Abstract
Background:
The long-term effects of traumatic brain injury (TBI) with loss of consciousness (LOC) on magnetic resonance imaging (MRI) markers of brain health and on dementia risk are still debated.
Objective:
To investigate the associations of history of TBI with LOC with incident dementia and neuroimaging markers of brain structure and small vessel disease lesions.
Methods:
The analytical sample consisted in 4,144 participants aged 65 and older who were dementia-free at baseline from the Three City –Dijon study. History of TBI with LOC was self-reported at baseline. Clinical Dementia was assessed every two to three years, up to 12 years of follow-up. A subsample of 1,675 participants <80 years old underwent a brain MRI at baseline. We investigated the associations between history of TBI with LOC and 1) incident all cause and Alzheimer’s disease (AD) dementia using illness-death models, and 2) neuroimaging markers at baseline.
Results:
At baseline, 8.3% of the participants reported a history of TBI with LOC. In fully-adjusted models, participants with a history of TBI with LOC had no statistically significant differences in dementia risk (HR = 0.90, 95% CI = 0.60–1.36) or AD risk (HR = 1.03, 95% CI = 0.69–1.52), compared to participants without TBI history. History of TBI with LOC was associated with lower white matter volume (β= –4.58, p = 0.048), but not with other brain volumes, white matter hyperintensities volume, nor covert brain infarct.
Conclusion:
This study did not find evidence of an association between history of TBI with LOC and dementia or AD dementia risks over 12-year follow-up, brain atrophy, or markers of small vessel disease.
Keywords: Alzheimer’s disease, brain MRI, dementia, traumatic brain injury
INTRODUCTION
Dementia is a burdensome syndrome with around 43.8 million cases estimated worldwide and 878,000 cases in France in 2016 [1]. In the absence of curative treatments, identifying modifiable factors that may reduce dementia risk and maintain cognitive functions is critical to establish prevention strategies. Recently, there has been an increased interest regarding traumatic brain injury (TBI) as a potential risk factor for neurodegenerative diseases [2]. TBI is defined as an alteration in brain function, or other evidence of brain pathology, caused by an external force [3]. It varies in severity from mild TBI (concussion) that may result in an alteration of mental status and/or brief loss of consciousness (LOC) (less than 30 min) [4, 5], to moderate and severe TBI that can result in prolonged disorders of consciousness or death. TBIs represent an important economic burden and are often associated with psychological and physical disabilities [6, 7]. A 3.6% increase in incidence rates worldwide from 1990 to 2016 has been reported by the Global Burden of Disease study [8], and the annual prevalence rate of TBI in France in 2016 has been estimated to be between 535 and 593 per 100,000 people [8].
Several reports from prospective studies have shown an association between history of TBI and increased risk of dementia [9–18] or Alzheimer’s disease (AD) dementia [9, 11, 12, 15–17, 19, 20], especially for severe TBI. Two recent cohort studies using nationwide hospitalization records from Denmark and Sweden reported strong increased risk of dementia in the first year following TBI (Hazard Ratio (HR)<6 months = 4.06 (95% Confidence Interval (CI): 3.79–4.34) in the Danish study and HR <1 year = 3.53 (95% CI: 3.23–3.84) in the Swedish study). These associations became weaker with increasing time from TBI (HR 10–12 years = 1.21 (95% CI: 1.10–1.32) in the Danish study and HR 10–20 years = 1.48 (95% CI: 1.39–1.58) in the Swedish study) [17, 18]. However, no association was evidenced in other studies, most of which were population-based studies with self-reported TBIs [21–26]. Self-reported TBIs include TBIs of milder severity that did not result in hospitalization or other medical treatment and this could lead to lower risk estimates compared to study that identified TBI history only based on medical records.
In terms of physiopathology, the potential mechanisms that could link TBI to dementia are still unclear. One hypothesis states that TBI induces axonal injury that could accelerate both propagation of AD-related pathological proteins and neuronal loss [27]. This hypothesis is supported by studies reporting associations between TBI and markers of neuronal loss and brain atrophy such as hippocampal volume or brain volume loss, suggesting that TBI may promote neurodegeneration [27–29]. The contribution of TBI to neurodegeneration may in part be explained by TBIs being linked to AD-related pathology, but results on this association are inconsistent [25, 26, 30–35]. Another hypothesis is that TBI may induce cerebrovascular pathology, such as small vessel disease, which could accelerate dementia onset [36, 37].
The primary aim of this work was thus to investigate the association between history of TBI with LOC and risk of all dementia and AD dementia in the Three City (3C) –Dijon study, a French population-based cohort. A secondary aim was to examine the associations between history of TBI with LOC and MRI markers of neurodegeneration and cerebrovascular disease.
METHODS
Study population
The 3C –Dijon study is a population-based cohort including 4,931 participants from Dijon, France. To be eligible for recruitment into the study, persons had to be 1) registered on the electoral rolls of Dijon city, 2) aged 65 years and over, and 3) not institutionalized. Baseline examinations took place between March 1999 and March 2001. Participants were then followed over 12 years, every 2 or 3 years. Repeated cognitive evaluations, as well as active assessment of dementia cases have been realized at each follow-up. At each study wave, a standardized questionnaire assessing socio-demographic, medical, cognitive, and functional characteristics was administered at home by trained neuropsychologists during face-to-face interviews. At baseline, MRI scans were proposed to participants aged ≤80 years; the MRI scan consent rate was 83%. A total of 1,923 baseline MRI examinations have been performed. Full details of the study have been previously published [38]. This research adhered to the principles of the Declaration of Helsinki. The ethics committee of the Kremlin-Bicêtre University Hospital and Sud-Méditerranée III (France) approved the 3C study protocol. All participants gave written informed consent.
Among the 4,862 dementia-free participants included in the 3C –Dijon study, 4,144 participants had non-missing data on TBI status at baseline and covariates. Among those, 1,675 participants underwent MRI examination before age 80, and have at least one of the studied MRI markers (Supplementary Figure 1). For analyses of incident dementia, participants were followed until death, dropout or until the end of follow-up (12 years).
Outcomes of interest
Dementia and its subtypes
Dementia diagnosis was ascertained using a standardized three-step procedure [38]. The first step was a cognitive evaluation by trained neuropsychologists using a series of psychometric tests. Participants who were suspected of dementia, based on their neuropsychological performance or decline relative to a previous examination were then examined for further medical assessments. Finally, each case was discussed by a validation committee composed of neurologists and geriatricians to classify etiology. The diagnosis of dementia was based on the Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition (DSM-IV) criteria. Dementia subtyping was based on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA) criteria for AD, and on the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia. Mixed dementia was defined as a diagnosis of AD with either cerebrovascular lesions on brain imaging when available or a documented history of stroke and the presence of prominent executive function deficits in addition to an AD-type cognitive profile. We considered all incident cases that occurred during the 12-year follow-up period for the current analyses.
Brain MRI markers
The protocol for brain MRI, using a 1.5-T Magnetom (Siemens, Erlangen, Germany), has been described in detail previously [39]. We selected five image-derived biomarkers: gray and white matter volumes (GM and WM, respectively), brain parenchymal fraction (BPF), hippocampal volume, white matter hyperintensities volume (WMHV), and covert brain infarct (CBI). Using voxel-based morphometry techniques, total intracranial volume (TIV) was computed by summing GM, WM, and cerebrospinal fluid (CSF) volumes. BPF was calculated as the sum of GM and WM volumes divided by TIV. Hippocampal volume was defined as the sum of left and right hemisphere volume [40]. A fully automatic image processing software was developed to detect and quantify WMH [39]. WMHV was calculated by summing the volumes of all the lesions detected. CBI of presumed vascular origin were visually rated on T1-, T2-, and proton density-weighted images. Characteristics of lesions were visualized simultaneously in axial, coronal, and sagittal planes. They were defined as focal lesions 3 to 15 mm in diameter with the same signal characteristics as CSF on all sequences, located in basal ganglia, brainstem, or cerebral WM [41].
Exposure of interest
TBI was assessed at baseline with the following question: “In your life, have you ever had a traumatic brain injury with loss of consciousness?”. The exposure was thus defined as history of TBI with LOC: yes/no.
Covariates
At baseline, sociodemographic information was collected and included date of birth, sex, height (measured), and education level (categorized as less than high school level versus high school level and higher). Potential confounding factors of interest included: APOE ɛ4 status, history of stroke, history of cardiovascular disease (CVD), hypertension, diabetes, physical impairment, and depressive symptoms levels. APOE ɛ4 status was defined as at least one ɛ4 allele carried versus none. The measurement methods for the APOE genotype have been described previously [39]. History of stroke and CVD were self-reported. Hypertension was defined by either measured systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or antihypertensive drug intake. Diabetes was defined as either the presence of fasting blood glucose ≥7 mmol/L or antidiabetic drug intake. Depressive symptomatology was assessed with the Center for Epidemiological Studies-Depression (CESD) scale, using scores of >16 as indicators of a clinically relevant level of depressive symptomatology [42]. Finally, fractures within two years prior to baseline were self-reported. Instrumental activities of daily living (IADL) impairment was defined as having difficulty with at least one activity. Low Mini-Mental State Examination (MMSE) score was defined as a MMSE score <24.
Statistical analysis
Regarding the dementia outcome, the analytical sample comprised 4,144 participants. Comparison of participants included vs excluded due to missing TBI status and covariates was performed. Regarding the MRI outcomes, 1,675 participants were included in the analytical sample and participants included vs excluded due to missing MRI assessment, TBI status or covariates were compared. Participants excluded from analyses were older, were less educated, and had more vascular diseases, more IADL disability, and lower MMSE scores (Supplementary Tables 1 and 2). Participants’ characteristics at baseline according to history of TBI with LOC status were reported using median and interquartile range, or mean and standard deviation. Comparisons were tested using t tests for continuous variables and χ2 statistics for categorical variables. Incidence rates of dementia according to history of TBI with LOC status were also computed.
The relationship between history of TBI with LOC and dementia incidence over 12-year follow-up was investigated using an illness-death model [43]. This model has the advantage to take into account interval censoring of age at dementia owing to the fact that dementia is assessed only at the study visits and dementia onset could have occurred between visits. It also accounts for competing risk of death, right censoring, and left truncation due to the selection of subjects alive and non-demented at inclusion. In this multistate model, individuals start out as healthy (state 0), they may become demented (move to state 1), and afterward they may die (state 2). Individuals may also die without first becoming demented (transition from state 0 to state 2). Transition intensity 01 from state 0 (healthy) to state 1 (dementia) represents the age-specific incidence adjusted for covariates.
In this analysis, participants’ age was considered as the time scale, and we applied a parametric approach with a Weibull distribution on the baseline transition intensities. Participants who remained free of dementia were censored at the age of their last follow-up before drop-out or at the end of follow up. To examine the association between history of TBI with LOC and the risk of dementia, two models were computed: a model only adjusted for sex and a second model additionally adjusted for APOE ɛ4 status, height, hypertension, stroke history, cardiovascular disease history, diabetes, and high depressive symptoms. Similar models were performed with AD dementia as the outcome (non-AD dementia cases were censored at the last visit seen). Then, effect modification by sex, APOE ɛ4 status, and education level were tested using interactions, and stratified analyses were further presented. To further explore potential selection bias due to exclusion of participants with missing data, missing values of TBI history and covariates were imputed by multiple imputation (MI) using chained equations with a fully conditional specification (10 imputed data sets) [44], and the primary analysis was rerun on complete datasets. In a sensitivity analysis, the primary analysis was rerun after excluding participants with a MMSE <27 at baseline in order to assess the impact of potential misclassification on the exposure of interest, as recall bias may arise from subclinical dementia.
The relationship between history of TBI with LOC and the different brain MRI markers (BPF, WM and GM volume, hippocampal volume, log-transformed WMHV, and CBI) was investigated using linear or logistic regression models, as appropriate. The basic model included age at baseline, delay between baseline and MRI assessment, sex, and TIV (except when BPF and CBI were the outcomes of interest). An adjusted model additionally included APOE ɛ4 status, height, hypertension, stroke history, cardiovascular disease history, diabetes, and high depressive symptoms. Effect modification by sex, APOE ɛ4 status, and education level was also examined using interaction terms and stratified analyses were further presented. To account for selection into the MRI subsample, we also applied inverse probability of attrition weighting. The weight (assessing the probability of being included in the analysis) of each participant was obtained by fitted values of a multivariable logistic regression modelling inclusion status (yes/no) as a dependent variable and including history of TBI status, the covariates used in the primary analysis, as well as other covariates (IADL dependency, fracture history), and cognitive test (MMSE and Benton tests [45]). Linear and logistic regressions between history of TBI with LOC and MRI markers were then weighted by the inverse of the stabilized probability of remaining in the sample.
Analyses were conducted in R (version 3.6.0) using SmoothHazard (version 1.4.1) [43].
RESULTS
Baseline characteristics of the analytic samples are presented overall and according to TBI status in Table 1. Among the 4,144 participants, 343 (8.3%) reported a history of TBI with LOC. Overall, mean age was 74.3 years old, with 61.4% women. Compared to unexposed participants, participants reporting a history of TBI with LOC had less often hypertension, and more often non-stroke CVD history. The incidence rate of dementia within the group with history of TBI was 12.3 per 1000 person-years (95% CI: 7.9, 16.7) compared to 12.7 per 1000 person-years (95% CI: 11.3, 14.0) in the group without history of TBI (p = 0.87).
Table 1.
Baseline characteristics of study participants according to TBI status at study entry, the Three City–Dijon study, 1999–2000
| Total Sample | No TBI with LOC | History of TBI | p | |
| (n = 4,144) | (n = 3,801) | with LOC (n = 3,43) | ||
| Women, n (%) | 2,543 (61.4) | 2,370 (62.4) | 173 (50.4) | <0.001 |
| Age, mean (SD) | 74.3 (5.5) | 74.3 (5.5) | 74.0 (5.3) | 0.42 |
| Education level | 0.68 | |||
| Less than high school, n (%) | 2,634 (63.6) | 2,412 (63.5) | 222 (64.7) | |
| High school and above, n (%) | 1,510 (36.4) | 1,389 (36.5) | 121 (35.3) | |
| APOE ɛ4, n (%) | 869 (21.0) | 800 (21.0) | 69 (20.1) | 0.74 |
| Height (cm), mean (SD) | 162 (0.9) | 162 (0.9) | 163 (0.9) | 0.03 |
| Hypertension, n (%) | 3,296 (79.5) | 3,037 (79.9) | 259 (75.5) | 0.06 |
| Stroke history, n (%) | 192 (4.6) | 175 (4.6) | 17 (5.0) | 0.87 |
| CVD history, n (%) | 605 (14.6) | 532 (14.0) | 73 (21.3) | <0.001 |
| Diabetes, n (%) | 387 (9.3) | 356 (9.4) | 31 (9.0) | 0.92 |
| High depressive symptoms, n (%) | 865 (20.9) | 786 (20.7) | 79 (23.0) | 0.34 |
| IADL dependency, n (%) | 370 (8.9) | 337 (8.9) | 33 (9.7) | 0.72 |
| Low baseline MMSE score, n (%) | 193 (4.7) | 175 (4.6) | 18 (5.3) | 0.68 |
TBI, traumatic brain injury; LOC, loss of consciousness; CVD, cardiovascular disease; MMSE, Mini-Mental State Examination.
In both sex adjusted and fully adjusted models, we did not observe any association between history of TBI with LOC and dementia risk (HR = 0.98 (95% CI: 0.68, 1.43) and HR = 0.90 (95% CI: 0.60, 1.36), respectively) (Table 2). When AD dementia was the outcome, a slightly higher hazard in the sex adjusted model was observed but remained non-significant (HR = 1.14 (95% CI: 0.74, 1.74)), and there was no evidence of an association in fully adjusted models (HR = 1.03 (95% CI: 0.69, 1.52)). Results using imputed missing variables are presented in Supplementary Table 3 and yielded similar estimates for dementia risk and estimates closer to the null for AD risk. After exclusion of participants with MMSE scores lower than 27, estimates were slightly higher than for the main findings (dementia HR = 0.99 (95% CI: 0.45–2.18); AD dementia HR = 1.21 (95% CI: 0.65–2.27) for fully adjusted models), yet still non-significant (Supplementary Table 4).
Table 2.
Associations* between history of TBI and risk of dementia and Alzheimer’s disease, the Three City –Dijon study, 1999–2011
| All Dementia | Alzheimer’s disease dementia | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Model 1 | ||||
| TBI history versus not | 0.98 (0.68–1.43) | 0.94 | 1.14 (0.74–1.74) | 0.55 |
| Model 2 | ||||
| TBI history versus not | 0.90 (0.60–1.36) | 0.62 | 1.03 (0.69–1.52) | 0.90 |
TBI, traumatic brain injury. Model 1 adjusted for sex. Model 2: model 1+ education, APOE ɛ4 status, height, stroke history, non-stroke CVD history, diabetes, hypertension, and high depressive symptoms. *Using illness-death models.
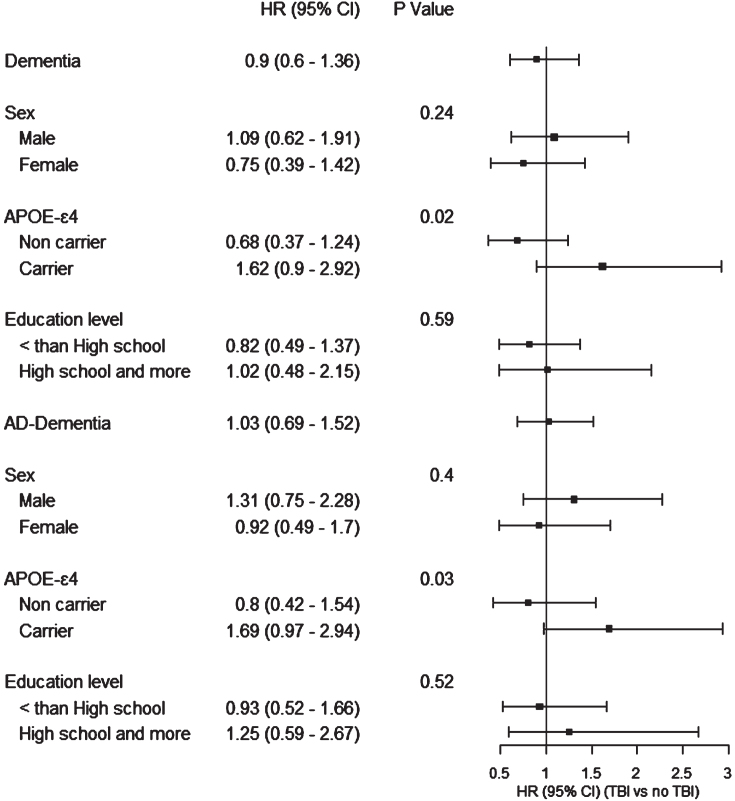
Stratified analysis presenting the association of TBI with dementia or AD dementia by sex, APOE ɛ4 status, and education level are presented in Fig. 1. Interaction was not significant across sex and education level. For APOE ɛ4 status, interaction was significant (p < 0.05), yet with non-significant point estimates in the harmful direction among APOE ɛ4 carriers for dementia risk (HR = 1.62 (95% CI: 0.90–2.92)) and AD dementia (HR = 1.69 (95% CI: 0.97–2.94)).
Fig. 1.
Association between history of TBI and risk of dementia, stratified by sex, APOE ɛ4 status, and education level, the Three City –Dijon study, 1999–2011.
Associations between history of TBI with LOC and brain MRI markers are reported in Table 3 for unweighted and weighted models. Individuals with history of TBI with LOC presented on average significantly lower WM volume compared with individuals without (adjusted unweighted model: beta = –4.58 (95% CI: –9.12, –0.04), p = 0.048). BPF, GM volume, hippocampal volume, WMHV, and CBI did not differ according to history of TBI with LOC status, in both sex and fully adjusted models. Weighted analyses yielded similar results. We did not find evidence any significant effect modification by sex, education, or APOE ɛ4 in analyses of neuroimaging markers (Supplementary Table 5).
Table 3.
Associations between history of TBI and MRI brain markers, the Three City –Dijon study, 1999–2011 (N = 1,675)
| Model 1 | Model 2 | Weighted* model 1 | Weighted* model 2 | |||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| BPF | ||||||||
| TBI history versus not | –0.36 (–0.88, 0.16) | 0.17 | –0.34 (–0.86, 0.17) | 0.19 | –0.41 (–0.92, 0.11) | 0.12 | –0.36 (–0.87, 0.15) | 0.16 |
| White matter volume | ||||||||
| TBI history versus not | –4.91 (–9.46, –0.38) | 0.03 | –4.58 (–9.12, –0.04) | 0.05 | –4.94 (–9.39, –0.49) | 0.03 | –4.59 (–9.04, –0.14) | 0.04 |
| Grey matter volume | ||||||||
| TBI history versus not | 0.21 (–4.66, 5.08) | 0.93 | 0.11 (–4.71, 4.92) | 0.96 | –0.24 (–5.02, 4.54) | 0.92 | –0.04 (–4.77, 4.68) | 0.99 |
| Hippocampal volume | ||||||||
| TBI history versus not | 0.003 (–0.10, 0.11) | 0.95 | 0.006 (–0.10, 0.11) | 0.90 | 0.05 (–0.06, 0.16) | 0.36 | 0.05 (–0.05, 0.16) | 0.32 |
| WMHV | ||||||||
| TBI history versus not | –0.07 (–0.19, 0.04) | 0.19 | –0.07 (–0.18, 0.04) | 0.20 | –0.10 (–0.21, 0.01) | 0.08 | –0.10 (–0.21, 0.01) | 0.09 |
| CBI** | ||||||||
| TBI history versus not | 1.02 (0.55–1.77) | 0.94 | 0.97 (0.51–1.71) | 0.91 | 1.27 (0.76–2.04) | 0.34 | 1.19 (0.69–1.95) | 0.52 |
BPF, brain parenchymal fraction; WMHV, white matter hyperintensities volume; CBI, covert brain infarct. Model 1 adjusted for sex, age at baseline, and delay between age at baseline and age at MRI. Model 2: model 1+ education, APOE ɛ4 status, height, stroke history, non-stroke CVD history, diabetes, hypertension, fracture, and high depressive symptoms. Each model was adjusted for total intracranial volume, except for brain parenchymal fraction and covert brain infarct. Brain volumes unit is cm3, WMHV was log-transformed. *Analysis using IPW to account for selection into the MRI subsample. **Estimates shown are odd ratios with 95% confidence intervals.
DISCUSSION
In a French prospective cohort of older adults free of dementia at baseline, we did not find support for an association between history of TBI with LOC and risk of dementia or AD over 12 years of follow-up. Our data showed a trend for an increased risk of dementia and AD dementia limited to APOE ɛ4 carriers. Moreover, history of TBI with LOC was only related to lower WM volume, but not with hippocampal volume, nor with markers of small vessel disease, as measured by brain MRI; these associations were not modified by APOE ɛ4 status.
Our results are consistent with several studies [22, 24–26, 30]. In particular, Crane et al. found no association between TBI with LOC and incident dementia, incident AD, or AD neuropathologic outcomes from autopsy in a pooled analysis of over 7,000 participants from three US cohort studies [25]. A recent study among autopsy subjects from the National Alzheimer’s Coordinating Center also did not show association between TBI history and neither dementia nor AD neuropathologic changes [26]. Finally, our previous work on 2,718 participants of the Health and Retirement Study also failed to evidence an association between TBI and dementia incidence [24]. This body of literature is conflicting with other well-powered studies showing higher risks of dementia or AD for individuals with history of TBI [9, 10, 16–18, 46].
These inconsistent associations’ reports may be related to heterogeneous definition of TBI exposure across studies. Indeed, in most studies that reported links between TBI and higher dementia risk, TBI exposure status was attributed from hospitalization/medical records, i.e., restricted to more severe TBI cases that require hospitalization or other medical treatment. TBI exposure defined from medical records may miss some self-reported TBI cases that did not seek any medical care and were therefore milder. This is in line with the higher prevalence of TBI observed in population-based studies with self-reported definition of TBI exposure in comparison with studies relying on national registries or medical claims [21, 22, 24–26]. Yet, mild TBIs have also been shown to be associated with higher dementia risk [46, 47], even if these studies were also often based on clinically-diagnosed TBIs, identifying mild TBIs that are severe enough to require hospitalization or notable care. Definitions for mild TBI often vary across studies, which may further contribute to inconsistencies in the literature. Moreover, the two nationwide studies reported the highest risk of dementia in the first year after TBI, suggesting claims datasets could conflate acute TBI-related cognitive change with the progressive development of dementia. For studies based on self-reported TBIs, information most often lacks regarding severity, delay since the exposure and number of TBIs sustain, therefore preventing to explore the specific impact of TBI types on dementia risk. In addition, differences across studies may also be related to the different types of assessment of dementia, with studies identifying dementia cases using medical records potentially missing undiagnosed cases among non TBI cases, thus potentially overestimating the associations. Studies settings may also influence results. History of TBI has consistently been showed to be associated with higher dementia risk among US military veterans [12–14], who are more likely to have severe TBI history. However, focus on this specific population also raise concerns about unmeasured confounding. For example, psychiatric comorbidities, such as post-traumatic stress disorder, depression, or presence of multiple traumas, are frequent among veterans and are also risk factors for dementia, and could account partly for the observed increased risk.
We found a non-significant increased risk of dementia and AD dementia in TBI participants carrying APOE ɛ4. This trend is in agreement with previous evidence of an impact of genetic factors on TBI-related neurodegenerative diseases [48–51]. The non-significance of our result may be due to a limited power due to the APOE ɛ4 subsample size (n = 801). In addition, our results did not show significant differences according to educational level nor sex, although previous studies reported mixed results regarding a potentially higher risk of dementia in men with TBI [10, 14, 19, 52].
Investigating the impact of TBI exposure on intermediate markers of dementia risk, such as AD pathology, neurodegeneration, or cerebrovascular lesions, is essential to better understand the impact of TBI on pathological aging. In this work, we evidenced lower WM brain volume for participants reporting a history of TBI with LOC, while we did not find any significant associations or trends with GM volume, hippocampal volume, nor cerebrovascular lesions. TBI can result in focal brain injury such as contusions. Our findings of an association between TBI with LOC history and lower WM volumes are in line with diffusion tensor imaging studies that are more sensitive than standard magnetic resonance techniques to WM damage following TBI [53]. These studies suggest cortical contusion can be followed by axonal damage. Tissue injury evolves over time with the development of macrophage infiltration, tissue edema, and demyelination [53]. These WM changes could lead to WM tract and neural network disruptions that could increase dementia risk. Whether TBI may also trigger AD-specific neuropathological processes or cerebrovascular disease development need further investigations. Indeed, results from well powered autopsy-based studies have been mixed regarding the cross-sectional association between TBI history and neuropathological measures [25, 26, 31, 35]. Although some studies reported lower hippocampal volume [54], higher amyloid deposition [32–35], as well as higher cerebrovascular load following a TBI with LOC [25, 35, 55], others did not evidence higher hippocampal atrophy [25, 30–32, 35], higher AD neuropathological changes [25, 26, 30, 31] or higher WM lesions load or more frequent brain infarcts among participants with history of TBI with LOC [26, 30, 31, 35].
The principal limitation of this study lies in the definition of TBI exposure, which is self-reported, with missing information regarding the duration of the LOC, as well as the date and severity of the TBIs. Our TBI exposure relies on participants recall and may be subject to misclassification bias. Some participants with no self-reported TBI with LOC may have had TBI without LOC, possibly driving the associations toward the null as a few studies evidenced negative effect of concussive TBI without LOC [46]. Studies using self-reported TBIs may thus under-estimate the true TBI prevalence. However, the sensitivity analysis excluding participants with low cognitive performances at baseline led to similar results, limiting concerns about differential misclassification. Finally, the number of TBI sustained was not collected in the 3C study, and individuals reporting a history of TBI may have sustained a single mild TBI, potentially explaining the lack of associations. These differences in TBI definitions may explain the conflicting findings across the literature, and future studies should attempt to address this gap by using more accurate TBI assessment. Selection bias is another potential limitation of our study, especially within the MRI subsample. However, analysis using multiple imputation and IPW to account for selection yielded similar results. In addition, our study included participants aged 65 years and older (mean age at baseline = 74), leading to a sample of older age compared to many studies reporting significant associations between TBI history and dementia. The inclusion of older participants may have led to the selection of people with history of TBI who survived longer and were free of dementia before the age of 65, had milder TBI, and are therefore healthier. In the sample, participants with history of TBI only differed from participants with no TBI regarding higher history of cardiovascular disease, whereas other health characteristics did not differ. The selection of healthier participants with history of TBI with LOC could lead to an underestimation of the association of history of TBI with LOC with dementia. Finally, although major risk factors for dementia were adjusted for in these analyses, residual confounding may remain.
Despite these limitations, this study has different strengths. Our study population is a prospective, population-based cohort with sufficient follow-up and sample size, providing an adequate setting to detect the long-term association between history of TBI with LOC and risk of dementia. Additionally, this work relies on well-defined dementia cases, where each participant was evaluated at home for cognitive and physical impairment, and each case was reviewed by a validation committee. Finally, the availability of MRI markers of brain health allowed us to investigate different intermediate markers which could further our understanding of the long-lasting effect of TBI with LOC exposure.
In conclusion, our results did not evidence a global association between history of TBI with LOC and dementia, AD risk, or MRI markers of brain health, yet with a trend toward higher risk of dementia associated with history of TBI among APOE ɛ4 carriers only. Future studies in the general population with a more comprehensive assessment of TBI exposure would be needed to confirm those results, and determine whether other potential risk factors may influence the association of TBI with dementia.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220658.
FUNDING
The 3C study is conducted under a partnership agreement between the Institut National de la Sante et de la Recherche Medicale (INSERM), the Victor Segalen-Bordeaux II University, and Sanofi-Aventis. The Fondation pour la Recherche Medicale funded the preparation and initiation of the study. The Fondation Plan Alzheimer partly funded the follow-up of the study. The 3C study is also supported by the Caisse Nationale Maladie des Travailleurs Salaries, Direction Generale de la Sante, Mutuelle Générale de l’Education Nationale, Institut de la Longevite, Conseils Regionaux of Aquitaine and Bourgogne, Fondation de France, la Caisse Nationale de Solidarité et d’Autonomie, and the Ministry of Research-INSERM Programme Cohortes et collections de donnees biologiques.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data will be made available upon request to E3C.CoordinatingCenter@gmail.com through a specific research proposal.
REFERENCES
- [1]. GBD 2016 Dementia Collaborators (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. LoBue C, Cullum CM, Didehbani N, Yeatman K, Jones B, Kraut MA, Hart J Jr (2018) Neurodegenerative dementias after traumatic brain injury. J Neuropsychiatry Clin Neurosci 30, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Soderberg J, Stanworth SJ, Stein MB, von Steinbuchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K, In TP, Investigators (2017) Traumatic braininjury: Integrated approaches to improve prevention, clinical care,and research. Lancet Neurol 16, 987–1048. [DOI] [PubMed] [Google Scholar]
- [4]. National Center for Injury Prevention and Control (2003) Report to Congress on Mild Traumatic Brain Injury in hte United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention, Atlanta, GA.
- [5]. Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, Dahlberg C, Gerber D, Goka R, Harley P, Hilt J, Horn L, Lehmkuhl D, Malec J (1993) Definition of mild traumatic brain injury. J Head Trauma 8, 86–87. [Google Scholar]
- [6]. Schneider ALC, Wang D, Gottesman RF, Selvin E (2021) Prevalence of disability associated with head injury with loss of consciousness in adults in the United States: A population-based study. Neurology 97, e124–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS (2010) Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Injury GBDTB, Spinal Cord Injury C (2019) Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC (2000) Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55, 1158–1166. [DOI] [PubMed] [Google Scholar]
- [10]. Schneider ALC, Selvin E, Latour L, Turtzo LC, Coresh J, Mosley T, Ling G, Gottesman RF (2021) Head injury and 25-year risk of dementia. Alzheimers Dement 17, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Wang HK, Lin SH, Sung PS, Wu MH, Hung KW, Wang LC, Huang CY, Lu K, Chen HJ, Tsai KJ (2012) Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 83, 1080–1085. [DOI] [PubMed] [Google Scholar]
- [12]. Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K (2014) Traumatic brain injury and risk of dementia in older veterans. Neurology 83, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K (2014) Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol 71, 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Yaffe K, Lwi SJ, Hoang TD, Xia F, Barnes DE, Maguen S, Peltz CB (2019) Military-related risk factors in female veterans and risk of dementia. Neurology 92, e205–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Perry DC, Sturm VE, Peterson MJ, Pieper CF, Bullock T, Boeve BF, Miller BL, Guskiewicz KM, Berger MS, Kramer JH, Welsh-Bohmer KA (2016) Association of traumatic brain injury with subsequent neurological and psychiatric disease: A meta-analysis. J Neurosurg 124, 511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Schaffert J, LoBue C, White CL, Chiang HS, Didehbani N, Lacritz L, Rossetti H, Dieppa M, Hart J, Cullum CM (2018) Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology 32, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Fann JR, Ribe AR, Pedersen HS, Fenger-Gron M, Christensen J, Benros ME, Vestergaard M (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: A population-based observational cohort study. Lancet Psychiatry 5, 424–431. [DOI] [PubMed] [Google Scholar]
- [18]. Nordstrom A, Nordstrom P (2018) Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med 15, e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J Jr, Womack KB, Didehbani N, Lacritz LH, Rossetti HC, Cullum CM (2017) Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol 31, 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK (2013) Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: A population-based study. J Neurol Neurosurg Psychiatry 84, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, Breteler MM (1999) Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology 53, 1959–1962. [DOI] [PubMed] [Google Scholar]
- [23]. Julien J, Joubert S, Ferland MC, Frenette LC, Boudreau-Duhaime MM, Malo-Veronneau L, de Guise E (2017) Association of traumatic brain injury and Alzheimer disease onset: A systematic review. Ann Phys Rehabil Med 60, 347–356. [DOI] [PubMed] [Google Scholar]
- [24]. Grasset L, Glymour MM, Yaffe K, Swift SL, Gianattasio KZ, Power MC, Zeki Al Hazzouri A (2020) Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimers Dement 16, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ, Bennett DA, Leurgans S, Schneider JA, Larson EB (2016) Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 73, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Sugarman MA, McKee AC, Stein TD, Tripodis Y, Besser LM, Martin B, Palmisano JN, Steinberg EG, O’Connor MK, Au R, McClean M, Killiany R, Mez J, Weiner MW, Kowall NW, Stern RA, Alosco ML (2019) Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement 15, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. LoBue C, Munro C, Schaffert J, Didehbani N, Hart J, Batjer H, Cullum CM (2019) Traumatic brain injury and risk of long-term brain changes, accumulation of pathological markers, and developing dementia: A review. J Alzheimers Dis 70, 629–654. [DOI] [PubMed] [Google Scholar]
- [28]. Cole JH, Jolly A, de Simoni S, Bourke N, Patel MC, Scott G, Sharp DJ (2018) Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain 141, 822–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Warner MA, Youn TS, Davis T, Chandra A, Marquez de la Plata C, Moore C, Harper C, Madden CJ, Spence J, McColl R, Devous M, King RD, Diaz-Arrastia R (2010) Regionally selective atrophy after traumatic axonal injury. Arch Neurol 67, 1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, Tosun D, Veitch DP, Jack CR Jr, Decarli C, Saykin AJ, Grafman J, Neylanthe TC, Department of Defense Alzheimer’s Disease Neuroimaging Initiative (2017) Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam Veterans using the Alzheimer’s Disease Neuroimaging Initiative: Preliminary Report. Alzheimers Dement (N Y) 3, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Chosy EJ, Gross N, Meyer M, Liu CY, Edland SD, Launer LJ, White LR (2020) Brain injury and later-life cognitive impairment and neuropathology: The Honolulu-Asia Aging Study. J Alzheimers Dis 73, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Mielke MM, Savica R, Wiste HJ, Weigand SD, Vemuri P, Knopman DS, Lowe VJ, Roberts RO, Machulda MM, Geda YE, Petersen RC, Jack CR Jr (2014) Head trauma and measures of amyloid and neurodegeneration in a population-based study. Neurology 82, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Mohamed AZ, Nestor PJ, Cumming P, Nasrallah FA, Alzheimer’s Disease Neuroimaging Initiative (2022) Traumatic brain injury fast-forwards Alzheimer’s pathology: Evidence from amyloid positron emission tomorgraphy imaging. J Neurol 269, 873–884. [DOI] [PubMed] [Google Scholar]
- [34]. Schneider ALC, Selvin E, Liang M, Latour L, Turtzo LC, Koton S, Coresh J, Mosley T, Whitlow CT, Zhou Y, Wong DF, Ling G, Gottesman RF (2019) Association of head injury with brain amyloid deposition: The ARIC-PET Study. J Neurotrauma 36, 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Agrawal S, Leurgans SE, James BD, Barnes LL, Mehta RI, Dams-O’Connor K, Mez J, Bennett DA, Schneider JA (2022) Association of traumatic brain injury with and without loss of consciousness with neuropathologic outcomes in community-dwelling older persons. JAMA Netw Open 5, e229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Ramos-Cejudo J, Wisniewski T, Marmar C, Zetterberg H, Blennow K, de Leon MJ, Fossati S (2018) Traumatic brain injury and Alzheimer’s disease: The cerebrovascular link. EBioMedicine 28, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, Diamandis T, Borlongan MC, Tajiri N, Borlongan CV (2013) Vascular damage: A persisting pathology common to Alzheimer’s disease and traumatic brain injury. Med Hypotheses 81, 842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. The 3C Study Group (2003) Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 22, 316–325. [DOI] [PubMed] [Google Scholar]
- [39]. Maillard P, Delcroix N, Crivello F, Dufouil C, Gicquel S, Joliot M, Tzourio-Mazoyer N, Alperovitch A, Tzourio C, Mazoyer B (2008) An automated procedure for the assessment of white matter hyperintensities by multispectral (T1, T2, PD) MRI and an evaluation of its between-centre reproducibility based on two large community databases. Neuroradiology 50, 31–42. [DOI] [PubMed] [Google Scholar]
- [40]. Crivello F, Lemaitre H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B (2010) Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage 53, 1064–1069. [DOI] [PubMed] [Google Scholar]
- [41]. Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C (2012) Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 78, 720–727. [DOI] [PubMed] [Google Scholar]
- [42]. Radloff L (1977) The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1, 385–401. [Google Scholar]
- [43]. Touraine C, Helmer C, Joly P (2016) Predictions in an illness-death model. Stat Methods Med Res 25, 1452–1470. [DOI] [PubMed] [Google Scholar]
- [44]. van Buuren S, Groothuis-Oudshoorn K (2011) mice: Multivariate imputation by chained equations in R. J Stat Softw 45, 1–67. [Google Scholar]
- [45]. Benton A (1965) Manuel Pour L’Application du Test de Retention Visuelle. Applications Cliniques et Expérimentales. Deuxième édition française. Centre de Psychology Appliquée,, Paris, France.
- [46]. Mielke MM, Ransom JE, Mandrekar J, Turcano P, Savica R, Brown AW (2022) Traumatic brain injury and risk of Alzheimer’s disease and related dementias in the population. }. J Alzheimers Dis 88, 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Snowden TM, Hinde AK, Reid HMO, Christie BR (2020) Does mild traumatic brain injury increase the risk for dementia? A systematic review and meta-analysis. J Alzheimers Dis 78, 757–775. [DOI] [PubMed] [Google Scholar]
- [48]. Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M (1995) Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 45, 555–557. [DOI] [PubMed] [Google Scholar]
- [49]. Sundstrom A, Marklund P, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L (2004) APOE influences on neuropsychological function after mild head injury: Within-person comparisons. Neurology 62, 1963–1966. [DOI] [PubMed] [Google Scholar]
- [50]. Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L (2007) Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr 19, 159–165. [DOI] [PubMed] [Google Scholar]
- [51]. Ariza M, Pueyo R, Matarin Mdel M, Junque C, Mataro M, Clemente I, Moral P, Poca MA, Garnacho A, Sahuquillo J (2006) Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry 77, 1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Farace E, Alves WM (2000) Do women fare worse: A metaanalysis of gender differences in traumatic brain injury outcome. J Neurosurg 93, 539–545. [DOI] [PubMed] [Google Scholar]
- [53]. Mendez MF (2017) What is the relationship of traumatic brain injury to dementia? . J Alzheimers Dis 57, 667–681. [DOI] [PubMed] [Google Scholar]
- [54]. Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B (1997) Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 18, 11–23. [PMC free article] [PubMed] [Google Scholar]
- [55]. Berginstrom N, Nordstrom P, Nyberg L, Nordstrom A (2020) White matter hyperintensities increases with traumatic brain injury severity: Associations to neuropsychological performance and fatigue. Brain Inj 34, 415–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request to E3C.CoordinatingCenter@gmail.com through a specific research proposal.