Abstract
Various countries have reported a decrease in breast cancer surgeries during the coronavirus disease 2019 (COVID-19) pandemic; however, inconsistent results have been reported in Japan. This study revealed changes in the number of surgeries during the pandemic using the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB) from January 2015 to January 2021, where insurance claims data from Japan as a whole are comprehensively accumulated. The number of breast-conserving surgeries (BCS) without axillary lymph node dissection (ALND) significantly decreased in July (− 846; 95% confidence interval (CI) − 1190 to − 502) and October 2020 (− 540; 95% CI − 861 to − 218). No decrease was observed for other types of surgery, BCS with ALND, and mastectomy with or without ALND. In the age-specific subgroup analysis, significant and transient reduction in BCS without ALND was observed in all age groups (0–49, 50–69, and ≥ 70 years). The number of BCS without ALND significantly decreased for a relatively short period in the early pandemic stages, suggesting reduced surgery for patients with a relatively low stage of cancer. Some patients with breast cancer might have been left untreated during the pandemic, and an unfavorable prognosis would be a concern.
Subject terms: Breast cancer, Cancer epidemiology, Public health, Surgical oncology
Introduction
Coronavirus disease 2019 (COVID-19) was initially identified in Wuhan City, China, in December 2019, and has spread worldwide. In Japan, since a person infected with severe acute respiratory syndrome coronavirus 2 was identified on January 15, 2020, eight waves of COVID-19 cases have been experienced, and three emergency declarations have been issued. The periods of the first, second, and third declarations were between April 7, 2020, and May 25, 2020, between January 7, 2021, and March 18, 2021, and between April 23, 2021, and September 28, 2021, respectively.
The COVID-19 pandemic has placed a heavy burden on the healthcare system worldwide and has changed the diagnosis pathways for various diseases, including breast cancer. The national screening programs for breast cancer had been suspended in the early stages of the pandemic1–5, and the number of screenings had drastically decreased in many countries6–13. In addition, expert committees issued recommendations to triage patients with breast cancer to accommodate the limited hospital resources during the COVID-19 pandemic14–16. Physicians may have avoided diagnostic practices in patients with low priority, conforming to the recommendations. Furthermore, some symptomatic patients may have avoided visiting the clinic due to fear of infection. Under these situations, breast cancer diagnosis had decreased worldwide7,17–22. Given these facts, a decrease in the number of surgeries for breast cancer during the pandemic was expected, and this has already been confirmed in various countries, including the United States8,23–25, the Netherlands26, Turkey27, Lithuania11, South India9, and South Korea19.
In Japan, the Ministry of Health, Labor, and Welfare (MHLW) temporarily requested the postponement of cancer screening at the beginning of the pandemic. Therefore, the number of breast cancer screenings decreased in 2020 compared with 201928. Furthermore, the number of diagnoses for breast cancer detected by screening decreased in 2020 compared with the average number during 2016–2019. In contrast, the number of diagnoses in symptomatic cases did not change29. However, the impact of the COVID-19 pandemic on the number of breast cancer surgeries has been inconsistent. For example, a study conducted in Gunma Prefecture and another using Diagnosis Procedure Combination (DPC) data reported that the number of breast cancer surgeries did not change during the pandemic30,31. In contrast, a study using the National Clinical Database, which is a nationwide web-based surgical patient registration system, reported that the number of breast-conserving surgeries (BCS) significantly decreased in 2020 compared to 2019, although total mastectomy did not32. Therefore, these discrepancies in results prompted us to conduct a study with exhaustive data to reveal the impact of the COVID-19 pandemic on breast cancer surgery in Japan. Additionally, the accumulation of results from various countries, including Japan, is necessary to understand the global impact of COVID-19.
To achieve this goal, we used sampling datasets from the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), which are administered by MHLW and contain comprehensive insurance claims data. These data allow us to observe changes in the number of breast cancer surgeries in Japan as a whole. Additionally, interrupted time-series analysis with seasonal autoregressive integrated moving average (SARIMA) models was used to control underlying trends, autocorrelation, moving average, and seasonality, which is one of the best designs for establishing causality when randomized controlled trials are not feasible33. The novelty of this study is evaluating the impact of the pandemic on breast cancer surgery using generalizable data in Japan and adopting the robust method. This study provides insights into the impact of the COVID-19 pandemic, inferring breast cancer prognosis in Japan, and contributes to understanding the global impact.
Results
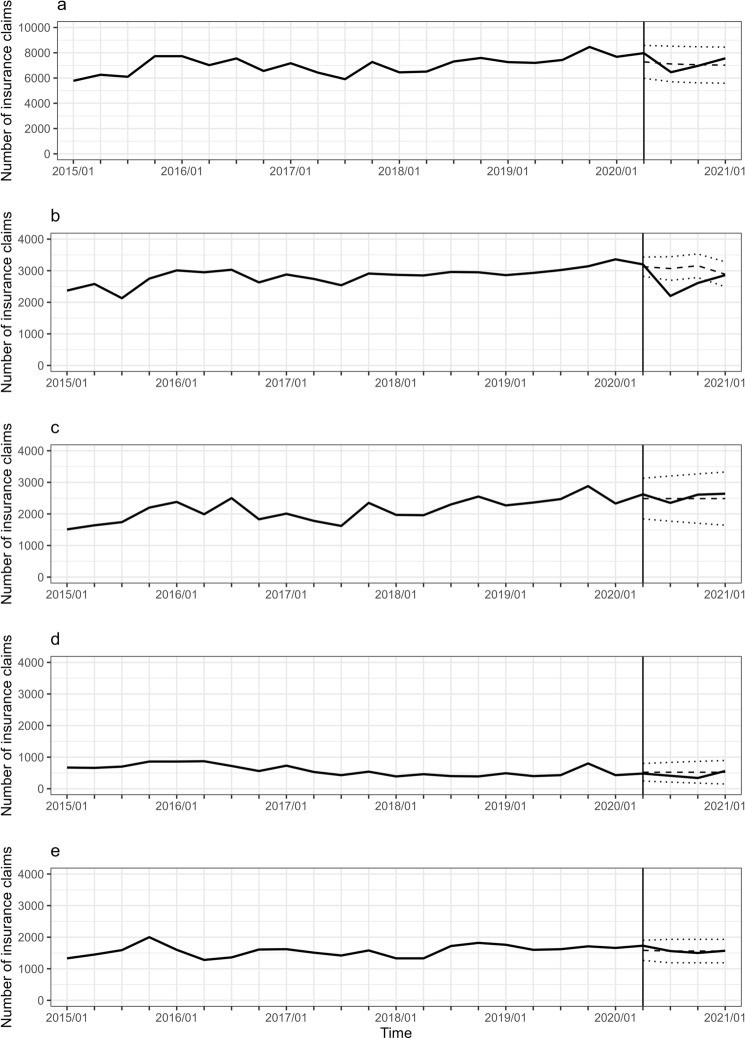
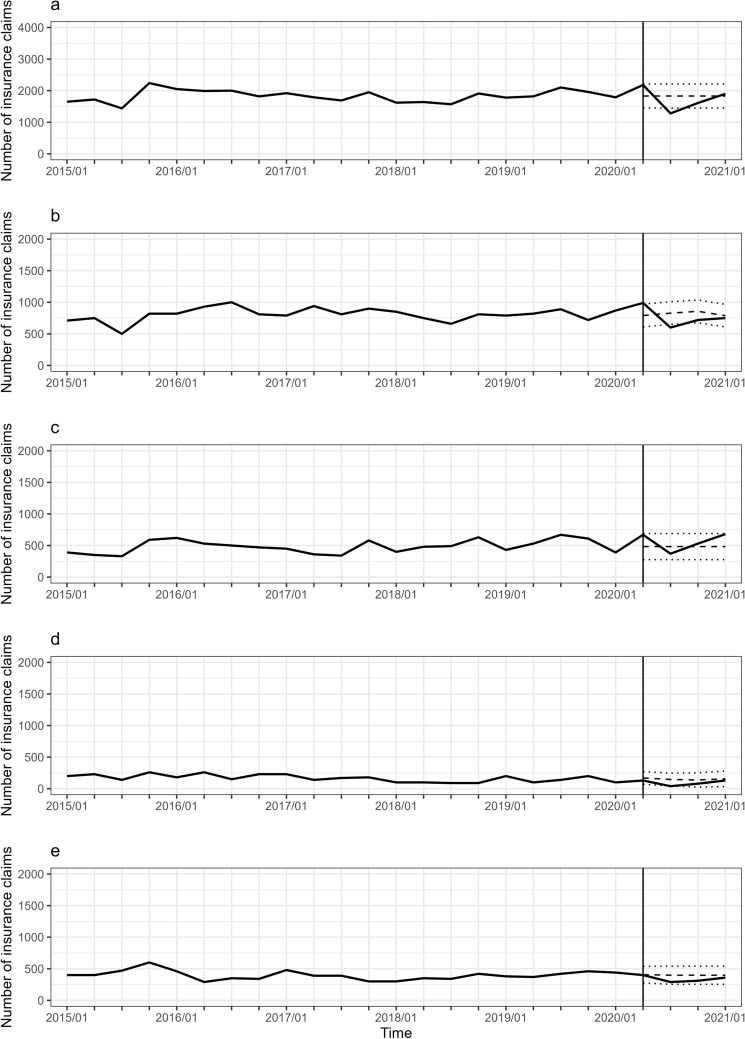
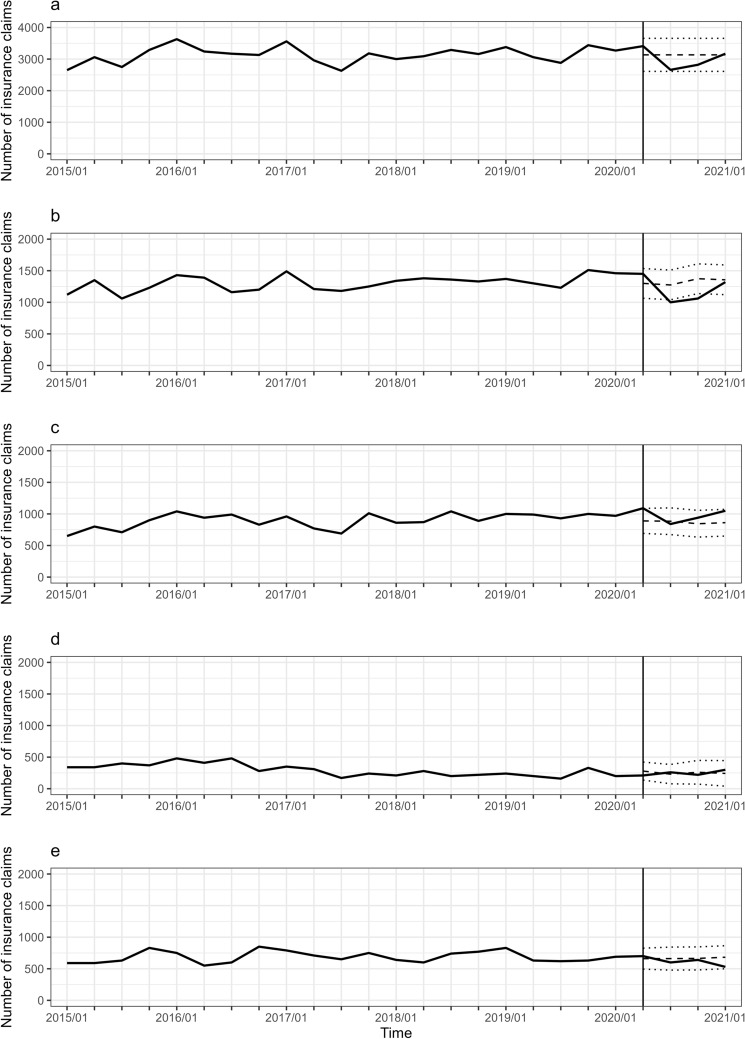
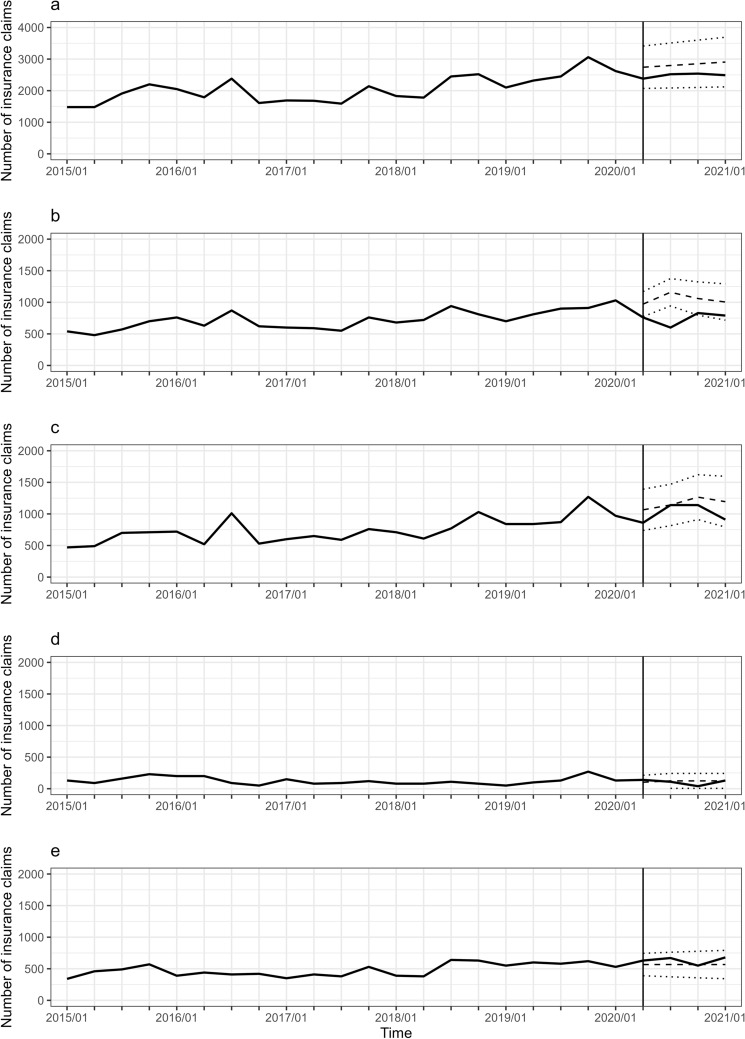
Estimated changes in the number and rate of each type of breast cancer surgery are listed in Table 1, whereas the observed number and expected counterfactual number with 95% CI are shown in Fig. 1. BCS without ALND significantly decreased in July (− 846 with 95% CI of − 1190 to − 502) and October 2020 (− 540 with 95% CI of − 861 to − 218). In contrast, the other types of surgery, BCS with ALND and mastectomy with and without ALND, did not significantly change. The results of the subgroup analysis by age are shown in Figs. 2, 3, 4 and Supplementary Table S1. The number of BCS without ALND significantly decreased in all age groups during the pandemic; in the age group 0–49 years in July 2020; in 50–69 years in July and October 2020; and in ≥ 70 years in April, July, and October 2020. Additionally, in the age groups 0–49 and 50–69 years, the number of mastectomies with and without ALND decreased in July 2020, although not significantly. Consequently, the total number of breast cancer surgeries decreased significantly in July 2020 in the 0–49 years age group (p = 0.002) and tended to decrease in the 50–69 years age group (p = 0.052). In contrast, in the ≥ 70 years age group, the number of mastectomies with and without ALND increased in July 2020, although not significantly, and the total number of surgeries did not change during the pandemic.
Table 1.
Estimated change of number and rate for breast cancer surgery during COVID-19 pandemic.
| Time | Estimated change of number1 | Estimated change of rate2 | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Number | 95% CI | % | 95% CI | |||||
| Total | Apr-2020 | 689 | − 499 | 1877 | 9 | − 7 | 26 | 0.256 |
| Jul-2020 | − 657 | − 1947 | 634 | − 9 | − 27 | 9 | 0.319 | |
| Oct-2020 | − 79 | − 1385 | 1227 | − 1 | − 20 | 17 | 0.906 | |
| Jan-2021 | 539 | − 770 | 1849 | 8 | − 11 | 26 | 0.420 | |
| BCS without ALND | Apr-2020 | 97 | − 188 | 381 | 3 | − 6 | 12 | 0.506 |
| Jul-2020 | − 846 | − 1190 | − 502 | − 28 | − 39 | − 16 | < 0.001 | |
| Oct-2020 | − 540 | − 861 | − 218 | − 17 | − 27 | − 7 | 0.001 | |
| Jan-2021 | 8 | − 384 | 401 | 0 | − 13 | 14 | 0.967 | |
| Mastectomy without ALND | Apr-2020 | 134 | − 438 | 707 | 5 | − 18 | 28 | 0.645 |
| Jul-2020 | − 136 | − 771 | 500 | − 5 | − 31 | 20 | 0.676 | |
| Oct-2020 | 124 | − 568 | 817 | 5 | − 23 | 33 | 0.725 | |
| Jan-2021 | 154 | − 591 | 900 | 6 | − 24 | 36 | 0.685 | |
| BCS with ALND | Apr-2020 | − 42 | − 289 | 205 | − 8 | − 55 | 39 | 0.741 |
| Jul-2020 | − 112 | − 389 | 166 | − 21 | − 75 | 32 | 0.431 | |
| Oct-2020 | − 182 | − 487 | 124 | − 35 | − 93 | 24 | 0.244 | |
| Jan-2021 | 38 | − 292 | 369 | 7 | − 56 | 71 | 0.820 | |
| Mastectomy with ALND | Apr-2020 | 147 | − 138 | 431 | 9 | − 9 | 27 | 0.312 |
| Jul-2020 | − 1 | − 339 | 337 | 0 | − 22 | 22 | 0.995 | |
| Oct-2020 | − 61 | − 399 | 277 | − 4 | − 26 | 18 | 0.723 | |
| Jan-2021 | 9 | − 329 | 347 | 1 | − 21 | 22 | 0.959 | |
ALND axillary lymph node dissection, BCS breast-conserving surgery, CI confidence interval.
1Estimated change of number represents the change during the COVID-19 pandemic under the control of underlying trends, autocorrelation, moving average, and seasonality.
2Estimated change of rate was calculated by dividing the estimated change of number by the predicted number in the absence of the pandemic (counterfactual number).
Figure 1.
Trajectory of the number of breast cancer surgeries. (a) The total number of breast cancer surgeries. (b) The number of breast-conserving surgeries without axillary lymph node dissection. (c) The number of mastectomies without axillary lymph node dissection. (d) The number of breast-conserving surgeries with axillary lymph node dissection. (e) The number of mastectomies with axillary lymph node dissection. The numbers were calculated using medical inpatient and Diagnosis Procedure Combination insurance claims. The observed numbers during all observation periods (solid line) and the expected counterfactual numbers with 95% confidence intervals during the COVID-19 pandemic (dashed and dotted lines) were plotted. Data are represented by quarterly series, January, April, July, and October.
Figure 2.
Trajectory of the number of breast cancer surgeries in patients aged 0–49 years. Note is the same as Fig. 1.
Figure 3.
Trajectory of the number of breast cancer surgeries in patients aged 50–69 years. Note is the same as Fig. 1.
Figure 4.
Trajectory of the number of breast cancer surgeries in patients aged ≥ 70 years. Note is the same as Fig. 1.
Discussion
We revealed that BCS without ALND significantly decreased in the early stage of the COVID-19 pandemic. In contrast, the other types of breast cancer surgery did not change using the sampling datasets of NDB, which included comprehensive insurance claims data from Japan as a whole.
Various countries have reported a decrease in the number of breast cancer surgeries during the COVID-19 pandemic8,9,11,19,23–27. Additionally, several studies have examined changes in the number according to the type of surgery; however, the results remain inconsistent. A single-center study in India and a multicenter study with five hospitals in the Netherlands reported that the number of BCS decreased during the pandemic. In contrast, the total number of breast surgeries or mastectomies did not change9,26. A study using the Kaiser Permanente Northern California Breast Cancer Tracking System database in the United States reported that the percentage of partial mastectomy decreased in 2020 compared with 2019 among patients who underwent surgery as the first treatment18. A single-center study in the United States and a multicenter study in two hospitals in Turkey reported that surgery type did not change among patients who underwent surgery23,27. A multicenter study with six hospitals in Korea revealed that the number of mastectomies, rather than lumpectomies, decreased in 2020 compared to 201919. In Japan, three studies compared the number of breast cancer surgeries performed before and during the pandemic. A multicenter study with 17 hospitals in Gunma Prefecture and another using DPC data reported that the number of breast cancer surgeries did not significantly change before and during the pandemic30,31. In contrast, a study using the National Clinical Database of Japan found that BCS decreased in 2020 compared with 2018 or 2019, rather than total mastectomy32. Our results were consistent with those of the latter. A common point between our study and the previous study is the high degree of exhaustiveness. NDB we used comprehensively accumulated insurance claims in Japan, with a registration rate of 98.5% in July 2022. The National Clinical Database used in the previous study registered more than 90% of all surgeries performed in Japan between 2019 and 2020, which was recorded by approximately 5,000 hospitals32. Consistent results in both studies using different database with exhaustive data strengthen the reliability of the conclusion that BCS decreased in Japan during the pandemic; however, mastectomy did not.
According to the Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer 2022, BCS is frequently performed in patients with stage I or II cancer. In contrast, ALND is recommended for patients with clinically evident axillary lymph node metastases. Therefore, patients who underwent BCS without ALND were presumed to have a relatively low stage without overt axillary lymph node metastasis. Although the reason why only this type of surgery declined during the pandemic remains unknown, we propose the following explanation: First, the decline can be attributed to the suspension of cancer screening, as suggested by a previous study in the Netherlands26. Breast cancer screening enables the early detection of cancer34. According to a study using hospital-based cancer registries in Japan, the number of diagnoses of breast cancer detected by screening decreased in 2020 compared with the average number during 2016–2019, and early-stage cancer detection significantly decreased29. Similar results were observed in a multicenter study in Japan, which had a relatively small sample size30. Therefore, a decrease in BCS without ALND may reflect a decline in the number of cancer diagnoses detected by cancer screening at a relatively early stage. Second, BCS may have been reduced to avoid radiotherapy, as previously suggested32, since BCS is accompanied by postoperative radiotherapy, which requires frequent outpatient visits. If this is the case, mastectomy is expected to increase instead. In the age group of ≥ 70 years, the number of mastectomies had an increasing trajectory in July 2020, unlike other age groups. Thus, the decrease in BCS might be offset by the increase in mastectomies, and the total number of surgeries remained unchanged during the pandemic. This result suggested that BCS had been partly replaced by mastectomies in this age group. Elderly adults are more likely to develop severe COVID-19 symptoms and are at higher risk of death. Therefore, they may have avoided BCS to avoid making frequent hospital visits for radiotherapy. Instead, mastectomy may have been performed as an alternative treatment. Third, neoadjuvant therapy may have been adopted to protect against pandemic-induced surgical delays due to limited hospital resources. Several societies, including the Japan Breast Cancer Society, recommend considering this treatment for early-stage patients with breast cancer in the guidelines for treating breast cancer during the COVID-19 pandemic14,16,35. Neoadjuvant therapy has been implemented in the United States17,23 and Canada36; however, the situation in Japan remains to be confirmed.
Patient prognosis differs depending on the reason for the reduction in surgery. If the reduction reflects undiagnosed patients during the pandemic, it implies that some individuals with breast cancer were left untreated. A systematic review and meta-analysis showed that delaying surgery for 12 weeks reduced the overall survival in breast cancers37. The total number of breast cancer surgeries decreased in the age groups of 0–49 years and 50–69 years in July 2020; therefore, worsening prognosis in these age groups is a source of concern. In contrast, if the reason for the reduction in BCS was to avoid radiotherapy or replace neoadjuvant therapy, patients are less likely to have a worse prognosis since they received alternative treatments to BCS. In the age group, ≥ 70 years, mastectomy might have been performed as an alternative to BCS and thus the total number of surgeries did not change. Therefore, the prognosis of breast cancer in elderly person may not be unfavorable compared with that in younger person.
This study has some limitations. First, information on the stage of breast cancer was unavailable since insurance claims data were for billing purposes rather than diagnostic or treatment purposes. Second, because the sampling dataset of NDB is a quarterly date, it is impossible to determine the monthly trends. Therefore, the beginning and ending points of the decline in BCS without ALND were not precisely identified. In addition, if the number of surgeries decreased in months that were not included in the sampling dataset, they would not be captured. Third, the observation period during the pandemic might have been too short to determine the entire impact of the COVID-19 pandemic on breast cancer surgery. Fourth, we could not evaluate the adoption of neoadjuvant therapy during the pandemic in Japan, although this is important information for inferring prognosis. NDB does not have information on the date of diagnosis or the first treatment after diagnosis. Additionally, as many of the drugs used before and after surgery overlapped, we could not identify neoadjuvant treatment with the drug. However, this study has the advantage of being highly generalizable owing to the use of a highly exhaustive database. Using interrupted time-series analysis with SARIMA is also our advantage, which is a more robust method to control for pre-existing underlying short- and long-term trends and enables us to provide the hypothetical scenario under which the intervention had not taken place, which is referred to as being “counterfactual”33,38.
In conclusion, we revealed that the number of BCS without ALND significantly and temporarily decreased in the early stages of the COVID-19 pandemic rather than in other types of surgery. Furthermore, this trajectory was observed in all age groups. These results are consistent with those of a previous study that used other exhaustive databases in Japan; therefore, the reliability of the results was enhanced. The reduction in the total number of surgeries in the age groups 0–49 years and 50–69 years indicates that some patients remained untreated, thus raising concerns about worsened prognosis.
Methods
Data sources
This was a repeated cross-sectional study using sampling datasets of NDB, which is a database of insurance claims data since 2009 and specific health checkup data since 2008, based on the Act on Assurance of Medical Care for Elderly People. The number of medical insurance claims recorded in July 2022 was 59,509,947, corresponding to 98.5% of all claims in Japan. As of March 31, 2020, 18,768 million insurance claims data had been accumulated in NDB. NDB data have been provided to researchers after reviewing the appropriateness of the data management plans and study protocol in MHLW since 2011. Three types of data were provided, depending on the extraction method. Of these, we used sampling datasets with the following features: 1. A dataset was created four times per year with a 3-month interval: January, April, July, and October; 2. Data were extracted randomly with an extraction rate of 1% for medical outpatient and pharmacy claims and that of 10% for medical inpatient and DPC claims; 3. the dataset does not contain personally identifiable information, such as name, address, or birthday; 4. Insurance claims with extremely high costs were excluded before extraction (7,000,000 JPY for medical inpatient and DPC claims and 500,000 JPY for medical outpatient and pharmacy claims, which correspond to 46,684 USD and 3335 USD, respectively, with a rate of October 20, 2022); 5. Data were extracted independently for each month; therefore, individuals in each month could not be linked. Sampling datasets of NDB between January 2015 and January 2021 were provided by MHLW, which included 20,452,831 medical outpatient claims, 3,115,714 medical inpatient claims, and 2,507,790 DPC claims.
Create time-series data
We counted only women and used medical inpatient and DPC insurance claims data since breast cancer surgery is generally performed under inpatient management. Breast cancer surgery is classified into the following four types: 1. BCS without axillary lymph node dissection (ALND); 2. mastectomy without ALND; 3. BCS with ALND; 4. mastectomy with ALND. The medical practice codes for the extraction are listed in Supplementary Table S2. Nipple-sparing mastectomy was included in BCS rather than mastectomy since this surgery is adapted for patients with preoperative stage II or lower, in which BCS is usually performed, according to the Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer 2022.
Time-series data with 25 points for each type of surgery were created using the following procedure: First, we counted the number of claims by type of claims (medical inpatient and DPC), type of surgery (BCS without ALND, mastectomy without ALND, BCS with ALND, mastectomy with ALND, and all breast cancer surgeries), and time (25 points), second, we multiplied each count by 10 (the reciprocal of the extraction rate), finally, we summed the number of medical inpatients and DPC claims by type of surgery and time.
We defined the period between January 2015 and January 2020 as before the pandemic (21 points) and between April 2020 and January 2021 as during the pandemic (4 points). The number of surgeries per month is provided in Supplementary Tables S3 and S4 for all patients and subgroup analysis by age, respectively.
Statistical analysis
Interrupted time-series analysis with SARIMA models was used to evaluate the impact of COVID-19 on breast surgical volume based on the method reported by Scheffe et al.33. All analyses were performed using R version 4.2.0, which is a free software environment for statistical computing and graphics. First, we performed auto.arima in the forecast package for R to identify the SARIMA model terms (p, d, q) × (P, D, Q)s, with the lowest Akaike’s information criterion. Seasonality (s) was determined to be 4 since the data were quarterly. In this step, all 25-point data were used, and step-change variables for each point during the pandemic were included in the model as external regressors. The step-change variables for each month (Si) take the value of 1 for each month during the pandemic (i = 2020/4, 2020/7, 2020/10, and 2021/1) and 0 otherwise. For example, the step-change variable for April 2020 () took a value of 1 in April 2020 and 0 in the other 24 points. The estimates of the step-change variable represent the change in number for each month during the pandemic. Second, using only the pre-pandemic points (21 points), the number during the pandemic was predicted by the SARIMA model with the same terms (p, d, q, P, D, and Q) determined by the first step, which represents the expected counterfactual number. The observed number and the counterfactual number with a 95% confidence interval (CI) are compared in figures. The change in the rate was calculated by dividing the estimated change in number by the counterfactual number. Subgroup analysis by age category was performed; the categories were 0–49, 50–69, and ≥ 70 years. Statistical significance was set at P < 0.05.
Ethics statement
All personally identifiable information was excluded from the sampling datasets of NDB during the creation process in MHLW. Therefore, individuals cannot be identified from the dataset. The Research Ethics Committees of the Chiba Foundation for Health Promotion and Disease Prevention concluded that informed consent was not necessary and approved this study protocol (approval number R3-4). This study was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Author contributions
All authors contributed to the conception and design of the study. Offers to provide NDB sampling dataset were M.F. and A.H. Data extraction conditions were determined by M.F., H.H., K.S., T.K., K.Y., D.S., T.F., and A.H.. M.F. and K.N. determined the data analysis plan, and M.F. performed the data analysis. The first draft of the manuscript was written by M.F., and all the authors commented on the previous versions of the manuscript. All the authors have read and approved the final manuscript.
Data availability
The data that support the findings of this study were obtained from the MHLW of Japan. However, restrictions apply to the accessibility of these data, which were used under license for the current study. Therefore, they are not publicly available. Nevertheless, these data are available from the author (Misuzu Fujita: mi-hujita@kenko-chiba.or.jp) upon reasonable request and with permission of MHLW.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-32317-w.
References
- 1.Jones D, et al. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: The view from primary care. Lancet Oncol. 2020;21:748–750. doi: 10.1016/S1470-2045(20)30242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinmohamed AG, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Vecchio Blanco G, Calabrese E, Biancone L, Monteleone G, Paoluzi OA. The impact of COVID-19 pandemic in the colorectal cancer prevention. Int. J. Colorectal Dis. 2020;35:1951–1954. doi: 10.1007/s00384-020-03635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zadnik V, et al. Impact of COVID-19 on cancer diagnosis and management in Slovenia—preliminary results. Radiol. Oncol. 2020;54:329–334. doi: 10.2478/raon-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maio F. et al., Breast cancer screening during COVID-19 emergency: Patients and department management in a local experience. J. Pers. Med.11, (2021). [DOI] [PMC free article] [PubMed]
- 6.Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7:878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, Mcnair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin. Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patt D, et al. Impact of COVID-19 on cancer care: How the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijaykumar DK, et al. Impact of COVID-19 on the diagnosis and surgical care of patients with breast cancer-a retrospective observational cohort study from Kerala South India. Indian J. Surg. Oncol. 2022;2:1–5. doi: 10.1007/s13193-022-01610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakouny Z, et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7:458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabkeviciene D. et al., The impact of the COVID-19 pandemic on cancer patient's management-Lithuanian Cancer Center experience. Healthcare (Basel, Switzerland)9, (2021). [DOI] [PMC free article] [PubMed]
- 12.Park H. et al., The impact of COVID-19 on the screening for colorectal, gastric, breast and cervical cancer in Korea. Epidemiol. Health e2022053 (2022). [DOI] [PMC free article] [PubMed]
- 13.Brugel M, et al. Dramatic changes in oncology care pathways during the COVID-19 pandemic: The French ONCOCARE-COV study. Oncologist. 2021;26:e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz J. R. et al., Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat.181, 487–497 (2020). [DOI] [PMC free article] [PubMed]
- 15.De Azambuja E, et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Breast Cancer. ESMO Open. 2020;5:e000793. doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawate T, et al. Recommendations for the management of breast cancer patients during the COVID-19 pandemic from the Japan Breast Cancer Society. Breast Cancer. 2021;28:247–253. doi: 10.1007/s12282-020-01214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caswell-Jin JL, et al. Breast cancer diagnosis and treatment during the COVID-19 pandemic in a nationwide, insured population. Breast Cancer Res. Treat. 2022;194:475–482. doi: 10.1007/s10549-022-06634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang A, et al. Care in the time of COVID-19: impact on the diagnosis and treatment of breast cancer in a large, integrated health care system. Breast Cancer Res. Treat. 2022;191:665–675. doi: 10.1007/s10549-021-06468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang YJ, et al. Impact of the COVID-19 pandemic on the diagnosis and surgery of breast cancer: A multi-institutional study. J. Breast Cancer. 2021;24:491–503. doi: 10.4048/jbc.2021.24.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrdoljak E, et al. COVID-19 pandemic effects on breast cancer diagnosis in Croatia: A population- and registry-based study. Oncologist. 2021;26:e1156–e1160. doi: 10.1002/onco.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob L. et al., Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers (Basel)13, (2021). [DOI] [PMC free article] [PubMed]
- 22.Morais S, et al. The impact of the coronavirus disease 2019 pandemic on the diagnosis and treatment of cancer in Northern Portugal. Eur. J. Cancer Prev. 2022;31:204–214. doi: 10.1097/CEJ.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonneson JE, et al. Impact of the COVID-19 pandemic on breast cancer stage at diagnosis, pesentation, and patient management. Ann. Surg. Oncol. 2022;29:2231–2239. doi: 10.1245/s10434-021-11088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenstein RN, et al. Effects of COVID-19 on mastectomy and breast reconstruction rates: A national surgical sample. J. Surg. Oncol. 2022;126:205–213. doi: 10.1002/jso.26889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin K, Singh P, Drohan B, Hughes KS. Breast imaging, breast surgery, and cancer genetics in the age of COVID-19. Cancer. 2020;126:4466–4472. doi: 10.1002/cncr.33113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filipe MD, et al. Effect of the COVID-19 pandemic on surgical breast cancer care in the Netherlands: A multicenter retrospective cohort study. Clin. Breast Cancer. 2020;20:454–461. doi: 10.1016/j.clbc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kara H, et al. Has the COVID-19 pandemic affected breast cancer stage and surgical volume? Front. Surg. 2022;9:811108. doi: 10.3389/fsurg.2022.811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health, Labour and Welfare, Government of Japan, (2022). Report on Regional Public Health Services and Health Promotion Services 2020. https://www.mhlw.go.jp/toukei/saikin/hw/c-hoken/20/index.html.
- 29.Okuyama A, et al. Impact of the COVID-19 pandemic on the diagnosis of cancer in Japan: analysis of hospital-based cancer registries. Jpn. J. Clin. Oncol. 2022;52:1215–1224. doi: 10.1093/jjco/hyac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeki H, et al. Decreased numbers of gastric, colorectal, lung, and breast cancer surgeries performed in 17 cancer-designated hospitals in Gunma Prefecture of Japan during the COVID-19 pandemic. Surg. Today. 2022;52:1714–1720. doi: 10.1007/s00595-022-02501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuno T, et al. Surgical volume reduction and the announcement of triage during the 1st wave of the COVID-19 pandemic in Japan: A cohort study using an interrupted time series analysis. Surg. Today. 2021;51:1843–1850. doi: 10.1007/s00595-021-02286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda N, et al. The impact of COVID-19 on surgical procedures in Japan: Analysis of data from the National Clinical Database. Surg. Today. 2022;52:22–35. doi: 10.1007/s00595-021-02406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffer AL, Dobbins TA, Pearson SA. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: A guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 2021;21:58. doi: 10.1186/s12874-021-01235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman C. Early detection and screening for breast cancer. Semin. Oncol. Nurs. 2017;33:141–155. doi: 10.1016/j.soncn.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Curigliano G, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habbous S, et al. Comparison of use of neoadjuvant systemic treatment for breast cancer and short-term outcomes before vs during the COVID-19 era in Ontario Canada. JAMA Netw. Open. 2022;5:e2225118. doi: 10.1001/jamanetworkopen.2022.25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson BA, Waddimba AC, Ogola GO, Fleshman JW, Jr., Preskitt J. T., A systematic review and meta-analysis of surgery delays and survival in breast, lung and colon cancers: Implication for surgical triage during the COVID-19 pandemic. Am. J. Surg. 2021;222:311–318. doi: 10.1016/j.amjsurg.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017;46:348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study were obtained from the MHLW of Japan. However, restrictions apply to the accessibility of these data, which were used under license for the current study. Therefore, they are not publicly available. Nevertheless, these data are available from the author (Misuzu Fujita: mi-hujita@kenko-chiba.or.jp) upon reasonable request and with permission of MHLW.