An aspect that has emerged in the last decade relevant to the development of prospective animal models of IVDD is the issue of the potentially confounding impact that sex hormones may have on such models. Although the effects of sex hormones on the metabolism of IVD cells was first identified in 1969 59 it is only in the last decade that these have been shown to significantly impact on degenerative processes in the IVD. 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 In order to avoid biological spread and cyclical variation in data that would complicate data analysis and development of a prospective IVDD model, female animals may be examined in a separate data set from male data to ensure any pathophysiological sexual dimorphism is identified. Ideally this would include both actively cycling and “post‐menopausal” (likely gonadectomized) female cohorts to include study of the cyclical fluctuations in circulating female sex hormone levels. Male sex hormones also effect IVD cells but cyclical circulatory hormonal fluctuations do not occur to the same degree. 61
LBP is a common symptom of premenstrual syndrome, experienced by most women during menstruation and may be exacerbated by premenstrual dysphoric disorder and dysmenorrhea or may be a symptom of endometriosis. 78 , 79 Female sex hormones play an important role in the etiology and pathophysiology of a number of musculoskeletal degenerative diseases, around 70% of perimenopausal women will experience LBP symptoms due to estrogen deficiency, estrogen decrease may be a risk factor for lumbar disc degeneration. 80 , 81 , 82 , 83 , 84 , 85 , 86 Postmenopausal women show accelerated IVDD due to relative estrogen deficiency, increased prevalence of spondylolisthesis, and facet joint osteoarthritis, in the first 15 years post menopause. 80 , 84 , 85 Continued progression of lumbar disc degeneration in postmenopausal women has been observed. 85
Estrogen signals through two classic nuclear receptors, estrogen receptor (ER)‐α and ‐β, and a membrane bound G‐protein‐coupled receptor 30 (GPR30). 17β‐estradiol (E2) enhances cell proliferation and prevents IL‐1β‐induced cell death in IVD cells, but this effect was partially blocked by G36, a GPR30 antagonist and completely abrogated by a combination of ER antagonists ICI 182 780 and G36. 73 This demonstrated that GPR30 was expressed in human IVD cells and transmitted signals triggering E2‐induced NP cell proliferation and also had a protective effect against IL‐1β‐induced disc cell apoptosis. The effects of E2 on NP cells required both GPR30 and classic estrogen receptors. During IVDD apoptotic effects are key factors responsible for a reduction in NP cell numbers and significantly effects the viability of IVD tissues. 87 Prevention of NP cell apoptosis thus represents an important preventative measure that could slow down the progression of IVDD. E2 promotes human AF cell proliferation, 88 and exerts anti‐apoptotic effects in rat disc cells 71 , 75 , 76 thus it represents a possible therapeutic that could significantly reduce the development of IVDD and generation of LBP. Estrogen can, thus, prevent the development of IVDD through its anti‐apoptotic properties inhibiting the production of the inflammatory cytokines IL‐1β and TNF‐α by disc cells. 89 , 90 This reduces catabolic events in the IVD by preventing the up‐regulation of MMPs induced by these inflammatory mediators. Estrogen also induces anabolic processes in the IVD by activating the PI3K/Akt pathway and also decreases oxidative damage. 89 By inhibiting IVDD, estrogen exerts protective effects that prevent degradative structural changes in the IVD that would otherwise pre‐dispose the IVD to nerve ingrowth and production of neurotrophic factors and inflammatory mediators by IVD cells that contribute to IVD nociceptor activation and mechano‐sensitization of disc afferent nerve fibers leading to the generation of LBP. 91 , 92 Additional factors such as geometry and the influence of the inflammatory system beyond hormonal differences can also have a significant influence on IVDD and LBP.
Further studies with functional foods and neutraceutical supplements under evaluation for their abilities to alleviate pain may also prove to be a useful non‐drug treatment for post‐menopausal back pain. 93 A recent study has reviewed the extensive range of natural compounds which display anti‐inflammatory NP cell protective anti‐catabolic properties with potential roles in IVD regenerative processes. 94
8. IVDD, ESTABLISHMENT OF NOCICEPTOR AND MECHANO‐RECEPTORS AND PERCEPTION OF LBP
IVDD is a pre‐requisite for neural development and receptors with the ability to perceive pain in the mechanically incompetent IVD. The dogmatic view of the IVD for a long time was that it is an immune privileged tissue due to immune cells being excluded from the intact IVD structure. The same cannot be said of the degenerate IVD which can contain defects in the CEP and clefts in the AF which can allow communication with the exterior environment and movement of immune cells into the IVD. The resultant increase in inflammatory cytokine and chemokine mediators, degradative proteases, neurotrophic and angiogenic factors in the degenerate IVD and catabolic events produces a structurally compromised IVD incapable of acting as a weight bearing viscoelastic musculoskeletal supportive tissue and since it is now a mechanosensive structure is also a major source of LBP. 95 However, even in the intact IVD inflammatory mediators and catabolic agents can be produced by the resident disc cells so the immune status of the IVD is long due a re‐appraisal. 96 Comprehensive bioinformatics analyses of the IVD has revealed that immune genes are responsible for an altered immune microenvironment in IVDD. 97 T cells, B cells and neutrophils are implicated in an auto immune response in the NP in IVDD. 98 Natural killer cell numbers are significantly lower in patients with lumbar herniations indicating a reduced immune clearance of foreign material in IVDD, 99 however, infiltrating macrophages may have compensatory roles to play in IVDD. 100 , 101 These immune cells produce a range of cytokines that contribute to the IVD degenerative process and pain generation in IVDD. 95
Thus, while the normal intact healthy IVD is not exposed to the immune system 102 in IVDD a number of inflammatory immune cells that gain entry to the IVD 103 are a source of inflammatory mediators which contribute to the further deterioration of the IVD environment and to an influx of nerves, and blood vessels into the IVD. 104 These nerves have nociceptive functions contributing to the LBP associated with IVDD. Almost five decades ago Naylar et al 105 proposed that since normal IVDs were not exposed to the immune system, if disc disruption and herniation occurred release of IVD material was liable to elicit an auto‐immune response as the disc contents would be identified as non‐self. Since then many studies have shown that released IVD material is highly immunogenic and stimulates a significant influx of immune cells (lymphocytes, macrophages, mast cells) into the degenerate IVD. 101 , 106 , 107 , 108 IVDD is considered a predisposing factor in the generation of discogenic LBP. 58 , 109 , 110 This is a complex process involving mechanical stimulation, a number of nerve receptors, ion‐channels, cytokines and inflammatory mediators, neurotrophins that stimulate nociceptors and mechanoreceptors which respond to abnormal spinal loading to generate pain signals. 92 , 111 , 112 , 113 , 114 Three categories of nerve fibers have been identified in the IVD, perivascular nerves, sensory nerves independent of blood vessels, and mechanoreceptors. 115 These are localized in the outer layers of the AF in normal IVDs but with IVDD and deterioration of the IVD ECM, nerves penetrate deeper into the inner regions of the AF and NP. 104 The occurrence of Class 3 Semaphorin axonal guidance receptors and growth factors in the healthy IVD may have a protective role repelling axons surrounding the healthy disc, maintaining the IVD as an aneural structure and regulating nerve in‐growth into the degenerate IVD acting as a barrier to neuronal ingrowth in the healthy disc. 116 Degeneration of the IVD results in an increase in nociceptor and mechanoreceptor numbers in the AF. Nociceptors may be stimulated mechanically or by local inflammatory conditions leading to abnormal spinal motion and the resulting mechanical stimulation that occurs further exacerbates pain generation through mechanoreceptors which are sensitive to mechanical stimulation. 91 , 117 , 118 Ion channels also have important roles to play in the IVD degenerative process. A large number of inflammatory and signaling molecules, such as TNF, IL‐1β, IL‐6, IL‐8 and neurotrophins (1B4, CGRP, Substance‐P, NGF, BDNF) 95 , 119 stimulate a number of receptors in the IVD leading to pain generation (Table 2). These pain signals are transmitted to the DRGs through myelinated A (σ, β) and unmyelinated C fibers then to the gray matter of the dorsal horn of the spinal cord which has a number of sensory and autonomic layers. 58 , 118 , 120 , 121 Two subtypes of voltage‐activated calcium channels have major roles to play in signal transmission: a low voltage‐activated CaV3.2 channel and a high voltage‐activated CaV2.2 channel. The CaV3.2 channel participates in signal conductance along nociceptive neurons while CaV2.2 channels provide synaptic transmission at the dorsal horn of the spinal cord. 122
TABLE 2.
IVD receptors, ion channels, neurotransmitters and nerve types relevant to LBP
| Receptors and ion channels | Ref | |
| RET | Proto‐oncogene “rearranged during transfection” receptor | 109 , 137 , 138 |
| GFRa1, 2, 3 | Glial cell‐derived neurotrophic factor (GDNF) receptor family | 157 |
| TRKA, B, C | Tropomyosin kinase receptor family | 157 |
| VR‐1 | Vanilloid receptor‐1 | 158 , 159 |
| P2X family | P2X purinoreceptor family | 55 , 160 |
| 5‐HTR | 5‐Hydroxy tryptamine receptor | 139 , 161 |
| TRVP‐1 | Transient receptor potential vanilloid‐1 | 162 |
| TRP ion‐channels | Transient receptor potential ion channel family | 163 |
| ENaC | Epithelial Na+ channel | 164 |
| DEG | Degenerin, an epithelial Na+ channel | 165 |
| ASIC family | Acid‐sensing ion channels 1a, 1b, 2a, 2b, 3, 4 | 131 , 132 , 133 , 134 , 135 , 136 , 165 , 166 |
| CB‐1 and CB‐2 | Cannabinoid receptors 1 and 2 | 167 , 168 |
| Mechano | Mechanoreceptors | 115 , 118 |
| RGD integrins | α5β1 integrin, potential mechanoreceptor | 117 |
| Sem 3A | Semaphorin receptor 3A | 116 |
| S100 positive receptors | S100 positive mechano receptors | 121 , 164 |
| Cav3.2 and CaV2.2 ion channels | Low voltage‐activated CaV3.2 channel and a high voltage‐activated CaV2.2 channel | 122 |
| Neurotransmitters | ||
| 1B4 | 1B4 neurotransmitter, also known as Synaptotagmin I | 169 , 170 |
| CGRP | Calcitonin gene‐related peptide | 171 |
| SP | Substance‐P | 172 , 173 |
| NGF | Nerve growth factor | 118 , 174 , 175 , 176 |
| BDNF | Brain derived neurotrophic factor (abrineurin) | 159 , 174 , 177 , 178 |
| GDNF | Glial cell‐derived neurotrophic factor | 157 |
| Nerve fibers | ||
| A (σ, β) fiber | Myelinated A nerve fibers | 58 , 118 , 121 |
| C fiber | Unmyelinated nerve fibers | 179 |
During IVDD, IVD cells secrete increased levels of proinflammatory cytokines, 95 , 123 and ECM degradation occurs due to elevated levels of active MMPs leading to a loss of aggrecan from the IVD and a reduction of disc height occurs and internal hydrostatic pressure due to the loss of this proteoglycan's space filling and hydrophilic properties. Structural changes to the IVD alters its biomechanical properties 124 and is a leading cause of increased inflammation, nerve ingrowth 125 , 126 and release of mediators of pain in a complex multicomponent process. 127 Proinflammatory conditions mediators and signaling pathways have important roles to play in the onset and progression of IVDD. 128 Inflammation mediated by immune cells significantly contributes to IVDD, through secretion of IL‐4, IL‐6, IL‐12, IFN‐γ, and MMPs. This leads to a reduction in NP cell numbers and a deterioration of the normal IVD microenvironment. 129 Long‐term inflammation recruits inflammatory cells into the degenerate IVD further exacerbating conditions in the IVD. 129 Inflammatory mediators such as TNF‐α and IL‐1β induce the expression of pain‐related factors such as nitric oxide (NO), cyclooxygenase 2 (COX‐2), and nerve growth factors (NGF), which promote nerve ingrowth. 127 , 130 All of these factors collectively contribute to the occurrence and severity of LBP.
Acid‐sensitive ion channels (ASICs) mediate IVDD via various pathways. 131 Acid‐sensitive ion currents in sensory neurons, can be triggered by a decrease in pH. ASICs of the degenerin/epithelial Na+ channel family, regulate this process in the IVD. 132 , 133 Six ASIC subunits have been identified: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4, which form homomeric or heteromeric ion channel complexes. ASIC1a has attracted much attention since it can regulate the Ca2+ influx process in the IVD and endoplasmic reticulum (ER) stress, which in turn regulates apoptosis in the IVD thus ASIC1a regulates the survival of NP cells in the hostile environment of the hypoxic, acidic degenerate IVD. 132 , 133 ASICs have important regulatory roles in many physiological or pathological processes in the IVD in a specific microenvironment of hypoxia and acidity. 134 , 135 The NP represents the largest hypoxic tissue in the human body. ASIC3 also regulates NP cells in a hypoxic environment. 136 The ASICs have roles in osmosensing, osmosignaling and inflammation under hypoxic conditions. 135 LBP and IVDD are decreased when TLR‐4 is inhibited in a mouse model of IVDD. 137 Inhibition of TLR‐4 also protects against inflammation‐induced mechanobiological alterations to IVD cells, 138 and reduces IVDD, LBP. 109 Noxious stimuli in the IVD such as an acidic pH, ECM degeneration, inflammatory mediators and neurotrophins that generate inflammatory conditions in the IVD result in membrane depolarization of peripheral nociceptive nerve endings. This results in the generation of an action potential in such nerves, axonal conductance of electrical signals in nociceptive neurons to somatic DRGs then to the sensory gray matter dorsal horn of the spinal cord occurs. Signal transduction through chemical synapses in neural networks carries pain signals from IVD nociceptors to the brain. Two subtypes of voltage‐activated calcium channels have major roles to play in signal transmission: a low voltage‐activated CaV3.2 channel and a high voltage‐activated CaV2.2 channel. The CaV3.2 channel participates in signal conductance along nociceptive neurons while CaV2.2 channels provide synaptic transmission at the dorsal horn of the spinal cord. 122
Interaction of the 5‐hydroxytryptamine receptor with the inflammatory mediator TNF prolongs pain related behavior emanating from the IVD. 48 , 139 Three G protein‐coupled opioid receptors of the Gi subtype have been identified, μ‐, δ, and κ, the μ receptor (Mu) is present in the IVD. μ‐Opioid receptor expression in the IVD, spinal cord and DRGs is associated with pain‐related behavior following IVD herniation in rats. 48 , 139 Mu is a receptor for endogenous opioids such as beta‐endorphin and endomorphin and it also responds to the synthetic opioids morphine, heroin ([D‐Ala2, N‐MePhe4, Gly‐ol]‐enkephalin) (DAMGO), fentanyl, etorphine, buprenorphin and methadone. 140 , 141 , 142 , 143 , 144 The nucleotide‐binding oligomerization domain‐like receptor family pyrin domain‐containing‐3 (NLRP3) inflammasome, is associated with IVD inflammation, pyroptosis, ECM degradation and apoptosis of IVD cells in IVDD. 145 Inflammation is a key feature of the degenerate IVD, the P2X7R/NLRP3 axis contributes to these inflammatory processes and is a potential therapeutic target to alleviate this condition. 146 CB‐1 and CB‐2 cannabinoid receptors mediate antinociception of neuropathic pain. 147 , 148 , 149 The pain alleviating properties of cannabidiol (CBD) stem from its binding to CB‐1R and CB‐2R, G protein coupled‐receptors adenosine receptor subtype 2A, serotonin receptor subtype 1A and G protein‐coupled receptor 55; ligand‐gated ion channel TRPV‐1 and nuclear factor PPAR. CBD has been used in pain management in MS, PD and chronic pain syndromes including LBP. 150 , 151 , 152 , 153 , 154 Signaling through CB2R prevents anti‐oxidative stress, inducing protective anti‐inflammatory and anti‐senescent effects on NP cells, inhibits IVDD in‐vitro and in‐vivo in a rat acupuncture model and human IVD. Activation of CB2R suppresses MMP‐9, 13, induces type II collagen and SOX‐9 (SRY‐box transcription factor‐9) expression restoring ECM integrity through the AMPK/GSK3β pathway. 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156
9. IVDD AND HOW IT CONTRIBUTES TO THE GENERATION OF DISCOGENIC LBP
Many animal studies have shown that IVDD significantly contributes to the development of LBP, damage to normal IVD structure compromises its biomechanical competence and the viability of IVD cell populations. The resultant mechanical destabilization and altered cell‐ECM signaling results in the production of inflammatory cytokines and active MMPs which promotes disc degeneration. Degenerative changes in the disc ECM conducive to neovascularization and the ingrowth of nerves and an elevation in the production of inflammatory cytokines and neurotrophic factors all lead to a significant deterioration in the normal cellular microenvironment of the IVD conducive to the production of mechano‐and pain receptors. The appearance of glial fibrillary acidic protein‐immunopositive cells in diseased IVDs is closely associated with nerve ingrowth and suggests that Schwann cells have a role to play in regulating disc innervation and nerve function in the disc. 180 Neurotrophins, ion channels, inflammatory cytokines all contribute to detrimental changes in the IVD that promote the ingrowth of nerves into the previously aneural IVD. The generation of inflammatory cytokines and neurotrophins by disc cells produces an environment that stimulates nociceptive nerves generating painful responses that are signaled to the sensory DRG and sympathetic ganglia that serve the IVD and these signal to the sensory layers of the dorsal horns of the spinal cord. 175 Controlled annular incision or supraphysiological compression can induce IVD destabilization, production of MMPs and inflammatory mediators and deterioration in normal IVD organization in experimental models of IVDD. With the elevation in MMP levels in these models the IVD ECM becomes depleted of aggrecan and in‐growth of blood vessels and nerves can occur 104 similar to that observed in human IVDD 181 and increased innervation of the IVD occurs. 182 A mouse model of IVDD and LBP induced by supraphysiologal compression 114 displays increased innervation levels. 104 Overt compression in murine IVDs induces long‐lasting increases in inflammatory mediators, nerve injury and regeneration of afferent nerve fibers serving the IVD providing a pathomechanism for chronic discogenic LBP. 114 , 175 , 183
The normal IVD is poorly innervated by sensory and sympathetic perivascular nerve fibers (nociceptive) and postganglionic sympathetic nerve fibers in its outermost annular layer. Upon degeneration, depletion of the IVD aggrecan levels lowers its hydrostatic pressure and the IVD becomes susceptible to the ingrowth of nerves and blood vessels from its periphery. The CS chains of aggrecan in the normal IVD strongly inhibit nerve ingrowth into the IVD furthermore, the high hydrostatic pressure provided by aggrecan‐HA aggregates physically prevents the ingrowth of blood vessels. In‐vitro culture experiments conducted with aggrecan isolated from lumbar IVDs shows this proteoglycan significantly inhibits neuronal growth. 184 Many studies have also shown that lectican CSPGs inhibit neurite outgrowth in‐vitro using a range of neural cell populations. 185 , 186 , 187 , 188 Furthermore, in traumatized neural tissues lectican CSPGs up‐regulated in stabilizing glial scars also strongly inhibit neural outgrowth and functional neural recovery. 189 , 190 CSPGs have long been known as inhibitors of neural growth preventing functional axonal regeneration after injury. Therapeutic use of chondroitinase ABC to remove CS chains from CSPGs in the gliotic scars improves functional neuronal recovery 191 clearly establishing the inhibitory regulatory properties of the CS side chains of lectican PGs. Endogenous ADAMTS‐4 activity in the spinal cord also promotes functional neural recovery after spinal cord injury by removing lecticans from the lesion site. 192 Thus in IVDD, cell mediated changes result in an elevation in catabolic inflammatory cytokine expression, 95 , 123 , 130 , 193 , 194 , 195 elevated production of MMPs, 196 , 197 , 198 , 199 and neurotropic and angiogenic factors 28 , 119 , 125 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 which promote nerve and blood vessel ingrowth into the degenerate IVD. 116 , 126 , 178 , 182 , 209 , 210 , 211 , 212
While the causes of LBP remain to fully determined, peripheral, spinal, and supraspinal biochemical and electrophysiological studies in animal models indicate that peripheral pro‐inflammatory mediators and neuropeptides sensitize nociceptors in the IVD in a similar manner to how they generate pain in knee OA. 213 Degenerative changes in the IVD ECM and alterations in its biomechanical properties are also major contributors to the generation of LBP. 202 Many studies have documented increases in the number of nerve fibers in the degenerate IVD with pain influenced by nerve activation due to the inflammatory conditions and the destabilization that prevails in the degenerate disc. Altered biomechanics and production of neurotrophins (NGF, BDNF) and inflammatory mediators (iNOS, IL‐1β, TNF‐α) influence cell signaling pathways resulting in nerve activation in the degenerate IVD and perceptions of pain. 208 , 210 , 214 Major intracellular signaling pathways regulated by NF‐κB, MAPK, and Wnts mediate the molecular events responsible for the initiation and progression of IVD degeneration and perception of pain and these represent potential therapeutic targets for the treatment of LBP. 202 Expression of the pain receptors Trk‐A (Tropomyosin receptor kinase A, high affinity nerve growth factor receptor, neurotrophic tyrosine kinase receptor type 1, TRK1‐transforming tyrosine kinase protein) and Trk‐B by cells of the non‐degenerate and degenerate IVD suggests an autocrine role for neurotrophins in the regulation of disc cell pain biology. 208 The IVD has also been reported to contain Semaphorin 3A which has regulatory roles in the development and function of neural networks. 215 , 216
TRP channels are also present in the IVD and these are potential sensors and transducers of inflammatory pain. Transient receptor potential ankyrin‐1 (TRPA1) and TRP vanilloid −1, 2, 4 (TRPV1, 2, and 4) have all been detected in the IVD. 162 , 217 Knockout of TRPA1 in mice is associated with degenerative changes in the NP and CEP, however, knockout of TRPV1 did not show obvious IVD changes. Elevated cytokine levels in the degenerate IVD increases gene expression of TRPV2 and TRPV4. 162 , 217 Inhibition of TRPV4 reduces inflammation induced by hyperphysiological stretching of AF cells. 218 The reduced osmolarity in the degenerate IVD due to depletion of aggrecan increases TRPV4 expression and pro‐inflammatory cytokine production by IVD cells. 219 Activation of the TRPV1 channel by the PGE2/EP4 cell signaling pathway induces spinal hypersensitivity in a mouse model of CEP degeneration. 220
9.1. Altered cell signaling and mechanobiological generation of LBP in IVDD
Neuropathic pain is associated with inflammation at sites of tissue damage resulting in a cascade of events concentrating and activating innate immune cells by immuno‐stimulatory cytokines, neurotrophic factors, and chemokines. 221 , 222 Neuroinflammatory environments activate glial cells in the spinal cord and brain. Astrocytes and oligodendrocytes modulate synaptic neurotransmission and potentiation of neuropathic pain. Following peripheral nociceptive activation via nerve injury, activated microglia release pro‐inflammatory TNFα, IL‐1β, and IL‐6, producing inflammatory pain and neuroinflammation. 108 , 223 This recruits other microglia and eventually activates nearby astrocytes. This prolongs the inflammatory state and leads to chronic neuropathic pain. 120 , 223 Inflammation and immune responses by mast cells, neutrophils, macrophages, T lymphocytes, microglia and astrocytes are all implicated in inflammatory processes producing neuropathic pain. IVDD results in mechanical or inflammatory effects and stimulation of AF nociceptors and mechanoreceptors causing discogenic pain. 224 A consequence of the abnormal spinal motion that occurs in biomechanically incompetent degenerate IVDs is an elevation in mechanical stimulation of IVD receptors. 225 Ingrowth of blood vessels and nerve fibers into deeper regions of the AF in IVDD and an elevation in inflammatory mediator levels also contribute to the generation of LBP. 223 These pain signals are conducted by myelinated A δ fibers and unmyelinated C fibers to the DRG and then to the somatosensory dorsal horn of the gray matter of the spinal cord. Elevation in nociceptor stimulation by IVDD may increase the sensitivity of the somatosensory system with peripheral sensitization leading to normally innocuous stimuli elliciting an amplified response. 221 IVDD can thus effect adjacent nerve roots or DRG resulting in neuropathic pain of mechanical or biochemical origin. Feed‐on effects of IVDD on other spinal structures, such as the facet joints, ligaments, and muscles can also result in them becoming centers of pain generation. Thus, IVDD may be responsible for the development of nociceptive and neuropathic pain without being the actual pain focus. Noxious stimuli in the IVD such as an acidic pH, ECM degeneration, inflammatory mediators and neurotrophins that generate inflammatory conditions in the IVD result in membrane depolarization of peripheral nociceptive nerve endings. 120 , 223 This results in the generation of an action potential in such nerves, axonal conductance of electrical signals in nociceptive neurons to somatic DRGs then to the sensory gray matter dorsal horn of the spinal cord and then to the brain occurs. Signal transduction through chemical synapses in neural networks carries pain signals from IVD nociceptors to the brain. 122 Stimulation of aberrant neuronal activity by inflammatory mediators induces protein kinases A and C, calcium/calmodulin‐dependent protein kinase, and MAPK signaling in primary sensory and dorsal horn neurons mediating the induction and maintenance of neuropathic Activation of MAPKs (p38, extracellular signal‐regulated kinase, and c‐Jun N‐terminal kinase) in spinal cord microglia or astrocytes results in the production of inflammatory mediators and sensitization of dorsal horn neurons and activation of spinal glia. Such neuron‐glia interactions enhance and prolong neuropathic pain. 226
9.2. Mouse immune cells destroy nerve perineuronal nets, causing hypersensitization of sensory nerves that perceive chronic pain
Neuropathic pain is the most difficult type of pain to treat clinically because it is not known how nerve injuries cause chronic pain. A recent study has shown that neurons in the spinal cord which process pain signals can be attacked by activated spinal glia and the perineuronal nets (PNNs) which normally protect these neurons providing neural plasticity become degraded. 222 Aggrecan is lost from the PNNs and the hypersensitivity to heat and spontaneous pain occurs. Damage to the PNNs affects the transmittance of pain signals to the brain, heightens pain sensitivity and represents a new mechanism of chronic neuropathic pain generation. 222
10. ANIMAL MODELS OF IVD DEGENERATION AND LBP
Literature reviews in PubMed and Google were used to collect information on the cited studies in Table 3 using search terms such as “IVD degeneration,” “low back pain,” “models of IVD degeneration,” “small animal models of IVD degeneration,” “large animal models of IVD degeneration.” The studies cited were selected by the authors to illustrate examples of large and small animal models of IVDD and the diversity of the models developed and was not intended to provide comprehensive coverage of all IVD models of IVDD that have so far been developed which are extensive. A complete coverage was considered outwith the scope of this review; however, additional information is available on these in two further studies. 94 , 227
TABLE 3.
Illustrative examples of large and small animal models of IVD degeneration
| Animal model | Degenerative mechanism | Features of model | Ref |
|---|---|---|---|
|
24G needle stick AF lesion rat IVDD model |
Elevation in TNFα, IL‐6 and NGF in injured IVD and increases in CGRP, Ca‐binding adaptor protein DRG GFAP | Persistent increases in pain neuropeptides in DRGs and spinal cord glia in the sensory dorsal root horns | 228 |
|
Needle stick injury plus dynamic compression rat IVDD model Potential patho‐mechanism of LBP |
Transient elevation in TNFα, IL‐1β, IL‐6 levels and IVDD, compression induces elevated neuropeptide levels in DRGs and SC dorsal horn | Increase in IVD inflammatory mediator levels. IVD injury/dynamic compression produces a long‐lasting inflammatory response and CGRP in DRGs/SC dorsal horn. | 114 , 183 |
|
Rat bent tail sustained static compression model of IVDD Induces mild degeneration |
Upregulation of MMPs and ADAMTSs and down regulation of TIMPs leads to ECM degradation and IVDD in C7‐C10 IVDs | Static IVD compression alters collagen and MMPs, induces IVDD and β1 integrin expression. Ilizarov device used on 7–10th caudal VBs | 229 , 230 , 246 |
|
Murine tail IVDD (i) External tail IVD static compression device (ii) Mouse compressive suture model of IVDD |
Compression of IVDs leads to reduced NP hydration, and a reduction in IVD type II collagen and aggrecan content. | IVDD is induced by increased compression on cells in the tail IVDs | 247 , 248 |
| Controlled IVD destabilization induced by a large AF lesion, modification of the Ovine Osti annular rim lesion model of IVDD. | Mechanical destabilization of the IVD using a controlled annular lesion increases MMP and ADAMTS expression by IVD cells, leading to degradation of type II collagen and aggrecan and a decrease in the biomechanical competence of the IVD. | IVDD impacts endplate vascularity, VB remodeling, degeneration of facet joint, neovascularization and nerve ingrowth into IVD. MSCs regenerate degenerate IVD. Reduces spinal flexibility, neuromuscular control of spinal muscle, multifidis muscle changes and LBP with IVDD. | 37 , 38 , 39 , 40 , 41 , 42 , 43 , 104 , 249 , 250 , 251 , 252 , 253 , 254 |
| Rabbit intermittent cyclic mechanical tension model elliciting damage to CEP. | CEP damage occurs via the Nuclear Factor‐κB cell signaling pathway and the up‐regulation of MMP‐13 to induce IVDD. | Cyclic mechanical tension induces CEP calcification, decreases type II collagen, aggrecan and Sox9 expression and impacts nutrition of IVD | 231 , 232 , 233 |
|
Spontaneous IVDD models (i) Sand rat (ii) Chondrodystrophoid (ChD) canine (iii) non‐ChD canine (iv) SM/J Mouse |
Age dependant reduction in PGs, IVD hydration, ECM biomechanical competence. Early IVDD in ChD canine compared to non‐ChD due to relative decline in notochordal cell numbers. SM/J mouse early onset spontaneous IVDD. NP cell death, degeneration of NP, AF, CEP. Elevated expression of Col10a1, Ctgf, Runx2 and chondrocyte hypertrophy. | Degeneration of NP & AF, osteophytosis, cell death, CEP sclerosis, decline in notochordal cells in sand rat and ChD canine. Non‐ChD canine has less IVDD. Prominent ARGxx Agg'ase neo‐epitope shows higher aggrecan turnover, abberant expression of collagen X and MMP‐13. High expression of Enpp1 and Alpl indicates increased dystrophic mineralization of CEP. | |
|
Rat multifidus resection Model of IVDD Murine paraspinal muscle surgical lesion IVDD model |
Spinal destabilization | These IVDD models show the interdependence of spinal muscles and the IVD for the maintenance of spinal stability and efficient spinal weight bearing. | |
| Evaluation of GDF‐6 in the attenuation pro‐inflammatory conditions on a rabbit AF puncture model of IVDD and pain generation in a rat xenograft radiculopathy model |
Rabbit annular puncture induced biomechanical destabilization Rat xenograft radiculopathy model |
Evaluation of GDF6 on: (i) gene expression of inflammatory/pain‐related molecules and structural integrity in a rabbit IVDD model, and (ii) sensory dysfunctional changes leading to pain‐marker expression in a rat DRG xenograft radiculopathy model. | 243 |
| Evaluation of the efficacy of GDF6 in a rat posterior disc puncture model conducted at a single and three consecutive IVD levels | Rat posterior annular puncture destabilization model of IVDD induced at a single and at three consecutive IVD levels | GDF6 lowers production of inflammatory mediators and pain peptides, improves IVD structural organization and benefits animal pain related behavior. | 244 |
A number of rodent models have been developed to specifically assess various aspects of IVDD and the development of LBP (Table 3). Several rodent models of IVDD have been developed by altering the normal biomechanical forces experienced by the IVD using (i) annular needle puncture, 228 (ii) Ilozarov type cages which maintain tails in a bent position, 229 , 230 (iii) use of IVD sutures which compress the IVD and (iv) by compressively loading the IVD. Each of these procedures produce IVDD to variable degree. Procedure (i) and (iv) have also been used in combination to induce a more extended degenerative response in the IVD. 183 Procedures (ii) and (iii) were developed to provide milder forms of IVDD. The ovine large annular lesion (6 × 20 mm) of IVDD is a versatile model that reproduces many of the degenerative features described in human IVD. 37 , 38 , 39 The relatively large IVDs of this model allows zonal analyses to be undertaken, a feature not possible for the rodent models of IVDD. The effects of IVDD on erector spinae muscles have also been investigated using this model demonstrating deleterious effects on spinal flexibility and neuromuscular control of spinal muscle groups which result in a diminished control of co‐ordinated bodily movement. 33 , 40 , 41 , 42 , 43 The resultant effects on altered loading of degenerate IVDs results in further mechanical stimulation of nociceptors and mechanoreceptors and generation of LBP. A rabbit model of intermittent cyclical loading to the IVD results in damage to the CEP and an increase in the calcification of this tissue with a negative impact on the nutritional status of disc cells contributing to IVDD. 231 , 232 , 233 Several spontaneous models of IVDD have also been developed in the sand rat, which is a gerbil. 234 , 235 , 236 , 237 The chondrodystrophoid and non‐chondrodystrophoid canine are interesting IVDD models with the former displaying early degenerative IVD changes correlating with an early disappearance of notochordal cells while in the latter IVDD occurs much later and is less prominent and notochordal cell numbers are maintained into old age. 238 , 239 The SM/J poor healer mouse is an inbred small mouse strain that displays age dependent early‐onset, spontaneous disc degeneration characterized by a lack of cells in the NP, a clear increase in hypertrophic chondrocyte‐like cells in the CEPs, and clefts throughout the IVD. 240 Rat and mouse multifidus resection models induce spinal instability and IVDD demonstrating the interdependence of the IVD and spinal muscles for spinal stability, flexibility and optimal weight bearing. 241 , 242
A single level annular puncture model of IVDD, rat xenograft radiculopathy model 243 and rat multi‐level models of IVDD have been developed and used to evaluate the properties of growth and differentiation factor‐6 (GDF6) as a therapeutic agent to combat IVD degenerative processes. 244 GDF6 significantly inhibited the expression of TNFα and IL‐1β in rat IVDs by 8 weeks after annular puncture and had a protective effect on IVD structure and morphology 32 weeks post induction of IVDD. 243 , 245 GDF6 also reduced mechanically mediated pain behavior and inhibited the expression of the inflammatory mediators TNFα and IL‐1β and CGRP in the DRG model. 243 Furthermore, a rat inflammatory protein array showed GDF6 reduced the expression of IL‐6, intercellular adhesion molecule‐1 (ICAM‐1), matrix metalloproteae‐13 (MMP‐13), TNFα, IL‐1β and increased the expression of transforming growth factor β2 (TGF‐β2), IL‐10 and the adipokine resistin (RETN, adipose tissue‐specific secretory factor or C/EBP‐epsilon‐regulated myeloid‐specific secreted cysteine‐rich protein) in a TNFα and IL‐1β stimulated disc cell culture system. 243 , 244 , 245 RETN is a modulatory peptide that regulates inflammation in pathological human tissues. 76 Thus, GDF6 can improve the structure of the IVD, inhibit the expression of inflammatory and pain related factors, and improve pain behavior in rats. 243 , 244 , 245 Human clinical trials with GDF6 are eagerly awaited since it has considerable potential in the prevention of further deterioration in degenerate IVDs, has regenerative properties that promote recovery of normal IVD architectural functional organization and inhibits the generation of inflammatory mediators that lead to IVDD and the generation of LBP. 243 , 244 , 245 This is evident in rat IVDD models by a reduction in pain related factors and in mechanically induced pain behavior. The rat multi‐level IVDD model that has also been used to evaluate the beneficial properties of GDF6 is a novel development in animal models of IVDD. 244
10.1. IVDD models for the examination of IVD mechano‐pathobiology and LBP
IVD cells have evolved to exist in a weighted environment and biomechanical forces have important regulatory effects on disc cells. 224 , 259 The IVD is subject to cycles of compression/relaxation imposed through the axial skeleton and normal bodily movements such as flexion/extension and torsional twisting and bending movements. Indeed, these cycles of compression/relaxation are an important pumping mechanism that promotes diffusion of nutrients to IVD cells and removal of their metabolic waste products in the normal IVD. 260 , 261 Structural alterations in the IVD which impede this nutritional pathway can lower disc cell viability and contribute to the development of IVDD. 225 , 262 , 263 The animal IVDD studies we have cited provide valuable insights into the complexities of IVDD and the generation of LBP. Structural analysis of ECM organization in the normal IVD shows that NP cells predominantly receive compression with some shear component when the IVD is in torsion while AF cells occupy a fibrocartilaginous ECM designed to accommodate radial tensional hoop stresses arising from axial compression and bulging of the NP resulting in outward bulging of the AF. 225 In the degenerate IVD depleted of its space‐filling aggrecan which normally provides weight bearing properties, the normal weight bearing/tensional stresses the IVD cells are exposed to result in increased bulging of the less supportive NP, a reduction in disc height, greater bulging of the AF and a reduction in disc cell viability. 263 The lamellar collagenous structure is weak in compression and may become inverted, adjacent collagenous lamellae may even separate (de‐lammelation) resulting in the formation of internal clefts and fissures in the IVD. 264 When these clefts communicate with the outer AF herniation of the NP may occur, and ingrowth of nerves and blood vessels may occur through these clefts into the normally avascular aneural IVD setting up a scenario where the IVD can no longer adequately withstand axial compression. 104 The increased nociceptive nerve and mechanoreceptor numbers in the degenerate IVD also makes this a structure which is sensitive to mechanical compression and a major contributor to the generation of LBP. 265
Compared to large animal models of IVDD the husbandry and handling of mice is straight forward and these are a popular and very useful animal model amenable to genetic manipulation. 227 This is reflected in the 1000+ mouse models which have been developed in the last decade aiding in the elucidation of the multifactorial components that contribute to the complexity of IVDD. 227 It was deemed beyond the scope of this review to provide a comprehensive coverage of all of these IVD models; however, a number of publications can be consulted for further information in this area. 266 , 267 , 268 , 269 , 270 , 271 , 272 The laboratory mouse is the premier animal for investigations on IVD molecular and cellular systems. 266 , 268 Experimental genetic tools have been developed for the mouse, including unique inbred strains, a complete reference genome, deep sequencing data for inbred lines, 270 extensive genome variation maps (e.g., SNPs), and technologies for genome manipulation. 271 , 272 An international collaborative effort to generate targeted mutations in all murine protein‐coding genes was initiated in 2007. 269 The Mouse Genome Database (http://www.informatics.jax.org) is the primary community database for the laboratory mouse and a key source of gene biological reference data, gene functions, phenotypes, disease models relevant to human biology and disease freely accessible to all researchers. 267
Large animal models of IVDD such as the canine spontaneous and ovine mechanical de‐stabilization models of IVDD have important attributes compared to small IVDD models developed in mice, rats, and rabbits. 37 , 38 , 238 , 258 These large animal models of IVDD have a more similar size and cellular composition to that of the human IVD with notochordal cell populations disappearing in early development whereas notochordal cell populations persist in the small animal models and their influence on pathological changes developing in the degenerate IVD need to be considered, however, these are not a prominent feature in human IVDD. 273 The development of degenerate lesions in the ovine model leads to the development of pathological features in multiple IVD tissues similar to those observed in human IVD degeneration. 37 , 39 Studies using the ovine IVD model have been awarded 9 ISSLS and 3 Grammer prizes which may be considered tacit acceptance by a broad peer review of this model for relevant extrapolations to degenerative features that occur in human IVDD. Large IVDs allow multidisciplinary approaches to be used to examine multiple features of IVDD including zonal analyses of IVD composition, gene expression, biochemical examinations of focal changes in IVD structural organization, biomechanical behavior of specific IVD tissue regions and focal histopathological scoring across a broad range of structural features of functional significance over the entire IVD. 37 , 38 , 39 , 239 , 249 , 258 , 273 , 274 This allows specific questions to be asked with the large animal models of IVDD relevant to human IVDD which cannot be asked with the small animal models. However, small animal models of IVDD have other attributes relating mainly to gene manipulation effects on disc pathobiology 227 , 266 , 267 , 268 , 269 , 270 , 271 , 272 or the development of biomechanically induced whole animal models of IVDD. Mouse and rat models of IVDD have been developed where the IVD is left intact but the supportive paraspinal muscles receive a discrete surgical lesion. 241 , 242 This destabilizes the spine and induces degenerative changes in the IVD and emphasizes the inter‐relationship between the IVD and other spinal components in normal spinal loading. Small animal models of IVDD are also amenable to quantitative assessment of pain using facial recognition methodology 275 , 276 , 277 , 278 , 279 , 280 , 281 , 282 , 283 although this has also now been applied to canine and ovine studies. 284 , 285 , 286 , 287 Significant improvements in the analysis of small animal pain behavior in IVDD through the application of AI in the assessment of ultra‐high speed images documenting changes in animal gait, locomotion, kinematics, facial images for pain has significantly improved the interpretation and quantitation of data generated from small animal LBP models. 278 , 281 , 283 With these methodological improvements the door has been opened for more meaningful evaluations of therapeutic agents and treatment protocols that can potentially inhibit pain stimuli emanating from the IVDD and paradiscal spinal structures. 288 , 289
10.2. Relevance of static and dynamic compressive loading in the development of physiologically relevant animal models to assess IVD mechano‐pathobiology and LBP
IVD cells are not subject solely to static compression but as already stated receive a dynamic balance of tension/compression which not only effects nutritive pathways 260 , 262 , 263 but also has mechanotransductive properties that regulate disc cell behavior. 224 , 259 , 265 Thus, it is essential that a physiologically relevant model is designed to mimic these dynamic in‐vivo micromechanical environmental conditions to better understand their roles in the IVD degenerative process. IVDD is a complex multifactorial process, mouse models have been invaluable in providing information on IVD pathobiology. 227 Static compression murine models of IVDD have provided information on changes in IVD composition and organization that occur with IVDD and the changes in MMP expression that occur in this process. 229 , 246 , 290 , 291 , 292 Static compression also has interesting effects on the cellular dynamics of notochordal cell populations in the murine IVD. This has shown that vital instructional cues held by this cell type aid in the establishment and maintenance of the other resident disc cell populations and possibly could be harnessed in the regeneration of the IVD. 293 Moreover, a premature decline in notochordal cell numbers in the murine IVD is a forerunner of degenerative changes in the murine IVD ECM. Compression‐induced degeneration of the murine IVD has led to the development of a finite‐element model which describes these degenerative processes. 248 The rabbit IVD also contains a prominent notochordal cell population. In a rabbit IVD explant model of unconfined uniaxial compression, static compression of 0.5 and 1 MPa and dynamic compression of 0.5 and 1 MPa were applied at a frequency of 0.1 and 1 Hz for 6 h, respectively. 294 Static compressive loads suppressed aggrecan and collagen gene expression, however, dynamic compression produced significant increases in gene expression for Type I and II collagen and aggrecan and regional differences between the AF and NP with marked changes in ECM organization evident histologically. An up‐regulation in IL‐1β and TNF‐α expression, and decreased viability of IVD cells was also evident with the most significant changes evident in statically loaded IVDs. Static and dynamic compression induced different biologic responses, static compression was catabolic, whereas dynamic loading at near physiological levels apparently induced synthetic activity and an anabolic response in the IVD. 294 The increased compressive load experienced by the AF in degenerate IVDs also leads to a down regulation in type I collagen expression. 295 Examination of isolated human and bovine NP cells seeded into 3D type I collagen matrices exposed to variable loading regimens in pressure chambers also display differing gene expression profiles depending on loading with a high hydrostatic pressure (2.5 MPa) resulting in decreased anabolic gene expression. 296 AF and NP cells subjected to static unconfined compression in an alginate culture system also displayed differential effects on gene expression. 297 AF cells responded to mechanical deformation by increased expression of types I and II collagen, aggrecan, biglycan, decorin, and lumican. NP cells were not responsive to mechanical loading with changes in gene expression of matrix proteins not observed at any time in this system. 297 Differential changes in cytoskeletal organization by AF and NP cells in response to static compression were observed with increased expression of vimentin mRNA and polymerization of vimentin subunits by AF cells but no detectable changes in NP cells. 297 These observations support the differential mechanotransductive effects on disc cell behavior by mechanical loading and the complexities of events that lead to degenerative changes in the IVD. 259 Examination of in‐vivo remodeling of IVDs in response to short‐ and long‐term dynamic compression using rat IVDs instrumented with an Ilizarov‐type device has shown that dynamic compression should be considered a “healthy” loading regimen that maintains or promotes matrix biosynthesis without substantially disrupting disc structural integrity. 298 A slow accumulation of degenerative changes similar to those observed in human IVDD occurs when dynamic compression was applied for prolonged durations. This effect was mild, however, when compared to effects induced by static compression and bending that created greater structural disruption to the IVD 3D structural organization. 298 The effect of immobilization and dynamic compression on IVD cell gene expression profiles has been examined in rat tail‐IVDs instrumented with an Ilizarov‐type device. Immobilization and dynamic compression of IVDs downregulated type I and type II collagen but upregulated aggrecanase, collagenase, and stromelysin expression in the AF but not in the NP. 299 Dynamic compressive effects on IVD mechanics and cellular responses have been examined in a bovine organ culture IVD model using caudal IVDs. 300 This study showed that remodeling of the IVD occurred in response to biomechanical loading and that this was an important regulator of IVD composition and ECM homeostasis. However, when loading regimens resulted in excessive IVD remodeling degenerative changes in IVD structural organization may detrimentally affect its function as a visco‐elastic weight bearing cushion. This study thus reinforced the dynamic inter‐relationship that exists between disc cellular behavior and mechanotransductive regulatory effects induced by static and dynamic compressive loading. 300
10.3. DRG compression models of LBP
Understanding the complexities of neuropathic pain is an important clinical challenge; however, the molecular mechanism remains elusive. Chronic DRG compression models of neuropathic pain suggest the Wnt/β‐catenin pathway plays a critical role in the pathogenesis of neuropathic pain and may be an appropriate therapeutic target. 301 , 302 Proinflammatory factors such as TNF‐α and IL‐18 are significantly elevated in neuropathic pain models. Levels of these mediators are significantly lower when the Wnt/β‐catenin pathway is inhibited using a Wnt/β‐catenin pathway inhibitor such as XAV939. XAV939 is a potent, small molecule inhibitor of tankyrase (TNKS) 1 and 2 (IC₅₀ = 11 and 4 nM, respectively). 303
11. FUTURE RESEARCH AND CONCLUDING REMARKS
This review has outlined the complexities of IVDD and the multiple IVD receptors and inflammatory mediators and neurotrophins that have roles in the development of nociceptor and mechanoreceptors in the degenerate IVD that produce pain signals transported to the brain by the CNS for interpretation. Inflammation in the IVD has powerful effects on the resident disc cell populations and is the impetus for the ingrowth of nerves and blood vessels into the normal IVD leading to its degeneration. Clearly, in order to prevent events that lead to pain generation, preservation of a healthy functional IVD is important, prevention of inflammation and oxidative conditions also prevents ER stress and mitochondrial dysfunction. Animal models of inflammatory and neuropathic pain indicate that inflammation regulates the resolution of pain by producing pro‐resolving mediators such as resolvin D1. 304 Resolvins are derived from, eicosapentaenonic, docosahexanoic, docosapentaenoic, and clupanodonic omega‐3 fatty acids. 305 , 306 , 307 Resolvins have cell regulatory properties similar to prostaglandins promoting the restoration of normal cellular functional properties following the inflammatory conditions that occur in tissue injury. It remains to be established how resolvins are induced in the CNS but resolvin studies nevertheless offer exciting possibilities in the development of potential methods for the alleviation of intractable neuropathic pain in chronically affected patients. 305 , 306 , 307 In the last three decades, a number of studies on bioactive peptides that are opioid receptor ligands, have also been undertaken. 308 Hemorphins are endogenous 4–10 amino acid peptides released during proteolysis of the beta subunit of hemoglobin. The hemorphins exhibit diverse therapeutic effects in both humans and animal models including regulation of blood pressure, mood regulation, enhancement in memory and cognitive learning and analgesic effects. 309 , 310 , 311 Such effects occur through the ability of these peptides to modulate a diverse range of proteins including enzymes and G‐protein coupled opioid receptors. 310 The resolvins and hemomorphins offer considerable promise as agents that can be potentially developed into therapeutic protocols for the alleviation of chronic neuropathic as well as nociceptive pain and deserve further evaluation in future studies in the improved animal models of IVDD that have been developed.
A question has been raised as to whether nutritional intervention can prevent chronic pain development. 312 , 313 , 314 , 315 A number of dietary phytochemicals possess anti‐oxidant and anti‐inflammatory properties and have been shown to have tissue and disc cell protective properties in‐vitro. Flavonoids have potent anti‐oxidant and anti‐inflammatory cell and tissue protective properties. 316 , 317 , 318 Alleviation of inflammation by flavonoids provides relief from nociceptive and neuropathic pain 319 through modulation of interactions with pain receptors, 320 ion channels, 321 , 322 , 323 inhibition of inflammatory cell signaling, 324 , 325 , 326 neuroglial activation, 327 and a reduction in inflammatory cytokine levels. 328 , 329 , 330 The vagus nerve and its branches have an extensive distribution in the body and its afferent and efferent fibers have motor and sensory properties that regulate many organ systems and the gut microbiome. Following spinal cord injury, in male Wistar rats the neurochemical characteristics of vagal sensory neurons distant from the spinal cord display changes in P2X3 receptor, Substance P and isolectin B4 (1B4) expression, known neuronal injury‐responsive markers. This demonstrates communication between the vagal nerve and spinal cord. 331 The vagus nerve innervating the cervical spine has been used in spinal cord‐brain stimulation procedures to treat neurological disorders of cognitive decline (epilepsy, depression). 332 The vagus nerve provides communication between the gut microbiome and linked organ systems (brain, liver, lung, stomach) and transports regulatory gut metabolites to these organs. 268 The recently identified gut‐IVD axis 269 warrants further investigation in the context of control of discogenic LBP and repair of the degenerate IVD. If a means can be found to deliver therapeutic levels of bioactive regulatory compounds to IVDs in‐vivo then this may prevent the development of inflammatory conditions in the IVD that lead to the generation of LBP. Inflammatory conditions that occur in the gut associated with obesity suggests a low‐saturated fat, low sugar diet may decrease ER oxidative stress and Toll‐like receptor and glial cell activation in the IVD and afferent vagal nerve fiber stimulation via the stomach‐brain and gut‐brain axes. 314 Dietary phytochemicals processed by the gut microbiome release prebiotic metabolites that are therapeutic. 333 The vagal nerve is a regulatory delivery system for such metabolites in the gut‐brain, gut‐lung and gut‐liver axes in a number of diseases and may represent a new therapeutic frontier. The gut‐IVD axis has also recently been identified as a potential route of communication to the IVD. 334 This is an area that warrants future investigation as a potential means of either protecting the IVD or of delivering bioactive factors to prevent potentiation of pain signals in the IVD and associated spinal pain centers.
AUTHOR CONTRIBUTIONS
This study was conceived and written by Ashish D. Diwan and James Melrose. None of the above listed companies had any input into the design, implementation or interpretation of the study.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by NHMRC Project Grant 1004032. Ashish D. Diwan received research grants from Nuvasive Australia & Baxter Inc, research support from Cartago Biotech, and educational support from Globus Medical (PA). James Melrose has received consultancy fees from Arthropharm and Sylvan Pharmaceutical Company Pty Ltd. Ashish D. Diwan has received educational consultancy fees from 3M & Nuvasive, Inc.
Diwan, A. D. , & Melrose, J. (2023). Intervertebral disc degeneration and how it leads to low back pain. JOR Spine, 6(1), e1231. 10.1002/jsp2.1231
Funding information National Health and Medical Research Council, Grant/Award Number: 1004032; Nuvasive Australia & Baxter Inc
REFERENCES
- 1. Chou R. Low back pain (chronic). BMJ Clin Evid. 2010;2010:1116. [PMC free article] [PubMed] [Google Scholar]
- 2. Briggs A, Buchbinder R. Back pain: a National Health Priority Area in Australia? Med J Aust. 2009;190:499‐502. [DOI] [PubMed] [Google Scholar]
- 3. Chou R. In the clinic. Low back pain. Ann Intern Med. 2014;150:ITC6‐1. [DOI] [PubMed] [Google Scholar]
- 4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2010;380:2163‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehrlich G. Low back pain. Bull World Health Organ. 2003;81:671‐676. [PMC free article] [PubMed] [Google Scholar]
- 6. Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95‐103. [DOI] [PubMed] [Google Scholar]
- 7. Walker B, Muller R, Grant WD. Low back pain in Australian adults: the economic burden. Asia Pac J Public Health. 2003;15:79‐87. [DOI] [PubMed] [Google Scholar]
- 8. Yelin E, Weinstein S, King T. The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum. 2016;46:259‐260. [DOI] [PubMed] [Google Scholar]
- 9. Ravindra V, Senglaub SS, Rattani A, et al. Degenerative lumbar spine disease: estimating global incidence and worldwide volume. Global Spine J. 2018;8:784‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vernon‐Roberts B, Moore RJ, Fraser RD. The natural history of age‐related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976). 2007;32:2797‐2804. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Han X, Zhang X, Wang S. Spatiotemporal evolution of global population ageing from 1960 to 2017. BMC Public Health. 2019;19:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee R, Mason A, Cotlear D. Some economic consequences of global aging: A discussion note for the World Bank. Health, Nutrition and Population (HNP) discussion paper. World Bank, . https://openknowledge.worldbank.org/handle/10986/13603. License: CC BY 3.0 IGO; 2010. [Google Scholar]
- 13. Malsawma Z. A guide to population‐related home pages on the World Wide Web. Popul Today. 1996;24:4‐5. [PubMed] [Google Scholar]
- 14. Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968‐974. [DOI] [PubMed] [Google Scholar]
- 15. Anonymous . The global burden of low back pain. IASP back‐pain fact sheet . 2021.
- 16. Anonymous . Low back pain. NIH Publication No. 20‐NS‐5161. 2020.
- 17. Ronald L, Mason A, Cotlear D. Some Economic Consequences of Global Aging: A Discussion Note for the World Bank. Health, Nutrition and Population (HNP) Discussion Paper. World Bank, . https://openknowledge.worldbank.org/handle/10986/13603; 2010. [Google Scholar]
- 18. Anonymous . Back problems. Australian Institute of Health and Welfare. Cat. no. PHE 231. http://www.aihw.gov.au/reports/chronic-musculoskeletal-conditions/back-problems; 2020. [Google Scholar]
- 19. United Nations, Department of Economic and Social Affairs . Population Division World Population Ageing. United Nations, ST/ESA/SER.A/444; 2020. [Google Scholar]
- 20. Casazza B. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85:343‐350. [PubMed] [Google Scholar]
- 21. Walton D, Macdermid JC, Giorgianni AA, et al. Risk factors for persistent problems following acute whiplash injury: update of a systematic review and meta‐analysis. J Orthop Sports Phys Ther. 2013;43:31‐43. [DOI] [PubMed] [Google Scholar]
- 22. Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain. 2009;147:17‐19. [DOI] [PubMed] [Google Scholar]
- 23. Menezes Costa LDC, Maher CG, Hancock MJ, et al. The prognosis of acute and persistent low‐back pain: a meta‐analysis. CMAJ. 2012;184:E613‐E624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koes B, van Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. Eur Spine J. 2010;19:2075‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manusov E. Evaluation and diagnosis of low back pain. Prim Care. 2012;39:471‐479. [DOI] [PubMed] [Google Scholar]
- 26. Hoy DEA. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028‐2037. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi Y, Chiba T, Kurokawa M, Aoki Y, Takahashi K, Yamagata M. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine (Phila Pa 1976). 2003;28:871‐880. [DOI] [PubMed] [Google Scholar]
- 28. García‐Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwaszkiewicz K, Schneider JJ, Hua S. Targeting peripheral opioid receptors to promote analgesic and anti‐inflammatory actions. Front Pharmacol. 2013;4:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones C, Lin CC, Day RO, et al. OPAL: a randomised, placebo‐controlled trial of opioid analgesia for the reduction of pain severity in people with acute spinal pain‐a statistical analysis plan. Trials. 2022;23:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nury E, Schmucker C, Nagavci B, et al. Efficacy and safety of strong opioids for chronic noncancer pain and chronic low back pain: a systematic review and meta‐analyses. Pain. 2022;163:610‐636. [DOI] [PubMed] [Google Scholar]
- 32. Wu L, Zhang S, Shkhyan R, et al. Kappa opioid receptor signaling protects cartilage tissue against posttraumatic degeneration. JCI Insight. 2017;2:e88553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James G, Stecco C, Blomster L, et al. Muscle spindles of the multifidus muscle undergo structural change after intervertebral disc degeneration. Eur Spine J. 2022;31:1879‐1888. doi: 10.1007/s00586-022-07235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drake R, Vogl AW, Mitchell AW. Gray's Anatomy for Students E‐Book. Elsevier Health Sciences; 2009. [Google Scholar]
- 35. Zheng Y, Ke S, Lin C, et al. Effect of core stability training monitored by rehabilitative ultrasound image and surface electromyogram in local Core muscles of healthy people. Pain Res Manag. 2019;2019:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kliziene I, Sipaviciene S, Klizas S, Imbrasiene D. Effects of core stability exercises on multifidus muscles in healthy women and women with chronic low‐back pain. J Back Musculoskelet Rehabil. 2015;28:841‐847. [DOI] [PubMed] [Google Scholar]
- 37. Melrose J, Shu C, Young C, et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976). 2012;37:18‐25. [DOI] [PubMed] [Google Scholar]
- 38. Shu C, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int J Mol Sci. 2017;18:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shu C, Dart A, Bell R, et al. Efficacy of administered mesenchymal stem cells in the initiation and co‐ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine. 2018;1:e1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. James G, Sluka KA, Blomster L, et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J. 2018;27:1744‐1756. [DOI] [PubMed] [Google Scholar]
- 41. Hodges P, James G, Blomster L, et al. Multifidus muscle changes after back injury are characterized by structural remodeling of muscle, adipose and connective tissue, but not muscle atrophy: molecular and morphological evidence. Spine (Phila Pa 1976). 2015;40:1057‐1071. [DOI] [PubMed] [Google Scholar]
- 42. Hodges P, James G, Blomster L, et al. Can proinflammatory cytokine gene expression explain multifidus muscle fiber changes after an intervertebral disc lesion? Spine (Phila Pa 1976). 2014;39:1010‐1017. [DOI] [PubMed] [Google Scholar]
- 43. James G, Blomster L, Hall L, et al. Mesenchymal stem cell treatment of intervertebral disc lesion prevents fatty infiltration and fibrosis of the multifidus muscle, but not cytokine and muscle fiber changes. Spine (Phila Pa 1976). 2016;41:1208‐1217. [DOI] [PubMed] [Google Scholar]
- 44. Colloca C, Keller TS, Moore RJ, Harrison DE, Gunzburg R. Validation of a noninvasive dynamic spinal stiffness assessment methodology in an animal model of intervertebral disc degeneration. Spine (Phila Pa 1976). 2009;34:1900‐1905. [DOI] [PubMed] [Google Scholar]
- 45. Colloca C, Keller TS, Moore RJ, Gunzburg R, Harrison DE. Intervertebral disc degeneration reduces vertebral motion responses. Spine (Phila Pa 1976). 2007;32:E544‐E550. [DOI] [PubMed] [Google Scholar]
- 46. Colloca C, Keller TS, Moore RJ, Gunzburg R, Harrison DE. Effects of disc degeneration on neurophysiological responses during dorsoventral mechanical excitation of the ovine lumbar spine. J Electromyogr Kinesiol. 2008;18:829‐837. [DOI] [PubMed] [Google Scholar]
- 47. Colloca C, Keller TS, Harrison DE, Moore RJ, Gunzburg R, Harrison DD. Spinal manipulation force and duration affect vertebral movement and neuromuscular responses. Clin Biomech (Bristol, Avon). 2006;21:254‐262. [DOI] [PubMed] [Google Scholar]
- 48. Keller T, Colloca CJ, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part I. Vertebral motion. J Manipulative Physiol Ther. 2003;26:567‐578. [DOI] [PubMed] [Google Scholar]
- 49. Colloca C, Keller TS, Gunzburg R. Neuromechanical characterization of in vivo lumbar spinal manipulation. Part II. Neurophysiological response. J Manipulative Physiol Ther. 2003;26:579‐591. [DOI] [PubMed] [Google Scholar]
- 50. Colloca C, Gunzburg R, Freeman BJ, Szpalski M, Afifi M, Moore RJ. Biomechanical quantification of pathologic manipulable spinal lesions: an in vivo ovine model of spondylolysis and intervertebral disc degeneration. J Manipulative Physiol Ther. 2012;35:354‐366. [DOI] [PubMed] [Google Scholar]
- 51. Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21:695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Santos F, Silva JT, Giardini AC, et al. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol Pain. 2012;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kallewaard J, Terheggen MA, Groen GJ, et al. 15. Discogenic low back pain. Pain Pract. 2010;10:560‐579. [DOI] [PubMed] [Google Scholar]
- 54. Peng Y, Lv FJ. Symptomatic versus asymptomatic intervertebral disc degeneration: is inflammation the key? Crit Rev Eukaryot Gene Expr. 2015;25:13‐21. [DOI] [PubMed] [Google Scholar]
- 55. Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74:2627‐2642. [DOI] [PubMed] [Google Scholar]
- 56. Ohtori S, Takahashi K, Yamagata M, et al. Neurones in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. A study using diI, an anterograde neurotracer. J Bone Joint Surg Br. 2001;83:1191‐1194. [DOI] [PubMed] [Google Scholar]
- 57. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2001;26:946‐950. [DOI] [PubMed] [Google Scholar]
- 58. Yang G, Liao W, Shen M, Mei H. Insight into neural mechanisms underlying discogenic back pain. J Int Med Res. 2018;46(11):4427‐4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paatsama S, Rissanen P, Rokkanen P. Effect of estradiol, testosterone, cortisone acetate, somatotropin, thyrotropin and parathyroid hormone on the lumbar intervertebral disc in growing dogs. J Small Anim Pract. 1969;10:351‐354. [DOI] [PubMed] [Google Scholar]
- 60. Ao P, Huang W, Li J, et al. 17β‐estradiol protects nucleus pulposus cells from serum deprivation‐induced apoptosis and regulates expression of MMP‐3 and MMP‐13 through promotion of autophagy. Biochem Biophys Res Commun. 2018;503:791‐797. [DOI] [PubMed] [Google Scholar]
- 61. Bertolo A, Baur M, Aebli N, Ferguson SJ, Stoyanov J. Physiological testosterone levels enhance chondrogenic extracellular matrix synthesis by male intervertebral disc cells in vitro, but not by mesenchymal stem cells. Spine J. 2014;14:455‐468. [DOI] [PubMed] [Google Scholar]
- 62. Gao X, Su XT, Lu ZH, Ou J. 17β‐Estradiol prevents extracellular matrix degradation by downregulating MMP3 expression via PI3K/Akt/FOXO3 pathway. Spine (Phila Pa 1976). 2020;45:292‐299. [DOI] [PubMed] [Google Scholar]
- 63. Guo H, Yang SD, Zhang F, et al. 17β Estradiol protects against interleukin 1β induced apoptosis in rat nucleus pulposus cells via the mTOR/caspase 3 pathway. Mol Med Rep. 2019;20:1523‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jeong H, Bae EK, Kim H, et al. Estrogen attenuates the spondyloarthritis manifestations of the SKG arthritis model. Arthritis Res Ther. 2017;19:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jin L, Lv ZD, Wang K, et al. Estradiol alleviates intervertebral disc degeneration through modulating the antioxidant enzymes and inhibiting autophagy in the model of menopause rats. Oxid Med Cell Longev. 2018;2018:7890291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li P, Xu Y, Gan Y, et al. Estrogen enhances matrix synthesis in nucleus pulposus cell through the estrogen receptor β‐p38 MAPK pathway. Cell Physiol Biochem. 2016;29:2216‐2226. [DOI] [PubMed] [Google Scholar]
- 67. Li P, Gan Y, Xu Y, et al. 17beta‐estradiol attenuates TNF‐α‐induced premature senescence of nucleus pulposus cells through regulating the ROS/NF‐κB pathway. Int J Biol Sci. 2017;13:145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu H, Yang SD, Xu Y, et al. Protective role of 17β‐estradiol on tumor necrosis factor‐α‐induced apoptosis in human nucleus pulposus cells. Mol Med Rep. 2017;16:1093‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu S, Yang SD, Huo XW, Yang DL, Ma L, Ding WY. 17β‐Estradiol inhibits intervertebral disc degeneration by down‐regulating MMP‐3 and MMP‐13 and up‐regulating type II collagen in a rat model. Artif Cells Nanomed Biotechnol. 2018;46:182‐191. [DOI] [PubMed] [Google Scholar]
- 70. Sheng B, Yuan Y, Liu X, et al. Protective effect of estrogen against intervertebral disc degeneration is attenuated by miR‐221 through targeting estrogen receptor α. Acta Biochim Biophys Sin (Shanghai). 2018;50:345‐354. [DOI] [PubMed] [Google Scholar]
- 71. Wang H, Ding W, Yang D, Gu T, Yang S, Bai Z. Different concentrations of 17β‐estradiol modulates apoptosis induced by interleukin‐1β in rat annulus fibrosus cells. Mol Med Rep. 2014;10:2745‐2751. [DOI] [PubMed] [Google Scholar]
- 72. Wang T, Yang SD, Liu S, Wang H, Liu H, Ding WY. 17β‐Estradiol inhibits tumor necrosis factor‐α induced apoptosis of human nucleus pulposus cells via the PI3K/Akt pathway. Med Sci Monit. 2016;22:4312‐4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wei A, Shen B, Williams LA, et al. Expression and functional roles of estrogen receptor GPR30 in human intervertebral disc. J Steroid Biochem Mol Biol. 2016;158:46‐55. [DOI] [PubMed] [Google Scholar]
- 74. Yang D, Zhu D, Zhu S, et al. 17β‐Estradiol/extrogen receptor β alleviates apoptosis and enhances matrix biosynthesis of nucleus pulposus cells through regulating oxidative damage under a high glucose condition. Biomed Pharmacother. 2018;107:1004‐4009. [DOI] [PubMed] [Google Scholar]
- 75. Yang S, Ma L, Gu TX, et al. 17β‐Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis. 2014;19:789‐800. [DOI] [PubMed] [Google Scholar]
- 76. Yang S, Yang DL, Sun YP, et al. 17β‐estradiol protects against apoptosis induced by interleukin‐1β in rat nucleus pulposus cells by down‐regulating MMP‐3 and MMP‐13. Apoptosis. 2015;20:348‐357. [DOI] [PubMed] [Google Scholar]
- 77. Zhao C, Chen Q, Zhang WJ, et al. 17β‐Estradiol protects rat annulus fibrosus cells against apoptosis via α1 integrin‐mediated adhesion to type I collagen: an In‐vitro study. Med Sci Monit. 2016;22:1375‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahajan A, Patni R, Verma S. Low back pain and menopause. J Midlife Health. 2019;10:163‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muscat Baron Y. Menopause and the intervertebral disc. Menopause. 2017;24:1118‐1121. [DOI] [PubMed] [Google Scholar]
- 80. Lou C, Chen HL, Feng XZ, et al. Menopause is associated with lumbar disc degeneration: a review of 4230 intervertebral discs. Climacteric. 2014;17:700‐704. [DOI] [PubMed] [Google Scholar]
- 81. Lou C, Chen H, Mei L, et al. Association between menopause and lumbar disc degeneration: an MRI study of 1,566 women and 1,382 men. Menopause. 2017;24:1136‐1144. [DOI] [PubMed] [Google Scholar]
- 82. Wang Y. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J Orthop Translat. 2015;3:205‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y. Menopause as a potential cause for higher prevalence of low back pain in women than in age‐matched men. J Orthop Translat. 2016;8:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang Y, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender‐specific and age‐specific prevalence. J Orthop Translat. 2016;11:39‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wáng Y. Continued progression of lumbar disc degeneration in postmenopausal women. Climacteric. 2015;18:435. [DOI] [PubMed] [Google Scholar]
- 86. Wáng Y, Wáng JQ, Káplár Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quant Imaging Med Surg. 2016;6:199‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18:777‐785. [DOI] [PubMed] [Google Scholar]
- 88. Gruber H, Yamaguchi D, Ingram J, et al. Expression and localization of estrogen receptor‐beta in annulus cells of the human intervertebral disc and the mitogenic effect of 17‐beta‐estradiol in vitro. BMC Musculoskelet Disord. 2002;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jin L, Song XX, Li XF. The role of estrogen in intervertebral disc degeneration. Steroids. 2020;154:108549. [DOI] [PubMed] [Google Scholar]
- 90. Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. [DOI] [PubMed] [Google Scholar]
- 91. Park E, Moon SW, Suh HR, et al. Disc degeneration induces a mechano‐sensitization of disc afferent nerve fibers that associates with low back pain. Osteoarthr Cartil. 2019;27:1608‐1617. [DOI] [PubMed] [Google Scholar]
- 92. Zhang S, Hu B, Liu W, et al. The role of structure and function changes of sensory nervous system in intervertebral disc‐related low back pain. Osteoarthr Cartil. 2021;29:17‐27. [DOI] [PubMed] [Google Scholar]
- 93. Casale R, Symeonidou Z, Ferfeli S, Micheli F, Scarsella P, Paladini A. Food for special medical purposes and nutraceuticals for pain: A narrative review. Pain Ther. 2021;10:225‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alini M, Diwan AD, Erwin WM, Little CB, Melrose J. An update on animal models of intervertebral disc degeneration and low back pain: exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Risbud M, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc‐content. Nat Rev Rheumatol. 2014;10:44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bermudez‐Lekerika P, Crump KB, Tseranidou S, et al. Immuno‐modulatory effects of intervertebral disc cells. Front Cell Dev Biol. 2022;10:924692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hai B, Song Q, Du C, et al. Comprehensive bioinformatics analyses reveal immune genes responsible for altered immune microenvironment in intervertebral disc degeneration. Mol Genet Genomics. 2022;297:1229‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang H, Samartzis D. Clarifying the nomenclature of intervertebral disc degeneration and displacement: from bench to bedside. Int J Clin Exp Pathol. 2014;7:1293‐1298. [PMC free article] [PubMed] [Google Scholar]
- 99. Sato N, Kikuchi S, Sato K. Quantifying the stress induced by distress in patients with lumbar disc herniation in terms of natural killer cell activity measurements: chromium release assay versus multiparameter flow cytometric assay. Spine (Phila Pa1976). 2002;27:2095‐2100. [DOI] [PubMed] [Google Scholar]
- 100. Silva AEA. Macrophages down‐regulate gene expression of intervertebral disc degenerative markers under a pro‐inflammatory microenvironment. Front Immunol. 2019;10:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang L, He T, Liu J, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. 2021;12:666355. doi: 10.3389/fimmu.2021.666355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sun Z, Liu B, Luo ZJ. The immune privilege of the intervertebral disc: implications for intervertebral disc degeneration treatment. Int J Med Sci. 2020;17:685‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ye F, Lyu FJ, Wang H, Zheng Z. The involvement of immune system in intervertebral disc herniation and degeneration. JOR Spine. 2022;5:e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976). 2002;27:1278‐1285. [DOI] [PubMed] [Google Scholar]
- 105. Naylor A, Happey F, Turner RL, Shentall RD, West DC, Richardson C. Enzymic and immunological activity in the intervertebral disk. Orthop Clin North Am. 1975;6:51‐58. [PubMed] [Google Scholar]
- 106. Murai K, Sakai D, Nakamura Y, et al. Primary immune system responders to nucleus pulposus cells: evidence for immune response in disc herniation. Eur Cell Mater. 2010;19:13‐21. [DOI] [PubMed] [Google Scholar]
- 107. Sun Z, Zhang M, Zhao XH, et al. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6:1009‐1014. [PMC free article] [PubMed] [Google Scholar]
- 108. Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J Roy Soc Interface. 2015;12:20141191. doi: 10.1098/rsif.2014.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bisson D, Mannarino M, Racine R, Haglund L. For whom the disc tolls: intervertebral disc degeneration, back pain and toll‐like receptors. Eur Cell Mater. 2021;41:355‐369. [DOI] [PubMed] [Google Scholar]
- 110. Lyu F, Cui H, Pan H, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li W, Gong Y, Liu J, et al. Peripheral and central pathological mechanisms of chronic low back pain: A narrative review. J Pain Res. 2021;14:1483‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Millecamps M, Tajerian M, Naso L, Sage EH, Stone LS. Lumbar intervertebral disc degeneration associated with axial and radiating low back pain in ageing SPARC‐null mice. Pain. 2012;153:1167‐1179. [DOI] [PubMed] [Google Scholar]
- 113. Millecamps M, Czerminski JT, Mathieu AP, Stone LS. Behavioral signs of axial low back pain and motor impairment correlate with the severity of intervertebral disc degeneration in a mouse model. Spine J. 2015;15:2524‐2537. [DOI] [PubMed] [Google Scholar]
- 114. Miyagi M, Millecamps M, Danco AT, Ohtori S, Takahashi K, Stone LS. ISSLS prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976). 2014;39:1345‐1354. [DOI] [PubMed] [Google Scholar]
- 115. Groh A, Fournier DE, Battié MC, Séguin CA. Innervation of the human intervertebral disc: A scoping review. Pain Med. 2021;22:1281‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tolofari S, Richardson SM, Freemont AJ, Hoyland JA. Expression of semaphorin 3A and its receptors in the human intervertebral disc: potential role in regulating neural ingrowth in the degenerate intervertebral disc. Arthritis Res Ther. 2010;12:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kurakawa T, Kakutani K, Morita Y, et al. Functional impact of integrin α5β1 on the homeostasis of intervertebral discs: a study of mechanotransduction pathways using a novel dynamic loading organ culture system. Spine J. 2015;15:417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976). 1995;20:2645‐2651. [DOI] [PubMed] [Google Scholar]
- 119. Binch A, Cole AA, Breakwell LM, et al. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res Ther. 2014;16:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88:68‐71. [DOI] [PubMed] [Google Scholar]
- 121. Dimitroulias A, Tsonidis C, Natsis K, et al. An immunohistochemical study of mechanoreceptors in lumbar spine intervertebral discs. J Clin Neurosci. 2010;17:742‐745. [DOI] [PubMed] [Google Scholar]
- 122. Hoppanova L, Lacinova L. Voltage‐dependent CaV3.2 and CaV2.2 channels in nociceptive pathways. Pflugers Arch. 2022;474:421‐434. [DOI] [PubMed] [Google Scholar]
- 123. Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob Spine J. 2013;3:175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Adams M, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31:2151‐2161. [DOI] [PubMed] [Google Scholar]
- 125. Richardson S, Purmessur D, Baird P, Probyn B, Freemont AJ, Hoyland JA. Degenerate human nucleus pulposus cells promote neurite outgrowth in neural cells. PLoS One. 2012;7:e47735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stefanakis M, Al‐Abbasi M, Harding I, et al. Annulus fissures are mechanically and chemically conducive to the ingrowth of nerves and blood vessels. Spine (Phila Pa 1976). 2012;37:1883‐1891. [DOI] [PubMed] [Google Scholar]
- 127. Navone SEA. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 2017;32:523‐542. [DOI] [PubMed] [Google Scholar]
- 128. Cunha CEA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther. 2018;20:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Molinos MEA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20141191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khan A, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann N Y Acad Sci. 2017;1410:68‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Guo Y, Meng Y, Liu H, et al. Acid‐sensing ion channels mediate the degeneration of intervertebral disc via various pathways‐A systematic review. Channels (Austin). 2019;13:367‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cai F, Wang F, Hong X, et al. Acid‐sensing ion channel 1a regulates the survival of nucleus pulposus cells in the acidic environment of degenerated intervertebral discs. Iran J Basic Med Sci. 2016;19:812‐820. [PMC free article] [PubMed] [Google Scholar]
- 133. Li X, Wu FR, Xu RS, et al. Acid‐sensing ion channel 1a‐mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opin Ther Targets. 2014;18:1‐14. [DOI] [PubMed] [Google Scholar]
- 134. Cuesta A, Del Valle ME, Garcia‐Suarez O, et al. Acidsensing ion channels in healthy and degenerated human intervertebral disc. Connect Tissue Res. 2014;55:197‐204. [DOI] [PubMed] [Google Scholar]
- 135. Sadowska A, Kameda T, Krupkova O, et al. Osmosensing, osmosignalling and inflammation: how intervertebral disc cells respond to altered osmolarity. Eur Cell Mater. 2018;36:231‐250. [DOI] [PubMed] [Google Scholar]
- 136. Wang D, Zhu H, Cheng W, Lin S, Shao R, Pan H. Effects of hypoxia and ASIC3 on nucleus pulposus cells: from cell behavior to molecular mechanism. Biomed Pharmacother. 2019;117:109061. [DOI] [PubMed] [Google Scholar]
- 137. Krock E, Millecamps M, Currie JB, Stone LS, Haglund L. Low back pain and disc degeneration are decreased following chronic toll‐like receptor 4 inhibition in a mouse model. Osteoarthr Cartil. 2018;26:1236‐1246. [DOI] [PubMed] [Google Scholar]
- 138. Jacobsen T, Hernandez PA, Chahine NO. Inhibition of toll‐like receptor 4 protects against inflammation‐induced mechanobiological alterations to intervertebral disc cells. Eur Cell Mater. 2021;41:576‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kobayashi H, Kikuchi S, Konno S, Kato K, Sekiguchi M. Interaction of 5‐hydroxytryptamine and tumor necrosis factor‐α to pain‐related behavior by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976). 2011;36:210‐218. [DOI] [PubMed] [Google Scholar]
- 140. Wang J, Johnson PS, Persico AM, Hawkins AL, Griffin CA, Uhl GR. Human mu opiate receptor. cDNA and genomic clones, pharmacologic characterization and chromosomal assignment. FEBS Lett. 1994;338:217‐222. [DOI] [PubMed] [Google Scholar]
- 141. Bare L, Mansson E, Yang D. Expression of two variants of the human mu opioid receptor mRNA in SK‐N‐SH cells and human brain. FEBS Lett. 1994;354:213‐216. [DOI] [PubMed] [Google Scholar]
- 142. Mestek A, Hurley JH, Bye LS, et al. The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin‐dependent protein kinase and protein kinase C. J Neurosci. 1995;15:2396‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pan Y, Xu J, Mahurter L, Xu M, Gilbert AK, Pasternak GW. Identification and characterization of two new human mu opioid receptor splice variants, hMOR‐1O and hMOR‐1X. Biochem Biophys Res Commun. 2003;301:1057‐1061. [DOI] [PubMed] [Google Scholar]
- 144. Bond C, LaForge KS, Tian M, et al. Single‐nucleotide polymorphism in the human mu opioid receptor gene alters beta‐endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608‐9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chao‐Yang G, Peng C, Hai‐Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr Cartil. 2021;29:793‐801. [DOI] [PubMed] [Google Scholar]
- 146. Penolazzi L, Bergamin LS, Lambertini E, et al. The P2X7 purinergic receptor in intervertebral disc degeneration. J Cell Physiol. 2022;237:1418‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gruber S, Smith RT, Dahlgren MK, Lambros AM, Sagar KA. No pain, all gain? Interim analyses from a longitudinal, observational study examining the impact of medical cannabis treatment on chronic pain and related symptoms. Exp Clin Psychopharmacol. 2021;29:147‐156. [DOI] [PubMed] [Google Scholar]
- 148. Köstenberger M, Nahler G, Jones TM, Neuwersch S, Likar R. The role of cannabis, cannabidiol and other cannabinoids in chronic pain. The perspective of physicians. J Neuroimmune Pharmacol. 2021. doi: 10.1007/s11481-021-10010-x [DOI] [PubMed] [Google Scholar]
- 149. Scott D, Wright CE, Angus JA. Evidence that CB‐1 and CB‐2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124‐131. [DOI] [PubMed] [Google Scholar]
- 150. Langford R, Mares J, Novotna A, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984‐997. [DOI] [PubMed] [Google Scholar]
- 151. Rieder C. Cannabidiol in Parkinson's disease. Braz J Psychiatry. 2020;42:126‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Xantus G, Zavori L, Matheson C, Gyarmathy VA, Fazekas LM, Kanizsai P. Cannabidiol in low back pain: scientific rationale for clinical trials in low back pain. Expert Rev Clin Pharmacol. 2021;14:671‐675. [DOI] [PubMed] [Google Scholar]
- 153. Yimam M, O'Neal A, Horm T, et al. Antinociceptive and anti‐inflammatory properties of cannabidiol alone and in combination with standardized bioflavonoid composition. J Med Food. 2021;24:960‐967. [DOI] [PubMed] [Google Scholar]
- 154. Zhao J, Gao X, Zhao L, Wang Y, Zhang J, Liu S. Effects of cannabidiol on Parkinson's disease in a transgenic mouse model by gut‐brain metabolic analysis. Evid Based Complement Alternat Med. 2022;2022:1525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Du J, Xu M, Kong F, et al. CB2R attenuates intervertebral disc degeneration by delaying nucleus pulposus cell senescence through AMPK/GSK3β pathway. Aging Dis. 2022;13:552‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rodriguez C, Ouyang L, Kandasamy R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav Pharmacol. 2022;33:130‐157. [DOI] [PubMed] [Google Scholar]
- 157. Yamada J, Akeda K, Sano T, Iwasaki T, Takegami N, Sudo A. Expression of glial cell line‐derived neurotrophic factor in the human intervertebral disc. Spine (Phila Pa 1976). 2020;45:E768‐E775. [DOI] [PubMed] [Google Scholar]
- 158. Aoki Y, Ohtori S, Takahashi K, et al. Expression and co‐expression of VR1, CGRP, and IB4‐binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine (Phila Pa 1976). 2005;30:1496‐1500. [DOI] [PubMed] [Google Scholar]
- 159. Ohtori S, Takahashi K, Moriya H. Existence of brain‐derived neurotrophic factor and vanilloid receptor subtype 1 immunoreactive sensory DRG neurons innervating L5/6 intervertebral discs in rats. J Orthop Sci. 2003;8:84‐87. [DOI] [PubMed] [Google Scholar]
- 160. Aoki Y, Ohtori S, Takahashi K, et al. P2X3‐immunoreactive primary sensory neurons innervating lumbar intervertebral disc in rats. Brain Res. 2003;989:214‐220. [DOI] [PubMed] [Google Scholar]
- 161. Kato K, Kikuchi S, Konno S, Sekiguchi M. Participation of 5‐hydroxytryptamine in pain‐related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine (Phila Pa 1976). 2008;33:1330‐1336. [DOI] [PubMed] [Google Scholar]
- 162. Kameda T, Zvick J, Vuk M, et al. Expression and activity of TRPA1 and TRPV1 in the intervertebral disc: association with inflammation and matrix remodeling. Int J Mol Sci. 2019;20:1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sadowska A, Hitzl W, Karol A, et al. Differential regulation of TRP channel gene and protein expression by intervertebral disc degeneration and back pain. Sci Rep. 2019;9:18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sun X, Jin J, Zhang JG, et al. Expression of acid‐sensing ion channels in nucleus pulposus cells of the human intervertebral disk is regulated by non‐steroid anti‐inflammatory drugs. Acta Biochim Biophys Sin (Shanghai). 2014;46:774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yingjun G, Xun Q. Acid‐sensing ion channels under hypoxia. Channels. 2013;7:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Uchiyama Y, Cheng CC, Danielson KG, et al. Expression of acid‐sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J Bone Miner Res. 2007;22:1996‐2006. [DOI] [PubMed] [Google Scholar]
- 167. Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685‐688. [DOI] [PubMed] [Google Scholar]
- 168. Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor‐ and glial cell‐derived neurotrophic factor‐responsive primary sensory neurones. Neuroscience. 2002;10:747‐753. [DOI] [PubMed] [Google Scholar]
- 169. Chowdhury D, Travis GH, Sutcliffe JG, Burton FH. Synaptotagmin I and 1B4 are identical: implications for synaptotagmin distribution in the primate brain. Neurosci Lett. 1995;190:9‐12. [DOI] [PubMed] [Google Scholar]
- 170. Sugita S, Shin OH, Han W, Lao Y, Südhof TC. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. EMBO J. 2002;21:270‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sun K, Zhu J, Yan C, et al. CGRP regulates nucleus pulposus cell apoptosis and inflammation via the MAPK/NF‐κB signaling pathways during intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021:2958584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Coppes M, Marani E, Thomeer RT, Groen GJ. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997;22:2342‐2350. [DOI] [PubMed] [Google Scholar]
- 173. Zheng J, Zhang J, Zhang X, et al. Reactive oxygen species mediate low back pain by upregulating substance P in intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021:6681815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gruber H, Hoelscher GL, Ingram JA, Hanley EN Jr. Genome‐wide analysis of pain‐, nerve‐ and neurotrophin ‐related gene expression in the degenerating human annulus. Mol Pain. 2012;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015;15:1347‐1355. [DOI] [PubMed] [Google Scholar]
- 176. Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor‐dependent neurons related to inflammatory pain. Spine (Phila Pa 1976). 2004;29:1007‐1081. [DOI] [PubMed] [Google Scholar]
- 177. Mo S, Liu C, Chen L, et al. KEGG‐expressed genes and pathways in intervertebral disc degeneration: protocol for a systematic review and data mining. Medicine (Baltimore). 2019;98:e15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gruber H, Jones B, Marrero E, Hanley EN. Proinflammatory cytokines IL‐1β and TNF‐α influence human annulus cell signaling cues for neurite growth: In vitro Coculture studies. Spine (Phila Pa 1976). 2017;42:1529‐1537. [DOI] [PubMed] [Google Scholar]
- 179. Dahia C, Mahoney EJ, Durrani AA, Wylie C. Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976). 2009;34:456‐462. [DOI] [PubMed] [Google Scholar]
- 180. Johnson W, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976). 2001;26:2550‐2557. [DOI] [PubMed] [Google Scholar]
- 181. Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28:2570‐2576. [DOI] [PubMed] [Google Scholar]
- 182. Freemont AP, Peacock TE, Goupille P, Hoyland J, O'Brien J, Jayson M. Nerve ingrowth into diseased intervertebral disc in chronic backpain. Lancet. 1997;350:178‐181. [DOI] [PubMed] [Google Scholar]
- 183. Miyagi M, Ishikawa T, Kamoda H, et al. ISSLS prize winner: disc dynamic compression in rats produces long‐lasting increases in inflammatory mediators in discs and induces long‐lasting nerve injury and regeneration of the afferent fibers innervating discs: a pathomechanism for chronic discogenic low back pain. Spine (Phila Pa 1976). 2012;37:1810‐1818. [DOI] [PubMed] [Google Scholar]
- 184. Johnson W, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658‐2664. [DOI] [PubMed] [Google Scholar]
- 185. Jin J, Tilv ES, Huang Z, Zhou L, Geller HM, Yu P. Effect of chondroitin sulfate proteoglycans on neuronal cell adhesion, spreading and neurite growth in culture. Neural Regen Res. 2018;13:289‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kuffler D, Sosa IJ, Reyes O. Schwann cell chondroitin sulfate proteoglycan inhibits dorsal root ganglion neuron neurite outgrowth and substrate specificity via a soma and not a growth cone mechanism. J Neurosci Res. 2009;87:2863‐2871. [DOI] [PubMed] [Google Scholar]
- 187. Morgenstern D, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313‐332. [DOI] [PubMed] [Google Scholar]
- 188. Siebert J, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119:176‐188. [DOI] [PubMed] [Google Scholar]
- 189. Snow D, Mullins N, Hynds DL. Nervous system‐derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: a light, epifluorescence, and electron microscopy study. Microsc Res Tech. 2001;54:273‐286. [DOI] [PubMed] [Google Scholar]
- 190. Yamada H, Fredette B, Shitara K, et al. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J Neurosci. 1997;17:7784‐7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Muir E, De Winter F, Verhaagen J, Fawcett J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp Neurol. 2019;321:113032. [DOI] [PubMed] [Google Scholar]
- 192. Tauchi R, Imagama S, Natori T, et al. The endogenous proteoglycan‐degrading enzyme ADAMTS‐4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Phillips K, Cullen K, Chiverton N, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: Interleukin‐1 is a master regulator of catabolic processes. Osteoarthr Cartil. 2015;23:1165‐1177. [DOI] [PubMed] [Google Scholar]
- 195. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL‐1 and TNF in matrix degradation in the intervertebral disc. Rheumatology. 2008;47:809‐814. [DOI] [PubMed] [Google Scholar]
- 196. Li Y, Li K, Han X, et al. The imbalance between TIMP3 and matrix‐degrading enzymes plays an important role in intervertebral disc degeneration. Biochem Biophys Res Commun. 2016;469:507‐214. [DOI] [PubMed] [Google Scholar]
- 197. Crean J, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine (Phila Pa 1976). 1997;22:2877‐2884. [DOI] [PubMed] [Google Scholar]
- 198. Vo N, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pockert A, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482‐491. [DOI] [PubMed] [Google Scholar]
- 200. Binch A, Cole AA, Breakwell LM, et al. Class 3 semaphorins expression and association with innervation and angiogenesis within the degenerate human intervertebral disc. Oncotarget. 2015;6:18338‐18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Gruber H, Hoelscher G, Bethea S, Hanley E. Interleukin 1‐beta upregulates brain‐derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech Histochem. 2012;87:506‐511. [DOI] [PubMed] [Google Scholar]
- 202. Hiyama A, Sakai D, Mochida J. Cell signaling pathways related to pain receptors in the degenerated disk. Glob Spine J. 2013;3:165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jung W‐W, Kim H‐S, Shon J‐R, et al. Intervertebral disc degeneration‐induced expression of pain‐related molecules: glial cell ‐derived neurotropic factor as a key factor. J Neurosurg Anesthesiol. 2011;23:329‐334. [DOI] [PubMed] [Google Scholar]
- 204. Krock E, Rosenzweig DH, Chabot‐Doré A‐J, et al. Painful, degenerating intervertebral discs up‐regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213‐1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lee J, Song JY, Baek M, et al. Interleukin‐1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29:265‐269. [DOI] [PubMed] [Google Scholar]
- 206. Navone S, Marfia G, Canzi L, et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J Orthop Res. 2012;30:1470‐1477. [DOI] [PubMed] [Google Scholar]
- 207. Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg Relat Res. 2018;2:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthrit Res Ther. 2008;10:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Baumgartner L, Wuertz‐Kozak K, Le Maitre CL, et al. Multiscale regulation of the intervertebral disc: achievements in experimental, in silico, and regenerative research. Int J Mol Sci. 2021;22:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Freemont A, Watkins A, Le Maitre C, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286‐292. [DOI] [PubMed] [Google Scholar]
- 211. Lama P, Le Maitre CL, Harding IJ, Dolan P, Adams MA. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J Anat. 2018;233:86‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wu B, Yang L, Peng B. Ingrowth of nociceptive receptors into diseased cervical intervertebral disc is associated with discogenic neck pain. Pain Med. 2019;20:1072‐7077. [DOI] [PubMed] [Google Scholar]
- 213. Zhang R, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthr Cartil. 2013;21:1308‐1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Anbar M, Gratt BM. Role of nitric oxide in the physiopathology of pain. J Pain Symptom Manage. 1997;14:225‐254. [DOI] [PubMed] [Google Scholar]
- 215. Carulli D, de Winter F, Verhaagen J. Semaphorins in adult nervous system plasticity and disease. Front Synaptic Neurosci. 2021;13:672891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Fard D, Tamagnone L. Semaphorins in health and disease. Cytokine Growth Factor Rev. 2021;57:5‐63. [DOI] [PubMed] [Google Scholar]
- 217. Kim M, Ramachandran R, Séguin CA. Spatiotemporal and functional characterisation of transient receptor potential vanilloid 4 (TRPV4) in the murine intervertebral disc. Eur Cell Mater. 2021;41:194‐203. [DOI] [PubMed] [Google Scholar]
- 218. Cambria E, Arlt MJE, Wandel S, et al. TRPV4 inhibition and CRISPR‐Cas9 knockout reduce inflammation induced by hyperphysiological stretching in human annulus fibrosus cells. Cells. 2020;9:1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Walter B, Purmessur D, Moon A, et al. Reduced tissue osmolarity increases TRPV4 expression and pro‐inflammatory cytokines in intervertebral disc cells. Eur Cell Mater. 2016;32:123‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Liu S, Wang Q, Li Z, et al. TRPV1 channel activated by the PGE2/EP4 pathway mediates spinal hypersensitivity in a mouse model of vertebral endplate degeneration. Oxid Med Cell Longev. 2021;2021:9965737‐9965716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Peng B, Hao J, Hou S, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006;31:560‐566. [DOI] [PubMed] [Google Scholar]
- 222. Tansley S, Gu N, Guzman AB, et al. Microglia‐mediated degradation of perineuronal nets promotes pain. Science. 2022;377:80‐86. doi: 10.1126/science.abl6773 [DOI] [PubMed] [Google Scholar]
- 223. Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240‐264. [DOI] [PubMed] [Google Scholar]
- 224. Setton L, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88:52‐57. [DOI] [PubMed] [Google Scholar]
- 225. Fearing B, Hernandez PA, Setton LA, Chahine NO. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine. 2018;1:e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Ji R, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004:reE14. [DOI] [PubMed] [Google Scholar]
- 227. Melrose J, Tessier S, Risbud MV. Genetic murine models of spinal development and degeneration provide valuable insights into intervertebral disc pathobiology. Eur Cell Mater. 2021;41:52‐72. [DOI] [PubMed] [Google Scholar]
- 228. Miyagi M, Ishikawa T, Orita S, et al. Disk injury in rats produces persistent increases in pain‐related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976). 2011;36:2260‐2266. [DOI] [PubMed] [Google Scholar]
- 229. Xia W, Zhang LL, Mo J, et al. Effect of static compression loads on intervertebral disc: an in vivo bent rat tail model. Orthop Surg. 2018;10:134‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Yan Z, Pan Y, Wang S, et al. Static compression induces ECM remodeling and integrin α2β1 expression and signaling in a rat tail caudal intervertebral disc degeneration model. Spine (Phila Pa 1976). 2017;42:E448‐E458. [DOI] [PubMed] [Google Scholar]
- 231. Xiao L, Xu HG, Wang H, et al. Intermittent cyclic mechanical tension promotes degeneration of endplate cartilage via the nuclear factor‐κB signaling pathway: an in vivo study. Orthop Surg. 2016;8:393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Xu H, Hu CJ, Wang H, et al. Effects of mechanical strain on ANK, ENPP1 and TGF‐beta1 expression in rat endplate chondrocytes in vitro. Mol Med Rep. 2011;4:831‐835. [DOI] [PubMed] [Google Scholar]
- 233. Xu H. Autophagy protects endplate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone. 2015;75:242‐243. [DOI] [PubMed] [Google Scholar]
- 234. Gruber H, Johnson T, Norton HJ, Hanley EN Jr. The sand rat model for disc degeneration: radiologic characterization of age‐related changes: cross‐sectional and prospective analyses. Spine (Phila Pa 1976). 2002;27:230‐234. [DOI] [PubMed] [Google Scholar]
- 235. Gruber H, Gordon B, Williams C, Norton HJ, Hanley EN Jr. Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross‐sectional analyses of a large male and female population. Spine (Phila Pa 1976). 2007;32:2529‐2536. [DOI] [PubMed] [Google Scholar]
- 236. Gruber H, Phillips R, Ingram JA, Norton HJ, Hanley EN Jr. Spontaneous age‐related cervical disc degeneration in the sand rat. Clin Orthop Relat Res. 2014;472:1936‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Gruber H, Hanley EN Jr. Morphologic features of spontaneous annular tears and disc degeneration in the aging sand rat (Psammomys obesus obesus). Biotech Histochem. 2017;92:402‐410. [DOI] [PubMed] [Google Scholar]
- 238. Bergknut N, Rutges JP, Kranenburg HJ, et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976). 2012;37:351‐358. [DOI] [PubMed] [Google Scholar]
- 239. Bergknut N, Smolders LA, Grinwis GC, et al. Intervertebral disc degeneration in the dog. Part 1: anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet J. 2013;195:282‐291. [DOI] [PubMed] [Google Scholar]
- 240. Novais E, Tran VA, Miao J, et al. Comparison of inbred mouse strains shows diverse phenotypic outcomes of intervertebral disc aging. Aging Cell. 2020;19:e13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Davies M, Kaur G, Liu X, et al. Paraspinal muscle degeneration and regenerative potential in a murine model of lumbar disc injury. N Am Spine Soc J. 2021;6:100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhu D, Miao Z, Dong M, et al. Development of A novel rat intervertebral disc degeneration model by surgical multifidus resection induced instability. World Neurosurg. 2022;165:e357‐e364. [DOI] [PubMed] [Google Scholar]
- 243. Miyazaki S, Diwan AD, Kato K, et al. ISSLS PRIZE IN BASIC SCIENCE 2018: growth differentiation factor‐6 attenuated pro‐inflammatory molecular changes in the rabbit anular‐puncture model and degenerated disc‐induced pain generation in the rat xenograft radiculopathy model. Eur Spine J. 2018;27:739‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Cui H, Zhang J, Li Z, et al. Growth differentiation factor‐6 attenuates inflammatory and pain‐related factors and degenerated disc‐induced pain behaviors in rat model. J Orthop Res. 2021;39:959‐970. [DOI] [PubMed] [Google Scholar]
- 245. Miyazaki K, Miyazaki S, Yurube T, et al. Protective effects of growth differentiation factor‐6 on the intervertebral disc: an in vitro and in vivo study. Cell. 2022;11:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Yurube T, Takada T, Suzuki T, et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012;14:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Liu Z, Zhou Q, Zheng J, Li C, Zhang W, Zhang X. A novel in vivo mouse intervertebral disc degeneration model induced by compressive suture. Exp Cell Res. 2021;398:112359. [DOI] [PubMed] [Google Scholar]
- 248. Lotz J, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression‐induced degeneration of the intervertebral disc: an in vivo mouse model and finite‐element study. Spine (Phila Pa 1976). 1998;23:2493‐2506. [DOI] [PubMed] [Google Scholar]
- 249. Melrose J, Smith SM, Little CB, Moore RJ, Vernon‐Roberts B, Fraser RD. Recent advances in annular pathobiology provide insights into rim‐lesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur Spine J. 2008;17:1131‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Moore R, Osti OL, Vernon‐Roberts B, Fraser RD. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine (Phila Pa 1976). 1992;17:874‐878. [DOI] [PubMed] [Google Scholar]
- 251. Moore R, Vernon‐Roberts B, Osti OL, Fraser RD. Remodeling of vertebral bone after outer anular injury in sheep. Spine (Phila Pa 1976). 1996;21:936‐940. [DOI] [PubMed] [Google Scholar]
- 252. Moore R, Crotti TN, Osti OL, Fraser RD, Vernon‐Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine (Phila Pa 1976). 1999;24:519‐525. [DOI] [PubMed] [Google Scholar]
- 253. Osti O, Vernon‐Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976). 1990;15:762‐767. [DOI] [PubMed] [Google Scholar]
- 254. Constant C, Hom WH, Nehrbass D, et al. Comparison and optimization of sheep in vivo intervertebral disc injury model. JOR Spine. 2022;5:e1198. doi: 10.1002/jsp2.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Erwin W, DeSouza L, Funabashi M, et al. The biological basis of degenerative disc disease: proteomic and biomechanical analysis of the canine intervertebral disc. Arthritis Res Ther. 2015;17:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lee N, Kramer JS, Stoker AM, et al. Canine models of spine disorders. JOR Spine. 2020;3:e1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Smolders L, Bergknut N, Grinwis GC, et al. Intervertebral disc degeneration in the dog. Part 2: chondrodystrophic and non‐chondrodystrophic breeds. Vet J. 2013;195:292‐299. [DOI] [PubMed] [Google Scholar]
- 258. Thompson K, Moore S, Tang S, Wiet M, Purmessur D. The chondrodystrophic dog: A clinically relevant intermediate‐sized animal model for the study of intervertebral disc‐associated spinal pain. JOR Spine. 2018;1:e1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Vergroesen P, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23:1057‐1070. [DOI] [PubMed] [Google Scholar]
- 260. Magnier C, Boiron O, Wendling‐Mansuy S, Chabrand P, Deplano V. Nutrient distribution and metabolism in the intervertebral disc in the unloaded state: a parametric study. J Biomech. 2009;42:100‐108. [DOI] [PubMed] [Google Scholar]
- 261. Urban J, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29:2700‐2709. [DOI] [PubMed] [Google Scholar]
- 262. De Geer C. Intervertebral disk nutrients and transport mechanisms in relation to disk degeneration: A narrative literature review. J Chiropr Med. 2018;17:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Zhu Q, Jackson AR, Gu WY. Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3d finite element analysis. J Biomech. 2012;45:2769‐2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Urban J, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Urban J, Fairbank JCT. Current perspectives on the role of biomechanical loading and genetics in development of disc degeneration and low back pain; a narrative review. J Biomech. 2020;102:109573. [DOI] [PubMed] [Google Scholar]
- 266. Blake JEA. Mouse Genome Database (MGD)‐2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res. 2017;45:D723‐D729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Brown S, Moore MW. The international mouse phenotyping consortium: past and future perspectives on mouse phenotyping. Mamm Genome. 2012;23:632‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Bult C, Eppig JT, Blake JA, Kadin JA, Richardson JE. Mouse genome database 2016. Nucleic Acids Res. 2016;44:D840‐D847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Collins F, Rossant J, Wurst W. A mouse for all reasons. Cell. 2007;128:9‐13. [DOI] [PubMed] [Google Scholar]
- 270. Keane T, Goodstadt L, Danecek P, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Mali PEA. RNA‐guided human genome engineering via Cas9. Science. 2013;339:823‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Wang HEA. One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell. 2013;153:910‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Schollum M, Appleyard RC, Little CB, Melrose J. A detailed microscopic examination of alterations in normal anular structure induced by mechanical destabilization in an ovine model of disc degeneration. Spine (Phila Pa 1976). 2010;35:1965‐1973. [DOI] [PubMed] [Google Scholar]
- 275. De Rantere D, Schuster CJ, Reimer JN, Pang DS. The relationship between the Rat Grimace Scale and mechanical hypersensitivity testing in three experimental pain models. Eur J Pain. 2016;20:417‐426. [DOI] [PubMed] [Google Scholar]
- 276. Heinsinger N, Spagnuolo G, Allahyari RV, et al. Facial grimace testing as an assay of neuropathic pain‐related behavior in a mouse model of cervical spinal cord injury. Exp Neurol. 2020;334:113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Matsumiya L, Sorge RE, Sotocinal SG, et al. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci. 2012;51:42‐49. [PMC free article] [PubMed] [Google Scholar]
- 278. Miller A, Leach MC. The Mouse Grimace Scale: A clinically useful tool? PLoS One. 2015;10:e0136000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Philips B, Weisshaar CL, Winkelstein BA. Use of the Rat Grimace Scale to evaluate neuropathic pain in a model of cervical radiculopathy. Comp Med. 2017;67:34‐42. [PMC free article] [PubMed] [Google Scholar]
- 280. Schneider L, Henley KY, Turner OA, Pat B, Niedzielko TL, Floyd CL. Application of the Rat Grimace Scale as a marker of supraspinal pain sensation after cervical spinal cord injury. J Neurotrauma. 2017;34:2982‐2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Sotocinal S, Sorge RE, Zaloum A, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Tuttle A, Molinaro MJ, Jethwa JF, et al. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol Pain. 2018;14:1744806918763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Whittaker A, Liu Y, Barker TH. Methods used and application of the mouse grimace scale in biomedical research 10 years on: A scoping review. Animals (Basel). 2021;11:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Cohen S, Beths T. Grimace scores: tools to support the identification of pain in mammals used in research. Animals (Basel). 2020;10:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Evangelista M, Monteiro BP, Steagall PV. Measurement properties of grimace scales for pain assessment in nonhuman mammals: a systematic review. Pain. 2022;163:e697‐e714. [DOI] [PubMed] [Google Scholar]
- 286. McLennan K, Mahmoud M. Development of an automated pain facial expression detection system for sheep (Ovis aries). Animals (Basel). 2019;9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Steagall P, Bustamante H, Johnson CB, Turner PV. Pain management in farm animals: focus on cattle, sheep and pigs. Animals (Basel). 2021;11:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. D'Antoni F, Russo F, Ambrosio L, et al. Artificial intelligence and computer aided diagnosis in chronic low back pain: A systematic review. Int J Environ Res Public Health. 2022;19:5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Tagliaferri S, Angelova M, Zhao X, et al. Artificial intelligence to improve back pain outcomes and lessons learnt from clinical classification approaches: three systematic reviews. NPJ Digit Med. 2020;3:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Hirata H, Yurube T, Kakutani K, et al. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res. 2014;32:455‐463. [DOI] [PubMed] [Google Scholar]
- 291. Yurube T, Nishida K, Suzuki T, et al. Matrix metalloproteinase (MMP)‐3 gene up‐regulation in a rat tail compression loading‐ind uced disc degeneration model. J Orthop Res. 2010;28:1026‐1032. [DOI] [PubMed] [Google Scholar]
- 292. Yurube T, Hirata H, Kakutani K, et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression‐induced disc degeneration model. Arthritis Res Ther. 2014;16:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Matta A, Erwin WM. Current status of the instructional cues provided by notochordal cells in novel disc repair strategies. Int J Mol Sci. 2021;23:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Wang D, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila Pa 1976). 2007;32:2521‐2528. [DOI] [PubMed] [Google Scholar]
- 295. Wenger K, Woods JA, Holecek A, Eckstein EC, Robertson JT, Hasty KA. Matrix remodeling expression in anulus cells subjected to increased compressive load. Spine (Phila Pa 1976). 2005;30:1122‐1126. [DOI] [PubMed] [Google Scholar]
- 296. Neidlinger‐Wilke C, Würtz K, Urban JP, et al. Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J. 2006;15:S372‐S378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Chen J, Yan W, Setton LA. Static compression induces zonal‐specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573‐583. [DOI] [PubMed] [Google Scholar]
- 298. Wuertz K, Godburn K, MacLean JJ, et al. In vivo remodeling of intervertebral discs in response to short‐ and long‐term dynamic compression. J Orthop Res. 2009;27:1235‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. MacLean J, Lee CR, Grad S, Ito K, Alini M, Iatridis JC. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine (Phila Pa 1976). 2003;28:973‐981. [DOI] [PubMed] [Google Scholar]
- 300. Korecki C, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine (Phila Pa 1976). 2008;33:1403‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Zhang Y, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123:2268‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhang Y, Zhao D, Li X, et al. The Wnt/β‐catenin pathway regulated cytokines for pathological neuropathic pain in chronic compression of dorsal root ganglion model. Neural Plast. 2021;2021:6680192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Huang S, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614‐620. [DOI] [PubMed] [Google Scholar]
- 304. Tao X, Luo X, Zhang T, Hershey B, Esteller R, Ji RR. Spinal cord stimulation attenuates mechanical allodynia and increases central Resolvin D1 levels in rats with spared nerve injury. Front Physiol. 2021;12:687046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Balta M, Loos BG, Nicu EA. Emerging concepts in the resolution of periodontal inflammation: A role for Resolvin E1. Front Immunol. 2017;8:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Gerlach B, Marinello M, Heinz J, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27:525‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Moro K, Nagahashi M, Ramanathan R, Takabe K, Wakai T. Resolvins and omega three polyunsaturated fatty acids: clinical implications in inflammatory diseases and cancer. World J Clin Cases. 2016;4:155‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Mielczarek P, Hartman K, Drabik A, et al. Hemorphins‐from discovery to functions and pharmacology. Molecules. 2021;26:3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ali A, Alzeyoudi SAR, Almutawa SA, Alnajjar AN, Vijayan R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol Res. 2020;158:104855. [DOI] [PubMed] [Google Scholar]
- 310. Ayoub M, Vijayan R. Hemorphins targeting G protein‐coupled receptors. Pharmaceuticals (Basel). 2021;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Wei F, Zhao L, Jing Y. Hemoglobin‐derived peptides and mood regulation. Peptides. 2020;127:170268. [DOI] [PubMed] [Google Scholar]
- 312. Rampin A, Carrabba M, Mutoli M, et al. Recent advances in KEAP1/NRF2‐targeting strategies by phytochemical antioxidants, nanoparticles, and biocompatible scaffolds for the treatment of diabetic cardiovascular complications. Antioxid Redox Signal. 2022;36:707‐728. [DOI] [PubMed] [Google Scholar]
- 313. Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA. Withania somnifera L.: insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci. 2020;23:1501‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167‐184. [DOI] [PubMed] [Google Scholar]
- 315. Weinzierl A, Ampofo E, Menger MD, Laschke MW. Tissue‐protective mechanisms of bioactive phytochemicals in flap surgery. Front Pharmacol. 2022;13:864351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Cao H, Yang L, Tian R, Wu H, Gu Z, Li Y. Versatile polyphenolic platforms in regulating cell biology. Chem Soc Rev. 2022;51:4175‐4198. [DOI] [PubMed] [Google Scholar]
- 317. Malla A, Dar BA, Isaev AB, Lone Y, Banday MR. Flavonoids: A reservoir of drugs from nature. Mini Rev Med Chem. 2022;22. doi: 10.2174/1389557522666220420102545 [DOI] [PubMed] [Google Scholar]
- 318. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. [DOI] [PubMed] [Google Scholar]
- 319. Ferraz C, Carvalho TT, Manchope MF, et al. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre‐clinical and clinical data, and pharmaceutical development. Molecules. 2020;25:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Kaswan N, Mohammed Izham NAB, Tengku Mohamad TAS, Sulaiman MR, Perimal EK. Cardamonin modulates neuropathic pain through the possible involvement of serotonergic 5‐HT1A receptor pathway in CCI‐induced neuropathic pain mice model. Molecules. 2021;26:3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Gu X, Yang L, Wang S, et al. A rat model of radicular pain induced by chronic compression of lumbar dorsal root ganglion with SURGIFLO. Anesthesiology. 2008;108:113‐121. [DOI] [PubMed] [Google Scholar]
- 322. Pinho‐Ribeiro F, Hohmann MS, Borghi SM, et al. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF‐κB. Chem Biol Interact. 2015;288:88‐99. [DOI] [PubMed] [Google Scholar]
- 323. Zhou Y, Cai S, Moutal A, et al. The natural flavonoid Naringenin elicits analgesia through inhibition of NaV1.8 voltage‐gated sodium channels. ACS Chem Nerosci. 2019;10:4834‐4846. [DOI] [PubMed] [Google Scholar]
- 324. Sun Y, Jia D, Xue M, Huang Z, Huang C. Trifluoro‐icaritin alleviates chronic inflammatory pain through α7nAChR‐mediated suppression of HMGB1/NF‐κB signaling in the spinal cord of rats. Brain Res Bull. 2022;183:13‐26. [DOI] [PubMed] [Google Scholar]
- 325. Ullah R, Ali G, Subhan F, et al. Attenuation of nociceptive and paclitaxel‐induced neuropathic pain by targeting inflammatory, CGRP and substance P signaling using 3‐Hydroxyflavone. Neurochem Int. 2021;144:104981. [DOI] [PubMed] [Google Scholar]
- 326. Zhang X, Zhao W, Liu X, Huang Z, Shan R, Huang C. Celastrol ameliorates inflammatory pain and modulates HMGB1/NF‐κB signaling pathway in dorsal root ganglion. Neurosci Lett. 2019;692:83‐89. [DOI] [PubMed] [Google Scholar]
- 327. Jin G, He SD, Lin SM, et al. Koumine attenuates neuroglia activation and inflammatory response to neuropathic pain. Neural Plast. 2018;2018:9347696‐9347613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Jin H, Wang Q, Wu J, et al. Baicalein inhibits the IL‐1β‐induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration In vivo. Inflammation. 2019;42:1032‐1044. [DOI] [PubMed] [Google Scholar]
- 329. Maayah Z, Takahara S, Ferdaoussi M, Dyck JRB. The anti‐inflammatory and analgesic effects of formulated full‐spectrum cannabis extract in the treatment of neuropathic pain associated with multiple sclerosis. Inflamm Res. 2020;69:549‐558. [DOI] [PubMed] [Google Scholar]
- 330. Xu J, Wang W, Zhong XX, Feng Y, Wei X, Liu XG. EXPRESS: Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol Pain. 2016;12:1744806916657089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Herrity A, Petruska JC, Stirling DP, Rau KK, Hubscher CH. The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1021‐R1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Hammer N, Glätzner J, Feja C, et al. Human vagus nerve branching in the cervical region. PLoS One. 2015;10:e0118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Nijs J, Tumkaya Yilmaz S, Elma Ö, et al. Nutritional intervention in chronic pain: an innovative way of targeting central nervous system sensitization? Expert Opin Ther Targets. 2020;24:793‐803. [DOI] [PubMed] [Google Scholar]
- 334. Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut‐disc axis: A cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917‐925. [DOI] [PubMed] [Google Scholar]


