Summary
Obesity is an epidemic and a public health threat. Medical weight management remains one of the options for the treatment of excess weight and recent advances have revolutionized how we treat, and more importantly how we will be treating obesity in the near future. Metreleptin and Setmelanotide are currently indicated for rare obesity syndromes, and 5 other medications (orlistat, phentermine/topiramate, naltrexone/bupropion, liraglutide, semaglutide) are approved for non-syndromic obesity. Tirzepatide is about to be approved, and other drugs, with exciting novel mechanisms of action primarily based on incretins, are currently being investigated in different phases of clinical trials. The majority of these compounds act centrally, to reduce appetite and increase satiety, and secondarily, in the gastrointestinal tract to slow gastric emptying. All anti-obesity medications improve weight and metabolic parameters, with variable potency and effects depending on the specific drug. The currently available data do not support a reduction in hard cardiovascular outcomes, but it is almost certain that such data are forthcoming in the very near future. The choice of the anti-obesity medication needs to take into consideration the patient's clinical and biochemical profile, co-morbidities, and drug contra-indications, as well as expected degree of weight loss and improvements in cardio-renal and metabolic risk. It also remains to be seen whether precision medicine may offer personalized solutions to individuals with obesity, and whether it may represent the future of medical weight management along with the development of novel, very potent, anti-obesity medications currently in the pipeline.
Funding
None.
Keywords: Obesity, Weight management, Anti-obesity medications
Research in context.
Evidence before this study
This manuscript represents an update of a previous comprehensive review on the pharmacotherapy of obesity (Pilitsi et al., 2019). We searched Medline and Embase to identify relevant systematic reviews on the topic in the last 5 years (period 2018–2022). We identified 15 articles that systematically reviewed the literature on medical weight management (Appendix A). They all searched one or more databases, and described the evidence on weight parameters. In addition to weight and body mass index, only 4 articles included data on metabolic and cardiovascular parameters and adverse events. None of the identified reviews presented an algorithm for drug therapy consideration based on the available safety and efficacy data. None of them included an overview of the medications in the horizon.
Added value of this study
This manuscript represents an overview on the currently available and FDA-approved anti-obesity medications (AOM) and those under development. For the FDA-approved AOM, we review the evidence on weight and cardio-metabolic parameters while trying to identify, when applicable, whether the beneficial impact on each parameter is a direct effect of the medication per se, or secondary to total body weight loss. In addition, we summarize the available evidence on AOM in individuals with mental illness. We also shed light on tirzepatide which is currently under a fast track review by the FDA, and promises to be the most effective anti-obesity medications to be approved in 2023. We suggest a treatment algorithm taking into consideration patient's profile and medications efficacy and safety data.
Implications of all the available evidence
This review sheds light on the scarcity of the currently available data describing the long-term effects of anti-obesity medications, and in particular, the lack of evidence for an impact on all-cause and cardio-vascular mortality risk, which is urgently needed. Large long-term trials are required to demonstrate the benefit of obesity pharmacotherapy on clinically relevant hard outcomes. We also provide a glimpse on drugs in the pipeline, which promise to revolutionize the way we will be treating obesity in the near future.
Introduction
Obesity is a major public health threat, and obesity rates have been alarmingly increasing in the last few decades.1 The highest prevalence is reported in the Pacific Islands states, where obesity affects more than 50% of the population.2 In United States, individuals with obesity constitute almost one third of adults, with a prevalence ranging between 23% and 38% across various states.2 The worldwide situation has worsened recently following the COVID-19 pandemic.3,4
Obesity is associated with an increased risk for various metabolic, cardiovascular, skeletal co-morbidities, and cancer,5 in addition to a significant impact on psychosocial health.6 Furthermore, obesity is linked to increased mortality.7 The latest analysis of the Global Burden of Disease (GBD) study in 2017 showed that a body mass index (BMI) ≥25 kg/m2 was associated with 2.4 and 2.3 million deaths in women and men, respectively.7
Monogenic obesity syndromes are rare and constitute <5% of all obesity cases.8 Most commonly, obesity is multifactorial, with several factors contributing to the excess weight, including dietary pattern, physical activity, sleep patterns, medications, in addition to genetic, epigenetic, and environmental determinants.9 Therefore, the treatment of obesity is very challenging, and one size does not fit all.
Obesity treatment guidelines agree that the appropriate approach for weight management should be multidisciplinary, including lifestyle modifications, behavioral therapy, pharmacotherapy and/or bariatric surgery.10,11 Anti-obesity medications (AOM) are indicated in individuals with a BMI ≥30 kg/m2 or if ≥27 kg/m2 in the presence of one or more co-morbidities (Appendix B). However, the history of AOM was marked by the failure of several ones after their widespread use in the market, secondary to serious adverse effects, namely cardio-vascular events, suicidality, risk for abuse and dependence12 and recently cancer.13 Therefore, the Food Drug Administration (FDA) and European Medicines Agency (EMA) revised their regulatory approval criteria of AOM, highlighting in particular the importance of cardiovascular and central nervous system safety.14,15 Importantly, this is also what most insurance companies would need to see to get AOM approved. The last decade observed the approval of 2 drugs for syndromic obesity, one of them indicated to treat the complications of leptin deficiency in patients with generalized lipodystrophy, and 6 drugs for the long term management of non-syndromic obesity.10,16, 17, 18 One of them, Lorcaserin, was withdrawn in 2021, secondary to a signal for an increased risk of cancer, although it was not clarified whether the association was causal or due to early detection of cancer cases due to weight loss. Thus, this signal might have been related to the excess weight and/or increased screening for cancer in this population.19,20
The aim of this manuscript is to review the pharmacologic management of obesity in adults, suggest an algorithm for the treatment approach of excess weight, and describe potential drugs that are currently under investigation.
Methods
For FDA approved AOM, we conducted a systematic search on Medline and Embase to identify relevant trials (period 2017–2022) and systematic reviews/meta-analyses (SR/MAs) (period 2012–2022) on specific outcomes/parameters, to update a previous review on the topic.21 We used Medical Subject Heading (MeSH) terms and keywords related to obesity or fat, adipose tissue, body composition and those related to FDA approved AOM (naltrexone/bupropion, liraglutide, orlistat, phentermine/topiramate (combination), semaglutide) (Appendix C). Three reviewers (M.G., R.H., R.T.) screened the title and abstract and full texts articles. We included randomized controlled trials (RCTs) and SR/MAs on adults (18 years and older), with obesity (BMI ≥ 30 kg/m2), treated with an FDA-approved AOM, and reporting on one of the following: weight, glycemic parameters, blood pressure, fatty liver, sleep apnea, cardiovascular outcomes and mental illnesses. Three reviewers (M.G., R.H., R.T.) performed data abstraction in duplicate and independently, on population baseline characteristics, intervention dose and frequency, and outcome of interest.
For weight loss medications under development, we searched the clinicaltrials.gov (search update June 4, 2022), using the condition “obesity”, and we included ongoing (active or recruiting) phase 1, 2 or 3 trials on adults and older adults. We also manually screened the citations of previous reviews on the topic.
In the narrative and tables, we report mean and standard deviation (SD) of continuous parameters. When SD was not reported, we derived it from the standard errors or the 95% confidence interval, if available, or stated not available if SD derivation was not possible.
Role of the funding source
There was no funding source for this study.
Results
The search strategy yielded 519 records, after removing duplicates. After title and abstract screening, we included 201 papers for full text screening. We included in our manuscript 46 original RCTs (including landmark phase 3 trials and other trials in individuals with obesity (with or without comorbidities), assessing various cardio-metabolic parameters) and 8 SR/MAs on FDA-approved AOM.
FDA approved medications for monogenic syndromes of obesity
Setmelanotide
Setmelanotide, is a melanocortin-4 (MC4) receptor agonist that was FDA approved in 2020 as a subcutaneous injectable formulation for chronic weight management in patients 6 years and older with obesity resulting from proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) or leptin receptor (LEPR) deficiency, confirmed by genetic testing.18 These conditions are associated with insufficient activation of the MC4 receptor resulting in hyperphagia and severe childhood-onset obesity. Setmelanotide re-establishes the activity of the MC4 receptor pathway, thus reducing hunger and promoting body weight loss, by lowering caloric intake, and increasing energy expenditure in animal models.18,22,23
In a multicentre single arm trial, 11 patients with obesity secondary to LEPR deficiency (mean age 23.7 (8.4) years and a mean baseline BMI of 48.2 (10.4) kg/m2), treated with Setmelanotide, showed a significant decrease in hunger score by 43.7% (p < 0.0001) and bodyweight by 12.5% (p < 0.0001), at one year compared to baseline.24 In addition, 45% of patients achieved ≥10% total body weight loss at one year.24 Similarly, 10 patients with obesity secondary to POMC deficiency (mean age of 18.4 (6.2) years and a mean BMI of 40.4 (9) kg/m2), treated with Setmelanotide, showed a significant decrease in hunger score by 27.1% (p 0.0005) and bodyweight of 25.6% (p < 0.0001) at one year compared to baseline; 80% of those patients achieved ≥10% total body weight loss.24 The most common adverse events observed with Setmelanotide were injection site reactions (100% of patients with POMC or LEPR deficiency) and hyperpigmentation disorders (100% in POMC deficiency and 45% in LEPR deficiency).24 A total body weight loss of 7.6% was observed with Setmelanotide in another 1-year trial in patients with Bardet-Biedl or Alstrom Syndrome (p 0.0005).25
Metreleptin
Leptin is an adipokine that was investigated not only for its metabolic effect, but also for potential immune, neuroendocrine and neurocognitive functions.26, 27, 28, 29, 30, 31 Metreleptin is a leptin analog that was FDA approved in 2014 as a replacement therapy for leptin deficiency in patients with congenital or acquired lipodystrophy and associated co-morbidities.17 It is administered as a subcutaneous injection once daily, with a dose depending on the patient weight (starting at 0.06 mg/kg/day with a maximum of 0.13 mg/kg/day for patients with baseline weight ≤40 kg, and 2.5 mg (for males) or 5 mg (for females) once daily with a maximum of 10 mg/day for patients with baseline weight >40 kg).17 In a prospective non-randomized crossover study of 17 patients (mean age of 29 (16) years, a mean weight of 70.1 (17.3) kg), with generalized or partial lipodystrophy and low leptin levels, Metreleptin resulted in a significant improvement in metabolic parameters at 6 months compared to baseline: a decrease in total cholesterol by 73 mg/dL (p 0.04), a decrease in triglycerides by 240 mg/dL (p 0.02), a decrease in fasting glucose by 26 mg/dL (p 0.01), a decrease in urinary glucose excretion of 5 g/24 h (p 0.007) and an increase of 3.3 mg/kgFFM/min in insulin sensitivity (p 0.004). Metreleptin also resulted in a significant decrease in 24-h energy expenditure, by 5.0% (121 (152) kcal/day; p 0.006) and 7.9% (190 (272) kcal/day; p 0.04) at 2 weeks and 6 months, respectively, compared to baseline.32 It also resulted in a reduction in lean body mass of 2 (10) kg (p 0.005) at 6 months compared to baseline.32 In a post-hoc analysis combining data from 4 studies, it appeared that individuals with very low leptin levels at baseline could possibly respond to Metreleptin with a reduction of their body weight. However, these findings are limited by the small number of participants in the specific sub-group of interest, and therefore, the statistical significance and the clinical importance of this report need to be confirmed and further elucidated.33 The development of antibodies against Metreleptin was reported, although the consequences are not well understood due to the small number or reports.34
FDA approved medications for non-syndromic obesity
Mechanism of action
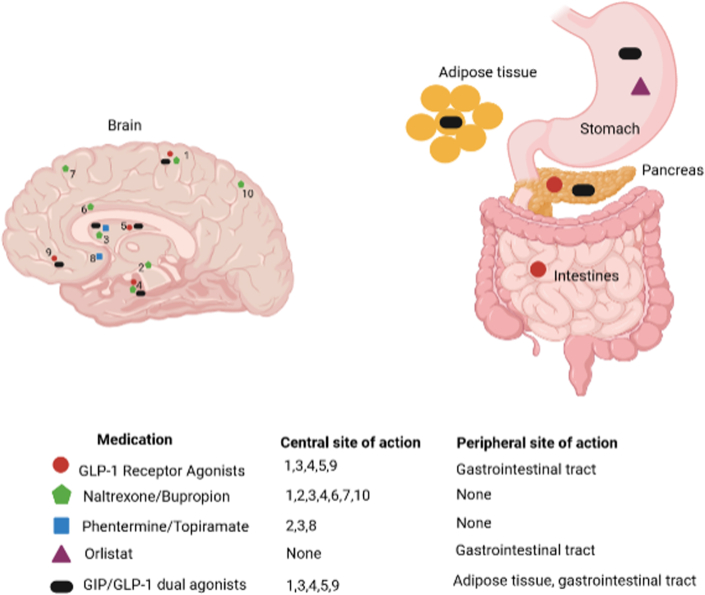
The currently approved AOM target peripheral and central pathways to decrease energy intake by reducing appetite and increasing satiety35, 36, 37, 38, 39, 40, 41, 42, 43 (Fig. 1; Table 1).
Fig. 1.
Site of action of FDA approved anti-obesity medications. (1): parietal cortex, (2) hippocampus, (3) hypothalamus, (4) insula, (5) putamen, (6) dorsal anterior cingulate, (7) superior frontal cortex, (8) nucleus accumbens, (9) orbitofrontal cortex, (10) superior parietal cortex. For GLP-1 receptor agonists and naltrexone/bupropion, the central sites of action are derived from animal studies and brain functional MRI studies in humans; For phentermine/topiramate, the central sites of action are derived from animal studies. Animal studies showed that GLP-1 agonists act on the hypothalamus and the nucleus of the solitary tract.35 GLP-1 agonists act also on the parietal cortex, insula, putamen, and orbitofrontal cortex in response of food images, as demonstrated in functional MRI studies.37,38 Naltrexone/bupropion acts on the hypothalamus, superior parietal cortex, posterior insula, dorsal anterior cingulate, hippocampus. There are inconsistent data on its role in the superior frontal cortex, the amygdala and nucleus accumbens.36,41, 42, 43 Phentermine acts on the hypothalamus. Topiramate acts on the hypothalamus and the hippocampus, as shown in animal models39 and the nucleus accumbens, in a proof-of-concept study for the treatment of alcohol dependence, not for weight management.40 GLP-1, glucagon-like peptide-1; HCl, hydrochloric acid. This figure was created using BioRender (https://biorender.com/).
Table 1.
Anti-obesity medications: approval, mechanism of action, adverse events, and contraindications.
| Drug (trade name) | Approval FDA/EMA (year) | Mechanism of action | Adverse eventsa | Contraindicationsb |
|---|---|---|---|---|
| Orlistat (Xenical, Alli) | FDA 1999 EMA 1998 |
Gastric and pancreatic lipase inhibitor | Oily rectal leakage, abdominal distress, abdominal pain, flatulence with discharge, fecal urgency, steatorrhea, fecal incontinence, increased defecation | Patients with chronic malabsorption syndrome or cholestasis, pregnancy |
| Phentermine/Topiramate (Qsymia) | FDA 2012 | NE agonist/GABA agonist, glutamate antagonist | Elevation in heart rate, mood and sleep disorders, cognitive impairment, metabolic acidosis, paresthesia, dry mouth | Glaucoma, hyperthyroidism, during or within 14 days following the administration of monoamine oxidase inhibitors, hypersensitivity to sympathomimetic amines, pregnancy |
| Naltrexone/Bupropion (Contrave/Mysimba) | FDA 2014 EMA 2015 |
Opioid receptor antagonist/DA and NE reuptake inhibitor | Nausea, constipation, headache, vomiting, dizziness, insomnia, dry mouth, diarrhea, sleep disorder | Chronic opioid use, acute opioid withdrawal, uncontrolled hypertension, seizure disorder, bulimia or anorexia nervosa, abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiseizure drugs; concomitant use of MAOIs, patient receiving linezolid or IV methylene blue, pregnancy |
| Liraglutide (Saxenda) | FDA 2014 EMA 2015 |
GLP-1 analogue | Increased heart rate, hypoglycemia, constipation, diarrhea, nausea, vomiting, headache | Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, pregnancy |
| Semaglutide (Wegovy) | FDA 2021 EMA 2021 |
GLP-1 analogue | Nausea, vomiting, diarrhea, abdominal pain, constipation, headache | Personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2, pregnancy |
| Setmelanotide (Imcivree) | FDA 2020 EMA 2021 |
MC4R agonist | Injection site reactions, hyperpigmentation, nausea, headache, diarrhea, vomiting, abdominal pain | None |
| Tirzepatidec | Under consideration by FDA | GIP/GLP-1 dual agonist | Nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain | Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, known serious hypersensitivity to tirzepatide or any of the excipients |
Abbreviations: DA, dopamine; EMA, European Medicines Agency; FDA, Food and Drug Administration; GABA, gamma-aminobutyric acid; GI, gastrointestinal; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; IV, intravenous; MAOIs, monoamine oxidase inhibitors; MC4R: melanocortin-4 receptor; NE, norepinephrine.
Adverse events presented here are those that are present in more than 10% of the population, based on the FDA approval leaflet.
Contraindications are based on the FDA approval leaflet.
Under expedited consideration for FDA approval.
Orlistat has mainly a peripheral effect; it inhibits gastric and pancreatic lipases, thus decreasing dietary fat absorption.44 Phentermine is a sympathomimetic that is less potent than other amphetamine on dopamine release, and therefore, is associated with a lower risk of substance abuse.45,46 It stimulates serotonin release, but only at very high doses.45,47 Animal data showed increased energy expenditure with phentermine,48 although inconsistently,49 and this has never been confirmed in humans. As a standalone therapy, it is only approved for short term treatment (<12 weeks).10,12,50, 51, 52 Phentermine combined with topiramate is approved for long term treatment of obesity. Topiramate is a gamma-aminobutyric acid agonist, glutamate antagonist and carbonic anhydrase inhibitor that has been shown to suppress appetite, through mechanisms that are still unclear.39 Animal studies have shown increased energy expenditure53 and improved insulin sensitivity,54 but this has not been yet confirmed in humans.55 Naltrexone (opioid receptor antagonist that inhibits the POMC pathway inhibitor) and Bupropion (antidepressant, norepinephrine and dopamine reuptake inhibitor that directly stimulate POMC cells) work synergistically to increase POMC peptide production, and therefore decrease food intake.36,56 In addition, the combination Naltrexone/Bupropion (NB) acts on the reward pathways, as demonstrated in functional magnetic resonance imaging (fMRI) studies.36 NB increased activity in various cortical areas in response to food cues,41, 42, 43 implying increased self-control and awareness of internal signals of fullness. Finally, glucagon like peptide receptor (GLP1-R) agonists, Liraglutide and Semaglutide, act centrally by decreasing appetite, and peripherally, on the pancreas by increasing insulin secretion and on the gastro-intestinal tract leading to decreased intestinal motility and delayed gastric emptying.57
Effect on weight
Orlistat (Xenical, Alli)
The XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) study enrolled men and women with obesity and without type 2 diabetes who were either assigned to take Orlistat 120 mg ter in die (TID) or placebo TID for a 4-year period.58 All patients were prescribed a reduced calorie diet (−800 calories/day) with 30% from fat and not more than 300 mg cholesterol per day. They were also advised to walk 1 km per day. The range of mean age of all participants was 43.0–43.7 years, and 55% were women.58 The baseline mean BMI was 37 kg/m2.58 At 1-year, mean total body weight loss was 10.6 kg in the intervention group (n = 1487) and 6.2 kg for the placebo group (n = 1295), SD not available (Fig. 2). At 4 years, mean total body weight loss, compared to baseline was 5.8 kg for the intervention group (n = 851) and 3.0 kg for the placebo group (n = 567) (SD not available) (Fig. 2). The compliance with the drug administration from the first dose until treatment dissolution was 93% for both orlistat patients and placebo patients,58 and the overall dropout rate was 8%.21, 58 The XENDOS trial did not include patients with DM. However, several trials investigated the efficacy of orlistat, alone or in combination with Metformin or insulin treatment, in patients with overweight or obesity and DM with suboptimal control. In these studies, participants taking orlistat achieved a significantly greater percent in total body weight loss (range of mean percent weight loss 3.9–6.2), when compared to patients on placebo (mean percent weight loss 1.3–4.3).59, 60, 61, 62 The adverse events observed in more than 10% of the population in the main orlistat trials include gastrointestinal (GI) symptoms such as rectal leakage, abdominal pain, abdominal stress, flatulence with discharge, fecal urgency, steatorrhea, fecal incontinence, and increased defecation (Table 1).63
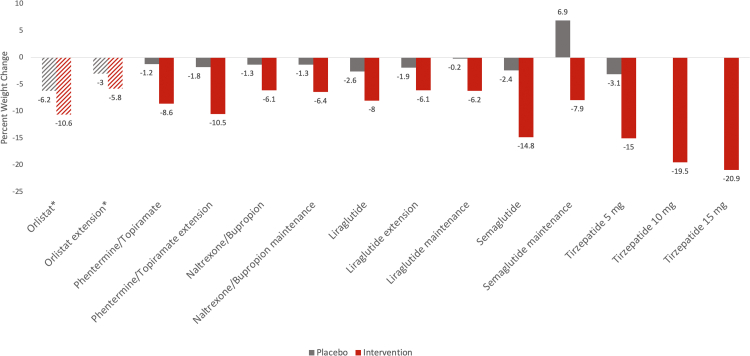
Fig. 2.
Mean percent (%) weight change reported in the main phase 3 and extension trials of the FDA approved anti-obesity medications. Orlistat: XENDOS trial (years 1 and 4). Phentermine/topiramate: CONQUER and SEQUEL trials. Naltrexone/bupropion: COR-I and COR-II trials. Liraglutide: SCALE Obesity, SCALE Obesity and Prediabetes Extension, and SCALE maintenance trials. Semaglutide: STEP 1 and STEP 4 trial. All trials are listed in order as seen in the figure from left to right. The grey color represents placebo arms; the red color represents intervention arms. aThe mean weight change in the orlistat group is in kg not in percent (stripped bar charts). bUnder expedited consideration for FDA approval.
Phentermine/Topiramate (Qsymia)
Three phase 3 RCTs assessed the efficacy of phentermine/topiramate on total body weight loss: EQUIP, CONQUER and SEQUEL.64, 65, 66 In the EQUIP trial, patients with obesity (BMI ≥ 35 kg/m2) were randomly assigned to receive phentermine/topiramate 3.75 mg/23 mg or 15 mg/92 mg or placebo, in addition to a reduced calorie diet for 56 weeks. The mean age of participants was 42.7 years, and 83% were women.64 The mean baseline BMI was 42 kg/m2.64 The percent weight reductions were −5.1 (8.6) % for the low dose group (n = 234), −10.9 (8.5) % for the high dose group (n = 498), and −1.5 (8.5) % for the placebo group (n = 498). In CONQUER, men and women with overweight/obesity were randomly assigned to receive phentermine/topiramate 7.5 mg/46 mg or 15 mg/92 mg or placebo, for 56 weeks. All patients received standardized counseling for diet and lifestyle modification. The mean age of participants was 51.1 years, and 70% were women.65 The range of mean baseline BMI was 36.2–36.7 kg/m2.65 Weight reductions of 6.6 (9.2) % was achieved for the low dose group (n = 498), 8.6 (10.4) % for the high dose group (n = 995), and 1.2 (8.8) % for the placebo group (n = 994) (Fig. 2).65 The SEQUEL trial was a 52-week extension of CONQUER trial. Compared to baseline, the weight reduction was 9.3% in the low dose (n = 153), 10.5% in the high dose (n = 295) and 1.8% with the placebo (n = 227), SD not available (Fig. 2). A sub-group analysis according to diabetes mellitus (DM) status showed a higher weight loss in the intervention arms, compared to placebo arms, with a trend for a higher efficacy in patients without DM, compared to those with DM.65 Furthermore, a trial on 130 patients with obesity and DM from the OB-202/DM-230 study, assessed weight change at 56 weeks when comparing phentermine/topiramate to placebo, and showed a significant decrease in mean percent body weight of 9.6% vs. 2.6% (p < 0.0001, SD not available), and a drop in HbA1c of 1.6% vs. 1.2% (p < 0.05, SD not available), respectively.67 The dropout rate ranged between 31 and 47% in the EQUIP and CONQUEUR trials and was of 5–10% among those who remained in the extension trial. The adverse events observed in more than 10% of the population in the main phentermine/topiramate trials include increased heart rate; central nervous system symptoms such as mood and sleep disorders, cognitive impairment, paresthesia, headaches, and dizziness; GI symptoms such as constipation, dysgeusia, and dry mouth; infections and infestations such as upper respiratory tract infections, nasopharyngitis, and sinusitis; metabolism and nutrition disorders such as metabolic acidosis (Table 1).64, 65, 66,68
A SR/MA of 6 RCTs on phentermine/topiramate compared to placebo showed a weighted mean difference in total body weight loss of 7.7 (6.6, 8.8) kg, favoring the intervention at 56–108 weeks, with a response that is dose dependent.69
Naltrexone/bupropion (Contrave/Mysimba)
The Contrave Obesity Trials (COR) program included three main RCTs investigating the effect of NB on total body weight loss in patients with obesity.70, 71, 72 These RCTs administered a combined oral sustained-release formulation of naltrexone and bupropion (NB32) (8 mg naltrexone/90 mg bupropion in each tablet, two tablets taken twice a day) or matching placebo twice daily, in addition to lifestyle modification, over a period of 56 weeks.70, 71, 72 The COR-I trials, included a third arm with a lower total dose of 16 mg/day naltrexone with 360 mg/day bupropion (NB16) (4 mg naltrexone/90 mg bupropion in each tablet, two tablets taken twice a day).70 The COR-II trial had the co-primary efficacy outcomes at 28 weeks, and re-randomized participants achieving <5% total body weight loss to NB32 or placebo.71 The study population consisted of men and women with overweight/obesity and controlled co-morbidities, namely hypertension and/or dyslipidemia.70, 71, 72 Across arms, the range of mean age of participants was 43.7–45.9 years, and the range of percentage of women was 84.6–91.6%.70, 71, 72 The range of mean baseline BMI was 36.1–37.0 kg/m2.70, 71, 72 In the COR-I trial, the percent total body weight loss was 5 (6.5) %, 6.1 (6.5) %, and 1.3 (6.8) % in the NB16 (n = 578), NB32 (n = 583), placebo (n = 581) arms, respectively (Fig. 2). Total body weight loss was significantly different between the 2 NB doses in an exploratory analysis of the primary analysis.70 The COR-II trial showed a weight reduction of 6.4 (8.6) % in the intervention group NB32 (n = 1001), compared to 1.2 (6.41) % (n = 495) in the placebo group (Fig. 2).71 The COR-Behavioral Modification (COR-BMOD) trial included an intensive behavioral therapy co-intervention in both arms and showed a weight reduction of 9.3 (9.7) % in the NB group (n = 591) compared to 5.1 (8.5) % in the placebo group (n = 202).72 In patients with DM, with a baseline characteristics profile similar to other COR trials (with the exception of the presence of DM), at 56 weeks, NB (32/360 mg) compared to placebo, resulted in a significant total body weight loss (percent total body weight loss 5 (4.9)% in NB, 1.8 (5)% in placebo, p < 0.001).73 The dropout rate in the COR program RCTs ranged between 11% and 27% for the intervention group and was around 11% for the placebo group.70, 71, 72 The adverse events observed in more than 10% of the population in the main trials for approval of this drug include GI symptoms such as nausea, dry mouth, constipation, diarrhea, and vomiting; symptoms of the nervous system such as headaches and dizziness; or psychiatric disorders such as insomnia, and sleep disorders (Table 1).74
The pooled effect of 4 NB RCTs in patients with obesity showed that the mean difference in total body weight loss in NB arms was 2.5 (1.9–3.2) kg, compared with placebo.75
Liraglutide (Saxenda)
The Satiety and Clinical Adiposity Liraglutide Evidence (SCALE) program included four RCTs on liraglutide 3 mg once daily (QD), compared to placebo, each extending over 56 weeks,76, 77, 78 except for the SCALE Obesity and Prediabetes Extension trials that spanned over 160 weeks.79 The study population included patients with overweight/obesity, and the range of mean age of all participants was 45.0–49.0 years, and majority women (76–84% of participants).76, 77, 78, 79 The range of mean baseline BMI was 37.5–39.3 kg/m2.76, 77, 78, 79 In the SCALE Obesity and Prediabetes trial, participants with overweight/obesity without type 2 diabetes (n = 3731) were randomized to liraglutide, 3.0 mg/d (n = 2487) or placebo (n = 1244), in addition to lifestyle modifications.76 These lifestyle modifications included advising patients to increase their physical activity to at least 150 min per week and reduce their daily energy intake by 500 kcal below their individualized energy requirements.76 At 56 weeks, the weight reduction was 8 (6.7) % in the Liraglutide arm and 2.6 (5.7) % in the placebo arm (Fig. 2).76 At 160 weeks, weight reductions, compared to baseline, were 6.1 (7.3) % and 1.9 (6.3) % (Fig. 2) for patients on liraglutide 3 mg (n = 1472) and placebo (n = 738), respectively, implying a small weight regain after the first year of treatment. The SCALE maintenance trial included a run-in period aiming at achieving a least 5% total body weight loss, with a 500 kcal per day deficit diet, based on estimated 24-h energy expenditure. Participants were then randomized to liraglutide 3 mg (n = 207) or placebo (n = 206) and had thereafter additional percent weight reduction of 6.2 (7.3) % on and 0.2 (7.0) %, respectively.77 The SCALE-Intensive Behavioral Therapy (SCALE-IBT) compared liraglutide 3 mg (n = 142) to placebo (n = 140), on top of a co-intervention in both arms, consisting of intensive behavioral therapy (IBT), an exercise plan, and a caloric prescription depending on the participant baseline weight.78 Weight reduction was of 7.5% and 4.0% in liraglutide and placebo, respectively, at 56 weeks of treatment (SD not available). In patients with DM, with a baseline characteristics profile similar to other SCALE trials (with the exception of the presence of DM), at 56 weeks, a significant total body weight loss was achieved (percent total body weight loss of 6% in liraglutide 3 mg, 4.7% in liraglutide 1.8 mg, 2.0% in the placebo, p < 0.001 between both arms and placebo, SD not available).80 The dropout rate in the SCALE studies was 8–10% in the Liraglutide group and 11–20% in the placebo groups,76,77 with an exceptionally high dropout rate of 65% in the extension study.79 The adverse events observed in more than 10% of the population in the main trials for approval of this drug include a somewhat increased heart rate; GI symptoms such as constipation, diarrhea, nausea, dyspepsia, decreased appetite, and vomiting; infections such as nasopharyngitis and upper respiratory tract infection and influenza; hypoglycemia; symptoms of the nervous system such as dizziness and headaches (Table 1).76,77,79,81
Semaglutide (Wegovy)
The Semaglutide Treatment Effect in People with Obesity (STEP) trials evaluated the efficacy and safety of Semaglutide 2.4 mg weekly compared to placebo, for a duration of 68 weeks each.82, 83, 84 The study population included men and women with overweight/obesity with at least one treated or untreated weight-related comorbidity. The range of mean BMI was 37.8–38.4 kg/m2.82, 83, 84 For STEP 1 trial, the mean age of all participants was 46.0 years, and 74% were women.82 The STEP 1 trial included individual counseling sessions every 4 weeks to help with the adherence to a reduced calorie diet of 500 kcal deficit and an increased physical activity prescription of 150 min per week. STEP 1 trial reported a weight reduction of 14.8% in the intervention group (n = 1306), compared to a reduction of 2.4% in the placebo group (n = 655); SD not available (Fig. 2).82 Dropout rates was not reported. In STEP 4-Maintenance trial, mean age was 46.0 years, and 79% were women.83 Participants were prescribed a reduced-calorie diet (500-kcal/d deficit relative to estimated energy expenditure) and increased physical activity (150 min/wk) in addition to Semaglutide 2.4 mg weekly for 20 weeks, after which mean body weight of participants dropped by 10.6%. Participants were then randomized to semaglutide 2.4 mg (n = 535) or placebo (n = 268). After randomization, Semaglutide group achieved a weight reduction of 7.9 (8.3) % while the placebo group had a weight gain of 6.9 (8.8) % (Fig. 2).83 Dropout rate for both arms was very low of 0.8%.83 STEP 3- IBT trial included 611 participants, mean age 46 years, and 81% were women.84 All participants received a low-calorie diet prescription of 1000–1200 kcal followed by a hypocaloric diet of 1200–1800 kcal, in addition to a physical activity prescription. Participants also received individual IBT visits with a registered dietitian. In the intervention group (n = 407), weight reduction was 16.0%, while in the placebo group (n = 204) weight reduction was 5.7%, SD not available.84 Patients with DM in the STEP 2 trial had a baseline characteristics profile similar to other STEP trials in terms of mean age, range of percent females, mean body weight, mean BMI, mean blood pressure (systolic and diastolic), and coexisting conditions at the time of screening. At 68 weeks, the STEP 2 trial achieved a significant total body weight loss (percent total body weight loss 9.6 (8.0) % in semaglutide 2.4 mg; 7.0 (8.0) % in semaglutide 1.0 mg, 3.0 (8.0) % in placebo, p < 0.0001 between both arms and between semaglutide 1.0 mg and placebo).85 Compliance data was not reported, and the dropout rate was remarkably low in the STEP studies, ranging from 0 to 6%.82, 83, 84 The adverse events observed in more than 10% of the population in the main trials for approval of this drug include GI symptoms such as nausea, dyspepsia, vomiting, flatulence, diarrhea, abdominal pain, abdominal distention, or constipation; infections and infestations such as upper respiratory tract infections, urinary tract infection, and nasopharyngitis; musculoskeletal and connective tissue disorders such as back pain; and symptoms of the nervous system such as dizziness and headaches (Table 1).16,82,84
Pooled data from 3 RCTs on weekly semaglutide (2.4 mg) to placebo showed a mean difference in total body weight loss 12.6% (95% CI 10.3–14.8) at 68 weeks, favoring the intervention.86
Head to head comparison between FDA approved anti-obesity medications effect on weight
A network meta-analysis of RCTs on FDA approved AOM, until 2016, showed that compared to placebo, at 1 year, phentermine/topiramate was associated with the highest total body weight loss of 8.8 (7.4–10.2) kg, while liraglutide and NB had a similar total body weight loss, 5.3 (4.5–6.1) kg for liraglutide and 5.0 (4.0–5.9) kg for NB. The lowest total body weight loss was reported with orlistat of 2.6 (2.2–3.0) kg.87 On the other hand, liraglutide and NB were associated with the highest risk of discontinuation secondary to adverse events.87
Two RCTs compared Liraglutide to Orlistat and Semaglutide, respectively.88,89
A 20-week RCT, followed by a 1-year extension, compared increasing doses of daily Liraglutide (1.2, 1.8, 2.4 or 3.0 mg) to placebo or open label Orlistat (120 mg TID).89 After 1 year, all Liraglutide and placebo groups were switched to Liraglutide 2.4 or 3 mg.89 At 1 year, total body weight loss was higher with liraglutide compared to placebo and Orlistat, with a difference in the achieved weight of 5.8 (3.7–8.0) kg and 3.8 (1.6–6.0) kg, respectively, favoring liraglutide; at 2 years, total body weight loss remained significantly higher in liraglutide groups (5.3 kg) compared to the orlistat group (2.3 kg) (p < 0.001).89
The STEP 8 trial compared semaglutide (2.4 mg weekly) to Liraglutide (3 mg daily) with matching daily and weekly placebo in patients with obesity, mean BMI 37.5 (6.8) kg/m2.88 Total body weight loss was higher in Semaglutide compared to Liraglutide, with a mean difference in total body weight loss of 9.4 (6.8–12.0) % at 68 weeks.88 The adverse events were reported in more than 90% of participants of all groups, and they were mostly related to GI symptoms.88 Treatment discontinuation secondary to adverse events was higher with Liraglutide (12.6%), compared to Semaglutide and placebo (3.5%).88
Combination of FDA approved anti-obesity medications and effect on weight
One 12-week trial compared the effect of phentermine (37.5 mg daily) and orlistat (120 mg TID) combined to Phentermine (37.5 mg daily) and placebo in 51 participants with overweight/obesity. The primary outcome was sterol metabolism, which was assessed by measuring the change in the serum levels of sterols from baseline to 12 weeks, and weight was one of the secondary outcomes.90 At 12 weeks, the drop in weight was 8.6 (0.8) kg with phentermine and orlistat, compared to 6.9 (0.6) kg with phentermine and placebo (p 0.04). Adverse events were not reported.90
The benefit of adding phentermine to liraglutide was explored in a pilot study of 45 participants with obesity (mean BMI 34.3 (4.7) kg/m2).91 After achieving 12% total body weight loss with liraglutide (3 mg daily) and behavioral therapy over 1 year, participants were re-randomized to liraglutide (3 mg daily) and Phentermine (15 mg daily) or liraglutide (3 mg daily) and placebo.91 There was a trend for a modest additional total body weight loss in the combination group compared to liraglutide and placebo group, but the difference did not reach statistical significance (difference 1.4 (−0.1 to 3.0) %, p 0.073).91 Interestingly, hunger was significantly lower in the former compared to the latter group.91 The most common adverse events were musculoskeletal and gastroesophageal reflux, without a significantly increased risk of hypertension or palpitations.91
A 26-week RCT compared phentermine, canagliflozin, as monotherapy or in combination to placebo in patients (n = 335) with obesity (mean BMI 37.3 (5.2) kg/m2) with or without hypertension or dyslipidemia.92 The combination canagliflozin and phentermine led to a significant reduction in weight compared to placebo with a difference in the percent total body weight loss of 6.9 (5.2–8.6) %; total body weight loss was also significantly higher in the combination arm compared to monotherapy.92
Effect on central obesity
Orlistat (Xenical, Alli)
In XENDOS, at 1-year, and compared to baseline, the waist circumference (WC) decreased by 9.6 cm with orlistat and by 7.0 cm in the placebo group (p < 0.01). At 4 years, WC dropped by 6.4 cm and 4.4 cm, in the orlistat and placebo groups, compared to baseline (p < 0.01)58 (Appendix D).
One trial evaluated the effect of orlistat on visceral adiposity.93 Patients were randomized to receive orlistat 60 mg TID (n = 61) or placebo (n = 62), in addition to instructions on lifestyle modification and advice on distribution of fat intake and multivitamin intake for all participants for a total duration of 24 weeks. The investigators used computerized tomography (CT) scans to assess the visceral adipose tissue (VAT) mass in kilograms. Both interventions lead to a decrease in VAT mass, with a significantly higher drop in patients taking orlistat; mean drop of 0.6 (0.7) kg in orlistat arm, compared to a mean drop of 0.4 (0.6) kg in the placebo arm (p < 0.05).93 Interestingly, in sub-group analysis according to total body weight loss, there was no difference between arms when total body weight loss was >5%, while the drop was larger with orlistat compared to placebo, in those who lost <5% of their weight.93
Phentermine/Topiramate (Qsymia)
In EQUIP, at 56 weeks, mean WC reductions, were 5.6 (9.8) % for the low dose group, 10.9 (10.3) % for the high dose group, and 3.1 (10.3) % for the placebo group (p < 0.0001 between high dose group and placebo and between the two doses).64 Similarly, at 56 weeks, CONQUER trial reported a similar pattern in the drop in WC65 (Appendix D).
Naltrexone/Bupropion (Contrave/Mysimba)
After 56 weeks, the COR-I trial a mean WC reduction of 5 (10.3) cm with NB16, 6.2 (10.4) cm with NB32, and 2.5 (10.4) cm in the placebo70 (p < 0.0001 for both doses vs. placebo). The findings in the COR-II trial were of a similar magnitude compared to COR-I,71 while the drop in WC was larger in the COR-BMOD trial; mean WC reduction of 10.0 (11.7) cm with NB16 compared to 6.8 (10.9) cm in the placebo arm72 (Appendix D).
Liraglutide (Saxenda)
In the SCALE Obesity and Prediabetes trial, at 56 weeks, the mean WC reduction was 8.2 (7.3) cm in the liraglutide arm and 3.9 (6.6) cm in the placebo arm (p < 0.001).76 At 160 weeks, at the Extension trial, compared to baseline, mean WC reductions of 6.9 (8.3) cm and 3.4 (7.5) cm were achieved in liraglutide 3 mg and placebo groups (n = 738), respectively (p < 0.0001).79 In the SCALE maintenance trial, the change in WC followed the same pattern but of a lower magnitude.77 In the SCALE IBT, mean WC reductions of 9.4 cm and 6.7 cm in the liraglutide 3 mg and placebo groups, respectively (p 0.0063, SDs not available), reflecting a higher total body weight loss in both groups with behavioral therapy at 56 weeks of treatment78 (Appendix D).
One trial assessed the percent change in VAT in patients taking liraglutide 3.0 mg (n = 73) compared to placebo (n = 55) for 40 weeks, where all participants received recommendations for diet and physical activity.94 Participants underwent magnetic resonance imaging (MRI) scanning where retrieved images were analysed for several measures, including VAT. A significantly higher reduction in percent VAT was seen among patients on liraglutide with a mean drop of 12.5 (9.3) %, as compared to patients on placebo with a mean drop of 1.6 (12.3) % (p < 0.0001).94
Semaglutide (Wegovy)
At 68 weeks, the STEP 1 trial reported a mean WC reduction of 13.5 (SD not available) cm in the intervention group and 4.1 (SD not available) cm in the placebo group (p < 0.001).82 In STEP 4-Maintenance trial, and after the run-in period, a mean WC reduction of 6.4 (8.3) cm was achieved with semaglutide group and an increase of 3.3 (8.3) cm in the placebo after 68 weeks (p < 0.001).83 STEP 3- IBT trial showed the same pattern of change in WC as reported in STEP 184 (Appendix D).
Effect on glycemic parameters
Orlistat (Xenical, Alli)
At 1 and 4 years of treatment, the XENDOS trial did not report on any glycemic parameter (Table 2).58 The trials that investigated the efficacy of orlistat in patients with overweight/obesity and DM (baseline HbA1c 8–9%) showed a significant decrease in glycemic parameters in participants taking orlistat compared to patients on placebo.59, 60, 61, 62
Table 2.
Summary of landmark randomized controlled trials of the FDA approved anti-obesity medications (excluding trials in diabetes mellitus).
| Drug (trade name)a | Main phase 3 trial (duration) | Arms (N) Co-intervention Drop-out rate (%) |
Age Mean (SD) Gender Women (%) |
Proportion (%) of participants losing ≥5% or ≥10% of baseline weight | HbA1c % change from baseline Mean (SD) |
Lipid % change from baseline Mean (SD) |
SBP/DBP Change from baseline (mmHg) |
|---|---|---|---|---|---|---|---|
| Orlistat (Xenical, Alli) | XENDOS (1 y)b58 | I: 120 mg TID (1487) C: placebo TID (1295) + reduced calorie diet and physical activity (1 km walk per day) Drop-out: NA |
I: 43.0 (8.0) C: 43.7 (8.0) I: 55.2 C: 55.3 |
≥5% loss I: 73 C: 45 ≥10% loss I: 41 C: 21 |
NA | HDL-C/LDL-C I: +3.4 (NA)/−11.4 (NA) C: +8.5 (NA)/−1.6 (NA) TC/TG I: −8.8 (NA)/−6.2 (NA) C: −1.3 (NA)/−6.3 (NA) |
I: −7.3/−3.6 C: −5.2/−2.6 |
| XENDOS (4 y)b58 | I: 120 mg TID (851) C: placebo TID (567) + reduced calorie diet and physical activity (1 km walk per day) Drop-out:I: 48% C: 66% |
I: 43.0 (8.0) C: 43.7 (8.0) I: 55.2 C: 55.3 |
≥5% loss I: 53 C: 37 ≥10% loss I: 26 C: 16 |
NA | HDL-C/LDL-C I: +6.5 (NA)/−12.8 (NA) C: +9.1 (NA)/−5.1 (NA) TC/TG I: −7.9 (NA)/+2.4 (NA) C: −2.3 (NA)/+2.9 (NA) |
I: −4.9/−2.6 C: −3.4/−1.9 |
|
| Phentermine/Topiramate (Qsymia) | CONQUER (56 weeks)c65 | I1: 7.5 mg/46 mg (498) I2: 15 mg/92 mg (995) C: placebo (994) + standard LSM Drop-out: 31% |
I1: 51.1 (10.4) I2: 51.0 (10.7) C: 51.2 (10.3) I1: 70 I2: 70 C: 70 |
≥5% loss I1: 62 I2: 70 C: 21 ≥10% loss I1: 37 I2: 48 C: 7 |
I1: 0.0 (0.7) I2: −0.1 (0.9) C: +0.1 (0.9) |
HDL-C/LDL-C I1: +5.2 (19.4)/−3.7 (25.6) I2: +6.8 (20.8)/−6.9 (27.4) C: +1.2 (20.8)/−4.1 (27.4) TC/TG I1: −4.9 (15.4)/−8.6 (49.5) I2: −6.3 (17.0)/−10.6 (52.9) C: −3.3 (17.0)/+4.7 (52.9) |
I1: −4.7/−3.4 I2: −5.6/−3.8 C: −2.4/−2.7 |
| SEQUEL (56-week extension of CONQUER)c66 | I1: 7.5 mg/46 mg (153) I2: 15 mg/92 mg (295) C: placebo (227) + standard LSM Drop-out: I1: 8.4% I2: 10.5% C: 4.9% |
I1: 52.2 (10.6) I2: 51.2 (10.4) C: 52.7 (9.8) I1: 69.3 I2: 66.1 C: 64.8 |
≥5% loss I1: 75 I2: 79 C: 30 ≥10% loss I1: 50 I2: 54 C: 12 |
I1: +0.01 (0.6) I2: 0.0 (0.6) C: +0.2 (0.4) |
HDL-C/LDL-C I1: +7.3 (NA)/−4.6 (NA) I2: +11.9 (NA)/−5.6 (NA) C: +4.7 (NA)/−10.7 (NA) TC/TG I1: NA/−12.5 (NA) I2: NA/−13.7 (NA) C: NA/+0.4 (NA) |
I1: −4.7/−3.7 I2: −4.3/−3.5 C: −3.2/−3.9 |
|
| EQUIP (56 weeks)c64 | I1: 3.75 mg/23 mg (234) I2: 15 mg/92 mg (498) C: placebo (498) + standard LSM Drop-out: I1: 39.0% I2: 33.6% C: 47.1% |
I1: 43.0 (11.0) I2: 41.9 (12.2) C: 43.0 (11.8) I1: 83.4 I2: 82.8 C: 82.7 |
≥5% loss I1: 45 I2: 67 C: 17 ≥10% loss I1: 19 I2: 47 C: 7 |
NA | HDL-C/LDL-C I1: +0.5 (17.1)/−7.7 (19.8) I2: +3.5 (18.3)/−8.4 (20.9) C: 0.0 (18.3)/−5.5 (20.9) TC/TG I1: −5.4 (13.3)/+5.2 (46.8) I2: −6.0 (14.3)/−5.2 (49.9) C: −3.5 (14.3)/+9.1 (49.9) |
I1: −1.8/−0.1 I2: −2.9/−1.5 C: +0.9/+0.4 |
|
| Naltrexone/Bupropion (Contrave) | COR-I (56 weeks)c70 | I1: 4 mg/90 mg, 2 tablets BID (578) I2: 8 mg/90 mg, 2 tablets BID (583) C: placebo BID (581) + standard LSM Drop-out: I1: 24% I2: 21% C: 27% |
I1: 44.4 (11.3) I2: 44.4 (11.1) C: 43.7 (11.1) I1: 85 I2: 85 C: 85 |
≥5% loss I1: 39 I2: 48 C: 16 ≥10% loss I1: 20 I2: 25 C: 7 |
NA | HDL-C/LDL-C I1: +7.6 (21.6)/−1.5 (25.7) I2: +8.0 (21.0)/−2.0 (21.3) C: +0.8 (21.7)/−0.5 (25.8) TC/TG I1: NA/−8.0 (43.0) I2: NA/−12.7 (155.7) C: NA/−3.1 (44.3) |
% change I1: +0.3/+0.1 I2: −0.1/0.0 C: −1.9/−0.9 |
| COR-II (56 weeks)c71 | I: 8 mg/90 mg, 2 tablets BID (1001) C: placebo BID (495) + standard LSM Drop-out: I: 15% C: 21% |
I: 44.3 (11.2) C: 44.4 (11.4) I: 84.6 C: 84.8 |
≥5% loss I: 50 C: 17 ≥10% loss I: 28 C:6 |
NA | Mean change (mg/dl) HDL-C/LDL-C I: +3.6 (10.6)/−6.2 (23.8) C: −0.9 (10.7)/−2.1 (27.7) % change TC/TG I: NA/−9.8 (4.97) C: NA/−0.5 (2.09) |
I: +0.6/+0.4 C: −0.5/+0.3 |
|
| COR-BMOD (56 weeks)c72 | I: 8 mg/90 mg, 2 tablets BID (591) C: placebo BID (202) + intensive group behavior modification Drop-out: I: 11% C: 20% |
I: 45.9 (10.4) C: 45.6 (11.4) I: 89.3 C: 91.6 |
≥5% loss I: 66 C: 42 ≥10% loss I: 42 C: 20 |
NA | HDL-C/LDL-C I: +9.4 (24.7)/+7.1 (34.0) C: +2.8 (22.8)/+10.0 (31.2) TC/TG I: NA/−16.6 (38.4) C: NA/−8.5 (38.8) |
NA | |
| Liraglutide (Saxenda) | SCALE Obesity and Prediabetes (56 weeks)c76 | I: 3.0 mg QD (2487) C: placebo (1244) + standard LSM Drop-out: I: 12% C: 21% |
I: 45.2 (12.1) C: 45.0 (12.0) I: 78.7 C: 78.1 |
≥5% loss I: 63 C: 27 ≥10% loss I: 33 C: 11 |
I: −0.3 (0.3) C: −0.1 (0.3) |
Mean change (mg/dl) HDL-C/LDL-C I: +2.3 (NA)/−3.0 (NA) C: +0.7 (NA)/−1.0 (NA) TC/TG I: −3.1 (NA)/−13.3 (NA) C: −1.0 (NA)/−5.5 (NA) |
I: −4.2/−2.6 C: −1.5/−1.9 |
| SCALE Obesity and Prediabetes Extension (160 weeks)c79 | I: 3.0 mg QD (1505) C: placebo (749) + standard LSM Drop-out: 65% |
I: 47.5 (11.7) C: 47.3 (11.8) I: 76 C: 77 |
≥5% loss I: 50 C: 24 ≥10% loss I: 23 C: 10 |
I: −0.3 (0.3) C: +0.1 (0.3) |
NA | I: −3.2/−2.3 C: −0.5/−1.9 |
|
| SCALE Maintenance (56 weeks)c77 | I: 3.0 mg QD (207) C: placebo (206) All participants had weight loss of ≥5% from a low-calorie diet before randomization Drop-out: I: 8% C: 11% |
I: 45.9 (11.9) C: 46.5 (11.0) I: 84 C: 79 |
≥5% loss I: 51 C: 22 ≥10% loss I: 26 C: 6 |
I: −0.1 (0.3) C: 0.1 (0.3) | HDL-C/LDL-C I: +7.7 (7.7)/+7.7 (23.2) C: +3.9 (7.7)/+11.6 (23.2) TC/TG I: +7.7 (27.1)/0.0 (44.3) C: +11.6 (27.1)/+0.1 (44.3) |
I: +0.2/+1.4 C: +2.8/+1.2 |
|
| SCALE IBT (56 weeks)78 | I: 3.0 mg QD (142) C: placebo (140) + intensive behavioral therapyd Drop-out: I: 0.7% C: 7.1% |
I: 45.4 (11.6) C: 49.0 (11.2) I: 83.8 C: 82.9 |
≥5% loss I: 62 C: 39 ≥10% loss I: 31 C: 20 |
I: −0.2 (NA) C: −0.1 (NA) |
Mean change (mg/dl) HDL-C/LDL-C I: +1.9 (NA)/−1.5 (NA) C: +1.2 (NA)/+1.5 (NA) TC/TG I: −1.5 (MA)/−15.0 (NA) C: +2.3 (NA)/−4.4 (NA) |
I: −2.8/−10 C: −0.6/−0.8 |
|
| Semaglutide (Wegovy) | STEP 1 (68 weeks)82 | I: 2.4 mg weekly (1306) C: placebo (655) + standard LSM Drop-out: NA |
I: 46 (13.0) C: 47 (12.0) I: 73.1 C: 76 |
≥5% loss I: 86 C: 32 ≥10% loss I: 69 C: 12 |
I: −0.4 (NA) C: −0.1 (NA) |
HDL-C/LDL-Ce I: 1.0 (NA)/0.9 (NA) C: 1.0 (NA)/1.0 (NA) TC/TGe I: 0.9 (NA)/0.7 (NA) C: 1.0 (NA)/0.9 (NA) |
I: −6.2/−1.1 C: −0.4/−0.4 |
| STEP 4 – maintenance (68 weeks)83 | I: 2.4 mg weekly (535) C: placebo (268) All participants received Semaglutide 2.4 mg weekly for 20 weeks and were randomized to continued drug or placebo afterwards Drop-out: I: 1% C: 1% |
I: 47 (12.0) C: 46 (12.0) I: 80.2 C: 76.5 |
≥5% loss I: 89 C: 48 ≥10% loss I: 79 C: 20 |
I: −0.1 (0.7) C: +0.1 (0.0) |
HDL-C/LDL-C I: +18 (17.8)/+1 (23.6) C: +18 (25.0)/+8 (20.9) TC/TG I: +5 (11.7)/−6 (41.4) C: +11 (12.6)/+15 (66.8) |
I: +0.5/+0.3 C: +4.4/+0.9 |
|
| STEP 3 - IBT (68 weeks)84 | I: 2.4 mg weekly (407) C: placebo (204) + lifestyle interventionf Drop-out: I: 6% C: 5% |
I: 46 (13.0) C: 46 (13.0) I: 77.4 C: 88.2 |
≥5% loss I: 87 C: 48 ≥10% loss I: 75 C: 27 |
I: −0.5 (NA) C: −0.3 (NA) |
HDL-C/LDL-C I: +6.5 (NA)/−4.7 (NA) C: +5.0 (NA)/+2.6 (NA) TC/TG I: −3.8 (NA)/−22.5 (NA) C: +2.1 (NA)/−6.5 (NA) |
I: −5.6/−3.0 C: −1.6/−0.8 |
|
| Setmelanotide (Imcivree) | Placebo for a short period, then all took treatments (52 weeks)g24 | 2 single-arm trials: Trial 1: patients with POMC deficiency receiving Setmelanotide Trial 2: patients with LEPR deficiency receiving Setmelanotide |
Trial 1:18 (6.2) Trial 2: 23.7 (8.4) Trial 1: 50% Trial 2: 73% |
≥5% loss Trial 1: 90 Trial 2: 64 ≥10% loss Trial 1: 80 Trial 2: 45 |
Trial 1: −0.3 (NA) Trial 2: −0.2 (NA) |
HDL-C/LDL-C Trial 1: 45.0 (43.8)/−7.6 (23.1) Trial 2: 19.6 (24.0)/−10.0 (12.1) TC/TG Trial 1: NA (NA)/−36.6 (30.4) Trial 2: NA (NA)/−7.0 (26.6) |
Trial 1: −1.8/−1.6 Trial 2: −6.6/−1.2 |
| Tirzepatideh | SURMOUNT-1 (72 weeks)95 | I1: 5 mg weekly (630) I2: 10 mg weekly (636) I3: 15 mg weekly (630) C: placebo (643) All participants received tirzepatide or placebo, administered subcutaneously once weekly as an adjunct to lifestyle intervention. Drop-out: Total: 14% I: 10% |
I1: 45.6 (12.7) I2: 44.7 (12.4) I3: 44.9 (12.3) C: 44.4 (12.5) I1: 67.6 I2: 67.1 I3: 67.5 C: 67.8 |
≥5% loss I1: 85 I2: 89 I3: 91 C: 35 ≥10% loss I1: 69 I2: 78 I3: 84 C: 19 |
NA | Pooled Tirzepatide Groups HDL-C/LDL-C I: 8.0/−5.8 C: −0.7/−1.7 TC/TG I: −4.8/−24.8 C: −1.8/−5.6 |
Pooled Tirzepatide Groups I: −7.2/−4.8 C: −1.0/−0.8 |
Abbreviations: BID, twice daily; DBP; Diastolic Blood Pressure; HbA1c, glycosylated hemoglobin; HDL-C: high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LEPR, Leptin receptor; LSM, lifestyle modification; NA, not available; POMC, Pro-opiomelanocortin; QD, once per day; RD, registered dietitian; SBP, systolic blood pressure; TC, total cholesterol; TGs, triglycerides; TID, three times daily; y, year.
Participants weighing less than 91 kg at randomization were prescribed 1200 kcal per day, while a caloric prescription was calculated for participants weighing between 91 and 136 kg by body weight in pounds multiplied by 6. Participants weighing more than 136 kg, they were prescribed an 1800 kcal diet per day. All were prescribed 100 min of physical activity per week, and it was increased by 25 min every four weeks, with an ultimate goal of 250 min per week.
Participants who dropped out were excluded from the primary analysis.
Non-compliant participants were excluded from the analysis.
Adapted from the Diabetes Prevention Program and delivered by registered dieticians. The program included recommendations for diet, physical activity, and behavior change.
Ratio of measurement at 68 weeks over baseline.
All participants received a diet prescription and physical activity prescription. Participants had 30 individual intensive behavioral therapy visits with a registered dietician.
Trial consisted of a dose titration phase, then an 8-week placebo-controlled withdrawal phase, then a 32 additional weeks of open-label treatment.
Under expedited consideration for FDA approval.
Phentermine/Topiramate (Qsymia)
Both CONQUER and SEQUEL trials reported small changes in mean HbA1c levels; although they differed significantly between arms, favoring the intervention groups, the clinical implication of such findings is questionable (Table 2). In the CONQUER trial, there was an improvement in insulin resistance with a mean drop in homeostatic model assessment of insulin resistance (HOMA-IR) of 0.9 (8.5) and 1.1 (8.8) in low and high dose phentermine/topiramate groups, and a gain of 0.5 (7.6) in the placebo group (p 0.0007 for low dose vs. placebo and p < 0.0001 for high dose vs. placebo, p comparing high to low dose not reported).65
Naltrexone/Bupropion (Contrave/Mysimba)
In patients without DM, the COR-I trial reported a mean HOMA-IR reduction of 14.3 (73.6) % in the NB16 group, 20.2 (64.7) % in the NB32 group, compared to a 5.9 (78.8) % in the placebo group (p 0.044 for NB16 vs. placebo and p 0.0003 for NB32 vs. placebo).70 The COR-II trial showed a change in mean HOMA-IR of a similar magnitude.71 The COR-BMOD trial reported larger drop in HOMA-IR of 29.9 (60.1) % in the NB32 group compared to 16.6 (65.0) % in patients on placebo (p 0.003), probably reflecting the larger weight loss in this trial.72
In patient with obesity and DM (baseline mean HbA1c of 8.0%), there was a significant decrease in HbA1c by 0.6 (1.3) in NB32 group compared to 0.1 (1.6) in the placebo group, p < 0.001, reflecting the weight loss changes in the corresponding arms.73
Liraglutide (Saxenda)
In the SCALE Obesity and Prediabetes trial, at 56 weeks, the mean drop in HbA1c was 0.3 (0.3) % in the liraglutide arm and 0.1 (0.3) % in the placebo arm (p < 0.001).76 At 160 weeks from baseline in the Extension trial, the mean drop in HbA1c was 0.4 (0.3) % in liraglutide group, while there was an increase of 0.1 (0.3) % in the placebo group, p < 0.0001.79 The SCALE maintenance and the SCALE-IBT trials reported small changes in HbA1c that differed significantly between arms, favoring the intervention (Table 2).78
In patients with obesity and DM (mean baseline HbA1c 8%), there was a significant drop in HbA1c of 1.1–1.3% in liraglutide arms, compared to 0.3% in the placebo arm (p < 0.001).80
Semaglutide (Wegovy)
At 68 weeks, the STEP 1 trial reported a mean HbA1c reduction of 0.5% in the intervention group, compared to 0.2% in the placebo group (SDs not available; p-value not reported).82 A similar change was reported in the STEP 3-IBT trial.84 In STEP 4-Maintenance trial, from randomization after the run-in period at week 20 to week 68, there was a drop in HbA1c by 0.1 (0.7) % with semaglutide 2.4 mg while there was an increase of 0.1 (0) % with the placebo group (p < 0.001) (Table 2).83
In patients with obesity and DM (mean baseline HbA1c 8.1%), there was a drop in HbA1c by 1.5–1.6% in semaglutide arms, and by 0.4% in the placebo arms.85 There was no significant difference between semaglutide doses.85
Effect on lipid profile
Orlistat (Xenical, Alli)
After 1 year of treatment, patients taking Orlistat showed an increase in High-density lipoprotein cholesterol (HDL-C) and a decrease in Low-density lipoprotein cholesterol (LDL-C); changes of a small magnitude but significantly different than placebo (p < 0.01 for both HDL-C and LDL-C). Triglycerides level decreased in both arms (Table 2).58 A similar pattern was seen at the 4-year mark in both HDL and LDL, while there was no difference in triglycerides level between arms (Table 2).58
Phentermine/Topiramate (Qsymia)
In the EQUIP trial, at 56 weeks, the increase in HDL-C was dose dependent (p 0.0005 between high dose group and placebo and 0.0158 between the two doses). LDL decreased in all arms, with a significant difference between the high dose phentermine/topiramate and placebo (p 0.015), and no difference between doses (p 0.6). Finally, triglycerides dropped only in the high dose arm, while it increased in the low dose and placebo arms (p < 0.0001 between high dose group and placebo and 0.0027 between the two doses) (Table 2).64
The CONQUER trial showed similar, though larger, effects on lipid parameters after 56 weeks of treatment, possibly due to the addition of lifestyle modifications to all participants. The changes in the intervention arms were significantly different than the placebo arm for HDL-C, LDL-C and triglycerides levels (Table 2).65 The changes in lipid level reported in the SEQUEL trial were very close to those in the CONQUER trial, and there was a trend for a dose dependent increase in HDL and decrease in TG (Table 2).66
Naltrexone/Bupropion (Contrave/Mysimba)
The COR-I, COR-II, and COR-BMOD trials report data on HDL-C, LDL-C, and triglycerides after 56 weeks of treatment. In the COR-I trial, HDL-C increased significantly in intervention arms compared to placebo (p < 0.0001 for comparison of both doses with placebo). LDL-C did not differ significantly between arms, while the drop in triglycerides was higher in the intervention arms compared to placebo (p 0.0461 and <0.0001for NB16 and NB32 with placebo, respectively) (Table 2).70 The changes in the COR-II trial followed the same pattern (Table 2).71
The COR-BMOD trial showed an increase in HDL-C and triglycerides for both arms, since all participants were receiving intensive behavioral therapy, but the change was significantly higher in the NB arm (p < 0.001 for HDL-C and p 0.004 for triglycerides). There was no difference in LDL-C between arms (Table 2).72
Liraglutide (Saxenda)
The SCALE Obesity and Prediabetes reported mean change in lipid parameters at 56 weeks of treatment. Although the changes in HDL-C, LDL-C, and triglycerides were significantly different in liraglutide arm compared to placebo (p 0.001, p 0.002, p < 0.001, respectively) the magnitude of these changes was small.76 The 160-week SCALE extension trial did not report on lipid parameters (Table 2).79
After 56 weeks of treatment, the SCALE maintenance trial showed no significant difference in lipid parameters between treatment arms, except for triglycerides level that did not change in the intervention arm, but increased by 8.86 (44.29) mg/dL in the placebo group (p 0.03) (Table 2).77 Similarly, there was no significant difference in lipid parameters between arms in the SCALE-IBT trial (Table 2).78
Semaglutide (Wegovy)
Across all STEP trials, there was no significant difference in the HDL level between arms after the intervention.82, 83, 84 The STEP 1 trial reported the ratio of measurement at 68 weeks over baseline for all lipid parameters. LDL-C level was similar between both arms, with ratios of 0.97 and 1.01 for the intervention compared to control, respectively. The change in triglycerides differed between arms, with a ratio of change over baseline of 0.78 for patients taking semaglutide compared to a ratio of 0.93 in patients on placebo (Table 2).82 The STEP 4 maintenance trial reported mean percent changes in lipid parameters at 68 weeks of treatment. LDL-C increased in both arms, but to a lower extent in semaglutide compared to placebo (p < 0.001). Triglycerides decreased in patients on semaglutide, while they increased in patients on placebo (p < 0.001) (Table 2).83
In the STEP 3 trial LDL-C level dropped with semaglutide by 4.7%, while it increased by 2.6% with placebo (p < 0.001). Triglyceride levels decreased in both arms but favored semaglutide (p < 0.001) (Table 2).84
Effect on blood pressure
Orlistat (Xenical, Alli)
At 1-year, systolic (SBP) and diastolic blood pressure (DBP) dropped by 7.3 mmHg and 3.6 mmHg respectively, for the intervention group, and 5.2 mmHg and 2.6 mmHg, respectively for the placebo group (p < 0.01 for comparison for both SBP and DBP; SD not available). At 4 years, the drop was at a lower extent but differences between arms remained significant (Table 2).58
Phentermine/Topiramate (Qsymia)
In EQUIP, the reported reductions in SBP and DBP, at 56 weeks, were of a small magnitude, for both low dose and high dose groups. Conversely, there was a trend for an increase in SBP and DBP, in the placebo groups (p < 0.01 for high dose compared to placebo and for comparing doses for DBP only).64 At 56 weeks, CONQUER trial reported mean SBP and DBP reductions of 4.7 (13.6) mmHg and 3.4 (9.2) mmHg respectively for the low dose phentermine/topiramate, 5.6 (15.1) mmHg and 3.8 (9.8) mmHg respectively for the high dose phentermine/topiramate, and 2.4 (14.5) mmHg and 2.7 (9.8) mmHg for the placebo group (p < 0.01 for any dose compared to placebo for SBP and only for high dose compared to placebo for DBP).65 The decrease in BP seen in the EQUIP trial was relatively small compared to the changes reported in the CONQUER trial. In fact, the EQUIP trial enrolled normotensive participants while the CONQUER trial recruited a population with hypertension and other cardio-metabolic disorders. At 52 weeks, SEQUEL, the extension of CONQUER trial reported similar changes in SBP and DBP (Table 2).66
Naltrexone/Bupropion (Contrave/Mysimba)
After 56 weeks, the COR-I trial reported minimal reductions in SBP and DBP in the NB and placebo groups (p < 0.01 for comparisons between any dose and placebo).70 The reported changes in the COR-II trial were similar in magnitude.71 The COR-BMOD trial didn't assess for changes in SBPs or DBPs (Table 2).72
Liraglutide (Saxenda)
In the SCALE Obesity and Prediabetes trial, at 56 weeks, the reductions in SBP and DPB were 4.2 (2.2) mmHg and 2.6 (8.7) mmHg respectively in the liraglutide arm, and 1.5 (12.4) mmHg and 1.9 (8.7) mmHg respectively in the placebo arm (p < 0.001 for both SBP and DBP).76 At 160 weeks, at the Extension trial, the drop in SBP and DBP was similar to the original trial.79 The SCALE maintenance and SCALE IBT trials reported smaller changes in blood pressure parameters, that were not significant (Table 2).77,78
Semaglutide (Wegovy)
At 68 weeks, the STEP 1 trial reported mean SBP and DBP reductions of 6.2 mmHg and 2.8 mmHg respectively in the intervention group, compared to 1.1 mmHg and 0.4 mmHg respectively in the placebo group (p < 0.001 for SBP; p not available for DBP; SDs not available).82 In STEP 4-Maintenance trial reported mean SBP and DBP elevations of 0.5 (13.0) mmHg and 0.3 (8.8) mmHg respectively in the intervention group, compared to 4.4 (13.0) mmHg and 0.9 (10.5) mmHg, respectively, in the placebo group (p < 0.001 for SBP; p 0.46 for DBP), possibly explained by weight regain.83 STEP 3-IBT trial resulted in reductions in SBP and DBP of 5.6 mmHg and 3.0 mmHg, respectively, for participants receiving semaglutide 2.4 mg, compared to 1.6 mmHg and 0.8 mmHg, respectively, for those receiving placebo at 68 weeks (p 0.001 for SBP; p 0.008 for DBP) (Table 2).84
Effect on fatty liver
Data on fatty liver are available for all AOM except phentermine/topiramate.
Orlistat (Xenical, Alli)
In a 6-month trial in 170 Chinese patients (76% with nonalcoholic fatty liver disease (NAFLD)), orlistat (at a dose of 120 mg TID) compared to placebo showed no difference in liver enzymes levels, but a higher drop in liver fat, measured by MRI, in the orlistat arm, compared to placebo (mean difference in total fat of 4%, drop of 5.5% in orlistat and drop of 2.0% in the placebo, p < 0.001). Steatosis grade improved by four grades, two grades, and one grade in 1.5%, 11.8%, 44.1% of both study groups, respectively; a significant improvement in steatosis grades was seen when comparing arms (57.3% improvement in orlistat arm vs. 23.5% in placebo arm, p < 0.001).96 The treatment effect remained significant even after adjusting for total body weight loss.96 Conversely, in another RCT, the effect of orlistat on liver fibrosis parameters, assessed by the fibrosis-4 index and NAFLD fibrosis score, was studied.97 The fibrosis-4 index did not show a significant decrease after 12 weeks of treatment with placebo (p 0.959) or orlistat (p 0.510). As for the NAFLD fibrosis score, a significant increase was seen in placebo treated group (p 0.025) compared to a non-significant decrease in orlistat treated group (p 0.715), suggesting a potential protective effect with orlistat, yet not confirmed.97 The main limitation of the study was the lack of liver biopsy data, considered the gold standard method to evaluate liver fibrosis.97
Naltrexone/Bupropion (Contrave/Mysimba)
A post-hoc analysis of the COR trials showed a significant improvement in liver enzymes in the NB group compared to placebo; this effect was directly driven by total body weight loss. The only exception was the fibrosis-4 index, which incorporates data on age, platelet count and hepatic enzymes level, that was not only associated with total body weight loss but also with treatment arm.98
Liraglutide (Saxenda)
Several small studies explored the impact of liraglutide on NAFLD.99 Two small (n = 30) short term (16–26 weeks) studies in patients with obesity and NAFLD investigated the impact of liraglutide (3 mg daily), compared to placebo, on fatty liver, measured on MRI100 or ultrasound.101 The improvement in liver fat parameters was correlated with weight rather than treatment arms.100,101 There was no improvement in liver fibrosis, possibly explained by the short intervention duration.101 A 48-week small multicentre RCT on liraglutide (n = 26) compared to placebo (n = 26) in patients with non-alcoholic steatohepatitis, showed a significant improvement in liver histology.102 Patients on liraglutide had a 4.3 higher chance of resolution of NAFLD on liver biospy, compared to placebo (p 0.019).102
Semaglutide (Wegovy)
In a 1-year phase 1 trial, patients with NAFLD disease (n = 67) were randomized to receive semaglutide (0.4 mg daily) or placebo,103 and liver parameters were assessed using MRI. There was no difference in liver stiffness, the primary outcome, measured using magnetic resonance elastography.103 Conversely, the proportion of participants who had at least 30% reduction in liver steatosis in the semaglutide group was almost 2-fold the proportion in the placebo group, 73% and 33%, respectively at 72 weeks (p 0.0006).103 Total body weight loss was higher with semaglutide, with an estimated difference of −9.6% (p ≤ 0.0001), compared to placebo, and whether total body weight loss was the driver of the benefit on liver steatosis was not explored.
In a phase 2 trial, 320 patients with biopsy proven non-alcoholic steatohepatitis (NASH), using the definition of the NASH Clinical Research Network, were randomized to receive semaglutide (0.1, 0.2 or 0.4 mg daily) or placebo.104 Histologic findings at 72 weeks showed that the proportion of NASH resolution was higher in semaglutide groups, and the highest proportion 59% reported in the 0.4 mg semaglutide group and only 17% of the placebo group (p < 0.001).104 However, there was no difference in the fibrosis parameters between groups.104 Whether total body weight loss, rather than the intervention per se, is responsible of the beneficial impact of NASH still needs further investigation.104
Effect on obstructive sleep apnea
We identified three studies evaluating the effect of FDA approved AOM on obstructive sleep apnea (OSA), one trial on each of phentermine/topiramate,105 and liraglutide106 and a pooled analysis of 5 trials on NB.107
Phentermine/Topiramate (Qsymia)
In one trial, participants with obesity and moderate OSA were randomized to receive the combination treatment of phentermine/topiramate (15/92) (n = 22) or placebo (n = 23) with a standardized advice on lifestyle modification for a duration of 28 weeks.105 The primary outcome was the apnea-hypopnea index (AHI) with a score of 5–14 events per hour considered mild, 15–29 moderate, and 30 or more considered severe. The reduction in apnea-hypopnea events was more favorable in patients taking phentermine/topiramate compared to placebo; a reduction of 31.5 (19.9) events per hour in the intervention and 16.6 (19.9) events per hour in the placebo (p 0.0084).105
Naltrexone/Bupropion (Contrave/Mysimba)
A pooled analysis of 5 RCTs on NB32 (n = 2545) compared to placebo (n = 1515) assessed the prevalence of OSA at 24–56 weeks and showed no significant difference between groups.107
Liraglutide (Saxenda)
The change in AHI was studied in one trial on patients with moderate to severe OSA (AHI of 15–29 or 30 or more events per hour) taking liraglutide 3.0 mg (n = 180) or placebo (n = 179).106 After 32 weeks of treatment, there was a significant reduction in AHI in liraglutide group (drop of 12.2 (1.8) events per hour) compared to placebo (drop of AHI 6.1 (2.0) events per hour), p 0.015.106
Effect on cardiovascular outcomes
Naltrexone/Bupropion (Contrave/Mysimba)
The LIGHT trial was a non-inferiority cardiovascular (CV) outcome trial aiming to investigate the safety of NB in patients with obesity and a high CV risk.108 CV outcomes were defined as major adverse cardiovascular events (MACE), which includes cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. The interim analysis at 25% of the total sample size showed a risk reduction in CV risk, HR 0.6 (0.4–0.9).108 However, the trial was stopped prematurely after the release of the interim analysis results to the public, threatening the scientific integrity of the trial.108 The CV safety was recently evaluated in a network meta-analysis of phase 3 trials on NB, using each drug separately or in combination, for smoking cessation or for weight management, and showed no increased risk of major adverse cardiovascular events, defined as MACE.109
Liraglutide (Saxenda)
A post-hoc analysis combined data from the phases 2 and 3 SCALE RCTs where liraglutide was compared to placebo, in various patient populations pooled together (n = 5980), including those with pre-diabetes, DM and OSA, and investigated the time to first occurrence of a composite CV outcome, defined as death, non-fatal myocardial infarct or non-fatal stroke. Although it did not reach statistical significance, there was a trend for a lower risk for CV outcomes (hazard ratio (HR) for composite outcome 0.4 (0.2–1.1).110
Semaglutide (Wegovy)
The Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity – (SELECT) ongoing trial enrolls 17,500 participants and investigates the effect of Semaglutide compared to placebo on a composite CV outcome, defined as MACE, including CV death, non-fatal myocardial infarction, or non-fatal stroke, as a primary outcome; expected completion in October 2023.111
A recent SR/MA of RCTs (2010–2020) on AOM and mortality (all-cause and cardiovascular) and cardiovascular events (myocardial infarction, stroke, heart failure) identified 28 RCTs comparing one AOM (orlistat, NB, phentermine/topiramate, liraglutide and lorcaserin) to placebo.112 Pooling all RCTs together showed no significant reduction in overall mortality, cardiovascular mortality nor cardiovascular outcomes, with the use of AOM compared to placebo.112 Interestingly, the meta-regression showed that total body weight loss was inversely associated with mortality risk.112
Anti-obesity drugs in patients with mental illnesses
While the main trials of FDA approved AOM excluded participants with mental illness, the EQUIP, CONQUER and SEQUEL included 16–22% of participants with a history of depression or on anti-depressant medications, suggesting the potential safe use of phentermine/topiramate in this specific population.64, 65, 66
Four small pilot RCTs (N = 10–25) on patients with obesity and binge eating disorder, extending over 3–6 months showed that NB and phentermine/topiramate are safe, with a significant reduction in binge eating episodes and in weight, without an increase in adverse events, providing preliminary data for larger trials to confirm such findings.113, 114, 115, 116 A 3-month RCT administered NB or placebo to patients with schizophrenia for weight reduction or smoking cessation and did not show any difference in the measured parameters between the 2 treatment arms.117
A 16-week RCT from Denmark investigated the effect of liraglutide (1.8 mg daily; n = 52) compared to placebo (n = 51) in patients with obesity (mean BMI 33 kg/m2) and schizophrenic disorders on clozapine or olanzapine.118 There was a significant improvement in glycemic parameters (64% in the liraglutide and 16% in the placebo group had normal glucose tolerance, p < 0.001) and weight (mean difference in weight reduction 5.3 (3.7–7.0) kg, favoring liraglutide).118 There was no significant difference in adverse events.118 Another 6-month small RCT (N = 47) compared the safety and efficacy of liraglutide 3 mg daily to placebo in patients with obesity (range of mean BMI 37–41 kg/m2) and schizophrenia or schizoaffective disorder.119 The mean difference in the percent total body weight loss was 4.6 (0.7–8.4)% and there was no difference in the adverse event profile between the 2 arms.119
A post-hoc analysis of the LIGHT trial provided evidence regarding the efficacy and safety of the use of NB in patients with anti-depressants (N = 2277 on antidepressants; N = 6617 without antidepressants).120 The effect of NB on weight reduction was the same regardless of the presence or absence of anti-depressant use.120 In patients on anti-depressants, there was a significantly larger total body weight loss in the NB group compared to the placebo group at 56 weeks, while at 102 weeks there was a trend favoring the NB group that did not reach statistical significance (a drop of 6.3% vs. 4.3% in the NB and placebo, respectively).120
A pooled analysis of liraglutide phase 2 and 3 trials showed no difference in the incidence of depression and anxiety, but a potential increased risk of suicidality ideation.121
Medication under consideration for FDA-Approval
Tirzepatide
Tirzepatide is a gastric inhibitory polypeptide (GIP)/GLP-1 dual agonist, that works centrally, in the hypothalamus to decrease food intake and possibly increase energy expenditure by desensitizing the GIP receptor, through chronic GIP agonism, in preclinical models. However, animal and human data on the effect of GIP on energy expenditure has been controversial and it is expected that increased energy expenditure will not be seen in humans.122, 123, 124, 125, 126, 127 Tirzepatide also works peripherally by delaying gastric emptying.128 It was approved in 2022 for the treatment of type 2 DM.129 In a 40-week phase 3 trial-SURPASS -2-, patients with DM (n = 1879) were randomized to receive tirzepatide at doses 5, 10 and 15 mg or semaglutide 1 mg.130 Tirzepatide, at any dose, was superior to semaglutide in reducing HbA1c.130 The drop in weight was 6.7% for semaglutide, while it was 8.5, 11.0 and 13.0% in each of Tirzepatide doses (p < 0.01).130 It should be noted however that the dose of semaglutide used in this study is not the approved anti-obesity dose 2.4 mg weekly. The SURMOUNT-1 trial compared different doses of Tirzepatide to placebo in patients with obesity but without DM.95 Tirzepatide at weekly doses 5 mg, 10 mg and 15 mg, led to a mean total body weight loss of 15, 19 and 21%, respectively, compared to only 3% in the placebo arm, at 72 weeks (p < 0.001 for all comparisons with placebo) (Fig. 2).95 In this trial, WC at 72 weeks, decreased by 14.0 cm with Tirzepatide 5 mg, 17.7 cm with the 10 mg dose, 18.5 cm with the 15 mg dose, and by 4.0 cm in the placebo group (p < 0.001)95 (Appendix D). A recent trial sequential analysis exploring the optimal dose of tirzepatide in patients with DM, showed a dose dependent effect on glycemic control and weight reduction, without a significant increase in the rate of adverse events with higher doses.131 Interestingly, pooled data from SURMOUNT-1 and SURPASS clinical trials programs showed a significant relative risk reduction in major adverse CV events and CV death, by 48 and 49% respectively, with Tirzepatide, compared to control.132
Tirzepatide is currently being investigated in several phase 3 trials (n = 210–900 participants) in patients with overweight/obesity.133, 134, 135, 136, 137, 138 It may be associated with less gastro-intestinal side effects compared to GLP-1 agonists. The FDA has recently granted tirzepatide a Fast Track designation to investigate its effect as a treatment for individuals with obesity or overweight with metabolic co-morbidities.139
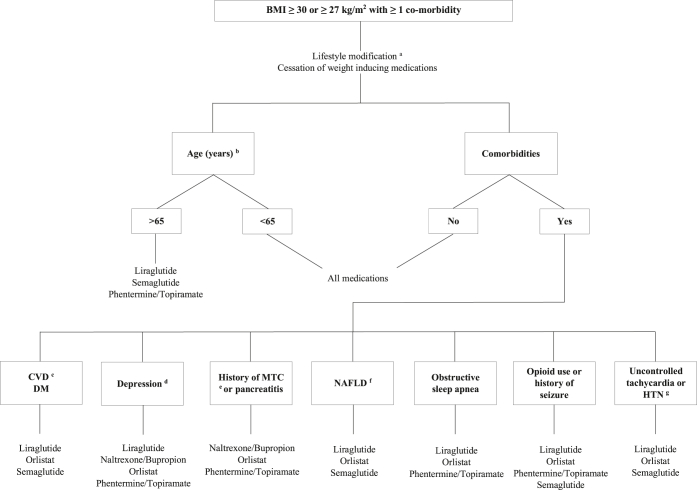
Suggested algorithm
All obesity treatment guidelines recommend pharmacotherapy, in addition to lifestyle modification and behavioral therapy, when BMI is ≥30 kg/m2, or ≥27 kg/m2 in the presence of comorbidities related to excessive weight.10,140, 141, 142, 143, 144, 145, 146, 147, 148, 149 Lifestyle modifications are the first line treatment for all. Given that the short150,151 and long152 term efficacy of different diets are the same, the prescribed diet should be a one with a proved efficacy, taking into consideration safety data, patient preference and health status.153 In addition, reviewing the patient's medications is crucial, to identify the ones that might be contributing to weight gain, and potentially withhold them, after communication with the primary physician.10 In fact, data from the National Health and Nutrition Examination Survey (2017–2018) showed that 20% of US adults are on weight inducing medications.154
In older patients and those with co-morbidities and polypharmacy, the choice of AOM needs to account for the availability of safety and efficacy data in this specific population, and the potential interaction with other drugs (Fig. 3). For instance, in patients with tachycardia or hypertension, phentermine/topiramate and NB should not be considered. As per the FDA leaflets, uncontrolled hypertension is a contra-indication for the use of Phentermine and NB; arrhythmias are also a contra-indication for the use of phentermine and tachycardia is listed under adverse events for NB.68,74 For liraglutide and semaglutide, increased heart rate is considered under “Warning and Precautions”, with a suggestion to monitor as the clinical implication of the increase in heart rate by few beats per minutes is not known.16,81 No adverse effects related to heart rate were reported with Orlistat.63 For other cardio-metabolic co-morbidities, such as OSA, DM and CVD, the suggestion for AOM take into consideration the safety and/or direct or indirect evidence on efficacy with specific drugs. Phentermine/topiramate, NB and liraglutide were investigated in patients with depression. The data on the safety of phentermine/topiramate in participants with a history of depression or on anti-depressive medications was derived from the main trials that did not reveal any signal for increased risk of adverse events.64, 65, 66 In a post-hoc analysis from the LIGHT trial, in the sub-group of participants who were on anti-depressants, the adverse events related to mental illness/depression were similar in NB and placebo arms.120 A pooled analysis of Liraglutide trials, on participants with psychiatric diseases (depression and others) showed a signal for increased suicidal ideation, but no increase in the incidence of mental diseases. For phentermine/topiramate, NB and liraglutide, the FDA includes “Suicidal Behavior and Ideation” under “Warning and Precautions” and recommends to monitor patients for depression and suicidal thoughts, and to stop the drug in case of symptoms development.68,74,81 Data on orlistat and semaglutide in patients with mental diseases is still lacking. The safety and efficacy of AOM in patients with schizophrenia is scarce. Small trials on NB and Phentermine/Topiramate have shown some safety and efficacy on total body weight loss. Lisdexamphetamine is FDA approved for moderate and severe binge eating disorder.160 The AOM suggestions for patients with history of medullary thyroid cancer, pancreatitis, seizures and opioid users take into consideration AOM contra-indications, as described in Table 1.
Fig. 3.
Suggested algorithm for the selection of anti-obesity medications, taking into consideration the availability of drug safety, contra-indications, and preliminary efficacy data for a specific patient profile.a CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension; MTC, medullary thyroid cancer; NAFLD, non-alcoholic fatty liver disease. aWith the exception of data in patients with NAFLD, Orlistat was not investigated in patients with CVD, mental diseases and sleep apnea. However, given its safety profile, Orlistat can be considered in these conditions. None of the drugs is approved for use during pregnancy. Additional contra-indications for specific medications are described in Table 1. bAge >65 years: Liraglutide and Phentermine/Topiramate trials included 7% of participants ≥65 years. For Semaglutide, SUSTAIN trials in patients with diabetes mellitus showed that efficacy is preserved regardless of age.155 The COR program included only 2% of participants from the elderly population,156 and the XENDOS included none. cCVD includes patient with previous history of CVD and those at high risk for CVD. The suggestion for the use of GLP-1 receptors agonist is based on the availability of indirect evidence on cardiovascular risk reduction in patients with diabetes mellitus, LEADER trial for liraglutide157 and SUSTAIN trial for semaglutide.158 There was no signal of increased CVD in patient with obesity on liraglutide. The suggestion for the use of Liragluide and Semaglutide in patients with obesity and DM is based on the fact that GLP-1 receptor agonists are anti-diabetic medications. dDepression: For Phentermine/topiramate, NB and liraglutide, the FDA includes “Suicidal Behavior and Ideation” under “Warning and Precautions” and recommend to monitor patients for depression and suicidal thoughts, and to stop the drug in case of symptoms development.68,74,81eMTC: personal or family history of MTC, a contra-indication for Liraglutide and Semaglutide. fSmall trials on Orlistat, Liraglutide and Semaglutide showed improvement in steatosis, but none of them showed improvement in liver fibrosis; only post-hoc data are available on NB. gThe FDA leaflet of Phentermine states the following under contra-indications “History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension).159 The FDA leaflet of NB states that uncontrolled hypertension is a contra-indication.74 For liraglutide and semaglutide, increased heart rate is considered under “Warning and Precautions” in the FDA leaflets, with a suggestion to monitor as the clinical implication of the increase in heart rate by few beats per minutes is not known.16,81
After starting AOM, responders are those who lose at least 5% of their baseline weight after 3 months of therapy and should continue their treatment.10 Conversely, those who don't achieve this target are considered as non-responders and should be switched to another drug.10
Medications in the horizon
Several drugs in pipeline are currently being investigated as potential AOM. Except for sodium glucose cotransporter-2 (SGLT2) inhibitors, vitamin E and gladribin analogues, these drugs act centrally to reduce appetite and increase satiety (Fig. 4, Table 3).
Fig. 4.
Site of action of drugs under development for treatment of obesity. (1) parietal cortex, (2) hypothalamus, (3) insula, (4) putamen, (5) nucleus accumbens, (6) striatum, (7) orbitofrontal cortex, (8) hindbrain, (9) mesolimbic area. GLP-1/glucagon dual agonists, GIP/GLP-1 dual agonists, Y2R, amylin receptor agonists, methylphenidate, oxytocin, and leptin sensitizers act centrally to reduce food intake. Few drugs act solely peripherally: SGLT-2 inhibitors on the kidney; Gladribin on liver and muscle; Vitamin E on adipose tissue. GIP, Gastric inhibitory polypeptide; GLP-1, Glucagon receptor agonist; SGLT2, Sodium-glucose Cotransporter-2 (SGLT2) Inhibitors; Y2R, Y2-receptor. Tesofensine is not included in the figure as we did not identify any related ongoing study. This figure was created using BioRender (https://biorender.com/).
Table 3.
Anti-obesity medicationsa in the horizon identified from Clinicaltrials.gov.
| Drug | Site of action | Development stage | Dose | Indication | Sample size | Expected completion | Registration number |
|---|---|---|---|---|---|---|---|
| GLP1/glucagon dual agonists | Parietal cortex, insula, putamen, orbitofrontal cortex, gastrointestinal tract, adipose tissue, liver | ||||||
| BI 456906 | Phase II | Very low, low, medium and high SQ dose groups once weekly | Obesity | 387 | October-22 | NCT04667377 | |
| IBI362 | Phase II | Low, moderate, and high SQ dose groups once weekly | Obesity | 320 | September-23 | NCT04904913 | |
| Phase II | 5 different SQ doses | Obesity | 66 | January-24 | NCT04440345 | ||
| GIP/GLP1 dual agonists | Arcuate nucleus and other hypothalamic regions, parietal cortex, insula, putamen, orbitofrontal cortex, adipose tissue, gastrointestinal tract | ||||||
| Tirzepatide | Phase III | 10 mg and 15 mg SQ once weekly | Obesity and DM | 900 | April-23 | NCT04657003 | |
| Phase III | Regimen A and B SQ once weekly | Obesity | 261 | June-23 | NCT04844918 | ||
| Phase III | N/A | Obesity | 750 | May-23 | NCT04660643 | ||
| Phase III | N/A | Obesity and weight maintenance after intensive lifestyle | 800 | May-23 | NCT04657016 | ||
| CT-868 | Phase II | Low and maximally tolerated SQ daily doses | Obesity and DM | 96 | February-23 | NCT05110846 | |
| GMA106 | Phase I | Single SQ injection | Obesity | 48 | April-23 | NCT05054530 | |
| CT-388 | Phase I | SQ injection | Obesity and DM | 96 | October-23 | NCT04838405 | |
| GIP/GLP1/glucagon tri-agonists | Parietal cortex, insula, putamen, orbitofrontal cortex, arcuate nucleus and other hypothalamic regions, gastrointestinal tract, adipose tissue, liver | ||||||
| LY3437943 | Phase II | 4 different SQ doses | Obesity | 388 | November-22 | NCT04881760 | |
| GLP1R agonists | Parietal cortex, insula, putamen, orbitofrontal cortex, gastrointestinal tract | ||||||
| Liraglutide vs. Exenatide | Phase III | Liraglutide: 0.6 mg up to 1.8 mg SQ injection daily; Exenatide: 10 mcg daily for 4 weeks then twice a day for 8 weeks, and 2 mg SQ injection once weekly | Obesity | 150 | September-22 | NCT03671733 | |
| XW003 Vs Liraglutide | Phase II | XW003: up-titrated to 1.2, 1.8, or 2.4 mg SQ once weekly starting with 0.2 mg; Liraglutide: uptitrated over 4 weeks to 3 mg SQ once daily | Obesity | 200 | October-22 | NCT05111912 | |
| PF-06882961 | Phase II | 5 doses (40, 80, 120, 160 or 200 mg oral twice daily) | Obesity | 497 | October-23 | NCT04707313 | |
| Oral LY3502970 | Phase II | 4 different oral doses | Overweight and obesity | 272 | November-22 | NCT05051579 | |
| SHR20004 Noiiglutide | Phase II | Low, medium and high SQ doses | Obesity | 254 | May-22 | NCT04799327 | |
| Y2R agonist | Hypothalamus | ||||||
| NNC0165-1875 with semaglutide | Phase II | Semaglutide 2.4 mg SQ and NNC0165-1875 1 or 2 mg SQ | Obesity | 119 | December-22 | NCT04969939 | |
| Amylin receptor agonist | Area postrema of the caudal hindbrain, hypothalamus, gastrointestinal tract, pancreas | ||||||
| NNC0174-0833 | Phase I | 0.3 mg, 0.9 mg or 1.8 mg SQ | Obesity | 24 | September-22 | NCT05254158 | |
| Cagrilintide with Semaglutide | Phase III | Cagrilintide: 2.4 mg SQ weekly Semaglutide: 2.4 mg SQ weekly | Obesity and DM | 1200 | January-25 | NCT05394519 | |
| SGLT-2 inhibitors | Kidney, adipose tissue | ||||||
| Dapagliflozin with metformin vs. metformin | Phase III | Dapagliflozin: 10 mg orally daily Metformin: 850 mg orally twice daily | Obesity and DM or prediabetes | 90 | July-21 | NCT03968224 | |
| Dopamine reuptake inhibitor | |||||||
| Methylphenidate | Nucleus accumbens, striatum | Phase III | One tablet twice daily | Obesity | 40 | December-18 | NCT02754258 |
| Acetylcholine blockers | |||||||
| Botulinum toxin type A | gastrointestinal tract | Phase III | 20 injections of 0.5 ml each | Obesity | 18 | July-22 | NCT04274608 |
| Phase II | Injection in muscles of stomach wall | Morbid obesity | 20 | December-22 | NCT02035397 | ||
| Glabridin analogue | Muscles, liver | ||||||
| HSG4112 | Phase II | 200, 400 and 600 mg orally daily | Obesity | 81 | December-22 | NCT05197556 | |
| Oxcytocin | Muscles, liver, adipocytes, pancreas, paraventricular nucleus, nucleus accumbens, striatum, mesolimbic area | ||||||
| Phase II | 24 IU nasal spray, 4 times per day | Obesity | 61 | July-22 | NCT03043053 | ||
| Vitamin E | Adipose tissue | ||||||
| Tocotrienols | Phase II | One 430 mg tocotrienol softgel daily | Obesity in postmenopausal women | 60 | December-23 | NCT03705845 | |
| Taste receptors activator | Taste buds | ||||||
| ARD-101 | Phase II | 200 mg orally twice daily | Obesity | 20 | November-22 | NCT05121441 | |
| Leptin sensitizers | Adipose tissue, liver, hypothalamus | ||||||
| ERX1000 | Phase I | 4 mg orally | Obesity | 48 | June-23 | NCT04890873 | |
| Others | |||||||
| PPAR gamma modular: AMG 133 | Phase I | N/A | Obesity | 110 | November-22 | NCT04478708 | |
| MBL949 | Phase II | 5 different SQ doses | Obesity | 127 | June-23 | NCT05199090 | |
| NO-13065 | Phase I | N/A | Obesity | 84 | September-22 | NCT04838639 | |
| NNC0247-0829 | Phase I | Single or multiple SQ doses | Obesity | 103 | June-22 | NCT04010786 | |
| LY3841136 | Phase I | Single or multiple SQ ascending doses | Obesity | 160 | September-23 | NCT05295940 | |
| BMS-986172 | Phase I | Specified oral dose on specified days | Obesity | 40 | May-22 | NCT04926051 |
Abbreviations: DM, diabetes mellitus; IU, international unit; SQ, subcutaneous.
Indication included obesity with or without DM.
Medications in phase 3 trials
Methylphenidate
Methylphenidate is a stimulant approved for the treatment of attention deficit hyperactivity disorders (ADHD).161 It is a dopamine reuptake inhibitor that is suggested to reduce energy intake.162 A prospective study on 78 adults with ADHD and severe obesity (mean BMI 42 kg/m2) who received psychostimulants, including amphetamine, methylphenidate or dextroamphetamine, showed a total body weight loss of 12% in treated participants over a mean time of 466 days of observation.163 A cross-over placebo-controlled trial of 14 adults (mean BMI 25.0 (4.0) kg/m2) with ADHD, compared methylphenidate to placebo, and showed a significant reduction in fat and energy intake 1 h after the ingestion of a buffet styled lunch.164 In a small trial, 9 adult men with obesity and without ADHD, were randomized to receive placebo, moderate (0.5 mg/kg) or high (1 mg/kg) dose methylphenidate. There was a significant reduction in energy intake by 23% in the intervention arms, compared to placebo (p < 0.02) as measured by pizza consumption in an acute laboratory setting 1 h after ingestion of the medication.162 The most common side effects associated with the use of methylphenidate are headache, insomnia, upper abdominal pain, tachycardia and anorexia.161
A currently ongoing 2-month phase 3 trial compares the effect of methylphenidate at a dose of 0.50 mg/kg BID (n = 20) to placebo (n = 20) in adolescents and adults aged 16–40 years with obesity, on energy intake as a primary outcome, energy expenditure and body weight as secondary outcomes.165 The study completion date was December 2018, but no results are available online yet.165
Tesofensine
Tesofensine is a noradrenaline, serotonin and dopamine reuptake inhibitor that acts as an appetite suppressant and may increase dopaminergic activity in the forebrain.166,167 A phase 2 RCT in 203 patients with obesity (BMI 30–40 kg/m2) evaluated the effect of an increasing dose of Tesfensine 0.25 mg, 0.5 mg, and 1 mg to placebo, on top of caloric restriction.168 At 24 weeks, there was a dose dependent drop in weight of 4.5%, 9.2% and 10.6% in Tesofensine arms, and only 2.0% in the placebo arm.168 A longer term RCT including 2 phases, each extending over 24 weeks, investigated the effect of increasing doses of Tesofensine on appetite and showed a dose dependent response.169 The main concern with Tesofensine was the risk of increased sympathetic activity with elevations in blood pressure and heart rate. The Viking Trial extended over 24 weeks and enrolled 372 participants, randomized to Tesofensine 0.25 mg or 0.5 mg or placebo.170 There was on average 10% total body weight loss at 24 weeks in the intervention arm, and no significant adverse events.170 However, the peer-reviewed publication of the trial has not been available online yet.
Exenatide
Exenatide, a GLP1-RA, was explored in the treatment of patients with obesity, without DM. A 24-week RCT in patients with a range of mean baseline weight 107–109 kg, compared Exenatide (n = 73) with placebo (n = 79), on top of lifestyle modification as a co-intervention for both arms.171 A significant difference in total body weight loss of 3.3 (0.5) % (p < 0.001), favoring Exenatide.171 Conversely, a more recent study did not confirm the same findings.172 One ongoing study with 3 arms explores the effects of exenatide 10 mcg twice daily, exenatide 2 mg once weekly and liraglutide 1.8 mg once daily, on weight change at 3 months, as a primary outcome.173
The combination of weekly exenatide (2 mg) with dapagliflozin 10 mg was explored in a small (n = 50) placebo-controlled trial of patients with obesity (mean BMI 35 kg/m2), 73% of whom had prediabetes.174 At 24 weeks, there was a difference in total body weight loss of 4 kg, favoring the combination arm.174 A 16-week RCT is currently ongoing, investigating the effect of dapagliflozin and exenatide, administered as single agents or combined.175 The primary outcome is food-related neural activity in the reward system using fMRI and weight is being assessed as a secondary outcome.175
Dapagliflozin + Metformin
One ongoing RCT compares dapagliflozin combined with metformin to metformin alone in patients with obesity (BMI > 40 kg/m2) and newly diagnosed DM or prediabetes and controlled on Metformin. The primary outcome is weight change at 12 months.176
Cagrilintide
Cagrilintide is an amylin analogue that increases satiety signals centrally and decreases gastric emptying peripherally; it is currently being investigated for total body weight loss in a phase 3 trial in combination with semaglutide compared to placebo in patients with obesity and DM (n = 1200).177
Medications with completed phase 2 trials
Several potential drugs for total body weight loss have completed phase 2 trials but have not been yet investigated in phase 3 trials (Appendix E), and these include: GSK1521498,178 LAPS-Exendin,179 LIK 066,180 MEDI0382,181 nasal PYY3-36,182 and RZL 012.183
Medications in phase 1 and 2 trials
Several drugs in the horizon, investigated in phase 1 or phase 2 trials, target different mechanisms.
New GLP1-RA reached phase 2 trials for patients with obesity: XW003,184 in comparison to liraglutide; PF-06882961,185 LY3502970,186 and SHR20004 Noiiglutide,187 in comparison to placebo.
In addition to GLP-1 RA and GIP agonists, a glucagon agonist is an attractive option for weight management as it stimulates thermogenesis by acting on adipose tissue, in addition to its lipolytic effects in the liver.188 Several dual (GLP1/glucagon; GIP/GLP1) agonists BI 456906,189 IBI362.190,191 CT-868,192 GMA106,193 and CT-388,194 and one tri-agonist (GIP/GLP1/glucagon LY3437943)195 are being explored.
Y2R inhibits food intake by acting centrally on the hypothalamus,196 and one Y2R agonist NNC0165-1875 is being explored for weight management in comparison to semaglutide.197
Amylin, co-secreted with insulin from pancreatic β cells in response to food intake, acts as a satiety signal on homeostatic and hedonic brain regions, slows gastric emptying, and suppresses post-prandial glucagon surge.198 NNC0174-0833 is an amylin receptor agonist being studied in a phase 1 trial in patients with obesity.199
Glabridin analogue HSG4112 regulates metabolic gene expression by increasing energy expenditure in the liver and muscles200 and is being studied in a phase 2 trial.201
Oxytocin has hypophagic effects, by acting centrally, and leads to a reduction in the intake of appetizing food.202 It is being investigated for weight management in a phase 2 trial.203
Tocotrienols can increase adiponectin expression and reduce adipose tissue inflammation, allowing improvement in insulin resistance.204 They are under investigation for postmenopausal women with obesity.205 Taste buds’ activators alter nutrient-sensing mechanisms and therefore activate anorexigenic pathways.206 ARD-101, a taste activator receptor is being evaluated in an ongoing phase 2 trial for obesity.207
Additional molecules being tested for their potential use in medical weight management include: leptin sensitizer,208,209 PPAR gamma modulator AMG,210 MBL949,211 NO-13065,212 NNC0247-0829,213 LY3841136214 and BMS-986172.215
Medications for weight loss in specific conditions
Several ongoing trials investigate the role of FDA approved AOM in patients with obesity and specific conditions (Table 4). Phase 3 RCTs on Semaglutide, in its subcutaneous formulation, evaluate its effect on weight in patients with obesity and prediabetes (n = 201216), obesity and heart failure (phase 3, n = 516217), obesity and knee osteoarthritis (n = 375218), obesity and cardiovascular disease (n = 17,500219), obesity and albuminuria (n = 98220) obesity, diabetes and kidney transplant candidacy (n = 50221).
Table 4.
Anti-obesity medications for special conditions identified from clinicaltrials.gov.
| Drug | Development stage | Dose | Indication | Sample size | Expected completion | Registration number |
|---|---|---|---|---|---|---|
| Tirzepatide | Phase III | Doses 1 and 2 SQ once weekly | Obesity in Chinese | 210 | December-22 | NCT05024032 |
| Phase III | N/A | Obesity and heart failure with preserved ejection fraction | 700 | July-24 | NCT04847557 | |
| Semaglutide | Phase III | 2.4 mg SQonce weekly | Obesity and prediabetes | 201 | July-23 | NCT05040971 |
| Phase III | 0.25 mg up to 2.4 mg SQ once weekly | Obesity and heart failure | 516 | April-23 | NCT04788511 | |
| Phase III | 2.4 mg SQ once weekly | Obesity and knee osteoarthritis | 407 | September-23 | NCT05064735 | |
| Phase III | 50 mg orally once daily | East Asian obese | 198 | August-23 | NCT05132088 | |
| Phase III | 3 mg up to 50 mg orally once daily | Obesity (oral tablet) | 660 | May-23 | NCT05035095 | |
| Phase III | 0.24 mg up to 2.4 mg SQ once weekly | Obesity and cardiovascular disease | 17,500 | September-23 | NCT03574597 | |
| Phase III | escalated up to 2.4 mg SQ once weekly | Obesity (±DM) | 375 | August-22 | NCT04251156 | |
| Phase III | 0.24 mg up to 2.4 mg SQ once weekly | Obesity and reduction in microalbuminuria | 98 | May-23 | NCT04889183 | |
| Phase III | 0.25 mg up to 1 mg SQ once weekly | Obesity, DM and kidney transplant candidacy | 15 | January-23 | NCT04741074a | |
| Liraglutide | Phase III | 0.6 mg up to 3 mg SQ daily | Obesity and comorbidities | 300 | June-22 | NCT04487743 |
| Phase III | 0.6 mg up to 3 mg SQ daily | Obesity and comorbidities | 414 | December-22 | NCT04605861 | |
| Phase II | 0.6 mg/day SQ on week 1 then 1.2 mg/day SQ on week 2 then to 1.8 mg/day SQ | Obesity and DM on hemodialysis | 30 | July-23 | NCT04529278 | |
| Contrave | Phase III | Naltrexone sustained-release (32 mg/day) with bupropion SR (360 mg/day) orally daily | Obesity and binge eating | 136 | December-22 | NCT03045341 |
| Setmelanotide | Phase III | SQ injection once daily | Obesity associated with defects in leptin-melanocortin pathway | 300 | December-23 | NCT03651765 |
| Phase III | 0.5 mg up to 2 mg SQ daily | Bardet-Biedl Syndrome, POMC Deficiency, PCSK1 Deficiency, LEPR Deficiency | 12 | September-23 | NCT04966741 | |
| Phase III | 2 mg, 3 mg, 20 mg and 30 mg SQ daily | Bardet-Biedl Syndrome, POMC Deficiency | 30 | August-23 | NCT05194124 | |
| Phase III | SQ injection once daily | Rare genetic disorders | 213 | March-22 | NCT03013543 | |
| Phase II | 1, 2, 3 mg SQ daily for patients 6 to <16 years of age, and 2–3 mg SQ daily for patients ≥16 years of age | Hypothalamic Obesity | 18 | June-22 | NCT04725240 | |
| Cannabidiol RAD011 (Oral Solution) | Phase III | Low, medium and high oral doses | Prader–Willi Syndrome | 7 | October-22 | NCT05098509a |
| Somatropin | Phase III | 0.245 or 0.084 mg/kg/week SQ | Prader–Willi Syndrome | 32 | May-24 | NCT04697381 |
| Oxytocin | Phase II | Intranasal 3 times per day, dosage based on weight: 16 IU–24 IU; dose escalation, if appropriate at 2 weeks | Hypothalamic Obesity | 18 | May-22 | NCT02849743 |
| Phase II | Intranasal 16 IU daily | Prader Willi | 50 | August-23 | NCT03197662 | |
| Taste receptors activator ARD-101 | Phase II | 200 mg orally twice daily | Prader Willi | 12 | May-23 | NCT05153434 |
Abbreviations: BID, twice daily; IU, International Unit; LEPR, Leptin receptor; PCSK, Proprotein convertase subtilisin/kexin type 9 serine protease; POMC, Proopiomelanocortin; SQ, subcutaneous.
Trial terminated by the sponsor for reasons other than safety.
Semaglutide, in its oral formulation is being investigated in phase 3 trials for total body weight loss purposes in East Asian patients with obesity (n = 198222), and patients with obesity (n = 660223).
Two phase 3 ongoing trials on liraglutide on patients with obesity and comorbidities (n = 300224 and n = 414225) and one phase 2 trial in patients with obesity and type 2 diabetes on hemodialysis (n = 30226) investigate total body weight loss at week 26, as a primary outcome.
One phase 3 trial on NB on patients with obesity and binge eating (n = 136227), assesses binge eating frequency and BMI as co-primary outcomes.
Setmelanotide is being studied in ongoing phase 3 trials of patients with rare genetic disorders,228, 229, 230, 231 and an ongoing phase 2 trial in 15 patients with hypothalamic obesity.232
Discussion
While early total body weight loss of at least 5% labels patients as responders to AOM, a higher response should be targeted; 5–10% total body weight loss is associated with a reduction in the risk of various metabolic, skeletal, and anatomical complications of obesity,11 and a total body weight loss >15% may be needed to result in improvement of CV outcomes, as observed in studies assessing effects of weight loss in response to lifestyle modifications.233 The effectiveness of the currently available weight loss monotherapies is still modest, with the exception of GLP-1 RA, and in particular semaglutide, which in combination with behavioral modification therapy, may achieve more than 15% weight loss.82 The combination of more than one molecule may be needed to achieve a larger total body weight loss; for instance, tirzepatide, a GIP/GLP-1 dual agonist, led to ∼23% weight loss with the highest dose.95 New drugs seem thus have a much higher potency and are expected to change the medical weight management landscape in the not-so-distant future. Trials on AOM showed improvement in various metabolic parameters, some of which were of a small magnitude with a limited clinical implication. Furthermore, whether this effect has been driven solely by total body weight loss, rather than a direct effect of the drug per se, and whether it differs between metabolically healthy and metabolically unhealthy individuals with obesity, has been poorly investigated.234 While some available data showed improvement in liver steatosis with GLP1-RA, there is no evidence of reduction in liver fibrosis with any AOM, a parameter that requires longer term studies to show benefit. To-date, data on the effect of AOM on CV outcomes are still lacking. However, indirect evidence from trials in patients with DM is promising. The LEADER trial compared liraglutide (1.8 mg/d) to placebo in patients with DM on a composite CV outcome (cardiovascular death, non-fatal myocardial infarct and non-fatal stroke).157, 235 After a median follow up of 3.8 years, there was a 13% relative risk reduction in the composite CV outcomes and 22% relative risk reduction in cardiovascular death, in liraglutide group, compared to placebo.157, 235 In a similar study design in patients with DM, semaglutide showed a 26% relative risk reduction the composite CV outcome, compared to placebo, while there was no difference in CV death.158 Interestingly, new findings suggest a potential neuroprotective effect of GLP-1 agonist, with a reduction of the risk of dementia and age-related cognitive decline, associated with diabetes and obesity236,237; such a promising effect needs to be demonstrated in large prospective studies, extending over several years to decades.
The evidence to-date on the efficacy of AOM is derived from Western studies, conducted in middle-aged women. Data in older individuals and those from non-Western countries, in particular Middle Eastern and African populations, are still lacking. Furthermore, the long-term safety and efficacy of various AOM is scarce.238
One of the main challenges in weight management is the inability to predict patient's response to medications, with a wide variability in individuals weight change in response to a given medication.239 With the exception of semaglutide trials where the majority of participants had a significant weight loss, in the AOM trials, 20–50% of participants lost <5% of their total weight loss, and the reasons for non-response has been poorly investigated. Precision medicine may be the future of weight management, implying a tailored approach depending on patient profile.240,241 Such approach requires the availability of large cohorts to collect data on genetics, epigenetics, and genomics, and therefore derive a therapeutic algorithm.241 The OBEsity Diverse Interventions Sharing (OBEDIS) – focusing on dietary and other interventions - aims at streamlining and harmonizing variables and endpoints of AOM clinical trials, to allow data pooling and derivation of prediction models.242
Conclusion
In summary, accumulating evidence indicates that the next few years will be a period during which novel pharmacotherapies for obesity will revolutionize the way we treat obesity, and through improvements in body weight, the way we treat cardio-renal and metabolic complications of obesity including, diabetes, cardiometabolic and liver comorbidities of obesity.
Contributors
M.C. and C.S.M. designed the study, reviewed, and wrote and edited the manuscript. R.H. and M.G. screened the citations retrieved from the search, abstracted data, designed figures and tables and wrote the manuscript. C.R. abstracted data and wrote the manuscript. R.T. screened the citations from the search and abstracted data. M.C. and C.S.M. had access to the data and had final responsibility for the decision to submit for publication.
Declaration of interests
C.S.M. has been a shareholder of and reports grants through his institution from Merck, grants through his Institution and personal consulting fees from Coherus Inc. and AltrixBio, he reports grants through his institution and personal consulting fees from Novo Nordisk, reports personal consulting fees and support with research reagents from Ansh Inc., reports personal consulting fees from Genfit, Lumos, Amgen, Corcept, Intercept, Astra Zeneca, 89bio and Regeneron, reports support (educational activity meals at and through his institution) from Amarin, Novo Nordisk and travel support and fees from TMIOA, Elsevier, the California Walnut Commission, College Internationale Researche Servier and the Cardio Metabolic Health Conference. None is related to the work presented herein. All other authors declare no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101882.
Appendix A. Supplementary data
References
- 1.Tiwari A., Balasundaram P. StatPearls Publishing; 2021. Public health considerations regarding obesity. [PubMed] [Google Scholar]
- 2.Ritchie H., Roser M. Obesity. 2017. https://ourworldindata.org/obesity
- 3.Lange S.J., Kompaniyets L., Freedman D.S., Kraus E.M., Porter R. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2–19 years—United States, 2018–2020. MMWR Morb Mortal Wkly Rep. 2021;70(37):1278–1283. doi: 10.15585/mmwr.mm7037a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restrepo B.J. Obesity prevalence among US adults during the COVID-19 pandemic. Am J Prev Med. 2022;63(1):102–106. doi: 10.1016/j.amepre.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh D.P., Zhang W., Bansback N., Amarsi Z., Birmingham C.L., Anis A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):1–20. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwer D.B., Polonsky H.M. The psychosocial burden of obesity. Endocrinol Metab Clin North Am. 2016;45(3):677–688. doi: 10.1016/j.ecl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai H., Alsalhe T.A., Chalghaf N., Riccò M., Bragazzi N.L., Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 2020;17(7) doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foucan L., Larifla L., Durand E., et al. High prevalence of rare monogenic forms of obesity in obese Guadeloupean Afro-Caribbean children. J Clin Endocrinol Metab. 2018;103(2):539–545. doi: 10.1210/jc.2017-01956. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad N.N., Butsch W.S., Aidarous S. Clinical management of obesity in women: addressing a lifecycle of risk. Obstet Gynecol Clin North Am. 2016;43(2):201–230. doi: 10.1016/j.ogc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Apovian C.M., Aronne L.J., Bessesen D.H., et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 11.Garvey W.T., Mechanick J.I., Brett E.M., et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines formedical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 12.Müller T.D., Blüher M., Tschöp M.H., DiMarchi R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201–223. doi: 10.1038/s41573-021-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Administration USFaD FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market. 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requests-withdrawal-weight-loss-drug-belviq-belviq-xr-lorcaserin-market#:∼:text=On%20February%2013%2C%202020%20FDA,an%20increased%20occurrence%20of%20cancer
- 14.Administration FaD Guidance for industry developing products for weight management. Draft guidance. 2007. https://www.federalregister.gov/documents/2007/02/15/E7-2581/draft-guidance-for-industry-on-developing-products-for-weight-management-availability
- 15.Heal D.J., Gosden J., Smith S.L. Regulatory challenges for new drugs to treat obesity and comorbid metabolic disorders. Br J Clin Pharmacol. 2009;68(6):861–874. doi: 10.1111/j.1365-2125.2009.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA WEGOVY (semaglutide) injection, for subcutaneous use. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf
- 17.FDA MYALEPT (metreleptin) for injection for subcutaneous use. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125390s004lbl.pdf
- 18.FDA IMCIVREE (setmelanotide) injection, for subcutaneous use. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213793s000lbl.pdf
- 19.De Andrade Mesquita L., Fagundes Piccoli G., Richter da Natividade G., Frison Spiazzi B., Colpani V., Gerchman F. Is lorcaserin really associated with increased risk of cancer? A systematic review and meta-analysis. Obes Rev. 2021;22(3) doi: 10.1111/obr.13170. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R., Ryan D. Lorcaserin departs, leaving more questions than answers. Obesity (Silver Spring) 2020;28(7):1167. doi: 10.1002/oby.22789. [DOI] [PubMed] [Google Scholar]
- 21.Pilitsi E., Farr O.M., Polyzos S.A., et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170–192. doi: 10.1016/j.metabol.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Blüher S., Ziotopoulou M., Bullen J.W., Jr., et al. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes. 2004;53(1):82–90. doi: 10.2337/diabetes.53.1.82. [DOI] [PubMed] [Google Scholar]
- 23.Pierroz D.D., Ziotopoulou M., Ungsunan L., Moschos S., Flier J.S., Mantzoros C.S. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes. 2002;51(5):1337–1345. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 24.Clément K., van den Akker E., Argente J., et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 25.Haws R.M., Gordon G., Han J.C., Yanovski J.A., Yuan G., Stewart M.W. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp Clin Trials Commun. 2021;22:100780. doi: 10.1016/j.conctc.2021.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J.L., Matarese G., Shetty G.K., et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan J.L., Wong S.L., Orlova C., Raciti P., Mantzoros C.S. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab. 2007;92(6):2307–2311. doi: 10.1210/jc.2006-2864. [DOI] [PubMed] [Google Scholar]
- 28.Chan J.L., Wong S.L., Mantzoros C.S. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet. 2008;47(11):753–764. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon H.-S., Huh J.Y., Dincer F., Schneider B.E., Hasselgren P.-O., Mantzoros C.S. Identification and saturable nature of signaling pathways induced by metreleptin in humans: comparative evaluation of in vivo, ex vivo, and in vitro administration. Diabetes. 2015;64(3):828–839. doi: 10.2337/db14-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon H.-S., Matarese G., Brennan A.M., et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60(6):1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr O.M., Tsoukas M.A., Mantzoros C.S. Leptin and the brain: influences on brain development, cognitive functioning and psychiatric disorders. Metabolism. 2015;64(1):114–130. doi: 10.1016/j.metabol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Grover A., Quaye E., Brychta R.J., et al. Leptin decreases energy expenditure despite increased thyroid hormone in patients with lipodystrophy. J Clin Endocrinol Metab. 2021;106(10):e4163–e4178. doi: 10.1210/clinem/dgab269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depaoli A., Long A., Fine G.M., Stewart M., O'Rahilly S. Efficacy of metreleptin for weight loss in overweight and obese adults with low leptin levels. Diabetes. 2018;67(Supplement_1):296. [Google Scholar]
- 34.Chan J.L., Koda J., Heilig J.S., et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol. 2016;85(1):137–149. doi: 10.1111/cen.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabery S., Salinas C.G., Paulsen S.J., Ahnfelt-Rønne J., Alanentalo T., Baquero A.F., et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billes S.K., Sinnayah P., Cowley M.A. Naltrexone/bupropion for obesity: an investigational combination pharmacotherapy for weight loss. Pharmacol Res. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Farr O.M., Tsoukas M.A., Triantafyllou G., et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: a randomized, placebo-controlled, crossover study. Metabolism. 2016;65(7):945–953. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farr O.M., Upadhyay J., Rutagengwa C., et al. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes Metab. 2019;21(11):2459–2464. doi: 10.1111/dom.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Husum H., Van Kammen D., Termeer E., Bolwig T.G., Mathé A.A. Topiramate normalizes hippocampal NPY-LI in flinders sensitive line ‘depressed’rats and upregulates NPY, galanin, and CRH-LI in the hypothalamus: implications for mood-stabilizing and weight loss-inducing effects. Neuropsychopharmacology. 2003;28(7):1292–1299. doi: 10.1038/sj.npp.1300178. [DOI] [PubMed] [Google Scholar]
- 40.Johnson B.A. Progress in the development of topiramate for treating alcohol dependence: from a hypothesis to a proof-of-concept study. Alcohol Clin Exp Res. 2004;28(8):1137–1144. doi: 10.1097/01.alc.0000134533.96915.08. [DOI] [PubMed] [Google Scholar]
- 41.Rabiner E.A., Beaver J., Makwana A., et al. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol Psychiatry. 2011;16(8):826–835. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Tomasi D., Volkow N., et al. Effect of combined naltrexone and bupropion therapy on the brain's reactivity to food cues. Int J Obes. 2014;38(5):682–688. doi: 10.1038/ijo.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G.-J., Zhao J., Tomasi D., et al. Effect of combined naltrexone and bupropion therapy on the brain's functional connectivity. Int J Obes. 2018;42(11):1890–1899. doi: 10.1038/s41366-018-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanovski S.Z., Yanovski J.A. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumann M., Ayestas M., Dersch C., Brockington A., Rice K., Rothman R. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse. 2000;36(2):102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Nelson D.L., Gehlert D.R. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29(1):49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- 47.Tao R., Fray A., Aspley S., Brammer R., Heal D., Auerbach S. Effects on serotonin in rat hypothalamus of D-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol. 2002;445(1–2):69–81. doi: 10.1016/s0014-2999(02)01751-x. [DOI] [PubMed] [Google Scholar]
- 48.Arch J.R. The contribution of increased thermogenesis to the effect of anorectic drugs on body composition in mice. Am J Clin Nutr. 1981;34(12):2763–2769. doi: 10.1093/ajcn/34.12.2763. [DOI] [PubMed] [Google Scholar]
- 49.Ferrer-Lorente R., Cabot C., Fernández-López J.-A., Remesar X., Alemany M. Effects of oleoyl-estrone with dexfenfluramine, sibutramine or phentermine on overweight rats. Eur J Pharmacol. 2005;513(3):243–248. doi: 10.1016/j.ejphar.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 50.Farr O.M., Sofopoulos M., Tsoukas M.A., et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–965. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ten Kulve J.S., Veltman D.J., van Bloemendaal L., et al. Liraglutide reduces CNS activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care. 2016;39(2):214–221. doi: 10.2337/dc15-0772. [DOI] [PubMed] [Google Scholar]
- 52.Van Bloemendaal L., IJzerman R.G., Ten Kulve J.S., et al. GLP-1 receptor activation modulates appetite-and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–4196. doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 53.Richard D., Ferland J., Lalonde J., Samson P., Deshaies Y. Influence of topiramate in the regulation of energy balance. Nutrition. 2000;16(10):961–966. doi: 10.1016/s0899-9007(00)00452-4. [DOI] [PubMed] [Google Scholar]
- 54.Abo-Elmatty D., Zaitone S. Topiramate induces weight loss and improves insulin sensitivity in dietary obese rats: comparison to sibutramine. Eur Rev Med Pharmacol Sci. 2011;15(10):1187–1195. [PubMed] [Google Scholar]
- 55.Tremblay A., Chaput J.-P., Bérubé-Parent S., et al. The effect of topiramate on energy balance in obese men: a 6-month double-blind randomized placebo-controlled study with a 6-month open-label extension. Eur J Clin Pharmacol. 2007;63(2):123–134. doi: 10.1007/s00228-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 56.Tak Y.J., Lee S.Y. Anti-obesity drugs: long-term efficacy and safety: an updated review. World J Mens Health. 2021;39(2):208–221. doi: 10.5534/wjmh.200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jepsen M.M., Christensen M.B. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin Emerg Drugs. 2021;26(3):231–243. doi: 10.1080/14728214.2021.1947240. [DOI] [PubMed] [Google Scholar]
- 58.Torgerson J.S., Hauptman J., Boldrin M.N., SjÖStrÖM L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 59.Hanefeld M., Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2002;4(6):415–423. doi: 10.1046/j.1463-1326.2002.00237.x. [DOI] [PubMed] [Google Scholar]
- 60.Hollander P.A., Elbein S.C., Hirsch I.B., et al. Role of orlistat in the treatment of obese patients with type 2 diabetes: a 1-year randomized double-blind study. Diabetes Care. 1998;21(8):1288–1294. doi: 10.2337/diacare.21.8.1288. [DOI] [PubMed] [Google Scholar]
- 61.Kelley D.E., Bray G.A., Pi-Sunyer F.X., et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2002;25(6):1033–1041. doi: 10.2337/diacare.25.6.1033. [DOI] [PubMed] [Google Scholar]
- 62.Miles J.M., Leiter L., Hollander P., et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care. 2002;25(7):1123–1128. doi: 10.2337/diacare.25.7.1123. [DOI] [PubMed] [Google Scholar]
- 63.FDA Xenical - orlistat capsule. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf
- 64.Allison D.B., Gadde K.M., Garvey W.T., et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gadde K.M.D., Allison D.B.P., Ryan D.H.M.D., et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 66.Timothy Garvey W., Ryan D.H., Look M., et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garvey W.T., Ryan D.H., Bohannon N.J., et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care. 2014;37(12):3309–3316. doi: 10.2337/dc14-0930. [DOI] [PubMed] [Google Scholar]
- 68.FDA QSYMIA (phentermine and topiramate extended-release) capsules, for oral use, CIV. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf
- 69.Lei X.G., Ruan J.Q., Lai C., Sun Z., Yang X. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity. 2021;29(6):985–994. doi: 10.1002/oby.23152. [DOI] [PubMed] [Google Scholar]
- 70.Greenway F.L.P., Fujioka K.M.D., Plodkowski R.A.P., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 71.Apovian C.M., Aronne L., Rubino D., et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wadden T.A., Foreyt J.P., Foster G.D., et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hollander P., Gupta A.K., Plodkowski R., et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.FDA CONTRAVE (naltrexone HCl and bupropion HCl) extended-release tablets. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf
- 75.Onakpoya I.J., Lee J.J., Mahtani K.R., Aronson J.K., Heneghan C.J. Naltrexone-bupropion (Mysimba) in management of obesity: a systematic review and meta-analysis of unpublished clinical study reports. Br J Clin Pharmacol. 2020;86(4):646–667. doi: 10.1111/bcp.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pi-Sunyer X., Astrup A., Fujioka K., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 77.Wadden T.A., Hollander P., Klein S., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;39(1):187. doi: 10.1038/ijo.2014.88. [DOI] [PubMed] [Google Scholar]
- 78.Wadden T.A., Tronieri J.S., Sugimoto D., et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring) 2020;28(3):529–536. doi: 10.1002/oby.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Roux C.W.P., Astrup A.P., Fujioka K.M.D., et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 80.Davies M.J., Bergenstal R., Bode B., et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 81.FDA SAXENDA (liraglutide) injection, for subcutaneous use. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206321s007lbl.pdf
- 82.Wilding J.P.H., Batterham R.L., Calanna S., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 83.Rubino D., Abrahamsson N., Davies M., et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wadden T.A., Bailey T.S., Billings L.K., et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies M., Færch L., Jeppesen O.K., et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 86.Zhong P., Zeng H., Huang M., Fu W., Chen Z. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis. Endocrine. 2022;75(3):718–724. doi: 10.1007/s12020-021-02945-1. [DOI] [PubMed] [Google Scholar]
- 87.Khera R., Murad M.H., Chandar A.K., et al. Association of pharmacological treatments for obesity withweight loss and adverse events a systematic review and meta-analysis. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubino D.M., Greenway F.L., Khalid U., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without siabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Astrup A., Carraro R., Finer N., et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwon Y.-J., Kwon G.E., Lee H.S., Choi M.H., Lee J.-W. The effect of orlistat on sterol metabolism in obese patients. Front Endocrinol. 2022;13:824269. doi: 10.3389/fendo.2022.824269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tronieri J.S., Wadden T.A., Walsh O.A., et al. Effects of liraglutide plus phentermine in adults with obesity following 1 year of treatment by liraglutide alone: a randomized placebo-controlled pilot trial. Metabolism. 2019;96:83–91. doi: 10.1016/j.metabol.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hollander P., Bays H.E., Rosenstock J., et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 93.Smith S.R., Stenlof K.S., Greenway F.L., et al. Orlistat 60 mg reduces visceral adipose tissue: a 24-week randomized, placebo-controlled, multicenter trial. Obesity. 2011;19(9):1796–1803. doi: 10.1038/oby.2011.143. [DOI] [PubMed] [Google Scholar]
- 94.Neeland I., Marso S.P., Ayers C., Pandey A., Joshi P.H. Effects of liraglutide on visceral and ectopic fat in adults with overweight/obesity at high cardiovascular risk: a randomized clinical trial. Diabetes. 2021;70(Supplement 1):595–605. doi: 10.1016/S2213-8587(21)00179-0. [DOI] [PubMed] [Google Scholar]
- 95.Jastreboff A.M., Aronne L.J., Ahmad N.N., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 96.Ye J., Wu Y., Li F., et al. Effect of orlistat on liver fat content in patients with nonalcoholic fatty liver disease with obesity: assessment using magnetic resonance imaging-derived proton density fat fraction. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819879047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Esmail V.A.W., Mohammed M.O., Al-Nimer M.S. Short-term orlistat therapy improves fatty infiltration indices and liver fibrosis scores in patients with non-alcoholic fatty liver disease and metabolic syndrome. Arab J Gastroenterol. 2021;22(1):1–5. doi: 10.1016/j.ajg.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Bajaj H.S., Burrows M., Blavignac J., et al. Extended-release naltrexone/bupropion and liver health: pooled, post hoc analysis from four randomized controlled trials. Diabetes Obes Metab. 2021;23(3):861–865. doi: 10.1111/dom.14284. [DOI] [PubMed] [Google Scholar]
- 99.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipose tissue, obesity and non-alcoholic fatty liver disease. Minerva Endocrinol. 2017;42(2):92–108. doi: 10.23736/S0391-1977.16.02563-3. [DOI] [PubMed] [Google Scholar]
- 100.Khoo J., Hsiang J.C., Taneja R., et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019;39(5):941–949. doi: 10.1111/liv.14065. [DOI] [PubMed] [Google Scholar]
- 101.Wang X.J., Gong P., Zhou C., et al. Liraglutide reduces attenuation coefficient as a measure of hepatic steatosis during 16 weeks' treatment in nondiabetic obese patients: a pilot trial. JGH Open. 2021;5(2):193–198. doi: 10.1002/jgh3.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Armstrong M.J., Gaunt P., Aithal G.P., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 103.Flint A., Andersen G., Hockings P., et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2021;54(9):1150–1161. doi: 10.1111/apt.16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newsome P.N., Buchholtz K., Cusi K., et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 105.Winslow D.H., Bowden C.H., DiDonato K.P., McCullough P.A. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35(11):1529–1539. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blackman A., Foster G., Zammit G., et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes. 2016;40(8):1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pi-Sunyer X., Apovian C.M., McElroy S.L., Dunayevich E., Acevedo L.M., Greenway F.L. Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: a pooled analysis. Int J Obes. 2019;43(10):2085–2094. doi: 10.1038/s41366-018-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nissen S.E., Wolski K.E., Prcela L., et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 109.Sposito A.C., Bonilha I., Luchiari B., et al. Cardiovascular safety of naltrexone and bupropion therapy: systematic review and meta-analyses. Obes Rev. 2021;22(6) doi: 10.1111/obr.13224. [DOI] [PubMed] [Google Scholar]
- 110.Davies M.J., Aronne L.J., Caterson I.D., Thomsen A.B., Jacobsen P.B., Marso S.P. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734–739. doi: 10.1111/dom.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.A/S NN Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT) 2018. https://clinicaltrials.gov/ct2/show/NCT03574597?term=Semaglutide+Effects+on+Cardiovascular+Outcomes+in+People+With+Overweight+or+Obesity&draw=2&rank=1
- 112.Capristo E., Maione A., Lucisano G., Russo M.F., Mingrone G., Nicolucci A. Effects of weight loss medications on mortality and cardiovascular events: a systematic review of randomized controlled trials in adults with overweight and obesity. Nutr Metab Cardiovasc Dis. 2021;31(9):2587–2595. doi: 10.1016/j.numecd.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 113.Grilo C.M., Lydecker J.A., Morgan P.T., Gueorguieva R. Naltrexone + bupropion combination for the treatment of binge-eating disorder with obesity: a randomized, controlled pilot study. Clin Ther. 2021;43(1):112–122.e1. doi: 10.1016/j.clinthera.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guerdjikova A.I., Walsh B., Shan K., Halseth A.E., Dunayevich E., McElroy S.L. Concurrent improvement in both binge eating and depressive symptoms with naltrexone/bupropion therapy in overweight or obese subjects with major depressive disorder in an open-label, uncontrolled study. Adv Ther. 2017;34(10):2307–2315. doi: 10.1007/s12325-017-0613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guerdjikova A.I., Williams S., Blom T.J., Mori N., McElroy S.L. Combination phentermine–topiramate extended release for the treatment of binge eating disorder: an open-label, prospective study. Innov Clin Neurosci. 2018;15(5–6):17–21. [PMC free article] [PubMed] [Google Scholar]
- 116.Safer D.L., Adler S., Dalai S.S., et al. A randomized, placebo-controlled crossover trial of phentermine-topiramate ER in patients with binge-eating disorder and bulimia nervosa. Int J Eat Disord. 2020;53(2):266–277. doi: 10.1002/eat.23192. [DOI] [PubMed] [Google Scholar]
- 117.Lyu X., Du J., Zhan G., et al. Naltrexone and bupropion combination treatment for smoking cessation and weight loss in patients with schizophrenia. Front Pharmacol. 2018;9:181. doi: 10.3389/fphar.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larsen J.R., Vedtofte L., Jakobsen M.S., et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine-or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(7):719–728. doi: 10.1001/jamapsychiatry.2017.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Whicher C.A., Price H.C., Phiri P., et al. The use of liraglutide 3.0 mg daily in the management of overweight and obesity in people with schizophrenia, schizoaffective disorder and first episode psychosis: results of a pilot randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2021;23(6):1262–1271. doi: 10.1111/dom.14334. [DOI] [PubMed] [Google Scholar]
- 120.McIntyre R.S., Paron E., Burrows M., et al. Psychiatric safety and weight loss efficacy of naltrexone/bupropion as add-on to antidepressant therapy in patients with obesity or overweight. J Affect Disord. 2021;289:167–176. doi: 10.1016/j.jad.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 121.O'Neil P.M., Aroda V.R., Astrup A., et al. Neuropsychiatric safety with liraglutide 3.0 mg for weight management: results from randomized controlled phase 2 and 3a trials. Diabetes Obes Metab. 2017;19(11):1529–1536. doi: 10.1111/dom.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hansotia T., Maida A., Flock G., et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117(1):143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyawaki K., Yamada Y., Ban N., et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 124.Bates H.E., Campbell J.E., Ussher J.R., et al. Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr−/− mice. Diabetes. 2012;61(1):40–48. doi: 10.2337/db11-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Q., Delessa C.T., Augustin R., et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021;33(4):833–844.e5. doi: 10.1016/j.cmet.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daousi C., Wilding J., Aditya S., et al. Effects of peripheral administration of synthetic human glucose-dependent insulinotropic peptide (GIP) on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin Endocrinol. 2009;71(2):195–201. doi: 10.1111/j.1365-2265.2008.03451.x. [DOI] [PubMed] [Google Scholar]
- 127.Bergmann N.C., Lund A., Gasbjerg L.S., et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia. 2019;62(4):665–675. doi: 10.1007/s00125-018-4810-0. [DOI] [PubMed] [Google Scholar]
- 128.Fukuda M. The role of GIP receptor in the CNS for the pathogenesis of obesity. Diabetes. 2021;70(9):1929–1937. doi: 10.2337/dbi21-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.FDA FDA approves novel, dual-targeted treatment for type 2 diabetes 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes
- 130.Frías J.P., Davies M.J., Rosenstock J., et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 131.Yu Y., Hu G., Yin S., Yang X., Zhou M., Jian W. Optimal dose of tirzepatide for type 2 diabetes mellitus: a meta-analysis and trial sequential analysis. Front Cardiovasc Med. 2022;9:990182. doi: 10.3389/fcvm.2022.990182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patoulias D., Papadopoulos C., Fragakis N., Doumas M. Updated meta-analysis assessing the cardiovascular efficacy of tirzepatide. Am J Cardiol. 2022;181:139–140. doi: 10.1016/j.amjcard.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 133.Eli Lilly and Company A study of tirzepatide (LY3298176) in participants with type 2 diabetes who have obesity or are overweight (SURMOUNT-2) 2020. https://clinicaltrials.gov/ct2/show/NCT04657003?term=NCT04657003&draw=2&rank=1
- 134.Eli Lilly and Company A study of tirzepatide (LY3298176) in participants with obesity disease (SURMOUNT-J) 2021. https://clinicaltrials.gov/ct2/show/NCT04844918?term=NCT04844918&draw=2&rank=1
- 135.Eli Lilly and Company A study of tirzepatide (LY3298176) in participants with obesity or overweight for the maintenance of weight loss (SURMOUNT-4) 2020. https://clinicaltrials.gov/ct2/show/NCT04660643?term=NCT04660643&draw=2&rank=1
- 136.Eli Lilly and Company A study of tirzepatide (LY3298176) in participants after a lifestyle weight loss program (SURMOUNT-3) 2020. https://clinicaltrials.gov/ct2/show/NCT04657016?term=NCT04657016&draw=2&rank=1
- 137.Eli Lilly and Company A study of tirzepatide (LY3298176) in Chinese participants without type 2 diabetes who have obesity or overweight (SURMOUNT-CN) (SURMOUNT-CN) 2022. https://clinicaltrials.gov/ct2/show/NCT05024032?term=NCT05024032&draw=2&rank=1
- 138.Eli Lilly and Company A study of tirzepatide (LY3298176) in participants with heart failure with preserved ejection fraction and obesity (SUMMIT) 2022. https://clinicaltrials.gov/ct2/show/NCT04847557?term=NCT04847557&draw=2&rank=1
- 139.Lilly receives U.S. FDA fast track designation for tirzepatide for the treatment of adults with obesity, or overweight with weight-related comorbidities. 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-fast-track-designation-tirzepatide
- 140.Acosta A., Streett S., Kroh M.D., et al. White paper AGA: POWER - practice guide on obesity and weight management, education, and resources. Clin Gastroenterol Hepatol. 2017;15(5):631–649.e10. doi: 10.1016/j.cgh.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 141.Durrer Schutz D., Busetto L., Dicker D., et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12(1):40–66. doi: 10.1159/000496183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mckinney L., Skolnik N., Chrusch A. American Academy of Family Physicians; 2013. Diagnosis and management of obesity. [Google Scholar]
- 143.Mechanick J.I., Apovian C., Brethauer S., et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures–2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis. 2020;16(2):175–247. doi: 10.1016/j.soard.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 144.Millen B.E., Wolongevicz D.M., Nonas C.A., Lichtenstein A.H. 2013 American Heart Association/American College of Cardiology/the Obesity Society guideline for the management of overweight and obesity in adults: implications and new opportunities for registered dietitian nutritionists. J Acad Nutr Diet. 2014;114(11):1730–1735. doi: 10.1016/j.jand.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 145.Moyer V.A., Force∗ UPST Screening for and management of obesity in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(5):373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 146.NHaMR C Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. National Health and Medical Research Council. 2013. https://www.nhmrc.gov.au/about-us/publications/clinical-practice-guidelines-management-overweight-and-obesity
- 147.Overweight Mo. Group OW VA/DoD clinical practice guideline for screening and management of overweight and obesity. Department of Veterans Affairs, Department of Defense Washington DC. 2020. https://www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
- 148.Stegenga H., Haines A., Jones K., Wilding J. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ. 2014;349:g6608. doi: 10.1136/bmj.g6608. [DOI] [PubMed] [Google Scholar]
- 149.Wharton S., Lau D.C.W., Vallis M., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ge L., Sadeghirad B., Ball G.D., et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369:m696. doi: 10.1136/bmj.m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnston B.C., Kanters S., Bandayrel K., et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 152.Jabbour J., Rihawi Y., Khamis A.M., et al. Long term weight loss diets and obesity indices: results of a network meta-analysis. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.821096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S139–S140. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hales C.M., Gu Q., Ogden C.L., Yanovski S.Z. Use of prescription medications associated with weight gain among US adults, 1999-2018: a nationally representative survey. Obesity. 2022;30(1):229–239. doi: 10.1002/oby.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Warren M., Chaykin L., Trachtenbarg D., Nayak G., Wijayasinghe N., Cariou B. Semaglutide as a therapeutic option for elderly patients with type 2 diabetes: pooled analysis of the SUSTAIN 1-5 trials. Diabetes Obes Metab. 2018;20(9):2291–2297. doi: 10.1111/dom.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Batsis J.A., Zagaria A.B. Addressing obesity in aging patients. Med Clin North Am. 2018;102(1):65–85. doi: 10.1016/j.mcna.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marso S.P., Bain S.C., Consoli A., et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 159.FDA ADIPEX-P ® (phentermine hydrochloride USP) CIV for oral use. 1959. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/085128s065lbl.pdf
- 160.FDA VYVANSE ® (lisdexamfetamine dimesylate) capsules, for oral use, CII. 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208510lbl.pdf
- 161.FDA Ritalin hydrochloride, methylphenidate hydrochloride. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/010187s073lbl.pdf
- 162.Leddy J.J., Epstein L.H., Jaroni J.L., et al. Influence of methylphenidate on eating in obese men. Obes Res. 2004;12(2):224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- 163.Levy L., Fleming J., Klar D. Treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int J Obes. 2009;33(3):326–334. doi: 10.1038/ijo.2009.5. [DOI] [PubMed] [Google Scholar]
- 164.Goldfield G.S., Lorello C., Doucet E. Methylphenidate reduces energy intake and dietary fat intake in adults: a mechanism of reduced reinforcing value of food? Am J Clin Nutr. 2007;86(2):308–315. doi: 10.1093/ajcn/86.2.308. [DOI] [PubMed] [Google Scholar]
- 165.Eric D., Philippe R. The effects of methylphenidate on energy intake and energy expenditure (MPH) 2016. https://clinicaltrials.gov/ct2/show/record/NCT02754258?view=record
- 166.Sjödin A., Gasteyger C., Nielsen A.-L., et al. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes. 2010;34(11):1634–1643. doi: 10.1038/ijo.2010.87. [DOI] [PubMed] [Google Scholar]
- 167.Angelidi A.M., Belanger M.J., Kokkinos A., Koliaki C.C., Mantzoros C.S. Novel non-invasive approaches to the treatment of obesity: from pharmacotherapy to gene therapy. Endocr Rev. 2021:bnab034. doi: 10.1210/endrev/bnab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Astrup A., Madsbad S., Breum L., Jensen T.J., Kroustrup J.P., Larsen T.M. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9653):1906–1913. doi: 10.1016/S0140-6736(08)61525-1. [DOI] [PubMed] [Google Scholar]
- 169.Gilbert J.A., Gasteyger C., Raben A., Meier D.H., Astrup A., Sjödin A. The effect of tesofensine on appetite sensations. Obesity. 2012;20(3):553–561. doi: 10.1038/oby.2011.197. [DOI] [PubMed] [Google Scholar]
- 170.GlobeNewswire Saniona's tesofensine meets primary and secondary endpoints in phase 3 obesity registration trial. 2018. https://www.globenewswire.com/news-release/2018/12/17/1667781/0/en/Saniona-s-tesofensine-meets-primary-and-secondary-endpoints-in-Phase-3-obesity-registration-trial.html
- 171.Rosenstock J., Klaff L.J., Schwartz S., et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33(6):1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Basolo A., Burkholder J., Osgood K., et al. Exenatide has a pronounced effect on energy intake but not energy expenditure in non-diabetic subjects with obesity: a randomized, double-blind, placebo-controlled trial. Metabolism. 2018;85:116–125. doi: 10.1016/j.metabol.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu J. Effects of GLP-1 RAs on weight and metabolic indicators in obese patients. 2018. https://clinicaltrials.gov/ct2/show/record/NCT03671733
- 174.Lundkvist P., Sjöström C.D., Amini S., Pereira M.J., Johnsson E., Eriksson J.W. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19(1):49–60. doi: 10.1111/dom.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Richard I. Dapagliflozin plus exenatide on central regulation of appetite in diabetes type 2 (DECREASE) 2017. https://clinicaltrials.gov/ct2/show/NCT03361098
- 176.Aldo F.-H. Effectiveness of dapagliflozin for weight loss. 2019. https://clinicaltrials.gov/ct2/show/NCT03968224
- 177.Novo Nordisk AS A research study to see how well CagriSema helps people losing weight in people who have a body weight above the healthy range and type 2 diabetes (REDEFINE 2) 2022. https://clinicaltrials.gov/ct2/show/NCT05394519?term=NCT05394519&draw=2&rank=1
- 178.GlaxoSmithKline a 35 day study to investigate the effects of GSK1521498 on bodyweight in obese subjects with over-eating behaviours. 2010. https://clinicaltrials.gov/ct2/show/NCT01195792?term=NCT01195792&draw=2&rank=1
- 179.Hanmi Pharmaceutical Company Limited Effect of LAPS-exendin on body weight in obese population. 2014. https://clinicaltrials.gov/ct2/show/NCT02075281?term=NCT02075281&draw=2&rank=1
- 180.Novartis Pharmaceuticals A dose-finding study to evaluate the change in weight after treatment with LIK066 in Japanese patients with obesity. 2017. https://clinicaltrials.gov/ct2/show/NCT03320941?term=NCT03320941&draw=2&rank=1
- 181.MedImmune LLC A study to investigate different doses of 0382 in overweight and obese subjects with type 2 diabetes mellitus. 2019. https://clinicaltrials.gov/ct2/show/NCT03244800?term=NCT03244800&draw=2&rank=1
- 182.Brandt G. A study of nasal PYY3-36 and placebo for weight loss in obese subjects. 2007. https://clinicaltrials.gov/ct2/show/NCT00537420?term=NCT00537420&draw=2&rank=1
- 183.Raziel Therapeutics Ltd Evaluation of safety, efficacy and thermogenesis-induction of RZL-012 in overweight and obese volunteers. 2017. https://clinicaltrials.gov/ct2/show/NCT03171415?term=NCT03171415&draw=2&rank=1
- 184.Qin H. Effects of XW003 versus liraglutide on body weight of adult participants with obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT05111912?term=NCT05111912&draw=2&rank=1
- 185.Pfizer A 26-week, 2-part study to evaluate the efficacy and safety of PF-06882961 in adults with obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT04707313?term=NCT04707313&draw=2&rank=1
- 186.Eli Lilly and Company A study of LY3502970 in participants with obesity or overweight with weight-related comorbidities. 2021. https://clinicaltrials.gov/ct2/show/NCT05051579?term=NCT05051579&draw=2&rank=1
- 187.Jiangsu HengRui Medicine Co L The effect of SHR20004 (Noiiglutide) on body weight in obese subjects without diabetes. 2021. https://clinicaltrials.gov/ct2/show/NCT04799327?term=NCT04799327&draw=2&rank=1
- 188.Sánchez-Garrido M.A., Brandt S.J., Clemmensen C., Müller T.D., DiMarchi R.D., Tschöp M.H. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60(10):1851–1861. doi: 10.1007/s00125-017-4354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ingelheim B. A study to test whether different doses of BI 456906 help people with overweight or obesity to lose weight. 2020. https://clinicaltrials.gov/ct2/show/NCT04667377?term=NCT04667377&draw=2&rank=1
- 190.Yan B. A study of IBI362 in participants with obesity or overweight for weight loss. 2021. https://clinicaltrials.gov/ct2/show/NCT04904913?term=NCT04904913&draw=2&rank=1
- 191.InnoventBiologics (Suzhou) CoLtd Evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of IBI362 in overweight or obesity subjects. 2020. https://clinicaltrials.gov/ct2/show/NCT04440345?term=NCT04440345&draw=2&rank=1
- 192.Michael E. A study of CT-868 in overweight and obese participants with type 2 diabetes mellitus. 2021. https://clinicaltrials.gov/ct2/show/NCT05110846?term=NCT05110846&draw=2&rank=1
- 193.CMAX Clinical Research Assessment of the safety, tolerability, and pharmacokinetic of GMA106. 2021. https://clinicaltrials.gov/ct2/show/NCT05054530?term=NCT05054530&draw=1&rank=1
- 194.Michael E. A study of CT-388 in otherwise healthy overweight and obese adults and patients with type 2 diabetes mellitus. 2021. https://clinicaltrials.gov/ct2/show/NCT04838405?term=NCT04838405&draw=2&rank=1
- 195.Eli Lilly and Company A study of LY3437943 in participants who have obesity or are overweight. 2021. https://clinicaltrials.gov/ct2/show/NCT04881760?term=NCT04881760&draw=2&rank=1
- 196.Kopelman P., Grace C. New thoughts on managing obesity. Gut. 2004;53(7):1044–1053. doi: 10.1136/gut.2003.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Novo Nordisk AS A research study to investigate how well NNC0165-1875 in combination with semaglutide works in people with obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT04969939?term=NCT04969939&draw=2&rank=1
- 198.Dehestani B., Stratford N.R., le Roux C.W. Amylin as a future obesity treatment. J Obes Metab Syndr. 2021;30(4):320–325. doi: 10.7570/jomes21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Novo Nordisk AS A research study of how NNC0174-0833 behaves in Chinese volunteers who are normal weight, overweight or with obesity. 2022. https://clinicaltrials.gov/ct2/show/NCT05254158?term=NCT05254158&draw=2&rank=1
- 200.Choi L.S., Jo I.G., Kang K.S., et al. Discovery and preclinical efficacy of HSG4112, a synthetic structural analog of glabridin, for the treatment of obesity. Int J Obes. 2021;45(1):130–142. doi: 10.1038/s41366-020-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim Y. A study to evaluate the safety and efficacy of HSG4112 in overweight and obese patients. 2022. https://clinicaltrials.gov/ct2/show/NCT05197556?term=NCT05197556&draw=2&rank=1
- 202.Hong S.M., Ko J.-K., Moon J.-J., Kim Y.-R. Oxytocin: a potential therapeutic for obesity. J Obes Metab Syndr. 2021;30(2):115–123. doi: 10.7570/jomes20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Elizabeth L. The effects of oxytocin in obese adults. 2017. https://clinicaltrials.gov/ct2/show/NCT03043053?term=NCT03043053&draw=2&rank=1
- 204.Gray B., Swick J., Ronnenberg A.G. Vitamin E and adiponectin: proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr Rev. 2011;69(3):155–161. doi: 10.1111/j.1753-4887.2011.00377.x. [DOI] [PubMed] [Google Scholar]
- 205.Shen C.-L. Tocotrienols for obesity of postmenopausal women (VitE-obesity) 2018. https://clinicaltrials.gov/ct2/show/NCT03705845?term=NCT03705845&draw=2&rank=1
- 206.Ishaq M., Tran D., Wu Y., et al. Asperuloside enhances taste perception and prevents weight gain in high-fat fed mice. Front Endocrinol. 2021;12:201. doi: 10.3389/fendo.2021.615446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Niethammer A. Study to evaluate ARD-101 in adults with obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT05121441?term=NCT05121441&draw=2&rank=1
- 208.Crunkhorn S. Leptin sensitizer reverses obesity. Nat Rev Drug Discov. 2016;15(9):601. doi: 10.1038/nrd.2016.166. [DOI] [PubMed] [Google Scholar]
- 209.Uysal T. ERX1000 - safety, tolerability, pharmacokinetic, and pharmacodynamic study in male and female subjects with obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT04890873?term=NCT04890873&draw=2&rank=1
- 210.Amgen Single and multiple ascending dose study of AMG 133 in participants with obesity. 2020. https://clinicaltrials.gov/ct2/show/NCT04478708?term=NCT04478708&draw=2&rank=1
- 211.Novartis Pharmaceuticals Study to assess safety, tolerability and efficacy of SC administered MBL949 in obese participants with or without T2DM. 2022. https://clinicaltrials.gov/ct2/show/NCT05199090?term=NCT05199090&draw=2&rank=1
- 212.Iwata K. Study to evaluate the safety, tolerability, pharmacokinetics, and food effect of NO-13065 in healthy and obese adult subjects. 2021. https://clinicaltrials.gov/ct2/show/NCT04838639?term=NCT04838639&draw=2&rank=1
- 213.Novo Nordisk AS A research study investigating NNC0247-0829 for weight management in people with overweight or obesity. 2019. https://clinicaltrials.gov/ct2/show/NCT04010786?term=NCT04010786&draw=1&rank=1
- 214.Eli Lilly and Company A study of LY3841136 in healthy and overweight participants. 2022. https://clinicaltrials.gov/ct2/show/NCT05295940?term=NCT05295940&draw=2&rank=1
- 215.Squibb B.-M. A study to assess the safety, tolerability and drug levels of BMS-986172 in healthy and obese participants, including an assessment of the effects of food on BMS-986172 absorption. 2021. https://clinicaltrials.gov/ct2/show/NCT04926051?term=NCT04926051&draw=2&rank=1
- 216.Novo Nordisk AS Research study looking at how well semaglutide works in people living with obesity and prediabetes (STEP 10) 2021. https://clinicaltrials.gov/ct2/show/NCT05040971?term=NCT05040971&draw=2&rank=1
- 217.Novo Nordisk AS Research study to investigate how well semaglutide works in people living with heart failure and obesity (STEP-HFpEF) 2021. https://clinicaltrials.gov/ct2/show/NCT04788511?term=NCT04788511&draw=2&rank=1
- 218.Novo Nordisk AS Research study looking at how well semaglutide works in people suffering from obesity and knee osteoarthritis. 2021. https://clinicaltrials.gov/ct2/show/NCT05064735?term=NCT05064735&draw=2&rank=1
- 219.Novo Nordisk AS Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT) 2018. https://clinicaltrials.gov/ct2/show/NCT03574597?term=NCT03574597&draw=2&rank=1
- 220.Hiddo Lambers Heerspink Semaglutide and albuminuria reduction trial in obese individuals without diabetes (SMART) 2021. https://clinicaltrials.gov/ct2/show/NCT04889183?term=NCT04889183&draw=2&rank=1
- 221.Gummo L. Effect of subcutaneous semaglutide on kidney transplant candidacy (RAISE-KT) 2021. https://clinicaltrials.gov/ct2/show/NCT04741074?term=NCT04741074&draw=2&rank=1
- 222.Novo Nordisk AS Research study to investigate how well semaglutide tablets taken once daily work in East Asian people who are overweight or living with obesity (OASIS 2) 2021. https://clinicaltrials.gov/ct2/show/NCT05132088?term=NCT05132088&draw=2&rank=1
- 223.Novo Nordisk AS Research study to investigate how well semaglutide tablets taken once daily work in people who are overweight or living with obesity (OASIS 1) 2021. https://clinicaltrials.gov/ct2/show/NCT05035095?term=NCT05035095&draw=2&rank=1
- 224.Wu J. Efficacy and safety of liraglutide on body weight in obese subjects or overweight subjects with co-morbidities. 2020. https://clinicaltrials.gov/ct2/show/NCT04487743?term=NCT04487743&draw=2&rank=1
- 225.Li X. The efficacy and safety of liraglutide on body weight loss in obese and overweight patients. 2020. https://clinicaltrials.gov/ct2/show/NCT04605861?term=NCT04605861&draw=2&rank=1
- 226.Touzot M., Beaussier H. Efficacy and tolerance of liraglutide for weight loss in obese type 2 diabetic hemodialysis patients (LIRADIAL) 2020. https://clinicaltrials.gov/ct2/show/NCT04529278?term=NCT04529278&draw=2&rank=1
- 227.Yale University Behavioral and pharmacologic treatment of binge eating and obesity: acute treatment. 2017. https://clinicaltrials.gov/ct2/show/NCT03045341?term=NCT03045341&draw=2&rank=1
- 228.Srinivasan M. Long term extension trial of setmelanotide. 2018. https://clinicaltrials.gov/ct2/show/NCT03651765?term=NCT03651765&draw=2&rank=1
- 229.Rhythm Pharmaceuticals Setmelanotide in pediatric rare genetic diseases of obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT04966741?term=NCT04966741&draw=2&rank=1
- 230.Rhythm Pharmaceuticals A phase 3 crossover trial of two formulations of setmelanotide in patients with specific gene defects in the melanocortin-4 receptor pathway. 2022. https://clinicaltrials.gov/ct2/show/NCT05194124?term=NCT05194124&draw=1&rank=1
- 231.Rhythm Pharmaceuticals Setmelanotide phase 2 treatment trial in patients with rare genetic disorders of obesity. 2017. https://clinicaltrials.gov/ct2/show/NCT03013543?term=NCT03013543&draw=2&rank=1
- 232.Pilley S., Jost-Price E. Open-label study of setmelanotide in hypothalamic obesity. 2021. https://clinicaltrials.gov/ct2/show/NCT04725240?term=NCT04725240&draw=2&rank=1
- 233.Group L.A.R. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4(11):913–921. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mathew H., Farr O.M., Mantzoros C.S. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65(1):73–80. doi: 10.1016/j.metabol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Verma S., Poulter N.R., Bhatt D., et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke. Circulation. 2018;138(25):2884–2894. doi: 10.1161/CIRCULATIONAHA.118.034516. [DOI] [PubMed] [Google Scholar]
- 236.Nørgaard C.H., Friedrich S., Hansen C.T., et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. 2022;8(1) doi: 10.1002/trc2.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Grieco M., Giorgi A., Gentile M.C., et al. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci. 2019;13:1112. doi: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mirabelli M., Chiefari E., Caroleo P., et al. Long-term effectiveness of liraglutide for weight management and glycemic control in type 2 diabetes. Int J Environ Res Public Health. 2019;17(1):207. doi: 10.3390/ijerph17010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dent R., McPherson R., Harper M.-E. Factors affecting weight loss variability in obesity. Metabolism. 2020;113:154388. doi: 10.1016/j.metabol.2020.154388. [DOI] [PubMed] [Google Scholar]
- 240.Frühbeck G., Kiortsis D.N., Catalán V. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018;6(3):164–166. doi: 10.1016/S2213-8587(17)30312-1. [DOI] [PubMed] [Google Scholar]
- 241.Hurtado A.M., Acosta A. Precision medicine and obesity. Gastroenterol Clin North Am. 2021;50(1):127–139. doi: 10.1016/j.gtc.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Alligier M., Barrès R., Blaak E.E., et al. OBEDIS core variables project: European expert guidelines on a minimal core set of variables to include in randomized, controlled clinical trials of obesity interventions. Obes Facts. 2020;13(1):1–28. doi: 10.1159/000505342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.