Abstract
Coronavirus disease 2019 (COVID-19) is an ongoing global pandemic that has affected nearly 600 million people to date across the world. While COVID-19 is primarily a respiratory illness, cardiac injury is also known to occur. Cardiovascular magnetic resonance (CMR) imaging is uniquely capable of characterizing myocardial tissue properties in-vivo, enabling insights into the pattern and degree of cardiac injury. The reported prevalence of myocardial involvement identified by CMR in the context of COVID-19 infection among previously hospitalized patients ranges from 26 to 60%. Variations in the reported prevalence of myocardial involvement may result from differing patient populations (e.g. differences in severity of illness) and the varying intervals between acute infection and CMR evaluation. Standardized methodologies in image acquisition, analysis, interpretation, and reporting of CMR abnormalities across would likely improve concordance between studies. This consensus document by the Society for Cardiovascular Magnetic Resonance (SCMR) provides recommendations on CMR imaging and reporting metrics towards the goal of improved standardization and uniform data acquisition and analytic approaches when performing CMR in patients with COVID-19 infection.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12968-023-00933-0.
Keywords: Cardiovascular magnetic resonance, COVID-19, SARS-CoV-2, Cardiac complications, Myocarditis, Myocardial infarction, Microinfarctions, Thrombotic complications, Multisystem inflammatory syndrome, Diagnostic criteria
Background
The coronavirus disease 2019 (COVID-19) pandemic is a major cause of morbidity and death worldwide. Individuals with pre-existing cardiovascular disease are at increased risk of severe illness and death in association with COVID-19 [1, 2]. Furthermore, growing evidence has highlighted COVID-19 as a multisystem disease, with an array of potential cardiovascular manifestations in the acute and post-acute phases of the illness [3]. Multiple studies report evidence of ischemic and non-ischemic myocardial injury [4–7], as well as myocardial and immune response, as part of a systemic inflammatory response in the context of acute COVID-19. Ischemic injury may relate to typical acute plaque rupture and other etiologies, such as myocardial ischemia precipitated by critical illness. However, a pro-thrombotic state and coronary vasculitis associated with COVID-19 have also been observed [8–11]. Non-ischemic etiologies of myocardial injury include myocarditis [12] and less often, stress-induced (Takotsubo) cardiomyopathy [13], both of which can manifest as acute heart failure [14] (Fig. 1). In children, while acute COVID symptoms are generally mild, a multisystem inflammatory syndrome in children (MIS-C) assumed to be a delayed hyperimmune response to SARS-COV-2 infection/exposure has been reported [15]. Cardiovascular manifestations in MIS-C can range from vasodilatory or cardiogenic shock, acute heart failure, myocarditis and/or coronary artery involvement akin to Kawasaki disease [16–18].
Fig. 1.
Cardiovascular manifestations of COVID-19 on cardiovascular magnetic resonance (CMR). Clockwise from the top: (1) A patient diagnosed with acute myocarditis, found to have midmyocardial late gadolinium enhancement (LGE) in the inferior and inferoseptal walls, with increased T2 relaxation times in the inferior wall (white arrows). (2) A patient with subendocardial LGE in the mid to distal septum and apex, found to have an occlusion in the mid left anterior descending artery on coronary angiography (red arrows). (3) Globally increased native T1 and T2 relaxation times in a patient with multisystem inflammatory syndrome. (4) A patient diagnosed with stress cardiomyopathy, with thickening of the basal segments (white arrows) and akinesis of apex (asterisk) seen on cine imaging. (5) A patient diagnosed with acute pericarditis, found to have diffuse LGE in the pericardium (red arrows) and a pericardial effusion (asterisk)
The long-term cardiovascular manifestations of SARS-COV-2 infection remain unknown. Several studies report protracted non-ischemic myocardial injury and/or ongoing myocarditis after apparent recovery from the acute phase of COVID-19 [19–22]. However, the longer-term significance of such observations is uncertain. Due to a high prevalence of cardiovascular disease in the general population, studies of COVID-19 patients must include appropriate control groups to improve reliability and clinical interpretation of their studies [23–25]. CMR has also been used to evaluate rare reports of myocardial injury associated with COVID-19 vaccination, particularly in male adolescents and young adults [26–30].
The clinical complexity of patients with cardiovascular involvement in the setting of COVID-19 presents unique challenges in diagnosis, clinical management, and longer-term risk stratification to optimize clinical outcomes. Cardiovascular magnetic resonance (CMR) imaging has become a leading imaging modality to detect both acute and long-term cardiovascular sequelae of COVID-19 infection due to its unique capability of detecting myocardial injury and characterizing myocardial tissue properties in-vivo. The number of reports of myocardial involvement in COVID-19 using CMR is rapidly increasing. However, comparisons between studies are hindered by variation in methodology used in acquisition and analysis methods. Previous reports by the Society for Cardiovascular Magnetic Resonance (SCMR) have provided guidance on the use of CMR during and after COVID-19 infection [31–33]. This consensus document focuses on recommendations on CMR imaging and reporting metrics, toward improved standardization, uniform data acquisition, and analytic approaches for assessing cardiac involvement in COVID-19. In accordance with SCMR guidance [34], the writing panel comprised of experts with a broad range of expertise in CMR and COVID-19 related cardiovascular manifestations and a wide geographical and subspecialty background. The panel reviewed existing literature and in accordance with available scientific evidence developed consensus recommendations for clinical CMR practice. These recommendations were then further modified following external review and approved by final consensus of the writing panel.
Histopathological cardiac findings in COVID-19
Histopathological findings of the heart in patients with COVID-19 may advance our understanding of the underlying pathophysiology of cardiac involvement in this disease. It has been postulated that both systemic inflammatory response as well as direct organ damage by infiltration of SARS-CoV-2 virus are the putative mechanisms for myocardial injury in COVID-19. However, to date, there is little evidence supporting direct damage to cardiomyocytes due to virus-mediated lysis and the virus has been detected in the myocytes in only a few cases [35]. Although SARS-CoV-2 mRNA has been detected in myocardium in 25–50% of COVID-19 patients during autopsy, it is predominantly found within the pericytes and in the subendothelium rather than myocytes [36]. Other possible mechanisms for myocardial injury in this disease include cytokine storm, microvascular angiopathy, endothelial dysfunction and a hypercoagulable state which causes coronary arterial thrombosis [37].
A systematic pathological analysis of 40 hearts from an autopsy series of hospitalized patients who died of COVID-19 showed that the most common pathological cause of myocyte necrosis was microthrombi or small focal areas of myocardial necrosis [38]. Overall, 35% (14/40) had evidence of myocyte necrosis, predominantly of the left ventricle (LV), with no significant difference in the incidence of severe coronary artery disease (CAD) between those with and without necrosis. Of those with myocyte necrosis, 21% showed acute myocardial infarction (≥ 1 cm2 area of necrosis) whereas 79% showed small areas of focal myocyte necrosis (> 20 necrotic myocytes with an area of ≥ 0.05 mm2 but < 1 cm2). Further, 79% (11/14) showed cardiac thrombi; 14% (2/14) had epicardial coronary artery thrombi, whereas 64% (9/14) had microthrombi in myocardial capillaries, arterioles, and small muscular arteries. Interestingly, microthrombi from COVID-19-positive autopsy cases were different in composition from intramyocardial thromboemboli from COVID-19-negative subjects, and from coronary thrombi retrieved from COVID-19-positive and -negative patients with ST-segment–elevation myocardial infarction.
A systematic review of the post-mortem pathological findings in COVID-19, the major microscopic findings were myocardial necrosis, interstitial macrophages, lymphocytic infiltration of the myocardium and thrombosis of coronary microvasculature [39]. On immunohistochemistry, the myocardium demonstrates inflammation with infiltration of CD68 + macrophages as well as CD3 + , CD4 + and CD8 + cytotoxic lymphocytes [39]. These findings demonstrate that COVID-19 leads to an inflammatory and a prothrombotic state in the myocardium. Presence of CD3 + lymphocytes is consistent with the fact that cellular immunity plays a key role in the host response mounted during COVID-19 infection. Another review of 277 cardiac autopsies across 22 studies suggested that classical myocarditis (confluent myocyte necrosis or diffuse lymphocytic infiltration) was identified only in 7.2% with the prevalence of non-myocarditis inflammatory infiltrate, single-cell ischemia and acute myocardial infarction being 12.6%, 13.7% and 4.7%, respectively [40]. As per the current evidence, unlike other forms of viral myocarditis, fulminant myocarditis as a cause of death is rare in COVID-19 and non-specific cardiac inflammation is more common [35, 36].
Cardiovascular imaging in the evaluation of patients with COVID-19
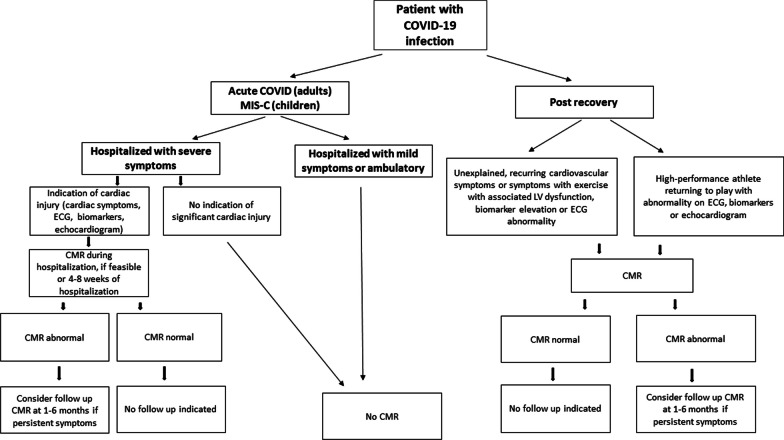
Although the reference standard for diagnosis of myocarditis is histopathology, routine endomyocardial biopsy for the diagnosis of myocarditis in the setting of COVID-19 is not currently recommended. In clinical practice, alongside cardiac troponin levels, cardiovascular imaging is key to diagnosis and clinical decision-making in patients with suspected myocarditis and other forms of cardiac injury, including after COVID-19 [35, 36]. Transthoracic echocardiography (TTE) is typically the first line cardiac imaging modality, and is highly valuable for functional assessment. However, TTE has limited capability for myocardial tissue characterization for disease classification related to COVID-19. Indeed, in a global survey of clinical TTE use in 1216 patients hospitalized with COVID-19, LV abnormalities were detected in 39% of patients; however, in most cases, a specific underlying cause was not identified [41]. Flurodeoxyglucose (FDG)-positron emission tomography (PET) findings have been described in patients with suspected myocarditis and after COVID-19 infection, although routine use would not be recommended, due to ionizing radiation exposure as well as availability [42, 43]. CMR offers both morphological and functional assessment and the ability to detect myocardial inflammation and injury with high accuracy, making it the ideal imaging modality to study cardiac involvement in COVID-19. In acute COVID-19 infection, CMR has the potential to improve diagnostic and prognostic assessment among patients with severe COVID-19 infection and clinical evidence of myocardial injury. Among convalescent patients, CMR is of highest utility among patients with ongoing cardiopulmonary symptoms and abnormal cardiovascular testing including electrocardiogram (ECG) and TTE (Fig. 2).
Fig. 2.
Recommendations for the use of CMR in COVID-19. As shown, CMR may be considered among patients with severe COVID-19 infection and clinical evidence of myocardial injury. Among convalescent patients with COVID-19 infection, CMR is of highest utility among patients with abnormal echocardiogram, electrocardiogram (ECG), and biomarkers as well as ongoing cardiopulmonary symptoms. MIS-C multisystem inflammatory syndrome in children
CMR methods for the evaluation of patients with COVID-19
Cine CMR is the imaging reference standard for assessing cardiac structure and function; it provides high spatial and good temporal resolution with whole heart coverage, allowing precise assessment of both the left and right heart that can inform potential mechanism(s) of clinical symptoms, therapeutic responses, and potentially prognosis post COVD-19, like in other cardiac diseases [44]. Strain imaging (such as by myocardial tagging or feature tracking) can detect subclinical cardiac functional abnormalities [45, 46].
CMR uniquely offers non-invasive myocardial tissue characterization, and can detect a range of ischemic, non-ischemic, and inflammatory etiologies not accessible to other imaging modalities. Late gadolinium enhancement (LGE) highlights areas within the myocardium that have expanded interstitial space, typically in areas of focal fibrosis, as well as myocyte necrosis in the acute setting [47–49]. CMR patterns of LGE can distinguish ischemic from non-ischemic etiologies of myocardial injury, such as infarction versus myocarditis [50]. Of note, gadolinium-based media is the preferred contrast agent for LGE assessment. T2-weighted CMR images allows detection of focal and global myocardial edema that accompany acute myocarditis and infarction [51–55]. Thus T2-weighted CMR is particularly helpful in assessing acute myocardial injury and the acute myocardial response to systemic illnesses.
Parametric mapping techniques, such as T1-, T2- and extracellular volume (ECV) mapping, offer quantitative and pixel-wise characterization of the myocardial tissue. These methods have the potential to be more sensitive than LGE CMR for the detection of both acute and chronic myocardial disease [45, 56].
CMR can also assess large and small coronary vessels using CMR stress perfusion imaging [61] and perfusion mapping [62]. CMR evaluation of the pulmonary transit time can be used to detect subtle cardiac dysfunction [63]. Imaging of the pulmonary vessels and lungs at the time of CMR is also feasible, as part of the evaluation of organ involvement in COVID-19 [64, 65]. Taken together, these capabilities provide a powerful multiparametric approach by which CMR can identify both acute and chronic cardiovascular alterations in patients affected by COVID-19.
CMR findings in COVID-19
Prior reports of the extent and degree of CMR findings in patients with COVID-19 have been heterogeneous. The literature to-date on CMR findings in patients with COVID-19 has recently been reviewed in detail elsewhere, and varying prevalence of CMR abnormalities has been reported (e.g., ranging from 26 to 60% among previously hospitalized patients) [66, 67]. The potential contributing factors include variability in study design, patient selection (e.g. disease severity, presence of pre-existing comorbidities or SARS-CoV2 subtypes), the phase of the COVID-19 illness when the CMR was performed (acute infection, during index hospitalization, or outpatient convalescence), imaging protocols, and diagnostic criteria. The inclusion of appropriate control groups was frequently omitted in early studies of patients with COVID-19 for logistical reasons. However, the inclusion of control groups is important due to the high prevalence of cardiovascular disease in the general population.
COVID-HEART [68, 69] is the largest prospective, observational, longitudinal cohort CMR study to date with 342 confirmed COVID-19 and elevated troponin (COVID + /troponin +) patients across 25 hospital in the United Kingdom. Importantly, the study included two prospective control groups, comprising patients with COVID-19 and normal troponin levels (COVID + /troponin−) and patients without COVID-19 or elevated troponin but matched by age and cardiovascular co-morbidities (COVID-/comorbidity +). COVID + /troponin + patients underwent CMR within 28 days of hospital discharge and at 6-months, serum biomarkers, and genetics, amongst other investigations. Overall, COVID + /troponin + patients had a significantly two-fold higher frequency of LV dysfunction and LGE in early convalescence, compared to contemporary controls; however, the proportion with CMR imaging evidence of myocardial inflammation (6.7%) was low, and scar etiology was diverse, including a newly described pattern of probable micro-infarction (see Fig. 3). Myocardial scar, but not prior COVID-19 infection or troponin, was an independent predictor of major cardiovascular adverse event (MACE) (OR 2.25; 95% CI 1.12–4.57, p = 0.02).
Fig. 3.
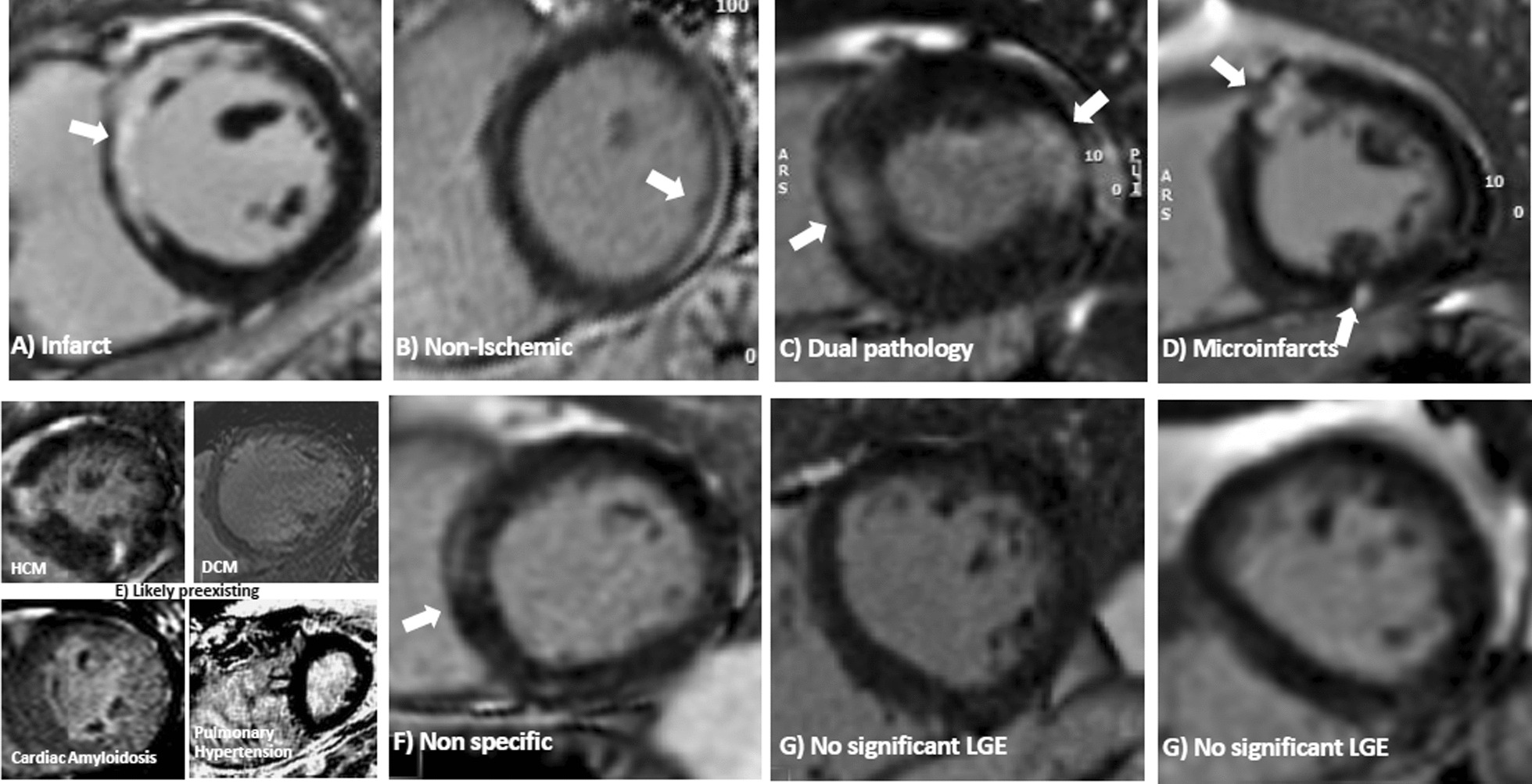
Microinfarction in COVID-19 infection. Patterns of LGE (in brackets the features of each): (A) Infarct (bright, subendocardial, territorial); B Non-ischemic (mid myocardial, less bright, more diffuse); C Dual pathology (both a and b); D Microinfarcts (bright spots—e.g. a gram or so- of LGE often but not exclusively subendocardial and potentially in more than one territory); E Chronic, likely pre-existent disease (only 4 cases total) and non-specific (E1: Dilated cardiomyopathy, E2: amyloidosis, E3) Non-specific (unequivocal LGE that both cannot be considered normal and has insufficient volume to assign with certainty to any other category). F Nonspecific (unequivocal LGE that cannot be considered normal and has insufficient volume to assign with certainty to any other category). G Nonsignificant LGE (minor right ventricle insertion point LGE alone; trabecular LGE alone; or septal perforator LGE alone, which can be considered normal variant) [as originally published in the COVID-HEART Study by Artico et al. [68]
This consensus document focuses on recommendations on CMR imaging and reporting metrics, toward improved standardization, uniform data acquisition and analytic approaches for assessing cardiac involvement in COVID-19. Depending on the individual case, the following CMR findings may be detected in patients who have contracted COVID-19 (see also Fig. 3):
Left ventricular (LV) involvement
LV dilatation and dysfunction, including impaired peak global longitudinal (GLS) and global circumferential strain (GCS) parameters, have been reported in some patients with COVID-19. This may be secondary to myocardial infarction, myocarditis, myocardial inflammation without lymphocytic myocarditis, or global myocardial injury secondary to hypoperfusion in the context of critical illness.
Myocardial infarction
Myocardial infarction may occur, which may be due to coronary plaque rupture in the context of acute illness, coronary occlusion promoted by up-regulation of pro-coagulant signaling pathways in COVID-19, embolization, endotheliitis, or systemic hypoperfusion. Additionally, small, punctate infarcts (microinfarctions) may be seen (Fig. 3).
Myocarditis and myocarditis-like CMR abnormalities
The presence of histopathologically-confirmed myocarditis in relation to SARS-CoV2 infection is thought to be low. CMR physicians are thus cautioned that clinical presentation must be considered prior to diagnoses of myocarditis related to COVID-19. Nevertheless, both typical and atypical clinical presentation may similarly be associated with non-ischemic CMR patterns in the context of COVID-19, including midwall, subepicardial, patchy, or a scattered distribution of LGE. Although a non-ischemic pattern of LGE at the right ventricular (RV) insertion points has been described, the pattern is not specific for COVID-19 [70]. Focal/global elevation of myocardial T1 and/or T2 signals have been widely reported in survivors of COVID-19 [19–21, 23, 25, 65, 70–81]. Although these signals may reflect histopathologic myocarditis (with lymphocytic infiltration and myocyte necrosis), they may also reflect upregulation of extracellular inflammation in the context of a systemic infection like COVID-19. SARS-CoV2 can involve pericytes of the myocardium independent of myocyte involvement (i.e., without myocarditis) [82]. Increased myocardial blood volume (MBV) has also been reported in patients with systemic inflammatory illnesses [83, 84]. These pathophysiologic processes may lead to acute myocardial edema that can increase myocardial T1 and T2 values, which are non-specific, and may or may not be accompanied by LGE findings. Nevertheless, the clinical significance of the observed CMR imaging abnormalities in the context of COVID-19 may be important, particularly their long-term significance in relation to symptomatology and prognosis; more longitudinal studies are required to further the understanding of these observed CMR changes.
Pericardial effusion and pericarditis
A pericardial effusion may be present in association with myo-pericarditis, or in the context of myocardial inflammatory response as part of a systemic illness [85].
Intraventricular thrombi
Both LV and RV thrombi have been described in patients with COVID-19, likely due to the prothrombotic nature of the disease [86].
Myocardial perfusion deficits
Inducible regional stress perfusion deficits have been described in patients who had COVID-19, some of which are thought to reflect occult pre-existing CAD [87, 88]. Global inducible perfusion deficits and LV injury may be caused by systemic hypoperfusion during moderate-severe acute COVID-19 illness. SARS-CoV-2 can directly infect the vascular endothelium, where microthrombosis and endotheliitis can result in endothelial dysfunction, microinfarctions and perfusion deficits. It is unclear at this time whether there may be long-term coronary microcirculatory abnormalities as a direct result of COVID-19.
Right ventricular (RV) involvement
RV dilatation and dysfunction (which may manifest as impaired RV peak GLS and GCS) may occur in patients with acute COVID-19 infection. RV dilation may be an initial compensatory adaptation to increased RV afterload and/or the augmented pulmonary circulatory requirements and parenchymal injury in context of COVID-mediated hypoxia, and may ultimately lead to increased RV wall stress and subsequent fibrosis. Adjunctive imaging of the pulmonary vasculature (via CMR angiography) and measurement of blood pool oxygenation (via T2 or susceptibility mapping approaches, and pulmonary parenchyma) may provide additive diagnostic utility in elucidating mechanism of RV injury.
CMR findings in multisystem inflammatory syndrome in children (MIS-C) related to COVID-19
Children typically have milder acute COVID-19 symptoms, and do not have cardiac manifestations related to acute COVID-19 disease itself when compared to adults [89]. Instead, the cardiac complications seen in children are a result of a systemic delayed hyperimmune response to SARS-COV-2 (MIS-C), presenting a few weeks after the initial infection/exposure. In an initial report of the use of CMR in MIS-C from the United States, children presenting with ventricular dysfunction were studied during the acute phase of illness [90]. Although ventricular function recovered rapidly with treatment prior to discharge, there was evidence of myocardial edema, both on T2-weighted imaging and native T1 and T2 mapping, hyperemia/capillary leak on early gadolinium enhancement, and myocardial injury detected by the presence of subepicardial LGE. These findings were consistent with other reports [91–93], including one which reported ongoing ventricular dysfunction and coronary artery changes in their patients. In MIS-C, CMR detected abnormal strain in patients with global dysfunction in 35%, myocardial edema in 50%, and a subendocardial infarction in 1 patient [93].
The overall risk of myocardial involvement in children is lower than reported in the adult literature, as shown by a recent international multicenter CMR study [94], in which 82% of the sickest MIS-C patients had no evidence of CMR abnormalities. Among these patients, 18% (20/111) met the Lake Louise CMR criteria [94] for acute myocarditis. Studies evaluating early and mid-term outcomes in MIS-C have also shown that myocardial abnormalities on CMR resolve [74, 95] suggesting a favorable long-term prognosis in the pediatric population.
CMR Findings in myocarditis following COVID-19 vaccination
Myocarditis is an established but rare adverse event following administration of mRNA-based COVID-19 vaccines. The risk of myocarditis following mRNA-based COVID-19 vaccination is highest in males between 12 and 40 years of age following administration of the second dose [28, 96–103]. The risk after the third dose is lower than following the second dose [104–106], which could be related to a longer inter-dose interval. When CMR was systematically employed to study the first sizeable pediatric cohort with vaccine-associated myocarditis in the United States, 88% of the patients fulfilled the Lake Louise myocarditis criteria [28]. A high incidence of LGE (88%) was noted in these adolescents with vaccine-associated myocarditis when compared with patients with MIS-C myocarditis [28, 94]. In adults, the incidence appears to be lower, and the extent of imaging abnormalities less severe when compared to myocarditis with acute SARS-CoV-2 infection, including having higher LV ejection fraction and less frequent involvement of the septum in vaccine-associated myocarditis [107]. Typical CMR findings include subepicardial LGE and high T2 at the basal to mid inferolateral wall.
As myocardial injury and inflammation can be present in preserved ventricular function, CMR increases diagnostic sensitivity, and should be considered in patients with suspected myocarditis following vaccination. Although most patients with myocarditis after COVID-19 vaccination have a mild initial clinical course, there are limited long-term follow-up data. In a case series of 13 adults with acute myocarditis following COVID-19 vaccination, intermediate term follow-up CMR at a median of 5 months demonstrated resolution of myocardial edema, normalization of LV function and interval decrease in LGE extent [108]. However, minimal residual LGE without edema has been documented in a proportion of patients at follow-up, likely reflecting myocardial fibrosis [108–110]. Further studies with long-term clinical and imaging follow-up are needed. Further studies are also needed to determine the risk with subsequent vaccine doses and other risk factors including prior history of myocarditis.
Recommendations for CMR protocols for the assessment of patients with COVID-19
As for the use of CMR in evaluating other cardiac conditions, the imaging protocol should be tailored to the specific clinical question(s) and targeted towards the underlying pathophysiology. We provide a summary of common pulse sequences used for conventional and advanced myocardial characterization of these diagnostic targets and their technical considerations. Standardization of CMR protocols is important for assuring the quality, consistency, and completeness in the evaluation of cardiac involvement in patients with COVID-19. An example CMR imaging protocol is provided in Tables 1 and 2. Additional files 1 and 2 provide further details on imaging sequences and acquisition.
Table 1.
Recommended CMR-protocols in adult patients with active/post COVID-19
| Recommended CMR sequences | Answering most clinical questions |
|---|---|
| Survey | Recommended |
|
Cine sequences: Short axis (full biventricular coverage) Long axis (HLA, VLA, LVOT) RV views (RVOT, RV 2Ch, 3Ch) |
Recommended Recommended Optional |
| T2-weighted imaging (e.g. STIR) (myocardium/pericardium) | Optionala |
|
Parametric Mappingc: Native T1-mapping Native T2-mapping Post-contrast T1-mapping (for ECV) |
Recommended Recommended Recommended |
| Acquisition based myocardial strain (Tagging, DENSE, fSENC)b | Optional |
| Stress perfusion (vasodilator)d | Optional |
| Early gadolinium enhancement (EGE)e | Optional |
|
Late gadolinium enhancement (LGE) Short axis full coverage and long axis views RV LGE |
Recommended |
| Real-time cine (to assess for ventricular inter-dependence, if applicable)f | Optional |
| 2D-flow (aorta and pulmonary arteries)g | Optional |
| Angiography (pulmonary vessels)g | Optional |
2Ch two-chamber; 4Ch four-chamber; ECV extracellular volume fraction; HLA horizontal long axis; LV left ventricle/left ventricular; LVOT left ventricular outflow tract; RV right ventricle/right ventricular; RVOT right ventricular outflow tract; VLA vertical long axis
aWhere available, T2-mapping may circumvent some of the technical limitations of conventional T2-weighted imaging (see Additional file 1)
bStrain imaging may be considered if assessment for subclinical myocardial dysfunction is warranted
cFor tissue characterization techniques, whole LV coverage will increase the diagnostic yield of detecting regions of myocardial inflammation, although this will lengthen scan time. At least 3 short-axis slices covering the LV should be obtained, recognizing that incomplete coverage will increase the potential of missing areas of myocardial inflammation
dIn patients with cardiovascular risk factors, chest pain during COVID-19 illness may be an indication of significant underlying CAD; in these cases, it may be reasonable to include stress perfusion into the CMR protocol, to assess for signs of both obstructive CAD and myocarditis, as well as other cardiovascular changes potentially encountered in COVID-19, in a single examination
eEGE may be considered for thrombi detection with extension of short-axis coverage to include the atria for screening of thrombi in the atria and LV/RV
fReal-time cine may be considered if there is suspicion for constrictive physiology
gDedicated pulmonary vascular imaging may be considered if involvement of pulmonary vasculature is suspected
Table 2.
Recommended CMR-protocols in pediatric patients with COVID-19/MIS-C, Vaccine associated myocarditis
| Recommended CMR sequences | Answering most clinical questions |
|---|---|
| Survey | Recommended |
|
Cine sequences: Short axis (full biventricular coverage) Long axis (HLA, VLA, LVOT) RV views (RVOT, RV 2CH, 3Ch) |
Recommended Recommended Optional |
| T2-weighted imaging (e.g. STIR) (myocardium/pericardium) | Recommended |
|
Parametric Mapping: Native T1-mapping Native T2-mapping Post-contrast T1-mapping (for ECV) |
Recommended (if available) Recommended (if available) Recommended (if available) |
| Early gadolinium enhancement (EGE) | Recommended (if available) |
|
Late gadolinium enhancement (LGE) Short axis full coverage and long axis views |
Recommended |
| 2D-flow (aorta and pulmonary arteries) | Optional |
| Coronary artery (3D-navigator) imaging | Optional |
| Acquisition based myocardial strain | Optional |
| Stress perfusion | Optional |
| 4D-flow | Optional |
| Angiography (pulmonary vessels) | Optional |
DENSE displacement encoding with stimulated echoes, ECV extracellular volume fraction, EGE early gadolinium enhancement, HLA horizontal long axis, LGE late gadolinium enhancement, LVOT left ventricular outflow tract, RV right ventricle/right ventricular, RVOT right ventricular outflow tract, STIR short tau inversion recovery, VLA vertical long axis
Recommendations for clinical reporting of CMR findings in COVID-19
A standardized approach to CMR image analysis, the diagnostic criteria and reporting of the CMR findings in COVID-19 is highly recommended to establish a consistent approach to the communication of the findings, including for comparison between centers, and reporting in the literature. This may facilitate a more uniform approach to the study of COVID-19 cardiac involvement using CMR as an advanced and reliable imaging modality.
Image analysis
CMR image quality directly affects diagnostic performance, and must be evaluated before clinical interpretation. The SCMR publishes guidelines on CMR image post-processing and interpretation [111], including the SCMR Mapping Consensus Statement (2017) [45]. A summary of these documents focusing on the myocardial tissue characterization techniques is provided here (Additional file 2).
Diagnostic criteria for detecting myocardial edema or inflammation on CMR in COVID-19
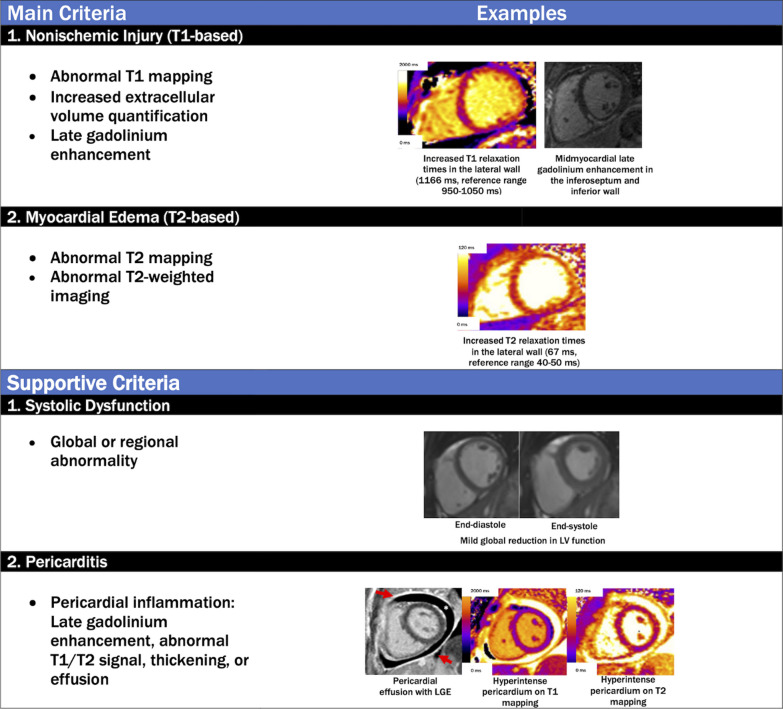
Diagnostic criteria for imaging evidence that may be consistent with non-ischemic myocardial edema and/or inflammation in COVID-19 patients can be applied using a conceptual framework provided by the “Lake Louise Criteria,” [112] most recently updated in 2018 [113] to include parametric mapping techniques (Fig. 4). It is important to note that these criteria were developed before the COVID-19 pandemic and await further validation in patients with COVID-19; nevertheless, this approach is useful for non-invasively detecting myocardial edema as a final common pathway for many forms of myocardial involvement. The specific CMR diagnostic criteria and suggested diagnostic cut-offs are summarized in Fig. 4 and Table 2. At the time when these recommendations were written, there are no pathognomonic patterns on CMR that are specific for “COVID-19 myocarditis”, but this is an evolving area in which continued accumulation of data may shed new light that will further the understanding of this disease process and its impact on the cardiovascular system. The CMR findings relating to the COVID-19 illness reported in the literature thus far are included in this document, and below.
Fig. 4.
Revised Lake Louise Criteria for the diagnosis of nonischemic myocardial inflammation in patients with COVID-19. The specificity of a diagnosis of nonischemic myocardial inflammation is increased in patients meeting at least one T1-based criterion and one T2-based criterion. Supportive criteria include (1) global or regional ventricular systolic dysfunction and (2) pericardial inflammation. Red arrows indicate pericardial LGE, and the asterisk indicates the pericardial effusion
Clinical reporting
For clinical reporting of CMR findings in patients with COVID-19, in addition to a general assessment of cardiovascular structure, function and tissue characterization, particular attention should be paid to the possible findings reported in COVID-19, as discussed earlier and in Table 3. These include: imaging signs of myopericarditis and associated pericardial effusions; small, punctate micro infarctions (Fig. 3), including the RV; associated RV dysfunction and/or dilatation; and intraventricular thrombi (within both the LV and RV). Chest pain in patients with cardiovascular risk factors during COVID-19 illness may be an indication of significant underlying CAD; in these cases, it may be reasonable to include stress perfusion into the CMR protocol, to assess for signs of both obstructive CAD and myocarditis, as well as other cardiovascular changes potentially encountered in COVID-19, in a single examination.
Table 3.
Evaluation of CMR images and parameters for reporting cardiac findings in COVID-19
| CMR parameters for reporting cardiovascular findings in COVID-19 | |
|---|---|
| Ventricular structure and function |
• Presence/location of global or regional LV and RV systolic dysfunction • LV & RV end-diastolic volume (LVEDV, RVEDV) • LV & RV end-systolic volume (LVESV, RVESV) • LV & RV ejection fraction (LVEF, RVEF) • LV & RV stroke volume (SV) and stroke volume index (SVI) • LV wall thicknesses • LV mass and mass index (LVMI) • Signs of RV volume or pressure overload |
| T2-weighted imaging |
• Visual analysis: presence, extent and localization of visually apparent global or regional edema on T2-weighted imaging • Semi-quantitative analysis: global T2 SI ratio ≥ 2.0a or regionalb high T2 SI |
| T1/T2 mapping |
Focal/global elevation of myocardial T1 and/or T2 signals, their location and extent, which may or may not be accompanied by LGE findings or functional abnormalities • Pulse sequence (e.g. MOLLI, ShMOLLI, and relevant method version) • Field strength of CMR system • Reference normal range (mean ± SD, 2SD range) • Use only good quality parametric maps for clinical reporting • Number of slices and orientation (e.g. 3 SAx slices) • Global T1/T2 values • Segmental T1/T2 values and range may be helpful for spatial characterization • Very small regions of interest (< 20 pixels) should be avoided • The Z-score (number of SDs by which the patient findings differs from the local normal mean can help convey the degree of abnormality). A T1 or T2 value ≥ 2SD above the normal mean is generally accepted to be abnormally elevated • Clinical interpretation of whether the findings may be consistent with myocardial edema, and/or a differential diagnosis of the imaging findings within the clinical context of the referral |
| Edema |
• Acute infarction: abnormally elevated T2 (T2-weighted or T2-mapping) in areas of infarction on LGE would support acute myocardial infarction • Non-ischemic myocardial inflammation/edema: the Updated Lake Louise Criteria (2018) recommends that one T2-based criteria (T2-weighted or T2-mapping) plus one T1-based criteria (non-ischemic LGE pattern, elevated native T1-mapping or ECVc) would support imaging criteria for probable non-ischemic myocardial inflammation/edema |
| Necrosis and fibrosis |
• Presence, extent and localization of visually apparent lesions on LGE imaging • Myocardial infarctions, and if present, the location, transmurality and extent, possible coronary territory • In patients with COVID-19, small, punctate infarcts may be seen, which should be verified on perpendicular views • RV infarctions should be actively assessed for and reported • Any non-ischemic type LGE, including “myocarditis-like” type LGE patterns, such as midwall and subepicardial patterns, “scattered” or “patchy” type LGE, their extent and distribution • LGE at the RV insertion point have been described, although may have similar frequencies in individuals without COVID-19 |
| Pericardium |
• Presence, extent and localization of effusion in cine images. In general, a pericardial width > 4 mm should be regarded as abnormal • Pericardial thickness (normal ≤ 2 mm) • Signal increase in LGE, T2-weighted, T2-mapping or T1-mapping • Any hemodynamic effects or imaging evidence of constriction (such as right atrial or RV free wall collapse, ventricular inter-dependence during free-breathing cine imaging) |
| Thrombus |
• Presence or absence of LV and RV intraventricular thrombi • Presence of thrombus in the main pulmonary artery or main branches and other cardiac chambers, if visible |
| 2D Flow of aorta and pulmonary arteries |
• Forward, backward and net flow in the ascending aorta and main pulmonary artery • Can be used to calculate mitral and tricuspid regurgitant volume and fraction along with LV & RV stroke volumes if needed • Evaluation of pulmonary emboli and lung opacities |
| Perfusion deficits |
• Regional perfusion deficits may suggest underlying obstructive CAD • Global inducible perfusion deficits (based on quantitative analysis of myocardial blood flow) may result from systemic hypoperfusion, microvascular dysfunction from microthrombosis or endotheliitis |
aPublished or local normal values should be used; degree of LV coverage should be reported
b“Regional” refers to an area of at least 10 contiguous pixels
cNative T1 and ECV are also sensitive to, although not specific for, acute myocardial inflammation and edema, because these parameters are also sensitive to detecting chronic changes, such as in areas of focal and diffuse myocardial fibrosis
CAD coronary artery disease, SI signal intensity. Other abbreviations as in Table 1
Conclusion
Existing published work documents a heterogenous spectrum of COVID-19 related cardiovascular manifestations depending on COVID-19 disease severity and individual patient characteristics. Multiparametric CMR allows a safe and non-invasive assessment of cardiac structure, function and, importantly, myocardial tissue characterization in COVID-19 patients. As such, CMR permits elucidation of specific diagnoses, including myocardial edema, myocardial infarction and microinfarctions, myo-pericarditis, ischemia, fibrosis, intracavitary thrombi, and non-ischemic cardiac dysfunction. Furthermore, the high reproducibility of CMR permits reliable longitudinal tracking of any observed cardiovascular changes, response to potential therapy, and association with clinical outcomes. This SCMR consensus document provides guidance on the acquisition, interpretation, and analysis of CMR images in the context of COVID-19 infection, to improve standardization of methods globally. Further research is needed to determine the biological basis of the CMR abnormalities that are observed, to enable greater understanding of underlying disease mechanisms, as well as their clinical significance with regards to function, quality of life, and long-term cardiovascular risk.
Supplementary Information
Additional file 1.CMR Imaging Protocol—details on imaging sequences and acquisition.
Additional file 2. Clinical Reporting of CMR findings in COVID-19—Image analysis.
Acknowledgements
None.
Abbreviations
- bSSFP
Balanced steady-state free precession
- CAD
Coronary artery disease
- CMR
Cardiovascular magnetic resonance
- COVID-19
Coronavirus disease 2019
- ECG
Electrocardiogram
- ECV
Extracellular volume fraction
- FoV
Field of view
- GCS
Global circumferential strain
- GLS
Global longitudinal strain
- LGE
Late gadolinium enhancement
- LV
Left ventricle/left ventricular
- MACE
Major adverse cardiovascular events
- MBV
Myocardial blood volume
- MIS-C
Multisystem inflammatory syndrome in children
- MOCO
Motion correction
- ROI
Region of interest
- RV
Right ventricle/right ventricular
- SCMR
Society for Cardiovascular Magnetic Resonance
- SD
Standard deviation
- SI
Signal intensity
- T1
Spin–lattice or longitudinal relaxation time
- T2
Spin–spin or transverse relaxation time
- TTE
Transthoracic echocardiography
Author contributions
All authors were major contributors in writing this manuscript. All authors read and approved the final manuscript.
Authors' information
VMF: British Heart Foundation Associate Professor of Cardiovascular Medicine, Deputy Director of the Oxford Centre for Clinical Magnetic Resonance Research (OCMR), Honorary Consultant Cardiologist, University of Oxford, United Kingdom. SP: British Heart Foundation Professor of Cardiovascular Imaging, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, LS2 9JT, United Kingdom. TW:Assistant Professor of Medicine, Director-UPMC Cardiovascular Magnetic Resonance Center, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA. QT: Assistant Professor of Medical Imaging, Department of Imaging Physics, Delft University of Technology, Delft, the Netherlands. ZRE: NIHR Academic Clinical Lecturer in Cardiology (MD, PhD), Queen Mary University of London, Barts Health NHS Trust, London, UK. SJ: Associate Professor of Pediatrics and Radiology, Division of Pediatric Cardiology, Director of Pediatric Cardiac MRI, New York Medical College, Maria Fareri Children’s Hospital at Westchester Medical Center, New York, USA. YH: Professor of Medicine, Director of Cardiac Imaging, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA. JW: Professor of Medicine, Director of Cardiac Imaging Program, Weill Cornell Medical College, New York, New York, USA. VO: Assistant Professor of Cardiac Radiology, Department of Cardiovascular Radiology and Endovascular Interventions, All India Institute of Medical Sciences, New Delhi, India. DAB: Professor, Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA. KH: Associate Professor of Radiology, Director of Cardiac Imaging Research, Toronto General Hospital, University of Toronto, Canada. MKV: Assistant Professor of Medicine, Division of Cardiovascular Medicine, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA. NN: Professor of Medicine, University of Cape Town, Cape Town, South Africa. JSM: Professsor of Cardiology, Director Outpatient research Department ECRC, Charité University Medicine Berlin at Humboldt University and Head Noninvasive Imaging Helios Clinics, Germany. JK: Associate Professor of Medicine and Medicine in Radiology, Co-Director Cardiac MRI Program, Associate Director Adult Echocardiography Program, Weill Cornell Medical College, New York, New York, USA; Chair SCMR COVID-19 Task Force.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
VMF: VMF acknowledges support from the British Heart Foundation (BHF, CH/16/1/32013), the Oxford BHF Centre of Research Excellence, the National Institute for Health Research Oxford Biomedical Research Centre at the Oxford University Hospitals NHS Foundation Trust. VMF has authorship rights for patent WO/2021/044153 “Method and apparatus for enhancing medical images”, WO/2020/161481 “Method and Apparatus for quality prediction” and WO/2020/234570 “A method for identity validation and quality assurance of quantitative magnetic resonance imaging protocols.” TW: TW declares he has no competing interest to declare. QT: QT declares that she has no competing interests. ZRE: ZR-E acknowledges the National Institute for Health Research (NIHR) Integrated Academic Training program which supports her Academic Clinical Lectureship post and was also supported by British Heart Foundation Clinical Research Training Fellowship No. FS/17/81/33318. SJ: SJ acknowledges support from the U.S. Food and Drug Administration, COVID-19 Vaccine-associated Myocarditis (FDA-21F19004-T0006, FDA-75F40122C00148). YH: YH acknowledges funding from NIH R01 HL148103. VO: VO declares that she has no competing interests. DAB: DAB declares no competing interests. KH: KH acknowledges honoraria from Sanofi Genzyme, Amicus and Medscape. JW:JW acknowledges funding from NIH R01 HL159055. SP: SP is funded by a British Heart Foundation Personal Chair (CH/16/2/32089). NN: NN declares he has no competing interest to declare. NN acknowledges support from the South African Medical Research Council, National Research Foundation and the Lily and Ernst Hausmann Trust. JK: JK acknowledges funding from NIH R01 HL159055, NIH K23 HL140092.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107:373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 2.Kong KA, Jung S, Yu M, Park J, Kang IS. Association between cardiovascular risk factors and the severity of coronavirus disease 2019: nationwide Epidemiological Study in Korea. Front Cardiovasc Med. 2021;8:732518. doi: 10.3389/fcvm.2021.732518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, Danilov T, Kukar N, Shaban N, Kini A, Camaj A, Bienstock SW, Rashed ER, Rahman K, Oates CP, Buckley S, Elbaum LS, Arkonac D, Fiter R, Singh R, Li E, Razuk V, Robinson SE, Miller M, Bier B, Donghi V, Pisaniello M, Mantovani R, Pinto G, Rota I, Baggio S, Chiarito M, Fazzari F, Cusmano I, Curzi M, Ro R, Malick W, Kamran M, Kohli-Seth R, Bassily-Marcus AM, Neibart E, Serrao G, Perk G, Mancini D, Reddy VY, Pinney SP, Dangas G, Blasi F, Sharma SK, Mehran R, Condorelli G, Stone GW, Fuster V, Lerakis S, Goldman ME. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V, Mount Sinai CIC. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkert FR, Niederreiter L, Dichtl W, Mayr A, Virgolini I, Klauser A, Weiss G, Bellmann-Weiler R. Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: the mystery of the phantom embolus or local endothelitis. Eur Heart J Case Rep. 2021;5:ytaa521. doi: 10.1093/ehjcr/ytaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meizinger C, Klugherz B. Focal ST-segment elevation without coronary occlusion: myocardial infarction with no obstructive coronary atherosclerosis associated with COVID-19—a case report. Eur Heart J Case Rep. 2021;5:532. doi: 10.1093/ehjcr/ytaa532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedeschi D, Rizzi A, Biscaglia S, Tumscitz C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID-19. Catheter Cardiovasc Interv. 2021;97:272–277. doi: 10.1002/ccd.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorecka M, Thirunavukarasu S, Levelt E, Greenwood JP. Multiple etiologies to myocardial injury in COVID-19. JACC Case Rep. 2021;3:971–972. doi: 10.1016/j.jaccas.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Osch D, Asselbergs FW, Teske AJ. Takotsubo cardiomyopathy in COVID-19: a case report. Haemodynamic and therapeutic considerations. Eur Heart J Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong N, Cai J, Zhou Y, Liu J, Li F. End-stage heart failure with COVID-19: strong evidence of myocardial injury by 2019-nCoV. JACC Heart Fail. 2020;8:515–517. doi: 10.1016/j.jchf.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, Delacourt C, Iriart X, Ovaert C, Bader-Meunier B, Kone-Paut I and Levy-Bruhl D. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25. [DOI] [PMC free article] [PubMed]
- 16.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H, New York S, Centers for Disease C and Prevention Multisystem Inflammatory Syndrome in Children Investigation T Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wacker J, Malaspinas I, Aggoun Y, Bordessoule A, Vallee JP, Beghetti M. Coronary artery dilatation in a child with hyperinflammatory syndrome with SARS-CoV-2-positive serology. Eur Heart J. 2020;41:3103. doi: 10.1093/eurheartj/ehaa536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 19.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng MY, Ferreira VM, Leung ST, Yin Lee JC, Ho-Tung Fong A, To Liu RW, Man Chan JW, Wu AKL, Lung KC, Crean AM, Fan-Ngai Hung I, Siu CW. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2476–2478. doi: 10.1016/j.jcmg.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, Leith D, Shetye A, Ariff B, Bell R, Captur G, Coleman M, Goldring J, Gopalan D, Heightman M, Hillman T, Howard L, Jacobs M, Jeetley PS, Kanagaratnam P, Kon OM, Lamb LE, Manisty CH, Mathurdas P, Mayet J, Negus R, Patel N, Pierce I, Russell G, Wolff A, Xue H, Kellman P, Moon JC, Treibel TA, Cole GD, Fontana M. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raisi-Estabragh Z, McCracken C, Cooper J, Fung K, Paiva JM, Khanji MY, Rauseo E, Biasiolli L, Raman B, Piechnik SK, Neubauer S, Munroe PB, Harvey NC, Petersen SE. Adverse cardiovascular magnetic resonance phenotypes are associated with greater likelihood of incident coronavirus disease 2019: findings from the UK Biobank. Aging Clin Exp Res. 2021;33:1133–1144. doi: 10.1007/s40520-021-01808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, Oliveira MF, Adam RD, Aziminia N, Menacho K, Chacko L, Brown JT, Patel RK, Shiwani H, Bhuva A, Augusto JB, Andiapen M, McKnight A, Noursadeghi M, Pierce I, Evain T, Captur G, Davies RH, Greenwood JP, Fontana M, Kellman P, Schelbert EB, Treibel TA, Manisty C, Moon JC. Prospective case–control study of cardiovascular abnormalities 6 months following mild COVID-19 in Healthcare Workers. JACC Cardiovasc Imaging. 2021;14:2155–2166. doi: 10.1016/j.jcmg.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT., Jr Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosner CM, Atkins M, Saeed IM, de Lemos JA, Khera A, Maghsoudi A, Min J, Tehrani BN, O'Connor CM, deFilippi CR. Patients with myocarditis associated with COVID-19 vaccination. J Am Coll Cardiol. 2022;79:1317–1319. doi: 10.1016/j.jacc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SA, Buddhe S, Misra N, Ramachandran P, Gaur L, Eshtehardi P, Anwar S, Kaushik N, Han F, Chaudhuri NR and Grosse-Wortmann L. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148. [DOI] [PubMed]
- 29.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez Tijmes F, Thavendiranathan P, Udell JA, Seidman MA, Hanneman K. Cardiac MRI assessment of nonischemic myocardial inflammation: state of the art review and update on myocarditis associated with COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3:e210252. doi: 10.1148/ryct.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen BD, Wong TC, Bucciarelli-Ducci C, Bryant J, Chen T, Dall'Armellina E, Finn JP, Fontana M, Francone M, Han Y, Hays AG, Jacob R, Lawton C, Manning WJ, Ordovas K, Parwani P, Plein S, Powell AJ, Raman SV, Salerno M, Carr JC. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for re-activation of cardiovascular magnetic resonance practice after peak phase of the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22:58. doi: 10.1186/s12968-020-00654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, Elliott MD, Ferrari VA, Friedrich MG, Lawton C, Manning WJ, Ordovas K, Plein S, Powell AJ, Raman SV, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22:26. doi: 10.1186/s12968-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelle S, Bucciarelli-Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S, Raimondi F, Weinsaft JW, Wong TC, Carr J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson. 2020;22:61. doi: 10.1186/s12968-020-00656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uretsky S, Aggarwal N, van Heeswijk RB, Rajpal S, Rowin E, Taylor MD, Verjans JW, Wokhlu A, Markl M, Raman SV, Shah DJ. Standards for writing Society for Cardiovascular Magnetic Resonance (SCMR) endorsed guidelines, expert consensus, and recommendations: a report of the publications committee. J Cardiovasc Magn Reson. 2021;23:129. doi: 10.1186/s12968-021-00801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Guagliumi G, Virmani R, Finn AV. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Writing C, Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, Baggish AL, Bozkurt B, Cornwell WK, 3rd, Harmon KG, Kim JH, Lala A, Levine BD, Martinez MW, Onuma O, Phelan D, Puntmann VO, Rajpal S, Taub PR, Verma AK. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, Faggi L, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Virmani R, Finn AV. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 39.Maiese A, Frati P, Del Duca F, Santoro P, Manetti AC, La Russa R, Di Paolo M, Turillazzi E and Fineschi V. Myocardial pathology in COVID-19-associated cardiac injury: a systematic review. Diagnostics (Basel). 2021;11. [DOI] [PMC free article] [PubMed]
- 40.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE, Popescu BA, Donal E, Cosyns B, Edvardsen T, Mills NL, Haugaa K. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perreto G, Busnardo E, Ferro P, Palmisano P, Vignale D, Espoosito A, De Luca G, Campochiaro C, Sartorelli S, De Gaspari M, Rizzo S, Dagna L, Basso C, Gianolli L, Della Bella P and Sala S. Applications of FDG-PET scan in arrhythmic myocarditis. JACC: Cardiovasc Imaging. 2022. [DOI] [PubMed]
- 43.Hanneman K, Houbois C, Schoffel A, Gustafson D, Iwanochko RM, Wintersperger BJ, Chan R, Fish JE, Howe KL, Thavendiranathan P. Combined cardiac fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging assessment of myocardial injury in patients who recently recovered from COVID-19. JAMA Cardiol. 2022;7:298–308. doi: 10.1001/jamacardio.2021.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P, Parker M, Judd RM, Kim RJ. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–619. doi: 10.1161/CIRCIMAGING.111.964965. [DOI] [PubMed] [Google Scholar]
- 45.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: part I. J Cardiovasc Magn Reson. 2010;12:71. doi: 10.1186/1532-429X-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36:1985–1991. doi: 10.1016/S0735-1097(00)00958-X. [DOI] [PubMed] [Google Scholar]
- 48.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.CIR.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 49.Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 50.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 51.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.CIR.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 53.Aquaro GD, GhebruHabtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, PerazzoloMarra M, Barison A, Di Bella G. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74:2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 54.Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991;68:1089–1091. doi: 10.1016/0002-9149(91)90501-B. [DOI] [PubMed] [Google Scholar]
- 55.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 56.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 58.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 59.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre-Mongeon F, Blankstein R, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013;6:955–962. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Patel AR, Salerno M, Kwong RY, Singh A, Heydari B, Kramer CM. Stress cardiac magnetic resonance myocardial perfusion imaging: JACC review topic of the week. J Am Coll Cardiol. 2021;78:1655–1668. doi: 10.1016/j.jacc.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotecha T, Martinez-Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, Patel D, Coghlan G, Moon J, Plein S, Lockie T, Rakhit R, Patel N, Xue H, Kellman P, Fontana M. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12:1958–1969. doi: 10.1016/j.jcmg.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seraphim A, Knott KD, Menacho K, Augusto JB, Davies R, Pierce I, Joy G, Bhuva AN, Xue H, Treibel TA, Cooper JA, Petersen SE, Fontana M, Hughes AD, Moon JC, Manisty C, Kellman P. Prognostic value of pulmonary transit time and pulmonary blood volume estimation using myocardial perfusion CMR. JACC Cardiovasc Imaging. 2021;14:2107–2119. doi: 10.1016/j.jcmg.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassar MP, Tunnicliffe EM, Petousi N, Lewandowski AJ, Xie C, Mahmod M, Samat AHA, Evans RA, Brightling CE, Ho LP, Piechnik SK, Talbot NP, Holdsworth D, Ferreira VM, Neubauer S, Raman B. Symptom persistence despite improvement in cardiopulmonary health-insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021;41:101159. doi: 10.1016/j.eclinm.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, Mozes FE, Lewandowski AJ, Ohuma EO, Holdsworth D, Lamlum H, Woodman MJ, Krasopoulos C, Mills R, McConnell FAK, Wang C, Arthofer C, Lange FJ, Andersson J, Jenkinson M, Antoniades C, Channon KM, Shanmuganathan M, Ferreira VM, Piechnik SK, Klenerman P, Brightling C, Talbot NP, Petousi N, Rahman NM, Ho LP, Saunders K, Geddes JR, Harrison PJ, Pattinson K, Rowland MJ, Angus BJ, Gleeson F, Pavlides M, Koychev I, Miller KL, Mackay C, Jezzard P, Smith SM, Neubauer S. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen SE, Friedrich MG, Leiner T, Elias MD, Ferreira VM, Fenski M, Flamm SD, Fogel M, Garg R, Halushka MK, Hays AG, Kawel-Boehm N, Kramer CM, Nagel E, Ntusi NAB, Ostenfeld E, Pennell DJ, Raisi-Estabragh Z, Reeder SB, Rochitte CE, Starekova J, Sucha D, Tao Q, Schulz-Menger J and Bluemke DA. Cardiovascular magnetic resonance for patients with COVID-19. JACC Cardiovasc Imaging. 2021. [DOI] [PMC free article] [PubMed]
- 67.Raman B, Bluemke DA, Luscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022. [DOI] [PMC free article] [PubMed]
- 68.Artico J, Shiwani H, Moon J, Gorecka M, McCann G, Roditi G, Morrow A, Mangion K, Lukaschuk E, Shanmuganathan M, Miller C, Chiribiri A, Prasad S, Adam R, Singh T, Bucciarelli-Ducci C, Dawson D, Knight D, Fontana M, Manisty C, Treibel T, Levelt E, Arnold R, Macfarlane P, Young R, McConnachie A, Neubauer S, Piechnik S, Davies R, Ferreira V, Dweck M, Berry C, Greenwood J. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter, observational study. Circulation. 2022. [DOI] [PMC free article] [PubMed]
- 69.Gorecka M, McCann GP, Berry C, Ferreira VM, Moon JC, Miller CA, Chiribiri A, Prasad S, Dweck MR, Bucciarelli-Ducci C, Dawson D, Fontana M, Macfarlane PW, McConnachie A, Neubauer S, Greenwood JP, Investigators C-H Demographic, multi-morbidity and genetic impact on myocardial involvement and its recovery from COVID-19: protocol design of COVID-HEART—a UK, multicentre, observational study. J Cardiovasc Magn Reson. 2021;23:77. doi: 10.1186/s12968-021-00752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, Li H, Xu L. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, Liu B, Yu Y, Han Y. Elevated extracellular volume fraction and reduced global longitudinal strains in participants recovered from COVID-19 without clinical cardiac findings. Radiology. 2021;299:E230–E240. doi: 10.1148/radiol.2021203998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, Wolff A, Moon JC, Xue H, Kellman P, Patel N, Fontana M. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Webster G, Patel AB, Carr MR, Rigsby CK, Rychlik K, Rowley AH, Robinson JD. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. 2021;23:86. doi: 10.1186/s12968-021-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urmeneta Ulloa J, Martinez de Vega V, Salvador Montanes O, Alvarez Vazquez A, Sanchez-Enrique C, Hernandez Jimenez S, Sancho Garcia FD, Lopez Ruiz L, Recio Rodriguez M, Pizarro G, Carnevali Ruiz D, Angel Cabrera J. Cardiac magnetic resonance in recovering COVID-19 patients. Feature tracking and mapping analysis to detect persistent myocardial involvement. Int J Cardiol Heart Vasc. 2021;36:100854. doi: 10.1016/j.ijcha.2021.100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szabo L, Juhasz V, Dohy Z, Fogarasi C, Kovacs A, Lakatos BK, Kiss O, Sydo N, Csulak E, Suhai FI, Hirschberg K, Becker D, Merkely B, Vago H. Is cardiac involvement prevalent in highly trained athletes after SARS-CoV-2 infection? A cardiac magnetic resonance study using sex-matched and age-matched controls. Br J Sports Med. 2021;56:553. doi: 10.1136/bjsports-2021-104576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galea N, Marchitelli L, Pambianchi G, Catapano F, Cundari G, Birtolo LI, Maestrini V, Mancone M, Fedele F, Catalano C, Francone M. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a Cardiovascular Magnetic Resonance study. J Cardiovasc Magn Reson. 2021;23:68. doi: 10.1186/s12968-021-00764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, Seetharam K, Riveros D, Beto RJ, Balla S, Monseau AJ, Sengupta PP. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14:541–555. doi: 10.1016/j.jcmg.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breitbart P, Koch A, Schmidt M, Magedanz A, Lindhoff-Last E, Voigtlander T, Schmermund A, Mehta RH, Eggebrecht H. Clinical and cardiac magnetic resonance findings in post-COVID patients referred for suspected myocarditis. Clin Res Cardiol. 2021;110:1832–1840. doi: 10.1007/s00392-021-01929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustin A, Sridi S, Gravinay P, Legghe B, Gosse P, Ouattara A, Roze H, Coste P, Gerbaud E, Desclaux A, Boyer A, Prevel R, Gruson D, Bonnet F, Issa N, Montaudon M, Laurent F, Stuber M, Camou F, Cochet H. High-resolution Free-breathing late gadolinium enhancement Cardiovascular magnetic resonance to diagnose myocardial injuries following COVID-19 infection. Eur J Radiol. 2021;144:109960. doi: 10.1016/j.ejrad.2021.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6:116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, Blankenberg S, Püschel K, Westermann D. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siddiqui Y, Crouser ED, Raman SV. Nonischemic myocardial changes detected by cardiac magnetic resonance in critical care patients with sepsis. Am J Respir Crit Care Med. 2013;188:1037–1039. doi: 10.1164/rccm.201304-0744LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 85.Furqan MM, Verma BR, Cremer PC, Imazio M, Klein AL. Pericardial diseases in COVID19: a contemporary review. Curr Cardiol Rep. 2021;23:90. doi: 10.1007/s11886-021-01519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Philip AM, George LJ, John KJ, George AA, Nayar J, Sahu KK, Selvaraj V, Lal A, Mishra AK. A review of the presentation and outcome of left ventricular thrombus in coronavirus disease 2019 infection. J Clin Transl Res. 2021;7:797–808. [PMC free article] [PubMed] [Google Scholar]
- 87.Drakos S, Chatzantonis G, Bietenbeck M, Evers G, Schulze AB, Mohr M, Fonfara H, Meier C, Yilmaz A. A cardiovascular magnetic resonance imaging-based pilot study to assess coronary microvascular disease in COVID-19 patients. Sci Rep. 2021;11:15667. doi: 10.1038/s41598-021-95277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornton GD, Shetye A, Knight DS, Knott K, Artico J, Kurdi H, Yousef S, Antonakaki D, Razvi Y, Chacko L, Brown J, Patel R, Vimalesvaran K, Seraphim A, Davies R, Xue H, Kotecha T, Bell R, Manisty C, Cole GD, Moon JC, Kellman P, Fontana M, Treibel TA. Myocardial perfusion imaging after severe COVID-19 infection demonstrates regional ischemia rather than global blood flow reduction. Front Cardiovasc Med. 2021;8:764599. doi: 10.3389/fcvm.2021.764599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 90.Jain S, Nolan S, Biller R, Pinto A, Fuisz A, Gewitz M. Cardiovascular magnetic resonance in myocarditis related to multisystem inflammatory syndrome in children associated with COVID-19. Congenital Cardiol Today. 2020;18:20–22. [Google Scholar]
- 91.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, DucoulePointe H. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology. 2020;297:E283–E288. doi: 10.1148/radiol.2020202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sirico D, Basso A, Reffo E, Cavaliere A, Castaldi B, Sabatino J, Meneghel A, Martini G, Da Dalt L, Zulian F, Di Salvo G. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 93.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, Cleary A, Stewart K, Adhvaryu K, Savis A, Kabir SR, Uy MP, Heard H, Peacock K, Miller O. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22:896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aeschlimann FA, Misra N, Hussein T, Panaioli E, Soslow JH, Crum K, Steele JM, Huber S, Marcora S, Brambilla P, Jain S, Navallas M, Giuli V, Rucker B, Angst F, Patel MD, Azarine A, Caro-Dominguez P, Cavaliere A, Di Salvo G, Ferroni F, Agnoletti G, Bonnemains L, Martins D, Boddaert N, Wong J, Pushparajah K, Raimondi F. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J Cardiovasc Magn Reson. 2021;23:140. doi: 10.1186/s12968-021-00841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bermejo IA, Bautista-Rodriguez C, Fraisse A, Voges I, Gatehouse P, Kang H, Piccinelli E, Rowlinson G, Lane M, Semple T, Moscatelli S, Dwornik M, Lota A, Di Salvo G, Wage R, Prasad SK, Mohiaddin R, Pennell DJ, Thavendiranathan P, Krupickova S. Short-term sequelae of multisystem inflammatory syndrome in children assessed by CMR. JACC Cardiovasc Imaging. 2021;14:1666–1667. doi: 10.1016/j.jcmg.2021.01.035. [DOI] [PubMed] [Google Scholar]
- 96.Hajjo R, Sabbah DA, Bardaweel SK and Tropsha A. Shedding the light on post-vaccine myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients. Vaccines (Basel). 2021;9. [DOI] [PMC free article] [PubMed]
- 97.Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, Lee MS. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, Edwards K, Soslow JH, Dendy JM, Schlaudecker E, Lang SM, Barnett ED, Ruberg FL, Smith MJ, Campbell MJ, Lopes RD, Sperling LS, Baumblatt JA, Thompson DL, Marquez PL, Strid P, Woo J, Pugsley R, Reagan-Steiner S, DeStefano F, Shimabukuro TT. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, Channon KM, Mills NL, Sheikh A, Hippisley-Cox J. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, Channon KM, Mills NL, Sheikh A, Hippisley-Cox J. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation. 2022;146:743–754. doi: 10.1161/CIRCULATIONAHA.122.059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naveed Z, Li J, Wilton J, Spencer M, Naus M, Velasquez Garcia HA, Kwong JC, Rose C, Otterstatter M, Janjua NZ, Canadian Immunization Research Network Provincial Collaborative Network I Comparative risk of myocarditis/pericarditis following second doses of BNT162b2 and mRNA-1273 coronavirus vaccines. J Am Coll Cardiol. 2022;80:1900–1908. doi: 10.1016/j.jacc.2022.08.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friedensohn L, Levin D, Fadlon-Derai M, Gershovitz L, Fink N, Glassberg E, Gordon B. Myocarditis following a third BNT162b2 vaccination dose in military recruits in Israel. JAMA. 2022;327:1611. doi: 10.1001/jama.2022.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ryan Ruiyang Ling KR, Felicia Liying Tan, Bee Choo Tai, Jyoti Somani, Dale Fisher, Graeme MacLaren. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med 2022. 2022. [DOI] [PMC free article] [PubMed]
- 106.Ling RR, Ramanathan K, Tan FL, Tai BC, Somani J, Fisher D, MacLaren G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022. [DOI] [PMC free article] [PubMed]
- 107.Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, Hong R, Hanneman K. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology. 2022;304:554. doi: 10.1148/radiol.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fronza M, Thavendiranathan P, Karur GR, Abdel-Qadir H, Udell JA, Wald RM, Hanneman K. Cardiac MRI and clinical follow-up in COVID-19 vaccine-associated myocarditis. Radiology. 2022;304:220802. doi: 10.1148/radiol.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schauer J, Buddhe S, Gulhane A, Sagiv E, Studer M, Colyer J, Chikkabyrappa SM, Law Y, Portman MA. Persistent cardiac MRI findings in a cohort of adolescents with post COVID-19 mRNA vaccine myopericarditis. J Pediatr. 2022. [DOI] [PMC free article] [PubMed]
- 110.JL C, KE S, M G. Cardiac magnetic resonance imaging midterm follow up of COVID-19 vaccine-associated myocarditis. JACC Cardiovasc Imaging. 2022. [DOI] [PMC free article] [PubMed]
- 111.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 114.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22:17. doi: 10.1186/s12968-020-00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferreira VM, Wijesurendra RS, Liu A, Greiser A, Casadei B, Robson MD, Neubauer S, Piechnik SK. Systolic ShMOLLI myocardial T1-mapping for improved robustness to partial-volume effects and applications in tachyarrhythmias. J Cardiovasc Magn Reson. 2015;17:77. doi: 10.1186/s12968-015-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Q, Werys K, Popescu IA, Biasiolli L, Ntusi NAB, Desai M, Zimmerman SL, Shah DJ, Autry K, Kim B, Kim HW, Jenista ER, Huber S, White JA, McCann GP, Mohiddin SA, Boubertakh R, Chiribiri A, Newby D, Prasad S, Radjenovic A, Dawson D, Schulz-Menger J, Mahrholdt H, Carbone I, Rimoldi O, Colagrande S, Calistri L, Michels M, Hofman MBM, Anderson L, Broberg C, Andrew F, Sanz J, Bucciarelli-Ducci C, Chow K, Higgins D, Broadbent DA, Semple S, Hafyane T, Wormleighton J, Salerno M, He T, Plein S, Kwong RY, Jerosch-Herold M, Kramer CM, Neubauer S, Ferreira VM, Piechnik SK. Quality assurance of quantitative cardiac T1-mapping in multicenter clinical trials—a T1 phantom program from the hypertrophic cardiomyopathy registry (HCMR) study. Int J Cardiol. 2021;330:251–258. doi: 10.1016/j.ijcard.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Popescu IA, Werys K, Zhang Q, Puchta H, Hann E, Lukaschuk E, Ferreira VM, Piechnik SK. Standardization of T1-mapping in cardiovascular magnetic resonance using clustered structuring for benchmarking normal ranges. Int J Cardiol. 2021;326:220–225. doi: 10.1016/j.ijcard.2020.10.041. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira VM, Piechnik SK. CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ J. 2020;50:658–676. doi: 10.4070/kcj.2020.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carapella V, Puchta H, Lukaschuk E, Marini C, Werys K, Neubauer S, Ferreira VM, Piechnik SK. Standardized image post-processing of cardiovascular magnetic resonance T1-mapping reduces variability and improves accuracy and consistency in myocardial tissue characterization. Int J Cardiol. 2020;298:128–134. doi: 10.1016/j.ijcard.2019.08.058. [DOI] [PubMed] [Google Scholar]
- 121.Hann E, Popescu IA, Zhang Q, Gonzales RA, Barutcu A, Neubauer S, Ferreira VM, Piechnik SK. Deep neural network ensemble for on-the-fly quality control-driven segmentation of cardiac MRI T1 mapping. Med Image Anal. 2021;71:102029. doi: 10.1016/j.media.2021.102029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Q, Hann E, Werys K, Wu C, Popescu I, Lukaschuk E, Barutcu A, Ferreira VM, Piechnik SK. Deep learning with attention supervision for automated motion artefact detection in quality control of cardiac T1-mapping. Artif Intell Med. 2020;110:101955. doi: 10.1016/j.artmed.2020.101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puyol-Anton E, Ruijsink B, Baumgartner CF, Masci PG, Sinclair M, Konukoglu E, Razavi R, King AP. Automated quantification of myocardial tissue characteristics from native T1 mapping using neural networks with uncertainty-based quality-control. J Cardiovasc Magn Reson. 2020;22:60. doi: 10.1186/s12968-020-00650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vergani V, Razavi R, Puyol-Anton E, Ruijsink B. Deep learning for classification and selection of cine CMR images to achieve fully automated quality-controlled CMR analysis from scanner to report. Front Cardiovasc Med. 2021;8:742640. doi: 10.3389/fcvm.2021.742640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruijsink B, Puyol-Anton E, Oksuz I, Sinclair M, Bai W, Schnabel JA, Razavi R, King AP. Fully automated, quality-controlled cardiac analysis from CMR: validation and large-scale application to characterize cardiac function. JACC Cardiovasc Imaging. 2020;13:684–695. doi: 10.1016/j.jcmg.2019.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. doi: 10.1186/1532-429X-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baessler B, Schaarschmidt F, Dick A, Stehning C, Schnackenburg B, Michels G, Maintz D, Bunck AC. Mapping tissue inhomogeneity in acute myocarditis: a novel analytical approach to quantitative myocardial edema imaging by T2-mapping. J Cardiovasc Magn Reson. 2015;17:115. doi: 10.1186/s12968-015-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Robison S, Karur GR, Wald RM, Thavendiranathan P, Crean AM, Hanneman K. Noninvasive hematocrit assessment for cardiovascular magnetic resonance extracellular volume quantification using a point-of-care device and synthetic derivation. J Cardiovasc Magn Reson. 2018;20:19. doi: 10.1186/s12968-018-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.CMR Imaging Protocol—details on imaging sequences and acquisition.
Additional file 2. Clinical Reporting of CMR findings in COVID-19—Image analysis.
Data Availability Statement
Not applicable.