Abstract
Animal models have been invaluable in the identification of molecular events occurring in and contributing to intervertebral disc (IVD) degeneration and important therapeutic targets have been identified. Some outstanding animal models (murine, ovine, chondrodystrophoid canine) have been identified with their own strengths and weaknesses. The llama/alpaca, horse and kangaroo have emerged as new large species for IVD studies, and only time will tell if they will surpass the utility of existing models. The complexity of IVD degeneration poses difficulties in the selection of the most appropriate molecular target of many potential candidates, to focus on in the formulation of strategies to effect disc repair and regeneration. It may well be that many therapeutic objectives should be targeted simultaneously to effect a favorable outcome in human IVD degeneration. Use of animal models in isolation will not allow resolution of this complex issue and a paradigm shift and adoption of new methodologies is required to provide the next step forward in the determination of an effective repairative strategy for the IVD. AI has improved the accuracy and assessment of spinal imaging supporting clinical diagnostics and research efforts to better understand IVD degeneration and its treatment. Implementation of AI in the evaluation of histology data has improved the usefulness of a popular murine IVD model and could also be used in an ovine histopathological grading scheme that has been used to quantify degenerative IVD changes and stem cell mediated regeneration. These models are also attractive candidates for the evaluation of novel anti‐oxidant compounds that counter inflammatory conditions in degenerate IVDs and promote IVD regeneration. Some of these compounds also have pain‐relieving properties. AI has facilitated development of facial recognition pain assessment in animal IVD models offering the possibility of correlating the potential pain alleviating properties of some of these compounds with IVD regeneration.
Keywords: animal models of disc degeneration, artificial intelligence and deep machine learning, intervertebral disc, intervertebral disc degeneration, intervertebral disc regeneration, low back‐pain
Animal models of IVD deneration have yielded invaluable information on the pathobiology of this degenerative condition and identified prospective therapeutic targets.The complexity of the degenerative changes and multiple therapeutic targets identified by these models suggests artificial intelligence methodology may be required to unravel these complexities and provide a rationale way forward in the development of effective repair strategies.
1. INTRODUCTION
The present review was undertaken to provide an update of the highly cited publication by Alini and colleagues: Are animal models useful for studying human disc disorders/degeneration published in 2008 in The European Spine Journal. 1 This paper with >750 citations (Google Scholar August 2022) illustrates the importance and high level of interest in animal models of intervertebral‐disc degeneration (IVDD). Animal models have been invaluable in increasing our understanding of disc biology, however because of differences between species, care is required in the formulation of specific questions and selection of appropriate animal models if the intention is to validly extrapolate findings to human targets and therapeutically target IVDD in patients. In line with this, the previous review advocated that much more effort was needed to undertake research on human disc material and administrators/legislators were requested to improve access to human tissues for research purposes. Notwithstanding the need for human‐animal comparative studies and validation of model translational utility, there has been a veritable explosion of data in the area of IVDD pathobiology. While recognizing the potential posturally related biomechanical advantage, the use of primate and bipedal mouse or rat models are considered inappropriate on ethical grounds and the previous review recommended that these models should be discontinued. Studies however have continued to be published using bipedal rodent models particularly in scoliosis studies (Table 1), 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 with limited new information generated applicable to human IVDD.
TABLE 1.
Studies using bipedal rodent spinal models
| Model | Features | References |
|---|---|---|
| Bipedal rat scoliosis model | Melatonin antagonist (Luzindole) and improvement in the treatment of the bipedal scoliotic rat | 7 |
| Bipedal mouse scoliosis model | Enhanced central leptin activity in a scoliosis model in bipedal mice | 5 |
| Bipedal mouse scoliosis model | Assessment of whether elevated OPN has a role in the development of scoliosis in bipedal mice | 6 |
| Bipedal rat/mouse scoliosis models | Animal models of scoliosis | 2 |
| Bipedal mouse model | The effect of sympathectomy on the development and progression of scoliosis in bipedal mice | 9 |
| Bipedal mouse model | Radiologic and histomorphometric study on a bipedal C57Bl6 mouse model. Modulation of estrogen receptor expression prevents scoliotic curve progression | 3 |
| Rat bipedal kyphoscoliosis model | A model of kyphoscoliosis created by a scapula‐to‐contralateral ilium tethering procedure in bipedal rats | 4 |
| Bipedal standing mouse model | Novel bipedal standing mouse model of intervertebral disc and facet joint degeneration | 11 |
| Bipedal rat model | Effect of axial vertical vibration on degeneration of lumbar intervertebral discs | 10 |
| Bipedal mouse model of IVD and facet joint degeneration | Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration | 8 |
Since the prior review three new large animal species (alpaca/llama, equine and kangaroo) have been advocated for IVD studies 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ongoing studies will determine how useful these new models actually are and whether they offer advantages or novel insights compared with existing more widely explored species. A major aim of using large animal models is to produce data that can be informative to comparative size and biomechanical events occurring in human IVD. Beyond these considerations however, animal IVDs to be analyzed should also approximate human IVDs in terms of structure and the cell types they contain. This immediately indicates some limitations with animals that have been used for IVD studies which unlike humans contain abundant notochordal cell populations and gelatinous NPs late into IVD maturation, for example, pigs and non‐chondrodystrophoid (ChD) dog breeds. 27 , 28 , 29 In 2008, it was advocated that the non‐ChD‐canines were unsuitable for the development of a translationally relevant animal models of human IVDD. 1 Non‐ChD‐canine breeds show how notochordal cells delay IVDD and regulate resident disc cell populations to improve their regenerative properties (see detailed discussion later in this review). With the completion of the canine genome (see Appendix S1) and establishment of differences between canine breeds, it is now abundantly clear that ChD‐breeds should be used to study degeneration of the IVD. 30 , 31 Canines display more phenotypic variation than any other mammals and are affected by a wide variety of diseases of a genetic origin. 32 , 33 , 34 This reinforces that while mixed breed dogs may be more accessible for research purposes, canine breeds of a well‐defined pedigree should be used for spinal studies (see supplemental information). Development of the current day domestic dog represents a dramatic unprecedented long‐term evolutionary experiment on a large wolf‐like progenitor, with unparalleled phenotypic diversity. 35
Animal genetics also need to be considered when selecting sheep breeds for IVD studies (see Appendix S1). The pedigree Iberian Merino was first transported to South Africa then Australia by General Macarthur in 1797 with The First Fleet. This was a relatively small animal compared with the modern day Merino but like its ChD‐canine counterpart, reproduced many aspects of the degenerative pathology of human IVDs including ingrowth of blood vessels and nerves into annular lesions, 36 , 37 remodeling of vertebral bone, 38 osteoarthritic changes in facet joints, 39 , 40 degenerative changes in end‐plate vascularization, 41 generation of radial and concentric tears in the IVD 42 and consequential changes in IVD composition and biomechanical performance. 43
2. THE INVOLVEMENT OF SEX HORMONES IN INTERVERTEBRAL DISC PATHOBIOLOGY
An aspect that has emerged in the last decade relevant to the development of prospective animal models of IVDD is the role that sex hormones may have. Although the effects of sex hormones on the metabolism of IVD cells was first identified in 1969 44 it is only in the last decade that these have been shown to significantly impact on degenerative processes in the IVD. 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 It is therefore critical that both male and female animals be examined and data collected and separately analyzed to ensure any pathophysiological sexual dimorphism is identified. Ideally this would include both actively cycling and “post‐menopausal” (likely gonadectomized) female cohorts to mimic and model clinically relevant human populations. Male sex hormones also effect IVD cells but age‐related decline in these does not occur to the same degree as females, suggesting that unless there is a specific research question, routine inclusion of gonadectomized male cohorts may not be ethically justified from a translational perspective. 46
Low back pain (LBP) is a common symptom of premenstrual syndrome (PMS), experienced by most women during menstruation and may be exacerbated by premenstrual dysphoric disorder and dysmenorrhea or may be a symptom of endometriosis. 63 , 64 Female sex hormones play an important role in the etiology and pathophysiology of a number of musculoskeletal degenerative diseases, around 70% of perimenopausal women will experience LBP symptoms due to estrogen deficiency and estrogen decrease may be a risk factor for lumbar disc degeneration. 65 , 66 , 67 , 68 , 69 , 70 Postmenopausal women show accelerated IVDD due to relative estrogen deficiency, increased prevalence of spondylolisthesis, and facet joint osteoarthritis, in the first 15 years post menopause. 46 Continued progression of lumbar disc degeneration in postmenopausal women has been observed. 46 Further studies with functional foods and neutraceutical supplements under evaluation for their abilities to alleviate pain may prove to be a useful non‐drug treatment for post‐menopausal back pain. 71
Estrogen can prevent the development of IVDD through its anti‐apoptotic properties inhibiting the production of the inflammatory cytokines IL‐1β and TNF‐α by disc cells. 72 , 73 This reduces catabolic events in the IVD by preventing the up‐regulation of MMPs induced by these inflammatory mediators. Estrogen also induces anabolic processes in the IVD by activating the PI3K/Akt pathway and also decreases oxidative damage. 74 By inhibiting IVDD, estrogen exerts protective effects that prevent degradative structural changes in the IVD that would otherwise pre‐dispose the IVD to nerve ingrowth and production of neurotrophic factors and inflammatory mediators by IVD cells that contribute to IVD nociceptor activation and mechano‐sensitization of disc afferent nerve fibers leading to the generation of LBP. 75 , 76 A model of IVDD developed in rats has shown that inflammatory mediators and neurotrophic factors secreted by IVD cells in the degenerate IVD produces an environment in this tissue that promotes nerve ingrowth 77 and the sensitization of mechanoreceptors and nociceptors by dynamic compression of the IVD. 78 Dynamic compression of the degenerate IVD produces long‐lasting increases in IVD inflammatory mediators and nociceptor activation leading to the generation of LBP. 78
3. SMALL ANIMAL MODELS OF IVDD
Despite the high disability rates of LBP and high socioeconomic impact in adult humans, the discovery of new drug treatments for the alleviation of chronic mechanical LBP is lacking due to a paucity of knowledge of LBP pathobiology. The use of animal models of LBP aims to alleviate this deficiency. Compared with large animal models of IVDD the husbandry and handling of mice is straight forward and their relatively short generational period and ability to manipulate murine genes has made these a popular and very useful animal model. This is reflected in the large number of mouse studies which have been conducted in the last decade to elucidate the multifactorial components that contribute to IVDD. 79
The bipedal rat and mouse models continue to be used despite ethical issues expressed in our review of 2008 (Table 1). Quadruped rodent models have also continued to be a popular animal model for IVD studies. The sheer numbers of rodent spinal studies that have appeared in the last decade points to their accessibility and utility through genetic manipulation. Whether this popularity equates with translational relevance and value remains to be determined. Studies using rodent IVD models outnumber any other animal model in the last decade. The mouse is a well‐established medical‐research experimental model, genes can be easily found in the mouse genome sequence, and it is also possible to test experimentally the function of those genes and to test possible therapeutic agents and evaluate their precise effects. The Mouse ENCODE (ENCyclopedia Of DNA Elements) project is building a comprehensive catalogue of functional elements in the mouse genome, comparing these to the human. 80 , 81 These include protein and non‐protein coding genes and selection elements that regulate which genes are turned on or off, and when this happens in development. Mice and humans share about 97.5 per cent of their working DNA; on average, the protein‐coding regions of the mouse and human genomes are 85 percent identical; some genes are 99 percent identical while others may share only 60 percent identity. The mouse genome was published in 2002 and found to be comparable to the human genome in terms of its size and the genes it encompassed. 82 This has enabled the development of thousands of mouse strains with mutations mirroring those seen in human genetic diseases. 83 These models have been an invaluable resource in determining the potential roles of specific genes in human diseases. 79
An extensive review on the mouse IVD was published in 2021 79 and in order to avoid duplication, the interested reader is referred to this review for specific information on models of the mouse IVD. Some supplemental information on the mouse is also provided at the end of this review (see supplemental information).
4. GENETIC STUDIES USING ANIMAL MODELS
5. CANINE AND OVINE GENOMIC RESEARCH
Considerable progress has been made in the elucidation of the canine genome, 30 , 84 this highlights canine breeds which evolved from the ancestral wolf genome and the susceptibility of different canine breeds to specific diseases 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 (see Appendix S1).
The ovine genome has also been published by The International Sheep Genomics Sequencing Consortium and illustrates the complexities of a four stomach ruminant animal and its evolution compared with other mammals 93 (Figure 1; Appendix S1).
FIGURE 1.
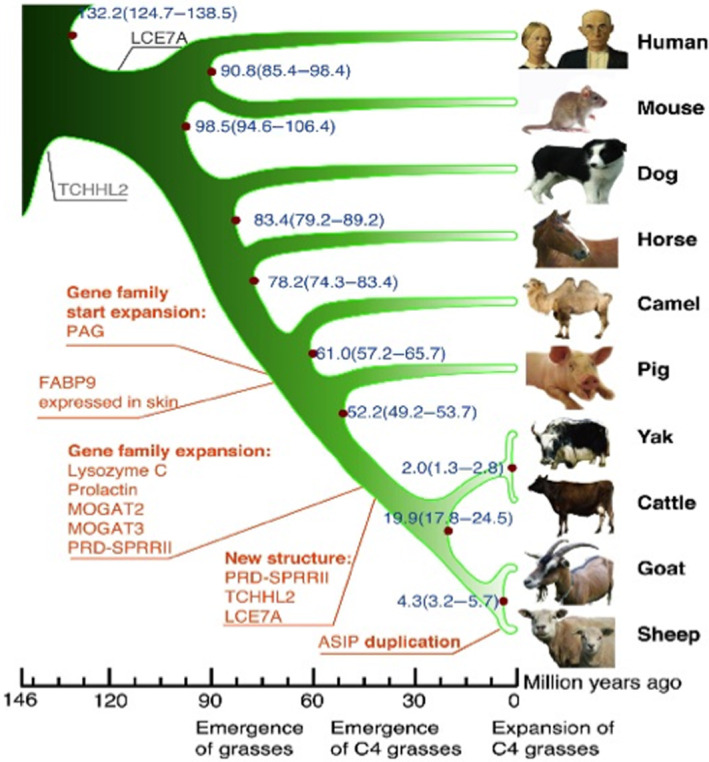
Phylogenetic tree of sheep and related animals. generated using single‐copy orthologous genes. Origins (black) and amplificationgenes (red). Scale is in millions of years ago (Mya). Blue numbers on the nodes are calculated divergence times from present (Mya) and its confidence interval. Figure reproduced from 93 under Open Access
6. NEW LARGE ANIMAL MODELS OF IVDD
Macropod, Equine and Cammelid species have been proposed for IVD studies and these represent interesting developments. 12 , 13 , 14 , 15 , 16 , 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 94 , 95 , 96 The equine IVD is anatomically quite different from other species in having a ball and cup type structure. The kangaroo tail IVD is unique in being weight‐bearing and these and other macropdods are the only animals with five appendages. 18 Although macropods assume a quadrupedal gate at very slow speeds, they are bipedal at higher speed and thus their spine is loaded vertically more akin to humans. The thoracolumbar alpaca disc has a similar cross‐sectional profile to bovine caudal IVDs (Figure 2) and is reported to undergo degenerative changes. 20 , 21 Further studies are required to determine if they contain notochordal cell populations in adulthood. It remains to be seen how cost effective these new models will be, availability issues may also be disincentives to their routine use as models of IVDD.
FIGURE 2.
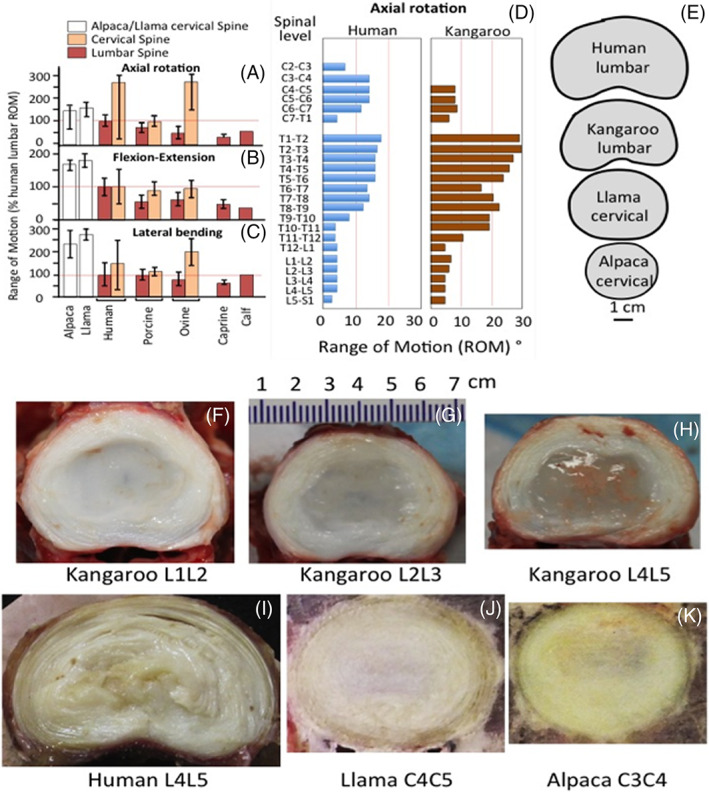
Approximate range of motion (ROM) (A‐C) of animal cervical and lumbar spine segments in axial rotation (A) flexion‐extension, (B) lateral‐bending (C) as a percentage of human lumbar ROM and axial rotation at different spinal levels for the kangaroo and humans (D). 15 , 97 , 98 , 99 , 100 , 101 References for benchmark data are: Human Lumbar, 101 , 102 Porcine Lumbar and Porcine Cervical, 98 Ovine Lumbar and Ovine Cervical, 99 Caprine Lumbar, 15 Calf Lumbar. 103 Relative sizes of human and kangaroo lumbar and cervical Llama and Alpaca IVDs (E). Macroscopic views of horizontally bisected kangaroo L1L2 (F), L2L3 (G) L4L5 (H), human L4L5 (I) and Llama C4C5 (J) and Alpaca C3C4 (K) IVDs. Images F‐H were kindly supplied by Dr Uphar Chamoli, Director of Engineering, Kunovus Pty Ltd, School of Biomedical Engineering, University of Technology, and St. George Clinical School, University of NSW, Sydney, Australia. Images J, K reproduced from 21 with permission.
7. CAMMELIDS
Cammelids (Alpacas and Llamas) have large IVDs with some similarities to human spinal anatomy, 21 size and biomechanical flexibility, 20 and natural history of spontaneously occurring IVDD. 19 , 25 However, they have a dissimilar cross‐sectional area closer to that of bovine caudal IVDs. 20 , 21 The prevalence of age‐dependant IVDD in the alpaca cervical spine 21 suggests it may be worthy of further examination. Compressive myelopathy due to IVD herniation in a llama (Lama glama) has been reported. 25 It is difficult to envisage the widespread use of the cammelids as models of disc degeneration given that they are not widely available, are expensive and have more difficult husbandry (e.g., fencing requirements). The cervical cammelid IVD is significantly more flexible than human IVDs displaying higher ROM in flexion extension and lateral bending. 21
8. EQUINE SPINAL STUDIES
The normal and degenerate equine spine has undergone radiographic and MRI analyses. 22 , 94 , 95 Biochemical and biomechanical studies have also examined cervical facet joint cartilage, 96 and IVD tissues 13 , 24 in normal IVDs and those which have undergone prolapse and diskospondylitis. 12 , 17 The morphology and mobility of normal and prolapsed equine IVDs have also been examined. 16 , 23 , 26 These thoracic and cervical IVDs have characteristic concave and convex cartilaginous endplates (Figure 3). Equine IVDs appear more like a fibrous articulating “ball and cup joint” rather than a typical IVD as seen in other animals. The equine IVD nevertheless displays degenerative tears and lesions consistent with a major weight bearing structure.
FIGURE 3.
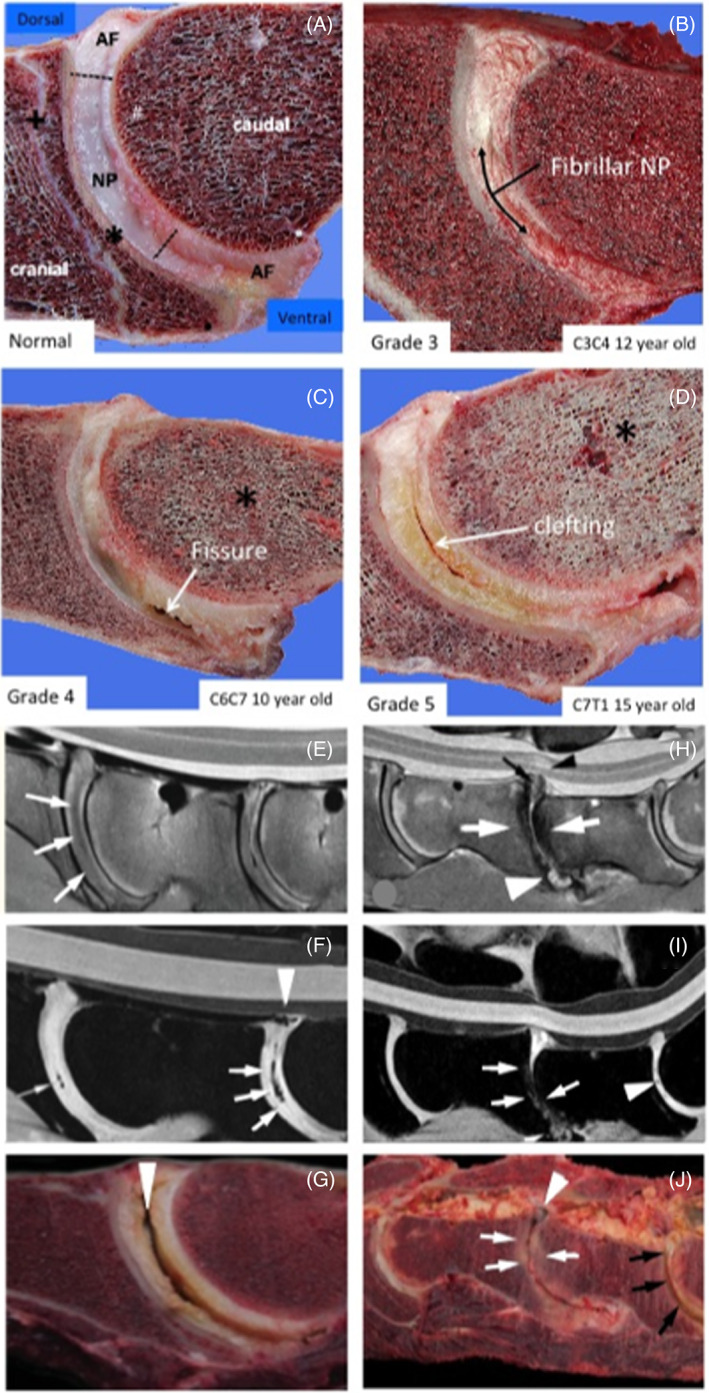
Vertical sections through equine spinal segments showing the characteristic ball and socket appearance of an equine IVD and characteristic tears and lesions that occur with IVDD (A–D). MRI images of equine spinal segments showing reduced MRI signal in the IVD with the onset of IVDD (E, H, F, I) correlating with lesions evident on gross examination of spinal segments (G, J). IVD features are annotated and degenerative clefts and fissures indicated with arrows and decreased bone density changes with an asterisk in (A–D). Images A–D reproduced from 13 under Open Access Attribution‐noncommercial 4.0 International (CC BY‐NC 4.0) license. Diffuse hypo intense regions and signal voids and diffuse hypointense areas are noted throughout the intervertebral discs with loss of definition of the nucleus (white arrows); B, sagittal water selective cartilage image; noted clefts (white arrows) and regions of the dorsal longitudinal ligament of C6‐C7 (white arrow head); and diffuse hypointense areas were noted throughout the IVDs with loss of definition of the nucleus (white arrows); Clefts and loss of NP definition are indicated with white arrows and defects in the longitudinal ligament attachments with arrow heads (E–J). A large central cleft in a C6C7 IVD is indicated by an arrowhead in (G). Severe remodeling of CEPs and discolouration of protruding dorsal IVDs (white arrows) in the C6C7 IVD (J). The C7S1 IVD displays severe cleft formation and yellow discolouration (black arrows)
9. KANGAROO
Kangaroo and human lumbar IVDs have similar flexibility profiles. 14 Similarities in thoracic spinal vertebral geometry in the vertebrae, pedicles and facet joints, makes the kangaroo a clinically relevant human surrogate for testing spinal implants. The thoracic kangaroo spine however is significantly more flexible than the human (Figure 2d). The kangaroo is a pseudo‐biped (at higher ambulatory speeds) but it also the only animal with “5 load‐bearing limbs”, the tail IVDs contribute to spinal weight bearing when walking. Forces through the pelvis to the lumbar IVDs in the kangaroo differ when the tail is stabilizing the spine through the pelvis. The juncture of the mobile lumbar IVDs with immobile pelvis is a major determinant of the metabolism of the disc cell populations in human lumbar IVDs contributing to the higher incidence of disc degeneration at the L4L5 as well as the lumbosacral intervertebral disc. The kangaroo's tail acts as a counterbalance to the body during hopping 18 but has complementary roles during walking where it is used as a muscular appendage and additional leg to support, propel and power their motion. The muscles supporting the vertebrae and tail IVDs in the kangaroo are very rich in mitochondria indicating they have important roles in the energetics of this very capable support structure. The tail is planted on the ground when the kangaroo is in a walking mode and their front and hind legs assume a distinctive gait referred to as “pentapedal” locomotion, 14 , 104 , 105 with the fifth point of contact being the tail. Kangaroo tails contain more than 20 vertebrae and adjacent IVDs and are biomechanically and physiologically capable structures. 18 The dense tail muscles are much larger than the muscles of the front limbs and their abundant mitochondria indicate they have a large aerobic capacity. 106 Studies of the structure of the tail IVDs of the kangaroo have yet to be undertaken, it would be interesting to ascertain how similar they were to bovine caudal IVDs which some researchers have shown to be useful investigative tissues. However, since kangaroo tail IVDs are weight bearing they may be even more relevant for such studies, as it is a uniquely adapted axial support structure. 18 , 107 , 108
10. THE CANINE MODEL OF IVDD
A number of studies have been conducted demonstrating the applicability of the ChD‐canine as a model of IVDD (Table 2). A number of therapeutic agents have been evaluated using these models including mesenchymal stem cells and a number of bioscaffolds and hydrogels employed using a number of tissue engineering approaches.
TABLE 2.
The canine as a model of IVDD
| Model | Features | References |
|---|---|---|
| ChD canine | A clinically relevant intermediate‐sized animal model of IVD‐associated spinal pain | 109 |
| ChD and non‐ChD canine IVD | Cervical IVD herniation in ChD and non‐ChD small breed dogs | 110 |
| Canine | IVDD in dogs: consequences, diagnosis, treatment. | 111 |
| Canine | Canine IVDD model | 28, 112 |
| Canine | Comparison of magnetic resonance imaging, and histological findings in surgically treated IVDs | 113 |
| Canine | Incidence of IVDD‐related diseases and associated mortality rates | 114 |
| Canine | Canine model of IVDD | 27 |
| Canine | Macroscopic grading of IVDD degeneration using the Thompson system and comparison with low‐field MRI | 115 |
| ChD and non‐ChD canine IVD | Evaluation of IVDD in dogs by use of Pfirrmann grading and low‐field magnetic resonance imaging | 116 |
| Canine | A review of IVDD in dogs | 117 |
| Canine | Retrospective study of IVD herniation in dogs in Japan | 118 |
| Canine thoracolumbar IVD | Canine thoracolumbar IVDD: diagnosis, prognosis, and treatment | 119 |
| ChD and non‐ChD canine IVD | A histopathological comparison of intervertebral disc degeneration in chondrodystrophic and non‐chondrodystrophic dogs | 120 |
| Therapeutic agents examined for canine disc regeneration | |
|---|---|
| Procedure | References |
| Human bone morphogenetic protein 7 transfected NP cells delay the degeneration of the canine IVD | 121 |
| Effects of osteogenic protein‐1 on intervertebral disc regeneration in animal models | 122 |
| Intradiscal application of rhBMP‐7 does not induce regeneration in a canine model of spontaneous IVDD | 123 |
| NP cells expressing hBMP7 can prevent the degeneration of allogenic IVD in a canine transplantation model | 124 |
| NTG‐101 molecular therapy halts progression of canine DDD | 125, 126 |
| Restoration of IVD integrity by intradiscal delivery of Celecoxib‐loaded microspheres | 127 |
| IVD repair using Link‐N | 128 |
| Extradural morphine use in postoperative analgesia in dogs undergoing IVD surgery | 129 |
| Use of MSCs, notochordal cells/notochordal conditioned media in regenerative procedures on the canine IVD | |
|---|---|
| Procedure | References |
| Treatment of naturally degenerated canine lumbosacral IVDs with autologous MSCs | 130, 131 |
| Use of notochordal cell‐derived matrix for biological repair of the IVD | 132 |
| Therapeutic use of bone marrow MSCs to treat canine IVDD | 130, 131 |
| Notochordal‐cell derived extracellular vesicles exert regenerative effects on canine and human NP cells | 133 |
| Treatment of canine IVDD using notochordal rich NP | 126 |
| BMP‐2, but not MSCs, exert regenerative effects on Canine and Human NP cells | 134 |
| A review of MSC and bioscaffold use in disc repair and regeneration | 135 |
| A review of the use of MSCs in the treatment of IVDD in companion animals | 136 |
| Transplantation of adipose derived MSCs for acute thoracolumbar disc disease | 137 |
| Human Wharton's jelly cell transplantation for the treatment of canine IVDD | 138 |
| Coculture of notochordal, NP and MSCs for canine IVD regeneration | 139 |
| Notochord conditioned medium stimulates ECM production by NP and MSCs | 140 |
| Regenerative treatment strategies for IVDD in dogs | 141 |
| Canine notochordal cell‐secreted factors protect murine and human NP cells from apoptosis by inhibition of activated caspase‐9 and caspase‐3/7. | 142 |
| IVD‐derived stem cells: implications for regenerative medicine and neural repair | 143 |
| Cell number effects on MSC transplantation in canine IVDD | 144 |
| MSCs arrest IVDD through chondrocytic differentiation and resident IVD cell stimulation | 145 |
| Cell transplantation for the treatment of lumbar IVDD | 146 |
| MSCs for the treatment of canine IVDD | 147 |
| Bioscaffolds evaluated for the repair of the canine IVD | ||
|---|---|---|
| Scaffold | Functional properties | References |
| Thermoreversible HA‐ poly(N‐isopropylacrylamide) (HA‐pNIPAM) hydrogels | Injectable thermoreversible HA‐hydrogel NP cell microbeads. Suitable for NP repair as an injectable carrier, maintained cell phenotype, ECM production. | 148 |
| Implantation of bullet fragments made from copper, lead, and aluminum alloys in lumbar IVDs | Impact of surgically implanted copper, lead, and aluminum alloy bullets in lumbar IVDs. Significant IVD tissue reactions noted with copper and lead. Copper produced severe IVDD aluminum alloy did not. | 149 |
| Poly(ε‐caprolactone‐co‐lactide)‐β‐poly(ethylene glycol)‐β poly(ε‐caprolactone‐co‐lactide) PCLA‐PEG‐PCLA hydrogel slow release celecoxib | Intradiscal application of a PCLA‐PEG‐PCLA celecoxib loaded hydrogel for the treatment of LBP. LBP in 9 of 10 dogs, evident by clinical examination and owner questionnaires. | 127 |
| Total disc replacement | Tissue‐engineered (TE) canine cervical IVDs from adult canine AF and NP cells seeded in collagen and alginate hydrogel IVD molds. At 4 and 16‐weeks NP cells surrounded by PG‐rich ECM, fibrocartilaginous ECM in AF. IVDs stable, stable disc height, hydration over 16 weeks and integration with host tissue. | 150 |
| Polyetheretherketone (PEEK) | Anterior canine cervical IVD fusion using bioactive polyetheretherketone. Bony fusion rate of TiO2‐coated PEEK ~40% by μCT, better fusion rates and bone‐bonding ability than uncoated PEEK implant. | 151 |
| Injection of polyester amide microspheres | Safety and biocompatibility of intradiscal injection of polyester amide (PEA) microspheres in a canine model of IVDD. Good cyto‐ and biocompatibility, safe sustained release of intradiscal delivery of biologics. | 152 |
| Injection of gelified ethanol into lumbosacral IVDs of healthy dogs | Short and long term effects of gelified ethanol in lumbosacral IVDs to treat IVD protrusions in humans via NP injection. | 153 |
| PEG and methylprednisolone sodium succinate (MPSS) in the treatment of herniated IVDs | Placebo‐controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate (MPSS) in the treatment of herniated IVDs, 47.6% dogs recovered ambulation | 154 |
| Celecoxib‐loaded poly‐N‐isopropylacrylamide hydrogel | Intradiscal application of a thermoreversible celecoxib‐loaded poly‐N‐isopropylacrylamide MgFe‐layered double hydroxide hydrogel | 155 |
| MSC loaded injectable microcryogel reinforced alginate | Microcryogel reinforced alginate encapsulation of MSCs for leak‐proof delivery alleviated canine IVDD | 156 |
| TE IVD allograft | Tissue‐engineered allograft IVD transplantation | 157 |
| NP hydrogel prosthesis: canine IVD. | Evaluation of a hydrogel NP prosthesis ex vivo | 158 |
| TE IVD composite | Evaluation of a TE IVD composite for disc regeneration | 159 |
| Alginate/chitosan hybrid scaffold | Alginate/chitosan hybrid fiber scaffold for AF cells | 160 |
11. THE OVINE MODEL OF IVDD AND REGENERATION
Of the canine, goat, ovine models the latter stands out as being particularly significant in terms of its similarity in structure to human IVDs and the spectrum of IVD degenerative pathobiological features reproduced in ovine models of experimental IVDD (Table 3). The large annular lesion mechanical destabilizing ovine model of experimental disc degeneration is an aggressive model utilizing a large 6 mm deep and 20 mm wide outer annular lesion. 161 This lesion is of such a size that it severely disrupts normal annular architecture and annular biomechanical properties destabilizing the entire disc but it does not result in prolapse of the nucleus pulposus through the annular defect. The resultant mechanical destabilization results in dramatic changes in the gene expression profiles of the resident disc cell populations. Expression of MMP‐1 and 13, and ADAMTS4 and ADAMTS5 are elevated in lesion affected IVDs. Increased expression of type I collagen and type II collagen accompany the elevated MMP expression patterns indicating that anabolic and catabolic gene expression occur hand in hand with IVDD. Initially the annular defect site is filled with granulation tissue and an influx of blood vessels and nerves into the inner margins of the AF is evident. This does not occur in control IVDs, where sparse blood vessels and nerves are confined to the outermost lamella even in aged IVDs. Blood vessels and nerves encroach further into the destabilized disc when the NP becomes depleted of space‐filling proteoglycan as the disc degenerate and display a reduced disc height. However by 6 months post‐treatment of the degenerate IVD with bone marrow derived stromal mesenchymal stem cells (BMMSCs) a replenishment in NP proteoglycans was evident and a significant recovery (95%+) of normal disc heights was achieved. 162 Blood vessels and nerves regressed from the repaired annular defect site as NP proteoglycan levels began to rise with the defect granulation tissue eventually being replaced entirely by new annular lamellae of increased size by 6 months post operation. 37 This annular repair process was replicated by administration of HA oligosaccharides to the defect site which is consistent with the depolymerisation of HA known to occur under the inflammatory conditions present during IVDD. 163 Furthermore, testing of the mechanical properties of MSC repaired IVDs showed they had re‐attained similar biomechanics to age matched ovine control discs. 162 Thus, the repair of large annular lesion affected discs was very significant and strong evidence of the potential efficacy of BMMSCs for repair of degenerate human IVDs. No other animal model of disc degeneration has used such a large defect to induce disc degeneration. The successful repair of such a massive 6 × 20 mm defect is particularly significant given that this defect is not stabilized by fixation thus internal disc micro‐movement occurring during normal body locomotion would be expected to inhibit re‐attachment of damaged surfaces to one another during the annular repair process. This positive response with BMMSCs is an outstanding achievement not replicated in any other animal model of disc degeneration. The mechanism that drives IVD repair is probably due to local release of growth factors by the administered stem cells and the direction of resident IVD cell populations to participate in tissue repair processes. There is no evidence that the administered stem cells become engrafted long‐term despite the detection of some residual viable stem cells 1 month after administration. It is difficult to envisage that the resident nutritive system in the IVD would be capable of maintaining the viability of such a large number of administered cells. A growth factor mediated repair process has also been proposed in canine IVD studies. 125 , 126 , 164 Earlier claims in rodent IVD studies using needle punctures to induce IVDD and MSCs to repair the IVD 165 , 166 do not compare with the significance of the findings produced with the ovine model (Figure 4). The ovine model has been awarded nine ISSLS 36 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 and two Grammer Prizes. 175 , 176 This ovine model reproduces many of the degenerative features which characterize human IVDD. A number of studies have been conducted with the ovine model to examine spatiotemporal changes in discal and paradiscal components such as the NP, 177 CEPs, 41 facet joints 40 and vertebral bone that occur adjacent to and distant from the lesion site. 38 When the degenerate IVD becomes depleted of aggrecan it becomes susceptible to the ingrowth of blood vessels and nerves, 37 focal expression of fibroblast growth factor (FGF)‐2, TGF‐β1 and α‐smooth muscle cell actin 178 by cell populations associated with annular remodeling and attempted repair of the lesion site. In 2003 The ISSLS Prize was awarded for a study on the quantitation of nerve ingrowth into the degenerate ovine IVD (Table 3). 36
TABLE 3.
The Ovine model of IVDD and regeneration
| Model | Features | References |
|---|---|---|
| Percutaneous nucleotomy model of IVDD | Lumbar spine degeneration following injury via percutaneous minimally invasive partial nucleotomy | 180 |
| Cervical IVDD and Regeneration Model | Cervical IVDD and regeneration model using a ventral surgical approach | 181 |
| Lumbar IVDD Models | Lumbar IVDD Models for evaluation and development of novel regenerative therapies | 182 |
| Stepwise model of IVDD | Stepwise model of IVDD with intact AF to test regenerative strategies | 183 |
| IVDD failure induced by complex posture/loading rate | Model of IVD failure induced by concordant complex posture and loading rate on motion segment failure | 184 |
| Far‐lateral disc herniation model | Far‐lateral disc herniation induced by a percutaneous posterolateral approach | 180 |
| Herniation model of IVDD | Herniation model induced by compression, flexion and facet‐constrained shear | 171, 175 |
| IVDD model used to assess regeneration | Transpedicular surgical approach for regenerative strategies for repair of IVDD | 185 |
| Ovine IVDD model | Disc disruption induced by low frequency cyclic loading | 186 |
| Drill bit injury of IVDD | IVDD model induced by a lateral retroperitoneal drill bit injury | 187 |
| Histopathological grading scheme for IVDD/regeneration | Histopathological grading of IVDD and the therapeutic utility of adult MSCs for IVD Regeneration. | 179 |
| Posterolateral disc prolapse model | Posterolateral disc prolapse in flexion initiated by lateral inner annular failure: | 188 |
| Nucleotomy model | Nucleotomy model with intact AF to test the efficacy of IVD disc regenerative strategies | 189 |
| IVD regeneration model | An assessment of the transpedicular approach as an alternative route for intervertebral disc regeneration | 190 |
| IVDD induced by a lateral lesion | Lateral surgical lumbar IVDD model | 191 |
| Large annular lesion destabilization model of IVDD | Mechanical destabilization induced by controlled annular incision of IVD dysregulates MMP and anabolic genes | 161 |
| Nucleotomy model of IVDD | Partial nucleotomy ovine model of IVDD | 192 |
| Bioscaffolds used in Regenerative strategies for the ovine IVD model | ||
|---|---|---|
| Model | Feature | References |
| Minimally invasive repair model of IVDD | Minimally invasive repair of IVDD using injectable polymethyl‐methacrylate and bovine collagen | 193 |
| TE 3D IVD Biocomposite | IVD engineering of bio‐mimetic biocomposite mimicking the AF lamellae structure | 194 |
|
Electrospun polycaprolactone |
Electrospun‐aligned microfibrous implant for Annulus fibrosus repair | 195 |
| High‐density collagen gel | In‐vivo AF repair using high‐density collagen gel | 196 |
| Cervical IVD prosthesis | Cervical IVD prosthesis based on the physiological curvature of endplate for cervical disc replacement | 197 |
| Polyglycolic acid/polyvinylidene fluoride annulus implant | Repair in AF defects using a bio‐integrative implant in an in‐vivo ovine model | 198 |
| HA hydrogel, ionically crosslinked metacrylated gellan gum hydrogels | In vivo biofunctional evaluation of hydrogels for disc regeneration | 199 |
| Hydrogel, suture, NP tissue replacement, biological glue combinations | Hydrogels for nucleus replacement with an intact; annulus incision repaired by suture and glue; annulus incision with removal and re‐implantation of nucleus tissue; and two different hydrogels repaired by suture and glue. | 200 |
| Resorbable PGA‐HA implants | Regeneration of NP tissue in an ovine IVDD model by cell‐free resorbable polymer scaffolds | 201 |
| Oxidized HA gelatin implant | An injectable NP implant restores compressive range of motion in the ovine disc | 202 |
| IVD transplant | Preparation of intact bovine tail IVDs for organ culture | 203 |
| Construct of collagen gels seeded with ovine AF cells | Self‐assembly of aligned tissue‐engineered AF and IVD composite via collagen gel contraction | 204 |
| MSCs for repair of IVDD evaluated using the ovine model | ||
|---|---|---|
| Model | Features | References |
| Large destabilizing lesion model of experimental IVDD | MSCs initiate and co‐ordinate repair of a large destabilizing IVD lesion | 162 |
| Methodological review | Strategies for IVD repair repair using MSCs and bioscaffolds | 135 |
| IVDD induction by a postero‐lateral annular IVD lesion | Use of allogeneic mesenchymal precursor cells to promote healing in a postero‐lateral annular IVD lesion | 205 |
| Microdiscectomy IVDD model | MSCs plus PPS for regeneration of the degenerate IVD | 206 |
| Lumbar IVDD model | Immunoselected STRO‐3 + MSCs for IVD repair | 207 |
| Cervical model of IVDD | Use of MSCs + PPS to preserve cervical motion segments. | 208 |
| Other therapeutic procedures evaluated for repair of the ovine IVD and spinal defects | ||
|---|---|---|
| Model | Features | References |
| In‐vitro and in‐vivo study | HA oligosaccharides stimulate MMP and anabolic gene expression in vitro and annular repair | 163 |
| Thoracic spine fusion model. | Development of an open mini‐thoracotomy surgical approach to an ovine thoracic spine fusion model. | 209 |
| Lumbar interbody spine fusion model | Augmented bone graft products demonstrated to compare favorably with autologous bone graft in an ovine model of lumbar interbody spine fusion | 210 |
| Anterior cervical discectomy and fusion models | Failure of resorbable plates and screws in an ovine model of anterior cervical discectomy and fusion. | 211 |
FIGURE 4.
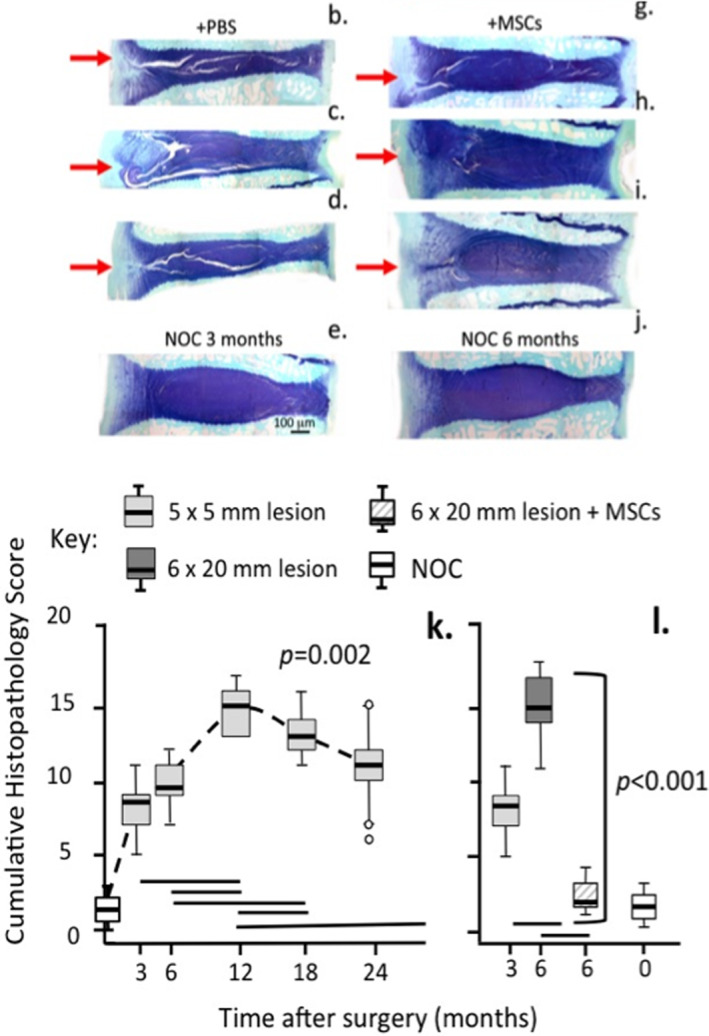
Depiction of how a large 6 × 20 mm outer anterolateral annular lesion (red arrow) destabilizes the ovine IVD and induces disc degeneration with propagation of the lesion into the IVD (b, c, d) and demonstration of the utility of bone marrow derived stromal stem cells for the regeneration of lesion affected IVDs (g, h, i). Toluidine blue‐fast green stained vertical IVD sections. Non‐operated control IVDs (e, j), freshly made lesion in a cadaver IVD (f). Disc degeneration was induced for 4 (b, c, g, h) or 12 weeks (d, i) then injected with PBS (b, c, d) carrier or MSCs (g, h, i) and recovery allowed to proceed for 8 (g, h) or 22 weeks (i). Annular lesions severely reduced the disc heights in the degenerate IVDs (b, c, d) but disc heights were recovered to ~95% of normal values in MSC treated IVDs (g, h, i) where repair of the IVD lesion occurred. In the corresponding PBS carrier treated discs (b, c, d) lesion development was extensive. Histopathological scoring of IVDs 179 in which degeneration had been induced by a 5 × 5 (k) or 6 × 20 mm lesion (l) based on IVD structure, proteoglycan content, disc height, lesion progression, cellular infiltration showed a steady increase in the cumulative degenerate histopathology index in the 5 × 5 mm lesion over 24 months (k) and development of a similar histopathology score by 6 months in the 6 × 20 mm lesion (l) however the administration of MSCs reduced this degeneracy index to levels similar to those evident in non‐operated control IVDs. Figure modified by Open Access under an Attribution 4.0 International CC BY 4.0 license from 162 , 179
12. ANIMAL MODELS DEVELOPED TO STUDY LOW BACK‐PAIN
Animal models have provided invaluable information of molecular events occurring during IVDD, and importantly have demonstrated how changes in the IVD microenvironment can lead to the development of discogenic LBP and pain in other spinal structures such as muscle, CEP, facet joint and spinal ligaments. 212 Paraspinal muscles affected by IVDD are major pain centers in the spine. 212 A rat multifidus transection model of IVDD shows the inter‐dependance of spinal muscles and the IVD. 213 This is a useful model which utilizes an intact IVD and shows how induction of spinal instability can initiate IVDD without violating IVD structure directly. The altered biomechanical loading on the degenerate IVD and mechanical destabilization alters cell‐ECM signaling resulting in the production of inflammatory cytokines and active MMPs which further promotes disc degenerative process. 212 Degenerative changes in the disc ECM conducive to neovascularization and the ingrowth of nerves and an elevation in the production of inflammatory cytokines and neurotrophic factors all lead to a significant deterioration in the normal cellular microenvironment in the IVD resulting in increased numbers of mechano‐and nociceptive pain receptors. 214 Noxious stimuli in the IVD such as an acidic pH, ECM degeneration, inflammatory mediators and neurotrophins generate inflammatory conditions in the IVD that promote this increase in noci‐ and mechanoreceptors. These environmental changes in the degenerate IVD results in membrane depolarization of peripheral nociceptive nerve endings 215 , 216 and nerve activation and axonal transduction of such signals to somatic DRGs then to the dorsal horn sensory gray matter of the spinal cord and the brain. Signal transduction through chemical synapses in neural networks carries pain signals from IVD nociceptors to the brain. 217 Activation of IVD neuronal activity by inflammatory mediators also induces protein kinase A and C, calcium/calmodulin‐dependent protein kinase, and MAPK signaling in dorsal horn neurons and the induction and maintenance of neuropathic pain. Activation of MAPKs (p38, ERK, and c‐Jun N‐terminal kinase) in spinal cord microglia or astrocytes results in the production of inflammatory mediators, sensitization of dorsal horn neurons and activation of spinal glia. Such neuron–glia interactions enhance and prolong neuropathic pain. 218
LBP is a condition recognized as the leading musculoskeletal condition of major socioeconomic impact and a major cause of years lived with disability. 219 , 220 , 221 , 222 LBP can be ellicited by painful stimuli emanating from the spinal muscles, nerves, or spinal bones and is often exacerbated by IVDD. 223 LBP can vary in intensity from a dull constant ache to a sudden sharp pain 224 and is classified by its duration time as acute (pain duration <6 weeks), sub‐chronic (pain duration 6–12 weeks), or chronic (pain duration>12 weeks). 225 , 226 , 227 LBP may be further classified by its underlying cause as mechanical, non‐mechanical, or referred pain. 228 About 40% of the worlds human population suffer from LBP some time in their lifetime 229 and this may be as high as a value frequently quoted for Western societies of 80%. 221 It is estimated that 9–12% of the global general population (632 million) have LBP at any one time. LBP symptoms are often first evident between 20 and 40 years of age 223 with men and women being equally affected and it is more common in individuals aged 40–80 years of age with the incidence of LBP increasing with advancing age. 229 , 230
Animal models continue to be developed for specific applications focussing on low LBP, mainly dominated by rodent studies however developments in the interpretation of pain responses in large animals has also emerged. 231 , 232 , 233 , 234 , 235 , 236 , 237 A number of procedures have also been developed to assess pain responses in other large animal models of appendicular osteoarthritis. 232 , 238 , 239 , 240 , 241 Significant advances in facial recognition technology with the application of AI and DML has also emerged as useful methodology in animal pain models, although it may be more suitable for acute rather than chronic painful conditions. 242 , 243 , 244 , 245 , 246 The mouse continues to be a popular animal model for such IVD studies (see supplemental information) and a multitude of genetically modified mice have been developed to ask specific questions relevant to disc pathobiology (see supplemental information). A recent review of these mouse strains and their usefulness in studies on IVDD pathobiology demonstrates the invaluable contributions they have made to our understanding of molecular pathophysiology. 79 Knowledge on the biology and potential usefulness in IVD regenerative procedures of notochordal cells gleaned from specific animal models of disc degeneration including mice has also increased significantly in the last 10 years. 132 , 247 , 248 , 249 , 250 , 251 , 252
13. APPLICATION OF AI IN THE ANALYSIS OF IVDD, LBP AND PATIENT TREATMENTS
AI is a collection of digital technologies that have found widespread application in data analysis in many aspects of healthcare. 253 Machine learning is a statistical technique that models data and has widespread application in precision medicine, predicting what treatments are likely to succeed on an individualized patient basis and can also be used in the assessment of outcomes from surgical procedures or the development of personalized rehabilitation protocols. 254 DML or neural network analysis mimic the interactivity and cooperativity of the signaling evident in neural networks in the brain and are more complicated forms of machine learning. These have predictive capability and are amenable to automation increasing the throughput of spinal data analysis. From its inception in the late 1900 s AI has found application in a diverse range of areas in healthcare. Becker 255 proposed that AI has proved useful in (i) risk assessments of disease onset and evaluation of the success of treatments, (ii) management or elimination of complications, (iii) patient oncare assessments, and (iv) research into the efficacy of treatments and the pathological evaluation of disease parameters. Merali and colleagues have used AI to predict outcomes from surgery to treat degenerative myelopathy. 256 Karhade et al. 257 developed machine learning algorithms to predict surgical outcomes of patients who received surgery to treat lumbar degenerative disc disorders. Deep learning techniques can predict the outcome of patients treated for lumbar disc herniation after a 6 month recovery period and can also be used to identify early stage patients likely to benefit from conservative therapy aiding in decision‐making for patient treatment options. 258 , 259
14. APPLICATION OF AI TO BETTER UNDERSTAND DEGENERATIVE PROCESSES IN PARKINSON'S DISEASE
AI has been applied in the assessment of Parkinson's disease (PD) in 1000 fibroblast cell lines from normal and PD affected individuals using robotic technology, automated cell culture and cell painting techniques to evaluate cellular structural changes in PD affected cells. 260 AI has significantly improved evaluation of the complex molecular events occurring in PD. 260 This is a good example of how AI may be potentially applied to aid in the resolution of a complex disease process and in the development of prospective therapeutic drugs. A similar approach could potentially be applied to better understand the complexities of IVDD and development of therapeutic drugs.
15. HOW AI AND DML HAVE IMPROVED CLINICAL SPINAL IMAGING
Clinical imaging of degenerative pathological features in disease processes yields invaluable information relevant to the diagnosis, management or development of therapeutic measures to treat such diseases. 261 AI and DML are particularly well suited to the evaluation of large data sets in clinical imaging. A deep convolutional neural network trained on a large, manually evaluated data set of 1599 patients and 7948 IVDs has been developed and outperforms human evaluations of MRI data. 262 A semantic segmentation network (BianqueNet) composed of three innovative modules achieves high‐precision segmentation of IVDD‐related regions. 263 This quantitative method calculates the signal intensity and geometric features of IVDD strongly correlated with IVDD grade. This fully automated quantitation system provides precise clinical information for clinical trials, and investigations into the mechanism of IVDD, and the increased spinal imaging capability increases the throughput of patient data. 263 A deep learning based program (Spine Explorer) has been developed for automated segmentation and quantification of the vertebrae, spinal muscles and IVDs in lumbar spine MRIs. 264 , 265 , 266 Regions of vertebrae and discs are manually segmented on T2W sagittal MRIs used to train a convolutional neural network for automated segmentation and quantitative morphometric and signal measurements of lumbar vertebrae and discs. MRIs in this dataset can be automatically measured and manually checked with Image J. 264 , 265 , 266 Integrating machine learning techniques have been developed to provide detailed and objective clinical assessments of IVDD effects on patient mobility. 267 The five‐repetition sit‐to‐stand (5R‐STS) test is an objective tool for the testing of everyday flexibility and muscular co‐ordination in spinal movements and has found application in the evaluation of the validity and reliability of the 5R‐STS test in patients with degenerative pathologies of the lumbar spine and functional impairment induced by IVDD. 268
Modic changes (MCs) are used to describe MRI signal intensity changes in vertebrae and correlated with disease pathology in degenerative spinal disorders. However, there is a growing need for novel quantitative and standardized methods to improve the interpretation of such Modic spinal changes in disease processes. A deep learning‐based approach to the analysis of Modic images has provided findings that are substantially in agreement with spinal evaluations by radiologists with the potential to improve inter‐rater reliability of such evaluations of MRI assessments. 269 AI and DML are thus revolutionizing spinal imaging, including visualization of vertebrae and IVDs in diagnostic and surgical outcome and rehabilitation assessments, biomechanics, and motion analysis. 270
DML has also improved the analysis of clinical spinal images in the last 6 years in the segmentation, pathology detection, diagnosis, and quantitative evaluation of MRIs. 271 Positron emission tomography/computed tomography (PET/CT) of the lumbar spine using 18 F‐fluoro‐deoxyglucose (FDG) and 18 F‐sodium fluoride (NaF) tracers have imaged spinal inflammation and microcalcification, in degenerate spinal structures. 272 Functional MRI and 18 F‐FDG PET imaging have helped to define pain‐relevant, physiologically active brain regions. 273 An increased uptake of 18 F‐FDG in the caudal aspect of the LBP thoracic spinal cord locates metabolic activity in the spinal cord 273 , 274 This could aid in the treatment of LBP by localizing physiologically active cord regions to guide minimally invasive delivery of analgesics or stimulators to these regions. Changes in cerebral metabolic activity and multi‐frame static brain 18 F FDG PET imaging after L2 DRG stimulation for discogenic LBP has also shown increased metabolic activity in nociceptive brain matrices and an increase in F 18 F FDG uptake following DRG stimulation. 275 Based on how DML has improved MRI of the spine its application to PET/CT imaging would also be expected to further improve on what is already a powerful imaging methodology.
16. APPLICATION OF AI IN ANIMAL MODELS OF IVDD
Mice are popular preclinical models to elucidate mechanisms of IVDD and the testing of potential therapeutics. Artificial intelligence and supervised and unsupervised machine learning algorithms, including artificial neural networks have been applied to quantitate degenerative changes identified using a new quantitative 14 category histopathological scoring scheme in murine IVDD, with high sensitivity and specificity. 276 A 27 point 6 category histopathological scoring scheme has also been developed to quantitate specific degenerative features in an ovine experimental animal model of IVDD. 179 This scheme demonstrated the efficacy of mesenchymal stem cells for the repair of AF lesions and regeneration of the IVD, 162 and would be amenable for AI based evaluation similar to that used in the murine model. Implementation of AI methodology with the ovine IVDD model would improve its sensitivity and accuracy by obviating inter‐observer variation, and increase its utility for evaluation of novel therapeutic compounds. With ongoing development of more complex multi‐tissue pathology scoring schemes, evaluation of multiple pain outcome measures, generation of genome‐wide expressian data from numerous tissues, and use of new imaging technologies, application of AI and DML methods in animal models of IVDD will become increasingly important both to interrogate and interpret research findings and to translate these to equivalent human measures.
A recent publication employing AI has been applied to computational imaging of painful and pain free human IVDs. 277 Using this approach in‐growth of blood vessels and nerves have been imaged and quantified in painful IVDs vindicating an earlier publication where it was demonstrated that an ingrowth of blood vessels and nerves occurred into degenerate IVDs when they became depleted of their space filling aggrecan proteoglycans. 37 Furthermore this AI approach was used to develop a predictive tool and pain index for the assessment of macroscopic images of normal and degenerate human IVDs. These findings were confirmed using conventional confocal histology and histopathological scoring of the degeneracy grade of the tissues. 277 An influx of inflammatory cells along fissures in the outer and inner AF associated with vessel ingrowth but only in painful IVDs was also demonstrated 277 confirming earlier observations obtained using an ovine model of experimental disc degeneration. 37 , 178 This new AI methodology has thus improved on conventional imaging of the IVD. 277
17. PROMISING NEW THERAPIES FOR IVDD AND LBP
Many compounds have shown considerable promise in countering the inflammatory conditions that occur in the degenerate IVD 278 and which stimulate IVD repair processes 279 in in‐vitro laboratory experiments and pre‐clinical studies (Table 4). These compounds warrant further evaluation in‐vivo and the aforementioned murine and ovine models would be suitable for this purpose and improved by the application of AI technology.
TABLE 4.
Therapeutic compounds that show promise in the prevention of IVDD and LBP
| Naturally occurring NP protective phytochemicals with an ability to inhibit IVDD | ||
|---|---|---|
| Gefitinib | Gefitinib, a tyrosine kinase inhibitor, suppresses EGFR and has protective properties against IVDD in the rat IVD. | 280 |
|
Atsttrin TNFα inhibitor of IVDD |
Atsttrin, an engineered protein identified from studies on progranulin, inhibits TNF‐α‐induced inflammation and suppresses IVDD. | 281 |
| SIRT1, Sirtuin family member NAD‐dependent deacetylase | SIRT1 has protective effects in a puncture‐induced IVDD model. Resveratrol, a SIRT1 activator also protects against puncture‐induced IVDD. | 282 |
|
Lycorine, alkaloid of lily family and daffodilsLycorine (Lycoris radiate) pyrrolophenanthridine |
Suppresses MMP‐3, 13, ADAMTS‐4, 5 expression by CEP cells through prevention of NFκB signaling and IL‐1β‐induced endplate degeneration. Reduces proinflammatory cytokines, suppresses expression of MMP‐3, 13, ADAMTS‐4, 5 by CEP cells via inhibition of NFκB signaling. | 283, 284 |
| Tanshinone IIA | Reduces inflammation and radiculopathic pain, inhibits IRAK‐1 and NF‐κB/p38/JNK signaling and pro‐inflammatory mediator production, MMP expression, and radiculopathic pain. | 285 |
| Icariin | Attenuates IL‐1β‐induced inflammatory response in human NP cells. | 286 |
| Wogonin | Inhibits IVDD through upregulation of Nrf2/ARE and MAPK signaling. | 287 |
| Epigallocatechin 3‐gallate | Suppresses IL‐1β‐induced inflammatory responses in IVD cells in vitro and reduces radiculopathic pain in‐vivo. | 288 |
| Quercetin | Inhibits apoptosis of NP cells, alleviates IVDD by modulating p38 MAPK‐mediated SIRT‐1 autophagy pathway and the Nrf2/NF‐κB axis. | 289, 290, 291 |
| Diosmin, citrus flavonoid | Trialed for treatment of LBP, reduces neuropathic pain in mice/rat models. | 292, 293, 294 |
| Procyanidin B3 dimer | Aleviates IVDD via interaction with the TLR4/MD‐2 complex. | 295 |
| Luteolin | Inhibits IL‐1β‐induced NP apoptosis /catabolism, ameliorates IVDD. | 296 |
| Baicalein | Inhibits IL‐1β‐induced inflammation in NP cells in‐vitro and IVDD. | 297 |
| Hesperidin | Anti‐hyperalgesic properties in a rat neuropathic pain model, has anti‐oxidant and anti‐inflammatory properties. | 298 |
| Curcumol | Alleviates inflammation in NP cells via PI3K/Akt/NF‐κB signaling. | 299 |
| Mangiferin | Alleviates NP mitochondrial ROS, suppresses NF‐κB signaling pathway. | 300 |
| Naringin, Naringenin | Protects human NP cells from TNF‐α‐induced inflammation, oxidative stress, enhanced AMPK/SIRT1 autophagy, IVD repair, reduces LBP. | 301, 302, 303 |
| Cannabidiol (CBD) | Alleviates pain via CB‐1, 2R, G protein serotonin R; TRPV‐1, PPAR, prevents anti‐oxidative stress, inflammation, senescent effects in NP. Suppresses MMP‐9, 13, induces Coll II, SOX‐9 via AMPK/GSK3β pathway. | 304, 305, 306, 307, 308, 309, 310, 311, 312, 313 |
| Celastrol pentacyclic quinone of T. wilfordii | Decreases MMP‐3, 9, 13, ADAMTS‐4, 5, COX‐2, iNOS, IL‐6 and TNF‐α in a rat IVDD model, inhibits the NF‐κB pathway in NP cells | 314 |
| Ligustilide (Ligustrazine) essential oil from Ligusticum striatum | Inhibits apoptosis, iNOS, COX‐2, TNF‐α/IL‐6 expression in NP cells, IVDD, suppresses aberrant TGFβ activation in NP cells, anti‐inflammatory, reduces IVDD and neurogenic pain. | 314, 315 |
| Ganoderic acid A, hetero‐ cyclic triterpenoid of Ganoderma lucidum fungi | Suppresses activation of TLR4/NLRP3 signaling, inhibits H2O2 induced apoptosis, inflammatory cytokines and oxidative stress, up‐regulates Col II and aggrecan production by NP cells. | 316 |
| Animal metabolites with pain relieving properties | ||
| Resolvin D1 (RvD1) | RvD1 is a pro‐resolving lipid mediator that reduces neuropathic pain. SC stimulation reduces CSF IL‐1β levels and increases RvD1 levels alleviating IL‐1β induced neuropathic pain and neuroinflammation. | 317, 318 |
| Hemorphins | Hemorphin LVV‐H7 has analgesic effects in a model of SC injury, through IL‐1 blockade by IL‐R antagonist protein and activation of opioid receptors. | 319 |
Abbreviations: Akt, serine/threonine‐specific protein kinase; AMPK, 5′ AMP‐activated protein kinase; CSF, cerebrospinal fluid; EGFR, epidermal growth factor receptor; GSK3β, Glycogen synthase kinase‐3 beta; IRAK‐1, interleukin‐1 receptor‐associated kinase‐1; JNK, c‐Jun N‐terminal kinase; NFκB, Nuclear factor kappa B; Nrf2, Nuclear factor erythroid 2‐related factor 2/ARE and MAPK, mitogen‐activated protein kinase; MD‐2, lymphocyte antigen 96; PI3K, phosphatidyl‐3 kinase; PPAR, peroxisome proliferator‐activated receptor; ROS, reactive oxygen species; SIRT‐1, NAD‐dependent deacetylase sirtuin‐1; SC, spinal cord; TLR4, Toll‐like receptor‐4; TRPV‐1, transient receptor potential cation channel subfamily V member 1.
18. ANTI‐INFLAMMATORY PHYTOCHEMICALS DISPLAYING IVD CELL PROTECTIVE IVD REGENERATIVE PROPERTIES AND THAT HAVE PAIN RESOLVING CAPABILITY APPLICABLE TO THE TREATMENT OF LBP
Flavonoids are a widely distributed family of polyphenolic dietary plant compounds that possess anti‐oxidant and anti‐inflammatory properties through their abilities to inhibit LOX, COX, NFκB, and iNOS activity [reviewed in 320 ]. They also induce nuclear factor‐erythroid factor 2‐related factor 2 (Nrf2) gene expression in cells. Nrf2 is a master transcription factor that regulates the expression of over 1000 genes in the cell under normal and stressed conditions. Nrf2 induces expression of an array of antioxidant response element‐dependent genes that regulate physiological and pathophysiological outcomes of oxidant exposure providing cell and tissue protective properties during damaging oxidizing cellular environments. Flavonoids may well turn out to be useful neutraceutical supplements for IVD protection but further research is required to properly assess this possibility. A selection of flavonoids and related phytochemicals with anti‐oxidant and anti‐inflammatory properties and in some cases an ability to promote IVD regeneration and alleviate pain are presented in Table 4. The recent identification of the gut microbiome‐IVD axis may be a potential delivery system for potent anti‐oxidant and anti‐inflammatory gut generated phytochemical metabolites with cell and tissue protective properties 321 , 322 , 323 and potential roles in the prevention of chronic pain. 324 , 325 , 326 , 327 Further studies are warranted to examine this possibility.
Flavonoids have anti‐oxidant and anti‐inflammatory properties that modulate the production and action of inflammatory cytokines and provide benefit in the treatment of OA and RA. 328 , 329 , 330 , 331 Several flavonoids have also shown promise in the treatment of IVDD 287 , 291 , 297 , 301 and in pain alleviation. 74 , 332 , 333 , 334 , 335 , 336 , 337 , 338 Wogonin mitigates IVDD through the Nrf2/ARE and MAPK signaling pathways. 287 Quercetin alleviates IVDD by modulating p38 MAPK‐mediated autophagy. 291 Baicalein inhibits the IL‐1β‐induced inflammatory response in NP cells and attenuates disc degeneration in vivo. 297 Lycorine is a major alkaloid pyrrolophenanthridine component of the traditional medicinal Chinese herb amaryllidaceae family Lycoris radiate. Lycorine displays strong anti‐leukemia, anti‐tumor, anti‐angiogenic, anti‐viral, anti‐bacterial, anti‐inflammatory, and antimalarial pharmacological properties. 321 Lycorine suppresses the expression of MMP‐3, 13, ADAMTS‐4, 5 by cells of the CEP via the inhibition of NFκB signaling preventing IL‐1β‐induced CEP and NP degeneration in‐vitro. 283 Lycorine reduces proinflammatory cytokine production and protects cartilage from degradation by MMPs in a mouse model of OA. 339 , 340 Diosmin is a citrus flavonoid (diosmetin 7‐O‐rutinoside) non‐prescription dietary supplement that has been trialed for the treatment of LBP. 294 Diosmin is reported to reduce chronic constriction injury‐induced neuropathic pain in mice 292 and provides central and peripheral anti‐hyperalgesic effects in a neuropathic pain model in rats. 293 The related flavonoid hesperidin has anti‐hyperalgesic properties in a neuropathic pain model in rats. 298 Further studies on flavonoids are warranted to determine their optimal mode of administration, mechanism of action, specific molecular targets and how efficacious drug levels in specific tissues can best be obtained. Tanshinone IIA represses inflammatory responses and reduces radiculopathic pain by inhibiting IRAK‐1 and NF‐κB/p38/JNK signaling. 285 Tanshinone IIA displays potent anti‐inflammatory and anti‐catabolic activities, and is an abundant component of the root of the Chinese sage (Salvia miltiorrhiza) highly prized in traditional medicine. Tanshinone IIA inhibits the expression of pro‐inflammatory mediators and MMPs in‐vitro, and radiculopathic pain in‐vivo, and displays potential in the treatment of inflammation and the generation of LBP In IVDD. Quercetin inhibits senescence associated secreted phenotypic factor expression in NP cells and ameliorates IVDD via the Nrf2/NF‐κB axis, 289 suppresses NP cell apoptosis and attenuates IVDD via the SIRT1‐Autophagy Pathway. 290 Procyanidin B3 (Pro‐B3), a catechin dimer biflavonoid is a common component of the human diet which has anti‐inflammatory properties, inhibiting LPS‐mediated ECM degradation and the activation of the NF‐κB/TLR‐4 pathway in NP cells. 295 Naringin and naringenin are flavonoids that regulate cytokine, MMP, ECM protein gene expression and genes that promote apoptosis of NP cells. Molecular docking studies have demonstrated binding of naringin and naringenin to genes identified as potent inhibitors of inflammation. Collectively these traits show the beneficial properties of naringin and naringenin in ECM and NP cell protection in IVDD and LBP. 302 , 303
Mitochondrial dysfunction promotes IVDD by affecting oxidative stress, mitophagy, mitochondrial homeostasis, cellular senescence, cell death and metabolic dysfunction. 341 Flavonoids modulate antioxidant cellular responses, apoptosis, mitochondrial biogenesis, autophagy, and have roles in mitochondrial ion channels that can be cytoprotective. 342 , 343 Flavonoids have beneficial effects on mitochondrial homeostasis through their inherent anti‐oxidant properties. 342 The cell protective properties of flavonoids is partly due to their ability to counter mitochondrial mysfunction. 320 , 344 Urolithin A is a benzo‐coumarin metabolite produced by the gut microbiome by digestion of ellagic acid and ellagitannins found in dietary pomegranates, strawberries, raspberries and wallnuts. Urolithin A does not occur free in dietary foods and is not produced by mammalian enzyme systems. 345 , 346 Urolithin A is a natural pro‐biotic that promotes mitophagy, mitochondrial biogenesis and metabolic function impacting on muscle health in preclinical models of aging and in the elderly and middle‐aged. Urolithin A improves mitochondrial function in the articular chondrocytes of diarthrodial joints, reduces disease progression in a mouse OA model and inhibits cartilage degeneration, synovial inflammation, and the pain associated with this condition. 347 Urolithin A thus has properties that would also be beneficial in the treatment of IVDD and LBP. The gut microbiome‐IVD axis proposed by Li et al. 348 and Rajasekaran et al. 349 may serve as an intrinsic delivery system that could be utilized to deliver efficacious gut metabolites such as urolithin A to the IVD in a similar manner to how bioactive gut metabolites are transported by the gut brain axis. 350 , 351 , 352 This also gives some credibility to the consumption of diets enriched in probiotic compounds as a potential therapeutic option to promote tissue repair. This proposal warrants further investigation.
Cannabidiol (CBD) displays potential in the management of neuropathic LBP. CBD has antidepressant, anxiolytic, anticonvulsant, antipsychotic and pain alleviating properties. CBD acts through the cannabinoid CB1 and CB‐2 receptors, three G protein coupled‐receptors (adenosine receptor subtype 2A, serotonin receptor subtype 1A and G protein‐coupled receptor 55), one ligand‐gated ion channel (transient receptor potential vanilloid channel‐1, TRPV‐1) and one nuclear factor (peroxisome proliferator‐activated receptor γ, PPAR). CBD has been used in the management of epilepsy, 353 , 354 Alzheimer's disease, 355 pain alleviation in MS, 307 neurological responses in Parkinson's disease, 308 , 313 chronic pain 305 , 306 , 309 , 312 including LBP. 311 CBD has been evaluated in a double blind randomized clinical trial for the relief of central neuropathic pain in MS. 307
19. MAMMALIAN AGENTS WITH PAIN ALLEVIATING PROPERTIES
Progranulin has roles in the regeneration of peripheral nerves following trauma 356 , 357 its overexpression in sensory neurons attenuates neuropathic pain in mice 356 through effects on autophagy. Increased autophagic activity in dorsal root ganglia also attenuates neuropathic pain following peripheral nerve injury. 358 Animal models of inflammatory and neuropathic pain show inflammation regulates pain resolution by producing pro‐resolving mediators such as resolvin D1 (RvD1). 318 Resolvins are derived from, eicosapentaenonic, docosahexanoic, docosapentaenoic, and clupanodonic omega‐3 fatty acids 317 , 359 , 360 and have cell regulatory properties similar to prostaglandins promoting the restoration of normal cellular functional properties following resolution of inflammatory conditions that occur with tissue injury. Resolvins are induced in the CNS following trauma and offer exciting possibilities in the development of potential methods for the alleviation of intractable neuropathic pain in chronically affected patients. 317 , 359 , 360 The inter‐relationship between inflammation and neuropathic pain needs to be resolved to provide pain relief in musculoskeletal disorders. 361 Resolvins are lipid mediators that are released during the resolution phase of acute inflammation. The resolvins are active at pico to nanogram levels and have the ability to regulate proinflammatory processes actively promoting monocyte and macrophage uptake of cellular and ECM debris, apoptotic PMNs, and the killing and clearing of invading microbes. 362 The resolvins also reduce the activation of CD4 and CD8 cell activation and prevent Th1 and Th17 cell differentiation 363 and have potent anti‐inflammatory and pain resolving properties. 364 Aspirin can initiate resolvin production as part of its pain resolving properties. 365 The antiinflammatory and pro‐resolution properties of RvD1 offers novel therapeutic possibilities in the management of neuropathic pain. 366 Resolvins alleviate neuropathic pain by regulating inflammatory mediators involved in the NF‐κB/p65 and p‐ERK cell signaling pathways and inhibit TRPA1, TRPV3 and TRPV4 activity correlating with changes in acute pain behavior in animal models consistent with pain relief. 367 Resolvin D1 and fish oil n‐3 polyunsaturated fatty acids stimulate neurite outgrowth from primary DRG neuron cultures in normal mice. Dietary supplementation with fish oil or daily RvD1 in a mouse model of type 2 diabetes significantly improved diabetic neuropathy. 368 Electrostimulation of the spinal cord induces RvD1 production, lowers production of inflammatory mediators and contributes to pain relief. 318
In the last three decades, a number of studies on bioactive peptides that are opioid receptor ligands, have been undertaken. 369 Hemorphins are endogenous 4–10 amino acid peptides released during proteolysis of the beta subunit of hemoglobin. The hemorphins exhibit diverse therapeutic effects in both humans and animal models including regulation of blood pressure, mood regulation, enhancement in memory, and cognitive learning and analgesic effects. 370 , 371 , 372 Such effects occur through the ability of these peptides to modulate a diverse range of proteins including enzymes and G‐protein coupled opioid receptors. 371 The resolvins and hemomorphins offer considerable promise as potential agents that can be developed for the alleviation of chronic neuropathic and nociceptive LBP in the future.
20. NOTOCHORDAL CELL‐BASED THERAPEUTICS FOR THE TREATMENT OF IVDD
20.1. The notochord and IVD development
Vertebrates are classified within the phylum Chordata, and are named because during later embryonic development, the notochord is replaced by the vertebral column with, depending on species, remnants of the notochord persisting within the IVD nucleus pulposus (NP) for variable periods. 373 Lineage tracing studies have demonstrated that all cells within the NP originate from the notochord. 374 With maturity in many mammals including humans, the cells within the IVD NP become chondrocyte‐like and lose their notochordal phenotype, presumably due to differentiation. 375 , 376 During development a multitude of signals from the notochord influence the fate of undifferentiated mesenchymal tissues (paraxial mesoderm), particularly the spinal cord that is formed as a consequence of condensation of neural crest cells that reside dorsal to the notochord that form the neural tube and ultimately the spinal cord. 377 , 378 Central to dorsal embryonic cellular and tissue fate, sonic hedgehog (SHH) concentration‐dependent differentiation of dorsal structures figures prominently however there are other mitogens and morphogens at play such as BMP2 and BMP4. 379 In support of these vital developmental decisions on the part of undifferentiated cells/tissue, grafting of ectopic notochordal tissues dorsal to the neural tube leads to inhibition of normal dorsal structure tissue formation. 379 Nonetheless and importantly with respect to the IVD, the terminal stage of vertebrogenesis in higher animals is marked by segmentation of the notochord where notochordal remnants persist within the center of the NP. 380
The brachyury gene “T” codes for the T‐box transcription factor that along with SHH, Noggin and Paired box (Pax)1 is involved with sclerotomal differentiation and is necessary for notochord maintenance. 381 , 382 SRY‐related HMG‐box (Sox) transcription factors, notably Sox5, 6 and 9 significantly influence the notochord post sclerotome formation with their expression similar to that occuring with chondrogenesis. 383 Amongst the host of differentiation‐inducing factors, Sox5 and 6 are considered to be crucial to the survival of notochordal cells within the NP such that homozygous Sox5−/− and 6−/− mice develop abnormal, notochordal cell‐poor NPs with deformed vertebral columns. 383 The precise molecular mechanisms involved with the ultimate destination of notochordal cells within the NP remain unknown, however much has been determined with respect to the function of these cells.
20.2. Importance of notochordal cells to IVD health
Interestingly, pigs, rabbits and non‐ChD dogs retain their notochordal cell‐rich NP throughout life and concomitantly do not normally develop IVDD. On the other hand, ChD dogs (beagles, Shih Tzus, Welsh Corgis, French Bulldogs and others) like humans, lose their notochordal cells in early life and are known to suffer early and significant IVDD. A recent paper has reported that ChD dogs have a CDDY mutation involving a second fibroblast growth factor (FGF)‐4 retrogene insertion in chromosome 12 that results in their short leg, longer torso phenotype. 384 The different ChD and non‐ChD canine subspecies provides an opportunity to determine the relative contribution of the notochordal cell‐rich NP to protection from IVDD as compared with one that loses most if not all notochordal cells. Since the original paper by Aguiar et al. in 1999, a number of publications have emerged suggesting that notochordal‐cell secreted factors may be at least partially responsible for the protection from IVDD in non‐ChD canines. 125 , 126 , 248 , 385 , 386 , 387 , 388 , 389 Recently the secretome of the non‐ChD canine notochordal cell‐rich NP was analyzed. 126 This study identified Connective Tissue Growth Factor‐(CTGF) and Transforming Growth Factor Beta‐1 (TGF‐β1) as key molecules capable of reducing the progression of IVDD in a small animal needle‐puncture induced model of IVDD. Most recently a single injection of these same molecules (suspended within an excipient solution), was shown to modify the progression of IVDD in small, and large animal models in vivo, and induce a regenerative effect in human IVD NP cells in vitro. 132
It is interesting to note that it has been recently reported that injection of notochordal cell‐derived matrix (obtained from porcine IVD NPs rich in notochordal cells) into moderately degenerative ChD canines had a protective effect. In this report although the details of the injected notochordal cell‐derived matrix are lacking, the in vitro work utilized NP tissue that was lyophilized overnight, pulverized and then suspended within growth media and supplemented with antibiotics, L‐proline, ITS, ascorbic acid and bovine serum albumin. 390 The essential element from this report is that capturing notochordal cell‐secreted products contributes to NP homeostatic regulation. Interestingly, the notion of understanding notochordal cell biology with respect to potential influence upon chondrocyte‐like cells within the NP to improve extra‐cellular matrix (ECM) integrity was postulated two decades ago by Oegema. 390 Other animals are known to retain notochordal cells within their NPs such as rats, rabbits, cats and pigs and these animals also do not suffer from early IVDD, an observation that furthers the association of the notochordal cell‐rich NP and resistance to degeneration. 1 , 140 , 391
Notochordal cells reside within the IVD NP as tightly packed physaliferous cells within a well hydrated, mucoid ECM rich in proteoglycans. 29 , 125 , 126 , 132 , 385 , 386 , 387 , 388 , 389 , 392 On the other hand, the cells within the ChD canine IVD exist as clusters of chondrocyte‐like cells within a more fibrocartilaginous ECM. Non‐ChD and ChD canines produce and assemble proteoglycans differently. 392 Notochordal‐cell‐secreted glycoproteins in non‐ChD IVDs rapidly migrate to the inter‐cellular compartment prior to forming large sized aggregates. On the other hand, PGs synthesized by chondrocyte‐like cells in the ChD IVD are synthesized at a lower rate and form large aggregates within the pericellular region and only slowly moving to the inter‐cellular space over many days thereafter. One important conclusion from this work of Cappello et al. is that the cellular phenotype of the IVD NP has significant impact upon tissue composition and integrity. 392
20.3. Clinical translation of notochordal based therapeutics
The burden of IVDD and associated spinal pain is significant with chronic back pain causing the highest years lost to disability globally of any condition. 221 While IVDD is present in asymptomatic patients, large population studies involving patients suffering from back pain demonstrate significant positive correlation between their back pain and severity of IVDD on MRI. 393 A recent report indicated a single injection of rhTGF‐β1 plus rhCTGF/CCN‐2 within an excipient conditioned medium offered good pre‐clinical evidence that this combination of molecules (identified from the notochordal‐rich IVD NP secretome) may be effective in modifying the course of IVDD. 125 , 126 Appropriate toxicological and phase 1 and 2 studies must be performed to further evaluate the efficacy and safety of this intervention in humans.
20.4. Translational challenges
In order to treat the degenerative human IVD, the most likely route of administration of any biologic would be a trans‐annular injection directly into the NP. Central to the notion of intradiscal injection is an accurate diagnosis of the symptomatic disc since most cases of IVDD have adjacent sites showing degenerative change that may or may not also be symptomatic. An emerging technology that may mitigate potential risks associated with discography and needle puncture concerns the use of magnetic resonance spectroscopy (MRS). This technology is reported to have an accuracy of 85%, sensitivity of 82%, and specificity of 88% with respect to identifying the painful disc as compared with provocative discography. 394 Although still investigative, MRS may prove to be a preferred method (along with other clinical, laboratory and imaging methods) to noninvasively diagnose the symptomatic disc and aid in therapeutic decision making. Biologic therapy for the painful IVD would revolutionize the treatment of this disabling and expensive condition.
21. CONCLUSIONS
While there is no question that animal models have provided insightful information on the pathobiology of IVDD and the identification of therapeutic molecular targets applicable to the human condition, it is clear that this is a very complex and multifactorial disease process. It is also clear that animal models in isolation are incapable of providing an answer to the many complex features of human IVDD as a structural disease and an illness with variable pain types and states, and functional disability. AI can deal with complex large data sets, optimal results are obtained from big data where outcome measures can be clearly set. AI may represent the next step forward in the development of an IVDD algorithm which makes sense of IVDD, a very complex disease. AI and DML have already improved the analysis of clinical spinal imaging used to diagnose and assess the development of IVDD. AI may lead to the development of effective therapeutic measures for IVDD and a means of evaluating the success or failure of any prospective treatment procedure for a major healthcare problem in modern day society. AI has also been used in the development of screening procedures for the many potential therapeutic compounds listed in Table 4 and in the identification of their molecular targets. AI is also being used in the development of novel therapeutic drugs of high efficacy. The identification of the utility of murine and ovine IVD models for therapeutic drug evaluations, may thus find application using AI and DML in LBP and IVDD research. There is considerable promise that AI methodology could find application in unraveling the considerable complexities of IVDD and how best to treat this debilitating condition.
While currently there is a disconnect to some extent between AI and the novel therapies covered in this review the sheer depth of these studies outlining the beneficial tissue and cell protective properties of the many listed compounds and the illustration of how they might potentially be applied to IVD regeneration is highly suggestive of the likelihood that they may find future application. For this to become a reality more reliable evaluative methods are required for the assessment of IVD tissues and aspects of their regeneration in animal models. We consider that the AI methodology we have described in this review will provide such an improvement that may well allow the therapeutic potential of these novel compounds to be realized in future studies.
AUTHOR CONTRIBUTIONS
This study was conceptualized by Mauro Alini and James Melrose with intellectual input from Chirstopher B. Little, Ashish D. Diwan, and W. Mark Erwin. All authors contributed to manuscript writing and review and all approved the final version of the manuscript.
FUNDING INFORMATION
James Melrose has received consultancy fees from Arthropharm and Sylvan Pharmaceutical companies. ADD's institutions receive unrestricted research grants from Nuvasive Australia & Baxter Inc., Education support from Globus Medical (PA). ADD receives payments from Cartago Biotech, educational consultant payments from 3M & Nuvasive, research service payments from Kunovus Technologies & Merunova. These companies had no impact or say in the design or content of this study.
CONFLICT OF INTEREST
The authors declare no conflict interest.
Supporting information
APPENDIX S1: Supporting Information.
ACKNOWLEDGMENTS
This study was funded by National Health and Medical Research Council project grants 910508, 1004032 and 10010163.
Alini, M. , Diwan, A. D. , Erwin, W. M. , Little, C. B. , & Melrose, J. (2023). An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine, 6(1), e1230. 10.1002/jsp2.1230
Funding information National Health and Medical Research Council, Grant/Award Numbers: 10010163, 1004032, 910508
REFERENCES
- 1. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2‐19. doi: 10.1007/s00586-007-0414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bobyn JD, Little DG, Gray R, Schindeler A. Animal models of scoliosis. J Orthop Res. 2015;33:458‐467. doi: 10.1002/jor.22797 [DOI] [PubMed] [Google Scholar]
- 3. Demirkiran G, Dede O, Yalcin N, Akel I, Marcucio R, Acaroglu E. Selective estrogen receptor modulation prevents scoliotic curve progression: radiologic and histomorphometric study on a bipedal C57Bl6 mice model. Eur Spine J. 2014;23:455‐462. doi: 10.1007/s00586-013-3072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu L, Zhu Y, Han X, Wu Y. The creation of scoliosis by scapula‐to‐contralateral ilium tethering procedure in bipedal rats: a kyphoscoliosis model. Spine (Phila Pa 1976). 2011;36:1340‐1349. doi: 10.1097/BRS.0b013e3181f3d164 [DOI] [PubMed] [Google Scholar]
- 5. Wu T, Sun X, Zhu Z, et al. Role of enhanced central leptin activity in a scoliosis model created in bipedal amputated mice. Spine (Phila Pa 1976). 2015;40:E1041‐E1045. doi: 10.1097/brs.0000000000001060 [DOI] [PubMed] [Google Scholar]
- 6. Xie N, Li M, Wu T, Liu J, Wang B, Tang F. Does elevated osteopontin level play an important role in the development of scoliosis in bipedal mice? Spine J. 2015;15:1660‐1664. doi: 10.1016/j.spinee.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 7. Yang S, Zheng C, Jiang J, et al. The value of applying a melatonin antagonist (Luzindole) in improving the success rate of the bipedal rat scoliosis model. BMC Musculoskelet Disord. 2017;18:137. doi: 10.1186/s12891-017-1500-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ao X, Wang L, Shao Y, et al. Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin Orthop Relat Res. 2019;477:1492‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo J, Liu Z, Wu T, et al. The effect of sympathectomy on the development and progression of scoliosis in bipedal mice model. Zhonghua Wai Ke Za Zhi. 2013;51:1030‐1033. [PubMed] [Google Scholar]
- 10. Liang X, Shen H, Shi WD, et al. Effect of axial vertical vibration on degeneration of lumbar intervertebral discs in modified bipedal rats: An in‐vivo study. Asian Pac J Trop Med. 2017;10:714‐717. [DOI] [PubMed] [Google Scholar]
- 11. Pelletier M. CORR insights®: development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin Orthop Relat Res. 2019;477:1505‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams SB, Steckel R, Blevins W. Diskospondylitis in five horses. J Am Vet Med Assoc. 1985;186:270‐272. [PubMed] [Google Scholar]
- 13. Veraa S, Bergmann W, Wijnberg ID, Back W, Vernooij H, Nielen M, van den Belt AM. Equine cervical intervertebral disc degeneration is associated with location and MRI features. Vet Radiol Ultrasound. 2019;60:696‐706. doi: 10.1111/vru.12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chamoli U, Umali J, Kleuskens MWA, Chepurin D, Diwan AD. Morphological characteristics of the kangaroo lumbar intervertebral discs and comparison with other animal models used in spine research. Eur Spine J. 2019;29:652‐662. doi: 10.1007/s00586-019-06044-8 [DOI] [PubMed] [Google Scholar]
- 15. Detiger SE, Hoogendoorn RJW, van der Veen AJ, et al. Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J Orthop Res. 2013;31:703‐709. doi: 10.1002/jor.22296 [DOI] [PubMed] [Google Scholar]
- 16. Foss RR, Genetzky RM, Riedesel EA, Graham C. Cervical intervertebral disc protrusion in two horses. Can Vet J. 1983;24:188‐191. [PMC free article] [PubMed] [Google Scholar]
- 17. Furr MO, Anver M, Wise M. Intervertebral disk prolapse and diskospondylitis in a horse. J Am Vet Med Assoc. 1991;198:2095‐2096. [PubMed] [Google Scholar]
- 18. O'Connor SM, Dawson TJ, Kram R, Donelan JM. The kangaroo's tail propels and powers pentapedal locomotion. Biol Lett. 2014;10:20140381. doi: 10.1098/rsbl.2014.0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sickinger M, Hirz M, Schmidt MJ, Reinacher M. Dysuria due to discospondylitis and intervertebral disc herniation in a male alpaca (Vicugna pacos). Acta Vet Scand. 2016;58:33. doi: 10.1186/s13028-016-0216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stolworthy DK, Bowden AE, Roeder BL, et al. MRI evaluation of spontaneous intervertebral disc degeneration in the alpaca cervical spine. J Orthop Res. 2015;33:1776‐1783. doi: 10.1002/jor.22968 [DOI] [PubMed] [Google Scholar]
- 21. Stolworthy DK, Fullwood RA, Merrell TM, Bridgewater LC, Bowden AE. Biomechanical analysis of the camelid cervical intervertebral disc. J Orthop Translat. 2015;3:34‐43. doi: 10.1016/j.jot.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sweers L, Carstens A. Imaging features of discospondylitis in two horses. Vet Radiol Ultrasound. 2006;47:159‐164. doi: 10.1111/j.1740-8261.2006.00123.x [DOI] [PubMed] [Google Scholar]
- 23. Townsend HG, Leach DH. Relationship between intervertebral joint morphology and mobility in the equine thoracolumbar spine. Equine Vet J. 1984;16:461‐465. doi: 10.1111/j.2042-3306.1984.tb01981.x [DOI] [PubMed] [Google Scholar]
- 24. Townsend HG, Leach DH, Doige CE, Kirkaldy‐Willis WH. Relationship between spinal biomechanics and pathological changes in the equine thoracolumbar spine. Equine Vet J. 1986;18:107‐112. doi: 10.1111/j.2042-3306.1986.tb03559.x [DOI] [PubMed] [Google Scholar]
- 25. Valentine BA, Saulez MN, Cebra CK, Fischer KA. Compressive myelopathy due to intervertebral disk extrusion in a llama (Lama glama). J Vet Diagn Invest. 2006;18:126‐129. doi: 10.1177/104063870601800122 [DOI] [PubMed] [Google Scholar]
- 26. Yovich JV, Powers BE, Stashak TS. Morphologic features of the cervical intervertebral disks and adjacent vertebral bodies of horses. Am J Vet Res. 1985;46:2372‐2377. [PubMed] [Google Scholar]
- 27. Bergknut N, Rutges JPHJ, Kranenburg HJC, et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976). 2012;37:351‐358. doi: 10.1097/BRS.0b013e31821e5665 [DOI] [PubMed] [Google Scholar]
- 28. Bergknut N, Smolders LA, Grinwis GCM, et al. Intervertebral disc degeneration in the dog. Part 1: anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration. Vet J. 2013;195:282‐291. doi: 10.1016/j.tvjl.2012.10.024 [DOI] [PubMed] [Google Scholar]
- 29. Hunter CJ, Matyas JR, Duncan NA. The three‐dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279‐291. doi: 10.1046/j.1469-7580.2003.00162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostrander E, Wayne RK. The canine genome. Genome Res. 2005;15:1706‐1716. [DOI] [PubMed] [Google Scholar]
- 31. Wayne R, Ostrander EA. Origin, genetic diversity, and genome structure of the domestic dog. Bioessays. 1999;21:247‐257. [DOI] [PubMed] [Google Scholar]
- 32. Cruz F, Vilà C, Webster MT. The legacy of domestication: accumulation of deleterious mutations in the dog genome. Mol Biol Evol. 2008;25:2331‐2336. [DOI] [PubMed] [Google Scholar]
- 33. Plassais J, Kim J, Davis BW, et al. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat Commun. 2019;10:1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang C, Wallerman O, Arendt ML, et al. A novel canine reference genome resolves genomic architecture and uncovers transcript complexity. Commun Biol. 2021;4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostrander E, Wayne RK, Freedman AH, Davis BW. Demographic history, selection and functional diversity of the canine genome. Nat Rev Genet. 2017;18:705‐720. [DOI] [PubMed] [Google Scholar]
- 36. Fagan A, Moore R, Vernon Roberts B, Blumbergs P, Fraser R. ISSLS prize winner: the innervation of the intervertebral disc: a quantitative analysis. Spine (Phila Pa 1976). 2003;28:2570‐2576. [DOI] [PubMed] [Google Scholar]
- 37. Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976). 2002;27:1278‐1285. [DOI] [PubMed] [Google Scholar]
- 38. Moore R, Vernon‐Roberts B, Osti OL, Fraser RD. Remodeling of vertebral bone after outer anular injury in sheep. Spine (Phila Pa 1976). 1996;21:936‐940. [DOI] [PubMed] [Google Scholar]
- 39. Gries N, Berlemann U, Moore RJ, Vernon‐Roberts B. Early histologic changes in lower lumbar discs and facet joints and their correlation. Eur Spine J. 2000;9:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moore R, Crotti TN, Osti OL, Fraser RD, Vernon‐Roberts B. Osteoarthrosis of the facet joints resulting from anular rim lesions in sheep lumbar discs. Spine (Phila Pa 1976). 1999;24:519‐525. [DOI] [PubMed] [Google Scholar]
- 41. Moore R, Osti OL, Vernon‐Roberts B, Fraser RD. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine (Phila Pa 1976). 1992;17:874‐878. [DOI] [PubMed] [Google Scholar]
- 42. Fazzalari N, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine (Phila Pa 1976). 2001;26:2575‐2581. [DOI] [PubMed] [Google Scholar]
- 43. Latham J, Pearcy MJ, Costi JJ, Moore R, Fraser RD, Vernon‐Roberts B. Mechanical consequences of annular tears and subsequent intervertebral disc degeneration. Clin Biomech (Bristol, Avon). 1994;9:211‐219. [DOI] [PubMed] [Google Scholar]
- 44. Paatsama S, Rissanen P, Rokkanen P. Effect of estradiol, testosterone, cortisone acetate, somatotropin, thyrotropin and parathyroid hormone on the lumbar intervertebral disc in growing dogs. J Small Anim Pract. 1969;10:351‐354. [DOI] [PubMed] [Google Scholar]
- 45. Ao P, Huang W, Li J, et al. 17β‐estradiol protects nucleus pulposus cells from serum deprivation‐induced apoptosis and regulates expression of MMP‐3 and MMP‐13 through promotion of autophagy. Biochem Biophys Res Commun. 2018;503:791‐797. [DOI] [PubMed] [Google Scholar]
- 46. Bertolo A, Baur M, Aebli N, Ferguson SJ, Stoyanov J. Physiological testosterone levels enhance chondrogenic extracellular matrix synthesis by male intervertebral disc cells in vitro, but not by mesenchymal stem cells. Spine J. 2014;14:455‐468. [DOI] [PubMed] [Google Scholar]
- 47. Gao X, Su XT, Lu ZH, Ou J. 17β‐estradiol prevents extracellular matrix degradation by downregulating MMP3 expression via PI3K/Akt/FOXO3 pathway. Spine (Phila Pa 1976). 2020;45:292‐299. [DOI] [PubMed] [Google Scholar]
- 48. Guo H, Yang SD, Zhang F, et al. 17β estradiol protects against interleukin 1β induced apoptosis in rat nucleus pulposus cells via the mTOR/caspase 3 pathway. Mol Med Rep. 2019;20:1523‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeong H, Bae EK, Kim H, et al. Estrogen attenuates the spondyloarthritis manifestations of the SKG arthritis model. Arthritis Res Ther. 2017;19:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin L, Lv ZD, Wang K, et al. Estradiol alleviates intervertebral disc degeneration through modulating the antioxidant enzymes and inhibiting autophagy in the model of menopause rats. Oxid Med Cell Longev. 2018;2018:7890291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li P, Xu Y, Gan Y, et al. Estrogen enhances matrix synthesis in nucleus Pulposus cell through the estrogen receptor β‐p38 MAPK pathway. Cell Physiol Biochem. 2016;29:2216‐2226. [DOI] [PubMed] [Google Scholar]
- 52. Li P, Gan Y, Xu Y, et al. 17beta‐estradiol attenuates TNF‐α‐induced premature senescence of nucleus Pulposus cells through regulating the ROS/NF‐κB pathway. Int J Biol Sci. 2017;13:145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu H, Yang SD, Xu Y, et al. Protective role of 17β‐estradiol on tumor necrosis factor‐α‐induced apoptosis in human nucleus pulposus cells. Mol Med Rep. 2017;16:1093‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu S, Yang SD, Huo XW, Yang DL, Ma L, Ding WY. 17β‐estradiol inhibits intervertebral disc degeneration by down‐regulating MMP‐3 and MMP‐13 and up‐regulating type II collagen in a rat model. Artif Cells Nanomed Biotechnol. 2018;46:182‐191. [DOI] [PubMed] [Google Scholar]
- 55. Sheng B, Yuan Y, Liu X, et al. Protective effect of estrogen against intervertebral disc degeneration is attenuated by miR‐221 through targeting estrogen receptor α. Acta Biochim Biophys Sin (Shanghai). 2018;50:345‐354. [DOI] [PubMed] [Google Scholar]
- 56. Wang H, Ding W, Yang D, Gu T, Yang S, Bai Z. Different concentrations of 17β‐estradiol modulates apoptosis induced by interleukin‐1β in rat annulus fibrosus cells. Mol Med Rep. 2014;10:2745‐2751. [DOI] [PubMed] [Google Scholar]
- 57. Wang T, Yang SD, Liu S, Wang H, Liu H, Ding WY. 17β‐estradiol inhibits tumor necrosis factor‐α induced apoptosis of human nucleus Pulposus cells via the PI3K/Akt pathway. Med Sci Monit. 2016;22:4312‐4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wei A, Shen B, Williams LA, et al. Expression and functional roles of estrogen receptor GPR30 in human intervertebral disc. J Steroid Biochem Mol Biol. 2016;158:46‐55. [DOI] [PubMed] [Google Scholar]
- 59. Yang D, Zhu D, Zhu S, et al. 17β‐estradiol/extrogen receptor β alleviates apoptosis and enhances matrix biosynthesis of nucleus pulposus cells through regulating oxidative damage under a high glucose condition. Biomed Pharmacother. 2018;107:1004‐4009. [DOI] [PubMed] [Google Scholar]
- 60. Yang S, Ma L, Gu TX, et al. 17β‐estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis. 2014;19:789‐800. [DOI] [PubMed] [Google Scholar]
- 61. Yang S, Yang DL, Sun YP, et al. 17β‐estradiol protects against apoptosis induced by interleukin‐1β in rat nucleus pulposus cells by down‐regulating MMP‐3 and MMP‐13. Apoptosis. 2015;20:348‐357. [DOI] [PubMed] [Google Scholar]
- 62. Zhao C, Chen Q, Zhang WJ, et al. 17β‐estradiol protects rat annulus Fibrosus cells against apoptosis via α1 integrin‐mediated adhesion to type I collagen: An In‐vitro study. Med Sci Monit. 2016;22:1375‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahajan A, Patni R, Verma S. Low Back pain and menopause. J Midlife Health. 2019;10:163‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Muscat Baron Y. Menopause and the intervertebral disc. Menopause. 2017;24:1118‐1121. [DOI] [PubMed] [Google Scholar]
- 65. Lou C, Chen HL, Feng XZ, et al. Menopause is associated with lumbar disc degeneration: a review of 4230 intervertebral discs. Climacteric. 2014;17:700‐704. [DOI] [PubMed] [Google Scholar]
- 66. Lou C, Chen H, Mei L, et al. Association between menopause and lumbar disc degeneration: an MRI study of 1,566 women and 1,382 men. Menopause. 2017;24:1136‐1144. [DOI] [PubMed] [Google Scholar]
- 67. Wang Y. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J Orthop Translat. 2015;3:205‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y. Menopause as a potential cause for higher prevalence of low back pain in women than in age‐matched men. J Orthop Translat. 2016;8:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: a systematic review with a focus on gender‐specific and age‐specific prevalence. J Orthop Translat. 2016;11:39‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wáng Y. Continued progression of lumbar disc degeneration in postmenopausal women. Climacteric. 2015;18:435. [DOI] [PubMed] [Google Scholar]
- 71. Casale R, Symeonidou Z, Ferfeli S, Micheli F, Scarsella P, Paladini A. Food for special medical purposes and nutraceuticals for pain: a narrative review. Pain Ther. 2021;10:225‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jin L, Song XX, Li XF. The role of estrogen in intervertebral disc degeneration. Steroids. 2020;154:108549. [DOI] [PubMed] [Google Scholar]
- 73. Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. [DOI] [PubMed] [Google Scholar]
- 74. Jin J, Xie Y, Shi C, et al. Lipoxin A4 inhibits NLRP3 Inflammasome activation in rats with non‐compressive disc herniation through the JNK1/Beclin‐1/PI3KC3 pathway. Front Neurosci. 2020;14:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang S, Hu B, Liu W, et al. The role of structure and function changes of sensory nervous system in intervertebral disc‐related low back pain. Osteoarthr Cartil. 2021;29:17‐27. [DOI] [PubMed] [Google Scholar]
- 76. Park E, Moon SW, Suh HR, et al. Disc degeneration induces a mechano‐sensitization of disc afferent nerve fibers that associates with low back pain. Osteoarthr Cartil. 2019;27:1608‐1617. [DOI] [PubMed] [Google Scholar]
- 77. Miyagi M, Ishikawa T, Kamoda H, et al. ISSLS prize winner: disc dynamic compression in rats produces long‐lasting increases in inflammatory mediators in discs and induces long‐lasting nerve injury and regeneration of the afferent fibers innervating discs: a pathomechanism for chronic discogenic low back pain. Spine (Phila Pa 1976). 2012;37:1810‐1818. [DOI] [PubMed] [Google Scholar]
- 78. Miyagi M, Millecamps M, Danco AT, Ohtori S, Takahashi K, Stone LS. ISSLS prize winner: increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976). 2014;39:1345‐1354. [DOI] [PubMed] [Google Scholar]
- 79. Melrose J, Tessier S, Risbud MV. Genetic murine models of spinal development and degeneration provide valuable insights into intervertebral disc pathobiology. Eur Cell Mater. 2021;41:52‐72. [DOI] [PubMed] [Google Scholar]
- 80. Qu H, Fang X. A brief review on the human Encyclopedia of DNA elements (ENCODE) project. Genomics Proteomics Bioinformatics. 2013;11:135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yavartanoo M, Choi JK. ENCODE: a sourcebook of Epigenomes and chromatin language. Genomics Inform. 2013;11:2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mouse Genome Sequencing Consortium , Waterston, RH , Lindblad‐Toh, K , Birney E, Rogers, J , Abril, JF , Agarwal, P , Agarwala, R , Ainscough, R , Alexandersson, M , An, P , Antonarakis, SE , Attwood, J , Baertsch, R , Bailey, J , Barlow, K , Beck, S , Berry, E , Birren, B , Bloom, T , Bork, P , Botcherby, M , Bray, N , Brent, MR , Brown, DG , Brown, SD , Bult, C , Burton, J , Butler, J , Campbell, RD , Carninci, P , Cawley, S , Chiaromonte, F , Chinwalla, AT , Church, DM , Clamp, M , Clee, C , Collins, FS , Cook, LL , Copley, RR , Coulson, A , Couronne, O , Cuff, J , Curwen, V , Cutts, T , Daly, M , David, R , Davies, J , Delehaunty, KD , Deri, J , Dermitzakis, ET , Dewey, C , Dickens, NJ , Diekhans, M , Dodge, S , Dubchak, I , Dunn, DM , Eddy, SR , Elnitski, L , Emes, RD , Eswara, P , Eyras, E , Felsenfeld, A , Fewell, GA , Flicek, P , Foley, K , Frankel, WN , Fulton, LA , Fulton, RS , Furey, TS , Gage, D , Gibbs, RA , Glusman, G , Gnerre, S , Goldman, N , Goodstadt, L , Grafham, D , Graves, TA , Green, ED , Gregory, S , Guigó, R , Guyer, M , Hardison, RC , Haussler, D , Hayashizaki, Y , Hillier, LW , Hinrichs, A , Hlavina, W , Holzer, T , Hsu, F , Hua, A , Hubbard, T , Hunt, A , Jackson, I , Jaffe, DB , Johnson, LS , Jones, M , Jones, TA , Joy, A , Kamal, M , Karlsson, EK , Karolchik, D , Kasprzyk, A , Kawai, J , Keibler, E , Kells, C , Kent, WJ , Kirby A, Kolbe, DL , Korf I, Kucherlapati RS, Kulbokas EJ, Kulp, D , Landers, T , Leger, JP , Leonard, S , Letunic, I , Levine, R , Li J, Li, M , Lloyd, C , Lucas, S , Ma B, Maglott DR, Mardis ER, Matthews, L , Mauceli, E , Mayer, JH , McCarthy, M , McCombie, WR , McLaren, S , McLay, K , McPherson, JD , Meldrim, J , Meredith, B , Mesirov, JP , Miller, W , Miner, TL , Mongin, E , Montgomery, KT , Morgan, M , Mott, R , Mullikin, JC , Muzny, DM , Nash, WE , Nelson, JO , Nhan, MN , Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton‐Larty E, Pachter, L , Parra, G , Pepin, KH , Peterson, J , Pevzner, P , Plumb, R , Pohl, CS , Poliakov, A , Ponce, TC , Ponting, CP , Potter, S , Quail, M , Reymond, A , Roe, BA , Roskin, KM , Rubin, EM , Rust, AG , Santos, R , Sapojnikov, V , Schultz, B , Schultz, J , Schwartz, MS , Schwartz, S , Scott, C , Seaman, S , Searle, S , Sharpe, T , Sheridan, A , Shownkeen, R , Sims, S , Singer, JB , Slater, G , Smit, A , Smith, DR , Spencer, B , Stabenau, A , Stange‐Thomann, N , Sugnet, C , Suyama, M , Tesler, G , Thompson, J , Torrents, D , Trevaskis, E , Tromp, J , Ucla, C , Ureta‐Vidal, A , Vinson, JP , Von Niederhausern, AC , Wade, CM , Wall, M , Weber, RJ , Weiss, RB , Wendl, MC , West, AP , Wetterstrand, K , Wheeler, R , Whelan, S , Wierzbowski, J , Willey, D , Williams, S , Wilson, RK , Winter, E , Worley, KC , Wyman, D , Yang, S , Yang, SP , Zdobnov, EM , Zody, MC , Lander, ES . Initial sequencing and comparative analysis of the mouse genome. Nature; 420, 520–562 (2002). [DOI] [PubMed] [Google Scholar]
- 83. Brown S, Hancock JM. The mouse genome. Genome Dyn. 2006;2:33‐45. [DOI] [PubMed] [Google Scholar]
- 84. van Steenbeek FG, Hytonen MK, Leegwater PA, Lohi H. The canine era: the rise of a biomedical model. Anim Genet. 2016;47:519‐527. doi: 10.1111/age.12460 [DOI] [PubMed] [Google Scholar]
- 85. Boyko A, Quignon P, Li L, et al. A simple genetic architecture underlies morphologic variation in dogs. PLoS Biol. 2010;8:e1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Freedman AH, Gronau I, Schweizer RM, et al. Genome sequencing highlights the dynamic early history of dogs. PLoS Genet. 2014;10:e1004016. doi: 10.1371/journal.pgen.1004016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huson H, Parker HG, Runstadler J, Ostrander EA. A genetic dissection of breed composition and performance enhancement in the Alaskan sled dog. BMC Genet. 2010;11:71. doi: 10.1186/1471-2156-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Parker HG. Genomic analyses of modern dog breeds. Mamm Genome. 2012;23:19‐27. doi: 10.1007/s00335-011-9387-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Parker HG, Kim LV, Sutter NB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304:1160‐1164. doi: 10.1126/science.1097406 [DOI] [PubMed] [Google Scholar]
- 90. Parker HG, Kukekova AV, Akey DT, et al. Breed relationships facilitate fine‐mapping studies: a 7.8‐kb deletion cosegregates with collie eye anomaly across multiple dog breeds. Genome Res. 2007;17:1562‐1571. doi: 10.1101/gr.6772807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sutter NB, Eberle MA, Parker HG, et al. Extensive and breed‐specific linkage disequilibrium in Canis familiaris. Genome Res. 2004;14:2388‐2396. doi: 10.1101/gr.3147604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vonholdt BM, Pollinger JP, Lohmueller KE, et al. Genome‐wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature. 2010;464:898‐902. doi: 10.1038/nature08837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang Y, Xie M, Chen W, et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science. 2014;344:1168‐1173. doi: 10.1126/science.1252806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berner D, Winter K, Brehm W, Gerlach K. Influence of head and neck position on radiographic measurement of intervertebral distances between thoracic dorsal spinous processes in clinically sound horses. Equine Vet J Suppl. 2012;21‐26:21‐26. doi: 10.1111/j.2042-3306.2012.00678.x [DOI] [PubMed] [Google Scholar]
- 95. Hahn CN, Handel I, Green SL, Bronsvoort MB, Mayhew IG. Assessment of the utility of using intra‐ and intervertebral minimum sagittal diameter ratios in the diagnosis of cervical vertebral malformation in horses. Vet Radiol Ultrasound. 2008;49:1‐6. doi: 10.1111/j.1740-8261.2007.00308.x [DOI] [PubMed] [Google Scholar]
- 96. O'Leary SA, White JL, Hu JC, Athanasiou KA. Biochemical and biomechanical characterisation of equine cervical facet joint cartilage. Equine Vet J. 2018;50:800‐808. doi: 10.1111/evj.12845 [DOI] [PubMed] [Google Scholar]
- 97. Kumar N, Kukreti S, Ishaque M, Mulholland R. Anatomy of deer spine and its comparison to the human spine. Anat Rec. 2000;260:189‐203. doi: [DOI] [PubMed] [Google Scholar]
- 98. Wilke HJ, Geppert J, Kienle A. Biomechanical in vitro evaluation of the complete porcine spine in comparison with data of the human spine. Eur Spine J. 2011;20:1859‐1868. doi: 10.1007/s00586-011-1822-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine (Phila Pa 1976). 1997;22:2365‐2374. doi: 10.1097/00007632-199710150-00009 [DOI] [PubMed] [Google Scholar]
- 100. Oxland TR. Fundamental biomechanics of the spine‐‐what we have learned in the past 25 years and future directions. J Biomech. 2016;49:817‐832. doi: 10.1016/j.jbiomech.2015.10.035 [DOI] [PubMed] [Google Scholar]
- 101. Panjabi MM, White AA 3rd. Basic biomechanics of the spine. Neurosurgery. 1980;7:76‐93. doi: 10.1227/00006123-198007000-00014 [DOI] [PubMed] [Google Scholar]
- 102. Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three‐dimensional load‐displacement curves. J Bone Joint Surg Am. 1994;76:413‐424. doi: 10.2106/00004623-199403000-00012 [DOI] [PubMed] [Google Scholar]
- 103. Kettler A, Liakos L, Haegele B, Wilke HJ. Are the spines of calf, pig and sheep suitable models for pre‐clinical implant tests? Eur Spine J. 2007;16:2186‐2192. doi: 10.1007/s00586-007-0485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dawson J. Kangaroos. 2nd ed. CSIRO Publishing; 2012. [Google Scholar]
- 105. Windsor D, Dagg AI. The gaits of the Macropodinae (Marsupialia). J Zool. 1971;163:165‐175. doi: 10.1111/j.1469-7998.1971.tb04530.x [DOI] [Google Scholar]
- 106. Dawson TJ, Mifsud B, Raad MC, Webster KN. Aerobic characteristics of red kangaroo skeletal muscles: is a high aerobic capacity matched by muscle mitochondrial and capillary morphology as in placental mammals? J Exp Biol. 2004;207:2811‐2821. doi: 10.1242/jeb.01115 [DOI] [PubMed] [Google Scholar]
- 107. Hickman G. The mammalian tail: a review of functions. Mamm Rev. 1979;9:143‐157. doi: 10.1111/j.1365-2907.1979.tb00252.x [DOI] [Google Scholar]
- 108. Schmitt D, Rose MD, Turnquist JE, Lemelin P. Role of the prehensile tail during ateline locomotion: experimental and osteological evidence. Am J Phys Anthropol. 2005;126:435‐446. doi: 10.1002/ajpa.20075 [DOI] [PubMed] [Google Scholar]
- 109. Thompson K, Moore S, Tang S, Wiet M, Purmessur D. The chondrodystrophic dog: a clinically relevant intermediate‐sized animal model for the study of intervertebral disc‐associated spinal pain. JOR Spine. 2018;1:e1011. doi: 10.1002/jsp2.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hakozaki T, Iwata M, Kanno N, et al. Cervical intervertebral disk herniation in chondrodystrophoid and nonchondrodystrophoid small‐breed dogs: 187 cases (1993‐2013). J Am Vet Med Assoc. 2015;247:1408‐1411. doi: 10.2460/javma.247.12.1408 [DOI] [PubMed] [Google Scholar]
- 111. Jeffery ND, Levine JM, Olby NJ, Stein VM. Intervertebral disk degeneration in dogs: consequences, diagnosis, treatment, and future directions. J Vet Intern Med. 2013;27:1318‐1333. doi: 10.1111/jvim.12183 [DOI] [PubMed] [Google Scholar]
- 112. Smolders LA, Bergknut N, Grinwis GCM, et al. Intervertebral disc degeneration in the dog. Part 2: chondrodystrophic and non‐chondrodystrophic breeds. Vet J. 2013;195:292‐299. doi: 10.1016/j.tvjl.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 113. Kranenburg HJ, Grinwis GCM, Bergknut N, et al. Intervertebral disc disease in dogs: part 2: comparison of clinical, magnetic resonance imaging, and histological findings in 74 surgically treated dogs. Vet J. 2013;195:164‐171. doi: 10.1016/j.tvjl.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 114. Bergknut N, Egenvall A, Hagman R, et al. Incidence of intervertebral disk degeneration‐related diseases and associated mortality rates in dogs. J Am Vet Med Assoc. 2012;240:1300‐1309. doi: 10.2460/javma.240.11.1300 [DOI] [PubMed] [Google Scholar]
- 115. Bergknut N, Grinwis G, Pickee E, et al. Reliability of macroscopic grading of intervertebral disk degeneration in dogs by use of the Thompson system and comparison with low‐field magnetic resonance imaging findings. Am J Vet Res. 2011;72:899‐904. doi: 10.2460/ajvr.72.7.899 [DOI] [PubMed] [Google Scholar]
- 116. Bergknut N, Auriemma E, Wijsman S, et al. Evaluation of intervertebral disk degeneration in chondrodystrophic and nonchondrodystrophic dogs by use of Pfirrmann grading of images obtained with low‐field magnetic resonance imaging. Am J Vet Res. 2011;72:893‐898. doi: 10.2460/ajvr.72.7.893 [DOI] [PubMed] [Google Scholar]
- 117. Brisson BA. Intervertebral disc disease in dogs. Vet Clin North Am Small Anim Pract. 2010;40:829‐858. doi: 10.1016/j.cvsm.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 118. Itoh H et al. A retrospective study of intervertebral disc herniation in dogs in Japan: 297 cases. J Vet Med Sci. 2008;70:701‐706. [DOI] [PubMed] [Google Scholar]
- 119. Griffin JF, Levine J, Kerwin S, Cole R. Canine thoracolumbar invertebral disk disease: diagnosis, prognosis, and treatment. Compend Contin Educ Vet. 2009;31:E3. [PubMed] [Google Scholar]
- 120. Hansen T, Smolders LA, Tryfonidou MA, et al. The myth of fibroid degeneration in the canine intervertebral disc: a histopathological comparison of intervertebral disc degeneration in Chondrodystrophic and Nonchondrodystrophic dogs. Vet Pathol. 2017;54:945‐952. doi: 10.1177/0300985817726834 [DOI] [PubMed] [Google Scholar]
- 121. Gu T, Shi Z, Wang C, et al. Human bone morphogenetic protein 7 transfected nucleus pulposus cells delay the degeneration of intervertebral disc in dogs. J Orthop Res. 2017;35:1311‐1322. doi: 10.1002/jor.22995 [DOI] [PubMed] [Google Scholar]
- 122. Li P, Zhang R, Gan Y, et al. Effects of osteogenic protein‐1 on intervertebral disc regeneration: a systematic review of animal studies. Biomed Pharmacother. 2017;88:260‐266. doi: 10.1016/j.biopha.2016.12.137 [DOI] [PubMed] [Google Scholar]
- 123. Willems N, Bach FC, Plomp SGM, et al. Intradiscal application of rhBMP‐7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res Ther. 2015;17:137. doi: 10.1186/s13075-015-0625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chaofeng W, Chao Z, Deli W, et al. Nucleus pulposus cells expressing hBMP7 can prevent the degeneration of allogenic IVD in a canine transplantation model. J Orthop Res. 2013;31:1366‐1373. doi: 10.1002/jor.22369 [DOI] [PubMed] [Google Scholar]
- 125. Matta A, Karim MZ, Gerami H, et al. NTG‐101: a novel molecular therapy that halts the progression of degenerative disc disease. Sci Rep. 2018;8:16809. doi: 10.1038/s41598-018-35011-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Matta A, Karim MZ, Isenman DE, Erwin WM. Molecular therapy for degenerative disc disease: clues from Secretome analysis of the Notochordal cell‐rich nucleus Pulposus. Sci Rep. 2017;7:45623. doi: 10.1038/srep45623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tellegen AR, Willems N, Beukers M, et al. Intradiscal application of a PCLA‐PEG‐PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: What's in it for humans? J Tissue Eng Regen Med. 2018;12:642‐652. doi: 10.1002/term.2483 [DOI] [PubMed] [Google Scholar]
- 128. Bach FC, Laagland LT, Grant MP, et al. Link‐N: the missing link towards intervertebral disc repair is species‐specific. PLoS One. 2017;12:e0187831. doi: 10.1371/journal.pone.0187831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Aprea F, Cherubini GB, Palus V, Vettorato E, Corletto F. Effect of extradurally administered morphine on postoperative analgesia in dogs undergoing surgery for thoracolumbar intervertebral disk extrusion. J Am Vet Med Assoc. 2012;241:754‐759. doi: 10.2460/javma.241.6.754 [DOI] [PubMed] [Google Scholar]
- 130. Steffen F, Bertolo A, Affentranger R, Ferguson SJ, Stoyanov J. Treatment of naturally degenerated canine lumbosacral intervertebral discs with autologous mesenchymal stromal cells and collagen microcarriers: a prospective clinical study. Cell Transplant. 2018;963689718815459:201‐211. doi: 10.1177/0963689718815459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Steffen F, Smolders LA, Roentgen AM, Bertolo A, Stoyanov J. Bone marrow‐derived mesenchymal stem cells as autologous therapy in dogs with naturally occurring intervertebral disc disease: feasibility, safety, and preliminary results. Tissue Eng Part C Methods. 2017;23:643‐651. doi: 10.1089/ten.TEC.2017.0033 [DOI] [PubMed] [Google Scholar]
- 132. Bach FC, Tellegen AR, Beukers M, et al. Biologic canine and human intervertebral disc repair by notochordal cell‐derived matrix: from bench towards bedside. Oncotarget. 2018;9:26507‐26526. doi: 10.18632/oncotarget.25476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bach F, Libregts S, Creemers L, et al. Notochordal‐cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget. 2017;8:88845‐88856. doi: 10.18632/oncotarget.21483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bach FC, Miranda‐Bedate A, van Heel FWM, et al. Bone morphogenetic protein‐2, but not mesenchymal stromal cells, exert regenerative effects on canine and human nucleus pulposus cells. Tissue Eng Part A. 2017;23:233‐242. doi: 10.1089/ten.TEA.2016.0251 [DOI] [PubMed] [Google Scholar]
- 135. Melrose J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen Med. 2016;11:705‐724. doi: 10.2217/rme-2016-0069 [DOI] [PubMed] [Google Scholar]
- 136. Hoffman AM, Dow SW. Concise review: stem cell trials using companion animal disease models. Stem Cells. 2016;34:1709‐1729. doi: 10.1002/stem.2377 [DOI] [PubMed] [Google Scholar]
- 137. Kim Y, Lee SH, Kim WH, Kweon OK. Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs. J Vet Sci. 2016;17:123‐126. doi: 10.4142/jvs.2016.17.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang Y, Tao H, Gu T, et al. The effects of human Wharton's jelly cell transplantation on the intervertebral disc in a canine disc degeneration model. Stem Cell Res Ther. 2015;6:154. doi: 10.1186/s13287-015-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Arkesteijn IT, Smolders LA, Spillekom S, et al. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res Ther. 2015;17:60. doi: 10.1186/s13075-015-0569-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. de Vries SA et al. Conditioned medium derived from notochordal cell‐rich nucleus pulposus tissue stimulates matrix production by canine nucleus pulposus cells and bone marrow‐derived stromal cells. Tissue Eng Part A. 2015;21:1077‐1084. doi: 10.1089/ten.TEA.2014.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bach FC, Willems N, Penning LC, Ito K, Meij BP, Tryfonidou MA. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet Res. 2014;10:3. doi: 10.1186/1746-6148-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mehrkens A, Karim M, Kim S, Hilario R, Fehlings M, Erwin W. Canine notochordal cell‐secreted factors protect murine and human nucleus pulposus cells from apoptosis by inhibition of activated caspase‐9 and caspase‐3/7. Evid Based Spine Care J. 2013;4:154‐156. doi: 10.1055/s-0033-1357363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Erwin WM, Islam D, Eftekarpour E, Inman RD, Karim MZ, Fehlings MG. Intervertebral disc‐derived stem cells: implications for regenerative medicine and neural repair. Spine (Phila Pa 1976). 2013;38:211‐216. doi: 10.1097/BRS.0b013e318266a80d [DOI] [PubMed] [Google Scholar]
- 144. Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28:1267‐1275. doi: 10.1002/jor.21147 [DOI] [PubMed] [Google Scholar]
- 145. Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17:1959‐1966. doi: 10.1038/mt.2009.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17(Suppl 4):492‐503. doi: 10.1007/s00586-008-0750-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hiyama A, Mochida J, Iwashina T, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589‐600. doi: 10.1002/jor.20584 [DOI] [PubMed] [Google Scholar]
- 148. Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan‐based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2012;21(Suppl 6):S839‐S849. doi: 10.1007/s00586-011-1976-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tindel NL, Reiter MF, Cohen‐Levy WB, Zafonte B, Banovac K, Eismont FJ. The effect of surgically implanted metallic bullet fragments on the intervertebral disc using a canine model. Spine J. 2019;19:755‐761. doi: 10.1016/j.spinee.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 150. Moriguchi Y, Mojica‐Santiago J, Grunert P, et al. Total disc replacement using tissue‐engineered intervertebral discs in the canine cervical spine. PLoS One. 2017;12:e0185716. doi: 10.1371/journal.pone.0185716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shimizu T, Fujibayashi S, Yamaguchi S, et al. In vivo experimental study of anterior cervical fusion using bioactive polyetheretherketone in a canine model. PLoS One. 2017;12:e0184495. doi: 10.1371/journal.pone.0184495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Willems N, Mihov G, Grinwis GCM, et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration. J Biomed Mater Res B Appl Biomater. 2017;105:707‐714. doi: 10.1002/jbm.b.33579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mackenzie SD, Brisson BA, Gaitero L, et al. Distribution and short‐ and long‐term effects of injected GELIFIED ethanol into the lumbosacral intervertebral disc IN healthy dogs. Vet Radiol Ultrasound. 2016;57:180‐190. doi: 10.1111/vru.12316 [DOI] [PubMed] [Google Scholar]
- 154. Olby NJ, Muguet‐Chanoit AC, Lim JH, et al. A placebo‐controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J Vet Intern Med. 2016;30:206‐214. doi: 10.1111/jvim.13657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Willems N, Yang HY, Langelaan MLP, et al. Biocompatibility and intradiscal application of a thermoreversible celecoxib‐loaded poly‐N‐isopropylacrylamide MgFe‐layered double hydroxide hydrogel in a canine model. Arthritis Res Ther. 2015;17:214. doi: 10.1186/s13075-015-0727-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zeng Y, Chen C, Liu W, et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak‐proof delivery and alleviation of canine disc degeneration. Biomaterials. 2015;59:53‐65. doi: 10.1016/j.biomaterials.2015.04.029 [DOI] [PubMed] [Google Scholar]
- 157. Xin H, Zhang C, Wang D, et al. Tissue‐engineered allograft intervertebral disc transplantation for the treatment of degenerative disc disease: experimental study in a beagle model. Tissue Eng Part A. 2013;19:143‐151. doi: 10.1089/ten.TEA.2012.0255 [DOI] [PubMed] [Google Scholar]
- 158. Bergknut N, Smolders LA, Koole LH, et al. The performance of a hydrogel nucleus pulposus prosthesis in an ex vivo canine model. Biomaterials. 2010;31:6782‐6788. doi: 10.1016/j.biomaterials.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 159. Ruan DK, Xin H, Zhang C, et al. Experimental intervertebral disc regeneration with tissue‐engineered composite in a canine model. Tissue Eng Part A. 2010;16:2381‐2389. doi: 10.1089/ten.TEA.2009.0770 [DOI] [PubMed] [Google Scholar]
- 160. Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82:701‐710. doi: 10.1002/jbm.a.31030 [DOI] [PubMed] [Google Scholar]
- 161. Melrose J, Shu C, Young C, et al. Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976). 2012;37:18‐25. doi: 10.1097/BRS.0b013e31820cd8d5 [DOI] [PubMed] [Google Scholar]
- 162. Shu CC, Dart A, Bell R, et al. Efficacy of administered mesenchymal stem cells in the initiation and co‐ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine. 2018;1:e1037. doi: 10.1002/jsp2.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Fuller ES, Shu C, Smith MM, Little CB, Melrose J. Hyaluronan oligosaccharides stimulate matrix metalloproteinase and anabolic gene expression in vitro by intervertebral disc cells and annular repair in vivo. J Tissue Eng Regen Med. 2018;12:e216‐e226. doi: 10.1002/term.2319 [DOI] [PubMed] [Google Scholar]
- 164. Matta A, Karim MZ, Gerami H, Benigno B, Erwin WM. A comparative study of mesenchymal stem cell transplantation and NTG‐101 molecular therapy to treat degenerative disc disease. Sci Rep. 2021;11:14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long‐term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31:1276‐1282. doi: 10.1002/jor.22355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ohnishi T, Sudo H, Iwasaki K, Tsujimoto T, Ito YM, Iwasaki N. In vivo mouse intervertebral disc degeneration model based on a new histological classification. PLoS One. 2016;11:e0160486. doi: 10.1371/journal.pone.0160486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Osti O, Vernon‐Roberts B, Fraser RD. 1990 Volvo award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976). 1990;15:762‐767. [DOI] [PubMed] [Google Scholar]
- 168. Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part I: a microscopic investigation of the translamellar bridging network. Spine (Phila Pa 1976). 2008;33:2702‐2710. doi: 10.1097/BRS.0b013e31817bb92c [DOI] [PubMed] [Google Scholar]
- 169. Shan Z, Wade KR, Schollum ML, Robertson PA, Thambyah A, Broom ND. A more realistic disc herniation model incorporating compression, flexion and facet‐constrained shear: a mechanical and microstructural analysis. Part II: high rate or “surprise” loading. Eur Spine J. 2017;26:2629‐2641. doi: 10.1007/s00586-017-5253-x [DOI] [PubMed] [Google Scholar]
- 170. Veres SP, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine (Phila Pa 1976). 2008;33:2711‐2720. doi: 10.1097/BRS.0b013e31817bb906 [DOI] [PubMed] [Google Scholar]
- 171. Veres SP, Robertson PA, Broom ND. ISSLS prize winner: how loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine (Phila Pa 1976). 2010;35:1897‐1908. doi: 10.1097/BRS.0b013e3181d9b69e [DOI] [PubMed] [Google Scholar]
- 172. Wade KR, Schollum ML, Robertson PA, Thambyah A, Broom ND. ISSLS prize winner: vibration really does disrupt the disc: a microanatomical investigation. Spine (Phila Pa 1976). 2016;41:1185‐1198. doi: 10.1097/brs.0000000000001594 [DOI] [PubMed] [Google Scholar]
- 173. Wade KR, Schollum ML, Robertson PA, Thambyah A, Broom ND. A more realistic disc herniation model incorporating compression, flexion and facet‐constrained shear: a mechanical and microstructural analysis. Part I: low rate loading. Eur Spine J. 2017;26:2616‐2628. doi: 10.1007/s00586-017-5252-y [DOI] [PubMed] [Google Scholar]
- 174. Yu J, Schollum ML, Wade KR, Broom ND, Urban JP. ISSLS prize winner: a detailed examination of the elastic network leads to a new understanding of annulus Fibrosus organization. Spine (Phila Pa 1976). 2015;40:1149‐1157. [DOI] [PubMed] [Google Scholar]
- 175. James G, Sluka KA, Blomster L, et al. Macrophage polarization contributes to local inflammation and structural change in the multifidus muscle after intervertebral disc injury. Eur Spine J. 2018;27:1744‐1756. [DOI] [PubMed] [Google Scholar]
- 176. Shen B, Melrose J, Ghosh P, Taylor TKF. Induction of matrix metalloproteinase‐2 and ‐3 activity in ovine nucleus pulposus cells grown in three‐dimensional agarose gel culture by interleukin‐1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66‐75. [DOI] [PubMed] [Google Scholar]
- 177. Melrose J, Ghosh P, Taylor TK, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992;10:665‐676. [DOI] [PubMed] [Google Scholar]
- 178. Melrose J, Smith S, Little CB, Kitson J, Hwa SY, Ghosh P. Spatial and temporal localization of transforming growth factor‐beta, fibroblast growth factor‐2, and osteonectin, and identification of cells expressing alpha‐smooth muscle Actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756‐1764. [DOI] [PubMed] [Google Scholar]
- 179. Shu C, Smith MM, Smith SM, Dart AJ, Little CB, Melrose J. A histopathological scheme for the quantitative scoring of intervertebral disc degeneration and the therapeutic utility of adult mesenchymal stem cells for intervertebral disc regeneration. Int J Mol Sci. 2017;18:1049. doi: 10.3390/ijms18051049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Schwan S, Ludtka C, Wiesner I, Baerthel A, Friedmann A, Göhre F. Percutaneous posterolateral approach for the simulation of a far‐lateral disc herniation in an ovine model. Eur Spine J. 2018;27:222‐230. doi: 10.1007/s00586-017-5362-6 [DOI] [PubMed] [Google Scholar]
- 181. Deml MC, Benneker LM, Schmid T, et al. Ventral surgical approach for an intervertebral disc degeneration and regeneration model in sheep cervical spine: anatomic technical description, strengths and limitations. Vet Comp Orthop Traumatol. 2019;32:389‐393. doi: 10.1055/s-0039-1688988 [DOI] [PubMed] [Google Scholar]
- 182. Daly CD, Ghosh P, Badal T, et al. A comparison of two ovine lumbar intervertebral disc injury models for the evaluation and development of novel regenerative therapies. Global Spine J. 2018;8:847‐859. doi: 10.1177/2192568218779988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Vadala G, Russo F, de Strobel F, et al. Novel stepwise model of intervertebral disc degeneration with intact annulus fibrosus to test regeneration strategies. J Orthop Res. 2018;36:2460‐2468. doi: 10.1002/jor.23905 [DOI] [PubMed] [Google Scholar]
- 184. Schollum ML, Wade KR, Shan Z, Robertson PA, Thambyah A, Broom ND. The influence of concordant complex posture and loading rate on motion segment failure: a mechanical and microstructural investigation. Spine (Phila Pa 1976). 2018;43:E1116‐E1126. doi: 10.1097/brs.0000000000002652 [DOI] [PubMed] [Google Scholar]
- 185. le Fournier L, Fusellier M, Halgand B, et al. The transpedicular surgical approach for the development of intervertebral disc targeting regenerative strategies in an ovine model. Eur Spine J. 2017;26:2072‐2083. doi: 10.1007/s00586-017-5199-z [DOI] [PubMed] [Google Scholar]
- 186. Schollum ML, Wade KR, Robertson PA, Thambyah A, Broom ND. A microstructural investigation of disc disruption induced by low frequency cyclic loading. Spine (Phila Pa 1976). 2018;43:E132‐E142. doi: 10.1097/brs.0000000000002278 [DOI] [PubMed] [Google Scholar]
- 187. Lim K, Daly CD, Ghosh P, et al. Ovine lumbar intervertebral disc degeneration model utilizing a lateral retroperitoneal drill bit injury. J Vis Exp. 2017;123:55753. doi: 10.3791/55753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. van Heeswijk VM, Thambyah A, Robertson PA, Broom ND. Does an annular puncture influence the herniation path?: An In vitro mechanical and structural investigation. Spine (Phila Pa 1976). 2018;43:467‐476. doi: 10.1097/brs.0000000000002336 [DOI] [PubMed] [Google Scholar]
- 189. Vadala G, Russo F, Pattappa G, et al. A nucleotomy model with intact annulus fibrosus to test intervertebral disc regeneration strategies. Tissue Eng Part C Methods. 2015;21:1117‐1124. doi: 10.1089/ten.TEC.2015.0086 [DOI] [PubMed] [Google Scholar]
- 190. Vadala G, Russo F, Pattappa G, et al. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976). 2013;38:E319‐E324. doi: 10.1097/BRS.0b013e318285bc4a [DOI] [PubMed] [Google Scholar]
- 191. Oehme D, Goldschlager T, Rosenfeld J, et al. Lateral surgical approach to lumbar intervertebral discs in an ovine model. ScientificWorldJournal. 2012;2012:873726. doi: 10.1100/2012/873726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Guder E, Hill S, Kandziora F, Schnake KJ. Partial nucleotomy of the ovine disc as an in vivo model for disc degeneration. Z Orthop Unfall. 2009;147:52‐58. doi: 10.1055/s-2008-1039139 [DOI] [PubMed] [Google Scholar]
- 193. Hoshide R, Feldman E, Narayan A, Taylor W. A novel, minimally‐invasive approach to repair degenerative disk disease in an ovine model using injectable Polymethyl‐methacrylate and bovine collagen (PMMA/BC). Cureus. 2016;8:e729. doi: 10.7759/cureus.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sharabi M, Wade KR, Galbusera F, Rasche V, Haj‐Ali R, Wilke HJ. Three‐dimensional microstructural reconstruction of the ovine intervertebral disc using ultrahigh field MRI. Spine J. 2018;18:2119‐2127. doi: 10.1016/j.spinee.2018.06.356 [DOI] [PubMed] [Google Scholar]
- 195. Gluais M, Clouet J, Fusellier M, et al. In vitro and in vivo evaluation of an electrospun‐aligned microfibrous implant for annulus fibrosus repair. Biomaterials. 2019;205:81‐93. doi: 10.1016/j.biomaterials.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 196. Pennicooke B, Hussain I, Berlin C, et al. Annulus Fibrosus repair using high‐density collagen gel: An In vivo ovine model. Spine (Phila Pa 1976). 2018;43:E208‐E215. doi: 10.1097/brs.0000000000002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yu CC, Hao DJ, Huang DG, et al. Biomechanical analysis of a novel prosthesis based on the physiological curvature of endplate for cervical disc replacement. PLoS One. 2016;11:e0158234. doi: 10.1371/journal.pone.0158234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hegewald AA, Medved F, Feng D, et al. Enhancing tissue repair in annulus fibrosus defects of the intervertebral disc: analysis of a bio‐integrative annulus implant in an in‐vivo ovine model. J Tissue Eng Regen Med. 2015;9:405‐414. doi: 10.1002/term.1831 [DOI] [PubMed] [Google Scholar]
- 199. Reitmaier S, Kreja L, Gruchenberg K, et al. In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur Spine J. 2014;23:19‐26. doi: 10.1007/s00586-013-2998-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Reitmaier S, Wolfram U, Ignatius A, et al. Hydrogels for nucleus replacement‐‐facing the biomechanical challenge. J Mech Behav Biomed Mater. 2012;14:67‐77. doi: 10.1016/j.jmbbm.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 201. Woiciechowsky C, Abbushi A, Zenclussen ML, et al. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell‐free resorbable polymer scaffolds. J Tissue Eng Regen Med. 2014;8:811‐820. doi: 10.1002/term.1582 [DOI] [PubMed] [Google Scholar]
- 202. Malhotra NR, Han WM, Beckstein J, Cloyd J, Chen W, Elliott DM. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine (Phila Pa 1976). 2012;37:E1099‐E1105. doi: 10.1097/BRS.0b013e31825cdfb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Stieber JR, Quirno M, Kang M, Valdevit A, Errico TJ. The facet joint loading profile of a cervical intervertebral disc replacement incorporating a novel saddle‐shaped articulation. J Spinal Disord Tech. 2011;24:432‐436. doi: 10.1097/BSD.0b013e3182027297 [DOI] [PubMed] [Google Scholar]
- 204. Bowles RD, Williams RM, Zipfel WR, Bonassar LJ. Self‐assembly of aligned tissue‐engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A. 2010;16:1339‐1348. doi: 10.1089/ten.TEA.2009.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Freeman BJ, Kuliwaba JS, Jones CF, et al. Allogeneic mesenchymal precursor cells promote healing in postero‐lateral annular lesions and improve indices of lumbar intervertebral disc degeneration in an ovine model. Spine (Phila Pa 1976). 2016;41:1331‐1339. doi: 10.1097/brs.0000000000001528 [DOI] [PubMed] [Google Scholar]
- 206. Oehme D, Ghosh P, Shimmon S, et al. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine. 2014;20:657‐669. doi: 10.3171/2014.2.spine13760 [DOI] [PubMed] [Google Scholar]
- 207. Ghosh P, Moore R, Vernon‐Roberts B, et al. Immunoselected STRO‐3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479‐488. doi: 10.3171/2012.1.spine11852 [DOI] [PubMed] [Google Scholar]
- 208. Goldschlager T, Ghosh P, Zannettino A, et al. Cervical motion preservation using mesenchymal progenitor cells and pentosan polysulfate, a novel chondrogenic agent: preliminary study in an ovine model. Neurosurg Focus. 2010;28:E4. doi: 10.3171/2010.3.focus1050 [DOI] [PubMed] [Google Scholar]
- 209. Yong MR et al. Establishment and characterization of an open mini‐thoracotomy surgical approach to an ovine thoracic spine fusion model. Tissue Eng Part C Methods. 2014;20:19‐27. doi: 10.1089/ten.TEC.2012.0746 [DOI] [PubMed] [Google Scholar]
- 210. Solchaga LA, Hee CK, Aguiar DJ, et al. Augment bone graft products compare favorably with autologous bone graft in an ovine model of lumbar interbody spine fusion. Spine (Phila Pa 1976). 2012;37:E461‐E467. doi: 10.1097/BRS.0b013e31823b01dc [DOI] [PubMed] [Google Scholar]
- 211. Lyons AS, Sherman BP, Puttlitz CM, et al. Failure of resorbable plates and screws in an ovine model of anterior cervical discectomy and fusion. Spine J. 2011;11:876‐883. doi: 10.1016/j.spinee.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 212. Diwan A, Melrose J. Intervertebral disc degeneration and how it leads to low Back pain. Jor Spine (in press). 2022:e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhu D, Miao Z, Dong M, et al. Development of a novel rat intervertebral disc degeneration model by surgical multifidus resection induced instability. World Neurosurg. 2022;165:e357‐e364. [DOI] [PubMed] [Google Scholar]
- 214. Ozawa T, Ohtori S, Inoue G, Aoki Y, Moriya H, Takahashi K. The degenerated lumbar intervertebral disc is innervated primarily by peptide‐containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31:2418‐2422. [DOI] [PubMed] [Google Scholar]
- 215. Brisby H. Pathology and possible mechanisms of nervous system response to disc degeneration. J Bone Joint Surg Am. 2006;88:68‐71. [DOI] [PubMed] [Google Scholar]
- 216. Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240‐264. [DOI] [PubMed] [Google Scholar]
- 217. Hoppanova L, Lacinova L. Voltage‐dependent CaV3.2 and CaV2.2 channels in nociceptive pathways. Pflugers Arch. 2022;474:421‐434. [DOI] [PubMed] [Google Scholar]
- 218. Ji R, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004:reE14. [DOI] [PubMed] [Google Scholar]
- 219. Crow W, Willis DR. Estimating cost of care for patients with acute low back pain: a retrospective review of patient records. J Am Osteopath Assoc. 2009;109:229‐233. [PubMed] [Google Scholar]
- 220. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8‐20. [DOI] [PubMed] [Google Scholar]
- 221. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163‐2196. doi: 10.1016/s0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Yelin E, Weinstein S, King T. The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum. 2016;46:259‐260. [DOI] [PubMed] [Google Scholar]
- 223. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85:343‐350. [PubMed] [Google Scholar]
- 224. Walton DM, MacDermid JC, Giorgianni AA, Mascarenhas JC, West SC, Zammit CA. Risk factors for persistent problems following acute whiplash injury: update of a systematic review and meta‐analysis. J Orthop Sports Phys Ther. 2013;43:31‐43. doi: 10.2519/jospt.2013.4507 [DOI] [PubMed] [Google Scholar]
- 225. da C. Menezes Costa L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low‐back pain: a meta‐analysis. CMAJ. 2012;184:E613‐E624. doi: 10.1503/cmaj.111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Koes BW, van Tulder M, Lin CWC, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non‐specific low back pain in primary care. Eur Spine J. 2010;19:2075‐2094. doi: 10.1007/s00586-010-1502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non‐specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27:2791‐2803. doi: 10.1007/s00586-018-5673-2 [DOI] [PubMed] [Google Scholar]
- 228. Manusov EG. Evaluation and diagnosis of low back pain. Prim Care. 2012;39:471‐479. doi: 10.1016/j.pop.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 229. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028‐2037. doi: 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 230. Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131:293‐301. doi: 10.1016/j.pain.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Guesgen MJ, Beausoleil NJ, Leach M, Minot EO, Stewart M, Stafford KJ. Coding and quantification of a facial expression for pain in lambs. Behav Processes. 2016;132:49‐56. doi: 10.1016/j.beproc.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 232. Häger C, Biernot S, Buettner M, et al. The sheep grimace scale as an indicator of post‐operative distress and pain in laboratory sheep. PLoS One. 2017;12:e0175839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Leung V, Zhang E, Pang DS. Real‐time application of the rat grimace scale as a welfare refinement in laboratory rats. Sci Rep. 2016;6:31667. doi: 10.1038/srep31667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Matsumiya LC, Sorge RE, Sotocinal SG, et al. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci. 2012;51:42‐49. [PMC free article] [PubMed] [Google Scholar]
- 235. Miller AL, Leach MC. The mouse grimace scale: a clinically useful tool? PLoS One. 2015;10:e0136000. doi: 10.1371/journal.pone.0136000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Mogil J, Pang DSJ, Silva Dutra GG, Chambers CT. The development and use of facial grimace scales for pain measurement in animals. Neurosci Biobehav Rev. 2020;116:480‐493. [DOI] [PubMed] [Google Scholar]
- 237. Mota‐Rojas D, Olmos‐Hernández A, Verduzco‐Mendoza A, Hernández E, Martínez‐Burnes J, Whittaker AL. The utility of grimace scales for practical pain assessment in laboratory animals. Animals (Basel). 2020;10:1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Gleerup KB. Assessing pain in horses. Vet Rec. 2019;184:124. doi: 10.1136/vr.l385 [DOI] [PubMed] [Google Scholar]
- 239. Gleerup KB, Forkman B, Lindegaard C, Andersen PH. An equine pain face. Vet Anaesth Analg. 2015;42:103‐114. doi: 10.1111/vaa.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Mclennan K, Miller AL, Dalla Costa E, et al. Conceptual and methodological issues relating to pain assessment in mammals. Appl Anim Behav Sci. 2019;217:1‐15. [Google Scholar]
- 241. Piel M, Kroin JS, van Wijnen AJ, Kc R, Im HJ. Pain assessment in animal models of osteoarthritis. Gene. 2014;537:184‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Andresen N, Wöllhaf M, Hohlbaum K, et al. Towards a fully automated surveillance of well‐being status in laboratory mice using deep learning: starting with facial expression analysis. PLoS One. 2020;15:e0228059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Dalla Costa E, Pascuzzo R, Leach MC, et al. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS One. 2018;13:e0200339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the horse grimace scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS One. 2014;9:e92281. doi: 10.1371/journal.pone.0092281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. de Grauw J, van Loon JP. Systematic pain assessment in horses. Vet J. 2016;209:14‐22. [DOI] [PubMed] [Google Scholar]
- 246. Yamamoto K, Tatsutani S, Ishida T. Detection of nausea‐like response in rats by monitoring facial expression. Front Pharmacol. 2016;7:534. doi: 10.3389/fphar.2016.00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Brown S, Matta A, Erwin M, et al. Cell clusters are indicative of stem cell activity in the degenerate intervertebral disc: can their properties Be manipulated to improve intrinsic repair of the disc? Stem Cells Dev. 2018;27:147‐165. doi: 10.1089/scd.2017.0213 [DOI] [PubMed] [Google Scholar]
- 248. Korecki C, Taboas JM, Tuan RS, et al. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Kwon W, Moon HJ, Kwon TH, Park YK, Kim JH. Influence of rabbit notochordal cells on symptomatic intervertebral disc degeneration: anti‐angiogenic capacity on human endothelial cell proliferation under hypoxia. Osteoarthr Cartil. 2017;25:1738‐1746. [DOI] [PubMed] [Google Scholar]
- 250. Liu Y, Fu S, Rahaman MN, Mao JJ, Bal BS. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A. 2015;103:1053‐1059. [DOI] [PubMed] [Google Scholar]
- 251. Purmessur D, Schek RM, Abbott RD, Ballif BA, Godburn KE, Iatridis JC. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Turner S, Balain B, Caterson B, Morgan C, Roberts S. Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies. Eur Spine J. 2014;23:2462‐2472. [DOI] [PubMed] [Google Scholar]
- 253. Bohr A, Memarzadeh K. The rise of artificial intelligence in healthcare applications. Artificial Intelligence in Healthcare. 2020;2020:25‐60. [Google Scholar]
- 254. Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J. 2019;6:94‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Becker A. Artificial intelligence in medicine: what is it doing for us today? Health Policy Technol. 2019;9:198‐205. [Google Scholar]
- 256. Merali Z, Witiw CD, Badhiwala JH, Wilson JR, Fehlings MG. Using a machine learning approach to predict outcome after surgery for degenerative cervical myelopathy. PLoS One. 2019;14:e0215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Karhade A, Ogink P, Thio Q, et al. Development of machine learning algorithms for prediction of discharge disposition after elective inpatient surgery for lumbar degenerative disc disorders. Neurosurg Focus. 2018;45:E6. [DOI] [PubMed] [Google Scholar]
- 258. Wirries A, Geiger F, Hammad A, Oberkircher L, Blümcke I, Jabari S. Artificial intelligence facilitates decision‐making in the treatment of lumbar disc herniations. Eur Spine J. 2021;30:2176‐2184. [DOI] [PubMed] [Google Scholar]
- 259. Staartjes V, de Wispelaere MP, Vandertop WP, Schröder ML. Deep learning‐based preoperative predictive analytics for patient‐reported outcomes following lumbar discectomy: feasibility of center‐specific modeling. Spine J. 2019;19:853‐861. [DOI] [PubMed] [Google Scholar]
- 260. Schiff L, Migliori B, Chen Y, et al. Integrating deep learning and unbiased automated high‐content screening to identify complex disease signatures in human fibroblasts. Nat Commun. 2022;13:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Gorelik N, Chong J, Lin DJ. Pattern recognition in musculoskeletal imaging using artificial intelligence. Semin Musculoskelet Radiol. 2020;24:38‐49. [DOI] [PubMed] [Google Scholar]
- 262. Niemeyer F, Galbusera F, Tao Y, Kienle A, Beer M, Wilke HJ. A deep learning model for the accurate and reliable classification of disc degeneration based on MRI data. Invest Radiol. 2021;56:78‐85. [DOI] [PubMed] [Google Scholar]
- 263. Zheng H, Sun YL, Kong DW, et al. Deep learning‐based high‐accuracy quantitation for lumbar intervertebral disc degeneration from MRI. Nat Commun. 2022;13:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. D'Antoni F, Russo F, Ambrosio L, et al. Artificial intelligence and computer vision in low Back pain: a systematic review. Int J Environ Res Public Health. 2021;18:10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Huang J, Shen H, Wu J, et al. Spine explorer: a deep learning based fully automated program for efficient and reliable quantifications of the vertebrae and discs on sagittal lumbar spine MR images. Spine J. 2020;20:590‐599. [DOI] [PubMed] [Google Scholar]
- 266. Shen H, Huang J, Zheng Q, et al. A deep‐learning‐based, fully automated program to segment and quantify Major spinal components on axial lumbar spine magnetic resonance images. Phys Ther. 2021;101:pzab041. [DOI] [PubMed] [Google Scholar]
- 267. Staartjes V, Klukowska AM, Vieli M, et al. Machine learning‐augmented objective functional testing in the degenerative spine: quantifying impairment using patient‐specific five‐repetition sit‐to‐stand assessment. Neurosurg Focus. 2021;51:E8. [DOI] [PubMed] [Google Scholar]
- 268. Staartjes V, Schröder ML. The five‐repetition sit‐to‐stand test: evaluation of a simple and objective tool for the assessment of degenerative pathologies of the lumbar spine. J Neurosurg Spine. 2018;29:380‐387. [DOI] [PubMed] [Google Scholar]
- 269. Gao K, Tibrewala R, Hess M, et al. Automatic detection and voxel‐wise mapping of lumbar spine Modic changes with deep learning. JOR Spine. 2022;5:e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research. JOR Spine. 2019;2:e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Qu B, Cao J, Qian C, et al. Current development and prospects of deep learning in spine image analysis: a literature review. Quant Imaging Med Surg. 2022;12:3454‐3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Piri R, Nøddeskou‐Fink AH, Gerke O, et al. PET/CT imaging of spinal inflammation and microcalcification in patients with low back pain: a pilot study on the quantification by artificial intelligence‐based segmentation. Clin Physiol Funct Imaging. 2022;42:225‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zhou X, Cipriano P, Kim B, et al. Detection of nociceptive‐related metabolic activity in the spinal cord of low back pain patients using 18F‐FDG PET/CT. Scand J Pain. 2017;15:53‐57. [DOI] [PubMed] [Google Scholar]
- 274. Gordh T. A possible biomarker of low back pain: 18F‐FDeoxyGlucose uptake in PETscan and CT of the spinal cord. Scand J Pain. 2017;15:79‐80. [DOI] [PubMed] [Google Scholar]
- 275. Mehta V, Bouchareb Y, Ramaswamy S, Ahmad A, Wodehouse T, Haroon A. Metabolic imaging of pain matrix using 18 F Fluoro‐deoxyglucose positron emission tomography/computed tomography for patients undergoing L2 dorsal root ganglion stimulation for low Back pain. Neuromodulation. 2020;23:222‐233. [DOI] [PubMed] [Google Scholar]
- 276. Melgoza I, Chenna SS, Tessier S, et al. Development of a standardized histopathology scoring system using machine learning algorithms for intervertebral disc degeneration in the mouse model‐An ORS spine section initiative. JOR Spine. 2021;4:e1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Tiwari J, Sharma SR, Chauhan S, Adams M, Lama P. Computational image analysis of painful and pain‐free intervertebral disc. In: Gupta M, Ghatak S, Gupta A, Mukherjee AL, eds. Artificial Intelligence on Medical Data. Lecture Notes in Computational Vision and Biomechanics. Vol 37. Springer; 2023. [Google Scholar]
- 278. Baumgartner L, Wuertz‐Kozak K, Le Maitre CL, et al. Multiscale regulation of the intervertebral disc: achievements in experimental, In Silico, and regenerative research. Int J Mol Sci. 2021;22:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Park T, Kuo A, Smith MT. Chronic low back pain: a mini‐review on pharmacological management and pathophysiological insights from clinical and pre‐clinical data. Inflammopharmacology. 2018;26:881‐898. doi: 10.1007/s10787-018-0493-x [DOI] [PubMed] [Google Scholar]
- 280. Pan Z, Sun H, Xie B, et al. Therapeutic effects of gefitinib‐encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration. Biomaterials. 2018;160:56‐68. [DOI] [PubMed] [Google Scholar]
- 281. Ding H, Wei J, Zhao Y, Liu Y, Liu L, Cheng L. Progranulin derived engineered protein Atsttrin suppresses TNF‐alpha‐mediated inflammation in intervertebral disc degenerative disease. Oncotarget. 2017;8:109692‐109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Xia X, Guo J, Lu F, Jiang J. SIRT1 plays a protective role in intervertebral disc degeneration in a puncture‐induced rodent model. Spine (Phila Pa 1976). 2015;40:E515‐E524. [DOI] [PubMed] [Google Scholar]
- 283. Wang G, Huang K, Dong Y, et al. Lycorine suppresses endplate‐chondrocyte degeneration and prevents intervertebral disc degeneration by inhibiting NF‐kappaB Signalling pathway. Cell Physiol Biochem. 2018;45:1252‐1269. doi: 10.1159/000487457 [DOI] [PubMed] [Google Scholar]
- 284. Cao Z, Yang P, Zhou Q. Multiple biological functions and pharmacological effects of lycorine Sci . China Chem. 2013;56:1382‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Li W, Zhang Y, Xing C, Zhang M. Tanshinone IIA represses inflammatory response and reduces radiculopathic pain by inhibiting IRAK‐1 and NF‐kappaB/p38/JNK signaling. Int Immunopharmacol. 2015;28:382‐389. doi: 10.1016/j.intimp.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 286. Hua W, Zhang Y, Wu X, et al. Icariin attenuates interleukin‐1β‐induced inflammatory response in human nucleus Pulposus cells. Curr Pharm des. 2018;23:6071‐6078. [DOI] [PubMed] [Google Scholar]
- 287. Fang W, Zhou X, Wang J, et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int Immunopharmacol. 2018;65:539‐549. [DOI] [PubMed] [Google Scholar]
- 288. Krupkova O, Sekiguchi M, Klasen J, et al. Epigallocatechin 3‐gallate suppresses interleukin‐1β‐induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372‐386. [DOI] [PubMed] [Google Scholar]
- 289. Shao Z, Wang B, Shi Y, et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF‐κB axis. Osteoarthr Cartil. 2021;29:413‐422. [DOI] [PubMed] [Google Scholar]
- 290. Wang D, He X, Wang D, et al. Quercetin suppresses apoptosis and attenuates intervertebral disc degeneration via the SIRT1‐autophagy pathway. Front Cell Dev Biol. 2020;8:613006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Zhang S, Liang W, Abulizi Y, et al. Quercetin alleviates intervertebral disc degeneration by modulating p38 MAPK‐mediated autophagy. Biomed Res Int. 2021;2021:6631562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Bertozzi M, Rossaneis AC, Fattori V, et al. Diosmin reduces chronic constriction injury‐induced neuropathic pain in mice. Chem Biol Interact. 2017;273:180‐189. [DOI] [PubMed] [Google Scholar]
- 293. Carballo‐Villalobos A, González‐Trujano ME, Pellicer F, Alvarado‐Vásquez N, López‐Muñoz FJ. Central and peripheral anti‐hyperalgesic effects of diosmin in a neuropathic pain model in rats. Biomed Pharmacother. 2018;97:310‐320. [DOI] [PubMed] [Google Scholar]
- 294. Wang Y, Fang X, Ye L, Li Y, Shi H, Cao Y. A randomized controlled trial evaluating the effects of Diosmin in the treatment of radicular pain. Biomed Res Int. 2017;2017:6875968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Shang P, Tang Q, Hu Z, et al. Procyanidin B3 alleviates intervertebral disc degeneration via interaction with the TLR4/MD‐2 complex. J Cell Mol Med. 2020;24:3701‐3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Lin J, Chen J, Zhang Z, et al. Luteoloside inhibits IL‐1β‐induced apoptosis and catabolism in nucleus Pulposus cells and ameliorates intervertebral disk degeneration. Front Pharmacol. 2019;10:868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Jin H, Wang Q, Wu J, et al. Baicalein inhibits the IL‐1β‐induced inflammatory response in nucleus Pulposus cells and attenuates disc degeneration In vivo. Inflammation. 2019;42:1032‐1044. [DOI] [PubMed] [Google Scholar]
- 298. Carballo‐Villalobos A, González‐Trujano ME, Alvarado‐Vázquez N, López‐Muñoz FJ. Pro‐inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology. 2017;25:265‐269. [DOI] [PubMed] [Google Scholar]
- 299. He S, Fu Y, Yan B, et al. Curcumol alleviates the inflammation of nucleus Pulposus cells via the PI3K/Akt/NF‐κB signaling pathway and delays intervertebral disk degeneration. World Neurosurg. 2021;155:e402‐e411. [DOI] [PubMed] [Google Scholar]
- 300. Yu H, Hou G, Cao J, Yin Y, Zhao Y, Cheng L. Mangiferin alleviates mitochondrial ROS in nucleus Pulposus cells and protects against intervertebral disc degeneration via suppression of NF‐κB signaling pathway. Oxid Med Cell Longev. 2021;2021:6632786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Chen R, Gao S, Guan H, et al. Naringin protects human nucleus pulposus cells against TNF‐α‐induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid Med Cell Longev. 2022;2022:7655142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Devraj V, Vemuri SK, Banala RR, Gunda SK, Av GR, Gpv S. Evaluation of anti‐inflammatory and regenerative efficiency of Naringin and Naringenin in degenerated human nucleus Pulposus cells: biological and molecular modeling studies. Asian Spine J. 2019;13:875‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Li N, Whitaker C, Xu Z, Heggeness M, Yang SY. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016;16:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 304. Du J, Xu M, Kong F, et al. CB2R attenuates intervertebral disc degeneration by delaying nucleus Pulposus cell senescence through AMPK/GSK3β pathway. Aging Dis. 2022;13:552‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Gruber S, Smith RT, Dahlgren MK, Lambros AM, Sagar KA. No pain, all gain? Interim analyses from a longitudinal, observational study examining the impact of medical cannabis treatment on chronic pain and related symptoms. Exp Clin Psychopharmacol. 2021;29:147‐156. [DOI] [PubMed] [Google Scholar]
- 306. Köstenberger M, Nahler G, Jones TM, Neuwersch S, Likar R. The role of cannabis, Cannabidiol and other cannabinoids in chronic pain. The perspective of physicians. J Neuroimmune Pharmacol. 2021. doi: 10.1007/s11481-021-10010-x [DOI] [PubMed] [Google Scholar]
- 307. Langford R, Mares J, Novotna A, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984‐997. [DOI] [PubMed] [Google Scholar]
- 308. Rieder C. Cannabidiol in Parkinson's disease. Braz J Psychiatry. 2020;42:126‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Rodriguez C, Ouyang L, Kandasamy R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in cannabis. Behav Pharmacol. 2022;33:130‐157. [DOI] [PubMed] [Google Scholar]
- 310. Scott D, Wright CE, Angus JA. Evidence that CB‐1 and CB‐2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124‐131. [DOI] [PubMed] [Google Scholar]
- 311. Xantus G, Zavori L, Matheson C, Gyarmathy VA, Fazekas LM, Kanizsai P. Cannabidiol in low back pain: scientific rationale for clinical trials in low back pain. Expert Rev Clin Pharmacol. 2021;14:671‐675. [DOI] [PubMed] [Google Scholar]
- 312. Yimam M, O'Neal A, Horm T, et al. Antinociceptive and anti‐inflammatory properties of Cannabidiol alone and in combination with standardized bioflavonoid composition. J Med Food. 2021;24:960‐967. [DOI] [PubMed] [Google Scholar]
- 313. Zhao J, Gao X, Zhao L, Wang Y, Zhang J, Liu S. Effects of Cannabidiol on Parkinson's disease in a transgenic mouse model by gut‐brain metabolic analysis. Evid Based Complement Alternat Med. 2022;2022:1525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Chen J, Xuan J, Gu YT, et al. Celastrol reduces IL‐1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed Pharmacother. 2017;91:208‐219. [DOI] [PubMed] [Google Scholar]
- 315. Wang K, Chen T, Ying X, et al. Ligustilide alleviated IL‐1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. Int Immunopharmacol. 2019;69:398‐407. [DOI] [PubMed] [Google Scholar]
- 316. Wang D, Cai X, Xu F, Kang H, Li Y, Feng R. Ganoderic acid A alleviates the degeneration of intervertebral disc via suppressing the activation of TLR4/NLRP3 signaling pathway. Bioengineered. 2022;13:11684‐11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Moro K, Nagahashi M, Ramanathan R, Takabe K, Wakai T. Resolvins and omega three polyunsaturated fatty acids: clinical implications in inflammatory diseases and cancer. World J Clin Cases. 2016;4:155‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Tao X, Luo X, Zhang T, Hershey B, Esteller R, Ji RR. Spinal cord stimulation attenuates mechanical allodynia and increases central Resolvin D1 levels in rats with spared nerve injury. Front Physiol. 2021;12:687046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Cheng B, Tao PL, Cheng YY, Huang EY. Lvv‐hemorphin 7 and angiotensin iv in correlation with antinociception and anti‐thermal hyperalgesia in rats. Peptides. 2012;36:9‐16. [DOI] [PubMed] [Google Scholar]
- 320. Smith MM, Melrose J. Natural and semi‐synthetic flavonoid anti‐SARS‐CoV‐2 agents are compounds for the treatment of long COVID‐19 disease and neurodegenerative disorders of cognitive decline. Frontiers in Bioscience –Elite. 2022;14:27‐34. [DOI] [PubMed] [Google Scholar]
- 321. Cao H, Yang L, Tian R, Wu H, Gu Z, Li Y. Versatile polyphenolic platforms in regulating cell biology. Chem Soc Rev. 2022;51:4175‐4198. [DOI] [PubMed] [Google Scholar]
- 322. Malla A, Dar BA, Isaev AB, Lone Y, Banday MR. Flavonoids: a reservoir of drugs from nature. Mini Rev Med Chem. 2022;22. doi: 10.2174/1389557522666220420102545 [DOI] [PubMed] [Google Scholar]
- 323. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531. [DOI] [PubMed] [Google Scholar]
- 324. Rampin A, Carrabba M, Mutoli M, et al. Recent advances in KEAP1/NRF2‐targeting strategies by phytochemical antioxidants, nanoparticles, and biocompatible scaffolds for the treatment of diabetic cardiovascular complications. Antioxid Redox Signal. 2022;36:707‐728. [DOI] [PubMed] [Google Scholar]
- 325. Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA. Withania somnifera L.: insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci. 2020;23:1501‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Vallejo R, Tilley DM, Cedeño DL, Kelley CA, DeMaegd M, Benyamin R. Genomics of the effect of spinal cord stimulation on an animal model of neuropathic pain. Neuromodulation. 2016;19:576‐586. [DOI] [PubMed] [Google Scholar]
- 327. Weinzierl A, Ampofo E, Menger MD, Laschke MW. Tissue‐protective mechanisms of bioactive phytochemicals in flap surgery. Front Pharmacol. 2022;13:864351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Behl T, Upadhyay T, Singh S, et al. Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules. 2021;26:6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Gandhi G, Jothi G, Mohana T, et al. Anti‐inflammatory natural products as potential therapeutic agents of rheumatoid arthritis: a systematic review. Phytomedicine. 2021;93:153766. [DOI] [PubMed] [Google Scholar]
- 330. Goh Y, Jalil J, Lam KW, Husain K, Premakumar CM. Genistein: a review on its anti‐inflammatory properties. Front Pharmacol. 2022;13:820969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Mahmoud A, Sayed AM, Ahmed OS, Abdel‐Daim MM, Hassanein EHM. The role of flavonoids in inhibiting IL‐6 and inflammatory arthritis. Curr Top Med Chem. 2022;22:746‐768. doi: 10.2174/1568026622666220107105233 [DOI] [PubMed] [Google Scholar]
- 332. Sood A, Kumar B, Singh SK, et al. Flavonoids as potential therapeutic agents for the management of diabetic neuropathy. Curr Pharm des. 2020;26:5468‐5487. [DOI] [PubMed] [Google Scholar]
- 333. Ferraz C, Carvalho TT, Manchope MF, et al. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre‐clinical and clinical data, and pharmaceutical development. Molecules. 2020;25:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Uddin M, Mamun AA, Rahman MA, et al. Exploring the promise of flavonoids to combat neuropathic pain: from molecular mechanisms to therapeutic implications. Front Neurosci. 2020;14:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Siddiqui M, Abdellatif B, Zhai K, Liskova A, Kubatka P, Büsselberg D. Flavonoids alleviate peripheral neuropathy induced by anticancer drugs. Cancers (Basel). 2021;13:1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Stella B, Baratta F, Della Pepa C, Arpicco S, Gastaldi D, Dosio F. Cannabinoid formulations and delivery systems: current and future options to treat pain. Drugs. 2021;81:1513‐1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Uddin M, Mamun AA, Sumsuzzman DM, et al. Emerging promise of cannabinoids for the management of pain and associated neuropathological alterations in Alzheimer's disease. Front Pharmacol. 2020;11:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Singla R, Guimarães AG, Zengin G. Editorial: application of plant secondary metabolites to pain Neuromodulation. Front Pharmacol. 2021;11:623399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Chen S, Fang XQ, Zhang JF, et al. Lycorine protects cartilage through suppressing the expression of matrix metalloprotenases in rat chondrocytes and in a mouse osteoarthritis model. Mol Med Rep. 2016;14:3389‐3396. [DOI] [PubMed] [Google Scholar]
- 340. Zhang W, Yang J, Chen Y, et al. Lycorine hydrochloride suppresses stress‐induced premature cellular senescence by stabilizing the genome of human cells. Aging Cell. 2021;20:e13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Zhang C, Peng X, Wang F, Xie Z, Chen L, Wu X. Update on the correlation between mitochondrial dysfunction and intervertebral disk degeneration. DNA Cell Biol. 2022;41:257‐261. [DOI] [PubMed] [Google Scholar]
- 342. Kicinska A, Jarmuszkiewicz W. Flavonoids and mitochondria: activation of Cytoprotective pathways? Molecules. 2020;25:3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Koklesova L, Liskova A, Samec M, et al. Protective effects of flavonoids against Mitochondriopathies and associated pathologies: focus on the predictive approach and personalized prevention. Int J Mol Sci. 2021;22:8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Lagoa R, Graziani I, Lopez‐Sanchez C, Garcia‐Martinez V, Gutierrez‐Merino C. Complex I and cytochrome c are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim Biophys Acta. 2011;1807:1562‐1572. [DOI] [PubMed] [Google Scholar]
- 345. Cerdá B, Tomás‐Barberán FA, Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak‐aged wine in humans: identification of biomarkers and individual variability. J Agr Food Chem. 2005;53:227‐235. [DOI] [PubMed] [Google Scholar]
- 346. Selma M, Beltrán D, Luna MC, et al. Isolation of human intestinal bacteria capable of producing the bioactive metabolite Isourolithin a from Ellagic acid. Front Microbiol. 2017;8:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. D'Amico D, Olmer M, Fouassier AM, et al. Urolithin A improves mitochondrial health, reduces cartilage degeneration, and alleviates pain in osteoarthritis. Aging Cell. 2022;21(8):e13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut‐disc axis: a cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31:917‐925. [DOI] [PubMed] [Google Scholar]
- 349. Rajasekaran S, Soundararajan DCR, Tangavel C, et al. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J. 2020;29:1621‐1640. [DOI] [PubMed] [Google Scholar]
- 350. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain‐gut Axis in psychiatric and inflammatory disorders. Front Psych. 2018;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Cryan J, O'Mahony SM. The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23:187‐192. [DOI] [PubMed] [Google Scholar]
- 352. Giridharan V, de Quevedo CEB, Petronilho F. Microbiota‐gut‐brain axis in the Alzheimer's disease pathology: an overview. Neurosci Res. 2022;181:17‐21. [DOI] [PubMed] [Google Scholar]
- 353. Pagano C, Navarra G, Coppola L, Avilia G, Bifulco M, Laezza C. Cannabinoids: therapeutic use in clinical practice. Int J Mol Sci. 2022;23:3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Peng J, Fan M, An C, Ni F, Huang W, Luo J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin Pharmacol Toxicol. 2022;130:439‐456. [DOI] [PubMed] [Google Scholar]
- 355. Zhang X, Li J, Gu J, Zeng YQ. Roles of Cannabidiol in the treatment and prevention of Alzheimer's disease by multi‐target actions. Mini Rev Med Chem. 2022;22:43‐51. [DOI] [PubMed] [Google Scholar]
- 356. Altmann C, Hardt S, Fischer C, et al. Progranulin overexpression in sensory neurons attenuates neuropathic pain in mice: role of autophagy. Neurobiol Dis. 2016;96:294‐311. [DOI] [PubMed] [Google Scholar]
- 357. Altmann C, Vasic V, Hardt S, et al. Progranulin promotes peripheral nerve regeneration and reinnervation: role of notch signaling. Mol Neurodegener. 2016;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Guo J, Jing PB, Wang JA, et al. Increased autophagic activity in dorsal root ganglion attenuates neuropathic pain following peripheral nerve injury. Neurosci Lett. 2015;599:158‐163. [DOI] [PubMed] [Google Scholar]
- 359. Balta M, Loos BG, Nicu EA. Emerging concepts in the resolution of periodontal inflammation: a role for Resolvin E1. Front Immunol. 2017;8:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Gerlach B, Marinello M, Heinz J, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27:525‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Lim J, Park CK, Hwang SW. Biological roles of Resolvins and related substances in the resolution of pain. Biomed Res Int. 2015;2015:83093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Serhan C, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega‐3 fatty acid transformation circuits initiated by aspirin treatment that counter pro‐inflammation signals. J Exp Med. 2002;196:1025‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Chiurchiù V, Leuti A, Dalli J, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8:353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Ji R, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Serhan C, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. J Clin Invest. 2018;128:2657‐2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Liu Z, Miao GS, Wang JN, Yang CX, Fu ZJ, Sun T. Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor‐κB, Phospho‐extracellular signal‐regulated kinase, and pro‐ and Antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology. 2016;124(4):934‐944. [DOI] [PubMed] [Google Scholar]
- 367. Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti‐nociception. Br J Pharmacol. 2010;161:707‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Shevalye H, Yorek MS, Coppey LJ, et al. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol. 2015;114:199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Mielczarek P, Hartman K, Drabik A, et al. Hemorphins‐from discovery to functions and pharmacology. Molecules. 2021;26:3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Ali A, Alzeyoudi SAR, Almutawa SA, Alnajjar AN, Vijayan R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol Res. 2020;158:104855. [DOI] [PubMed] [Google Scholar]
- 371. Ayoub M, Vijayan R. Hemorphins targeting G protein‐coupled receptors. Pharmaceuticals (Basel). 2021;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Wei F, Zhao L, Jing Y. Hemoglobin‐derived peptides and mood regulation. Peptides. 2020;127:170268. [DOI] [PubMed] [Google Scholar]
- 373. Satoh N, Tagawa K, Takahashi H. How was the notochord born? Evol Dev. 2012;14:56‐75. doi: 10.1111/j.1525-142X.2011.00522.x [DOI] [PubMed] [Google Scholar]
- 374. McCann MR, Tamplin OJ, Rossant J, Seguin CA. Tracing notochord‐derived cells using a Noto‐cre mouse: implications for intervertebral disc development. Dis Model Mech. 2012;5:73‐82. doi: 10.1242/dmm.008128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129‐137. doi: 10.1006/excr.1998.4287 [DOI] [PubMed] [Google Scholar]
- 376. Hansen HJ. A pathologic‐anatomical study on disc degeneration in dog, with special reference to the so‐called enchondrosis intervertebralis. Acta Orthop Scand Suppl. 1952;11:1‐117. [DOI] [PubMed] [Google Scholar]
- 377. Echelard Y, Epstein DJ, St‐Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417‐1430. doi: 10.1016/0092-8674(93)90627-3 [DOI] [PubMed] [Google Scholar]
- 378. Hirano S, Hirako R, Kajita N, Norita M. Morphological analysis of the role of the neural tube and notochord in the development of somites. Anat Embryol (Berl). 1995;192:445‐457. doi: 10.1007/bf00240377 [DOI] [PubMed] [Google Scholar]
- 379. Fleming A, Keynes RJ, Tannahill D. The role of the notochord in vertebral column formation. J Anat. 2001;199:177‐180. doi: 10.1046/j.1469-7580.2001.19910177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503‐2512. doi: 10.1242/dev.01812 [DOI] [PubMed] [Google Scholar]
- 381. Barrionuevo F, Taketo MM, Scherer G, Kispert A. Sox9 is required for notochord maintenance in mice. Dev Biol. 2006;295:128‐140. doi: 10.1016/j.ydbio.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 382. Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280‐286. doi: 10.1016/0168-9525(90)90011-t [DOI] [PubMed] [Google Scholar]
- 383. Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135‐1148. doi: 10.1242/dev.00331 [DOI] [PubMed] [Google Scholar]
- 384. Brown EA, Dickinson PJ, Mansour T, et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc Natl Acad Sci U S A. 2017;114:11476‐11481. doi: 10.1073/pnas.1709082114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Erwin WM, Ashman K, O'Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up‐regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859‐3867. doi: 10.1002/art.22258 [DOI] [PubMed] [Google Scholar]
- 386. Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine (Phila Pa 1976). 2006;31:1094‐1099. doi: 10.1097/01.brs.0000216593.97157.dd [DOI] [PubMed] [Google Scholar]
- 387. Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FW. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. McCann M, Bacher CA, Seguin CA. Exploiting notochord cells for stem cell‐based regeneration of the intervertebral disc. J Cell Commun Signal. 2011;5:39‐43. doi: 10.1007/s12079-010-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Mehrkens A, Matta A, Karim MZ, et al. Notochordal cell‐derived conditioned medium protects human nucleus pulposus cells from stress‐induced apoptosis. Spine J. 2017;17:579‐588. doi: 10.1016/j.spinee.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 390. Oegema TR Jr. The role of disc cell heterogeneity in determining disc biochemistry: a speculation. Biochem Soc Trans. 2002;30:839‐844. doi: 10.1042/bst0300839 [DOI] [PubMed] [Google Scholar]
- 391. McCann MR, Seguin CA. Notochord cells in intervertebral disc development and degeneration. J Dev Biol. 2016;4(1):3. doi: 10.3390/jdb4010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976). 2006;31:873‐882. doi: 10.1097/01.brs.0000209302.00820.fd [DOI] [PubMed] [Google Scholar]
- 393. Cheung KM et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty‐three individuals. Spine (Phila Pa 1976). 2009;34:934‐940. doi: 10.1097/BRS.0b013e3181a01b3f [DOI] [PubMed] [Google Scholar]
- 394. Gornet MG, Peacock J, Claude J, et al. Magnetic resonance spectroscopy (MRS) can identify painful lumbar discs and may facilitate improved clinical outcomes of lumbar surgeries for discogenic pain. Eur Spine J. 2019;28:674‐687. doi: 10.1007/s00586-018-05873-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1: Supporting Information.


