Graphical abstract
Keywords: Traditional Huangjiu, Flavors dynamics, Microbial community, Fermentation process, Correlation analysis
Highlights
-
•
Concentrations of alcohols decreased mostly while esters changed inversely during traditional Huangjiu fermentation.
-
•
Saccharomyces, Aspergillus and Saccharopolyspora were predominant during fermentation.
-
•
Core microorganisms were positively correlated with most characteristic aroma compounds.
-
•
Winter brewing facilitates psychrophilic microbes growth affecting flavors formation.
Abstract
Traditional Huangjiu produced around Winter Solstice has higher quality and a more harmonious aroma. To investigate the variations of volatile metabolites and microbial communities during fermentation, gas chromatography–ion migration chromatography (GC–IMS), gas chromatography–mass spectroscopy (GC–MS) and high–throughput sequencing were employed. Aroma compounds results showed that alcohols and phenols increased before 45 days of fermentation and then decreased after 45 days, while esters gradually increased. Fungal genera Saccharomyces, Aspergillu, and Rhizomucor were dominant, whereas Staphylococcus, Pediococcus and Weissella were the dominant bacterial genera in the late stage. In addition, 11 genera such as Lactobacillus, Saccharopolyspora and Aspergillus (|r| > 0.6, p < 0.05) may contributed to traditional Huangjiu ecosystem stability. Moreover, correlation analysis indicated the dominant microorganisms (Saccharopolyspora, Staphylococcus, Lactobacillus, Saccharomyces and Aspergillus) were positively correlated with key compounds. These results provided theoretical guidance for further study on the flavor regulation of traditional Huangjiu via microbial community level and microbial augmentation.
1. Introduction
Traditional Huangjiu (a kind of Chinese rice wine) is extremely popular among consumers in East Asia due to its flavor characteristics of mellow, soft, sweet, fresh and clean (Mao, 2018). The old saying that “Traditional Huangjiu starts from the winter begins, and ends in the spring begins” (Fu, 2018) intuitively reveals that it is brewed under a low ambient temperature and its brewing process lasts for almost 90 days, namely winter brewing. Traditional Huangjiu is fermented in an open and non–sterile environment and therefore low ambient temperature conditions during winter brewing could be conducive to controlling the fermentation process and inhibiting the proliferation of infective bacteria which can cause rancidity and decrease the production of flavor compounds (Mao, 2018). Moreover, winter brewing is also beneficial to the long–term action of yeasts, bacteria and other microorganisms at low temperature to accumulate unique flavor substances (Mao, & Xuan, 2006). Traditional Huangjiu put into production near Winter Solstice, the coldest time of year, had better quality and more harmonious flavor according to the production experience. In our previous study (Yu et al., 2021), aroma characteristics of finished traditional Huangjiu (after fermentation) produced under different ambient temperature conditions were revealed and proved that the traditional Huangjiu produced around Winter Solstice had better aroma quality. However, there are no scientific explanations for this phenomenon and the potential relationships between microbial community and flavor compounds during fermentation are still unknown.
Aroma characteristics is an important factor affecting the perceived flavor quality of Huangjiu and consumers’ preferences. The aroma characteristics are the result of the joint action of various volatile compounds (Yang et al., 2020). Esters, alcohols, acids and aldehydes are the skeleton components of aroma in Huangjiu (Chen, Xu, & Qian, 2013), which are mostly generated during fermentation. During mechanized Huangjiu fermentation, most alcohols increased at first and then decreased, most esters increased continuously and then decreased slightly, while acids increased firstly and then decreased rapidly (Wang et al., 2014, Chen et al., 2018). The changes of aroma compounds in the whole fermentation process of millet Chinese rice wine were also described (Ye, Wang, Zhan, Tian, & Liu, 2022), in which the contents of alcohols and esters gradually increased, and acids increased firstly and then decreased with increasing fermentation time, while the aldehydes were inversely proportional to the fermentation time. Moreover, gas chromatography–ion mobility spectrometry (GC–IMS) recently has gradually been applied to characterize the aroma compounds variations and distinguish subtle differences by means of volatile fingerprint in Chinese rice wine (Yu et al., 2021, Zhang et al., 2022). Nevertheless, there are few studies on the analysis of aroma profiles in the fermentation process of traditional Huangjiu.
Complex microbial metabolism played a decisive role in the production of volatile compounds (Liu et al., 2019). Saccharomyces was responsible for the production of alcohol and contributed to aroma compounds in Chinese rice wine (Tian, Zeng, Fang, Zhou, & Du, 2022). Bioaugmentation inoculation of Saccharomyces cerevisiae could increase the content of alcohol and ester (Su, Zhang, Cao, & Yang, 2020). Some genera of moulds including Aspergillus and Rhizopus contributed to the saccharification process through their extracellular enzymes, including amylase and saccharifying enzymes. (Cai et al., 2018, Zhang et al., 2020). Additionally, bacteria also acted on the flavor formation of Huangjiu. The primary role of the bacteria was to produce various flavoring compounds or precursor of volatile compounds (Yang et al., 2020, Zou et al., 2018). In particular, Bacillus played an important role in the formation of esters and pyrazines (Huang et al., 2018). The genera Lactobacillus, Candida and Enterobacter were the major contributors of organic acids (Wang et al., 2014). And Saccharopolyspora was found to be the dominant bacterium in the fermentation process of mechanized Huangjiu (Liu et al., 2019). The microbial succession in Huangjiu revealed that Leuconostoc, Pediococcus, Bacillus, and Lactobacillus dominanted in the late stage, while Rhizopus and Saccharomyces were the predominant fungal genera throughout fermentation (Zhao et al., 2020). To our knowledge, the dynamics of microbial community, and relationships between microbial community and flavor compounds during the long–term fermentation process at low temperature are still not completely clear. Therefore, it is necessary to reveal the succession rule of microbial community during fermentation and to further study on their potential correlations.
The objectives of this study were (1) to analyze the dynamics of aroma compounds in traditional Huangjiu put into production around Winter Solstice by using a combination of GC–IMS and GC–MS analysis, (2) to compare the microbial community diversity and dynamic by using high–throughput sequence, (3) to further reveal the correlation between the dominant community and key aroma compounds during fermentation. This study provided detailed information on the dynamic of flavor compounds and microbial community during traditional Huangjiu fermentation, and might help better clarify the complex relationship between microbial community and aroma compounds, expecting to improve the quality of traditional Huangjiu by means of bioaugmentation with the identified beneficial strains in the future.
2. Materials and methods
2.1. Samples and chemicals
All the samples were provided by Zhejiang Pagoda Brand Huangjiu Co., Ltd. (a representative enterprise focusing on traditional Huangjiu), Shaoxing city, Zhejiang Province, China. According to the actual brewing process of the brewing factory and the requirements of the national standard of China (GB/T 17946–2008), the traditional Huangjiu samples in this research were brewed using glutinous rice, Jianhu water, wheat Qu (a saccharification starter) and Jiuyao (a fermentation starter) as the materials, and put into production with unique brewing technology of simultaneous fermentation and saccharification around Winter Solstice. And the traditional Huangjiu manufacturing involved soaking rice (15–20 days for water absorption), steaming rice, cooling to indoor temperature, mixing with saccharification and fermentation starters (wheat Qu and Jiuyao), and fermenting in pottery jars (primary fermentation at 28 °C for 3–5 days, then secondary fermentation in an open environment at medium–low temperatures (5.76 ± 0.68 °C-18.06 ± 0.66 °C) for about 90 days until the fermentation is complete). The fermenting mash samples were collected during the above long time period of secondary fermentation at different fermentation days (0d, 22d, 45d, 67d, and 90d, respectively), corresponding to sample C1, C2, C3, C4 and C5 in sequences. After mixing uniformly the pottery jars, three different fermentation pottery jars were sampled and mixed evenly as one sample (approximately 1,000 g), and triplicate above samples were collected at each sampling point. The collected samples were divided into two parts: one was immediately stored at –20°C for flavor analysis, and the other was kept at − 80°C for the analysis of microbial communities. Each example was sealed in the sterile sampling bag with a mark.
The regents including ethanol (≥99.7%), 2–octanol (≥99.0%, internal standard), dichloromethane (≥99.5%) and n–alkane mixture (C5–C30) were of chromatographic grade and obtained from Sigma–Aldrich (Shanghai, China). TIANamp Stool DNA Kit was purchased from Sangon Biotech Co., Ltd (Shanghai, China).
2.2. GC–IMS analysis
The GC–IMS (Flavorspec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) equipped with an MXT–WAX column (30 m × 0.53 mm × 0.1 μm, Restek, Beijing, China) was applied to analyze the volatile fingerprints of the samples. The parameters and procedures were described in our previous study (Yu et al., 2021). Traditional Huangjiu sample (1 mL) was introduced into a 20 mL headspace sample vial and incubated at 50 °C for 10 min, and then the headspace gas was injected into the inlet by heated syringes (85°C). Nitrogen (99.99% purity) was used as the carrier gas and the flow rate was 1.0 mL/min. The programmed temperature procedure was set as follows: the column was held at 60 °C, then 2 mL/min for 2 min, then an increase to 100 mL/min in the next 18 min, finally maintained at 100 mL/min until 40 min. All analysis was conducted in triplicate. The GC × IMS Library Search software equipped with NIST and IMS database was applied to qualitatively analyze the volatile compounds by matching the retention indexes and the drift time to standards in the GC–IMS library.
2.3. GC–MS analysis
Volatile compounds of the samples were extracted by headspace solid phase microextraction (HS–SPME) and solvent assisted flavor evaporation (SAFE), and then they were analyzed by GC–MS. The HS–SPME analysis was carried out according to our previous study (Yu, Xie, Xie, Ai, & Tian, 2019). The samples (5 g) and 20 μL internal standard (2–octanol, 315 μg/mL) were put into a 20 mL vial. After equilibrating at 50 °C for 5 min, the SPME fiber (100 μm, Supelco, Inc., Pennsylvania, USA) coated with DVB/CAR/PDMS was exposed to the headspace of the vial for 50 min at continuous stirring of 250 r/min. Specific SAFE conditions and parameters referred to our previous study (Yu, Guo, Ai, Chen, & Tian, 2022). The samples (60 mL), 200 μL internal standard (2–octanol, 315 μg/mL) and dichloromethane (60 mL) were placed in a 250 mL conical flask. After extraction, separation and centrifugation, the collected dichloromethane extract (about 180 mL) was dried and separated. Finally, the SAFE fraction was concentrated to 1 mL by nitrogen and stored at –20°C.
The procedure parameters for GC–MS were described in our previous research (Yu et al., 2021). A 7890 GC equipped with an MS (model 5973C, Agilent Technologies, Santa Clara, CA) was used. An HP–Innowax column (60 m × 0.25 mm × 0.25 μm) from Agilent Technologies was applied. The GC temperature procedure was set as follows: the oven temperature was held at 40 °C for 3 min at first, then increased at 3 °C/min to 120 °C and held for 5 min, and finally raised at 3 °C/min to 200 °C and held for 4 min. The temperature of the detector was 250 °C, the carrier gas was helium (99.999%) at a flow rate of 1 mL/min. The electron ionization energy was set at 70 eV, and the mass range was from 30 to 450 amu. The analyses were conducted in triplicate.
The MS spectra and retention indexes (RI) were compared with those in the NIST11 libraries and literatures to identify aroma compounds, where RIs were calculated according to the C5–C30 alkane standards. The aroma compounds were quantitatively analyzed by external standard method.
2.4. Total DNA extraction, PCR amplification and sequencing
Total DNA of the samples were extracted using TIANamp Stool DNA Kit (Sangon Biotech, Shanghai, China) according to manufacturer’s protocol. Primers 338F/806R and ITS1F/ITS2R were used to amplify the V3-V4 hypervariable region of the bacterial 16S rRNA genes and fungal ITS1 regions, respectively. The PCR products were extracted and purified, and then the purified amplicons were used to construct a PCR amplicon library and sequenced on an Illumina MiSeq platform (Illumina, San Diego, USA) at Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The experiments were conducted in triplicate.
2.5. Bioinformatics and statistical analysis
Raw reads were processed and analyzed via QIIME software package (V1.9.1) (Yang et al., 2022). The valid sequences with similarities > 97% were clustered into an OTU (operational taxonomic units) for microbial classification using Uparse software (V7.0.1090). Representative sequences for each OTU were screened for further annotation. For the representative bacterial OTU sequences, they were annotated and classified using the SILVA's SSUrRNA database. And UNITE fungal ITS database was used to annotate and compare the representative fungal OTU sequences. The amplicon databases were submitted to NCBI Sequence Read Archive (SRA) and were available under accession numbers PRJNA880416.
The alpha-diversity indices including Chao1, Shannon, Simpson and ACE in each sample were analyzed for comparing the species diversity and richness via Mothur software (V.1.30.1). And R software (V2.15.3) was used to visualize those data of alpha diversity. For β–diversity analysis, QIIME software (V1.7.0) was applied to calculate UniFrac distances based on weighted and unweighted. Cluster analysis was performed using ggplot2 in R software. Principal coordinate analysis (PCoA) was performed using SIMCA (V14.1) to capture principal coordinates and visualize complex multidimensional data.
2.6. Statistical data analysis
The data were analyzed by Duncan’s multiple range tests using SPSS Statistics 21 (SPSS Inc., Chicago, USA) and differences at a significant level of 0.05 were considered significant. The heatmap was generated and Pearson correlation coefficient between dominant bacteria and major aroma compounds were generated using R (V2.15.3), and then visualized with Cytoscape 3.4.0 (NIGMS, Seattle, USA).
3. Results and discussion
3.1. Variation tendency of the volatile fingerprints during traditional Huangjiu fermentation
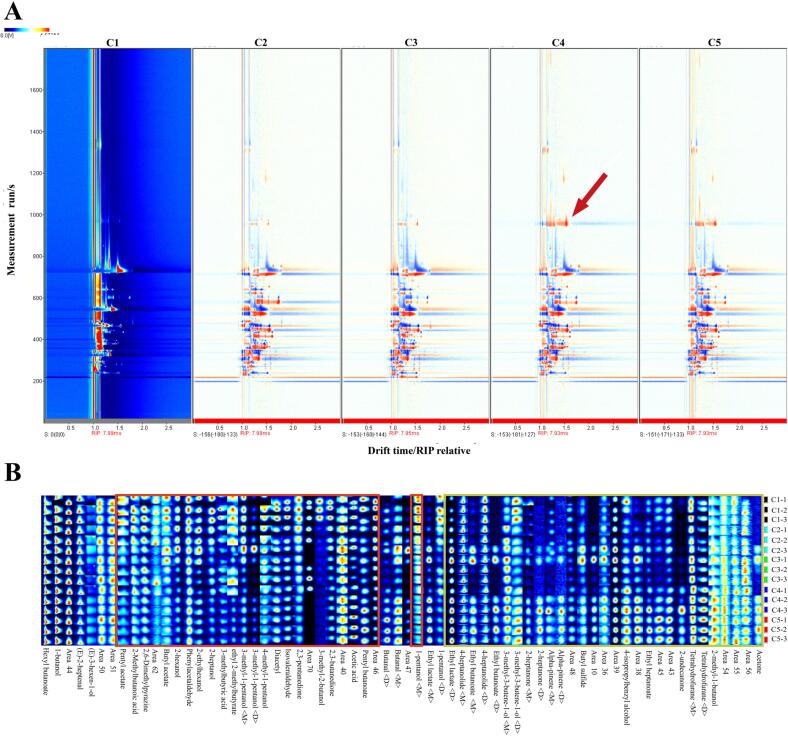
To reveal the differences and dynamics of volatile compounds during fermentation, GC–IMS was applied to analyze the volatile fingerprints of the traditional Huangjiu samples. Fig. 1A is a comparison plot of the differences in volatile compounds in the samples, in which the vertical coordinate represents the retention time of the gas chromatographic peaks, and the horizontal coordinate represents the ion migration time (normalized). The topographic plot of the sample (C1) was selected as a reference, it can be seen that the types of volatile compounds in the samples were basically the same, whereas the contents were different. To visually reveal the dynamic changes in each substance, all peaks were extracted to form a characteristic fingerprint for comparison (Fig. 1B). It can be seen from Fig. 1B that the trends of compounds at different stages of fermentation were different. Most of the detected alcohols (2–hexanol, 2–ethylhexanol, 3–methyl–1–pentanol, 1–heptanol, 1–pentanol, and so on) in the red frame gradually decreased, while the contents of esters (ethyl lactate, ethyl butanoate, ethyl heptanoate and pentyl acetate) and ketones in the yellow frame increased in general with increasing fermentation time, which were similar to those observed in the fermentation process of Chinese rice wine (Liu, Wang, Sun, & Ni, 2020). This result suggested that alcohols were accumulated at the early stage of fermentation and may gradually convert into esters and other flavor compounds as the fermentation process progressed.
Fig. 1.
2D–topographic plots of volatile organic compounds in traditional Huangjiu during fermentation (A); The fingerprint of volatile profiles in traditional Huangjiu during fermentation (B) (Each row represents all the signal peaks selected in a traditional Huangjiu sample, and each column represents the signal peaks of the same volatile compounds in different samples. C1, C2, C3, C4 and C5 correspond to the Huangjiu samples fermented for 0d, 22d, 45d, 67d, and 90d, respectively).
3.2. Analysis of the changes in aroma compounds during traditional Huangjiu fermentation by GC–MS
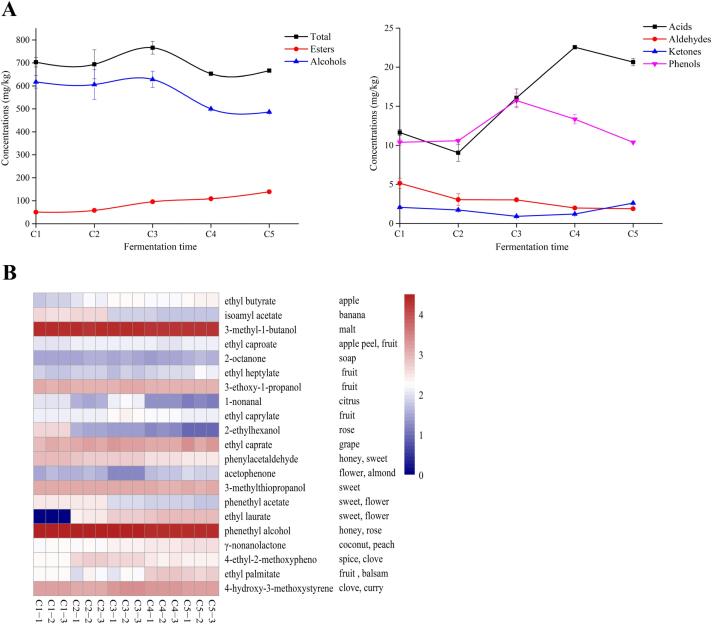
The aroma compounds in the samples collected with different fermentation time points were analyzed by GC–MS, which were listed in Table S1. A total of 54 aroma compounds were detected, including 21 esters, 14 alcohols, 6 acids, 4 aldehydes, 3 ketones and 4 phenols. Among the detected aroma compounds, esters were the most diverse and the contents of alcohols were the highest. The variations of the total content of the aroma compounds including alcohols, esters, acids, aldehydes, ketones and phenols during fermentation are shown in Fig. 2A. On the whole, the total concentrations of aroma compounds increased at C3 period, then slightly declined and tended to stable at the end of fermentation. This trend was also observed in mechanized Huangjiu (Chen et al., 2018). The total content of alcohols (73.01%–87.83%) was the highest, followed by esters, acids, phenols, aldehydes and ketones. Additionally, alcohols and aldehydes at first increased at C3 period and then decreased with increasing fermentation time, acids and phenols decreased slightly at first, then increased and finally decreased as the fermentation process progressed. Moreover, the concentrations of esters gradually increased while ketones decreased slightly and then gradually increased with increasing fermentation time. These results were generally consistent with those described by GC–IMS. According to the result of OAV values, twenty–one compounds with OAV > 1 were identified as characteristic aroma compounds, and their aroma descriptions were used to form a heat map (Fig. 2B) that characterized the distribution of these important aroma compounds during fermentation. And these compounds were basically consistent with the aroma active compounds determined by gas chromatography olfactometry in our previous study of traditional Huangjiu (Yu, Xie, Xie, Ai, & Tian, 2019).
Fig. 2.
The total content of aroma compounds and the variation of various compounds in the fermentation process of Huangjiu (A); Heatmap of key aroma compounds in traditional Huangjiu during fermentation (B) (C1, C2, C3, C4 and C5 correspond to the Huangjiu samples fermented for 0d, 22d, 45d, 67d, and 90d, respectively).
Alcohols in fermented wines are mainly produced by two pathways: sugar metabolism and dehydrogenation decarboxylation of amino acids (Hernandez-Orte et al., 2008). Among the alcohols detected, phenylethanol and 3–methyl butanol had the highest content, followed by isobutanol, n–propanol, 2,3–butanediol and 3–methylthiopropanol. The sum of these six alcohols reached 99.69% of the total alcohol content in the sample. The high content of higher alcohols gradually decreased with increasing fermentation time, which might be due to the decrease of metabolic activity of microorganisms to produce higher alcohols, such as phenylethanol, 3–methylbutanol, isobutanol, and 1–propanol (Mou, Mao, Meng, & Liu, 2016). Additionally, phenylethanol had the highest concentration at C1 period (326.21 mg/kg), while that of 3–methylbutanol, isobutanol and n–propanol was the highest at C3 stage. Among them, the OAV values of phenylethanol (OAV: 24–33), 3–methylbutanol (3–4) and 3–methylthiopropanol (11–13) were allgreater than 1 (Fig. 2B). The OAVs of these three characteristic aroma compounds overall decreased with increasing fermentation time and they had the lowest concentrations at the end of fermentation (C5 period), which may be ascribed to the relatively low temperature slowing down the metabolic activity of yeast cells to produce alcohol (Beltran et al., 2006).
Phenylethanol, with the aroma of rose and honey, was the most important higher alcohol in Huangjiu, which can be mainly produced through the ehrlich pathway or glycolysis and pentose phosphoric acid pathway (Wang et al., 2020). The compound 3–methylbutanol formed by corresponding leucine contributed to malt aroma. And the compound 3–methylthiopropanol with sweet and potato aromas probably came from the degradation of sulfur–containing amino acids (Mestres, Busto, & Guasch, 2000).
The ester compounds were associated with floral and fruity aroma, which could be formed by the microbial metabolism or esterification of carboxylic acids and alcohols during fermentation (Li et al., 2022). Among the detected esters, ethyl lactate and ethyl myristate had the highest contents, followed by ethyl 3–hydroxybutyrate, ethyl decanoate, ethyl acetate, ethyl lauricate, diethyl succinate and ethyl palmitate. These eight esters accounted for 86.69%–93.95% of the total content of esters, which was consistent with our previous study (Yu et al., 2022). As shown in Fig. 2A, most esters showed an upward trend overall, such as ethyl lactate, ethyl myristate, ethyl acetate and ethyl laurate, which was generally consistent with that of GC–IMS. Among them, ethyl lactate had the largest growth rate, which increased about five times from C1 (12.52 mg/kg) to C5 (61.76 mg/kg). It was also found from Fig. 4 that there were 10 esters with OAV > 1, which were ethyl caprate (OAV: 9–19), ethyl laurate (1–8), ethyl palmitate (1–2), γ–nonanolactone (21–57), ethyl butyrate (8–41), ethyl octanoate (203–370), ethyl hexanoate (58–82), ethyl heptanoate (1–2), phenylethyl acetate (1–5) and isoamyl acetate (5–57), indicating that they had different degrees of aroma contribution including fruit and flower aroma to the samples. The OAVs of these esters (ethyl caproate, isoamyl acetate and phenylethyl acetate) increased at first and then decreased, which can be hypothesized that the microorganism had high diversity and activity at the beginning of fermentation stage while mass microorganisms can not adapt to the environment of high acids and alcohols with the fermentation continuing (Chen et al., 2021). Additionally, the concentration of phenylethyl acetate was highest at the initial stages of fermentation and then decreased, indicating that phenylethyl acetate may derive from the raw materials and metabolites of the microorganisms (Yang et al., 2020). And ethyl octanoate reached the peak at C3 period and its OAV was the highest, which provided abundant fruit aroma for the samples. Followed by ethyl caproate (apple aroma) and γ–nonanolactone (peach aroma), the concentration of the γ–nonolactone reached the lowest at C2 period, but then increased gradually with the extension of fermentation time.
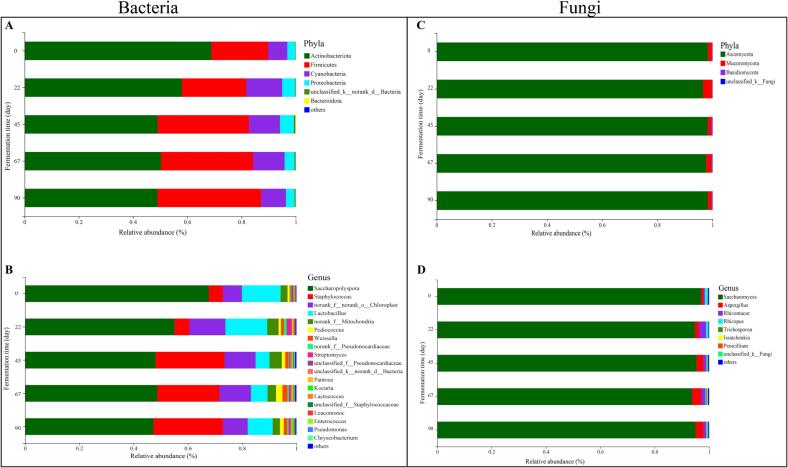
Fig. 4.
Relative abundance of microbial at phylum (A and C) and genus level (B and D) in traditional Huangjiu samples. (Phylum and genus with relative abundance < 0.1% were classified as others.
Aldehydes and ketones could make the aroma incline to be soft and harmonious. Most aldehydes were produced by deamination and decarboxylation of amino acids (Wang et al., 2020). Phenylacetaldehyde had the highest content and it increased first and then decreased continuously during fermentation. However, due to its low threshold concentration, the OAV value still reached 1006 at C5 stage, which endowed the samples with sweet and honey aroma (Qian & Reineccius, 2002). Benzaldehyde with bitter almond flavor was an important aroma compound, which fluctuated but generally increased during fermentation, and reached the highest content at C5 stage. Studies have shown that benzaldehyde mainly came from the oxidation of benzyl alcohol and the metabolic synthesis of various microorganisms using aromatic amino acids (Genovese, Gambuti, Piombino, & Moio, 2007).
Acids are important components contributing to the aroma and taste of Chinese rice wine and they can be produced by the yeasts during fermentation (Ugliano & Moio, 2005). Among them, the content of acetic acid was the highest, and it increased at first and then decreased slightly. Its content reached the highest (19.77 mg/kg) at the C4 stage. Acetic acid strengthened the strong feeling of the wine body and reacted with the corresponding alcohols to esters (Xu et al., 2018). Therefore, acetic acid was an important compound to reconcile the taste and aroma of traditional Huangjiu, which also corresponded to the saying “no acid, no taste”. The content of propionic acid and isobutyric acid followed, which increased during fermentation while declined at the middle of the fermentation. Among the phenols detected, the content of 4–vinyl–2–methoxyphenol was the highest, followed by 4–ethylphenol and 4–ethyl–2–methoxyphenol. The compound 4–ethylphenol with smoke aroma showed an increasing trend during fermentation, which was described as medicinal aroma in Guyue Longshan rice wine (Chen et al., 2013). The compounds 4–ethyl–2–methoxyphenol (OAV: 13–48) and 4–vinyl–2–methoxyphenol (348–642) had higher OAV values, indicating they contributed greatly to the aroma of traditional Huangjiu. With the extension of fermentation time, these substances increased first and then decreased as a whole. The compound 4–vinyl–2–methoxyphenol with clove and curry aroma was formed by ferulic acid at high temperature or under the action of ferulic acid decarboxylase (Chen et al., 2018) and contributed greatly to the flavor characteristics of Huangjiu.
3.3. Microbial community diversity during traditional Huangjiu fermentation
After the quality control process of raw sequences, the alpha diversity analysis was conducted with the clean sequences (Fig. S1). Alpha diversity analysis can reflect diversity and richness of microbial communities. During fermentation, the Ace and Chao index of the samples except C2 samples showed an upward trend on the whole while the scores of Ace and Chao index in the bacterial community were far lower than those in the fungal community during fermentation, indicating that the richness of bacterial and fungal communities increased overall with increasing fermentation time and the interaction between microbial also existed. Owing to the efficient fermentation catabolism ability of fungi such as saccharomyces cerevisiae to produce alcohol, they competed with other populations and inhibited the growth of bacteria that were not tolerant to alcohol (Liang et al., 2020). Moreover, the Shannon index mostly showed an upward trend while the Simpson index was opposite, indicating that the microbial richness gradually increased, which might be due to the introduction of some microorganisms in the environment into the fermentation system natural environment without intervention. Wheat Qu (a saccharification starter) contained various microorganisms including Saccharopolyspora, Staphylococcus, Aspergillus and Rhizopus (Cao et al., 2022), while the core microorganisms in Jiuyao (a mixture of yeasts, molds and bacteria) were Saccharomycopsis and Rhizopus fungi and Pediococcus and Weissella bacteria (Chen et al., 2020). Overall, the richness and diversity of fungi microorganisms were higher than those of bacteria according to the results of alpha diversity analysis during fermentation.
PCoA analysis can be used to study the similarity or difference of sample community composition. Based on the weighted Unifrac distance algorithm, the microbial community structure of the samples during fermentation was analyzed by PCoA (Fig. S1). At the bacterial and fungal level, respectively, the difference between different fermentation time points was obvious, indicating that there were significant indigenous differences in bacterial and fungal community structure among these samples (p < 0.05).
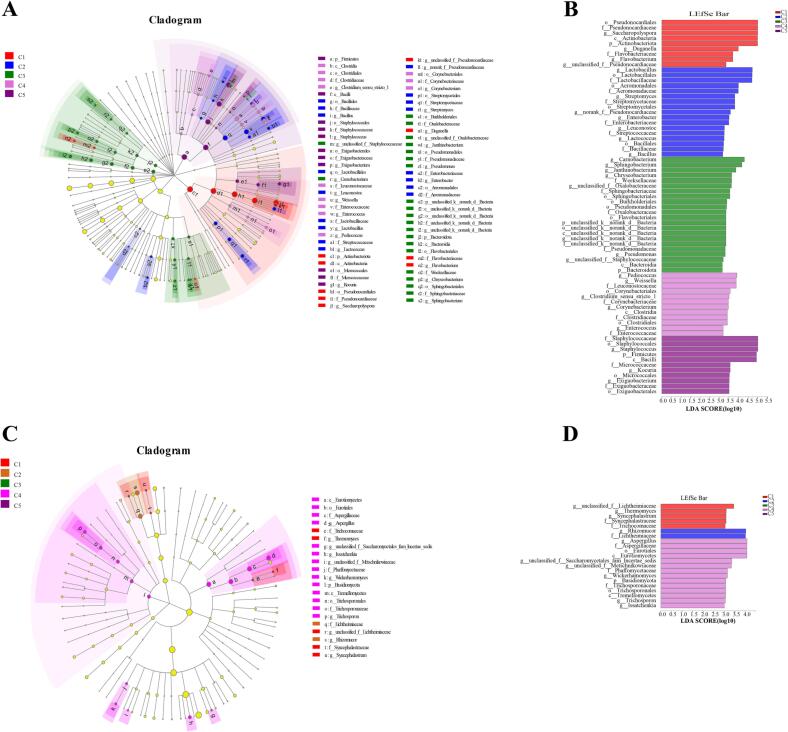
3.4. Microbial profile and core microbiota of traditional Huangjiu
To better understand the predominate microbiota composition in the samples during fermentation, LefSe was applied to identify bacterial and fungal taxa that had significantly different effects on sample division. For bacteria composition (Fig. 3A and B), 71 bacterial clades were observed with LDA scores equal to or above 3.0, which consisted of 4 phyla, 5 classes, 15 orders, 20 families, and 27 genera. The evolutionary relationship of the 71 important bacteria is shown in Fig. 3A. According to Fig. 3B, Actinobacteriota and Firmicutes were the predominant phyla across the entire fermentation process. At genera level, Saccharopolyspora, Lactobacillus, Carnobacterium, Pediococcus, and Staphylococcus were found to be the most abundant at C1, C2, C3, C4 and C5 period, respectively.
Fig. 3.
Landscape of bacterial (A) and fungal (C) clades and differences of LEfSe in bacteria (B) and fungi (D) in traditional Huangjiu samples during fermentation (C1, C2, C3, C4 and C5 correspond to the Huangjiu samples fermented for 0d, 22d, 45d, 67d, and 90d, respectively).
For fungi composition (Fig. 3C and D), 21 fungal clades were found with LDA scores equal or > 3.0, indicating those were the most important fungal clades to distinguish the samples. Aspergillus and Rhizomucor were the predominant genera across the entire fermentation process. Samples with at least three differentially abundant fungal clades were day 5 and day 72 samples (Fig. 3D), which suggested that fungal taxa at these two fermentation time points were most likely to explain the differences between the samples. Overall, samples during fermentation had more diversified bacterial communities than fungal communities (71 vs. 21 clades).
3.5. Dynamics of microbial community in traditional Huangjiu during fermentation
As shown in Fig. 4A, Actinobacteriota (49.01%–68.81%), Firmicutes (21.04%–38.22%), Cyanobacteria (7.03%–13.30%) and Proteobacteria (2.89%–5.13%) are major bacterial phyla found in the fermentation process of traditional Huangjiu samples with relative abundance > 1%. Levels of Firmicutes increased over the period of fermentation, whereas the abundance of Actinobacteriota decreased. Additionally, the abundance of Cyanobacteria rapidly increased during the first 22 days of fermentation but declined as the fermentation progressed. And the Proteobacteria showed an upward trend, but decreased slightly in the middle and late stages of fermentation. At the genus level (Fig. 4B), a total of 13 dominant bacteria were observed, the genus with relative abundance > 0.1% were Saccharopolyspora, Staphylococcus, Lactobacillus, Pediococcus, Weissella, Streptomyces, Pantoea, Kocuria, Lactococcus, Leuconostoc, Enterococcus, Pseudomonas and Chryseobacterium. This result was generally in agreement with those in Shaoxing mechanized rice wine (Liu et al., 2019, Liu et al., 2015). The absolute dominant genus was Saccharopolyspora that accounted for 47.28%–67.71% while slowly decreased with increasing fermentation time. Saccharopolyspora was a safe biofunctional bacterium and produced important active substances, such as enzymes, antibiotics and vitamins (Liu et al., 2015). However, most of the initially major genera increased rapidly during the first 22 day of fermentation. Specifically, Lactobacillus, Streptomyces, Pantoea, Lactococcus and Leuconostoc increased from 14.25%, 0.09%, 0.21%, 0.11% and 0.11% to 15.43%, 1.32%, 0.35%, 0.30% and 0.26%, respectively. In the meantime, these genus started to decline after 22 days and increased from 45 days until the end of fermentation. Lactobacillus was a facultative anaerobic acid–tolerant bacteria, which had a good growth under low oxygen and high acid environment (Zhao et al., 2020). And lactic acid and bacteriocins generated by Lactobacillus played a role in ininhibiting the spoilage microorganisms (Cappello, Zapparoli, Logrieco, & Bartowsky, 2017). Pantoea was a common endophytic bacterium in rice, which widely existed in fermented cereals (Fang, Dong, Chen, & Chen, 2015). The decreasing of Pantoea content at the middle stages of fermentation might be related to the enrichment of lactic acid bacteria. Moreover, the abundance of Staphylococcus, Pediococcus, Weissella, Kocuria and Enterococcus showed an overall upward trend. Among them, the abundance of Staphylococcus reached the highest of 25.63% by the end of fermentation. For Lactococcus, Leuconostoc and Pseudomonas, they increased at first and then decreased. Leuconostoc generally dominate in the initial stage of fermentation before being succeeded by more acid–resistant Lactobacillus, which was consistent with the variation trend of Lactobacillus.
Fungi have strong ability to secrete a variety of enzymes, which is conducive to the fermentation of fermented food. As shown in Fig. 4C and D, a total of 4 phyla and 76 genera were identified across all samples. At the phylum level, the fungal community was mainly dominated by Ascomycota (96.60%–98.33%) across the whole fermentation process, whereas Mucoromycota (1.36%–3.24%) and Basidiomycota (0.09%–0.32%) accounted for only a small portion. With regards to genus level, eight fungal genera were detected with relative abundance > 0.1%. Saccharomyces (97.03%) was the most abundant genera, followed by Aspergillus (0.90%), Rhizopus (0.89%) and Rhizomucor (0.54%) at the initial stage of fermentation, whereas at the end of fermentation was mainly dominated by Saccharomyces (95.09%), Aspergillus (2.82%), Rhizomucor (1.00%) and Rhizopus (0.33%). Saccharomyces, Rhizopus, and Aspergillus were also determined as the core functional microorganisms during Wuyi Hongqu Huangjiu fermentation (Huang et al., 2018). During fermentation, Saccharomyces dominated the entire fermentation process. The abundance of Aspergillus and Issatchenkia increased during the first 67 days of fermentation but declined at the end of fermentation. Aspergillus could secrete amylase, protease, peptidase and other enzymes into the environment, which promoted the hydrolysis of residual starch in post–fermentation (Yang et al., 2020) and offered precursors for the synthesis of flavor compounds (Chang et al., 2015). However, the levels of Rhizopus generally decreased, and remained at a relatively stable level at the late stage of fermentation. Adequate nutrition favored the formation of fungal communities at the early fermentation while gradually decreased as nutrients expended. Rhizopus contributed to saccharification process of Chinese rice wine and could produce some flavor substances, such as ethyl acetate, ethyl lactate, isobutanol, isoamyl alcohol, acetaldehyde, and so on (Londoño-Hernández et al., 2017, Lücke et al., 2019). During the first 22 days of fermentation, Rhizomucor increased quickly but decreased and then remained relatively stable at the middle and late stages of fermentation. Among them, the relative abundance of Rhizomucor reached the highest (2.53%) at fermentation periods of 22 days, while that of Aspergillus (3.46%), Trichosporon (0.24%) and Issatchenkia (0.21%) reached the highest on day 67. In addition, the trend of Trichosporon and Penicillium was similar that decreased at the initial stage of fermentation while increased after 22 days of fermentation.
To summarize, in the fermentation process of traditional Huangjiu produced around Winter Solstice, Actinobacteriota, Firmicutes, Cyanobacteria and Proteobacteria were the dominant bacteria at phyla while Saccharopolyspora, Staphylococcus and Lactobacillus were the dominant genus of bacteria. Saccharomyces, Aspergillus, Rhizomucor and Ascomycota dominanted the fungal community. The greater microbial diversity and complex microbial interactaions directly affect the production of flavor compounds (Tian et al., 2022). In this study, winter brewing was beneficial to the growth of low temperature microorganisms (yeast and lactic acid bacteria, etc.), and then promoted the good flavor of Huangjiu in the slow fermentation process.
3.6. Correlations among microbial communities in traditional Huangjiu during fermentation
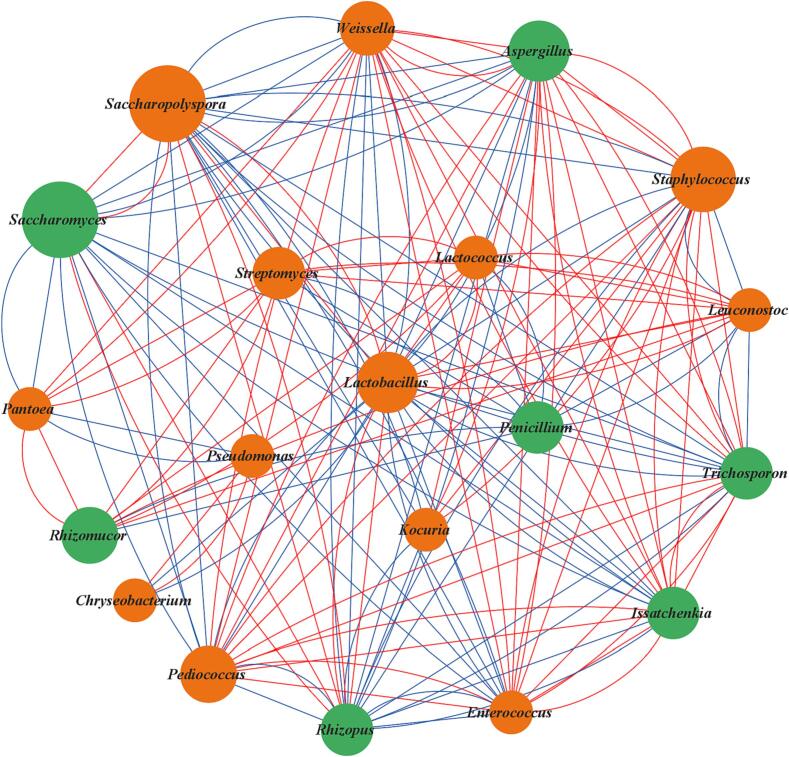
The interaction between microorganisms is one of the important factors influencing the structure of microbial (Zhao et al., 2020). To identify the relationships between different microbia genera in the microbial community, correlation networks between dominant bacteria and fungi were constructed using Pearson’s correlation coefficients and p value (Fig. 5). The results of correlation among different bacterial genera showed that Saccharopolyspora was positively correlated with Lactobacillus, (|r| > 0.6, p < 0.05) whereas negatively correlated with Pediococcus, Enterococcus, Kocuria, Staphylococcus and Weissella. And Staphylococcus was positively correlated with Kocuria, Enterococcus, Weissella, Pediococcus, Lactococcus, Leuconostoc and Pantoea, but negatively correlated with Leuconostoc and Lactobacillus. Furthermore, Lactobacillus was positively correlated with Leuconostoc, Lactococcus and Streptomyces, whereas negatively correlated with Weissella, Pediococcus and Enterococcus. For the fungi, Saccharomyces was positively correlated with Rhizopus, but negatively correlated with Aspergillus, Issatchenkia and Trichosporon. Aspergillus may not be able to adapt to the fermentation environment of high ethanol concentration and acidity caused by Saccharomyces (Tian et al., 2022). Aspergillus was positively correlated with Issatchenkia and Trichosporon, whereas negatively correlated with Rhizopus. In addition, Saccharopolyspora was positively correlated with Rhizopus and Saccharomyces,but negatively correlated with Aspergillus, Issatchenkia and Trichosporon. Overall, the stronger connection nodes (≥10 edges) were mostly distributed in Lactobacillus, Saccharopolyspora, Staphylococcus, Weissella, Aspergillus, Rhizopus, Trichosporon, Pediococcus, Issatchenkia, Enterococcus and Saccharomyces. The microbes could be mutually coordinated and restricted during fermentation, which had a significant contribution to improving the flavor of Chinese rice wine (Liang et al., 2020).
Fig. 5.
Association network diagram of fungi and bacteria. The orange and green circles refer to bacteria and fungi, respectively, and the red and blue lines refer to positive correlations and negative correlations, respectively (|r| > 0.6, p < 0.05).
3.7. Correlations between microorganisms and volatile flavor compounds during fermentation
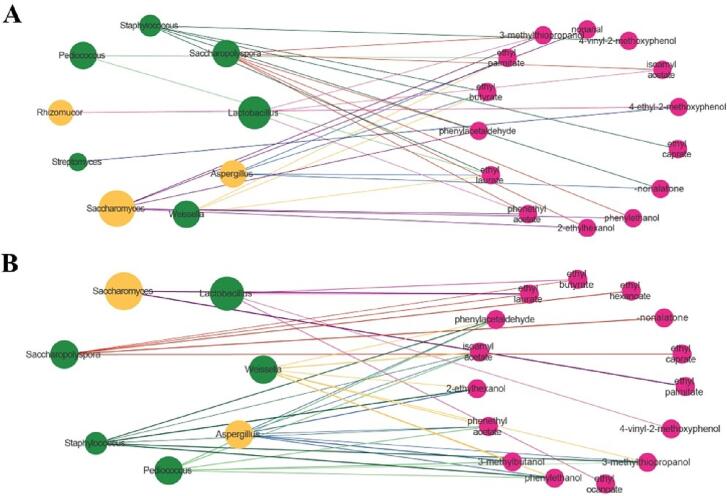
The Pearson correlation coefficients between 21 kinds of aroma compounds with OAV > 1 and dominant genera including 6 kinds of bacteria and 3 kinds of fungi (relative abundance > 1%) were calculated, and chose the coefficient |r| > 0.6 and significance at p < 0.05 as indicating strongly correlated nodes of the network (Fig. 6). Six bacterial and three fungal genera chosen were strongly positively correlated (p < 0.05) with 18 kinds of characteristic volatile flavor compounds (Fig. 6A), indicating the significance of these nine genera in flavor formation during traditional Huangjiu fermentation. Among them, Saccharomyces, the fungi with the highest relative abundance, had a significantly positive correlation with 2–ethylhexanol, phenylacetaldehyde, 3–methylthiopropanol, phenylethanol, nonanal, and phenylethyl acetate. There was also a positive correlation between Saccharomyces and phenylethanol in Chinese rice wine (Zheng et al., 2020). Aspergillus was positively correlated with ethyl laurate, ethyl butyrate, ethyl palmitate and γ–nonolactone, while Rhizopus and Streptomyces significantly and positively correlated with the formation of 4–ethyl–2–methoxyphenol alone. The main enzyme producing microorganisms in wheat Qu were Aspergillus and Rhizopus (Liu et al., 2020), and Aspergillus was involved in the production of esterase (Xu et al., 2016). The compound 4–ethyl–2–methoxyphenol with herbal and warm spicy taste has been identified in wines and could be related to various bacterial genera in millet Chinese rice wine (Yan et al., 2022). In bacterial genera, Saccharopolyspora had significant positive correlation with 2–ethylhexanol, phenylacetaldehyde, 3–methylthiopropanol, phenylethanol, phenylethyl acetate and isoamyl acetate. The formation of acetate esters was related to the catalytic enzymes present in Saccharopolyspora (Liu et al., 2019) and Saccharopolyspora was also identified as the dominant bacteria in Shaoxing mechanized rice wine and Moutai starter (Gan et al., 2019). Furthermore, Staphylococcus was significantly positively correlated with ethyl butyrate, ethyl laurate, γ–nonlactone, ethyl decanoate and 4–vinyl–2–methoxyphenol. Similar to Saccharopolyspora, Lactobacillus was also positively correlated with isoamyl acetate, phenylethyl acetate and 3–methylthiopropanol. Moreover, Pediococcus and Weissella played a positive role in the formation of ethyl laurate and ethyl palmitate, which were consistent with those of Chen et al. (2020). And the Weissella was also positively correlated with ethyl butyrate. According to reports, Weissella can increase the contents of esters and organic acids during fermentation (Hu et al., 2021). Overall, five genera (Saccharomyces, Aspergillus, Saccharopolyspora, Staphylococcus and Lactobacillus) were the core functional microorganisms during traditional Huangjiu fermentation, which were consistent with those in Shaoxing mechanized rice wine that these genera were most closely related to the synthesis of esters, acids and alcohols (Liu et al., 2019).
Fig. 6.
Positive (A) and negative (B) network of the relationships between dominant genera and characteristic aroma compounds (OAV > 1) of traditional Huangjiu samples based on pearson’s correlation (|r | > 0.6, p < 0.05)). The green, orange and pink nodes represent bacterial genera, fungal genera and characteristic aroma compounds, respectively.
Compared to the positive effect, a total of 7 genera showed negative correlations with one or more volatiles of 10 volatiles (Fig. 6B), in which of them there were at least 3 genera correlated with 3–methylbutanol, 2–ethylhexanol, phenylethanol, phenylacetaldehyde, 3–methylthiopropanol, ethyl laurate, isoamyl acetate, phenylethyl acetate and the negative effects mainly occurred in 4 genera including Weissella, Aspergillus, Staphylococcus and Pediococcus.
4. Conclusions
The dynamic of aroma compounds and microbial communities during traditional Huangjiu fermentation around Winter Solstice were studied. The results showed that there were distinct differences in aroma profiles and microbial community among the samples. With increasing fermentation time, alcohols and aldehydes mostly decreased while esters changed inversely, and the genera Saccharomyces, Aspergillu, Staphylococcus and Pediococcus dominated during fermentation. This study found that 11 genera may play a crucial role in maintaining the balance of the complex ecosystem in traditional Huangjiu. Furthermore, the correlation network indicated that five core functional microorganisms contributed greatly to the formation of most characteristic aroma compounds. Our study proved that winter brewing was beneficial to the growth of the core microorganisms, promoting the accumulation of characteristic compounds responsible for the harmonious aroma of traditional Huangjiu. The comprehensive research provided a theoretical guidance for the flavor regulation of traditional Huangjiu via microbial community level and microbial augmentation. However, the exact contributions of the core microorganisms are still unclear, and more in-depth studies are required to explore the specific metabolic pathways.
CRediT authorship contribution statement
Haiyan Yu: Conceptualization, Methodology, Formal analysis, Resources, Writing – original draft, Writing – review & editing. Qiaowei Li: Methodology, Formal analysis, Investigation, Writing – original draft. Wei Guo: Methodology, Formal analysis, Investigation, Writing – original draft. Chen Chen: Methodology. Lianzhong Ai: Resources, Supervision. Huaixiang Tian: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank all those who contributed directly or indirectly to the project. The research was supported by the National Natural Science Foundation of China (No. 32172336) and Capacity Project of Local Colleges and Universities of the Science and Technology Commission of Shanghai, China (21010504100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100620.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Beltran G., Novo M., Leberre V., Sokol S., Labourdette D., Guillamon J.M.…Rozes N. Integration of transcriptomic and metabolic analyses for understanding the global responses of low-temperature winemaking fermentations. FEMS Yeast Research. 2006;6(8):1167–1183. doi: 10.1111/j.1567-1364.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Cai H., Zhang T., Zhang Q., Luo J., Cai C., Mao J. Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiology. 2018;73:319–326. doi: 10.1016/j.fm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Cao, Y., Xia, Q., Chen, J., & Jin, Z. (2022). Understanding of microbial diversity in three representative Qu in China and characterization of the volatile compounds in the corresponding Chinese rice wine. LWT, 164, 113680. doi: 10.1016/j.lwt.2022.113680.
- Cappello M., Zapparoli G., Logrieco A., Bartowsky E. Linking wine lactic acid bacteria diversity with wine aroma and flavour. International Journal of Food Microbiology. 2017;243:16–27. doi: 10.1016/j.ijfoodmicro.2016.11.025. [DOI] [PubMed] [Google Scholar]
- Chang P., Scharfenstein L., Solorzano C., Abbas H., Hua S., Jones W., Zablotowicz R. High sequence variations in the region containing genes encoding a cellular morphogenesis protein and the repressor of sexual development help to reveal origins of Aspergillus oryzae. International Journal of Food Microbiology. 2015;200:66–71. doi: 10.1016/j.ijfoodmicro.2015.01.021. [DOI] [PubMed] [Google Scholar]
- Chen C., Liu Y., Tian H., Ai L., Yu H. Metagenomic analysis reveals the impact of JIUYAO microbial diversity on fermentation and the volatile profile of Shaoxing–jiu. Food Microbiology. 2020;86 doi: 10.1016/j.fm.2019.103326. [DOI] [PubMed] [Google Scholar]
- Chen, G., Huang, Z., Wu, L., Wu, Q., Guo, W., Zhao, H., ... & Sun, B. (2021). Microbial diversity and flavor of Chinese rice wine (Huangjiu): An overview of current research and future prospects. Current Opinion in Food Science, 42, 37-50. doi: 10.1016/j.cofs.2021.02.017.
- Chen Q., Liu S., Tang Y., Han X., Zhou Z., Wang Z., Ji Z., Mao J. Changes in flavor components during fermentation process of mechanically produced Huangjiu. Food Science. 2018;39(14):221–228. doi: 10.7506/spkx1002-6630-201814033. [DOI] [Google Scholar]
- Chen S., Xu Y., Qian M. Aroma characterization of Chinese rice wine by gas chromatography–olfactometry, chemical quantitative analysis, and aroma reconstitution. Journal of Agricultural and Food Chemistry. 2013;61(47):11295–11302. doi: 10.1021/jf4030536. [DOI] [PubMed] [Google Scholar]
- Fang R., Dong Y., Chen F., Chen Q. Bacterial diversity analysis during the fermentation processing of traditional Chinese yellow rice wine revealed by 16S rDNA 454 pyrosequencing. Journal of Food Science. 2015;80(10):2265–2271. doi: 10.1111/1750-3841.13018. [DOI] [PubMed] [Google Scholar]
- Fu, L. (2018). Spring, summer, autumn and winter of Huangjiu. China Wine, 10 (09), 54–55. doi: CNKI:SUN:ZGJU.0.2018–09–007.
- Gan S., Yang F., Sahu S., Luo R., Liao S., Wang H., Jin T., Wang L., Zhang P., Liu X. Deciphering the composition and functional profile of the microbial communities in Chinese Moutai liquor starters. Frontiers in Microbiology. 2019;10:1540. doi: 10.3389/fmicb.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese A., Gambuti A., Piombino P., Moio L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chemistry. 2007;103(4):1228–1236. doi: 10.1016/j.foodchem.2006.10.027. [DOI] [Google Scholar]
- Hernandez-Orte P., Cersosimo M., Loscos N., Cacho J., Garcia-Moruno E., Ferreira V. The development of varietal aroma from non–floral grapes by yeasts of different genera. Food Chemistry. 2008;107(3):1064–1077. doi: 10.1016/j.foodchem.2007.09.032. [DOI] [Google Scholar]
- Hu X., Tian R., Wang K., Cao Z., Yan P., Li F., Li X., Li S., He P. The prokaryotic community, physicochemical properties and flavors dynamics and their correlations in fermented grains for Chinese strong–flavor Baijiu production. Food Research International. 2021;148 doi: 10.1016/j.foodres.2021.110626. [DOI] [PubMed] [Google Scholar]
- Huang Z., Hong J., Xu J., Li L., Guo W., Pan Y., Chen S., Bai W., Rao P., Ni L. Exploring core functional microbiota responsible for the production of volatile flavour during the traditional brewing of Wuyi Hong Qu glutinous rice wine. Food Microbiology. 2018;76:487–496. doi: 10.1016/j.fm.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Li D., Yang M., Wen X., Wu Y., Wang Z., Geng J. Research progress on volatile flavor components of Huangjiu. Food Research and Development. 2022;43(02):202–207. doi: 10.12161/j.issn.1005-6521.2022.02.030. [DOI] [Google Scholar]
- Liang Z., Su H., Lin X., He Z., Li W., Deng D. Microbial communities and amino acids during the fermentation of Wuyi Hong Qu Huangjiu. LWT. 2020;130 doi: 10.1016/j.lwt.2020.109743. [DOI] [Google Scholar]
- Liu S., Chen Q., Zou H., Yu Y., Zhou Z., Mao J., Zhang S. A metagenomic analysis of the relationship between microorganisms and flavor development in Shaoxing mechanized huangjiu fermentation mashes. International Journal of Food Microbiology. 2019;303:9–18. doi: 10.1016/j.ijfoodmicro.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Liu S., Hu J., Xu Y., Xue J., Zhou J., Han X., Ji Z., Mao J. Combined use of single molecule real–time DNA sequencing technology and culture–dependent methods to analyze the functional microorganisms in inoculated raw wheat Qu. Food Research International. 2020;132 doi: 10.1016/j.foodres.2020.109062. [DOI] [PubMed] [Google Scholar]
- Liu, S., Mao, J., Liu, Y., Meng, X., Ji, Z., Zhou, Z., & Ai–lati, A. (2015). Bacterial succession and the dynamics of volatile compounds during the fermentation of Chinese rice wine from Shaoxing region. World Journal of Microbiology and Biotechnology, 31 (12), 1907–1921. doi: 10.1007/s11274–015–1931–1. [DOI] [PubMed]
- Liu Z., Wang Z., Sun J., Ni L. The dynamics of volatile compounds and their correlation with the microbial succession during the traditional solid–state fermentation of Gutian Hong Qu glutinous rice wine. Food Microbiology. 2020;86 doi: 10.1016/j.fm.2019.103347. [DOI] [PubMed] [Google Scholar]
- Londoño-Hernández L., Ramírez-Toro C., Ruiz H., Ascacio-Valdés J.A., Aguilar-Gonzalez M., Rodríguez-Herrera R., Aguilar C. Rhizopus oryzae–Ancient microbial resource with importance in modern food industry. International Journal of Food Microbiology. 2017;257:110–127. doi: 10.1016/j.ijfoodmicro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Lücke F., Fritz V., Tannhäuser K., Arya A. Controlled fermentation of rapeseed presscake by Rhizopus, and its effect on some components with relevance to human nutrition. Food Research International. 2019;120:726–732. doi: 10.1016/j.foodres.2018.11.031. [DOI] [PubMed] [Google Scholar]
- Mao Q. Winter wine spring squeeze Huangjiu. Liquor Making. 2018;45(01):106–108. doi: 10.3969/j.issn.1002-8110.2018.01.034. [DOI] [Google Scholar]
- Mao Q., Xuan X. Brewing characteristics of Huangjiu. China Brewing. 2006;9(04):5–8. doi: 10.3969/j.issn.0254-5071.2006.04.002. [DOI] [Google Scholar]
- Mestres M., Busto O., Guasch J. Analysis of organic sulfur compounds in wine aroma. Journal of chromatography A. 2000;881(1–2):569–581. doi: 10.1016/S0021-9673(00)00220-X. [DOI] [PubMed] [Google Scholar]
- Mou R., Mao J., Meng X., Liu Y. Analysis of fungi diversity and volatile flavor compounds in Chinese rice wine fermentation process. Journal of Food Science and Biotechnology. 2016;35(03):303–309. doi: 10.3969/j.issn.1673-1689.2016.03.013. [DOI] [Google Scholar]
- Qian M., Reineccius G. Identification of aroma compounds in Parmigiano-Reggiano cheese by gas chromatography/olfactometry. Journal of Dairy Science. 2002;85(6):1362–1369. doi: 10.3168/jds.S0022-0302(02)74202-1. [DOI] [PubMed] [Google Scholar]
- Su C., Zhang K., Cao X., Yang J. Effects of Saccharomycopsis fibuligera and Saccharomyces cerevisiae inoculation on small fermentation starters in Sichuan–style Xiaoqu liquor. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109425. [DOI] [PubMed] [Google Scholar]
- Tian S., Zeng W., Fang F., Zhou J., Du G. The microbiome of Chinese rice wine (Huangjiu) Current Research in Food Science. 2022 doi: 10.1016/j.crfs.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugliano M., Moio L. Changes in the concentration of yeast–derived volatile compounds of red wine during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. Journal of Agricultural and Food Chemistry. 2005;53(26):10134–10139. doi: 10.1021/jf0514672. [DOI] [PubMed] [Google Scholar]
- Wang J., Yuan C., Gao X., Kang Y., Huang M., Wu J., Liu Y., Zhang J., Li H., Zhang Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory–directed flavor analysis. Food Research International. 2020;134 doi: 10.1016/j.foodres.2020.109238. [DOI] [PubMed] [Google Scholar]
- Wang P., Mao J., Meng X., Li X., Liu Y., Feng H. Changes in flavour characteristics and bacterial diversity during the traditional fermentation of Chinese rice wines from Shaoxing region. Food Control. 2014;44:58–63. doi: 10.1016/j.foodcont.2014.03.018. [DOI] [Google Scholar]
- Xu, J., Wu, H., Wang, Z., Zheng, F., Lu, X., Li, Z., & Ren, Q. (2018). Microbial dynamics and metabolite changes in Chinese Rice Wine fermentation from sorghum with different tannin content. Scientific reports, 8 (1), 1–11. doi: 10.1038/s41598–018–23013–1. [DOI] [PMC free article] [PubMed]
- Xu, N., Liu, Y., Hu, Y., Zhou, M., Wang, C., & Li, D. (2016). Autolysis of Aspergillus oryzae mycelium and effect on volatile flavor compounds of soy sauce. Journal of Food Science, 81 (8), C1883–C1890. doi: 10.1111/1750–3841.13396. [DOI] [PubMed]
- Yan Y., Chen H., Sun L., Zhang W., Lu X., Li Z., Xu J., Ren Q. The changes of microbial diversity and flavor compounds during the fermentation of millet Huangjiu, a traditional Chinese beverage. Plos One. 2022;17(1):e0262353. doi: 10.1371/journal.pone.0262353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Fan Y., Li T., Yang Y., Zeng F., Wang H., Suo H., Song J., Zhang Y. Microbial composition and correlation between microbiota and quality–related physiochemical characteristics in chongqing radish paocai. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130897. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hu W., Xia Y., Mu Z., Tao L., Song X., Zhang H., Ni B., Ai L. Flavor formation in Chinese rice wine (Huangjiu): Impacts of the flavor–active microorganisms, raw materials, and fermentation technology. Frontiers in Microbiology. 2020;11 doi: 10.3389/fmicb.2020.580247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Wang L., Zhan P., Tian H., Liu J. Characterization of the aroma compounds of Millet Huangjiu at different fermentation stages. Food Chemistry. 2022;366 doi: 10.1016/j.foodchem.2021.130691. [DOI] [PubMed] [Google Scholar]
- Yu H., Guo W., Ai L., Chen C., Tian H. Unraveling the difference in aroma characteristics of Huangjiu from Shaoxing region fermented with different brewing water, using descriptive sensory analysis, comprehensive two–dimensional gas chromatography–quadrupole mass spectrometry and multivariate data analysis. Food Chemistry. 2022;372 doi: 10.1016/j.foodchem.2021.131227. [DOI] [PubMed] [Google Scholar]
- Yu H., Guo W., Xie T., Ai L., Tian H., Chen C. Aroma characteristics of traditional Huangjiu produced around Winter Solstice revealed by sensory evaluation, gas chromatography–mass spectrometry and gas chromatography–ion mobility spectrometry. Food Research International. 2021;145 doi: 10.1016/j.foodres.2021.110421. [DOI] [PubMed] [Google Scholar]
- Yu H., Xie T., Xie J., Ai L., Tian H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography–mass spectrometry and gas chromatography–olfactometry. Food Chemistry. 2019;293:8–14. doi: 10.1016/j.foodchem.2019.03.071. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Y., Feng X., Iftikhar M., Meng X., Wang J. The Analysis of Changes in Nutritional Components and Flavor Characteristics of Wazu Rice Wine During Fermentation Process. Food Analytical Methods. 2022;15(4):1132–1142. doi: 10.1016/j.foodchem.2019.03.071. [DOI] [Google Scholar]
- Zhang K., Wu W., Yan Q. Research advances on sake rice, koji, and sake yeast: A review. Food Science & Nutrition. 2020;8(7):2995–3003. doi: 10.1002/fsn3.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Su W., Mu Y., Jiang L., Mu Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109800. [DOI] [PubMed] [Google Scholar]
- Zheng N., Jiang S., He Y., Chen Y., Zhang C., Guo X., Ma L., Xiao D. Production of low–alcohol Huangjiu with improved acidity and reduced levels of higher alcohols by fermentation with scarless ALD6 overexpression yeast. Food Chemistry. 2020;321 doi: 10.1016/j.foodchem.2020.126691. [DOI] [PubMed] [Google Scholar]
- Zou W., Zhao C., Luo H. Diversity and function of microbial community in Chinese strong–flavor baijiu ecosystem: A review. Frontiers in Microbiology. 2018;9:671. doi: 10.3389/fmicb.2018.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.